
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 27 September 2017
Sec. Plant Pathogen Interactions
Volume 8 - 2017 | https://doi.org/10.3389/fpls.2017.01622
This article is part of the Research TopicInduction of new cell types or organs in plants during biotic interactionsView all 16 articles
The development of gall shapes has been attributed to the feeding behavior of the galling insects and how the host tissues react to galling stimuli, which ultimately culminate in a variable set of structural responses. A superhost of galling herbivores, Copaifera langsdorffii, hosts a bizarre “horn-shaped” leaflet gall morphotype induced by an unidentified species of Diptera: Cecidomyiidae. By studying the development of this gall morphotype under the anatomical and physiological perspectives, we demonstrate the symptoms of the Cecidomyiidae manipulation over plant tissues, toward the cell redifferentiation and tissue neoformation. The most prominent feature of this gall is the shifting in shape from growth and development phase toward maturation, which imply in metabolites accumulation detected by histochemical tests in meristem-like group of cells within gall structure. We hypothesize that the development of complex galls, such as the horn-shaped demands the reacquisition of cell meristematic competence. Also, as mature galls are green, their photosynthetic activity should be sufficient for their oxygenation, thus compensating the low gas diffusion through the compacted gall parenchyma. We currently conclude that the galling Cecidomyiidae triggers the establishment of new sites of meristematic tissues, which are ultimately responsible for shifting from the young conical to the mature horn-shaped gall morphotype. Accordingly, the conservative photosynthesis activity in gall site maintains tissue homeostasis by avoiding hypoxia and hipercarbia in the highly compacted gall tissues.
For millions of years, insects and plants have developed strategies of attack and counter attack that culminate in the establishment of unique ecological relationships between living organisms (Howe and Jander, 2008). One of the most intriguing of such interactions leads to the development of galls, with several morphotypes that generally result from species-specific relationships (Stone and Schönrogge, 2003; Isaias et al., 2013). Therefore, specialized insects are capable of manipulating plant tissues and metabolism to induce a neo-formed plant organ, the gall (Mani, 1964; Shorthouse et al., 2005; Giron et al., 2016, Oliveira et al., 2016). Additionally, a cascade of subcellular events related to the increased oxidative stress and the mechanisms of stress control, cell signaling, division, and elongation should play significant roles in gall development and determination of gall shape (Mittapalli et al., 2007; Oliveira and Isaias, 2010; Oliveira et al., 2010, 2016; Bedetti et al., 2014; Isaias et al., 2015).
In the past two decades, there has been an effort to elucidate the cellular mechanisms of determination of gall shapes commonly observed among Neotropical galls (cf. Isaias et al., 2013). Even simple-shaped galls such as the globoid, lenticular, and fusiform seem to develop by a variety of somewhat divergent cellular dynamics (Oliveira et al., 2010, 2016; Carneiro et al., 2014, 2015; Isaias et al., 2014a; Suzuki et al., 2015), but complex bizarre-shaped gall morphotypes demand much deeper alterations in the fates of plant host cells and tissues.
The best way to reprogram cell cycles seems to be by turning cells back to the meristematic condition, which has not been observed in anatomical studies concerning the globoid, fusiform and lenticular galls (Oliveira et al., 2010; Carneiro et al., 2013; Isaias et al., 2013, 2014b; Vecchi et al., 2013). However, in the bivalve-shaped gall induced by Euphalerus ostreoides on Lonchocarpus muehlbergianus (Isaias et al., 2011) groups of redifferentiated cells are responsible for the development of two valves that protrude from the leaf lamina to form the larval chamber. Herein, we propose that alterations on the degree of cell redifferentiation in galls depend on refined stimulation by galling insects, and the reacquisition of meristematic condition, i.e., dedifferentiation (sensu Buvat, 1989). We hypothesize that a profound cell redifferentitation enable some galls to assume complex forms, sometimes very distinct from the most common gall morphotypes observed in nature (Isaias et al., 2013, 2014b), such as the bizarre horn-shaped galls induced on leaflets of Copaifera langsdorffii.
Leaves are the most common oviposition sites for galling herbivores (Mani, 1964; Isaias et al., 2013; Fernandes and Santos, 2014), which alter the structure and function of the tissues, sometimes, in detriment of their photosynthetic capacity. For instance, some galls may photosynthesize as much as their host leaves, as reported for the galls induced by Pseudophacopteron aspidospermii (Malenovsky et al., 2015) on Aspidosperma australe, and a Cecidomyiidae on Aspidosperma spruceanum (Oliveira et al., 2011), or may have depleted photosynthetic apparatus (Larson, 1998; Kar et al., 2013). The horn-shaped galls of C. langsdorffii seem to belong to the group of photosynthesis deficient galls, for their levels of total chlorophyll are about 27-fold lower than the non-galled leaflets, while the maximum electron transport rates decrease about seven times (Castro et al., 2012). However, even at low rates, the photosynthesis in green galls may provide oxygen to avoid hypoxia, and consume carbon dioxide to avoid hypercarbia, thus helping to maintain the stability of the organ (Pincebourde and Casas, 2016; Oliveira et al., 2017).
Current study focuses on the “horn-shaped” leaflet gall morphotype induced by a Cecidomyiidae (Diptera) on the superhost of galling herbivores, C. langsdorffii (Fabaceae). Field observations revealed a strong alteration in shape from young to mature galls (Isaias et al., 2014a), besides great increase in size. We expect that, by studying the development of such gall morphotype under the anatomical and physiological perspectives, we can elucidate key steps involving the transition from the laminar structure of non-galled leaves to the conical shape of young galls, and toward the mature horn-shaped galls. In addition, we expect that both outer and inner gall tissue compartments (Bragança et al., 2017) should maintain some level of photosynthesis, which is important to the metabolic stability of the gall.
Samples of the galls and non-galled leaflets were collected in a population of C. langsdorffii Desf. (Fabaceae) growing on ferruginous rocky outcrops (canga) at Retiro das Pedras (20°05“35”S, 43°59“01”W), Serra da Calçada, municipality of Brumadinho, southeastern Brazil. The population (15 plant individuals bearing hundreds of galls, each) was monitored and sampled monthly (n ≥ 5 galls and non-galled leaves) from January 2007 to March 2008, to define the different developmental stages of the horn-shaped gall.
For structural studies, galls in successive stages of development (n = 5 per stage) were separated according to their sizes and morphological features. Induction stage was defined by a swelling on the leaf lamina (1.0 ± 0.2 mm). Growth and development stage was defined by the appearance of a volcano-like structure followed by the protrusion of two appendices (2.0 mm until 7.0 mm of height). Maturation stage was defined by the gall maximum size, and acquisition of the horn-shape (9.5 ± 2.1 mm). Senescent stage was characterized by the brown color and presence of an escape channel (9.0 ± 1.2 mm, open galls). Samples of galls were fixed in FAA (Johansen, 1940), dehydrated in n-butyl series, and embedded in Paraplast® (Kraus and Arduin, 1997). The samples were cross-sectioned (8–12 μm) and stained with astra blue and safranin solution (8:2, v/v) (Bukatsch, 1972, modified to 0.5%).
For scanning electron microscopy (SEM), samples of galls in all developmental stages were fixed in 4% Karnovsky (1965) (modified to phosphate buffer), post fixed in 1% osmium tetroxide, gradually dehydrated in ethanol series, CO2 critical point dried, and covered with 35 nm of gold (O’Brien and McCully, 1981). The samples were analyzed using the scanning electron microscope Jeol – JEM 6060.
