
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Plant Sci. , 26 February 2016
Sec. Plant Physiology
Volume 7 - 2016 | https://doi.org/10.3389/fpls.2016.00229
This article is part of the Research Topic Interplay between NO signalling, ROS and the antioxidant system in plants View all 20 articles
Plants treated with chemical compounds can develop an enhanced capacity to resist long after being subjected to (a)biotic stress, a phenomenon known as priming. Evidence suggests that reactive oxygen species (ROS) and reactive nitrogen species (RNS) coordinately regulate plant stress responses to adverse environmental conditions; however, the mechanisms underlying this function remain unknown. Based on the observation that pre-exposure of citrus (Citrus aurantium L.) roots to the NO donor sodium nitroprusside (SNP) or to H2O2 prior to NaCl application can induce acclimation against subsequent stress we characterized the changes occurring in primed citrus tissues using several approaches. Herein, using this experimental model system, we provide an overview of our current knowledge of the possible mechanisms associated with NO and H2O2 priming to abiotic stresses, particularly concerning salinity and drought. The data and ideas presented here introduce six aspects of priming behavior in citrus under abiotic stress that provide knowledge necessary to exploit priming syndrome in the context of sustainable agriculture.
Environmental stress factors, such as drought and salinity, strongly affect plant growth and pose a growing threat to sustainable agriculture (Golldack et al., 2014). This has become a hot issue due to concerns about the effects of climate change on plant resources, biodiversity and global food security (Ahuja et al., 2010). Consequently, understanding the mechanisms underlying plant abiotic stress acclimation helps us to develop fruitful new agricultural strategies (Beckers and Conrath, 2007). Evidence suggests that plants are capable of inducing some stress “memory,” or “stress imprinting” following a first stress exposure that leads to acclimation to a later (a)biotic stress. Through priming (also known as hardening), plants are able to induce responses to a range of stresses, providing low-cost protection in relatively high stress-pressure conditions (Borges et al., 2014). Despite priming phenomena have previously been widely described under biotic stress (Prime-A-Plant Group et al., 2006; Conrath et al., 2015) and in the invigoration of seeds (Rajjou et al., 2012), the mechanisms of long-lasting priming are still unclear, notably under abiotic stress (Tanou et al., 2012b). It has been suggested that hormone-dependent pathways and availability of signal transduction proteins along with epigenetic mechanisms, such as histone modifications and DNA methylation, are involved in priming against abiotic stress (Bruce et al., 2007; Conrath, 2011). Recently, Jiménez-Arias et al. (2015) showed that Arabidopsis seed-based priming against salt stress involves epigenetic changes (DNA hypomethylation) in genes controlling proline metabolism. In this regard, it has been proposed that priming stimulates salicylic acid (SA), abscisic acid (ABA), and jasmonic acid (JA) signaling that could facilitate the transcriptional induction of defense genes and epigenetic changes; this trans-generational induced resistance is elicited by the RNA-directed DNA methylation (RdDM) pathway, which triggers heritable changes in DNA methylation that can direct priming-inducing chromatin modifications at defense gene promoters (Pastor et al., 2013).
Almost all abiotic stressors generate reactive oxygen species (ROS) and reactive nitrogen species (RNS), resulting in oxidative and nitrosative stress in plants (Molassiotis and Fotopoulos, 2011). It has been increasingly evident that RNS (in the form of nitric oxide, NO) and ROS (in the form of H2O2) play important roles in priming phenomena in various annual plants (Del Río, 2015). Nevertheless, a priming approach has a great potential if studied in non-model long-lived fruit trees species (Molassiotis et al., 2010). Citrus is widely cultivated in Mediterranean-type ecosystems where climatic change is expected to amplify drought and salinity stress (Gordo and Sanz, 2010). Basal resistance by itself is too weak to protect citrus against environmental stimuli, since it is sensitive to oxidative and nitrosative stress induced by various abiotic stress conditions (Ziogas et al., 2013). Hence, the possible implications of NO and H2O2 in the acclimation of citrus plants to adverse environmental conditions, as well as the interactions between the two molecules, were studied. In this regard, we initially documented at physiological level that NO and H2O2 are able to induce priming against salt stress by pre-treating the roots of sour orange (Citrus aurantium L.) seedlings either with the NO donor sodium nitroprusside (SNP) or with H2O2 prior to NaCl application (see Tanou et al., 2009b). Using this experimental system, the mechanisms by which citrus plants respond to salinity were investigated in order to gain a wide understanding of oxidative- and nitrosative-associated priming in plants. In this review, we summarize our current knowledge of the possible mechanisms associated with NO- and H2O2-induced salinity and drought acclimation in citrus plants. Overall, this approach reveals the following six aspects regarding the mechanism of NO- and H2O2-associated priming events in citrus against abiotic challenges.
