- Department of Biotechnology, University of Verona, Verona, Italy
The ABC1K family of atypical kinases (activity of bc1 complex kinase) is represented in bacteria, archaea, and eukaryotes. In plants they regulate diverse physiological processes in the chloroplasts and mitochondria, but their precise functions are poorly defined. ABC1K7 and ABC1K8 are probably involved in oxidative stress responses, isoprenyl lipid synthesis and distribution of iron within chloroplasts. Because reactive oxygen species take part in abscisic acid (ABA)-mediated processes, we investigated the functions of ABC1K7 and ABC1K8 during germination, stomatal movement, and leaf senescence. Both genes were upregulated by ABA treatment and some ABA-responsive physiological processes were affected in abc1k7 and abc1k8 mutants. Germination was more severely affected by ABA, osmotic stress and salt stress in the single and double mutants; the stomatal aperture was smaller in the mutants under standard growth conditions and was not further reduced by exogenous ABA application; ABA-induced senescence symptoms were more severe in the leaves of the single and double mutants compared to wild type leaves. Taken together, our results suggest that ABC1K7 and ABC1K8 might be involved in the cross-talk between ABA and ROS signaling.
Introduction
The ABC1K family of atypical kinases is characterized by a highly conserved ABC1 domain and one or more protein kinase domains (Leonard et al., 1998). This ancient family is highly conserved among bacteria, archaea and eukaryotes, and has undergone substantial expansion in photosynthetic organisms (Lundquist et al., 2013). The strong conservation of ABC1K proteins suggests they play a fundamental biological role. The prototypical member is ABC1/COQ8, a nuclear-encoded protein identified in the yeast Saccharomyces cerevisiae which is required for ubiquinone biosynthesis in the mitochondria. In yeast, this protein is needed for aerobic respiration at the mitochondrial bc1 complex level, and its inactivation makes the bc1 complex unstable resulting in a respiratory defect (Bousquet et al., 1991). The biological role of ABC1/COQ8 homologs has been investigated in other species revealing a conserved functional role in ubiquinone synthesis in bacteria, archaea and the mitochondria of eukaryotes (Ernster and Forsmark-Andrée, 1993; Macinga et al., 1998; Poon et al., 2000; Do et al., 2001; Iiizumi et al., 2002; Mollet et al., 2008). However, little is known about the role of ABC1K homologs in chloroplasts.
The Arabidopsis thaliana genome contains 17 members of the ABC1K family. The first to be characterized, the mitochondrial ABC1At protein, differs only slightly from S. cerevisiae ABC1/COQ8 and partially restores the respiratory defect in abc1- mutant yeast (Cardazzo et al., 1998). AtOSA1 was the first chloroplast-localized ABC1K protein to be investigated; it is induced by cadmium (Cd) and oxidative stress, and it does not complement the yeast abc1- mutant, suggesting a functional difference between this protein and mitochondrial ABC1 (Jasinski et al., 2008). Another ABC1K protein, AtACDO1, was also shown to be localized in chloroplasts, and is associated with chlorophyll degradation and oxidative stress responses under high-light (Yang et al., 2012a). Two plastoglobule-localized ABC1K proteins, ABC1K1 and ABC1K3, regulate prenylquinone metabolism, which plays an important role in plant stress responses and chloroplast morphology (Lundquist et al., 2013; Martinis et al., 2013). ABC1K1 is needed to stabilize chlorophyll-binding proteins in photosynthetic complexes, and abc1k1 knockdown plants show defects in sugar metabolism suggesting that ABC1K1 may integrate photosynthesis with associated metabolic pathways in chloroplasts (Martinis et al., 2014). Recently, it was reported that another chloroplast localized ABC1 gene, AtSIA1 together with AtOSA1, with which it shows high sequence conservation, participates in iron distribution inside the chloroplast and in plant response to oxidative stress (Manara et al., 2014).
The A. thaliana plastoglobule proteome was shown to include six of the eight ABC1K proteins currently known to be localized in chloroplasts (Ytterberg et al., 2006; Vidi et al., 2007; Lundquist et al., 2012b, 2013). Similarly, the proteomic analysis of maize (Zea mays) leaves indicated that eight ABC1K proteins are located in the chloroplasts (Majeran et al., 2010). Indeed, in silico predictions of protein localization indicate that most of the maize, rice (Oryza sativa) and A. thaliana ABC1K proteins are located in either the chloroplasts or the mitochondria, and this may potentially be the case in all plants (Lundquist et al., 2012a). A systematic nomenclature based on phylogeny has been proposed for the ABC1K family to avoid the assignment of arbitrary names, and we have adopted this nomenclature herein (Lundquist et al., 2012a).
In a previous study, we characterized ABC1K7 (formerly AtSIA1) and ABC1K8 (formerly AtOSA1) to determine their physiological functions (Manara et al., 2014). Among the A. thaliana ABC1K proteins, ABC1K7 was most closely related to ABC1K8 (46% identity) and showed 50% identity with cyanobacterial ABC1K proteins such as Crocosphaera watsonii ZP00517317 suggesting this protein was also a member of the chloroplast ABC1K group. The strong sequence conservation between ABC1K7 and ABC1K8 prompted us to study the phenotypes of abc1k7 and abc1k8 single mutants and abc1k7/abc1k8 double mutant (to investigate potential functional redundancy) as well as transgenic lines overexpressing ABC1K7 and ABC1K8 in their mutant backgrounds (Manara et al., 2014). This confirmed the chloroplast localization of ABC1K7 as previously reported for ABC1K8 (Jasinski et al., 2008) and revealed its role in oxidative stress responses, isoprenyl lipid synthesis and (together with ABC1K8) iron distribution within the chloroplast (Manara et al., 2014). Plant ABC1K proteins have previously been associated with different forms of abiotic stress tolerance (Jasinski et al., 2008; Gao et al., 2010, 2012; Wang et al., 2011). The phenotypes of the abc1k7 and abc1k8 single mutants and abc1k7/abc1k8 double mutants supported this hypothesis because they were less tolerant to ROS and the antioxidant network was activated even under standard growth conditions (Manara et al., 2014). In addition, an untargeted lipidomic analysis demonstrated that ABC1K7 and ABC1K8 are required for chloroplast lipid synthesis or accumulation and modulate chloroplast membrane lipid composition (Manara et al., 2015). abc1k7 and abc1k8 single mutants produced lower levels of the highly unsaturated lipid digalactosyldiacylglycerol (DGDG) than WT plants and also different forms of monogalactosyldiacylglycerol (MGDG) and kaempferol. The abc1k8 mutant is also characterized by higher levels of oxylipin-conjugated DGDG and sinapates, and the abc1k7/abc1k8 double mutant produced even higher levels of oxylipin-conjugated MGDG and DGDG (Manara et al., 2015).
Reactive oxygen species can modulate ABA-induced seed dormancy (Liu et al., 2010) and stomatal closure (Neill et al., 2008; Simontacchi et al., 2013). The accumulation of ROS may also cause the decline in ABA levels during germination, e.g., H2O2 accumulates rapidly during seed imbibition and can induce ABA catabolism and the biosynthesis of gibberellins (Liu et al., 2010). ROS, especially H2O2, act as secondary messengers during ABA-induced stomatal closure (Zhang et al., 2001). ABA is a hormonal trigger of leaf senescence (Gepstein and Thimann, 1980) and ABA levels in leaves increase during this process (Zhao et al., 2010), which can be promoted by the application of exogenous ABA (Weaver et al., 1998).
To gain insight into the role of ABC1K7 and ABC1K8 in the regulation of ABA signaling during seed dormancy, stomatal responses and leaf senescence, we studied the hormonal regulation of ABC1K7 and ABC1K8 and their ability to regulate other genes. We found that the ABC1K7 and ABC1K8 genes are upregulated by ABA treatment after 24 h and that the proteins play a significant role in ABA-mediated physiological processes. ABC1K7 and ABC1K8 regulate several ABA-responsive genes that control germination, abiotic stress responses, stomatal closure and leaf senescence. There were no significant differences in endogenous ABA levels among abc1k7 and abc1k8 mutants, abc1k7/abc1k8 double mutants and WT plants, but the mutants were more sensitive to exogenous ABA treatments. Our results suggest there is substantial crosstalk between ROS-mediated and ABA-mediated signaling pathways in plants.
Materials and Methods
Plant Material and Growth Conditions
The abc1k7 (atsia1) mutant (SALK_020431), the generation of the abc1k7/abc1k8 (atsia1/atosa1) double mutant and the generation of transgenic lines OX-ABC1K7 and OX-ABC1K8 overexpressing ABC1K7 and ABC1K8, respectively, were reported in an earlier publication (Manara et al., 2014). The A. thaliana abc1k8 (atosa1) mutant seeds (GABI_132G06) were kindly provided by Prof. E. Martinoia (City University of Zurich, Switzerland; Jasinski et al., 2008). The OX-ABC1K7 and OX-ABC1K8 transgenic lines were used in the complementation experiments to confirm differences between mutant and WT plants. Three independent lines for both OX-ABC1K7 and OX-ABC1K8 were chosen and the expression of the recombinant fusion proteins was confirmed by western blot (Manara et al., 2014). These overexpressing lines were obtained in mutant backgrounds and they show complementation of mutant phenotype. As described in Manara et al. (2014) the different overexpressing lines complemented the mutant phenotype in a similar manner and, for simplicity only one overexpressing line for each gene was showed in this work. A. thaliana ecotype Col-0 was used as the WT control.
Plants were grown under controlled conditions in a phytochamber (16 h light/8 h dark, illumination 100–120 μmol m-2s-1, day/night temperature 22°C/18°C). Seeds were sown in Petri dishes on water-soaked Whatman filter paper and incubated for 3 days at 4°C in the dark. The plants were then transferred to soil in a phytochamber under the controlled conditions described above.
Abiotic Stress, ABA, and ABA Synthesis Inhibitor Treatments
To analyze the effect of other abiotic stresses on gene expression, seedlings grown in vitro on MS medium (Murashige and Skoog, 1962) with 0.7% agar and 3% sucrose were transferred to the following different conditions for 0, 1, 2, 5, and 24 h and kept under the growth conditions described: (i) ½-strength Hoagland solution (Hoagland and Arnon, 1938) supplemented with 10 μM CdSO4; (ii) plates directly kept at 4°C; (iii) ½-strength Hoagland solution supplemented with 300 mM mannitol. Leaf samples were collected after 5 and 24 h treatment, and immediately frozen in liquid nitrogen. The effect of ABA treatment on the ABC1K7 and ABC1K8 expression levels was analyzed in A. thaliana WT seedlings grown in ½-strength Hoagland solution supplemented with ABA (20 μM). As in the previous experiments, leaf samples were collected after 5 and 24 h treatment, and immediately frozen in liquid nitrogen. The analysis of ABC1K7 and ABC1K8 expression upon treatment with the ABA synthesis inhibitor NDGA was conducted in A. thaliana in vitro grown WT plantlets, which were transferred into Petri dishes containing disks of Whatman paper soaked with liquid Murashige and Skoog (MS) medium solution (control condition) and liquid MS containing 100 μM nordihydroguaiaretic acid (NDGA, Sigma-Aldrich; Ren et al., 2007). Treated plantlets were incubated under controlled conditions in a phytochamber (16 h light/8 h dark, illumination 100–120 μmol m-2s-1, day/night temperature 22°C/18°C). Leaf samples were collected after 2, 5, and 24 h treatment, and immediately frozen in liquid nitrogen.
