
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci. , 11 December 2017
Sec. Virology
Volume 8 - 2017 | https://doi.org/10.3389/fpls.2017.02093
This article is part of the Research Topic Update on Plant Virus Infection - a Cell Biology Perspective View all 13 articles
Regulation of post-transcriptional gene expression on mRNA level in eukaryotic cells includes translocation, translation, translational repression, storage, mRNA decay, RNA silencing, and nonsense-mediated decay. These processes are associated with various RNA-binding proteins and cytoplasmic ribonucleoprotein complexes many of which are conserved across eukaryotes. Microscopically visible aggregations formed by ribonucleoprotein complexes are termed RNA granules. Stress granules where the translationally inactive mRNAs are stored and processing bodies where mRNA decay may occur present the most studied RNA granule types. Diverse RNP-granules are increasingly being assigned important roles in viral infections. Although the majority of the molecular level studies on the role of RNA granules in viral translation and replication have been conducted in mammalian systems, some studies link also plant virus infection to RNA granules. An increasing body of evidence indicates that plant viruses require components of stress granules and processing bodies for their replication and translation, but how extensively the cellular mRNA regulatory network is utilized by plant viruses has remained largely enigmatic. Antiviral RNA silencing, which is an important regulator of viral RNA stability and expression in plants, is commonly counteracted by viral suppressors of RNA silencing. Some of the RNA silencing suppressors localize to cellular RNA granules and have been proposed to carry out their suppression functions there. Moreover, plant nucleotide-binding leucine-rich repeat protein-mediated virus resistance has been linked to enhanced processing body formation and translational repression of viral RNA. Many interesting questions relate to how the pathways of antiviral RNA silencing leading to viral RNA degradation and/or repression of translation, suppression of RNA silencing and viral RNA translation converge in plants and how different RNA granules and their individual components contribute to these processes. In this review we discuss the roles of cellular RNA regulatory mechanisms and RNA granules in plant virus infection in the light of current knowledge and compare the findings to those made in animal virus studies.
Endogenous mRNAs produced in the nucleus of eukaryotic cells are subsequently exported to the cytoplasm for protein synthesis. There they are exposed to a complex RNA regulatory network. This network is able to adjust the amount of individual mRNAs within the total RNA population and to keep translationally active and inactive mRNAs in a delicate balance. Regulation of RNA allocation and turnover concerns not only mRNAs but also other RNA types in the cells including intruding viral RNAs (vRNAs). vRNAs possess features of aberrant RNAs which are often recognized as targets of the RNA decay machinery. Typically virus infection is initiated by the production of viral replication proteins and establishment of the replication complex in which progeny genomes and subgenomic RNAs (sgRNAs) are transcribed. Newly synthesized vRNAs and sgRNAs are translated into various viral proteins which carry out all viral functions together with compatible host factors. They also fight against the host's defense pathways. Therefore, to achieve a robust and productive infection, the vRNA molecules need to be able to cope with the cellular degradation and translation machineries. The eukaryotic RNA regulatory network including antiviral defense involves several complex pathways that have been shown to interact, compete and overlap on many levels (Figure 1) and altogether have a central role in maintaining cellular homeostasis. This review aims to illuminate the role of the RNA regulatory network in plant virus infection from different angles. We will overview the essential processes, cover the sites for these processes, the RNA granules, and highlight the ways some plant viruses modify and take advantage of the RNA regulatory network and its components.
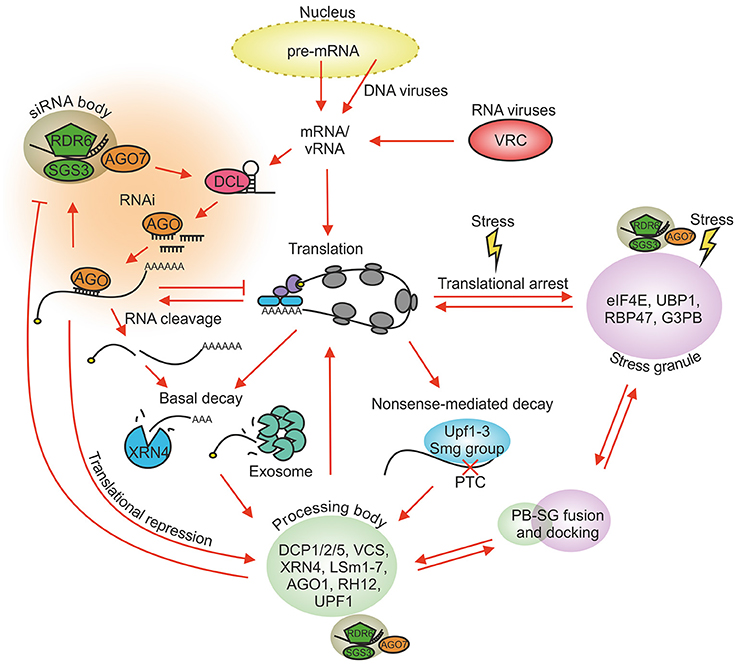
Figure 1. Major RNA regulatory processes in plant cells. Host mRNAs carrying the 5′ cap and the 3′ poly(A) tail are recruited to ribosomes for translation. vRNAs have evolved means to regulate host translation for their benefit. Various cellular stresses create SGs and increase the size and number of PBs. Translationally stalled pre-initiation complexes condense into SGs and can be released back to translation when stress conditions disappear. RNAs lacking the 5′ cap and the 3′ poly(A) tail are targeted for decay whereas translationally repressed RNAs can be redirected from PBs back to translation. PBs and SGs dock, fuse and exchange material. vRNAs and mRNAs recognized by the RNA silencing and NMD machineries are targeted for degradation. The RNA silencing functions are partially located to siRNA bodies and NMD to PBs. siRNA bodies can associate with PBs and SGs during stress. Only the main components of each granule type are shown. PB, processing body; SG, stress granule; NMD, nonsense-mediated decay; PTC, premature termination codon; VRC, virus replication complex.
Functional endogenous mRNAs carrying the 5′ cap and the 3′ poly(A) tail are in general stable and translatable. They all have their individual turnover rates, some being long-lived and some taken to degradation within minutes. A constant exchange of protein factors within the messenger ribonucleoprotein (mRNP)-complexes assembling around the transcripts ensures the correct fate for each mRNA molecule during their dynamic life span (Mitchell and Parker, 2014). mRNA decay is typically initiated by shortening of the 3′ poly(A) tail by deadenylases and removal of the 5′ cap by decapping protein 2 (DCP2). The core mRNA decay machinery involves 5′–3′ decay by an XRN family exoribonuclease and 3′–5′ degradation by the exosome, which is a protein complex combining both exo- and endoribonuclease activities (reviewed in Garneau et al., 2007). The interaction and aggregation domains of the mRNA binding proteins enable the assembly of mRNPs into higher-order structures (Mitchell and Parker, 2014). When these assemblies become visible by light microscopy they are called RNA granules. Deadenylation, decapping, and degradation activities coalesce in RNA granules known as processing bodies (PBs; reviewed in Eulalio et al., 2007a). While PB components are indispensable for mRNA decay, aggregation into microscopically visible PBs is not a prerequisite for their function, also smaller entities can function in the diffuse cytoplasm (Eulalio et al., 2007b). Arabidopsis XRN exoribonucleases participate not only in mRNA decay but also in the degradation and processing pathways of rRNAs, miRNAs and other small RNAs (reviewed in Kurihara, 2017) and in vRNA degradation. Overexpression of XRN1 leads to enhanced degradation of tomato bushy stunt virus (genus Tombusvirus) RNA in yeast and cucumber necrosis virus (genus Tombusvirus) RNA in Nicotiana benthamiana (Cheng et al., 2007), whereas XRN4 knock-down facilitated systemic tobacco mosaic virus (genus Tobamovirus) infection in N. benthamiana (Peng et al., 2011). Based on these results XRN4 can be proposed to contribute to the antiviral defense in planta.
In eukaryotic organisms endogenous aberrant mRNAs containing premature in-frame stop codons are caught by the cellular quality control and degradation pathway known as nonsense-mediated decay (NMD; reviewed in He and Jacobson, 2015). The helicase Up frameshift 1 (UPF1) is a key component of the NMD machinery. In addition the complex comprises UPF2, UPF3 and at least one member of the Smg-group of proteins recruited to the activated complex to mediate target mRNA degradation. NMD complexes form co-translationally but also after the translocation of the targeted transcript to PBs for decay (Sheth and Parker, 2003). In addition to its surveillance function NMD functions in cellular stress responses. Many viruses express part of their open reading frames from sgRNAs and because of this expression strategy the genomic RNAs contain stop codons followed by atypically long 3′UTRs. Such vRNAs can be recognized and restricted by NMD not only in animals but also in plants. Accordingly, an increase in potato virus X (PVX; genus Potexvirus) and turnip crinkle virus (genus Carmovirus) infection was detected in N. benthamiana plants expressing transiently a dominant negative mutant UPF1 protein (Garcia et al., 2014). These two viruses belong to different superfamiles, PVX to Alphavirus-like and turnip crinkle virus to Flavivirus-like viruses. Studies with another (+)RNA virus from the Alphavirus-like superfamily, the human pathogen Semliki Forest virus (genus Alphavirus), indicated that depletion of NMD components UPF1, Smg5 and Smg7 increase the level of replication and viral protein production in human cells (Balistreri et al., 2014). Some (+)RNA virus genera, such as potyviruses, belonging to Picornavirus-like superfamily, lack sgRNAs and other NMD-eliciting signals from their genomes and therefore are most likely not recognized by this pathway (Garcia et al., 2014). NMD was proposed to affect vRNA early in the infection when RNA silencing is not yet induced and to be later inhibited by the infection with the aid of a viral protein or by some other cis- and trans-acting factors suppressing NMD (Garcia et al., 2014). In line with this suggestion, the C-terminal part of the Semliki Forest virus nsP3 protein was found to be required for protection of vRNA against UPF1-mediated degradation (Balistreri et al., 2014). Further investigations are required to elucidate the viral mechanisms for avoiding recognition and degradation of vRNA by NMD.
The third pathway actively in use in planta to regulate gene expression and RNA stability is RNA silencing (reviewed recently in Csorba et al., 2015). This evolutionarily conserved mechanism in eukaryotes is triggered by double-stranded RNAs (dsRNA). Although RNA silencing contributes to many cellular functions, antiviral defense has been proposed to be its indigenous function (Pumplin and Voinnet, 2013). RNA silencing involving endonucleolytic cleavage and/or translational repression of the target RNAs is regulated by small RNAs (sRNAs) consisting of many types of 20–24 nucleotide-long dsRNA molecules. sRNAs produced during viral infection, viral small interfering RNAs (vsiRNAs), restrict the corresponding virus in a sequence-specific manner. VsiRNA production is catalyzed by DICER-LIKE (DCL) endonucleases and dsRNA-binding (DRB) proteins. During the effector step of RNA silencing vsiRNAs form RNA-induced silencing complexes (RISCs) with ARGONAUTE (AGO) proteins to cleave or translationally repress the target RNAs guided by the complementarity of the vsiRNA (Figure 1). Plant-specific RNA-dependent RNA polymerases (RDRs) together with cofactors SUPPRESSOR OF GENE SILENCING 3 (SGS3) and SILENCING DEFECTIVE 5 convert single-stranded RNA to double-stranded RNA (dsRNA) for their subsequent cleavage to siRNAs. RDR6 and SGS3 contribute also to the amplification step of RNA silencing by catalyzing dsRNA synthesis from the RISC cleavage products. RDR6, SGS3, and AGO7, which function in trans-acting siRNA (tasiRNA) synthesis, all have roles in plant defense against virus infections (Mourrain et al., 2000; Qu et al., 2008) and they aggregate in cytoplasmic bodies called siRNA bodies (Jouannet et al., 2012; Moreno et al., 2013). As RNA silencing is the major plant antiviral mechanism all plant viruses need to have mechanisms to overcome it. Viral suppressors of RNA silencing VSRs have been identified from both plant DNA and RNA viruses and they provide protection for vRNA by multiple mechanisms including sequestration of vsiRNAs to prevent RISC assembly and by interfering with DCL and RISC activities, AGO loading, systemic silencing and amplification of silencing (Csorba et al., 2015).
