- 1BioDiscovery Institute and Department of Biological Sciences, University of North Texas, Denton, TX, United States
- 2BioEnergy Science Center, US Department of Energy, Oak Ridge, TN, United States
Unlike animals, plants, being sessile, cannot escape from exposure to severe abiotic stresses such as extreme temperature and water deficit. The dynamic structure of plant cell wall enables them to undergo compensatory changes, as well as maintain physical strength, with changing environments. Plant hormones known as brassinosteroids (BRs) play a key role in determining cell wall expansion during stress responses. Cell wall deposition differs between grasses (Poaceae) and dicots. Grass species include many important food, fiber, and biofuel crops. In this article, we focus on recent advances in BR-regulated cell wall biosynthesis and remodeling in response to stresses, comparing our understanding of the mechanisms in grass species with those in the more studied dicots. A more comprehensive understanding of BR-mediated changes in cell wall integrity in grass species will benefit the development of genetic tools to improve crop productivity, fiber quality and plant biomass recalcitrance.
Introduction
During its whole life cycle, a plant’s physical survival is threatened by exposure to various biotic and abiotic stresses, which can cause morphological and physiological changes that limit growth and productivity (Bajguz and Hayat, 2009). A thin and tough layer called the cell wall surrounds plant cells to provide structural strength and act as a protective barrier against both biotic and abiotic stresses, such as pathogen attack and salinity (osmotic stress) (Taiz and Zeiger, 1998).
Phytohormones are chemical mediators that enable plants to coordinate a variety of cellular processes such as rapid responses to external stimuli (Deb et al., 2016); regulation by phytohormones is required for cell wall sensing and reconstruction during adaptive responses to adverse conditions (Didi et al., 2015; Houston et al., 2016). Brassinosteroids (BR) are a family of plant steroid hormones that elicit cell expansion (Vriet et al., 2013). Plant cell expansion and differentiation are inherently accompanied by a series of dynamic changes in cell wall composition (Hofte, 2015). The BR signaling pathway is fine-tuned to determine cell wall loosening or stiffening to assure the appropriate cell wall properties under various environmental conditions (Voxeur and Hofte, 2016). Application of exogenous BR has been proven to enhance crop tolerance to unfavorable conditions (Sharma et al., 2013), and genetic manipulation of genes that control endogenous BR levels can promote crop tolerance and improve biomass yield under a wide arrange of abiotic stress conditions (Wu et al., 2008; Ahammed et al., 2015).
The grass family (the Poaceae), one of the largest flowering plant families, covers one fifth of the earth’s land (Fincher, 2009). Grasses, including rice, maize, wheat, switchgrass, ryegrass and related species, dominate the majority of human food, livestock feed, biofuel resource and lawn and ornamental use. Grass cell walls constitute a major portion of the plant biomass and present unique features compared with those of dicots (Vogel, 2008). Therefore, understanding hormonal regulation of grass cell wall construction under adverse conditions is important for future manipulation of food and biofuel crops under climate change. Recent reviews have suggested how BR signaling underlies how plant cell walls sense pathogens and operate an active defense against pathogen attack (Underwood, 2012; Bellincampi et al., 2014; Malinovsky et al., 2014). In this review, we focus on the mechanisms of cell wall remodeling in grasses under the control of BRs as a response to abiotic stresses.
Grass Cell Wall Structure
Plant cell walls mainly consist of the polysaccharide polymers cellulose, hemicellulose and pectin, along with lignin and a small amount of structural protein (Bashline et al., 2014). Individual cellulose microfibrils are organized to form a highly ordered (crystalline) matrix via hydrogen bonds. Hemicellulose binds to the surface of cellulose to prevent cellulose microfibrils from clasping together. Pectin and structural proteins are embedded into the cellulose-hemicellulose network to enhance the correct assembly of cell wall components and, along with lignin, to provide additional mechanical strength (Taiz and Zeiger, 1998; Cosgrove and Jarvis, 2012).
There are two major types of cell wall according to composition and structure. Type I walls consist of a xyloglucan matrix into which cellulose microfibrils are embedded, with high levels of pectin and structural proteins and low levels of arabinoxylans, glucomannans, and galacto-glucomannans. In contrast, type II walls have a cross-linked network of glucuronoarabinoxylans bound to cellulose fibers, with various minor portions of pectin and structural proteins (Vogel, 2008). Type I cell walls are present in dicots, non-grass monocots and gymnosperms, and type II cell walls are present in grasses (Fincher, 2009). Additional notable features of grass cell walls include the presence of mixed-linkage β-glucans and the abundance of xylan and lignin deposited in secondary cell walls (Vogel, 2008). These unique aspects indicate the existence of genes specifically involved in grass cell wall biogenesis, many of which remain to be explored (Lin et al., 2016).
Damage to Cell Walls Under Abiotic Stress
Among abiotic stresses, water-deficiency through drought, osmotic stress and salinity are the most challenging for crop growth and food production (Tenhaken, 2015; Feng et al., 2016). The water potential of the plant cell is in accordance with that of the environment surrounding the cell (Bray, 2007). Drought, osmotic stress and salinity decrease the water potential of the soil solution and water leaks from the cell to the external solution (Bray, 2007). As a consequence, this can lead to reduction of cell turgor and physical damage to the cell wall including disconnection of binding sites for wall components, loss of fragments from the wall, and decreased associations between the wall and the plasma membrane (Hamann, 2015b).
