- 1Institute of Tropical Agriculture and Forestry, Hainan University, Haikou, China
- 2The Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, China
- 3Key Laboratory of Biology and Genetic Resources of Tropical Crops, Ministry of Agriculture, Haikou, China
Cassava, being one of the top three tuberous crops, features highly efficient starch accumulation in the storage root to adapt the tropical resources and environments. The molecular mechanism for the process, however, is still unclear. ADP-glucose pyrophosphorylase, the first and rate-limited enzyme in starch biosynthesis pathway, is a heterotetramer comprised of two small/catalytic and two large/modulatory subunits. To understand the regulation of MeAGPase, the promoter of a highly expressed small subunit, MeAGPs1a, was used as bait for a yeast one-hybrid assay to screen storage root cDNA library. One cDNA, coding for a small auxin-up RNA protein, named MeSAUR1, was isolated from cassava. MeSAUR1 could bind to the promoter of MeAGPS1a in yeast one-hybrid test and in vitro, and was located in cell nucleus. MeSAUR1 displayed a higher transcript level in cassava root cortex, and its expression was induced by indole-3-acetic acid, gibberellin and ethylene, but repressed by abscisic acid. A dual-luciferase interaction test further convinced that MeSAUR1 could bind to the promoter of MeAGPS1a, and positively regulate the transcription of MeAGPS1a in cassava.
Introduction
Cassava (Manihot esculenta Crantz), originated from Amazon tropical rainforest in South America, is now one of the top three tuberous crops in the global world. Cassava storage root/starch is not only the main staple for African and South American people, but also an important source for food processing and bio-energy industry. Starch is an insoluble polymer of glucose residues produced by the majority of higher plant species, and mainly stored in seeds and storage organs (Tetlow et al., 2004). ADP-glucose pyrophosphorylase (AGPase) is a rate-limited enzyme by utilizing ADP-glucose as the glucosyl donor for the elongation of α-1,4-glucosidic chains in starch biosynthesis pathway, and it consists of two small/catalytic and two large/modulatory subunits as a heterotetramer in higher plants (Ballicora et al., 2004; Saripalli and Gupta, 2015). The AGPase gene family has been characterized in several higher plants, e.g., two small subunits (SS) and several large subunits (LS) in rice and sweet potato, respectively (Akihiro et al., 2005; Zhou et al., 2016). Over-expressing SS gene or/and LS gene could enhance AGPase activity, increase seed weight and starch content (Li et al., 2011).
Auxin is an important plant hormone which can regulate cell elongation and division, root development and the formation of vascular bundle, flower and other organs. In plant, the Auxin responsive genes are mainly divided into three groups: auxin/indole-3-acetic acid (Aux/IAA), gretchenhagen-3 (GH3) and small auxin-up RNA (SAUR). The SAUR genes are the largest auxin responsive gene family, normally with the characteristics of no intron, and one or several auxin responsive elements in their promoter regions (Hagen and Guilfoyle, 2002). SAUR proteins are generally located in plasma membrane, cytoplasm and nucleus, and over-expressing SAUR genes could cause the phenotypic changes in leaf size, hypocotyl and root length, and distortion of inflorescence (Chae et al., 2012; Hou et al., 2013; Stamm and Kumar, 2013; Spartz et al., 2017). Moreover, SAUR proteins, e.g., OsSAUR39 and OsSAUR45, also acted as a negative regulator of auxin synthesis and transport in rice, possibly through the repression of OsYUCCA and OsPIN gene expression (Kant et al., 2009; Xu et al., 2017).
In this study, an Auxin responsive factor gene from cassava, named MeSAUR1, was identified through a yeast one-hybrid screening by using the promoter of MeAGPS1a as bait and the cDNA expression library of storage root as prey. The MeSAUR1 protein could bind to the bait sequence in yeast and their interaction was also confirmed in vitro. Furthermore, a dual-luciferase reporter assay demonstrated that MeSAUR1 could up-regulate the promoter activity of MeAGPS1a, suggesting that MeSAUR1 protein might be a positive regulator of MeAGPS1a and involved in cassava starch biosynthesis.
Materials and Methods
Plant Material
Manihot esculenta Crantz cv.KU50 was used for construction of full-length cDNA library and gene expression analysis. The plants were cultivated in the experimental station of the Institute of Tropical Biosciences and Biotechnology, Chinese Academy of Tropical Agricultural Sciences (Chengmai County, Hainan Province, China). Eight tissues, including tip and mature leaves, flowers, petiole, stems rind, root stele, root cortex, tuber roots and primal roots were collected at 180 days after planting. Tissue cultured plantlets of KU50 were grown in MS medium, and the culture condition was 25°C and 16/8 h in light/dark photoperiod. One-month-old plantlets were treated with 100 μM abscisic acid (ABA), or 10 mM Ethephon (2-chloroethyl phosphoric acid, ET), or 100 μM Gibberellin A3 (GA3) or 10 μM 3-Indole acetic acid (IAA, Sigma, United States). Leaves were harvested at 1, 3, 6, and 12 h after hormone treatments, respectively. As control, plantlets were collected from 0 h and each sample included nine plantlets. All materials were immediately stored at -80°C after liquid nitrogen freeze.
Yeast One-Hybrid
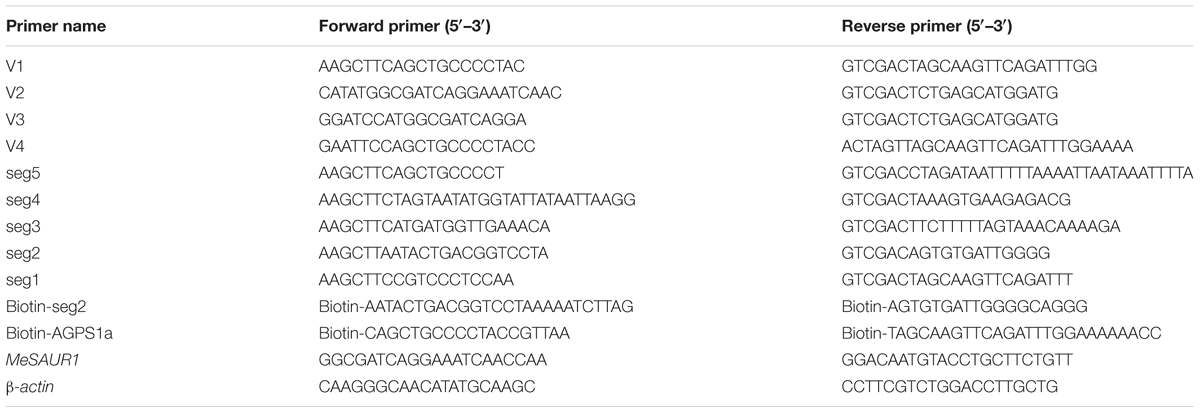
Yeast One-Hybrid assay was conducted according to the method described by Li et al. (2016). The MeAGPS1a promoter (1025 bp) was amplified by PCR with V1 primer pair (Table 1), and then ligated into the pAbAi vector (Clontech, United States) via the Hind III and Sal I restriction sites, constructed yeast one-hybrid bait vector pAbAi-MeAGPS1a. Cassava storage root total RNA, samples from root early development, enlargement and mature stages, was extracted using plant RNeasy extraction kit (Tiangen, CHN). The mRNA was purified using the NucleoTrap mRNA Mini Kit (Macherey Nage, GER) and the single-strand cDNA was synthesized using the RevertAid First-Strand cDNA Synthesis Kit (Fermentas, GER) following the steps provided by manufacturer. The double-stranded cDNA was amplified by long-distance PCR and size selected (>200 bp) using Chroma SpinTM TE-400 columns (Clontech, United States). Cassava double-stranded cDNAs, the prey vector pGADT7-Rec with Sma I-linearized and the bait vector pAbAi-MeAGPS1a were transformed into the yeast strain Y1HGold (Clontech, United States), then cultivated on SD/-Leu medium with 300 ng/ml Aureobasidin A (AbA) at 30°C for 3 days. The drug-resistant colonies were identified by plasmid PCR and sequence analysis.
In order to confirm the interaction between MeSAUR1 and MeAGPS1a promoter, we amplified MeSAUR1 coding sequence (CDS) with V2 primer pair (Table 1). The CDS was ligated into pGADT7 vector through Nde I and Sal I, and named pGADT7-MeSAUR1. pGADT7-MeSAUR1 and pAbAi-MeAGPS1a were co-transformed into the Y1HGold yeast strain, pGADT7-Rec53+p53-AbAi, pAbAi-MeAGPS1a, pGADT7-MeSAUR1 and pGADT7-MeSAUR1+pAbAi were used as controls. Transformed cells were grown on SD/-Leu selective medium with300 ng/ml AbA at 30°C for 3 days.