One of the mechanisms actively employed by primed plants to survive under abiotic stress is the induction of the antioxidant defense system (Hossain et al., 2015). In citrus plants it was evidenced that pre-treatments with NO and H2O2 prior to NaCl stress induced antioxidative defense-related enzymatic activity [e.g., superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR)] (Tanou et al., 2009b) and mRNA expression [(eg., Cu/Zn-SOD, Mn-SOD and Fe-SOD, xanthine oxidoreductase (XO), and alternative oxidase (AOX)] (Tanou et al., 2012a). Another antioxidant function of NO arises from the observation that NO protects citrus leaves from DNA strand cleavage caused by hydroxyl radical •OH produced following NaCl application (Tanou et al., 2009b), thereby avoiding oxidative damage induced by most harmful ROS. It has been proposed that NO protects from •OH-stimulated oxidative stress in two possible ways: directly by scavenging ROS, and indirectly by NO-mediated induction of ferritin proteins that contributes to diminishing free-Fe2+ levels and Fenton-type reactions within the organelles (Martin et al., 2009). It had been demonstrated that •OH effectively targeted DNA methylation and regulatory genes (Shen et al., 2014), implying that NO may have an important role in priming process, at least in part, by DNA methylation-based epigenetic modifications.
Systemic signals are perceived in distant plant tissues and initiate systemic stress responses through priming (Gaupels and Corina Vlot, 2012). Knowledge on such long-distance signaling has been recently documented in various plant systems (Frost et al., 2008; Chaturvedi et al., 2012; Shah et al., 2014). According to the above studies, phloem is the likely path for systemic transmission or movement of signals associated with the acclimation process (Chaturvedi et al., 2012; Ruiz-Medrano et al., 2012). Similarly, root-applied NO or H2O2 remarkably increased NO and H2O2 steady-level in the leaves of citrus, indicating that these two molecules are systemic priming elicitors at the whole-plant level (Tanou et al., 2012a). Histochemical localization of H2O2 and 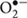 production in citrus leaves using 3,3′-diaminobenzidine (DAB) and nitroblue tetrazolium (NBT) staining indicated that ROS specifically accumulated in the vicinity of the primary vein (Tanou et al., 2012a). Such preferentially topological distribution of ROS reflects the greater exposure of periveinal cells to systemic signals, and that, when diffusing out of the veins, the concentration is diluted and therefore the cells near the vascular bundles are more likely to react (Alvarez et al., 1998). Further evidence for the long-distance and long-lasting nature of NO and H2O2 arose from the observation that both NO and H2O2-derived 4,5-diaminofluorescin diacetate (DAF-2DA) and 2,′7′-dichlorofluorescin diacetate (DCF-DA) fluorescence were detected in the vascular tissues (xylem and particularly phloem) and in the upper and lower epidermal cells under normal and NaCl stress conditions and occurred following 8 days of NO/H2O2 application (Tanou et al., 2012a). In citrus we also observed that the local application of NO in roots induced drought acclimation in leaves for at least 35 days after exposure to PEG stress (Ziogas et al., 2015) further suggesting that NO priming signal is memorized and systemically transduced. However, the evidence for in planta NO and H2O2 long-lasting signaling is not strong enough to conclude their priming roles due to the short half-lives of NO and H2O2 (Neill et al., 2002). One mode of long-distance/lasting NO and H2O2 action may be associated with the auto-propagation of ROS and RNS waves throughout the plant (Mittler et al., 2011; Molassiotis and Fotopoulos, 2011). The initial abiotic stress-induced burst of ROS/RNS in a local group of plant cells triggers cell to cell communication that propagates throughout different tissues of the plant and carries a systemic signal over long distances (Miller et al., 2009). Another scenario might be the systemic NO and H2O2 signaling through binding to specific stress-related enzymes, such as mitogen-activated protein kinases (MAPKs) (Mishra et al., 2006) and S-nitrosoglutathione reductase (GSNOR) (Leterrier et al., 2011), thereby modifying their activity. In this regard, GSNOR transcripts in leaves and roots of salt-primed citrus plants were down-regulated showing that GSNOR could be considered a mechanism by which NO and H2O2 orchestrate priming signaling (Tanou et al., 2012a).
production in citrus leaves using 3,3′-diaminobenzidine (DAB) and nitroblue tetrazolium (NBT) staining indicated that ROS specifically accumulated in the vicinity of the primary vein (Tanou et al., 2012a). Such preferentially topological distribution of ROS reflects the greater exposure of periveinal cells to systemic signals, and that, when diffusing out of the veins, the concentration is diluted and therefore the cells near the vascular bundles are more likely to react (Alvarez et al., 1998). Further evidence for the long-distance and long-lasting nature of NO and H2O2 arose from the observation that both NO and H2O2-derived 4,5-diaminofluorescin diacetate (DAF-2DA) and 2,′7′-dichlorofluorescin diacetate (DCF-DA) fluorescence were detected in the vascular tissues (xylem and particularly phloem) and in the upper and lower epidermal cells under normal and NaCl stress conditions and occurred following 8 days of NO/H2O2 application (Tanou et al., 2012a). In citrus we also observed that the local application of NO in roots induced drought acclimation in leaves for at least 35 days after exposure to PEG stress (Ziogas et al., 2015) further suggesting that NO priming signal is memorized and systemically transduced. However, the evidence for in planta NO and H2O2 long-lasting signaling is not strong enough to conclude their priming roles due to the short half-lives of NO and H2O2 (Neill et al., 2002). One mode of long-distance/lasting NO and H2O2 action may be associated with the auto-propagation of ROS and RNS waves throughout the plant (Mittler et al., 2011; Molassiotis and Fotopoulos, 2011). The initial abiotic stress-induced burst of ROS/RNS in a local group of plant cells triggers cell to cell communication that propagates throughout different tissues of the plant and carries a systemic signal over long distances (Miller et al., 2009). Another scenario might be the systemic NO and H2O2 signaling through binding to specific stress-related enzymes, such as mitogen-activated protein kinases (MAPKs) (Mishra et al., 2006) and S-nitrosoglutathione reductase (GSNOR) (Leterrier et al., 2011), thereby modifying their activity. In this regard, GSNOR transcripts in leaves and roots of salt-primed citrus plants were down-regulated showing that GSNOR could be considered a mechanism by which NO and H2O2 orchestrate priming signaling (Tanou et al., 2012a).
