
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 22 December 2015
Sec. Plant Development and EvoDevo
Volume 6 - 2015 | https://doi.org/10.3389/fpls.2015.01156
This article is part of the Research Topic Root Apex Zones: From Growth to Behaviour View all 13 articles
Auxin/Indole-3-Acetic Acid (Aux/IAA) genes are early auxin response genes ecoding short-lived transcriptional repressors, which regulate auxin signaling in plants by interplay with Auxin Response Factors (ARFs). Most of the Aux/IAA proteins contain four different domains, namely Domain I, Domain II, Domain III, and Domain IV. So far all Aux/IAA mutants with auxin-related phenotypes identified in both Arabidopsis and rice (Oryza sativa) are dominant gain-of-function mutants with mutations in Domain II of the corresponding Aux/IAA proteins, suggest that Aux/IAA proteins in both Arabidopsis and rice are largely functional redundantly, and they may have conserved functions. We report here the functional characterization of a rice Aux/IAA gene, OsIAA9. RT-PCR results showed that expression of OsIAA9 was induced by exogenously applied auxin, suggesting that OsIAA9 is an auxin response gene. Bioinformatic analysis showed that OsIAA9 has a repressor motif in Domain I, a degron in Domain II, and the conserved amino acid signatures for protein-protein interactions in Domain III and Domain IV. By generating transgenic plants expressing GFP-OsIAA9 and examining florescence in the transgenic plants, we found that OsIAA9 is localized in the nucleus. When transfected into protoplasts isolated from rosette leaves of Arabidopsis, OsIAA9 repressed reporter gene expression, and the repression was partially released by exogenously IAA. These results suggest that OsIAA9 is a canonical Aux/IAA protein. Protoplast transfection assays showed that OsIAA9 interacted ARF5, but not ARF6, 7, 8 and 19. Transgenic Arabidopsis plants expressing OsIAA9 have increased number of lateral roots, and reduced gravitropic response. Further analysis showed that OsIAA9 transgenic Arabidopsis plants accumulated fewer granules in their root tips and the distribution of granules was also affected. Taken together, our study showed that OsIAA9 is a transcriptional repressor, and it regulates gravitropic response when expressed in Arabidopsis by regulating granules accumulation and distribution in root tips.
In Arabidopsis, Aux/IAA genes are one of the early auxin response gene families (Hagen and Guilfoyle, 2002). Aux/IAA proteins are short-lived transcription repressors that involve in the regulation of auxin signaling (Guilfoyle and Hagen, 2007; Guilfoyle, 2015). Most of the Aux/IAA proteins contain four conserved domains, namely, Domain I, Domain II, Domain III, and Domain IV. Domain I is an active repression domain containing a conserved LxLxL motif. Domain II contains a conserved degron and is responsible for the stability of Aux/IAA proteins. Domains III and IV are similar to the conserved C-terminal dimerization domain of Auxin Response Factors (ARFs), and are required for homo dimerization among Aux/IAA proteins, and hetero dimerization between Aux/IAA proteins and ARFs (Ulmasov et al., 1997, 1999; Ramos et al., 2001; Tiwari et al., 2001, 2003, 2004; Dreher et al., 2006; Nanao et al., 2014).
Aux/IAA proteins regulate auxin signaling by interplay with ARFs (Guilfoyle and Hagen, 2007; Guilfoyle, 2015). When cellular auxin levels are low, Aux/IAA proteins are stable, and they can form dimers with ARF activators that bound on the TGTCTC Auxin Response Elements (AuxREs) in the promoter regions of the auxin response genes, thus inhibiting the expression of auxin response genes. Elevated cellular auxin levels will result in the activation of auxin receptor TIR1 (Dharmasiri et al., 2005; Kepinski and Leyser, 2005), leading to the ubiquitylation and then degradation of Aux/IAA proteins via the 26S proteasome (Tan et al., 2007), thus allowing the ARF activators to activate auxin response genes (Ulmasov et al., 1999; Tiwari et al., 2001, 2004; Guilfoyle and Hagen, 2007).
So far all the aux/iaa mutants identified in Arabidopsis, including single T-DNA insertion mutants, double, and triple mutants of closely related Aux/IAA genes, showed no visible developmental defects (Overvoorde et al., 2005), suggesting that Aux/IAA genes are largely redundant functionally. On the other hand, several different types of phenotypes were observed in the dominant aux/iaa mutants. for example, the iaa18-1 mutant has aberrant cotyledon placement in embryos (Ploense et al., 2009), the axr2-1/iaa7 mutant has agravitropic root and shoot growth, and a short hypocotyl and stem (Nagpal et al., 2000), the iaa16-1 mutant has restricted adult plant growth and abolished fertility in homozygous (Rinaldi et al., 2012), the iaa18 and iaa28 mutants are severely defective in lateral root formation (Rogg et al., 2001; Uehara et al., 2008), and the slr-1/iaa14 mutant completely lacks lateral roots (Fukaki et al., 2002). Some of the dominant gain-of-function iaa mutants even have opposite phenotypes. For example, the axr3/iaa17 mutant has defects in root hair development, while shy2/iaa3 mutant has longer root hairs (Knox et al., 2003). The shy2/iaa3 mutant also has other phenotypes including enlarged cotyledons, short hypocotyls, and altered auxin-regulated root development (Tian and Reed, 1999; Tian et al., 2002). These results suggest that stability of Aux/IAA proteins is crucial for their functions in regulating plant growth and development.
Consistent with the fact that Aux/IAA protein are unstable proteins, Arabidopsis transgenic plants expressing wild type Aux/IAA genes from Arabidopsis and grape (Vitis vinifera) are also morphological indistinguishable from wild type plants (Park et al., 2002; Fujita et al., 2012; Kohno et al., 2012). However, auxin-related phenotypes were observed in Arabidopsis transgenic plants expressing mutated Aux/IAA gene with a mutation in Domain II, or Aux/IAA gene lacking Domain II (Park et al., 2002; Fukaki et al., 2005; Sato and Yamamoto, 2008).
Phenotypic changes were observed in knock-down mutants of Aux/IAA genes in tomato (Solanum lycopersicum) (Wang et al., 2005a; Bassa et al., 2012; Deng et al., 2012; Su et al., 2014), and expressing wild type PtrIAA14.1, a poplar Aux/IAA gene in Arabidopsis resulted in phenotypic changes (Liu et al., 2015), suggesting the functions of Aux/IAA from at least some plant species may different from that in Arabidopsis.
In rice (Oryza sativa), however, all the identified Aux/IAA mutants with auxin-related phenotypes are dominant gain-of-function mutants with mutations in the Domain II of corresponding Aux/IAA proteins (Jun et al., 2011; Zhu et al., 2011; Kitomi et al., 2012), and auxin-related phenotypes were also observed in transgenic rice plants expressing mutated or dominant mutation-type rice Aux/IAA genes (Nakamura et al., 2006; Song and Xu, 2013), suggesting that Aux/IAA proteins in rice may regulate auxin signaling in a way similar to those in Arabidopsis.
We report here the characterization of OsIAA9, a rice Aux/IAA gene. Expression of OsIAA9 was greatly induced by exogenously supplied IAA. Bioinformatics and protoplast transfection assay results showed that OsIAA9 is a canonical Aux/IAA protein. When expressed in Arabidopsis in a wild type form, however, OsIAA9 affected lateral root formation and root gravitropic response.
The Japonica rice (Oryza sativa) variety Nipponbare was used for Auxin treatment and OsIAA9 gene cloning. The Arabidopsis thaliana (Arabidopsis) ecotype Columbia-0 (Col-0) was used for plant transformation and protoplasts isolation. DR5:GUS transgenic plants were in Col background (Wang et al., 2005b).
