
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Plant Sci. , 28 April 2016
Sec. Plant Pathogen Interactions
Volume 7 - 2016 | https://doi.org/10.3389/fpls.2016.00565
This article is part of the Research Topic Biotrophic plant-microbe interactions View all 34 articles
Plant resistance to biotrophic pathogens is classically believed to be mediated through salicylic acid (SA) signaling leading to hypersensitive response followed by the establishment of Systemic Acquired Resistance. Jasmonic acid (JA) signaling has extensively been associated to the defense against necrotrophic pathogens and insects inducing the accumulation of secondary metabolites and PR proteins. Moreover, it is believed that plants infected with biotrophic fungi suppress JA-mediated responses. However, recent evidences have shown that certain biotrophic fungal species also trigger the activation of JA-mediated responses, suggesting a new role for JA in the defense against fungal biotrophs. Plasmopara viticola is a biotrophic oomycete responsible for the grapevine downy mildew, one of the most important diseases in viticulture. In this perspective, we show recent evidences of JA participation in grapevine resistance against P. viticola, outlining the hypothesis of JA involvement in the establishment of an incompatible interaction with this biotroph. We also show that in the first hours after P. viticola inoculation the levels of OPDA, JA, JA-Ile, and SA increase together with an increase of expression of genes associated to JA and SA signaling pathways. Our data suggests that, on the first hours after P. viticola inoculation, JA signaling pathway is activated and the outcomes of JA–SA interactions may be tailored in the defense response against this biotrophic pathogen.
Grapevine is one of the most valuable crops for fruit and wine production in a global scale, representing more than 7500 kHa of cultivated area in 2014 (data from the International Organization of Vine and Wine1). Downy mildew is one of the most economically significant grapevine diseases worldwide. It was introduced in Europe in the 1870s (Millardet, 1881) and quickly spread to all major grape-producing regions of the world (Galet, 1977; Gessler et al., 2011). The grapevine downy mildew causal agent, Plasmopara viticola (Berk. et Curt.) and De Toni, is a biotrophic obligatory oomycete that obtains nutrients from living cells of hosts in order to complete its life cycle. It infects all green parts of the plant, specifically leaves and clusters (Gessler et al., 2011). Under favorable conditions, motile zoospores are released from sporangia and swim toward the stomata. Subsequently, zoospores germinate and the germ tube penetrates into the substomatal cavity, primary hypha expand into the intercellular spaces of the mesophyll tissue differentiating specialized structures known as haustoria (Diez-Navajas et al., 2008). These highly specialized structures of biotrophic oomycetes and fungi play an essential role in nutrient acquisition from the plant cells and allow intense exchanges of signals that redirect the host metabolism and suppress the defense reaction (Diez-Navajas et al., 2008).
While American and Asiatic Vitis spp. present genetic resistance to this pathogen, domesticated grapevine Vitis vinifera, presently the most cultivated on a global scale, is sensitive to downy mildew. As a control measure, several fungicide applications are necessary every year and P. viticola resistance has already been found to the most common groups of site specific fungicides (Chen et al., 2007; Blum et al., 2010). Only in the past few decades, resistance breeding partly replaced the chemical plant protection applied against grapevine downy mildew. Partially resistant grapevine varieties resulted from breeding programs by introgression of resistant traits from wild Vitis spp. (e.g., V. labrusca, V. amurensis). However, recent reports have shown that P. viticola presents a high evolutionary potential as several isolates were able to break down plant resistance of interspecific hybrids (Peressotti et al., 2010; Casagrande et al., 2011). These findings have highlighted the need to fully understand grapevine resistance mechanisms against P. viticola.
The signaling pathways associated to grapevine and P. viticola interaction are still poorly understood. In plant defense against pathogens, phytohormones such as jasmonates and salicylic acid (SA) have received considerable attention (Bari and Jones, 2009). It is generally assumed that SA is involved in the activation of defense responses against biotrophic and hemi-biotrophic pathogens as well as the establishment of systemic acquired resistance, whereas inducible defense against leaf-chewing insects and necrotrophic microbes is mediated by jasmonic acid (JA)-dependent signaling (Glazebrook, 2005). These generalities are disputed in grapevine as JA signaling has been implicated in resistance against biotrophs, such as powdery and downy mildews (Hamiduzzaman et al., 2005; Belhadj et al., 2006, 2008; Trouvelot et al., 2008).