Bioinformatic Analyses of MeSAUR1
The amino acid sequences of SAUR gene families in cassava and Arabidopsis were downloaded from Phytozome v121 and NCBI2, respectively. A multiple alignment analysis was performed using DNAMAN software. The phylogenetic tree was constructed by MEGA 5.0 using neighbor-joining method (Tamura et al., 2011). Gene or protein accession numbers of cassava and Arabidopsis SAUR genes used in this study were listed in Supplementary Table S1.
Expression Profiles Analysis of MeSAUR1 by Quantitative PCR
Total RNA was extracted by using plant RNeasy extraction kit (Tiangen, CHN) and the first-strand cDNA was reverse-synthesized using the RevertAidTM First-Strand cDNA Synthesis Kit (Fermentas, GER). Quantitative PCR (qPCR) was performed according to Hu’s method (Hu et al., 2016) using Stratagene Mx3000P Real-Time PCR and SYBR® Premix Ex TaqTM (TaKaRa, JPN). The primer pairs of MeSAUR1 and β-actin were list in Table 1. The PCR amplification condition used for all reactions was implemented as follows: 95°C for 90 s, 40 cycles of 10 s at 95°C, 15 s at 55°C and 30 s at 72°C. Each sample was amplified in four independent biological replications, and the mean value was used for expression profile analysis. The relative gene expression data were calculated based on the 2-ΔΔCt method (Livak and Schmittgen, 2001). Significant difference analysis between different samples were tested with IBM SPSS Statistics 23 software.
Subcellular Localization
To confirm the subcelluar location of MeSAUR1, the coding sequence of MeSAUR1 was amplified with V3 primer pair which contained Bam HI and Sal I sites (Table 1). The coding sequence of MeSAUR1was introduced into the pCAMBIA1302 vector to generate CaMV35S::MeSAUR1-GFP. Then CaMV35S::MeSAUR1-GFP and pCAMBIA1302 were transformed into Agrobacterium tumefaciens and infected onion epidermal cells. The transformed onion epidermal was cultured on MS medium in darkness at 25°C for 24–36 h, and then observation was performed by using confocal microscopy.
MeSAUR1 Protein Expression and Extraction
The full-length coding sequence of MeSAUR1 was amplified with V2 primer pair (Table 1). The PCR product was ligated into the pCold Pros2 vector (TaKaRa, JPN) at the site of Nde I/Sal I, generating pCold Pros2-MeSAUR1. pCold Pros2-MeSAUR1 was introduced into Escherichia coli strain BL21 (DE3) for protein expression. E. coli cells containing pCold Pros2-MeSAUR1 were cultured in LB medium supplied with 100 mg/L Ampicillin at 37°C. When the OD600 of the culture reaches 0.4–0.8, quickly cool the culture to 15°C in ice water, and then 1.0 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) was added and the cultures were incubated at 15°C, 120 rpm for 24 h. The cells were collected and resuspended in the BugBuster® Protein Extraction Reagent (Novagen, GER) and incubated at 30°C for 1 h. The supernatant was collected and purified with Ni-Charged MagBeads (GenScript, United States). pCold Pros2 was expressed and purified as a control in accordance with the above methods. The purified protein was verified by SDS-PAGE and Western Blotting (Sambrook and Russell, 2001). Tag Anti-ProS2 (Takara, JPN) and Goat Anti-Mouse IgG/HRP (Boster, United States) were the primary and secondary antibodies used in Western Blot, respectively.
DNA-Protein-Interaction Enzyme-Linked Immunosorbent Assay (DPI-ELISA)
DNA-protein-interaction enzyme-linked immunosorbent assay was according to the method described by Brand et al. (2010). The MeAGPS1a promoter biotinylated probe was obtained by PCR with Biotin-MeAGPS1a primer pair (Table 1). Tag Anti-ProS2 (TaKaRa, JPN) and Goat Anti-Mouse IgG/HRP (Boster, United States) were the primary and secondary antibodies used in DPI-ELISA.
Dual-Luciferase (Dual-LUC) Assay
The assay was performed according to the method of Hellens et al. (2005). LUC and REN were derived from pGreen II 0800-LUC in this study. The MeAGPS1a promoter, obtained with V4 primer pair by PCR, was ligated with LUC through Spe I, generating MeAGPS1a pro::LUC. The MeSAUR1 CDS was fused with CaMV35 promoter through Sal I and ligated with MeAGPS1a pro::LUC to generate CaMV35S::MeSAUR1-MeAGPS1a pro::LUC. Two constructed vectors were introduced into A. tumefaciens strain BLA4404. The culture was infiltrated into the abaxial side of tobacco leaves when its OD600 reached to 0.6–1.0. Total protein was extracted from the infected area after culturing for 3 days. The fluorescent values of LUC and REN were detected according to the steps of the Dual-Luciferase Reporter Assay System (Promega, United States). The value of LUC was normalized to that of REN. Due to the sensitivity of this experiment, each experiment used 16 replicates and retaining the majority of 8–10 repetitions for analysis.
Results
Identification of MeSAUR1 in Cassava
AGPase is the rate-limiting enzyme in plant starch biosynthesis, and it is a heterotetramer, containing two small subunits and two large subunits. The amino acids sequences of Arabidopsis AGPase family genes were used to BLAST in cassava genome database. Three genes encoded small subunits and five genes encoded large subunits were found in cassava (Supplementary Table S2). The transcript level of MeAGPS1a was found to be the highest one in AGPase gene family among all tested tissues, and only the MeAGPS1a protein could interact with the large subunits of cassava AGPase by yeast two-hybrid assay (Supplementary Figure S1).
In order to screen certain transcription factors (TFs) which regulate MeAGPS1a, and understand more about the regulation mechanism of starch biosynthesis in cassava storage root, a yeast one-hybrid assay was performed with the MeAGPS1a promoter as bait and the storage root cDNA library as prey. Finally, over 300 positive colonies with inserted fragments longer than 750 bp were sequenced after re-checking the primary positive colonies on the same selective medium. Eight of them were annotated as candidate TFs by BLAST analysis. One of them was designated as MeSAUR1 (Manes.05G149400), coding for an Auxin responsive SAUR protein. The binding specificity between MeSAUR1 and MeAGPS1a promoter was further assayed by one to one interaction, only the yeast clones harboring MeSAUR1 and pAGPS1a-AbAi or positive control could grow on the SD/-Leu selective medium containing 300 ng/ml AbA (Figure 1). The results illustrated that MeSAUR1 was able to bind to the MeAGPS1a promoter specifically.
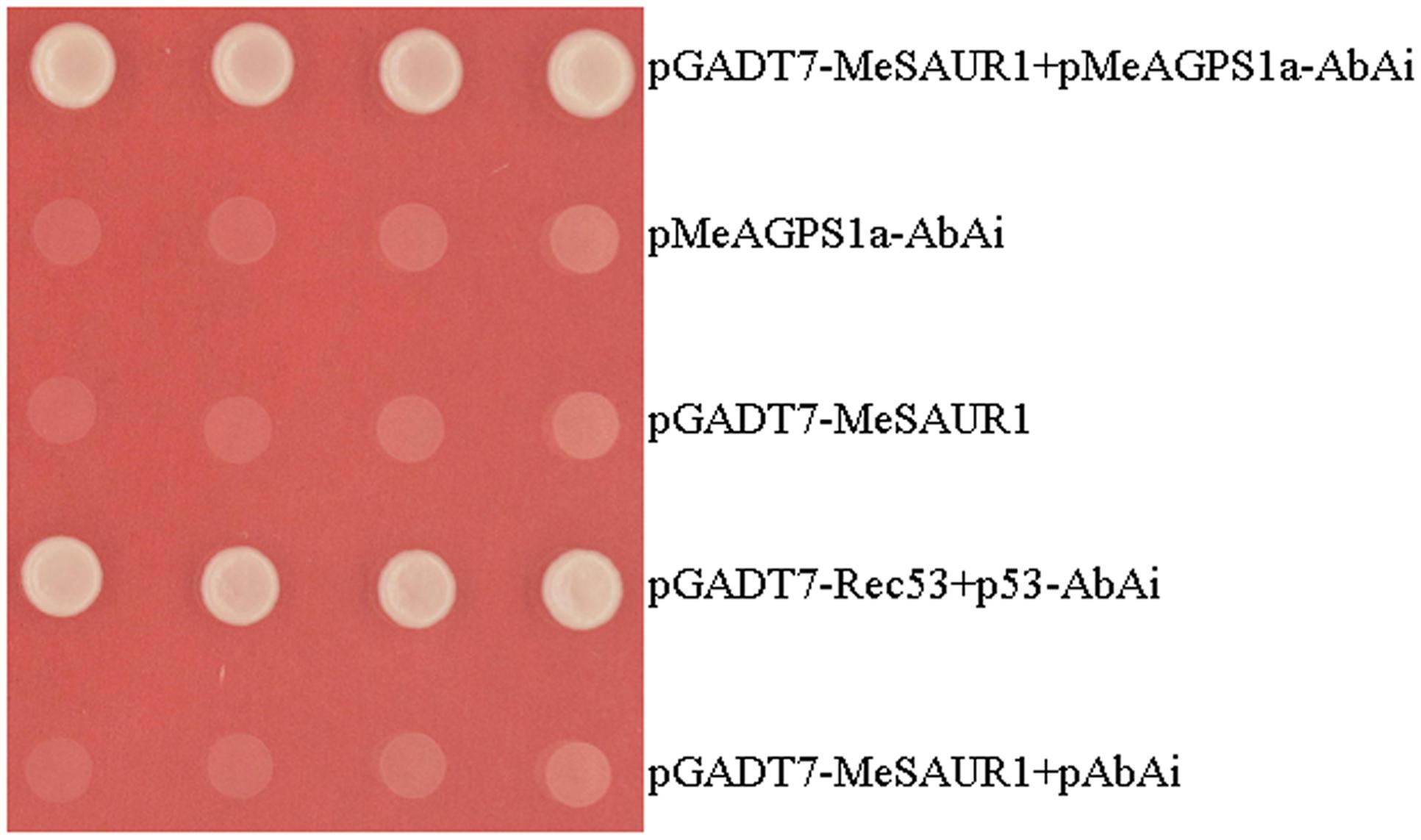
FIGURE 1. Activation of MeAGPS1a promoter in yeast by MeSAUR1. Yeast cells carried pGADT7-MeSAUR1+pMeAGPS1a-AbAi, pMeAGPS1a-AbAi, pGADT7-Rec53+p53-AbAi, pGADT7-MeSAUR1and pGADT7-MeSAUR1+pAbAi were grown in SD/-Leu selective medium containing 300 ng/ml AbA for 3 days at 30°C.