For auxin treatment and RNA isolation from rice seedlings, rice seeds were germinated and grown on water for 10 d, and treated with 10 μM IAA for 4 h before RNA was isolated. For plant transformation and protoplasts isolation, Arabidopsis seeds were sown directly into soil pots and kept in a growth room. For phenotypic analysis, Arabidopsis seeds were sterilized and sown on 0.8% (w/v) phytoagar solidified ½ MS (Murashige & Skoog) (Murashige and Skoog, 1962) plates with vitamins (PlantMedia) and 1% (w/v) sucrose, unless indicated otherwise. The plates were kept at 4°C in darkness for 2 days, and then moved into a growth room. Rice plants was grown at 28°C, and Arabidopsis plants at 20°C, with a 16 h/8 h (light/darkness) photoperiod.
Closely related Arabidopsis and rice Aux/IAA proteins to OsIAA9 were identified by BLAST searching Arabidopsis and rice proteome database1 using the entire amino acid sequence of OsIAA9. Full-length amino acid sequences of OsIAA9 and closely related Arabidopsis and rice Aux/IAA proteins were subjected to phylogenetic analysis using “One Click” mode with default settings on Phylogeny2.
To examine the expression of OsIAA9 in response to auxin, 10-day-old rice seedlings were treated with 10 μM IAA for 4 h in darkness on a shaker at 40 rpm. Samples were frozen in liquid N2 and kept at -80°C for RNA isolation.
Total RNA from rice and Arabidopsis seedlings was isolated by using the procedures described previously (Wang et al., 2014; Guo et al., 2015; Liu et al., 2015). cDNA was synthesized by Oligo(dT)-primed reverse transcription using the EazyScript First-Strand DNA Synthesis Super Mix (TransGen Biotech) according to the manufacturer’s instructions.
RT-PCR was used to examine the expression of OsIAA9, and Arabidopsis gene ACTIN2 (ACT2) or rice gene OsACT2 were used as controls for RT-PCR.
The reporters constructs LexA-Gal4:GUS and Gal4:GUS, and the effector constructs GD, LD-VP, CAT, and ARFs used for protoplast transfection were as described previously (Tiwari et al., 2003; Wang et al., 2005b, 2007; Liu et al., 2015).
To generate HA or GD tagged OsIAA9 constructs for protoplast transfection assays, the full-length open-reading frame (ORF) of OsIAA9 was amplified by RT-PCR using RNA isolated from rice seedlings, and cloned in frame with an N-terminal HA or GD tag into the pUC19 vector under the control of the 35S promoter (Tiwari et al., 2003; Wang et al., 2005b). The 35S:OsIAA9 construct in pUC19 was digested with proper enzymes, and subcloned into the binary vector pPZP211 (Hajdukiewicz et al., 1994) for plant transformation.
To generate GD tagged OsIAA9CTD for protoplast transfection, ORF sequence of OsIAA9CTD (corresponding to the amino acid residues 84–182) was amplified by PCR using 35S:OsIAA9 plasmids as template, and cloned in frame with an N-termianl GD tag into the pUC19 vector under the control of the 35S promoter.
To generate GFP tagged OsIAA9 for subcellular localization analysis of OsIAA9, GD tag in 35S:GD-OsIAA9 construct was replaced with a GFP tag, subcloned into the binary vector pPZP211, and used for plant transformation.
The primers used for gene cloning and gene expression analysis of OsIAA9 are OsIAA9-F, 5′-CAACATATGGAGCTGGAGCTTGGGCT-3′, OsIAA9-R, 5′- CAACTTAAGTTAACCCAGTATCTTCAGGC -3′, and OsIAA9CTD-F, 5′-CAACATATGTCGGCGCGGCGGGCGT-3′. The primers used to amplify ACT2 and OsACT2 were described previously (Guo et al., 2015).
About 5-week-old Arabidopsis plants with several mature flowers on the main inflorescence were transformed using the floral dip method (Clough and Bent, 1998). T1 seeds were sterilized and grown on 1/2 MS plates containing 50 μg/ml kanamycin to select transgenic plants. At least five transgenic lines with similar phenotypes were obtained. Phenotypes of transgenic plants were examined in the T1 generation, and confirmed in following several generations. Represent homozygous T3 or T4 transgenic plants were used for further analysis.
The procedures for plasmids preparation, protoplast isolation, transfection, and GUS activity assay have been described previously (Wang et al., 2005b, 2007, 2008, 2014, 2015; Zhou et al., 2014; Guo et al., 2015; Liu et al., 2015). Briefly, plasmids of the reporter and effector genes were prepared using the GoldHi EndoFree Plasmid Maxi Kit (Kangwei), and co-trasfected into protoplasts isolated from rosette leaves of ∼4-week-old Col wild type Arabidopsis plants. The transfected protoplasts were incubated under darkness at room temperature for 20–22 h before GUS activities were measured using a SynergyTM HT microplate reader (BioTEK).
Gravitropic response was measured as described by Li et al. (2011). Briefly, sterilized seeds were grown vertically on 1/5 MS plates for 4 days. The plates were photographed and the plates were then turned 900. The plates were photographed again 1 day later. Angles of the angle between gravity and the root were measured using NIH Image J3.
Starch granule was stained by following the procedures described by Li et al. (2011).
Photographs of Arabidopsis seedlings were taken under a Motic K dissection microscope equipped with an EOS 1100D camera. Root length was then measured using NIH Image J. GFP florescence of OsIAA9-GFP transgenic Arabidopsis seedlings was examined under an Olympus FV1000 confocal microscope.
Available experimental evidences suggest that functional mechanism of Aux/IAA may be conserved in rice and Arabidopsis. To test if this is the case, we decided to identify a canonical rice Aux/IAA gene, and study its functions in the regulation of auxin signaling and plant growth and development in Arabidopsis.
OsIAA9 was chosen because it is one of the Aux/IAA genes whose expression was highly induced by 2,4-D, a synthesized auxin (Jain et al., 2006). As shown in Figure 1A, expression of OsIAA9 was also highly induced by IAA, a natural occurred auxin. By using the entire amino acid sequence of OsIAA9 to BLAST rice and Arabidopsis protein database4, we identified and selected several Aux/IAA proteins that have relative higher amino acid sequence similarity to OsIAA9. Phylogenetic analysis showed that OsIAA9 and OsIAA20 are paralogs. The most closely related Arabidopsis Aux/IAA to OsIAA9 is IAA31 (Figure 1B), an Aux/IAA protein with mutated Domain II (Figure 1C). These results are largely consistent with that reported by Jain et al. (2006).