Germination Analysis
Sterilized seeds were germinated on MS agar medium supplemented with different concentrations of ABA (0, 20, 50, and 100 μM, for 2 and 4 days), mannitol (100 and 200 mM, for 2 days), and NaCl (100 mM for 3 days and 200 mM for 7 days). The plants were grown in the phytochamber under the controlled conditions described above. Approximately, 100 seeds of each genotype were sown on each plate and scored for germination. Each experiment was performed in triplicate.
RNA Extraction, cDNA Synthesis, and Real-Time RT-PCR
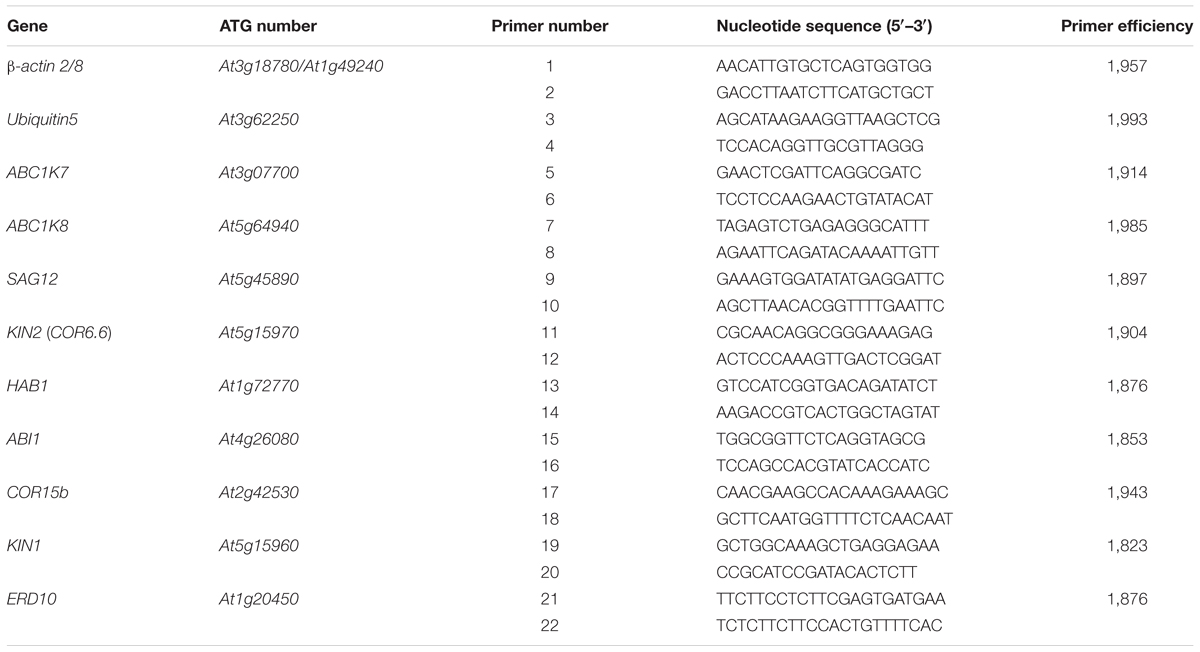
Total RNA was extracted from fresh or liquid nitrogen frozen tissues using TRIzol reagent (Invitrogen, Karlsruhe, Germany). After DNase treatment, first-strand cDNA was synthesized using SuperScriptTM III Reverse Transcriptase (Invitrogen). Real-time RT-PCR (40 amplification cycles) was carried out using the ABI PRISM® 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) with Platinum® SYBR® Green qPCRSuperMix UDG (Invitrogen). Each reaction was carried out in triplicate and melting curves were analyzed to ensure the amplification of a single product. Quantitative data were normalized to the mean of two endogenous reference genes: actin2/8 (At3g18780/At1g49240; primers 1 and 2) and ubiquitin 5 (At3g62250; primers 3 and 4). The 2-ΔΔCT method for the analysis of relative gene expression levels was used to organize the data (Livak and Schmittgen, 2001). ABC1K7 (At3g07700) and ABC1K8 (At5g64940) transcription levels were quantified using specific primers (primers 5 and 6 for ABC1K7, and primers 7 and 8 for ABC1K8). SAG12mRNA levels were also measured (primers 9 and 10). The levels of ABA-regulated gene transcripts KIN2 (COR6.6; At5g15970; primers 11 and 12), HAB1 (At1g72770; primers 13 and 14), ABI1 (At4g26080; primers 15 and 16), COR15b (At2g42530; primers 17 and 18), KIN1 (At5g15960; primers 19 and 20), and ERD10 (At1g20450; primers 21 and 22) were also measured. All primers are listed in Table 1 and their efficiency was determined using LinRegPCR v2012.2 (Ramakers et al., 2003).
Measurement of Stomatal Apertures
Detached rosette leaves from illuminated plants (100–120 μmol m-2s-1) grown in hydroponic solution with or without the addition of 20 μM ABA were fixed overnight (0.1% glutaraldehyde, 4% paraformaldehyde, 0.1 M sodium phosphate buffer pH 7.2) and the stomata were observed directly using a Leica DM RB microscope (Leica Microsystem GmbH) as previously described (Mustilli et al., 2002). At least 50 stomata were measured for each genotype and condition. The stomatal aperture was measured using ImageJ software (http://rsbweb.nih.gov/ij/).
Analysis of ABA-Induced Leaf Senescence
Detached rosette leaves were incubated in water or in ABA solution (20 and 50 μM) for 5 days under the conditions described above, as previously reported (Jia et al., 2013). Each experiment was performed in triplicate.
Determination of Chlorophyll Content
Leaves were weighed, frozen and ground to powder under liquid nitrogen. Chlorophyll was extracted in 80% acetone saturated with Na2CO3, and cell debris was removed by centrifugation (10,000 × g, 4°C, 10 min). Absorbance values were recorded at 750.0, 646.6, and 663.3 nm. The concentrations of chlorophyll a and b were determined using classical equations (Porra, 2002). Each experiment was performed in triplicate.
Measurements of ABA Content
Abscisic acid content was measured in leaves, dry seeds and imbibed seeds of plants grown in control conditions and treated for 7 days with 100 mM mannitol and 100 mM CdSO4. Plant material has harvested and immediately frozen in liquid nitrogen. Grinded tissues have been processed, and ABA quantification has been obtained, accordingly to the protocol previously described in Glauser et al. (2014).
Statistical Analysis
All experiments were carried out at least three times. Differences between WT, mutants and overexpressing lines were determined by one-way analysis of variance (ANOVA). Significant differences are indicated as follows: ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001.
Results
Analysis of ABC1K7 and ABC1K8 Expression in Response to Abiotic Stresses, Exogenous ABA, and upon Inhibition of Endogenous ABA Synthesis
We investigated the effect of different forms of abiotic stress on the expression of ABC1K7 and ABC1K8 by submitting plants to Cd treatment, cold and mannitol treatment. As reported in Figure 1A, although differently, both genes are upregulated in response to the stress: ABC1K7 and ABC1K8 show a substantial and clear upregulation upon Cd and mannitol treatments lasting also 24 h after exposure, while cold stress imposes only an early response (5 h upon treatment). Because ABA acts as an endogenous messenger in plant stress responses (Hirayama and Shinozaki, 2007) and induces changes in gene expression (Rabbani et al., 2003; Shinozaki et al., 2003), we also measured the levels of ABC1K7 and ABC1K8 mRNA in the leaves of WT plants with and without exposure to exogenous ABA. As control of ABA effect on gene expression, we included in the analysis ERD10, an ABA responsive gene. As highlighted in Figure 1B, both genes were induced by exposure to ABA for 24 h, with significance levels of P < 0.01 for ABC1K7 and P < 0.05 for ABC1K8. The ABC1Ks ABA-mediated induction was also reported in rice and poplar (Gao Q.S. et al., 2011; Wang et al., 2013). Interestingly, the application for 24 h of 100 μM NDGA, an inhibitor of the endogenous ABA biosynthesis (Ren et al., 2007; Gao G. et al., 2011), induced a downregulation in expression of both ABC1K7 and ABC1K8 (Figure 1C). Notably, the expression of ABC1K8 is responding to inhibition of ABA synthesis already after 2 h of treatment (Figure 1C).