Molecular Characterization of MeSAUR1
The full-length of MeSAUR1 cDNA is 930 bp long, including 318 bp CDS, 125 bp 5′UTR and 488 bp 3′UTR, without any intron. It encodes a protein of 105 amino acid residues with a predicted molecular mass of 12.04 kD. The deduced protein contains a conserved SAUR-specific domain (SSD, Figure 2). The MeSAUR protein family has 132 members in cassava genome, and can be divided into three clades based on a phylogenetic tree. MeSAUR1 is located in the clade I (Figure 3). In addition, there are eight auxin responsive elements (ARE: TGTCNN, Mironova et al., 2014) in the promoter of MeSAUR1, especially two TGTCNN elements (-37, -128) locate nearby its transcription start site (Supplementary Table S3).
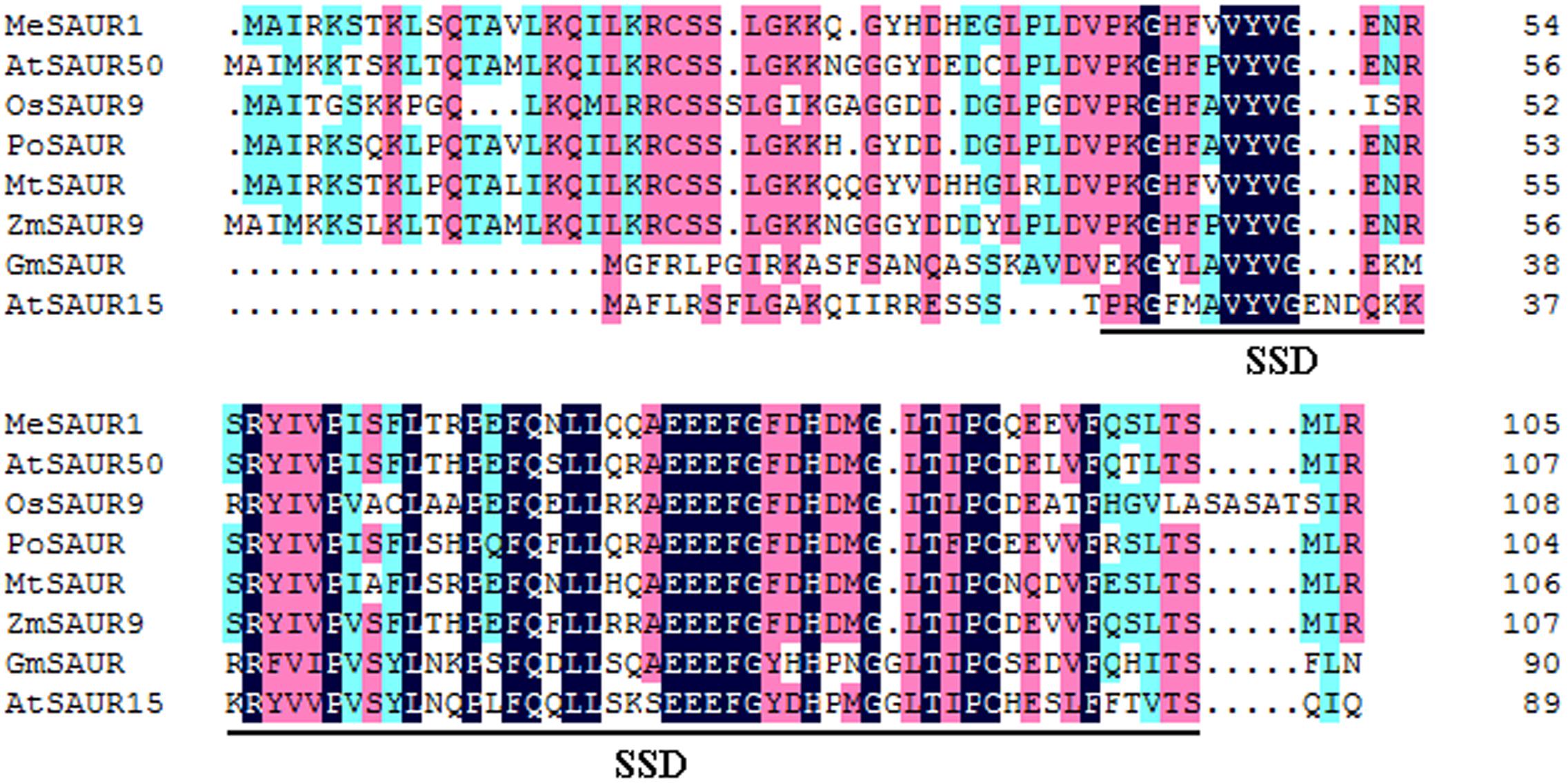
FIGURE 2. Sequence comparison of MeSAUR1 with related SAUR proteins. The sequences were aligned using DNAMAN6.0. Identical residues are highlighted in dark blue, and biochemically conserved substitutions are highlighted in three gradual darker colors according to the identity. The conserved SAUR-specific domain (SSD) is indicated by bracket. The aligned sequences include MeSAUR1 (Manes.05G149400), AtSAUR50 (At4g34760), AtSAUR15 (At4g38850), OsSAUR9 (Os02g24740), PoSAUR (AFT92005), MtSAUR (XP_013462204), ZmSAUR9 (ACG25834), and GmSAUR (S44175).
