- 1State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences, Beijing, China
- 2Chinese Academy of Meteorological Sciences, Beijing, China
Stomata control the flow of gases between plants and the atmosphere. This review is centered on stomatal responses to elevated CO2 concentration and considers other key environmental factors and underlying mechanisms at multiple levels. First, an outline of general responses in stomatal conductance under elevated CO2 is presented. Second, stomatal density response, its development, and the trade-off with leaf growth under elevated CO2 conditions are depicted. Third, the molecular mechanism regulating guard cell movement at elevated CO2 is suggested. Finally, the interactive effects of elevated CO2 with other factors critical to stomatal behavior are reviewed. It may be useful to better understand how stomata respond to elevated CO2 levels while considering other key environmental factors and mechanisms, including molecular mechanism, biochemical processes, and ecophysiological regulation. This understanding may provide profound new insights into how plants cope with climate change.
Introduction
Elevated atmospheric carbon dioxide concentration (elevated CO2) is a major component of climate change. It has increased from the pre-industrial level of 280 μmol mol-1 in 1750 to c. 400 μmol mol-1 at present and is expected to increase to c. 900 μmol mol-1 by the end of the 21st century. The global surface temperature is projected to rise 2.6–4.8°C by the end of this century, according to RCP8.5 (IPCC, 2013), a more undisciplined management scenario. Climate change, including elevated CO2, rising temperatures, and altered precipitation patterns, have markedly affected terrestrial ecosystem structure and function, carbon and water balance, and crop productivity (Lobell et al., 2011; Peñuelas et al., 2013; Ruiz-Vera et al., 2013; Bagley et al., 2015; Lavania et al., 2015). Moreover, a profound interaction between climate change and other critical environmental factors, including limited nutrition and air pollution, as well as some biotic factors, such as herbivorous insects, may intensify the adverse impacts (Gillespie et al., 2012; Peñuelas et al., 2013; Xu et al., 2013; Zavala et al., 2013; Sun et al., 2015; Xu et al., 2016).
Many studies have reported the biological responses to CO2 enrichment and their interaction with environmental change at various levels (Ainsworth and Rogers, 2007; Medeiros et al., 2015; Xu et al., 2015; Rodrigues et al., 2016). Elevated CO2 generally can enhance CO2 fixation and consequently plant growth and production (Ainsworth and Rogers, 2007; Xu et al., 2013). On the other hand, the decrease in stomatal conductance (gs) under elevated CO2 conditions may limit the CO2 fixation rate but promote water use efficiency (WUE) to benefit plant growth, especially within a climate change context where water shortage periods are expected to increase (Leakey et al., 2009; Sreeharsha et al., 2015).
Of these responses, the stomata are pivotal doors that control the gas exchange between vegetation and the atmosphere, i.e., CO2 entering from the atmosphere and water vapor releasing from plants into the atmosphere (Woodward, 1987). Carbon dioxide can reach the fixed Rubisco site through CO2 gas diffusions from the boundary layer, stomata, and intercellular airspaces near the chloroplast (Ball et al., 1987; Woodward, 1987; Warren, 2008). The main factors controlling stomatal opening processes include Ca2+ level, guard cell turgor, and hormones (Assmann, 1999; Lawson et al., 2014). Stomatal behavior may be affected by environmental factors, such as water status (e.g., soil water deficit, vapor pressure deficit [VPD]), temperature, CO2 concentrations, and light either alone and/or in combination (e.g., Lee et al., 2008; Perez-Martin et al., 2009; Hubbart et al., 2013; Laanemets et al., 2013; Šigut et al., 2015). Furthermore, stomatal short-term behavior (e.g., stomatal closure) and a long-term developmental (e.g., stomatal size and its density) responses to environmental changes might occur together, depending on species and genotypes (Gray et al., 2000; Ainsworth and Rogers, 2007; Haworth et al., 2013; DaMatta et al., 2016).
Our review focuses on the stomatal responses to elevated CO2 conditions with climatic change as well as the relevant metabolic processes and underlying mechanisms. The future perspectives for this study and possible implications are briefly presented and discussed. The present report may advance our current knowledge of the stomatal response to climatic change. It may also provide a new vision of its interdisciplinary and systematic synthesis to promote further relevant research.
Stomatal Response to Elevated Co2
Elucidating the stomatal response to CO2 concentrations is important for understanding the stomatal physiology and gas exchanges between vegetation and the atmosphere. To adapt CO2 intake for photosynthesis and water release for transpiration, plants need to mediate stomatal development and behavior to balance CO2 and water exchange through the leaf epidermis in a changing environment (Gray et al., 2000; Haworth et al., 2013; Lawson et al., 2014). Elevated CO2 generally causes reductions in stomatal density (SD, e.g., Woodward, 1987; Lin et al., 2001; Teng et al., 2009), stomatal conductance (Medlyn et al., 2001; Ainsworth and Rogers, 2007; Gao et al., 2015), leaf transpiration (Teng et al., 2009; Katul et al., 2010), and canopy/ecosystem evapotranspiration (Medlyn et al., 2001; Bernacchi et al., 2007; Leakey et al., 2009; Bernacchi and VanLoocke, 2015). However, some studies have challenged this response because the reverse response might occur when elevated CO2 interacts with other climatic factors (see the sections below).
Stomatal Conductance Response
Response Magnitude
The decreased magnitude of gs by CO2 enrichment greatly depends on environmental variables and species (Medlyn et al., 2001; Ainsworth and Rogers, 2007; Haworth et al., 2013; Ward et al., 2013). In an earlier report, doubled ambient CO2 decreased gs by c. 40% in almost all enclosure experiments, such as greenhouse and chamber experiments (Kimball et al., 1993; Morison and Lawlor, 1999). A 50% gs decrease induced by elevated CO2 was found (Jackson et al., 1994), and a synthesis report showed a 21% gs decrease in trees (Medlyn et al., 2001). A model scaling from leaf-level to canopy indicated that elevated CO2 might reduce canopy gs by 16% (Baldocchi and Harley, 1995). According to a meta-analysis, the elevated CO2-induced gs reduction in free air CO2 enrichment (FACE) experiments was averaged 22% across all plant species (n = c. 580). A significant variation among plant functional types (PFTs) was obtained: a maximum decease for C3 grass (30–40%) and a minimum decrease for shrub species (c. 15%; Ainsworth and Rogers, 2007). However, in a few experiments, gs did not respond to CO2 concentrations in an obvious way (Ellsworth et al., 2011; Haworth et al., 2013; Ward et al., 2013; Bernacchi and VanLoocke, 2015; DaMatta et al., 2016). The gs increase was even observed (Uddling et al., 2009) with short-term CO2 fertilization, for instance, in A. thaliana (Zinta et al., 2014). A recent experiment also found 23 and 18% gs increases from elevated CO2 conditions in during vegetative and reproductive growth phases, respectively, of the Pigeon pea (Cajanus cajan L.; Sreeharsha et al., 2015). In a recent finding, the Arabidopsis Tetraploid, Me-0, with larger stomata, still had a comparatively high gs when exposed to increased CO2 concentrations, suggesting that taller plants with larger stomatal size can better deal with rising CO2 by improving their stomatal behavior (Monda et al., 2016). Thus, the decrease in gs due to elevated CO2 is a general rather than a universal response due to some unexpected factors’ effects. This difference is particularly found in dramatic ecotypes-, species-, PFTs-, and development stages. As such, the underlying mechanism remains to be clarified further.
Interaction of gs, A, and WUE
The decrease in gs generally leads to a decrease in net assimilation rate (A) and is recognized as one of the two major limitations of photosynthesis; the other is non-stomatal limitation (Noormets et al., 2001). There was no obvious evidence from FACE that gs independently acclimated to elevated CO2 levels despite exposure time (Nijs et al., 1997; Leakey et al., 2006a; Ainsworth and Rogers, 2007; Gao et al., 2015). An earlier model by Ball et al. (1987) predicted that gs may be restricted when down-regulation in A occurs in response to CO2 enrichment. Although stomatal limitation to photosynthesis may decrease with elevated CO2 levels (e.g., Noormets et al., 2001), the uncoupling of gs with A has been confirmed in a transgenic tobacco plant due to its reduced Rubisco content (von Caemmerer et al., 2004). However, an experiment has shown that a high A caused by increasing gs can be maintained in a rice mutant that has a deficient slow anion channel 1 (SLAC1), that is, a guard cell anion channel protein that does not respond to rising CO2 levels (Kusumi et al., 2012). Furthermore, a recent experiment indicated that, with elevated CO2, Cajanus cajan leaves had 7–18% higher leaf instantaneous WUE (WUEi) due to simultaneously maintaining both higher A and gs. However, the former was higher than the latter (Sreeharsha et al., 2015). It is also noteworthy that at a high CO2 levels, a significant gs decrease in C4 plants, such as maize, may occur only during drought, leading to WUE promotion rather than enhanced photosynthetic capacity as a result of the gs decrease (Leakey et al., 2006b, 2009). A recent report by Lawson and Blatt (2014) has indicated that although stomatal responses to environmental changes may be closely associated with CO2 assimilation and water transpiration, a better balance between CO2 uptake and water loss may be improved by manipulating guard cell physical, anatomical, and transport characteristics to promote WUE (Lawson and Blatt, 2014). This may need further testing under elevated CO2 conditions.
Stomatal Development and Its Density
Response Magnitude of Stomatal Density
A decrease in SD is considered a general response to elevated CO2. As reported by Woodward (1987), as CO2 levels from the pre-industrial level of 280 μmol mol-1 rose to the ambient level of 340 μmol mol-1 in 1987, a dramatic (67%) decrease in SD was found in the leaves of herbarium specimens and in experiments under controlled environmental conditions. Based on a paleobotanic analysis of fossil Buxus (3775–3640 BC) by Rivera et al. (2014), the SD and stomatal index (SI) had significantly greater values than the current Buxus balearica and B. sempervirens species (297.6 vs. 227.8 stomata mm-2, 12.7 vs. 8.0%, respectively). The dramatic declines are closely associated with a drastic increase in atmospheric CO2 concentration that has been occurring since the mid-Holocene era (Joos et al., 2004; Rivera et al., 2014). However, only a 5% SD decrease due to elevated CO2 was obtained from a meta-analysis on stomatal response (Ainsworth and Rogers, 2007). Relatively few studies reported an unchanged (Tricker et al., 2005) or even increased SD (Reid et al., 2003). A recent report by Field et al. (2015) showed that SD in non-vascular land plants, such as hornwort (Anthoceros punctatus, Phaeoceros laevis) and some moss sporophytes, did not respond to CO2 enrichment. It was even slightly increased in Funaria hygrometrica sporophytes at elevated CO2. A recent report showed the appearance of SD responses to elevated CO2 depends on tropic coffee genotypes (Rodrigues et al., 2016). These findings imply that the magnitude of SD response to CO2 enrichment might easily vary according to the experimental facility, experimental duration, species/genotypes, and other environmental variables (e.g., Ainsworth and Rogers, 2007; Haworth et al., 2013; Rodrigues et al., 2016). Thus, considerable caution is required when using SD as an indicator of a stomatal adaptive process in response to elevated atmospheric CO2 concentration.