In contrast to the vast knowledge about RISC-mediated slicing of vRNA, much less is known about sRNA-guided repression of vRNA translation in plants. RISC-mediated translational repression by endogenous miRNAs is common in plants (Brodersen et al., 2008) and, similarly to the situation in animal cells, requires the association of sRNAs, their mRNA targets and AGO proteins with ribosomes (Lanet et al., 2009; Reynoso et al., 2013; Fatyol et al., 2016). sRNA-mediated translational repression may also restrict vRNA translation in planta. sRNA with a perfect complementarity to a target sequence in the 5′ untranslated region (UTR) of the tobacco etch virus (genus Potyvirus) inhibited translation (Iwakawa and Tomari, 2013). In this study the tobacco etch virus 5′UTR was part of a monocistronic reporter gene mRNA leaving the role of RISC-mediated translation repression in plant virus infection for future studies. AGO1 association with ribosomes is held as a hallmark of translational repression (Lanet et al., 2009). As HCPro, the RNA silencing suppressor of potyviruses, and AGO1 interact and associate with ribosomes in planta it was proposed that HCPro may counteract translational repression during potyvirus infection (Ivanov et al., 2016). AGO1 is involved in translational repression of RNA2 associated with recovery of Nicotiana benthamiana from tomato ringspot virus infection (Ghoshal and Sanfaçon, 2014; Karran and Sanfacon, 2014). Recovery from tobacco rattle virus infection which normally occurs in Arabidopsis does not take place in the presence of a strong VSR, P38, encoded by turnip crinkle virus, which also suggests involvement of RNA silencing in the translational repression of viral RNA in the recovered plants (Ma et al., 2015).
The cellular RNA regulatory network is able to react to various conditions such as oxidative and heat stress and nutrient depletion by reducing overall translational rate and storing selected mRNAs for further use after the cell has been released from stress. Translational shutdown is thought to be an ancient mechanism that enables cells to selectively redirect resources to survival in adverse conditions. In animal cells stress-induced phosphorylation of the eukaryotic initiation factor eIF2α is a central trigger for the formation of subcellular structures called stress granules (SGs). SGs are sites where the already assembled ribosomal 43S and 48S pre-initiation complexes are relocated for storage (reviewed in Lloyd, 2013). Among the kinases phosphorylating eIF2α during cellular stress in animal cells are general control nondepressible 2 kinase (GCN2), double-stranded RNA (dsRNA)-activated protein kinase R (PKR) and PKR-like endoplastic reticulum kinase (PERK). eIF2α phosphorylation interferes with eIF2α-GDP recycling leading to compromised availability of eIF2α-GTP-Met-tRNAiMet and an overall reduction of translation (Kedersha et al., 1999, 2002; Kimball et al., 2003). The ensuing translational shutdown rapidly triggers the aggregation of halted pre-initiation complexes into SGs for sorting and temporary storage. In planta eIF2α phosphorylation is induced e.g., upon UV-induced stress (Meteignier et al., 2016) and wounding (Lageix et al., 2008), by activation of GCN2 kinase in the latter case. Activation of PKR and subsequent eIF2α phosphorylation is a common antiviral response leading to global translational repression during many animal virus infections (see examples in Lloyd, 2013), but there is no direct evidence for PKR-mediated antiviral defense in plants. A mammalian protein P58, which contains several tetratricopeptide repeat motifs and a conserved J domain of the DnaJ chaperone, acts as an inhibitor of PKR. It is recruited by the influenza virus to limit PKR-mediated antiviral responses (Melville et al., 2000). Similarly to the mammalian P58 protein its plant homolog P58IPK was proposed to regulate PKR- or PERK-like kinases during virus infection (Bilgin et al., 2003). An increase in eIF2α phosphorylation was detected in P58IPK-silenced N. benthamiana plants upon virus infection (Bilgin et al., 2003), but apart from this study the evidence for a PKR-mediated antiviral response is non-existing. No sequence homologs of the animal PKR can be identified from the N. benthamiana genome. No eIF2α phosphorylation could be detected either in connection to PVX infection or during resistance gene-mediated antiviral defense response against PVX associated with repression of viral translation in N. benthamiana (Meteignier et al., 2016). Furthermore, eIF2α phosphorylation was not detected either in turnip crinkle virus or turnip yellow mosaic virus-infected Arabidopsis (Zhang Y. et al., 2008). Although evidence for involvement of SG components in plant virus infection exist (Hafrén et al., 2015; Krapp et al., 2017), the molecular basis of SG induction during virus infection is an uncovered area in planta.
Many RNA regulatory processes share common protein factors and often co-localize to the same subcellular sites. In addition to the mRNA decay-associated proteins PBs contain proteins involved in mRNA surveillance (NMD-associated proteins), RNA silencing-associated proteins and proteins regulating translational repression (Eulalio et al., 2007a). A spatial overlap of the RNA silencing proteins AGO1 and SILENCING DEFECTIVE 3 within Arabidopsis PBs was demonstrated (Xu and Chua, 2011). In animal cells PBs assemble upon induction of gene silencing and knock-down of the AGO proteins prevents PB formation (Eulalio et al., 2007b). The mechanisms of mRNA decay and RNA silencing are functionally coupled also in planta (Gazzani et al., 2004). The relationship is antagonistic since these pathways compete for the same RNA substrates lacking the 5′ cap or the 3′ poly-A signal (Moreno et al., 2013; Martinez de Alba et al., 2015). These RNA molecules can either undergo exonucleolytic RNA decay or re-enter the RNA silencing pathway through RDR6-catalyzed dsRNA synthesis. It has been proposed that RNA decay can prevent the initiation of threshold-dependent RNA silencing by degrading ssRNA substrates before aberrant RNAs accumulate to critical levels (Garcia et al., 2014). Indeed, many proteins of the RNA quality control machinery, involved both in 5′–3′ and 3′–5′ RNA degradation, have been identified as endogenous RNA silencing suppressors. For example, in Arabidopsis the PB components DCP1, DCP2, and VARICOSE (VCS) have been shown to suppress RDR6-dependent post-transcriptional gene silencing (PTGS) (Thran et al., 2012; Martinez de Alba et al., 2015). Endogenous silencing suppression activity has also been shown for Arabidopsis FIERY1, XRN2, and XRN3 (Gy et al., 2007) and the exosome core subunits RRP4 and RRP41 or exosome co-factors RRP44A, RRP6L1, and HEN2 (Moreno et al., 2013; Lange et al., 2014; Hematy et al., 2016). Mutations in XRN4 (Gazzani et al., 2004) or DCP2 (Thran et al., 2012) enhance PTGS. Furthermore, it has been shown that a dysfunctional decapping pathway leads to RDR6-dependent production of siRNAs against endogenous protein encoding mRNAs, causing considerable changes in the transcriptome. And in turn, Arabidopsis PTGS mutants were able to rescue the drastic transcriptome alterations caused by the dysfunctional mRNA decay pathway (Martinez de Alba et al., 2015). Garcia et al. (2014) also suggested for exogenous RNAs that saturation of NMD by vRNAs may switch the regulation toward RDR action and siRNA-mediated silencing of viral transcripts.
Plant protein ASYMMETRIC LEAVES 2 (AS2) is a PB component and an activator of DCP2-mediated decapping. It also inhibits siRNA production and functions as an endogenous PTGS suppressor (Ye et al., 2015). AS2 expression is upregulated by the cabbage leaf curl virus (genus Begomovirus) nuclear shuttle protein BV1 which also causes AS2 to exit the nucleus. In the cytoplasm AS2 activates decapping by DCP2, reduces siRNA production, weakens RNAi and promotes virus infection (Ye et al., 2015; Figure 2A). The authors proposed that this strategy could be used also by other viruses to weaken antiviral defense both in plant and animal hosts. A recent work (Conti et al., 2017) supports this proposal by providing evidence that both tobacco mosaic virus coat protein and movement protein may have evolved to interfere with RNA decay by increasing it in order to down-regulate RNA silencing against its genomic RNA.
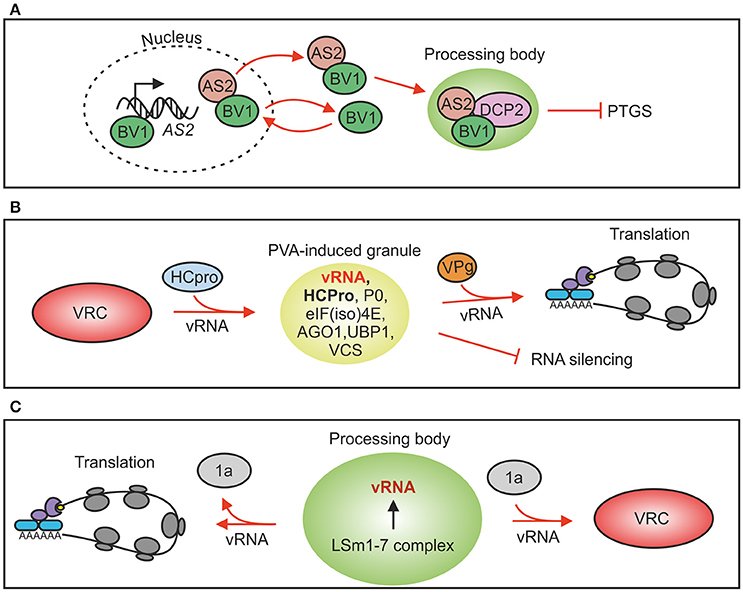
Figure 2. Plant virus interactions with RNP granules. (A) Cabbage leaf curl virus nuclear-cytosol shuttle protein BV1 can bind to the promoter region of AS2 gene and induce its expression. AS2 is transported with BV1 to PBs where it activates DCP2-mediated decapping. Increased RNA decay down-regulates RNA silencing and consequently virus infection gets advantage. (B) Potato virus A silencing suppressor protein HCPro induces formation of RNP granules that contain viral RNA, ribosomal protein P0 and several PB and SG markers. Viral protein genome-linked, VPg, abolishes PVA-induced granules and increases viral translation. Evidence to support silencing suppression-related PG functions exists. (C) Brome mosaic virus genomic RNAs 2 and 3 contain motifs for binding to the decapping activator LSm1-7 complex in PBs of yeast. This interaction is required both for brome mosaic virus translation and replication. In the absence of viral protein 1a vRNA is subjected to translation whereas in its presence vRNA is recruited to replication.
