
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci. , 31 March 2016
Sec. Plant Biotechnology
Volume 7 - 2016 | https://doi.org/10.3389/fpls.2016.00398
This article is part of the Research Topic Mechanisms of abiotic stress responses and tolerance in plants: physiological, biochemical and molecular interventions View all 121 articles
It is well known that intracellular signaling from chloroplast to nucleus plays a vital role in stress responses to survive environmental perturbations. The chloroplasts were proposed as sensors to heat stress since components of the photosynthetic apparatus housed in the chloroplast are the major targets of thermal damage in plants. Thus, communicating subcellular perturbations to the nucleus is critical during exposure to extreme environmental conditions such as heat stress. By coordinating expression of stress specific nuclear genes essential for adaptive responses to hostile environment, plants optimize different cell functions and activate acclimation responses through retrograde signaling pathways. The efficient communication between plastids and the nucleus is highly required for such diverse metabolic and biosynthetic functions during adaptation processes to environmental stresses. In recent years, several putative retrograde signals released from plastids that regulate nuclear genes have been identified and signaling pathways have been proposed. In this review, we provide an update on retrograde signals derived from tetrapyrroles, carotenoids, reactive oxygen species (ROS) and organellar gene expression (OGE) in the context of heat stress responses and address their roles in retrograde regulation of heat-responsive gene expression, systemic acquired acclimation, and cellular coordination in plants.
Taking into account the presence of genes encoding organellar proteins in different cellular compartments of the plant cell, intracellular communication is critical for regulation and coordination of a variety of physiological processes, including responses to biotic and abiotic stresses. By definition, retrograde signaling is a communication pathway whereby the transcriptional activities in the nucleus are regulated in part by signals derived from plastids and mitochondria. According to existing literatures, two categories can be largely classified in respect to retrograde signaling, including developmental control of organelle biogenesis, and operational control to adjust and acclimate to environmental stresses (Pogson et al., 2008). In general, the chloroplasts in plants and algae are assumed to be the descendants of the ancient photosynthetic bacteria. As an semi-autonomous organelle, the chloroplast maintains a similar circular genome and transcription and translation machinery like its evolutionary precursor (Watson and Surzycki, 1983; Sugiura et al., 1998). Because the components of the complex energetic reactions linked to photosynthesis are encoded by organelle and nuclear genomes, gene expression in these separate compartments require the existence of sophisticated regulatory mechanisms that ensure adequate synthesis of proteins functioning in common photosynthetic complexes. The tightly coordinated gene expression in both nucleus and chloroplast is required for the correct stoichiometric subunit composition of these complexes. It is generally accepted that anterograde signals originating from the nucleus and retrograde signals emerging from the chloroplast orchestrate this intracellular coordination (Woodson and Chory, 2008).
Historically, the first report describing the retrograde signaling was based on two barley chloroplast ribosome-deficient mutants, the barley (Hordeum vulgare) albostrians, whose defects in plastid functions result in downregulation of nuclear-encoded plastid proteins (Bradbeer et al., 1979). Since, this revolutionary discovery, the intensive studies have been focusing on the function of retrograde signaling in plastid development by coordinating chlorophyll biosynthesis with the expression of nuclear genes that encode plastid-localized chlorophyll-binding proteins using young seedlings of mustard, Arabidopsis, pea, or barley with lincomycin, chloramphenicol, or streptomycin, the inhibitors of plastid protein synthesis (Oelmuller et al., 1986; Susek et al., 1993; Yoshida et al., 1998; Sullivan and Gray, 1999). The gun (genome uncoupled) mutants where the communication between the chloroplast and the nucleus has been disrupted are very helpful in deciphering the retrograde signaling phenomenon (Woodson and Chory, 2008; Barajas-Lopez et al., 2013; Chi et al., 2013). The mutant seedlings express nuclear-encoded photosynthetic genes (PhANG) despite defective chloroplast physiology or inhibited biogenesis (Susek et al., 1993). According to restrictions in defined steps in tetrapyrrole biosynthesis, identification of the gun2, gun3, gun4, and gun5 mutants provided evidence that accumulation of the chlorophyll intermediate Mg-protoporphyrin IX (Mg-Proto IX) is involved in initiation of retrograde signaling whereas the gun1 mutant results from mutation in a gene encoding a chloroplast-localized pentatricopeptide repeat-containing protein (PPR; Susek et al., 1993; Mochizuki et al., 2001; Larkin et al., 2003; Strand et al., 2003; Koussevitzky et al., 2007).
The involvement of the key enzymes of the tetrapyrrole biosynthesis pathway in the gun phenotype led to numerous studies on tetrapyrroles, especially Mg-protoporphyrin IX (Mg-ProtoIX), as putative retrograde signals. Large changes in nuclear gene expression have been triggered by stress-induced perturbations of tetrapyrrole biosynthesis and specific accumulation of the chlorophyll biosynthetic intermediate Mg-ProtoIX and its methylester (Mg-ProtoIX-ME) have been shown to coincide with these changes in nuclear gene expression (Johanningmeier and Howell, 1984; Kropat et al., 1997, 2000; Strand et al., 2003; Alawady and Grimm, 2005; Pontier et al., 2007; von Gromoff et al., 2008). By taking advantage of the fluorescent properties of tetrapyrroles, Mg-ProtoIX could be visualized to accumulate both in the chloroplasts and the cytosol under stress conditions using confocal laser scanning spectroscopy (Ankele et al., 2007), suggesting a possibility that the signaling metabolite Mg-ProtoIX is exported from the chloroplast to the cytosol, thus to transmit the plastid signal to nucleus. However, the role of Mg-ProtoIX/Mg-ProtoIX-ME as a plastid signal was subsequently questioned because no correlation between the accumulation of Mg-ProtoIX and retrograde signaling was observed in two different studies (Mochizuki et al., 2008; Moulin et al., 2008). Given that Mg-ProtoIX is phototoxic, its accumulation within cytosol might induce problematic effects on cellular homeostasis. Thus, the identity of retrograde signals remains worthy of further investigation. Interestingly, a recent report identified heme as a strong candidate for mediating chloroplast-to-nucleus signaling (Woodson et al., 2011). Two plastid-derived isoprenoid derivatives, methylerythritol cyclodiphosphate, and β-cyclocitral, were reported to function as retrograde signal molecules although their receptor(s) or sites of action in the nucleus remain largely unknown (Ramel et al., 2012; Xiao et al., 2012). Importantly, as an intermediate of the methylerythritol phosphate (MEP) pathway, methylerythritol cyclodiphosphate accumulates in response to abiotic stresses (Xiao et al., 2012). Similarly, β-Cyclocitral, a volatile apocarotenoid derived from β-carotene, accumulates in response to singlet oxygen (1O2) and light stresses and regulates nuclear gene expression (Ramel et al., 2012, 2013). Moreover, 1O2-induced signaling and feedback was proposed to mediate the impact of tetrapyrrole biosynthesis on nuclear gene expression (Schlicke et al., 2014). These findings indicate that the nature of retrograde signaling remains largely undefined.
Plants have evolved complex signaling networks to sense and respond to environmental stresses. It has been assumed that chloroplasts act as a specific sensor of intra- and extracellular stimuli and can integrate a multitude of intracellular signals and pathways in order to sustain homeostasis at both the cellular and organismal levels. With respect to chloroplast-nuclear signaling in response to environmental stimuli, intensive studies have been focusing on the initiation of signaling cascades in the chloroplast and transcriptional changes in the nucleus. In the past few years, a number of retrograde signaling pathways were identified and/or proposed as stress-specific organelles-to-nucleus retrograde signaling cascades. This review focuses on the recent advancements in revealing the order of events that induce the activation of the acclimation response to abiotic stresses. Special attention was given to the retrograde regulatory networks identified in studies on cellular responses to heat stress.
According to existing literatures, a temperature upshift, usually 10–15°C above an optimum temperature for growth, is taken as heat stress for photosynthetic processes in higher plants (Wahid et al., 2007; Allakhverdiev et al., 2008). Considering that components of the photosynthetic apparatus in the chloroplast are susceptible targets of thermal damage in plants, the chloroplasts were proposed as sensors to heat stress. It should be noted that heat stress commonly causes severe thermal damages to photosystem II (PSII), the most heat-sensitive photosynthetic apparatus within the chloroplast thylakoid membrane protein complexes involved in photosynthetic electron transfer and ATP synthesis (Berry and Bjorkman, 1980; Havaux, 1993; Sharkey, 2005; Wahid et al., 2007; Allakhverdiev et al., 2008). As common indicators of heat stress-induced damages, chlorophyll fluorescence, the ratio of variable fluorescence to maximum fluorescence (Fv/Fm) and the base fluorescence (Fo), correlates with disruptions of photochemical reactions in thylakoid lamellae of chloroplasts (Yamada et al., 1996; Wise et al., 2004; Wahid et al., 2007; Allakhverdiev et al., 2008). Particularly, heat stress causes the dissociation of oxygen evolving complex (OEC) in PSII, which further results in an imbalance in the electron flow from OEC toward the acceptor side of PSII in the direction of PSI reaction center (Havaux and Tardy, 1996; Klimov et al., 1997; Wahid et al., 2007; Allakhverdiev et al., 2008). Moreover, the reaction center-binding protein D1 of PSII was cleaved and a manganese (Mn)-stabilizing 33-kDa proteins was dissociated from PSII reaction center complex in spinach thylakoids upon heat stress treatment (Yamane et al., 1997). In addition to damages on OEC in PSII, heat stress also causes dramatic reduction in the efficiency in carbon assimilation metabolism in the stroma of chloroplast (Sharkey, 2005).
In the prokaryotic and eukaryotic kingdoms, the heat stress response as a universal cellular response represents the first line of inducible defense against imbalances in cellular homeostasis. In higher plants, a rapid expression reprogramming of a set of proteins known as heat shock proteins (HSPs) is considered to be a marked activation of heat stress response (Kotak et al., 2007; von Koskull-Doring et al., 2007). Based on the analysis of Arabidopsis and crop plants genome-wide expression profiles, the transcripts of the well-characterized HSPs increased dramatically, including Hsp101, Hsp70s, and small HSPs, which are proposed to act as molecular chaperones in protein quality control under heat stress (Rizhsky et al., 2004; Busch et al., 2005; Lim et al., 2006; Schramm et al., 2006; Larkindale and Vierling, 2008; Matsumoto et al., 2014; Wang et al., 2014b; Frey et al., 2015).
In the unicellular green alga Chlamydomonas reinhardtii, Mg-ProtoIX and Mg-ProtoIX-ME were previously shown to transiently induce the expression of HEAT SHOCK PROTEIN 70A/B (HSP70A and HSP70B) encoding the cytosolic and the plastid-localized proteins, respectively (Kropat et al., 1997, 2000). However, the light-induction of HSP70 is impaired in the chlorophyll-deficient brs-1 mutant, indicating that the accumulation of Mg-ProtoIX is essential for the light induction of HSP70 (Hess et al., 1994). Using the four Chlamydomonas reinhardtii mutants in the Mg-chelatase that catalyzes the insertion of magnesium into protoporphyrin IX, the reduced levels of Mg-tetrapyrroles but increased levels of soluble heme were detected in the four mutants and the light-induction of HSP70A was preserved, although Mg-ProtoIX has been implicated in this induction (von Gromoff et al., 2008). More importantly, HSP70A was activated by feeding Hemin to algae cultures in the dark and this induction was mediated by the same plastid response element (PRE) in the HSP70A promoter that has also been shown to mediate induction by Mg-ProtoIX and light. Such communication likely involves both Hemin and Mg-ProtoIX, respectively, indicating that these signals converge on the same pathway (von Gromoff et al., 2008). Studies focused on the induction specificity of these two tetrapyrroles demonstrated that neither Proto, nor Pchlide or Chlide was able to induce the nuclear genes (Kropat et al., 1997). A model was derived according to accumulated literature (Beck, 2001, 2005): MgProto and/or MgProtoMe, produced in the plastid become(s) accessible on the cytoplasmic side of the chloroplast after light activation of the HSP70 genes within the chloroplast or its envelope. In cytoplasm or nucleus, putative factor(s) may recognize these tetrapyrroles, either regulating expression of the nuclear HSP70 genes directly or stimulating a signaling cascade that enters the nucleus.
Given that the tetrapyrroles Mg-ProtoIX and heme have been implicated in the retrograde control of nuclear gene expression in Chlamydomonas reinhardtii, the intensive studies have been focusing on genome-wide transcriptional profiling to explore the global impact of these tetrapyrroles on regulation of gene expression and the scope of the response. Upon feeding with Mg-ProtoIX and heme, almost 1,000 genes were shown to be changed transiently but significantly (Voss et al., 2011). Most of these genes encoded enzymes of the tricarboxylic acid cycle, heme-binding proteins, stress-response proteins, as well as proteins involved in protein folding and degradation whereas only a few genes were involved in photosynthetic processes. More than 50% of the latter class of genes was also regulated by heat shock. Significantly, 51% of the 982 tetrapyrrole-regulated genes were also activated in response to heat stress, indicating that both tetrapyrroles function as secondary messengers for adaptive physiological responses affecting the entire cell and not only organellar proteins.
