
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 22 May 2017
Sec. Plant Genetics and Genomics
Volume 8 - 2017 | https://doi.org/10.3389/fpls.2017.00794
 Yaduru Shasidhar1,2†
Yaduru Shasidhar1,2† Manish K. Vishwakarma1†
Manish K. Vishwakarma1† Manish K. Pandey1*
Manish K. Pandey1* Pasupuleti Janila1*
Pasupuleti Janila1* Murali T. Variath1
Murali T. Variath1 Surendra S. Manohar1
Surendra S. Manohar1 Shyam N. Nigam1
Shyam N. Nigam1 Baozhu Guo3
Baozhu Guo3 Rajeev K. Varshney1,4*
Rajeev K. Varshney1,4*Enhancing seed oil content with desirable fatty acid composition is one of the most important objectives of groundnut breeding programs globally. Genomics-assisted breeding facilitates combining multiple traits faster, however, requires linked markers. In this context, we have developed two different F2 mapping populations, one for oil content (OC-population, ICGV 07368 × ICGV 06420) and another for fatty acid composition (FA-population, ICGV 06420 × SunOleic 95R). These two populations were phenotyped for respective traits and genotyped using Diversity Array Technology (DArT) and DArTseq genotyping platforms. Two genetic maps were developed with 854 (OC-population) and 1,435 (FA-population) marker loci with total map distance of 3,526 and 1,869 cM, respectively. Quantitative trait locus (QTL) analysis using genotyping and phenotyping data identified eight QTLs for oil content including two major QTLs, qOc-A10 and qOc-A02, with 22.11 and 10.37% phenotypic variance explained (PVE), respectively. For seven different fatty acids, a total of 21 QTLs with 7.6–78.6% PVE were identified and 20 of these QTLs were of major effect. Two mutant alleles, ahFAD2B and ahFAD2A, also had 18.44 and 10.78% PVE for palmitic acid, in addition to oleic (33.8 and 17.4% PVE) and linoleic (41.0 and 19.5% PVE) acids. Furthermore, four QTL clusters harboring more than three QTLs for fatty acids were identified on the three LGs. The QTLs identified in this study could be further dissected for candidate gene discovery and development of diagnostic markers for breeding improved groundnut varieties with high oil content and desirable oil quality.
Groundnut or peanut (Arachis hypogaea L.) is one of the important oilseed crops in the world. It is grown in more than 100 countries with a global production of 42.4 Mt from an area of 25.7 Mha (FAO, 2014). More than 70% of the groundnut growing area comes under arid and semi-arid regions. Groundnut seeds contain edible oil (40–56%), protein (20–30%), carbohydrate (10–20%), and several nutritional components such as vitamin E, niacin, calcium, magnesium, phosphorus, zinc, iron, riboflavin, thiamine, and potassium (Dean et al., 2009). India and China are the largest groundnut producers and have a high consumption of groundnut, i.e., confectionery and as cooking oil. In western countries, groundnuts are majorly used in food industries for making peanut butter and confectionary and direct consumption as boiled, salted, and roasted nuts (Pandey et al., 2012; Varshney et al., 2013). It is estimated that 1% increase in the seed oil content increases the groundnut processer’s benefit by 7% (see Liao, 2003), indicating greater impact of oil content trait for farmers and traders.
Groundnut oil is a rich source of plant sterol especially β-sitosterol, which is known to have anticancer properties and reduce cholesterol levels upto 10–15% by inhibiting the cholesterol absorption (Awad and Fink, 2000). Groundnut oil is also a source of valuable antioxidants vitamin E, resveratrol, which neutralize the harmful free radicals, stresses and maintains cell membrane integrity (Meredith and Alfred, 2003). Along with the above beneficial features, groundnut oil is a high calorie diet, i.e., 100 g of oil provides 884 calories. The groundnut seed with high oil (≥50%) and high oleic acid (75–80%) contents can be an excellent alternative to canola and olive oil, which are currently costlier by 2.5 and 11 times, respectively. Currently selected countries such as Australia, Argentina, United States of America, Brazil, South Africa, and Israel are producing high oleic groundnuts on a large scale (Barkley et al., 2013) but such trend is likely to follow in other parts of the world.
The groundnut oil accounts 44–56% of the dry seed weight (Chen et al., 2010) and is composed of different fatty acids. Major fatty acids include oleic (C18:1), linoleic (C18:2), palmitic (C16:0), stearic (C18:0), arachidic (C20:0), behenic (C22:0), lignoceric (C24:0), and gadoleic acid (C20:1). Groundnut oil comprises about 80% unsaturated fatty acids (UFA) and 20% saturated fatty acids (SFA). UFA constitutes 42% mono UFA (MUFA), i.e., oleic acid while 37% poly UFA (PUFA), i.e., linoleic acid (Wang et al., 2015). Normal groundnut oil with high linoleic acid is vulnerable to oxidation, leading to unpleasant smell, taste and short shelf life of the oil and other groundnut products. The third major fatty acid palmitic acid (C16:0) is a SFA and contributes about 10% of the total oil content while the remaining 10% is contributed by minor fatty acids (Wang et al., 2015). In general, human consumption of the groundnut oil with high proportion of oleic acid and low proportion of linoleic acid (high oleic/linoleic acid ratio) is preferable because it cuts down the risk of cardiovascular disease (CVD) by reducing low-density lipoproteins (LDL) levels in the blood (Vassiliou et al., 2009). In this direction, identification of ‘F435,’ the first high oleic acid mutant, was the breakthrough for improving groundnut oil quality. The mutant ‘F435,’ with 80% oleic and 2% linoleic acid, has been deployed in breeding several groundnut cultivars with improved oil quality (Norden et al., 1987).
Fatty acid desaturase (FAD) enzyme facilitates the conversion of oleic acid to linoleic acid by adding a double bond to oleic acid. This enzyme is coded by two homologous genes (ahFAD2A and ahFAD2B) located on A and B subgenomes, respectively. Both FAD genes have 99% sequence homology and inactivation of this enzyme results in high oleic acid in mutants due to substitution (G:C to A:T) and insertion (A:T) of one base pair in FAD genes located on A and B subgenomes, respectively (Chen et al., 2010). These mutations lead to the accumulation of more oleic acid and decreasing linoleic and palmitic acid ensuring oil quality improvement by increasing shelf life. Keeping in mind the preferences of consumers and profitability to growers, groundnut breeding mainly focuses on developing improved varieties with desirable levels of oil content and other fatty acids, especially oleic, linoleic, and palmitic acids. Successful introgression of the FAD mutant alleles from SunOleic 95R with increased oleic acid has already been demonstrated using genomics-assisted breeding (GAB) approaches such as marker-assisted backcrossing (MABC) and marker-assisted selection (MAS) in both high and low oil containing genotypes (Chu et al., 2011; Janila et al., 2016b).
So far many studies have been conducted to decipher the biosynthesis pathway of fatty acids (Lung and Weselake, 2006) but very limited studies have been carried out in terms of genetics of fatty acids synthesis and its regulation (Wang et al., 2015). Previous genetic studies on groundnut oil trait suggested that it was highly influenced by genotype, environment, and genotype × environment interaction and was also positively correlated with soil pH, iron content, and oleic/linoleic acid (O/L) ratio (Dwivedi et al., 1993). Although the fatty acid composition varies with groundnut growth habits, the oil content is independent of them (Bansal et al., 1993). Oil content synthesis and accumulation is guided by complex pathways and it is considered a quantitative trait (Dwivedi et al., 1993).
In order to perform high resolution trait mapping, it is essential to generate dense genetic maps and good phenotyping data (Pandey et al., 2016). A high density linkage map plays a very crucial role in the identification of the QTLs for any crop, especially for complex polygenic traits (Guo et al., 2013, 2016; Varshney et al., 2013). To date, various QTL analysis studies for oil content and fatty acids have been conducted using early generation markers like SSR (Selvaraj et al., 2009; Sarvamangala et al., 2011; Pandey et al., 2014; Wang et al., 2015), but the marker density of these genetic maps were still low. With recent advances in availability of reference genomes for diploid progenitors of groundnut (Bertioli et al., 2016; Chen et al., 2016), the genotyping platforms and availability of sequencing technology along with array-based genotyping methods (Pandey et al., 2017), it has become easy to generate thousands of data points for conducting high resolution trait mapping (Pandey et al., 2016). Diversity Arrays Technology (DArT) and sequence based (DArTseq) platform are the advanced genotyping technologies, which were successfully used in diverse crops to study various traits (Raman et al., 2013). The power of this technique lies in the reduction of genome complexity by restriction enzymes followed by microarray hybridization to assay markers across the genome. This methodology clearly increases the probability to generate polymorphic data points in the present study. GAB has already demonstrated its strength in accelerated improvement of target traits in groundnut and therefore, availability of linked markers for oil content and other minor fatty acids will help in breeding the improved varieties with a desirable combination of oil content and fatty acid composition. The present study reports the development of two dense genetic maps and their use in identifying the QTLs for oil content and fatty acids in groundnut.
