
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 28 June 2017
Sec. Plant Breeding
Volume 8 - 2017 | https://doi.org/10.3389/fpls.2017.01099
This article is part of the Research TopicQuality of horticultural crops: a recurrent/new challenge for plant scientists in a changing worldView all 24 articles
 Meiru Jia
Meiru Jia Ning Ding
Ning Ding Qing Zhang
Qing Zhang Sinian Xing
Sinian Xing Lingzhi Wei
Lingzhi Wei Yaoyao Zhao
Yaoyao Zhao Ping Du
Ping Du Wenwen Mao
Wenwen Mao Jizheng Li
Jizheng Li Bingbing Li*
Bingbing Li* Wensuo Jia*
Wensuo Jia*Ripening of fleshy fruits is controlled by a series of intricate signaling processes. Here, we report a FERONIA/FER-like receptor kinase, FaMRLK47, that regulates both strawberry (Fragaria × ananassa) fruit ripening and quality formation. Overexpression and RNAi-mediated downregulation of FaMRLK47 delayed and accelerated fruit ripening, respectively. We showed that FaMRLK47 physically interacts with FaABI1, a negative regulator of abscisic acid (ABA) signaling, and demonstrated that FaMRLK47 regulates fruit ripening by modulating ABA signaling, a major pathway governing strawberry fruit ripening. In accordance with these findings, overexpression and RNAi-mediated downregulation of FaMRLK47 caused a decrease and increase, respectively, in the ABA-induced expression of a series of ripening-related genes. Additionally, overexpression and RNAi-mediated downregulation of FaMRLK47 resulted in an increase and decrease in sucrose content, respectively, as compared with control fruits, and respectively promoted and inhibited the expression of genes in the sucrose biosynthesis pathway (FaSS and FaSPS). Collectively, this study demonstrates that FaMRLK47 is an important regulator of strawberry fruit ripening and quality formation, and sheds light on the signaling mechanisms underlying strawberry fruit development and ripening.
Fleshy fruits are physiologically classified as climacteric or non-climacteric. Climacteric fruits show a sharp increase in respiration during the ripening process, while non-climacteric fruits do not (Nitsch, 1953; Coombe, 1976; Brady, 1987). Most basic studies of fruit development and ripening have focused on climacteric fruits, such as the model plant Solanum lycopersicum (tomato). Fragaria × ananassa (strawberry) is a typical non-climacteric fruit. Studies of strawberry fruit development and ripening are likely to provide insight into the regulatory mechanisms underlying non-climacteric fruit development and ripening.
The ripening of fleshy fruits is a complex process involving dramatic changes in physiological and biochemical metabolism, which trigger changes in color, texture, flavor, and aroma (Giovannoni, 2001; Seymour et al., 2013). In the past decades, studies of fruit ripening have mainly focused on these metabolic changes, particularly regarding their interactions with phytohormones. Ehylene has long been known to be the critical signal controlling ripening of climacteric fruits (Nitsch, 1953; Coombe, 1976; Brady, 1987; Klee and Giovannoni, 2011). Early studies suggested that auxin (IAA) is a key regulator of strawberry fruit growth and ripening (Veluthambi and Poovaiah, 1984; Given et al., 1988). Whereas overexpression of FaNCED1, which encodes a key enzyme in the abscisic acid (ABA) biosynthesis pathway, promoted strawberry fruit ripening, knock-down of this gene delayed it Jia et al. (2011). Furthermore, manipulating the expression of an ABA receptor, FaPYR1, and of its downstream signal members, ABI1 and SnRK2.6, affected the accumulation of anthocyanins and other fruit qualities (Chai et al., 2011; Jia et al., 2013a; Han et al., 2015). These studies suggested that ABA is an important signal controlling strawberry fruit ripening. Besides IAA and ABA, there is evidence that ethylene (White, 2002; Trainotti et al., 2005; Villarreal et al., 2010) and jasmonic acid (Concha et al., 2013) also regulate strawberry fruit ripening and quality formation. Collectively, it appears that strawberry fruit development and ripening are regulated by the synergistic effects of multiple phytohormones. We recently showed that sucrose also regulates anthocyanin accumulation in strawberry fruit (Jia et al., 2013b).
While the senescence-associated, deteriorative aspects of ripening have historically been emphasized, it is now commonly accepted that ripening is a complex process determined by a series of signaling events (Brady, 1987; Fischer and Bennett, 1991; Giovannoni, 2001). At the cellular level, the process spanning fruit set to ripening can be categorized into three major stages: cell division, cell differentiation and expansion, and cell degradation (Nitsch, 1953; Giovannoni, 2001; Seymour et al., 2013). Regulation of the cell wall’s physical properties is essential for plant growth and development, and a cell wall signaling pathway that reports on the status of the cell wall has long since been predicted to exist (Wolf et al., 2012). Recently, plasmalemma-anchored receptor-like kinases (RLKs) have attracted much attention due to their roles in sensing cell wall integrity (Humphrey et al., 2007; Cheung and Wu, 2011; Boisson-Dernier et al., 2011; Lindner et al., 2012). RLKs constitute a gene subfamily of over 600 members in Arabidopsis (Shiu and Bleecker, 2001a,b, 2003). Malectin is a membrane-anchored protein of the endoplasmic reticulum that recognizes and binds to Glc2-N-glycan, thereby regulating the production and secretion of N-glycosylated proteins (Schallus et al., 2008, 2010; Takeda et al., 2014). Interestingly, a group of RLKs harbors an extracellular sequence with a unique domain that is similar to malectin (Schulze-Muth et al., 1996; Boisson-Dernier et al., 2011; Lindner et al., 2012). The first malectin domain-containing RLK, CrRLK1, was identified in Catharanthus roseus. The Arabidopsis thaliana genome contains 17 CrRLK1-like RLK genes (Schulze-Muth et al., 1996), several of which have been functionally identified. FERONIA (FER) belongs to the CrRLK1-like subfamily and was first identified for its role in fertilization (Huck et al., 2003). FER directly interacts with guanine nucleotide exchange factors (RopGEFs), which activate downstream components that mediate the production of reactive oxygen species (ROS) at the entrance point of the female gametophyte, thereby inducing pollen tube rupture and sperm release (Duan et al., 2014; Ngo et al., 2014; Kessler et al., 2015). Furthermore, FER is a pivotal mediator of cross-talk between phytohormones, including ABA, brassinosteroids (BRs), and ethylene (Yu et al., 2012). The mechanism by which FER modulates ABA signaling was revealed in a study by Yu et al. (2012), which found that ROP11, a downstream component of FER signaling, physically interacts with ABI2, a key signal in the ABA signaling pathway. As described above, IAA, ABA, BR, and ethylene are all critical regulators of strawberry fruit development and ripening. Although Arabidopsis is intrinsically different from strawberry with respect to fruit development and ripening, the convergent roles of FER in phytohormone signaling prompted us to examine whether FER-like kinase is involved in strawberry fruit development and ripening. Besides FER, a few of the other malectin domain-containing RLKs have also been functionally characterized in Arabidopsis. Theseus1 (THE1), which was identified in a screen for suppressors that attenuated the short hypocotyl phenotype of dark-grown seedlings, functions as a cell wall integrity sensor that mediates the disruption of cellulose synthesis (Hematy et al., 2007; Hematy and Hofte, 2008; Boisson-Dernier et al., 2011). Anxur1 (ANX1) and Anxur2 (ANX2), two close relatives of FER, were also found to be pollen-specific and to regulate pollen tube rupture and sperm release (Boisson-Dernier et al., 2009). As a redundant homolog of THE1, HERCULES 1 (HERK1) was demonstrated to regulate plant growth and development (Guo et al., 2009a,b).
Malectin domain-containing RLKs (MRLKs) have been proposed to sense cell wall integrity and FER, a member of the malectin domain-containing RLK family, has been implicated in phytohormone cross-talk (Duan et al., 2010; Huang et al., 2013; Chen et al., 2016). We identified 62 MRLK members in strawberry, which we named FaMRLK1 to FaMRLK62 based on their chromosome location (Zhang et al., 2016). In this study, we found that FaMRLK47, the homolog of FER, is a negative regulator of strawberry fruit development and ripening. Overexpression and RNAi-mediated downregulation of FaMRLK47 delayed and accelerated fruit ripening, respectively. FaMRLK47 physically interacts with FaABI1, and regulates fruit ripening by modulating ABA signaling, which results in changes in fruit ripening and qualities, such as sugar content and pigment accumulation. These findings provide insights into the molecular basis for the regulation of strawberry fruit development and ripening.
Strawberry plants (Fragaria × ananassa ‘Benihoppe’) were grown on soil supplemented with nutrient soil, organic fertilizer, and vermiculite (7:2:1; v/v/v) in the greenhouse. The controlled condition of greenhouse was 12/12-h photoperiod at 450 μmol m-2 s-1 and 70% humidity, under day/night temperature of 25°C/15°C.
The cDNA sequences of full-length RLKs were obtained from the TAIR website1 and NCBI2. To identify CrRLK1-like RLK (CrRLK1L) genes in strawberry, the coding sequence of FERONIA (At3g51550) was used as query to BLAST the strawberry genome. Phylogenetic trees were constructed using the Neighbor-Joining (NJ) method in MEGA 4.0.2 software, with 1000 bootstrap replicates to evaluate the reliability of different phylogenetic groups. The deduced amino acid sequences of FaMRLKs were aligned using ClustalX 2.0.12 with default settings. The alignments were edited and marked using GeneDoc.
To isolate FaMRLK47 and FaMRLK50, total RNA was extracted from strawberry fruit using an E.Z.N.A.®Total RNA Kit (OMEGA). The cDNA was synthesized from 1 μg of total RNA using M-MLV Reverse Transcriptase (Promega) according to the manufacturer’s instruction. Full-length FaMRLK47 and FaMRLK50 were cloned by RT-PCR from cDNA using Q5 High-Fidelity DNA Polymerase (New England Biolabs) under the following conditions: 94°C/30 s for 1 cycle, 94°C/30 s, 56°C/25 s, and 72°C/5 min for 35 cycles, and a final extension of 72°C/5 min. The amplified fragments were subcloned into the pMD19-T vector and transformed into Escherichia coli DH5α. Then the selected positive colonies were sequenced by Invitrogen to confirm the full-length sequence. The Primer sequences and GenBank accession numbers are shown in Supplementary Table S1.
Quantitative reverse transcriptase PCR (RT-qPCR) was performed using SYBR Premix Ex TaqTM (TaKaRa) in a ABI7500 Real-Time PCR System. Primers used for RT-qPCR were designed using Primer3 Plus3. Three biological replicates were set up, and each sample was analyzed at least in triplicate. FaACTIN was used as an internal control and the 2-ΔΔCT method (where ΔCT represents the difference between the cycle threshold values of the target and reference genes) was used to calculate the relative transcript levels (Schmittgen and Livak, 2008).
The process from fruit set to ripening was classified into six stages as follows: small green fruit (abbreviated as SG), large green fruit (LG), white fruit (W), initially reddening (IR), and fully reddening (FR). For each stage, five fruits were combined as an individual sample. After fruits were frozen in liquid nitrogen, seeds (achenes) were removed with a needle, and the receptacles were used to analyze gene expression. The expression of FaMRLKs was assessed by RT-qPCR analysis, using the primer sequences shown in Supplementary Table S2.