The chloroplast in plants and algae maintains a circular genome and transcription and translation machinery similar to that of its evolutionary precursor, the descendants of the ancient photosynthetic bacteria (Sugiura et al., 1998). Accordingly, the majority of chloroplast proteins encoded in the nucleus are imported into the chloroplasts after biosynthesis in the cytoplasm (Jarvis, 2008). The existence of an organellar gene expression (OGE)-dependent retrograde signal pathway was first suggested more than 30 years ago (Bradbeer et al., 1979). Subsequently, numerous studies found that treatments with inhibitors of OGE, such as chloramphenicol, lincomycin, or erythromycin severely inhibited the expression of nuclear genes for photosynthesis-related proteins during early stages of plastid development (Oelmuller et al., 1986; Susek et al., 1993; Yoshida et al., 1998; Sullivan and Gray, 1999). One of working modules for the OGE-dependent retrograde signal pathway is that a plastid-localized pentatricopeptide repeat (PPR) protein, encoded by GUN1, integrates the signal cascades triggered by the aberrant plastid functions before the integrated signal is transmitted into the nucleus by a regulatory mechanism involving the transcription factor ABA INSENSITIVE4 (ABI4; Koussevitzky et al., 2007). It has been proposed that a disruption on protein synthesis in the chloroplast could give rise to a signal or that the affected plastids keep away from the stage at which they could send the appropriate signal while the identity of the components of the signaling cascade and, in particular, the primary target genes and transcriptional factors (TFs) mediating this response remain largely unknown (Kleine et al., 2009). A recent report revealed that the chloroplast translational capacity is a critical factor in generating the retrograde signal (s) to activate the heat-responsive expressions of the heat stress transcription factor HsfA2 and its target genes (Yu et al., 2012). In the study described above, the chloroplast ribosomal protein S1 (RPS1) was identified as a heat-responsive protein, functioning as a subunit protein of the plastid ribosome in synthesis of photosynthetic proteins in Arabidopsis. In general, mutations of RPS1 causes the translational defects in chloroplasts repress the nuclear heat-responsive gene expression upon heat treatments, revealing a novel regulatory mechanism whereby plant cells trigger heat-responsive activation of the nuclear gene expression to keep accordance with the current status of chloroplasts under heat stress.
Accumulated evidence has also shed light on the effect of chloroplast–mitochondria signaling interactions on stress responses. The tight communication between chloroplasts and mitochondria is required for balancing the activities of the two energy organelles under normal growth conditions or in adaptation to environmental stresses (Woodson and Chory, 2008; Blanco et al., 2014; Ng et al., 2014; Allahverdiyeva et al., 2015; Bobik and Burch-Smith, 2015; Gollan et al., 2015; Van Akent and Van Breusegem, 2015). It has been proposed that the metabolite exchange between the organelles via translocators located on envelope membranes of chloroplasts and mitochondria acts as an important channel of communication, which contributes to their central roles in energy capture and utilization (Blanco et al., 2014; Bobik and Burch-Smith, 2015). As for the intra-mitochondrial stress response, energy-dissipating components modulate the retrograde signaling pathways from mitochondria, which controls the cellular adaptation processes under stress conditions (Ng et al., 2014; Rurek, 2014). In Arabidopsis, AOX1a isoform is induced by heat stress (Elhafez et al., 2006) and seems to be also regulated by chloroplasts upon highlight treatment (Finnegan et al., 1997; Blanco et al., 2014). Recent studies suggest that AOX1a functions in optimizing photosynthesis by sustaining the chloroplastic redox state and regulating cellular redox homeostasis when electron transport through the COX pathway is disturbed at complex III (Pu et al., 2015; Vishwakarma et al., 2015). Interestingly, ABI4 was shown to regulate the responsive expression of both Lhcb and AOX1A genes, suggesting that ABI4 acts as a critical molecular link for coordinating the communication between chloroplasts and mitochondria (Koussevitzky et al., 2007; Giraud et al., 2009). A recent report suggests that the nuclear localized cyclin-dependent kinase E1 (CDKE1), a prerequisite for AOX induction, acts as a central nuclear component integrating mitochondrial and plastid retrograde signals, which could modulate energy metabolism under stress (Blanco et al., 2014). The mitochondrial retrograde signaling and auxin signaling are reported to be reciprocally regulated in balancing growth and environmental stresses (Ivanova et al., 2014) and a membrane-bound NAC transcription factor, ANAC017, was identified to mediate mitochondrial retrograde signaling by genetic screening regulators of alternative oxidase1a mutants in Arabidopsis (Ng et al., 2013). It has been known that mitochondria of plant cells modulate the cytosolic Ca2+ level by changing the potential at the inner mitochondrial membrane, functioning in the retrograde regulation of expression of heat-responsive genes (Pyatrikas et al., 2014; Rikhvanov et al., 2014). However, delineating these stress-interacting networks between chloroplasts and mitochondria involving the perception and integration of stress stimuli is important and most of network components remain largely unexplored.
Based on an update on recent findings related to plastid-to-nucleus metabolic signals in plants, it has been suggested that the metabolic reprogramming under a variety of environmental stresses relates to the functional alteration of essential cell compartments, such as chloroplasts (Estavillo et al., 2011, 2013; Xiao et al., 2012, 2013; Chi et al., 2015). Plastid retrograde signals could be generated from various sources, including the tetrapyrrole pathway, the level of ROS, or the related metabolic processes in the plastids as described in Table 1. Particularly, ROS among all these signals are thought to play a key role in modulating initial signal cascades in higher plants. Recently, new plastid metabolic signals within chloroplasts have been identified in Arabidopsis (Estavillo et al., 2013; Xiao et al., 2013; Chi et al., 2015), emphasizing the role of plastids in abiotic-stress sensing and signaling in a retrograde way.
As an unavoidable consequence of aerobic metabolism, plants permanently produce a variety of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), superoxide, hydroxyl radicals (⋅OH), and 1O2. It is well known that the chloroplast is a major producer of ROS during photosynthesis and contains a large array of ROS-scavenging mechanisms (Asada, 2006). A variety of abiotic stresses inhibit the excitation energy transfer in the PSII antenna complex and the electron transport in the PSII reaction center in algae and higher plants and the limitation in the excitation energy transfer and the electron transport is accompanied with the formation of ROS (Suzuki et al., 2012). ROS such as 1O2 is formed by the excitation energy transfer, whereas superoxide anion radical ( ), H2O2 and ⋅OH are formed by the electron transport (Pospisil and Prasad, 2014). ROS are proposed to diffuse away from their sites of production and consequently elicit a different set of signaling events under a wide range of biotic and abiotic stress conditions (Neill et al., 2002; Mittler et al., 2004; Miller et al., 2008; Suzuki et al., 2012). A variety of operational retrograde signaling pathways are thought to be triggered by ROS and photosynthesis redox imbalance during stress conditions and play an important role in the acclimation of plants (Pogson et al., 2008; Galvez-Valdivieso and Mullineaux, 2010; Suzuki et al., 2012). On the other hand, the current redox-status of chloroplasts, which correlates with the imbalance of ROS accumulation caused by abiotic stresses, may be transmitted by monitoring the state of the plastoquinone, ascorbate, and glutathione pools (Foyer et al., 2009; Suzuki et al., 2012; Foyer and Noctor, 2013; Petrov et al., 2015). In addition to chloroplasts, several other heat stress-dependent ROS production sites have been described. It has been suggested that the respiratory burst oxidase homolog D (RBOHD), acting as a ROS-generating NADPH oxidase in the plasma membrane, could function in the oxidative burst occurring during heat stress (Suzuki et al., 2011). The accumulation of H2O2 can be inhibited by an inhibitor of the enzyme NADPH oxidase in Arabidopsis and tobacco cell cultures, suggesting that RBOHD has a central role in heat stress signaling and thermotolerance (Larkindale et al., 2005; Volkov et al., 2006; Koenigshofer et al., 2008; Miller et al., 2009). It was reported that an impaired mitochondrial metabolism seems to be responsible for oxidative bursts occurring in tobacco cells undergoing heat-induced programmed cell death (PCD), suggesting that production of ROS during heat stress can also occur in mitochondria (Vacca et al., 2004; Valenti et al., 2007). Recent studies suggest that AOX1A plays a significant role in regulating ROS generation when electron transport is disrupted through the COX pathway (Vishwakarma et al., 2015). It was reported that the protonophore CCCP-induced depolarization of the mitochondrial membrane inhibited ROS generation upon heat treatments, suggesting that heat stress-induced mitochondrial membrane hyperpolarization causes the increased ROS production in plant cells (Fedyaeva et al., 2014). As a mitochondrial inner membrane protein, uncoupling protein one (UCP1) is able to uncouple the electrochemical gradient from adenosine-5′-triphosphate (ATP) synthesis, dissipating energy as heat. Overexpressing a plant UCP1 ortholog in the mitochondrial inner membrane triggered increased uncoupling respiration and inhibited ROS accumulation under abiotic stresses (Barreto et al., 2014). As a key phospholipid in mitochondrial membranes, cardiolipin (CL) is involved in maintaining the functional integrity and dynamics of mitochondria. The mutations of CARDIOLIPIN SYNTHASE (CLS) caused defects in mitochondrial morphogenesis and stress response to heat treatments in Arabidopsis, which has revealed a novel regulatory mechanism in adaptation to environmental stresses in plants (Pan et al., 2014). According to the plant mitochondrial literatures, a prominent theme is likely to link mitochondrial composition to environmental stress responses (Miller et al., 2008; Mittler et al., 2011; Jacoby et al., 2012; Suzuki et al., 2012; Noctor et al., 2014; Van Akent and Van Breusegem, 2015; You and Chan, 2015). Particularly, peroxisomes are another site of ROS generation under conditions that increase photorespiration, such as stomatal closure (Vanderauwera et al., 2011).
), H2O2 and ⋅OH are formed by the electron transport (Pospisil and Prasad, 2014). ROS are proposed to diffuse away from their sites of production and consequently elicit a different set of signaling events under a wide range of biotic and abiotic stress conditions (Neill et al., 2002; Mittler et al., 2004; Miller et al., 2008; Suzuki et al., 2012). A variety of operational retrograde signaling pathways are thought to be triggered by ROS and photosynthesis redox imbalance during stress conditions and play an important role in the acclimation of plants (Pogson et al., 2008; Galvez-Valdivieso and Mullineaux, 2010; Suzuki et al., 2012). On the other hand, the current redox-status of chloroplasts, which correlates with the imbalance of ROS accumulation caused by abiotic stresses, may be transmitted by monitoring the state of the plastoquinone, ascorbate, and glutathione pools (Foyer et al., 2009; Suzuki et al., 2012; Foyer and Noctor, 2013; Petrov et al., 2015). In addition to chloroplasts, several other heat stress-dependent ROS production sites have been described. It has been suggested that the respiratory burst oxidase homolog D (RBOHD), acting as a ROS-generating NADPH oxidase in the plasma membrane, could function in the oxidative burst occurring during heat stress (Suzuki et al., 2011). The accumulation of H2O2 can be inhibited by an inhibitor of the enzyme NADPH oxidase in Arabidopsis and tobacco cell cultures, suggesting that RBOHD has a central role in heat stress signaling and thermotolerance (Larkindale et al., 2005; Volkov et al., 2006; Koenigshofer et al., 2008; Miller et al., 2009). It was reported that an impaired mitochondrial metabolism seems to be responsible for oxidative bursts occurring in tobacco cells undergoing heat-induced programmed cell death (PCD), suggesting that production of ROS during heat stress can also occur in mitochondria (Vacca et al., 2004; Valenti et al., 2007). Recent studies suggest that AOX1A plays a significant role in regulating ROS generation when electron transport is disrupted through the COX pathway (Vishwakarma et al., 2015). It was reported that the protonophore CCCP-induced depolarization of the mitochondrial membrane inhibited ROS generation upon heat treatments, suggesting that heat stress-induced mitochondrial membrane hyperpolarization causes the increased ROS production in plant cells (Fedyaeva et al., 2014). As a mitochondrial inner membrane protein, uncoupling protein one (UCP1) is able to uncouple the electrochemical gradient from adenosine-5′-triphosphate (ATP) synthesis, dissipating energy as heat. Overexpressing a plant UCP1 ortholog in the mitochondrial inner membrane triggered increased uncoupling respiration and inhibited ROS accumulation under abiotic stresses (Barreto et al., 2014). As a key phospholipid in mitochondrial membranes, cardiolipin (CL) is involved in maintaining the functional integrity and dynamics of mitochondria. The mutations of CARDIOLIPIN SYNTHASE (CLS) caused defects in mitochondrial morphogenesis and stress response to heat treatments in Arabidopsis, which has revealed a novel regulatory mechanism in adaptation to environmental stresses in plants (Pan et al., 2014). According to the plant mitochondrial literatures, a prominent theme is likely to link mitochondrial composition to environmental stress responses (Miller et al., 2008; Mittler et al., 2011; Jacoby et al., 2012; Suzuki et al., 2012; Noctor et al., 2014; Van Akent and Van Breusegem, 2015; You and Chan, 2015). Particularly, peroxisomes are another site of ROS generation under conditions that increase photorespiration, such as stomatal closure (Vanderauwera et al., 2011).