Stomatal Development under Elevated CO2
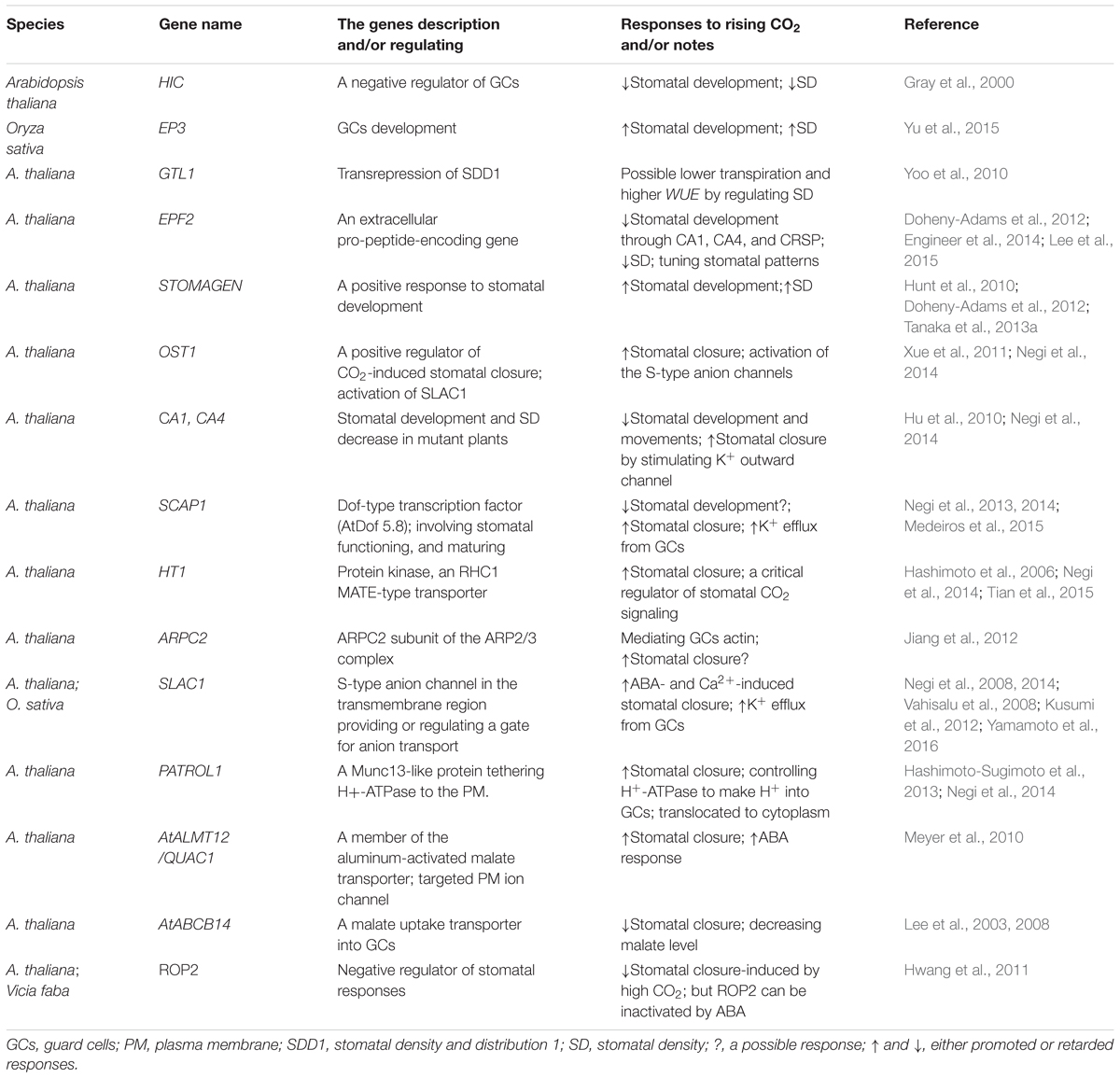
The relevant genes may be involved in stomatal development under elevated CO2 conditions (Gray et al., 2000). The Arabidopsis gene hic (high carbon dioxide) encodes a negative regulator of stomatal development that responds to CO2 concentrations and can be adversely regulated by elevated CO2. A 42% increase in SD in the mutant hic plants was evidence of a doubled CO2 level (Gray et al., 2000). Arabidopsis plants with the GTL1 gene have higher transpiration and lower WUE due to regulation of SD via transrepression of SD and distribution 1 (SDD1; Yoo et al., 2010). As reported by Engineer et al. (2014), the extracellular pro-peptide-encoding gene epidermal patterning factor 2 (EPF2) in wild-type Arabidopsis can be induced by elevated CO2, possibly providing an essential role for CO2 control of stomatal development. Furthermore, in the β-carbonic anhydrase double mutants (ca1, ca4), a secreted protease CRSP may cleave the EPF2 and then repress stomatal development, demonstrating an inverse response of the wild-type plants to elevated CO2. This partly elucidates the key mechanisms of how the sensing and transduction CO2 signals are linked to stomatal development (Engineer et al., 2014). This finding also indicates that some transduction signals between stomata and nearby pavement cells (PCs) may be involved in abscisic acid (ABA)-mediated inhibition of PC enlargement and may ultimately affect stomatal distribution and its density (Tanaka et al., 2013a). It has been suggested that the signals are peptide hormones (Hunt et al., 2010; Sugano et al., 2010; Jewaria et al., 2013; Lee et al., 2015; see below). However, a clear role of the response of stomatal development to elevated CO2 remains largely unknown. A description on the genes regarding stomatal development in response to CO2 concentration are listed in Table 1.
Stomatal Density and A under Elevated CO2
Photosynthetic capacity is closely linked to SD (Xu and Zhou, 2008). Leaf A was negatively correlated with SD when plants were exposed to elevated CO2 (Woodward, 1987; Ainsworth and Rogers, 2007), whereas a positive correlation occurred when grass was subjected to a water status gradient (Xu and Zhou, 2008). Moreover, photosynthetic potential might be enhanced with increased SD in Arabidopsis by a modulating gas diffusion function, as was recently reported by Tanaka et al. (2013b). In this case, the A increase at elevated CO2 is tightly associated with increased SD. Here, the stomagen gene overexpression confers a positive response to stomatal development in A. thaliana (Hunt et al., 2010; Doheny-Adams et al., 2012; Tanaka et al., 2013b). As recently reported, an EP3 gene in rice may be responsible for guard cell development, which may determine SD. This is due to the ep3 mutant plants exhibiting a smaller GC with low SD, gs, and A compared with their wild-type controls (Yu et al., 2015).
Trade-off between Stomatal Density and Leaf Growth under Elevated CO2
The response and feedback of SD with leaf growth to elevated CO2 may be described generally in a linkage network (Figure 1). A general SD decrease in CO2 enrichment may have several possible coherent explanations. (1) The promotion of a leaf area may contribute to a lower SD. For instance, the leaf area in grass plants markedly increased under elevated CO2 (Xu et al., 2014), possibly reducing the SD (Xu and Zhou, 2008; Xu et al., 2009b). An 11–23% decrease in SD by in Scots pine (Pinus sylvestris) needles by high CO2 conditions might result from an increase in needle thickness and needle width, i.e., the surface area of the entire needle. Thus, this structural plasticity may often occur in short-term elevated CO2 fertilization. (2) With long-term elevated CO2, the relevant gene expression levels may contribute to the diminishment of stomatal development, leading to a reduction in SD (e.g., Gray et al., 2000; Engineer et al., 2014). (3) The coordination of other key environmental factors may together regulate changes in SD. For instance, a moderate water deficit may increase SD due to a potential acclimated response, whereas excessive watering or severe water deficit stress decreases SD by inhibiting GCs (Xu and Zhou, 2008; Xu et al., 2009b). This suggests that the former would encounter an SD decline due to rising CO2, and the latter would accelerate its reduction further (Woodward, 1987; Xu and Zhou, 2008; Xu et al., 2009b).
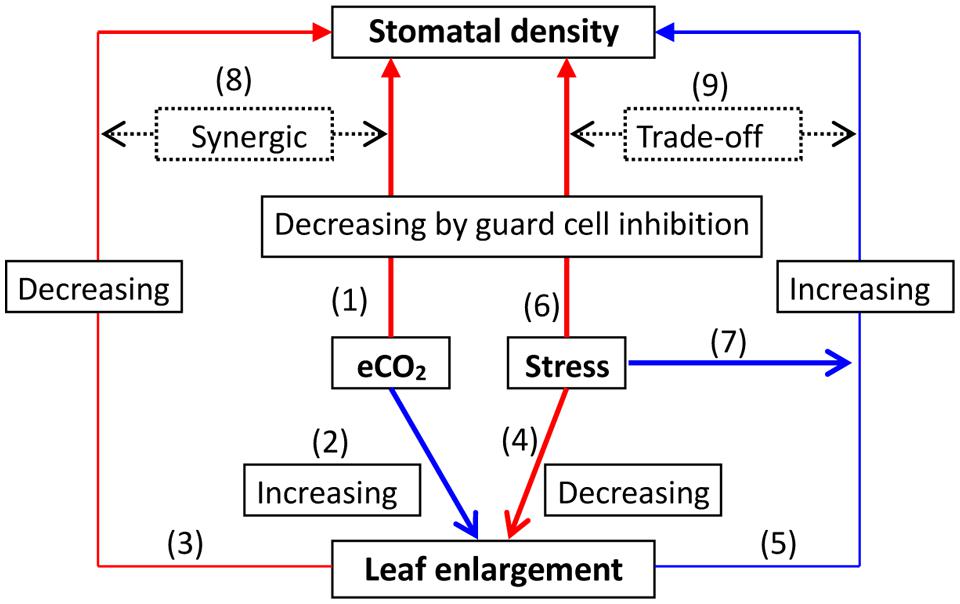
FIGURE 1. A representation of the response to elevated CO2 (eCO2) with abiotic factor stress on stomatal density (SD) under regulation by leaf growth. Elevated CO2 may lead to an acclimated reduction in SD by inhibition of guard cells, which involves the regulation of gene expression and adaptive evolution (1); meanwhile, eCO2 could promote leaf enlargement (2) consequently decreasing SD (3). A severe abiotic stress factor such as drought may diminish leaf enlargement (4), ultimately increasing SD (5); it may directly decrease SD due to inhibition of guard cell development (6). However, under moderate stress, SD may be increased possibly due to an acclimated response (7). In summary, this synergic increase (8) or trade-off interaction (9) may occur between the effects of both leaf growth and changes in SD toward the variations in eCO2 under abiotic stress, ultimately determining SD.
Similarly, a temperature higher than optimum may limit leaf enlargement, leading to increased SD, but moderate warming may do the opposite. In fact, both temperature and water status or their interaction might regulate stomatal development and distribution in response to CO2 enrichment. This would ultimately determine SD due to the trade-off, depending on environmental stresses or species-specific adaptation (Fraser et al., 2009; Xu et al., 2009b; Locosselli and Ceccantini, 2013; Figure 1), which still remains elusive to some extent. As reported by Pyakurel and Wang (2014), elevated CO2 can reduce the leaf area and increase the SD of birch plants, demonstrating high resistance to water deficit stress. Moreover, an interaction between elevated CO2 and light may also determine SD. For example, rice leaf SD was slightly decreased by elevated CO2 or by decreased light irradiance. However, the effect of light on SD may be diminished by elevated CO2 (Hubbart et al., 2013). Thus, multifaceted effects on SD responses to elevated CO2 need to be further clarified.
Molecular Mechanisms Controlling Guard Cell in Response to Elevated CO2
General Molecular Mechanism
Guard cell (GC) metabolism and the signal transduction network have been reviewed in several reports (e.g., Lawson et al., 2014; Negi et al., 2014). Here, we succinctly present the findings of these reports, particularly the explanations concerning the regulation of CO2 concentration (Figure 2). Generally, ion and organic solute concentration levels determine the turgor pressure of guard cells and subsequently affect stomatal aperture. Under elevated CO2, stomata tend to close because a greater depolarization seems to appear in GCs. The process may be controlled by (1) a decrease in K+ concentration, with enhanced activity in outward rectifying K+ channels and decreased inward activity, (2) decreased cytosolic Ca2+ in GCs, (3) decreased Cl- and malate (Mal2-) concentrations by stimulating the release of Cl- and Mal2- from GCs resulting from the activation of S-type anion channels, and (4) by decreases in the cytosolic zeaxanthin level and the pH value in GCs. Together, these factors lead to a decline in GC turgor, causing the GCs to shrink and the stomatal aperture to close (e.g., Webb et al., 1996; Zhu et al., 1998; Assmann, 1999; Schroeder et al., 2001; Fujita et al., 2013; Lawson et al., 2014). The potential messengers in the stomatal response to CO2 concentrations mainly include ion channel activity, cytosolic free calcium, ABA, malate levels, membrane potential, pH gradients, zeaxanthin content in chloroplasts, photosynthesis-derived ATP content, protein phosphorylation, and dephosphorylation processes (McAinsh et al., 1990; Schroeder et al., 2001; Ainsworth and Rogers, 2007; Kim et al., 2010; Wang et al., 2013; Lawson et al., 2014). For instance, the experiments have shown that elevated CO2 can enhance anion channel activity in GCs to induce stomatal closure. In this event, the SLAC1 protein provides or regulates a gate for anion transport (Raschke et al., 2003; Marten et al., 2008; Vahisalu et al., 2008; Negi et al., 2014; Yamamoto et al., 2016).
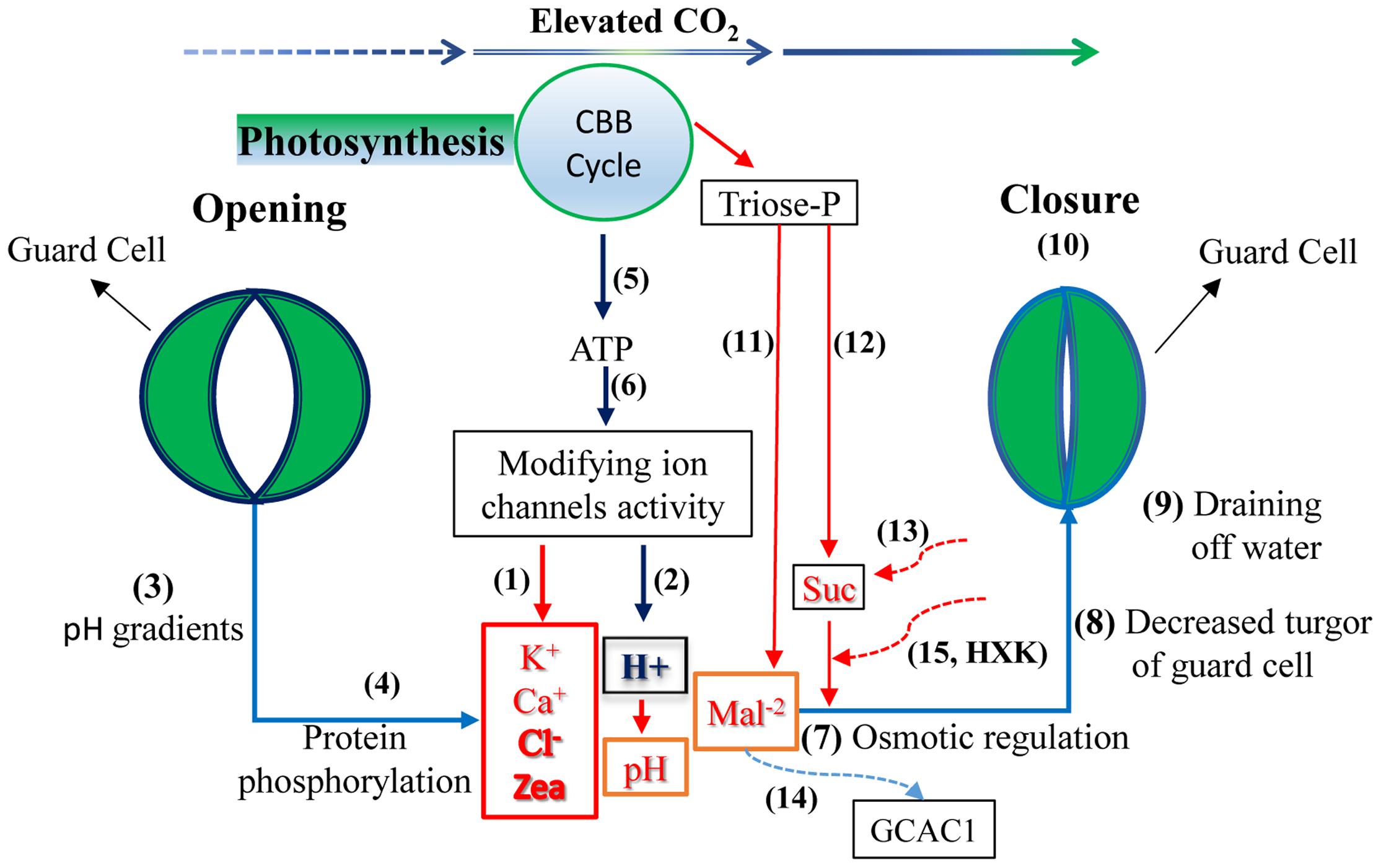
FIGURE 2. Possible stomatal response mechanisms controlling guard cells (GC) under elevated CO2. With rising CO2, a depolarization in GCs appears: the levels of K+, Ca2+, Cl-, and zeaxanthin (Zea) may decrease (1), whereas the H+ concentration may remain at a high level (2) leading to a lower pH value. The pH gradient (3), protein phosphorylation (4), and photosynthesis-derived ATP (5, 6) are involved in the regulation process by modifying channel activities; together, they promote osmotic regulation (7) and decrease GC turgor (8) consequently causing the GCs to drain water (9) leading to stomatal closure to some extent (10). Meanwhile, Calvin–Benson–Basshan (CBB) cycle and sugar metabolism in GC may produce less malate (Mal2-), (11) and sucrose (Suc) (12) with triose phosphate (triose-P) at eCO2, which also affects osmotic regulation. Furthermore, elevated CO2 may reduce Suc accumulation in the vicinity of the GC wall from the mesophyll due to the limitation of some apoplastic Suc in the transpiration stream toward GC (13) and enhance Mal2- transport from GCs into mesophyll cells by stimulating anion efflux through channels such as GCAG1 and the potential involvement of the AtABCB14 gene (14), also resulting in stomatal closure. Finally, hexokinase (HXK) involvement may limit sugar synthesis and its entrance into GCs from mesophyll cells (15) and then inducing stomatal closure (e.g., Webb et al., 1996; Assmann, 1999; Schroeder et al., 2001; Kang et al., 2007; Lee et al., 2008; Kim et al., 2010; Fujita et al., 2013; Kelly et al., 2013; Lawson et al., 2014; Negi et al., 2014).