Jasmonic acid signaling has been extensively studied in model plants such as Arabidopsis. Briefly, biosynthesis of JA takes place in three different cell compartments. In the chloroplast, α-linolenic acid is released from membranes and deoxygenated by 13-lipoxygenases (13-LOXs), followed by the sequential action of allene oxide synthase (AOS) and allene oxide cyclase (AOC), resulting in the synthesis of 12-oxophytodienoic acid (OPDA). OPDA is transported to the peroxisome where the cyclopentenone ring is reduced by a cis-OPDA reductase 3 (OPR3) and subsequently the carboxylic acid side chain is shortened by β-oxidation to generate (+)-7-iso-JA, which is again released into the cytosol and epimerizes to the less active (-)-JA (Dave and Graham, 2012). In 2004, it was found that the active phytohormone is not JA itself but its isoleucine conjugate (Staswick and Tiryaki, 2004). This conjugation is catalyzed by jasmonate resistant 1 (JAR1) using (+)-7-iso-JA as the substrate to form bioactive jasmonate (+)-7-iso-jasmonoyl-L-isoleucine (JA-Ile; Staswick and Tiryaki, 2004; Fonseca et al., 2009). JA-dependent gene activation involves binding of JA-Ile to the F-box protein coronatine insensitive 1 (COI1), which acts as a JA receptor in the E3 ubiquitin-ligase SKP1-Cullin-F-box complex (SCFCOI1). Further discovery of JASMONATE ZIM-DOMAIN (JAZ) proteins as negative regulators of JA-induced gene expression and as the true targets of SCFCOI1 complex represented a major breakthrough in analysis of JA signaling (Chini et al., 2007; Thines et al., 2007; Yan et al., 2007). In the absence of the JA-Ile, JAZ proteins block basic helix-loop-helix leucine zipper transcription factor (MYC2) activity by recruiting the general corepressors TOPLESS (TPL) and TPL-related proteins through an interaction with the adaptor protein Novel Interactor of JAZ (NINJA; Pauwels et al., 2010). In response to JA-Ile, JAZ proteins are targeted by SCFCOI1 for degradation, MYC2 is released activating JA-dependent gene expression and ultimately activating the regulation of various physiological processes (Figure 1). This model and the role of other proteins in JA perception and signaling has been widely discussed in many reviews (e.g., Wasternack, 2007; Avanci et al., 2010; Dave and Graham, 2012; Pieterse et al., 2012; Wasternack and Hause, 2013).

FIGURE 1. On the first hours after Plasmopara viticola inoculation, the levels of SA, JA, and JA-Ile increased in V. vinifera cv. Regent relatively to mock inoculated control plants. Rapid accumulation of bioactive JA-Ile promotes SCF-COI1 mediated ubiquitination and subsequent degradation of JAZ proteins and corepressors TLP and NINJA via the 26S proteasome, relieving the transcription factors such as MYC2 and promoting the expression of JA-responsive genes such as PR10. High levels of SA mediate a change in the cellular redox potential, resulting in the reduction of the NPR1 oligomer to its active monomeric form. Monomeric NPR1 is then translocated into the nucleus where it functions as a transcriptional co-activator of SA-responsive genes, such as PR-1. Both the SA and JA signaling pathways seem to be simultaneously activated on the first hours of interaction. See text for details on the molecular processes underlying both JA- and SA-signaling. Solid lines indicate established accumulation and dashed lines suggested activities.
The first cues of JA role in grapevine resistance to downy mildew emerged from elicitor-based studies where it was shown that following both β-aminobutyric acid (BABA) and sulfated laminarin (PS3) application, the expression of LOX and JA responsive genes increased (Hamiduzzaman et al., 2005). Other studies also reported, that after P. viticola inoculation, the expression of AOC and AOS (Polesani et al., 2010), LOXO and JAZ (Marchive et al., 2013) and JAZ1 and AOC increased (Gauthier et al., 2014). Other evidences pointing to the involvement of JA pathway came from the studies of Polesani et al. (2010) that showed an increase of JA and MeJA levels after inoculation, of Ali et al. (2012) that pointed out an increased α-linolenic acid content in resistant grapevine cultivars and Gauthier et al. (2014) that reported an transient increase in JA levels in b-1,3 glucan laminarin elicited plants.