Two F2 populations, i.e., one for oil content (ICGV 07368 × ICGV 06420) and the other for fatty acids (ICGV 06420 × SunOleic 95R), were developed at ICRISAT, Patancheru, India. These two populations have been referred to as OC-population and FA-population, respectively, throughout the article. The parent “ICGV 07368” is a high yielding variety with low oil content (45%) and the parent “ICGV 06420” is a drought tolerant variety with high oil content (55%) and normal fatty acid profile. The parent “SunOleic 95R” is the first high oleic groundnut variety released in the USA and carries FAD mutant alleles in A and B subgenomes (Gorbet and Knauft, 1997). The hybridity of F1 plants of both the populations was confirmed using SSR markers and were selfed to develop F2 populations.
DNA from both mapping populations was extracted from 10 to 15 days young F2 plants along with parents using the modified cetyltrimethylammonium bromide (CTAB) extraction method (Mace et al., 2003). The DNA quality and quantity were checked on 0.8% agarose and DNA was diluted to 5 ng/μl of working concentrations for genotyping work.
The genotyping with SSR markers was performed following the methods explained in Varshney et al. (2009). PCR products were resolved on 1.5% agarose gel for confirming the amplification. The forward primers were dye labeled with FAM, VIC, and NED, which were detected as blue, green, and black color peaks, respectively (Applied Biosystems, USA). The PCR products were denatured and capillary separated with ABI 3700 automatic DNA sequencer (Applied Biosystems, USA) and GeneMapper Software v (Applied Biosystems, USA) was used to analyze the results.
Both F2 populations along with their parents were also genotyped with a DArT array consisting of 15,360 features at Diversity Arrays Technology Pty Ltd. (DArT P/L), Australia. The detailed method of genotyping is available at the website1 and is also described in Pandey et al. (2014). DArTseq procedure is quite similar to DArT genotyping and involves sequencing based method along with some steps of DArT as described in Vishwakarma et al. (2016). All polymorphic sequences of the DArT and DArTseq markers generated from the parental lines of the F2 populations were scored as presence vs. absence and used to construct genetic maps.
Phenotyping data was generated on 184 individuals of OC-population for oil content while on 179 individuals of FA-population for different fatty acid compositions, namely oleic (C18:1), linoleic (C18:2), palmitic (C16:0), stearic (C18:0), arachidic (C20:0), behenic (C22:0), and lignoceric (C24:0) at ICRISAT, Patancheru, India. The oil content and fatty acid composition were estimated using near infrared reflectance spectroscopy (NIRS) (Model XDS RCA, FOSS Analytical AB, Sweden, Denmark) (Sundaram et al., 2010). Oil content estimation was also done using the Soxhlet method following the protocol of Sharma et al. (2002). In addition to NIRS, the fatty acids were also estimated by gas chromatography (GC) (Shimadzu GG-9A GLC unit, Tokyo, Japan), following the protocol of Metcalf et al. (1966).
Phenotyping data was analyzed using SPSS software2. Association among different fatty acids was established by Pearson correlation and calculating a two-tailed P-value with 95% confidence intervals. The Chi-square test was performed to check the goodness of fit of the segregation ratios of three major fatty acids, i.e., oleic, linoleic, and palmitic acids.
The genetic maps were constructed using JoinMap version 4 (Van Ooijen, 2006). The Kosambi map function was used for genetic map construction and calculation of map distance from recombination fractions. The markers were integrated into the framework genetic maps by applying the independence LOD (logarithm of the odds), with LOD threshold ranging from 2.0 to 20.0 with a minimum recombination frequency (∂) threshold of 40%. In the case of OC-population, initially the framework genetic map was constructed with polymorphic SSR markers and then DArT and DArTseq marker data were integrated for developing dense genetic maps. For FA-population, the genetic map was constructed using DArT and DArTseq polymorphic loci and then ahFAD2A and ahFAD2B mutant allele specific loci were also integrated. MapChart was used to draw a final genetic map for better visualization (Voorrips, 2002).
The genetic map information together with phenotyping data was used for the identification of QTLs for oil content and fatty acids using ICIM mapping software version 4.0 (Wang et al., 2012). Inclusive Composite Interval Mapping (ICIM) for QTLs with additive (one-dimensional scanning, ICIM-ADD) method with 1cM step and 0.001 probability mapping parameters in stepwise regression were employed in QTL analysis. The LOD threshold score of 3.0 as minimum significance level with 1000 permutations was set manually.
The OC-population derived from cross ICGV 07368 (45%) × ICGV 06420 (55%) showed good segregation for oil content ranging from 47.2 to 55.7% with an average oil content of 51.6% (Figure 1A). In FA-population, the parental genotype ‘SunOleic 95R’ possessed desirable major fatty acid composition (82% oleic acid, 3% linoleic acid, and 6% palmitic acid), while ‘ICGV 06420’ possessed undesirable major fatty acid composition (45% oleic acid, 31% linoleic acid, and 12% palmitic acid) along with minor fatty acids. The phenotypic variability of fatty acids in the F2 population ranged from 35.8 to 81.8% for oleic acid, 4.6 to 39.1% for linoleic acid, 0.2 to 3% for behenic acid, 0.2 to 4.5% for lignoceric acid, 6.6 to 13.9% for palmitic acid, 1.7 to 4.3% for stearic acid, and 1.0 to 2.6% for arachidic acid (Figures 1B–H). Phenotyping data on OC-population showed a normal distribution for oil content. In the case of oleic acid, linoleic acid, palmitic and stearic acids, although normal distribution is followed, but the curve is shifted to one side with few transgressive segregants (Figures 1B–E). In the case of behenic acid and lignoceric acids, although the phenotypic variability between the parental genotypes of the mapping population was very less but we have observed a significant number of transgressive segregants toward either side of the phenotypic extreme. Similarly, higher frequency of individuals with transgressive segregation was observed for arachidic acid in the FA-population (Figure 1G).
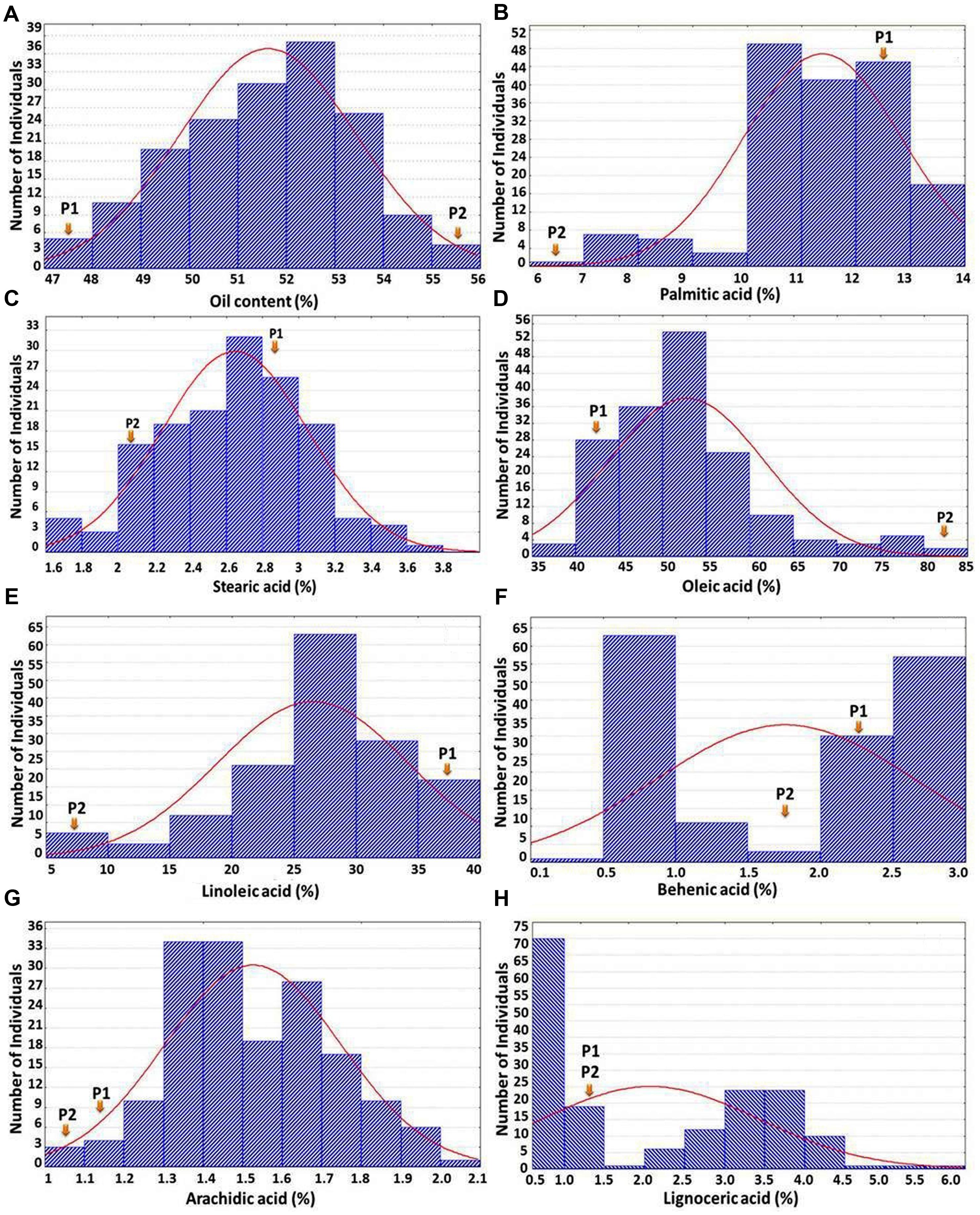
FIGURE 1. Frequency distribution of oil content and seven different fatty acids. The X-axis shows the percentage of the trait and the Y-axis represents the number of individuals in F2 population. P1 and P2 represent the parents ICGV 07368 and ICGV 06420, respectively, in (A); while P1 and P2 represent the parents ICGV 06420 and SunOleic 95R, respectively, in (B–H).