For phytohormone treatment, fruit disks (10 mm in diameter and 1 mm in thickness) were prepared and combined from 20 fruits in the large green stage. For each treatment, disk samples (5 g per sample) were equilibrated for 30 min in equilibration buffer (Archbold, 1988; 10 mM MgCl2, 5 mM CaCl2, 200 mM mannitol, 10 mM EDTA, 5 mM vitamin C, and 50 mM MES-Tris, pH 5.5) and then shaken for 6 h at 25°C in equilibration buffer containing 100 μM ABA or 200 μM IAA under darkness. After a 6-h incubation, the samples were washed with deionized water, frozen immediately in liquid nitrogen, and kept at -80°C until use. For temperature treatment, the fruit was split longitudinally into two even parts; one half was subjected to high (40°C, 8 h) or low (4°C, 24 h) temperature treatment, and the other (the control) was incubated for the same period at 25°C. After treatment, the fruits without seeds were frozen in liquid nitrogen and stored at -80°C until use. Each individual analysis was conducted in triplicate. Primers of ripening-related genes used for the RT-qPCR analysis are presented in Supplementary Table S3.
To construct vectors for overexpression of FaMRLK47 and FaMRLK50 (abbreviated hereafter as FaMRLK47-OE and FaMRLK50-OE), full-length FaMRLK47 and FaMRLK50 were cloned into the plant expression vector pCambia1304 using the XbaI and SacI restriction sites. To construct vectors for downregulating FaMRLK47 (abbreviated hereafter as FaMRLK47-RNAi), the plant expression vector pFGC5941 was used. pCambia1304, pFGC5941, FaMRLK47-OE, FaMRLK47-RNAi, and FaMRLK50-OE were transformed individually into Agrobacterium tumefaciens strain EHA105 (Lazo et al., 1991). The transformed strains were grown at 28°C in Luria-Bertani liquid medium containing 10 mM MES and 20 μM acetosyringone with appropriate antibiotics. When the culture reached an optical density at 600 nm of approximately 0.8, A. tumefaciens cells were harvested, resuspended in infection buffer [10 mM MgCl2, 10 mM MES (pH 5.6), and 200 mM acetosyringone], and shaken for 2 h at room temperature before being used for infiltration. Pairs of fruits at 18 DPA (day past anthesis) and with similar phenotypes were selected and, for each pair of fruits, one was transfected with FaMRLK47-OE, FaMRLK50-OE, or FaMRLK47-RNAi and the other (the control) was transfected with pCambia1304, pCambia1304 empty vector, or pFGC5941, respectively. For transfection, A. tumefaciens suspension was evenly injected into the fruits with a syringe until the whole fruit became hygrophanous (Jia et al., 2013a), and for each gene, 25 pairs of fruit were injected. To examine the expression of ripening-related genes, FaMRLK47-OE and FaMRLK50-OE transformed fruits were collected 12 days after infiltration and FaMRLK47-RNAi fruit was collected 8 days after infiltration. After removing seeds, fruit samples were frozen in liquid nitrogen and kept at -80°C until used. To investigate the effect of FaMRLK47-OE or FaMRLK47-RNAi on ABA signaling in fruits, detached fruits were transfected with the FaMRLK47-OE, FaMRLK47-RNAi, or empty pCambia1304 vector, and then incubated at 22°C and 100% humidity. Three days after the transfection and incubation, fruits were treated with 100 μM ABA for 6 h and expression of the selected genes was analyzed as described above.
Flesh firmness was measured after removing fruit skin on opposite sides of the fruit using a GY-4 fruit hardness tester (Zhejiang Top Instrument). The contents of anthocyanins, flavonoid, and total phenol in the fruit were evaluated using described methods (Fuleki and Francis, 1968; Lees and Francis, 1971). The soluble sugar content was examined as described by Jia et al. (2011). The total titratable acidity calculated, expressed as percent malic acid, was measured using the acid–base titration method (Kafkas et al., 2007). Volatile organic components were analyzed by headspace solid-phase microextraction and gas chromatography–mass spectrometry as described by Dong et al. (2013). ABA content was measured by an indirect enzyme-linked immunosorbent assay (ELISA) (Zhang et al., 2009). The ELISA procedures were performed according to the instructions provided by the manufacturer (China Agricultural University, Beijing, China) and the assay plates were read by the Thermo Electron (Labsystems) Multiskan MK3 (PIONEER, Co., Beijing).
Yeast two-hybrid assays were performed using the Matchmaker GAL4-based Two-Hybrid System 3 (Clontech), according to the manufacturer’s instructions. The coding sequence of the FaMRLK47 kinase domain (540–892 aa) was fused in-frame with the GAL4 DNA-binding domain (BD) in the pGBKT7 vector to generate the FaMRLK47-BD plasmid. The full-length cDNA sequences of FaABI1 were inserted into the pGADT7 vector. Four different combinations, pGADT7/pGBKT7, FaABI1-AD/pGBKT7, pGADT7/FaMRLK47-BD, and FaABI1-AD /FaMRLK47-BD were respectively transformed into AH109 strains using the lithium acetate method. After transfection, strains were steaked on -Leu/-Trp medium and further selected on minimal -Leu/-Trp/-His/-Ade medium, and then treated with 20 μg/mL X-Gal for interaction validation. Combinations of pGADT7/pGBKT7, FaABI1-AD/pGBKT7, and pGADT7/FaMRLK47-BD were used as negative controls. The primers used for yeast two-hybrid assays are provided in Supplementary Table S4.
For the bimolecular fluorescence complementation (BiFC) assay, the full-length cDNA sequence of FaMRLK47 or FaABI1 was cloned into pCambia1300-YFPn/c to generate the interaction vectors FaMRLK47-YFPc or FaABI1-YFPn. FaMRLK47-YFPc or FaABI1-YFPn plasmids were further transformed into A. tumefaciens strain EHA105, and cultured at 28°C. To examine the in vivo interaction, FaMRLK47-YFPc and FaABI1-YFPn were co-expressed in tobacco leaves (Nicotiana tabacum) by Agrobacterium-mediated infiltration (Schütze et al., 2009). The negative control was performed by co-expressing FaMRLK47-YFPc and empty pCambia1300-YFPn vector in tobacco leaves. Chimeric fluorescence was examined by confocal microscopy (Olympus Fluoview FV1000). For YFP and bright field imaging, excitation wavelengths of 488 and 543 nm were used, respectively.
For subcellular localization of FaMRLK47, full-length cDNA sequences were amplified by PCR using the forward primer 5′-TTAATTAAATGAAGTGTTTCTTTTTCTATATTTGGTTC-3′ and the reverse primer 5′-GGCGCGCCAACGTCCCTTTGGGTTCATGATTTGTGAG-3′. The PCR fragments were inserted into pMDC83 using Asc1 and Pac1 and the constructs were then introduced into A. tumefaciens strain EHA105 and transformed into tobacco leaves as described by Schütze et al. (2009). Transfected plants were grown in darkness for 24 h and in light for 48 h at 24°C. After 3 days, fluorescence was observed using a confocal laser-scanning microscope (Olympus Fluoview FV1000). The primers used for BiFC and localization are shown in Supplementary Table S5.
Samples were analyzed in triplicate, and the data were noted as the mean ± SD. Data were analyzed using Student’s t-test implemented in SAS software (version 8.1, United States), and the least significant difference at a 0.05 level of probability was used to explore the effect of P input on parameters. A P-value of ≤ 0.05 was considered to indicate a significant difference, and a P-value of ≤ 0.01 was considered to indicate a highly significant difference.
The FERONIA-like genes belong to a family of CrRLK1-like RLKs (CrRLK1Ls), which in turn belong to a super-family of malectin domain-containing RLKs. In a previous study (Zhang et al., 2016), we conducted a genome-wide screen of woodland strawberry, Fragaria vesica, for ‘Malectin domain-containing RLKs’ (accordingly designated as MRLKs) and identified 62 members (named FvMRLK1–62). In the present study, a screen of the F. vesca genome revealed a CrRLK1L family consisting of 20 members. Phylogenetic analysis of CrRLK1L homologs from various plant species revealed eight clades, with FERONIA, NAXURs, HERKs, and THESEUSs being distributed in different clades (Figure 1 and Supplementary Figure S1). Notably, FERONIA was located in Clade II and only one member of the FaCrRLK1L family, i.e., FaMRLK47, was located in this clade. FaMRLK47 showed a relatively high level of amino acid sequence identity (72.33%) with FERONIA, and can thus be viewed as a homolog of FER. Interestingly, clade I, which contains five members (FaMRLK50–54), was found to consist exclusively of proteins from the strawberry genome. Furthermore, members of clades I, II, and III exhibited relatively high levels of amino acid sequence identity with FERONIA (FER hereafter), and can thus be considered FEL-like receptor kinases. FaCrRLK1Ls and FER exhibited between 42.45 and 48.03% amino acid sequence identity for clade I proteins and between 38.02 and 50.66% for clade III proteins.
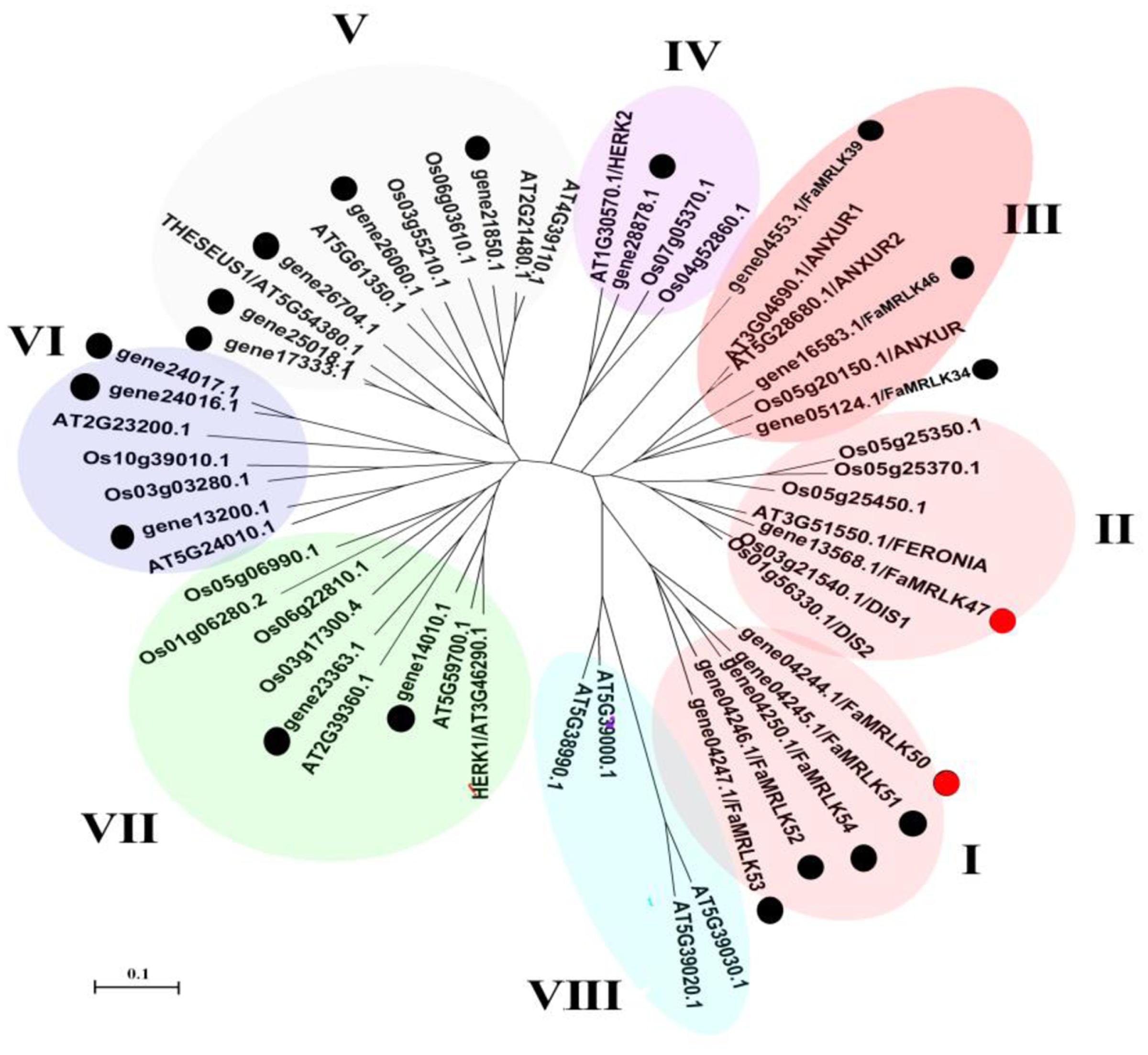
FIGURE 1. Phylogenetic tree of deduced FaMRLK amino acid sequences. Phylogenetic analysis of CrRLK1L homologs from various plant species. The phylogeny was constructed using the Neighbor-Joining method in MEGA4.0.2 with 1,000 bootstrap replicates. FER-like proteins in strawberry are marked with black circles. At, Arabidopsis thaliana; Os, Oryza sativa.