Changes in environmental conditions are likely to cause rapid changes in the level, composition, and structure of different metabolites, proteins, and RNA molecules that precede signal transduction or stress acclimation events in plants (Baxter et al., 2014). An interesting finding that emerged from the work in citrus is the fact that the NaCl-responsive leaf proteome (85 proteins) was remarkably affected by pre-exposure to NO or to H2O2. Indeed, NO or H2O2 pretreatment prior to salt stress imposition, reversed a large part of the NaCl-responsive proteins (53 and 55 proteins, respectively; Tanou et al., 2009a). The major set of these proteins (46.7%) participate in photosynthesis and particularly in the Calvin cycle (e.g., several isoforms of Rubisco activase, Rubisco large subunit, fructose 1,6-bisphosphate aldolase, phosphoglycerate kinase, glyceraldehyde-3-phosphate dehydrogenase, phosphoribulokinase, transketolase, and carbonic anhydrase; Tanou et al., 2012a), this being a probable attempt at sustaining the photosynthesis rate during salt stress. Regulatory mechanisms of photosynthetic proteins were also studied in other stress-affected plant species and crops. For example, Komatsu et al. (2014) pointed the major proteomic alternation in wheat under various abiotic stresses focused on photosynthesis-responsive proteins. In addition to NO- or to H2O2-mediated regulation of protein expression during salinity acclimation, a more recent study showed that pre-treatment with NO also modulates specifically several PEG-affected proteins (e.g., glycolate oxidase, NADP-isocitrate dehydrogenase, and UPF0603 protein Atlg54780) in citrus plants experiencing priming against drought (Ziogas et al., 2015). These findings indicate that the priming function of NO and H2O2 in citrus plants is a dynamic, photosynthetic activity-demanding process that might be, at least partially, attributed to proteome reprogramming.
It has been well documented that ROS and RNS exposure can prime plants against abiotic stresses through chemical reactions with specific target proteins that result in covalent posttranslational protein modifications (PTMs), altering protein function and activity (Lounifi et al., 2013). Therefore, the characterization of PTMs is crucial for a deeper understanding of NO and H2O2 priming. Particularly, protein carbonylation is a type of protein oxidation driven by oxidative stress, which occurs by direct attack on Lys, Arg, Pro, or Thr protein residues (Oracz et al., 2007). It has been shown that basal levels of protein carbonylation under control conditions increase sharply when citrus plants are grown under salinity (Tanou et al., 2009a, 2012a), inhibiting enzyme activities and increasing protein susceptibility toward proteolytic attack (Dunlop et al., 2002). Proteomic 2DE-OxyBlot-based analysis in citrus indicated that H2O2 and SNP pretreatments before salt stress prevented the NaCl-induced protein carbonylation to the levels of untreated control plants and allowed identifying 40 carbonylated proteins showing a reversal in accumulation level upon H2O2 or SNP application (Tanou et al., 2009a). Furthermore, tyrosine (Tyr) nitration, i.e., the addition of a nitro group (NO2) to one of the two equivalent ortho carbons of the aromatic ring of Tyr residues in the presence of excess levels of ROS and NO or NO-derived species, is recognized as an important redox PTM (Corpas et al., 2009). Protein Tyr nitration has been established as a biomarker of systemic “nitroxidative stress,” leading plant metabolism to a pro-oxidant status that disrupts NO signaling and induces protein structural and functional changes, some of which contribute to altered cell and tissue homeostasis (Corpas et al., 2013). Similar to the pattern of protein carbonylation, Tyr-nitration increased in citrus leaves exposed to salinity or to NO or H2O2 under stress-free conditions but diminished to control basal levels when these chemical treatments were applied before the imposition of NaCl stress (Tanou et al., 2012a). These results clearly show that citrus plants adopt a common oxy- and nitro-based stress-alleviating mechanism in their leaves. In both cases the results strengthen the notion that these PTMs are not just a ‘fingerprint’ of oxidative and nitrosative stress, but also they are essential components of citrus priming mechanism.