In sense RNA-mediated silencing in planta RdRs copy ssRNA to dsRNA, which are subsequently process into siRNAs by DCLs. This process takes place in cytoplasmic siRNA bodies (Fukunaga and Doudna, 2009; Kumakura et al., 2009). Interestingly, upon heat stress the number of siRNA bodies increases remarkably and these structures become positive for SG markers in addition to the SGS3 and AGO7 markers (Jouannet et al., 2012). This suggests that mRNAs stalled in translation may accumulate in cytoplasmic foci representing fusions between SGs and siRNA bodies. The tobacco etch virus 6K2 protein is an inducer of viral replication complex formation (Schaad et al., 1997). A partial (60%) spatial overlap of the tobacco etch virus 6K2 and AGO7 was detected proposing a link between potyviral replication-associated membranous structures and siRNA bodies (Jouannet et al., 2012). This finding could represent a point of convergence between viral replication and the host's defense mechanisms.
Rather than being fixed entities SGs have emerged as dynamic structures that actively exchange both proteins and mRNPs with the cellular environment (Mollet et al., 2008; Weber et al., 2008; Sorenson and Bailey-Serres, 2014). SGs could be the first location for mRNAs after translation has halted since mRNAs in SGs are still associated with translation initiation factors, but based on studies in yeast it is also possible that mRNPs from PBs may enter SGs (Buchan and Parker, 2009). Translationally inactive mRNAs together with their associated proteins and regulatory RNAs accumulates into PBs. The eukaryotic mRNA cycle model about sorting mRNAs between translation, storage and degradation in SGs and PBs, termed the mRNA cycle, was set forth by Balagopal and Parker (2009). PBs and SGs have been shown to fuse in animal cells (Kedersha et al., 2005; Thomas et al., 2011; Buchan et al., 2013), which suggests that markers commonly distinguishing PBs from SGs may overlap in RNA granules formed under certain stress conditions. For example potato virus A (PVA; genus Potyvirus) -induced RNA granules contain both OLIGOURIDYLATE BINDING-PROTEIN 1 (UBP1) of plant SGs and VCS and AGO1 of PBs (Hafrén et al., 2015) showing that these granules cannot be classed unambiguously into PBs or SGs but that they have a unique composition defined by PVA infection (Figure 2B). The main distinction between SGs and PBs is that SGs contain translation factors and PBs mRNA decay factors. These examples highlight the wide crosstalk between regulatory pathways and active exchange of protein components between the associated subcellular structures.
More than 70 different proteins have been identified in the SGs and over 20 in the PBs of eukaryotic cells. In addition many proteins are shared between different RNA granule types in yeast and mammals (Poblete-Durán et al., 2016). Although it is evident that plant, yeast and animal PBs and SGs have similar functions (Xu et al., 2006; Weber et al., 2008; Xu and Chua, 2009; Sorenson and Bailey-Serres, 2014), the composition of RNA granules is currently less studied in plants. Nevertheless, several hallmark-proteins have been identified from plant SGs, including UBP1, eukaryotic initiation factor 4E (eIF4E), poly-A binding protein (PABP), and PBs, including DCP1, DCP2, VCS, and AGO1 (Xu et al., 2006; Weber et al., 2008; Sorenson and Bailey-Serres, 2014).
T-cell intracellular antigen 1 (TIA-1), Ras-GAP SH3 domain-binding protein (G3BP), and tristetraprolin (TTP) are among the RNA-binding proteins promoting SG formation in animal cells (reviewed in Kedersha et al., 2013). In plants UBP1, RNA-binding proteins 45 and 47 (RBP45/47), and PABP represent proteins related to animal TIA-1 (Sorenson and Bailey-Serres, 2014). TIA-1's capacity to promote SG formation in animal cells depends on the phosphorylation of eIF2α as well as its high-affinity RNA-binding domain and a glutamine-rich prion-like domain with self-aggregation properties (Kedersha et al., 1999; Gilks et al., 2004). Likewise plant UBP1 contains several RNA-binding domains and a prion domain which are essential for SG aggregation. In Arabidopsis the removal of these domains from UBP1 or RBP47 crippled the assembly of SGs (Weber et al., 2008) and as can be expected from a SG protein, plant UBP1 is a protein mainly associated with translationally silent mRNAs (Sorenson and Bailey-Serres, 2014). Recent studies in plant systems have highlighted UBP1's functional role in stress-related post-transcriptional gene regulation. For example in low oxygen conditions UBP1 preferentially bind translationally repressed mRNAs selectively aggregating them into SGs. Transcript residence time in SG's is brief and once oxygen is resupplied halted pre-initiation complexes are rapidly recycled into actively translating polysomes (Sorenson and Bailey-Serres, 2014).
Adding to a foundation built on eIF4E, UBP1, RBP47, TTP, and G3BP, several plant proteins have been identified as potential SG components based mainly on their stress-induced co-localization into cytoplasmic foci with one or more of the traditional SG markers (Table 1). Recent examples include members of the Arabidopsis tandem CCCH zinc finger protein family (Pomeranz et al., 2010; Bogamuwa and Jang, 2013, 2016; Qu et al., 2014), a stress-responsive C-terminal binding protein ANGUSTIFOLIA (Bhasin and Hulskamp, 2017), the CML38 calcium sensor (Lokdarshi et al., 2016) and an RNA-binding TUDOR-SN homolog which is important for the stabilization of transcripts encoding stress-related proteins (Yan et al., 2014; Krapp et al., 2017).
In Arabidopsis the decapping complex comprises DCP1, DCP2 (TDT), DCP5, VCS, and possibly DEA(D/H)-box RNA helicase DHH1/AtRH12 (Xu et al., 2006; Xu and Chua, 2009). Human protein Ge-1/Hedls, an enhancer of mRNA decapping and a component of PBs, is thought to provide a scaffold for the decapping complex comprising DCP2 and the decapping co-activators DCP1, DEAD-box protein RCK/p54 and enhancer of decapping 3 (Fenger-Gron et al., 2005). VCS is the plant homolog of Ge-1/Hedls and serves as potential hub for protein-protein interactions in plants as well. VCS contains repeating WD-40 domains which typically coordinate the assembly of multiprotein complexes where they serve as scaffolds for protein-protein interactions. In PBs VCS's main role is to facilitate the interaction between the catalytic subunit DCP2 and its coactivator DCP1 (Xu et al., 2006). Similarly to animals VCS is also required for miRNA-guided translational repression in Arabidopsis (Brodersen et al., 2008). The plant-specific SUO is another PB-localized protein involved in translational repression (Yang et al., 2012). The SUO protein contains GW-repeats which potentially mediate interactions with AGO proteins that are central for miRNA-mediated translational repression. Mutations in SUO GW-repeats increased the abundance of several endogenous proteins without affecting their mRNA levels indicating release from translational repression (Yang et al., 2012). The functions of the PB-associated proteins thus suggest a role for PBs both in mRNA decapping and translational repression also in plants.
VCS/Ge1 appears to be an integral host factor for achieving virus infection. PVA infection redirects host VCS into RNA granules together with vRNA. The depletion of VCS disabled viral gene expression and reduced the amount of vRNA in infected cells suggesting that PVA uses VCS to its advantage (Hafrén et al., 2015). A similar effect was reported from human liver cells infected with Hepatitis C Virus: Ge-1 was required for optimal accumulation of vRNA and proteins (Pager et al., 2013).
In addition to the core components efficient decapping requires accessory proteins that associate with PBs to enhance or regulate mRNA turnover. In animals and plants alike the evolutionarily conserved Sm-like proteins (LSm1-7) form a heptameric structure that requires a protein-associated with topoisomerases (PAT1) to form a bridge between deadenylated mRNA and the decapping complex (Tharun, 2009; Golisz et al., 2013; Roux et al., 2015; Vindry et al., 2017). An Arabidopsis lsm5 mutant was sensitive to drought, heat and salt stress (Golisz et al., 2013; Okamoto et al., 2016) while a pat1 mutant displayed a constitutively activated innate immune response via an R-protein (Roux et al., 2015) emphasizing their considerable importance in optimal stress responses. The role of the tandem zinc finger protein TTP is not limited to SGs as it also promotes the assembly of PBs and activates decapping (Bogamuwa and Jang, 2016). Protein constituents of PBs in plant cells and their animal homologs are listed in Table 2.
Multifunctional G3BPs participate in SG assembly upstream of eIF2α phosphorylation. In animal cells stress-induced dephosphorylation of S149 in G3BP causes a conformational change enabling homo- and heteromultimerization with G3BP2 (Tourriere et al., 2003; Matsuki et al., 2013). Oligomerization of G3BPs causes the nucleation of SGs even in the absence of stress suggesting it might be a prerequisite for the eventual assembly of other SG components (Matsuki et al., 2013). SGs are regarded as antiviral compartments and many viruses have developed means to disrupt SG assembly via G3BP interactions. Some viruses, such as vaccinia virus and hepatitis C virus, have co-opted G3BP to serve as a potential component of viral factories and replication complexes (Yi et al., 2006; Katsafanas and Moss, 2007) or an assisting factor for virion assembly (Garaigorta et al., 2012). Polioviral 3C protease cleaves G3BP leading to the formation of SGs without G3BP (White et al., 2007; Piotrowska et al., 2010). A G3BP-like protein localizing to plant SGs was recently discovered in Arabidopsis. AtG3BP was identified through its interaction with a viral protein, the nuclear shuttle protein 3 (NSP3) of the abutilon mosaic virus (genus Begomovirus), in SGs (Krapp et al., 2017). The interaction between G3BP and viral proteins was mapped to a conserved FGDF-type motif. Also in animal cells G3BP has been shown to associate with the C-terminal FGDF motifs of ns3 protein of the alphaviruses Semliki Forest virus and Chikungunya virus (Panas et al., 2014; Scholte et al., 2015). The FGDF motif is relevant for G3BP-interactions of the host proteins as well (Panas et al., 2015). Interestingly, FGDF motifs are present in the proteases of potyviruses, waikaviruses, and closteroviruses suggesting that SG regulation via G3BP interactions may have greater influence in plant virus infections than currently known.
Animal RNA viruses make use of TIA1, the homolog of UBP1. A recent report showed that the knockdown of TIA-1 impaired the accumulation of Newcastle disease virus (family Paramyxovirdae) in HeLa cells (Sun et al., 2017). Other viruses such as the Dengue virus (genus Flavivirus) and Poliovirus (PV; genus Enterovirus) have evolved a different strategy: viral proteins sequester TIA1 so that it cannot form functional SGs. The Dengue virus interferes with SG formation by using nonstructural protein NS1 to sequester TIA1 to the perinuclear space (Xia et al., 2015). Likewise the poliovirus disrupts host defense by aggregating TIA1 into nonfunctional foci (White and Lloyd, 2011; Fitzgerald and Semler, 2013).