As the organelles in which photosynthesis occurs, chloroplasts are extremely susceptible to heat stress (Yamada et al., 1996; Wise et al., 2004; Wahid et al., 2007; Allakhverdiev et al., 2008). Several lines of evidence suggest that different environmental stresses, including also high temperature, can result in oxidative bursts of superoxide and/or hydrogen peroxide in plants (Foyer et al., 1997; Dat et al., 1998; Vallelian-Bindschedler et al., 1998). Exposure to high temperature stress can lead to the increased accumulation of ROS in PSI, PSII as well as in the Calvin–Benson cycle, which cause irreversible oxidative damage to cells (Asada, 2006; Suzuki et al., 2012). ROS that are produced in chloroplasts can function as plastid signals to inform the nucleus to activate the expression of genes encoding antioxidant enzyme and to adjust the stress-responsive machinery for more efficient adaptation to environmental stresses. Under high temperature conditions, tobacco cells produce large amounts of ROS, which are a prerequisite for triggering PCD signaling cascades since application of the antioxidants ascorbate or superoxide dismutase (SOD) to the cultures supports cell survival (Vacca et al., 2006). Upon heat stress treatment, hydrogen peroxide accumulated in the leaves of tobacco (Nicotiana tabacum) defective in ndhC–ndhK–ndhJ (Delta ndhCKJ), suggesting the function of the NAD(P) H dehydrogenase-dependent pathway in suppressing the accumulation of ROS in chloroplasts (Wang et al., 2006). These results also indicate that the cyclic photophosphorylation via the NDH pathway might play an important role in regulation of CO2 assimilation under heat-stressed condition, thus optimizing the photosynthetic electron transport and reducing the generation of ROS. Under moderate heat treatment conditions, cleavage of the reaction center-binding D1 protein of photosystem II was observed in spinach thylakoid membranes (Yamashita et al., 2008). In accordance with this, 1O2 and ⋅OH were detected in spinach PSII membranes, suggesting that the ROS are generated by heat-induced inactivation of a water-oxidizing manganese complex and through lipid peroxidation. In Arabidopsis, following heat treatment, the chlorophyll synthase mutant (chlg-1) accumulated a substantial level of chlorophyllide a, which resulted in a surge of phototoxic singlet oxygen, suggesting that chlorophyll synthase acts in maintenance of ROS homeostasis in response to heat stress (Lin et al., 2014).
Unlike plastid gene expression (PGE)-dependent signals, the ROS-dependent retrograde signaling pathways are thought to be primarily acting in adaptation to environmental stresses rather than genome coordination (Woodson and Chory, 2008). Among a variety of ROS-dependent retrograde signaling pathways, most of studies are focusing on the singlet oxygen pathway, which is independent of Mg-ProtoIX and GUN1-mediated signaling (Suzuki et al., 2012). Unlike H2O2, 1O2 is a highly reactive radical that is involved in signaling pathway leading to cell death or to acclimation (Wagner et al., 2004). Using the conditional flu mutants that accumulate protochlorophyllide, a potent photosensitizer and generate large amounts of 1O2 during dark adaptation and upon re-exposure to light, the singlet oxygen signaling pathway has been extensively studied in Arabidopsis (Meskauskiene et al., 2001; op den Camp et al., 2003; Apel and Hirt, 2004; Wagner et al., 2004; Laloi et al., 2007; Lee et al., 2007). Importantly, the accumulated 1O2 in the flu chloroplasts correlates with the induction of stress responses, including dramatic alterations in nuclear gene expression and enhanced biosynthesis of the stress hormones, SA, Et, and JA (op den Camp et al., 2003). Moreover, these 1O2-induced changes were regulated by the chloroplastic proteins EXECUTER1 (EX1) and EXECUTER 2 (EX2) through a distinct pathway (Wagner et al., 2004; Lee et al., 2007; Kim et al., 2012). Interestingly, D1 protein has been identified as a primary target of 1O2 and seems to act as a major scavenger of 1O2 because of being close to the site of 1O2 formation in the RC of PSII (Vass and Cser, 2009). D1 protein is well known for its high turnover rate, which has been attributed to the rapid degradation of the oxidized D1 protein after its interaction with 1O2 and its replacement by newly synthesized D1 polypeptides (Aro et al., 1993; Lindahl et al., 2000). In addition to the D1 protein, β-carotene, plastoquinol, and α-tocopherol have also been identified as scavenger of 1O2 and protect PSII against photo-oxidative damage (Telfer et al., 1994; Kruk and Trebst, 2007). Given that the half-life of 1O2 is very short (200 ns), it is unlikely to escape from the plastid compartment and transmit to nucleus. Thus, more stable second messengers derived from 1O2 within the plastid are assumed to activate a signaling pathway in controlling the expression of nuclear genes. As one of the main 1O2 quenchers in chloroplasts, β-carotene can be oxidized by 1O2 to produce β-cyclocitral that was found to be involved in singlet oxygen-dependent retrograde signaling (Ramel et al., 2012).
As the most stable ROS, H2O2 serves as a signaling molecule that plays a crucial role in environmental stress responses (Mittler et al., 2011; Suzuki et al., 2012). To survive under heat stress conditions, plants undergo a process of stress acclimation in which HSP expression is promptly activated by specific heat shock transcription factors (HSFs) binding the conserved sequence of the heat shock elements (HSEs) in the promoters of heat-responsive genes (Nover et al., 1996; Scharf et al., 1998; Kotak et al., 2007). As a universal cellular response to a shift up in temperature, the HSF–HSP network is a highly conserved molecular mechanism, representing the first line of inducible defense against imbalances in cellular homeostasis in the prokaryotic and eukaryotic kingdoms. Several lines of evidence support that the heat-responsive expression of HSP and chaperones can be induced by oxidative stress in plants, which can provide a protective function against oxidative stress. In tomato and rice, H2O2 induced the expression of mitochondrial HSP22 and chloroplastic HSP26, respectively (Banzet et al., 1998; Lee et al., 2000). In cyanobacteria and Arabidopsis, high light and H2O2, respectively, induced activation of some chaperones, HSP, and HSFs at mRNA levels (Desikan et al., 2001; Hihara et al., 2001). To dissect particular stress responses that are relative to H2O2 signals, various types of model systems including mutants and transgenic plants altered in the ROS levels are applied to investigate the signaling networks. Using genome-wide analysis of the Arabidopsis catalase deficient mutant, a series of genes encoding specific small HSPs, several transcription factors and candidate regulatory proteins were found to be regulated by H2O2 (Vandenabeele et al., 2004; Vanderauwera et al., 2005). Subsequently, H2O2 was reported to play a major signaling role in the activation of HSFs during the early phase of heat stress (Volkov et al., 2006). In Class A Hsfs, HsfA2 is highly inducible at the transcriptional level in response to heat stress and the treatments with H2O2 and ozone (Nishizawa et al., 2006). Intensive studies suggest a critical role of the mitogen-activated protein kinase (MAPK) in H2O2-mediated expression of Hsfs, including HsfA2 under heat stress (Kovtun et al., 2000; Link et al., 2002; Sangwan et al., 2002; Kotak et al., 2007; Saidi et al., 2011). According to existing literatures, it has been assumed that H2O2 is likely to diffuse freely across the chloroplast envelope to trigger a cytosolic MAPK cascade as a signal (Apel and Hirt, 2004). Significantly, treatments with ascorbate or DPI inhibited the heat-induced expression levels, suggesting that H2O2 acts as an essential component in the heat stress signaling pathway. Interestingly, heat shock promoter element (HSE) protein-binding complex of high molecular weight rapidly (15 min) formed in extracts of heat-stressed or H2O2-treated cells, indicating that oxidative stress affects gene expression via HSF activation. As described above, ROS act as molecular signals to activate downstream pathways, resulting in protective effects. So far, several redox-sensitive TFs have been identified since their activities rely on redox changes (Tron et al., 2002; Heine et al., 2004; Shaikhali et al., 2008, 2012). Notably, the heat shock factor HSFA4a can act as a sensor of ROS and function upstream of ZAT12 and APX1, two genes with an HSE in their promoters, suggesting that HSFA4a plays an key role in the ROS-mediated heat stress responses (Miller and Mittler, 2006).
To function as a signaling molecule on a cellular level, H2O2 has to be able to cross the inner and outer envelopes of the chloroplast and other membranes, yet its polar nature might limit its capacity to diffuse through hydrophobic membranes unassisted. In this context, the question arises as to what extent H2O2 is able to diffuse out of the compartments where it is generated. H2O2 can be released from isolated chloroplasts immediately (<1 min) upon illumination, as shown by an increase in resorufin fluorescence and detection of electron paramagnetic resonance signals from H2O2-derived hydroxyl radicals (Mubarakshina et al., 2010). H2O2 is thought to be able to diffuse through membranes, possibly through aquaporins (Henzler and Steudle, 2000; Bienert et al., 2007; Bienert and Chaumont, 2014), suggesting that channel-mediated membrane transport allows the fine adjustment of H2O2 levels in the cytoplasm, intracellular organelles, the apoplast, and the extracellular space, which are essential for it to function as a signal molecule. Using fluorescent probe Amplex red which forms fluorescent products in the reaction with H2O2, the increased production of hydrogen peroxide under high light conditions was observed within the thylakoid membrane, rather than outside the membranes (Borisova et al., 2012). Actually, only a small proportion of chloroplast H2O2 may cross the chloroplast envelope and directly propagate a cytosolic signal to the nucleus (Mubarakshina and Ivanov, 2010; Borisova et al., 2012). Alternatively, the redox state of cellular compounds like glutathione and ascorbate could be changed by H2O2 as an oxidant. Furthermore, H2O2 could affect the reduction state of the quinone pools in chloroplasts, thereby serving as a signal molecule (Pogson et al., 2008).
Although intensive studies have focused on hydrogen peroxide as a signaling molecule, a clear demonstration of this process is still missing from the literature. Generally, chloroplast is thought to be a major producer of ROS during photosynthesis under heat stress and contains a large array of ROS-scavenging mechanisms whereas ROS production also occurs in mitochondria. Accumulated evidence suggests that H2O2 and Ca2+ function as second messengers to activate the heat-responsive expression of genes with HSEs in their promoters, such as HSFs, HSPs, and cytosolic ascorbate peroxidase (APX), as described in Figure 1. Under heat stress, the maintenance of ROS homeostasis depends on redox enzymes and metabolites, including SOD and the ascorbate–glutathione (ASC–GSH) cycle, functioning in different cell compartments. Notably, heat stress induces activation of a NADPH oxidase (respiratory burst oxidase homolog RBOH) in the plasma membrane via an increased membrane fluidity and/or via a consequent increase in cytosolic levels of Ca2+ controlled by a Ca2+ permeable channel (CNGC). In turn, Ca2+ influx activates RBOH by promoting its phosphorylation, leading to the increase of ROS (Figure 1).
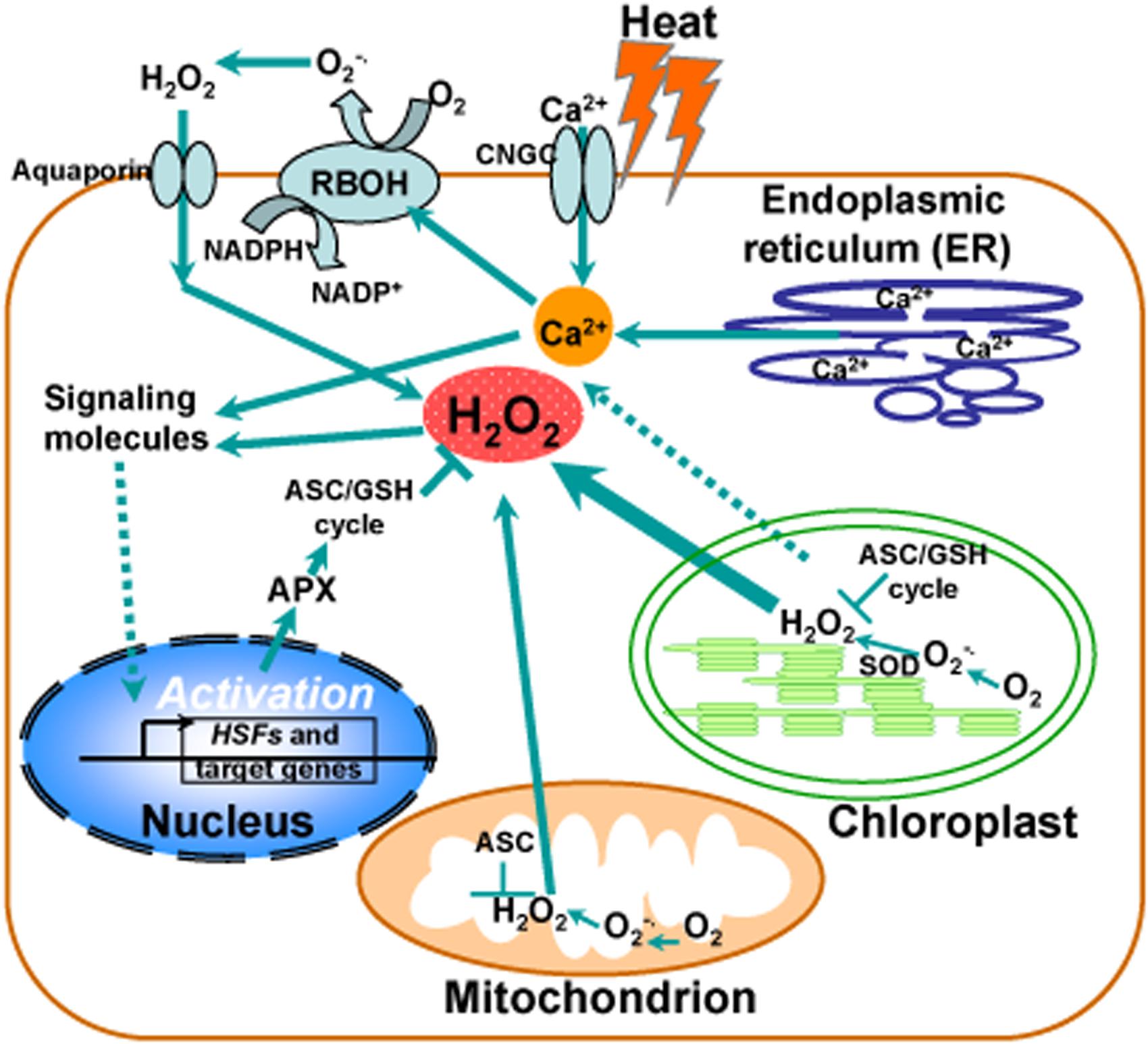
FIGURE 1. Calcium and reactive oxygen species (ROS) homeostasis in response to heat stress. Schematic representation of the major generation sites of ROS and transient calcium increase from different intracellular stores and the influx of extracellular calcium into the cell induced by the opening of cyclic nucleotide gated channels (CNGC) in the plasma membrane in response to heat stress. Heat stress induces activation of calcium channels in ER membranes, leading to the release of calcium in to the cytosol. Chloroplast is a major producer of ROS during photosynthesis under heat stress and contains a large array of ROS-scavenging mechanisms. ROS production also occurs in mitochondria. Hydrogen peroxide (H2O2) and Ca2+ serve as second messengers involved in heat-responsive activation of genes with heat shock elements in their promoters, such as heat shock transcription factors (HSFs), heat shock proteins (HSPs), and cytosolic ascorbate peroxidase (APX). Under heat stress, the maintenance of ROS homeostasis is involved in redox enzymes and metabolites, such as superoxide dismutase (SOD) and the ascorbate–glutathione (ASC–GSH) cycle, functioning in different cell compartments. A NADPH oxidase (respiratory burst oxidase homolog RBOH) in the plasma membrane becomes activated by heat stress via an increased membrane fluidity and/or via a consequent increase in cytosolic levels of Ca2+ controlled by a Ca2+ permeable channel (CNGC). Ca2+ influx activates RBOH by promoting its phosphorylation, leading to the increase of ROS.