Fresh samples of horn-shaped galls at the late growth and development stage (the most metabolically demanding stage) were submitted to histochemical tests. The presence of IAA, indol-acetaldehyde (IAld), flavonoid derivatives and phenolics was tested using free-hand sections (room temperature). For IAA and IAld detection, the sections were treated with Ehrlich’s reagent (Leopold and Plummer, 1961) and p-dimethylaminocinnamaldehyde – DMAC (Schneider et al., 1972), respectively, for 5 min. IAA are stained in pink and IAld are stained in green, according to assays using commercial standards applied to thin layer chromatography (Bedetti et al., 2014). Flavonoid derivatives were detected by deep blue stain in sections incubated in 0.5% caffeine, 0.5% sodium benzoate, and 90% butanol for 5 min, and post incubated in 1% p-dimethylaminocinnamaldehyde (DMACA) in water, hydrochloric acid and ethanol (5:1:5) for 2 h (Feucht et al., 1986). Phenolics were detected in samples fixed in 2% ferric chloride in 95% ethanol (Gahan, 1984).
Fluorescence quenching analysis was performed in non-galled leaflets and galls (n = 5) at mature phase (at 8:00 am) using a modulated fluorescence imaging apparatus, Handy FluorCam PSI (Photo Systems Instrument, Czechia). The fluorescence parameters were measured on the surface of whole galls (not sectioned), and in hemisectioned galls (to measure photosynthetic yield in the innermost cell layers). Both whole and hemisectioned galls were analyzed to show the gradient of photosynthetic activity along the outermost cell layers to the inner cortex, nearest to the larval chamber. The maintenance of electron chains integrity, and the photosynthetic capacity of non-galled and galled tissues were demonstrated after adaptation to dark (30 min), and exposition to various light treatments following the software protocol – Quenching (Photo Systems Instruments, Version 21). The following parameters were used in this study based on Genty et al. (1989), Lichtenthaler and Miehé (1997), and Oxborough (2004): Fv/Fm (maximum PSII quantum yield in dark-adapted state, where Fv = Fm-F0), (F′m-F′)/F′m (PSII operating efficiency, where F′m is the fluorescence signal when all PSII centers are closed in the light-adapted state and F′ is the measurement of the light-adapted fluorescence signal), Rfd (instantaneous fluorescence decline ratio in light, an empiric parameter used to assess tissue vitality), and NPQLss (steady-state non-photochemical quenching in light).
The galling Diptera: Cecidomyiidae induces a horn-shaped gall on the leaflets of C. langsdorffii, and has a univoltine life cycle along a year-time (Figure 1). Gall induction occurs from June to August concomitant to leaf flushing. The growth and development stage occurs from July to March and overlaps with the beginning of the maturation stage, which lasts from January to June. The senescent stage occurs during the heyday of the dry season in June and July.
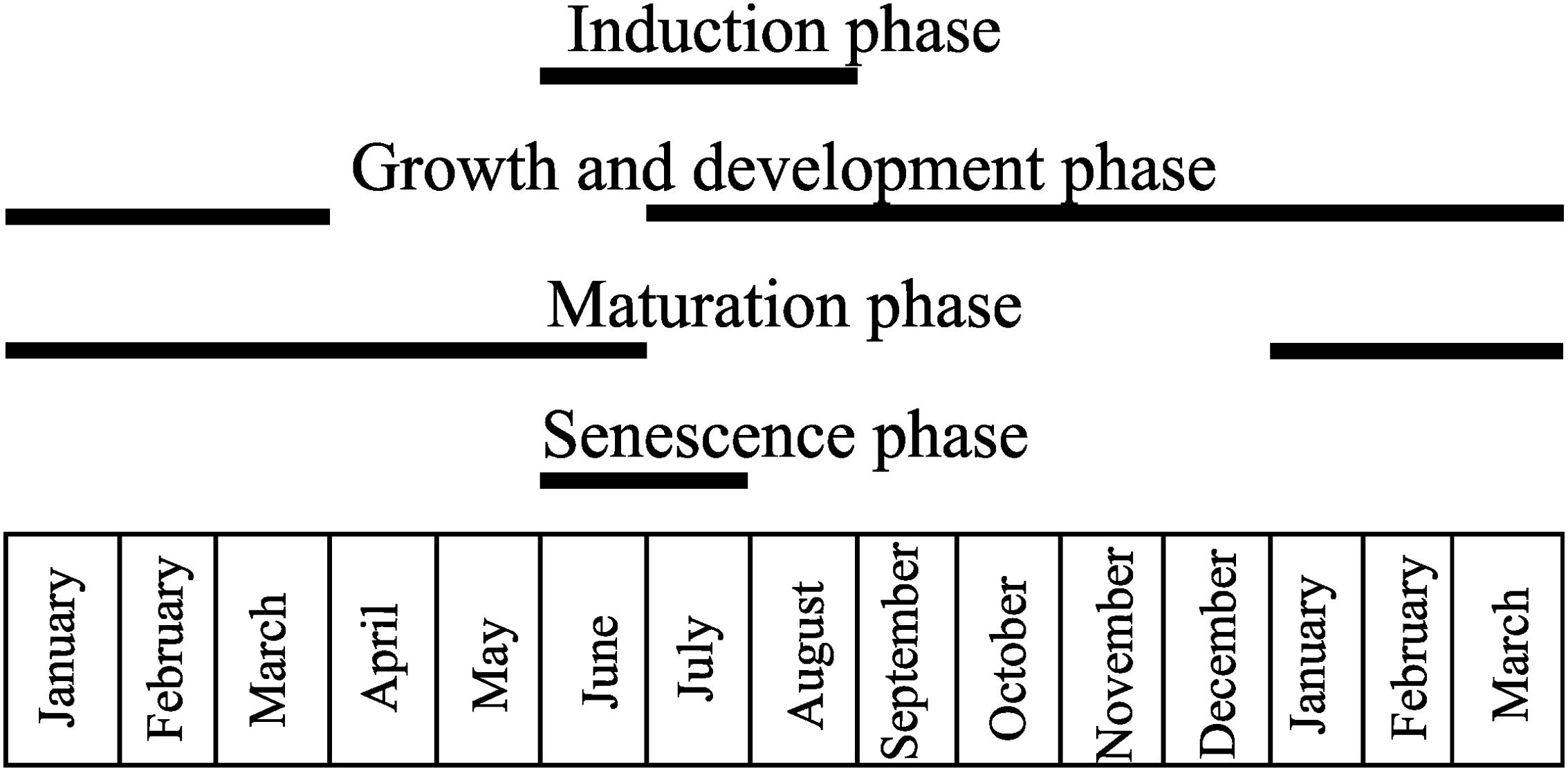
FIGURE 1. Univoltine life cycle of the horn-shaped gall induced by Diptera: Cecidomyiidae on the leaflets of Copaifera langsdorffii. Gall induction occurs from June to August, while the growth and development stage occurs from July to March, and overlaps with the beginning of the maturation stage, which lasts from January to June. The senescence stage occurs on June and July.
The stage of gall induction is morphologically defined by the swelling of the leaflet lamina, with redifferentiated trichomes at the center (Figure 2A). Gall growth and development involve intense cell division and elongation to form a volcano-like structure covered by neoformed trichomes (Figures 2B,C). From inside the volcano-like structure, two appendices covered with a dense indumentum of non-glandular trichomes develop (Figures 2D,E). At maturation, galls reach their maximum size and the appendices turn into two lateral horn-like structures (Figure 2F). Galls may be isolated or clustered, occurring on both surfaces of the leaflets; they are pedunculated, closed, varying from light green to brownish-red. Each gall has a single larval chamber, which hosts one galling insect.

FIGURE 2. Developmental stages of the horn-shaped gall induced by Diptera: Cecidomyiidae on leaflets of C. langsdorffii. (A) Induction stage of the gall determined by the swelling of the leaflet lamina (asterisk), and redifferentiation of trichomes at the center. (B) Macroscopic aspect of the galls at the early growth and developmental stage, with the formation of the volcano-like structure (asterisk), and neoformed trichomes at the top (arrow). (C) Scanning electron micrograph showing detail of the volcano-like structure covered by neoformed trichomes. (D) Scanning electron micrograph showing detail of two appendices with a dense indumentum (arrows) emerging from the volcano-like structure (asterisk). (E) Macroscopic aspect of the galls at the growth and developmental stage with two appendices (arrows). (F) Mature galls are green, with two “horns” derived from the appendices (arrows), and the volcano-like structure remains at the base, forming a peduncle (asterisk).