Another common reaction in plant responses to many stress conditions (such as heavy metal ions) is the generation of a burst of reactive oxygen species (ROS), which results in the toxicity of oxidative stress (Mittler, 2002). A rapid accumulation of ROS in plant cells inhibits the activity of antioxidants and antioxidative enzymes and can cause the degradation of lipids and even destruction of the cell membrane (Das and Roychoudhury, 2014). Moreover, the generation of OH by the Fenton reaction involving heavy metal ions or antioxidative enzymes is considered to cause plant cell wall loosening via breaking cross-linkages between ferulates and lignin (Karkonen and Kuchitsu, 2015).
A group of plasma membrane-localized receptor-like kinases (RLKs) and mechanosensitive ion channels (such as Ca2+ channels) are considered to directly or indirectly detect the impairment of cell wall integrity as a mechanical cue to sense adverse effects in the environment. They subsequently translate the physical changes in the cell surface to cellular signals (such as Ca2+ influx), which further trigger corresponding cascades of plant defense responses for stress management (Hamann and Denness, 2011; Feng et al., 2016). These responses are better understood in yeast than in plants (Hamann and Denness, 2011).
Transduction of BR Signaling in Grasses
When perceiving environmental cues, plants translate them into physiological signals through coordination of the levels of phytohormones such as gibberellic acid (GA), abscisic acid (ABA), and BRs that will further trigger cellular responses to maintain integrity and remodeling of the cell wall (Ahammed et al., 2015). Recent findings have characterized the molecular machinery of BR signal reception and transduction in Arabidopsis (Wolf et al., 2012, 2014; Zhu et al., 2013). However, the BR signaling pathway in monocots still remains to be explored, although a few conserved components have been identified in rice (Yamamuro et al., 2000; Bai et al., 2007; Li et al., 2009; Tong et al., 2012; Vriet et al., 2013; Zhang et al., 2014; Zhang B. et al., 2016), maize (Zhang Y. et al., 2016) and Brachypodium distachyon (Feng et al., 2015). Here, we show a proposed model for the BR signaling pathway in grasses generally based on the information for rice (Figure 1). BR signaling is perceived by cells through its binding to the extracellular domain of a plasma membrane-bound receptor kinase, BRASSINOSTEROID INSENSITIVE 1 (OsBRI1) (Yamamuro et al., 2000; Zhu et al., 2013). BR-binding prevents BRI1 from associating with the negative regulator OsGSK and promotes BRI1 to interact with the co-receptor kinase BRI1-ASSOCIATED RECEPTOR KINASE1 (OsBAK1) (Li et al., 2009; Zhang et al., 2014). The trans-phosphorylation between OsBRI1 and OsBAK1 activates the kinase activity of BRI1, the intracellular domain of which initiates the signal transduction cascade within cells (Vriet et al., 2013). BR SIGNALING KINASE (BSK1) located in the cytoplasm is phosphorylated and activated by BRI1 leading to the activation of BRI1 SUPPRESSOR1 (BSU1) (Zhang B. et al., 2016). Through desphosphorylation, BSU1 negatively regulates OsGSK2, an inhibitor of the BRASSINAZOLE-RESISTANT1 (BZR) family of transcription factors (Tong et al., 2012). Upon BR signaling, OsBZR1 is activated by dephosphorylation and inhibition of its interaction with 14-3-3 proteins. This leads to a rapid accumulation of OsBZR1 in the nucleus to directly control the expression of BR target genes (Bai et al., 2007). In Arabidopsis, the BR-activated transcription factor BZR1 and its homologous gene BZR2/BES1 have been shown to directly bind to promoter regions of a large number of cell wall-related genes (Jiang et al., 2015), including the majority of cellulose synthase genes (Xie et al., 2011), and NAC and MYB transcription factors associated with regulatory pathways for lignin synthesis (Zhao and Dixon, 2011; Benatti et al., 2012). Though direct evidence is absent, grasses may operate a similar BR-mediated signal cascade to regulate the expression of genes involved in cell wall biogenesis.