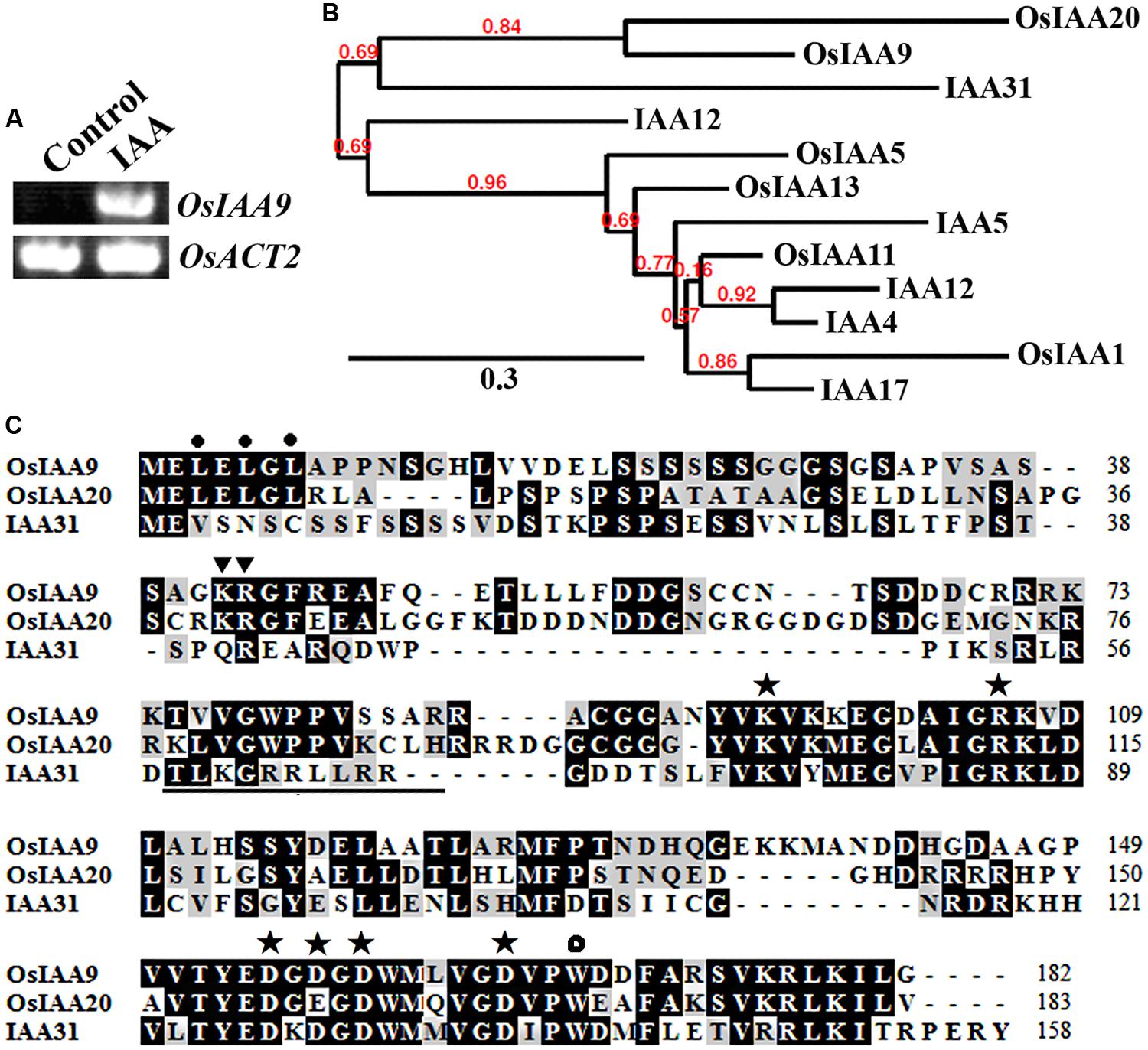
FIGURE 1. OsIAA9 is a canonical Aux/IAA protein. (A) Expression of OsIAA9 in rice seedlings in response to IAA treatment. RNA was isolated from IAA treated rice seedlings and RT-PCR was used to examine the expression of OsIAA9. Expression of OsACT2 was used as a control. (B) Phylogenetic analysis of OsIAA9 and some Arabidopsis and rice Aux/IAA proteins that have relative higher amino acid similarities with OsIAA9. The entire amino acid sequences were used to generate phylogenetic tree on Phylogeny (http://www.phylogeny.fr) by using the “One Click” mode with default settings. Branch support values are indicated above the branches. (C) Amino acid sequence alignment of OsIAA9, OsIAA20 and IAA31. Domain II is underlined. The KR residues between Domain I and II that are crucial for protein degradation are indicated by triangles, the L residues in the LxLxL repression motif are indicated by closed circles, the conserved residues in Domain III and IV that are crucial for protein–protein interaction are indicated by asterisks, and the conserved W residue in OsIAA23 that is crucial for protein–protein interaction is indicated by open circle.
Previously experiment showed that canonical Aux/IAA proteins contain an LxLxL repressor motif in Domain I, a GWPPV degron core sequence in Domain II, conserved KR residues between Domain I and Domain II that are crucial for the degradation of Arabidopsis Aux/IAA proteins, some conserved amino acid residues in Domain III and Domain IV that are require for protein–protein interaction among Aux/IAA proteins or between Aux/IAA proteins and ARFs (Tiwari et al., 2001, 2003, 2004; Dreher et al., 2006; Nanao et al., 2014). As shown in Figure 1C, OsIAA9 has all the features for a canonical Aux/IAA protein. In addition, OsIAA9 also has the conserved W residue found in OsIAAs and OsARFs that is crucial for protein-protein interaction (Ni et al., 2014).
In Arabidopsis, canonical Aux/IAA proteins are short-lived proteins whose stability is affected by auxin, and they function as transcription repressors. To examine if OsIAA9 is a transcription repressor and its stability is affected by auxin, we first examine it subcellular localization by examining transgenic Arabidopsis plants expressing GFP-OsIAA9 under the control of the 35S promoter. As shown in Figure 2, OsIAA9 is predominantly localized in nucleus.
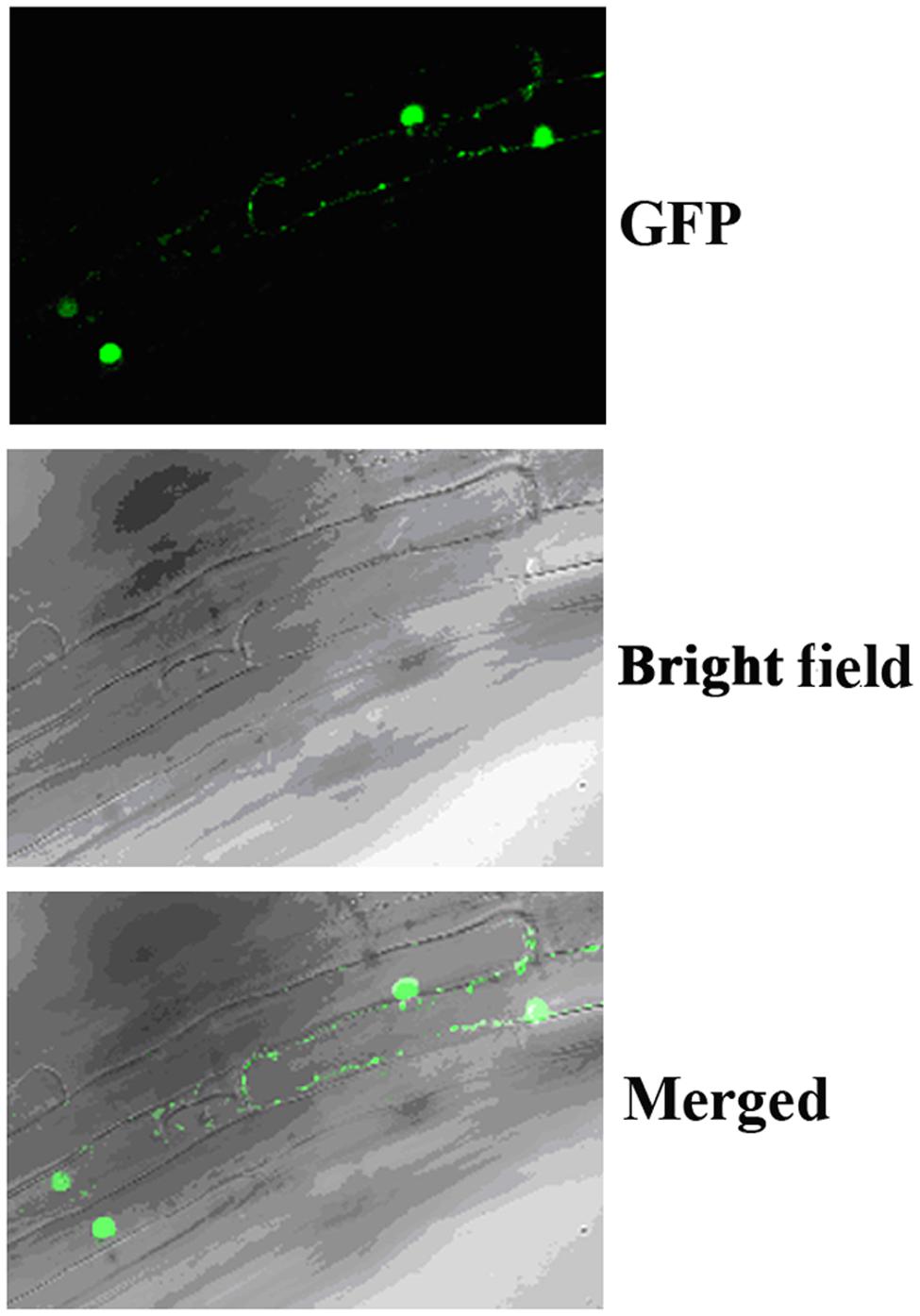
FIGURE 2. Subcellular localization of OsIAA9. Root tips of transgenic Arabidopsis seedlings expression GFP-OsIAA9 under the control of the 35S promoter were examined under a florescence microscope. (Upper) GFP channel, (middle) bright field image, (lower) merged.