To date, there is no general agreement on the H2O2 production rate and concentration in different compartments of the cell (Veljovic-Jovanovic et al., 2002; Queval et al., 2008). Moreover, the molecular mechanisms of H2O2 diffusion through, however, is still under debate and the necessity for aquaporins for H2O2 movement in vivo is yet to be determined (Foyer and Noctor, 2005, 2013; Pogson et al., 2008; Mittler et al., 2011).
How retrograde signals are perceived in the cytosol and communicated to the nucleus remains largely unknown. Due to its importance, much research effort has been expended to understand how plants integrate these retrograde signals via a signaling network, which involves second messenger molecules as well as signal-sensing proteins in the cytosol. Thus, we both discuss recent advances in the intermediate signaling components such as calcium ions (Ca2+), HSP90 associated complex and PTM between the nucleus and the plastid as well as sensing and signaling cascades in regulation of plant resistance to environmental stresses, particularly to heat stress.
Intracellular changes in Ca2+ in response to different biotic and abiotic stimuli are detected by various sensor proteins in the plant cell. It is a significant signature that heat shock can trigger a transient increase in [Ca2+]cyt in the cytosol of plants (Biyaseheva et al., 1993; Gong et al., 1998; Liu et al., 2003, 2006). Although the molecular mechanisms in regulation of the heat-induced increase in [Ca2+]cyt in plant cells remain largely unknown, it is known that the major sources of increased cytosolic free Ca2+ are released from intracellular Ca2+ pools and extracellular Ca2+ stores under heat stress. In Nicotiana tabacum, it was observed that heat stress mobilizes cytosolic Ca2+ from both intracellular and extracellular sources (Gong et al., 1998). In Arabidopsis, phospholipase C (PLC)/inositol 1,4,5-trisphosphate (IP3) mediates the heat-induced increase in [Ca2+]cyt (Liu et al., 2006; Zheng et al., 2012). In the moss Physcomitrella patens, it was shown that heat is sensed at the plasma membrane and causes a transient opening of Ca2+-permeable channels possibly by modulating the membrane fluidity (Saidi et al., 2009). Using reverse genetic analysis and the whole-cell patch-clamp technique, CNGC6, a member of CNGC family in Arabidopsis, was identified as a heat- and cAMP-activated PM Ca2+-permeable channel that is involved in heat stress responses (Gao et al., 2012). Interestingly, nitric oxide (NO) acts as a Ca2+ upstream signal in heat stress responses (Wang L. et al., 2014). In a recent report, OsANN1 was identified as a Ca2+-dependent phospholipid-binding protein, which is involved in heat and drought stress responses by modulating the levels of H2O2 and redox homeostasis (Qiao et al., 2015). More than 40 putative calcium channels are predicted based on the Arabidopsis genome and many of candidate channels have a C-terminus with a putative calmodulin (CaM)-binding domain, suggesting that CaMs are possible components involving the ensuing steps of the heat stress responses (Ward et al., 2009).
Identification and characterization of CaM-modulated proteins in relation to heat stress could prove to be essential for a deeper understanding of the molecular mechanisms involved in heat stress tolerance in plants. It is proposed that the Ca2+–CaM complex activates multiple kinases and regulates the activity of heat stress transcription factors in plants (Liu et al., 2003, 2005, 2008; Zhang et al., 2009). For instance, AtCaM3 regulates the expression of HSPs by activating calcium/CaM-binding protein kinase (CBK; Liu et al., 2008). These results suggest that CaM may act as an integrator of different stress signaling pathways, allowing plants to maintain homeostasis among different cellular processes (Bokszczanin and Fragkostefanakis, 2013; Virdi et al., 2015). On the other hand, the activation of extensive calcium-dependent protein kinase cascades could provide a mechanism whereby a chloroplast-derived ROS signal would merge into a regulatory network. The transiently increased levels of cytosolic calcium can activate the ROS-producing enzyme RBOHD through the activation of a calcium-dependent protein kinases that phosphorylates RBOHD (Suzuki et al., 2011). The RBOHD-derived ROS can trigger the ROS/redox signaling network that would activate downstream pathways via the important components involving heat stress responses such as MBF1c, certain HSFs and MAPKs (Mittler et al., 2004; Miller et al., 2009). The heat-induced accumulation of ROS and an increase of the cytosolic Ca2+ concentration can be sensed by the calmodulin CaM3, which leads to the MPK6-mediated activation of vacuolar processing enzyme that regulates heat-induced PCD (Li et al., 2012).
Not surprisingly, operational control of chloroplast retrograde regulation of heat stress responses is initiated by a combination of factors. In general, Ca2+ is a key player in heat stress signal transduction pathways where transient, spiking or oscillatory changes in cytosolic Ca2+ levels help to couple environmental cues to appropriate cellular responses. Thus, understanding whether and how much Ca2+ signaling contributes to defining stimulus–response specificity has long been a challenge (Monshausen, 2012). Although chloroplasts contain high concentrations (i.e., 4–23 mM) of total Ca2+ (Brand and Becker, 1984; Evans et al., 1991), it is not clear that they have the capacity to sequester Ca2+ or are involved in the generation of Ca2+ signals in response to environmental stresses. The challenge is to design physiologically relevant strategies to define the Ca2+-dependent functions of chloroplasts during heat stress responses (Figure 1).
The 90-kDa HSP (HSP90) is an abundant, evolutionarily conserved molecular chaperone in eukaryotic cells, functioning in the folding and activation of proteins involved in signal transduction, control of the cell cycle and disease resistance (Krishna and Gloor, 2001; Sangster and Queitsch, 2005). In Arabidopsis, this HSP90 family includes seven members. The AtHsp90-1 through AtHsp90-4 proteins are classified into the cytoplasmic subfamily, whereas the AtHsp90-5, AtHsp90-6, and AtHsp90-7 proteins are predicted to be chaperones functioning in plastid, mitochondria, and endoplasmic reticulum, respectively (Krishna and Gloor, 2001). It has been suggested that the environmentally sensitive HSP90 complex may initiate signaling cascades in response to environmental perturbations (Sangster and Queitsch, 2005). In a recent proteomic study using an affinity column containing Mg-ProtoIX covalently linked to an Affi-Gel matrix, ligands of the putative signaling metabolite Mg-ProtoIX were identified as three cytosolic heat shock 90-type proteins (HSP90), and interactions between Mg-ProtoIX and were investigated (Kindgren et al., 2011). To test whether the identified interaction between HSP90 and Mg-ProtoIX is biologically relevant, the transgenic lines with repressed HSP90 levels were generated. Data based on theses transgenic lines suggest that HSP90 proteins respond to the Mg-ProtoIX signal, providing insight into better understanding that how the tetrapyrrole-mediated plastid signal is recognized in the cytosol and further transduced into the nucleus in regulation of nuclear gene expression (Kindgren et al., 2012).
Interestingly, ROF1 (AtFKBP62), a peptidyl prolyl cis/trans isomerase, was shown to be involved in long term acquired thermotolerance by interacting with HSP90.1 and modulating the heat shock transcription factor, HsfA2 (Meiri and Breiman, 2009). Importantly, heat treatments induce nuclear localization of the ROF1-HSP90.1 complex, which depends on HsfA2 by interacting with HSP90.1 but not with ROF1 (Meiri and Breiman, 2009). These results suggest a role for ROF1 as a cytosolic component in mediating heat stress signal transduction and functions in prolongation of thermotolerance by sustaining the levels of small HSPs that are essential for survival at high temperatures (Figure 2). Recently, a direct physical interaction between cytosolic HSP90A/HSP70A and heat shock factor 1 was detected, but surprisingly this interaction persisted after the onset of stress (Schmollinger et al., 2013). It has been accepted that unlike other heat-shock proteins, HSP90 proteins appear not to be involved in protein folding, HSP90 rather interacts with proteins in the near native state and HSP90 is essential for maintaining the activity of numerous signaling proteins (Young et al., 2001; Sangster and Queitsch, 2005).
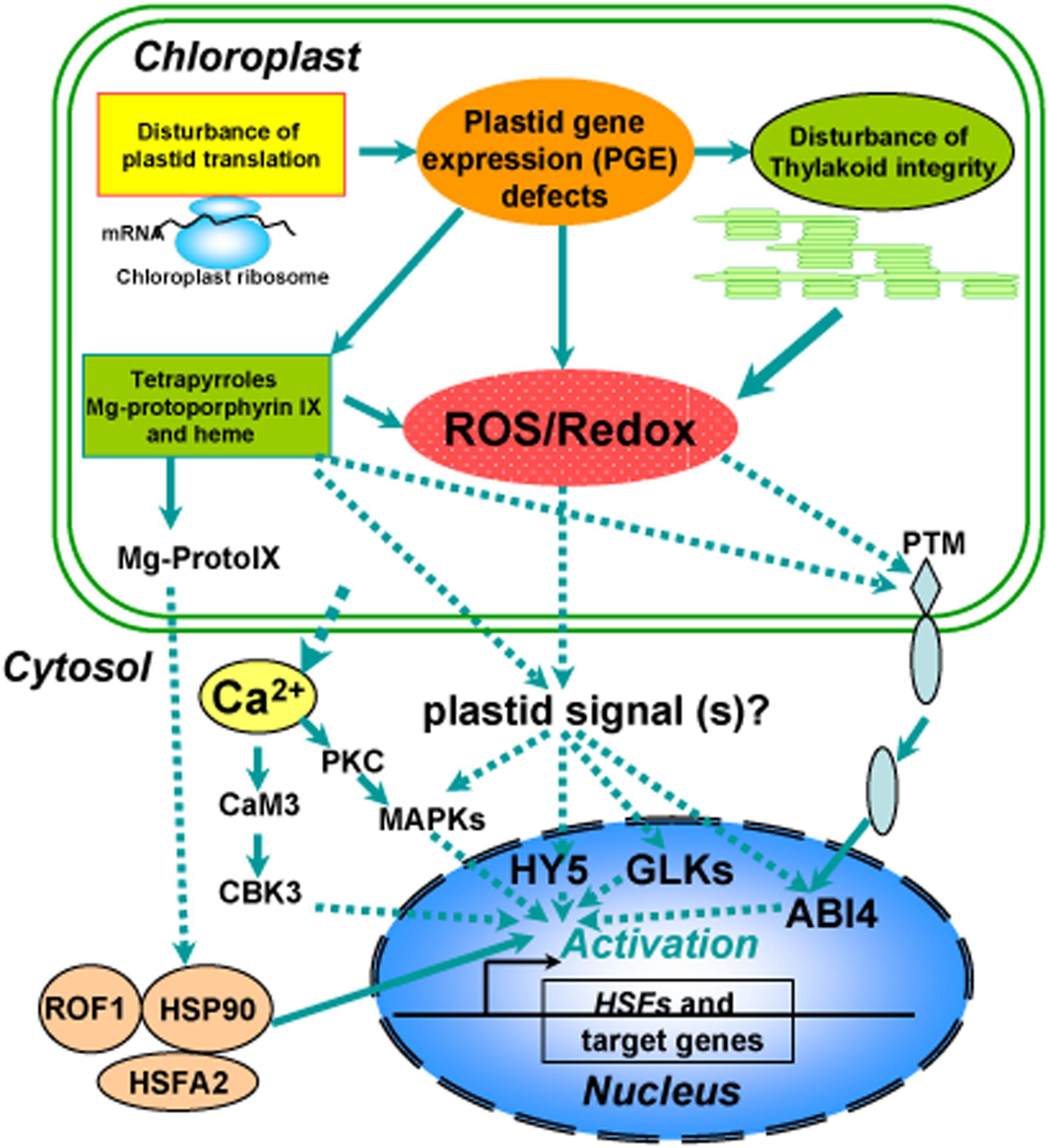
FIGURE 2. Overview of a proposed model for chloroplast retrograde regulation of heat stress responses. Thylakoid membranes are the primary susceptible targets of thermal damage in plants. Heat stress induces disturbance on the photosynthetic machinery and defects in plastid gene expression (PGE), leading to generation of ROS and accumulation of tetrapyrroles such as Mg-protoporphyrin IX (Mg-ProtoIX) and heme. The resulting ROS and disturbance of redox homeostasis in chloroplasts could serve as retrograde signals to activate downstream signal cascades via GLKs, HY5, or ABI4 in the nucleus. Ca2+, sequestered from chloroplasts under heat stress, via calmodulin activates calmodulin-binding protein kinase 3 (CBK3) and also activates protein kinase C (PKC), which via phosphorylation regulates MAP kinase (MAPK). The stressed chloroplasts may sequester Mg-ProtoIX, that binds to HSP90, which can form a complex with ROF1 (AtFKBP62), a peptidyl prolyl cis/trans isomerase. The resulting ROF1-HSP90 complex mobilizes into the nucleus with the help of the heat stress transcription factor HsfA2, which could allow them to drive the transcription of target genes such as HSPs required for establishing cellular heat tolerance. A possible retrograde regulatory pathway has also been proposed under heat stress in which PTM, a chloroplast envelope-bound plant homeodomain transcription factor, is cleaved during signaling transduction and the amino-terminal PTM is transferred into the nucleus, where histone modifications to the ABI4 promoter occur.
