- 1Département de Phytologie–Centre de Recherche et d’Innovation sur les Végétaux, Université Laval, Québec, QC, Canada
- 2Centre for Biomolecular Engineering, Australian Institute for Bioengineering and Nanotechnology, The University of Queensland, Brisbane, QLD, Australia
- 3Department of Plant Production and Soil Science, Forestry and Agricultural Biotechnology Institute, University of Pretoria, Pretoria, South Africa
The use of plants as expression hosts for recombinant proteins is an increasingly attractive option for the production of complex and challenging biopharmaceuticals. Tools are needed at present to marry recent developments in high-yielding gene vectors for heterologous expression with routine protein purification techniques. In this study, we designed the Cysta-tag, a new purification tag for immobilized metal affinity chromatography (IMAC) of plant-made proteins based on the protein-stabilizing fusion partner SlCYS8. We show that the Cysta-tag may be used to readily purify proteins under native conditions, and then be removed enzymatically to isolate the protein of interest. We also show that commonly used protease recognition sites for linking purification tags are differentially stable in leaves of the commonly used expression host Nicotiana benthamiana, with those linkers susceptible to cysteine proteases being less stable then serine protease-cleavable linkers. As an example, we describe a Cysta-tag experimental scheme for the one-step purification of a clinically useful protein, human α1-antitrypsin, transiently expressed in N. benthamiana. With potential applicability to the variety of chromatography formats commercially available for IMAC-based protein purification, the Cysta-tag provides a convenient means for the efficient and cost-effective purification of recombinant proteins from plant tissues.
Introduction
As the practice of using plants to produce recombinant proteins matures in both industrial and academic contexts (Davies, 2010; Paul and Ma, 2011; Xu et al., 2012), the development of bespoke tags for protein purification has now become of particular relevance (Buyel et al., 2015). Fusion protein tags can permit effective recovery of high purity recombinant proteins from both prokaryotic and eukaryotic expression hosts (Pina et al., 2014), and even improve the stability and solubility of some labile or ‘difficult to express’ proteins (Terpe, 2003). A current trend in plant-based protein expression is the use of polypeptides, such as hydrophobins (Joensuu et al., 2010), elastin-like polypeptides (Conley et al., 2009; Floss et al., 2010) and the γ-zein motif ZERA (Torrent et al., 2009), that allow for the stabilization and non-chromatographic purification of recombinant protein fusion partners by the induction of insoluble aggregates. Fusion tags for the stabilization and chromatographic purification of recombinant proteins have also been devised, generally involving biologically active antibody fragments as affinity ligands. An example was provided by Obregon et al. (2006), who showed the α2 and α3 constant regions of a human IgG α-chain fusion partner to increase the accumulation of human immunodeficiency virus-p24 antigen by 13-fold in transgenic tobacco leaves and to allow for its affinity purification with anti-human IgG antisera. More recently, human IgG Fc fragments were used to improve the yields of anthrax toxin receptor (Andrianov et al., 2010) and camelid Nanobodies® (De Buck et al., 2013) in transgenic plants, and to allow for the one-step purification of these proteins by protein A affinity chromatography.
Our general objective in this study was to engineer a widely applicable affinity chromatography tag for which non-biological –and cheap– affinity ligands are readily available in a variety of formats. Metal-chelating tags such as the popular poly-histidine (poly-His) tag have proved useful over the years to purify recombinant proteins from a variety of expression hosts by immobilized metal affinity chromatography (IMAC). Poly-His tags grafted at the C- or N-terminus of recombinant proteins have notably been used as purification ligands for protein recovery from different plant tissues (e.g., Leelavathi and Reddy, 2003; Valdez-Ortiz et al., 2005; Vardakou et al., 2012; de Souza et al., 2014), protocols and reagents for IMAC are available from numerous commercial suppliers, and IMAC procedures represent a generally convenient and cost-effective approach for the affinity purification of recombinant proteins at a lab scale (Lichty et al., 2005; Saraswat et al., 2013; Pina et al., 2014). Poly-His tags, however, may negatively affect the expression or activity of certain proteins (Woestenenk et al., 2004; Chant et al., 2005; Amor-Mahjoub et al., 2006; Renzi et al., 2006; Horchani et al., 2009; Sainsbury et al., 2009) and may sometimes be ineffective in native conditions due to their small size and variable accessibility at protein termini (Eschenfeldt et al., 2010). IMAC can be performed under denaturing conditions to make the poly-His motif more accessible (Terpe, 2003), but this is not applicable to those numerous proteins that cannot tolerate denaturation. Likewise, enzymatic procedures –such as the TAGZyme™ system– have been devised for the post-IMAC removal of His tag motifs (Pedersen et al., 1999; Schäfer et al., 2002), but the use of such systems remains costly and hardly accessible to most laboratories (Arnau et al., 2006; Waugh, 2011).
With these limitations in mind, we developed a chimeric poly-His tag, the ‘Cysta-tag,’ based on our recent observation that translational fusion to tomato multicystatin domain SlCYS8 can sustain, and even enhance, recombinant protein accumulation in leaves of the widely used expression host Nicotiana benthamiana (Sainsbury et al., 2013; Robert et al., 2016). We show the insertion of a poly-His motif in a solvent-exposed loop of SlCYS8 to produce an effective tag for IMAC purification of human α1-antitrypsin (α1AT), an anti-inflammatory protein with potential for the augmentation therapy of emphysema and other chronic obstructive pulmonary diseases (Stockley, 2015). We also document the variable stability of common protease cleavage sites for His tag removal, in the specific context of fusion proteins transiently expressed in N. benthamiana.
Materials and Methods
Structural Analyses
Structural models were generated in silico for SlCYS8 (GenBank accession no. AF198390) and tentative Cysta-tag hybrids to predict the impact of inserting a (His)6 [or 6x His] hexapeptide motif in the original cystatin structure. Twenty possible models were built for each possible variant using Modeller v. 9.7 (Eswar et al., 2006), with the NMR solution structure coordinates of oryzacystatin I (Nagata et al., 2000) as a template (Protein Data Bank accession no. 1EQK). The stereochemical quality of each model was assessed by comparison with the oryzacystatin structure using the PROCHECK program, v.3.5.4 (http://www.ebi.ac.uk/thornton-srv/ software/PROCHECK/; Laskowski et al., 1993). The best model for SlCYS8 and the best model for a tentative Cysta-tag variant were selected for visualization purposes, Cysta-tag engineering and heterologous expression in Escherichia coli or N. benthamiana.
Gene Constructs and Cloning
An α1AT (SERPINA1; Accession No. NM_000295)-encoding DNA sequence was synthesized by GeneArt (Life Technologies) with an internal synonymous substitution in the original sequence to remove an undesired BsaI restriction site. Sequences encoding SlCYS8, including a secreted version bearing the alfalfa protein disulphide isomerase (PDI) N-terminal signal peptide, were sourced from previously described constructs (Sainsbury et al., 2013). A (His)6 motif was introduced within SlCYS8 by extension overlap PCR between residues alanine (Ala)-62 and glycine (Gly)-63. Constructs for protein expression were assembled using a modified version of GoldenGate cloning (Engler et al., 2008), where counter selection against the ccdB gene yields only recombined expression plasmids (Sainsbury et al., 2012, 2013). Protein-encoding PCR fragments with appropriate GoldenGate recombination sites were blunt-end ligated into SmaI-digested pUC18strep in the presence of SmaI to limit plasmid self-ligation. Complementary oligonucleotides encoding various linker sequences with terminal extensions for recombination were annealed and similarly ligated into pUC18strep. For assembly into expression vectors, recombination reactions between expression plasmids and donor clones were driven by the simultaneous use of BsaI and a T4-DNA ligase. Non-recombined pUC18strep and expression plasmid clones were eliminated by expression of the vector-selecting antibiotic or of the ccdB gene, respectively. For expression in N. benthamiana, a pEAQ plasmid (Sainsbury et al., 2009) modified to act as an acceptor plasmid for GoldenGate recombination was used (Sainsbury et al., 2013). For expression in E. coli, the expression vector pGEX-3X (GE Healthcare) was similarly modified to act as a GoldenGate acceptor (Sainsbury et al., 2012). To construct the green fluorescent protein (GFP) fusion, PCR fragments encoding GFP and the Cysta-tag with complementary overlaps of 20 nucleotides were assembled into pEAQ-HT (Sainsbury et al., 2009) linearized with AgeI and StuI, using the Gibson Assembly Master Mix (New England Biolabs). All constructs were verified by Sanger sequencing before heterologous protein expression.