The correlation analysis clearly indicated a significant and negative correlation of oleic acid [C18:1] with linoleic acid [C18:2] (r = -0.701, P < 0.0001), stearic acid [C18:0] (r = -0.218, P < 0.001), and arachidic acid [C20:0] (r = -0.331, P < 0.0001) (Table 1 and Figure 2). A significant positive correlation was observed for linoleic with palmitic acid [C16:0] (r = 0.888, P < 0.0001) and stearic acid [C18:0] (r = 0.239, P < 0.001). Palmitic acid was negatively correlated with oleic acid (r = -0.475, P < 0.001) and behenic acid [C22:0] (r = -0.155, P < 0.01) (Table 1 and Figure 2). Chi square (χ2) analysis for oleic acid on F2 progeny revealed digenic 15:1 (low:high) segregations. Similarly, linoleic acid and palmitic acid also followed digenic 15:1 (high:low) ratio (Table 2).
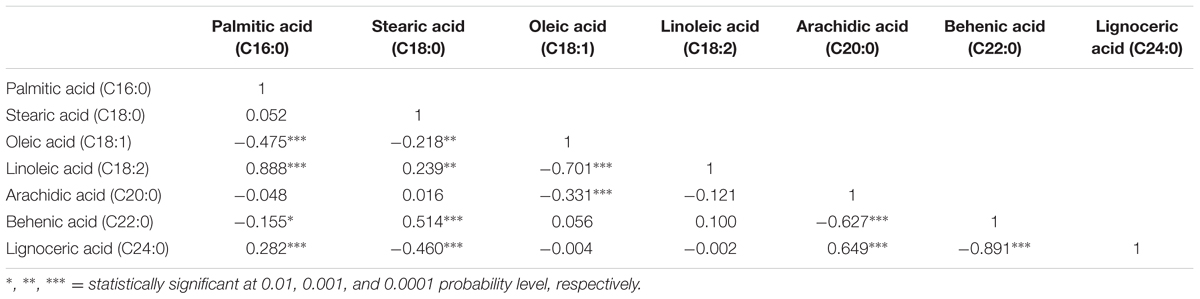
TABLE 1. Pairwise correlation among the six different fatty acids in FA-population (ICGV 06420 × SunOleic 95R).
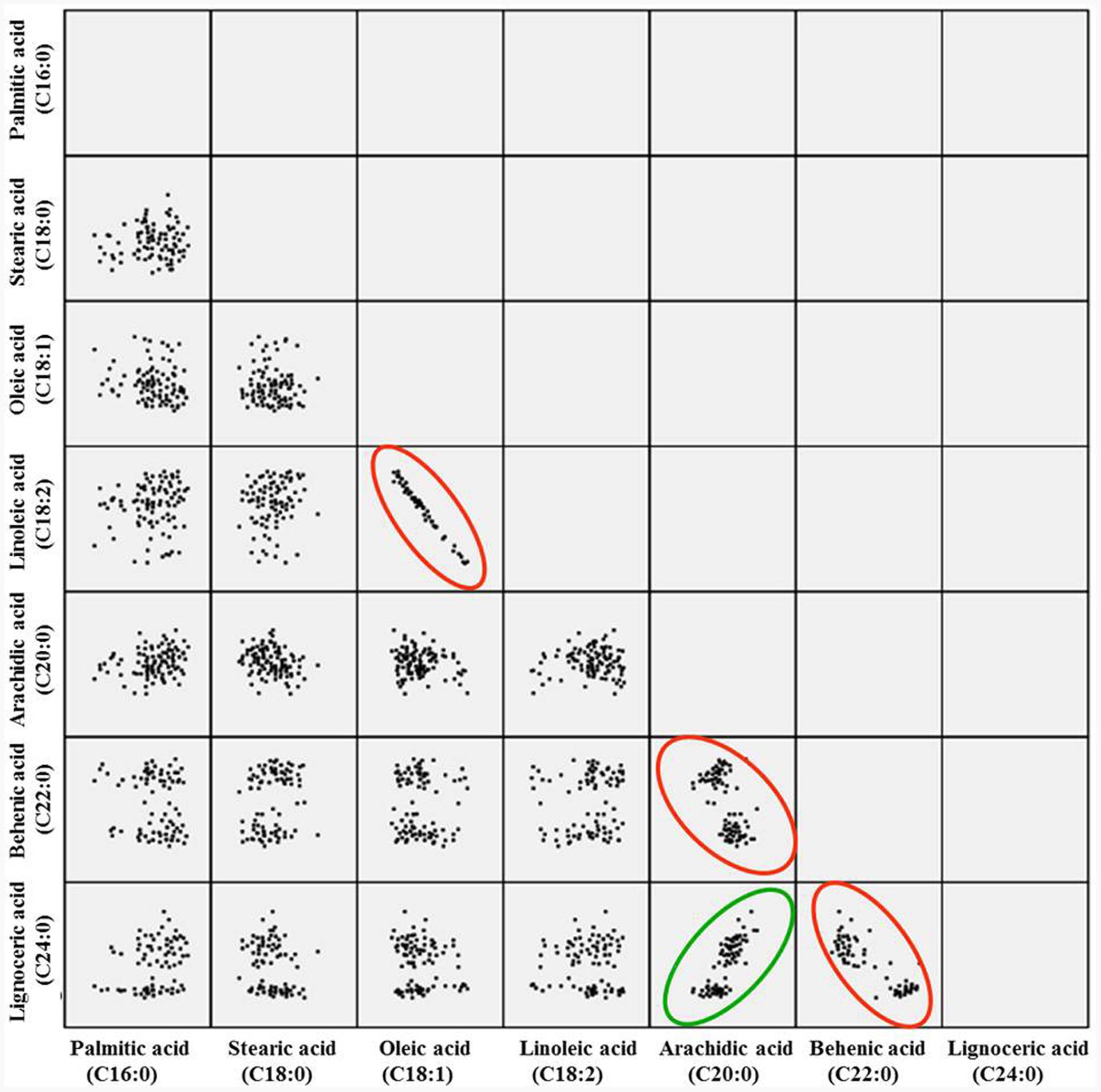
FIGURE 2. Pairwise correlation of the seven different fatty acids in F2 population. The red circle indicates negative correlation, while green indicates the positive correlation.
In this study, the parent genotype ‘ICGV 06420’ had heterozygous mutant FAD allele (ol1Ol1) in the A subgenome and homozygous dominant mutant FAD allele in the B subgenome (Ol2Ol2), and the other parent ‘SunOleic 95R’ had homozygous recessive mutant FAD alleles at both the loci of two subgenomes (ol1ol1ol2ol2). Genotyping of the FA-population for ahFAD mutant alleles yielded six allelic combinations (ol1ol1 Ol2Ol2, ol1ol1 ol2ol2, ol1ol1 Ol2ol2, ol1Ol1 Ol2Ol2, ol1Ol1 ol2ol2, and ol1Ol1 Ol2ol2) (Table 3). As expected the homozygous mutant combination (ol1ol1 ol2ol2) showed the highest average of oleic acid (65%) and the lowest for linoleic (15.6%) and palmitic (9.7%) acids. It was also observed that the heterozygous mutant ahFAD2B (Ol2ol2) allele also contributed to the significant increase in oleic acid (60%) and reduced linoleic (20.8%), and palmitic (9.7%) acids from the normal fatty acid profile.
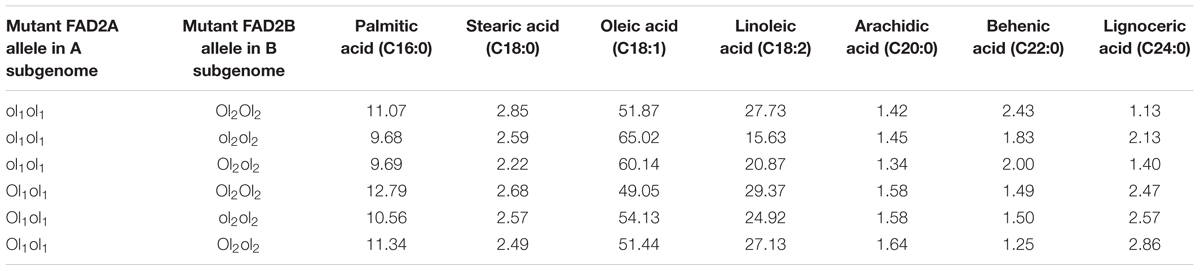
TABLE 3. The fatty acid mean composition (%) for the genotypes detected in the F2 segregating population (ICGV 06420 × SunOleic 95R).