Very recently, Figueiredo et al. (2015) characterized gene expression profile for the first steps of JA biosynthesis (LOX2, AOC, AOS, and OPR3), activation (JAR1) and signaling (COI1) in two Vitis vinifera cultivars with different degrees of resistance to P. viticola. These authors have shown that, following P. viticola inoculation, there was an early (6 and 12 hpi) up-regulation of JA biosynthesis-related enzymes (LOXO, AOS, AOC, and OPR3) and a later activation (18 and 24 hpi) of two of the key components of JA signaling, JAR1 and COI1 in the resistant cultivar. Simultaneously, an up-regulation of LOX, JAZ, and PR14 genes and a higher content of JA (at 12 and 24 hpi) and SA (at 24, 48, and 72 hpi) was described for the incompatible Vitis amurensis cv. ‘Shuanghong’–P. viticola interaction (Li et al., 2015).
Altogether these studies highlighted the potential role of JA in this particular plant-biotrophic pathogen interaction. To further investigate this hypothesis we have determined OPDA, JA, JA-Ile, and SA levels and conducted a qPCR expression analysis of JA-signaling associated genes [MYC2, JAZ1, and JAZ3, TOPLESS, NINJA, and PR10 (pathogenesis-related protein 10)], SA-signaling markers [NPR1 (non-expressor of PR1); PR1 (pathogenesis-related protein 1)] and genes involved in the crosstalk between SA and JA signaling [WRKY70 and MYB44 (MYB domain protein 44)]. The V. vinifera cv. Regent, bred at the JKI-Institute for Grapevine Breeding Geilweilerhof (Akkurt et al., 2007) presenting a high degree of resistance to both downy and powdery mildew (Anonymous, 2000) was chosen as a model. Early inoculation time-points (6, 12, and 24 hpi) were considered in order to account for signaling events related to pathogen recognition in V. vinifera. Briefly, between 6 and 12 hpi stomatal penetration and development of stomatal vesicles with primary hyphae occur and at 24 hpi elongated hyphae invade the intercellular space of the mesophyll progressing to the branching stage in susceptible plants and stopping the development in resistant plants (Kortekamp and Zyprian, 2003; Unger et al., 2007).