Role of Sugar
In the guard cell itself, major reports have provided evidence that GCs may play only a trivial role in the regulation of the stomatal aperture, including osmotic adjustments. As such, GSs have a limited photosynthetic capacity, fewer chloroplasts, low electron transport, and relatively lower levels of relevant metabolites, such as those related to ATP and NADPH, sucrose (Suc), and malate (Outlaw, 1989; Reckmann et al., 1990; Lawson et al., 2003; Lawson et al., 2014). Some studies support that the apoplastic Suc, where occurs in some cell walls of GCs from mesophyll cells as an osmoticum, may be responsible for the stomatal opening (Lawson et al., 2003; Kang et al., 2007). An early starch-sugar hypothesis suggested sugars, such as Suc, which is the end product of photosynthesis, may be derived from starch degradation in mesophyll cells and may play an important role in linking mesophyll photosynthesis to GC function (Pallas, 1964; Tallman and Zeiger, 1988; Ni, 2012). However, this hypothesis is still not well tested. With the ambiguous role of sugars in stomatal regulation, the role of Suc as a major osmoticum driving stomatal movement has been debated. However, Suc may still play a critical role in interrelating mesophyll and stomatal behavior, possibly via apoplasts. Thus, Suc role is implicated in a feedback-inhibition mechanism with an expression of hexokinase (HXK) in GCs when the Suc production rate exceeds the efflux rate at which Suc is loaded into the phloem (Kelly et al., 2013) under elevated CO2 conditions (Cheng et al., 1998; Long et al., 2004; Ainsworth and Rogers, 2007). A HXK-induced expression of ABA-related genes leads to a decrease in the influx of apoplastic sugar entering the GCs from the mesophyll, which may coordinate photosynthesis with transpiration by coupling with a stomatal closure (Kang et al., 2007; Kelly et al., 2013). It highlights the pivotal role of HXK. Moreover, a limitation to a carbon sink or transportation of sugar from shoot to root via the phloem leads to the accumulation of sugar in shoots and/or leaves and results in stomatal closure. This strengthens the hypothesis of sugar-driven stomatal movement (Domec and Pruyn, 2008; Silber et al., 2013).
Gene Involvement
The negative regulation of elevated CO2-induced stomatal closure may be closely linked to an impaired Ca2+ priming sensor, a HT1 protein kinase, and an RHC1 MATE-type transporter in Arabidopsis plants (Hashimoto et al., 2006; Young et al., 2006; Negi et al., 2014; Tian et al., 2015) (Table 1). However, the underlying mechanism concerning the precise signal transduction molecular pathways that regulate the stomatal closure upstream still remains elusive. This needs to be explored further, particularly for different genetic types, species, and even PFTs. A repression of the ABC transporter AtABCB14 may play a considerable role in stomatal closure in response to elevated CO2 levels (Lee et al., 2003, 2008; Laanemets et al., 2013). This SLAC1 may also be involved in stomatal closure induced by elevated CO2 levels (Negi et al., 2008; Laanemets et al., 2013). A recent report indicated that SLAC1 perception of CO2 signals may be located in a transmembrane region by an ABA-independent pathway (Yamamoto et al., 2016). Phosphorylation of KAT1 on the C-terminal region, which is expressed primarily in GCs in A. thaliana plants, might modulate the activity of K+ channels involved in the signal transduction cascade (Sato et al., 2009), which might be negatively regulated by nitric oxide (NO)—an active signaling molecule in plants (Gayatri et al., 2013). ABA may trigger its generation (Neill et al., 2008; Shi et al., 2014; Xia et al., 2015) through the modulation of vitamin B6 homeostasis (Xia et al., 2014). Furthermore, because blue light photoreception may also be involved in light–CO2 interactions in GCs, the changes in zeaxanthin levels may correspond to changes in the CO2 level, which are linked to the pH sensitivity of the relevant enzymes (Zeiger and Zhu, 1998; Zhu et al., 1998). A recent report indicated that NADPH oxidases and respiratory burst oxidase homologs (RBOHs) were closely associated with the network of reactive oxygen species (ROS) production, which may regulate the stomatal aperture (Baxter et al., 2014).
Mesophyll-Derived Signal (MDS)
Malate generated in GCs, through the metabolite of triose phosphate (triose-P) from the Calvin–Benson–Basshan (CBB) cycle, may directly involve stomatal aperture regulation as an osmoticum and as a sink for the end products of GC electron transport involving phosphoenolpyruvate carboxylase (PEPC; Cousins et al., 2007; Lawson et al., 2014). A component of malate may also originate from mesophyll cells because when the tricarboxylic acid (TCA) cycle function has been limited, e.g., by the inhibition of fumarase (Nunes-Nesi et al., 2007), there is a decline in GC malate, as it is one of the metabolites derived from the TCA cycle in mesophyll cells (Fernie and Martinoia, 2009; Araújo et al., 2011). It might confirm that malate could be the mesophyll-derived signal (MDS) linking stomatal behavior. A negative correlation between the fumarate level in mesophyll and gs indicated that fumarate, as an MDS, may also be involved in stomatal closure, although its influence seems to be less than that of malate (Araújo et al., 2011; Medeiros et al., 2015). Moreover, high CO2 concentration-induced stomatal closure may be attributable to an increase in the concentrations of malate produced in the mesophyll stimulating anion efflux through, for example, the R-type channel (ALMT). This is a malate-sensitive anion channel operating as a CO2 sensor in GCs and is linked to mesophyll photosynthesis (Hedrich and Marten, 1993; Sasaki et al., 2010; De Angeli et al., 2013; Lawson et al., 2014; Medeiros et al., 2015).
However, whether mesophyll and/or guard cell photosynthesis is involved in the GC response to CO2 concentrations remains controversial (von Caemmerer et al., 2004; Messinger et al., 2006; Lawson et al., 2014). Early reports show that a specific blue light response involving H+-ATPase activation is independent of A, whereas the red light response may be associated with A, which might be induced by the intercellular CO2 concentration (Ci) reduction resulting from the mesophyll consumption of CO2 (Roelfsema et al., 2002; Messinger et al., 2006). A recent study showed that Arabidopsis plants with an overexpression of plasma membrane H+-ATPase under the control of a guard cell-specific promoter may facilitate the coordinative capacity between stomatal opening, A, and growth rate (Wang et al., 2014). Thus, the role of photosynthesis in regulating GC movement in response to elevated CO2 remains elusive (Roelfsema et al., 2002; Ainsworth and Rogers, 2007; Engineer et al., 2014). The relative role of photosynthesis in guard cells and the nearby related cells, such as mesophyll cells, in response to elevated CO2 may require further testing.
There is no clear evidence for or against the existence of MDS and the involved signals. Some potential signals, such as chloroplastic ATP, zeaxanthin, NADPH, RuBP, and stomatin, have been suggested (cf. Lawson et al., 2014). Support for the role of MDS has been found in some excellent experiments, such as those by epidermal peels vs. the intact leaves methods. These experiments yield strong evidence that MDS might occur (e.g., Roelfsema et al., 2002; Mott et al., 2008; Fujita et al., 2013). Some reports indicate the MDS may exist in modern seed plants rather than in ferns and lycophytes (e.g., McAdam and Brodribb, 2012). Additionally, MDS may need certain transduction medium conditions, such as a vapor phase (Sibbernsen and Mott, 2010) or aqueous phase (Fujita et al., 2013). With increasing evidence that Ci may play only a trivial role (von Caemmerer et al., 2004; Hanson et al., 2013), the biological activities closely related to MDS often refer to electron transport, the redox state, metabolites in the transpiration stream, vapor phase ion, and electrical signals (Lawson et al., 2014). A report indicated that stomatal opening linked to apoplast transfer from mesophyll signals is dependent on photosynthesis at lower levels of CO2 (Fujita et al., 2013). Moreover, the stomatal closure is relatively independent of photosynthesis at elevated CO2, i.e., without ATP involvement in mesophyll photosynthesis (Roelfsema et al., 2002; Fujita et al., 2013). The S-type anion channels activated at elevated CO2 may contribute to stomatal closure (Roelfsema et al., 2002; Fujita et al., 2013). A study using chlorophyll fluorescence imaging showed spatiotemporal decoupling of stomata and mesophyll in response to the cutting of leaf veins, which weakens further support for the appearance of MDS (Hanson et al., 2013).
Integrated Signaling Processes
The changes in stomatal development and its aperture induced by elevated CO2 and involving mesophyll conductance (gm; Mizokami et al., 2015; Youshi and Santrucek, 2015) might be mediated by ABA levels (Giday et al., 2014; Youshi and Santrucek, 2015). In a recent study, genetic analysis using mutants in the ABA signaling pathway on GC-specific transcriptional memory for the related genes indicated that SnRK2.6 is more important for overall stomatal control. The SnRK2.2 and SnRK2.3 are more important for implementing GC stress memory in the subsequent dehydration response (Virlouvet and Fromm, 2015). However, the involvement of SnRK2.2 and SnRK2.3 in elevated CO2 regulation on the stomatal response and feedback remains largely unclear. The long-distance signaling cascades (Lake et al., 2002), e.g., from mature leaves to immature leaves, may also contribute to the GC behavior response to CO2 levels. ABA, ethylene, salicylic acid (SA), jasmonic acid (JA), NO, some peptides, and sugar levels might be involved in the integrated signaling processes’ response to environmental changes (e.g., Neill et al., 2008; Poór et al., 2011; Silber et al., 2013; Xia et al., 2014, 2015; Grienenberger and Fletcher, 2015; Medeiros et al., 2015).
Interactions with Other Factors
Elevated CO2 with Drought
Soil water deficit and high VPD often reduce the stomatal opening, depending on the species (Warren, 2008; Perez-Martin et al., 2009; Peak and Mott, 2011). Generally, water status has a stronger impact on gs than changes in CO2 concentration. A relatively small effect of elevated CO2 on gs generally appears as water deficit stress occurs, possibly because the drought-induced reduction dramatically outweighs the reduction caused by elevated CO2 (Morgan et al., 2004; Leakey et al., 2006b). Flexas et al. (2004) indicated that decreases in gs and gm, but not biochemical activities, may limit the photosynthetic capacity in drought-stressed leaves, depending on the species (Bota et al., 2004; Flexas et al., 2014). Even for drought-severely stressed plants, the biochemical limitation can be negligible (Galmés et al., 2007). A non-stomatal limitation appears only when gs is below 250 mmol m-2s-1 in grass plants grown in drought conditions (Xu et al., 2009a). In tall fescue (Festuca arundinacea) plants exposed to elevated CO2, an increased A with a low gs but high Rubisco activity during both drought and rewatering may also indicate the alleviation of metabolic limitations caused by drought damages rather than stomatal limitations imposed by elevated CO2 (Chen et al., 2015). CO2 enrichment may relieve non-stomatal limitations by protecting the photosynthetic apparatus during severe drought (Xu et al., 2014). However, a recent report showed that Ramonda nathaliae plants with smaller stomata have higher resistance to drought than R. serbica, which have larger stomata (Rakić et al., 2015). This highlights the role of the stomatal size.