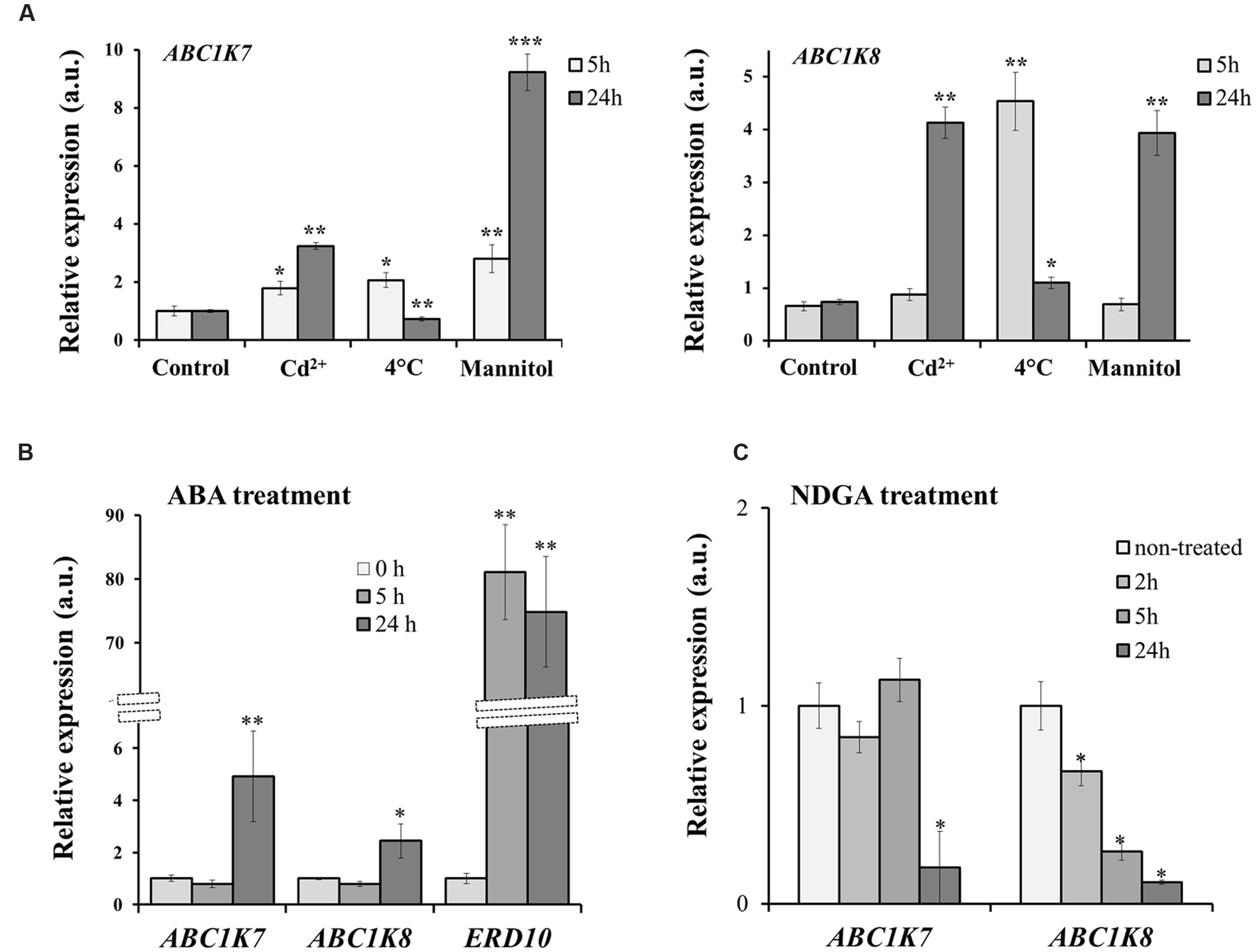
FIGURE 1. Analysis of ABC1K7 and ABC1K8 mRNA levels. (A) Real-time RT-PCR analysis of ABC1K7 and ABC1K8 in the leaves of WT plants exposed to a variety of abiotic stress. (B) Real-time RT-PCR analysis of ABC1K7, ABC1K8, and ERD10 in the leaves of WT plants exposed to 20 μM ABA for different durations. (C) Real-Time RT-PCR analysis of RNA abundance of ABC1K7 and ABC1K8 genes upon treatment with the ABA synthesis inhibitor NDGA for 2, 5, and 24 h. The expression levels were calculated using the 2-ΔΔCT method and are relative to the control leaf expression level (0 h). In C, the expression levels are relative to the expression in control plantlets, which was set to 1 for each gene. Each value represents the mean ± SE. Significant differences relative to the control treatment are shown as follows: ∗P < 0.05 and ∗∗P < 0.01, ∗∗∗P < 0.001.
The Role of ABC1K7 and ABC1K8 during Germination
We investigated the role of ABC1K7 and ABC1K8 in the ABA-mediated regulation of germination by comparing the effect of increasing ABA concentrations (Umezawa et al., 2009) on the germination of abc1k7 and abc1k8 single mutants, abc1k7/abc1k8 double mutants, overexpressing lines and WT plants. After 2 days of exposure to 20 μM ABA, the percentage of germinating plants was significantly lower in the abc1k7 (P < 0.01), abc1k8 (P < 0.05), and abc1k7/abc1k8 (P < 0.05) lines compared to WT plants, and this effect was more significant in all three mutant lines (P < 0.01) when the ABA concentration was increased to 50 and 100 μM (Figure 2A). After 4 days of exposure to ABA, the mutant plants showed partial recovery and the only significant difference in germination frequency (P < 0.05) was found between the abc1k7/abc1k8 double mutant and WT plants at the highest ABA concentration tested (Figure 2B). OX-ABC1K7 and OX-ABC1K8 overexpressing lines show a WT-like behavior confirming the complementation of the phenotype (Figure 2). There were no differences between WT and mutant plants in terms of leaf or root growth on media supplemented with either 50 or 100 μM ABA (data not shown). However, these conditions resulted in general growth inhibition and leaf yellowing in all genotypes (data not shown). Next we tested the effect of osmotic stress and salt stress on the germination of mutant and WT plants by exposing the seeds to mannitol or sodium chloride. Osmotic stress induced by 100 or 200 mM mannitol did not affect the germination of WT plants but the lower of the two concentrations significantly (P < 0.05) reduced the frequency of germination in the double mutant. The higher concentration significantly (P < 0.05) reduced the frequency of germination in both the abc1k7 and abc1k8 single mutants, and had a more severe effect (P < 0.01) on the double mutant (Figure 2C). Moderate salt stress (100 mM NaCl) affected the frequency of germination in all genotypes 3 days after sowing although there was a significantly greater effect in the abc1k7 and abc1k8 mutants (P < 0.05) which became more severe in the abc1k7/abc1k8 double mutant (P < 0.01). Higher salt stress intensity (200 mM NaCl) affected the frequency of germination in all genotypes 7 days after sowing although again there was a significantly greater effect in the abc1k7 and abc1k8 mutants (P < 0.05) which became more severe (P < 0.01) in the abc1k7/abc1k8 double mutant (Figure 2D).
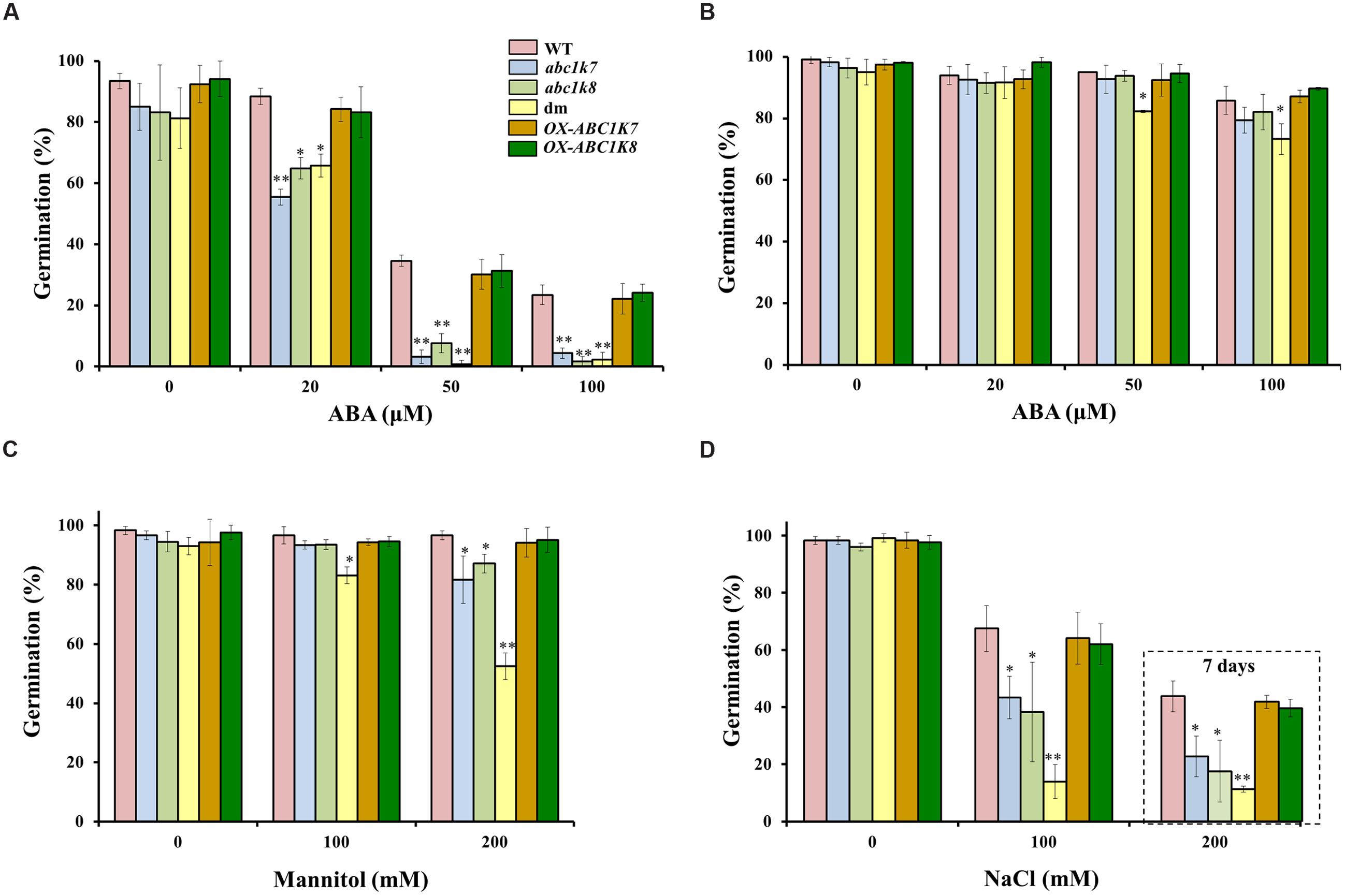
FIGURE 2. Analysis of ABA, mannitol and NaCl treatment on the germination of WT, mutant, and overexpressing lines. (A,B) Effect of ABA (0, 20, 50, and 100 μM) exposure for (A) 2 days and (B) 4 days after sowing. (C) Effect of mannitol (0, 100, and 200 mM) 2 days after sowing. (D) Effect of NaCl (0, 100 mM) 3 days after sowing and (200 mM) 7 days after sowing (dashed box). Each value represents the mean ± SD. Approximately, 100 seeds from each genotype were analyzed in three independent experiments. Significant differences relative to the WT are shown as follows: ∗P < 0.05 and ∗∗P < 0.01.
The Role of ABC1K7 and ABC1K8 during Stomatal Closure
The role of ABC1K7 and ABC1K8 in the ABA-mediated stomatal response was tested in abc1k7 and abc1k8 mutants, abc1k7/abc1k8 double mutants, transgenic lines overexpressing each of the proteins (OX-ABC1K7 and OX-ABC1K8) and WT controls (Manara et al., 2014). Following exposure to light (100–120 μmol m-2s-1) in the absence of ABA, the stomatal aperture of the mutant and double mutant lines was significantly smaller (P < 0.01) than that of WT plants and overexpressing lines (Figures 3A,B). ABA treatment (20 μM for 5 h) induced stomatal closure in the WT and overexpressing lines, whereas the stomata aperture in abc1k7 and abc1k8 mutants and abc1k7/abc1k8 double mutants was not further reduced by ABA application (Figures 3A,B). The stomatal density and size under normal conditions were similar in the WT, abc1k7, abc1k8, and abc1k7/abc1k8 plants (data not shown).