After P. viticola inoculation, both JAZ genes analyzed also increased their expression at 6 hpi (JAZ3: 2.03 ± 0.33) and 12 hpi (JAZ1: 3.85 ± 0.98), the co-repressor TOPLESS and NINJA also increased their expression at 6 hpi (NINJA: 2.77 ± 0.29) and 24 hpi (TOPLESS: 1.72 ± 0.01). These results are coherent with the release of JAZ-bound transcription factors resulting in the activation of downstream JA responses (Figure 2B) and with the feed-back loop model where de novo synthesis of JAZ repressors is described for a negative feedback control. Moreover, in the interaction of V. amurensis with P. viticola, Li et al. (2015) have also shown an up-regulation of JAZ related genes from 24 hpi and after JA-elicitor treatment Gauthier et al. (2014) have reported an up-regulation of JAZ1 at 12 hpi.
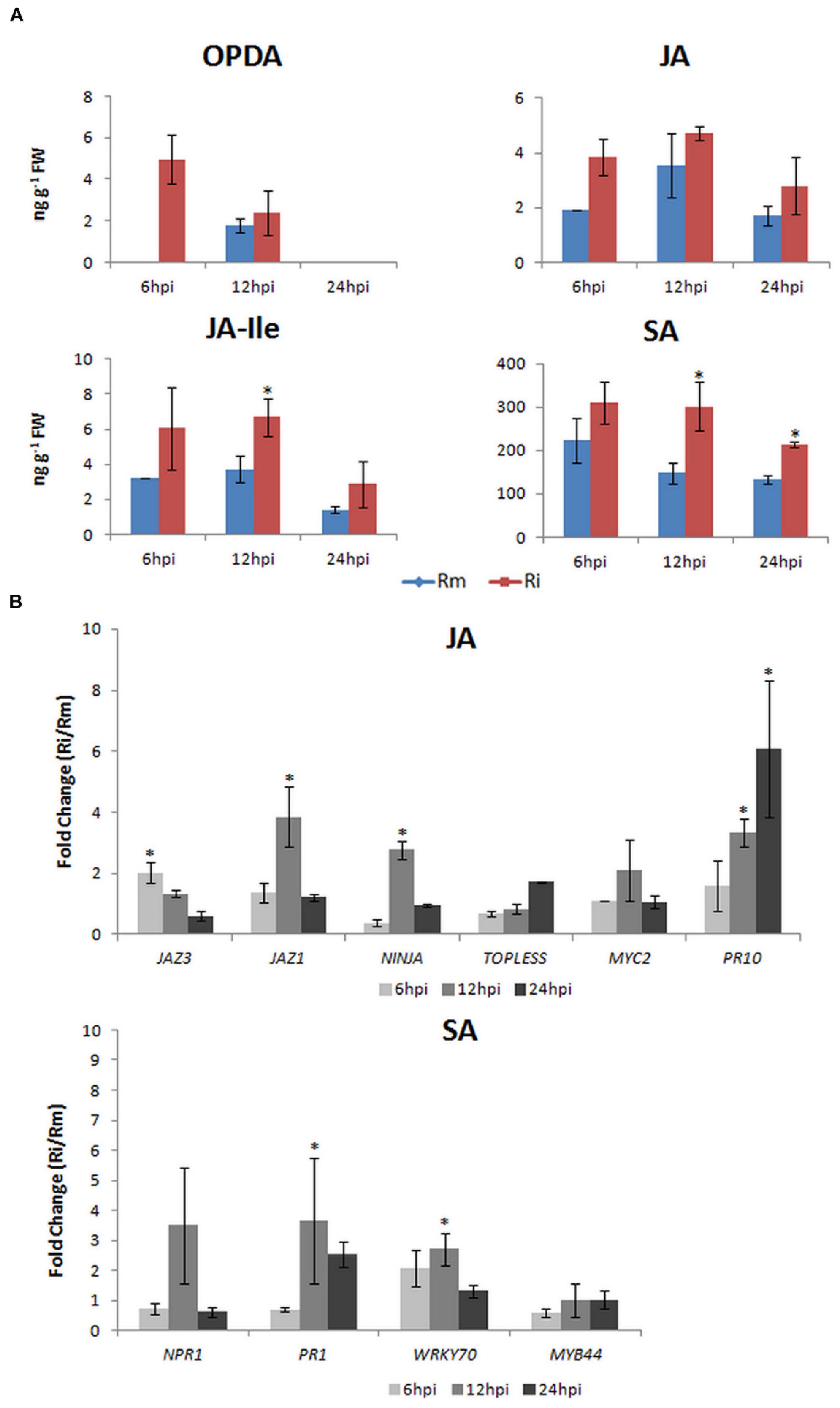
FIGURE 2. Vitis vinifera cv. Regent plants were inoculated with P. viticola (Ri) as described in Figueiredo et al. (2012). Plant material was harvested at 6, 12, and 24 hpi. Mock inoculated samples (Rm) were done for each time-point. (A) Determination of the endogenous levels (ng g-1 FW) of OPDA, JA, JA-ILE, and SA. Briefly, 50 mg of lyophilized samples were used for phytohormone quantification in a 4000 QTRAP LC/MS/MS system (AB Sciex) at the Proteomics & Mass Spectrometry Facility at the Danforth Plant Science Center (USA). Phytohormone levels are represented as the mean and standard deviation of three biological replicates. (B) qPCR expression analysis of JA- and SA-signaling associated genes. Total RNA extraction, cDNA synthesis, and qPCR experiments were done according to Monteiro et al. (2013). Primer sequences, amplicon size, amplification efficiency, annealing and melting temperatures for each gene studied are given in Supplementary Table 1. To normalize expression data, ubiquitin conjugating enzyme (UBQ), Elongation factor 1α (EF1α) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used (Monteiro et al., 2013). Transcript abundance of inoculated samples relative to mock inoculated controls at each time point is represented as the mean and standard deviation of five biological replicates. Expression between 0 and 1 represents a down-regulation. Asterisks (*) represent significant difference (p ≤ 0.05) between inoculated and control samples at the same time point (Mann–Whitney U test; SPSS Inc., USA, V20).