We then examined if OsIAA9 functions as transcriptional repressor by using protoplast transfection assays. Plasmids of effector gene GD-OsIAA9 or control gene GD, activator gene LD-VP, and the reporter gene LexA-GAL4:GUS were contransfected into protoplasts, and GUS activities were measured after the transfected protoplasts were incubated in the presence and absent of 1.0 μM IAA. As shown in Figure 3A, in the absence of IAA, co-transfection of control gene GD and activator gene LD-VP activated the reporter gene, while co-transfection of effector gene GD-OsIAA9 and activator gene LD-VP resulted in repression of the reporter gene. In the presence of IAA, the repression on the expression of the reporter gene by co-transfected OsIAA9 gene was partially released (Figure 3A), indicating that OsIAA9 is a transcription repressor, and it is unstable in the presence of auxin. When transfected into protoplasts with an integrated auxin response reporter gene DR5:GUS, OsIAA9 repressed the expression of the reporter gene (Figure 3B), suggesting that OsIAA9 regulates auxin response gene expression.
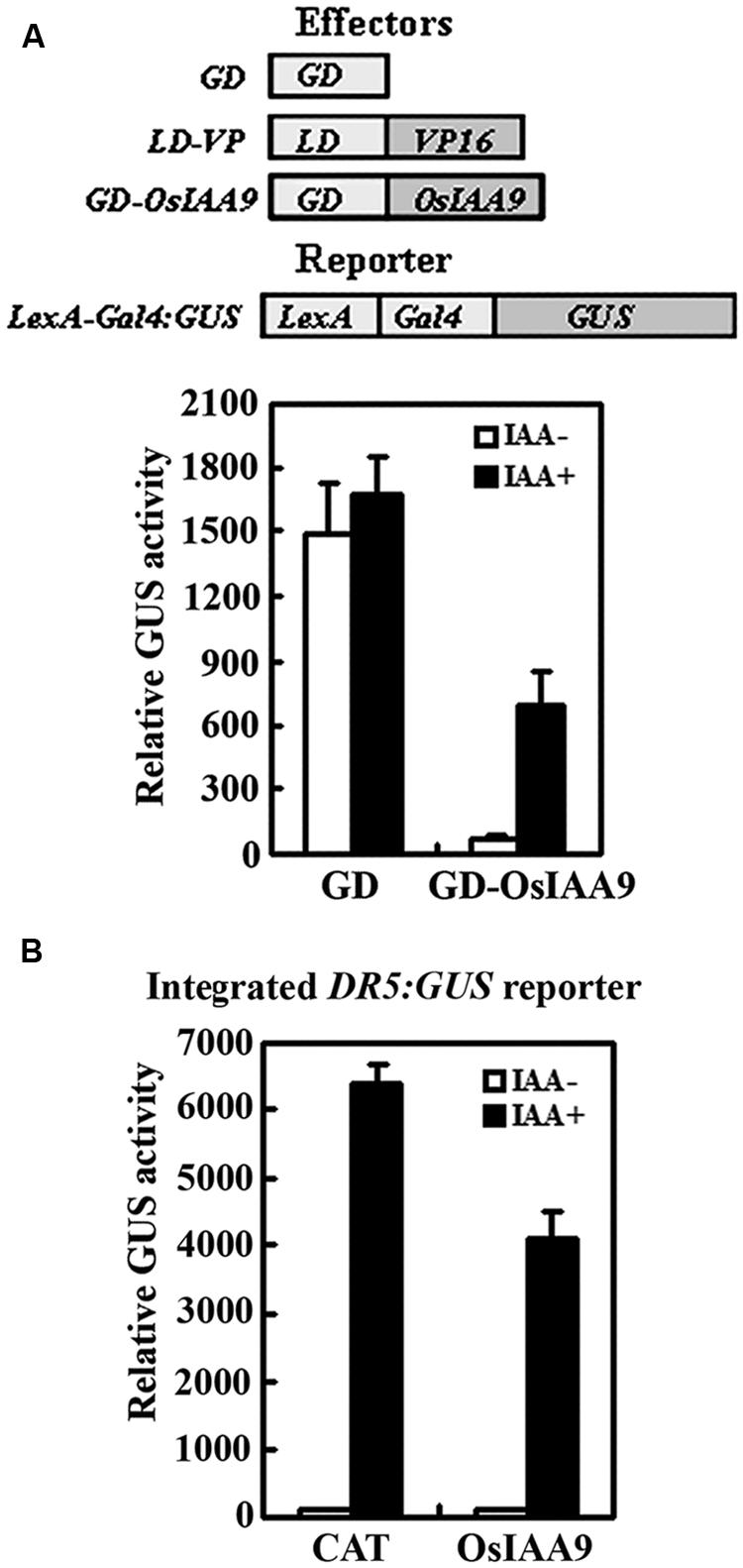
FIGURE 3. OsIAA9 is a transcriptional repressor and its stability is affected by auxin. Repression of LexA-Gal4:GUS reporter gene (A), and the integrated DR5:GUS reporter gene (B) by OsIAA9. Effectors and reporters (drawn on the top of A) or effectors plasmids alone were transfected into protoplasts isolated from Col wild type, or DR5:GUS transgenic plants, and incubated in the presence and absence of 1 μM IAA for 20–22 h, then GUS activity was assayed. The 35S:CAT (chloramphenicol acetyltransferase) plasmids were used as a control. Data represent the mean ± SD of three replicates.
In Arabidopsis, Aux/IAA proteins regulate auxin signaling through interacting with ARF activators. So far only five ARF activators in Arabidopsis have been shown to be transcriptional activators (Wang et al., 2005b). Having shown that OsIAA9 is a transcriptional repressor and it regulates auxin reporter gene expression (Figure 3), we wanted to further examine if OsIAA9 interacts with any of the ARF activators by using protoplast transfection assays.
Because Aux/IAA proteins have been shown to be active transcription repressor, and Domain III and IV are the domains that required for the interaction among Aux/IAA proteins and between Aux/IAA protein and ARFs, we made a GD-OsIAA9CTD construct by fusing OsIAA9 C-terminal Domain (Domain III and IV) with GD, and cotransfected it with ARF activator effector genes and the reporter gene Gal4:GUS into protoplasts. GD gene was cotransfected as a control. GUS activity was measured after incubation. As shown in Figure 4, cotransfection of GD gene with all the ARF activator genes does not have any effects on the expression of the reporter gene, while cotransfection of GD-OsIAA9CTD gene with ARF5 gene, but not other ARF activator genes activated Gal4:GUS reporter, suggesting that OsIAA9 specifically interacts with ARF5.