Strawberry fruit development, from fruit set to ripening, can be divided into several substages, i.e., the small green fruit (SG), middle green fruit (MG), large green fruit (LG), white fruit (W), initially reddening fruit (IR), and fully reddening fruit (FR) substages (Figure 2A). Given that FaMRLK47 is a homolog of FER and the only FaMRLK member in clade II (Figure 1), we focused on this protein in the present study. As the members of clade I only existed in the strawberry genome and members of clade II exhibited relatively high levels of amino acid sequence identity with FERONIA, we also evaluated their expression in relation to strawberry fruit development and ripening (Figure 2B). Whereas FaMRLK34, FaMRLK39, and FaMRLK6 transcripts were not detected in strawberry fruit, the relative levels of the other six members differed, all tending to decrease from the SG to W substages. Notably, FaMRLK47 expression was higher than that of other members from clade I and II. Furthermore, while the expression levels of FaMRLK47 started to drop during the MG substage, those of all other members examined started to decline during the SG substage, which suggests that FaMRLK47 is more tightly associated with the onset of fruit ripening.
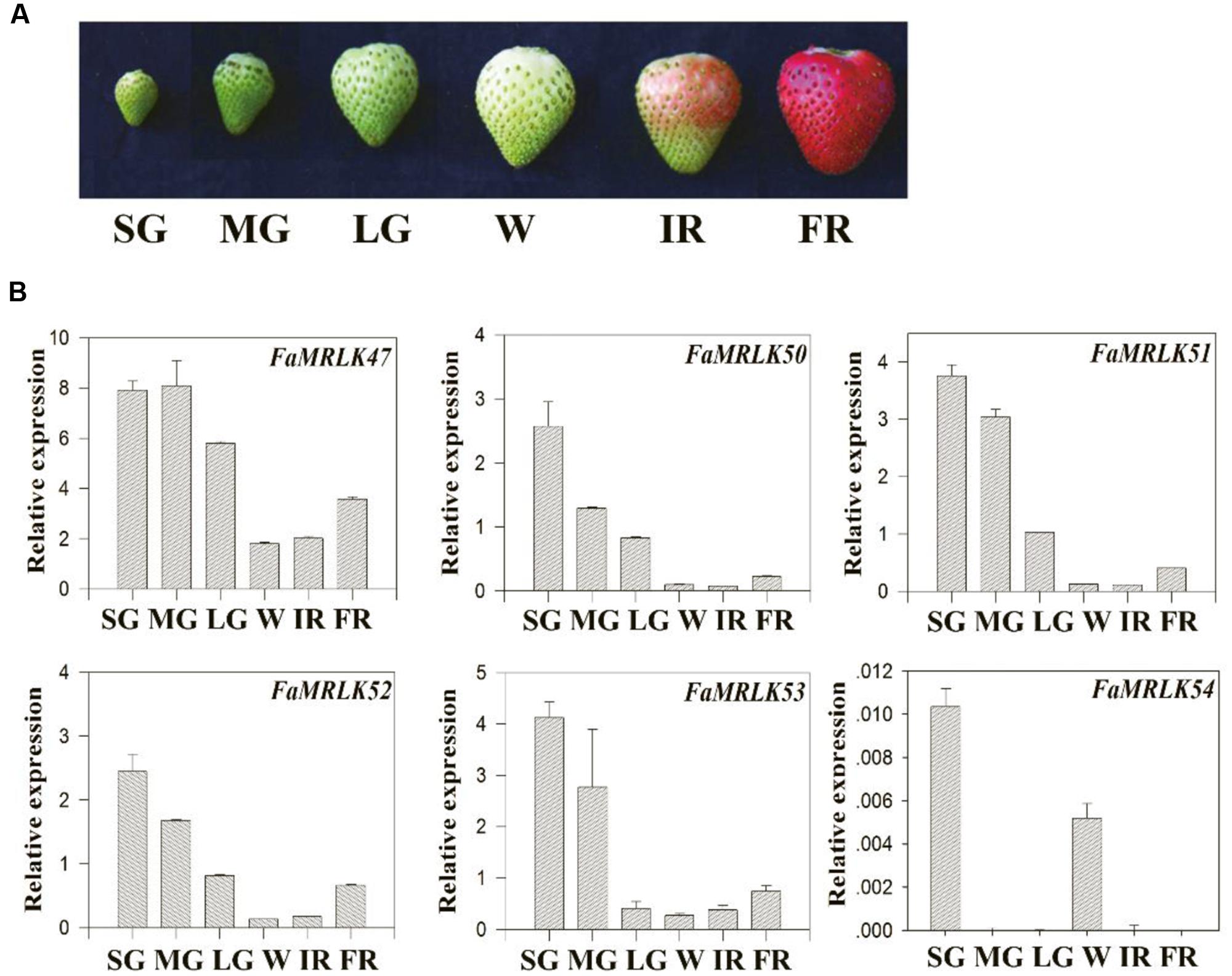
FIGURE 2. Temporospatial pattern of FaMRLK expression in strawberry fruits. (A) Phenotypes of strawberry fruit at different developmental stages: small green (SG), middle green (MG), large green (LG), white (W), initial reddening (IR), and fully reddening (FR). (B) Quantitative reverse transcriptase PCR (RT-qPCR) analysis of the expression of various FaMRLK genes at the indicated developmental stages. Labels below bars denote the corresponding developmental stages, as in (A). FaACTIN was used as an internal control. Values are means ± SD of three biological replicates.
Strawberry fruit development and ripening are regulated by both internal and external factors, including IAA, ABA, and temperature (Giovannoni, 2001; Seymour et al., 2013). While the application of IAA delays strawberry fruit ripening, application of ABA promotes it Given et al. (1988) and Jia et al. (2011, 2013a). In our previous studies, we found that ripening of strawberry fruit was stimulated by high temperatures and delayed by low temperatures (Han et al., 2015). We therefore studied the expression profiles of the FER-like FaMRLKs in response to IAA, ABA, and low/high temperature treatment. While the transcription of all of these genes was sensitive to IAA, ABA, and temperature treatments, their responses differed. FaMRLK47, FaMRLK50, and FaMRLK53 expression were inhibited by ABA treatment, whereas FaMRLK51 and FaMRLK52 expression were upregulated by ABA treatment. FaMRLK47, FaMRLK50, FaMRLK52, and FaMRLK53 expression were also sensitive to temperature stress; however, while FaMRLK47 expression was promoted by both low and high temperature treatment, FaMRLK52 expression was inhibited and promoted, respectively, by low and high temperature treatments (Figure 3).
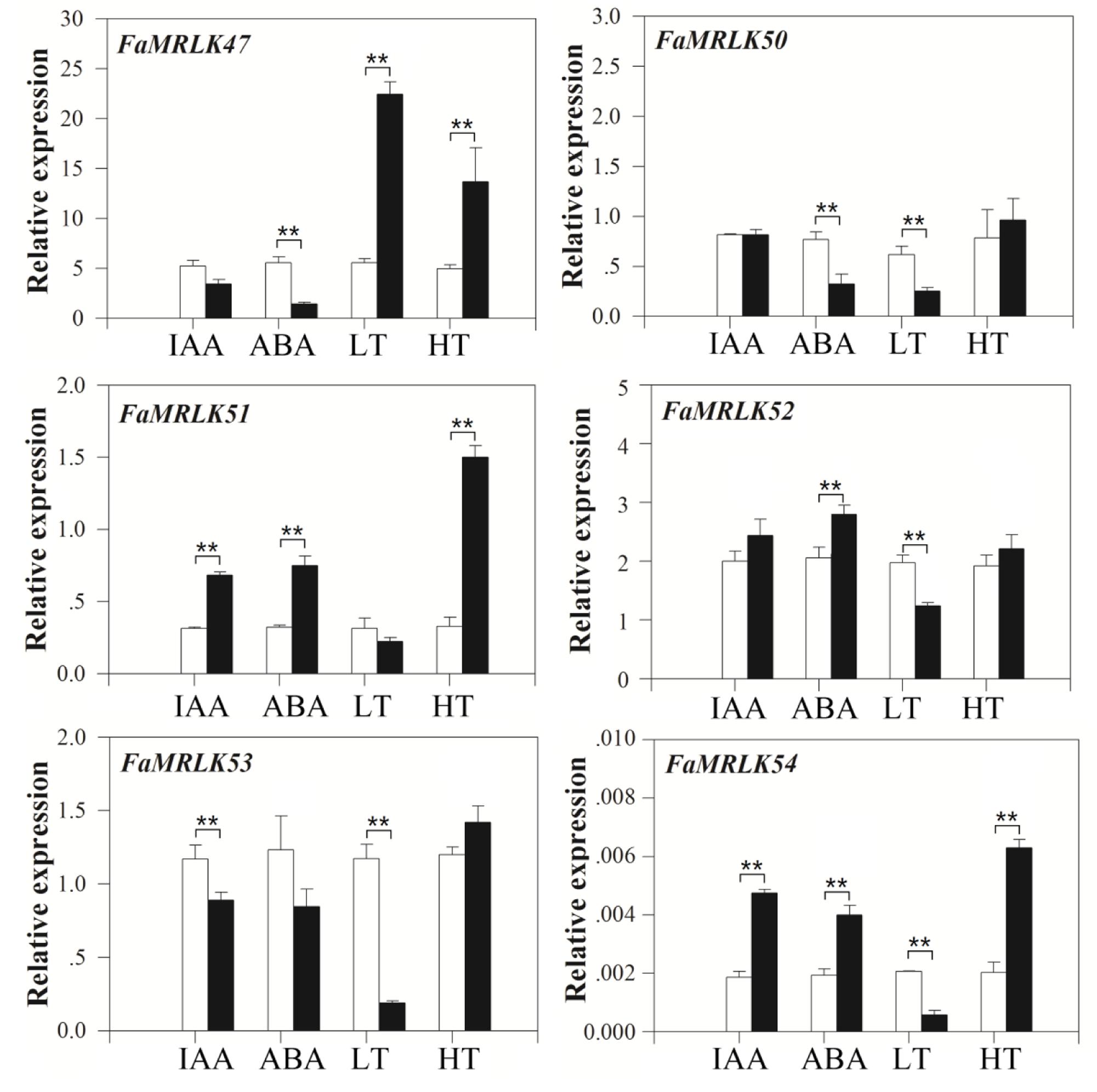
FIGURE 3. Quantitative reverse transcriptase PCR analysis of FaMRLK expression in response to IAA, ABA, low temperature (LT), and high temperature (HT) treatments in fruits at the LG stage. RT-qPCR was conducted using FaACTIN as an internal control. Values are means ± SD of three biological replicates. Asterisks denote significant differences compared with the control sample (i.e., the 0 concentration for hormone treatment and 25°C for temperature treatment) at ∗P < 0.05 and ∗∗P < 0.01, according to Student’s t-test. White bars indicate control samples; black bars indicate treated samples.
