- 1Research and Innovation Centre, Genomics and Biology of Fruit Crop Department, Fondazione Edmund Mach, Trento, Italy
- 2ToolGen Inc., Seoul, Republic of Korea
- 3Center for Genome Engineering, Institute for Basic Science, Seoul, Republic of Korea
- 4Department of Chemistry, Seoul National University, Seoul, Republic of Korea
The combined availability of whole genome sequences and genome editing tools is set to revolutionize the field of fruit biotechnology by enabling the introduction of targeted genetic changes with unprecedented control and accuracy, both to explore emergent phenotypes and to introduce new functionalities. Although plasmid-mediated delivery of genome editing components to plant cells is very efficient, it also presents some drawbacks, such as possible random integration of plasmid sequences in the host genome. Additionally, it may well be intercepted by current process-based GMO regulations, complicating the path to commercialization of improved varieties. Here, we explore direct delivery of purified CRISPR/Cas9 ribonucleoproteins (RNPs) to the protoplast of grape cultivar Chardonnay and apple cultivar such as Golden delicious fruit crop plants for efficient targeted mutagenesis. We targeted MLO-7, a susceptible gene in order to increase resistance to powdery mildew in grape cultivar and DIPM-1, DIPM-2, and DIPM-4 in the apple to increase resistance to fire blight disease. Furthermore, efficient protoplast transformation, the molar ratio of Cas9 and sgRNAs were optimized for each grape and apple cultivar. The targeted mutagenesis insertion and deletion rate was analyzed using targeted deep sequencing. Our results demonstrate that direct delivery of CRISPR/Cas9 RNPs to the protoplast system enables targeted gene editing and paves the way to the generation of DNA-free genome edited grapevine and apple plants.
Introduction
Grape and apple fruit crop plants are a major source of fiber, nutrients, and antioxidants, all essential for a healthy diet. These crops play a key role in the economy of many developed and developing countries and considerable efforts are being made to improve commercial traits using both conventional breeding and genetic engineering. However, growing social distrust in genetically modified (GM) crops in many countries has resulted in the adoption of very stringent and costly regulations disciplining the authorization of these crops, with the result that they have become very difficult or impossible to commercialize successfully (Sprink et al., 2016). Although genetic transformation of grape and apple crops has been used for the past two decades to enhance primarily biotic and abiotic tolerance, there are only a few examples of field evaluation or commercialization of transgenic plants worldwide (Kanchiswamy et al., 2015). A transition is needed toward more efficient and productive use of available genome sequences to meet growing demands for sustainable and safe intensification of food production. Here we explore the possibility of adopting next-generation plasmid-independent CRISPR/Cas9 genome editing approaches to develop improved grape and apple varieties that will probably avoid current GM regulations, and thus broaden the utility of this technology, with greater global acceptance levels. US Department of Agriculture (USDA) does not impose any GMO regulations to the plants with targeted mutagenesis generated by self-repair mechanisms, if they are free from Agrobacterium or any transgenic or foreign genetic materials; consequently, we assume there is high probability that CRISPR/Cas9 RNPs could be exempted from current GMO regulations (Waltz, 2012; Ledford, 2013; Jones, 2015). Nevertheless, the EU is uncertain to approve them and has yet to provide information on whether targeted mutation made by CRISPR/Cas9 or CRISPR/Cas9 RNPs fall outside regulatory criteria (Waltz, 2012; Jones, 2015).
CRISPR/Cas9 editing tools are efficiently exploited for gene mutation, repression, activation and epigenome editing in several model and crop plants, such as Arabidopsis, tobacco, rice, sorghum, maize, wheat, poplar, tomato, soya bean, petunia, citrus and recently grape and apple (Nishitani et al., 2016; Ren et al., 2016; Song et al., 2016). Meanwhile CRISPR/Cas9 RNPs DNA-free genome editing tools are successfully demonstrated in Arabidopsis, tobacco, lettuce, rice, petunia, and recently in wheat (Woo et al., 2015; Subburaj et al., 2016; Zhang et al., 2016).
To date, CRISPR/Cas9 RNPs editing tools have not been applied to genetic modification of grape and apple crops. Here, we demonstrate adoption of next-generation CRISPR/Cas9 RNPs technology for these fruit species to establish an efficient DNA-free method for the site-directed mutagenesis system. In the grapevine, PM (Gadoury et al., 2012; Pessina et al., 2016) is caused by the destructive fungal pathogen Erysiphe necator, an obligate biotroph infecting all green tissues and berries, resulting in drastic losses in yield and berry quality. Currently PM can be effectively controlled by frequent applications of fungicides in the field. However, the rapid emergence of new fungal strains and the hazardous effect of fungicides on the environment, combined with additional costs to growers (which can reach up to 20% of total production costs), demand the development of sustainable alternative strategies. Recently, it has been demonstrated that RNAi-mediated silencing of the susceptible gene (S-gene) MLO-7 significantly increases resistance to PM in the grapevine (Pessina et al., 2016). Here we targeted MLO-7 for mutagenesis in order to increase resistance to PM in commercially important cultivar such as Chardonnay.
The enterobacterial phytopathogen Erwinia amylovora causes fire blight, an invasive disease that threatens the apple and a wide range of commercial and ornamental Rosaceae host plants. Although, many studies have identified candidate genes as suitable targets for increasing fire blight resistance via genetic engineering, currently no resistant cultivars are commercially available, due to the social and regulatory hurdles associated with GMO plants (Singh et al., 2006). Here we selected important fruit producing apple cultivar Golden delicious to target DIPM-1, DIPM-2, and DIPM-4, in order to increase resistance to fire blight disease (Meng et al., 2006). DIPM sequence structures are closely aligned with leucine-rich repeat receptor-like kinase receptors from several organisms, and furthermore DIPMs show direct physical interaction with the disease-specific (dsp) gene of Erwinia amylovora, which may act as a susceptible factor. Mutagenesis of DIPM-1, 2, and 4 could provide apple cultivars resistant to fire blight disease.
We successfully show direct delivery of CRISPR/Cas9 RNPs to the grape and apple protoplast and efficient mutation of the targeted candidate genes. Targeted gene mutation, such as indel (insertion or deletion), was observed in 2 out of 2 specific sites of MLO-7 in grapevine cultivars and single specific sites of each DIPM-1, 2, and 4 in apple cultivars.