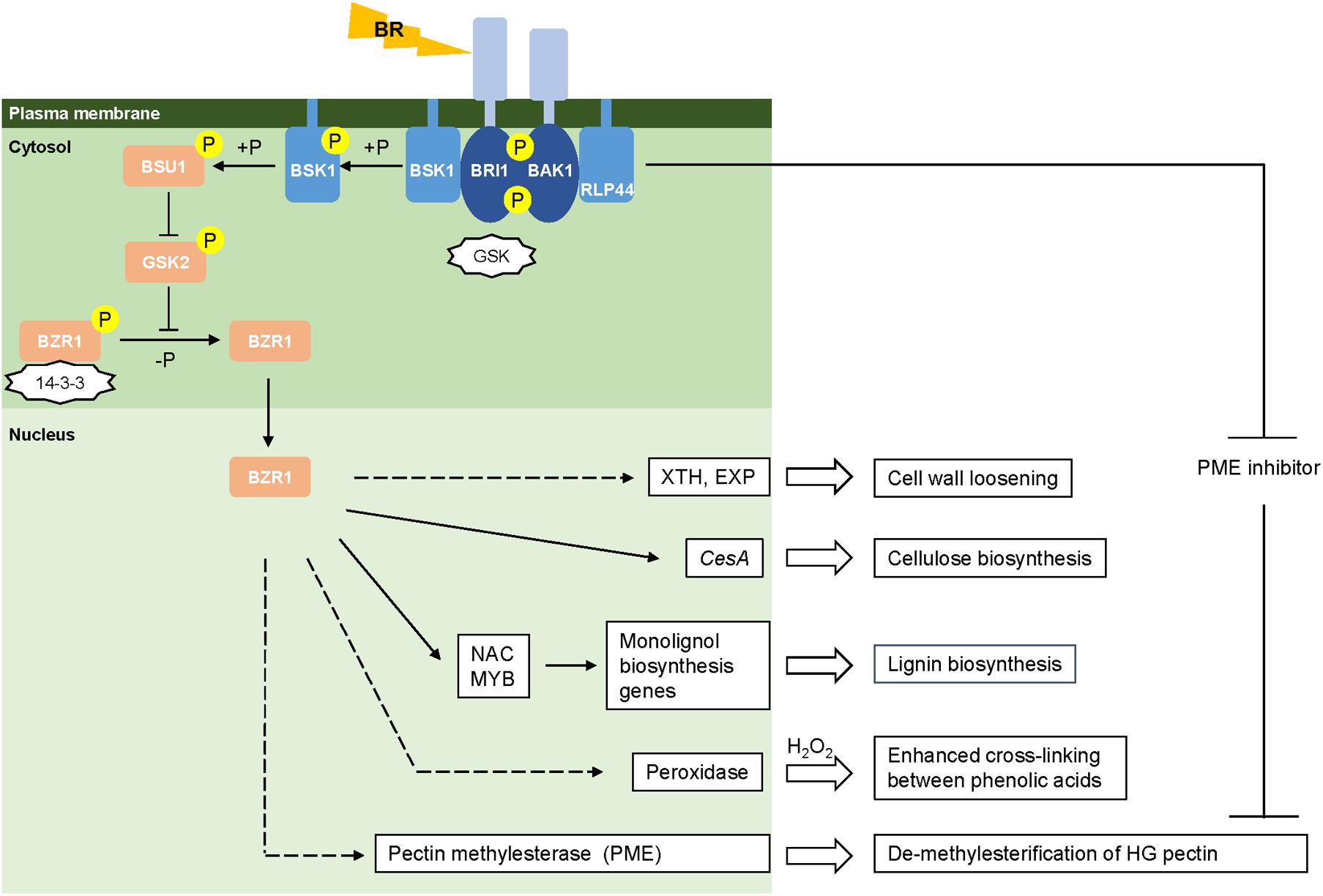
FIGURE 1. Brassinosteroid mediated cell wall remodeling. Models of the BR signaling pathway are drawn based on the information from rice (Vriet et al., 2013). Activated and repressed reactions are represented by arrows and blunt-ended lines, respectively. Negative regulators (GSK and 14-3-3) are in the shape of stars. Phosphorylation and de-phosphorylation are represented by “+P” and “-P,” respectively. Direct and indirect regulation are represented by solid and dashed lines, respectively.
BR-Mediated Cell Wall Remodeling
Recent evidence suggests the association of BR signaling pathways with cell wall remodeling. Here, we discuss effects of BRs on cell wall loosening proteins and major structural cell wall components including cellulose, lignin and pectin, according to recent advances in both grasses and dicots.
BR-Mediated Cell Wall Loosening Proteins
Modification of the structure of the plant cell wall is required as a defense response upon the perception of abiotic stresses (Tenhaken, 2015). Two groups of enzymes, xyloglucan transferase/hydrolase (XTHs) and expansins, are involved in cell wall loosening. XTHs catalyze the internal cleavage of xylogulcan polymers and transfer the newly generated ends to other xyloglucan chains (Uozu et al., 2000; Eklof and Brumer, 2010), whereas expansins loosen the linkages between cellulose microfibrils through non-covalent rearrangement of their targets (Yennawar et al., 2006). A subset of XTH and expansin genes is significantly up-regulated by BL treatment in Arabidopsis and soybean (Zurek and Clouse, 1994; Kozuka et al., 2010; Abuqamar et al., 2013). Similarly, the expression of a number of XTHs and expansin genes has been reported to be regulated by BR in rice, maize and wheat (Uozu et al., 2000; Yokoyama et al., 2004; Liu et al., 2007; Genovesi et al., 2008). Considering that grasses contain lower levels of xyloglucan in their cell walls than do dicots, it has been suggested that XTH isoforms in grass species may contribute to building xyloglucan-(β-1,3:1,4-glucan) links, rather than rearrangement of xyloglucan chains (Eklof and Brumer, 2010). BR-mediated regulation of XTH and expansin mRNA levels may lead to alteration of the interaction between xyloglucan and cellulose microfibrils to alter cell wall stiffness.
BR-Mediated Cellulose Deposition
Cellulose microfibrils, composed of β-1,4-glucan chains (Hill et al., 2014), contribute to the majority of plant above-ground biomass and their synthesis and deposition is responsive to changing environmental conditions (Wang et al., 2016). Cellulose synthesis requires multiple members of the cellulose synthase (CesA) gene superfamily, which encode catalytic subunits that form hexameric complexes localized on the plasma membrane (Hill et al., 2014). In Arabidopsis, BR signaling has been shown to increase cellulose accumulation through upregulation of CesA genes at both the transcriptional and post-transcriptional levels. The expression of most CesA genes is induced by BR-mediated activation of the transcription factor BES1, which directly binds to the CANNTG E-box in the promoter region of CesA genes (Xie et al., 2011), while the activity of CESA1 kinase is increased by the degradation of its inhibitor protein BRASSINOSTEROID INSENSITIVE2 (BIN2) (Sánchez-Rodríguez et al., 2017). Some observations suggest that grasses may share a similar BR-mediated pathway for CesA gene regulation. The BR receptor kinase gene OsBRI1 shows co-expression with OsCESA3 in a genome-scale gene network for rice (Lee et al., 2011). An associated up-regulation of BRI1, CESA3 and other genes involved in BR signaling is observed in a wild wheat species (Agropyron elongatum) compared with domesticated genotypes during water stress. The enhanced BR-signaling pathway in A. elongatum may contribute to its higher water-stress tolerance and significant increase of root and shoot biomass compared with the domesticated line under water-deficient conditions (Placido et al., 2013).