Cysteine Protease Inhibitory Activity
Bacterial expression and purification of recombinant cystatins were carried out as described previously (Sainsbury et al., 2013). Protein concentrations were determined by densitometry of Coomassie blue-stained gels after SDS-PAGE using the Phoretix 2-D Expression software, v. 2005 (Nonlinear Dynamics) and bovine serum albumin (Sigma–Aldrich) as a protein standard. Anti-papain activity of SlCYS8 and the Cysta-tag was determined by the monitoring of papain proteolysis progress curves with the fluorigenic synthetic peptide Z-Phe–Arg-methylcoumarin as a substrate, as described earlier (Goulet et al., 2008).
Plant-Based Expression
pEAQ vectors for in planta expression were maintained in Agrobacteria tumefaciens strain AGL1 following transformation by electroporation. Bacterial cultures were first grown in lysis broth medium supplemented with appropriate antibiotics, and the bacteria then collected by gentle centrifugation. Bacterial pellets were resuspended in leaf infiltration medium (10 mM MES, pH 5.6, containing 10 mM MgCl2 and 100 μM acetosyringone) and incubated for 2–4 h at room temperature prior to transfection. Leaf infiltration was performed using a needle-less syringe as described earlier (D’Aoust et al., 2009), after mixing each protein-encoding (or empty vector) agrobacterial suspension with an equal volume of bacteria carrying the pEAQ express vector for transgene silencing suppression (Sainsbury et al., 2009). Infiltrated leaf tissue was collected 7 days post-infiltration for recombinant protein extraction and analysis, after incubating the plants at 23°C under a 16 h:8 h day–night photoperiod.
Protein Extraction and Gel Electrophoresis
Leaf disks representing 160 mg of control (empty vector)-infiltrated tissue were harvested to determine protein expression rates following heterologous expression. The leaf disks were homogenized by disruption with a bead mill in three volumes of phosphate-buffered saline (PBS), pH 7.3, containing 5 mM EDTA, 0.05% (v/v) Triton X-100 (Sigma) and the cOMPLETE protease inhibitor cocktail for endogenous protease neutralization (Roche). Cell lysates were clarified by centrifugation at 20,000 × g for 5 min at 4°C and protein concentrations determined using the Bradford assay reagent (Thermo Scientific) with bovine serum albumin as a protein standard. Protein extracts were resolved by SDS-PAGE prior to Coomassie blue staining or immunodetection.
Immunoblotting
Proteins for immunoblotting were resolved by 12% (w/v) SDS-PAGE and electrotransferred onto nitrocellulose sheets. Non-specific binding sites after electrotransfer were saturated with 5% (w/v) skim milk powder in PBS containing 0.025% (v/v) Tween-20, which also served as a dilution buffer for the antibodies. Human α1AT was detected with commercial polyclonal IgG raised in rabbit against this protein (US Biologicals) and alkaline phosphatase-conjugated goat anti-rabbit IgG secondary antibodies (Sigma–Aldrich). SlCYS8 and the Cysta-tag were detected with commissioned polyclonal IgG (Agrisera) raised in rabbit against a bacterially expressed SlCYS8 (Girard et al., 2007) and alkaline phosphatase-conjugated goat anti-rabbit IgG secondary antibodies (Sigma–Aldrich). The Cysta-tag was also detected with mouse anti-poly-His IgG (Cell Signaling Technologies) and horseradish peroxidase-conjugated IgG secondary antibodies (Sigma–Aldrich). GFP was detected with mouse anti-GFP antibodies (Cell Signaling Technologies) and horseradish peroxidase-conjugated secondary antibodies. Colorimetric signals for phosphatase-conjugated antibodies were developed with nitro blue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate as a substrate (Sigma–Aldrich). Electrochemiluminescent signals for peroxidase-conjugated antibodies were generated with the Clarity Western ECL Substrate™ (Bio-Rad).
Quantitative ELISA
Enzyme-linked immunosorbent assays (ELISA) were performed to quantify α1AT based on a procedure described earlier for human α1-antichymotrypsin (α1ACT; Sainsbury et al., 2013). Immulon 2HB ELISA plates (Thermo Scientific) were coated with duplicate samples of soluble protein extract diluted 1:50 to 27–30 μg/mL in PBS, pH 7.3. Non-specific binding sites were blocked with 1% (w/v) casein in PBS before application of anti-human α1AT diluted in PBS with 0.25% (w/v) casein. Anti-rabbit IgG conjugated to horseradish peroxidase were used as secondary antibodies, followed by colour signal development with the 3,3′,5,5′-tetramethylbenzidine SureBlue™ peroxidase substrate (KPL). The absorbance was read at 450 nm after adding 1 N HCl to stop color development. A standard curve was generated for each plate with human α1AT (EMD Chemicals) diluted in control extracts from tissue infiltrated with an empty vector, to account for possible matrix effects.
Fusion Protein Purification
Cysta-tagged proteins were purified from crude protein extracts using the ÄKTA Prime Plus Liquid Chromatography System (GE Healthcare). Cysta-tagged α1AT was purified from 5 g of infiltrated tissue ground in liquid nitrogen and resuspended in three volumes of extraction buffer (20 mM sodium phosphate, pH 7.4, 0.5 M NaCl) containing EDTA-free cOMPLETE protease inhibitor cocktail (Roche). Leaf tissue expressing the Cysta-tag–GFP fusion was disrupted in three volumes of the same buffer using a PT1200 Polytron homogenizer (Kinematica). The leaf lysates were clarified by centrifugation at 20,000 × g for 10 min at 4°C, the supernatants frozen overnight at –80°C, and the mixtures centrifuged again to remove insoluble debris. Dithiothreitol (DTT; to 1 mM) and imidazole (to 20 mM) were added to the extracts, and the mixtures submitted to a final centrifugation round to remove precipitates. The resulting extracts were passed through a 0.45 μm syringe filter and approximately 12 ml was injected into a 5-ml sample loop in order to fill the loop completely. Samples were loaded onto 1-ml HisTrap columns for IMAC (GE Healthcare) and washed with extraction buffer containing 1 mM DTT and 20 mM imidazole. Immobilized proteins were eluted with 400 mM imidazole in extraction buffer containing 1 mM DTT, and the recovered fractions stored at –80°C or immediately prepared for SDS-PAGE. Yield and purity of the α1AT eluates were calculated relative to starting amount of α1AT and total protein content, respectively.
Cysta-Tag Proteolytic Removal
Cysta-tag removal was done by protease treatment with the common linker processing enzyme human factor Xa (New England Biolabs). Purified protein samples were dialyzed overnight in 20 mM Tris-HCl, pH 8.0, containing 100 mM NaCl and 2 mM CaCl2. The Cysta-tag–protein (GFP) fusion was adjusted to a working concentration of 500 ng/μl and digested with factor Xa at molar ratios of 1:20 or 1:50. Protease reactions were performed at 20°C in total volumes of 100 μl. Samples were collected at different time points, and factor Xa activity stopped by the addition of SDS-PAGE sample loading buffer and heating for 5 min at 95°C.
Results and Discussion
Cysta-Tag Design, Structure, and Expression
We took a rational approach to the design of the Cysta-tag, taking into account proximity of the poly-His tag insertion site to (1) the two inhibitory loops and N-terminal trunk of SlCYS8, which both contribute to the biological activity of the protein (Benchabane et al., 2010); and (2) the C-terminus of the cystatin, given the need to avoid steric interference from the fusion partner on IMAC substrate binding. Modeling attempts with these considerations in mind led us to select a structurally unconstrained site for the insertion of a (His)6 motif, between residues alanine (Ala)-62 and glycine (Gly)-63 (Figure 1A). In silico modeling of the chimeric protein resulted in a putative Cysta-tag variant with a predicted tertiary structure closely matching the tertiary structure of SlCYS8, aside from an extended surface loop with the poly-His motif away from the protease inhibitory loops (Figure 1B). Ramachandran plots were produced with the inferred structural coordinates of SlCYS8 (not shown) and the ‘Ala-62–(His)6–Gly-63’ Cysta-tag (Figure 1C) to confirm the stereochemical quality of our structural models (Laskowski et al., 1993). For both two proteins, more than 90% of the amino acid residues (black dots on Figure 1C) fell within the ‘most favored’ (red) and ‘additional allowed regions’ (yellow) areas of the graph, indicating adequate stereochemical quality of the predicted structures (Morris et al., 1992) and eventual robustness of the chimeric protein tag.