Few genotypes with the homozygous combination of both mutant FAD alleles (ahFAD2A and ahFAD2B) showed a high oleic acid content of 81.7%, which is similar to ‘SunOleic 95R,’ the high oleic parent. The dosage of the FAD mutant alleles clearly defined the composition of three major fatty acids, namely oleic, linoleic, and palmitic acids (Figure 3 and Table 3). Genotypes with homozygous ahFAD allele mutation in A and B subgenomes (ol1ol1 ol2ol2) showed an average increase in the oleic acid by 20.5%, decrease in the linoleic acid by 79% and in palmitic acid by 20% (Figure 3). Although heterozygous (Ol1ol1 Ol2ol2) mutant FAD allele combination was found influencing the major fatty acids, but in lesser amounts because of decrease in dosage of number of mutant alleles. Further, the variation in the other minor fatty acids was less significantly recorded. None of the FAD allele combinations influenced the stearic acid levels significantly.
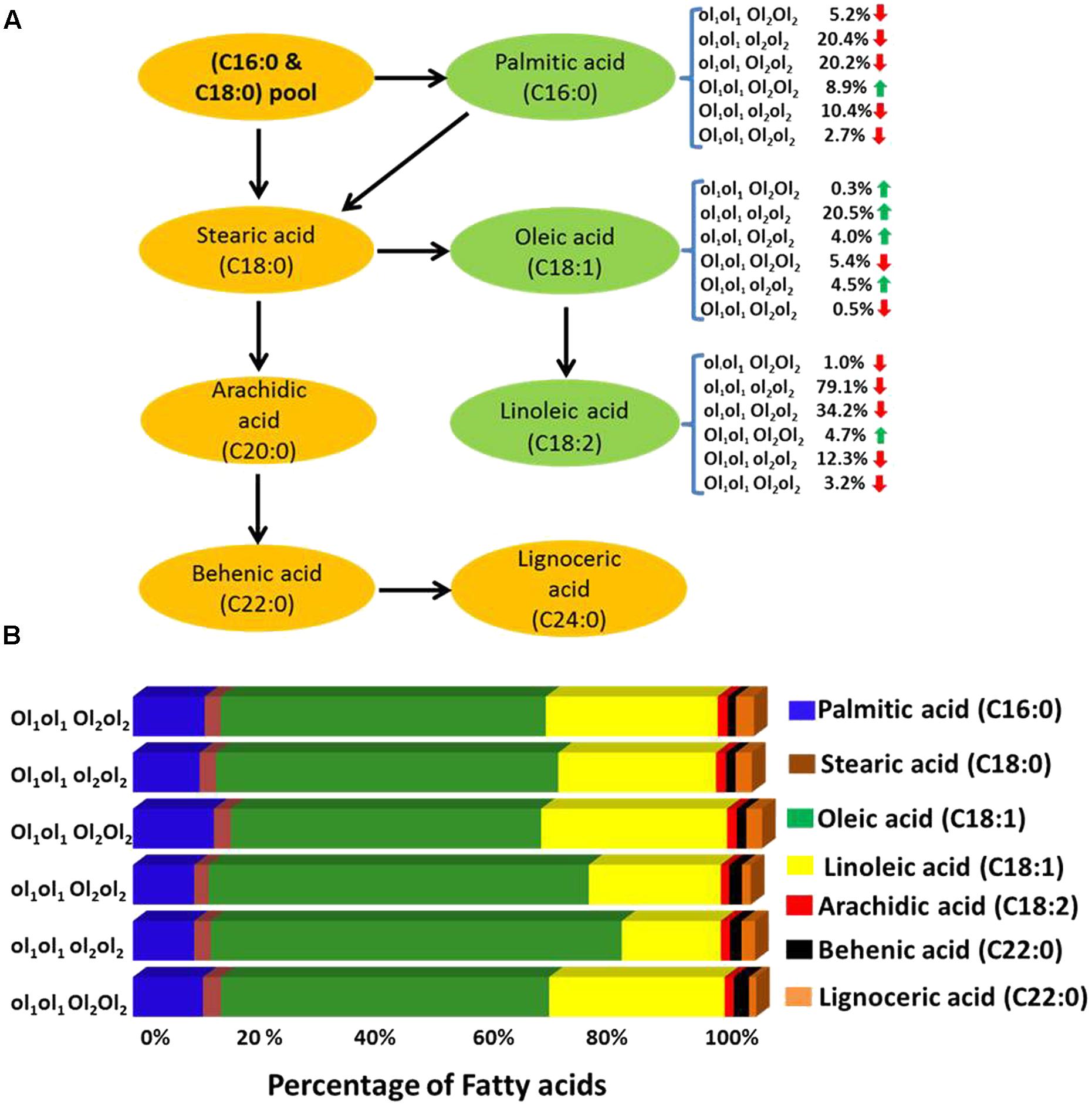
FIGURE 3. (A) The effect of FAD mutant allele combinations of different fatty acids. The ol1 and Ol1 represent the mutant and the wild type allele, respectively, of A subgenome while ol2 and Ol2 represent the mutant and the wild type allele, respectively, of B subgenome. Red and green arrow indicates the decrease and increase of the fatty acid percentage levels, respectively. (B) Mean fatty acid composition (%) in plants possessing different mutant FAD allele (mutant and wild) combinations in fatty acid (FA)-population.
A polymorphic survey of parental lines of OC-population with 250 SSR markers yielded 55 polymorphic markers. Genotyping data generated for these polymorphic SSR markers were used for development of framework genetic map for OC-population. In addition to SSRs, further genotyping of OC-population with 15,360 DArT and DArTseq yielded genotyping data for 2,238 polymorphic loci. A total of 1,384 polymorphic loci were either highly distorted or were having missing data, therefore, not included in constructing the genetic map. Genetic map construction with these polymorphic markers resulted in the development of a genetic map with 854 mapped loci (799 DArT and DArTseq markers and 55 SSRs) into 20 linkage groups (LGs) (Table 4, Supplementary Figure S1, and Table S1). This genetic map covered a total genome distance of 3,525.8 cM and achieved a density of 4.1 cM/loci ranging from 20 (A10) to 79 (B04) loci per LG. Similarly, the length of the LGs ranged from 94.6 cM (B06) to 288.3 cM (B09). In case of FA-population, genotyping data with 15,360 DArT/DArTseq features yielded 1,725 (655 DArT and 1,070 DArTseq) polymorphic loci. Of these polymorphic loci, 1,435 DArT/DArTseq marker loci, including two ahFAD2A and ahFAD2B alleles, were mapped on 20 LGs spanning a total map distance of 1,869.17 cM with an average inter marker distance of 1.3 cM. The number of mapped loci ranged from 16 (B07) to 281 (A05) loci and length of the LGs ranged from 26.0 cM (B07) to 153.8 cM (B04) (Table 4, Supplementary Figure S2, and Table S2).
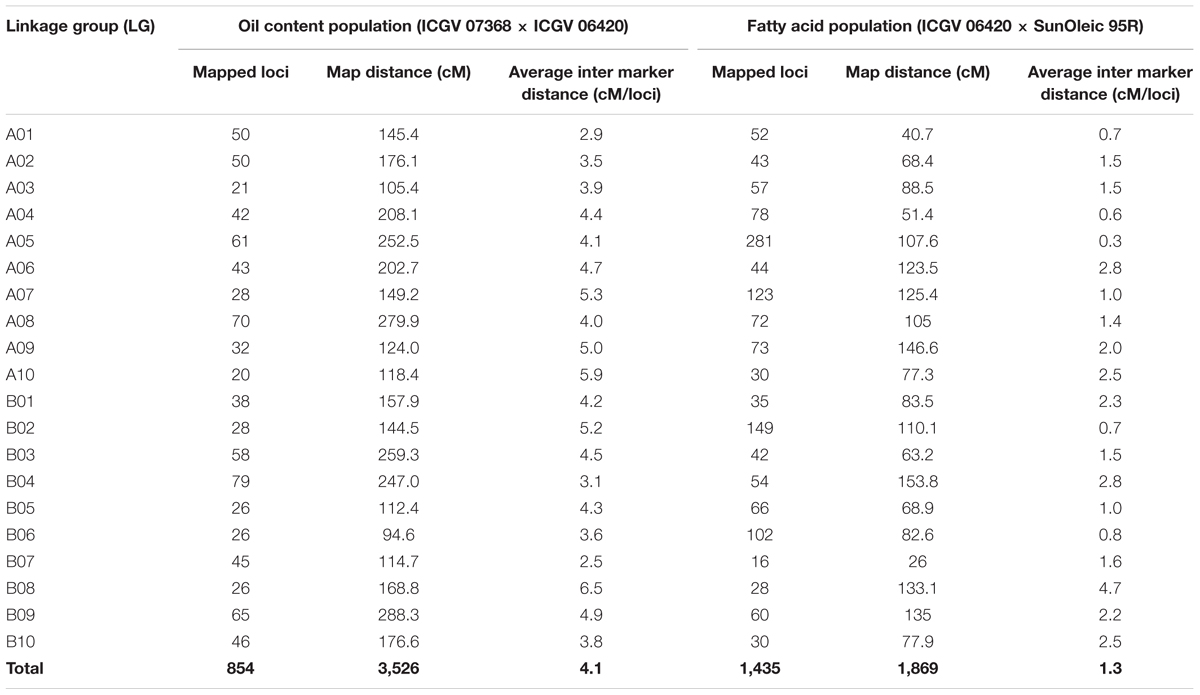
TABLE 4. Details of the linkage maps for populations ICGV 07368 × ICGV 06420 (oil content) and ICGV 06420 × SunOleic 95R (fatty acids).