Either exogenous application of BRs or overexpression of BR receptor genes could benefit cellulose deposition and accumulation, especially to compensate for cellulose loss caused by abiotic stresses (Sun et al., 2005; Li et al., 2009; Zhang et al., 2014). Some evidence has suggested that BR signaling may not directly determine the total content of cellulose (Schrick et al., 2012) but rather be more involved in the orientation of cellulose microfibril deposition through the control of the cortical microtubular organization in cells (Bashline et al., 2014).
BR-Mediated Lignin Accumulation
The second most abundant carbon sink in plants, lignin is absent from the primary cell wall and deposited in the secondary cell wall surrounding specific cell types to enhance cell wall rigidity and provide structural support (Boerjan et al., 2003; Karkonen and Koutaniemi, 2010). Lignin is a phenolic heteropolymer, which mainly consists of three types of 4-hydroxycinnamyl alcohol units, guaiacyl (G), syringyl (S) and p-hydroxyphenyl (H), derived from the monolignols coniferyl alcohol, sinapyl alcohol and p-coumaryl alcohol, respectively (Boerjan et al., 2003; Chen et al., 2012). An induction of lignin biosynthesis is often observed under biotic and abiotic stresses as a defense response (Dixon and Paiva, 1995; Moura et al., 2010). For example, excess heavy metal (Cu, Zn, Al) causes an elevated accumulation of lignin in cell walls of rice and wheat (Moura et al., 2010).
Brassinosteroids have been reported to play a crucial role in secondary cell wall deposition. Application of the BR biosynthesis inhibitor (BRz) in cotton ovules causes severe inhibition of secondary cell wall development in the fibers (Sun et al., 2005). Tracheary element formation and secondary cell wall thickening can be observed in suspension cell cultures of Arabidopsis and banana following exogenous BR-supplementation (Oda et al., 2005; Negi et al., 2015). Furthermore, loss of function of a BR biosynthesis protein (DIM1) in Arabidopsis leads to a significant reduction in lignin content and a lower lignin S/G ratio (Hossain et al., 2012). Consistent with this finding, BR treatment induces the accumulation of lignin with predominantly S units in switchgrass suspension cells (Shen et al., 2013). A regulatory mechanism for BR signaling and secondary cell wall development has been proposed in Arabidopsis; the BR-activated transcription factor BES1 promotes the expression of VND6 and VND7, which determine the transition of xylem cells to form tracheary elements, and alters the expression of MYB transcription factors involved in regulating lignin biosynthesis (Zhong et al., 2008; Yamaguchi et al., 2010; Zhao and Dixon, 2011; Didi et al., 2015; Li et al., 2016).
Besides the regulation of genes involved in monolignol biosynthesis, BRs may also have effects on the bonds between monolignol polymers and phenolic acids in the cell wall through controlling antioxidant enzymes at the transcriptional and post-transcriptional level. The exogenous application of BR significantly increases the activity of antioxidant enzymes (such as catalase, superoxide dismutase, ascorbate peroxidase, and peroxidase) through up-regulation of the expression of the corresponding genes in maize, wheat, and rice exposed to metal stress (Vardhini and Anjum, 2015; Yan et al., 2015; Sharma et al., 2016). Peroxidases mediate the formation of phenolic radicals, leading to both lignin polymerization and cross-linking between the ferulic acid units esterified to arabinoxylans which occur especially in grasses (Hamann, 2015a; Tenhaken, 2015). The increased activity of peroxidases and the formation of ROS together enhance the covalent cross-linking of components in the cell wall and strengthen the mechanical properties of the wall (Lamb and Dixon, 1997; Tenhaken, 2015). Therefore, it is possible that BRs enhance the antioxidant defense system as well as increasing the cross-linking of phenolic compounds in the cell wall to alleviate oxidative damage caused by the ROS burst (O’Brien et al., 2012).
BR-Mediated Pectin Modification
Pectins play a critical role in enabling cell walls to remain firm but extensible (Harholt et al., 2010). Pectic polysaccharides bind to the cellulose and hemicellulose network, forming hydrated gels to inhibit collapse of the cellulose matrix and to monitor changes in polymer residues and pH (Harholt et al., 2010; Voxeur and Hofte, 2016). Pectic polysaccharides consist of various galacturonic acid (GalUA)-containing polymers, including homogalacturonan (HG), xylogalacturonan (XGA), rhamnogalacturonan I (RGI), and rhamnogalacturonan II (RGII) as backbone units, of which GalUA residues can be substituted by arabinan, galactan, and arabinogalactan as branch chains (Harholt et al., 2010; Hofte, 2015). The degree of methylesterification in HGs determines looseness1 the stiffness of the pectic matrix and is precisely controlled by the balance of activity between pectin methylesterase enzymes (PMEs) and PME inhibitors (PMEIs) (Wolf et al., 2012). PMEs de-methyl esterify HG chains in the cell wall, which leads to a decrease in stiffness of the wall and acceleration of cell growth under Ca2+ limited conditions (Hofte, 2015) or promotes the formation of a HG-Ca2+ gel to lock the cell wall into an inextensible state under Ca2+ abundant conditions (Cosgrove, 2016). Ca2+ fluxes/levels complement BR signaling by contributing to the fine-tuned control of cell wall integrity under normal or adverse conditions. For example, BRs have been shown to upregulate the level of one PME transcript and trigger PME activity to increase the stiffness of cell walls in response to cold and freezing in Arabidopsis (Qu et al., 2011). The BR-receptor kinase BAK1 in Arabidopsis can directly interact with a plasma membrane receptor-like protein (RLP44) to repress the activity of PME inhibitors and therefore reduce the stiffness of the pectic matrix and promote cell wall loosening under both normal and stress conditions (Wolf et al., 2012, 2014). Therefore, BR signaling in Arabidopsis is coupled with the modification of methyl-esterified HGs to control pectin-dependent cell wall integrity (Wolf et al., 2012). Knowledge of BR-meditated pectin methylesterase activity so far is lacking in grasses and is an important area for future research.