S-nitrosylation is another PTM that has been validated as signaling mechanism mediated by nitrosative/oxidative stress that occurs on cysteine (Cys) residues, being redox reversible with high spatial and temporal specificity modifying protein activity and accumulation (Astier and Lindermayr, 2012). In citrus leaves subjected to NaCl, S-nitrosylation decreased whereas NO or H2O2 pre-treatments before salt stress substantially increased protein S-nitrosylation. Remarkably, this response was in contrast to protein carbonylation and Tyr-nitration patterns under the same experimental conditions (Tanou et al., 2009a, 2012a), denoting differences of PTMs regulation during salinity acclimation. By studying the ROS and RNS priming input against salinity we performed a comparative analysis of carbonylated, nitrated and nitrosylated proteome in citrus plants (Tanou et al., 2012a). This approach revealed that the majority of the PTM-targeted proteins in leaves were involved in Calvin–Benson cycle followed by disease/defense mechanisms and protein destination. More interestingly, among the 92 carbonylated, 88 Try-nitrated and 82 S-nitrosylated proteins, approximately one third of them, namely 34, 26 and 36 proteins respectively were specifically carbonylated or Try-nitrated or S-nitrosylated (Tanou et al., 2012a). On the contrary, 22 citrus proteins, including Rubisco large subunit, GAPDH subunits A, B, the photosystem II 44 kDa reaction center, sedoheptulose-1,7-bisphosphatase, phosphoribulokinase, carbonic anhydrase, light-harvesting chlorophyll a/b binding protein, ribulose-5-phosphate 3-epimerase, fructose-1,6-bisphosphate aldolase, glycolate oxidase and sinapyl alcohol dehydrogenase, simultaneously targeted by these three PTMs (Tanou et al., 2012a). It is also interesting that in a previous study we observed an overlap (17 proteins) among the NO– or H2O2–targeted carbonylated (n = 40) and S-nitrosylated (n = 49) proteins in citrus leaves experiencing priming effects against salinity (Tanou et al., 2009a), thereby disclosing that these proteins are common markers of NO and H2O2 signaling. Possible explanations for the co-modulated pattern of protein carbonylation and Tyr-nitration in contrast to S-nitrosylation have been proposed. The co-occurrence of more than one PTMs could be explained by the fact that the first PTM allosterically triggers the occurrence of other(s), thereby fine-tuning the degradation of damaged proteins by the proteasomes and/or offering protection against irreversible damage (Lounifi et al., 2013). It is therefore possible that S-nitrosylation might prevent the irreversible loss of function of proteins by protein oxidation due to carbonylation and/or Tyr-nitration by locking the structure of these proteins in a state under which they are no more sensitive to ROS/RNS attack (Sun et al., 2006). Altogether, the results support the existence of a link between oxidative and nitrosative regulatory events through PTMs characterizing priming phenomena in citrus plants.
When examining the expression of genes involved in NO production [e.g., nitric oxide synthase (NOS)-like proteins, nitrate reductase (NR), nitrite reductase (NiR)] as well as H2O2 generation [e.g., diamine oxidases (DAO) and polyamine oxidases (PAO)] we observed a distinctive complementary pattern in leaves and roots of citrus exposed to NaCl (Tanou et al., 2012a), likely reflecting locally and systemically differences in oxy/nitro priming signaling. Consistent with these tissue-specific transcriptional patterns, we also uncovered the existence of a tissue-dependent regulation of S-nitrosylation induced by NO and H2O2 stimuli (Tanou et al., 2012a). The S-nitrosylation was induced in leaves and depressed in roots following NO and H2O2 priming treatments, which is in agreement with the differential spatial distribution of oxidative and nitrosative stress in leaves versus roots documented in Lotus japonicus (Signorelli et al., 2013). By contrast, chemical pre-treatments with NO or H2O2 suppressed protein carbonylation and nitration in both leaves and roots exposed to NaCl, suggesting that some PTM responses are commonly regulated in the different types of citrus tissues (Tanou et al., 2012a). This is further strengthened by the fact that SNP stimulated Tyr-nitration in leaves and roots during acclimation to drought stress (Ziogas et al., 2015). Such functional differences between leaves and roots may be partially attributed to the tissue differences in the types of NO and ROS generation systems. Superoxide (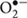 ) and H2O2 produced through SODs up-regulation and polyamine degradation in roots of citrus just following pre-treatments with NO or H2O2 can activate specific signaling pathways distinct from those perceived by leaves (Tanou et al., 2012a). Analogously, NO accumulation via NR activation in citrus leaves subjected to NaCl or PEG stress (Tanou et al., 2012a) accompanies several different NO-signaling events which could regulate downstream pathways and stress acclimation (Baudouin and Hancock, 2014).
) and H2O2 produced through SODs up-regulation and polyamine degradation in roots of citrus just following pre-treatments with NO or H2O2 can activate specific signaling pathways distinct from those perceived by leaves (Tanou et al., 2012a). Analogously, NO accumulation via NR activation in citrus leaves subjected to NaCl or PEG stress (Tanou et al., 2012a) accompanies several different NO-signaling events which could regulate downstream pathways and stress acclimation (Baudouin and Hancock, 2014).