Materials and Methods
Grapevine Protoplast Preparation
Grapevine protoplast was isolated from 15 to 20 days old embryogenic calli and 15–20 days old in vitro micro propagated young and healthy leaves. Embryogenic calli or young and healthy leaves (10–15) were used for protoplast isolation and transformation. These plant materials were immersed in cell-wall digestion enzyme solution mix containing macerozyme R-10 (0.1–0.5%) and cellulase R-10 (1–2%) in 20 mM MES, 0.5 M mannitol, 20 mM KCL, and 10 mM CaCl2. Vacuum infiltration of plant materials took place with cell-wall digestion enzyme for 20 mins before incubating them at room temperature in a rotary shaker at 40 rpm for 4 h (embryogenic calli) or overnight (leaves). After digestion, protoplasts were filtered through Nylon mesh (75 μM), with the addition of 1:1 protoplast enzyme solution and W5 washing solution (5 mM glucose, 2 mM MES (pH 5.7), 154 mM NaCl, 125 mM CaCl2, and 5mM KCl), harvesting the protoplast by centrifugation at 100 × g for 5 mins. The supernatant was discarded and the protoplast re-suspended in 5 ml of W5 solution. A wide mouthed or point cut pipette tip was used to slowly transfer the protoplast to 5 ml of sucrose solution (21%), then centrifuged at 50 × g for 5 mins. A Pasteur pipette was used to suck the interface protoplast layer (viable and healthy protoplast), then re-suspended in 25 ml of W5 solution and incubated at 4°C for 1 h. This was centrifuged at 50 × g for 5 mins, the supernatant discarded and the protoplasts re-suspended in MMG solution (0.5 M mannitol, 4 mM MES (pH 5.7) and 15 mM MgCl2). The protoplast was counted using a hemocytometer and 2 × 105 cells used for each CRISPR RNPs transformation. At least two biological replications and three technical replication sets were used to optimize and measure enzyme concentration and protoplast yield.
Apple Protoplast Preparation
Apple protoplast was isolated from 20 to 25 days old in vitro micro propagated young and healthy leaves (10–15). The protocol for apple protoplast preparation was similar to that for the grapevine, except for the addition of hemicellulase to the cell-wall digestion enzyme solution (1–2%). The viability and density of grape and apple protoplast were determined using a haemocytometer, by staining the protoplast with fluorescein diactetate (FDA) as described elsewhere (Lei et al., 2015). At least two biological replications and three technical replication sets were used to optimize and measure enzyme concentration and protoplast yield.
In vitro sgRNA Cleavage Assay
Commercially available ready to use recombinant Cas9 protein (160 kDa) and sgRNAs were purchased from ToolGen, Inc. (Seoul, Republic of Korea). The sgRNAs were designed for target-specific sites which have higher out-of-frame scores, to achieve maximum knock out efficiency in the MLO-7 coding regions of the grapevine and the DIPM1, DIPM 2 and 4 of the apple and highly efficient sgRNAs are selected via CRISPR RGEN Tools website1 (Bae et al., 2014; Figure 1; Table 1). For assessment of activity of CRISPR/Cas9 system, in vitro cleavage assay was performed as described elsewhere (Cho et al., 2013). Corresponding target sites were amplified by specific primer sets (Table 2), amplified PCR product (300 ng) is incubated for 60 