Conclusion
The possible roles of BR signaling that contribute to cell wall remodeling are summarized in Figure 1. Few BR response targets have been established and much remains to be discovered about how BRs regulate the expression of cell wall related genes and corresponding enzymatic activity in grasses. In addition, the crosstalk between BRs and other phytohomones in controlling cell wall integrity is another area that requires more investigation (Bai et al., 2012; Huang et al., 2015; Deb et al., 2016). A better understanding of BR-mediated cell wall homeostasis will guide the design of genetic modification strategies to improve biomass and stress tolerance in grasses.
Author Contributions
XR collected data from literature and wrote the manuscript. RD revised the article.
Funding
The research from the authors’ laboratory was supported by the US Department of Energy Bioenergy Sciences Center (BESC, grant # BER DE-AC05-00OR2727), through the Office of Biological and Environmental Research in the DOE Office of Science.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The research from the authors’ laboratory was supported by the US Department of Energy Bioenergy Sciences Center (BESC, grant # BER DE-AC05-00OR2727), through the Office of Biological and Environmental Research in the DOE Office of Science.
References
Abuqamar, S., Ajeb, S., Sham, A., Enan, M. R., and Iratni, R. (2013). A mutation in the expansin-like A2 gene enhances resistance to necrotrophic fungi and hypersensitivity to abiotic stress in Arabidopsis thaliana. Mol. Plant Pathol. 14, 813–827. doi: 10.1111/mpp.12049
Ahammed, G. J., Xia, X. J., Li, X., Shi, K., Yu, J. Q., and Zhou, Y. H. (2015). Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Curr. Protein Pept. Sci. 16, 462–473. doi: 10.2174/1389203716666150330141427
Bai, M. Y., Shang, J. X., Oh, E., Fan, M., Bai, Y., Zentella, R., et al. (2012). Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat. Cell Biol. 14, 810–817. doi: 10.1038/ncb2546
Bai, M. Y., Zhang, L. Y., Gampala, S. S., Zhu, S. W., Song, W. Y., Chong, K., et al. (2007). Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice. Proc. Natl. Acad. Sci. U.S.A. 104, 13839–13844. doi: 10.1073/pnas.0706386104
Bajguz, A., and Hayat, S. (2009). Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 47, 1–8. doi: 10.1016/j.plaphy.2008.10.002
Bashline, L., Lei, L., Li, S. D., and Gu, Y. (2014). Cell wall, cytoskeleton, and cell expansion in higher plants. Mol. Plant. 7, 586–600. doi: 10.1093/mp/ssu018
Bellincampi, D., Cervone, F., and Lionetti, V. (2014). Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Front. Plant Sci. 5:228. doi: 10.3389/fpls.2014.00228
Benatti, M. R., Penning, B. W., Carpita, N. C., and McCann, M. C. (2012). We are good to grow: dynamic integration of cell wall architecture with the machinery of growth. Front. Plant Sci. 3:187. doi: 10.3389/Fpls.2012.00187
Boerjan, W., Ralph, J., and Baucher, M. (2003). Lignin biosynthesis. Annu. Rev. Plant Biol. 54, 519–546. doi: 10.1146/annurev.arplant.54.031902.134938
Bray, E. A. (2007). Plant response to water deficit stress. Encycl. Life Sci. doi: 10.1002/9780470015902.a0001298.pub2
Chen, F., Tobimatsu, Y., Havkin-Frenkel, D., Dixon, R. A., and Ralph, J. (2012). A polymer of caffeyl alcohol in plant seeds. Proc. Natl. Acad. Sci. U.S.A. 109, 1772–1777. doi: 10.1073/pnas.1120992109
Cosgrove, D. J. (2016). Plant cell wall extensibility: connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 67, 463–476. doi: 10.1093/jxb/erv511
Cosgrove, D. J., and Jarvis, M. C. (2012). Comparative structure and biomechanics of plant primary and secondary cell walls. Front. Plant Sci. 3:204. doi: 10.3389/fpls.2012.00204
Das, K., and Roychoudhury, A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2:53. doi: 10.3389/fenvs.2014.00053
Deb, A., Grewal, R. K., and Kundu, S. (2016). Regulatory cross-talks and cascades in rice hormone biosynthesis pathways contribute to stress signaling. Front. Plant Sci. 7:1303. doi: 10.3389/Fpls.2016.01303
Didi, V., Jackson, P., and Hejatko, J. (2015). Hormonal regulation of secondary cell wall formation. J. Exp. Bot. 66, 5015–5027. doi: 10.1093/jxb/erv222