The feeding activity of the galling larva stimulates the first detectable symptom of the induction phase of the horn-shaped gall, cell hypertrophy (Figure 3A) In addition, the hypertrophied cells have unidentified vacuolar substances (Figure 3B).
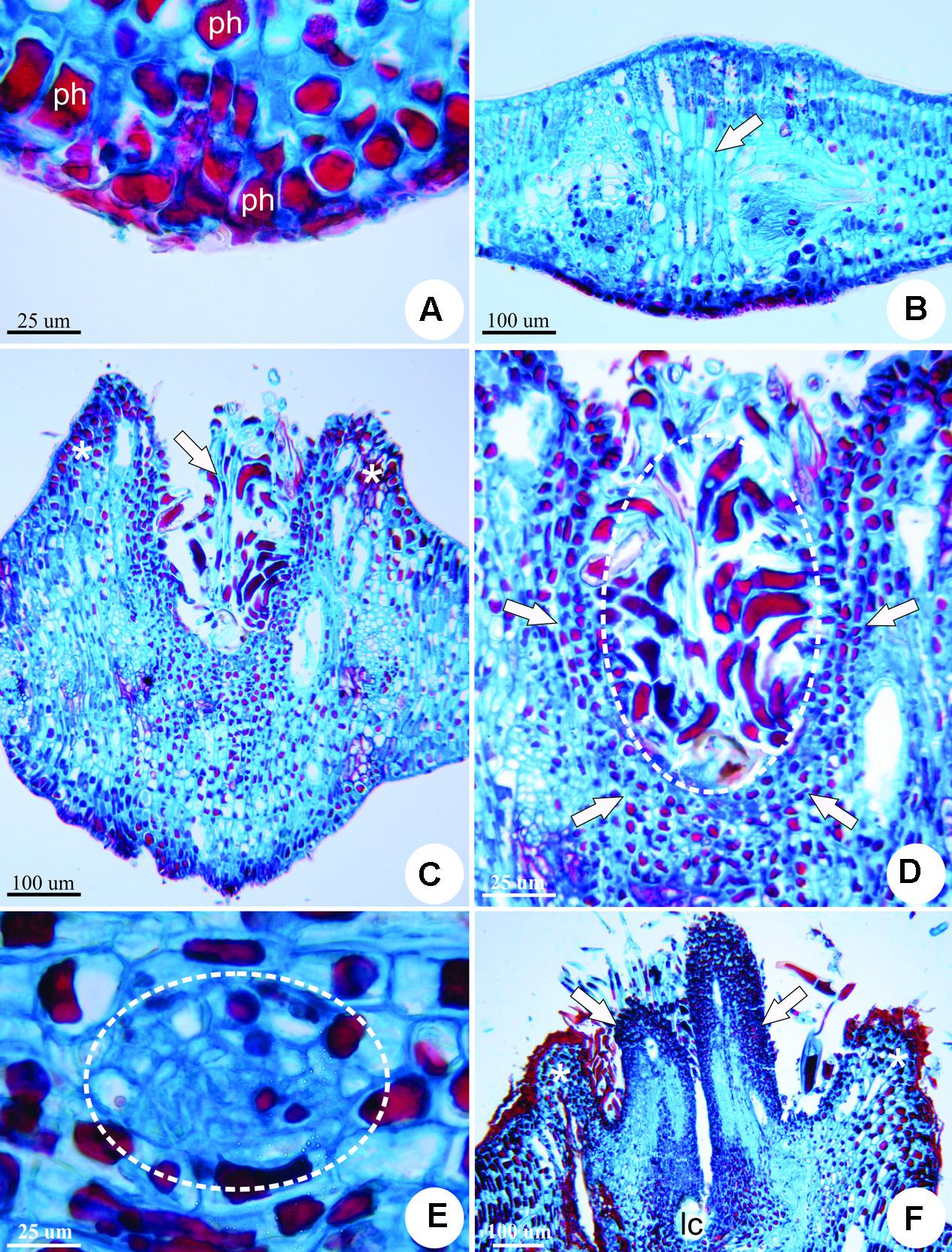
FIGURE 3. Anatomy of the horn-shaped gall induced by Diptera: Cecidomyiidae on the leaflets of C. langsdorffii in transverse sections. (A) Detail of phenolic substances (ph) accumulated at the site of induction. (B) Gall induction site defined by the swelling of the leaflet lamina, hypertrophy and elongation of mesophyll cells (arrow) and the accumulation of phenolics. (C) Early growth and developmental stage, with intense cell division and hypertrophy during the formation of a volcano-like structure (asterisks), and neoformation of trichomes (arrow). (D) Detail of small cells (arrows) nearest the larval chamber covered by trichomes (dotted line). (E) Detail of neoformed phloem bundle (dotted line) redifferentiated from the cortical parenchyma cells next to the larval chamber. (F) Redifferentiation of two appendices laterally to the larval chamber (lc) (arrows) that emerge from inside the volcano-like structure (asterisk).
The stage of growth and development occurs along 9 months and is featured by several important morphological events toward the establishment of the final shape of the gall. At the early phase of the growth and development stage, there is an increased periclinal division of mesophyll cells adjacent to the induction site. This hyperplasia, together with cell hypertrophy is responsible for the development of a volcano-like structure with neoformed trichomes (Figure 3C). These trichomes shelter the galling insect during this phase, for the larval chamber is yet to be completely closed. The cells nearest to the larval chamber are smaller than the cells of the outer layers of the volcano-like structure (Figure 3D). Neo-formed vascular bundles redifferentiate from parenchymatic cells in the cortex around the larval chamber (Figure 3E). Two groups of meristem-like cells redifferentiate inside the volcano-like structure and originate two appendices laterally to the larval chamber (Figure 3F). Epidermal cells accompany the growth of the appendices, and fuse on the adaxial portion of the structure closing a small larval chamber (Figure 4A). Below the larval chamber, a third meristem-like group of cells redifferentiates (Figure 4B) from parenchymatic cells, divide and elongate, protruding gall tissues upward from inside the volcano-like structure, and forming a peduncle (Figure 4C). Thus, the divisions of the meristem-like group of cells pushes the larval chamber up, together with the two lateral appendages in a continuum to determine the horn-shaped structure of the gall. The volcano-like structure remains at the base of the gall, where numerous secretory cavities occur (Figure 4D). Similar secretory cavities can also be observed at the outer cortex of the gall.