At 12 hpi with P. viticola MYC2 expression increased (6 hpi: 1.10 ± 0.01; 12 hpi: 2.48 ± 1.00; 24 hpi: 1.06 ± 0.20), together with the expression of PR10 (6 hpi: 1.59 ± 0.83; 12 hpi: 3.35 ± 0.45; 24 hpi: 6.19 ± 2.24), suggesting an activation of JA signaling. This activation is corroborated by the increase of JA at 6 and 12 hpi and by the significantly increase of JA-Ile levels at 12 hpi (Figure 2A). After P. viticola inoculation it was also reported a significant increase of JA levels at 12 and 24 hpi (Li et al., 2015) in V. amurensis and a later increase (48 hpi) of both JA and MeJA levels in V. riparia (Polesani et al., 2010). PR10 levels were also shown to increase after P. viticola inoculation in both Benzothiadiazole-primed and control V. vinifera plants (Dufour et al., 2013) and in V. vinifera cv. Regent plants (Figueiredo et al., 2012). Altogether, our results on this pathosystem suggest that in the resistant V. vinifera cv. Regent, an increase in α-linolenic acid content occurs after P. viticola inoculation (Ali et al., 2012) which is used for the biosynthesis of JA. The conversion of JA to its bioactive form JA-Ile is corroborated by both the increase of JAR1 expression, described by Figueiredo et al. (2015), and the increase of JA-Ile levels at 12 hpi (Figure 2A). The activation of JA-dependent defense responses is suggested by the increase of MYC2 and PR10 expression.
It is generally accepted that SA activates resistance against biotrophic pathogens, while JA is critical for activation of defense against herbivorous insects and necrotrophic pathogens. Both signaling pathways are interdependent and although most reports indicate a mutually antagonistic interaction between SA- and JA-dependent signaling, synergistic interactions have been described as well (reviewed in Pieterse et al., 2012).
Signaling downstream of SA is largely controlled by the regulatory protein NPR1 that acts as a transcriptional co-activator of a large set of defense related genes, namely PR proteins (Dong, 2004) of which PR-1 is often used as a robust marker for SA-responsive gene expression (Pieterse et al., 2012). WRKY transcription factors are important regulators of SA-dependent defense responses (reviewed in Koornneef and Pieterse, 2008) and some of them have been implicated in SA/JA cross talk, namely WRKY70 (Li et al., 2004). WRKY70 positively regulates SA-mediated defenses while repressing the JA response (Li et al., 2004) and in turn is transcriptionally activated by MYB44 (Shim et al., 2013), thus both genes may be considered integrators of the cross-talk between SA and JA in plant defense responses (Figure 1).
After inoculation of V. vinifera cv. Regent with P. viticola, the expression of NPR1 increased at 12 hpi (3.44 ± 1.81) decreasing afterward, when compared to mock inoculated plants (Figure 2B). This peak of expression at 12 hpi is accompanied by the expression of PR1 (6 hpi: 0.70 ± 0.08; 12 hpi: 3.67 ± 2.09; 24 hpi: 2.53 ± 0.43). The levels of SA were significantly increased at both 12 and 24 hpi (Figure 2A). After P. viticola inoculation, high PR1 levels were also described by Dufour et al. (2013) in both Benzothiadiazole-primed and control V. vinifera plants. Moreover, in V. amurensis a significant increase in SA content was also shown to occur from 24 hpi, coordinated with an increase in PR1 expression (Li et al., 2015). Interestingly, these authors have also reported a significant increase in JA content from 12 hpi, thus both SA and JA were significantly altered at the first hours after inoculation with P. viticola. Although many reports describe an antagonistic interaction between the SA and JA pathways, neutral and synergistic interactions have been described as well (Mur et al., 2006). It was shown that at low concentrations SA and JA may act synergistically and at higher concentrations the effects are antagonistic, demonstrating that the outcome of the SA-JA interaction is dependent upon the relative concentration of each hormone (Mur et al., 2006).
Although WRKY70 has been implicated in SA/JA cross talk by positively regulating SA-mediated defenses while repressing the JA response (Li et al., 2004), MYB44 shows no altered expression and WRKY70 is slightly regulated at 6 and 12 hpi (6 hpi: 2.08 ± 0.61; 12 hpi: 2.71 ± 0.53). The expression of WRKY70 seems to be coordinated with an increase of NPR1 and PR1 expression at 12 hpi but it does not repress the expression of PR10. Altogether our results suggest that at the first hours after inoculation both SA and JA pathways seem to be activated (Figure 1), but an antagonistic mechanism between the two pathways may be present at later inoculation time-points. The employment of synergistic/antagonistic mechanisms may represent positive and negative feedback loops allowing the tailoring of V. vinifera cv. Regent response to the biotrophic oomycete P. viticola.