Nicotiana benthamiana UBP1 was identified as a component of PVA-induced RNA granules (Hafrén et al., 2015). Although these granules do not represent canonical SGs, UBP1 is important for the formation of PVA-induced granules and seems to be one of the many host factors PVA employs to ensure a successful infection. Potyviral genome-linked protein VPg specifically promotes PVA translation (Eskelin et al., 2011; Hafrén et al., 2013). Interestingly, the granulation-prone UBP1 does not promote PVA translation together with VPg, whereas many other components co-localizing to PVA-induced granules, e.g., VCS and eIF(iso)4E, do. The role of these granule proteins in PVA translation in turn propose a link between the granules and viral translation. Supporting this, VPg is able to disrupt the PVA-induced granules while promoting translation (Hafrén et al., 2015). Additional evidence suggested a functional link between granule formation and RNA silencing suppression. PVA-induced granules were neither formed in the absence of potyviral silencing suppressor HCPro nor by HCPro silencing suppression-deficient mutants suggesting that PVA-induced granules could be the sites for HCPro-guided silencing-suppression function (Hafrén et al., 2015). Similar granule-like foci, observed in the vicinity of the ER and the microtubule cytoskeleton, were induced by the HCPro of potato virus Y in Nicotiana benthamiana (del Toro et al., 2014).
Cysteine3Histidine (CCCH)-type zinc finger proteins and tandem zinc finger proteins (TZF) comprise a large protein family well conserved across eukaryotes. The PB and SG component Tristetraprolin (TTP) belongs to this protein family. It has a critical role in mRNA metabolism in recruiting enzymes involved in deadenylation, decapping, and exonucleolytic activities to specific mRNAs related to innate immunity and inflammatory responses in animals proposing a role in virus infection (Hamid and Makeyev, 2016). In plants the CCCH-motif is preceded by an arginine-rich (RR)-motif and therefore these proteins are called RR-TZF proteins (reviewed in Bogamuwa and Jang, 2014). Although plant RR-TZF are involved in biotic stress responses potentially by binding to specific elements of the corresponding mRNAs and recruiting the assembled mRNP complexes into PBs and SGs, no report has linked them to regulation of virus infection in plant cells so far.
Although PBs have housekeeping functions and are always present in the cells, formation of visible PBs is activated for example as a consequence of RNA silencing leading to an increase in translationally repressed mRNAs in the cell (Eulalio et al., 2007b). PB assembly upon induction of gene silencing was shown in plants using an RNA hairpin targeting one specific transcript, the Sulphur mRNA (Meteignier et al., 2016) and in connection to virus recovery (Ma et al., 2015). Viruses can affect PBs either by dispersing PB structures and components or by interfering with the mechanism of PB formation. For example poliovirus and human Coxsackie virus, both belonging to family Picornaviridae, interfere with PB formation during their replication cycle (reviewed in Malinowska et al., 2016). In addition, polioviral 3C protease processes XRN1 and DCP1 (Dougherty et al., 2011). Some viruses exploit PB components for their benefit. The LSm1-7 complex, which is an activator of decapping in the 5′–3′ exoribonucleolytic pathway (Tharun et al., 2000) and a component of PBs (Ingelfinger et al., 2002), is a central regulator of brome mosaic virus (genus Bromovirus) translation and replication (Galao et al., 2010). Studies in yeast revealed that the cis-acting RNA replication signals within RNA 2 and RNA3 were found to direct these brome mosaic virus RNAs into PBs (Beckham et al., 2007). PBs create an environment which lacks the components of the translation machinery and subsequently may facilitate replication complex assembly. On this basis, a role for PBs in the transition of brome mosaic virus RNAs from translation to replication was suggested in Galao et al. (2010; Figure 2C).
Plant antiviral defense based on the PB component AGO1 and its slicer activity can be vulnerable to viral silencing suppressors. For example Arabidopsis AGO1 has been shown to be a target of the 2b silencing suppressor of the cucumber mosaic virus (genus Cucumovirus). Cucumber mosaic virus 2b co-localizes with AGO1 to PBs and directly blocks AGO1's slicer activity both in vitro and in vivo (Zhang et al., 2006). AGO1 inactivation is mediated also by the turnip crinkle virus capsid P38 which can mimic AGO-binding GW-repeat proteins (Azevedo et al., 2010) and sweet potato mild mottle virus (genus Ipomovirus) P1 protein which blocks target RNA binding to preassembled AGO1 (Kenesi et al., 2017). AGO1 degradation has been reported to be mediated by PVX P25 (Chiu et al., 2010), beet western yellows virus (genus Polerovirus) P0 (Baumberger et al., 2007; Bortolamiol et al., 2007) and tomato ringspot virus (genus Nepovirus) coat protein (Karran and Sanfacon, 2014). In spite of the important role of AGO2 in antiviral RNA silencing in planta (reviewed in Carbonell and Carrington, 2015), it is not known whether it associates e.g., with PBs or SGs. Different types of stresses direct human AGO2 to SGs (Detzer et al., 2011) and it is a component of mammalian PBs (Sen and Blau, 2005; Hubstenberger et al., 2017).
Some recent studies link the antiviral defense response both to translational repression and PBs. Recovery is a phenomenon during which host plants recover from viral symptoms. The amount of polysome-associated viral transcripts is reduced and the amount of PBs is increased in plants recovered from tobacco rattle virus (genus Tobravirus) infection proposing an important role for PBs in the elimination of vRNAs (Ma et al., 2015). Translational repression is involved also in recovery from tomato ringspot virus infection (Ghoshal and Sanfaçon, 2014). Activation of a plant nucleotide-binding site leucine-rich repeat (NB-LRR) protein by the tobacco mosaic virus p50 replicase protein fragment can induce an antiviral response against PVX and turnip crinkle virus in N. benthamiana (Bhattacharjee et al., 2009). During this antiviral response PVX transcripts are translationally repressed and the number of PBs increases significantly in the affected cells (Meteignier et al., 2016). Although the NB-LRR response, UV stress and RNAi against certain transcripts all induce translational repression and PB formation in plants, the mechanisms leading to enhanced PB assembly are distinct. While induction of RNAi increases the number of PBs and the presence of silencing suppressors like P19 inhibits PB induction, PB formation during the NB-LRR response against PVX is not dependent on RNA silencing (Meteignier et al., 2016). Regardless of the mechanism that promotes PB formation, their increased assembly most likely reflects the high concentration of translationally repressed mRNAs.
siRNA bodies are RNA granules associated with the synthesis of tasiRNA and the amplification of the RNA silencing signal. Plant proteins found in siRNA bodies are listed in Table 3. Many plant viruses interfere with siRNA body functions in order to suppress their contribution to siRNA production. Some viruses such as rock bream iridovirus (genus Megalocytivirus), a fish DNA virus, (Zenke and Kim, 2008) and sweet potato chlorotic stunt virus (genus Closterovirus; Kreuze et al., 2005), encode class I RNase III enzymes which have RNA silencing suppression capacity. Class 1 RNase III endoribonuclease (RNase3) of sweet potato chlorotic stunt virus contains a dsRNA binding domain and a Mg2+-dependent catalytic domain for dsRNA cleavage (Kreuze et al., 2005). Its expression in sweet potato as a transgene promotes the accumulation of sweet potato feathery mottle virus (genus Potyvirus; Cuellar et al., 2009). Sweet potato chlorotic stunt virus RNase3 co-localizes to siRNA bodies via interaction with SGS3 (Weinheimer et al., 2016). It is likely that the virus uses SGS3 to target its RNase3 to dsRNA synthesized by RDR6 to cleave them into sRNAs which are too small to be used in RNAi. The potyviral protein VPg is, among its other functions, a suppressor of sense-mediated silencing that localizes to siRNA bodies and interacts with SGS3 to benefit virus infection (Rajamäki et al., 2014; Cheng and Wang, 2017). The turnip mosaic virus VPg carries out its silencing suppression function by targeting SGS3 and its binding partner RDR6 directly to degradation via the 20S proteasome (Cheng and Wang, 2017). As a result the production of vsiRNA in the siRNA-bodies will be inhibited. SGS3 functions are disrupted also by other viruses. For example, tomato yellow leaf curl virus (genus Begomovirus) V2 protein interacts directly with SGS3. Mutational analysis showed that this interaction is required for V2 silencing suppression function (Glick et al., 2008). Plantago asiatica mosaic virus (genus Potexvirus) TGBp1 (P25) co-localizes to siRNA bodies and inhibits dsRNA synthesis by the SGS3/RDR6 complex (Okano et al., 2014). Furthermore, yeast two-hybrid and bimolecular fluorescence assays revealed that the p2 silencing suppressor protein of the rice stripe virus (genus Tenuivirus) interacts with SGS3 suggesting that it could act through inactivation of the RDR6/SGS3 pathway (Du et al., 2011).
Some viruses interfere with the RNAi amplification step by inhibiting RDR6 functions. For example, the βC1 protein of tomato yellow leaf curl China betasatellite (family Geminiviridae) up-regulates the expression of N. benthamiana calmodulin-like protein REGULATOR OF RNA SILENCING (Nbrgs-CaM) which is an endogenous silencing suppressor. In turn, up-regulation of Nbrgs-CaM leads to the suppression of RDR6 gene expression resulting in both PTGS suppression and symptom induction (Li et al., 2014). Downregulation of RDR6 mRNA accumulation is also achieved by the silencing suppressor HCPro of sugarcane mosaic virus (genus Potyvirus; Zhang X. et al., 2008). The P6 protein of rice yellow stunt rhabdovirus (genus Nucleorhabdovirus) interacts with rice and Arabidopsis RDR6 in vivo and affects the production of secondary siRNAs by RDR6 (Guo et al., 2013). In conclusion, interference with siRNA body functions to inhibit the RNAi amplification phase seems to be widely used by both RNA and DNA viruses.
The nucleocytoplasmic compartmentalization of eukaryotic cells paved the way to the development of an extensive post-transcriptional regulatory network at the level of RNA decay. In addition to the core mRNA decay machinery, regulation is provided by several sRNA pathways (siRNAs, miRNAs), NMD and mechanisms targeting RNAs displaying specific secondary structures. An interesting hypothesis of the exaptive origins of regulated mRNA decay in eukaryotes has been set forward by Hamid and Makeyev (2016). They propose that the emergence of RNAi, NMD, and mRNA decay based on the recognition of specific RNA structural elements to aid in non-self RNA recognition coincided with the expansion of RNA viruses. Only later these mechanisms adapted to regulate cellular mRNAs. Out of the currently recognized plant virus genera roughly 70 % represent (+)RNA viruses, 9% (-)RNA and 9% dsRNA viruses (Dolja and Koonin, 2011). RNA viruses are overrepresented in plants as only about 12% of plant virus genera are DNA viruses and retroelements and viroids still add to the number of pathogenic RNA molecules within plant cells. Taking into account the vast number of RNA viruses in plants it is likely that more discoveries linking plant virus infection, antiviral defense, innate immune signaling and cellular RNA biology will be made in the future. This is exemplified by a recent finding of a novel type of RNA-targeting antiviral defense by Drosha-type Class 2 RNase III nuclease (Aguado et al., 2017). Drosha, which has evolved to function in the biogenesis of miRNAs in eukaryotic organisms, and related RNase III enzymes are translocated into the cytoplasm upon RNA virus infection. There they elicit unique RNA-targeting antiviral activity against viruses belonging to families Togaviridae and Flaviviridae via binding to certain stem-loop structures on vRNA thus hindering the activity of the RNA-dependent RNA polymerases (Aguado et al., 2017). When a pre-miRNA sequence is nested into the flavivirus-like turnip crinkle virus RNA it also becomes processed by an RNase III activity indicating that this enzyme is translocated from the nucleus to the cytoplasm upon virus infection also in higher plants. The authors propose that this particular type of RNase III-mediated antiviral defense conserved in mammals, arthropods, fish and plants is independent of the other known eukaryotic antiviral defense mechanisms and may have preceded them. Furthermore, Martinez-Perez et al. (2017) recently discovered a novel mechanism that regulates plant viral infection via N6-methyladenosine (m6A) modifications of vRNA. Human immunodeficiency virus (genus Lentivirus) and hepatitis C virus (genus Hepacivirus) use this mechanism to regulate viral gene expression and infection (Tirumuru et al., 2016) and infectious particle production (Gokhale et al., 2016), respectively. The m6A abundance on alfalfa mosaic virus (genus Alfamovirus) genomic RNA is regulated by Arabidopsis AtALKBH9B, which carries m6A demethylase activity (Martinez-Perez et al., 2017). Its silencing restricts the alfalfa mosaic virus infection. In vivo AtALKBH9B co-localizes with siRNA body component SGS3 and the NMD component UPF1 and shows a partial association with the PB component DCP1 both in healthy and infected N. benthamiana tissues. An NMD-mediated regulation of alfalfa mosaic virus infection was proposed by the authors. Infection by another bromovirus, the cucumber mosaic virus, was not affected by the knockdown of AtALKBH9B. Interestingly, AtALKBH9B interacts with alfalfa mosaic virus coat protein whereas there is no interaction with the cucumber mosaic virus coat protein. Therefore, the authors found it possible that AtALKBH9B exerts its regulatory function on virus infection via coat protein interaction.