So far, very few cytosolic and nuclear components involved in retrograde communication have been identified. The nuclear TF ABI4 (ABA-INSENTIVE 4) belongs to the DREBA3 subgroup of a large family of plant-specific transcription factors known as AP2/EREBP (Sakuma et al., 2002). In a screen for ABA-insensitive (abi) mutants, the isolation of ABI4 was the first evidence linking this factor with ABA signaling (Finkelstein et al., 1998). It has been proposed that ABI4 functions as a node of convergence for multiple plastid retrograde signaling pathways in response to GUN1-derived chloroplast signals (Koussevitzky et al., 2007). Moreover, ABI4 binds to this CCAC motif, which has been found to be enriched in promoters of genes that are derepressed during lincomycin treatment in gun1 and abi4 seedlings (Koussevitzky et al., 2007). However, its mode of action that relays the chloroplast signals through the cytosol to the nucleus is not fully understood (Leon et al., 2013). Interestingly, treatments with norflurazon and lincomycin induce the high-level production of 1O2 that has been considered to be a putative signal for the modulation of nuclear-encoded plastid proteins (NEPPs) in response to these inhibitors. However, this regulation is severely inhibited in the mutants of GUN1 and ABI4, indicating that a disruption of tetrapyrrole synthesis may result in localized ROS production or an altered redox state of the plastid, which could mediate retrograde signaling (Moulin et al., 2008). One of the challenging questions is how chloroplast signals are transmitted to the nucleus through the cytosol. The recent identification of PTM, a chloroplast envelope-bound plant homeodomain (PHD) transcription factor with transmembrane domains, potentially links ABI4 and retrograde chloroplast signaling (Sun et al., 2011). Notably, PTM is processed by an unidentified intramembrane peptidase and released from the plastid envelope to the cytoplasm in response to treatments that initiate retrograde signals such as norflurazon, lincomycin, and high light. In turn, the processed PTM transmits into the nucleus, where it directly activates the expression of ABI4. Consistence with the role of PTM in retrograde signal transduction, the expression of ABI4 is much reduced in the ptm mutant (Sun et al., 2011). In a recent report, heat-responsive activation of PTM was significantly inhibited in the mutant of plastid CASEIN KINASE 2 (CK2), encoding a major Ser/Thr-specific enzyme regulated by redox signals for protein phosphorylation in the chloroplast stroma (Wang et al., 2014a). These results provide a molecular basis for a chloroplast envelope-bound transcription factor in retrograde chloroplast signaling and shed new light on the mechanism whereby chloroplast signals are transmitted to the nucleus through the cytosol (Figure 2). Further experiments focused on identification of the endopeptidase responsible for PTM activation and environmental stimuli that regulate its activity would yield important insight into how the physiological state of the chloroplasts is communicated into the nucleus.
In addition, the transcription factors long hypocotyl 5 (HY5) and Golden 2-like (GLK1/2) are suggested to participate in retrograde signaling pathways and have been shown to respond to plastid signals (Ruckle et al., 2007; Ruckle and Larkin, 2009; Waters et al., 2009). HY5 is converted from a positive to a negative regulator of photosynthesis-associated nuclear genes (PhANG) in response to an unknown plastid signal (Ruckle et al., 2007; Ruckle and Larkin, 2009), demonstrating a convergence between plastid and light signaling networks. A recent study reveals that HY5, together with HSP90 proteins, responds to the tetrapyrrole-mediated plastid signal to control the expression of PhANG during the response to oxidative stress, supporting that Mg-ProtoIX, cytosolic HSP90, and HY5 are all part of the same retrograde signaling pathway that is modified by tetrapyrroles in response to environmental stresses (Kindgren et al., 2012). As members of the GARP superfamily, the GLK proteins are partially redundant and are required for normal chloroplast development (Riechmann et al., 2000). The GLK proteins regulate the expression of genes involved in chlorophyll biosynthesis, light harvesting, and electron transport (Waters et al., 2009). Based on the expression analysis of some retrograde signaling marker genes in the glk1/glk2 double mutant, the GLK proteins have been implicated in retrograde signaling, specifically in the plastid protein import pathway (Kakizaki et al., 2009; Waters et al., 2009).
Taken together, these findings described above show that the remodeling of chloroplast retrograde signaling networks by these transcription factors is a mechanism by which plants integrate signals describing the functional and developmental state of chloroplasts with signals triggered by a variety of environmental stresses when coordinating the expression of the nuclear and chloroplast genomes during plant adaptation to stresses. These cytosolic mediators can perceive changes of ROS/redox at cellular levels and thus activate rapid and specific responses to environmental cues involving changes in choloroplastic dynamics as well as ROS-dependent signaling networks, although the mechanisms involved remain to be fully established. Chloroplasts can therefore be regarded as a highly important decision-making platform in the cell under heat stress, where ROS and redox play a determining role (Figure 2).
The chloroplasts act as sensors of environmental changes and complex networks of plastid signals coordinate cellular activities and function in stress responses to survive environmental perturbations. Therefore, chloroplast retrograde regulation is essential not only for coordinating the gene expression involving photosynthetic processes in both the nucleus and in the chloroplasts but also for mediating plant stress responses. Compared with extensive studies on retrograde regulation of drought and high light stress responses (Fernandez and Strand, 2008; Suzuki et al., 2012; Dietz, 2015; Nagahatenna et al., 2015), our knowledge is very limited in respect to the chloroplast-dependent regulatory mechanisms by which plants respond to heat stress. It is generally accepted that the chloroplasts generates multiple signals in response to a variety of environmental stresses. To respond optimally to environmental stresses, the retrograde signals derived from chloroplasts must transmit the information to the nucleus by cytosolic second messengers or distinct signal cascade pathways. Chloroplasts are metabolically active organelles that used to be regarded as a major source for ROS generated in response to abiotic stress. ROS production can be perceived by the cell as an alarm that triggers stress responses. Although H2O2 is proposed as a possible retrograde signal molecule, the challenge with this model lies in that how H2O2 could function as a specific messenger to communicate information on the state of chloroplasts to the nucleus because H2O2 is generated at different cellular compartments and in response to various different stresses and stimuli in higher plants. In future, identifying the novel interconnecting components of retrograde signaling by innovative genetic or biochemical approaches will significantly advance our understanding of the complicated retrograde pathways in response to a variety of abiotic stresses.
F-QG has designed the research topic of this review paper and provided the outline of paper contents; F-QG and A-ZS wrote the manuscript and made the schematic representation figures.
This work was supported by the Ministry of Science and Technology of China (2012CB944802) and the National Natural Science Foundation of China (91317305, 31570260 and 31170244).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We apologize to colleagues whose work we could not cite due to space limitations.
Alawady, A. E., and Grimm, B. (2005). Tobacco Mg protoporphyrin IX methyltransferase is involved in inverse activation of Mg porphyrin and protoheme synthesis. Plant J. 41, 282–290. doi: 10.1111/j.1365-313X.2004.02291.x
Allahverdiyeva, Y., Battchikova, N., Brosche, M., Fujii, H., Kangasjarvi, S., Mulo, P., et al. (2015). Integration of photosynthesis, development and stress as an opportunity for plant biology. New Phytol. 208, 647–655. doi: 10.1111/nph.13549
Allakhverdiev, S. I., Kreslavski, V. D., Klimov, V. V., Los, D. A., Carpentier, R., and Mohanty, P. (2008). Heat stress: an overview of molecular responses in photosynthesis. Photosynth. Res. 98, 541–550. doi: 10.1007/s11120-008-9331-0
Ankele, E., Kindgren, P., Pesquet, E., and Strand, A. (2007). In vivo visualization of Mg-ProtoporphyrinIX, a coordinator of photosynthetic gene expression in the nucleus and the chloroplast. Plant Cell 19, 1964–1979. doi: 10.1105/tpc.106.048744
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Aro, E. M., McCaffery, S., and Anderson, J. M. (1993). Photoinhibition and D1 Protein-degradation in peas acclimated to different growth irradiances. Plant Physiol. 103, 835–843.
Asada, K. (2006). Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 141, 391–396. doi: 10.1104/pp.106.082040
Banzet, N., Richaud, C., Deveaux, Y., Kazmaier, M., Gagnon, J., and Triantaphylides, C. (1998). Accumulation of small heat shock proteins, including mitochondrial HSP22, induced by oxidative stress and adaptive response in tomato cells. Plant J. 13, 519–527. doi: 10.1046/j.1365-313X.1998.00056.x
Barajas-Lopez, J. D., Blanco, N. E., and Strand, A. (2013). Plastid-to-nucleus communication, signals controlling the running of the plant cell. Biochim. Biophys. Acta Mol. Cell Res. 1833, 425–437. doi: 10.1016/j.bbamcr.2012.06.020
Barreto, P., Okura, V. K., Pena Neshich, I. A., Maia, I. D. G., and Arruda, P. (2014). Overexpression of UCP1 in tobacco induces mitochondrial biogenesis and amplifies a broad stress response. BMC Plant Biol. 14:144. doi: 10.1186/1471-2229-14-144
Beck, C. F. (2001). Signaling pathways in chloroplast-to-nucleus communication. Protist 152, 175–182. doi: 10.1078/1434-4610-00056
Beck, C. F. (2005). Signaling pathways from the chloroplast to the nucleus. Planta 222, 743–756. doi: 10.1007/s00425-005-0021-2
Berry, J., and Bjorkman, O. (1980). Photosynthetic response and adaptation to temperature in higher-plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 31, 491–543. doi: 10.1146/annurev.pp.31.060180.002423
Bienert, G. P., and Chaumont, F. (2014). Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta Gen. Sub. 1840, 1596–1604. doi: 10.1016/j.bbagen.2013.09.017
Bienert, G. P., Moller, A. L. B., Kristiansen, K. A., Schulz, A., Moller, I. M., Schjoerring, J. K., et al. (2007). Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 282, 1183–1192. doi: 10.1074/jbc.M603761200
Biyaseheva, A. E., Molotkovskii, Y. G., and Mamonov, L. K. (1993). Increase of free Ca2+ in the cytosol of plant-protoplasts in response to heat-stress as related to Ca2+ homeostasis. Russ. Plant Physiol. 40, 540–544.