Elevated CO2 may improve plant water status by reducing gs and thereby raising WUE, ameliorating the adverse effects of stressful factors on plant growth and physiological processes (Ainsworth and Rogers, 2007; Xu et al., 2013, 2014). A decrease in soil water availability under elevated CO2 may be closely linked to an increase in leaf area, which offsets a decline in gs and promotes plant growth (Manea and Leishman, 2015). Studies have clearly shown that water status mediates rising CO2 effectiveness through the coupling of processes between gas exchange and leaf enlargement. Nevertheless, the pros and cons of acclimation to changes in water conditions may coexist in response to elevated CO2. Leaf area enlargement, i.e., canopy enhancement induced by CO2, may exaggerate water use, whereas decreased gs would promote WUE (e.g., Woodward, 1990; Ward et al., 2013; Manea and Leishman, 2015), depending on canopy density and its homogeneity (Bernacchi and VanLoocke, 2015). However, an intrinsic WUE decline might appear during severe drought in some relict species plants exposed to elevated CO2 (Linares et al., 2009). Thus, future research is necessary to focus on the linkage among leaf area, gs, and both WUEi and total plant biomass water use efficiency (WUEt) under climatic change. Furthermore, some results indicated that although WUEt and WUEi showed a similar response to elevated CO2, the former seemed to have a higher level of sensitivity, implying that WUEt may be a better indicator than WUEi of the response to climate change (Duan et al., 2014). WUE and the root: shoot biomass ratio increased significantly with decreased precipitation but decreased with elevated CO2 levels (Li et al., 2014). Thus, besides the regulation of leaf growth, root development may also involve stomatal movement behavior and WUE changes under climatic change. The possible primary stomatal closure induced by elevated CO2 may be offset by positive indirect effects on gs, possibly caused by root system promotion and hydraulic capacity under rising CO2 conditions (Uddling et al., 2009). Forest canopy evapotranspiration can be reduced under high CO2 concentration levels (Medlyn et al., 2001), possibly due to leaf gs slowdown. Thus, water loss is diminished. However, a lower response to elevated CO2 in the canopy evapotranspiration rate relative to leaf gs was found in a rice field (Shimono et al., 2013). Nevertheless, the canopy carbon fixation and its association with gs at the leaf and canopy scales during climatic change remains to be tested. A succinct description on the trade-off between gs, leaf enlargement, and WUE under elevated CO2 and drought conditions is summarized in Figure 3.
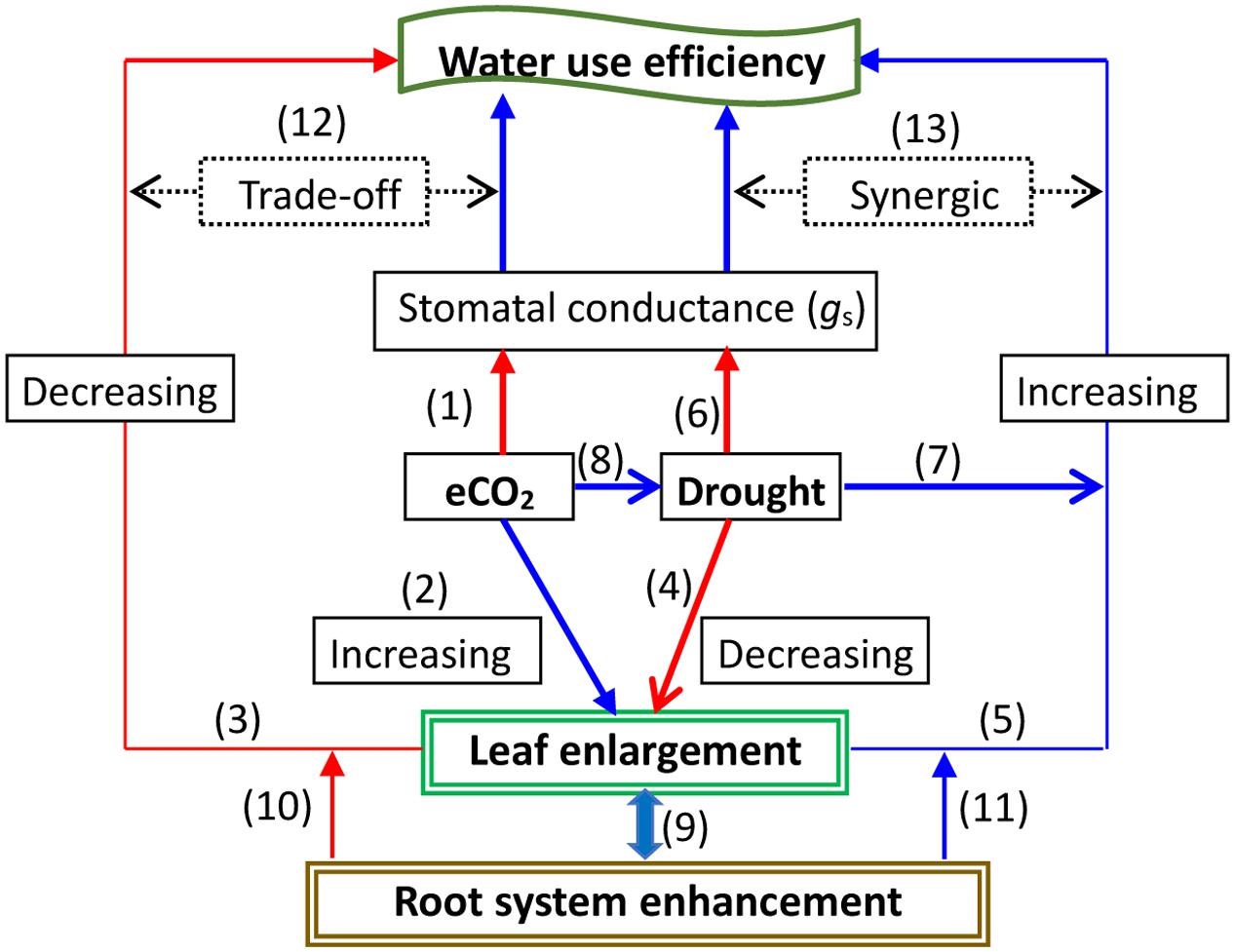
FIGURE 3. A representation of the response to elevated CO2 (eCO2) with drought on water use efficiency (WUE) under regulations by balancing stomatal conductance (gs) and leaf growth. Elevated CO2 may lead to an acclimated reduction in gs, which involves signaling sensing and transduction, biophysical and biochemical processes, and gene expression (1); meanwhile, eCO2 could promote leaf enlargement (2), possibly increasing transpiration (E) of the total leaf subsequently reducing WUE (3). A severe drought stress may shrink leaf growth (4), consequently decreasing E and finally increasing WUE (5); gs can directly be reduced by drought (6). However, a moderate drought may directly enhance WUE by some adaptive responses such as a relative increase in the root systems (7), which can be further improved by elevated CO2 (8). Root systems may be enhanced by eCO2, particularly under drought through alterations in carbon allocation between above- and belowground parts (9), which may lead to either decreased WUE at eCO2 (10), or increased WUE under drought conditions (11). Consequently, this trade-off interaction (12) or synergic increase (13) may occur with leaf growth and gs changes at eCO2 under drought, ultimately affecting WUE.
Moreover, most studies have confirmed that elevated CO2 may improve the water status of drought-stressed plants by reducing gs (e.g., Brodribb et al., 2009; Katul et al., 2010; Chen et al., 2015; Easlon et al., 2015), but these findings were species-dependent (Beerling et al., 1996; Bernacchi et al., 2007; Liu et al., 2016). However, this case may not occur under severe or extreme drought conditions, possibly due to the depression of stomatal regulatory ability (Xu and Zhou, 2008). Furthermore, plant size and root distribution may override the expected direct physiological effects of elevated CO2 (Duursma et al., 2011; Liu et al., 2016).
Elevated CO2 with Salinity
Generally, stomata may exert a similar response to salt stress relative to drought (Clough and Sim, 1989; Wang et al., 2003; Flexas et al., 2004; Chaves et al., 2009). Stomatal conductance often decreases remarkably with increased salinity and/or aridity, such as leaf to air VPD, depending on the species and its habits (e.g., Clough and Sim, 1989; Chaves et al., 2009; Ashraf and Harris, 2013; Nguyen et al., 2015; Sanoubar et al., 2016). Enhanced salt stress and elevated CO2 concentrations are projected to co-occur in the future (Chaves et al., 2009; Pérez-López et al., 2009; Hoque et al., 2016). Generally, stomatal conductance was decreased by severe salt stress and elevated CO2 alone or in combination (Pérez-López et al., 2012; Nguyen et al., 2015; Stavridou et al., 2016). For example, as barley (Hordeum vulgare) plants are grown in high salinity soil, the rate of CO2 diffusion to the carboxylating site and photochemical electron sink capacity increased under elevated CO2 conditions, despite stomatal and internal conductance being decreased (Pérez-López et al., 2012). Similar to the severe desiccation effect, high salinity stress may lead to oxidative damage in plant tissue (Shalata et al., 2001; Sanoubar et al., 2016). However, elevated CO2 may alleviate the oxidative stress-induced by salinity with lower ROS level and a higher A, thus improving plant growth under high salinity conditions (Nicolas et al., 1993; Pérez-López et al., 2009). Studies have indicated that the rising-CO2 protection from salt-inhibited plants alleviates the metabolic limitations rather than the stomatal limitations. Moreover, although there was a gs decrease of 1–2 factors by high soil salinity in wetland grass Phragmites australis plants, the salinity effect hardly occurred with the combination of elevated CO2 and temperature (plus 310 μmolmol-1 CO2, and plus 5°C relative to ambient variables; Eller et al., 2014). The non-species expansion into saline areas may be promoted because the salinity-caused non-stomatal limitations (i.e., carboxylation rates of Rubisco or electron transport rates) may be mitigated under the elevated climatic conditions (Eller et al., 2014). However, the alleviated effect of elevated CO2 on severe salt stress strongly depends on species and cultivars/ecotypes (Eller et al., 2014; Geissler et al., 2015). Nevertheless, the responses of stomatal characteristics to the combination of elevated CO2 on salt stress are scarcely reported and need to be explored further.
Elevated CO2 with High Temperatures
The combined effects of elevated CO2 and high temperatures have also been reported in some studies. While there are exceptional cases (e.g., Bernacchi et al., 2007), elevated CO2 decreases gs, thus increasing leaf temperature because lower transpiration releases less heat (Kim et al., 2006; Negi et al., 2014; Šigut et al., 2015). As a consequence, elevated CO2 with high temperatures may play an antagonistic role by exaggerating heat damage partly due to decreased gs (Warren et al., 2011). However, an elevated CO2-induced 13–30% decline in gs induced a 2°C increase in leaf temperature, leading to a 2.9–6.0°C increase in the temperature optima for the light-saturated rate of CO2 assimilation (Amax). Thus, this would enhance heat stress tolerance in beech and spruce saplings (Šigut et al., 2015). The increased adaptation to heat stress may be due to reduced photorespiration and the limitation of photosynthesis by RuBP regeneration under elevated CO2 (Šigut et al., 2015). A recent report also confirmed the heat-tolerance enhancement due to elevated CO2 for coffee crops (Rodrigues et al., 2016). Thus, the negative effect of elevated CO2 on heat stress due to reduced gs was not confirmed. In contrast, a beneficial adaptation may occur. Yet, this may depend on the species and the range of temperature variation.
Elevated CO2 with Nutrition Status and Air Pollution
Based on a recent report (Easlon et al., 2015), better plant growth and photosynthesis in the low gs in A. thaliana lines under N-limitation, rather than sufficient N supply under elevated CO2, may imply an adaptive coupling between lowered gs and improved N utilization. Increased conservative N investment in photosynthetic biochemistry in order to acclimate to CO2 fertilization highlights a positively synergistic relationship between stomatal regulation and nutrition status. However, a lower gs in elevated CO2 concentrations but a higher gs with an abundant N supply have been found in Liquidambar styraciflua plants (Ward et al., 2013), suggesting that these factors may play opposite roles in the gs response. A recent study has indicated that improved phosphorus (P) nutrition can enhance drought tolerance in the field pea due to the CO2-induced decrease in gs and the promotion of root systems (Jin et al., 2015). A general decline in gs by elevated CO2 and ozone (O3) alone or their combination has been extensively reported, suggesting that rising CO2 may alleviate the injury caused by high O3 pollution decreasing gs (Kellomäki and Wang, 1997; Mansfield, 1998; Warren et al., 2006; Hoshika et al., 2015). However, some species, such as aspen (Populus tremuloides Michx.) and birch (Betula papyrifera Marsh.), have a high gs under both high CO2 and high O3 concentrations (Uddling et al., 2009). This indicates that the interactive effects between elevated CO2 and O3 on stomatal behavior may depend on species, plant/leaf ages, and treatment regimens, such as time and sites (Uddling et al., 2009; Hoshika et al., 2015; Matyssek et al., 2015). Thus, it again highlights the complex/specific response.