The QTL analysis identified eight QTLs for oil content (Table 5 and Figure 4) with PVE ranging from 5.67 to 22.11%. Of these eight QTLs, two are having major effect. The first major QTL ‘qOc-A10’ explained 22.11% PVE with a LOD of 13.21 located on A10 and the second major QTL ‘qOc-A02’ explained 10.37% PVE with a LOD 4.8 mapped on A02. Of the six minor-effect QTLs, two QTLs, qOc-B09-1 and qOc-B09-2, were mapped on B09 explaining PVE 8.40 and 7.83%, respectively. Two QTLs, qOc-B06-1 and qOc-B06-2, were detected on B06 explaining 6.19 and 7.13% PVE, respectively. The remaining two QTLs, qOc-A08 and qOc-B03, were mapped on A08 and B03 with 7.05 and 5.67% PVE, respectively.
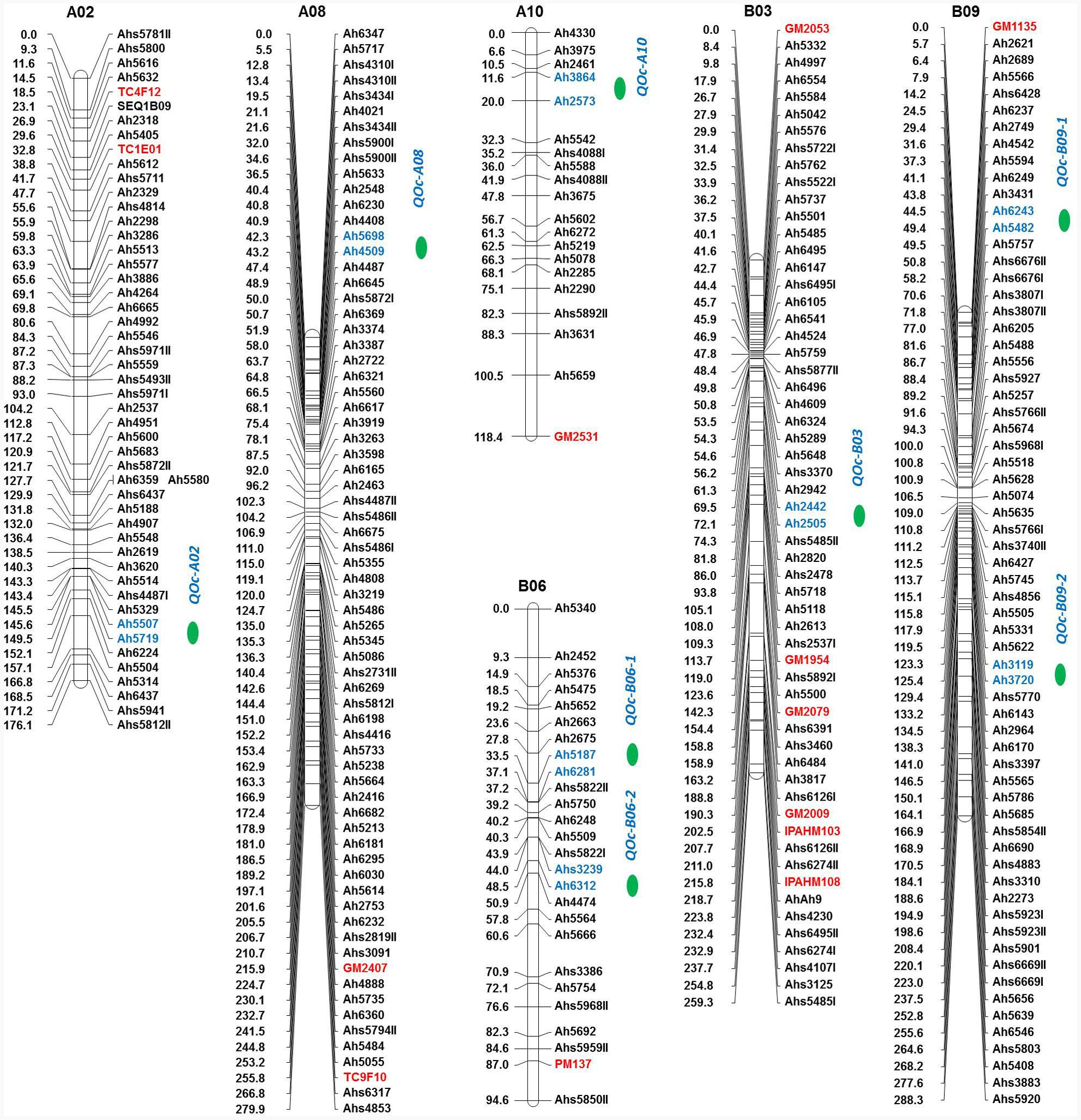
FIGURE 4. Genetic map of the oil content (OC) population showing quantitative trait locus (QTLs) for oil content. For each linkage group (LG), marker loci are given on the right side of the bar and marker positions (in cM) are mentioned on the left side. Markers with red color indicate the SSRs while markers in blue color represent the flanking markers of the identified QTL region. QTL location is indicated with a green color circle.
The QTL analysis identified 21 QTLs for seven fatty acids in FA-population, of which 20 were the major effect (Table 5 and Figure 5). For oleic acid, three major QTLs namely, qOle-A09-1 (A09), qOle-A09-2 (A09), and qOle-B09 (B09), were identified explaining 17.4, 34.2, and 33.8% PVE, respectively. For linoleic acid, two major QTLs were identified on A09 viz. qLin-A09-1 and qLin-A09-2 explaining 19.5 and 12.1% PVE. The third major QTL qLin-B09 mapped on B09 could explain 41% PVE. For palmitic acid, five QTLs, with a combined 69.2% PVE, were identified on five different LGs (A08, A09, A10, B04, and B09). For arachidic acid, two QTLs were detected, i.e., one each on A05 and A07 explaining 23.4 and 12.4% PVE, respectively. For behenic acid, one minor and one major effect QTL were detected on A09 and A05 with PVE 8.4 and 12.0%, respectively. In the case of lignoceric acid, five QTLs were identified on four LGs (A05, A07, A09, and B01) explaining altogether 58% PVE. Interestingly, for steric acid, one major QTL namely qSte-A09 with 78.6% PVE was mapped on A09.
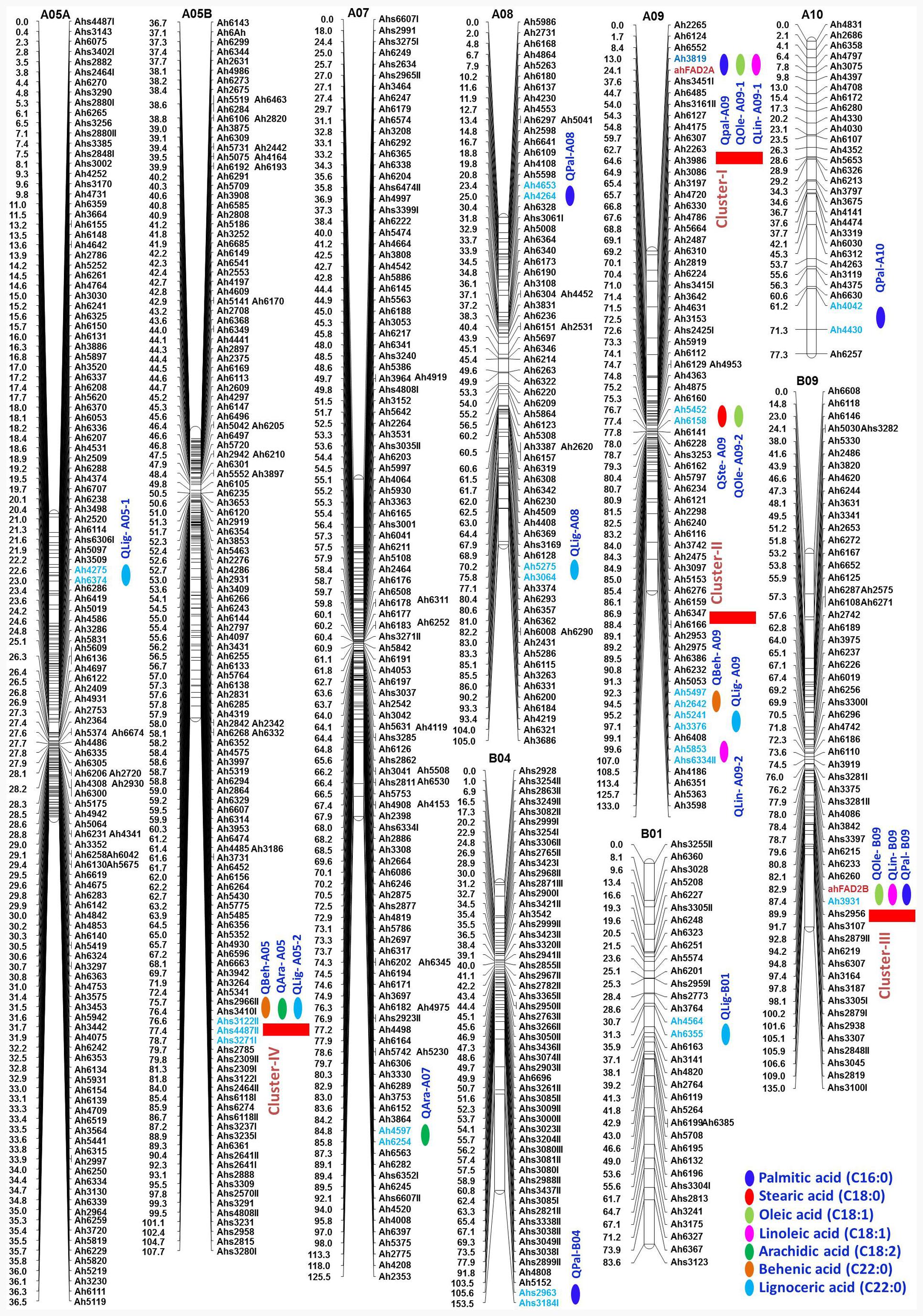
FIGURE 5. Genetic map of the FA-population showing QTLs for different fatty acids. For each LG, marker loci are given on the right side of the bar and marker positions (in cM) are mentioned on the left side. Markers with red color indicate the SSRs while markers in blue color represent the flanking markers of the identified QTL region. QTL location is indicated with a green color circle.