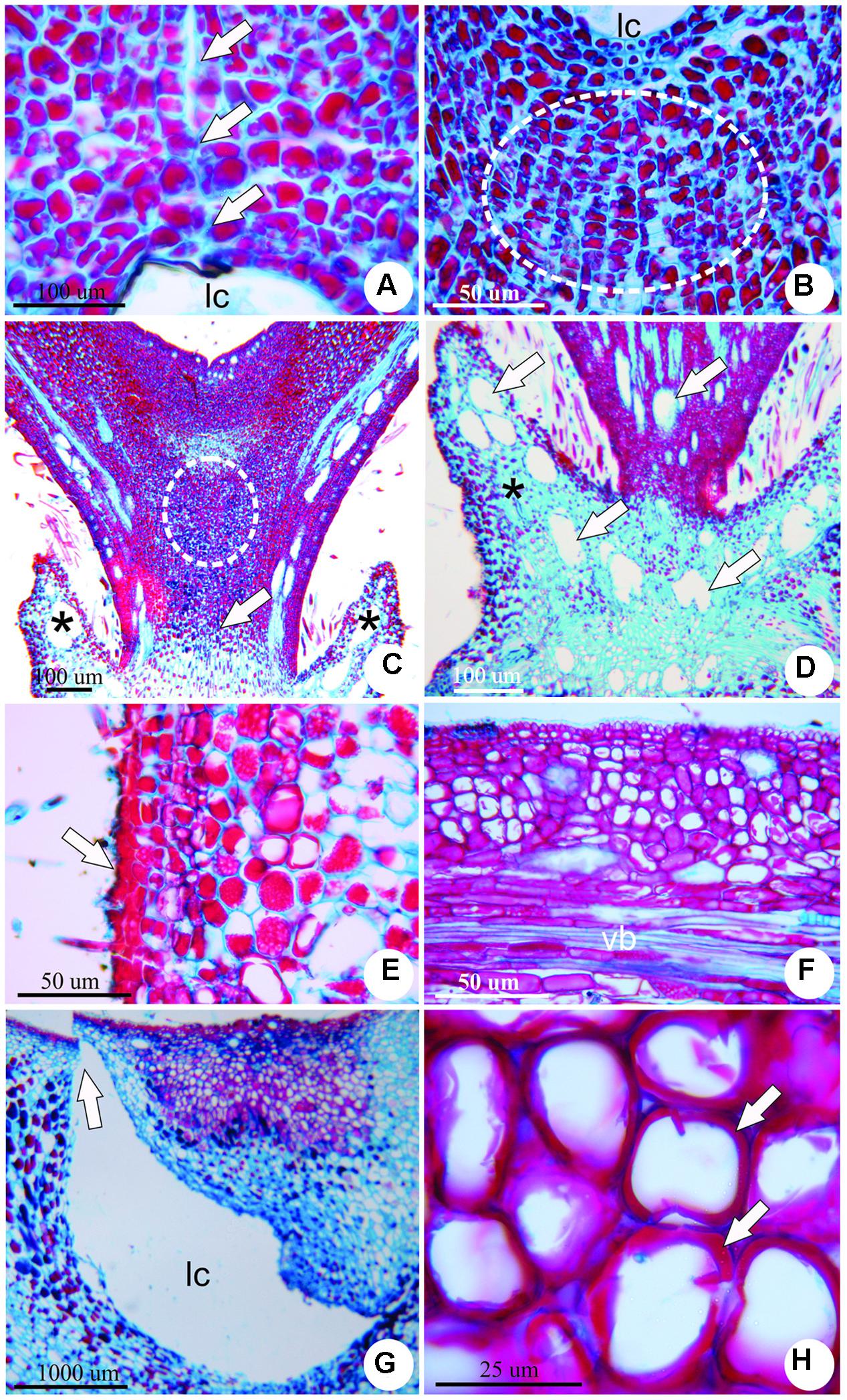
FIGURE 4. Anatomy of the horn-shaped gall induced by Diptera: Cecidomyiidae on the leaflets of C. langsdorffii. (A) Epidermal cells of the appendages fuse on the adaxial portion to close the larval chamber (arrows). (B) Meristem-like group of cells redifferentiate from parenchyma cells below the larval chamber (dotted line). (C) Gall at late growth and development stage, evidencing increment in cell divisions and elongation due to the activity of the meristem-like group of cells (dotted line), that protrude the gall body from inside the volcano-like structure (asterisks), forming a peduncle (arrow). (D) Detail of the volcano-like structure with numerous secretory cavities (arrows). (E) Detail of suberized tissue spot (arrow) on the outer surface of the mature gall. (F) Vascular bundle (vb) in the outer cortex of a mature gall. (G) Detail of the escape channel (arrow) built at the side of the larval chamber (lc) by the end of the maturation stage. (H) Detail of lignified cell walls (arrows) of nutritive cells in senescent gall.
During the stage of maturation, gall size reaches its maximum size (9.5 ± 2.1 mm), and the horn-shaped structure is defined. Mature galls are green (sometimes with red or brown pigmentation), covered by simple epidermis, with sparse patches of periderm (Figure 4E). Gall cortex is eminently parenchymatic, with inconspicuous intercellular spaces, and a single larval chamber with one galling insect. Vascular bundles are distributed at the outer cortex of the gall (Figure 4F). Nutritive cells redifferentiate from the dermal system around the larval chamber.
By the end of maturation stage, galls enter the stage of senescence, which is characterized by the presence of an escape channel built from the expansion of the larval chamber toward the apex of the gall (Figure 4G). When the galling insect emerges through the escape channel, the walls of the nutritive cells around the larval chamber lignify (Figure 4H), and a suberized tissue totally replaces the epidermis.
The sites of IAA accumulation were detected by the pink staining with Ehrlich reagent, specially concentrated in the vascular bundles at the base of the larval chamber (Figure 5A), in the meristem-like cells (Figure 5B), and in the appendices (Figure 5C). The sites of IAld accumulation are evidenced by the green stain with DMAC in the meristem-like cells (Figure 5D), appendices (Figure 5E), and vascular bundles next to the larval chamber (Figure 5F). Flavonoid derivatives (Figures 5G–I) and phenolics (Figures 5J–L) detected by DMACA and 2% ferric chloride in 95% ethanol, respectively, overlapped at the sites of IAld and IAA accumulation.
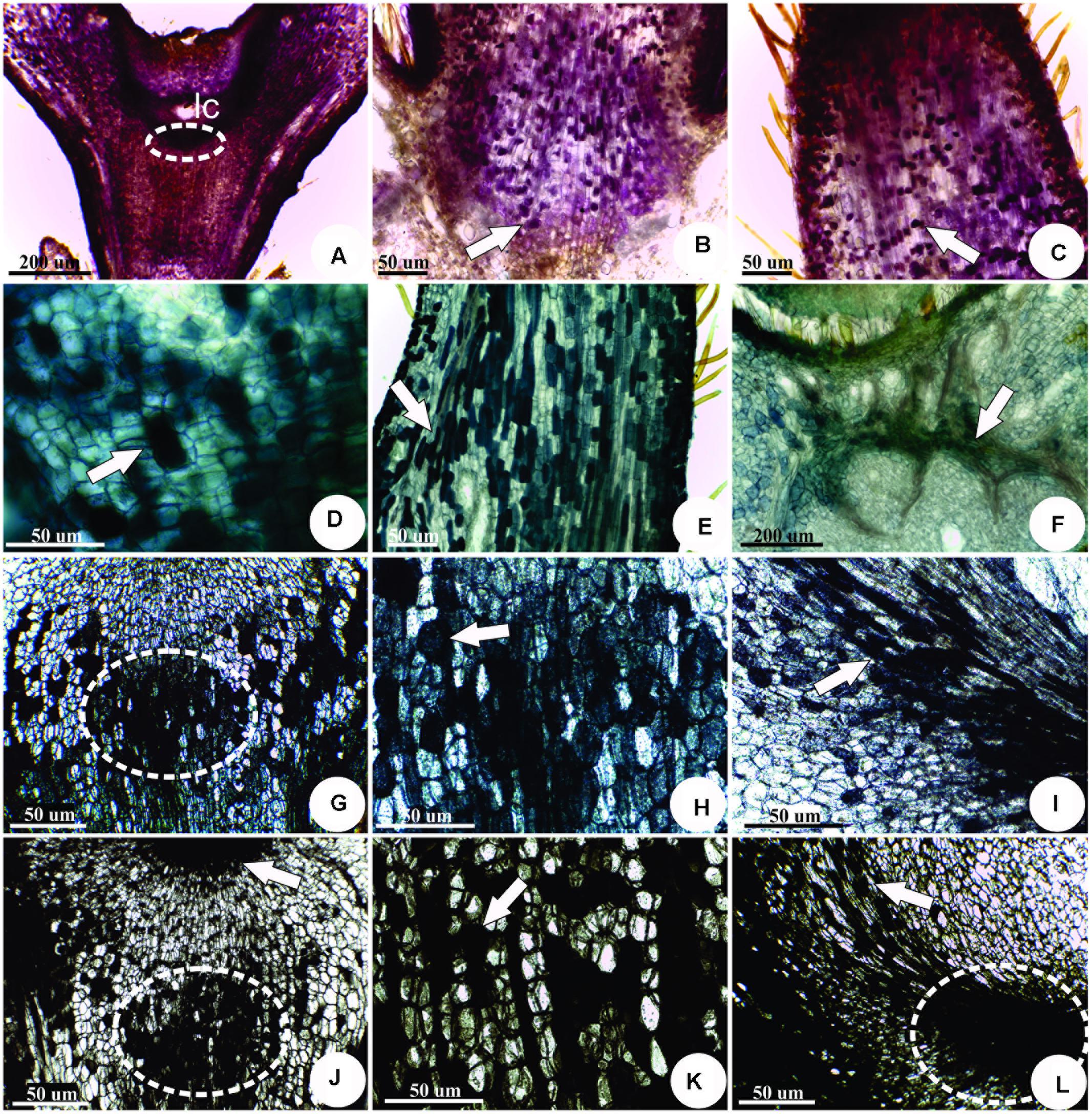