Dixon, R. A., and Paiva, N. L. (1995). Stress-induced phenylpropanoid metabolism. Plant Cell 7, 1085–1097. doi: 10.1105/tpc.7.7.1085
Eklof, J. M., and Brumer, H. (2010). The XTH gene family: an update on enzyme structure, function, and phylogeny in xyloglucan remodeling. Plant Physiol. 153, 456–466. doi: 10.1104/pp.110.156844
Feng, W., Lindner, H., Robbins, N. E. II, and Dinneny, J. R. (2016). Growing out of stress: the role of cell- and organ-scale growth control in plant water-stress responses. Plant Cell 28, 1769–1782. doi: 10.1105/tpc.16.00182
Feng, Y., Yin, Y. H., and Fei, S. Z. (2015). Down-regulation of BdBRI1, a putative brassinosteroid receptor gene produces a dwarf phenotype with enhanced drought tolerance in Brachypodium distachyon. Plant Sci. 234, 163–173. doi: 10.1016/j.plantsci.2015.02.015
Fincher, G. B. (2009). Revolutionary times in our understanding of cell wall biosynthesis and remodeling in the grasses. Plant Physiol. 149, 27–37. doi: 10.1104/pp.108.130096
Genovesi, V., Fornale, S., Fry, S. C., Ruel, K., Ferrer, P., Encina, A., et al. (2008). ZmXTH1, a new xyloglucan endotransglucosylase/hydrolase in maize, affects cell wall structure and composition in Arabidopsis thaliana. J. Exp. Bot. 59, 875–889. doi: 10.1093/jxb/ern013
Hamann, T. (2015a). The plant cell wall integrity maintenance mechanism-concepts for organization and mode of action. Plant Cell Physiol. 56, 215–223. doi: 10.1093/pcp/pcu164
Hamann, T. (2015b). The plant cell wall integrity maintenance mechanism - a case study of a cell wall plasma membrane signaling network. Phytochemistry 112, 100–109. doi: 10.1016/j.phytochem.2014.09.019
Hamann, T., and Denness, L. (2011). Cell wall integrity maintenance in plants: lessons to be learned from yeast? Plant Signal. Behav. 6, 1706–1709. doi: 10.4161/psb.6.11.17782
Harholt, J., Suttangkakul, A., and Scheller, H. V. (2010). Biosynthesis of pectin. Plant Physiol. 153, 384–395. doi: 10.1104/pp.110.156588
Hill, J. L., Hammudi, M. B., and Tien, M. (2014). The arabidopsis cellulose synthase complex: a proposed hexamer of CESA trimers in an equimolar stoichiometry. Plant Cell 26, 4834–4842. doi: 10.1105/tpc.114.131193
Hofte, H. (2015). The yin and yang of cell wall integrity control: brassinosteroid and FERONIA Signaling. Plant Cell Physiol. 56, 224–231. doi: 10.1093/pcp/pcu182
Hossain, Z., McGarvey, B., Amyot, L., Gruber, M., Jung, J., and Hannoufa, A. (2012). DIMINUTO 1 affects the lignin profile and secondary cell wall formation in Arabidopsis. Planta 235, 485–498. doi: 10.1007/s00425-011-1519-4
Houston, K., Tucker, M. R., Chowdhury, J., Shirley, N., and Little, A. (2016). The plant cell wall: a complex and dynamic structure as revealed by the responses of genes under stress conditions. Front. Plant Sci. 7:984. doi: 10.3389/Fpls.2016.00984
Huang, D. B., Wang, S. G., Zhang, B. C., Shang-Guan, K. K., Shi, Y. Y., Zhang, D. M., et al. (2015). A gibberellin-mediated DELLA-NAC signaling cascade regulates cellulose synthesis in rice. Plant Cell 27, 1681–1696. doi: 10.1105/tpc.15.00015
Jiang, J. J., Zhang, C., and Wang, X. L. (2015). A recently evolved isoform of the transcription factor BES1 promotes brassinosteroid signaling and development in Arabidopsis thaliana. Plant Cell 27, 361–374. doi: 10.1105/tpc.114.133678
Karkonen, A., and Koutaniemi, S. (2010). Lignin biosynthesis studies in plant tissue cultures. J. Integr. Plant Biol. 52, 176–185. doi: 10.1111/j.1744-7909.2010.00913.x
Karkonen, A., and Kuchitsu, K. (2015). Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 112, 22–32. doi: 10.1016/j.phytochem.2014.09.016
Kozuka, T., Kobayashi, J., Horiguchi, G., Demura, T., Sakakibara, H., Tsukaya, H., et al. (2010). Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol. 153, 1608–1618. doi: 10.1104/pp.110.156802
Lamb, C., and Dixon, R. A. (1997). The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. 48, 251–275. doi: 10.1146/annurev.arplant.48.1.251
Lee, I., Seo, Y. S., Coltrane, D., Hwang, S., Oh, T., Marcotte, E. M., et al. (2011). Genetic dissection of the biotic stress response using a genome-scale gene network for rice. Proc. Natl. Acad. Sci. U.S.A. 108, 18548–18553. doi: 10.1073/pnas.1110384108