To investigate a potential role of FaMRLK47 in the regulation of strawberry fruit development and ripening, we transiently manipulated its expression in strawberry fruits. As a representative member of the FaMRLK family that specifically exists in the strawberry genome, FaMRLK50 was also investigated. Since FaMRLK47 and FaMRLK50 transcript levels dramatically decreased from the SG to LG substages, we first sought to examine the function of FaMRLK47 and FaMRLK50 by transiently overexpressing these two genes in strawberry plants. As shown in Figure 4A, overexpression of FaMRLK47 and FaMRLK50 resulted in a great increase in their transcript levels in fruits. While overexpression of FaMRLK50 did not affect fruit development and ripening, overexpression of FaMRLK47 delayed fruit ripening, as reflected by pigment accumulation. Conversely, RNAi-mediated downregulation of FaMRLK47 accelerated fruit ripening (Figures 4A,B). Collectively, these experiments indicate that FaMRLK47 is an important regulator of strawberry fruit development and ripening.
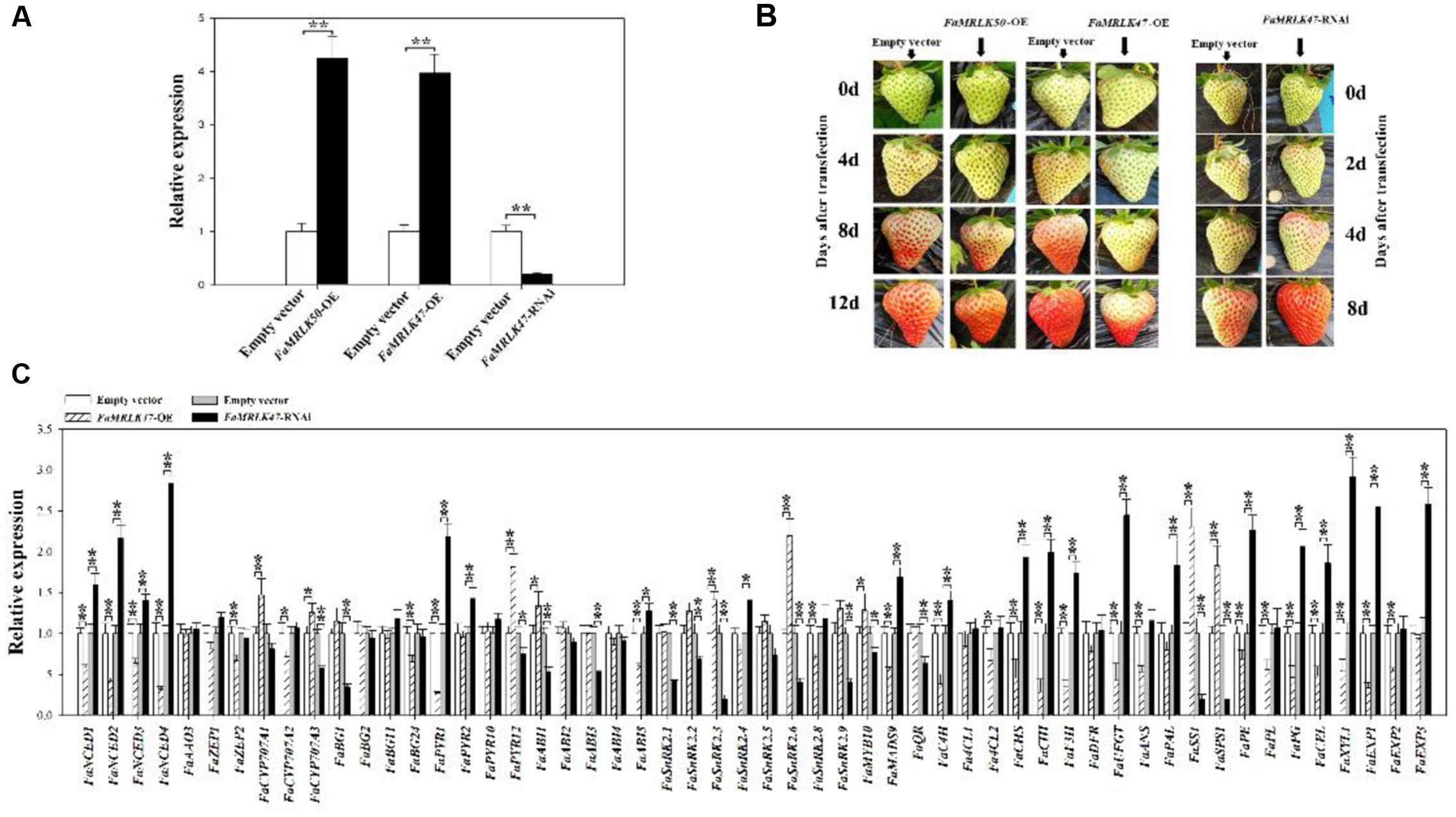
FIGURE 4. Effect of FaMRLK47 or FaMRLK50 overexpression (OE) and RNAi-mediated silencing of FaMRLK47 on strawberry (Fragaria × ananassa) fruit ripening. (A) RT-qPCR analysis of FaMRLK47 and FaMRLK50 expression in OE, RNAi, and control fruits. FaMRLK47 was overexpressed or silenced, and FaMRLK50 was overexpressed as described in Section “Materials and Methods.” The overexpression or RNAi constructs were injected into the fruits at 18 DPA and gene expression was analyzed 12 days after transfection with the OE vector or 8 days after transfection with the RNAi vector. Control samples were transfected with the empty vector (pCambia1304). RT-qPCR was conducted using FaACTIN as an internal control. Values are means ± SD of three biological replicates. Double asterisks denote significant difference at P < 0.01 using Student’s t-test. (B) The influence of FaMRLK47-OE, FaMRLK47-RNAi, and FaMRLK50-OE on the time course of strawberry fruit development and ripening. (C) The effect of FaMRLK47-OE and FaMRLK47-RNAi on the expression of ripening-related genes. Values are means ± SD of three biological replicates. Asterisks denote significant differences compared with the control sample (i.e., Empty vector) at ∗P < 0.05 and ∗∗P < 0.01, using Student’s t-test.
To further explore the FaMRLK47-mediated mechanisms underlying the regulation of strawberry fruit development and ripening, we examined the effects of FaMRLK47 overexpression and downregulation on the expression of a series of ripening-related genes (Figure 4C). Most of these ripening-related genes are important structure genes and transcription factors that are involved in the formation of fruit qualities such as color, texture, aroma, and sugar (Jia et al., 2011, 2013a; Seymour et al., 2011; Lin-Wang et al., 2014; Han et al., 2015). Given that fruit ripening is highly affected by ABA, we also detected the expression of genes involved in ABA biosynthesis, metabolism, and signal transduction. As shown in Figure 4C, manipulating FaMRLK47 expression altered the expression patterns of both ripening-related and ABA-related genes, which implied that FaMRLK47 is an important regulator of diverse processes in fruit ripening, including fruit quality formation, ABA production, and signal transduction. Further detection of the ripening-related physiological parameters demonstrated that overexpression and downregulation of FaMRK47 resulted in a decrease and increase, respectively, in most of the fruit quality parameters that were expected to increase and decrease during fruit development and ripening (Table 1). Moreover, the ABA content showed a decline in FaMRLK47-OE fruit (Figure 5A). Comprehensive analysis of the changing patterns of gene expression and physiological parameters showed that FaMRLK47 mainly plays a role in the regulation of anthocyanins accumulation, flavonoid metabolism and fruit softness, and FaCHS, FaCHI, FaUFGT, FaPAL, FaPE, FaPG, FaXYL1, and FaEXP1 might function as the important downstream structure genes of FaMRLK47 in these processes. In addition, as shown in Figure 4C, FaNCED1-4, FaCYP707A, FaPYL1/12, FaABI1/5, and FaSnRK2.3/2.6 might participate in FaMRLK47- mediated ABA production and signal transduction.
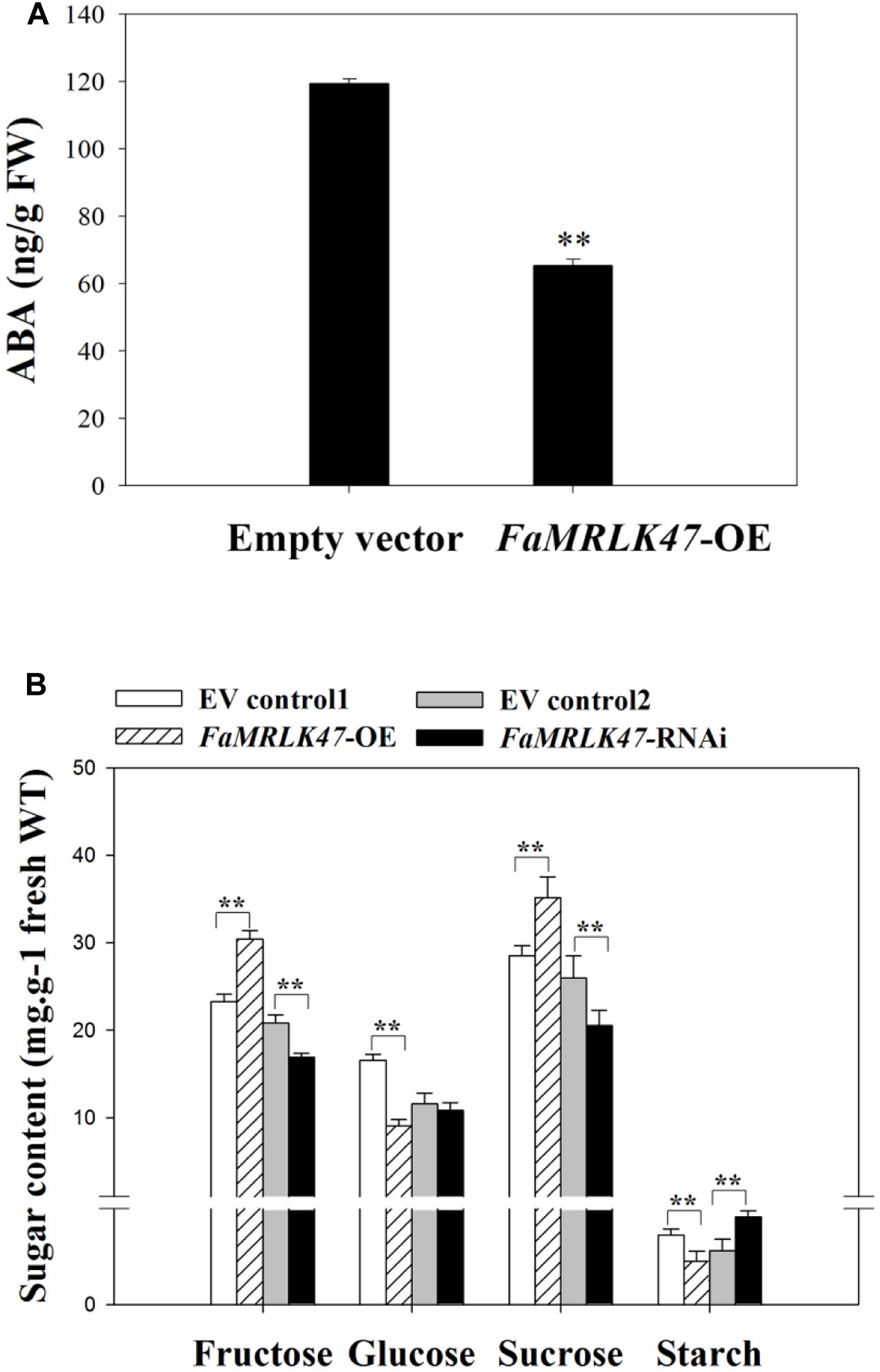
FIGURE 5. Changes in ABA content and sugar metabolism during strawberry fruit growth and development. (A) Changes of ABA content in FaMRLK47-OE fruits. Values are means ± SD of three biological replicates. Asterisks denote significant differences compared with the control sample (i.e., OE-C) at ∗∗P < 0.01, using Student’s t-test. (B) Changes in sugar metabolism during fruit development (glucose, open circles; fructose, open squares; sucrose, open triangles). Values are means ± SD of three samples. ∗∗P < 0.01 (Student’s t-test), when compared with control values.