While many investigations have paid attention to the amplification of vRNA in replication complexes and post-replication fate of vRNA in relation to gene silencing, the virus-host interactions regulating the replicated vRNA through the rest of the cellular RNA regulatory network is a research area that only recently gained attention in plant biology. Viruses have evolved to regulate host RNA metabolism and SGs, PBs, siRNA bodies and other subcellular structures associated with it in order to maintain the cellular environment optimal for robust viral gene expression and avoid the elimination of vRNA. Apart from the core proteins, the protein composition of RNA granules formed by different plant viruses likely varies. Methods based on the enrichment of the RNA granules via centrifugation and subsequent affinity purification via antibodies or specific affinity tags have been developed for plant (Hafrén et al., 2015), animal and yeast (Wheeler et al., 2017) RNA granules. A recent study in which PBs were purified by fluorescence-activated particle sorting from human epithelial cells followed by characterization of the associated mRNAs revealed that PBs mainly accumulate translationally repressed but not decayed mRNAs (Hubstenberger et al., 2017), suggesting a more pronounced role for PBs in accumulating translationally repressed mRNAs and less so in mRNA decay than previously anticipated. This study shows the importance of biochemical purification of the RNA granules in obtaining more detailed information about their protein and RNA composition as well as functions, giving one possible direction were to go to understand also the pro- and antiviral activities associated with RNA granules of plant origin.
Even with the best studied animal viruses there are still substantial knowledge gaps in understanding the viral interactions with RNA granule components as discussed by Lloyd (2016). For example the molecular mechanisms how enteroviral proteases control the translational apparatus, SGs, PBs and the activation of innate immune response by cleaving their key regulatory components have been worked out extensively but many open questions related to the mechanistic roles of these key components in RNA granule assembly and mRNP recruitment as well as in innate immune activation still remain. While many SG, PB, and siRNA body proteins of plant origin are known and some plant viral interactions with them have been described there is much to discover in this insufficiently studied area. Similarly the ways how plant viruses utilize RNA granule components for their benefit, how plants defend themselves against viruses via RNA granules and how viruses counter the RNA granule-associated antiviral actions are likely versatile. As an example of these complex interactions, a recent study indicated the presence of an autophagy-associated cargo adaptor protein NBR1 in potyvirus-induced RNA granules in turnip mosaic virus infection (Hafrén et al., 2017). Bulk autophagy was found essential for plant fitness and virus accumulation, whereas an antiviral role was identified for selective NBR1-mediated autophagy targeting the RNA silencing suppressor HCPro. NBR1 co-localizes together with HCPro and AGO1 to granule structures which proposes that HCPro is likely targeted to the RNA granule context. Interestingly, a specific form of autophagy called granulophagy is targeting both SGs and PBs in yeast (Buchan et al., 2013). Potyvirus-induced granules, which contain both SG and BP markers (Hafrén et al., 2015), were proposed to be targeted by granulophagy (Hafrén et al., 2017). Potyviral proteins VPg and to a lesser extent 6K2 were found to resist autophagy-related HCPro turnover. The authors propose that these complex interactions between potyvirus-induced granules, potyviral proteins and autophagic degradation form another layer to the co-evolutionary arms race between potyviruses and their host plants. This example together with the others discussed in this review show that beyond RNA silencing and its counter defense there is a plethora of interactions connected to post-replication vRNA regulation in plant cells to be discovered during the coming years.
KM: Writing the text; AL: Writing the text, drawing the figures; MP: Writing the text, Compiling the tables.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the reviewers for their constructive and insightful comments which improved the outcome of this review and sincerely apologize to colleagues whose publications are not cited here. Financial support from the Academy of Finland (grant 298254) and from the Jane and Aatos Erkko Foundation to KM is gratefully acknowledged.
Aguado, L. C., Schmid, S., May, J., Sabin, L. R., Panis, M., Blanco-Melo, D., et al. (2017). RNase III nucleases from diverse kingdoms serve as antiviral effectors. Nature 547, 114–117. doi: 10.1038/nature22990
Azevedo, J., Garcia, D., Pontier, D., Ohnesorge, S., Yu, A., Garcia, S., et al. (2010). Argonaute quenching and global changes in Dicer homeostasis caused by a pathogen-encoded GW repeat protein. Genes Dev. 24, 904–915. doi: 10.1101/gad.1908710
Balagopal, V., and Parker, R. (2009). Polysomes, P bodies and stress granules: states and fates of eukaryotic mRNAs. Curr. Opin. Cell Biol. 21, 403–408. doi: 10.1016/j.ceb.2009.03.005
Balistreri, G., Horvath, P., Schweingruber, C., Zund, D., McInerney, G., Merits, A., et al. (2014). The host nonsense-mediated mRNA decay pathway restricts Mammalian RNA virus replication. Cell Host Microbe 16, 403–411. doi: 10.1016/j.chom.2014.08.007
Baumberger, N., Tsai, C. H., Lie, M., Havecker, E., and Baulcombe, D. C. (2007). The Polerovirus silencing suppressor P0 targets ARGONAUTE proteins for degradation. Curr. Biol. 17, 1609–1614. doi: 10.1016/j.cub.2007.08.039
Beckham, C. J., Light, H. R., Nissan, T. A., Ahlquist, P., Parker, R., and Noueiry, A. (2007). Interactions between brome mosaic virus RNAs and cytoplasmic processing bodies. J. Virol. 81, 9759–9768. doi: 10.1128/JVI.00844-07
Bhasin, H., and Hulskamp, M. (2017). ANGUSTIFOLIA, a plant homolog of CtBP/BARS localizes to stress granules and regulates their formation. Front. Plant Sci. 8:1004. doi: 10.3389/fpls.2017.01004
Bhattacharjee, S., Zamora, A., Azhar, M. T., Sacco, M. A., Lambert, L. H., and Moffett, P. (2009). Virus resistance induced by NB-LRR proteins involves Argonaute4-dependent translational control. Plant J. 58, 940–951. doi: 10.1111/j.1365-313X.2009.03832.x
Bilgin, D. D., Liu, Y., Schiff, M., and Dinesh-Kumar, S. P. (2003). P58(IPK), a plant ortholog of double-stranded RNA-dependent protein kinase PKR inhibitor, functions in viral pathogenesis. Dev. Cell 4, 651–661. doi: 10.1016/S1534-5807(03)00125-4
Bogamuwa, S., and Jang, J. C. (2013). The Arabidopsis tandem CCCH zinc finger proteins AtTZF4, 5 and 6 are involved in light-, abscisic acid- and gibberellic acid-mediated regulation of seed germination. Plant Cell Environ. 36, 1507–1519. doi: 10.1111/pce.12084
Bogamuwa, S., and Jang, J. C. (2016). Plant tandem CCCH zinc finger proteins interact with ABA, Drought, and stress response regulators in processing-bodies and stress granules. PLoS ONE 11:e0151574. doi: 10.1371/journal.pone.0151574
Bogamuwa, S. P., and Jang, J. C. (2014). Tandem CCCH zinc finger proteins in plant growth, development and stress response. Plant Cell Physiol. 55, 1367–1375. doi: 10.1093/pcp/pcu074
Bortolamiol, D., Pazhouhandeh, M., Marrocco, K., Genschik, P., and Ziegler-Graff, V. (2007). The Polerovirus F box protein P0 targets ARGONAUTE1 to suppress RNA silencing. Curr. Biol. 17, 1615–1621. doi: 10.1016/j.cub.2007.07.061
Brodersen, P., Sakvarelidze-Achard, L., Bruun-Rasmussen, M., Dunoyer, P., Yamamoto, Y. Y., Sieburth, L., et al. (2008). Widespread translational inhibition by plant miRNAs and siRNAs. Science 320, 1185–1190. doi: 10.1126/science.1159151
Buchan, J. R., Kolaitis, R.-M., Taylor, J. P., and Parker, R. (2013). Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 153, 1461–1474. doi: 10.1016/j.cell.2013.05.037
Buchan, J. R., and Parker, R. (2009). Eukaryotic stress granules: the ins and outs of translation. Mol. Cell 36, 932–941. doi: 10.1016/j.molcel.2009.11.020
Carbonell, A., and Carrington, J. C. (2015). Antiviral roles of plant ARGONAUTES. Curr. Opin. Plant Biol. 27, 111–117. doi: 10.1016/j.pbi.2015.06.013
Carroll, J. S., Munchel, S. E., and Weis, K. (2011). The DExD/H box ATPase Dhh1 functions in translational repression, mRNA decay, and processing body dynamics. J. Cell Biol. 194, 527–537. doi: 10.1083/jcb.201007151