To reduce the environmental impact of pesticide overuse, there is an increasing interest in the use of elicitors to induce resistance against pathogens in crop plants. Disease control measures for grapevine downy mildew are based on the preventive use of phytochemical compounds. Elicitors of grapevine immunity such as BABA or PS3 are being extensively studied as alternatives for pesticide application. Here, we have highlighted the involvement of jasmonic and SA in grapevine resistance against P. viticola.
Future research efforts have to be made to characterize the effectiveness of JA as an elicitor of grapevine immunity against biotrophic fungi, namely on physiological adjustments, growth, yield and reduction of disease incidence. Also, very recently the effect of the foliar application of methyl jasmonate to Tempranillo grapevines to improve wine quality was studied (Portu et al., 2015). It was shown that the phenolic composition, namely 3-O-glucosides of petunidin and peonidin, trans-p-coumaroyl derivatives of cyanidin and peonidin and trans-piceid content increase significantly. Thus exogenous application of JA and jasmonates may be not only important as elicitors of grapevine immunity but also be a simple and accessible practice to enhance grape and wine quality.
AF designed the study and planned the experiment. AG and JF performed the experiments. AF, MS, AG, and JF performed data analysis. AF and MS wrote the manuscript. All authors have read and approved the manuscript.
This work was supported by the FCT projects PTDC/AGR-GPL/112217/2009, EXPL/BBB-BIO/0439/2013, PEst-OE/BIA/UI4046/2014, PEst-OE/QUI/UI0612/2013 and UID/MULTI/00612/2013, and research grant SFRH/BPD/99712/2014.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer HK and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
The authors wish to acknowledge the Proteomics & Mass Spectrometry Facility at the Danforth Plant Science Center for its contribution on the quantification of the phytohormone levels (National Science Foundation under Grant No. DBI-1427621) and to Dr. Lisete Sousa from the Department of Statistics and Operational Research/FCUL for her advices on the statistical analysis.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.00565
Akkurt, M., Welter, L., Maul, E., Topfer, R., and Zyprian, E. (2007). Development of SCAR markers linked to powdery mildew (Uncinula necator) resistance in grapevine (Vitis vinifera L. and Vitis sp.). Mol. Breed. 29, 103–111. doi: 10.1007/s11032-006-9047-9
Ali, K., Maltese, F., Figueiredo, A., Rex, M., Fortes, A. M., Zyprian, E., et al. (2012). Alterations in grapevine leaf metabolism upon inoculation with Plasmopara viticola in different time-points. Plant Sci. 191, 100–107. doi: 10.1016/j.plantsci.2012.04.014
Avanci, N., Luche, D., Goldman, G., and Goldman, M. (2010). Jasmonates are phytohormones with multiple functions, including plant defense and reproduction. Genet. Mol. Res. 9, 484–505. doi: 10.4238/vol9-1gmr754
Bari, R., and Jones, J. (2009). Role of plant hormones in plant defence responses. Plant Mol. Biol. 69, 473–488. doi: 10.1007/s11103-008-9435-0
Belhadj, A., Saigne, C., Telef, N., Cluzet, S., Bouscaut, J., Corio-Costet, M., et al. (2006). Methyl jasmonate induces defense responses in grapevine and triggers protection against Erysiphe necator. J. Agric. Food Chem. 54, 9119–9125. doi: 10.1021/jf0618022
Belhadj, A., Telef, N., Saigne, C., Cluzet, S., Barrieu, F., Hamdi, S., et al. (2008). Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol. Biochem. 46, 493–499. doi: 10.1016/j.plaphy.2007.12.001
Blum, M., Waldner, M., and Gisi, U. (2010). A single point mutation in the novel PvCesA3 gene confers resistance to the carboxylic acid amide fungicide mandipropamid in Plasmopara viticola. Fungal Genet. Biol. 47, 499–510. doi: 10.1016/j.fgb.2010.02.009
Casagrande, K., Falginella, L., Castellarin, S., Testolin, R., and Di Gaspero, G. (2011). Defence responses in Rpv3-dependent resistance to grapevine downy mildew. Planta 234, 1097–1109. doi: 10.1007/s00425-011-1461-5