Interestingly, in contrast to the changes in expression profiles of other ripening-related genes following FaMRLK47 manipulation, overexpression and downregulation of FaMRLK47 respectively promoted and inhibited the expression of FaSS and FaSPS1, two key genes in the sucrose biosynthesis pathway (Figure 4C). Further analysis of sugar metabolism showed that, while RNAi-mediated downregulation of FaMRLK47 expression resulted in a dramatic increase in the major sugar components of the fruit, i.e., sucrose, fructose and glucose, overexpression of FaMRLK47 caused a significant decrease in sucrose and fructose content (Figure 5B). Additionally, overexpression and RNAi-mediated downregulation of FaMRLK47 resulted in a significant decrease and increase in starch content, respectively. Collectively, these results suggest that FaMRLK47 is an important regulator of sugar metabolism.
FaMYB10 was reported to be an important positive regulator of anthocyanin accumulation, whereas FaMADS9 was shown to regulate fruit development and ripening (Seymour et al., 2011; Lin-Wang et al., 2014). In this study, we also monitored the expression of FaMYB10 and FaMADS9 (Figure 4C), and found that the expression of FaMADS9 was repressed in FaMRLK47-OE fruit and upregulated in FaMRLK47-RNAi fruit. Although FaMYB10 expression was not expected to be altered by changes in FaMRLK47 expression, overexpression of FaMRLK47 resulted in an increase in FaMYB10 expression, and downregulation of FaMRLK47 resulted in a decrease in FaMYB10 expression. These results imply that FaMRLK47 and FaMYB10 are regulatory proteins with diverse functions in fruit ripening. Furthermore, their regulatory mechanisms are more complex than previously expected.
In Arabidopsis, FER is involved in ABA signaling (Yu et al., 2012). As FaMRLK47 shares 74% amino acid sequence identity with FER, which is known to modulate ABA signaling (Yu et al., 2012), we were interested in establishing whether FaMRLK47 was associated with ABA signaling in strawberry fruit. To investigate a possible role for FaMRLK47 in the modification of the fruit’s response to ABA treatment, we overexpressed and downregulated FaMRLK47 in strawberry fruit for a short time (72 h), and then examined the expression of a series of ripening-related genes following ABA treatment. As shown in Figure 6, overexpression of FaMRLK47 resulted in a great decrease in the ability of ABA to induce the expression of these genes in comparison with control fruits, and conversely, RNAi-mediated downregulation of FaMRLK47 resulted in a great increase in the ability of ABA to induce the expression of these genes. These results indicate that FaMRLK47 functions as a negative regulator of the ABA signaling cascade.
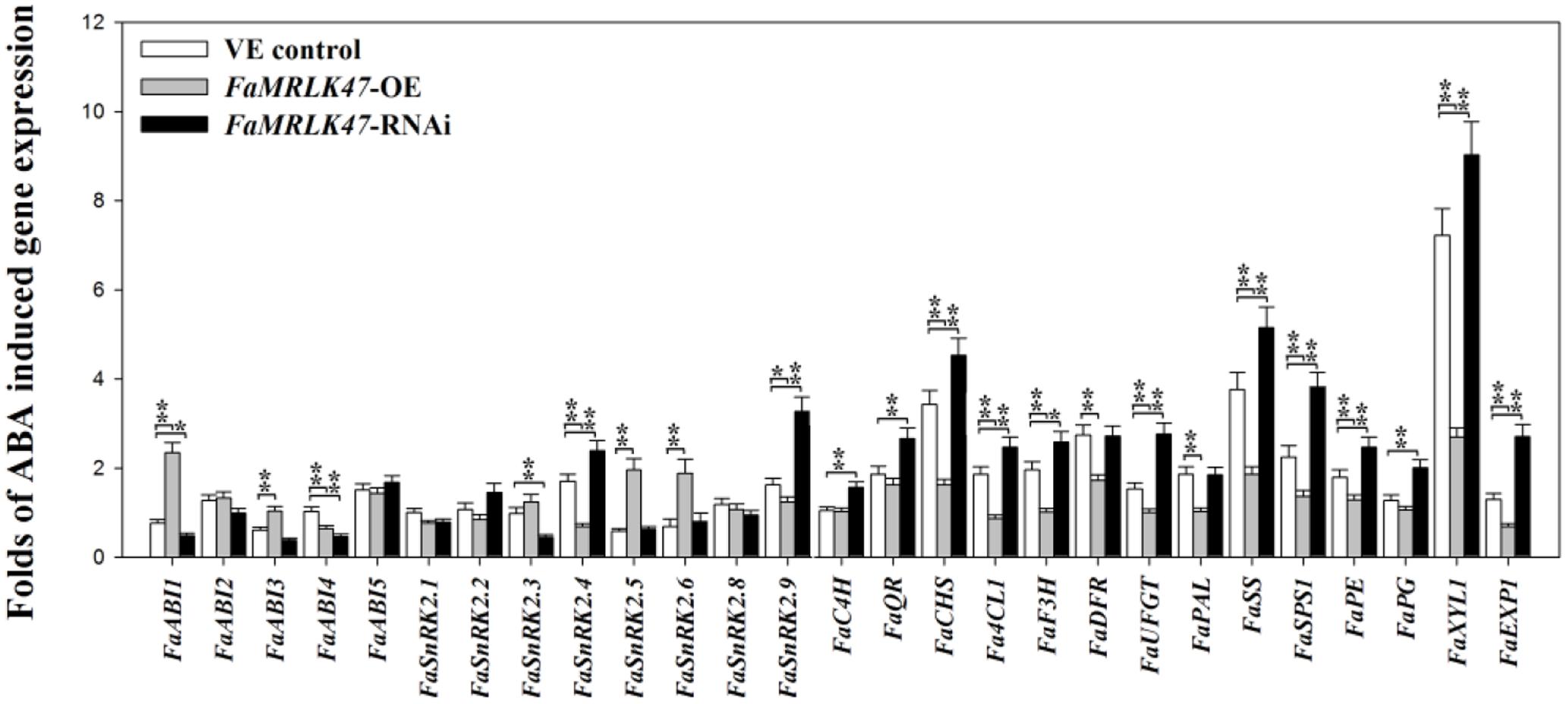
FIGURE 6. Effect of FaMRLK47-OE and FaMRLK47-RNAi on the sensitivity of ripening-related genes to ABA. Quantification of the sensitivity of ripening-related gene expression to ABA, with gene expression being expressed as a ratio of ABA treatment/non-treatment control. FaMRLK47-OE and FaMRLK47-RNAi samples were treated with or without ABA as described in Section “Materials and Methods.” Values are means ± SD of three replicates. ∗∗P < 0.01 and ∗P < 0.05 (Student’s t-test), when compared with control values.
Given that FaMRLK47 is capable of modifying ABA signaling, we examined whether FaMRLK47 could physically interact with important signal proteins in the ABA signaling pathway. As FaABI1 is a key signaling protein in the ABA signaling pathway and negative regulator of strawberry fruit development and ripening (Jia et al., 2013a), we examined the interaction between FaMRLK47 and FaABI1. Yeast two-hybrid analysis showed that by co-transformed of FaMRLK47 and FaABI1 into AH109, the transformed strain grew well on auxotrophic medium (SD-Ade-Leu-Trp-His), which indicated that FaMRLK47 interacts with FaABI1 (Figure 7A). To test whether FaMRLK47 could interact with ABI1 in living plant cells, we first observed the localization of FaMRLK47 in tobacco leaf cells by fusing it with eGFP. We found that FaMRLK47 localized to the membrane in tobacco leaf cells (Figure 7B). Furthermore, we co-transformed the BiFC vectors FaMRLK47-YFPc and FaABI1-YFPn into tobacco leaves, using co-transformation of FaMRLK47-YFPc and pCambia1300-YFPn as a control. The results showed that, while fluorescence was not observed in the control transformed leaves, strong fluorescence appeared when FaABI1-YFPn was combined with FaMRLK47-YFPc (Figure 7C), indicating that FaABI1 and FaMRLK47 indeed physically interact.