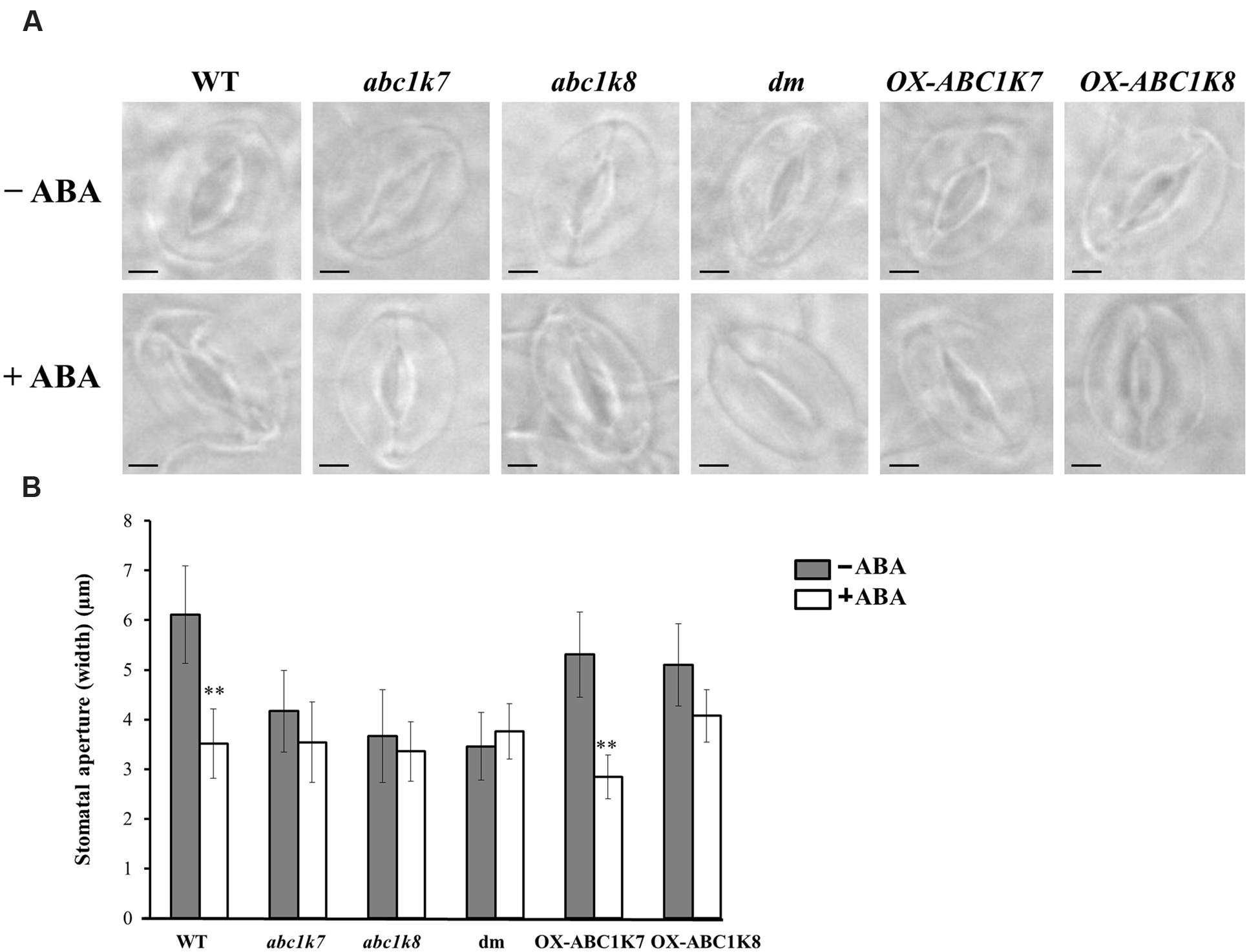
FIGURE 3. Effect of ABA treatment on the stomatal aperture. (A) Microscope images of stomata from WT, mutant and overexpressing plants under standard growth conditions (– ABA) or following 5 h exposure to 20 μM ABA (+ ABA). Bars = 5 μm. (B) Measurement of the stomatal apertures of WT, mutant and overexpressing plants under standard growth conditions (– ABA) or following 5 h exposure to 20 μM ABA (+ ABA). Each value represents the mean ± SD. Approximately 50 stomata from each genotype were analyzed in three independent experiments. Significant differences relative to the WT are shown as ∗∗P < 0.01.
The Role of ABC1K7 and ABC1K8 in ABA-Induced Leaf Senescence
The effect of ABA-induced leaf senescence was investigated using leaves detached from the mutant, double mutant and overexpressing plants as well as WT controls. Leaves were floated on water or 50 μM ABA for 5 days and senescence was assessed by observing leaf yellowing and measuring the chlorophyll content (Figure 4). Detached leaves floated on water showed no visible yellowing regardless of the genotype, whereas those floated on ABA solution developed yellow patches after 5 days. The leaves from abc1k7, abc1k8, and abc1k7/abc1k8 mutant plants development more extensive senescence symptoms than leaves from the WT and overexpressing plants, the latter behaving similar to WT (Figure 4A).
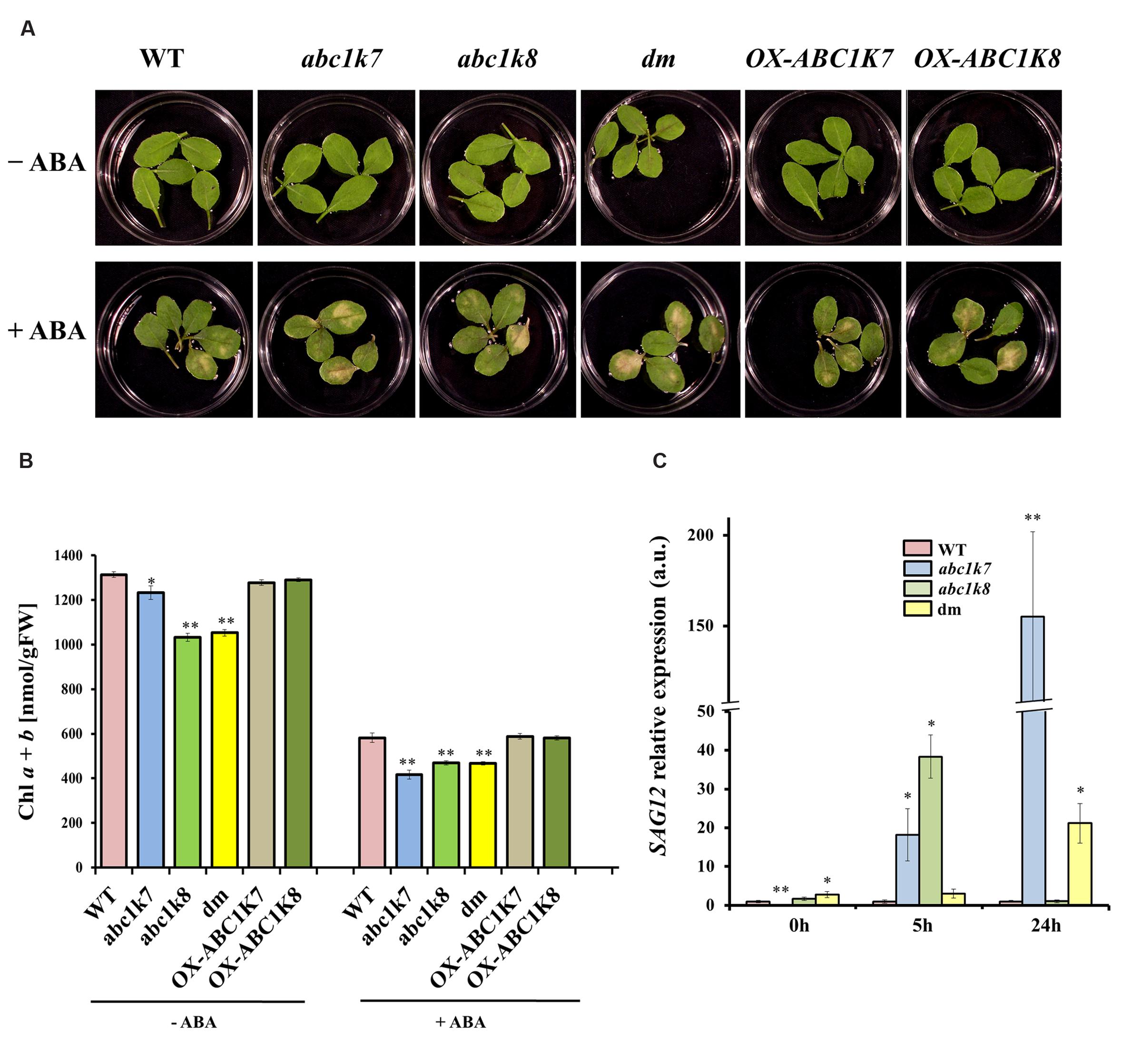
FIGURE 4. Analysis of ABA-induced leaf senescence. (A) Leaves detached from WT, mutant and overexpressing plants were floated on sterile water (– ABA) or 50 μM ABA for 5 days (+ ABA). (B) Chlorophyll content of detached leaves treated with sterile water (– ABA) or 50 μM ABA for 5 days (+ ABA). Each value represents the mean ± SD. (C) Real-time RT-PCR analysis of SAG12 in the leaves of WT and mutant plants under standard growth conditions (– ABA) or following 5 or 24 h exposure to 20 μM ABA (+ ABA). WT SAG12 expression was set to 1 in both control and ABA treatment. Each value represents the mean ± SE. Significant differences relative to the WT are shown as follows: ∗P < 0.05 and ∗∗P < 0.01.
These qualitative observations were measured objectively by determining the chlorophyll content of the leaves before and after ABA treatment. The total chlorophyll concentration was significantly lower (P < 0.01 and P < 0.05) in the mutant leaves compared to the leaves of WT and overexpressing plants, even when the leaves were floated on water (Figure 4B). This agrees with the previously observed phenotypes of abc1k8 and abc1k7/abc1k8 mutants, which were paler green and contained less chlorophyll than WT plants under controlled conditions (16 h light/8 h dark, 100–120 μmol m-2s-1; Manara et al., 2014). When the detached leaves were floated on 50 μM ABA, there was loss of chlorophyll in all genotypes, but the loss was highly significant (P < 0.01) in the abc1k7 and abc1k8 mutant and abc1k7/abc1k8 double mutant lines (Figure 4B). The impact of the abc1k7, abc1k8, and abc1k7/abc1k8 mutations on leaf senescence was evaluated further by measuring the abundance of the senescence-associated transcript SAG12 (Figure 4C). Under control condition SAG12 transcription is almost null in abc1k7 mutant respect to WT (P < 0.01) but significantly higher levels in the abc1k7/abc1k8 double mutant (P < 0.05). After exposure to ABA for 5 h, there was a significant increase in SAG12 mRNA levels in both the abc1k7 and abc1k8 mutant lines compared to WT (P < 0.05) and a highly significant increase (P < 0.01) in the abc1k7 mutant after exposure to ABA for 24 h (Figure 4C).