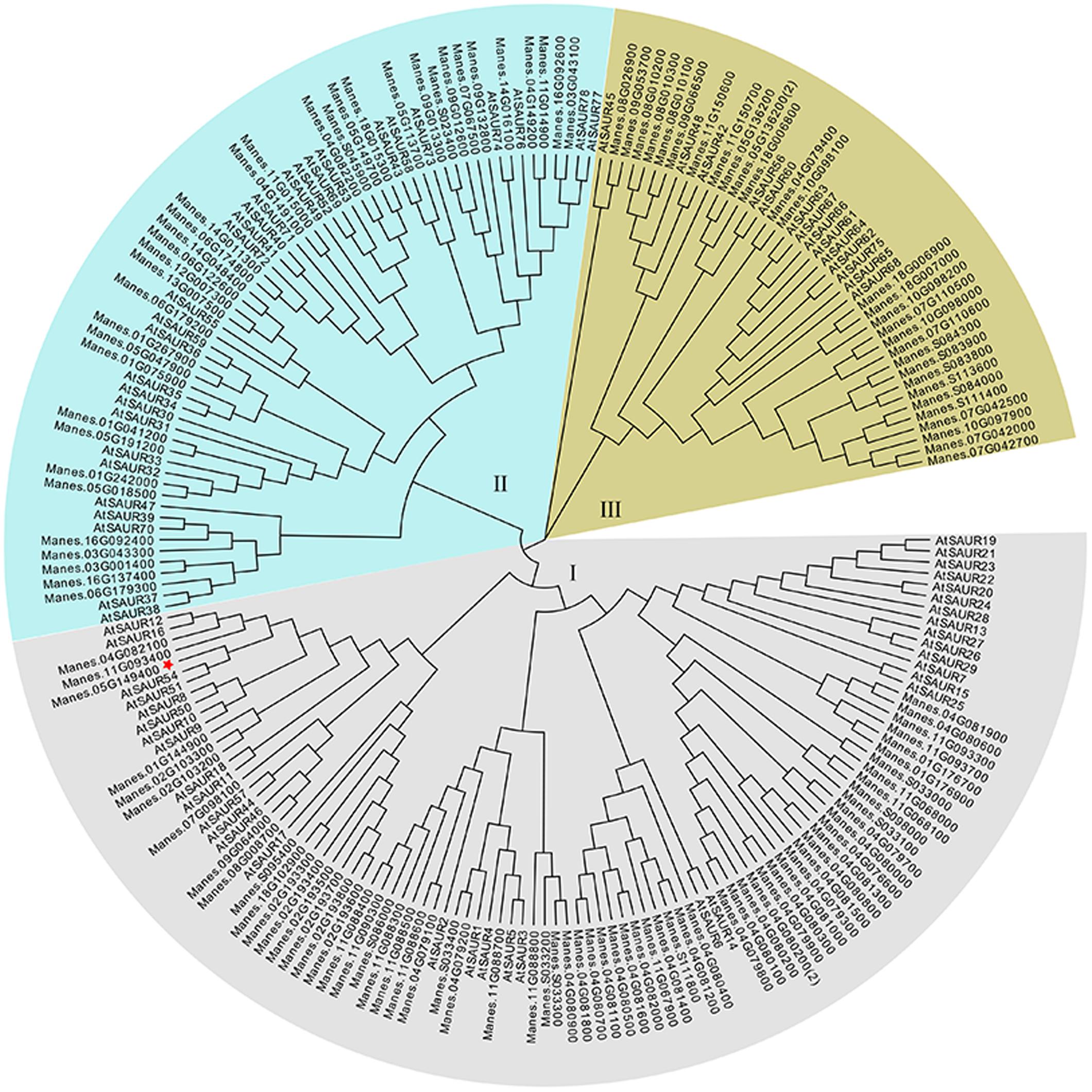
FIGURE 3. Phylogenetic analysis of 132 MeSAUR and 78 AtSAUR proteins MEGA version 5 from Cluster X2 alignment. The neighbor-joining method was used to construct the tree, the SAUR proteins used in the phylogenetic analysis are retrieved from Genebank and Phytozome, and their gene IDs are listed in Supplementary Table S1.
Expression Analysis of MeSAUR1
The transcript levels of MeSAUR1in different cassava tissues were measured by qPCR assay, and the results indicated that MeSAUR1 was highly expressed in root cortex, root stele and petiole, but relatively less expressed in other organs (Figure 4A). Furthermore, in order to examine the response to plant hormone for MeSAUR1, the cassava tissue cultured seedlings were treated with ABA, IAA, GA, and ET, respectively. The expression level of MeSAUR1 was reduced by ABA, but induced by IAA, GA, and ET, and reached to its peak all at 3 h after treatment, then remarkably decreased 6 h afterward (Figure 4B).
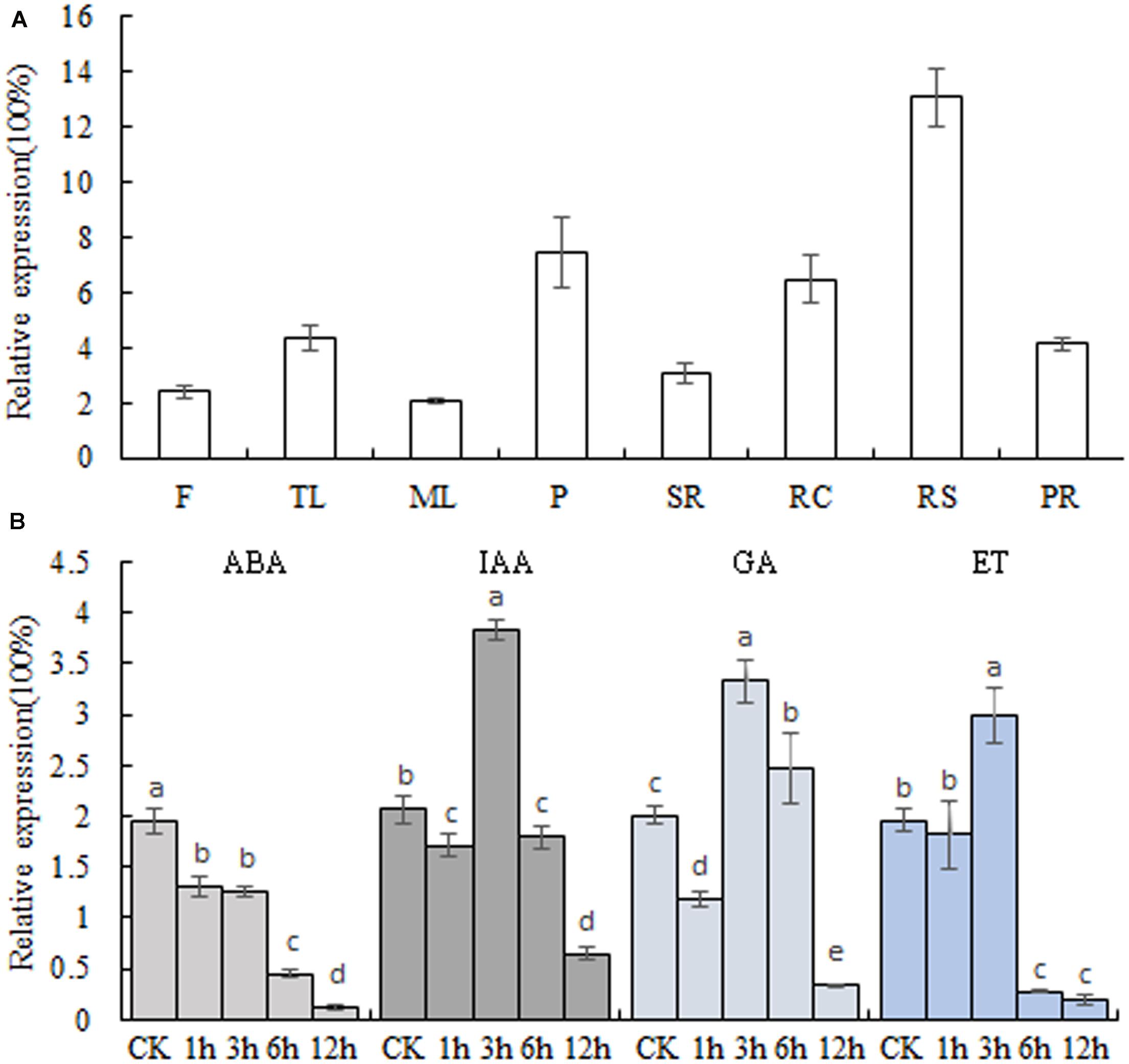
FIGURE 4. Transcription patterns of MeSAUR1. (A) Expression pattern of MeSAUR1 in different tissues of cassava plant. F, flower; TL, tip leaf; ML, mature leaf; P, petiole; SR, stem rind; RC, root cortex; RS, root stele; PR, primal root. (B) Transcription profiles of MeSAUR1 respond to ABA, IAA, GA, and ET. The y-axis is the scale of the relative transcript expression. Error bars represent the SD of four technical replicates, the significant difference is assessed by ANOVA at P < 0.05. a–e means the significant difference at p < 0.05 level.
Sub-cellular Location of MeSAUR1
To determine the sub-location of MeSAUR1 protein, the green fluorescent protein (GFP) reporter gene was fused in frame to the N-terminus of MeSAUR1 and transiently expressed into onion epidermal cells by particle bombardment and observed under a fluorescent microscope. The MeSAUR1-GFP fusion protein was located in the nucleus of onion epidermal cell, while the control GFP was dispersed in the onion epidermal cell (Figure 5). The result clearly indicated that MeSAUR1 is a nuclear-localized protein.