Of the 21 QTLs identified in FA-population, A09 holds the highest number of eight QTLs followed by four QTLs on A05, three QTLs on B09, two QTLs on A08, and one QTL each on A07, A10, B01, and B04. The ahFAD2A and ahFAD2B alleles were mapped onto A09 and B09, respectively, and showed high influence on many fatty acids. Four genomic regions were also identified, where a minimum of three QTLs for fatty acids were mapped. Two QTL clusters were identified on A09, i.e., Cluster-I between Ah3819-ahFAD2A and Cluster-II between Ah5497-Ah6334I. Cluster-I harbored QTLs for palmitic, oleic, and linoleic acids, while Cluster-II for lignoceric, linoleic, and behenic acids. Cluster-III identified on B09 between ahFAD2B-Ah3931 harboring QTLs for palmitic, oleic, and linoleic acids, while Cluster-IV on A05 between Ahs3122II-Ahs4487II harbored QTLs for arachidic, lignoceric, and behenic acids.
Genomics-assisted breeding has facilitated accelerated development of oil quality (Chu et al., 2011; Janila et al., 2016a) and disease resistance (Varshney et al., 2014) in groundnut. This study successfully developed dense genetic maps and identified genomic regions and linked markers for oil content and fatty acids. These genomic regions in the present study will facilitate further gene discovery and marker development for these traits in groundnut.
Polygenic traits are quantitative in nature and do not follow distinct or absolute values; instead, there will be a gradient of expression with little variation. The present study showed continuous variation for oil content, indicating its polygenic nature similar to the other major oilseed crops such as soybean, rapeseed, mustard, etc. (Singh et al., 2013; Wang et al., 2013; Akond et al., 2014). The polygenic nature of oil content was also earlier reported in different recombinant inbred line (RIL) populations in groundnut (Sarvamangala et al., 2011; Pandey et al., 2014).
Mutant FAD alleles are known for controlling oleic and linoleic acids, FAD2B being a higher phenotypic contributor as compared to FAD2A (Pandey et al., 2014). The present study showed that major fatty acids, namely oleic, linoleic, and palmitic acids, precisely followed the digenic 15:1 segregation pattern, i.e., two genes control the expression of the phenotype, here it refers to mutant ahFAD2A and ahFAD2B alleles. One previous study reported that normal oleic genotype (ol1ol1 Ol2Ol2 and Ol1Ol1 Ol2Ol2) when crossed with high oleic acid genotype (ol1ol1 ol2ol2) showed monogenic (3:1) and digenic (15:1) ratio, respectively (Barkley et al., 2013). Similar to oleic acid, two other major fatty acids, namely palmitic and linoleic acids, also showed the digenic inheritance. Also, the homozygous mutant combination of both A and B subgenomes showed higher levels of oleic acid and low levels of linoleic acid and palmitic acid in the population. Although, a subgenome mutant allele had less influence on the oleic acid levels in the present study, but the combination of both mutant alleles enhanced the oleic acid levels significantly.
The correlation study showed strong negative correlation between oleic and linoleic acid. The result was further confirmed with the genotyping for the ahFAD mutants in the population and their respective phenotyping effect for different fatty acids. It is a well-known fact that the high oleic acid accumulates due to mutation of the ahFAD alleles inhibiting the conversion of oleic acid to linoleic acid (Chen et al., 2010). The variations in the content of different fatty acids by various combinations of the ahFAD mutant alleles were shown on the biosynthetic pathway for better understanding of the correlation between different fatty acids.
The saturated acyl chains are formed by condensation of C2 units from malonyl-acyl carrier protein to acyl chains. Each cycle adds two carbons to the growing acyl chain leading to C18:0 (stearic acid) as a major deviating point. From this C18:0, successive addition of the acyl groups leads to the formation of C20:0 (arachidic acid), C22:0 (behenic acid), and C24:0 (lignoceric acid). Furthermore, C18:0 (stearic acid) upon desaturation at C9 produces C18:1 (oleic acid), on further desaturation forms C18:2 (linoleic acid). Plants with ahFAD mutant alleles resulted in the accumulation of higher oleic and lower linoleic acid. As the oleic acid desaturation to linoleic was decreased, other branch for minor fatty acids synthesis like arachidic, behenic, lignoceric were increased. Interestingly, behenic acid levels were increased in plants with only homozygous FAD mutant alleles in both subgenomes. Stearic acid levels were not much influenced by the FAD mutant allele combinations and this is correlated with detection of single major QTL qSte-I with 78.6% PVE. Introgression of the FAD mutant alleles had shown an increase in the oleic acid and clearly indicated major contribution ahFAD2B mutant allele than the ahFAD2A mutant allele in the total phenotype. A homozygous combination of both mutant FAD alleles (ahFAD2A and ahFAD2B) showed accumulation of oleic acid up to 81.7%, similar to ‘SunOleic 95R,’ the high oleic parent. Genotypes with ahFAD allele mutation in A and B subgenomes in the homozygous state (ol1ol1 ol2ol2) showed an average increase in the oleic acid levels of 20.5%, decrease in the linoleic acid levels by 79% and palmitic acid by 20%. Although heterozygous (Ol1ol1 Ol2ol2) mutant FAD allele combination was influencing the major fatty acids, but in lesser extent because of a decrease in dosage of number of mutant alleles.
Although the dosage is very crucial, the major influence of mutant allele located on B subgenome on oleic, linoleic, and palmitic is very clearly seen. The presence of ahFAD2B mutant allele with ahFAD2A mutant allele showed significant variation (20% higher oleic acid, 78% lower linoleic acid, and 20% lower palmitic acid), whereas, the wild type ahFAD2B with mutant ahFAD2A allele combination showed only 0.3% increase of oleic acid, 1% decrease of linoleic acid, and 5.2% decrease of palmitic acid. This clearly indicates the importance and impact of mutant allele located in B subgenome in controlling the different fatty acids profile as compared to ahFAD2A mutant allele. The above results are also in agreement with the QTLs identified in these genomic regions and their effect on controlling the oleic acid phenotype. The QTLs detected at ahFAD2B loci showed PVE of 33.8% for oleic, 41.0% for linoleic, and 18.4% for palmitic acid, whereas QTLs identified at ahFAD2A mutant loci showed PVE of 17.4% for oleic, 19.5% for linoleic, and 10.7% for palmitic acid. The F2 plants with wild type FAD alleles clearly showed a decline in oleic acid and increase in the linoleic and palmitic acids. The above results indicated the importance of the dosage of the mutant FAD alleles on major fatty acid levels and the composition of these major fatty acids could be considered in the development of the high oleic cultivars.
Groundnut has large and complex tetraploid genome and lack of diversity in the cultivated species decelerated the genomics studies. High marker density genetic maps are crucial for conducting high resolution QTL mapping. The first SSR based genetic map for cultivated groundnut was constructed with 135 loci (Varshney et al., 2009) and was later improved to 191 SSR loci (Ravi et al., 2011). With the application of next-generation sequencing technology, the first high density SNP (Single Nucleotide Polymorphism) based map for tetraploid groundnut with 1,685 marker loci has been constructed (Zhou et al., 2014). Recently in groundnut, a dense genetic map with the 1,152 DArT and DArTseq marker loci covering 2423.12 cM map distance was constructed for fresh seed dormancy (Vishwakarma et al., 2016). The present study reports the development of dense genetic map with 1,435 DArT/DArTseq marker loci spanning a total map distance of 1869.17 cM with an average inter marker distance of 1.3 cM. This is the most dense genetic map using DArT/DArTseq genotyping platform and the second most dense genetic map for cultivated groundnut using any of the other available marker systems.