Li, D., Wang, L., Wang, M., Xu, Y. Y., Luo, W., Liu, Y. J., et al. (2009). Engineering OsBAK1 gene as a molecular tool to improve rice architecture for high yield. Plant Biotechnol. J. 7, 791–806. doi: 10.1111/j.1467-7652.2009.00444.x
Li, Z., Omranian, N., Neumetzler, L., Wang, T., Herter, T., Usadel, B., et al. (2016). A transcriptional and metabolic framework for secondary wall formation in Arabidopsis. Plant Physiol. 172, 1334–1351. doi: 10.1104/pp.16.01100
Lin, F., Manisseri, C., Fagerstrom, A., Peck, M. L., Vega-Sanchez, M. E., Williams, B., et al. (2016). Cell wall composition and candidate biosynthesis gene expression during rice development. Plant Cell Physiolol. 57, 2058–2075. doi: 10.1093/pcp/pcw125
Liu, Y., Liu, D. C., Zhang, H. Y., Gao, H. B., Guo, X. L., Wang, D. M., et al. (2007). The alpha- and beta-expansin and xyloglucan endotransglucosylase/hydrolase gene families of wheat: molecular cloning, gene expression, and EST data mining. Genomics 90, 516–529. doi: 10.1016/j.ygeno.2007.06.012
Malinovsky, F. G., Fangel, J. U., and Willats, W. G. T. (2014). The role of the cell wall in plant immunity. Front. Plant Sci. 5:178. doi: 10.3389/Fpls.2014.00178
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. doi: 10.1016/S1360-1385(02)02312-9
Moura, J. C. M. S., Bonine, C. A. V., Viana, J. D. F., Dornelas, M. C., and Mazzafera, P. (2010). Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 52, 360–376. doi: 10.1111/j.1744-7909.2010.00892.x
Negi, S., Tak, H., and Ganapathi, T. R. (2015). In vitro xylem vessel elements formation from banana embryogenic cells and expression analysis of vessel development-related genes. Plant Biotechnol. Rep. 9, 47–54. doi: 10.1007/s11816-015-0342-y
O’Brien, J. A., Daudi, A., Butt, V. S., and Bolwell, G. P. (2012). Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236, 765–779. doi: 10.1007/s00425-012-1696-9
Oda, Y., Mimura, T., and Hasezawa, S. (2005). Regulation of secondary cell wall development by cortical microtubules during tracheary element differentiation in Arabidopsis cell suspensions. Plant Physiol. 137, 1027–1036. doi: 10.1104/pp.104.052613
Placido, D. F., Campbell, M. T., Folsom, J. J., Cui, X. P., Kruger, G. R., Baenziger, P. S., et al. (2013). Introgression of novel traits from a wild wheat relative improves drought adaptation in wheat. Plant Physiol. 161, 1806–1819. doi: 10.1104/pp.113.214262
Qu, T., Liu, R. F., Wang, W., An, L. Z., Chen, T., Liu, G. X., et al. (2011). Brassinosteroids regulate pectin methylesterase activity and AtPME41 expression in Arabidopsis under chilling stress. Cryobiology 63, 111–117. doi: 10.1016/j.cryobiol.2011.07.003
Sánchez-Rodríguez, C., Ketelaar, K., Schneider, R., Villalobos, J. A., Somerville, C. R., Persson, S., et al. (2017). BRASSINOSTEROID INSENSITIVE2 negatively regulates cellulose synthesis in Arabidopsis by phosphorylating cellulose synthase 1. Proc. Natl. Acad. Sci. U.S.A. 114, 3533–3538. doi: 10.1073/pnas.1615005114
Schrick, K., DeBolt, S., and Bulone, V. (2012). Deciphering the molecular functions of sterols in cellulose biosynthesis. Front. Plant Sci. 3:84. doi: 10.3389/Fpls.2012.00084
Sharma, I., Ching, E., Saini, S., Bhardwaj, R., and Pati, P. K. (2013). Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol. Biochem. 69, 17–26. doi: 10.1016/j.plaphy.2013.04.013
Sharma, P., Kumar, A., and Bhardwaj, R. (2016). Plant steroidal hormone epibrassinolide regulates heavy metal stress tolerance in Oryza sativa L. by modulating antioxidant defense expression. Environ. Exp. Bot. 122, 1–9. doi: 10.1016/j.envexpbot.2015.08.005
Shen, H., Mazarei, M., Hisano, H., Escamilla-Trevino, L., Fu, C. X., Pu, Y. Q., et al. (2013). A genomics approach to deciphering lignin biosynthesis in switchgrass. Plant Cell 25, 4342–4361. doi: 10.1105/tpc.113.118828
Sun, Y., Veerabomma, S., Abdel-Mageed, H. A., Fokar, M., Asami, T., Yoshida, S., et al. (2005). Brassinosteroid regulates fiber development on cultured cotton ovules. Plant Cell Physiol. 46, 1384–1391. doi: 10.1093/pcp/pci150
Taiz, L., and Zeiger, E. (1998). “Cell walls: structure, biogenesis, and expansion,” in Plant Physiology, eds L. Taiz and E. Zeiger (Sunderland, MA: Sinauer Associates), 409–443.