min at 37°C with Cas9 protein (25 nM) and sgRNA (25 nM) in 10 μl NEB 3 buffer (1×). Reactions were stopped with 6× stop solution containing 30% glycerol, 1.2% SDS, and100 mM EDTA. Products were resolved with 1% agarose gel electrophoresis and visualized with EtBr staining (Figure 3). Purified recombinant Cas9 protein and sgRNA were used in a ratio of 1:3, 3:1, and 1:1 (in μg) to optimize the highest mutation efficiency during protoplast transformation. We used same amount of Cas9 protein for 1:3 and 1:1 conditions and three times more Cas9 protein for 3:1 condition.
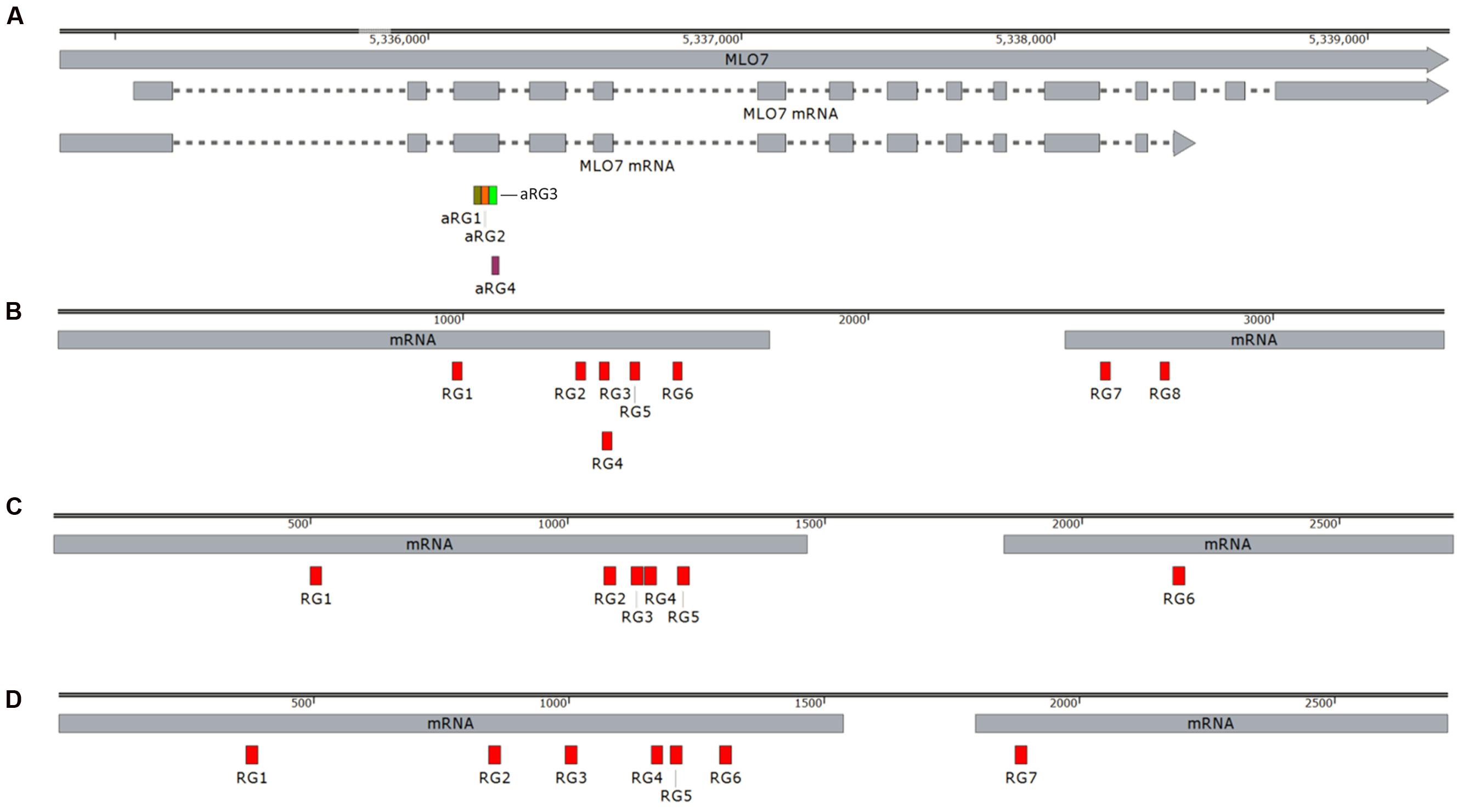
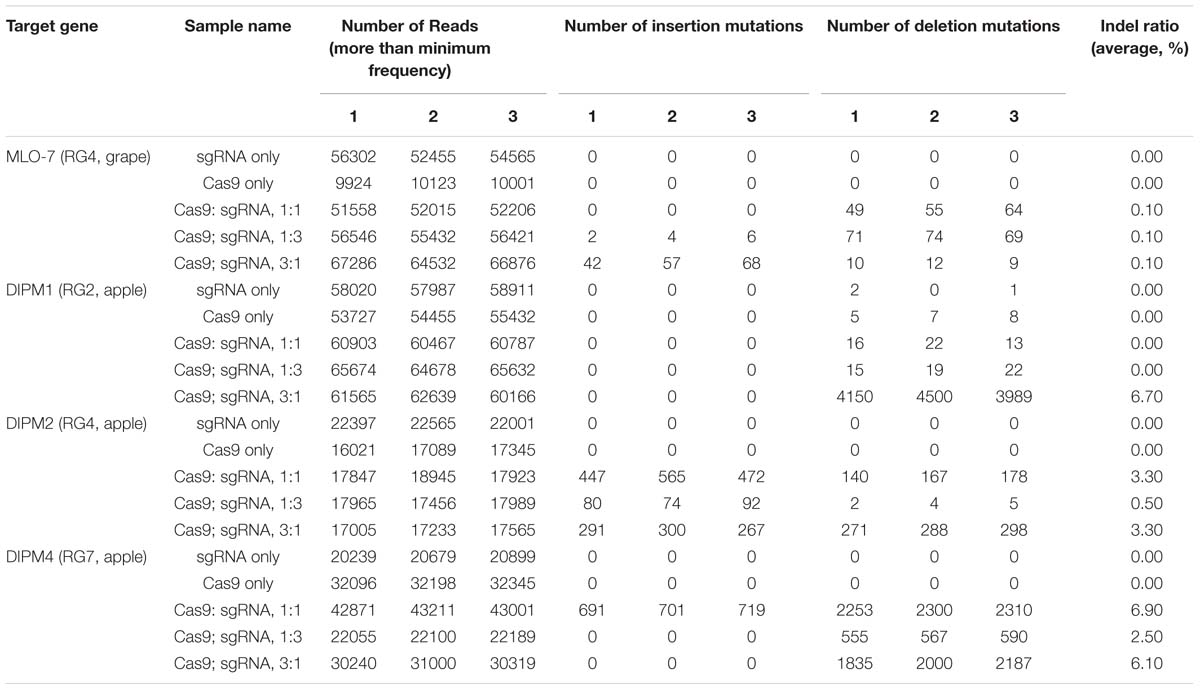
FIGURE 1. (A) Schematic diagram of the nucleotide sequence of the MLO-7 gene locus of the grapevine with sgRNA target sites. (B) Schematic diagram of the nucleotide sequence of DIPM 1. (C) Schematic diagram of the nucleotide sequence of DIPM 2. (D) Schematic diagram of the nucleotide sequence of DIPM 4. Boxes with label under the 5′-3′ gray line indicate the position of each sgRNAs.

TABLE 1. List of sgRNAs designed to target grape MLO-7 gene locus and apple DIPM-1, 2, and 4 gene loci.
Protoplast Transformation with CRISPR RNPs
In order to optimize efficient targeted mutagenesis of grapevine MLO-7 and the DIPM-1, 2, and 4 of apple gene loci, 2 × 105 re-suspended protoplasts were transformed with Cas9 protein and sgRNA in a ratio of 1:3, 3:1, and 1:1 (Woo et al., 2015; Subburaj et al., 2016). Protoplast volume 200 μl (2 × 105 cells) and RNPs for example 3:1 is Cas9 90 μg (stock 10 μg/μl) sgRNA 30 μg (stock 10 μg/μl) is used for transformation. Prior to the transformation, Cas9 and sgRNA were pre-mixed and incubated at room temperature for 10 mins. The protoplast, Cas9 and sgRNA mix were mixed and an equal volume of PEG 4000 added, gently but immediately mixing the tube before aggregation occurred and incubating it for 20 mins at room temperature. Four hundred microliter or an equal volume of W5 solution were added, mixed and incubated at room temperature for further 10 mins. An additional 800 μl were added or the volume of W5 solution doubled, mixed and incubated at room temperature for further 10 mins. This was centrifuged at 50 × g for 5 mins, the supernatant discarded and 1 ml of W5 solution added, followed by incubation at room temperature in the dark overnight. The lower sediments were collected for genomic DNA isolation. Three biological replications are performed for protoplast transformation and isolated genomic DNA from protoplast transfected cells are further used for targeted deep sequencing.
Targeted Deep Sequencing
Sequence at the sgRNA target sites were analyzed as described elsewhere (Woo et al., 2015). Corresponding target sites were PCR amplified using the primers listed in Table 2. Amplifications were performed using Phusion polymerase. Amplified PCR products were sequenced using the Illumina MiSeq platform (Quail et al., 2012). Mutations induced by CRISPR RNPs were calculated based on the indels around the CRISPR RNPs cleavage sites (3 bp upstream of PAM) using CRISPR RGEN Tools software2. Three biological replications are performed for targeted deep sequencing. Average of three biological replications are used for statistical analysis to determine percentage of indel ratio.