Analysis of ABA-Regulated Gene Expression
The role of ABC1K7 and ABC1K8 in ABA signaling was investigated by measuring the abundance of several ABA-modulated transcripts in abc1k7, abc1k8, and abc1k7/abc1k8 mutant lines compared to WT controls. The target genes comprised two type-2C phosphatases, ABI1 (Leung et al., 1994) and HAB1 (Rodriguez et al., 1998), which are negative regulators of ABA signaling; two non-seed LEA genes, Cor15b (LEA23; Wilhelm and Thomashow, 1993) and ERD10 (LEA5; Kiyosue et al., 1994); and two genes that are induced by cold, ABA, dehydration and mannitol, KIN1 (Wang et al., 1995) and KIN2 (Kurkela and Borg-Franck, 1992).
All six genes were induced by ABA in all genotypes (Figure 5). However, following 5 h of ABA exposure, HAB1 was more strongly induced (P < 0.01) in the abc1k8 and abc1k7/abc1k8 lines (Figure 5A) and ABI1 was more strongly induced all the mutant lines (P < 0.01 and P < 0.05) compared to WT plants (Figure 5B). These differences disappeared when ABA exposure was prolonged to 24 h (Figures 5A,B). Similarly, both LEA genes were significantly induced (P < 0.01) in the abc1k8 and abc1k7/abc1k8 lines following exposure for 5 h but the differences disappeared after 24 h (Figures 5C,D). Finally, KIN1 and KIN2 expression was significantly induced (P < 0.01) by ABA treatment for 5 h in the abc1k8 and in abc1k7/abc1k8 lines but significantly suppressed (P < 0.01 and P < 0.05) by exposure for 24 h (Figures 5E,F). No significant differences between mutant and WT plants were observed in the expression of LEA24 and LEA38, two other LEA genes with chloroplast-localized products (data not shown).
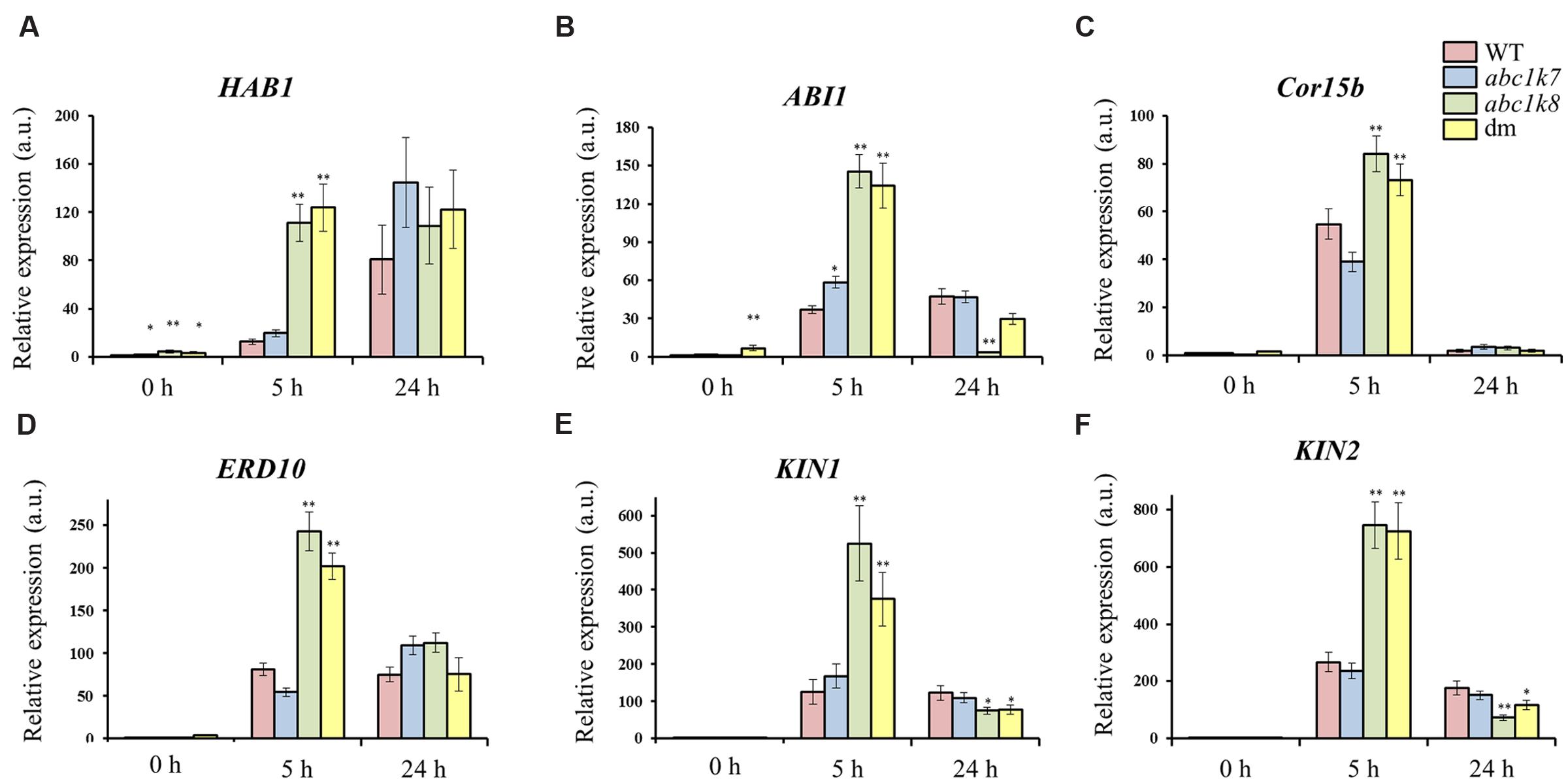
FIGURE 5. Analysis of ABA-regulated gene expression. Real-time RT-PCR analysis of (A) HAB1, (B) ABI1, (C) Cor15b, (D) ERD10, (E) KIN1, and (F) KIN2 expression in the leaves of WT and mutant plants before (0 h) and after (5 and 24 h) exposure to 20 μM ABA. Each value represents the mean ± SE. Significant differences relative to the WT are shown as follows: ∗P < 0.05 and ∗∗P < 0.01.
Analysis of Expression of ABA Metabolism Correlated Genes
To explore the link between the ABA-mediate response and the increased stress sensitivity showed by abc1k7 and abc1k8, the expression of genes involved in ABA metabolism was analyzed by Real Time PCR, comparing the expression level in control conditions and after a stress of 6 h dehydration (see Supplementary Material). Two genes involved in ABA synthesis, AAO3 (the Abscisic Aldehyde Oxidase 3) and NCED3 (9-cis-Epoxycarotenoid-Dioxygenase 3; Kanno et al., 2012) and two genes coding for proteins that were shown to have a role in ABA transport, AIT1 (ABA-Importing Transporter 1) and DTX50 (Detoxification Efflux Carrier 50; Zhang et al., 2014) were analyzed. As shown in Supplementary Figure S1, with the exception of AIT1, which is not modulated by dehydration, the other genes analyzed are overexpressed in WT by this stress condition. This stress, imposes an upregulation of DTX50, AAO3, and NCED3 also in abc1k7, abc1k8 mutant and abc1k7/abc1k8 double mutant plants. Notably the transporter protein DTX50 is upregulated in mutant lines already in control conditions (Supplementary Figure S1). Furthermore, NCED3, coding a key enzyme of ABA biosynthesis, is constitutively expressed in mutant backgrounds. AAO3 transcription level in abc1k7 and abc1k8 is similar to that of WT, whereas in the abc1k7/abc1k8 double mutant, the mRNA level upon standard growth conditions drops down, but it is normally up-regulated by dehydration.
Analysis of the Endogenous ABA Content
The results described above suggested that ABC1K7 and ABC1K8 are likely to play a role in ABA signaling. Therefore, the endogenous ABA content was measured in the leaves, dry seeds, and imbibed seeds (24 h) of each genotype to determine whether the observed phenotypes could be explained by differences in the availability of ABA (Figure 6). We found no differences in ABA content in the dry and imbibed seeds of the mutant and WT plants. The leaves of the abc1k8 mutant contained significantly more ABA than WT leaves (P < 0.05). Moreover, ABA levels were measured after Cd and mannitol treatments. Following Cd treatment, both single and double mutants showed an increased ABA content in comparison to WT, while mannitol did not influence ABA accumulation in leaves (Supplementary Figure S2).
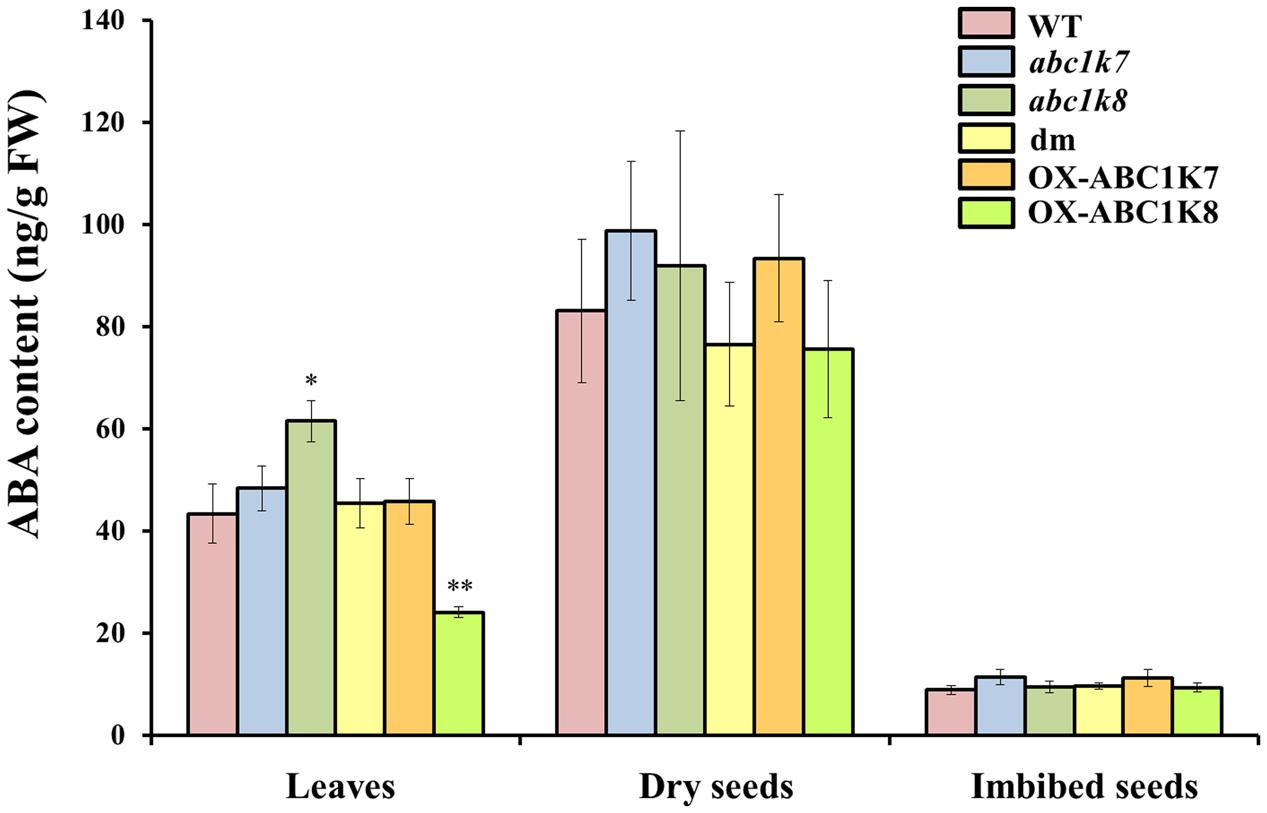
FIGURE 6. Analysis of the ABA content in plant tissues. The ABA content was quantified in leaves, dry seeds and imbibed seeds of WT, mutant and overexpressing plants grown under standard conditions. Each value represents the mean ± SD. Significant differences relative to the WT are shown as follows: ∗P < 0.05 and ∗∗P < 0.01.