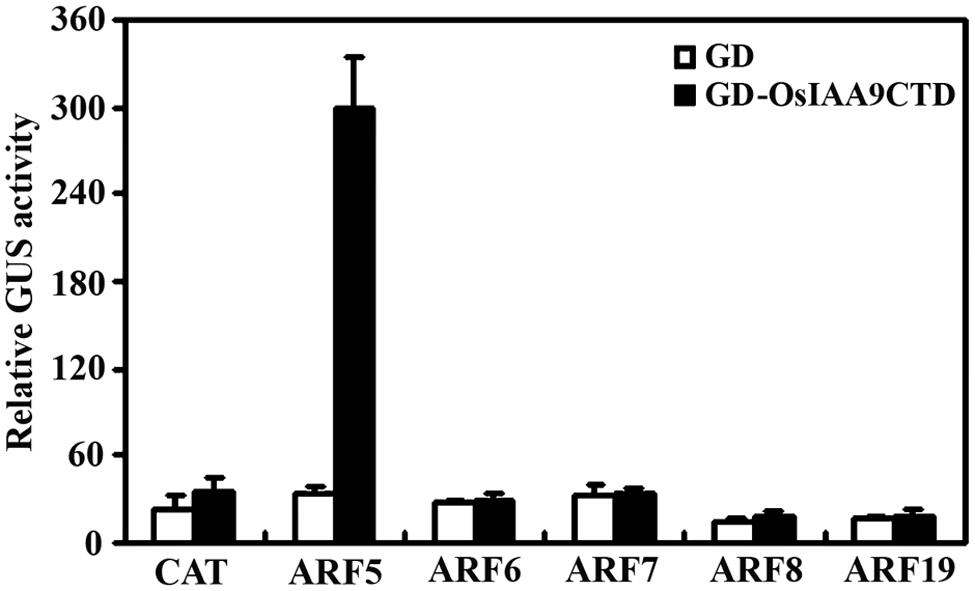
FIGURE 4. OsIAA9 interacts with ARF5 in plant cells. Plasmids of effectors GD-OsIAA9CTD and ARFs, and reporter Gal4:GUS were co-transfected into protoplasts and incubated for 20–22 h, then GUS activity was assayed. Data represent the mean ± SD of three replicates.
The results described above indicate that OsIAA9 is a canonical Aux/IAA protein. Arabidopsis transgenic plants overexpressing wild type Arabidopsis and grape Aux/IAA genes are morphologically similar to wild type plants (Park et al., 2002; Fujita et al., 2012; Kohno et al., 2012). However, expression of PtrIAA14.1, a canonical Aux/IAA protein encoding wild type poplar Aux/IAA gene in Arabidopsis resulted in auxin-related phenotypes including semi-draft with increased number of branches, down-curling leaves, and greatly reduced fertility (Liu et al., 2015). To further explore if OsIAA9 functions similar to canonical Aux/IAA proteins in Arabidopsis, we generated transgenic plant expressing OsIAA9 under the control of the 35S promoter (35S:OsIAA9). As shown in Figure 5A, transgenic Arabidopsis plants have reduced root gravitropic response as indicated by the root orientations. Expression of OsIAA9 in the transgenic plants was confirmed by RT-PCR (Figure 5B). Quantitative analysis showed that root elongation is largely unaffected in the Arabidopsis transgenic plants expressing OsIAA9 (Figure 5C). However, the transgenic plants produced more lateral roots (Figure 5D).
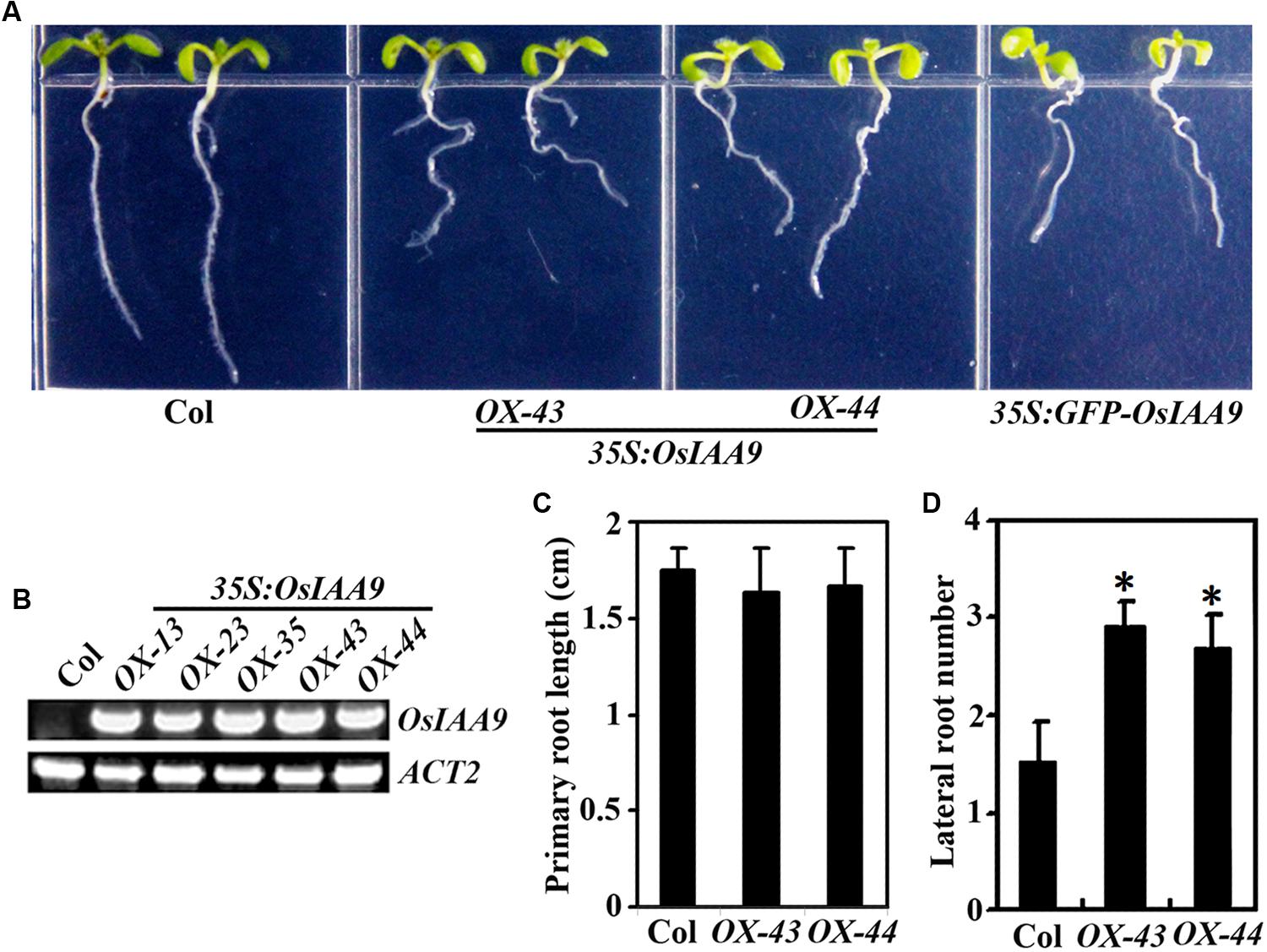
FIGURE 5. Morphologies of Arabidopsis transgenic plant seedlings expressing OsIAA9. (A) Seven-day-old Col wild type and transgenic plant seedlings. (B) Expression of OsIAA9 in transgenic plants. RNA was isolated from OsIAA9 transgenic Arabidopsis seedlings and RT-PCR was used to examine the expression of OsIAA9. Expression of ACT2 was used as a control. (C) Primary root length of 7-day-old wild type and transgenic plants. (D) Lateral root number of 10-day-old wild type and transgenic plants. Data in (C) and (D) represent the mean ± SD of 12–15 seedlings. ∗, significantly different from Col wild type seedlings (p < 0.0001).
Transgenic plants expressing GFP-OsIAA9 also resulted in abnormal root gravitropic response (Figure 5A), indicating that GFP-OsIAA9 protein is functional, thus the plants were used to examine subcellular localization of OsIAA9 (Figure 2).
To further examine the roles of OsIAA9 in regulating root gravitropic response, we tested the gravitropic response of Col wild type and OsIAA9 transgenic Arabidopsis seedlings to changed gravity. Seedlings were grown on vertical plates for 4 days, and pictured to measure the root directions. The plates were then turned 900 to change the gravity, photographs were taken again after 24 h. As shown in Figure 6A, wild type seedlings grew straight down toward the gravity direction before and after the plates were turned, whereas roots of transgenic seedling were in random directions before and after the plates were turned. Quantitative results of the root direction further confirmed our observation (Figure 6B).