FIGURE 5. Histochemistry of mature horn-shaped gall induced by Diptera: Cecidomyiidae on the leaflets of C. langsdorffii. (A–C) sites of IAA accumulation detected by the pink staining with Ehrlich reagent. (A) General view, showing intense staining around the larval chamber (lc), at the meristem-like group of cells (dotted line). (B) Detail of reaction in the cells of the gall peduncle (arrow). (C) Detail of reaction in the cells of the gall “horn” (arrow). (D–F) Indol-acetaldehyde (IAld) accumulation evidenced in green by the staining with DMAC. (D) Detail of the reaction in the meristem-like group of cells (arrow). (E) Detail of reaction in the cells of the gall “horn” (arrow). (F) Detail of reaction in the vascular cells at the base of gall peduncle (arrow). (G–I) Flavonoids detected in dark blue by DMACA. (G) Detail of the reaction in the meristem-like group of cells (dotted line). (H) Detail of reaction in the cells of the gall peduncle (arrow). (I) Detail of the reaction in the flanks of the meristem-like group of cells (arrow). (J–L) Phenolics detected in black by ferric chloride reaction. (J) Detail of the reaction in the meristem-like group of cells (dotted line). (K) Detail of reaction in the cells of the gall peduncle (arrow). (L) Detail of the reaction in the flanks (arrow) of the meristem-like group of cells (dotted line).
The mature horn-shaped galls on C. langsdorffii are green (Figures 6a,b) and have potential to photosynthesize due to the presence of chlorophylls in most of the cortical cell layers. The values of Fv/Fm (0.61 ± 0.02 and 0.55 ± 0.03; whole and sectioned galls, respectively) (Figures 6c,d), as well as values of (F′m–F′)/F′m (0.7 ± 0.01 and 0.62 ± 0.03; whole and sectioned galls, respectively) (Figures 6e,f), indicate that the chlorophyllous tissue is able to photosynthesize throughout the gall cortex, except for the larval chamber and nearby cells. The steady-state non-photochemical quenching in light (NPQLss) (Figures 6g,h) is high in the whole galls when compared to the sectioned ones (0.62 ± 0.03 and 0.34 ± 0.06, respectively). The instantaneous fluorescence ratio decline in light (Rfd) (Figures 6i,j) indicates the decrease of tissue stability toward larval chamber in galls (0.78 ± 0.07 and 0.53 ± 0.04; whole and sectioned galls, respectively).
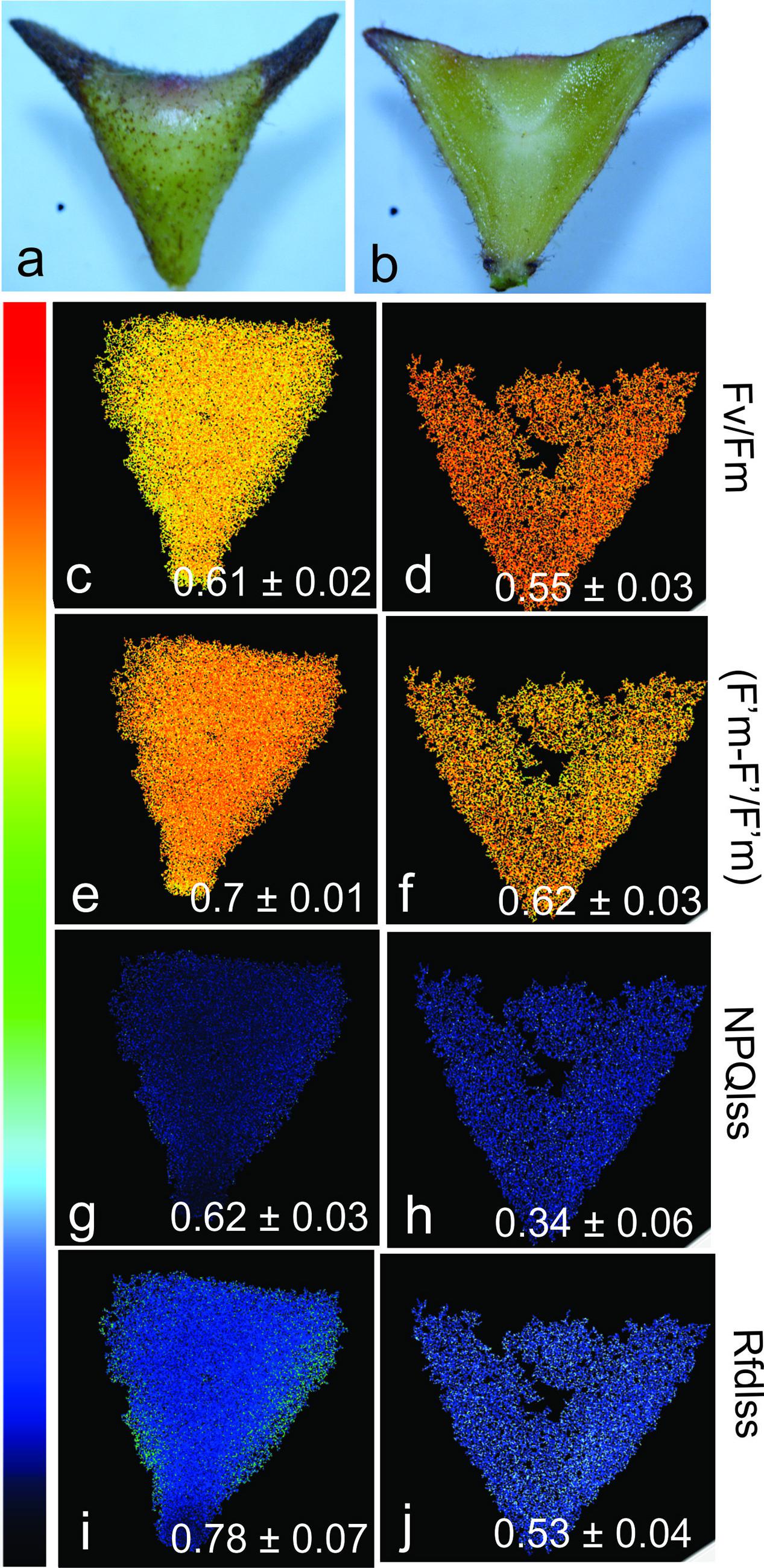
FIGURE 6. Chlorophyll fluorescence and photosynthetic activity in the horn-shaped gall induced by Diptera: Cecidomyiidae on Copaifera langsdorffii. (a,b) Whole gall and hemisectioned gall. (c,d) Fv/Fm (maximum PSII quantum yield in dark-adapted state) in outer and inner gall cortices. (e,f) (F′m–F′)/F′m (PSII operating efficiency) in outer and inner gall cortices. (g,h) Rfd (instantaneous fluorescence decline ratio in light, an empiric parameter used to assess tissue vitality) in outer and inner gall cortices. (i,j) NPQLss (steady-state non-photochemical quenching in light) in outer and inner gall cortices.