Results
Protoplast Isolation in Grapevine and Apple Cultivar
In the grapevine, embryogenic calli provided a higher yield of up to 3.6 × 106 with 90% viability when using 1.5% cellulase R-10 and 0.4% macerozyme R-10, with 20 min of vacuum infiltration followed by 3 h incubation with gentle shaking (Table 3). Conversely, leaves gave a lower protoplast yield and lower viability, with incubation periods of up to 24 h. In the apple, cell wall digestion with 1.5% cellulase R-10, 0.4% macerozyme R-10, and 1% hemicellulase provided a maximum yield of 1.0 × 106 with 80% viability, with 20 min of vacuum infiltration followed by 24 h incubation with gentle shaking, compared to the other ranges of various cell wall digesting enzyme concentrations (Table 1). In the apple, we selected leaves and avoided callus explants, due to their hard structure and lower protoplast yield.
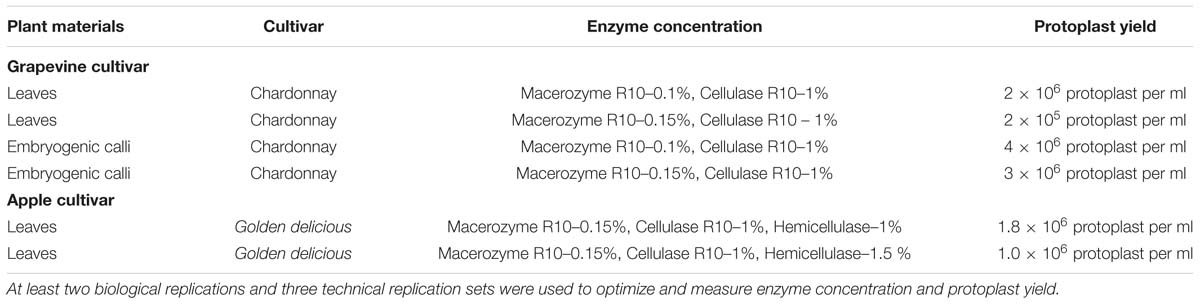
TABLE 3. Grape and apple protoplast yield with various concentrations of the cell-wall digestion enzymes from leaves (10-15 healthy leaves) and embryogenic calli (100 mg).
Targeted Mutagenesis of the Grapevine and the Apple Using CRISPR/Cas9 RNPs
To identify suitable sgRNAs for targeted mutagenesis, we designed several sgRNAs for each gene used in the present study and then cleavage frequencies of each sgRNAs were assessed using an in vitro digestion assay. All the sgRNAs used in this study were designed to pair with their corresponding 20 nucleotide target sites in MLO-7, DIPM-1, 2 and 4 gene loci and to assist Cas9 to create site-specific double strand breaks (DSBs) at 3 bp upstream of the PAM motifs (Table 1). As shown in Figure 2, sgRNAs in each gene showing the highest cleavage rate were selected for further study. To target the grapevine MLO-7 gene locus, we used sgRNA RG4 (Figure 2). Similarly, in the apple we used three specific sgRNAs for the DIPM-1 (RG2), DIPM-2 (RG4), and DIPM-4 (RG7) loci (Figure 2), respectively.
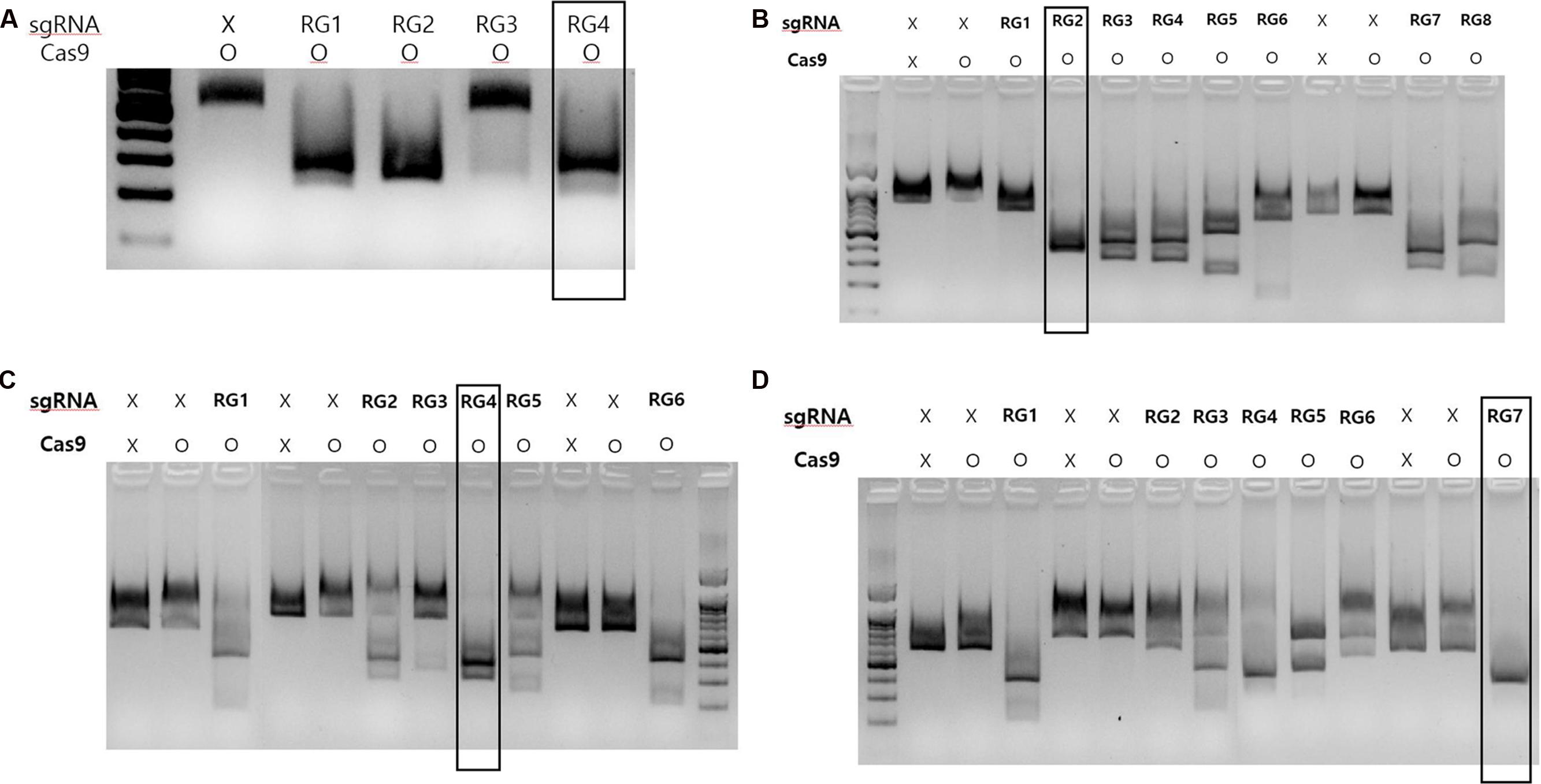
FIGURE 2. CRISPR/Cas9 RNPs in vitro digestion assay results of each sgRNAs used in the study. (A) In vitro digestion of targeted loci at MLO-7 gene. (B) In vitro digestion of targeted locus in the DIPM-2 gene. (C) In vitro digestion of targeted locus in the DIPM-2 gene. (D) In vitro digestion of targeted locus in the DIPM-4 gene. In each group, non-treated (sgRNA: x; Cas9: x) or Cas9-only (sgRNA: x; Cas9: o) samples were used as a control. After treatment with Cas9 with targeted sgRNA (RG1 to RG8 in each group) amplified target genomic DNA was digested and smaller bands were detected in gels after electrophoresis. Groups showing an intense band after digestion (indicated with black boxes) were selected and used for further experiments.