Blanco, N. E., Guinea-Diaz, M., Whelan, J., and Strand, A. (2014). Interaction between plastid and mitochondrial retrograde signalling pathways during changes to plastid redox status. Philos. Trans. R. Soc. B Biol. Sci. 369:20130231. doi: 10.1098/rstb.2013.0231
Bobik, K., and Burch-Smith, T. M. (2015). Chloroplast signaling within, between and beyond cells. Front. Plant Sci. 6:781. doi: 10.3389/fpls.2015.00781
Bokszczanin, K. L., and Fragkostefanakis, S. (2013). Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerance. Front. Plant Sci. 4:135. doi: 10.3389/fpls.2013.00315
Borisova, M. M., Kozuleva, M. A., Rudenko, N. N., Naydov, I. A., Klenina, I. B., and Ivanov, B. N. (2012). Photosynthetic electron flow to oxygen and diffusion of hydrogen peroxide through the chloroplast envelope via aquaporins. Biochim. Biophys. Acta Bioenerg. 1817, 1314–1321. doi: 10.1016/j.bbabio.2012.02.036
Bradbeer, J. W., Atkinson, Y. E., Borner, T., and Hagemann, R. (1979). Cytoplasmic synthesis of plastid polypeptides may be controlled by plastid-synthesized RNA. Nature 279, 816–817. doi: 10.1038/279816a0
Brand, J. J., and Becker, D. W. (1984). Evidence for direct roles of calcium in photosynthesis. J. Bioenerg. Biomembr. 16, 239–249. doi: 10.1007/BF00744278
Busch, W., Wunderlich, M., and Schoffl, F. (2005). Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. Plant J. 41, 1–14. doi: 10.1111/j.1365-313X.2004.02272.x
Chi, W., Feng, P., Ma, J., and Zhang, L. (2015). Metabolites and chloroplast retrograde signaling. Curr. Opin. Plant Biol. 25, 32–38. doi: 10.1016/j.pbi.2015.04.006
Chi, W., Sun, X. W., and Zhang, L. X. (2013). Intracellular signaling from plastid to nucleus. Annu. Rev. Plant Biol. 64, 559–582. doi: 10.1146/annurev-arplant-050312-120147
Dat, J. F., Lopez-Delgado, H., Foyer, C. H., and Scott, I. M. (1998). Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 116, 1351–1357. doi: 10.1104/pp.116.4.1351
Desikan, R., Mackerness, S. A. H., Hancock, J. T., and Neill, S. J. (2001). Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol. 127, 159–172. doi: 10.1104/pp.127.1.159
Dietz, K.-J. (2015). Efficient high light acclimation involves rapid processes at multiple mechanistic levels. J. Exp. Bot. 66, 2401–2414. doi: 10.1093/jxb/eru505
Elhafez, D., Murcha, M. W., Clifton, R., Soole, K. L., Day, D. A., and Whelan, J. (2006). Characterization of mitochondrial alternative NAD(P)H dehydrogenases in Arabidopsis: intraorganelle location and expression. Plant Cell Physiol. 47, 43–54. doi: 10.1093/pcp/pci221
Estavillo, G. M., Chan, K. X., Phua, S. Y., and Pogson, B. J. (2013). Reconsidering the nature and mode of action of metabolite retrograde signals from the chloroplast. Front. Plant Sci. 3:300. doi: 10.3389/fpls.2012.00300
Estavillo, G. M., Crisp, P. A., Pornsiriwong, W., Wirtz, M., Collinge, D., Carrie, C., et al. (2011). Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 23, 3992–4012. doi: 10.1105/tpc.111.091033
Evans, D. E., Briars, S. A., and Williams, L. E. (1991). Active calcium-transport by plant-cell membranes. J. Exp. Bot. 42, 285–303. doi: 10.1093/jxb/42.3.285
Fedyaeva, A. V., Stepanov, A. V., Lyubushkina, I. V., Pobezhimova, T. P., and Rikhvanov, E. G. (2014). Heat shock induces production of reactive oxygen species and increases inner mitochondrial membrane potential in winter wheat cells. Biochem. Moscow 79, 1202–1210. doi: 10.1134/S0006297914110078
Fernandez, A. P., and Strand, A. (2008). Retrograde signaling and plant stress: plastid signals initiate cellular stress responses. Curr. Opin. Plant Biol. 11, 509–513. doi: 10.1016/j.pbi.2008.06.002
Finkelstein, R. R., Wang, M. L., Lynch, T. J., Rao, S., and Goodman, H. M. (1998). The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA2 domain protein. Plant Cell 10, 1043–1054. doi: 10.1105/tpc.10.6.1043
Finnegan, P. M., Whelan, J., Millar, A. H., Zhang, Q. S., Smith, M. K., Wiskich, J. T., et al. (1997). Differential expression of the multigene family encoding the soybean mitochondrial alternative oxidase. Plant Physiol. 114, 455–466. doi: 10.1104/pp.114.2.455
Foyer, C. H., Bloom, A. J., Queval, G., and Noctor, G. (2009). Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annu. Rev. Plant Biol. 60, 455–484. doi: 10.1146/annurev.arplant.043008.091948
Foyer, C. H., LopezDelgado, H., Dat, J. F., and Scott, I. M. (1997). Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol. Plant. 100, 241–254. doi: 10.1034/j.1399-3054.1997.1000205.x
Foyer, C. H., and Noctor, G. (2005). Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 28, 1056–1071. doi: 10.1111/j.1365-3040.2005.01327.x
Foyer, C. H., and Noctor, G. (2013). Redox signaling in plants. Antioxid. Redox Signal. 18, 2087–2090. doi: 10.1089/ars.2013.5278
Frey, F. P., Urbany, C., Huettel, B., Reinhardt, R., and Stich, B. (2015). Genome-wide expression profiling and phenotypic evaluation of European maize inbreds at seedling stage in response to heat stress. BMC Genomics 16:123. doi: 10.1186/s12864-015-1282-1
Galvez-Valdivieso, G., and Mullineaux, P. M. (2010). The role of reactive oxygen species in signalling from chloroplasts to the nucleus. Physiol. Plant. 138, 430–439. doi: 10.1111/j.1399-3054.2009.01331.x
Gao, F., Han, X. W., Wu, J. H., Zheng, S. Z., Shang, Z. L., Sun, D. Y., et al. (2012). A heat-activated calcium-permeable channel - Arabidopsis cyclic nucleotide-gated ion channel 6 - is involved in heat shock responses. Plant J. 70, 1056–1069. doi: 10.1111/j.1365-313X.2012.04969.x
Giraud, E., Van Aken, O., Ho, L. H. M., and Whelan, J. (2009). The transcription Factor ABI4 is a regulator of mitochondrial retrograde expression of ALTERNATIVE OXIDASE1a. Plant Physiol. 150, 1286–1296. doi: 10.1104/pp.109.139782
Gollan, P. J., Tikkanen, M., and Aro, E. M. (2015). Photosynthetic light reactions: integral to chloroplast retrograde signalling. Curr. Opin. Plant Biol. 27, 180–191. doi: 10.1016/j.pbi.2015.07.006
Gong, M., van der Luit, A. H., Knight, M. R., and Trewavas, A. J. (1998). Heat-shock-induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol. 116, 429–437. doi: 10.1104/pp.116.1.429
Havaux, M. (1993). Characterization of thermal-damage to the photosynthetic electron-transport system in potato leaves. Plant Sci. 94, 19–33. doi: 10.1016/0168-9452(93)90003-I
Havaux, M., and Tardy, F. (1996). Temperature-dependent adjustment of the thermal stability of photosystem II in vivo: possible involvement of xanthophyll-cycle pigments. Planta 198, 324–333. doi: 10.1007/BF00620047
Heine, G. F., Hernandez, J. M., and Grotewold, E. (2004). Two cysteines in plant R2R3 MYB domains participate in REDOX-dependent DNA binding. J. Biol. Chem. 279, 37878–37885. doi: 10.1074/jbc.M405166200
Henzler, T., and Steudle, E. (2000). Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. J. Exp. Bot. 51, 2053–2066. doi: 10.1093/jexbot/51.353.2053
Hess, W. R., Muller, A., Nagy, F., and Borner, T. (1994). Ribosome-deficient plastids affect transcription of light-induced nuclear genes - genetic-evidence for a plastid-derived signal. Mol. Gen. Genet. 242, 305–312. doi: 10.1007/BF00280420
Hihara, Y., Kamei, A., Kanehisa, M., Kaplan, A., and Ikeuchi, M. (2001). DNA microarray analysis of cyanobacterial gene expression during acclimation to high light. Plant Cell 13, 793–806. doi: 10.1105/tpc.13.4.793
Ivanova, A., Law, S. R., Narsai, R., Duncan, O., Lee, J.-H., Zhang, B., et al. (2014). A functional antagonistic relationship between auxin and mitochondrial retrograde signaling regulates alternative oxidase1a expression in Arabidopsis. Plant Physiol. 165, 1233–1254. doi: 10.1104/pp.114.237495
Jacoby, R. P., Li, L., Huang, S., Pong Lee, C., Millar, A. H., and Taylor, N. L. (2012). Mitochondrial composition, function and stress response in plants. J. Integr. Plant Biol. 54, 887–906. doi: 10.1111/j.1744-7909.2012.01177.x
Jarvis, P. (2008). Targeting of nucleus-encoded proteins to chloroplasts in plants. New Phytol. 179, 257–285. doi: 10.1111/j.1469-8137.2008.02452.x
Johanningmeier, U., and Howell, S. H. (1984). Regulation of light-harvesting chlorophyll-binding protein messenger-rna accumulation in chlamydomonas-reinhardi - possible involvement of chlorophyll synthesis precursors. J. Biol. Chem. 259, 3541–3549.
Kakizaki, T., Matsumura, H., Nakayama, K., Che, F.-S., Terauchi, R., and Inaba, T. (2009). Coordination of plastid protein import and nuclear gene expression by plastid-to-nucleus retrograde signaling. Plant Physiol. 151, 1339–1353. doi: 10.1104/pp.109.145987
Kim, C., Meskauskiene, R., Zhang, S., Lee, K. P., Ashok, M. L., Blajecka, K., et al. (2012). Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell 24, 3026–3039. doi: 10.1105/tpc.112.100479
Kindgren, P., Eriksson, M.-J., Benedict, C., Mohapatra, A., Gough, S. P., Hansson, M., et al. (2011). A novel proteomic approach reveals a role for Mg-protoporphyrin IX in response to oxidative stress. Physiol. Plant. 141, 310–320. doi: 10.1111/j.1399-3054.2010.01440.x
Kindgren, P., Noren, L., Lopez, J. D. D. B., Shaikhali, J., and Strand, A. (2012). Interplay between HEAT SHOCK PROTEIN 90 and HY5 Controls PhANG Expression in Response to the GUN5 Plastid Signal. Mol. Plant 5, 901–913. doi: 10.1093/mp/ssr112
Kleine, T., Voigt, C., and Leister, D. (2009). Plastid signalling to the nucleus: messengers still lost in the mists? Trends Genet. 25, 185–190. doi: 10.1016/j.tig.2009.02.004
Klimov, V. V., Baranov, S. V., and Allakhverdiev, S. I. (1997). Bicarbonate protects the donor side of photosystem II against photoinhibition and thermoinactivation. FEBS Lett. 418, 243–246. doi: 10.1016/S0014-5793(97)01392-6
Koenigshofer, H., Tromballa, H.-W., and Loeppert, H.-G. (2008). Early events in signalling high-temperature stress in tobacco BY2 cells involve alterations in membrane fluidity and enhanced hydrogen peroxide production. Plant Cell Environ. 31, 1771–1780. doi: 10.1111/j.1365-3040.2008.01880.x
Kotak, S., Larkindale, J., Lee, U., von Koskull-Doring, P., Vierling, E., and Scharf, K. D. (2007). Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 10, 310–316. doi: 10.1016/j.pbi.2007.04.011
Koussevitzky, S., Nott, A., Mockler, T. C., Hong, F., Sachetto-Martins, G., Surpin, M., et al. (2007). Signals from chloroplasts converge to regulate nuclear gene expression. Science 316, 715–719. doi: 10.1126/science.%201140516
Kovtun, Y., Chiu, W. L., Tena, G., and Sheen, J. (2000). Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. U.S.A. 97, 2940–2945. doi: 10.1073/pnas.97.6.2940
Krishna, P., and Gloor, G. (2001). The Hsp90 family of proteins in Arabidopsis thaliana. Cell Stress Chaper. 6, 238–246. doi: 10.1379/1466-1268(2001)006<0238:THFOPI>2.0.CO;2
Kropat, J., Oster, U., Rudiger, W., and Beck, C. F. (1997). Chlorophyll precursors are signals of chloroplast origin involved in light induction of nuclear heat-shock genes. Proc. Natl. Acad. Sci. U.S.A. 94, 14168–14172. doi: 10.1073/pnas.94.25.14168
Kropat, J., Oster, U., Rudiger, W., and Beck, C. F. (2000). Chloroplast signalling in the light induction of nuclear HSP70 genes requires the accumulation of chlorophyll precursors and their accessibility to cytoplasm/nucleus. Plant J. 24, 523–531. doi: 10.1111/j.1365-313X.2000.00898.x
Kruk, J., and Trebst, A. (2007). Plastoquinol as a singlet oxygen scavenger in photosystem II. Photosynth. Res. 91, 315–315.