Recent studies have reported that many abiotic stress responses are coordinated by various signaling networks, particularly involving phytohormones, ROS, and RNS (Considine et al., 2015). In NaCl-treated citrus, NO and ROS accumulation in local and systemic tissues showed considerable overlap whereas a large part of the NaCl-sensitive proteins were commonly modulated by NO and H2O2 (Tanou et al., 2009a), suggesting that there is a dynamic interplay between the signals regulating priming. It is noted that comparative proteomic analysis in citrus leaves under physiological non-stressful conditions revealed (i) an interlinked NO- and H2O2-modulated protein network, (ii) the carbonylation status of a very large portion of the carbonylated citrus mitochondrial proteins, which remained constant or depressed by NO and H2O2 (Tanou et al., 2010), disclosing the parallels in action between NO and ROS. Here, we also provide examples demonstrating that NO could interact with other signaling pathways in modulating stress acclimation. For example, we observed temporal–spatial interactions between NO-specific PTMs and polyamines (PAs) homeostasis/metabolism in citrus challenged with salinity (Tanou et al., 2014), thus confirming that NO and PAs displayed some overlapping functions in plants (Parra-Lobato and Gomez-Jimenez, 2011). Targeted analysis of PA-affected S-nitrosylated citrus proteins led us to propose that PAs binding to specific proteins, such as dehydroascorbate reductase (DHAR) and monodehydroascorbate reductase (MDHAR), may affect protein conformational parameters and also the environment surrounding Cys residues of protein targets (Tanou et al., 2014). Such chemical modifications of Cys residues could lead either to Cys oxidation through ROS or to S-nitrosylation through NO. Drought stress can induce accumulation of hydrogen sulfide (H2S) and its biosynthetic enzyme L-cysteine desulfhydrase (LCD) in citrus. Moreover, in citrus roots H2S can induce the expression of NiR and mitochondrial NAD(P)H dehydrogenases that are involved in NO production (Gupta et al., 2011) while H2S accumulation acts downstream of NO in PEG-induced S-nitrosylation (Ziogas et al., 2015). Meanwhile, drought stress induction of ABA accumulation is a well-known fact (Tan et al., 2003). In citrus the fact that PEG stress-induced ABA accumulation and the expression of 9-cis-epoxycarotenoid dioxygenase (NCED), a key enzyme in ABA biosynthesis, were depressed in plants pre-exposed to NO or to H2S (Ziogas et al., 2015) suggests that the interplay among NO, H2S, and ABA could be considered a mechanism by which citrus orchestrates drought stress acclimation.
Research performed over the last years documented that NO and H2O2 induce priming toward salinity and drought in citrus plants and most importantly reveals key aspects of this phenomenon. Based on these results, we propose a signaling network through which NO and H2O2 provoke priming responses in leaves and roots of citrus (Figure 1). While several components of the priming mechanism have been proposed, we still lack a thorough understanding of the complex mode of action of specific signaling molecules in plant stress acclimation. In this regard, various -omics techniques investigating both roots and leaves should be combined to fully understand NO and ROS-induced priming. It will be perhaps the major challenge of priming research to test these chemical agents against multiple abiotic stresses that occur in field conditions. Such an approach would allow establishing priming technology as a tool to manage crop yield.
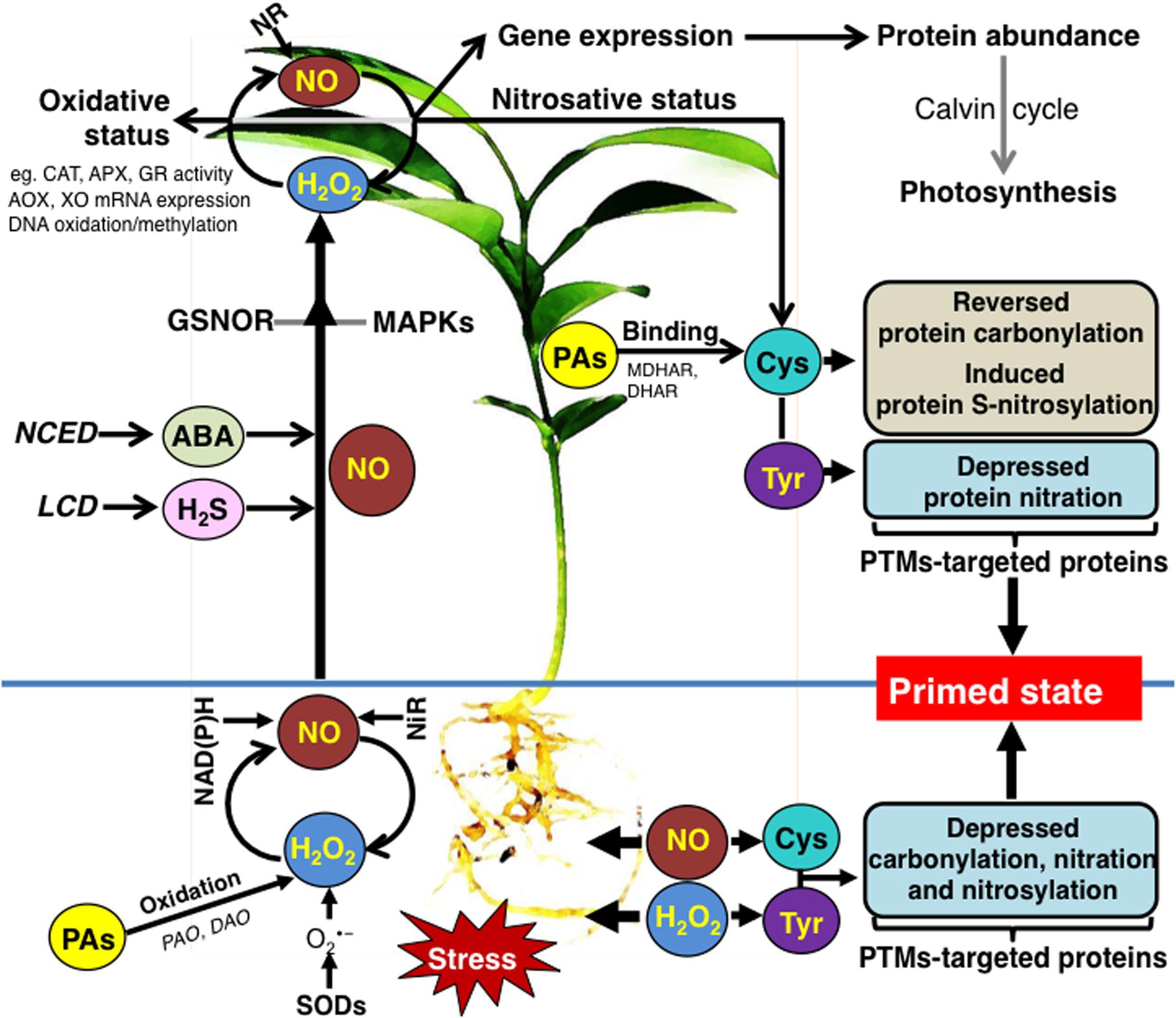