Tenhaken, R. (2015). Cell wall remodeling under abiotic stress. Front. Plant Sci. 5:771. doi: 10.3389/Fpls.2014.00771
Tong, H. N., Liu, L. C., Jin, Y., Du, L., Yin, Y. H., Qian, Q., et al. (2012). DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice. Plant Cell 24, 2562–2577. doi: 10.1105/tpc.112.097394
Underwood, W. (2012). The plant cell wall: a dynamic barrier against pathogen invasion. Front. Plant Sci. 3:85. doi: 10.3389/Fpls.2012.00085
Uozu, S., Tanaka-Ueguchi, M., Kitano, H., Hattori, K., and Matsuoka, M. (2000). Characterization of XET-related genes of rice. Plant Physiol. 122, 853–859. doi: 10.1104/Pp.122.3.853
Vardhini, B. V., and Anjum, N. A. (2015). Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front. Environ. Sci. 2:67. doi: 10.3389/fenvs.2014.00067
Vogel, J. (2008). Unique aspects of the grass cell wall. Curr. Opin. Plant Biol. 11, 301–307. doi: 10.1016/j.pbi.2008.03.002
Voxeur, A., and Hofte, H. (2016). Cell wall integrity signaling in plants: “To grow or not to grow that’s the question”. Glycobiology 26, 950–960. doi: 10.1093/glycob/cww029
Vriet, C., Russinova, E., and Reuzeau, C. (2013). From squalene to brassinolide: the steroid metabolic and signaling pathways across the plant kingdom. Mol. Plant. 6, 1738–1757. doi: 10.1093/mp/sst096
Wang, T., McFarlane, H. E., and Persson, S. (2016). The impact of abiotic factors on cellulose synthesis. J. Exp. Bot. 67, 543–552. doi: 10.1093/jxb/erv488
Wolf, S., Mravec, J., Greiner, S., Mouille, G., and Hofte, H. (2012). Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Curr. Biol. 22, 1732–1737. doi: 10.1016/j.cub.2012.07.036
Wolf, S., van der Does, D., Ladwig, F., Sticht, C., Kolbeck, A., Schurholz, A. K., et al. (2014). A receptor-like protein mediates the response to pectin modification by activating brassinosteroid signaling. Proc. Natl. Acad. Sci. U.S.A. 111, 15261–15266. doi: 10.1073/pnas.1322979111
Wu, C. Y., Trieu, A., Radhakrishnan, P., Kwok, S. F., Harris, S., Zhang, K., et al. (2008). Brassinosteroids regulate grain filling in rice. Plant Cell 20, 2130–2145. doi: 10.1105/tpc.107.055087
Xie, L. Q., Yang, C. J., and Wang, X. L. (2011). Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in Arabidopsis. J. Exp. Bot. 62, 4495–4506. doi: 10.1093/jxb/err164
Yamaguchi, M., Goue, N., Igarashi, H., Ohtani, M., Nakano, Y., Mortimer, J. C., et al. (2010). VASCULAR-RELATED NAC-DOMAIN6 and VASCULAR-RELATED NAC-DOMAIN7 effectively induce transdifferentiation into xylem vessel elements under control of an induction system. Plant Physiol. 153, 906–914. doi: 10.1104/pp.110.154013
Yamamuro, C., Ihara, Y., Wu, X., Noguchi, T., Fujioka, S., Takatsuto, S., et al. (2000). Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 12, 1591–1605. doi: 10.1105/tpc.12.9.1591
Yan, J. W., Guan, L., Sun, Y., Zhu, Y., Liu, L., Lu, R., et al. (2015). Calcium and ZmCCaMK are involved in brassinosteroid-induced antioxidant defense in maize leaves. Plant Cell Physiol. 56, 883–896. doi: 10.1093/pcp/pcv014
Yennawar, N. H., Li, L. C., Dudzinski, D. M., Tabuchi, A., and Cosgrove, D. J. (2006). Crystal structure and activities of EXPB1 (Zea m 1), alpha, beta-expansin and group-1 pollen allergen from maize. Proc. Natl. Acad. Sci. U.S.A. 103, 14664–14671. doi: 10.1073/pnas.0605979103
Yokoyama, R., Rose, J. K. C., and Nishitani, K. (2004). A surprising diversity and abundance of xyloglucan endotransglucosylase/hydrolases in rice. Classification and expression analysis. Plant Physiol. 134, 1088–1099. doi: 10.1104/pp.103.035261
Zhang, B., Wang, X. L., Zhao, Z. Y., Wang, R. J., Huang, X. H., Zhu, Y. L., et al. (2016). OsBRI1 activates BR signaling by preventing binding between the TPR and kinase domains of OsBSK3 via phosphorylation. Plant Physiol. 170, 1149–1161. doi: 10.1104/pp.15.01668
Zhang, C., Bai, M. Y., and Chong, K. (2014). Brassinosteroid-mediated regulation of agronomic traits in rice. Plant Cell Rep. 33, 683–696. doi: 10.1007/s00299-014-1578-7
Zhang, Y., Zhang, Y. J., Yang, B. J., Yu, X. X., Wang, D., Zu, S. H., et al. (2016). Functional characterization of GmBZL2 (AtBZR1 like gene) reveals the conserved BR signaling regulation in Glycine max. Sci. Rep. 6:31134. doi: 10.1038/Srep31134
Zhao, Q., and Dixon, R. A. (2011). Transcriptional networks for lignin biosynthesis: more complex than we thought? Trends Plant Sci. 16, 227–233. doi: 10.1016/j.tplants.2010.12.005
Zhong, R. Q., Lee, C. H., Zhou, J. L., McCarthy, R. L., and Ye, Z. H. (2008). A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell 20, 2763–2782. doi: 10.1105/tpc.108.061325
Zhu, J. Y., Sae-Seaw, J., and Wang, Z. Y. (2013). Brassinosteroid signalling. Development 140, 1615–1620. doi: 10.1242/dev.060590
Keywords: cell wall, cell wall remodeling, brassinosteroid, phytohormone, abiotic stress
Citation: Rao X and Dixon RA (2017) Brassinosteroid Mediated Cell Wall Remodeling in Grasses under Abiotic Stress. Front. Plant Sci. 8:806. doi: 10.3389/fpls.2017.00806
Received: 26 February 2017; Accepted: 28 April 2017;
Published: 17 May 2017.
Edited by:
Zhulong Chan, Huazhong Agricultural University, ChinaReviewed by:
Gea Guerriero, Luxembourg Institute of Science and Technology, LuxembourgZhaoqing Chu, Shanghai Chenshan Plant Science Research Center (CAS), China
Copyright © 2017 Rao and Dixon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolan Rao, eGlhb2xhbi5yYW9AdW50LmVkdQ==
 Xiaolan Rao
Xiaolan Rao Richard A. Dixon1,2
Richard A. Dixon1,2