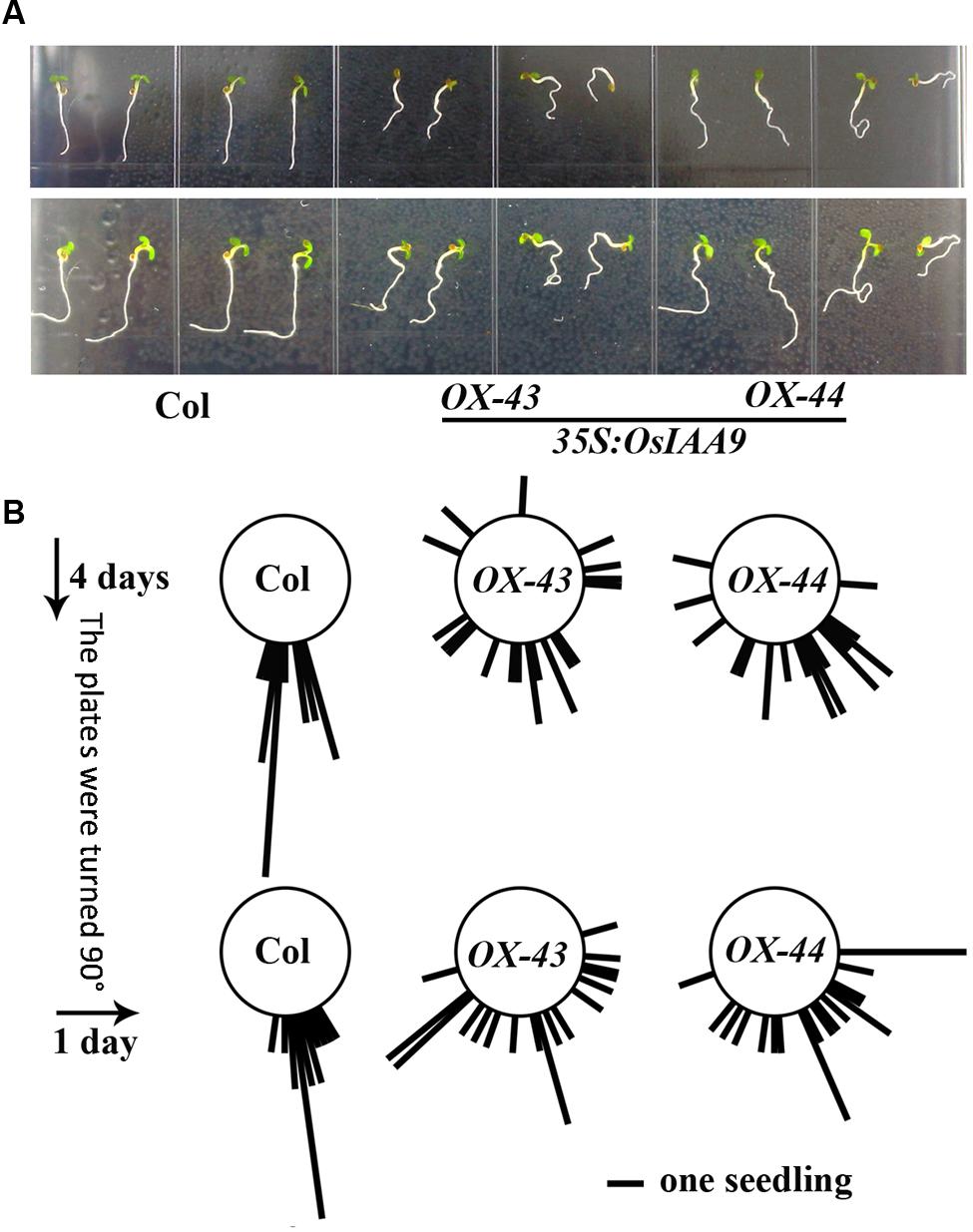
FIGURE 6. Expression of OsIAA9 in Arabidopsis affects root gravitropic response. (A) Response of Col wild type (left) and OsIAA9 transgenic seedlings (middle and right) to a directional change of gravity. Up panel: before the plates were turned, low panel: 1 day after the plates were turned. Seedlings were grown on vertically plates for 4 days, and then the plates were turned 900 and kept for 1 days. (B) Quantification of response of Col wild type and OsIAA9 transgenic plant seedlings to a directional change of gravity. The angle between the gravity vector and the root were measured when the seedlings were grown vertically for 4 days and 1 day after the plates were turn 900. Bar represents one seedling.
Starch granules in root tips play an important role during the sensing of gravity stimulus. Because the root gravitropic response was altered in the OsIAA9 transgenic plants, we suspected that starch granule formation in the transgenic plants may be disrupted. To test if this is the case, we compared starch granule formation in wild type and the transgenic Arabidopsis seedlings by staining the roots with Lugol’s solution, which contains iodine that can react with starch granules to generate a visible bright blue color. We found that in wild-type root tips, strong staining was observed in several different layers of columella cells, while in root tips of the OsIAA9 transgenic Arabidopsis seedlings, only slight staining was observed, and there is no clear stained cell layers could be observed (Figure 7).
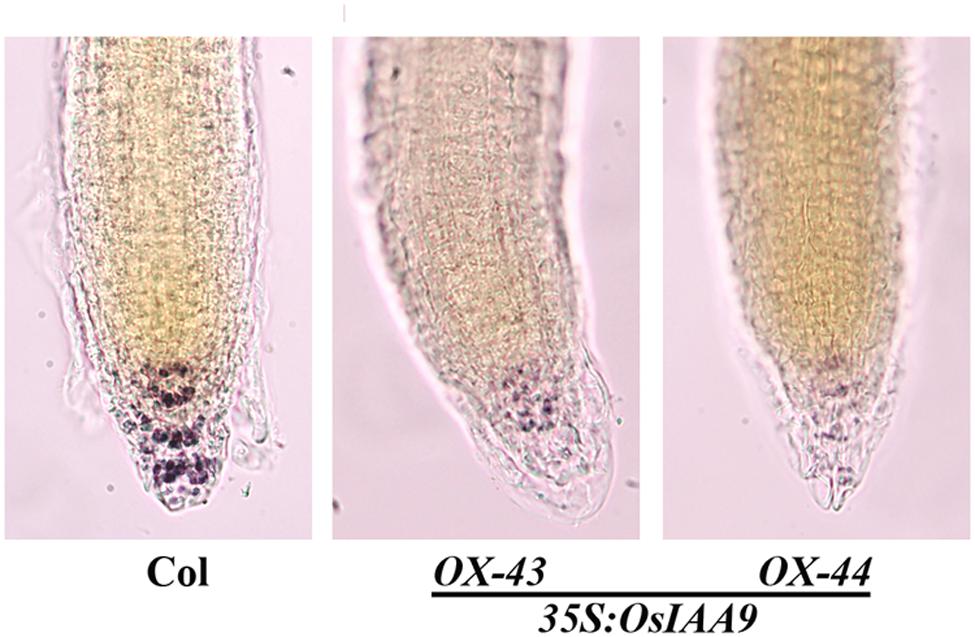
FIGURE 7. Starch Granules formation in root tips of OsIAA9 transgenic plant seedlings. Four-day-old seedlings of Col wild type (left) and OsIAA9 transgenic plants (middle, right) grown on vertically oriented plates were fixed with FAA overnight at 4°c, washed once with 50% ethanol, stained with Lugol’s solution for 1 min, and chloral hydrate solution for 2 min before photographs were taken immediately.
Interplay of Aux/IAA proteins and ARFs regulates auxin signaling in Arabidopsis (Hagen and Guilfoyle, 2002). Characterization of gain-of-function mutants in Arabidopsis revolved that Aux/IAA genes regulated many aspects of plant growth and development (Nagpal et al., 2000; Rogg et al., 2001; Fukaki et al., 2002; Uehara et al., 2008; Ploense et al., 2009; Rinaldi et al., 2012). So far all experimental evidences suggest that Aux/IAA proteins in Arabidopsis and rice may regulate auxin signaling and plant growth and development in a similar manner (Jain et al., 2006; Nakamura et al., 2006; Jun et al., 2011; Zhu et al., 2011; Kitomi et al., 2012; Song and Xu, 2013). Our results in this report show that OsIAA9 is a canonical Aux/IAA protein, it regulates auxin signaling in a way similar to that of the canonical Aux/IAA proteins in Arabidopsis. However, unlike the Arabidopsis canonical Aux/IAA proteins have been studied so far, OsIAA9 regulates lateral root formation and root gravitropic response when expressed in Arabidopsis in an unmutated form.