FIGURE 1. Schematic overview of the signaling networks involved in the NO- and ROS-induced priming against salininy and drought stress in citrus plants (see text for details). ABA, abscisic acid; AOX, alternative oxidase; APX, ascorbate peroxidise; CAT, catalase; Cys, cysteine; DAO, diamine oxidase; DHAR, dehydroascorbate reductase; GR, glutathione reductase; GSNOR, S-nitrosoglutathione reductase; H2S, hydrogen sulfide; LCD, L-cysteine desulfhydrase; MAPKs, mitogen-activated protein kinases; MDHAR, monodehydroascorbate reductase; NAD(P)H, mitochondrial NAD(P)H dehydrogenases; NCED, 9-cis-epoxycarotenoid dioxygenase; NiR, nitrite reductase; NR, nitrate reductase; PAO, polyamine oxidase; PTMs, posttranslational modifications; PAs, polyamines; SOD, superoxide dismutase; Tyr, tyrosine; XO, xanthine oxidoreductase.
AM, DJ, VZ, and GT wrote the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors apologize if some references related to the main theme of the current review could not be cited due to space constraints.
Ahuja, I., de Vos, R. C. H., Bones, A. M., and Hall, R. D. (2010). Plant molecular stress responses face climate change. Trends Plant Sci. 15, 664–674. doi: 10.1016/j.tplants.2010.08.002
Alvarez, M. E., Pennell, R. I., Meijer, P. J., Ishikawa, A., Dixon, R., and Lamb, C. (1998). Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92, 773–784. doi: 10.1016/S0092-8674(00)81405-1
Astier, J., and Lindermayr, C. (2012). Nitric oxide-dependent posttranslational modification in plants: an update. Int. J. Mol. Sci. 13, 15193–15208. doi: 10.3390/ijms131115193
Baudouin, E., and Hancock, J. T. (2014). Nitric oxide signaling in plants. Front. Plant Sci. 4:553. doi: 10.3389/fpls.2013.00553
Baxter, A., Mittler, R., and Suzuki, N. (2014). ROS as key players in plant stress signalling. J. Exp. Bot. 65, 1229–1240. doi: 10.1093/jxb/ert375
Beckers, G. J., and Conrath, U. (2007). Priming for stress resistance: from the lab to the field. Curr. Opin. Plant Biol. 10, 425–431. doi: 10.1016/j.pbi.2007.06.002
Borges, A. A., Jiménez-Arias, D., Expósito-Rodríguez, M., Sandalio, L. M., and Pérez, J. A. (2014). Priming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms. Front. Plant Sci. 5:642. doi: 10.3389/fpls.2014.00642
Bruce, T. J. A., Matthes, M. C., Napier, J. A., and Pickett, J. A. (2007). Stressful “memories” of plants: evidence and possible mechanisms. Plant Sci. 173, 603–608. doi: 10.1016/j.plantsci.2007.09.002
Chaturvedi, R., Venables, B., Petros, R. A., Nalam, V., Li, M., Wang, X., et al. (2012). An abietane diterpenoid is a potent activator of systemic acquired resistance. Plant J. 71, 161–172. doi: 10.1111/j.1365-313X.2012.04981.x
Conrath, U. (2011). Molecular aspects of defence priming. Trends Plant Sci. 16, 524–531. doi: 10.1016/j.tplants.2011.06.004
Conrath, U., Beckers, G. J. M., Langenbach, C. J. G., and Jaskiewicz, M. R. (2015). Priming for enhanced defense. Annu. Rev. Phytopathol. 53, 97–119. doi: 10.1146/annurev-phyto-080614-120132
Considine, M. J., María Sandalio, L., and Foyer, C. H. (2015). Unravelling how plants benefit from ROS and NO reactions, while resisting oxidative stress. Ann. Bot. 116, 469–473. doi: 10.1093/aob/mcv153
Corpas, F. J., Chaki, M., Leterrier, M., and Barroso, J. B. (2009). Protein tyrosine nitration: a new challenge in plants. Plant Signal. Behav. 4, 920–923. doi: 10.4161/psb.4.10.9466
Corpas, F. J., del Río, L. A., and Barroso, J. B. (2013). Protein tyrosine nitration in higher plants under natural and stress conditions. Front. Plant Sci. 4:29. doi: 10.3389/fpls.2013.00029
Del Río, L. A. (2015). ROS and RNS in plant physiology: an overview. J. Exp. Bot. 66, 2827–2837. doi: 10.1093/jxb/erv099
Dunlop, R. A., Rodgers, K. J., and Dean, R. T. (2002). Recent development in the intracellular degradation of oxidized proteins. Free Radic. Biol. Med. 33, 894–906. doi: 10.1016/S0891-5849(02)00958-9
Frost, C. J., Mescher, M. C., Carlson, J. E., and De Moraes, C. M. (2008). Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol. 146, 818–824. doi: 10.1104/pp.107.113027
Gaupels, F., and Corina Vlot, A. (2012). “Plant defense and long-distance signaling in the phloem,” in Phloem: Molecular Cell Biology, Systemic Communication, Biotic Interactions, eds G. A. Thompson and A. J. E. van Bel (Hoboken, NJ: John Wiley & Sons, Inc.), 227–247.