Discussion
In bacteria, archaea, and eukaryotic mitochondria, ABC1K proteins are essential for the biosynthesis of coenzyme Q, which functions as an electron carrier in the respiratory chain, and as a lipid-soluble antioxidant (Poon et al., 2000; Do et al., 2001; Mollet et al., 2008; Tauche et al., 2008). The A. thaliana chloroplast proteome contains members of the ABC1K family, which are involved in the regulation of prenylquinone metabolism (Lundquist et al., 2012a) and stress responses (Jasinski et al., 2008; Gao et al., 2010; Gao Q.S. et al., 2011; Wang et al., 2011). Indeed, the role of ABC1K proteins in abiotic stress tolerance is well documented and the transcription of ABC1K genes is often modulated by oxidative stress and salt (Jasinski et al., 2008; Gao et al., 2010, 2012; Wang et al., 2011; Yang et al., 2012a,b).
The involvement of ABC1K7 and ABC1K8 in oxidative stress responses, isoprenyl lipid biosynthesis and iron distribution within the chloroplast has recently been demonstrated (Manara et al., 2014, 2015). The abc1k7 and abc1k8 single mutants and the abc1k7/abc1k8 double mutant produce more ROS and antioxidants than WT plants even under standard growth conditions, and are less tolerant to ROS and oxidative stress (Jasinski et al., 2008; Manara et al., 2014). These mutants also have a different polar lipid composition of chloroplast membranes in comparison to WT plants (Manara et al., 2015). Nevertheless, the precise biological role of these proteins is unclear because the abc1k7 and abc1k8 mutants show no changes in growth or development under standard conditions and the only visible phenotype is the pale green leaves of the abc1k8 mutant line (Jasinski et al., 2008; Manara et al., 2014).
Even though ROS cause extensive cellular damage at high concentrations (Van Breusegem and Dat, 2006), they play an important physiological role in ABA signaling during seed development, maturation and dormancy, germination, seedling growth and seed aging (Liu et al., 2010). The transition from a quiescent to a vital seed is also associated with the generation of ROS (Gomes and Garcia, 2013). Antioxidant defense mechanisms usually maintain the intracellular concentration of ROS at a low level, known as the oxidative window, rather than eliminating them completely, so that their physiological functions can be executed (Bailly et al., 2008). In particular, the roles of ROS in seed dormancy, germination and the control of stomata movements depend on their interactions with phytohormones such as ABA (Neill et al., 2008; Simontacchi et al., 2013). Due to the fact that abc1k7 and abc1k8 showed an imbalanced cell redox state, we investigated the role of ABC1K7 and ABC1K8 in the regulation of ABA signaling to gain insight into their biological functions.
The ABC1K7 and ABC1K8 genes were both induced by ABA treatment and downregulated when endogenous ABA synthesis is inhibited by applying NDGA, suggesting that endogenous ABA content modulates their expression and that ABC1K7 and ABC1K8 proteins probably have a physiological role in ABA signaling. Considering the involvement of ABA in responses to abiotic stress, and taking into account that other ABC1K genes have been characterized by abiotic stress responsiveness (Wang et al., 2011, 2013; Lundquist et al., 2012a; Yang et al., 2012a), we investigated the effect of a variety of abiotic stresses, such as Cd treatment, cold and mannitol treatment, on the expression of ABC1K7 and ABC1K8. We found that both genes are upregulated in response to the stress: both ABC1K7 and ABC1K8 show a substantial and clear upregulation upon Cd and mannitol exposure lasting also 24 h after the treatment, while cold stress imposes an early response. These results indicate that, similarly to other ABC1K proteins, also ABC1K7 and ABC1K8 have a role in response to abiotic stress also mediated by ABA signal transduction.
We investigated the basis of these interactions in more detail by measuring the ABA content of seeds in mutant plants and WT controls. There were no genotype-dependent differences in ABA content in the dry and imbibed seeds. This, together with the fact that both ABC1K7 and ABC1K8 mRNAs are modulated by ABA content, suggests that in these organs the abc1k7, abc1k8, and abc1k7/abc1k8 mutations affect the perception of ABA. It appears that the loss of ABC1K7 and/or ABC1K8 activity increase ABA sensitivity. Indeed, even though the frequency of germination in the abc1k7, abc1k8 and abc1k7/abc1k8 mutants was similar to WT plants in the absence of ABA, it was delayed in mutant lines upon exposure to ABA. These differences were mostly eliminated following prolonged exposure to ABA, although the frequency of germination remained lower in the abc1k7/abc1k8 double mutants at the highest ABA concentrations tested. Considering that ABA is a key mediator of abiotic stress responses (Himmelbach et al., 2003; Hubbard et al., 2010), we also tested the germination frequency of abc1k7, abc1k8, and abc1k7/abc1k8 mutant lines under osmotic and salt stress. The presence of either mannitol or sodium chloride progressively inhibited germination in mutant lines. The ABA-sensitive phenotype of these mutants regarding seed germination and the upregulation of ABC1K7 and ABC1K8 upon abiotic stress, indicate that these ABC1K kinases may participate in ABA signaling during germination and play a role in the development of tolerance toward osmotic stress and salt stress.
Considering the expression of key players involved in the ABA signal transduction pathway, HAB1 and ABI1 genes were upregulated in the abc1k7, abc1k8, and abc1k7/abc1k8 double mutant even in standard growth conditions, and overexpressed upon ABA treatment. Their gene products are Type 2C protein phosphatases (also known as PP2Cs) which constitutively block ABA signal transduction and expression of ABA-induced genes (Umezawa et al., 2010). Once abiotic stresses or developmental cues up-regulate endogenous ABA (or in case of exogenous ABA application), ABA receptors such as PYR/PYL/RCAR bind ABA and interact with PP2C, inhibiting its activity of negative regulator. This results in the activation of the ABA-induced gene expression (Umezawa et al., 2010). Plants overexpressing PP2C proteins are characterized by ABA insensitivity, while loss-of-function mutants show increased ABA sensitivity, in terms of germination rate upon mannitol, NaCl or ABA treatment (Saez et al., 2004). Considering that in abc1k7, abc1k8 and abc1k7/abc1k8, HAB1 and ABI1 resulted up-regulated in both standard growth conditions and upon ABA treatment, the hypersensitivity to ABA, mannitol and NaCl of seed germination in abc1k7, abc1k8, and abc1k7/abc1k8 points to a perturbed ABA sensing in these lines.
Under standard conditions, stomatal opening was inhibited in the abc1k7, abc1k8 and abc1k7/abc1k8 mutants compared to WT plants, while no stomatal movements were observed in the abc1k7, abc1k8, and abc1k7/abc1k8 mutants upon ABA treatment. This may be explained taking into consideration that abc1k7, abc1k8 and abc1k7/abc1k8 mutants showed an imbalanced leaf redox state (Jasinski et al., 2008; Manara et al., 2014), which may influence ABA-mediated stomatal movements, e.g., H2O2 mediates the ABA-driven induction of Ca2+-permeable, non-selective cation current channels (Pei et al., 2000; Murata et al., 2001; Kwak et al., 2003). This, again, points to a higher sensitivity of abc1k7 and abc1k8 plants to the endogenous ABA.
Interestingly, the low germination rate and increased stomatal closure following treatment with ABA observed in abc1k7, abc1k8, and abc1k7/abc1k8 double mutants may also reflect the greater abundance of OPDA-esterified galactolipids observed in these mutant lines (Manara et al., 2015). Indeed, galactolipids conjugated with OPDA and dnOPDA may allow the storage of reactive oxylipins (Böttcher and Weiler, 2007) and free OPDA may acts synergistically with ABA to inhibit germination and regulate stomatal closure (Dave et al., 2011; Savchenko et al., 2014).
Abscisic acid content was investigated also in term of expression analysis of key members of ABA metabolism. This shows an upregulation, in mutant plants, of NCED3 and DTX50, involved in ABA synthesis and transport respectively, which may locally affect ABA content. ABA content was measured in leaves of mutant plants and WT: a moderate increase in ABA abundance was detected only in leaves of abc1k8 in control conditions. It may be that ABA distribution inside the leaf, considering stomata guard cell as a target site, may be affected and accounting for the detected stomata closure. Alternatively, the increased ROS and SOD activity characteristic of the mutant lines (Manara et al., 2014) may enhance H2O2 production, which can in turn contribute to stomatal closure without ABA application (Zhang et al., 2001).
The role of ABC1K7 and ABC1K8 in ABA signaling is also supported by the comparative transcriptional analysis of six different ABA-induced genes in mutant and WT plants. Other than the already mentioned ABI1 and HAB1, two LEA genes (Cor15b and ERD10) were upregulated in mutant backgrounds after ABA application. Cor15b is induced by low temperatures and exogenous ABA (Wilhelm and Thomashow, 1993) and ERD10 is induced by temperatures, exogenous ABA and dehydration (Kiyosue et al., 1994). The two stress-inducible KIN genes (KIN1 and KIN2) were induced more strongly in the abc1k8 and abc1k7/abc1k8 mutant plants than in WT, following 5 h of ABA treatment, whereas the abc1k7 mutant behaved in a similar manner to WT. These genes are known to be induced by low temperatures and exogenous ABA (Kurkela and Borg-Franck, 1992; Wang et al., 1995). The induction of all six genes was short-lived and differences between mutants and WT plants had diminished after 24 h exposure, although in the case of KIN1 and KIN2 the transcript levels fell below WT levels after 24 h.