Cheng, C. P., Jaag, H. M., Jonczyk, M., Serviene, E., and Nagy, P. D. (2007). Expression of the Arabidopsis Xrn4p 5'-3' exoribonuclease facilitates degradation of tombusvirus RNA and promotes rapid emergence of viral variants in plants. Virology 368, 238–248. doi: 10.1016/j.virol.2007.07.001
Cheng, X., and Wang, A. (2017). The potyvirus silencing suppressor protein VPg mediates degradation of SGS3 via ubiquitination and autophagy pathways. J. Virol. 91:e01478-16. doi: 10.1128/JVI.01478-16
Chiu, M.-H., Chen, I. H., Baulcombe, D. C., and Tsai, C.-H. (2010). The silencing suppressor P25 of Potato virus X interacts with Argonaute1 and mediates its degradation through the proteasome pathway. Mol. Plant Pathol. 11, 641–649. doi: 10.1111/j.1364-3703.2010.00634.x
Conti, G., Zavallo, D., Venturuzzi, A. L., Rodriguez, M. C., Crespi, M., and Asurmendi, S. (2017). TMV induces RNA decay pathways to modulate gene silencing and disease symptoms. Plant J. 89, 73–84. doi: 10.1111/tpj.13323
Csorba, T., Kontra, L., and Burgyan, J. (2015). viral silencing suppressors: tools forged to fine-tune host-pathogen coexistence. Virology 479–480, 85–103. doi: 10.1016/j.virol.2015.02.028
Cuellar, W. J., Kreuze, J. F., Rajamaki, M. L., Cruzado, K. R., Untiveros, M., and Valkonen, J. P. (2009). Elimination of antiviral defense by viral RNase III. Proc. Natl. Acad. Sci. U.S.A. 106, 10354–10358. doi: 10.1073/pnas.0806042106
Dalmay, T., Horsefield, R., Braunstein, T. H., and Baulcombe, D. C. (2001). SDE3 encodes an RNA helicase required for post-transcriptional gene silencing in Arabidopsis. EMBO J. 20, 2069–2078. doi: 10.1093/emboj/20.8.2069
del Toro, F., Fernandez, F. T., Tilsner, J., Wright, K. M., Tenllado, F., Chung, B. N., et al. (2014). Potato virus Y HCPro localization at distinct, dynamically related and environment-influenced structures in the cell cytoplasm. Mol. Plant Microbe Interact. 27, 1331–1343. doi: 10.1094/MPMI-05-14-0155-R
Detzer, A., Engel, C., Wunsche, W., and Sczakiel, G. (2011). Cell stress is related to re-localization of Argonaute 2 and to decreased RNA interference in human cells. Nucleic Acids Res. 39, 2727–2741. doi: 10.1093/nar/gkq1216
Dolja, V. V., and Koonin, E. V. (2011). Common origins and host-dependent diversity of plant and animal viromes. Curr. Opin. Virol. 1, 322–331. doi: 10.1016/j.coviro.2011.09.007
Dougherty, J. D., White, J. P., and Lloyd, R. E. (2011). Poliovirus-mediated disruption of cytoplasmic processing bodies. J. Virol. 85, 64–75. doi: 10.1128/JVI.01657-10
Du, Z., Xiao, D., Wu, J., Jia, D., Yuan, Z., Liu, Y., et al. (2011). p2 of rice stripe virus (RSV) interacts with OsSGS3 and is a silencing suppressor. Mol. Plant Pathol. 12, 808–814. doi: 10.1111/j.1364-3703.2011.00716.x
Eskelin, K., Hafren, A., Rantalainen, K. I., and Makinen, K. (2011). Potyviral VPg enhances viral RNA Translation and inhibits reporter mRNA translation in planta. J. Virol. 85, 9210–9221. doi: 10.1128/JVI.00052-11
Eulalio, A., Behm-Ansmant, I., and Izaurralde, E. (2007a). P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 8, 9–22. doi: 10.1038/nrm2080
Eulalio, A., Behm-Ansmant, I., Schweizer, D., and Izaurralde, E. (2007b). P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol. Cell. Biol. 27, 3970–3981. doi: 10.1128/MCB.00128-07
Fatyol, K., Ludman, M., and Burgyan, J. (2016). Functional dissection of a plant Argonaute. Nucleic Acids Res. 44, 1384–1397. doi: 10.1093/nar/gkv1371
Fenger-Gron, M., Fillman, C., Norrild, B., and Lykke-Andersen, J. (2005). Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping. Mol. Cell 20, 905–915. doi: 10.1016/j.molcel.2005.10.031
Fitzgerald, K. D., and Semler, B. L. (2013). Poliovirus infection induces the co-localization of cellular protein SRp20 with TIA-1, a cytoplasmic stress granule protein. Virus Res. 176, 223–231. doi: 10.1016/j.virusres.2013.06.012
Folkers, U., Kirik, V., Schobinger, U., Falk, S., Krishnakumar, S., Pollock, M. A., et al. (2002). The cell morphogenesis gene ANGUSTIFOLIA encodes a CtBP/BARS-like protein and is involved in the control of the microtubule cytoskeleton. EMBO J. 21, 1280–1288. doi: 10.1093/emboj/21.6.1280
Frei dit Frey, N., Muller, P., Jammes, F., Kizis, D., Leung, J., Perrot-Rechenmann, C., et al. (2010). The RNA binding protein Tudor-SN is essential for stress tolerance and stabilizes levels of stress-responsive mRNAs encoding secreted proteins in Arabidopsis. Plant Cell 22, 1575–1591. doi: 10.1105/tpc.109.070680
Fukunaga, R., and Doudna, J. A. (2009). dsRNA with 5' overhangs contributes to endogenous and antiviral RNA silencing pathways in plants. EMBO J. 28, 545–555. doi: 10.1038/emboj.2009.2
Galao, R. P., Chari, A., Alves-Rodrigues, I., Lobao, D., Mas, A., Kambach, C., et al. (2010). LSm1-7 complexes bind to specific sites in viral RNA genomes and regulate their translation and replication. RNA 16, 817–827. doi: 10.1261/rna.1712910
Garaigorta, U., Heim, M. H., Boyd, B., Wieland, S., and Chisari, F. V. (2012). Hepatitis C virus (HCV) induces formation of stress granules whose proteins regulate HCV RNA replication and virus assembly and egress. J. Virol. 86, 11043–11056. doi: 10.1128/JVI.07101-11
Garcia, D., Garcia, S., and Voinnet, O. (2014). Nonsense-mediated decay serves as a general viral restriction mechanism in plants. Cell Host Microbe 16, 391–402. doi: 10.1016/j.chom.2014.08.001
Garneau, N. L., Wilusz, J., and Wilusz, C. J. (2007). The highways and byways of mRNA decay. Nat. Rev. Mol. Cell Biol. 8, 113–126. doi: 10.1038/nrm2104
Gazzani, S., Lawrenson, T., Woodward, C., Headon, D., and Sablowski, R. (2004). A link between mRNA turnover and RNA interference in Arabidopsis. Science 306, 1046–1048. doi: 10.1126/science.1101092
Ghoshal, B., and Sanfaçon, H. (2014). Temperature-dependent symptom recovery in Nicotiana benthamiana plants infected with tomato ringspot virus is associated with reduced translation of viral RNA2 and requires ARGONAUTE 1. Virology 456–457, 188–197. doi: 10.1016/j.virol.2014.03.026
Gilks, N., Kedersha, N., Ayodele, M., Shen, L., Stoecklin, G., Dember, L. M., et al. (2004). Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell 15, 5383–5398. doi: 10.1091/mbc.E04-08-0715
Glick, E., Zrachya, A., Levy, Y., Mett, A., Gidoni, D., Belausov, E., et al. (2008). Interaction with host SGS3 is required for suppression of RNA silencing by tomato yellow leaf curl virus V2 protein. Proc. Natl. Acad. Sci. U.S.A. 105, 157–161. doi: 10.1073/pnas.0709036105
Goeres, D. C., Van Norman, J. M., Zhang, W., Fauver, N. A., Spencer, M. L., and Sieburth, L. E. (2007). Components of the Arabidopsis mRNA decapping complex are required for early seedling development. Plant Cell 19, 1549–1564. doi: 10.1105/tpc.106.047621
Gokhale, N. S., McIntyre, A. B. R., McFadden, M. J., Roder, A. E., Kennedy, E. M., Gandara, J. A., et al. (2016). N6-methyladenosine in flaviviridae viral RNA genomes regulates infection. Cell Host Microbe 20, 654–665. doi: 10.1016/j.chom.2016.09.015
Golisz, A., Sikorski, P. J., Kruszka, K., and Kufel, J. (2013). Arabidopsis thaliana LSM proteins function in mRNA splicing and degradation. Nucleic Acids Res. 41, 6232–6249. doi: 10.1093/nar/gkt296
Guo, H., Song, X., Xie, C., Huo, Y., Zhang, F., Chen, X., et al. (2013). Rice yellow stunt rhabdovirus protein 6 suppresses systemic RNA silencing by blocking RDR6-mediated secondary siRNA synthesis. Mol. Plant Microbe Interact. 26, 927–936. doi: 10.1094/MPMI-02-13-0040-R
Gy, I., Gasciolli, V., Lauressergues, D., Morel, J.-B., Gombert, J., Proux, F., et al. (2007). Arabidopsis FIERY1, XRN2, and XRN3 are endogenous RNA silencing suppressors. Plant Cell 19, 3451–3461. doi: 10.1105/tpc.107.055319
Hafrén, A., Eskelin, K., and Mäkinen, K. (2013). Ribosomal protein P0 promotes Potato virus A infection and functions in viral translation together with VPg and eIF(iso)4E. J. Virol. 87, 4302–4312. doi: 10.1128/JVI.03198-12
Hafrén, A., Lõhmus, A., and Mäkinen, K. (2015). Formation of potato virus A-induced RNA granules and viral translation are interrelated processes required for optimal virus accumulation. PLoS Pathog. 11:e1005314. doi: 10.1371/journal.ppat.1005314
Hafrén, A., Üstün, S., Hochmuth, A., Svenning, S., Johansen, T., and Hofius, D. (2017). Turnip mosaic virus counteracts selective autophagy of the viral silencing suppressor HCpro. Plant Physiol. doi: 10.1104/pp.17.01198. [Epub ahead of print].