Golldack, D., Li, C., Mohan, H., and Probst, N. (2014). Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front. Plant Sci. 5:151. doi: 10.3389/fpls.2014.00151
Gordo, O., and Sanz, J. J. (2010). Impact of climate change on plant phenology in mediterranean ecosystems. Global Change Biol. 16:3. doi: 10.1111/j.1365-2486.2009.02084.x
Gupta, K. J., Fernie, A. R., Kaiser, W. M., and van Dongen, J. T. (2011). On the origins of nitric oxide. Trends Plant Sci. 16, 160–168. doi: 10.1016/j.tplants.2010.11.007
Hossain, M. A., Bhattacharjee, S., Armin, S., Qian, P., Xin, W., Li, H., et al. (2015). Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front. Plant Sci. 6:420. doi: 10.3389/fpls.2015.00420
Jiménez-Arias, D., Borges, A. A., Luis, J. C., Valdés, F., Sandalio, L. M., and Pérez, J. A. (2015). Priming effect of menadione sodium bisulphite against salinity stress in Arabidopsis involves epigenetic changes in genes controlling proline metabolism. Environ. Exp. Bot. 120, 23–30. doi: 10.1016/j.envexpbot.2015.07.003
Komatsu, S., Kamal, A. H. M., and Hossain, Z. (2014). Wheat proteomics: proteome modulation and abiotic stress acclimation. Front. Plant Sci. 5:684. doi: 10.3389/fpls.2014.00684
Leterrier, M., Chaki, M., Airaki, M., Valderrama, R., Palma, J. M., Barroso, J. B., et al. (2011). Function of S-nitrosoglutathione reductase (GSNOR) in plant development and under biotic/abiotic stress. Plant Signal. Behav. 6:6. doi: 10.4161/psb.6.6.15161
Lounifi, I., Arc, E., Molassiotis, A., Job, D., Rajjou, L., and Tanou, G. (2013). Interplay between protein carbonylation and nitrosylation in plants. Proteomics 13, 568–578. doi: 10.1002/pmic.201200304
Martin, M., Colman, M. J. R., Gómez-Casati, D. F., Lamattina, L., and Zabaleta, E. J. (2009). Nitric oxide accumulation is required to protect against iron-mediated oxidative stress in frataxin-deficient arabidopsis plants. FEBS Lett. 583, 542–548. doi: 10.1016/j.febslet.2008.12.039
Miller, G., Schlauch, K., Tam, R., Cortes, D., Torres, M. A., Shulaev, V., et al. (2009). The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2:84. doi: 10.1126/scisignal.2000448
Mishra, N. S., Tuteja, R., and Tuteja, N. (2006). Signaling through MAP kinase networks in plants. Arch. Biochem. Biophys. 452, 55–68. doi: 10.1016/j.abb.2006.05.001
Mittler, R., Vanderauwera, S., Suzuki, N., Miller, G., Tognetti, V., Vandepoele, K., et al. (2011). ROS signaling: the new wave? Trends Plant Sci. 16, 300–309. doi: 10.1016/j.tplants.2011.03.007
Molassiotis, A., and Fotopoulos, V. (2011). Oxidative and nitrosative signaling in plants: two branches in the same tree? Plant Signal. Behav. 6, 2. doi: 10.4161/psb.6.2.14878
Molassiotis, A., Tanou, G., and Diamantidis, G. (2010). NO says more than ‘YES’ to salt tolerance salt priming and systemic nitricoxide signaling in plants. Plant Signal. Behav. 5, 209–212. doi: 10.4161/psb.5.3.10738
Neill, S. J., Desikan, R., Clarke, A., Hurst, R. D., and Hancock, J. T. (2002). Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 53, 1237–1247. doi: 10.1093/jexbot/53.372.1237
Oracz, K., Bouteau, H. E., Farrant, J. M., Cooper, K., Belghazi, M., Job, C., et al. (2007). ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J. 50, 452–465. doi: 10.1111/j.1365-313X.2007.03063.x
Parra-Lobato, M. C., and Gomez-Jimenez, M. C. (2011). Polyamine-induced modulation of genes involved in ethylene biosynthesis and signalling pathways and nitric oxide production during olive mature fruit abscission. J. Exp. Bot. 62, 4447–4465. doi: 10.1093/jxb/err124
Pastor, V., Luna, E., Mauch-Mani, B., Ton, J., and Flors, V. (2013). Primed plants do not forget. Environ. Exp. Bot. 94, 46–56. doi: 10.1016/j.envexpbot.2012.02.013
Prime-A-Plant Group, Conrath, U., Beckers, G. J., Flors, V., García-Agustín, P., Jakab, G., et al. (2006). Priming: getting ready for battle. Mol. Plant Microbe Interact. 19, 1062–1071. doi: 10.1094/MPMI-19-1062
Rajjou, L., Duval, M., Gallardo, K., Catusse, J., Bally, J., Job, C., et al. (2012). Seed germination and vigor. Annu. Rev. Plant Biol. 63, 507–533. doi: 10.1146/annurev-arplant-042811-105550
Ruiz-Medrano, R., Kragler, F., and Wolf, S. (2012). “Signaling and phloem-mobile transcripts,” in Short and Long Distance Signaling. Advances in Plant Biology, eds F. Kragler and M. Hulskamp (Berlin: Springer), 151–177.