All canonical Aux/IAA proteins contain four conserved domains. An LxLxL motif-containing domain, a degron-containing domain, and two domains required for protein–protein interaction (Ulmasov et al., 1997, 1999; Ramos et al., 2001; Tiwari et al., 2001, 2003, 2004; Dreher et al., 2006; Jain et al., 2006; Nanao et al., 2014).
Bioinformatics analysis showed that OsIAA9 is closely related to IAA31, a Domain II mutated type Arabidopsis Aux/IAA protein (Figure 1B). However, it contains all the features of a canonical Aux/IAA protein has, including the conserved KR residues crucial for 26 proteasome degradation of Aux/IAA proteins (Dreher et al., 2006), and the conserved W residue crucial for protein–protein interactions of OsIAAs and OsARFs (Ni et al., 2014) (Figure 1B). In consist with the canonical Aux/IAA protein features it has, OsIAA9 functions as a transcription repressor, its stability is affected by Auxin, it regulates auxin response repoter gene expression (Figure 3), and it interacted with ARF5 in transfected protoplasts (Figure 4). These results suggest that OsIAA9 is a canonical Aux/IAA protein, it regulates auxin signaling in a way similar to all other canonical Aux/IAA proteins.
So far all the aux/iaa mutants identified in Arabidopsis and rice with phenotypes are gain-of-function mutants with mutations occurred within Domain II of corresponding Aux/IAA proteins (Tian and Reed, 1999; Nagpal et al., 2000; Rogg et al., 2001; Fukaki et al., 2002; Tian et al., 2002; Knox et al., 2003; Uehara et al., 2008; Ploense et al., 2009; Jun et al., 2011; Zhu et al., 2011; Kitomi et al., 2012; Rinaldi et al., 2012). On the other hand, expression of mutated type, but not wild type Arabidopsis Aux/IAA genes in Arabidopsis resulted in auxin-related phenotypes (Park et al., 2002; Fukaki et al., 2005; Sato and Yamamoto, 2008). Expression of mutated or dominant mutation-type rice Aux/IAA genes in rice also resulted in auxin-related phenotypes (Nakamura et al., 2006; Song and Xu, 2013), suggest that stability of Aux/IAA proteins are crucial for their functions in regulating plant growth and development, and that function mechanisms of Arabidopsis and rice Aux/IAA proteins may be conserved.
Similar to other canonical Aux/IAA proteins, OsIAA9 functions as a transcription repressor, and its stability is affected by auxin as mentioned above. However, transgenic Arabidopsis plants expressing OsIAA9 showed several auxin-related phenotypes, including increased lateral root formation and reduced root gravitropic response (Figures 5 and 6). Previously we have showed that expression of wild type PtrIAA14.1, a canonical Aux/IAA gene from poplar, resulted in phenotypes changes in Arabidopsis (Liu et al., 2015), but the phenotypes are different from that of Arabidopsis transgenic plants expressing OsIAA9. On the other hand, among all the five ARF activators, both PtrIAA14.1 and OsIAA9 interacted only with ARF5 in transfected protoplasts (Liu et al., 2015; Figure 4), indicating that interaction between ARF5 and OsIAA9 may contribute little, if any to the resulted phenotypes in the transgenic plants.
In summary, we showed that OsIAA9 is a canonical Aux/IAA protein, and it causes auxin-related phenotypes including lateral root formation and gravitropic response when expressed in Arabidopsis.
SW conceived the study. SL and SW designed the experiments. SL, QL, SL, NMP, and HT performed the experiments. SL and SW analyzed the data. SW drafted the manuscript. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the Key Laboratory of Molecular Epigenetics of MOE (130014542), the Department of Human Resources and Social Security of Jilin Province (http://hrss.ji.gov.cn) and the Programme for Introducing Talents to Universities (B07017). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Bassa, C., Mila, I., Bouzayen, M., and Audran-Delalande, C. (2012). Phenotypes associated with down-regulation of Sl-IAA27 support functional diversity among Aux/IAA family members in tomato. Plant Cell Physiol. 53, 1583–1595. doi: 10.1093/pcp/pcs101
Clough, S. J., and Bent, A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. doi: 10.1046/j.1365-313x.1998.00343.x
Deng, W., Yang, Y., Ren, Z., Audran-Delalande, C., Mila, I., Wang, X., et al. (2012). The tomato SlIAA15 is involved in trichome formation and axillary shoot development. New Phytol. 194, 379–390. doi: 10.1111/j.1469-8137.2012.04053.x
Dharmasiri, N., Dharmasiri, S., and Estelle, M. (2005). The F-box protein TIR1 is an auxin receptor. Nature 435, 441–445. doi: 10.1038/nature03543
Dreher, K. A., Brown, J., Saw, R. E., and Callis, J. (2006). The Arabidopsis Aux/IAA protein family has diversified in degradation and auxin responsiveness. Plant Cell 18, 699–714. doi: 10.1105/tpc.105.039172
Fukaki, H., Nakao, Y., Okushima, Y., Theologis, A., and Tasaka, M. (2005). Tissue-specific expression of stabilized SOLITARY-ROOT/IAA14 alters lateral root development in Arabidopsis. Plant J. 44, 382–395. doi: 10.1111/j.1365-313X.2005.02537.x
Fukaki, H., Tameda, S., Masuda, H., and Tasaka, M. (2002). Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 29, 153–168. doi: 10.1046/j.0960-7412.2001.01201.x
Fujita, K., Horiuchi, H., Takato, H., Kohno, M., and Suzuki, S. (2012). Auxin-responsive grape Aux/IAA9 regulates transgenic Arabidopsis plant growth. Mol. Biol. Rep. 39, 7823–7829. doi: 10.1007/s11033-012-1625-9
Guilfoyle, T. J. (2015). The PB1 domain in auxin response factor and Aux/IAA proteins: a versatile protein interaction module in the auxin response. Plant Cell 27, 33–43. doi: 10.1105/tpc.114.132753
Guilfoyle, T. J., and Hagen, G. (2007). Auxin response factors. Curr. Opin. Plant Biol. 10, 453–460. doi: 10.1016/j.pbi.2007.08.014
Guo, H., Zhang, W., Tian, H., Zheng, K., Dai, X., Liu, S., et al. (2015). An auxin responsive CLE gene regulates shoot apical meristem development in Arabidopsis. Front. Plant Sci. 6:295. doi: 10.3389/fpls.2015.00295
Hagen, G., and Guilfoyle, T. J. (2002). Auxin-responsive gene expression: genes, promoters, and regulatory factors. Plant Mol. Biol. 49, 373–385. doi: 10.1023/A:1015207114117
Hajdukiewicz, P., Svab, Z., and Maliga, P. (1994). The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25, 989–994. doi: 10.1007/BF00014672
Jain, M., Kaur, N., Garg, R., Thakur, J. K., Tyagi, A. K., and Khurana, J. P. (2006). Structure and expression analysis of early auxin-responsive Aux/IAA gene family in rice (Oryza sativa). Funct. Integr. Genomics 6, 47–59. doi: 10.1007/s10142-005-0142-5
Jun, N., Gaohang, W., Zhenxing, Z., Huanhuan, Z., Yunrong, W., and Ping, W. (2011). OsIAA23-mediated auxin signaling defines postembryonic maintenance of QC in rice. Plant J. 68, 433–442. doi: 10.1111/j.1365-313X.2011.04698.x