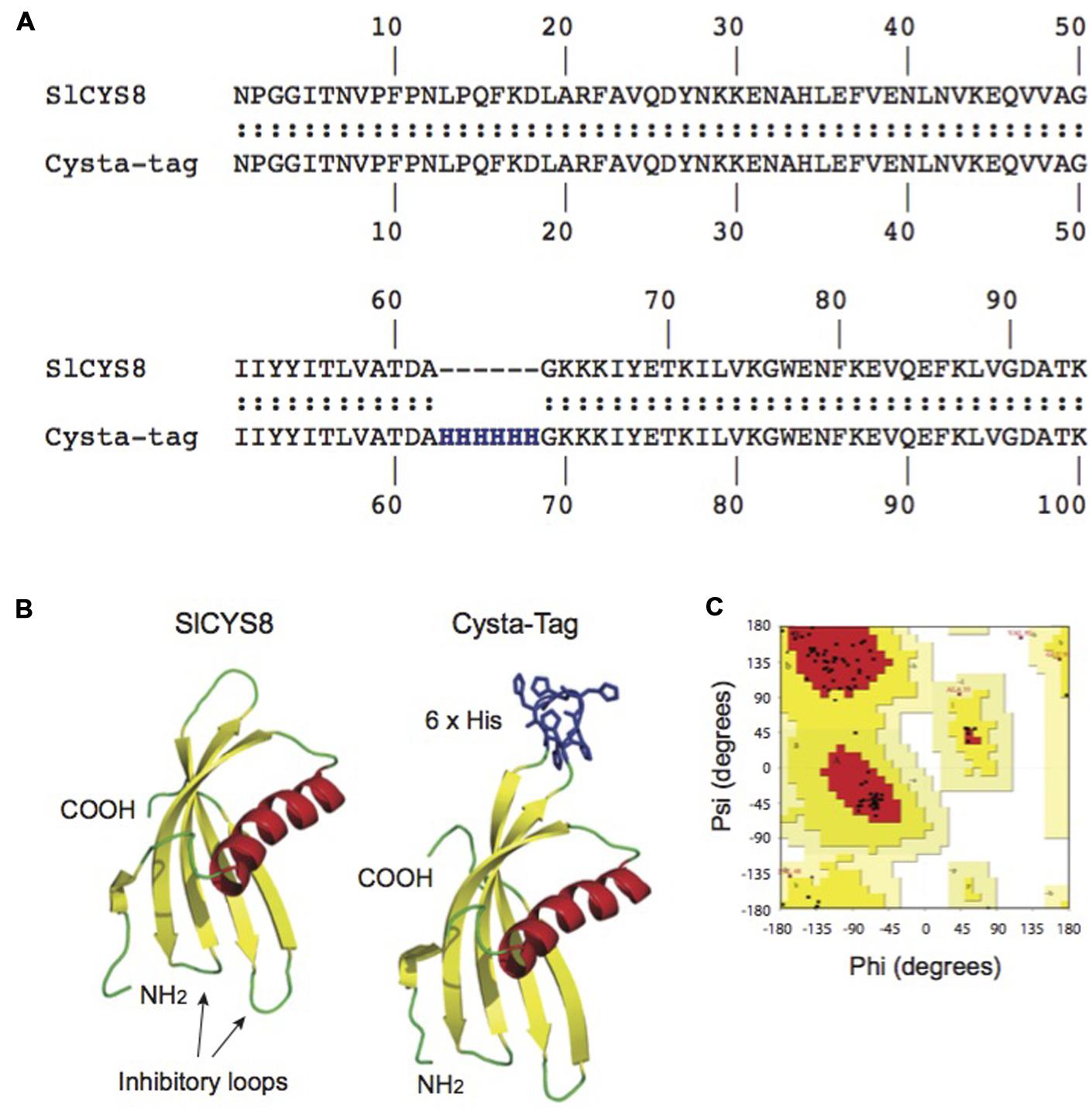
FIGURE 1. In silico modeling of the Cysta-tag. Computationally modeling putative His-motif variants of SlCYS8 based on the NMR solution structure of oryzacystatin-I (PDB 1EQK) identified surface loop residues Ala-62 and Gly-63 as a possible target site for (His)6 motif insertion with minimal impact on the template structure (A). Graphical representation of the in silico models shows location of the poly-His motif at this position, away from the N-terminus and inhibitory loops involved in protease inhibition (B). A Ramachandran plot produced with PROCHECK for the ‘Ala-62–(His)6–Gly-63’ Cysta-tag model (C) shows more than 90% of the amino acid residues (black dots) to fall within the ‘most favored’ (red) and ‘additional allowed regions’ (yellow) areas of the graph, as expected for good quality structural models (Morris et al., 1992).
We produced the –Ala-62–(His)6–Gly-63–Cysta-tag variant in E. coli to assess its overall stability and protease inhibitory activity compared to SlCYS8, using papain as a model target protease. Confirming a negligible impact for the inserted (His)6 motif on the cystatin template, an apparent dissociation constant of 42 nM was calculated for the Cysta-tag toward papain, similar to a dissociation constant of 43 nM determined for the original cystatin. Gene constructs were assembled to express SlCYS8 and the Cysta-tag in N. benthamiana leaves, either retained in the cytosol or targeted to the cell secretory pathway, to assess the impact of (His)6 motif insertion on stability of the cystatin in planta (Figure 2A). Coomassie blue-stained polyacrylamide slab gels following SDS-PAGE (Figure 2B) and immunoblots to confirm their identity (not shown) showed SlCYS8 and the Cysta-tag to accumulate at similar levels in both the cytosol and the apoplast, again indicating little impact of the poly-His motif on SlCYS8 overall stability and suggesting eventual robustness of the Cysta-tag as a fusion partner in planta.
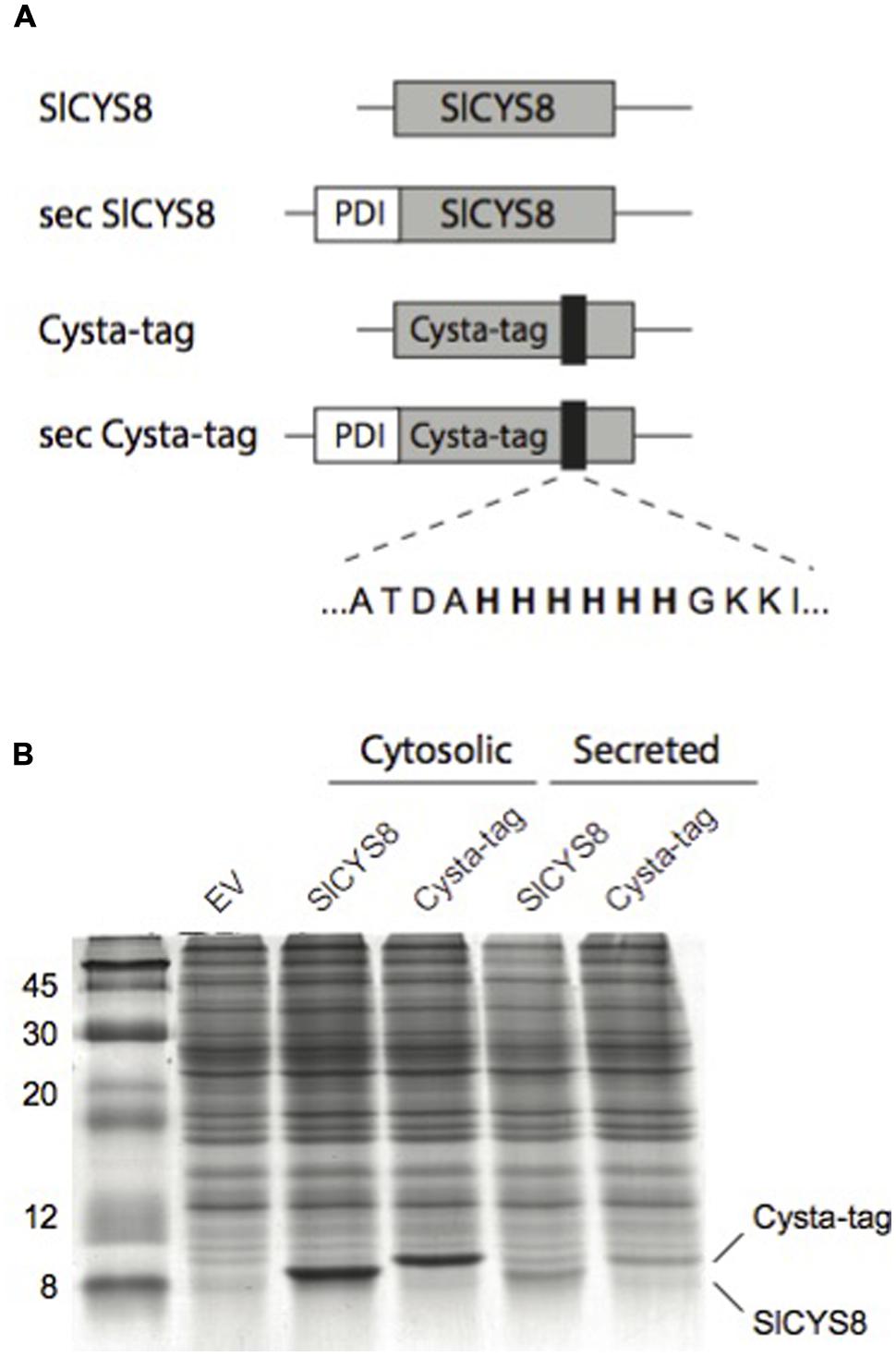
FIGURE 2. Assembly and expression of the Cysta-tag in N. benthamiana. (A) Schematic representation of gene constructs for expression of SlCYS8 and the Cysta-tag in planta, showing the relative location of poly-His motif (black areas) and flanking residues. PDI, signal peptide of alfalfa protein disulphide isomerase for targeting to the cell secretory pathway (sec). (B) Coomassie blue-stained polyacrylamide slab gel with protein extracts of leaf tissue expressing SlCYS8 and Cysta-tag in the cytosol or the secretory pathway. Protein bands for SlCYS8 and the Cysta-tag show similar steady-state levels in leaves and a slightly higher molecular weight for the Cysta-tag due to insertion of the (His)6 motif. EV stands for protein extract from leaf tissue infiltrated with agrobacteria harboring an ‘empty’ control vector.