Hamid, F. M., and Makeyev, E. V. (2016). Exaptive origins of regulated mRNA decay in eukaryotes. Bioessays 38, 830–838. doi: 10.1002/bies.201600100
He, F., and Jacobson, A. (2015). Nonsense-mediated mRNA decay: degradation of defective transcripts is only part of the story. Annu. Rev. Genet. 49, 339–366. doi: 10.1146/annurev-genet-112414-054639
Hematy, K., Bellec, Y., Podicheti, R., Bouteiller, N., Anne, P., Morineau, C., et al. (2016). The zinc-finger protein SOP1 is required for a subset of the nuclear exosome functions in Arabidopsis. PLoS Genet. 12:e1005817. doi: 10.1371/journal.pgen.1005817
Hubstenberger, A., Courel, M., Benard, M., Souquere, S., Ernoult-Lange, M., Chouaib, R., et al. (2017). P-body purification reveals the condensation of repressed mRNA regulons. Mol Cell 68, 144.e145–157. e145. doi: 10.1016/j.molcel.2017.09.003
Ingelfinger, D., Arndt-Jovin, D. J., Lührmann, R., and Achsel, T. (2002). The human LSm1-7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci. RNA 8, 1489–1501. doi: 10.1017/S1355838202021726
Ivanov, K. I., Eskelin, K., Basic, M., De, S., Lohmus, A., Varjosalo, M., et al. (2016). Molecular insights into the function of the viral RNA silencing suppressor HCPro. Plant J. 85, 30–45. doi: 10.1111/tpj.13088
Iwakawa, H. O., and Tomari, Y. (2013). Molecular insights into microRNA-mediated translational repression in plants. Mol. Cell 52, 591–601. doi: 10.1016/j.molcel.2013.10.033
Jouannet, V., Moreno, A. B., Elmayan, T., Vaucheret, H., Crespi, M. D., and Maizel, A. (2012). Cytoplasmic Arabidopsis AGO7 accumulates in membrane-associated siRNA bodies and is required for ta-siRNA biogenesis. EMBO J. 31, 1704–1713. doi: 10.1038/emboj.2012.20
Karran, R. A., and Sanfacon, H. (2014). Tomato ringspot virus coat protein binds to ARGONAUTE 1 and suppresses the translation repression of a reporter gene. Mol. Plant Microbe Interact. 27, 933–943. doi: 10.1094/MPMI-04-14-0099-R
Katsafanas, G. C., and Moss, B. (2007). Colocalization of transcription and translation within cytoplasmic poxvirus factories coordinates viral expression and subjugates host functions. Cell Host Microbe 2, 221–228. doi: 10.1016/j.chom.2007.08.005
Kedersha, N., Chen, S., Gilks, N., Li, W., Miller, I. J., Stahl, J., et al. (2002). Evidence that ternary complex (eIF2-GTP-tRN) -deficient preinitiation complexes are core constituents of mammalian stress granules. Mol. Biol. Cell 13, 195–210. doi: 10.1091/mbc.01-05-0221
Kedersha, N., Ivanov, P., and Anderson, P. (2013). Stress granules and cell signaling: more than just a passing phase? Trends Biochem. Sci. 38, 494–506. doi: 10.1016/j.tibs.2013.07.004
Kedersha, N. L., Gupta, M., Li, W., Miller, I., and Anderson, P. (1999). RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 147, 1431–1442. doi: 10.1083/jcb.147.7.1431
Kedersha, N., Stoecklin, G., Ayodele, M., Yacono, P., Lykke-Andersen, J., Fritzler, M. J., et al. (2005). Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 169, 871–884. doi: 10.1083/jcb.200502088
Kenesi, E., Carbonell, A., Lozsa, R., Vertessy, B., and Lakatos, L. (2017). A viral suppressor of RNA silencing inhibits ARGONAUTE 1 function by precluding target RNA binding to pre-assembled RISC. Nucleic Acids Res. 45, 7736–7750. doi: 10.1093/nar/gkx379
Kimball, S. R., Horetsky, R. L., Ron, D., Jefferson, L. S., and Harding, H. P. (2003). Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am. J. Physiol. Cell Physiol. 284, C273–C284. doi: 10.1152/ajpcell.00314.2002
Krapp, S., Greiner, E., Amin, B., Sonnewald, U., and Krenz, B. (2017). The stress granule component G3BP is a novel interaction partner for the nuclear shuttle proteins of the nanovirus pea necrotic yellow dwarf virus and geminivirus abutilon mosaic virus. Virus Res. 227, 6–14. doi: 10.1016/j.virusres.2016.09.021
Kreuze, J. F., Savenkov, E. I., Cuellar, W., Li, X., and Valkonen, J. P. (2005). Viral class 1 RNase III involved in suppression of RNA silencing. J. Virol. 79, 7227–7238. doi: 10.1128/JVI.79.11.7227-7238.2005
Kumakura, N., Takeda, A., Fujioka, Y., Motose, H., Takano, R., and Watanabe, Y. (2009). SGS3 and RDR6 interact and colocalize in cytoplasmic SGS3/RDR6-bodies. FEBS Lett. 583, 1261–1266. doi: 10.1016/j.febslet.2009.03.055
Kurihara, Y. (2017). Activity and roles of Arabidopsis thaliana XRN family exoribonucleases in noncoding RNA pathways. J. Plant Res. 130, 25–31. doi: 10.1007/s10265-016-0887-z
Lageix, S., Lanet, E., Pouch-Pelissier, M. N., Espagnol, M. C., Robaglia, C., Deragon, J. M., et al. (2008). Arabidopsis eIF2α kinase GCN2 is essential for growth in stress conditions and is activated by wounding. BMC Plant Biol. 8:134. doi: 10.1186/1471-2229-8-134
Lanet, E., Delannoy, E., Sormani, R., Floris, M., Brodersen, P., Crete, P., et al. (2009). Biochemical evidence for translational repression by Arabidopsis microRNAs. Plant Cell 21, 1762–1768. doi: 10.1105/tpc.108.063412
Lange, H., Zuber, H., Sement, F. M., Chicher, J., Kuhn, L., Hammann, P., et al. (2014). The RNA helicases AtMTR4 and HEN2 target specific subsets of nuclear transcripts for degradation by the nuclear exosome in Arabidopsis thaliana. PLoS Genet. 10:e1004564. doi: 10.1371/journal.pgen.1004564
Li, F., Huang, C., Li, Z., and Zhou, X. (2014). Suppression of RNA silencing by a plant DNA virus satellite requires a host calmodulin-like protein to repress RDR6 expression. PLoS Pathog. 10:e1003921. doi: 10.1371/journal.ppat.1003921
Lloyd, R. E. (2013). Regulation of stress granules and P-bodies during RNA virus infection. Wiley Interdiscipl. Rev. RNA 4, 317–331. doi: 10.1002/wrna.1162
Lloyd, R. E. (2016). Enterovirus control of translation and RNA granule stress responses. Viruses 8:93. doi: 10.3390/v8040093
Lokdarshi, A., Conner, W. C., McClintock, C., Li, T., and Roberts, D. M. (2016). Arabidopsis CML38, a calcium sensor that localizes to ribonucleoprotein complexes under hypoxia stress. Plant Physiol. 170, 1046–1059. doi: 10.1104/pp.15.01407
Lorkovic, Z. J., Wieczorek Kirk, D. A., Klahre, U., Hemmings-Mieszczak, M., and Filipowicz, W. (2000). RBP45 and RBP47, two oligouridylate-specific hnRNP-like proteins interacting with poly(A)+ RNA in nuclei of plant cells. RNA 6, 1610–1624. doi: 10.1017/S1355838200001163
Ma, X., Nicole, M. C., Meteignier, L. V., Hong, N., Wang, G., and Moffett, P. (2015). Different roles for RNA silencing and RNA processing components in virus recovery and virus-induced gene silencing in plants. J. Exp. Bot. 66, 919–932. doi: 10.1093/jxb/eru447
Malinowska, M., Niedzwiedzka-Rystwej, P., Tokarz-Deptula, B., and Deptula, W. (2016). Stress granules (SG) and processing bodies (PB) in viral infections. Acta Biochim. Pol. 63, 183–188. doi: 10.18388/abp.2015_1060
Martinez de Alba, A. E., Moreno, A. B., Gabriel, M., Mallory, A. C., Christ, A., Bounon, R., et al. (2015). In plants, decapping prevents RDR6-dependent production of small interfering RNAs from endogenous mRNAs. Nucleic Acids Res. 43, 2902–2913. doi: 10.1093/nar/gkv119
Martinez-Perez, M., Aparicio, F., Lopez-Gresa, M. P., Belles, J. M., Sanchez-Navarro, J. A., and Pallas, V. (2017). Arabidopsis m6A demethylase activity modulates viral infection of a plant virus and the m6A abundance in its genomic RNAs. Proc. Natl. Acad. Sci. U.S.A. 114, 10755–10760 doi: 10.1073/pnas.1703139114
Matsuki, H., Takahashi, M., Higuchi, M., Makokha, G. N., Oie, M., and Fujii, M. (2013). Both G3BP1 and G3BP2 contribute to stress granule formation. Genes Cells 18, 135–146. doi: 10.1111/gtc.12023
Melville, M. W., Katze, M. G., and Tan, S. L. (2000). P58IPK, a novel cochaperone containing tetratricopeptide repeats and a J-domain with oncogenic potential. Cell. Mol. Life Sci. 57, 311–322. doi: 10.1007/PL00000692
Merai, Z., Benkovics, A. H., Nyiko, T., Debreczeny, M., Hiripi, L., Kerenyi, Z., et al. (2013). The late steps of plant nonsense-mediated mRNA decay. Plant J. 73, 50–62. doi: 10.1111/tpj.12015
Meteignier, L. V., Zhou, J., Cohen, M., Bhattacharjee, S., Brosseau, C., Chan, M. G., et al. (2016). NB-LRR signaling induces translational repression of viral transcripts and the formation of RNA processing bodies through mechanisms differing from those activated by UV stress and RNAi. J. Exp. Bot. 67, 2353–2366. doi: 10.1093/jxb/erw042
Mitchell, S. F., and Parker, R. (2014). Principles and properties of eukaryotic mRNPs. Mol. Cell 54, 547–558. doi: 10.1016/j.molcel.2014.04.033
Mollet, S., Cougot, N., Wilczynska, A., Dautry, F., Kress, M., Bertrand, E., et al. (2008). Translationally repressed mRNA transiently cycles through stress granules during stress. Mol. Biol. Cell 19, 4469–4479. doi: 10.1091/mbc.E08-05-0499
Moreno, A. B., Martinez de Alba, A. E., Bardou, F., Crespi, M. D., Vaucheret, H., Maizel, A., et al. (2013). Cytoplasmic and nuclear quality control and turnover of single-stranded RNA modulate post-transcriptional gene silencing in plants. Nucleic Acids Res. 41, 4699–4708. doi: 10.1093/nar/gkt152
Mourrain, P., Beclin, C., Elmayan, T., Feuerbach, F., Godon, C., Morel, J. B., et al. (2000). Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell 101, 533–542. doi: 10.1016/S0092-8674(00)80863-6
Okamoto, M., Matsui, A., Tanaka, M., Morosawa, T., Ishida, J., Iida, K., et al. (2016). Sm-like protein-mediated RNA metabolism is required for heat stress tolerance in Arabidopsis. Front. Plant Sci. 7:1079. doi: 10.3389/fpls.2016.01079
Okano, Y., Senshu, H., Hashimoto, M., Neriya, Y., Netsu, O., Minato, N., et al. (2014). In planta recognition of a double-stranded RNA synthesis protein complex by a potexviral RNA silencing suppressor. Plant Cell 26, 2168–2183. doi: 10.1105/tpc.113.120535
Pager, C. T., Schutz, S., Abraham, T. M., Luo, G., and Sarnow, P. (2013). Modulation of hepatitis C virus RNA abundance and virus release by dispersion of processing bodies and enrichment of stress granules. Virology 435, 472–484. doi: 10.1016/j.virol.2012.10.027
Panas, M. D., Ahola, T., and McInerney, G. M. (2014). The C-terminal repeat domains of nsP3 from the Old World alphaviruses bind directly to G3BP. J. Virol. 88, 5888–5893. doi: 10.1128/JVI.00439-14
Panas, M. D., Schulte, T., Thaa, B., Sandalova, T., Kedersha, N., Achour, A., et al. (2015). Viral and cellular proteins containing FGDF motifs bind G3BP to block stress granule formation. PLoS Pathog. 11:e1004659. doi: 10.1371/journal.ppat.1004659