Shah, J., Chaturvedi, R., Chowdhury, Z., Venables, B., and Petros, R. A. (2014). Signaling by small metabolites in systemic acquired resistance. Plant J. 79, 645–658. doi: 10.1111/tpj.12464
Shen, L., Song, C. X., He, C., and Zhang, Y. (2014). Mechanism and function of oxidative reversal of DNA and RNA methylation. Annu. Rev. Biochem. 83, 585–614. doi: 10.1146/annurev-biochem-060713-035513
Signorelli, S., Corpas, F. J., Borsani, O., Barroso, J. B., and Monza, J. (2013). Water stress induces a differential and spatially distributed nitro-oxidative stress response in roots and leaves of Lotus japonicus. Plant Sci. 201–202, 137–146. doi: 10.1016/j.plantsci.2012.12.004
Sun, J., Steenbergen, C., and Murphy, E. (2006). S-nitrosylation: NO-related redox signaling to protect against oxidative stress. Antioxid. Redox Signal. 8, 1693–1705. doi: 10.1089/ars.2006.8.1693
Tan, B., Joseph, L. M., Deng, W., Liu, L., Li, Q., Cline, K., et al. (2003). Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 35, 44–56. doi: 10.1046/j.1365-313X.2003.01786.x
Tanou, G., Filippou, P., Belghazi, M., Job, D., Diamantidis, G., Fotopoulos, V., et al. (2012a). Oxidative and nitrosative-based signaling and associated post-translational modifications orchestrate the acclimation of citrus plants to salinity stress. Plant J. 72, 585–599. doi: 10.1111/j.1365-313X.2012.05100.x
Tanou, G., Fotopoulos, V., and Molassiotis, A. (2012b). Priming against environmental challenges and proteomics in plants: update and agricultural perspectives. Front. Plant Sci. 3:216. doi: 10.3389/fpls.2012.00216
Tanou, G., Job, C., Belghazi, M., Molassiotis, A., Diamantidis, G., and Job, D. (2010). Proteomic signatures uncover hydrogen peroxide and nitric oxide cross-talk signaling network in citrus plants. J. Proteome Res. 9, 5994–6006. doi: 10.1021/pr100782h
Tanou, G., Job, C., Rajjou, L., Arc, E., Belghazi, M., Diamantidis, G., et al. (2009a). Proteomics reveals the overlapping roles of hydrogen peroxide and nitric oxide in the acclimation of citrus plants to salinity. Plant J. 60, 795–804. doi: 10.1111/j.1365-313X.2009.04000.x
Tanou, G., Molassiotis, A., and Diamantidis, G. R. (2009b). Hydrogen peroxide- and nitric oxide-induced systemic antioxidant prime-like activity under NaCl-stress and stress-free conditions in citrus plants. J. Plant Physiol. 166, 1904–1913. doi: 10.1016/j.jplph.2009.06.012
Tanou, G., Ziogas, V., Belghazi, M., Christou, A., Filippou, P., Job, D., et al. (2014). Polyamines reprogram oxidative and nitrosative status and the proteome of citrus plants exposed to salinity stress. Plant Cell Environ. 37, 864–885. doi: 10.1111/pce.12204
Ziogas, V., Tanou, G., Belghazi, M., Filippou, P., Fotopoulos, V., Diamantidis, G. R., et al. (2015). Roles of sodium hydrosulfide and sodium nitroprusside as priming molecules during drought acclimation in citrus plants. Plant Mol. Biol. 89, 433–450. doi: 10.1007/s11103-015-0379-x
Keywords: abiotic stress, drought, priming, proteins, salinity
Citation: Molassiotis A, Job D, Ziogas V and Tanou G (2016) Citrus Plants: A Model System for Unlocking the Secrets of NO and ROS-Inspired Priming Against Salinity and Drought. Front. Plant Sci. 7:229. doi: 10.3389/fpls.2016.00229
Received: 21 December 2015; Accepted: 11 February 2016;
Published: 26 February 2016.
Edited by:
Violeta Velikova, Bulgarian Academy of Sciences, BulgariaReviewed by:
Giridara Kumar Surabhi, Regional Plant Resource Centre, IndiaCopyright © 2016 Molassiotis, Job, Ziogas and Tanou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgia Tanou, Z3Rhbm91QGFncm8uYXV0aC5ncg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.