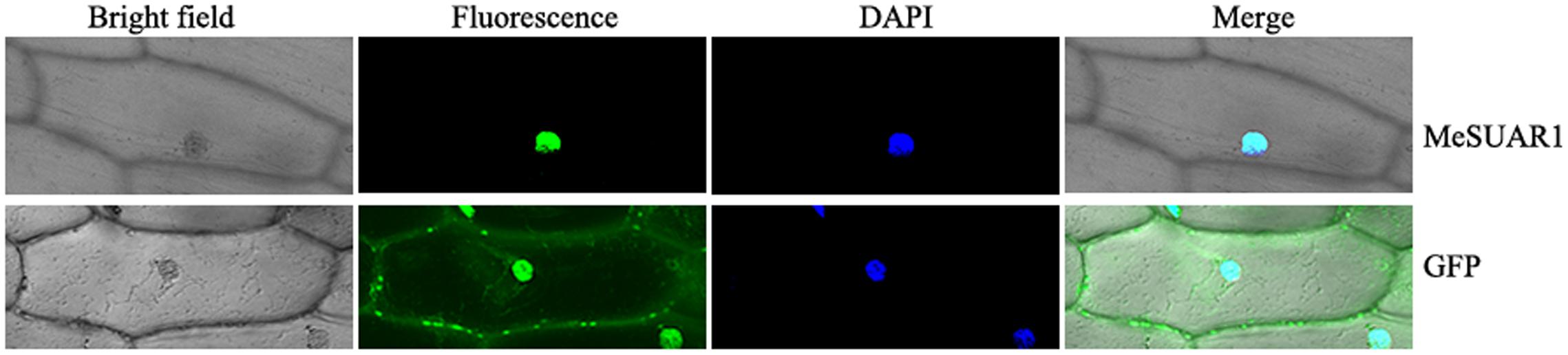
FIGURE 5. Nuclear localization of MeSAUR1. Top row/Bottom row: the corresponding bright field, fluorescence, merged fluorescence image, and DAPI image of MeSAUR1-GFP/GFP control.
MeSAUR1 Can Bind to the MeAGPS1a Promoter In Vitro
In order to determine whether MeSAUR1 interacts with the MeAGPS1a promoter in vitro, the purified recombinant MeSAUR1 protein was isolated from E. coli and its molecular mass was identical to the theoretical value when a 23 kD tagged protein ProS2 was added on (Figures 6A,B). Then, we performed DPI-ELISA assay using the recombinant protein and the double-strand biotinylated MeAGPS1a promoter DNA probes. The results suggested that MeSAUR1 bound to the MeAGPS1a promoter, as the absorbance of MeSAUR1+MeAGPS1a promoter was over fourfold higher than those of the two controls (Figure 6C). It demonstrated that there was an interaction between MeSAUR1 and MeAGPs1a promoter in vitro.
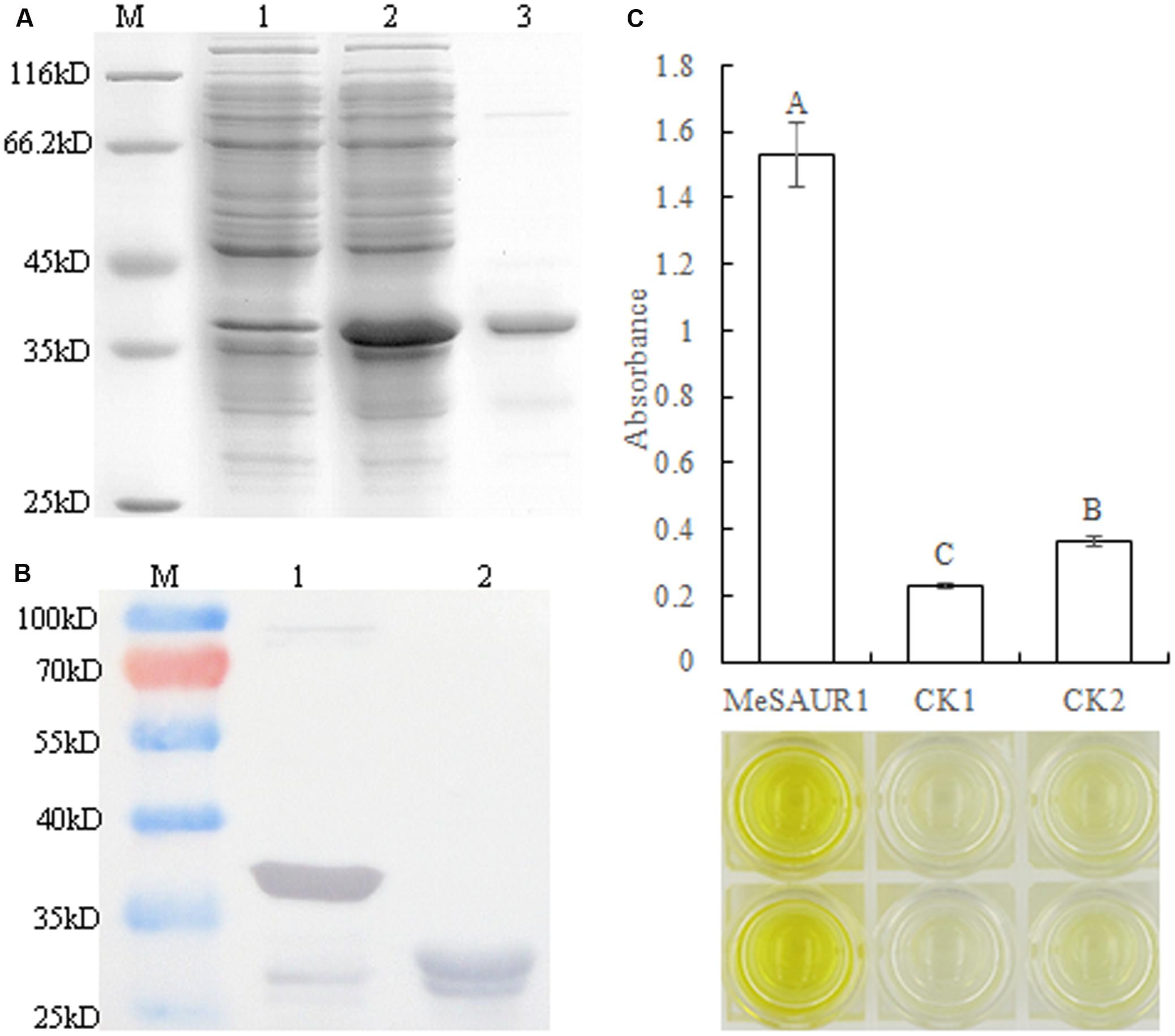
FIGURE 6. MeSAUR1 binding to the promoter of MeAGPS1a as analyzed DPI-ELISA. (A) Over-expression of MeSAUR1 in Escherichia coli. M: molecular markers, (1) E. coli cells harboring pCold Pros2-MeSAUR1 not induced; (2) E. coli cells harboring pCold Pros2-MeSAUR1 after 5 h of induction; (3) the purified MeSAUR1 fusion protein by E. coli cells harboring pCold Pros2-MeSAUR1 after 5 h of induction. (B) Western blot of purified MeSAUR1 fusion protein. M: Molecular markers; (1) MeSAUR1 fusion protein; (2) tagged protein ProS2. (C) MeSAUR1 interacts with the MeAGPS1a promoter by DPI-ELISA assay. MeSAUR1: double-strand biotinylated MeAGPS1a promoter DNA probe + purified MeSAUR1 fusion protein; CK1: double-strand biotinylated MeAGPS1a promoter DNA probe + tagged protein ProS2; CK2: purified water + purified MeSAUR1 fusion protein. Error bars represent the SD of four technical replicates, the significant difference is assessed by ANOVA at P < 0.01. A–C means the significant difference at p < 0.01 level.
MeSAUR1 Binds to the Region of -400 to -201 in the MeAGPS1a Promoter
Although we demonstrated that MeSAUR1 was able to interact with the MeAGPS1a promoter, its exact binding region was unclear. Therefore, the MeAGPS1a promoter was digested into five segments, with each segment about 200 bp, named as Seg1 to Seg5 (Figure 7A). Their PCR products were obtained with primer pairs seg1 to seg5 (Table 1). These segments were linked to pAbAi vector and transformed into Y1HGold yeast, and the SD/-Ura medium with 50 ng/ml AbA could effectively inhibit the growth of positive transformed Y1HGold yeasts. Then, the vector of pGADT7-AD-MeSUAR1 was transformed into pSeg1-AbAi to pSeg5-AbAi yeast cells, respectively, and pGADT7-AD-MeSUAR1+pSeg2-AbAi yeast cell could grow well in the SD/-Leu selective medium with 50 ng/ml AbA, but other four co-transformed yeast cells could not survive in the same medium (Figure 7B). Furthermore, we performed DPI-ELISA assay using the MeSAUR1 recombinant protein and the biotinylated Seg2 DNA probe again (Biotin-seg2, Table 1), the results indicated that MeSAUR1 could bind to the region of -400 to -201 in MeAGPS1a promoter accurately (Figure 7C).