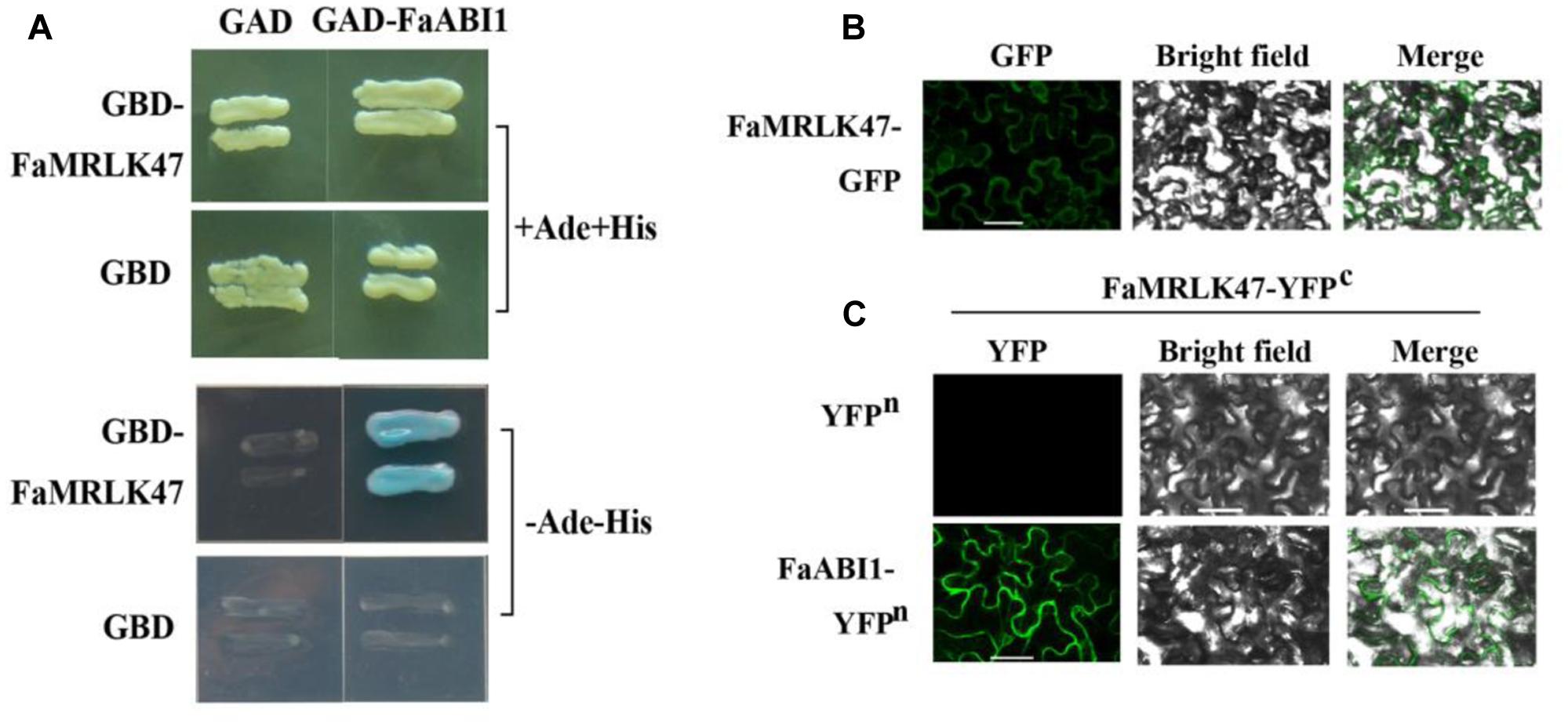
FIGURE 7. Subcellular localization of FaMRLK47 and physical interaction between FaMRLK47 and FaABI1. (A) Yeast two-hybrid analysis of the physical interaction between FaMRLK47 and FaABI1. Protein interactions were examined using combinations of prey and bait vectors. All tests were conducted on media containing adenine (+Ade+His; /–Leu/–Trp/+His/+Ade) or lacking adenine (–Ade–His; /–Leu/–Trp/–His/–Ade). Interactions were determined based on cell growth and were confirmed by an α-Gal assay on medium lacking adenine (/–Leu/–Trp/–His/–Ade). (B) Subcellular localization of FaMRLK47. pMDC83-FaMRLK47 was transformed into tobacco (Nicotiana tabacum) cells, and fluorescence was observed by confocal microscopy as described in Section “Materials and Methods.” Bars = 50 μm. (C) BiFC analysis of the physical interaction between FaMRLK47 and FaABI1. FaMRLK47 and FaABI1 were fused with the C and N terminus of yellow fluorescent protein (YFP; designated as YFPc and YFPn, respectively). Different combinations of the fused constructs were co-transformed into tobacco (Nicotiana tabacum) cells, and the cells were visualized using confocal microscopy as described in Section “Materials and Methods.” YFP and bright field were excited at 488 and 543 nm, respectively. Bars = 50 μm.
Ripening is a complex process, which involves dramatic changes in physiological and biochemical metabolism, with cell wall degradation considered to be the most important event (Fischer and Bennett, 1991; Giovannoni, 2001). Cell enlargement necessitates an increase in the surface of cell walls, and cell wall extension has been assumed to take place as a result of the loosening of intercrossing cellulose fibrils (Fischer and Bennett, 1991). Cell wall loosening and subsequent material deposition and rigidification must be tightly regulated, so that cell wall integrity and plant organ development can be coordinately maintained (Humphrey et al., 2007). Therefore, deciphering the mechanisms that sense and regulate cell wall integrity is of particular importance for understanding the process of strawberry fruit development and ripening.
Receptor like protein kinases (RLKs) are important candidate sensors of cell wall integrity and cell wall metabolism. Amongst the members of the RLK family, malectin domain-containing RLKs have attracted particular interest due to the presence of an extracellular sequence of the type thought to recognize and bind to oligosaccharides (Schulze-Muth et al., 1996; Schallus et al., 2008). In Arabidopsis, the malectin domain-containing RLKs are proposed to be encoded by a gene subfamily, named the CrRLK1L family, which has 17 members (Lindner et al., 2012). Numerous studies have aimed to identify the ligands of these RLKs, particularly the oligosaccharide-like ligands (Liu et al., 2009; Wolf et al., 2012; Engelsdorf and Hamann, 2014; Haruta et al., 2014; Kessler et al., 2015). While the malectin domain implies the existence of oligosaccharide-like ligands, one cannot exclude the possibility that the malectin domain functions to anchor the protein kinase to cell walls. Therefore, one would expect cell wall degradation to affect the behavior of these RLKs. In support of this notion, the present study indicated that the expression of all malectin domain-containing RLKs dramatically decreased during fruit development and ripening (Figure 2), implying that malectin domain-containing RLKs are tightly associated with strawberry fruit development and ripening. Direct evidence for this came from the finding that manipulation of FaMRLK47 expression altered the progression of fruit ripening.
FaMRLK47 shares 74% amino acid sequence identity with FER, a malectin domain-containing RLK from Arabidopsis. FER belongs to the CrRLK1L gene family, which consists of 17 members (Lindner et al., 2012). While little is known about most members of the CrRLK1 family, a few members have been functionally identified. FER was identified for its role in controlling pollen tube growth and fertilization (Escobar-Restrepo et al., 2007). Aside from FER, several other related members, such as THESEUS1, HERCULES, and ANXURs, have also been functionally identified (Hematy and Hofte, 2008; Miyazaki et al., 2009; Cheung and Wu, 2011). Intriguingly, studies suggest that all of these members are essentially associated with the sensing of cell wall integrity and thereby play important roles in regulating cell growth (Humphrey et al., 2007; Hematy and Hofte, 2008; Cheung and Wu, 2011; Lindner et al., 2012; Li and Zhang, 2014). Given that FaMRLK47 shares a relatively high level of amino acid sequence identity with FER and that FER-related protein kinases have been suggested to be important regulators of cell growth, it is possible that FaMRLK47 regulates early fruit growth in addition to the onset of fruit ripening. This assumption is consistent with the pattern of FaMRLK47 expression, i.e., the transcript levels of FaMRLK47 remain high throughout the early stages of fruit growth and decline dramatically during veraison.
It has been reported that ABA is an important regulator of strawberry fruit ripening (Jia et al., 2011; Han et al., 2015). In Arabidopsis, FER-mediated ABA signaling is based on a physical interaction between FER and guanine exchange factors (GEFs) (Yu et al., 2012). Specifically, FER physically interacts with GEFs, which results in activation of the GTPase ROP11. ROP11, in turn, physically interacts with ABI2, a critical signal downstream of the ABA receptor, thereby suppressing the ABA response. Recent reports also revealed that FER interacts directly with both ABI1 and ABI2 (Chen et al., 2016), but until now, the biological function of the interaction between FER and ABI1 was unknown. In this study, we showed that FaMRLK47 interacts directly with FaABI1 (Figure 7). Moreover, we found that FaMRLK47 changes the sensitivity of ripening-related genes to ABA treatment (Figure 6). These results imply that FaMRLK47 suppresses ABA-induced gene expression by interacting with FaABI1. In Arabidopsis, FER has been shown to control pollen tube growth and fertilization (Huck et al., 2003). Future studies should examine whether FaMRLK47 also controls pollen tube growth and fertilization in strawberry.
Fruit quality is primarily determined by the composition of organic constituents, including sugars, organic acids, pigments, and volatile compounds. Fruit ripening is tightly associated with fruit quality formation. The present study not only shows that FaMRLK47 plays a crucial role in regulating fruit ripening progression, but also that it plays a role in modifying fruit quality, as evidenced by its function in regulating sugar (especially sucrose) metabolism. As shown in Figure 5, RNAi-mediated downregulation of FaMRLK47 resulted in a large decrease in sucrose and fructose content, and overexpression of FaMRLK47 appeared to increase the content of sucrose, fructose, glucose, and starch. In Arabidopsis, FER was reported to regulate starch content via a physical interaction with glyceraldehyde-3-phosphate dehydrogenase (Yang et al., 2015). A recent study in rice showed that DRUS1/2, the ortholog FERONIA in rice, influences sugar utilization or conversion (Pu et al., 2017). In the present study, we found that FaMRLK47 is an important regulator of sucrose and starch metabolism, indicating that FER-like protein kinases have somewhat similar roles in different species. Given that FaMRLK47 functions in sucrose metabolism and that FER functions in starch metabolism (Yang et al., 2015), the FER-like protein kinases appear to regulate sugar metabolism via different mechanisms. The mechanism by which FaMRLK47 regulates sucrose metabolism merits further investigation.
The involvement of FaMRLK47 in the regulation of strawberry fruit development and ripening indicates that FER-related protein kinases are versatile regulators of plant growth and development. Consistent with this, it has been suggested that Arabidopsis FER proteins are involved in a variety of important processes, such as root hair elongation (Duan et al., 2010; Huang et al., 2013), ethylene biosynthesis (Mao et al., 2015), starch accumulation (Yang et al., 2015), seed development (Yu et al., 2014), pathogen resistance (Keinath et al., 2010; Kessler et al., 2010), and vegetative growth (Guo et al., 2009a,b; Deslauriers and Larsen, 2010). A recent study showed that FER was transcriptionally downregulated by ethylene during post-harvest ripening and senescence of apple fruit (Zermiani et al., 2015), implying that FER also affects the ripening of climacteric fruits. Ethylene and ABA were demonstrated to be the main regulators of climacteric and non-climacteric fruit ripening, and the results of both the Zermiani study and our study suggest that FER functions in the cross-talk between ethylene and ABA. The diverse signaling mechanisms of FER in fruit ripening need to be further explored.
While the results of the present study suggest that FaMRLK47 is a critical regulator of strawberry fruit development and ripening, it should be noted that FaMRLK47 may regulate different biological processes, such as early fruit growth and development, onset of fruit ripening, and fruit quality formation. It will be of great significance to establish how these different biological processes are mediated by the same signal, FaMRLK47. ABA has been demonstrated to promote sugar accumulation in strawberry fruits (Jia et al., 2011). Given that overexpression of FaMRLK47 suppresses the ABA response, the upregulation of FaSS and FaSPS1 and the increase in sucrose content observed in FaMRLK47 overexpression lines clearly do not occur via the ABA signaling pathway.