Expression of Cysta-Tag Fusions in N. benthamiana
We recently reported a strong positive effect of SlCYS8 used as a fusion partner on stability of an α1AT-related protein, α1ACT (Baker et al., 2007), in N. benthamiana leaves (Sainsbury et al., 2013). To demonstrate effectiveness of the Cysta-tag protein as a fusion partner moiety in plants, we chose to tag the clinically relevant α1AT, which has been the target for recombinant protein expression in plants where it can be produced in an active form (Terashima et al., 1999; Nadai et al., 2009; Zhang et al., 2012; Castilho et al., 2014). Because glycosylation of α1AT imparts increased stability to the normally secreted protein (Kwon and Yu, 1997), fusions were generated with a secreted version of the Cysta-tag (Figure 3A). For the gene constructs we used a mature form of α1AT lacking 23 amino acids at the N-terminus, as no well defined structure could be observed in this region by X-ray crystallography (Patschull et al., 2011) and because the corresponding N-terminal sequence in α1ACT, also presenting an undefined structure (Wei et al., 1994), was reported to undergo restricted proteolysis in a plant cell environment (Benchabane et al., 2009).
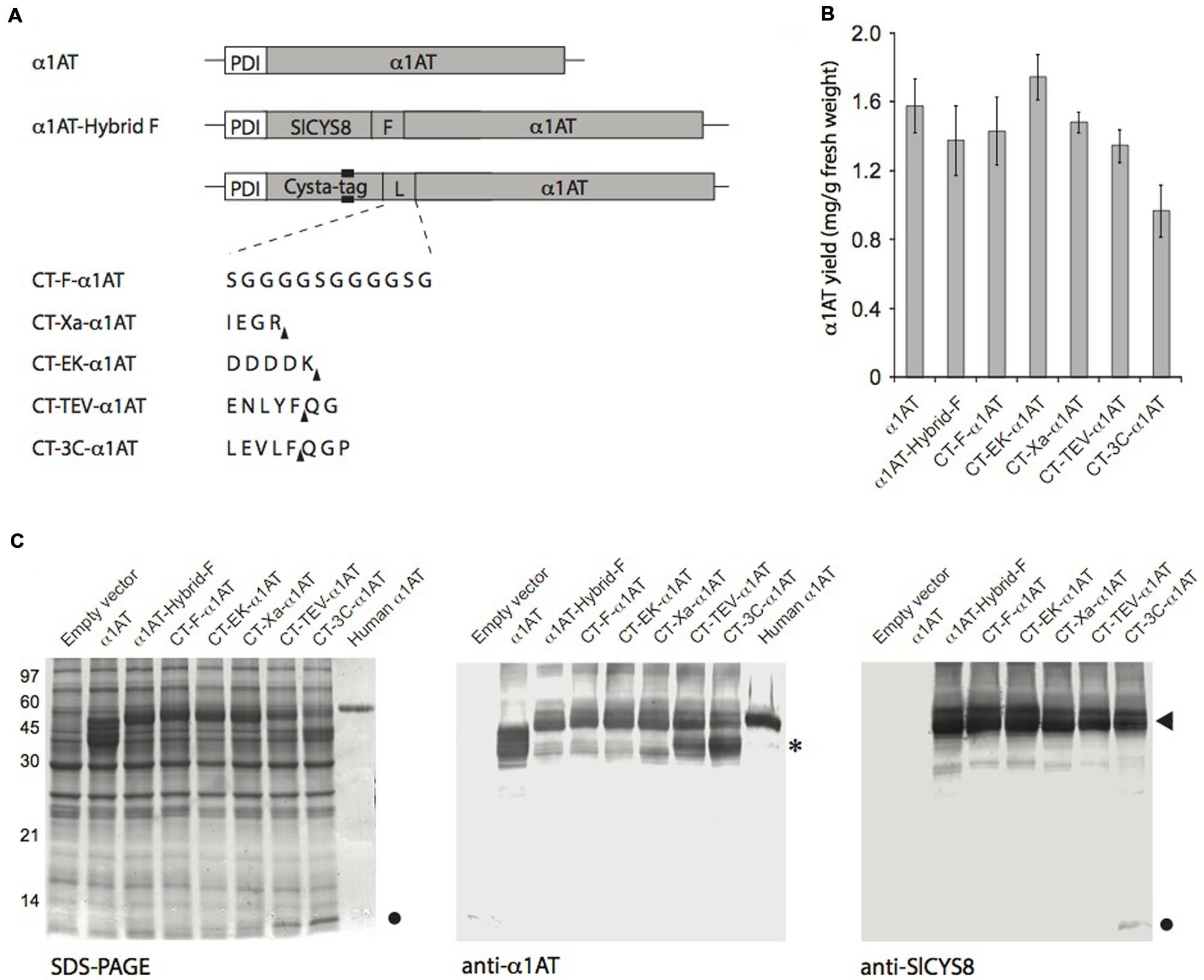
FIGURE 3. Transient expression of Cysta-tag–α1AT fusions with different protease-cleavable linkers in N. benthamiana leaves. (A) Schematic representation of the Cysta-tag (CT)–α1AT fusions, showing domain organization, location of the poly-His motif (black area) and identity of the inserted linker (L). F, flexible linker stable in planta (see Sainsbury et al., 2013); Xa, cleavage motif for human factor Xa; EK, cleavage motif for bovine enterokinase; TEV, cleavage motif for Tobacco etch virus protease; 3C, cleavage motif for Rhinovirus 3C protease. Arrowheads in upright position show protease cleavage sites. (B) ELISA for quantitation of α1AT in crude protein extracts of leaves expressing secreted α1AT or the different Cysta-tag–α1AT fusions. Each bar is the mean of three replicate values ±SD. (C) SDS-PAGE separation of α1AT and Cysta-tag hybrids and their immunodetection with anti-α1AT and anti-SlCYS8 polyclonal antibodies. Asterisk (∗) on the right highlights free α1AT in leaf extract, arrowhead Cysta-tag–α1AT fusion protein, and closed circle free Cysta-tag. Human α1AT on right lanes of the Coomassie blue-stained gel and anti-α1AT immunoblot corresponds to a commercially available, highly glycosylated form of the protein.