Elevated CO2 with Biotic Factors
The stomatal response to elevated CO2 with biotic factors has received much attention (e.g., Casteel et al., 2012; Zavala et al., 2013). For instance, a greater gs reduction in cabbage with decreased aphid (one of the most destructive insect pests in crops) colonization rates and total plant volatile emissions, such as terpene emissions, occurred when plants were exposed to elevated CO2 over the long-term (6–10 weeks) rather than the short-term (2 weeks; Klaiber et al., 2013). This indicates that, as hosts, plants may acclimatize to future increases in elevated CO2 by modifying stomatal behavior. Under elevated CO2, a decrease in micronutrients, such as calcium, magnesium, or phosphorus, due to the gs reduction may lead to poor aphid performance (Myzus persicae; Dáder et al., 2016). Furthermore, a recent report (Sun et al., 2015) showed that aphid infestation may synergistically promote the effects of elevated CO2 on stomatal closure, possibly by triggering the ABA signaling pathway. Therefore, the water status of the host plants of Medicago truncatula was improved, ultimately enhancing feeding efficiency and abundance of aphid (Zavala et al., 2013; Sun et al., 2015). Taken together, plant–insect interactions might be modified by stomatal closure under high levels of CO2. The metabolism and emission of plant biogenic volatile organic compounds may also be involved (Klaiber et al., 2013; Zavala et al., 2013). It is suggested that an enhanced accumulation of JA and SA may also be involved in signal transduction in relation to stomatal movement as plants are subjected to CO2 enrichment and herbivore attack. This highlights an important role in stomatal regulation to cope with a combination of climate change and biotic factors (Poór et al., 2011; Casteel et al., 2012; Zavala et al., 2013; Sun et al., 2015). Thus, the herbivore’s adaptive capacity to its host might be promoted when exposed to elevated CO2, at least partly through stomatal regulation.
Conclusion and Perspectives
Under high CO2 conditions, both stomatal conductance and its density generally decreased with a few exceptions. The decline in SD may be the result of a long-term genetic variation or short-term structural plasticity under elevated CO2. Elevated CO2 may induce the excessive depolarization of guard cells to cause stomatal closure when mesophyll-driven signals, such as malate, ATP, zeaxanthin, and NADPH, may be involved in stomatal movement. Their photosynthesis in both guard cells and mesophyll cells and their link to the stomatal response in elevated CO2 conditions may play an important role. However, challenges remain in elucidating the underlying mechanism. The differences and linkage in stomatal responses to elevated CO2 levels across the molecular, cellular, biochemical, eco-physiological, canopy, and vegetation levels (Zhu et al., 2012; Peñuelas et al., 2013; Shimono et al., 2013; Armstrong et al., 2016) should raise concerns about ecological and climatic management.
Several crucial aspects of research into the stomatal response may need to be strengthened in the future. (1) The underlying mechanism of responses to CO2 enrichment for key biological processes, including stomatal behavior; the critical metabolic bioprocesses, such as hormone-involved regulation; and relevant biochemical signal cascades must be further elucidated. (2) The diverse responses from different species and PFTs to elevated CO2 or its combination with other abiotic and biotic factors must be compared and clarified. (3) Various spatial–temporal scales from the molecular, biochemical, physiological, individual, and canopy to vegetation levels must be integrated. Instantaneous to annual or longer time-scales (e.g., Zhu et al., 2012; Shimono et al., 2013; Armstrong et al., 2016) must also be integrated. We should elucidate the underlying mechanism of the stomatal responses associated with key biological processes across the multiple scales under different climatic factors, including elevated CO2, warming, drought, and air pollution. (4) We need to investigate whether improving stomatal response to elevated CO2 by manipulating guard cell performance may yield a better balance between CO2 uptake and water loss through transpiration to enhance photosynthetic capacity with high WUE (e.g., Engineer et al., 2014; Lawson and Blatt, 2014; Grienenberger and Fletcher, 2015). Enhanced expression of some related genes, such as patrol1, may drastically increase both gs and plant growth under higher CO2 levels (Hashimoto-Sugimoto et al., 2013). This task needs to be implemented urgently. Finally, understanding how to improve or combine earth system models (ESMs), general circulation models (GCMs), and land surface models (LSMs) may help to correctly interpret the gs response to climate change (Sato et al., 2015). The integration issue should be solved urgently to precisely assess the response and feedback of terrestrial ecosystem to global change.
Author Contributions
YJ and BJ are co-first authors, ZX and GZ designed the study, ZX, YJ, and BJ collected and analyzed the data, all authors wrote and reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The study was funded by the National Natural Science Foundation of China (41330531, 31170456), and China Special Fund for Meteorological Research in the Public Interest (GYHY201506019).
References
Ainsworth, E. A., and Rogers, A. (2007). The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ. 30, 258–270. doi: 10.1111/j.1365-3040.2007.01641.x
Araújo, W. L., Nunes-Nesi, A., Osorio, S., Usadel, B., Fuentes, D., Nagy, R., et al. (2011). Antisense inhibition of the iron-sulphur subunit of succinate dehydrogenase enhances photosynthesis and growth in tomato via an organic acid–mediated effect on stomatal aperture. Plant Cell 23, 600–627. doi: 10.1105/tpc.110.081224
Armstrong, E., Valdes, P., House, J., and Singarayer, J. (2016). The role of CO2 and dynamic vegetation on the impact of temperate land use change in the HadCM3 coupled climate model. Earth Interact. 20. doi: 10.1175/EI-D-15-0036.1
Ashraf, M., and Harris, P. J. C. (2013). Photosynthesis under stressful environments: an overview. Photosynthetica 51, 163–190. doi: 10.1007/s11099-013-0021-6
Assmann, S. M. (1999). The cellular basis of guard cell sensing of rising CO2. Plant Cell Environ. 22, 629–637. doi: 10.1046/j.1365-3040.1999.00408.x
Bagley, J., Rosenthal, D. M., Ruiz-Vera, U. M., Siebers, M. H., Kumar, P., Ort, D. R., et al. (2015). The influence of photosynthetic acclimation to rising CO2 and warmer temperatures on leaf and canopy photosynthesis models. Glob. Biogeochem. Cycles 29, 194–206. doi: 10.1002/2014GB004848
Baldocchi, D. D., and Harley, P. C. (1995). Scaling carbon dioxide and water vapour exchange from leaf to canopy in a deciduous forest. II. Model testing and application. Plant Cell Environ. 18, 1157–1173. doi: 10.1111/j.1365-3040.1995.tb00626.x
Ball, J. T., Woodrow, I. E., and Berry, J. A. (1987). “A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions,” in Progress in Photosynthesis Research, ed. J. Biggens (Dordrecht: Martinus-Nijhoff Publishers), 221–224.
Baxter, A., Mittler, R., and Suzuki, N. (2014). ROS as key players in plant stress signalling. J. Exp. Bot. 65, 1229–1240. doi: 10.1093/jxb/ert375
Beerling, D. J., Heath, J., Woodward, F. I., and Mansfield, T. A. (1996). Drought-CO2 interactions in trees observations and mechanisms. New Phytol. 134, 235–242. doi: 10.1111/j.1469-8137.1996.tb04628.x
Bernacchi, C. J., Kimball, B. A., Quarles, D. R., Long, S. P., and Ort, D. R. (2007). Decreases in stomatal conductance of soybean under open-air elevation of [CO2] are closely coupled with decreases in ecosystem evapotranspiration. Plant Physiol. 143, 134–144. doi: 10.1104/pp.106.089557
Bernacchi, C. J., and VanLoocke, A. (2015). Terrestrial ecosystems in a changing environment: a dominant role for water. Annu. Rev. Plant Biol. 66, 599–622. doi: 10.1146/annurev-arplant-043014-114834
Bota, J., Medrano, H., and Flexas, J. (2004). Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 162, 671–681. doi: 10.1111/j.1469-8137.2004.01056.x
Brodribb, T. J., McAdam, S. A. M., Jordan, G. J., and Feild, T. S. (2009). Evolution of stomata responsiveness to CO2 and optimization of water-use efficiency among land plants. New Phytol. 183, 839–847. doi: 10.1111/j.1469-8137.2009.02844.x
Casteel, C. L., Segal, L. M., Niziolek, O. K., Berenbaum, M. R., and DeLucia, E. H. (2012). Elevated Carbon dioxide increases salicylic acid in Glycine max. Environ. Entomol. 41, 1435–1442. doi: 10.1603/EN12196
Chaves, M. M., Flexas, J., and Pinheiro, C. (2009). Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 103, 551–560. doi: 10.1093/aob/mcn125
Chen, Y., Yu, J., and Huang, B. (2015). Effects of elevated CO2 concentration on water relations and photosynthetic responses to drought stress and recovery during rewatering in tall fescue. J. Am. Soc. Hortic. Sci. 140, 19–26.
Cheng, S. H., Moore, B., and Seemann, J. R. (1998). Effects of short-and long-term elevated CO2 on the expression of ribulose-1, 5-bisphosphate carboxylase/oxygenase genes and carbohydrate accumulation in leaves of Arabidopsis thaliana (L.) Heynh. Plant Physiol. 116, 715–723. doi: 10.1104/pp.116.2.715
Clough, B. F., and Sim, R. G. (1989). Changes in gas exchange characteristics and water use efficiency of mangroves in response to salinity and vapour pressure deficit. Oecologia 79, 38–44. doi: 10.1007/BF00378237
Cousins, A. B., Baroli, I., Badger, M. R., Ivakov, A., Lea, P. J., Leegood, R. C., et al. (2007). The role of phosphoenolpyruvate carboxylase during C4 photosynthetic isotope exchange and stomatal conductance. Plant Physiol. 145, 1006–1017. doi: 10.1104/pp.107.103390
Dáder, B., Fereres, A., Moreno, A., and Trkębicki, P. (2016). Elevated CO2 impacts bell pepper growth with consequences to Myzus persicae life history, feeding behaviour and virus transmission ability. Sci. Rep. 6, 19120. doi: 10.1038/srep19120
DaMatta, F. M., Godoy, A. G., Menezes-Silva, P. E., Martins, S. C., Sanglard, L. M., Morais, L. E., et al. (2016). Sustained enhancement of photosynthesis in coffee trees grown under free-air CO2 enrichment conditions: disentangling the contributions of stomatal, mesophyll, and biochemical limitations. J. Exp. Bot. 67, 341–352. doi: 10.1093/jxb/erv463
De Angeli, A., Zhang, J., Meyer, S., and Martinoia, E. (2013). AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat. Commun. 4, 1804. doi: 10.1038/ncomms2815
Doheny-Adams, T., Hunt, L., Franks, P. J., Beerling, D. J., and Gray, J. E. (2012). Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philos. Trans. R. Soc. B Biol. Sci. 367, 547–555. doi: 10.1098/rstb.2011.0272
Domec, J. C., and Pruyn, M. L. (2008). Bole girdling affects metabolic properties and root, trunk and branch hydraulics of young ponderosa pine trees. Tree Physiol. 28, 1493–1504. doi: 10.1093/treephys/28.10.1493
Duan, H. L., Duursma, R. A., Huang, G. M., Smith, R. A., Choat, B., O’Grady, A. P., et al. (2014). Elevated [CO2] does not ameliorate the negative effects of elevated temperature on drought-induced mortality in Eucalyptus radiata seedlings. Plant Cell Environ. 37, 1598–1613. doi: 10.1111/pce.12260
Duursma, R. A., Barton, C. V., Eamus, D., Medlyn, B. E., Ellsworth, D. S., Forster, M. A., et al. (2011). Rooting depth explains [CO2] × drought interaction in Eucalyptus saligna. Tree Physiol. 31, 922–931. doi: 10.1093/treephys/tpr030
Easlon, H. M., Carlisle, E., McKay, J., and Bloom, A. (2015). Does low stomatal conductance or photosynthetic capacity enhance growth at elevated CO2 in Arabidopsis thaliana? Plant Physiol. 167, 793–799. doi: 10.1104/pp.114.245241
Eller, F., Lambertini, C., Nguyen, L. X., and Brix, H. (2014). Increased invasive potential of non-native Phragmites australis: elevated CO2 and temperature alleviate salinity effects on photosynthesis and growth. Glob. Change Biol. 20, 531–543. doi: 10.1111/gcb.12346
Ellsworth, D. S., Thomas, R., Crous, K. Y., Palmroth, S., Ward, E., Maier, C., et al. (2011). Elevated CO2 affects photosynthetic responses in canopy pine and subcanopy deciduous trees over 10 years: a synthesis from Duke FACE. Glob. Change Biol. 18, 223–242. doi: 10.1111/j.1365-2486.2011.02505.x
Engineer, C. B., Ghassemian, M., Anderson, J. C., Peck, S. C., Hu, H., and Schroeder, J. I. (2014). Carbonic anhydrases, EPF2 and a novel protease mediate CO2 control of stomatal development. Nature 513, 246–250. doi: 10.1038/nature13452
Fernie, A. R., and Martinoia, E. (2009). Malate. Jack of all trades or master of a few? Phytochemistry 70, 828–832. doi: 10.1016/j.phytochem.2009.04.023
Field, K. J., Duckett, J. G., Cameron, D. D., and Pressel, S. (2015). Stomatal density and aperture in non-vascular land plants are non-responsive to above-ambient atmospheric CO2 concentrations. Ann. Bot. 115, 915–922. doi: 10.1093/aob/mcv021
Flexas, J., Bota, J., Loreto, F., Cornic, G., and Sharkey, T. D. (2004). Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 6, 269–279. doi: 10.1055/s-2004-820867
Flexas, J., Diaz-Espejo, A., Gago, J., Gallé, A., Galmés, J., Gulías, J., et al. (2014). Photosynthetic limitations in Mediterranean plants: a review. Environ. Exp. Bot. 103, 12–23. doi: 10.1016/j.envexpbot.2013.09.002
Fraser, L. H., Greenall, A., Carlyle, C., Turkington, R., and Friedman, C. R. (2009). Adaptive phenotypic plasticity of Pseudoroegneria spicata: response of stomatal density, leaf area, and biomass to changes in water supply and increased temperature. Ann. Bot. 103, 769–775. doi: 10.1093/aob/mcn252
Fujita, T., Noguchi, K., and Terashima, I. (2013). Apoplastic mesophyll signals induce rapid stomatal responses to CO2 in Commelina communis. New Phytol. 199, 395–406. doi: 10.1111/nph.12261
Galmés, J., Medrano, H., and Flexas, J. (2007). Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 175, 81–93. doi: 10.1111/j.1469-8137.2007.02087.x
Gao, J., Han, X., Seneweera, S., Li, P., Zong, Y. Z., Dong, Q., et al. (2015). Leaf photosynthesis and yield components of mung bean under fully open-air elevated [CO2]. J. Integr. Agric. 14, 977–983. doi: 10.1016/S2095-3119(14)60941-2
Gayatri, G., Agurla, S., and Raghavendra, A. S. (2013). Nitric oxide in guard cells as an important secondary messenger during stomatal closure. Front. Plant Sci. 4:425. doi: 10.3389/fpls.2013.00425
Geissler, N., Hussin, S., El-Far, M. M., and Koyro, H. W. (2015). Elevated atmospheric CO2 concentration leads to different salt resistance mechanisms in a C3 (Chenopodium quinoa) and a C4 (Atriplex nummularia) halophyte. Environ. Exp. Bot. 118, 67–77. doi: 10.1016/j.envexpbot.2015.06.003
Giday, H., Fanourakis, D., Kjaer, K. H., Fomsgaard, I. S., and Ottosen, C. O. (2014). Threshold response of stomatal closing ability to leaf abscisic acid concentration during growth. J. Exp. Bot. 65, 4361–4370. doi: 10.1093/jxb/eru216
Gillespie, K. M., Xu, F., Richter, K. T., McGrath, J. M., Markelz, R. C., Ort, D. R., et al. (2012). Greater antioxidant and respiratory metabolism in field-grown soybean exposed to elevated O3 under both ambient and elevated CO2. Plant Cell Environ. 35, 169–184. doi: 10.1111/j.1365-3040.2011.02427.x
Gray, J. E., Holroyd, G. H., van der Lee, F. M., Bahrami, A. R., Sijmons, P. C., Woodward, F. I., et al. (2000). The HIC signalling pathway links CO2 perception to stomatal development. Nature 408, 713–716. doi: 10.1038/35047071
Grienenberger, E., and Fletcher, J. C. (2015). Polypeptide signaling molecules in plant development. Curr. Opin. Plant Biol. 23, 8–14. doi: 10.1016/j.pbi.2014.09.013
Hanson, D. T., Green, L. E., and Pockman, W. T. (2013). Spatio-temporal decoupling of stomatal and mesophyll conductance induced by vein cutting in leaves of Helianthus annuus. Front. Plant Sci. 4:365. doi: 10.3389/fpls.2013.00365
Hashimoto, M., Negi, J., Young, J., Israelsson, M., Schroeder, J. I., Iba, K., et al. (2006). Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat. Cell Biol. 8, 391–397. doi: 10.1038/ncb1387
Hashimoto-Sugimoto, M., Higaki, T., Yaeno, T., Nagami, A., Irie, M., Fujimi, M., et al. (2013). A Munc13-like protein in Arabidopsis mediates H+-ATPase translocation that is essential for stomatal responses. Nat. Commun. 4, 2215. doi: 10.1038/ncomms3215
Haworth, M., Elliott-Kingston, C., and McElwain, J. C. (2013). Co-ordination of physiological and morphological responses of stomata to elevated [CO2] in vascular plants. Oecologia 171, 71–82. doi: 10.1007/s00442-012-2406-9
Hedrich, R., and Marten, I. (1993). Malate-induced feedback regulation of anion channels could provide a CO2 sensor to guard cells. EMBO J. 12, k897–901.