Targeted Deep Sequencing to Analyze the Mutation Efficiency of CRISPR/Cas9 RNPs
In order to detect the mutation efficiency and mutation patterns at different sites in the grape gene locus MLO-7 and the apple gene loci DIPM-1, 2, and 4, we employed targeted deep sequencing of genomic DNA obtained from each protoplast pool during PCR amplification. Total genomic DNA was extracted from transformed protoplast, while CRISPR/Cas9 target sites in MLO-7, DIPM-1, 2, and 4 were amplified using site-specific primers (Table 2). PCR amplified products were subjected to targeted deep sequencing. Targeted deep sequencing results showed that there were various number indel mutation frequencies (%) for each CRISPR sgRNA sample (Table 4).
As shown in Table 4, various mutation patterns including indels were detected in all the different sgRNA RNPs complex transformed protoplast samples, whereas no mutations were detected in sgRNA-only or Cas9-only transformed protoplast samples. These results demonstrate direct delivery of CRISPR RNPs to grapevine and apple protoplast, and indel mutagenesis efficiency of 0.1% and 0.5 to 6.9% for targeted distinct sites of endogenous MLO-7 and DIPM-1, 2, and 4 via DSBs, respectively.
Discussion
Plant protoplasts constitute a dynamic and versatile system for CRISPR/cas9 genome editing in plants and has been widely adopted in several crop species for functional analysis of the traits concerned, cellular localization, and studies of multiple signaling cascades (Shan et al., 2013; Xie and Yang, 2013; Zhao et al., 2016). CRISPR/Cas9 or other genome editing tools mediated protoplast transfection system has been successfully adopted in Arabidopsis, rice, wheat, maize, tobacco, lettuce, and petunia (Jiang et al., 2013; Li et al., 2013; Shan et al., 2013; Wang et al., 2014; Gao et al., 2015; Woo et al., 2015; Subburaj et al., 2016), however, a similar system has not been developed for the grapevine and apple. In this study, we isolated protoplast from embryogenic calli and leaves of grapevine and apple cultivar in order to standardize an efficient protocol for the transient expression system of CRISPR/Cas9 RNPs. Protoplast isolation, transfection and transient gene expression system in grape and apple has been little explored and most of the available methods have not been updated for two decades (Doughty and Power, 1988; Patat-Ochatt et al., 1993; Patat-Ochatt, 1994; Reustle et al., 1995; Zhu et al., 1997; Saito and Suzuki, 1999; Fontes et al., 2010). Protoplast viability, yield, and efficient transfection depend on various factors, such as the concentration of cell wall digestion enzymes, buffer conditions, the osmotic status of protoplasts, the incubation period, and the type of explants used for protoplast isolation. In the current study, all these variables were updated and optimized in order to achieve a better yield. This is the first report of successful demonstration of CRISPR/Cas9 RNPs mediated protoplast transformation in grapevine and apple cultivars. Our method for transient expression of genome editing tools in the protoplast to target the gene of interest with specificity and higher efficiency should help grapevine and apple scientists to analyze the traits concerned in the host plant within a day or two. Furthermore, future work on regeneration of genome edited protoplast will provide an opportunity to develop DNA-free genome edited grapevine and apple fruit crop plants. One such example is regeneration of apple plants from meristem derived callus protoplast (Saito and Suzuki, 1999).
CRISPR/Cas9 is easy to prepare, scalable and affordable compared to ZFNs and TALENs. However, the broader application of plasmid mediated CRISPR/Cas9 to life sciences, biotechnology and medicine is limited by off-target effects, unwanted integration of plasmid vectors into the genome and possible GMO regulations (Hendel et al., 2015; Zhang et al., 2015). In order to overcome these limitations, we delivered CRISPR/Cas9 RNPs rather than plasmids directly into the protoplast cells and showed that RNPs enable efficient genome editing, while avoiding unwanted integration of plasmid DNA in the host genome, similar to other recent studies done in human, animal and plant cells (Figure 3; Kim et al., 2014; Lee et al., 2014; Liu et al., 2015; Woo et al., 2015; Subburaj et al., 2016).