Laloi, C., Stachowiak, M., Pers-Kamczyc, E., Warzych, E., Murgia, I., and Apel, K. (2007). Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 104, 672–677. doi: 10.1073/pnas.0609063103
Larkin, R. M., Alonso, J. M., Ecker, J. R., and Chory, J. (2003). GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science 299, 902–906. doi: 10.1126/science.1079978
Larkindale, J., Hall, J. D., Knight, M. R., and Vierling, E. (2005). Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 138, 882–897. doi: 10.1104/pp.105.062257
Larkindale, J., and Vierling, E. (2008). Core genome responses involved in acclimation to high temperature. Plant Physiol. 146, 748–761. doi: 10.1104/pp.107.112060
Lee, B. H., Won, S. H., Lee, H. S., Miyao, M., Chung, W. I., Kim, I. J., et al. (2000). Expression of the chloroplast-localized small heat shock protein by oxidative stress in rice. Gene 245, 283–290. doi: 10.1016/S0378-1119(00)00043-3
Lee, K. P., Kim, C., Landgraf, F., and Apel, K. (2007). EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 104, 10270–10275. doi: 10.1073/pnas.0702061104
Leon, P., Gregorio, J., and Cordoba, E. (2013). ABI4 and its role in chloroplast retrograde communication. Front. Plant Sci. 3:304. doi: 10.3389/fpls.2012.00304
Li, Z., Yue, H., and Xing, D. (2012). MAP Kinase 6-mediated activation of vacuolar processing enzyme modulates heat shock-induced programmed cell death in Arabidopsis. New Phytol. 195, 85–96. doi: 10.1111/j.1469-8137.2012.04131.x
Lim, C. J., Yang, K. A., Hong, J. K., Choi, A. S., Yun, D. J., Hong, J. C., et al. (2006). Gene expression profiles during heat acclimation in Arabidopsis thaliana suspension-culture cells. J. Plant Res. 119, 373–383. doi: 10.1007/s10265-006-0285-z
Lin, Y.-P., Lee, T.-Y., Tanaka, A., and Charng, Y.-Y. (2014). Analysis of an Arabidopsis heat-sensitive mutant reveals that chlorophyll synthase is involved in reutilization of chlorophyllide during chlorophyll turnover. Plant J. 80, 14–26. doi: 10.1111/tpj.12611
Lindahl, M., Spetea, C., Hundal, T., Oppenheim, A. B., Adam, Z., and Andersson, B. (2000). The thylakoid FtsH protease plays a role in the light-induced turnover of the photosystem II D1 protein. Plant Cell 12, 419–431. doi: 10.1105/tpc.12.3.419
Link, V., Sinha, A. K., Vashista, P., Hofmann, M. G., Proels, R. K., Ehness, R., et al. (2002). A heat-activated MAP kinase in tomato: a possible regulator of the heat stress response. FEBS Lett. 531, 179–183. doi: 10.1016/S0014-5793(02)03498-1
Liu, H. T., Gao, F., Cui, S. J., Han, J. L., Sun, D. Y., and Zhou, R. G. (2006). Primary evidence for involvementof IP3 in heat-shock signal transduction in Arabidopsis. Cell Res 16, 394–400. doi: 10.1038/sj.cr.7310051
Liu, H.-T., Gao, F., Li, G.-L., Han, J.-L., De-Long, L., Sun, D.-Y., et al. (2008). The calmodulin-binding protein kinase 3 is part of heat-shock signal transduction in Arabidopsis thaliana. Plant J. 55, 760–773. doi: 10.1111/j.1365-313X.2008.03544.x
Liu, H. T., Li, B., Shang, Z. L., Li, X. Z., Mu, R. L., Sun, D. Y., et al. (2003). Calmodulin is involved in heat shock signal transduction in wheat. Plant Physiol. 132, 1186–1195. doi: 10.1104/pp.102.018564
Liu, H. T., Sun, D. Y., and Zhou, R. G. (2005). Ca2+ and AtCaM3 are involved in the expression of heat shock protein gene in Arabidopsis. Plant Cell Environ. 28, 1276–1284. doi: 10.1104/pp.108.133744
Matsumoto, T., Morishige, H., Tanaka, T., Kanamori, H., Komatsuda, T., Sato, K., et al. (2014). Transcriptome analysis of barley identifies heat shock and HD-Zip I transcription factors up-regulated in response to multiple abiotic stresses. Mol. Breed. 34, 761–768. doi: 10.1007/s11032-014-0048-9
Meiri, D., and Breiman, A. (2009). Arabidopsis ROF1 (FKBP62) modulates thermotolerance by interacting with HSP90.1 and affecting the accumulation of HsfA2-regulated sHSPs. Plant J. 59, 387–399. doi: 10.1111/j.1365-313X.2009.03878.x
Meskauskiene, R., Nater, M., Goslings, D., Kessler, F., den Camp, R. O., and Apel, K. (2001). FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 98, 12826–12831. doi: 10.1073/pnas.221252798
Miller, G., and Mittler, R. (2006). Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann. Bot. 98, 279–288. doi: 10.1093/aob/mcl107
Miller, G., Schlauch, K., Tam, R., Cortes, D., Torres, M. A., Shulaev, V., et al. (2009). The Plant NADPH Oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal 2:ra45. doi: 10.1126/scisignal.2000448
Miller, G., Shulaev, V., and Mittler, R. (2008). Reactive oxygen signaling and abiotic stress. Physiol. Plant 133, 481–489. doi: 10.1111/j.1399-3054.2008.01090.x
Mittler, R., Vanderauwera, S., Gollery, M., and Van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498. doi: 10.1016/j.tplants.2004.08.009
Mittler, R., Vanderauwera, S., Suzuki, N., Miller, G., Tognetti, V. B., Vandepoele, K., et al. (2011). ROS signaling: the new wave? Trends Plant Sci. 16, 300–309. doi: 10.1016/j.tplants.2011.03.007
Mochizuki, N., Brusslan, J. A., Larkin, R., Nagatani, A., and Chory, J. (2001). Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc. Natl. Acad. Sci. U.S.A. 98, 2053–2058. doi: 10.1073/pnas.98.4.2053
Mochizuki, N., Tanaka, R., Tanaka, A., Masuda, T., and Nagatani, A. (2008). The steady-state level of Mg-protoporphyrin IX is not a determinant of plastid-to-nucleus signaling in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 105, 15184–15189. doi: 10.1073/pnas.0803245105
Monshausen, G. B. (2012). Visualizing Ca2+ signatures in plants. Curr. Opin. Plant Biol. 15, 677–682. doi: 10.1016/j.pbi.2012.09.014
Moulin, M., McCormac, A. C., Terry, M. J., and Smith, A. G. (2008). Tetrapyrrole profiling in Arabidopsis seedlings reveals that retrograde plastid nuclear signaling is not due to Mg-protoporphyrin IX accumulation. Proc. Natl. Acad. Sci. U.S.A. 105, 15178–15183. doi: 10.1073/pnas.0803054105
Mubarakshina, M. M., and Ivanov, B. N. (2010). The production and scavenging of reactive oxygen species in the plastoquinone pool of chloroplast thylakoid membranes. Physiol. Plant. 140, 103–110. doi: 10.1111/j.1399-3054.2010.01391.x
Mubarakshina, M. M., Ivanov, B. N., Naydov, I. A., Hillier, W., Badger, M. R., and Krieger-Liszkay, A. (2010). Production and diffusion of chloroplastic H2O2 and its implication to signalling. J. Exp. Bot. 61, 3577–3587. doi: 10.1093/jxb/erq171
Nagahatenna, D. S. K., Langridge, P., and Whitford, R. (2015). Tetrapyrrole-based drought stress signalling. Plant Biotechnol. J. 13, 447–459. doi: 10.1111/pbi.12356
Neill, S. J., Desikan, R., Clarke, A., Hurst, R. D., and Hancock, J. T. (2002). Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 53, 1237–1247. doi: 10.1093/jexbot/53.372.1237
Ng, S., De Clercq, I., Van Aken, O., Law, S. R., Ivanova, A., Willems, P., et al. (2014). Anterograde and retrograde regulation of nuclear genes encoding mitochondrial proteins during growth. Dev. Stress Mol. Plant 7, 1075–1093. doi: 10.1093/mp/ssu037
Ng, S., Ivanova, A., Duncan, O., Law, S. R., Van Aken, O., De Clercq, I., et al. (2013). A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. Plant Cell 25, 3450–3471. doi: 10.1105/tpc.113.113985
Nishizawa, A., Yabuta, Y., Yoshida, E., Maruta, T., Yoshimura, K., and Shigeoka, S. (2006). Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J. 48, 535–547. doi: 10.1111/j.1365-313X.2006.02889.x
Noctor, G., Mhamdi, A., and Foyer, C. H. (2014). The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol. 164, 1636–1648. doi: 10.1104/pp.113.233478
Nover, L., Scharf, K.-D., Gagliardi, D., Vergne, P., Czarnecka-Verner, E., and Gurley, W. B. (1996). The Hsf world: classification and properties of plant heat stress transcription factors. Cell Stress Chaper. 1, 215–223. doi: 10.1379/1466-1268(1996)001<0215:THWCAP>2.3.CO;2
Oelmuller, R., Levitan, I., Bergfeld, R., Rajasekhar, V. K., and Mohr, H. (1986). Expression of nuclear genes as affected by treatments acting on the plastids. Planta 168, 482–492. doi: 10.1007/BF00392267
op den Camp, R. G. L., Przybyla, D., Ochsenbein, C., Laloi, C., Kim, C. H., Danon, A., et al. (2003). Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15, 2320–2332. doi: 10.1105/tpc.014662
Pan, R. H., Jones, A. D., and Hu, J. P. (2014). Cardiolipin-mediated mitochondrial dynamics and stress response in Arabidopsis. Plant Cell 26, 391–409. doi: 10.1105/tpc.113.121095
Petrov, V., Hille, J., Mueller-Roeber, B., and Gechev, T. S. (2015). ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 6:69. doi: 10.3389/fpls.2015.00069
Pogson, B. J., Woo, N. S., Foerster, B., and Small, I. D. (2008). Plastid signalling to the nucleus and beyond. Trends Plant Sci. 13, 602–609. doi: 10.1016/j.tplants.2008.08.008
Pontier, D., Albrieux, C., Joyard, J., Lagrange, T., and Block, M. A. (2007). Knock-out of the magnesium protoporphyrin IX methyltransferase gene in Arabidopsis - effects on chloroplast development and on chloroplast-to-nucleus signaling. J. Biol. Chem. 282, 2297–2304. doi: 10.1074/jbc.M610286200
Pospisil, P., and Prasad, A. (2014). Formation of singlet oxygen and protection against its oxidative damage in Photosystem II under abiotic stress. J. Photochem. Photobiol. B Biol. 137, 39–48. doi: 10.1016/j.jphotobiol.2014.04.025
Pu, X., Lv, X., Tan, T., Fu, F., Qin, G., and Lin, H. (2015). Roles of mitochondrial energy dissipation systems in plant development and acclimation to stress. Ann. Bot. 116, 583–600. doi: 10.1093/aob/mcv063
Pyatrikas, D. V., Rikhvanov, E. G., Fedoseeva, I. V., Varakina, N. N., Rusaleva, T. M., Tauson, E. L., et al. (2014). Mitochondrial retrograde regulation of HSP101 expression in Arabidopsis thaliana under heat stress and amiodarone action. Russian J. Plant Physiol. 61, 80–89. doi: 10.1134/S1021443714010117
Qiao, B., Zhang, Q., Liu, D., Wang, H., Yin, J., Wang, R., et al. (2015). A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J. Exp. Bot. 66, 5853–5866. doi: 10.1093/jxb/erv294
Queval, G., Hager, J., Gakiere, B., and Noctor, G. (2008). Why are literature data for H2O2 contents so variable? A discussion of potential difficulties in the quantitative assay of leaf extracts. J. Exp. Bot. 59, 135–146. doi: 10.1093/jxb/erm193
Ramel, F., Birtic, S., Ginies, C., Soubigou-Taconnat, L., Triantaphylides, C., and Havaux, M. (2012). Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. U.S.A. 109, 5535–5540. doi: 10.1073/pnas.1115982109
Ramel, F., Ksas, B., Akkari, E., Mialoundama, A. S., Monnet, F., Krieger-Liszkay, A., et al. (2013). Light-induced acclimation of the Arabidopsis chlorina1 mutant to singlet oxygen. Plant Cell 25, 1445–1462. doi: 10.1105/tpc.113.109827
Riechmann, J. L., Heard, J., Martin, G., Reuber, L., Jiang, C. Z., Keddie, J., et al. (2000). Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290, 2105–2110. doi: 10.1126/science.290.5499.2105
Rikhvanov, E. G., Fedoseeva, I. V., Pyatrikas, D. V., Borovskii, G. B., and Voinikov, V. K. (2014). Role of mitochondria in the operation of calcium signaling system in heat-stressed plants. Russian J. Plant Physiol. 61, 141–153. doi: 10.1134/S1021443714020125
Rizhsky, L., Liang, H. J., Shuman, J., Shulaev, V., Davletova, S., and Mittler, R. (2004). When Defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 134, 1683–1696. doi: 10.1104/pp.103.033431
Ruckle, M. E., DeMarco, S. M., and Larkin, R. M. (2007). Plastid signals remodel light signaling networks and are essential for efficient chloroplast biogenesis in Arabidopsis. Plant Cell 19, 3944–3960. doi: 10.1105/tpc.107.054312
Ruckle, M. E., and Larkin, R. M. (2009). Plastid signals that affect photomorphogenesis in Arabidopsis thaliana are dependent on GENOMES UNCOUPLED 1 and cryptochrome 1. New Phytol. 182, 367–379. doi: 10.1111/j.1469-8137.2008.02729.x
Rurek, M. (2014). Plant mitochondria under a variety of temperature stress conditions. Mitochondrion 19, 289–294. doi: 10.1016/j.mito.2014.02.007
Saidi, Y., Finka, A., and Goloubinoff, P. (2011). Heat perception and signalling in plants: a tortuous path to thermotolerance. New Phytol. 190, 556–565. doi: 10.1111/j.1469-8137.2010.03571.x
Saidi, Y., Finka, A., Muriset, M., Bromberg, Z., Weiss, Y. G., Maathuis, F. J. M., et al. (2009). The heat shock response in moss plants is regulated by specific calcium-permeable channels in the plasma membrane. Plant Cell 21, 2829–2843. doi: 10.1105/tpc.108.065318
Sakuma, Y., Liu, Q., Dubouzet, J. G., Abe, H., Shinozaki, K., and Yamaguchi-Shinozaki, K. (2002). DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 290, 998–1009. doi: 10.1006/bbrc.2001.6299
Sangster, T. A., and Queitsch, C. (2005). The HSP90 chaperone complex, an emerging force in plant development and phenotypic plasticity. Curr. Opin. Plant Biol. 8, 86–92. doi: 10.1016/j.pbi.2004.11.012
Sangwan, V., Orvar, B. L., Beyerly, J., Hirt, H., and Dhindsa, R. S. (2002). Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J. 31, 629–638. doi: 10.1046/j.1365-313X.2002.01384.x