Very few studies have been conducted in the past for mapping QTLs for oil content in groundnut. With the bulk segregant analysis, PM36 SSR marker was found associated with the oil content (Selvaraj et al., 2009). Later on, based on a very less dense genetic map with 45 markers, four QTLs with PVE ranging 7.1–9.1% were reported (Sarvamangala et al., 2011). Recently for oil content in groundnut, six QTLs with 3.07–10.23% PVE were reported in the S-population and nine QTLs with 3.93–14.07% PVE were accounted in the T-population (Pandey et al., 2014). In an earlier study on six fatty acids, 164 main effect QTLs (M-QTLs) and 27 epistatic QTLs (E-QTLs) were reported in two RIL populations with the PVE ranging 0.16–40.56% (Wang et al., 2015). In our study, eight QTLs together explained 74.5% PVE and further study of these regions may provide insight into the interesting genes, which may be controlling oil content. The PVE ranging from 5.67 to 22.11% by these eight QTLs on six different LGs clearly indicated that oil content was influenced by multiple alleles spread across the genome. Similarly, 21 QTLs were detected for different fatty acids, eight of these QTLs were identified on two homologous LGs (A09 and B09) harboring the ahFAD mutant alleles (Pandey et al., 2014). These two LGs hold major QTLs for the three major fatty acids, namely oleic, linoleic, and palmitic acids. The clustering of the QTLs for fatty acids is common and is elucidated in groundnut (Pandey et al., 2014; Wang et al., 2015) and in several other crops, including rapeseed (Basnet et al., 2016) and soybean (Kim et al., 2010). In the present study, four clusters were reported with three QTLs each. The frequency distribution of oleic, linoleic, and palmitic acids were skewed toward one side, which showed the influence by a single factor, i.e., FAD alleles. The high frequency of transgressive segregations for arachidic acid and lignoceric acid might be due to complementary gene actions. The presence of transgressive segregants in lignoceric, arachidic, stearic, and behenic acids with identified QTLs on different LGs indicated the presence of specific genomic regions/genes which control the specific fatty acids. These genomic regions are very important in identifying key enzymes for targeting specific fatty acid, both to increase and decrease their content. Targeting of these clusters for further studies will be more helpful for genetic dissection, candidate gene discovery and accelerated groundnut improvement.
In summary, this study successfully developed two genetic maps based on DArT and DArTseq markers and one being the second most dense genetic map across all marker systems and most dense genetic map based on DArT and DArTseq markers in groundnut. Further, this study identified genomic regions for groundnut oil content and fatty acids. These genomic regions provide further opportunity towards gene discovery and marker development for marker-based selection to develop superior cultivars with the desired level of oil content and fatty acid profile.
Conceived and designed the experiment: RKV and MKP. Performed the experiments: YS and MKV. Analyzed data: YS, MKV, and MKP. Interpreted results: RKV, MKP, MKV, YS, and BG. Population development and phenotyping: PJ, MTV, SM, and SN. Wrote the manuscript: YS, MKV, and MKP.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The work presented in this article is the contribution from research projects sponsored by Department of Agriculture and Co-operation (DAC), Ministry of Agriculture, Government of India under Integrated Scheme of Oilseeds, Pulses, Oilpalm and Maize (ISOPOM) program. YS acknowledges the award of Junior Research Fellowship from Department of Biotechnology, Government of India. This work has been undertaken as part of the CGIAR Research Program on Grain Legumes. ICRISAT is a member of CGIAR Consortium.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.00794/full#supplementary-material
FIGURE S1 | Genetic map of F2 population (ICGV 07368 × ICGV 06420) segregating for oil content trait. The mapped loci are present on the left side and their positions (in cM) are mentioned on the right side of the linkage groups.
FIGURE S2 | Genetic map of F2 population (ICGV 06420 × SunOleic 95R) segregating for fatty acids. The mapped loci are present on the left side and their positions (in cM) are mentioned on the right side of the linkage groups.
TABLE S1 | Details of the genetic map of F2 population (ICGV 07368 × ICGV 06420) with information on mapped markers, linkage group, position and sequences.
TABLE S2 | Details of the genetic map of F2 population (ICGV 06420 × SunOleic 95R) with information on mapped markers, linkage group, position and sequences.
Akond, M., Liu, S., Boney, M., Kantartzi, S. K., Meksem, K., Bellaloui, N., et al. (2014). Identification of quantitative trait loci (QTL) underlying protein, oil and five major fatty acids contents in soybean. Am. J. Plant Sci. 5, 158–167. doi: 10.4236/ajps.2014.51021
Awad, A. B., and Fink, C. S. (2000). Phytosterols as anticancer dietary components: evidence and mechanism of action. J. Nutr. 130, 2127–2130.
Bansal, U. K., Satija, D. R., and Ahuja, K. L. (1993). Oil composition of diverse groundnut (Arachis hypogaea L.) genotypes relation to different environments. J. Sci. Food Agric. 63, 17–19. doi: 10.1002/jsfa.2740630104
Barkley, N. A., Isleib, T. G., Wang, M. L., and Pittman, R. N. (2013). Genotyping effect of ahFAD2 on fatty acid profiles in six segregating peanut (Arachis hypogaea L) populations. BMC Genet. 14:62. doi: 10.1186/1471-2156-14-62
Basnet, R. K., Carpio, D. P. D., Xiao, D., Bucher, J., Jin, M., Boyle, K., et al. (2016). A systems genetics approach identifies gene regulatory networks associated with fatty acid composition in Brassica rapa seed. Plant Physiol. 170, 568–585. doi: 10.1104/pp.15.00853
Bertioli, D. J., Cannon, S. B., Froenicke, L., Huang, G., Farmer, A. D., Cannon, E. K., et al. (2016). The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat. Genet. 48, 438–446. doi: 10.1038/ng.3517
Chen, X., Li, H., Pandey, M. K., Yang, Q., Wang, X., Garg, V., et al. (2016). Draft genome of the peanut A-genome progenitor (Arachis duranensis) provides insights into geocarpy, oil biosynthesis, and allergens. Proc. Natl. Acad. Sci. U.S.A. 113, 6785–6790. doi: 10.1073/pnas.1600899113
Chen, Z., Wang, M. L., Barkley, N. A., and Pittman, R. N. (2010). A simple allele-specific PCR assay for detecting FAD2 alleles in both A and B genomes of the cultivated peanut for high-oleate trait selection. Plant Mol. Biol. Rep. 28, 542–548. doi: 10.1007/s11105-010-0181-5
Chu, Y., Wu, C. L., Holbrook, C. C., Tillman, B. L., Person, G., and Ozias-Akins, P. (2011). Marker-assisted selection to pyramid nematode resistance and the high oleic trait in peanut. Plant Genome 4, 110–117. doi: 10.3835/plantgenome2011.01.0001
Dean, L. L., Hendrix, K. W., Holbrook, C. C., and Sanders, T. H. (2009). Content of some nutrients in the core of peanut germplasm collection. Peanut Sci. 36, 104–120. doi: 10.1007/s00122-011-1668-7
Dwivedi, S. L., Nigam, S. N., Jambunathan, R., Sahrawat, K. L., Nagabhushanam, G. V. S., and Raghunath, K. (1993). Effect of genotypes and environments on oil content and oil quality parameters and their correlation in peanut (Arachis hypogaea L.). Peanut Sci. 20, 84–89. doi: 10.3146/i0095-3679-20-2-5
Gorbet, D. W., and Knauft, D. A. (1997). Registration of ‘SunOleic 95R’ peanut. Crop Sci. 37:1392. doi: 10.2135/cropsci1997.0011183X003700040081x
Guo, B. Z., Khera, P., Wang, H., Peng, Z., Sudini, H., Wang, X., et al. (2016). “Annotation of trait loci on integrated genetic maps of Arachis species,” in Peanuts: Genetics, Processing, and Utilization, eds H. T. Stalker and R. F. Wilson (Academic Press and the American Oil Chemists’ Society (AOCS) Press), 163–207.
Guo, B. Z., Pandey, M. K., He, G., Zhang, X. Y., Liao, B., Culbreath, A. K., et al. (2013). Recent advances in molecular genetic linkage maps of cultivated peanut (Arachis hypogaea L.). Peanut Sci. 40, 95–106. doi: 10.1186/1471-2164-15-351
Janila, P., Pandey, M. K., Manohar, S. S., Variath, M. T., Nallathambi, P., Nadaf, H. L., et al. (2016a). Foliar fungal disease-resistant introgression lines of groundnut (Arachis hypogaea L.) record higher pod and haulm yield in multilocation testing. Plant Breed. 135, 355–366. doi: 10.1111/pbr.12358
Janila, P., Pandey, M. K., Shasidhar, Y., Variatha, M. T., Sriswathi, M., Khera, P., et al. (2016b). Molecular breeding for introgression of fatty acid desaturase mutant alleles (ahFAD2A and ahFAD2B) enhances oil quality in high and low oil containing peanut genotypes. Plant Sci. 242, 203–213. doi: 10.1016/j.plantsci.2015.08.013
Kim, H. K., Kim, Y. C., Kim, S. T., Son, B. G., Choi, Y. W., Kang, J. S., et al. (2010). Analysis of quantitative trait loci (QTLs) for seed size and fatty acid composition using recombinant inbred lines in soybean. J. Life Sci. 8, 1186–1192. doi: 10.5352/JLS.2010.20.8.1186
Lung, S. C., and Weselake, R. J. (2006). Diacylglycerol acyltransferase: a key mediator of plant triacylglycerol synthesis. Lipids 41, 1073–1088. doi: 10.1007/s11745-006-5057-y
Mace, E. S., Buhariwalla, H. K., and Crouch, J. H. (2003). A high-throughput DNA extraction protocol for tropical molecular breeding programs. Plant Mol. Biol. Rep. 21, 459–460. doi: 10.1007/BF02772596
Meredith, Z., and Alfred, K. A. (2003). Peanuts a source of medically important resveratrol. Nat. Prod. Radiance 2, 182–189.