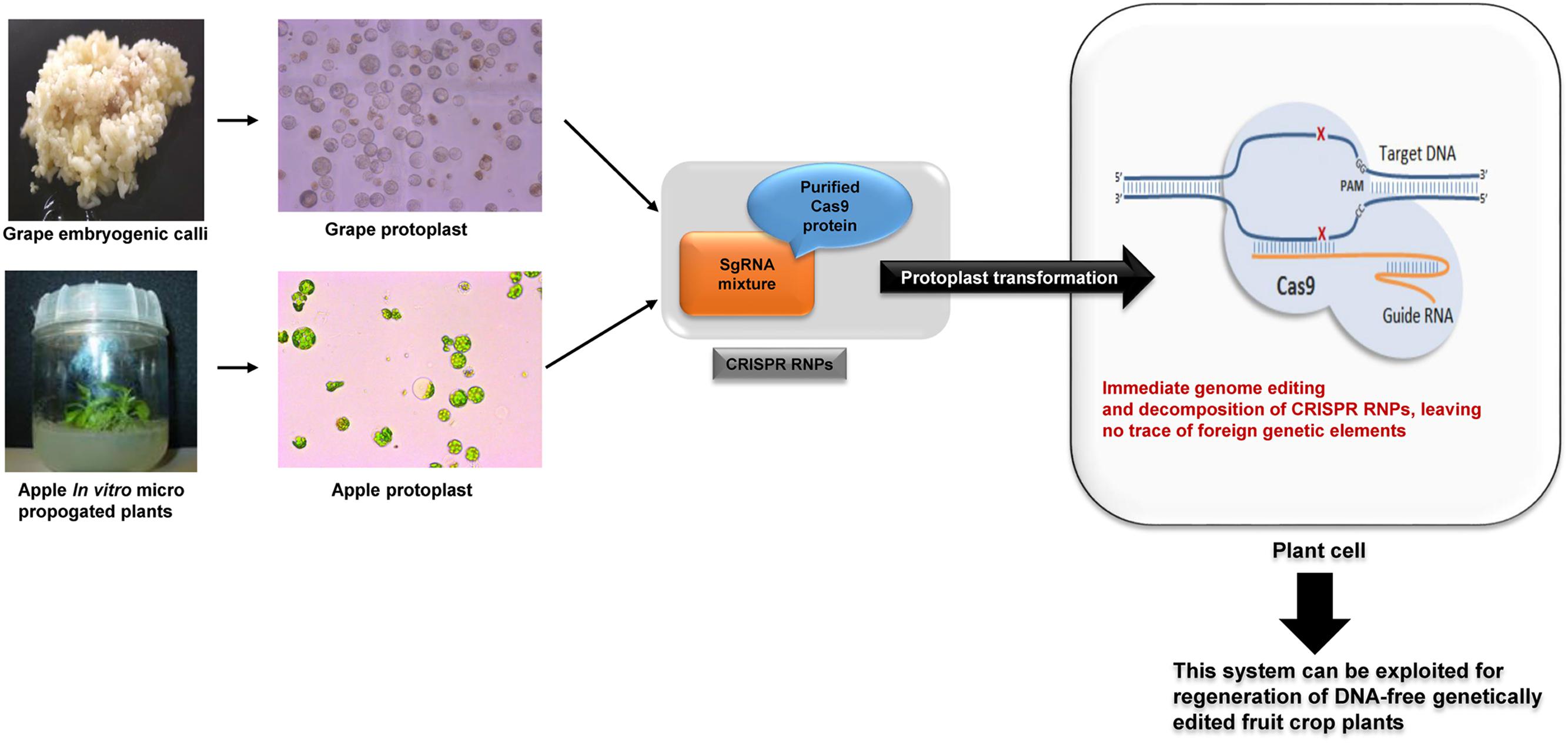
FIGURE 3. Schematic diagram of CRISPR RNPs direct delivery to grape and apple plant cells to produce DNA-free genetically edited crop plants.
Recently several groups have demonstrated that CRISPR/Cas9 can induce unwanted mutations in off-target sites, differing from on-target sites by up to 5 nt, leading to questions about their specificity (Cradick et al., 2013; Fu et al., 2013; Hsu et al., 2013; Tan et al., 2015). We and others have proposed various strategies to improve the on-target specificity of CRISPR/Cas9 (Koo et al., 2015; Kanchiswamy et al., 2016). Synthesis of unique sgRNA (Cho et al., 2014; Fu et al., 2014; Sung et al., 2014), Cas9 nickases and web based computer programs to identify unique target sites (Mali et al., 2013; Ran et al., 2013; Bae et al., 2014) and CRISPR/Cas9 RNPs (Woo et al., 2015). In order to facilitate the highest site-specific mutation frequency in grape and apple protoplast, we titrated the ratio of Cas9 and sgRNAs, in a similar way to our previous studies (Hsu et al., 2013; Woo et al., 2015). In this study, we employed three different Cas9: sgRNA ratios, i.e., 3:1, 1:1, and 1:3, for protoplast PEG mediated transformation in the grape and the apple. We determined that the 3:1 ratio for MLO-7 in the grapevine, the 3:1 ratio for DIPM 1, the 1:1 and 3:1 ratio for DIPM 2 and the 1:1 ratio for DIPM 4 in the apple resulted in highest mutation frequency. Here, we showed the critical advantage over plasmid mediated genome editing delivery by titrating the Cas9:sgRNA ratio to achieve maximum mutation frequency (Liu et al., 2015; Kanchiswamy et al., 2016).
This study demonstrated direct delivery of CRISPR/Cas9 RNPs to grape and apple protoplasts and site-directed mutation of the grape gene locus MLO-7 and the apple gene loci DIPM-1, 2, and 4.
Conclusion
We demonstrated efficient targeted mutagenesis in the grapevine gene locus MLO-7 and the apple gene loci DIPM-1, 2, and 4, using direct delivery of CRISPR RNPs. Although the mutation efficiency was found to vary with the targeted gene locus and the ratio of Cas9 and sgRNA, mutation patterns and frequency assays showed CRISPR RNPs to be an effective strategy for targeted mutagenesis of gene loci in grape and apple protoplasts. This method has already shown improved features compared to plasmid-mediated genome editing in humans, animals and plants, such as higher efficiency, significantly reduced off-target effects and more rapid editing activity after delivery (Liu et al., 2015; Woo et al., 2015; Kanchiswamy, 2016; Kanchiswamy et al., 2016). Furthermore, in plants, the new varieties obtained with this approach may be deregulated from current GMO legislations, as the Cas9 protein-guide RNA complexes will rapidly decompose in regenerating cell cultures. Further studies are now required to optimize plant regeneration from CRISPR RNPs transformed protoplast to explore the applications of this technology at field level.
Author Contributions
CN, O-JK, and M-HJ performed and designed experiments. RV, SK, J-SK, RiV, and MM provided materials, funding, plan for the experiment. CN wrote manuscript with the cooperation and support of all co-authors.
Funding
This work was supported by the research funding office of the Autonomous Province of Trento.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Stefano Piazzo, Valentino Poletti, and Lorenza Dallacosta from the Research and Innovation Centre, in the Genomics and Biology of Fruit Crop Department, Fondazione Edmund Mach, Trento, Italy for providing plant materials.