Chen, W., Delmotte, F., Richard-Cervera, S., Douence, L., Greif, C., and Corio-Costet, M. (2007). At least two origins of fungicide resistance in grapevine downy mildew Populations. Appl. Environ. Microbiol. 73, 5162–5172. doi: 10.1128/AEM.00507-07
Chini, A., Fonseca, S., Fernandez, G., Adie, B., Chico, J., Lorenzo, O., et al. (2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448, 666–671. doi: 10.1038/nature06006
Dave, A., and Graham, I. (2012). Oxylipin signaling: a distinct role for the jasmonic acid precursor cis-(+)-12-oxo-phytodienoic acid (cis-OPDA). Front. Plant Sci. 3:42. doi: 10.3389/fpls.2012.00042
Diez-Navajas, A., Wiedemann-Merdinoglu, S., Greif, C., and Merdinoglu, D. (2008). Nonhost versus host resistance to the grapevine downy mildew, Plasmopara viticola, studied at the tissue level. Phytopathology 98, 776–780. doi: 10.1094/PHYTO-98-7-0776
Dong, X. (2004). NPR1, all things considered. Curr. Opin. Plant Biol. 7, 547–552. doi: 10.1016/j.pbi.2004.07.005
Dufour, M., Lambert, C., Bouscaut, J., Merillon, J., and Corio-Costet, M. (2013). Benzothiadiazole-primed defence responses and enhanced differential expression of defence genes in Vitis vinifera infected with biotrophic pathogens Erysiphe necator and Plasmopara viticola. Plant Pathol. 62, 370–382. doi: 10.1111/j.1365-3059.2012.02628.x
Figueiredo, A., Monteiro, F., Fortes, A. M., Bonow-Rex, M., Zyprian, E., Sousa, L., et al. (2012). Cultivar-specific kinetics of gene induction during downy mildew early infection in grapevine. Funct. Integr. Genomics 12, 379–386. doi: 10.1007/s10142-012-0261-8
Figueiredo, A., Monteiro, F., and Sebastiana, M. (2015). First clues on a jasmonic acid role in grapevine resistance against the biotrophic fungus Plasmopara viticola. Eur. J. Plant Pathol. 142, 645–652. doi: 10.1007/s10658-015-0634-7
Fonseca, S., Chini, A., Hamberg, M., Adie, B., Porzel, A., Kramell, R., et al. (2009). ± 7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat. Chem. Biol. 5, 344–350. doi: 10.1038/nchembio.161
Gauthier, A., Trouvelot, S., Kelloniemi, J., Frettinger, P., Wendehenne, D., Daire, X., et al. (2014). The sulfated laminarin triggers a stress transcriptome before priming the SA- and ROS-dependent defenses during grapevine’s induced resistance against Plasmopara viticola. PLoS ONE 9:e88145. doi: 10.1371/journal.pone.0088145
Gessler, C., Pertot, I., and Perazzolli, M. (2011). Plasmopara viticola: a review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediter. 50, 3–44.
Glazebrook, J. (2005). Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227. doi: 10.1146/annurev.phyto.43.040204.135923
Hamiduzzaman, M., Jakab, G., Barnavon, L., Neuhaus, J., and Mauch-Mani, B. (2005). beta-Aminobutyric acid-induced resistance against downy mildew in grapevine acts through the potentiation of callose formation and jasmonic acid signaling. Mol. Plant Microbe Interact. 18, 819–829. doi: 10.1094/MPMI-18-0819
Koornneef, A., and Pieterse, C. (2008). Cross talk in defense signaling. Plant Physiol. 146, 839–844. doi: 10.1104/pp.107.112029
Kortekamp, A., and Zyprian, E. (2003). Characterization of Plasmopara-resistance in grapevine using in vitro plants. J. Plant Physiol. 160, 1393–1400. doi: 10.1078/0176-1617-01021
Li, J., Brader, G., and Palva, E. (2004). The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 16, 319–331. doi: 10.1105/tpc.016980
Li, X., Wu, J., Yin, L., Zhang, Y., Qu, J., and Lu, J. (2015). Comparative transcriptome analysis reveals defense-related genes and pathways against downy mildew in Vitis amurensis grapevine. Plant Physiol. Biochem. 95, 1–14. doi: 10.1016/j.plaphy.2015.06.016
Marchive, C., Leon, C., Kappel, C., Coutos-Thevenot, P., Corio-Costet, M., Delrot, S., et al. (2013). Over-Expression of VvWRKY1 in grapevines induces expression of jasmonic acid pathway-related genes and confers higher tolerance to the downy mildew. PLoS ONE 8:e54185. doi: 10.1371/journal.pone.0054185
Millardet, A. (1881). Notes sur les Vignes Américaines et Opuscules Divers sur le Même Sujet. Bordeaux: Féret & Fils.