Hoque, M. A., Scheelbeek, P. F. D., Vineis, P., Khan, A. E., Ahmed, K. M., and Butler, A. P. (2016). Drinking water vulnerability to climate change and alternatives for adaptation in coastal South and South East Asia. Clim. Change 136, 247–263. doi: 10.1007/s10584-016-1617-1
Hoshika, Y., Watanabe, M., Kitao, M., Häberle, K.-H., Grams, T. E. E., Koike, T., et al. (2015). Ozone induces stomatal narrowing in European and Siebold’s beeches: a comparison between two experiments of free-air ozone exposure. Environ. Pollut. 196, 527–533. doi: 10.1016/j.envpol.2014.07.034
Hu, H., Boisson-Dernier, A., Israelsson-Nordstrom, M., Bohmer, M., Xue, S., Ries, A., et al. (2010). Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat. Cell Biol. 12, 87–93. doi: 10.1038/ncb2009
Hubbart, S., Bird, S., Lake, J. A., and Murchie, E. H. (2013). Does growth under elevated CO2 moderate photoacclimation in rice? Physiol. Plant. 148, 297–306. doi: 10.1111/j.1399-3054.2012.01702.x
Hunt, L., Bailey, K. J., and Gray, J. E. (2010). The signalling peptide EPFL9 is a positive regulator of stomatal development. New Phytol. 186, 609–614. doi: 10.1111/j.1469-8137.2010.03200.x
Hwang, J. U., Jeon, B. W., Hong, D., and Lee, Y. (2011). Active ROP2 GTPase inhibits ABA- and CO2-induced stomatal closure. Plant Cell Environ. 34, 2172–2182. doi: 10.1111/j.1365-3040.2011.02413.x
IPCC (2013). “Summary for policymakers,” in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds D. Qin, T. F. Stocker, G.-K. Plattner, M. Tignor, S. K. Allen, et al. (Cambridge: Cambridge University Press).
Jackson, R. B., Sala, O. E., Field, C. B., and Mooney, H. A. (1994). CO2 alters water use, carbon gain, and yield for the dominant species in a natural grassland. Oecologia 98, 257–262. doi: 10.1007/BF00324212
Jewaria, P. K., Hara, T., Tanaka, H., Kondo, T., Betsuyaku, S., Sawa, S., et al. (2013). Differential effects of the peptides Stomagen, EPF1 and EPF2 on activation of MAP kinase MPK6 and the SPCH protein level. Plant Cell Physiol. 54, 1253–1262. doi: 10.1093/pcp/pct076
Jiang, K., Sorefan, K., Deeks, M. J., Bevan, M. W., Hussey, P. J., and Hetherington, A. M. (2012). The ARP2/3 complex mediates guard cell actin reorganization and stomatal movement in Arabidopsis. Plant Cell 24, 2031–2040. doi: 10.1105/tpc.112.096263
Jin, J., Lauricella, D., Armstrong, R., Sale, P., and Tang, C. (2015). Phosphorus application and elevated CO2 enhance drought tolerance in field pea grown in a phosphorus-deficient vertisol. Ann. Bot. 116, 975–985. doi: 10.1093/aob/mcu209
Joos, F., Gerber, S., Prentice, I. C., Otto-Bliesner, B. L., and Valdes, P. J. (2004). Transient simulations of Holocene atmospheric carbon dioxide and terrestrial carbon since the Last Glacial Maximum. Glob. Biogeochem. Cycles 18:GB2002. doi: 10.1029/2003GB002156
Kang, Y., Outlaw, W. H. Jr., Andersen, P. C., and Fiore, G. B. (2007). Guard-cell apoplastic sucrose concentration – a link between leaf photosynthesis and stomatal aperture size in the apoplastic phloem loader Vicia faba L. Plant Cell Environ. 30, 551–558. doi: 10.1111/j.1365-3040.2007.01635.x
Katul, G., Manzoni, S., Palmroth, S., and Oren, R. (2010). A stomatal optimization theory to describe the effects of atmospheric CO2 on leaf photosynthesis and transpiration. Ann. Bot. 105, 431–442. doi: 10.1093/aob/mcp292
Kellomäki, S., and Wang, K.-Y. (1997). Effects of elevated O3 and CO2 concentrations on photosynthesis and stomatal conductance in Scots pine. Plant Cell Environ. 20, 995–1006. doi: 10.1111/j.1365-3040.1997.tb00676.x
Kelly, G., Moshelion, M., David-Schwartz, R., Halperin, O., Wallach, R., Attia, Z., et al. (2013). Hexokinase mediates stomatal closure. Plant J. 75, 977–988. doi: 10.1111/tpj.12258
Kim, S. H., Sicher, R. C., Bae, H., Gitz, D. C., Baker, J. T., Timlin, D. J., et al. (2006). Canopy photosynthesis, evapotranspiration, leaf nitrogen, and transcription profiles of maize in response to CO2 enrichment. Glob. Change Biol. 12, 588–600. doi: 10.1111/j.1365-2486.2006.01110.x
Kim, T.-H., Böhmer, M., Hu, H., Nishimura, N., and Schroeder, J. I. (2010). Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 61, 561–591. doi: 10.1146/annurev-arplant-042809-112226
Kimball, B. A., Mauney, J. R., Nakayama, F. S., and Idso, S. B. (1993). Effects of increasing atmospheric CO2 on vegetation. Vegetatio 10, 65–75. doi: 10.1007/BF00048145
Klaiber, J., Najar-Rodriguez, A. J., Piskorski, R., and Dorn, S. (2013). Plant acclimation to elevated CO2 affects important plant functional traits, and concomitantly reduces plant colonization rates by an herbivorous insect. Planta 237, 29–42. doi: 10.1007/s00425-012-1750-7
Kusumi, K., Hirotsuka, S., Kumamaru, T., and Iba, K. (2012). Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. J. Exp. Bot. 63, 5635–5644. doi: 10.1093/jxb/ers216
Laanemets, K., Wang, Y. F., Lindgren, O., Wu, J., Nishimura, N., Lee, S., et al. (2013). Mutations in the SLAC1 anion channel slow stomatal opening and severely reduce K+ uptake channel activity via enhanced cytosolic [Ca2+] and increased Ca2+ sensitivity of K+ uptake channels. New Phytol. 197, 88–98. doi: 10.1111/nph.12008
Lake, J. A., Woodward, F. I., and Quick, W. P. (2002). Long-distance CO2 signalling in plants. J. Exp. Bot. 53, 183–193. doi: 10.1093/jexbot/53.367.183
Lavania, D., Dhingra, A., Siddiqui, M. H., Al-Whaibi, M. H., and Grover, A. (2015). Current status of the production of high temperature tolerant transgenic crops for cultivation in warmer climates. Plant Physiol. Biochem. 86, 100–108. doi: 10.1016/j.plaphy.2014.11.019
Lawson, T., and Blatt, M. R. (2014). Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 164, 1556–1570. doi: 10.1104/pp.114.237107
Lawson, T., Oxborough, K., Morison, J. I., and Baker, N. R. (2003). The responses of guard and mesophyll cell photosynthesis to CO2, O2, light, and water stress in a range of species are similar. J. Exp. Bot. 54, 1743–1752. doi: 10.1093/jxb/erg186
Lawson, T., Simkin, A. J., Kelly, G., and Granot, D. (2014). Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol. 203, 1064–1081. doi: 10.1111/nph.12945
Leakey, A. D., Ainsworth, E. A., Bernacchi, C. J., Rogers, A., Long, S. P., and Ort, D. R. (2009). Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J. Exp. Bot. 60, 2859–2876. doi: 10.1093/jxb/erp096
Leakey, A. D. B., Bernacchi, C. J., Ort, D. R., and Long, S. P. (2006a). Long-term growth of soybean at elevated [CO2] does not cause acclimation of stomatal conductance under fully open-air conditions. Plant Cell Environ. 29, 1794–1800. doi: 10.1111/j.1365-3040.2006.01556.x
Leakey, A. D. B., Uribelarrea, M., Ainsworth, E. A., Naidu, S. L., Rogers, A., Ort, D. R., et al. (2006b). Photosynthesis, productivity and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol. 140, 779–790. doi: 10.1104/pp.105.073957
Lee, J. S., Hnilova, M., Maes, M., Lin, Y. C. L., Putarjunan, A., Han, S. K., et al. (2015). Competitive binding of antagonistic peptides fine-tunes stomatal patterning. Nature 522, 439–443. doi: 10.1038/nature14561
Lee, M., Choi, Y., Burla, B., Kim, Y. Y., Jeon, B., Maeshima, M., et al. (2008). The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat. Cell Biol. 10, 1217–1223. doi: 10.1038/ncb1782
Lee, T. D., Tjoelker, M. G., Reich, P. B., and Russelle, M. P. (2003). Contrasting growth response of an N2-fixing and non-fixing forb to elevated CO2: dependence on soil N supply. Plant Soil 255, 475–486. doi: 10.1023/A:1026072130269
Li, Z., Zhang, Y., Yu, D., Zhang, N., Lin, J., Zhang, J., et al. (2014). The influence of precipitation regimes and elevated CO2 on photosynthesis and biomass accumulation and partitioning in seedlings of the rhizomatous perennial grass Leymus chinensis. PLoS ONE 9:e103633. doi: 10.1371/journal.pone.0103633
Lin, J., Jach, M. E., and Ceulemans, R. (2001). Stomatal density and needle anatomy of Scots pine (Pinus sylvestris) are affected by elevated CO2. New Phytol. 150, 665–674. doi: 10.1046/j.1469-8137.2001.00124.x
Linares, J. C., Delgado-Huertas, A., Camarero, J. J., Merino, J., and Carreira, J. A. (2009). Competition and drought limit the response of water-use efficiency to rising atmospheric carbon dioxide in the Mediterranean fir Abies pinsapo. Oecologia 161, 611–624. doi: 10.1007/s00442-009-1409-7
Liu, J. C., Temme, A. A., Cornwell, W. K., van Logtestijn, R. S., Aerts, R., and Cornelissen, J. H. (2016). Does plant size affect growth responses to water availability at glacial, modern and future CO2 concentrations? Ecol. Res. 31, 213–227. doi: 10.1007/s11284-015-1330-y
Lobell, D. B., Schlenker, W., and Costa-Roberts, J. (2011). Climate trends and global crop production since 1980. Science 333, 616–620. doi: 10.1126/science.1204531
Locosselli, G. M., and Ceccantini, G. (2013). Plasticity of stomatal distribution pattern and stem tracheid dimensions in Podocarpus lambertii: an ecological study. Ann. Bot. 110, 1057–1066. doi: 10.1093/aob/mcs179
Long, S. P., Ainsworth, E. A., Rogers, A., and Ort, D. R. (2004). Rising atmospheric carbon dioxide: plants FACE the future. Annu. Rev. Plant Biol. 55, 591–628. doi: 10.1146/annurev.arplant.55.031903.141610
Manea, A., and Leishman, M. R. (2015). Competitive interactions between established grasses and woody plant seedlings under elevated CO2 levels are mediated by soil water availability. Oecologia 177, 499–506. doi: 10.1007/s00442-014-3143-z
Mansfield, T. A. (1998). Stomata and plant water relations: does air pollution create problems? Environ. Pollut. 101, 1–11. doi: 10.1016/S0269-7491(98)00076-1
Marten, H., Hyun, T., Gomi, K., Seo, S., Hedrich, R., and Roelfsema, M. R. (2008). Silencing of NtMPK4 impairs CO2-induced stomatal closure, activation of anion channels and cytosolic Ca2+ signals in Nicotiana tabacum guard cells. Plant J. 55, 698–708. doi: 10.1111/j.1365-313X.2008.03542.x
Matyssek, R., Baumgarten, M., Hummel, U., Häberle, K. H., Kitao, M., and Wieser, G. (2015). Canopy-level stomatal narrowing in adult Fagus sylvatica under O3 stress–Means of preventing enhanced O3 uptake under high O3 exposure? Environ. Pollut. 196, 518–526. doi: 10.1016/j.envpol.2014.06.029
McAdam, S. A., and Brodribb, T. J. (2012). Fern and lycophyte guard cells do not respond to endogenous abscisic acid. Plant Cell 24, 1510–1521. doi: 10.1105/tpc.112.096404
McAinsh, M. R., Brownlee, C., and Hetherington, A. M. (1990). Abscisic acid-induced elevation of guard cell cytosolic Ca2+ precedes stomatal closure. Nature 343, 186–188. doi: 10.1038/343186a0
Medeiros, D. B., Daloso, D. M., Fernie, A. R., Nikoloski, Z., and Araújo, W. L. (2015). Utilizing systems biology to unravel stomatal function and the hierarchies underpinning its control. Plant Cell Environ. 38, 1457–1470. doi: 10.1111/pce.12517