We fused α1AT to the Cysta-tag using the generic flexible peptide linker Gly(4)Ser, reported earlier to be proteolysis-resistant in the secretory pathway of N. benthamiana leaf cells (Sainsbury et al., 2013). Expressing un-tagged α1AT as well as fusions to either SlCYS8 or the Cysta-tag showed no positive effect of the plant cystatin on steady-state levels of secreted α1AT 7 days post-infiltration (Figures 3B,C), in sharp contrast with the positive effect reported earlier for SlCYS8 used as a fusion partner for α1ACT (Sainsbury et al., 2013). This, however, could be expected given the very high accumulation rate of more than 1.5 mg/g fresh weight tissue measured for α1AT (Figure 3B), much higher than the accumulation rate obtained for α1ACT expressed in the same expression system (Sainsbury et al., 2013). Most importantly, fusion to the Cysta-tag resulted in expression levels comparable to those observed with SlCYS8–α1AT (Figures 3B,C).
Since the Cysta-tag is biochemically active and because it could, with a molecular mass of ∼11 kDa, physically interfere with stability and activity of the fusion partner, we investigated the resistance of commonly used cleavable linkers to degradation by host plant endogenous proteases. To this end, we assembled Cysta-tag–α1AT fusions with cleavage motifs for two Ser proteases, bovine enterokinase and human factor Xa, and for two Cys proteases, Tobacco etch virus (TEV) protease and Rhinovirus 3C protease (3C; Figure 3A). The four linkers showed variable stability in planta, with those acted on by Ser proteases being substantially more stable than those recognized by Cys proteases, which both led to an accumulation of free Cysta-tag detectable on Coomassie blue-stained gels (Figure 3C). In addition to being stable in the plant cell secretory pathway, the enterokinase and factor Xa cleavage motifs do not leave residual amino acids at the N-terminus of downstream-located proteins upon cleavage, unlike cleavage motifs of the TEV and 3C proteases expected to leave two or three non-native residues (see Figure 3A for expected cleavage sites). Our observations suggest that Ser protease-cleavable motifs such as those of enterokinase and factor Xa may be most useful in N. benthamiana expression platforms, both as stable linkers in planta before leaf processing and as convenient cleavable linkers for tag removal following recombinant protein purification.
Purification of Plant-Made α1AT via the Cysta-Tag
We submitted leaf tissue expressing Cysta-tag–α1AT to a routine IMAC procedure to confirm usefulness of the Cysta-tag as an affinity ligand for recovery of heterologous proteins in native conditions (Figure 4). A preliminary freeze/thaw treatment of clarified extracts resulted in the precipitation of a significant fraction of ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco), a major protein contaminant in crude leaf extracts (Robert et al., 2015), with no notable loss of the Cysta-tagged fusion (Figure 4A). Approximately 22% of total soluble proteins was lost during the process, compared to only a 1% average decrease in α1AT as measured by quantitative ELISA. Addition of imidazole to the protein extracts after freezing resulted in substantial protein precipitation leading to a further 18% loss of total protein and a concomitant 30% loss of α1AT relative to initial level in untreated extracts. These numbers represent an average across three independent purifications of factor Xa-linked fusion protein. Similar rates were obtained with the enterokinase-linked Cysta-tag–α1AT fusion (Supplementary Figure S1), which suggests consistency of protein precipitation rates across leaf pre-purification steps and no significant impact of the cleavage linker on protein loss during early downstream processing.
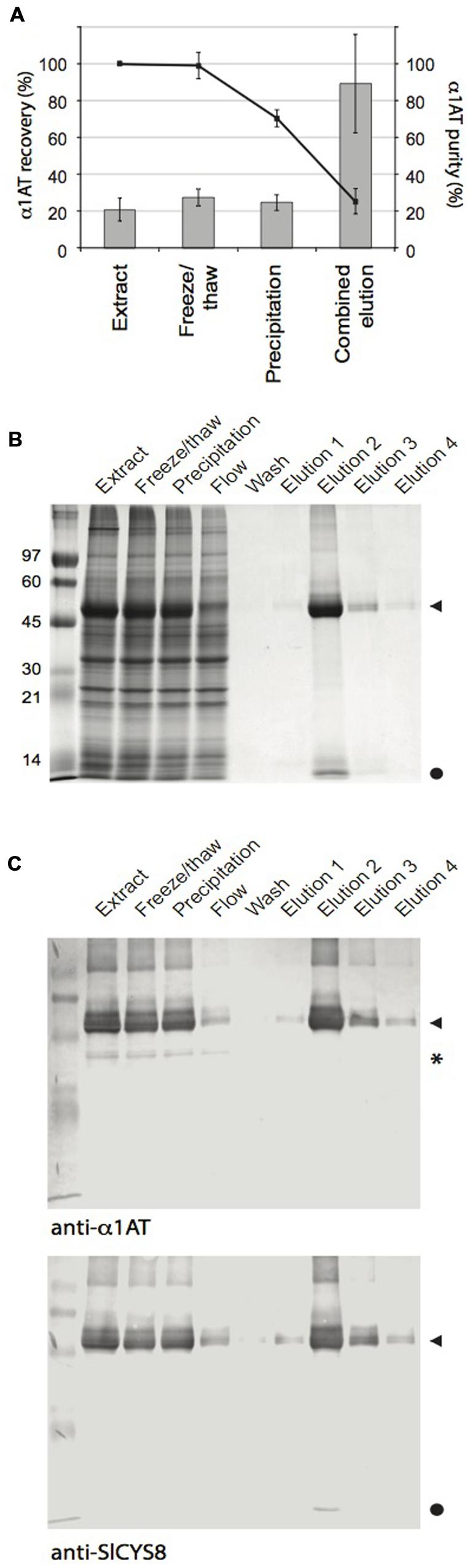
FIGURE 4. IMAC purification of the Cysta-tag–α1AT fusion. Cysta-tag and α1AT separated by the factor Xa-cleavable sequence were transiently expressed in N. benthamiana leaves. (A) α1AT recovery (line) and purity (columns) rates during purification. Calculations were made based on ELISA assays for α1AT and leaf total soluble protein determinations. Data are mean values of three independent purification rounds ±SD. (B) Coomassie blue-stained polyacrylamide gels following SDS-PAGE showing key protein fractions during the purification process. Identity of the Cysta-tag–α1AT fusion was confirmed by immunodetections with anti-α1AT and anti-SlCYS8 primary antibodies (C). Arrowheads point to the Cysta-tag–α1AT fusion, closed circles to free Cysta-tag and asterisk (∗) to free α1AT.
Coomassie blue-stained gels and immunoblots confirmed that most of the Cysta-tag–α1AT fusion in clarified extracts was retained in HisTrap columns, while un-tagged α1AT did not bind in the presence of 20 mM imidazole (Figure 4B). During optimization of the elution conditions, we found that lower concentrations of imidazole resulted in non-specific binding of RuBisCO into the column, and higher concentrations to reduced retention of the Cysta-tag fusion. The 55-kDa protein fusion expected for Cysta-tagged α1AT was effectively eluted by the addition of 400 mM imidazole, to give final recovery yield and purity rate of about 25 and 90%, respectively, for both factor Xa- and enterokinase-cleavable fusions (Figure 4 and Supplementary Figure S1). Since starting amounts of α1AT in leaves were around 1.6 mg per g fresh weight (see Figure 3B), this represents a recovery rate for the expressed protein of ∼0.4 mg per g fresh weight, equivalent to about 5% of total extracted leaf protein. A protein contaminant likely corresponding to free Cysta-tag was sometimes visible in Coomassie blue-stained gels after purification, which could in theory be removed along with released Cysta-tag subsequent to proteolytic tag removal by further IMAC or other techniques such as ion exchange or size exclusion chromatography.