Scharf, K. D., Heider, H., Hohfeld, I., Lyck, R., Schmidt, E., and Nover, L. (1998). The tomato Hsf system: HsfA2 needs interaction with HsfA1 for efficient nuclear import and may be localized in cytoplasmic heat stress granules. Mol. Cell. Biol. 18, 2240–2251. doi: 10.1128/MCB.18.4.2240
Schlicke, H., Hartwig, A. S., Firtzlaff, V., Richter, A. S., Glaesser, C., Maier, K., et al. (2014). Induced deactivation of genes encoding chlorophyll biosynthesis enzymes disentangles tetrapyrrole-mediated retrograde signaling. Mol. Plant 7, 1211–1227. doi: 10.1093/mp/ssu034
Schmollinger, S., Schulz-Raffelt, M., Strenkert, D., Veyel, D., Vallon, O., and Schroda, M. (2013). Dissecting the heat stress response in chlamydomonas by pharmaceutical and rnai approaches reveals conserved and novel aspects. Mol. Plant 6, 1795–1813. doi: 10.1093/mp/sst086
Schramm, F., Ganguli, A., Kiehlmann, E., Englich, G., Walch, D., and von Koskull-Doring, P. (2006). The heat stress transcription factor HsfA2 serves as a regulatory amplifier of a subset of genes in the heat stress response in Arabidopsis. Plant Mol. Biol. 60, 759–772. doi: 10.1007/s11103-005-5750-x
Shaikhali, J., Heiber, I., Seidel, T., Stroeher, E., Hiltscher, H., Birkmann, S., et al. (2008). The redox-sensitive transcription factor Rap2.4a controls nuclear expression of 2-Cys peroxiredoxin A and other chloroplast antioxidant enzymes. BMC Plant Biol. 8:48. doi: 10.1186/1471-2229-8-48
Shaikhali, J., Noren, L., Barajas-Lopez, J. D. D., Srivastava, V., Koenig, J., Sauer, U. H., et al. (2012). Redox-mediated mechanisms regulate DNA binding activity of the G-group of basic region leucine zipper (bZIP) transcription factors in Arabidopsis. J. Biol. Chem. 287, 27510–27525. doi: 10.1074/jbc.M112.361394
Sharkey, T. D. (2005). Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ. 28, 269–277. doi: 10.1111/j.1365-3040.2005.01324.x
Strand, A., Asami, T., Alonso, J., Ecker, J. R., and Chory, J. (2003). Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature 421, 79–83. doi: 10.1038/nature01204
Sugiura, M., Hirose, T., and Sugita, M. (1998). Evolution and mechanism of translation in chloroplasts. Annu. Rev. Genet. 32, 437–459. doi: 10.1146/annurev.genet.32.1.437
Sullivan, J. A., and Gray, J. C. (1999). Plastid translation is required for the expression of nuclear photosynthesis genes in the dark and in roots of the pea lip1 mutant. Plant Cell 11, 901–910. doi: 10.2307/3870823
Sun, X., Feng, P., Xu, X., Guo, H., Ma, J., Chi, W., et al. (2011). A chloroplast envelope-bound PHD transcription factor mediates chloroplast signals to the nucleus. Nat. Commun. 2:477. doi: 10.1038/ncomms1486
Susek, R. E., Ausubel, F. M., and Chory, J. (1993). Signal-transduction mutants of Arabidopsis uncouple nuclear cab and rbcs gene-expression from chloroplast development. Cell 74, 787–799. doi: 10.1016/0092-8674(93)90459-4
Suzuki, N., Koussevitzky, S., Mittler, R., and Miller, G. (2012). ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 35, 259–270. doi: 10.1111/j.1365-3040.2011.02336.x
Suzuki, N., Sejima, H., Tam, R., Schlauch, K., and Mittler, R. (2011). Identification of the MBF1 heat-response regulon of Arabidopsis thaliana. Plant J. 66, 844–851. doi: 10.1111/j.1365-313X.2011.04550.x
Telfer, A., Dhami, S., Bishop, S. M., Phillips, D., and Barber, J. (1994). Beta-carotene quenches singlet oxygen formed by isolated photosystem-ii reaction centers. Biochemistry 33, 14469–14474. doi: 10.1021/bi00252a013
Tron, A. E., Bertoncini, C. W., Chan, R. L., and Gonzalez, D. H. (2002). Redox regulation of plant homeodomain transcription factors. J. Biol. Chem. 277, 34800–34807. doi: 10.1074/jbc.M203297200
Vacca, R. A., de Pinto, M. C., Valenti, D., Passarella, S., Marra, E., and De Gara, L. (2004). Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco bright-yellow 2 cells. Plant Physiol. 134, 1100–1112. doi: 10.1104/pp.103.035956
Vacca, R. A., Valenti, D., Bobba, A., Merafina, R. S., Passarella, S., and Marra, E. (2006). Cytochrome c is released in a reactive oxygen species-dependent manner and is degraded via caspase-like proteases in tobacco bright-yellow 2 cells en route to heat shock-induced cell death. Plant Physiol. 141, 208–219. doi: 10.1104/pp.106.078683
Valenti, D., Vacca, R. A., de Pinto, M. C., De Gara, L., Marra, E., and Passarella, S. (2007). In the early phase of programmed cell death in Tobacco Bright Yellow 2 cells the mitochondrial adenine nucleotide translocator, adenylate kinase and nucleoside diphosphate kinase are impaired in a reactive oxygen species-dependent manner. Biochim. Biophys. Acta Bioenerg. 1767, 66–78. doi: 10.1016/j.bbabio.2006.11.004
Vallelian-Bindschedler, L., Schweizer, P., Mosinger, E., and Metraux, J. P. (1998). Heat-induced resistance in barley to powdery mildew (Blumeria graminis f.sp. hordei) is associated with a burst of active oxygen species. Physiol. Mol. Plant Pathol. 52, 185–199. doi: 10.1006/pmpp.1998.0140
Van Akent, O., and Van Breusegem, F. (2015). Licensed to kill: mitochondria. Chloropl. Cell Death Trends Plant Sci. 20, 754–766. doi: 10.1016/j.tplants.2015.08.002
Vandenabeele, S., Vanderauwera, S., Vuylsteke, M., Rombauts, S., Langebartels, C., Seidlitz, H. K., et al. (2004). Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J. 39, 45–58. doi: 10.1111/j.1365-313X.2004.02105.x
Vanderauwera, S., Suzuki, N., Miller, G., van de Cotte, B., Morsa, S., Ravanat, J.-L., et al. (2011). Extranuclear protection of chromosomal DNA from oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 108, 1711–1716. doi: 10.1073/pnas.1018359108
Vanderauwera, S., Zimmermann, P., Rombauts, S., Vandenabeele, S., Langebartels, C., Gruissem, W., et al. (2005). Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 139, 806–821. doi: 10.1104/pp.105.065896
Vass, I., and Cser, K. (2009). Janus-faced charge recombinations in photosystem II photoinhibition. Trends Plant Sci. 14, 200–205. doi: 10.1016/j.tplants.2009.01.009
Veljovic-Jovanovic, S., Noctor, G., and Foyer, C. H. (2002). Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiol. Biochem. 40, 501–507. doi: 10.1016/S0981-9428(02)01417-1
Virdi, A. S., Singh, S., and Singh, P. (2015). Abiotic stress responses in plants: roles of calmodulin-regulated proteins. Front. Plant Sci. 6:809. doi: 10.3389/fpls.2015.00809
Vishwakarma, A., Tetali, S. D., Selinski, J., Scheibe, R., and Padmasree, K. (2015). Importance of the alternative oxidase (AOX) pathway in regulating cellular redox and ROS homeostasis to optimize photosynthesis during restriction of the cytochrome oxidase pathway in Arabidopsis thaliana. Ann. Bot. 116, 555–569. doi: 10.1093/aob/mcv122
Volkov, R. A., Panchuk, I. I., Mullineaux, P. M., and Schoeffl, F. (2006). Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol. Biol. 61, 733–746. doi: 10.1007/s11103-006-0045-4
von Gromoff, E. D., Alawady, A., Meinecke, L., Grimm, B., and Beck, C. F. (2008). Heme, a plastid-derived regulator of nuclear gene expression in Chlamydomonas. Plant Cell 20, 552–567. doi: 10.1105/tpc.107.054650
von Koskull-Doring, P., Scharf, K. D., and Nover, L. (2007). The diversity of plant heat stress transcription factors. Trends Plant Sci. 12, 452–457. doi: 10.1016/j.tplants.2007.08.014
Voss, B., Meinecke, L., Kurz, T., Al-Babili, S., Beck, C. F., and Hess, W. R. (2011). Hemin and magnesium-protoporphyrin IX induce global changes in gene expression in Chlamydomonas reinhardtii. Plant Physiol. 155, 892–905. doi: 10.1104/pp.110.158683
Wagner, D., Przybyla, D., Camp, R. O. D., Kim, C., Landgraf, F., Lee, K. P., et al. (2004). The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306, 1183–1185. doi: 10.1126/science.1103178
Wahid, A., Gelani, S., Ashraf, M., and Foolad, M. R. (2007). Heat tolerance in plants: an overview. Environ. Exp. Bot. 61, 199–223. doi: 10.1016/j.envexpbot.2007.05.011
Wang, L., Guo, Y., Jia, L., Chu, H., Zhou, S., Chen, K., et al. (2014). Hydrogen peroxide acts upstream of nitric oxide in the heat shock pathway in Arabidopsis seedlings1[C][W]. Plant Physiol. 164, 2184–2196. doi: 10.1104/pp.113.229369
Wang, P., Duan, W., Takabayashi, A., Endo, T., Shikanai, T., Ye, J. Y., et al. (2006). Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiol. 141, 465–474. doi: 10.1104/pp.105.070490
Wang, Y., Chang, H., Hu, S., Lu, X., Yuan, C., Zhang, C., et al. (2014a). Plastid casein kinase 2 knockout reduces abscisic acid (ABA) sensitivity, thermotolerance, and expression of ABA- and heat-stress-responsive nuclear genes. J. Exp. Bot. 65, 4159–4175. doi: 10.1093/jxb/eru190
Wang, Y., Lin, S., Song, Q., Li, K., Tao, H., Huang, J., et al. (2014b). Genome-wide identification of heat shock proteins (Hsps) and Hsp interactors in rice: Hsp70s as a case study. BMC Genomics 15:344. doi: 10.1186/1471-2164-15-344
Ward, J. M., Maeser, P., and Schroeder, J. I. (2009). Plant ion channels: gene families, physiology, and functional genomics analyses. Annu. Rev. Physiol. 71, 59–82. doi: 10.1146/annurev.physiol.010908.163204
Waters, M. T., Wang, P., Korkaric, M., Capper, R. G., Saunders, N. J., and Langdale, J. A. (2009). GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell 21, 1109–1128. doi: 10.1105/tpc.108.065250
Watson, J. C., and Surzycki, S. J. (1983). Both the chloroplast and nuclear genomes of chlamydomonas-reinhardi share homology with Escherichia-coli genes for transcriptional and translational components. Curr. Genet. 7, 201–210. doi: 10.1007/BF00434891
Wise, R. R., Olson, A. J., Schrader, S. M., and Sharkey, T. D. (2004). Electron transport is the functional limitation of photosynthesis in field-grown Pima cotton plants at high temperature. Plant Cell Environ. 27, 717–724. doi: 10.1111/j.1365-3040.2004.01171.x
Woodson, J. D., and Chory, J. (2008). Coordination of gene expression between organellar and nuclear genomes. Nat. Rev. Genet. 9, 383–395. doi: 10.1038/nrg2348
Woodson, J. D., Perez-Ruiz, J. M., and Chory, J. (2011). Heme synthesis by plastid ferrochelatase I regulates nuclear gene expression in plants. Curr. Biol. 21, 897–903. doi: 10.1016/j.cub.2011.04.004
Xiao, Y., Savchenko, T., Baidoo, E. E. K., Chehab, W. E., Hayden, D. M., Tolstikov, V., et al. (2012). Retrograde signaling by the plastidial metabolite MEcPP Regulates expression of nuclear stress-response genes. Cell 149, 1525–1535. doi: 10.1016/j.cell.2012.04.038
Xiao, Y., Wang, J., and Dehesh, K. (2013). Review of stress specific organelles-to-nucleus metabolic signal molecules in plants. Plant Sci. 212, 102–107. doi: 10.1016/j.plantsci.2013.08.003
Yamada, M., Hidaka, T., and Fukamachi, H. (1996). Heat tolerance in leaves of tropical fruit crops as measured by chlorophyll fluorescence. Sci. Horticult. 67, 39–48. doi: 10.1016/S0304-4238(96)00931-4
Yamane, Y., Kashino, Y., Koike, H., and Satoh, K. (1997). Increases in the fluorescence F-0 level and reversible inhibition of Photosystem II reaction center by high-temperature treatments in higher plants. Photosynth. Res. 52, 57–64. doi: 10.1023/A:1005884717655
Yamashita, A., Nijo, N., Pospisil, P., Morita, N., Takenaka, D., Aminaka, R., et al. (2008). Quality control of photosystem II - Reactive oxygen species are responsible for the damage to photosystem II under moderate heat stress. J. Biol. Chem. 283, 28380–28391. doi: 10.1074/jbc.M710465200
Yoshida, R., Sato, T., Kanno, A., and Kameya, T. (1998). Streptomycin mimics the cool temperature response in rice plants. J. Exp. Bot. 49, 221–227. doi: 10.1093/jxb/49.319.221
You, J., and Chan, Z. (2015). ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 6:1092. doi: 10.3389/fpls.2015.01092
Young, J. C., Moarefi, I., and Hartl, F. U. (2001). Hsp90: a specialized but essential protein-folding tool. J. Cell Biol. 154, 267–273. doi: 10.1083/jcb.200104079
Yu, H.-D., Yang, X.-F., Chen, S.-T., Wang, Y.-T., Li, J.-K., Shen, Q., et al. (2012). Downregulation of chloroplast RPS1 negatively modulates nuclear heat-responsive expression of HsfA2 and its target genes in Arabidopsis. PLoS Genet. 8:e1002669. doi: 10.1371/journal.pgen.1002669
Zhang, W., Zhou, R.-G., Gao, Y.-J., Zheng, S.-Z., Xu, P., Zhang, S.-Q., et al. (2009). Molecular and genetic evidence for the key role of AtCaM3 in heat-shock signal transduction in Arabidopsis. Plant Physiol. 149, 1773–1784. doi: 10.1104/pp.108.133744
Keywords: chloroplast, retrograde signaling, heat stress responses, plastid signal molecules, reactive oxygen species (ROS)
Citation: Sun A-Z and Guo F-Q (2016) Chloroplast Retrograde Regulation of Heat Stress Responses in Plants. Front. Plant Sci. 7:398. doi: 10.3389/fpls.2016.00398
Received: 30 December 2015; Accepted: 14 March 2016;
Published: 31 March 2016.
Edited by:
Mohammad Anwar Hossain, Bangladesh Agricultural University, BangladeshReviewed by:
Paulo Arruda, Universidade Estadual de Campinas, BrazilCopyright © 2016 Sun and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang-Qing Guo, ZnFndW9Ac2licy5hYy5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.