Footnotes
References
Bae, S., Park, J., and Kim, J. S. (2014). Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30, 1473–1475. doi: 10.1093/bioinformatics/btu048
Cho, S. W., Kim, S., Kim, J. M., and Kim, J. S. (2013). Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 230–232. doi: 10.1038/nbt.2507
Cho, S. W., Kim, S., Kim, Y., Kweon, J., Kim, H. S., Bae, S., et al. (2014). Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 24, 132–141. doi: 10.1101/gr.162339.113
Cradick, T. J., Fine, E. J., Antico, C. J., and Bao, G. (2013). CRISPR/Cas9 systems targeting beta-globin and CCR5 genes have substantial off-target activity. Nucleic Acids Res. 41, 9584–9592. doi: 10.1093/nar/gkt714
Doughty, S., and Power, J. B. (1988). Callus formation from leaf mesophyll protoplasts of Malus Xdomestica Borkh. cv. Greensleeves. Plant cell Rep. 7, 200–201. doi: 10.1007/BF00269323
Fontes, N., Delrot, S., and Geros, H. (2010). A method for the isolation of protoplasts from grape berry mesocarp tissue. Recent Pat. Biotechnol. 4, 125–129. doi: 10.2174/187220810791110705
Fu, Y., Foden, J. A., Khayter, C., Maeder, M. L., Reyon, D., Joung, J. K., et al. (2013). High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 31, 822–826. doi: 10.1038/nbt.2623
Fu, Y., Sander, J. D., Reyon, D., Cascio, V. M., and Joung, J. K. (2014). Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat. Biotechnol. 32, 279–284. doi: 10.1038/nbt.2808
Gadoury, D. M., Cadle-Davidson, L., Wilcox, W. F., Dry, I. B., Seem, R. C., and Milgroom, M. G. (2012). Grapevine powdery mildew (Erysiphe necator): a fascinating system for the study of the biology, ecology and epidemiology of an obligate biotroph. Mol. Plant Pathol. 13, 1–16. doi: 10.1111/j.1364-3703.2011.00728.x
Gao, J., Wang, G., Ma, S., Xie, X., Wu, X., Zhang, X., et al. (2015). CRISPR/Cas9-mediated targeted mutagenesis in Nicotiana tabacum. Plant Mol. Biol. 87, 99–110. doi: 10.1007/s11103-014-0263-0
Hendel, A., Fine, E. J., Bao, G., and Porteus, M. H. (2015). Quantifying on- and off-target genome editing. Trends Biotechnol. 33, 132–140. doi: 10.1016/j.tibtech.2014.12.001
Hsu, P. D., Scott, D. A., Weinstein, J. A., Ran, F. A., Konermann, S., Agarwala, V., et al. (2013). DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832. doi: 10.1038/nbt.2647
Jiang, W., Zhou, H., Bi, H., Fromm, M., Yang, B., and Weeks, D. P. (2013). Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 41:e188. doi: 10.1093/nar/gkt780
Jones, H. D. (2015). Regulatory uncertainty over genome editing. Nat. Plants 1:14011. doi: 10.1038/nplants.2014.11
Kanchiswamy, C. N. (2016). DNA-free genome editing methods for targeted crop improvement. Plant Cell Rep. 35, 1469–1474. doi: 10.1007/s00299-016-1982-2
Kanchiswamy, C. N., Maffei, M., Malnoy, M., Velasco, R., and Kim, J. S. (2016). Fine-tuning next-generation genome editing tools. Trends Biotechnol. 34, 562–574. doi: 10.1016/j.tibtech.2016.03.007
Kanchiswamy, C. N., Sargent, D. J., Velasco, R., Maffei, M. E., and Malnoy, M. (2015). Looking forward to genetically edited fruit crops. Trends Biotechnol. 33, 62–64. doi: 10.1016/j.tibtech.2014.07.003
Kim, S., Kim, D., Cho, S. W., Kim, J., and Kim, J. S. (2014). Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 24, 1012–1019. doi: 10.1101/gr.171322.113
Koo, T., Lee, J., and Kim, J. S. (2015). Measuring and reducing off-target activities of programmable nucleases including CRISPR-Cas9. Mol. Cells 38, 475–481. doi: 10.14348/molcells.2015.0103
Lee, J. S., Kwak, S. J., Kim, J., Kim, A. K., Noh, H. M., Kim, J. S., et al. (2014). RNA-guided genome editing in Drosophila with the purified Cas9 protein. G3 (Bethesda) 4, 1291–1295. doi: 10.1534/g3.114.012179
Lei, R., Qiao, W., Hu, F., Jiang, H., and Zhu, S. (2015). A simple and effective method to encapsulate tobacco mesophyll protoplasts to maintain cell viability. MethodsX 2, 24–32. doi: 10.1016/j.mex.2014.11.004
Li, J. F., Norville, J. E., Aach, J., McCormack, M., Zhang, D., Bush, J., et al. (2013). Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 31, 688–691. doi: 10.1038/nbt.2654
Liu, J., Gaj, T., Yang, Y., Wang, N., Shui, S., Kim, S., et al. (2015). Efficient delivery of nuclease proteins for genome editing in human stem cells and primary cells. Nat. Protoc. 10, 1842–1859. doi: 10.1038/nprot.2015.117
Mali, P., Aach, J., Stranges, P. B., Esvelt, K. M., Moosburner, M., Kosuri, S., et al. (2013). CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 31, 833–838. doi: 10.1038/nbt.2675
Meng, X., Bonasera, J. M., Kim, J. F., Nissinen, R. M., and Beer, S. V. (2006). Apple proteins that interact with DspA/E, a pathogenicity effector of Erwinia amylovora, the fire blight pathogen. Mol. Plant Microbe Interact. 19, 53–61. doi: 10.1094/MPMI-19-0053
Nishitani, C., Hirai, N., Komori, S., Wada, M., Okada, K., Osakabe, K., et al. (2016). Efficient genome editing in apple using a CRISPR/Cas9 system. Sci. Rep. 6:31481. doi: 10.1038/srep31481
Patat-Ochatt, E. M. (1994). “Regeneration of plants from protoplasts of Malus Xdomestica Borkh. (Apple),” in Plant Protoplasts and Genetic and Engineering V, ed. Y. P. S. Bajaj (Berlin: Springer), 83–101. doi: 10.1007/978-3-662-09366-5_7
Patat-Ochatt, E. M., Boccon-Gibod, J., Duron, M., and Ochatt, S. J. (1993). Organogenesis of stem and leaf protoplasts of a haploid golden delicious apple clone (Malus Xdomestica Borkh.). Plant Cell Rep. 12, 118–120. doi: 10.1007/BF00241946