Enzymatic Removal of the Cysta-Tag
Initial attempts to digest factor Xa- and enterokinase-cleavable Cysta-tag–α1AT fusions were not successful, likely due to either steric hindrance at the protease cleavage site or to the well-documented inhibitory effect of α1AT against several Ser proteases including factor Xa (Morrissey, 1998). To confirm utility of the Cysta-tag approach to produce proteins free of their affinity partner, we designed a factor Xa-cleavable Cysta-tag fusion with GFP, also taking this opportunity to use a non-secreted version of the Cysta-tag to direct fusion protein accumulation in the cytosol. Cysta-tag–GFP was expressed in N. benthamiana leaves and purified as described above for the Cysta-tag–α1AT fusions (Figure 5). As expected, Coomassie blue-stained gels following SDS-PAGE (Figure 5A) and immunoblotting of both GFP and the Cysta-tag poly-His motif (Figure 5B) confirmed purification to high purity of a 38-kDa Cysta-tag–GFP fusion product, along with a product of higher molecular weight likely corresponding to Cysta-tag–GFP::GFP–Cysta-tag dimers as a result of GFP dimerization (Tsien, 1998). These observations demonstrate the potential of Cysta-tag-based expression for the affinity purification of recombinant proteins from leaf tissues regardless of their subcellular localization in transfected cells. They also show that the Cysta-tag could be detected with anti-poly-His antibodies (Figure 5B) and thus be used to monitor recombinant protein expression and purification processes.
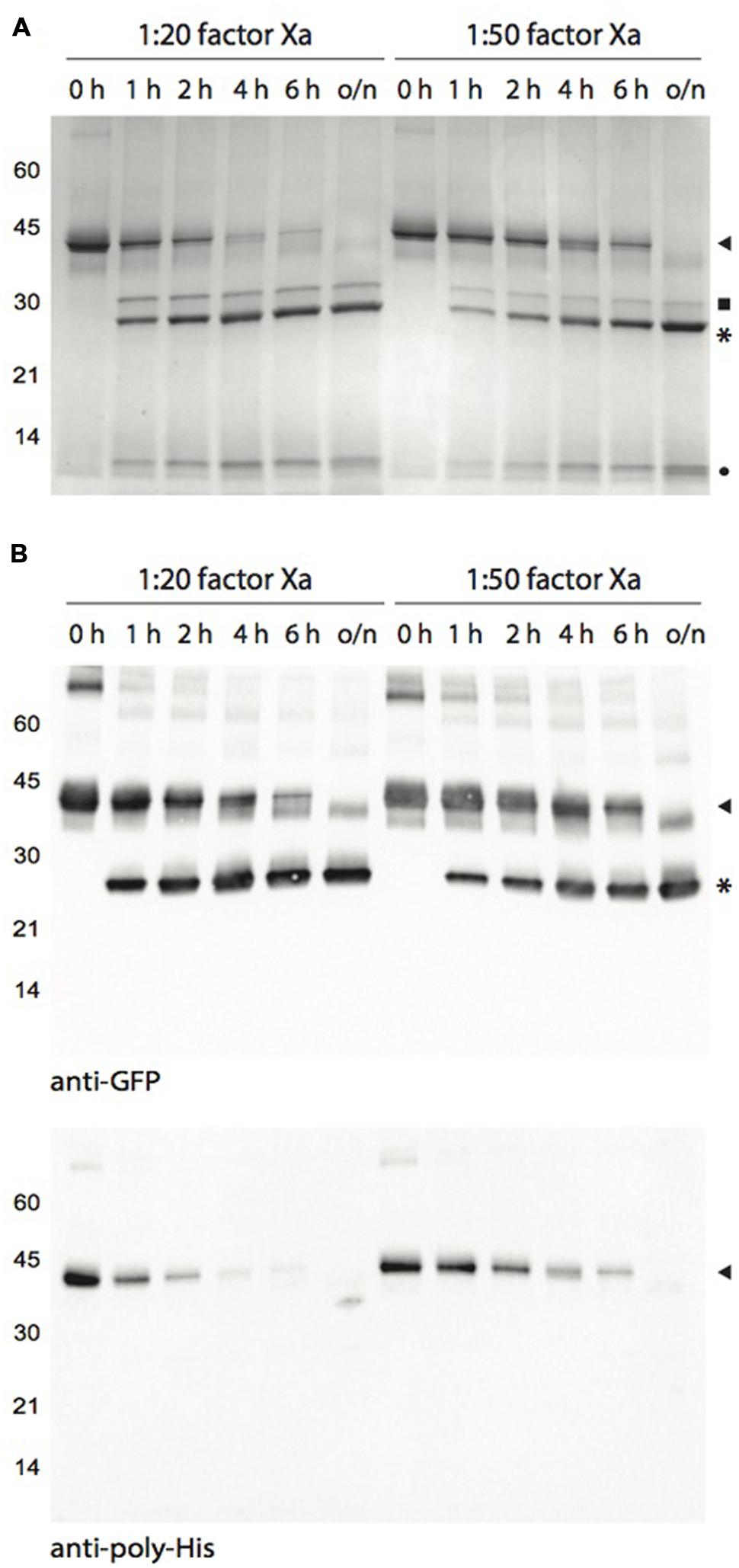
FIGURE 5. Human factor Xa-mediated cleavage of Cysta-tag–GFP at different incubation time points. (A) Coomassie blue-stained polyacrylamide gel for Cysta-tag-GFP fusion samples incubated with factor Xa at two enzyme::protein ratios for 1, 2, 4, 6 h, or overnight (o/n). Digestion was monitored by immunoblotting with polyclonal primary antibodies directed against GFP or the poly-His motif (B). Arrowheads point to the Cysta-tag–GFP fusion, asterisks (∗) to free GFP, closed circle to free Cysta-tag, and closed square to residual (contaminant) factor Xa.
Enzymatic removal of the Cysta-tag was performed with molar ratios of factor Xa fixed at 1:20 (as suggested by the manufacturer) and 1:50 relative to the recombinant protein (Figure 5). Both protease concentrations allowed for an effective cleavage of the fusion, readily reducing the amount of intact 38-kDa fusion protein –and dismantling the GFP fusion dimers– to generate a 27-kDa protein corresponding to free GFP. Cleavage at the 1:20 ratio was nearly complete after 4 h, confirming efficient removal of the affinity tag under standard processing conditions. The 1:50 ratio required an overnight incubation but the cleavage was also complete, pointing to the possibility of minimizing protein sample contamination with residual factor Xa after enzymatic cleavage for those proteins that support long incubation periods.
Conclusion
Our goal in this study was to devise a protease-removable fusion tag for the IMAC purification of plant-made proteins in native conditions. Building upon our finding that tomato cystatin SlCYS8 can act as a stabilizing fusion partner for secreted proteins in plant leaf biofactories (Sainsbury et al., 2013), we engineered a chimeric tag for IMAC that is also detectable using readily available anti-poly-His antibodies and thus useful to monitor the expression and purification of recombinant proteins. Through molecular modeling we identified a physically unconstrained site for poly-His motif insertion, located in an exposed surface loop of SlCYS8 distal to both the inhibitory loops and N-terminus involved in protease inhibitory activity. The expression rate, overall stability and anti-papain potency of the resulting chimeric protein were unaltered compared to the parent protein, SlCYS8. This novel tag can be linked to a protein of interest using peptide linkers encoding different endoprotease cleavage sites. Protein purification using the Cysta-tag results in efficient and reproducible recovery of high-quality protein products, regardless of their subcellular localization in planta. Our results demonstrate the general usefulness of Cysta-tag fusions for recombinant protein purification from plant sources under mild, non-denaturing conditions. Indeed, a recent study reported the use of the Cysta-tag for the heterologous expression and purification of a difficult to express plant β-glucosidase in native conditions, permitting a first functional characterization of this previously intractable enzyme (Mageroy et al., 2015). We here developed the Cysta-tag for the purification of plant-made proteins, but the diversity of tools and protocols already available for poly-His-based IMAC in various expression systems make our new approach potentially applicable to any prokaryotic or eukaryotic host.
Author Contributions
FS conceived the study with DM, took charge of the experimental design, performed lab experiments with PJ, and wrote a first draft of the manuscript. PJ contributed to the experimental design, performed lab experiments with FS, and contributed to the first draft of the manuscript. JV conceived the Cysta-tag with FS, performed the in silico (modeling) analyses, and contributed to the writing of the manuscript. M-CG contributed to the experimental design, coordinated the lab experiments, and contributed to the writing of the manuscript. DM conceived the study, contributed to the experimental design, coordinated the study, and prepared the last version of the manuscript.