In addition to abiotic stress, ABA is also a positive regulator of leaf senescence (Lee et al., 2011) and exogenous ABA application induced senescence, associated with the loss of chlorophyll (Jaradat et al., 2013). When floated on ABA solution, detached leaves from abc1k7, abc1k8, and abc1k7/abc1k8 mutant showed typical symptoms of yellowing and chlorosis, while WT leaves showed a limited senescence response. These distinct phenotypes were confirmed by measuring the chlorophyll content, which also highlighted lower chlorophyll content in standard growth conditions (as reported previously, Manara et al., 2014). Among the mutant plants, upon ABA treatment abc1k7 leaves showed the most severe symptoms of ABA-induced senescence and the strongest expression of the senescence-associated SAG12 (Grbić, 2003). These results are supported by the recent observations that ABC1K7 is induced by and potentially involved in senescence, which correlates with higher levels of oxidative stress and ROS production (Lundquist et al., 2012b) and that abc1k7 is characterized by increased plastoglobule dimension (Manara et al., 2014). The plastoglobule proteome contains ABC1K7 and SAG in a senescence-associated protein cluster, which reveals senescence-associated and senescence-induced proteases, and two chlorophyll degradation enzymes (Lundquist et al., 2012b). The latter are coexpressed with ABC1K7 and SAG, explaining the loss of chlorophyll content and the stronger induction of SAG12 in the abc1k7 mutant in response to ABA. Therefore, our data suggest that ABC1K7 and ABC1K8 may be involved in alleviating leaf senescence, which is enhanced in mutant plants. Moreover, the accumulation of OPDA-containing galactolipids, observed in abc1k7, abc1k8, and abc1k7/abc1k8 mutant plants (Manara et al., 2015), may contribute to the senescence-like phenotype, the reduction of chlorophyll content and the induction of SAG12 in response to ABA treatment.
Conclusion
The presented findings point to a role of ABC1K7 and ABC1K8 in handling the metabolism activated in response to abiotic stress (e.g., drought) or particular physiological process (i.e., senescence), which involve a cross-talk between ABA and ROS signaling. It could be reasonable to speculate that these proteins may play a role in the ABA signal transduction pathway or ROS production, being located in the plastid, the principal cellular compartment deputed to these anabolic reactions. However, further research is in progress to shed light on the mechanism of action of ABC1K7 and ABC1K8 together with other ABC1K proteins in Arabidopsis.
Author Contributions
AM, GDC, and AF designed the research, discussed the results and wrote the article; AM and GDC performed the research experiments. All the authors listed approved the work for publication.
Funding
This work was supported by MIUR (Italian Ministry of University and Research) for the provision of funding for AM.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MX and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgment
The authors acknowledge Prof. E. Martinoia (University of Zurich, Switzerland) for providing atosa1 mutant seeds.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.00366
Abbreviations
ABA, abscisic acid; ABC1K, activity of bc1 complex kinase; AtACDO1, ABC1-like kinase related to chlorophyll degradation and oxidative stress; AtOSA1, oxidative stress related ABC1-like protein; AtSIA1, salt induced ABC1 kinase 1; LEA, late embryogenesis abundant; ROS, reactive oxygen species; WT, wild type.
References
Bailly, C., El-Maarouf-Bouteau, H., and Corbineau, F. (2008). From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. CR Biol. 331, 806–814. doi: 10.1016/j.crvi.2008.07.022
Böttcher, C., and Weiler, E. W. (2007). Cyclo-Oxylipin-galactolipids in plants: occurrence and dynamics. Planta 226, 629–637. doi: 10.1007/s00425-007-0511-5
Bousquet, I., Dujardin, G., and Slonimski, P. P. (1991). ABC1, a novel yeast nuclear gene has a dual function in mitochondria: it suppresses a cytochrome b mRNA translation defect and is essential for the electron transfer in the bc1 complex. EMBO J. 10, 2023–2031.
Cardazzo, B., Hamel, P., Sakamoto, W., Wintz, H., and Dujardin, G. (1998). Isolation of an Arabidopsis thaliana cDNA by complementation of a yeast abc1 deletion mutant deficient in complex III respiratory activity. Gene 221, 117–125. doi: 10.1016/S0378-1119(98)00417-X
Dave, A., Hernández, M. L., He, Z., Andriotis, V. M., Vaistij, F. E., Larson, T. R., et al. (2011). 12-oxo-phytodienoic acid accumulation during seed development represses seed germination in Arabidopsis. Plant Cell 23, 583–599. doi: 10.1105/tpc.110.081489
Do, T. Q., Hsu, A. Y., Jonassen, T., Lee, P. T., and Clarke, C. F. (2001). A defect in coenzyme Q biosynthesis is responsible for the respiratory deficiency in Saccharomyces cerevisiae abc1 mutants. J. Biol. Chem. 276, 18161–18168. doi: 10.1074/jbc.M100952200
Ernster, L., and Forsmark-Andrée, P. (1993). Ubiquinol: an endogenous antioxidant in aerobic organisms. Clin. Investig. 71, S60–S65. doi: 10.1007/BF00226842
Gao, G., Zhang, S., Wang, C., Yang, X., Wang, Y., Su, X., et al. (2011). Arabidopsis CPR5 independently regulates seed germination and postgermination arrest of development through LOX pathway and ABA signaling. PLoS ONE 6:e19406. doi: 10.1371/journal.pone.0019406
Gao, Q. S., Yang, Z. F., Zhou, Y., Yin, Z., Qiu, J., Liang, G. H., et al. (2012). Characterization of an Abc1 kinase family gene OsABC1-2 conferring enhanced tolerance to dark-induced stress in rice. Gene 498, 155–163. doi: 10.1016/j.gene.2012.02.017
Gao, Q. S., Yang, Z. F., Zhou, Y., Zhang, D., Yan, C. H., Liang, G. H., et al. (2010). Cloning of an ABC1-like gene ZmABC1-10 and its responses to cadmium and other abiotic stresses in maize Zea mays L. Acta Agron. Sin. 12, 2073–2083. doi: 10.1016/S1875-2780(09)60089-4
Gao, Q. S., Zhang, D., Xu, L., and Xu, G. (2011). Systematic identification of rice ABC1 gene family and its response to abiotic stress. Rice Sci. 18, 167–177. doi: 10.1016/S1672-6308(11)60024-3
Gepstein, S., and Thimann, K. V. (1980). Changes in the abscisic acid content of oat leaves during senescence. Proc. Natl. Acad. Sci. U.S.A. 77, 2050–2053. doi: 10.1073/pnas.77.4.2050
Glauser, G., Vallat, A., and Balmer, D. (2014). “Hormone profiling,” in Arabidopsis Protocols, Methods in Molecular Biology, Vol 1062, eds J. J. Sanchez-Serrano and J. Salinas (New York, NY: Springer), 597–608.
Gomes, M. P., and Garcia, Q. S. (2013). Reactive oxygen species and seed germination. Biologia 68, 351–357. doi: 10.2478/s11756-013-0161-y
Grbić, V. (2003). SAG2 and SAG12 protein expression in senescing Arabidopsis plants. Physiol. Plant. 119, 263–269. doi: 10.1034/j.1399-3054.2003.00168.x
Himmelbach, A., Yang, Y., and Grill, E. (2003). Relay and control of abscisic acid signalling. Curr. Opin. Plant Biol. 6, 470–479. doi: 10.1016/S1369-5266(03)00090-6
Hirayama, T., and Shinozaki, K. (2007). Perception and transduction of abscisic acid signals: keys to the function of the versatile plant hormone ABA. Trends Plant Sci. 12, 343–351. doi: 10.1016/j.tplants.2007.06.013
Hoagland, D. R., and Arnon, D. I. (1938). The Water Culture Method for Growing Plants Without Soil. Berkeley, CA: University of California College Agriculture Experiment Station Circular, 347–353.
Hubbard, K. E., Nishimura, N., Hitomi, K., Getzoff, E. D., and Schroeder, J. I. (2010). Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev. 24, 1695–1708. doi: 10.1101/gad.1953910
Iiizumi, M., Arakawa, H., Mori, T., Ando, A., and Nakamura, Y. (2002). Isolation of a novel gene, CABC1, encoding a mitochondrial protein that is highly homologous to yeast activity of bc1 complex. Cancer Res. 62, 1246–1250.
Jaradat, M. R., Feurtado, J. A., Huang, D., Lu, Y., and Cutler, A. J. (2013). Multiple roles of the transcription factor AtMYBR1/AtMYB44 in ABA signaling, stress responses, and leaf senescence. BMC Plant Biol. 13:192. doi: 10.1186/1471-2229-13-192
Jasinski, M., Sudre, D., Schansker, G., Schellenberg, M., Constant, S., Martinoia, E., et al. (2008). AtOSA1, a member of the Abc1-Like family, as a new factor in cadmium and oxidative stress response. Plant Physiol. 147, 719–731. doi: 10.1104/pp.107.110247
Jia, Y., Tao, F., and Li, W. (2013). Lipid profiling demonstrates that suppressing Arabidopsis phospholipase Dd retards ABA-promoted leaf senescence by attenuating lipid degradation. PLoS ONE 8:e65687. doi: 10.1371/journal.pone.0065687
Kanno, Y., Hanada, A., Chiba, Y., Ichikawa, T., Nakazawa, M., Matsui, M., et al. (2012). Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc. Natl. Acad. Sci. U.S.A. 109, 9653–9658. doi: 10.1073/pnas.1203567109
Kiyosue, T., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1994). Characterization of two cDNAs (ERD10 and ERD14) corresponding to genes that respond rapidly to dehydration stress in Arabidopsis thaliana. Plant Cell Physiol. 35, 225–231.
Kurkela, S., and Borg-Franck, M. (1992). Structure and expression of kin2, one of two cold- and ABA-induced genes of Arabidopsis thaliana. Plant Mol. Biol. 19, 689–692. doi: 10.1007/BF00026794
Kwak, J. M., Mori, I. C., Pei, Z.-M., Leonhardt, N., Torres, M. A., Dangl, J. L., et al. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22, 2623–2633. doi: 10.1093/emboj/cdg277
Lee, I. C., Hong, S. W., Whang, S. S., Lim, P. O., Nam, H. G., and Koo, J. C. (2011). Age-dependent action of an ABA-inducible receptor kinase, RPK1, as a positive regulator of senescence in Arabidopsis leaves. Plant Cell Physiol. 52, 651–662. doi: 10.1093/pcp/pcr026
Leonard, C. J., Aravind, L., and Koonin, E. V. (1998). Novel families of putative protein kinases in bacteria and archaea: evolution of the “eukaryotic” protein kinase superfamily. Genome Res. 8, 1038–1047.
Leung, J., Bouvier-Durand, M., Morris, P. C., Guerrier, D., Chefdor, F., and Giraudat, J. (1994). Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science 264, 1448–1452. doi: 10.1126/science.7910981
Liu, Y., Ye, N., Liu, R., Chen, M., and Zhang, J. (2010). H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J. Exp. Bot. 61, 2979–2990. doi: 10.1093/jxb/erq125
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lundquist, P. K., Davis, J. I., and van Wijk, K. J. (2012a). ABC1K atypical kinases in plants: filling the organellar kinase void. Trends Plant Sci. 9, 546–555. doi: 10.1016/j.tplants.2012.05.010
Lundquist, P. K., Poliakov, A., Bhuiyan, N. H., Zybailov, B., Sun, Q., and van Wijk, K. J. (2012b). The functional network of the Arabidopsis thaliana plastoglobule proteome based on quantitative proteomics and genome-wide co-expression analysis. Plant Physiol. 158, 1172–1192. doi: 10.1104/pp.111.193144
Lundquist, P. K., Poliakov, A., Giacomelli, L., Friso, G., Appel, M., McQuinn, R. P., et al. (2013). Loss of plastoglobule kinases ABC1K1 and ABC1K3 causes conditional degreening, modified prenyl-lipids, and recruitment of the jasmonic acid pathway. Plant Cell 25, 1818–1839. doi: 10.1105/tpc.113.111120
Macinga, D. R., Cook, G. M., Poole, R. K., and Rather, P. N. (1998). Identification and characterization of aarF, a locus required for production of ubiquinone in Providencia stuartii and Escherichia coli and for expression of 2′-N-acetyltransferase in P. stuartii. J. Bacteriol. 180, 128–135.