Medlyn, B. E., Barton, C. V. M., Broadmeadow, M. S. J., Ceulemans, R., De Angelis, P., Forstreuter, M., et al. (2001). Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytol. 149, 247–264. doi: 10.1046/j.1469-8137.2001.00028.x
Messinger, S. M., Buckley, T. N., and Mott, K. A. (2006). Evidence for the involvement of photosynthetic processes in the stomatal response to CO2. Plant Physiol. 140, 771–778. doi: 10.1104/pp.105.073676
Meyer, S., Mumm, P., Imes, D., Endler, A., Weder, B., Al-Rasheid, K. A., et al. (2010). AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 63, 1054–1062. doi: 10.1111/j.1365-313X.2010.04302.x
Mizokami, Y., Noguchi, K., Kojima, M., Sakakibara, H., and Terashima, I. (2015). Mesophyll conductance decreases in the wild type but not in an ABA deficient mutant (aba1) of Nicotiana plumbaginifolia under drought conditions. Plant Cell Environ. 38, 388–398. doi: 10.1111/pce.12394
Monda, K., Araki, H., Kuhara, S., Ishigaki, G., Akashi, R., Negi, J., et al. (2016). Enhanced stomatal conductance by a spontaneous Arabidopsis Tetraploid, Me-0, results from increased stomatal size and greater stomatal aperture. Plant Physiol. 170, 1435–1444. doi: 10.1104/pp.15.01450
Morgan, J. A., Pataki, D. E., Körner, C., Clark, H., Del, Grosso SJ, Grünzweig, J. M., et al. (2004). Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 140, 11–25. doi: 10.1007/s00442-004-1550-2
Morison, J. I. L., and Lawlor, D. W. (1999). Interactions between increasing CO2 concentration and temperature on plant growth. Plant Cell Environ. 22, 659–682. doi: 10.1046/j.1365-3040.1999.00443.x
Mott, K. A., Sibbernsen, E. D., and Shope, J. C. (2008). The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ. 31, 1299–1306. doi: 10.1111/j.1365-3040.2008.01845.x
Negi, J., Hashimoto-Sugimoto, M., Kusumi, K., and Iba, K. (2014). New approaches to the biology of stomatal guard cells. Plant Cell Physiol. 55, 241–250. doi: 10.1093/pcp/pct145
Negi, J., Matsuda, O., Nagasawa, T., Oba, Y., Takahashi, H., Kawai-Yamada, M., et al. (2008). CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452, 483–486. doi: 10.1038/nature06720
Negi, J., Moriwaki, K., Konishi, M., Yokoyama, R., Nakano, T., Kusumi, K., et al. (2013). A Dof transcription factor, SCAP1, is essential for the development of functional stomata in Arabidopsis. Curr. Biol. 23, 479–484. doi: 10.1016/j.cub.2013.02.001
Neill, S., Barros, R., Bright, J., Desikan, R., Hancock, J., and Harrison, J. (2008). Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 59, 165–176. doi: 10.1093/jxb/erm293
Nguyen, H. T., Stanton, D. E., Schmitz, N., Farquhar, G. D., and Ball, M. C. (2015). Growth responses of the mangrove Avicennia marina to salinity: development and function of shoot hydraulic systems require saline conditions. Ann. Bot. 115, 397–407. doi: 10.1093/aob/mcu257
Ni, D. A. (2012). Role of vacuolar invertase in regulating Arabidopsis stomatal opening. Acta Physiol. Plant. 34, 2449–2452. doi: 10.1007/s11738-012-1036-5
Nicolas, M. E., Munns, R., Samarakoon, A. B., and Gifford, R. M. (1993). Elevated CO2 improves the growth of wheat under salinity. Funct. Plant Biol. 20, 349–360.
Nijs, I., Ferris, R., Blum, H., Hendrey, G., and Impens, I. (1997). Stomatal regulation in a changing climate: a field study using free air temperature increase (FATI) and free air CO2 enrichment (FACE). Plant Cell Environ. 20, 1041–1050. doi: 10.1111/j.1365-3040.1997.tb00680.x
Noormets, A., Sober, A., Pell, E. J., Dickson, R. E., Podila, G. K., Sôber, J., et al. (2001). Stomatal and non-stomatal limitation to photosynthesis in two trembling aspen (Populus tremuloides Michx.) clones exposed to elevated CO2 and/or O3. Plant Cell Environ. 24, 327–336. doi: 10.1046/j.1365-3040.2001.00678.x
Nunes-Nesi, A., Carrari, F., Gibon, Y., Sulpice, R., Lytovchenko, A., Fisahn Graham, J., et al. (2007). Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. Plant J. 50, 1093–1106. doi: 10.1111/j.1365-313X.2007.03115.x
Outlaw, W. H. (1989). Critical examination of the quantitative evidence for and against photosynthetic CO2 fixation by guard cells. Physiol. Plant. 77, 275–281. doi: 10.1111/j.1399-3054.1989.tb04981.x
Pallas, J. (1964). Guard cell starch retention and accumulation in the dark. Bot. Gaz. 125, 102–107. doi: 10.1086/336253
Peak, D., and Mott, K. A. (2011). A new, vapour-phase mechanism for stomatal responses to humidity and temperature. Plant Cell Environ. 34, 162–178. doi: 10.1111/j.1365-3040.2010.02234.x
Peñuelas, J., Sardans, J., Estiarte, M., Ogaya, R., Carnicer, J., Coll, M., et al. (2013). Evidence of current impact of climate change on life: a walk from genes to the biosphere. Glob. Change Biol. 19, 2303–2338. doi: 10.1111/gcb.12143
Pérez-López, U., Robredo, A., Lacuesta, M., Mena-Petite, A., and Muñoz-Rueda, A. (2012). Elevated CO2 reduces stomatal and metabolic limitations on photosynthesis caused by salinity in Hordeum vulgare. Photosynth. Res. 111, 269–283. doi: 10.1007/s11120-012-9721-1
Pérez-López, U., Robredo, A., Lacuesta, M., Sgherri, C., Muñoz-Rueda, A., Navari-Izzo, F., et al. (2009). The oxidative stress caused by salinity in two barley cultivars is mitigated by elevated CO2. Physiol. Plant. 135, 29–42. doi: 10.1111/j.1399-3054.2008.01174.x
Perez-Martin, A., Flexas, J., Ribas-Carbó, M., Bota, J., Tomàs, M., Infante, J. M., et al. (2009). Interactive effects of soil water deficit and air vapour pressure deficit on mesophyll conductance to CO2 in Vitis vinifera and Olea europaea. J. Exp. Bot. 60, 2391–2405. doi: 10.1093/jxb/erp145
Poór, P., Gémes, K., Horváth, F., Szepesi, A., Simon, M. L., and Tari, I. (2011). Salicylic acid treatment via the rooting medium interferes with stomatal response, CO2 fixation rate and carbohydrate metabolism in tomato, and decreases harmful effects of subsequent salt stress. Plant Biol. 13, 105–114. doi: 10.1111/j.1438-8677.2010.00344.x
Pyakurel, A., and Wang, J. R. (2014). Interactive effects of elevated [CO2] and soil water stress on leaf morphological and anatomical characteristic of paper birch populations. Am. J. Plant Sci. 5, 691–703. doi: 10.4236/ajps.2014.55084
Rakić, T., Gajic, G., Lazarevic, M., and Stevanovic, B. (2015). Effects of different light intensities, CO2 concentrations, temperatures and drought stress on photosynthetic activity in two paleoendemic resurrection plant species Ramonda serbica and R-nathaliae. Environ. Exp. Bot. 109, 63–72. doi: 10.1016/j.envexpbot.2014.08.003
Raschke, K., Shabahang, M., and Wolf, R. (2003). The slow and the quick anion conductance in whole guard cells: their voltage-dependent alternation, and the modulation of their activities by abscisic acid and CO2. Planta 217, 639–650. doi: 10.1007/s00425-003-1033-4
Reckmann, U., Scheibe, R., and Raschke, K. (1990). Rubisco activity in guard cells compared with the solute requirement for stomatal opening. Plant Physiol. 92, 246–253. doi: 10.1104/pp.92.1.246
Reid, C. D., Maherali, H., Johnson, H. B., Smith, S. D., Wullschleger, S. D., and Jackson, R. B. (2003). On the relationship between stomatal characters and atmospheric CO2. Geophys. Res. Lett. 30, 1983–1986. doi: 10.1029/2003GL017775
Rivera, L., Baraza, E., Alcover, J. A., Bover, P., Rovira, C. M., and Bartolomé, J. (2014). Stomatal density and stomatal index of fossil Buxus from coprolites of extinct Myotragus balearicus Bate (Artiodactyla, Caprinae) as evidence of increased CO2 concentration during the late Holocene. Holocene 24, 876–880. doi: 10.1177/0959683614530445
Rodrigues, W. P., Martins, M. Q., Fortunato, A. S., Rodrigues, A. P., Semedo, J. N., Simões-Costa, M. C., et al. (2016). Long-term elevated air [CO2] strengthens photosynthetic functioning and mitigates the impact of supra-optimal temperatures in tropical Coffea arabica and C. canephora species. Glob. Change Biol. 22, 415–431. doi: 10.1111/gcb.13088
Roelfsema, M. R. G., Hanstein, S., Felle, H. H., and Hedrich, R. (2002). CO2 provides an intermediate link in the red light response of guard cells. Plant J. 32, 65–75. doi: 10.1046/j.1365-313X.2002.01403.x
Ruiz-Vera, U. M., Siebers, M., Gray, S. B., Drag, D. W., Rosenthal, D. M., Kimball, B. A., et al. (2013). Global warming can negate the expected CO2 stimulation in photosynthesis and productivity for soybean grown in the Midwestern United States. Plant Physiol. 162, 410–423. doi: 10.1104/pp.112.211938
Sanoubar, R., Cellini, A., Veroni, A. M., Spinelli, F., Masia, A., Vittori Antisari, L., et al. (2016). Salinity thresholds and genotypic variability of cabbage (Brassica oleracea L.) grown under saline stress. J. Sci. Food Agric. 96, 319–330. doi: 10.1002/jsfa.7097
Sasaki, T., Mori, I. C., Furuichi, T., Munemasa, S., Toyooka, K., Matsuoka, K., et al. (2010). Closing plant stomata requires a homolog of an aluminum-activated malate transporter. Plant Cell Physiol. 51, 354–365. doi: 10.1093/pcp/pcq016
Sato, A., Taniguchi, M., Miyake, H., Umezawa, T., Shinozaki, K., Goto, D. B., et al. (2009). Phosphorylation of KAT1 C-terminus modulates K+ uptake activity. Biophys. J. 96, 171a. doi: 10.1016/j.bpj.2008.12.793
Sato, H., Kumagai, T. O., Takahashi, A., and Katul, G. (2015). Effects of different representations of stomatal conductance response to humidity across the African continent under warmer CO2-enriched climate conditions. J. Geophys. Res. Biogeosci. 120, 979–988. doi: 10.1002/2014JG002838
Schroeder, J. I., Allen, G. J., Hugouvieux, V., Kwak, J. M., and Waner, D. (2001). Guard cell signal transduction. Annu. Rev. Plant Biol. 52, 627–658. doi: 10.1146/annurev.arplant.52.1.627
Shalata, A., Mittova, V., Volokita, M., Guy, M., and Tal, M. (2001). Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: the root antioxidative system. Physiol. Plant. 112, 487–494. doi: 10.1034/j.1399-3054.2001.1120405.x
Shi, H., Ye, T., Zhu, J. K., and Chan, Z. (2014). Constitutive production of nitric oxide leads to enhanced drought stress resistance and extensive transcriptional reprogramming in Arabidopsis. J. Exp. Bot. 65, 4119–4131. doi: 10.1093/jxb/eru184
Shimono, H., Nakamura, H., Hasegawa, T., and Okada, M. (2013). Lower responsiveness of canopy evapotranspiration rate than of leaf stomatal conductance to open-air CO2 elevation in rice. Glob. Change Biol. 19, 2444–2453. doi: 10.1111/gcb.12214
Sibbernsen, E., and Mott, K. A. (2010). Stomatal responses to flooding of the intercellular air spaces suggest a vapor-phase signal between the mesophyll and the guard cells. Plant Physiol. 153, 1435–1442. doi: 10.1104/pp.110.157685