Pessina, S., Lenzi, L., Perazzolli, M., Campa, M., Dalla Costa, L., Urso, S., et al. (2016). Knockdown of MLO genes reduces susceptibility to powdery mildew in grapevine. Hortic. Res. 3:16016. doi: 10.1038/hortres.2016.16
Quail, M. A., Smith, M., Coupland, P., Otto, T. D., Harris, S. R., Connor, T. R., et al. (2012). A tale of three next generation sequencing platforms: comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genomics 13:341. doi: 10.1186/1471-2164-13-341
Ran, F. A., Hsu, P. D., Lin, C. Y., Gootenberg, J. S., Konermann, S., Trevino, A. E., et al. (2013). Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389. doi: 10.1016/j.cell.2013.08.021
Ren, C., Liu, X., Zhang, Z., Wang, Y., Duan, W., Li, S., et al. (2016). CRISPR/Cas9-mediated efficient targeted mutagenesis in Chardonnay (Vitis vinifera L.). Sci. Rep. 6:32289. doi: 10.1038/srep32289
Reustle, G., Harst, M., and Alleweldt, G. (1995). Plant regeneration of grapevine (Vitis sp.) protoplasts isolated from embryogenic tissue. Plant Cell Rep. 15, 238–241. doi: 10.1007/BF00193727
Saito, A., and Suzuki, M. (1999). Plant regeneration from meristem-derived callus protoplasts of apple (Malus3domestica cv. ‘Fuji’). Plant Cell Rep. 18, 549–553. doi: 10.1007/s002990050620
Shan, Q., Wang, Y., Li, J., Zhang, Y., Chen, K., Liang, Z., et al. (2013). Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 31, 686–688. doi: 10.1038/nbt.2650
Singh, O. V., Ghai, S., Paul, D., and Jain, R. K. (2006). Genetically modified crops: success, safety assessment, and public concern. Appl. Microbiol. Biotechnol. 71, 598–607. doi: 10.1007/s00253-006-0449-8
Song, G., Jia, M., Chen, K., Kong, X., Khattak, B., Xie, C., et al. (2016). CRISPR/Cas9: a powerful tool for crop genome editing. Crop J. 4, 75–82. doi: 10.1016/j.cj.2015.12.002
Sprink, T., Eriksson, D., Schiemann, J., and Hartung, F. (2016). Regulatory hurdles for genome editing: process- vs. product-based approaches in different regulatory contexts. Plant Cell Rep. 35, 1493–1506. doi: 10.1007/s00299-016-1990-2
Subburaj, S., Chung, S. J., Lee, C., Ryu, S. M., Kim, D. H., Kim, J. S., et al. (2016). Site-directed mutagenesis in Petunia x hybrida protoplast system using direct delivery of purified recombinant Cas9 ribonucleoproteins. Plant Cell Rep. 35, 1535–1544. doi: 10.1007/s00299-016-1937-7
Sung, Y. H., Kim, J. M., Kim, H. T., Lee, J., Jeon, J., Jin, Y., et al. (2014). Highly efficient gene knockout in mice and zebrafish with RNA-guided endonucleases. Genome Res. 24, 125–131. doi: 10.1101/gr.163394.113
Tan, E. P., Li, Y., Velasco-Herrera Mdel, C., Yusa, K., and Bradley, A. (2015). Off-target assessment of CRISPR-Cas9 guiding RNAs in human iPS and mouse ES cells. Genesis 53, 225–236. doi: 10.1002/dvg.22835
Wang, Y., Cheng, X., Shan, Q., Zhang, Y., Liu, J., Gao, C., et al. (2014). Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 32, 947–951. doi: 10.1038/nbt.2969
Woo, J. W., Kim, J., Kwon, S. I., Corvalan, C., Cho, S. W., Kim, H., et al. (2015). DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 33, 1162–1164. doi: 10.1038/nbt.3389
Xie, K., and Yang, Y. (2013). RNA-guided genome editing in plants using a CRISPR-Cas system. Mol. Plant 6, 1975–1983. doi: 10.1093/mp/sst119
Zhang, X. H., Tee, L. Y., Wang, X. G., Huang, Q. S., and Yang, S. H. (2015). Off-target Effects in CRISPR/Cas9-mediated Genome Engineering. Mol. Therapy Nucleic Acids 4:e264. doi: 10.1038/mtna.2015.37
Zhang, Y., Liang, Z., Zong, Y., Wang, Y., Liu, J., Chen, K., et al. (2016). Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat. Commun. 7:12617. doi: 10.1038/ncomms12617
Zhao, F.-L., Li, Y.-J., Hu, Y., Gao, Y.-R., Zang, X.-W., Ding, Q., et al. (2016). A highly efficient grapevine mesophyll protoplast system for transient gene expression and the study of disease resistance proteins. Plant Cell Tissue Organ Culture 125, 43–57. doi: 10.1007/s11240-015-0928-7
Zhu, Y.-M., Hoshino, Y., Nakano, M., Takahashi, E., and Mii, M. (1997). Highly efficient system of plant regeneration from protoplasts of grapevine (Vitis vinifera L.) through somatic embryogenesis by using embryogenic callus culture and activated charcoal. Plant Sci. 123, 151–157. doi: 10.1016/S0168-9452(96)04557-8
Keywords: genome editing, non-GMO, DNA-free, CRISPR/Cas9, apple, grapevine
Citation: Malnoy M, Viola R, Jung M-H, Koo O-J, Kim S, Kim J-S, Velasco R and Nagamangala Kanchiswamy C (2016) DNA-Free Genetically Edited Grapevine and Apple Protoplast Using CRISPR/Cas9 Ribonucleoproteins. Front. Plant Sci. 7:1904. doi: 10.3389/fpls.2016.01904
Received: 11 September 2016; Accepted: 01 December 2016;
Published: 20 December 2016.
Edited by:
Fabio Marroni, University of Udine, ItalyReviewed by:
Attila Molnar, University of Edinburgh, UKThorben Sprink, Julius Kühn-Institut, Germany
Luisa Bortesi, RWTH-Aachen University, Germany
Copyright © 2016 Malnoy, Viola, Jung, Koo, Kim, Kim, Velasco and Nagamangala Kanchiswamy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mickael Malnoy, bWlja2FlbC5tYWxub3lAZm1hY2guaXQ= Chidananda Nagamangala Kanchiswamy, Y2hpZGFuYW5kYS5uYWdhbWFuZ2FsYUBmbWFjaC5pdA==
 Mickael Malnoy
Mickael Malnoy Roberto Viola
Roberto Viola Min-Hee Jung2
Min-Hee Jung2 Ok-Jae Koo
Ok-Jae Koo Seokjoong Kim
Seokjoong Kim Riccardo Velasco
Riccardo Velasco Chidananda Nagamangala Kanchiswamy
Chidananda Nagamangala Kanchiswamy