Galling insects are highly sensitive to phenological and physiological changes on their hosts, which, in the case of C. langsdorffii, lead to the establishment of seasonal syndromes for the occurrence of different gall morphotypes (Oliveira et al., 2013). The horn-shaped gall is the most abundant gall morphotype on C. langsdorffii, and it is induced exclusively on young leaves, whose tissues are responsive (sensu Weis et al., 1988) to the galling Cecidomyiidae. Thus, the period of leaf flushing of C. langsdorffii during the dry season is a true window of opportunity (sensu Mendonça, 2001) for the development of this gall morphotype.
Gall morphology has been the focus of studies in the Neotropics, and is an important tool for assessing and understanding the diversity of host plant-gall systems. The morphology of the horn-shaped galls is unique, and is particularly interesting for this gall shifts shape along the development. Such morphogenetical feature of shape-shifting has also been reported for the cup-shaped galls on C. langsdorffii, which are globoid during the early developmental stages, and turns to cup-shaped during maturity (Isaias et al., 2013). Nevertheless, differently from the horn-shaped gall, the cup-shaped gall has a very fast life cycle, and the shape-shifting occurs along 1-day time. Herein, we observe changes on the external morphology of galls to mark the transition from the induction stage toward the stages of growth and development, and maturity (Rohfritsch and Shorthouse, 1982; sensu Meyer and Maresquelle, 1983). Such marked shifts in shape, especially during growth and development phase, must involve deep anatomical reprogramming, which occurs in galls via cell redifferentiation toward new cell fates.
Classically, gall shape has been understood as a response of plant cells to the feeding behavior of the gall inducer, which stimulates plant tissues to grow toward a new structure (Rohfritsch and Anthony, 1992). However, the high diversity of gall shapes found in nature indicates that such classic concept does not always explain the diversity of gall morphotypes found in nature. Cell hypertrophy and tissue hyperplasia are the most common plant responses to the stimuli of galling herbivores (Mani, 1964; Rohfritsch, 1992; Isaias et al., 2014b; Oliveira et al., 2016), and were also observed during the development of the horn-shaped galls on C. langsdorffii. The protrusion of the galls from the leaf lamina requires a set of periclinal cell divisions and anticlinal cell elongation, as observed herein, and in other Neotropical galls (cf. Isaias et al., 2014b). Such cell divisions are observed to occur especially due to the activity of meristem-like group of cells, which remain clustered below the larval chamber, while the cells next to it undergo differentiation. As formative plant tissues, meristems are composed by cells capable of dividing and giving rise to new cells (Scofield and Murray, 2006), with defined patterning and genetic regulation (Miwa et al., 2008), features of the meristem-like group of cells in the horn-shaped galls.
Each gall morphotype has specific patterns of cell division and elongation during the transition of the stage of induction toward the stage of maturation, when the final shape of the gall is defined. The intense activity of the meristem-like groups of cells determines the shape of the horn-shaped galls. Meristematic cells were previously reported in galls on Guapira opposita, forming multiple buds on the surface of the gall, and producing numerous leafy projections, which determine the rosette gall morphotype (Fleury et al., 2015). However, the developmental sites of the galls on G. opposita are the axillary buds, where meristem potentialities are naturally expressed. The horn-shaped galls are induced on leaflets, where meristematic tissues are lacking, and the presence of the meristem-like groups of cells in gall structure indicates cell redifferentiation (sensu Lev-Yadun, 2003) during gall development. The perspective of the reacquisition of the meristematic potentiality involves specific genetic control as proved for the establishment and maintenance of the apical meristem (Raghavan, 1986; Fosket, 1994). Also, the development of the two horn-like appendages resembles the general development of leaf primordia (Moon and Hake, 2011), with differences on the final structure.
The redifferentiation of tissues in gall developmental sites involves a cascade of events triggered by reactive oxygen species (ROS) and followed by the accumulation of phenolic derivatives. The enhanced levels of ROS impair the homeostasis of gall tissues, which may trigger the accumulation of ROS scavengers, such as the phenolics (Isaias et al., 2015). The accumulation of phenolics ends up inhibiting the activity of IAA-oxidases (Hori, 1992), thus increasing the cellular levels of auxins and their precursors (Isaias et al., 2015). In the leaflet galls on Piptadenia gonoacantha, sites of intense cell division and hyperplasia accumulate phenolics, IAA and its precursor, thus adding a topological evidence of the role of phenolics on the control of auxin levels during gall development (Bedetti et al., 2014). Similarly, the histochemical profiles of the horn-shaped galls on C. langsdorffii show that phenolics, IAA and IAld accumulate in the meristem-like group of cells below the larval chamber. Such group of cells can be recognized and its ectopic occurrence and abnormal activity originate the horn-shaped gall, a new plant organ with singular phenotype. Even though the host leaflet anatomy deeply changes toward this gall morphotype, some cell lineages express conservative functions, as the photosynthetic activity, which helps maintaining the stability of gall metabolism.
Alterations on the phenotype of the leaflet lamina toward the horn-shaped gall require dramatic changes on the structure and functions of C. langsdorffii cells and tissues. Leaves are organs designed to effectively exchange gasses with the surrounding environment, and to photosynthesize at high levels, thus functioning as a carbon source for plants. Conversely, galls are structures that protect and nurture the galling herbivores, often seen as carbon sinks of their host organs due to photosynthetic deficiency (Carneiro et al., 2014), or by the high demand of energy to sustain a vigorous growth and metabolism. Previous studies with the horn-shaped galls indicate that the photosynthetic activity occurs at low levels in gall tissues when compared to non-galled leaflets, despite the deep structural changes (Castro et al., 2012).
The reduction of the Fv/Fm and (F′m–F′)/F′m values in the sectioned horn-shaped galls indicates a decrease of photosynthetic potential from gall periphery toward the inner cortex. Additionally, the low intensity of light that reaches the innermost layers of the horn-shaped galls, and the underdeveloped photosynthetic apparatus of the meristem-like groups of cells should explain the low values of Fv/Fm, (F′m–F′)/F′m and Rfd, as proposed by Haitz and Lichtenthaler (1988) for other stress-generating factors. The photosynthesis in green galls provides oxygen to avoid hypoxia and consume carbon dioxide to avoid hypercarbia in their tissues, thus working as a metabolic strategy to maintain the stability of the new organ (Pincebourde and Casas, 2016; Oliveira et al., 2017). Even though the horn-shaped gall is large-sized, with highly compacted tissues, it seems to have a functional metabolism of carbon dioxide and oxygen. Such efficiency explains how abundant and large galls withstand their long life cycles on C. langsdorffii as their photosynthesis rates should help reducing intra-plant and inter-gall competition, as hypothesized for A. longifolia galls (Haiden et al., 2012).