Peng, J., Yang, J., Yan, F., Lu, Y., Jiang, S., Lin, L., et al. (2011). Silencing of NbXrn4 facilitates the systemic infection of Tobacco mosaic virus in Nicotiana benthamiana. Virus Res. 158, 268–270. doi: 10.1016/j.virusres.2011.03.004
Piotrowska, J., Hansen, S. J., Park, N., Jamka, K., Sarnow, P., and Gustin, K. E. (2010). Stable formation of compositionally unique stress granules in virus-infected cells. J. Virol. 84, 3654–3665. doi: 10.1128/JVI.01320-09
Poblete-Durán, N., Prades-Pérez, Y., Vera-Otarola, J., Soto-Rifo, R., and Valiente-Echeverría, F. (2016). Who regulates whom? an overview of RNA granules and viral infections. Viruses 8:E180. doi: 10.3390/v8070180
Pomeranz, M. C., Hah, C., Lin, P. C., Kang, S. G., Finer, J. J., Blackshear, P. J., et al. (2010). The Arabidopsis tandem zinc finger protein AtTZF1 traffics between the nucleus and cytoplasmic foci and binds both DNA and RNA. Plant Physiol. 152, 151–165. doi: 10.1104/pp.109.145656
Pumplin, N., and Voinnet, O. (2013). RNA silencing suppression by plant pathogens: defence, counter-defence and counter-counter-defence. Nat. Rev. Microbiol. 11, 745–760. doi: 10.1038/nrmicro3120
Qu, F., Ye, X., and Morris, T. J. (2008). Arabidopsis DRB4, AGO1, AGO7, and RDR6 participate in a DCL4-initiated antiviral RNA silencing pathway negatively regulated by DCL1. Proc. Natl. Acad. Sci. U.S.A. 105, 14732–14737. doi: 10.1073/pnas.0805760105
Qu, J., Kang, S. G., Wang, W., Musier-Forsyth, K., and Jang, J. C. (2014). The Arabidopsis thaliana tandem zinc finger 1 (AtTZF1) protein in RNA binding and decay. Plant J. 78, 452–467. doi: 10.1111/tpj.12485
Rajamäki, M.-L., Streng, J., and Valkonen, J. P. T. (2014). Silencing suppressor protein VPg of a potyvirus interacts with the plant silencing-related protein SGS3. Mol. Plant Microbe Interact. 27, 1199–1210. doi: 10.1094/MPMI-04-14-0109-R
Reynoso, M. A., Blanco, F. A., Bailey-Serres, J., Crespi, M., and Zanetti, M. E. (2013). Selective recruitment of mRNAs and miRNAs to polyribosomes in response to rhizobia infection in Medicago truncatula. Plant J. 73, 289–301. doi: 10.1111/tpj.12033
Roux, M. E., Rasmussen, M. W., Palma, K., Lolle, S., Regue, A. M., Bethke, G., et al. (2015). The mRNA decay factor PAT1 functions in a pathway including MAP kinase 4 and immune receptor SUMM2. EMBO J. 34, 593–608. doi: 10.15252/embj.201488645
Rymarquis, L. A., Souret, F. F., and Green, P. J. (2011). Evidence that XRN4, an Arabidopsis homolog of exoribonuclease XRN1, preferentially impacts transcripts with certain sequences or in particular functional categories. RNA 17, 501–511. doi: 10.1261/rna.2467911
Schaad, M. C., Jensen, P. E., and Carrington, J. C. (1997). Formation of plant RNA virus replication complexes on membranes: role of an endoplasmic reticulum-targeted viral protein. EMBO J. 16, 4049–4059. doi: 10.1093/emboj/16.13.4049
Scheller, N., Resa-Infante, P., de la Luna, S., Galao, R. P., Albrecht, M., Kaestner, L., et al. (2007). Identification of PatL1, a human homolog to yeast P body component Pat1. Biochim. Biophys. Acta 1773, 1786–1792. doi: 10.1016/j.bbamcr.2007.08.009
Scholte, F. E., Tas, A., Albulescu, I. C., Zusinaite, E., Merits, A., Snijder, E. J., et al. (2015). Stress granule components G3BP1 and G3BP2 play a proviral role early in Chikungunya virus replication. J. Virol. 89, 4457–4469. doi: 10.1128/JVI.03612-14
Sen, G. L., and Blau, H. M. (2005). Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat. Cell Biol. 7, 633–636. doi: 10.1038/ncb1265
Sheth, U., and Parker, R. (2003). Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 300, 805–808. doi: 10.1126/science.1082320
Sorenson, R., and Bailey-Serres, J. (2014). Selective mRNA sequestration by OLIGOURIDYLATE-BINDING PROTEIN 1 contributes to translational control during hypoxia in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 111, 2373–2378. doi: 10.1073/pnas.1314851111
Sun, Y., Dong, L., Yu, S., Wang, X., Zheng, H., Zhang, P., et al. (2017). Newcastle disease virus induces stable formation of bona fide stress granules to facilitate viral replication through manipulating host protein translation. FASEB J. 31, 1337–1353. doi: 10.1096/fj.201600980R
Sweet, T., Kovalak, C., and Coller, J. (2012). The DEAD-box protein Dhh1 promotes decapping by slowing ribosome movement. PLoS Biol. 10:e1001342. doi: 10.1371/journal.pbio.1001342
Tanaka, K. J., Ogawa, K., Takagi, M., Imamoto, N., Matsumoto, K., and Tsujimoto, M. (2006). RAP55, a cytoplasmic mRNP component, represses translation in Xenopus oocytes. J. Biol. Chem. 281, 40096–40106. doi: 10.1074/jbc.M609059200
Tharun, S. (2009). Lsm1-7-Pat1 complex: a link between 3' and 5'-ends in mRNA decay? RNA Biol. 6, 228–232. doi: 10.4161/rna.6.3.8282
Tharun, S., He, W., Mayes, A. E., Lennertz, P., Beggs, J. D., and Parker, R. (2000). Yeast Sm-like proteins function in mRNA decapping and decay. Nature 404, 515–518. doi: 10.1038/35006676
Thomas, M. G., Loschi, M., Desbats, M. A., and Boccaccio, G. L. (2011). RNA granules: the good, the bad and the ugly. Cell. Signal. 23, 324–334. doi: 10.1016/j.cellsig.2010.08.011
Thran, M., Link, K., and Sonnewald, U. (2012). The Arabidopsis DCP2 gene is required for proper mRNA turnover and prevents transgene silencing in Arabidopsis. Plant J. 72, 368–377. doi: 10.1111/j.1365-313X.2012.05066.x
Tirumuru, N., Zhao, B. S., Lu, W., Lu, Z., He, C., and Wu, L. (2016). N(6)-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. Elife 5:e15528. doi: 10.7554/eLife.15528
Tourriere, H., Chebli, K., Zekri, L., Courselaud, B., Blanchard, J. M., Bertrand, E., et al. (2003). The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 160, 823–831. doi: 10.1083/jcb.200212128
Vindry, C., Marnef, A., Broomhead, H., Twyffels, L., Ozgur, S., Stoecklin, G., et al. (2017). Dual RNA Processing Roles of Pat1b via Cytoplasmic Lsm1-7 and Nuclear Lsm2-8 Complexes. Cell Rep. 20, 1187–1200. doi: 10.1016/j.celrep.2017.06.091
Weber, C., Nover, L., and Fauth, M. (2008). Plant stress granules and mRNA processing bodies are distinct from heat stress granules. Plant J. 56, 517–530. doi: 10.1111/j.1365-313X.2008.03623.x
Weinheimer, I., Haikonen, T., Ala-Poikela, M., Moser, M., Streng, J., Rajamaki, M. L., et al. (2016). Viral RNase3 co-localizes and interacts with the antiviral defense protein SGS3 in plant cells. PLoS ONE 11:e0159080. doi: 10.1371/journal.pone.0159080
Wheeler, J. R., Jain, S., Khong, A., and Parker, R. (2017). Isolation of yeast and mammalian stress granule cores. Methods 126, 12–17. doi: 10.1016/j.ymeth.2017.04.020
White, J. P., Cardenas, A. M., Marissen, W. E., and Lloyd, R. E. (2007). Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe 2, 295–305. doi: 10.1016/j.chom.2007.08.006
White, J. P., and Lloyd, R. E. (2011). Poliovirus unlinks TIA1 aggregation and mRNA stress granule formation. J. Virol. 85, 12442–12454. doi: 10.1128/JVI.05888-11
Xia, J., Chen, X., Xu, F., Wang, Y., Shi, Y., Li, Y., et al. (2015). Dengue virus infection induces formation of G3BP1 granules in human lung epithelial cells. Arch. Virol. 160, 2991–2999. doi: 10.1007/s00705-015-2578-9
Xu, J., and Chua, N.-H. (2009). Arabidopsis decapping 5 is required for mRNA decapping, P-body formation, and translational repression during postembryonic development. Plant Cell 21, 3270–3279. doi: 10.1105/tpc.109.070078
Xu, J., and Chua, N.-H. (2011). Processing bodies and plant development. Curr. Opin. Plant Biol. 14, 88–93. doi: 10.1016/j.pbi.2010.10.003
Xu, J., Yang, J.-Y., Niu, Q.-W., and Chua, N.-H. (2006). Arabidopsis DCP2, DCP1, and VARICOSE form a decapping complex required for postembryonic development. Plant Cell 18, 3386–3398. doi: 10.1105/tpc.106.047605
Yan, C., Yan, Z., Wang, Y., Yan, X., and Han, Y. (2014). Tudor-SN, a component of stress granules, regulates growth under salt stress by modulating GA20ox3 mRNA levels in Arabidopsis. J. Exp. Bot. 65, 5933–5944. doi: 10.1093/jxb/eru334
Yang, L., Wu, G., and Poethig, R. S. (2012). Mutations in the GW-repeat protein SUO reveal a developmental function for microRNA-mediated translational repression in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 109, 315–320. doi: 10.1073/pnas.1114673109
Ye, J., Yang, J., Sun, Y., Zhao, P., Gao, S., Jung, C., et al. (2015). Geminivirus Activates ASYMMETRIC LEAVES 2 to Accelerate Cytoplasmic DCP2-Mediated mRNA Turnover and Weakens RNA Silencing in Arabidopsis. PLoS Pathog. 11:e1005196. doi: 10.1371/journal.ppat.1005196
Yi, Z., Fang, C., Pan, T., Wang, J., Yang, P., and Yuan, Z. (2006). Subproteomic study of hepatitis C virus replicon reveals Ras-GTPase-activating protein binding protein 1 as potential HCV RC component. Biochem. Biophys. Res. Commun. 350, 174–178. doi: 10.1016/j.bbrc.2006.09.027
Yu, S. F., Lujan, P., Jackson, D. L., Emerman, M., and Linial, M. L. (2011). The DEAD-box RNA helicase DDX6 is required for efficient encapsidation of a retroviral genome. PLoS Pathog. 7:e1002303. doi: 10.1371/journal.ppat.1002303
Zenke, K., and Kim, K. H. (2008). Functional characterization of the RNase III gene of rock bream iridovirus. Arch. Virol. 153, 1651–1656. doi: 10.1007/s00705-008-0162-2
Zhang, X., Du, P., Lu, L., Xiao, Q., Wang, W., Cao, X., et al. (2008). Contrasting effects of HC-Pro and 2b viral suppressors from Sugarcane mosaic virus and Tomato aspermy cucumovirus on the accumulation of siRNAs. Virology 374, 351–360. doi: 10.1016/j.virol.2007.12.045
Zhang, X., Yuan, Y.-R., Pei, Y., Lin, S.-S., Tuschl, T., Patel, D. J., et al. (2006). Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis argonaute1 cleavage activity to counter plant defense. Genes Dev. 20, 3255–3268. doi: 10.1101/gad.1495506
Keywords: RNA Interference, nonsense mediated mRNA decay, mRNA decay, processing bodies, stress granules, siRNA bodies, plant viruses
Citation: Mäkinen K, Lõhmus A and Pollari M (2017) Plant RNA Regulatory Network and RNA Granules in Virus Infection. Front. Plant Sci. 8:2093. doi: 10.3389/fpls.2017.02093
Received: 13 October 2017; Accepted: 24 November 2017;
Published: 11 December 2017.
Edited by:
Helene Sanfacon, Agriculture and Agri-Food Canada (AAFC), CanadaReviewed by:
Feng Qu, The Ohio State University, United StatesCopyright © 2017 Mäkinen, Lõhmus and Pollari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristiina Mäkinen, a3Jpc3RpaW5hLm1ha2luZW5AaGVsc2lua2kuZmk=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.