Funding
This work was supported by a Discovery grant from the Natural Science and Engineering Research Council (NSERC) of Canada to DM, and by an Australian Research Council (ARC) Discovery Early Career Research Award to FS (DE140101553).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
PJ was the recipient of an AgroPhytoSciences NSERC–FONCER scholarship and of a BMP graduate scholarship funded by NSERC, the Fonds de Recherche Québec Nature et Technologies and our private research partner Medicago Inc.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.00141
References
Amor-Mahjoub, M., Suppini, J. P., Gomez-Vrielyunck, N., and Ladjimi, M. (2006). The effect of the hexahistidine-tag in the oligomerization of HSC70 constructs. J. Chromatogr. B 844, 328–334. doi: 10.1016/j.jchromb.2006.07.031
Andrianov, V., Brodzik, R., Spitsin, S., Bandurska, K., McManus, H., Koprowski, H., et al. (2010). Production of recombinant anthrax toxin receptor (ATR/CMG2) fused with human Fc in planta. Protein Expr. Purif. 70, 158–162. doi: 10.1016/j.pep.2009.09.016
Arnau, J., Lauritzen, G., Petersen, G. E., and Pedersen, J. (2006). Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expr. Purif. 48, 1–13. doi: 10.1016/j.pep.2005.12.002
Baker, C., Belbin, O., Kalsheker, N., and Morgan, K. (2007). SERPINA3 (aka alpha-1-antichymotrypsin). Front. Biosci. 1:2275. doi: 10.2741/2275
Benchabane, M., Saint-Jore-Dupas, C., Bardor, M., Faye, L., Michaud, D., and Gomord, V. (2009). Targeting and post-translational processing of human alpha1-antichymotrypsin in BY-2 tobacco cultured cells. Plant Biotechnol. J. 7, 146–160. doi: 10.1111/j.1467-7652.2008.00382.x
Benchabane, M., Schlüter, U., Vorster, J., Goulet, M.-C., and Michaud, D. (2010). Plant cystatins. Biochimie 92, 1657–1666. doi: 10.1016/j.biochi.2010.06.006
Buyel, J. F., Twyman, R. M., and Fischer, R. (2015). Extraction and downstream processing of plant-derived recombinant proteins. Biotechnol. Adv. 33, 902–913. doi: 10.1016/j.biotechadv.2015.04.010
Castilho, A., Windwarder, M., Gattinger, P., Mach, L., Strasser, R., Altmann, F., et al. (2014). Proteolytic and N-glycan processing of human α1-antitrypsin expressed in Nicotiana benthamiana. Plant Physiol. 166, 1839–1851. doi: 10.1104/pp.114.250720
Chant, A., Kraemer-Pecore, C. M., Watkin, R., and Kneale, G. G. (2005). Attachment of a histidine tag to the minimal zinc finger protein of the Aspergillus nidulans gene regulatory protein AreA causes a conformational change at the DNA-binding site. Protein Expr. Purif. 39, 152–159. doi: 10.1016/j.pep.2004.10.017
Conley, A. J., Joensuu, J. J., Jevnikar, A. M., Menassa, R., and Brandle, J. E. (2009). Optimization of elastin-like polypeptide fusions for expression and purification of recombinant proteins in plants. Biotechnol. Bioeng. 103, 562–573. doi: 10.1002/bit.22278
D’Aoust, M.-A., Lavoie, P.-O., Belles-Isles, J., Bechtold, N., Martel, M., and Vézina, L.-P. (2009). Transient expression of antibodies in plants using syringe agroinfiltration. Methods Mol. Biol. 483, 41–50. doi: 10.1007/978-1-59745-407-0_3
Davies, H. M. (2010). Review article: commercialization of whole-plant systems for biomanufacturing of protein products: evolution and prospects. Plant Biotechnol. J. 8, 845–861. doi: 10.1111/j.1467-7652.2010.00550.x
De Buck, S., Nolf, J., De Meyer, T., Virdi, V., De Wilde, K., Van Lerberge, E., et al. (2013). Fusion of an Fc chain to a VHH boosts the accumulation levels in Arabidopsis seeds. Plant Biotechnol. J. 11, 1006–1016. doi: 10.1111/pbi.12094
de Souza, A., Hull, P. A., Gille, S., and Pauly, M. (2014). Identification and functional characterization of the distinct plant pectin esterases PAE8 and PAE9 and their deletion mutants. Planta 240, 1123–1138. doi: 10.1007/s0045-014-2139-6
Engler, C., Kandzia, R., and Marillonnet, S. (2008). A one pot, one step, precision cloning method with high throughput capability. PLoS ONE 3:e3647. doi: 10.1371/journal.pone.0003647
Eschenfeldt, W. H., Maltseva, N., Stols, L., Donnelly, M. I., Gu, M., Nocek, B., et al. (2010). Cleavable C-terminal His-tag vectors for structure determination. J. Struct. Funct. Genomics 11, 31–39. doi: 10.1007/s10969-010-9082-y
Eswar, N., Webb, B., Marti-Renom, M. A., Madhusudhan, M. S., Eramian, D., Shen, M., et al. (2006). “Comparative protein structure modeling using modeller,” in Current Protocols in Bioinformatics Suppl. 15 (New York: John Wiley & Sons), 5.6.1–5.6.30. doi: 10.1002/0471250953.bi0506s15
Floss, D. M., Schallau, K., Rose-John, S., Conrad, U., and Scheller, J. (2010). Elastin-like polypeptides revolutionize recombinant protein expression and their biomedical application. Trends Biotechnol. 28, 37–45. doi: 10.1016/j.tibtech.2009.10.004
Girard, C., Rivard, D., Kiggundu, A., Kunert, K., Gleddie, S. C., Cloutier, C., et al. (2007). A multicomponent, elicitor-inducible cystatin complex in tomato, Solanum lycopersicum. New Phytol. 173, 841–851. doi: 10.1111/j.1469-8137.2007.01968.x
Goulet, M.-C., Dallaire, C., Vaillancourt, L.-P., Khalf, M., Badri, A. M., Preradov, A., et al. (2008). Tailoring the specificity of a plant cystatin toward herbivorous insect digestive cysteine proteases by single mutations at positively selected amino acid sites. Plant Physiol. 146, 1010–1019. doi: 10.1104/pp.108.115741
Horchani, H., Quertani, S., Gargouri, Y., and Sayari, A. (2009). The N-terminal His-tag and the recombination process affect the biochemical properties of Staphylococcus aureus lipase produced in Escherichia coli. J. Mol. Catal. B Enzyme 61, 194–201. doi: 10.1016/j.molcatb.2009.07.002
Joensuu, J. J., Conley, A. J., Lienemann, M., Brandle, J. E., Linder, M. B., and Menassa, R. (2010). Hydrophobin fusions for high-level transient protein expression and purification in Nicotiana benthamiana. Plant Physiol. 152, 622–633. doi: 10.1104/pp.109.149021
Kwon, K. S., and Yu, M. H. (1997). Effect of glycosylation on the stability of alpha1-antitrypsin toward urea denaturation and thermal deactivation. Biochim. Biophys. Acta 1335, 265–272. doi: 10.1016/S0304-4165(96)00143-2
Laskowski, R. A., MacArthur, M. W., Moss, D. S., and Thornton, J. M. (1993). PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291. doi: 10.1107/S0021889892009944
Leelavathi, S., and Reddy, V. S. (2003). Chloroplast expression of His-tagged GUS-fusions: a general strategy to overproduce and purify foreign proteins using transplastomic plants as bioreactors. Mol. Breed. 11, 49–58. doi: 10.1023/A:1022114427971
Lichty, J. J., Malecki, J. L., Agnew, H. D., Michelson-Horowitz, D. J., and Tan, S. (2005). Comparison of affinity tags for protein purification. Protein Expr. Purif. 41, 98–105. doi: 10.1016/j.pep.2005.01.019
Mageroy, M. H., Parent, G., Germanos, G., Giguère, I., Delvas, N., Maaroufi, H., et al. (2015). Expression of the β-glucosidase gene Pgβglu-1 underpins natural resistance of white spruce against spruce budworm. Plant J. 81, 68–80. doi: 10.1111/tpj.12699
Morris, A. L., MacArthur, M. W., Hutchinson, E. G., and Thornton, J. M. (1992). Stereochemical quality of protein structure coordinates. Proteins 12, 345–364. doi: 10.1002/prot.340120407
Morrissey, J. H. (1998). “Coagulation factor X,” in Handbook of Proteolytic Enzymes, eds A. J. Barrett, N. D. Rawlings, and J. F. Woessner (London: Academic Press), 163–167.