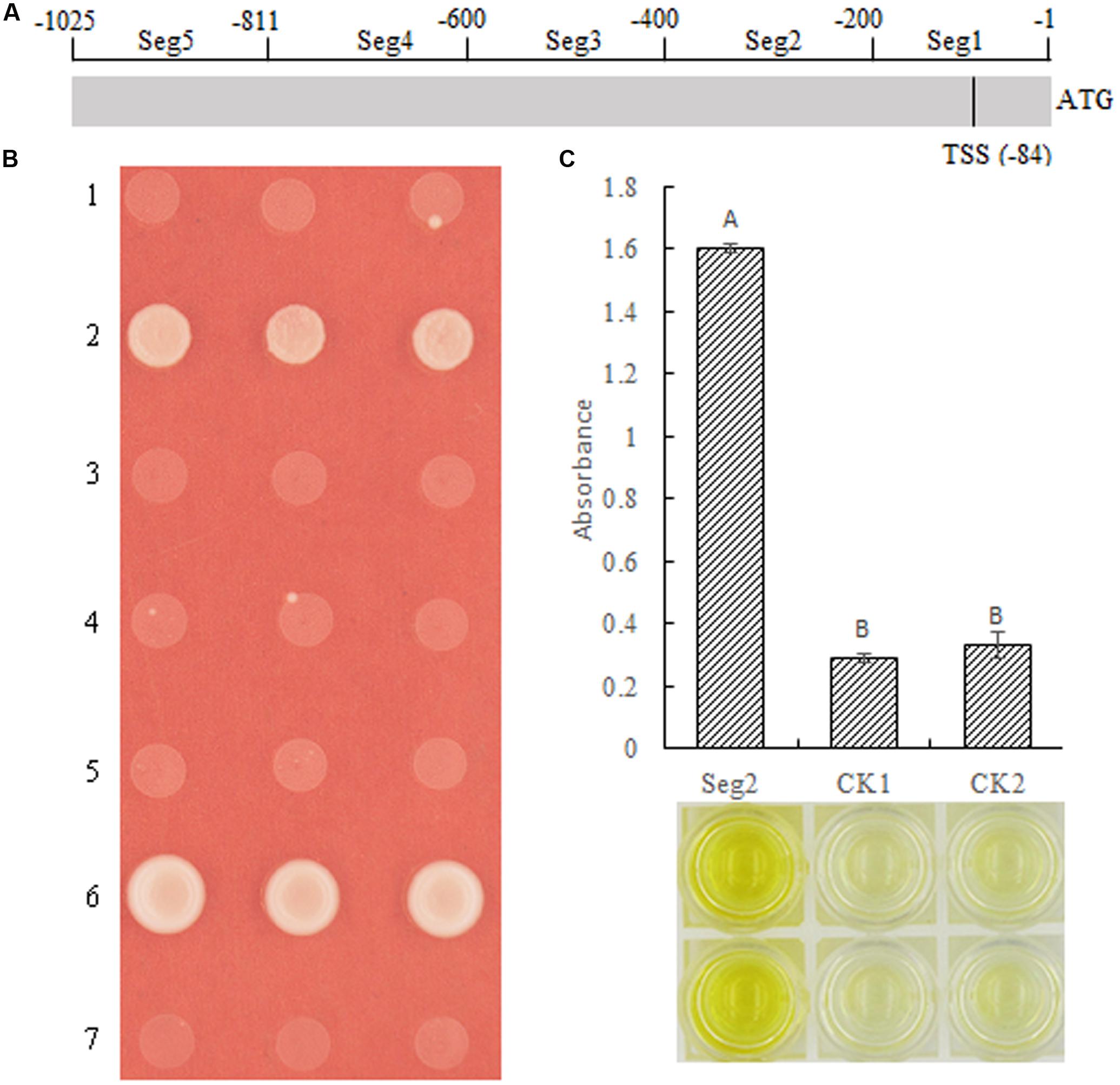
FIGURE 7. MeSAUR1 binding to the region of –400 to –201 in MeAGPS1a promoter. (A) The distribution of five segments in the MeAGPS1a promoter. TSS: transcription start site. (B) Activation of MeAGPS1a promoter in yeast by MeSAUR1. Yeast cells carried pGADT7-MeSAUR1 + p(Seg1 to Seg5)-AbAi (1–5), pGADT7-Rec53 + p53-AbAi (6) and pGADT7-MeSAUR1 + pAbAi (7) were grown in SD/-Leu selective medium with 50 ng/ml AbA for 3 days at 30°C; the three dots in each line mean three replications. (C) MeSAUR1 interacts with the Seg2 by DPI-ELISA assay. Seg2: double-strand biotinylated Seg2DNA probe + purified MeSAUR1 fusion protein; CK1: double-strand biotinylated Seg2 DNA probe + tagged protein ProS2; CK2: purified water + purified MeSAUR1 fusion protein. Error bars represent the SD of four technical replicates, the significant difference is assessed by ANOVA at P < 0.01. A,B means the significant difference at p < 0.01 level.
Activation of the MeAGPS1a Promoter by MeSAUR1
To provide evidence that MeSAUR1 protein regulates the transcription of MeAGPS1a, a dual-luciferase reporter assay was introduced. An integrated vector was created which contained 35S::MeSAUR1 and pMeAGPS1a::Luc (Figure 8A), then transformed into Agrobacterium line LBA4404 for transient expression in tobacco leaves. The relative luciferase activity of pCaMV35S::MeSAUR1-MeAGPS1a pro::LUC was higher than that of pMeAGPS1a pro::LUC (Figure 8B), indicating that MeSAUR1 could upregulate the transcript level of MeAGPS1a in cassava.
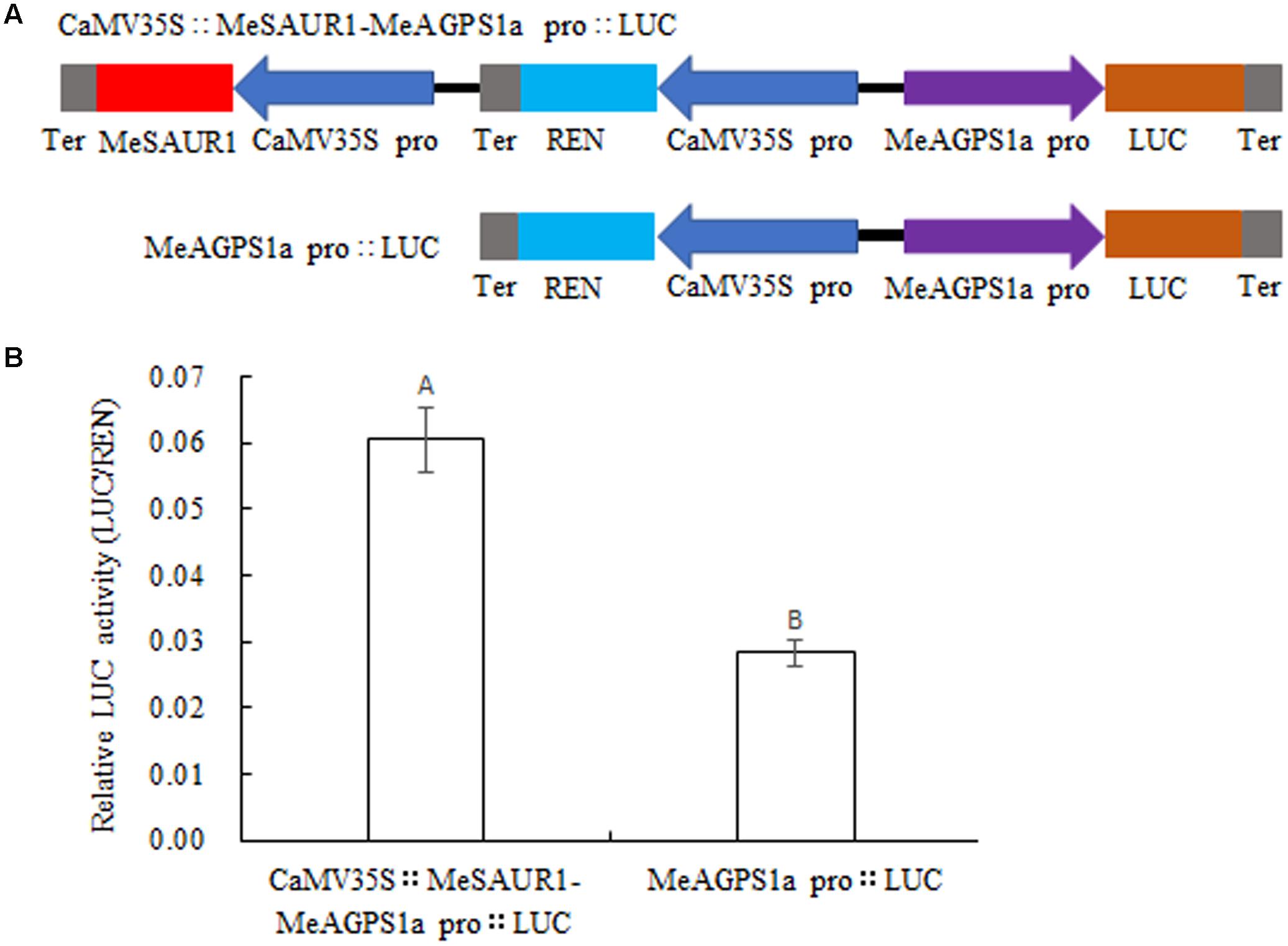
FIGURE 8. Activation of MeAGPS1a promoter in the transient expression system by MeSAUR1. (A) Schematic diagrams of the transient expression vectors used in the transient expression analysis. (B) Relative LUC activity of vector pCaMV35S::MeSAUR1-MeAGPS1a Pro::LUC and pMeAGPS1a Pro::LUC in tobacco leaves. Error bars represent the SD of nine technical replicates, the significant difference is assessed by ANOVA at P < 0.01. A,B means the significant difference at p < 0.01 level.