The biotic stress induced by the galling Cecidomyiidae on the structure and physiology of the leaflets of C. langsdorffii lead to distinct functionalities of plant cells and tissues, culminating in a new organogenetic pattern. During the univoltine life cycle of the galling Cecidomyiidae, we observe drastic changes on the external morphology of the galls with a marked transition from the induction stage toward the stages of growth and development, and maturity. Such marked shifts in shape require cell dedifferentiation and redifferentiation at the center of the gall, especially during growth and development phase, when the gall body emerges from inside the volcano-like structure. Subsequently, new meristematic centers at the flanks originate the parenchymatic horns. These meristematic zones drive deep anatomical reprogramming toward the reacquisition of the meristematic condition. The conservative functionalities of the host organ in galls, as the photosynthesis activity, maintain tissue homeostasis by avoiding hypoxia and hipercarbia in the highly compacted gall tissues. New frontiers on the functioning of ectopic meristematic sites, such as the meristem-like group of cells in the horn-shaped galls on C. langsdorffii should rely on the investigation of molecular mechanisms of cell signaling for the determination of the unusual structural patterns of complex galls.
RC, RI, AM, and DO designed the sample. RC, AM, and DO field sampling. RC, RI, AM, and DO data analysis. RC, RI, AM, and DO Wrote the manuscript.
We thank the Fundação de Apoio à Pesquisa do Estado de Minas Gerais – FAPEMIG, and the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (307011/2015-1) (301246/2016-5) for financial support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank BG Ferreira for the assistance with photomicrographs editing.
Bedetti, C. S., Modolo, L. V., and Isaias, R. M. S. (2014). The role of phenolics in the control of auxin in galls of Piptadenia gonoacantha (Mart.) MacBr (Fabaceae: Mimosoideae). Biochem. Syst. Ecol. 55, 53–59. doi: 10.1016/j.bse.2014.02.016
Bragança, G. P., Oliveira, D. C., and Isaias, R. M. S. (2017). Compartmentalization of metabolites and enzymatic mediation in nutritive cells of Cecidomyiidae galls on Piper arboreum Aubl. (Piperaceae). J. Plant Stud. 6, 11–19. doi: 10.5539/jps.v6n1p11
Buvat, R. (1989). Ontogeny, Cell Differentiation, and Structure of Vascular Plants. Berlin: Springer-Verlag, 560.
Carneiro, R. G., Pacheco, P., and Isaias, R. M. S. (2015). Could the extended phenotype extend to the cellular and subcellular levels in insect-induced galls? PLOS ONE 10:e0129331. doi: 10.1371/journal.pone.0129331
Carneiro, R. G. S., Burckhardt, D., and Isaias, R. M. S. (2013). Biology and systematics of gall-inducing triozids (Hemiptera: Psylloidea) associated with Psidium spp. (Myrtaceae). Zootaxa 3620, 129–146. doi: 10.11646/zootaxa.3620.1.6
Carneiro, R. G. S., Castro, A. C., and Isaias, R. M. S. (2014). Unique histochemical gradients in a photosynthesis-deficient plant gall. S. Afr. J. Bot. 92, 97–104. doi: 10.1016/j.sajb.2014.02.011
Castro, A. C., Oliveira, D. C., Moreira, A. S. F. P., Lemos Filho, J. P., and Isaias, R. M. S. (2012). Source sink relationship and photosynthesis in the hornshaped gall and its host plant Copaifera langsdorffii Desf. (Fabaceae). S. Afr. J. Bot. 83, 121–126. doi: 10.1016/j.sajb.2012.08.007
Feucht, W., Schmid, P. P. S., and Christ, E. (1986). Distribution of flavonols in meristematic and mature tissues of Prunus avium Shoots. J. Plant Physiol. 125, 1–8. doi: 10.1016/S0176-1617(86)80237-1
Fleury, G., Ferreira, B. G., Soares, G. L. G., Oliveira, D. C., and Isaias, R. M. (2015). Elucidating the determination of the rosette galls induced by Pisphondylia brasiliensis Couri and Maia 1992 (Cecidomyiidae) on Guapira opposita (Nyctaginaceae). Aust. J. Bot. 63, 608–617. doi: 10.1071/BT15106
Fosket, D. E. (1994). Plant Growth and Development: A Molecular Approach. San Diego, CA: Academic Press.
Gahan, P. B. (1984). Plant Histochemistry and Cytochemistry: An Introduction. Orlando, FL: Academic Press, 301.
Genty, B., Briantais, J.-M., and Baker, N. R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990, 87–92. doi: 10.1016/S0304-4165(89)80016-9
Giron, D., Huguet, E., Stone, G. N., and Body, M. (2016). Insect-induced effects on plants and possible effectors used by galling and leaf-mining insects to manipulate their host-plant. J. Insect Physiol. 84, 70–89. doi: 10.1016/j.jinsphys.2015.12.009
Haiden, S. A., Hoffmann, J. H., and Cramer, M. D. (2012). Benefits of photosynthesis for insects in galls. Oecologia 170, 987–997. doi: 10.1007/s00442-012-2365-1
Haitz, M., and Lichtenthaler, H. K. (1988). “The measurement of Rfd-values as plant vitality indices with the portable field chlorophyll fluorometer and the Pam-fluorometer,” in Applicattion of Chorophyll Fluorescence, ed. H. K. Lichtenthaler (Dordrecht: Springer), 249–254.
Hori, K. (1992). “Insect secretion and their effect on plant growth, with special reference to hemipterans,” in Biology of Insect-Induced Galls, eds J. D. Shorthouse and O. Rohfritsch (New York, NY: Oxford University Press), 157–170.
Howe, G. A., and Jander, G. (2008). Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 59, 41–66. doi: 10.1146/annurev.arplant.59.032607.092825
Isaias, R. M. S., Carneiro, R. G. S., Santos, J. C., and Oliveira, D. C. (2014a). “Gall morphotypes in the Neotropics and the need to standardize them,” in Neotropical Insect Galls, eds G. W. Fernandes and J. C. Santos (Dordrecht: Springer), 51–67.
Isaias, R. M. S., Carneiro, R. G. S., Oliveira, D. C., and Santos, J. C. (2013). Illustrated and annotated checklist of Brazilian gall morphotypes. Neotrop. Entomol. 42, 230–239. doi: 10.1007/s13744-013-0115-7
Isaias, R. M. S., Oliveira, D. C., and Carneiro, R. G. S. (2011). Role of Euphalerus ostreoides (Hemiptera: Psylloidea) in manipulating leaflet ontogenesis of Lonchocarpus muehlbergianus (Fabaceae). Botany 89, 581–592. doi: 10.1139/b11-048
Isaias, R. M. S., Oliveira, D. C., Carneiro, R. G. S., and Kraus, J. E. (2014b). “Developmental anatomy of galls in the Neotropics: arthropods stimuli versus host plant constraints,” in Neotropical Insect Galls, eds G. W. Fernandes and J. C. Santos (Dordrecht: Springer), 15–34.
Isaias, R. M. S., Oliveira, D. C., Moreira, A. S. F. P., Soares, G. L. G., and Carneiro, R. G. S. (2015). The imbalance of redox homeostasis in arthropod-induced plant galls: mechanisms of stress generation and dissipation. Biochim. Biophys. Acta 1850, 1509–1517. doi: 10.1016/j.bbagen.2015.03.007
Kar, P. K., Jena, K. B., Srivastava, A. K., Giri, S., and Sinha, M. K. (2013). Gall-inducing stress in the leaves of Terminalia arjuna, food plant of tropical tasarsilk worm, Antheraea mylitta. Emir. J. Food Agric. 25, 205–210. doi: 10.9755/ejfa.v25i3.10970
Karnovsky, M. J. (1965). A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J. Cell Biol. 27, 137–138.
Kraus, J. E., and Arduin, M. (1997). Manual Básico de Métodos em Morfologia Vegetal. Rio de Janeiro: Editora da Universidade Rural.
Larson, K. C. (1998). The impact of two gall-forming arthropods on the photosynthetic rates of their hosts. Oecologia 115, 161–166. doi: 10.1007/s004420050503
Leopold, A. C., and Plummer, T. H. (1961). Auxin–phenol complexes. Plant Physiol. 36, 589–591. doi: 10.1104/pp.36.5.589
Lev-Yadun, S. (2003). Stem cells in plants are differentiated too. Curr. Opin. Plant Biol. 4, 93–100.