Šigut, L., Holišová, P., Klem, K., Šprtová, M., Calfapietra, C., Marek, M. V., et al. (2015). Does long-term cultivation of saplings under elevated CO2 concentration influence their photosynthetic response to temperature? Ann. Bot. 116, 929–939. doi: 10.1093/aob/mcv043
Silber, A., Israeli, Y., Levi, M., Keinan, A., Chudi, G., Golan, A., et al. (2013). The roles of fruit sink in the regulation of gas exchange and water uptake: a case study for avocado. Agric. Water Manage. 116, 21–28. doi: 10.1016/j.agwat.2012.10.006
Sreeharsha, R. V., Sekhar, K. M., and Reddy, A. R. (2015). Delayed flowering is associated with lack of photosynthetic acclimation in Pigeon pea (Cajanus cajan L.) grown under elevated CO2. Plant Sci. 231, 82–93. doi: 10.1016/j.plantsci.2014.11.012
Stavridou, E., Hastings, A., Webster, R. J., and Robson, P. R. H. (2016). The impact of soil salinity on the yield, composition and physiology of the bioenergy grass Miscanthus × giganteus. GCB Bioenergy doi: 10.1111/gcbb.12351
Sugano, S. S., Shimada, T., Imai, Y., Okawa, K., Tamai, A., Mori, M., et al. (2010). Stomagen positively regulates stomatal density in Arabidopsis. Nature 463, 241–244. doi: 10.1038/nature08682
Sun, Y., Guo, H., Yuan, L., Wei, J., Zhang, W., and Ge, F. (2015). Plant stomatal closure improves aphid feeding under elevated CO2. Glob. Change Biol. 21, 2739–2748. doi: 10.1111/gcb.12858
Tallman, G., and Zeiger, E. (1988). Light quality and osmoregulation in Vicia guard cells: evidence for involvement of three metabolic pathways. Plant Physiol. 88, 887–895. doi: 10.1104/pp.88.3.887
Tanaka, Y., Nose, T., Jikumaru, Y., and Kamiya, Y. (2013a). ABA inhibits entry into stomatal-lineage development in Arabidopsis leaves. Plant J. 74, 448–457. doi: 10.1111/tpj.12136
Tanaka, Y., Sugano, S. S., Shimada, T., and Hara-Nishimura, I. (2013b). Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 198, 757–764. doi: 10.1111/nph.12186
Teng, N., Jin, B., Wang, Q., Hao, H., Ceulemans, R., Kuang, T., et al. (2009). No detectable maternal effects of elevated CO2 on Arabidopsis thaliana over 15 generations. PLoS ONE 4:e6035. doi: 10.1371/journal.pone.0006035
Tian, W., Hou, C., Ren, Z., Pan, Y., Jia, J., Zhang, H., et al. (2015). A molecular pathway for CO2 response in Arabidopsis guard cells. Nat. Commun. 6, 6057. doi: 10.1038/ncomms7057
Tricker, P. J., Trewin, H., Kull, O., Clarkson, G. J. J., Eensalu, E., Tallis, M. J., et al. (2005). Stomatal conductance and not stomatal density determines the long-term reduction in leaf transpiration of poplar in elevated CO2. Oecologia 143, 652–660. doi: 10.1007/s00442-005-0025-4
Uddling, J., Teclaw, R. M., Pregitzer, K. S., and Ellsworth, D. S. (2009). Leaf and canopy conductance in aspen and aspen-birch forests under free-air enrichment of carbon dioxide and ozone. Tree Physiol. 29, 1367–1380. doi: 10.1093/treephys/tpp070
Vahisalu, T., Kollist, H., Wang, Y. F., Nishimura, N., Chan, W. Y., Valerio, G., et al. (2008). SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452, 487–491. doi: 10.1038/nature06608
Virlouvet, L., and Fromm, M. (2015). Physiological and transcriptional memory in guard cells during repetitive dehydration stress. New Phytol. 205, 596–607. doi: 10.1111/nph.13080
von Caemmerer, S., Lawson, T., Oxborough, K., Baker, N. R., Andrews, T. J., and Raines, C. A. (2004). Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J. Exp. Bot. 55, 1157–1166. doi: 10.1093/jxb/erh128
Wang, W., Vinocur, B., and Altman, A. (2003). Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218, 1–14. doi: 10.1007/s00425-003-1105-5
Wang, Y., Noguchi, K., Ono, N., Inoue, S., Terashima, I., and Kinoshita, T. (2014). Overexpression of plasma membrane H+-ATPase in guard cells promotes light-induced stomatal opening and enhances plant growth. Proc. Natl. Acad. Sci. U.S.A. 111, 533–538. doi: 10.1073/pnas.1305438111
Wang, Y. F., Munemasa, S., Nishimura, N., Ren, H. M., Robert, N., Han, M., et al. (2013). Identification of cyclic GMP-activated nonselective Ca2+-permeable cation channels and associated CNGC5 and CNGC6 genes in Arabidopsis guard cells. Plant Physiol. 163, 578–590. doi: 10.1104/pp.113.225045
Ward, E. J., Oren, R., Bell, D. M., Clark, J. S., McCarthy, H. R., Kim, H. S., et al. (2013). The effects of elevated CO2 and nitrogen fertilization on stomatal conductance estimated from 11 years of scaled sap flux measurements at Duke FACE. Tree Physiol. 33, 135–151. doi: 10.1093/treephys/tps118
Warren, C. R. (2008). Soil water deficits decrease the internal conductance to CO2 transfer but atmospheric water deficits do not. J. Exp. Bot. 59, 327–334. doi: 10.1093/jxb/erm314
Warren, C. R., Matyssek, M. L. R., and Tausz, M. (2006). Internal conductance to CO2 transfer of adult Fagus sylvatica: variation between sun and shade leaves and due to free-air ozone fumigation. Environ. Exp. Bot. 59, 130–138. doi: 10.1016/j.envexpbot.2005.11.004
Warren, J. M., Norby, R. J., Wullschleger, S. D., and Oren, R. (2011) Elevated CO2 enhances leaf senescence during extreme drought in a temperate forest. Tree Physiol. 31, 117–130. doi: 10.1093/treephys/tpr002
Webb, A. A. R., McAinsh, M. R., Mansfield, T. A., and Hetherington, A. M. (1996). Carbon dioxide induces increases in guard cell cytosolic free calcium. Plant J. 9, 297–304. doi: 10.1046/j.1365-313X.1996.09030297.x
Woodward, F. I. (1987). Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 327, 617–618. doi: 10.1038/327617a0
Woodward, F. I. (1990). Global change: translating plant ecophysiological responses to ecosystems. Trends Ecol. Evol. 5, 308–311. doi: 10.1016/0169-5347(90)90087-T
Xia, J., Kong, D., Xue, S., Tian, W., Li, N., Bao, F., et al. (2014). Nitric oxide negatively regulates AKT1-mediated potassium uptake through modulating vitamin B6 homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 111, 16196–16201. doi: 10.1073/pnas.1417473111
Xia, X.-J., Zhou, Y.-H., Shi, K., Zhou, J., Foyer, C. H., and Yu, J.-Q. (2015). Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 66, 2839–2856. doi: 10.1093/jxb/erv089
Xu, Z. Z., Jiang, Y. L., and Zhou, G. S. (2015). Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Front. Plant Sci. 6:701. doi: 10.3389/fpls.2015.00701
Xu, Z. Z., Jiang, Y. L., and Zhou, G. S. (2016). Nitrogen cycles in terrestrial ecosystems: climate change impacts and mitigation. Environ. Rev. doi: 10.1139/er-2015-0066
Xu, Z. Z., Shimizu, H., Ito, S., Yagasaki, Y., Zou, C. J., Zhou, G. S., et al. (2014). Effects of elevated CO2, warming and precipitation change on plant growth, photosynthesis and peroxidation in dominant species from North China grassland. Planta 239, 421–435. doi: 10.1007/s00425-013-1987-9
Xu, Z. Z., Shimizu, H., Yagasaki, Y., Ito, S., Zheng, Y. R., and Zhou, G. S. (2013). Interactive effects of elevated CO2, drought, and warming on plants. J. Plant Growth Regul. 32, 692–707. doi: 10.1007/s00344-013-9337-5
Xu, Z. Z., and Zhou, G. S. (2008). Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 59, 3317–3325. doi: 10.1093/jxb/ern185
Xu, Z. Z., Zhou, G. S., and Shimizu, H. (2009a). Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J. Exp. Bot. 60, 3737–3749. doi: 10.1093/jxb/erp216
Xu, Z. Z., Zhou, G. S., and Shimizu, H. (2009b). Effects of soil drought with nocturnal warming on leaf stomatal traits and mesophyll cell ultrastructure of a perennial grass. Crop Sci. 49, 1843–1851. doi: 10.2135/cropsci2008.12.0725
Xue, S., Hu, H., Ries, A., Merilo, E., Kollist, H., and Schroeder, J. I. (2011). Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J. 30, 1645–1658. doi: 10.1038/emboj.2011.68
Yamamoto, Y., Negi, J., Wang, C., Isogai, Y., Schroeder, J. I., and Iba, K. (2016). The Transmembrane region of guard cell SLAC1 channels perceives CO2 signals via an ABA-independent pathway in Arabidopsis. Plant Cell 28, 557–567. doi: 10.1105/tpc.15.00583
Yoo, C. Y., Pence, H. E., Jin, J. B., Miura, K., Gosney, M. J., Hasegawa, P. M., et al. (2010). The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell 22, 4128–4141. doi: 10.1105/tpc.110.078691
Young, J. J., Mehta, S., Israelsson, M., Godoski, J., Grill, E., and Schroeder, J. I. (2006). CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independentphase, and CO2 insensitivity of the gca2 mutant. Proc. Natl. Acad. Sci. U.S.A. 103, 7506–7511. doi: 10.1073/pnas.0602225103
Youshi, T., and Santrucek, J. (2015). Superimposed behaviour of gm under ABA-induced stomata closing and low CO2. Plant Cell Environ. 38, 385–387. doi: 10.1111/pce.12437
Yu, H., Murchie, E. H., González-Carranza, Z. H., Pyke, K. A., and Roberts, J. A. (2015). Decreased photosynthesis in the erect panicle 3 (ep3) mutant of rice is associated with reduced stomatal conductance and attenuated guard cell development. J. Exp. Bot. 66, 1543–1552. doi: 10.1093/jxb/eru525
Zavala, J. A., Nabity, P. D., and DeLucia, E. H. (2013). An emerging understanding of mechanisms governing insect herbivory under elevated CO2. Annu. Rev. Entomol. 58, 79–97. doi: 10.1146/annurev-ento-120811-153544
Zeiger, E., and Zhu, J. (1998). Role of zeaxanthin in blue light photoreception and the modulation of light-CO2 interactions in guard cells. J. Exp. Bot. 49, 433–442. doi: 10.1093/jxb/49.Special_Issue.433
Zhu, L., Talbott, L. D., and Zeiger, E. (1998). The stomatal response to CO2 is linked to changes in guard cell zeaxanthin. Plant Cell Environ. 21, 813–820. doi: 10.1046/j.1365-3040.1998.00323.x
Zhu, X. G., Song, Q., and Ort, D. R. (2012). Elements of a dynamic systems model of canopy photosynthesis. Curr. Opin. Plant Biol. 15, 237–244. doi: 10.1016/j.pbi.2012.01.010
Zinta, G., AbdElgawad, H., Domagalska, M. A., Vergauwen, L., Knapen, D., Nijs, I., et al. (2014). Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob. Change Biol. 20, 3670–3685. doi: 10.1111/gcb.12626
Keywords: elevated CO2, drought, guard cell, global warming, mesophyll-driven signals, regulation mechanism, photosynthesis, stomatal behavior
Citation: Xu Z, Jiang Y, Jia B and Zhou G (2016) Elevated-CO2 Response of Stomata and Its Dependence on Environmental Factors. Front. Plant Sci. 7:657. doi: 10.3389/fpls.2016.00657
Received: 20 September 2015; Accepted: 29 April 2016;
Published: 13 May 2016.
Edited by:
Jan Kofod Schjoerring, University of Copenhagen, DenmarkReviewed by:
Iker Aranjuelo, Agrobiotechnology Institute–Consejo Superior de Investigaciones Científicas–Universidad Pública de Navarra, SpainMaite Lacuesta, Universidad del Pais Vasco – Euskal Herriko Unibertsitatea, Spain
Copyright © 2016 Xu, Jiang, Jia and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenzhu Xu, eHV6ekBpYmNhcy5hYy5jbg==; Guangsheng Zhou, Z3N6aG91QGliY2FzLmFjLmNu
 Zhenzhu Xu
Zhenzhu Xu Yanling Jiang
Yanling Jiang Bingrui Jia
Bingrui Jia Guangsheng Zhou
Guangsheng Zhou