Discussion
We used the promoter of MeAGPS1a as bait and the cDNA expression library of storage root as prey to perform a yeast one-hybrid screening. Eight of the positive colonies were annotated as candidate TFs. This study is focused on MeSAUR1, one of the candidates, which can bind to the -400 to -201 region in MeAGPS1a promoter in vitro and in vivo, and can up-regulate the promoter activity of MeAGPS1a. SAUR gene family is auxin responsive factor, and has been reported as functional genes involved in tissue development (Park et al., 2007; Spartz et al., 2012; Kong et al., 2013). MeSAUR1 protein is located in the cell nucleus. Combining all our results, we could conclude that MeSAUR1 is a TF despite the lack of the typical conserved domains of TFs. In addition, further mutation analysis should be used to demonstrate the specific DNA binding site of the SAUR protein.
MeSAUR1 expression can be induced by the application of exogenous auxin, GA and ET, and repressed by exogenous ABA in cassava. Interestingly, many plant hormone responsive elements are located in the promoter of MeSAUR1, e.g., eight AREs, two ERELEE4 (Rawat et al., 2005), three GAREs (Gubler and Jacobsen, 1992) and two ABREs (AATTATTA, Supplementary Table S3). It is worth mentioning that the ABRE could interact with ATHB6 protein, and the ATHB6 seemed to represent a negative regulator of ABA signal pathway (Himmelbach et al., 2002). Furthermore, other SAUR genes could also be induced or repressed by more than one type of plant hormones, for instance, AtSAUR36 was induced by auxin and repressed by GA; AtSAUR76, 77 and 78 were induced by auxin and ET, and their encoded proteins could interaction with ETR2 and EIN2 as crosstalk points between auxin and ethylene signaling pathway (Stamm and Kumar, 2013; Li et al., 2015). Therefore, MeSAUR1 might be at a crucial crosstalk point in plant hormone signaling pathways, also a mediate for plant hormone to affect starch biosynthesis in cassava.
AGPase is a key enzyme in starch biosynthesis, and over-expressing AGPase genes could increase the amount of its functional proteins, and further improve seed/root starch content and other related traits in cereal and root crops (Sweetlove et al., 1996; Tuncel and Okita, 2013). TF is a protein that can regulate the expression of genes by binding to a specific DNA sequence (Karin, 1990; Latchman, 1997), and many reports have shown that TF can function as transcriptional regulators for starch biosynthesis genes in plant. OsbZIP58 can directly bind to the promoter of six starch biosynthesis genes, OsAGPL3, Wx, OsSSIIa, OsSBE1, OsBEIIb, and OsISA2, and regulate their expression in rice (Wang et al., 2013). Some TFs have shown positive or negative co-expression pattern with starch biosynthesis genes, such as RSR1, a member of AP2/ERF family, could up-regulate starch biosynthesis genes and increase starch content in rice endosperm while it gets knocked out (Fu and Xue, 2010). Over-expression of ZmaNAC36 could up-regulate some starch biosynthesis genes, AGPL2, AGPS2, SSI, GBSSIIb, and SBEI, in maize endosperm, indicating that ZmaNAC36 is involved in the co-expression of many starch biosynthesis genes, and might play a role in regulating starch biosynthesis (Zhang et al., 2016). MeSAUR1 can bind to the MeAGPS1a promoter and activate its promoter activity, suggesting that MeSAUR1 can up-regulate the transcript level of MeAGPS1a in cassava. Currently, we are over-expressing or silencing MeSAUR1 in cassava by a transgenic approach to further investigate and analyze its influence to starch biosynthesis in cassava storage root.
Cassava is an important tuberous root crop with enriched starch in its storage root, and cassava starch is a main staple for over 700 million people in the world (Zhang et al., 2010). However, the molecular mechanism of high starch accumulation in cassava is still unclear. Many studies have been focused on genetic modification of AGPase and granule bound starch synthase (GBSS), few reports are involved in regulatory mechanism of starch biosynthesis in cassava (Ihemere et al., 2006; Zhao et al., 2011; Rolland-Sabaté et al., 2013). A TF, MeSAUR1, identified in this study, can up-regulate the transcript level of MeAGPS1a. This is our first step to discover the molecular mechanism of highly efficient starch accumulation in cassava. Subsequently, a series of studies on the TF and cassava starch biosynthesis genes, MeAGPS1a, MeAGPL3, MeSus1, and GBSSI, will be carried out. Eventually, we hope to reveal the regulation system, to find the core TFs which can up-regulate the entire starch biosynthesis pathway and to improve starch yield in cassava.
Conclusion
One cDNA, coding for a small auxin-up RNA protein, named MeSAUR1, was isolated from cassava. MeSAUR1 could bind to the promoter in yeast one-hybrid system and in vitro, and was located in cell nucleus. The expression level of MeSAUR1 was high in root cortex, and could be up-regulated under the treatment of IAA, GA, and ET but down-regulated by ABA. MeSAUR1 could activate the promoter of MeAGPS1a in tobacco leaves, indicating that it could positively regulate the transcript level of MeAGPS1a in cassava.
Author Contributions
WW and XC designed the research. PM, CLi, YM, ZX, and CLu performed the research. XC and PM wrote the paper. CZ and WW modified the paper. All authors read and approved the final manuscript.
Funding
This work was supported financially by Natural Science Foundation of China (31671748), Hainan Province Innovative Research Team Foundation (2016CXTD013), China Agriculture Research System (CARS-12) and Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (1630052016004).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
Dr. Yong-Mei Bi (Department of Molecular and Cellular Biology, University of Guelph, Canada) has modified the language for the manuscript and given us many constructive suggestions.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.01315/full#supplementary-material
FIGURE S1 | The interaction between small subunit and large subunit by yeast two-hybrid assay.
TABLE S1 | Listed members of 132 MeSAUR and 78 AtSAUR proteins.
TABLE S2 | Characteristics of AGPase family members in cassava.
TABLE S3 |Cis-elements distribution in the promoter of MeSAUR1 related to four plant hormones.
Footnotes
References
Akihiro, T., Mizuno, K., and Fujimura, T. (2005). Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA. Plant Cell Physiol. 46, 937–946. doi: 10.1093/pcp/pci101
Ballicora, M. A., Iglesias, A. A., and Preiss, J. (2004). ADP-glucose pyrophosphorylase: a regulatory enzyme for plant starch synthesis. Photosynth. Res. 79, 1–24. doi: 10.1023/B:PRES.0000011916.67519.58
Brand, L. H., Kirchler, T., Hummel, S., Chaban, C., and Wanke, D. (2010). DPI-ELISA: a fast and versatile method to specify the binding of plant transcription factors to DNA in vitro. Plant Methods 6:25. doi: 10.1186/1746-4811-6-25
Chae, K., Isaacs, C. G., Reeves, P. H., Maloney, G. S., Muday, G. K., Nagpal, P., et al. (2012). Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation. Plant J. 71, 684–697. doi: 10.1111/j.1365-313X.2012.05024.x
Fu, F. F., and Xue, H. W. (2010). Coexpression analysis identifies Rice Starch Regulator1, a rice AP2/EREBP family transcription factor, as a novel rice starch biosynthesis regulator. Plant Physiol. 154, 927–938. doi: 10.1104/pp.110.159517
Gubler, F., and Jacobsen, J. V. (1992). Gibberellin-responsive elements in the promoter of a barley high-pI alpha-amylase gene. Plant Cell 4, 1435–1441.