Nadai, M., Bally, J., Vitel, M., Job, C., Tissot, G., Botterman, J., et al. (2009). High-level expression of active human alpha1-antitrypsin in transgenic tobacco chloroplasts. Transgenic Res. 18, 173–183. doi: 10.1007/s11248-008-9209-0
Nagata, K., Kudo, N., Abe, K., Arai, S., and Tanokura, M. (2000). Three-dimensional solution structure of oryzacystatin-I, a cysteine proteinase inhibitor of the rice, Oryza sativa L. japonica. Biochemistry 39, 14753–14760. doi: 10.1021/bi0006971
Obregon, P., Chargelegue, D., Drake, P. M. W., Prada, A., Nuttall, J., Frigerio, L., et al. (2006). HIV-1 p24-immunoglobulin fusion molecule: a new strategy for plant-based protein production. Plant Biotechnol. J. 4, 195–207. doi: 10.1111/j.1467-7652.2005.00171.x
Patschull, A. O. M., Segu, L., Nyon, M. P., Lomas, D. A., Nobeli, I., Barrett, T. E., et al. (2011). Therapeutic target-site variability in α 1 -antitrypsin characterized at high resolution. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 67, 1492–1497. doi: 10.1107/S1744309111040267
Paul, M., and Ma, J. K.-C. (2011). Plant-made pharmaceuticals: leading products and production platforms. Biotechnol. Appl. Biochem. 58, 58–67. doi: 10.1002/bab.6
Pedersen, J., Lauritzen, C., Madsen, M. T., and Dahl, S. W. (1999). Removal of N-terminal polyhistidine tags from recombinant proteins using engineered aminopeptidases. Protein Expr. Purif. 15, 389–400. doi: 10.1006/prep.1999.1038
Pina, A. S., Lowe, C. R., and Roque, A. C. (2014). Challenges and opportunities in the purification of recombinant tagged proteins. Biotechnol. Adv. 32, 366–381. doi: 10.1016/j.biotechadv.2013.12.001
Renzi, F., Panetta, G., Vallone, B., Brunori, M., Arceci, M., Bozzoni, I., et al. (2006). Large-scale purification and crystallization of the endoribonuclease XendoU: trouble-shooting with His-tagged proteins. Acta Crystallogr. F 62, 298–301. doi: 10.1107/S1744309106006373
Robert, S., Goulet, M.-C., D’Aoust, M.-A., Sainsbury, F., and Michaud, D. (2015). Leaf proteome rebalancing in Nicotiana benthamiana for upstream enrichment of a transiently expressed recombinant protein. Plant Biotechnol. J. 13, 1169–1179. doi: 10.1111/pbi.12452
Robert, S., Jutras, P. V., Khalf, M., D’Aoust, M.-A., Goulet, M.-C., Sainsbury, F., et al. (2016). Companion protease inhibitors for the in situ protection of recombinant proteins in plants. Methods Mol. Biol. 1385, 115–126. doi: 10.1007/978-1-4939-3289-4_8
Sainsbury, F., Rhéaume, A.-J., Goulet, M.-C., Vorster, J., and Michaud, D. (2012). Discrimination of differentially inhibited cysteine proteases by activity-based profiling using cystatin variants with tailored specificities. J. Proteome Res. 11, 5983–5993. doi: 10.1021/pr300699n
Sainsbury, F., Thuenemann, E. C., and Lomonossoff, G. P. (2009). pEAQ: versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol. J. 7, 682–693. doi: 10.1111/j.1467-7652.2009.00434.x
Sainsbury, F., Varennes-Jutras, P., Goulet, M.-C., D’Aoust, M.-A., and Michaud, D. (2013). Tomato cystatin SlCYS8 as a stabilizing fusion partner for human serpin expression in plants. Plant Biotechnol. J. 11, 1058–1068. doi: 10.1111/pbi.12098
Saraswat, M., Musante, L., Ravida, A., Shortt, B., Byrne, B., and Holthofer, H. (2013). Preparative purification of recombinant proteins: current status and future trends. BioMed Res. Int. 2013, 312709. doi: 10.1155/2013/312709
Schäfer, F., Schäfer, A., and Steinert, K. (2002). A highly specific system for efficient enzymatic removal of tags from recombinant proteins. J. Biomol. Technol. 13, 158–171.
Stockley, R. A. (2015). The multiple facets of alpha-1-antitrypsin. Ann. Transl. Med. 3, 130. doi: 10.3978/j.issn.2305-5839.2015.04.25
Terashima, M., Murai, Y., Kawamura, M., Nakanishi, S., Stoltz, T., Chen, L., et al. (1999). Production of functional human alpha 1-antitrypsin by plant cell culture. Appl. Microbiol. Biotechnol. 52, 516–523. doi: 10.1007/s002530051554
Terpe, K. (2003). Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 60, 523–533. doi: 10.1007/s00253-002-1158-6
Torrent, M., Llompart, B., Lasserre-Ramassamy, S., Llop-Tous, I., Bastida, M., Marzabal, P., et al. (2009). Eukaryotic protein production in designed storage organelles. BMC Biol. 7:5. doi: 10.1186/1741-7007-7-5
Tsien, R. Y. (1998). The green fluorescent protein. Annu. Rev. Biochem. 67, 509–544. doi: 10.1146/annurev.biochem.67.1.509
Valdez-Ortiz, A., Rascón-Cruz, Q., Medina-Godoy, S., Sinagawa-García, S. R., Valverde-González, M. E., and Paredes-López, O. (2005). One-step purification and structural characterization of a recombinant His-tag 11S globulin expressed in transgenic tobacco. J. Biotechnol. 115, 413–423. doi: 10.1016/j.jbiotec.2004.09.013
Vardakou, M., Sainsbury, F., Rigby, N., Mulholland, F., and Lomonossoff, G. P. (2012). Expression of active recombinant human gastric lipase in Nicotiana benthamiana using the CPMV-HT transient expression system. Protein Expr. Purif. 81, 69–74. doi: 10.1016/j.pep.2011.09.005
Waugh, D. S. (2011). An overview of enzymatic reagents for the removal of affinity tags. Protein Expr. Purif. 80, 283–293. doi: 10.1016/j.pep.2011.08.005
Wei, A., Rubin, H., Cooperman, B. S., and Christianson, D. W. (1994). Crystal structure of an uncleaved serpin reveals the conformation of an inhibitory reactive loop. Nat. Struct. Biol. 1, 251–258. doi: 10.1038/nsb0494-251
Woestenenk, E. A., Hammarström, M., van den Berg, S., Härd, T., and Berglund, H. (2004). His tag effect on solubility of human proteins produced in Escherichia coli: a comparison between four expression vectors. J. Struct. Funct. Genom. 5, 217–229. doi: 10.1023/B:jsfg.0000031965.37625.0e
Xu, J., Dolan, M. C., Medrano, G., Cramer, C. L., and Weathers, P. J. (2012). Green factory: plants as bioproduction platforms for recombinant proteins. Biotechnol. Adv. 30, 1171–1184. doi: 10.1016/j.biotechadv.2011.08.020
Keywords: plant molecular farming, protein purification, immobilized metal affinity chromatography, tomato cystatin SlCYS8, human α1-antitrypsin
Citation: Sainsbury F, Jutras PV, Vorster J, Goulet M-C and Michaud D (2016) A Chimeric Affinity Tag for Efficient Expression and Chromatographic Purification of Heterologous Proteins from Plants. Front. Plant Sci. 7:141. doi: 10.3389/fpls.2016.00141
Received: 23 November 2015; Accepted: 27 January 2016;
Published: 15 February 2016.
Edited by:
Domenico De Martinis, Italian National Agency for New Technologies, Energy and Sustainable Economic Development, ItalyReviewed by:
Eva Stoger, University of Natural Resources and Life Sciences, Vienna, AustriaAudrey Yi-Hui Teh, St George’s, University of London, UK
Copyright © 2016 Sainsbury, Jutras, Vorster, Goulet and Michaud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dominique Michaud, ZG9taW5pcXVlLm1pY2hhdWRAZnNhYS51bGF2YWwuY2E=
 Frank Sainsbury
Frank Sainsbury Philippe V. Jutras
Philippe V. Jutras Juan Vorster3
Juan Vorster3 Marie-Claire Goulet
Marie-Claire Goulet Dominique Michaud
Dominique Michaud