In summary, the present study demonstrates that FaMRLK47 plays important roles not only in the regulation of strawberry ripening, but also in the regulation of fruit quality formation. Evidence for this is mainly derived from the following observations: (1) Overexpression and RNAi-mediated downregulation of FaMRLK47 delayed and accelerated fruit ripening, respectively; (2) FaMRLK47 function is associated with ABA signaling, which is a major mechanism regulating strawberry fruit ripening. Specifically, FaMRLK47 physically interacts with ABI1, a key signal in the ABA signaling pathway, and manipulation of FaMRLK47 expression modulated ABA-induced expression of ripening-related genes; (3) Manipulation of FaMRLK47 expression modulated sucrose content and the expression of genes encoding key enzymes in sucrose metabolism. As sucrose metabolism affects strawberry fruit quality formation, FaMRLK47 is a regulator of strawberry fruit quality formation. We propose that FaMRLK47 influences fruit ripening and quality via two distinct pathways, the ABA-dependent pathway and ABA-independent pathway (Figure 8). Thus, this study provides insight into the molecular mechanisms underlying the regulation of strawberry fruit development and ripening.
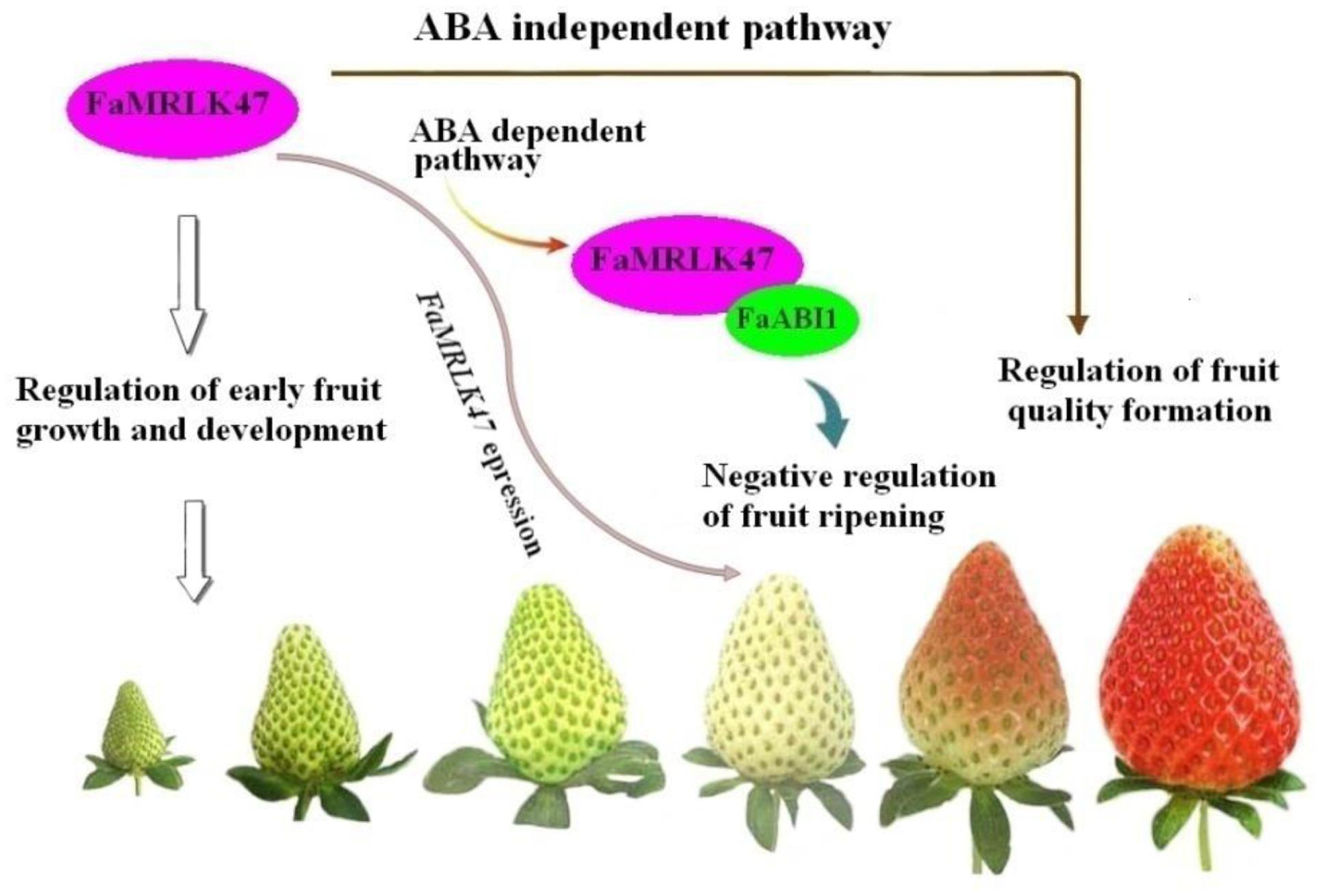
FIGURE 8. Proposed model for the role of FaMRLK47 in the regulation of strawberry fruit development and ripening. Acting as a negative signal, FaMRLK47 delays fruit ripening most probably by modifying the ABA signaling pathway. Furthermore, FaMRLK47 functions to regulate sugar metabolism, thereby regulating fruit quality formation in an ABA-independent pathway. Given the pivotal role of CrRLK1-related receptor kinases in the control of cell growth and the unique profile of FaMRLK47 expression during fruit development and ripening, FaMRLK47 is also expected to regulate early fruit growth and development.
MJ performed most of the experiments; ND, QZ, SX, and LW provided assistance with some of the experiments; YZ, PD, WM, and JL provided technical assistance; BL designed the experiments and analyzed the data; WJ conceived the project, supervised the experiments, and complemented the writing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the National Natural Science Foundation of China (Grant No. 31471851, 31672133, 31572104), Fok Ying-Tong Education Foundation, China (Grant No. 151027) and the 111 Project (Grant No. B17043).
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.01099/full#supplementary-material
Archbold, D. D. (1988). Abscisic acid facilitates sucrose import by strawberry fruit explants and cortex disks in vitro. Hortscience 23, 880–881.
Boisson-Dernier, A., Kessler, S. A., and Grossniklaus, U. (2011). The walls have ears: the role of plant CrRLK1Ls in sensing and transducing extracellular signals. J. Exp. Bot. 62, 1581–1591. doi: 10.1093/jxb/erq445
Boisson-Dernier, A., Roy, S., Kritsas, K., Grobei, M. A., Jaciubek, M., Schroeder, J. I., et al. (2009). Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development 136, 3279–3288. doi: 10.1242/dev.040071
Brady, C. J. (1987). Fruit ripening. Annu. Rev. Plant Physiol. 38, 155–178. doi: 10.1146/annurev.pp.38.060187.001103
Chai, Y. M., Jia, H. F., Li, C. L., Dong, Q. H., and Shen, Y. Y. (2011). FaPYR1 is involved in strawberry fruit ripening. J. Exp. Bot. 62, 5079–5089. doi: 10.1093/jxb/err207
Chen, J., Yu, F., Liu, Y., Du, C. Q., Li, X. S., Zhu, S., et al. (2016). FERONIA interacts with ABI2-type phosphatases to facilitate signaling cross-talk between abscisic acid and RALF peptide in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 113, E5519–E5527. doi: 10.1073/pnas.1608449113
Cheung, A. Y., and Wu, H. M. (2011). THESEUS 1, FERONIA and relatives: a family of cell wall-sensing receptor kinases? Curr. Opin. Plant Biol. 14, 632–641. doi: 10.1016/j.pbi.2011.09.001
Concha, C. M., Figueroa, N. E., Poblete, L. A., Oñate, F. A., Schwab, W., and Figueroa, C. R. (2013). Methyl jasmonate treatment induces changes in fruit ripening by modifying the expression of several ripening genes in Fragaria chiloensis fruit. Plant Physiol. Biochem. 70, 433–444. doi: 10.1016/j.plaphy.2013.06.008
Coombe, B. G. (1976). The development of fleshy fruits. Annu. Rev. Plant Physiol. 27, 207–228. doi: 10.1146/annurev.pp.27.060176.001231
Deslauriers, S. D., and Larsen, P. B. (2010). FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol. Plant 3, 626–640. doi: 10.1093/mp/ssq015
Dong, J., Zhang, Y., Tang, X., Jinb, W., and Hana, Z. (2013). Differences in volatile ester composition between Fragaria × ananassa and F. vesca and implications for strawberry aroma patterns. Sci. Hortic. 150, 47–53.
Duan, Q., Kita, D., Johnson, E. A., Aggarwal, M., Gates, L., Wu, H. M., et al. (2014). Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat. Commun. 5, 3129. doi: 10.1038/ncomms4129
Duan, Q., Kita, D., Li, C., Cheung, A. Y., and Wu, H. M. (2010). FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl. Acad. Sci. U.S.A. 107, 17821–17826. doi: 10.1073/pnas.1005366107
Engelsdorf, T., and Hamann, T. (2014). An update on receptor-like kinase involvement in the maintenance of plant cell wall integrity. Ann. Bot. 114, 1339–1347. doi: 10.1093/aob/mcu043
Escobar-Restrepo, J. M., Huck, N., Kessler, S., Gagliardini, V., Gheyselinck, J., Yang, W. C., et al. (2007). The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 317, 656–660. doi: 10.1126/science.1143562
Fischer, R. L., and Bennett, A. B. (1991). Role of cell wall hydrolases in fruit ripening. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 675–703. doi: 10.1146/annurev.pp.42.060191.003331
Fuleki, T., and Francis, F. J. (1968). Quantitative methods for anthocyanins. J. Food Sci. 33, 266–274. doi: 10.1111/j.1365-2621.1968.tb01365.x
Giovannoni, J. (2001). Molecular biology of fruit maturation and ripening. Annu. Rev. Plant Biol. 52, 725–749.