Hagen, G., and Guilfoyle, T. (2002). Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol. Biol. 49, 373–385. doi: 10.1023/A:1015207114117
Hellens, R. P., Allan, A. C., Friel, E. N., Bolitho, K., Grafton, K., Templeton, M. D., et al. (2005). Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1, 13. doi: 10.1186/1746-4811-1-13
Himmelbach, A., Hoffmann, T., Leube, M., Hohnener, B., and Grill, E. (2002). Homeodomain protein ATHB6 is a target of the protein phosphatase ABI1 and regulates hormone responses in Arabidopsis. EMBO J. 21, 3029–3038. doi: 10.1093/emboj/cdf316
Hou, K., Wu, W., and Gan, S. S. (2013). SAUR36, a small auxin up RNA gene, is involved in the promotion of leaf senescence in Arabidopsis. Plant Physiol. 161, 1002–1009. doi: 10.1104/pp.112.212787
Hu, M., Hu, W., Xia, Z., Zhou, X., and Wang, W. (2016). Validation of reference genes for relative quantitative gene expression studies in cassava (Manihot esculenta Crantz) by using quantitative real-time PCR. Front. Plant Sci. 7:680. doi: 10.3389/fpls.2016.00680
Ihemere, U., Arias-Garzon, D., Lawrence, S., and Sayre, R. (2006). Genetic modification of cassava for enhanced starch production. Plant Biotechnol. J. 4, 453–465. doi: 10.1111/j.1467-7652.2006.00195.x
Kant, S., Bi, Y. M., Zhu, T., and Rothstein, S. J. (2009). SAUR39, a small auxin-up RNA gene, acts as a negative regulator of auxin synthesis and transport in rice. Plant Physiol. 151, 691–701. doi: 10.1104/pp.109.143875
Karin, M. (1990). Too many transcription factors: positive and negative interactions. New Biol. 2, 126–131.
Kong, Y., Zhu, Y., Gao, C., She, W., Lin, W., Chen, Y., et al. (2013). Tissue-specific expression of SMALL AUXIN UP RNA41 differentially regulates cell expansion and root meristem patterning in Arabidopsis. Plant Cell Physiol. 54, 609–621. doi: 10.1093/pcp/pct028
Latchman, D. S. (1997). Transcription factors: an overview. Int. J. Biochem. Cell Biol. 29, 1305–1312. doi: 10.1016/S1357-2725(97)00085-X
Li, H. L., Wei, L. R., Guo, D., Wang, Y., Zhu, J. H., Chen, X. T., et al. (2016). HbMADS4, a MADS-box transcription factor from Hevea brasiliensis, negatively regulates HbSRPP. Front. Plant Sci. 7:1709. doi: 10.3389/fpls.2016.01709
Li, N., Zhang, S., Zhao, Y., Li, B., and Zhang, J. (2011). Over-expression of AGPase genes enhances seed weight and starch content in transgenic maize. Planta 233, 241–250. doi: 10.1007/s00425-010-1296-5
Li, Z. G., Chen, H. W., Li, Q. T., Tao, J. J., Bian, X. H., Ma, B., et al. (2015). Three SAUR proteins SAUR76, SAUR77 and SAUR78 promote plant growth in Arabidopsis. Sci. Rep. 5:12477. doi: 10.1038/srep12477
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-Δ Δ CT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Mironova, V. V., Omelyanchuk, N. A., Wiebe, D. S., and Levitsky, V. G. (2014). Computational analysis of auxin responsive elements in the Arabidopsis thaliana L. genome. BMC Genomics 15(Suppl. 12):S4. doi: 10.1186/1471-2164-15-S12-S4
Park, J. E., Kim, Y. S., Yoon, H. K., and Park, C. M. (2007). Functional characterization of a small auxin-up RNA, gene in apical hook development in Arabidopsis. Plant Sci. 172, 150–157. doi: 10.1371/journal.pone.0082596
Rawat, R., Xu, Z. F., Yao, K. M., and Chye, M. L. (2005). Identification of cis-elements for ethylene and circadian regulation of the Solanum melongena gene encoding cysteine proteinase. Plant Mol. Biol. 57, 629–643. doi: 10.1007/s11103-005-0954-7
Rolland-Sabaté, A., Sanchez, T., Buléon, A., Colonna, P., Ceballos, H., Zhao, S. S., et al. (2013). Molecular and supra-molecular structure of waxy starches developed from cassava (Manihot esculenta Crantz). Carbohydr. Polym. 92, 1451–1462. doi: 10.1016/j.carbpol.2012.10.048
Sambrook, J., and Russell, D. W. (2001). Molecular Cloning: A Laboratory Manual, 3rd Edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 1474–1478.
Saripalli, G., and Gupta, P. K. (2015). AGPase: its role in crop productivity with emphasis on heat tolerance in cereals. Theor. Appl. Genet. 128, 1893–1916. doi: 10.1007/s00122-015-2565-2
Spartz, A. K., Lee, S. H., Wenger, J. P., Gonzalez, N., Itoh, H., Inzé, D., et al. (2012). The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. Plant J. 70, 978–990. doi: 10.1111/j.1365-313X.2012.04946.x
Spartz, A. K., Lor, V. S., Ren, H., Olszewski, N. E., Miller, N. D., Wu, G., et al. (2017). Constitutive expression of Arabidopsis SMALL AUXIN UP RNA19 (SAUR19) in tomato confers Auxin-Independent hypocotyl elongation. Plant Physiol. 173, 1453–1462. doi: 10.1007/s00299-013-1406-5
Stamm, P., and Kumar, P. P. (2013). Auxin and gibberellin responsive Arabidopsis SMALL AUXIN UP RNA36 regulates hypocotyl elongation in the light. Plant Cell Rep. 32, 759–769. doi: 10.1007/s00299-013-1406-5
Sweetlove, L. J., Burrell, M. M., and ap Rees, T. (1996). Characterization of transgenic potato (Solanum tuberosum) tubers increased ADP-glucose pyrophosphorylase. Biochem. J. 320(Pt 2), 487–492. doi: 10.1042/bj3200487
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., and Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. doi: 10.1093/molbev/msr121
Tetlow, I. J., Morell, M. K., and Emes, M. J. (2004). Recent developments in understanding the regulation of starch metabolism in higher plants. J. Exp. Bot. 55, 2131–2145. doi: 10.1093/jxb/erh248
Tuncel, A., and Okita, T. W. (2013). Improving starch yield in cereals by over-expression of ADPglucose pyrophosphorylase: expectations and unanticipated outcomes. Plant Sci. 211, 252–260. doi: 10.1016/j.plantsci.2013.06.009
Wang, J. C., Xu, H., Zhu, Y., Liu, Q. Q., and Cai, X. L. (2013). OsbZIP58, a basic leucine zipper transcription factor, regulates starch biosynthesis in rice endosperm. J. Exp. Bot. 64, 3453–3466. doi: 10.1093/jxb/ert187
Xu, Y. X., Xiao, M. Z., Liu, Y., Fu, J. L., He, Y., and Jiang, D. A. (2017). The small auxin-up RNA OsSAUR45 affects auxin synthesis and transport in rice. Plant Mol. Biol. 94, 97–107. doi: 10.1007/s11103-017-0595-7
Zhang, J., Chen, J., Yi, Q., Hu, Y., Liu, H., Liu, Y., et al. (2016). Novel role of ZmaNAC36 in co-expression of starch synthetic genes in maize endosperm. Plant Mol. Biol. 84, 359–369. doi: 10.1007/s11103-013-0153-x
Zhang, P., Wang, W. Q., Zhang, G. L., Kaminek, M., Dobrev, P., Xu, J., et al. (2010). Senescence-inducible expression of isopentenyl transferase extends leaf life, increases drought stress resistance and alters cytokinin metabolism in cassava. J. Integr. Plant Biol. 52, 653–669. doi: 10.1111/j.1744-7909.2010.00956.x
Zhao, S. S., Dufour, D., Sánchez, T., Ceballos, H., and Zhang, P. (2011). Development of waxy cassava with different biological and physico-chemical characteristics of starches for industrial applications. Biotechnol. Bioeng. 108, 1925–1935. doi: 10.1002/bit.23120
Keywords: cassava, small auxin-up RNA gene, ADP-glucose pyrophosphorylase, starch biosynthesis, transcription factor
Citation: Ma P, Chen X, Liu C, Meng Y, Xia Z, Zeng C, Lu C and Wang W (2017) MeSAUR1, Encoded by a Small Auxin-Up RNA Gene, Acts as a Transcription Regulator to Positively Regulate ADP-Glucose Pyrophosphorylase Small Subunit1a Gene in Cassava. Front. Plant Sci. 8:1315. doi: 10.3389/fpls.2017.01315
Received: 02 May 2017; Accepted: 12 July 2017;
Published: 31 July 2017.
Edited by:
Keqiang Wu, National Taiwan University, TaiwanReviewed by:
Jin-Song Zhang, Institute of Genetics and Developmental Biology, CAS, ChinaJunxian He, The Chinese University of Hong Kong, Hong Kong
Copyright © 2017 Ma, Chen, Liu, Meng, Xia, Zeng, Lu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Chen, Y2hlbnhpbkBpdGJiLm9yZy5jbg== Wenquan Wang, d2FuZ3dlbnF1YW5AaXRiYi5vcmcuY24=
†These authors have contributed equally to this work.
 Ping’an Ma
Ping’an Ma Xin Chen
Xin Chen Chen Liu1,2,3
Chen Liu1,2,3 Zhiqiang Xia
Zhiqiang Xia Changying Zeng
Changying Zeng Wenquan Wang
Wenquan Wang