Majeran, W., Friso, G., Ponnala, L., Connolly, B., Huang, M., Reidel, E., et al. (2010). Structural and metabolic transitions of C4 leaf development and differentiation defined by microscopy and quantitative proteomics in maize. Plant Cell 22, 3509–3542. doi: 10.1105/tpc.110.079764
Manara, A., DalCorso, G., Guzzo, F., and Furini, A. (2015). Loss of the atypical kinases ABC1K7 and ABC1K8 changes the lipid composition of the chloroplast membrane. Plant Cell Physiol. 56, 1193–1204. doi: 10.1093/pcp/pcv046
Manara, A., DalCorso, G., Leister, D., Jahns, P., Baldan, B., and Furini, A. (2014). AtSIA1 and AtOSA1: two Abc1 proteins involved in oxidative stress responses and iron distribution within chloroplasts. New Phytol. 201, 452–465. doi: 10.1111/nph.12533
Martinis, J., Glauser, G., Valimareanu, S., and Kessler, F. (2013). A chloroplast ABC1-like kinase regulates vitamin E metabolism in Arabidopsis. Plant Physiol. 162, 652–662. doi: 10.1104/pp.113.218644
Martinis, J., Glauser, G., Valimareanu, S., Stettler, M., Zeeman, S. C., Yamamoto, H., et al. (2014). ABC1K1/PGR6 kinase: a regulatory link between photosynthetic activity and chloroplast metabolism. Plant J. 77, 269–283. doi: 10.1111/tpj.12385
Mollet, J., Delahodde, A., Serre, V., Chretien, D., Schlemmer, D., Lombes, A., et al. (2008). CABC1 gene mutations cause ubiquinone deficiency with cerebellar ataxia and seizures. Am. J. Hum. Genet. 82, 623–630. doi: 10.1016/j.ajhg.2007.12.022
Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x
Murata, Y., Pei, Z.-M., Mori, I. C., and Schroeder, J. (2001). Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13, 2513–2523. doi: 10.1105/tpc.13.11.2513
Mustilli, A. C., Merlot, S., Vavasseur, A., Fenzi, F., and Giraudat, J. (2002). Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14, 3089–3099. doi: 10.1105/tpc.007906
Neill, S., Barros, R., Bright, J., Desikan, R., Hancock, J., Harrison, J., et al. (2008). Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 59, 165–176. doi: 10.1093/jxb/erm293
Pei, Z.-M., Murata, Y., Benning, G., Thomine, S., Klüsener, B., Allen, G. J., et al. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. doi: 10.1038/35021067
Poon, W. W., Davis, D. E., Ha, H. T., Jonassen, T., Rather, P. N., and Clarke, C. F. (2000). Identification of Escherichia coli ubiB, a gene required for the first monooxygenase step in ubiquinone biosynthesis. J. Bacteriol. 182, 5139–5146. doi: 10.1128/JB.182.18.5139-5146.2000
Porra, R. J. (2002). The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res. 73, 149–156. doi: 10.1023/A:1020470224740
Rabbani, M. A., Maruyama, K., Abe, H., Khan, M. A., Katsura, K., Ito, Y., et al. (2003). Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol. 133, 1755–1767. doi: 10.1104/pp.103.025742
Ramakers, C., Ruijter, J. M., Deprez, R. H., and Moorman, A. F. (2003). Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339, 62–66. doi: 10.1016/S0304-3940(02)01423-4
Ren, H. B., Fan, Y. J., Gao, Z. H., Wei, K. F., Li, G. F., Liu, J., et al. (2007). Roles of a sustained activation of NCED3 and the synergistic regulation of ABA biosynthesis and catabolism in ABA signal production in Arabidopsis. Chin. Sci. Bull. 52, 484–491. doi: 10.1007/s11434-007-0072-9
Rodriguez, P. L., Leube, M. P., and Grill, E. (1998). Molecular cloning in Arabidopsis thaliana of a new protein phosphatase 2C (PP2C) with homology to ABI1 and ABI2. Plant Mol. Biol. 38, 879–883. doi: 10.1023/A:1006054607850
Saez, A., Apostolova, N., Gonzalez-Guzman, M., Gonzalez-Garcia, M. P., Nicolas, C., Lorenzo, O., et al. (2004). Gain-of-function and loss-of-function phenotypes of the protein phosphatase 2C HAB1 reveal its role as a negative regulator of abscisic acid signalling. Plant J. 37, 354–369. doi: 10.1046/j.1365-313X.2003.01966.x
Savchenko, T., Kolla, V. A., Wang, C. Q., Nasafi, Z., Hicks, D. R., Phadungchob, B., et al. (2014). Functional convergence of oxylipin and abscisic acid pathways controls stomatal closure in response to drought. Plant Physiol. 164, 1151–1160. doi: 10.1104/pp.113.234310
Shinozaki, K., Yamaguchi-Shinozaki, K., and Seki, M. (2003). Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol. 6, 410–417. doi: 10.1016/S1369-5266(03)00092-X
Simontacchi, M., Garcia-Mata, C., Bartoli, C. G., Santa-Maria, G. E., and Lamattina, L. (2013). Nitric oxide as a key component in hormone-regulated processes. Plant Cell Rep. 32, 853–866. doi: 10.1007/s00299-013-1434-1
Tauche, A., Krause-Buchholz, U., and Rödel, G. (2008). Ubiquinone biosynthesis in Saccharomyces cerevisiae: the molecular organization of O-methylase Coq3p depends on Abc1p/Coq8p. FEMS Yeast Res. 8, 1263–1275. doi: 10.1111/j.1567-1364.2008.00436.x
Umezawa, T., Nakashima, K., Miyakawa, T., Kuromori, T., Tanokura, M., Shinozaki, K., et al. (2010). Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant Cell Physiol. 51, 1821–1839. doi: 10.1093/pcp/pcq156
Umezawa, T., Sugiyama, N., Mizoguchi, M., Hayashi, S., Myouga, F., Yamaguchi-Shinozaki, K., et al. (2009). Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 106, 17588–17593. doi: 10.1073/pnas.0907095106
Van Breusegem, F., and Dat, J. F. (2006). Reactive oxigen species in plant cell death. Plant Physiol. 141, 384–390.
Vidi, P. A., Kessler, F., and Bréhélin, C. (2007). Plastoglobules: a new address for targeting recombinant proteins in the chloroplast. BMC Biotechnol. 7:4. doi: 10.1186/1472-6750-7-4
Wang, C., Jing, R., Mao, X., Chang, X., and Li, A. (2011). TaABC1, a member of the activity of bc1 complex protein kinase family from common wheat, confers enhanced tolerance to abiotic stresses in Arabidopsis. J. Exp. Bot. 62, 1299–1311. doi: 10.1093/jxb/erq377
Wang, H., Datla, R., Georges, F., Loewen, M., and Cutler, A. J. (1995). Promoters from kin1 and cor66, two homologous Arabidopsis thaliana genes: transcriptional regulation and gene expression induced by low temperature, ABA, osmoticum and dehydration. Plant Mol. Biol. 28, 605–617. doi: 10.1007/BF00021187
Wang, Z., Zhang, H., Yang, J., Chen, Y., Xu, X., Mao, X., et al. (2013). Phylogenetic, expression, and bioinformatic analysis of the ABC1 gene family in Populus trichocarpa. Sci. World J. 2013:785070. doi: 10.1155/2013/785070
Weaver, L. M., Gan, S. S., Quirino, B., and Amasino, R. M. (1998). A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment. Plant Mol. Biol. 37, 455–469. doi: 10.1023/A:1005934428906
Wilhelm, K. S., and Thomashow, M. F. (1993). Arabidopsis thaliana cor15b, an apparent homologue of cor15a, is strongly responsive to cold and ABA, but not drought. Plant Mol. Biol. 23, 1073–1077. doi: 10.1007/BF00021822
Yang, S., Zeng, X., Li, T., Liu, M., Zhang, S., Gao, S., et al. (2012a). AtACDO1, an ABC1-like kinase gene, is involved in chlorophyll degradation and the response to photooxidative stress in Arabidopsis. J. Exp. Bot. 15, 3959–3973. doi: 10.1093/jxb/ers072
Yang, S., Zhang, Q., Li, T., Du, D., Yang, S., and Yang, C. (2012b). AtSIA1, an ABC1-like kinase, regulates salt response in Arabidopsis. Biologia 67, 1107–1111. doi: 10.2478/s11756-012-0115-9
Ytterberg, A. J., Peltier, J. B., and van Wijk, K. J. (2006). Protein profiling of plastoglobules in chloroplasts and chromoplasts A surprising site for differential accumulation of metabolic enzymes. Plant Physiol. 140, 984–997. doi: 10.1104/pp.105.076083
Zhang, H., Zhu, H., Pan, Y., Yu, Y., Luan, S., and Li, L. (2014). A DTX/MATE-type transporter facilitates abscisic acid efflux and modulates ABA sensitivity and drought tolerance in Arabidopsis. Mol. Plant 7, 1522–1532. doi: 10.1093/mp/ssu063
Zhang, X., Zhang, L., Dong, F., Gao, J., Galbraith, D. W., and Song, C. P. (2001). Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 126, 1438–1448. doi: 10.1104/pp.126.4.1438
Keywords: ABA, ABC1K proteins, abiotic stress, germination, senescence, stomatal closure
Citation: Manara A, DalCorso G and Furini A (2016) The Role of the Atypical Kinases ABC1K7 and ABC1K8 in Abscisic Acid Responses. Front. Plant Sci. 7:366. doi: 10.3389/fpls.2016.00366
Received: 21 August 2015; Accepted: 09 March 2016;
Published: 24 March 2016.
Edited by:
Jin-Gui Chen, Oak Ridge National Laboratory, USAReviewed by:
Shucai Wang, Northeast Normal University, ChinaMeng Xie, Oak Ridge National Laboratory, USA
Copyright © 2016 Manara, DalCorso and Furini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonella Furini, YW50b25lbGxhLmZ1cmluaUB1bml2ci5pdA==
†These authors have contributed equally to this work.
 Anna Manara
Anna Manara Giovanni DalCorso
Giovanni DalCorso Antonella Furini
Antonella Furini