Given, N. K., Venis, M. A., and Gierson, D. (1988). Hormonal regulation of ripening in the strawberry, a non-climacteric fruit. Planta 174, 402–406. doi: 10.1007/BF00959527
Guo, H., Li, L., Ye, H., Yu, X., Algreen, A., and Yin, Y. (2009a). Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 106, 7648–7653. doi: 10.1073/pnas.0812346106
Guo, H., Ye, H., Li, L., and Yin, Y. (2009b). A family of receptor-like kinases are regulated by BES1 and involved in plant growth in Arabidopsis thaliana. Plant Signal. Behav. 4, 784–786. doi: 10.1073/pnas.0812346106
Han, Y., Dang, R. H., Li, J. X., Jiang, J. Z., Zhang, N., Jia, M. R., et al. (2015). SUCROSE NONFERMENTING1-RELATED PROTEIN KINASE2.6, an Ortholog of OPEN STOMATA1, is a negative regulator of strawberry fruit development and ripening. Plant Physiol. 167, 915–931. doi: 10.1104/pp.114.251314
Haruta, M., Sabat, G., Stecker, K., Minkoff, B. B., and Sussman, M. R. (2014). A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343, 408–411. doi: 10.1126/science.1244454
Hematy, K., and Hofte, H. (2008). Novel receptor kinases involved in growth regulation. Curr. Opin. Plant Biol. 11, 321–328. doi: 10.1016/j.pbi.2008.02.008
Hematy, K., Sado, P. E., Van, T. A., Rochange, S., Desnos, T., Balzergue, S., et al. (2007). A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol. 17, 922–931. doi: 10.1016/j.cub.2007.05.018
Huang, G. Q., Li, E., Ge, F. R., Li, S., Wang, Q., Zhang, C. Q., et al. (2013). Arabidopsis RopGEF4 and RopGEF10 are important for FERONIA-mediated developmental but not environmental regulation of root hair growth. New Phytol. 200, 1089–1101. doi: 10.1111/nph.12432
Huck, N., Moore, J. M., Federer, M., and Grossniklaus, U. (2003). The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development 130, 2149–2159. doi: 10.3410/f.1012937.188152
Humphrey, T. V., Bonetta, D. T., and Goring, D. R. (2007). Sentinels at the wall; cell wall receptors and sensors. New Phytol. 176, 7–21. doi: 10.1111/j.1469-8137.2007.02192.x
Jia, H. F., Chai, Y. M., Li, C. L., Lu, D., Luo, J. J., Qin, L., et al. (2011). Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 157, 188–199. doi: 10.1104/pp.111.177311
Jia, H. F., Dong, L., Sun, J. H., Li, C. L., Qin, L., and Shen, Y. Y. (2013a). Type 2C protein phosphatase ABI1 is a negative regulator of strawberry fruit ripening. J. Exp. Bot. 64, 1677–1687. doi: 10.1093/jxb/ert028
Jia, H. F., Wang, Y. H., Sun, M. Z., Li, B. B., Han, Y., Zhao, Y. X., et al. (2013b). Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytol. 198, 453–465. doi: 10.1111/nph.12176
Kafkas, E., Koşar, M., Paydaş, S., Kafkas, S., and Başer, K. H. C. (2007). Quality characteristics of strawberry genotypes at different maturation stages. Food Chem. 100, 1229–1236. doi: 10.1016/j.foodchem.2005.12.005
Keinath, N. F., Kierszniowska, S., Lorek, J., Bourdais, G., Kessler, S. A., Shimosato-Asano, H., et al. (2010). PAMP (pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. J. Biol. Chem. 285, 39140–39149. doi: 10.1074/jbc.M110.160531
Kessler, S. A., Lindner, H., Jones, D. S., and Grossniklaus, U. (2015). Functional analysis of related CrRLK1L receptor-like kinases in pollen tube reception. EMBO Rep. 16, 107–115. doi: 10.15252/embr.201438801
Kessler, S. A., Shimosato-Asano, H., Keinath, N. F., Wuest, S. E., Ingram, G., Panstruga, R., et al. (2010). Conserved molecular components for pollen tube reception and fungal invasion. Science 30, 968–971. doi: 10.1126/science.1195211
Klee, H. J., and Giovannoni, J. J. (2011). Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 45, 41–59. doi: 10.1146/annurev-genet-110410-132507
Lazo, G. R., Stein, P. A., and Ludwig, R. A. (1991). A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Nat. Biotechnol. 9, 963–967. doi: 10.1038/nbt1091-963
Lees, D. H., and Francis, F. J. (1971). Quantitative methods for anthocyanins. J Food Sci. 36, 1056–1060. doi: 10.1111/j.1365-2621.1971.tb03345.x
Li, S., and Zhang, Y. (2014). To grow or not to grow: FERONIA has her say. Mol. Plant 7, 1261–1263. doi: 10.1093/mp/ssu031
Lindner, H., Muller, L. M., Boisson-Dernier, A., and Grossniklaus, U. (2012). CrRLK1L receptor-like kinases: not just another brick in the wall. Curr. Opin. Plant Biol. 15, 659–669. doi: 10.1016/j.pbi.2012.07.003
Lin-Wang, K., McGhie, T. K., Wang, M., Liu, Y., Warren, B., Storey, R., et al. (2014). Engineering the anthocyanin regulatory complex of strawberry (Fragaria vesca). Front. Plant Sci. 5:651. doi: 10.3389/fpls.2014.00651
Liu, Y., Palma, A. S., and Feizi, T. (2009). Carbohydrate microsarrays: key developments in glycobiology. Biol. Chem. 390, 647–656. doi: 10.1515/BC.2009.071
Mao, D., Yu, F., Li, J., Van de Poel, B., Tan, D., Li, J., et al. (2015). FERONIA receptor kinase interacts with S-adenosylmethionine synthetase and suppresses S-adenosylmethionine production and ethylene biosynthesis in Arabidopsis. Plant Cell Environ. 38, 2566–2574. doi: 10.1111/pce.12570
Miyazaki, S., Murata, T., Sakurai-Ozato, N., Kubo, M., Demura, T., Fukuda, H., et al. (2009). ANXUR1 and 2, sister genes to FERONIA/SIRENE, are male factors for coordinated fertilization. Curr. Biol. 19, 1327–1331. doi: 10.1016/j.cub.2009.06.064
Ngo, Q. A., Vogler, H., Lituiev, D. S., Nestorova, A., and Grossniklaus, U. (2014). A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Dev. Cell 29, 491–500. doi: 10.1016/j.devcel.2014.04.008
Nitsch, J. P. (1953). The physiology of fruit growth. Annu. Rev. Plant Physiol. 4, 199–236. doi: 10.1146/annurev.pp.04.060153.001215
Pu, C. X., Han, Y. F., Zhu, S., Song, F. Y., Zhao, Y., Wang, C. Y., et al. (2017). The rice receptor-like kinases DWARF AND RUNTISH SPIKELET1 and 2 repress cell death and affect sugar utilization during reproductive development. Plant Cell 29, 70–89. doi: 10.1105/tpc.16.00218
Schallus, T., Feher, K., Sternberg, U., Rybin, V., and Muhle-Goll, C. (2010). Analysis of the specific interactions between the lectin domain of malectin and diglucosides. Glycobiology 20, 1010–1020. doi: 10.1093/glycob/cwq059
Schallus, T., Jaeckh, C., Feher, K., Palma, A. S., Liu, Y., Simpson, J. C., et al. (2008). Malectin: a novel carbohydrate-binding protein of the endoplasmic reticulum and a candidate player in the early steps of protein N-glycosylation. Mol. Biol. Cell 19, 3404–3414.
Schmittgen, T. D., and Livak, K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. doi: 10.1038/nprot.2008.73
Schulze-Muth, P., Irmler, S., Schroder, G., and Schroder, J. (1996). Novel type of receptor-like protein kinase from a higher plant (Catharanthusroseus). cDNA, gene, intramolecular autophosphorylation, and identification of a threonine important for auto- and substrate phosphorylation. J. Biol. Chem. 271, 26684–26689. doi: 10.1074/jbc.271.43.26684
Schütze, K., Harter, K., and Chaban, C. (2009). Bimolecular fluorescence complementation (BiFC) to study protein-protein interactions in living plant cells. Methods Mol. Biol. 479, 189–202. doi: 10.1007/978-1-59745-289-2_12
Seymour, G. B., Østergaard, L., Chapman, N. H., Knapp, S., and Martin, C. (2013). Fruit development and ripening. Annu. Rev. Plant. Physiol. 64, 219–241.
Seymour, G. B., Ryder, C. D., Cevik, V., Hammond, J. P., Popovich, A., King, G. J., et al. (2011). A SEPALLATA gene is involved in the development and ripening of strawberry (Fragaria × ananassa Duch.) fruit, a non-climacteric tissue. J. Exp. Bot. 62, 1179–1188. doi: 10.1093/jxb/erq360
Shiu, S. H., and Bleecker, A. B. (2001a). Plant receptor-like kinase gene family: diversity, function, and signaling. Sci STKE 2001:re22. doi: 10.1126/stke.2001.113.re22
Shiu, S. H., and Bleecker, A. B. (2001b). Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. U.S.A. 98, 10763–10768. doi: 10.1073/pnas.181141598
Shiu, S. H., and Bleecker, A. B. (2003). Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 132, 530–543. doi: 10.1104/pp.103.021964
Takeda, K., Qin, S. Y., Matsumoto, N., and Yamamoto, K. (2014). Association of malectin with ribophorin I is crucial for attenuation of misfolded glycoprotein secretion. Biochem. Biophys. Res. Commun. 454, 436–440. doi: 10.1016/j.bbrc.2014.10.102
Trainotti, L., Pavanello, A., and Casadoro, G. (2005). Different ethylene receptors show an increased expression during the ripening of strawberries: does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits? J. Exp. Bot. 56, 2037–2046. doi: 10.1093/jxb/eri202
Veluthambi, K., and Poovaiah, B. W. (1984). Auxin-regulated polypeptide changes at different stages of strawberry fruit development. Plant Physiol. 75, 349–353. doi: 10.1104/pp.75.2.349
Villarreal, N. M., Bustamante, C. A., Civello, P. M., and Martínez, G. A. (2010). Effect of ethylene and 1-MCP treatments on strawberry fruit ripening. J. Sci. Food Agric. 90, 683–689. doi: 10.1002/jsfa.3868
White, P. J. (2002). Recent advances in fruit development and ripening: an overview. J. Exp. Bot. 53, 1995–2000. doi: 10.1093/jxb/erf105
Wolf, S., Hématy, K., and Höfte, H. (2012). Growth control and cell wall signaling in plants. Annu. Rev. Plant Physiol. 63, 381–407. doi: 10.1146/annurev-arplant-042811-105449
Yang, T., Wang, L., Li, C., Liu, Y., Zhu, S., Qi, Y., et al. (2015). Receptor protein kinase FERONIA controls leaf starch accumulation by interacting with glyceraldehyde-3-phosphate dehydrogenase. Biochem. Biophys. Res. Commun. 465, 77–82. doi: 10.1016/j.bbrc.2015.07.132
Yu, F., Li, J., Huang, Y., Liu, L., Li, D., Chen, L., et al. (2014). FERONIA receptor kinase controls seed size in Arabidopsis thaliana. Mol. Plant 7, 920–922. doi: 10.1093/mp/ssu010
Yu, F., Qian, L., Nibau, C., Duan, Q., Kita, D., Levasseur, K., et al. (2012). FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc. Natl. Acad. Sci. U.S.A. 109, 14693–14698. doi: 10.1073/pnas.1212547109
Zermiani, M., Zonin, E., Nonis, A., Begheldo, M., Ceccato, L., Vezzaro, A., et al. (2015). Ethylene negatively regulates transcript abundance of ROP-GAP rheostat-encoding genes and affects apoplastic reactive oxygen species homeostasis in epicarps of cold stored apple fruits. J. Exp. Bot. 66, 7255–7270. doi: 10.1093/jxb/erv422
Zhang, M., Yuan, B., and Leng, P. (2009). The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J. Exp. Bot. 60, 1579–1588. doi: 10.1093/jxb/erp026
Keywords: FaABI1, FaMRLK47, FERONIA, fruit quality formation, strawberry (Fragaria × ananassa)
Citation: Jia M, Ding N, Zhang Q, Xing S, Wei L, Zhao Y, Du P, Mao W, Li J, Li B and Jia W (2017) A FERONIA-Like Receptor Kinase Regulates Strawberry (Fragaria × ananassa) Fruit Ripening and Quality Formation. Front. Plant Sci. 8:1099. doi: 10.3389/fpls.2017.01099
Received: 11 March 2017; Accepted: 07 June 2017;
Published: 28 June 2017.
Edited by:
Maarten Hertog, KU Leuven, BelgiumReviewed by:
Benedetto Ruperti, University of Padua, ItalyCopyright © 2017 Jia, Ding, Zhang, Xing, Wei, Zhao, Du, Mao, Li, Li and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bingbing Li, bGliaW5nYmluZ0BjYXUuZWR1LmNu Wensuo Jia, amlhd3NAY2F1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.