Metcalf, L. D., Schmitz, A. A., and Pelka, J. K. (1966). Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal. Chem. 38, 514–515. doi: 10.1021/ac60235a044
Norden, A. J., Gorbet, D. W., Knauft, D. A., and Young, C. T. (1987). Variability in oil quality among peanut genotypes in the Florida breeding program. Peanut Sci. 14, 7–11. doi: 10.3146/i0095-3679-14-1-3
Pandey, M. K., Agarwal, G., Kale, S. M., Clevenger, J., Nayak, S. N., Sriswathi, M., et al. (2017). Development and evaluation of a high density genotyping ‘Axiom_Arachis’ array with 58K SNPs for accelerating genetics and breeding in groundnut. Sci. Rep. 7:40577. doi: 10.1038/srep40577
Pandey, M. K., Monyo, E., Ozias-Akins, P., Liang, X., Guimarães, P., Nigam, S. N., et al. (2012). Advances in Arachis genomics for peanut improvement. Biotechnol. Adv. 30, 639–651. doi: 10.1016/j.biotechadv.2011.11.001
Pandey, M. K., Roorkiwal, M., Singh, V., Lingam, A., Kudapa, H., Thudi, M., et al. (2016). Emerging genomic tools for legume breeding: current status and future perspectives. Front. Plant Sci. 7:455. doi: 10.3389/fpls.2016.00455
Pandey, M. K., Wang, M. L., Qiao, L., Feng, S., Khera, P., Wang, H., et al. (2014). Identification of QTLs associated with oil content and mapping FAD2 genes and their relative contribution to oil quality in peanut (Arachis hypogaea L.). BMC Genet. 15:133. doi: 10.1186/s12863-014-0133-4
Raman, H., Raman, R., Kilian, A., Detering, F., Long, Y., Edwards, D., et al. (2013). A consensus map of rapeseed (Brassica napus L.) based on diversity array technology markers: applications in genetic dissection of qualitative and quantitative traits. BMC Genomics 14:277. doi: 10.1186/1471-2164-14-277
Ravi, K., Vadez, V., Isobe, S., Mir, R. R., Guo, Y., Nigam, S. N., et al. (2011). Identification of several small main-effect QTLs and a large number of epistatic QTLs for drought tolerance related traits in groundnut (Arachis hypogaea L.). Theor. Appl. Genet. 122, 1119–1132. doi: 10.1007/s00122-010-1517-0
Sarvamangala, C., Gowda, M. V. C., and Varshney, R. K. (2011). Identification of quantitative trait loci for protein content, oil content and oil quality for groundnut (Arachis hypogaea L.). Field Crops Res. 122, 49–59. doi: 10.3389/fpls.2013.00023
Selvaraj, M. G., Narayana, M., Schubert, A. M., Ayers, J. L., Baring, M. R., and Burow, M. D. (2009). Identification of QTLs for pod and kernel traits in cultivated peanut by bulked segregant analysis. Elec. J. Biotechnol. 12. doi: 10.2225/vol12-issue2-fulltext-13
Sharma, A., Khare, S. K., and Gupta, M. N. (2002). Enzyme-assisted aqueous extraction of peanut oil. J. Am. Oil Chem. Soc. 79, 215–218. doi: 10.1016/j.foodchem.2012.07.120
Singh, S., Mohapatra, T., Singh, R., and Hussain, Z. (2013). Mapping of QTLs for oil content and fatty acid composition in Indian mustard [Brassica juncea (L.) Czern. and Coss.]. J. Plant Biochem. Biotechnol. 22, 80–89. doi: 10.1007/s13562-012-0113-6
Sundaram, J., Kandala, C. V., Holser, R. A., Butts, C. L., and Windham, W. R. (2010). Determination of in-shell peanut oil and fatty acid composition using near infrared reflectance spectroscopy. J. Am. Oil Chem. Soc. 87, 1103–1114. doi: 10.1007/s11746-010-1589-7
Van Ooijen, J. W. (2006). JoinMap4, Software for the Calculation of Genetic Linkage Maps in Experimental Populations. Wageningen: Kyazma B.V.
Varshney, R. K., Bertioli, D. J., Moretzsohn, M. C., Vadez, V., Krishnamurty, L., Aruna, R., et al. (2009). The first SSR based genetic linkage map for cultivated groundnut (Arachis hypogaea L.). Theor. Appl. Genet. 118, 729–739. doi: 10.1007/s00122-008-0933-x
Varshney, R. K., Mohan, S. M., Gaur, P. M., Gangarao, N. V. P. R., Pandey, M. K., Bohra, A., et al. (2013). Achievements and prospects of genomics-assisted breeding in three legume crops of the semi-arid tropics. Biotechnol. Adv. 31, 1120–1134. doi: 10.1016/j.biotechadv.2013.01.001
Varshney, R. K., Pandey, M. K., Janila, P., Nigam, S. N., Sudini, H., Gowda, V. C., et al. (2014). Marker-assisted introgression of a QTL region to improve rust resistance in three elite and popular varieties of peanut (Arachis hypogaea L.). Theor. Appl. Genet. 127, 1771–1781. doi: 10.1007/s00122-014-2338-3
Vassiliou, E. K., Gonzalez, A., Garcia, C., Tadros, J. H., Chakraborty, G., and Toney, J. H. (2009). Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF-a both in vitro and in vivo system. Lipids Health Dis. 8:25. doi: 10.1186/1476-511X-8-25
Vishwakarma, M. K., Pandey, M. K., Shasidhar, Y., Manohara, S. S., Nagesh, P., Janila, P., et al. (2016). Identification of two major quantitative trait locus for fresh seed dormancy using the diversity arrays technology and diversity arrays technology-seq based genetic map in Spanish-type peanuts. Plant breed. 135, 367–375. doi: 10.1111/pbr.12360
Voorrips, R. E. (2002). Mapchart: software for the graphical presentation of linkage maps and QTLs. J. Hered. 93, 77–78. doi: 10.1093/jhered/93.1.77
Wang, J., Li, H., Zhang, L., and Meng, L. (2012). Users’ Manual of QTL IciMapping Version 3.3. Beijing: Chinese Academy of Agricultural Sciences (CAAS).
Wang, M. L., Khera, P., Pandey, M. K., Wang, H., Qiao, L., Feng, S., et al. (2015). Genetic mapping of QTLs controlling fatty acids provided insights into the genetic control of fatty acid synthesis pathway in peanut (Arachis hypogaea L.). PLoS ONE 10:e0119454. doi: 10.1371/journal.pone.0119454
Wang, X., Wang, H., Long, Y., Li, D., Yin, Y., Tian, J., et al. (2013). Identification of QTLs associated with oil content in a high-oil Brassica napus cultivar and construction of a high-density consensus map for QTLs comparison in B. napus. PLoS ONE 8:e80569. doi: 10.1371/journal.pone.0080569
Zhou, X., Xia, Y., Ren, X., Chen, Y., Huang, L., Huang, S., et al. (2014). Construction of a SNP-based genetic linkage map in cultivated peanut based on large scale marker development using next-generation double-digest restriction-site-associated DNA sequencing (ddRADseq). BMC Genomics 15:351. doi: 10.1186/1471-2164-15-351
Keywords: DArT/DArTseq, genetic map, QTLs, oil content, fatty acids, peanut
Citation: Shasidhar Y, Vishwakarma MK, Pandey MK, Janila P, Variath MT, Manohar SS, Nigam SN, Guo B and Varshney RK (2017) Molecular Mapping of Oil Content and Fatty Acids Using Dense Genetic Maps in Groundnut (Arachis hypogaea L.). Front. Plant Sci. 8:794. doi: 10.3389/fpls.2017.00794
Received: 03 February 2017; Accepted: 27 April 2017;
Published: 22 May 2017.
Edited by:
Dan MacLean, The Sainsbury Laboratory, UKReviewed by:
Steven B. Cannon, Corn Insects and Crop Genetics Research (ARS–USDA), USACopyright © 2017 Shasidhar, Vishwakarma, Pandey, Janila, Variath, Manohar, Nigam, Guo and Varshney. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajeev K. Varshney, ci5rLnZhcnNobmV5QGNnaWFyLm9yZw== Manish K. Pandey, bS5wYW5kZXlAY2dpYXIub3Jn Pasupuleti Janila, cC5qYW5pbGFAY2dpYXIub3Jn
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.