Monteiro, F., Sebastiana, M., Pais, M. S., and Figueiredo, A. (2013). Reference gene selection and validation for the early responses to downy mildew infection in susceptible and resistant Vitis vinifera cultivars. PLoS ONE 8:e72998. doi: 10.1371/journal.pone.0072998
Mur, L., Kenton, P., Atzorn, R., Miersch, O., and Wasternack, C. (2006). The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol. 140, 249–262. doi: 10.1104/pp.105.072348
Pauwels, L., Barbero, G., Geerinck, J., Tilleman, S., Grunewald, W., Perez, A., et al. (2010). NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464, 788–791. doi: 10.1038/nature08854
Peressotti, E., Wiedemann-Merdinoglu, S., Delmotte, F., Bellin, D., Di Gaspero, G., Testolin, R., et al. (2010). Breakdown of resistance to grapevine downy mildew upon limited deployment of a resistant variety. BMC Plant Biol. 10:147. doi: 10.1186/1471-2229-10-147
Pieterse, C., Van Der Does, D., Zamioudis, C., Leon-Reyes, A., Van Wees, S., and Schekman, R. (2012). Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28, 489–521. doi: 10.1146/annurev-cellbio-092910-154055
Polesani, M., Bortesi, L., Ferrarini, A., Zamboni, A., Fasoli, M., Zadra, C., et al. (2010). General and species-specific transcriptional responses to downy mildew infection in a susceptible (Vitis vinifera) and a resistant (V. riparia) grapevine species. BMC Genomics 11:117. doi: 10.1186/1471-2164-11-117
Portu, J., Santamaría, P., López-Alfaro, I., López, R., and Garde-Cerdán, T. (2015). Methyl jasmonate foliar application to Tempranillo vineyard improved grape and wine phenolic content. J. Agric. Food Chem. 63, 2328–2337. doi: 10.1021/jf5060672
Shim, J., Jung, C., Lee, S., Min, K., Lee, Y., Choi, Y., et al. (2013). AtMYB44 regulates WRKY70 expression and modulates antagonistic interaction between salicylic acid and jasmonic acid signaling. Plant J. 73, 483–495. doi: 10.1111/tpj.12051
Staswick, P., and Tiryaki, I. (2004). The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16, 2117–2127. doi: 10.1105/tpc.104.023549
Thines, B., Katsir, L., Melotto, M., Niu, Y., Mandaokar, A., Liu, G., et al. (2007). JAZ repressor proteins are targets of the SCFCO11 complex during jasmonate signalling. Nature 448, 661–665. doi: 10.1038/nature05960
Trouvelot, S., Varnier, A., Allegre, M., Mercier, L., Baillieul, F., Arnould, C., et al. (2008). A beta-1,3 glucan sulfate induces resistance in grapevine against Plasmopara viticola through priming of defense responses, including HR-like cell death. Mol. Plant Microbe Interact. 21, 232–243. doi: 10.1094/MPMI-21-2-0232
Unger, S., Bueche, C., Boso, S., and Kassemeyer, H. (2007). The course of colonization of two different vitis genotypes by Plasmopara viticola indicates compatible and incompatible host-pathogen interactions. Phytopathology 97, 780–786. doi: 10.1094/PHYTO-97-7-0780
Wasternack, C. (2007). Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 100, 681–697. doi: 10.1093/aob/mcm079
Wasternack, C., and Hause, B. (2013). Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. an update to the 2007 review in Annals of Botany. Bot. 111, 1021–1058. doi: 10.1093/aob/mct067
Keywords: Vitis vinifera, biotroph, downy mildew, salicylic acid, jasmonic acid
Citation: Guerreiro A, Figueiredo J, Sousa Silva M and Figueiredo A (2016) Linking Jasmonic Acid to Grapevine Resistance against the Biotrophic Oomycete Plasmopara viticola. Front. Plant Sci. 7:565. doi: 10.3389/fpls.2016.00565
Received: 29 December 2015; Accepted: 12 April 2016;
Published: 28 April 2016.
Edited by:
Ralph Panstruga, RWTH Aachen University, GermanyReviewed by:
Rensen Zeng, Fujian Agriculture and Forestry University, ChinaCopyright © 2016 Guerreiro, Figueiredo, Sousa Silva and Figueiredo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreia Figueiredo, YWFmaWd1ZWlyZWRvQGZjLnVsLnB0
†These authors are co-senior authors.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.