Lichtenthaler, H. K., and Miehé, J. (1997). Fluorescence imaging as a diagnostic tool for plant stress. Trends Plant Sci. 2, 316–320. doi: 10.1016/S1360-1385(97)89954-2
Malenovsky, I., Burckhardt, D., Queiroz, D. L., Isaias, R. M. S., and Oliveira, D. C. (2015). Descriptions of two new Pseudophacopteron species (Hemiptera: Psylloidea: Phacopteronidae) inducing galls on Aspidosperma (Apocynaceae) in Brazil. Acta Entomol. Musei Natl. Pragae 55, 513–538.
Mani, M. S. (1964). Ecology of Plant Galls. The Hague: Dr. W. Junk Publishers. doi: 10.1007/978-94-017-6230-4
Mendonça, M. S. (2001). Galling insect diversity patterns: the resource synchronization hypothesis. Oikos 95, 171–176. doi: 10.1034/j.1600-0706.2001.950120.x
Mittapalli, O., Neal, J. J., and Shukle, R. H. (2007). Antioxidant defense response in a galling insect. Proc. Natl. Acad. Sci. U.S.A. 104, 1889–1894. doi: 10.1073/pnas.0604722104
Miwa, H., Betsuyaku, S., Iwamoto, K., Kinoshita, A., Fukuda, H., and Sawa, S. (2008). The receptor-like kinase SOL2 mediates CLE signaling in Arabidopsis. Plant Cell Physiol. 49, 1752–1757. doi: 10.1093/pcp/pcn148
Moon, J., and Hake, S. (2011). How a leaf gets its shape. Curr. Opin. Plant Biol. 14, 24–30. doi: 10.1016/j.pbi.2010.08.012
O’Brien, T. P., and McCully, M. E. (1981). The Study of Plant Structure: Principles and Selected Methods. Melbourne, VIC: Termacarphi Pty.
Oliveira, D. C., and Isaias, R. M. S. (2010). Redifferentiation of leaflet tissues during midrib gall development in Copaifera langsdorffii (Fabaceae). S. Afr. J. Bot. 76, 239–248. doi: 10.1016/j.sajb.2009.10.011
Oliveira, D. C., Isaias, R. M. S., Fernandes, G. W., Ferreira, B. G., Carneiro, R. G. S., and Fuzaro, L. (2016). Manipulation of host plant cells and tissues by gall-inducing insects and adaptive strategies used by different feeding guilds. J. Insect Physiol. 84, 103–113. doi: 10.1016/j.jinsphys.2015.11.012
Oliveira, D. C., Isaias, R. M. S., Moreira, A. S. F. P., Magalhães, T. A., and Lemos-Filho, J. P. (2011). Is the oxidative stress caused by Aspidosperma spp. galls capable of altering leaf photosyntheis?. Plant Sci. 180, 489–495. doi: 10.1016/j.plantsci.2010.11.005
Oliveira, D. C., Magalhães, T. A., Carneiro, R. G. S., Alvim, M. N., and Isaias, R. M. S. (2010). Do Cecidomyiidae galls of Aspidosperma spruceanum (Apocynaceae) fit the pre-established cytological and histochemical patterns? Protoplasma 242, 81–93. doi: 10.1007/s00709-010-0128-6
Oliveira, D. C., Mendonça, M. S. Jr., Moreira, A. S. F. P., Lemos Filho, J. P., and Isaias, R. M. S. (2013). Water stress and phenological synchronism between Copaifera langsdorffii (Fabaceae) and multiple galling insects: formation of seasonal patterns. J. Plant Interact. 8, 225–233. doi: 10.1080/17429145.2012.705339
Oliveira, D. C., Moreira, A. S. F. P., Isaias, R. M. S., Martini, V., and Rezende, U. C. (2017). Sink status and photosynthetic rate of the leaflet galls induced by Bystracoccus mataybae (Eriococcidae) on Matayba guianensis (Sapindaceae). Front. Plant Sci. 8:1249. doi: 10.3389/fpls.2017.01249
Oxborough, K. (2004). Imaging of chlorophyll a fluorescence: theoretical and practical aspects of an emerging technique for the monitoring of photosynthetic performance. J. Exp. Bot. 55, 1195–1205. doi: 10.1093/jxb/erh145
Pincebourde, S., and Casas, J. (2016). Hypoxia and hypercarbia in endophagous insects: larval position in the plant gas exchange network is key. J. Insect Physiol. 84, 137–153. doi: 10.1016/j.jinsphys.2015.07.006
Raghavan, V. (1986). Embryogenesis in Angiosperms. A Developmental and Experimental Study. Cambridge: Cambridge University Press.
Rohfritsch, O. (1992). “Patterns in gall development,” in Biology of Insect-Induced Galls, eds J. D. Shorthouse and O. Rohfritsch (New York, NY: Oxford University Press), 60–86.
Rohfritsch, O., and Anthony, M. (1992). “Strategies in gal induction by two groups of hymenopterans,” in Biology of Insect-Induced Galls, eds J. D. Shorthouse and O. Rohfritsch (New York, NY: Oxford University Press), 102–117.
Rohfritsch, O., and Shorthouse, J. D. (1982). “Insect galls,” in Molecular Biology of Plant Tumors, eds G. Kahl and J. S. Schell (New York, NY: Academic Press), 131–152. doi: 10.1016/B978-0-12-394380-4.50011-6
Schneider, E. A., Gibson, R. A., and Wightman, F. (1972). Biosynthesis and metabolism of indol-3yl-acetic acid. J. Exp. Bot. 74, 152–170. doi: 10.1093/jxb/23.1.152
Scofield, S., and Murray, J. A. H. (2006). The evolving concept of the meristem. Plant Mol. Biol. 60, V–VII. doi: 10.1007/s11103-006-0061-4
Shorthouse, J. D., Wool, D., and Raman, A. (2005). Gall-inducing insects – nature’s most sophisticated herbivores. Basic Appl. Ecol. 6, 407–411. doi: 10.1016/j.baae.2005.07.001
Stone, G. N., and Schönrogge, K. (2003). The adaptive significance of insect gall morphology. Trends Ecol. Evol. 18, 512–522. doi: 10.1111/j.1558-5646.1998.tb02248.x
Suzuki, A. Y., Bedetti, C. S., and Isaias, R. M. S. (2015). Detection and distribution of cell growth regulators and cellulose microfibrils during the development of Lopesia sp. galls on Lonchocarpus cultratus (Fabaceae). Botany 93, 435–444. doi: 10.1139/cjb-2015-0012
Vecchi, C., Menezes, N. L., Oliveira, D. C., Ferreira, B. G., and Isaias, R. M. S. (2013). The redifferentiation of nutritive cells in galls induced by Lepidoptera on Tibouchina pulchra (Cham.) Cogn. reveals predefined patterns of plant development. Protoplasma 250, 1363–1368. doi: 10.1007/s00709-013-0519-6
Keywords: cell redifferentiation, gall shape, hystochemistry, photosynthesis
Citation: Carneiro RGS, Isaias RMS, Moreira ASFP and Oliveira DC (2017) Reacquisition of New Meristematic Sites Determines the Development of a New Organ, the Cecidomyiidae Gall on Copaifera langsdorffii Desf. (Fabaceae). Front. Plant Sci. 8:1622. doi: 10.3389/fpls.2017.01622
Received: 03 April 2017; Accepted: 05 September 2017;
Published: 27 September 2017.
Edited by:
Carolina Escobar, Universidad de Castilla-La Mancha, SpainReviewed by:
Fei Gao, University of Arkansas, United StatesCopyright © 2017 Carneiro, Isaias, Moreira and Oliveira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Denis C. Oliveira, ZGVuaXNvbGl2ZWlyYUB1ZnUuYnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.