Kepinski, S., and Leyser, O. (2005). The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435, 446–451. doi: 10.1038/nature03542
Kitomi, Y., Inahashi, H., Takehisa, H., Sato, Y., and Inukai, Y. (2012). OsIAA13-mediated auxin signaling is involved in lateral root initiation in rice. Plant Sci. 190, 116–122. doi: 10.1016/j.plantsci.2012.04.005
Knox, K., Grierson, C. S., and Leyser, O. (2003). AXR3 and SHY2 interact to regulate root hair development. Development 130, 5769–5777. doi: 10.1242/dev.00659
Kohno, M., Takato, H., Horiuchi, H., Fujita, K., and Suzuki, S. (2012). Auxin-nonresponsive grape Aux/IAA19 is a positive regulator of plant growth. Mol. Biol. Rep. 39, 911–917. doi: 10.1007/s11033-011-0816-0
Li, Y., Dai, X., Cheng, Y., and Zhao, Y. (2011). NPY genes play an essential role in root gravitropic responses in Arabidopsis. Mol. Plant 4, 171–179. doi: 10.1093/mp/ssq052
Liu, S., Hu, Q., Luo, S., Li, Q., Yang, X., Wang, X., et al. (2015). Expression of wild-type PtrIAA14.1, a poplar Aux/IAA gene causes morphological changes in Arabidopsis. Front. Plant Sci. 6:388. doi: 10.3389/fpls.2015.00388
Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x
Nagpal, P., Walker, L. M., Young, J. C., Sonawala, A., Timpte, C., Estelle, M., et al. (2000). AXR2 encodes a member of the Aux/IAA protein family. Plant Physiol. 123, 563–574. doi: 10.1104/pp.123.2.563
Nakamura, A., Umemura, I., Gomi, K., Hasegawa, Y., Kitano, H., Sazuka, T., et al. (2006). Production and characterization of auxin-insensitive rice by overexpression of a mutagenized rice IAA protein. Plant J. 46, 297–306. doi: 10.1111/j.1365-313X.2006.02693.x
Nanao, M. H., Vinos-Poyo, T., Brunoud, G., Thévenon, E., Mazzoleni, M., Mast, D., et al. (2014). Structural basis for oligomerization of auxin transcriptional regulators. Nat. Commun. 5:3617. doi: 10.1038/ncomms4617
Ni, J., Zhu, Z., Wang, G., Shen, Y., Zhang, Y., and Wu, P. (2014). Intragenic suppressor of osiaa23 revealed a conserved tryptophan residue crucial for protein-protein interactions. PLoS ONE 9:85358. doi: 10.1371/journal.pone.0085358
Overvoorde, P. J., Okushima, Y., Alonso, J. M., Chan, A., Chang, C., Ecker, J. R., et al. (2005). Functional genomic analysis of the AUXIN/INDOLE-3-ACETIC ACID gene family members in Arabidopsis thaliana. Plant Cell 17, 3282–3300. doi: 10.1105/tpc.105.036723
Park, J. Y., Kim, H. J., and Kim, J. (2002). Mutation in domain II of IAA1 confers diverse auxin-related phenotypes and represses auxin-activated expression of Aux/IAA genes in steroid regulator-inducible system. Plant J. 32, 669–683. doi: 10.1046/j.1365-313X.2002.01459.x
Ploense, S. E., Wu, M. F., Nagpal, P., and Reed, J. W. (2009). A gain-of-function mutation in IAA18 alters Arabidopsis embryonic apical patterning. Development 136, 1509–1517. doi: 10.1242/dev.025932
Ramos, J. A., Zenser, N., Leyser, O., and Callis, J. (2001). Rapid degradation of auxin/indoleacetic acid proteins requires conserved amino acids of domain II and is proteasome dependent. Plant Cell 13, 2349–2360. doi: 10.2307/3871512
Rinaldi, M. A., Liu, J., Enders, T. A., Bartel, B., and Strader, L. C. (2012). A gain-of-function mutation in IAA16 confers reduced responses to auxin and abscisic acid and impedes plant growth and fertility. Plant Mol. Biol. 79, 359–373. doi: 10.1007/s11103-012-9917-y
Rogg, L. E., Lasswell, J., and Bartel, B. (2001). A gain-of-function mutation in IAA28 suppresses lateral root development. Plant Cell 13, 465–480. doi: 10.1105/tpc.13.3.465
Sato, A., and Yamamoto, K. T. (2008). Overexpression of the non-canonical Aux/IAA genes causes auxin-related aberrant phenotypes in Arabidopsis. Physiol. Plant. 133, 397–405. doi: 10.1111/j.1399-3054.2008.01055.x
Song, Y., and Xu, Z. F. (2013). Ectopic overexpression of an AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) Gene OsIAA4 in rice induces morphological changes and reduces responsiveness to auxin. Int. J. Mol. Sci. 14, 13645–13656. doi: 10.3390/ijms140713645
Su, L., Bassa, C., Audran, C., Mila, I., Cheniclet, C., Chevalier, C., et al. (2014). The auxin Sl-IAA17 transcriptional repressor controls fruit size via the regulation of endoreduplication-related cell expansion. Plant Cell Physiol. 55, 1969–1976. doi: 10.1093/pcp/pcu124
Tan, X., Calderon-Villalobos, L. I., Sharon, M., Zheng, C., Robinson, C. V., Estelle, M., et al. (2007). Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446, 640–645. doi: 10.1038/nature05731
Tian, Q., and Reed, J. W. (1999). Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 126, 711–721.
Tian, Q., Uhlir, N. J., and Reed, J. W. (2002). Arabidopsis SHY2/IAA3 inhibits auxin-regulated gene expression. Plant Cell 14, 301–319. doi: 10.1105/tpc.010283
Tiwari, S. B., Hagen, G., and Guilfoyle, T. J. (2003). The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell 15, 533–543. doi: 10.1105/tpc.008417
Tiwari, S. B., Hagen, G., and Guilfoyle, T. J. (2004). Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 16, 533–543. doi: 10.1105/tpc.017384
Tiwari, S. B., Wang, X.-J., Hagen, G., and Guilfoyle, T. J. (2001). Aux/IAA proteins are active repressors and their stability and activity are modulated by auxin. Plant Cell 13, 2809–2822. doi: 10.1105/tpc.13.12.2809
Uehara, T., Okushima, Y., Mimura, T., Tasaka, M., and Fukaki, H. (2008). Domain II mutations in CRANE/IAA18 suppress lateral root formation and affect shoot development in Arabidopsis thaliana. Plant Cell Physiol. 49, 1025–1038. doi: 10.1093/pcp/pcn079
Ulmasov, T., Hagen, G., and Guilfoyle, T. J. (1997). ARF1, a transcription factor that binds auxin response elements. Science 276, 1865–1868. doi: 10.1126/science.276.5320.1865
Ulmasov, T., Hagen, G., and Guilfoyle, T. J. (1999). Activation and repression of transcription by auxin response factors. Proc. Natl. Acad. Sci. U.S.A. 96, 5844–5849. doi: 10.1073/pnas.96.10.5844
Wang, H., Jones, B., Li, Z., Frasse, P., Delalande, C., Regad, F., et al. (2005a). The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 17, 2676–2692. doi: 10.1105/tpc.105.033415
Wang, S., Tiwari, S. B., Hagen, G., and Guilfoyle, T. J. (2005b). AUXIN RESPONSE FACTOR7 restores the expression of auxin-responsive genes in mutant Arabidopsis leaf mesophyll protoplasts. Plant Cell 17, 1979–1993. doi: 10.1105/tpc.105.031096
Wang, S., Chang, Y., Guo, J., and Chen, J. G. (2007). Arabidopsis ovate family protein 1 is a transcriptional repressor that suppresses cell elongation. Plant J. 50, 858–872. doi: 10.1111/j.1365-313X.2007.03096.x
Wang, S., Hubbard, L., Chang, Y., Guo, J., Schiefelbein, J., and Chen, J. G. (2008). Comprehensive analysis of single-repeat R3 MYB proteins in epidermal cell patterning and their transcriptional regulation in Arabidopsis. BMC Plant Biol. 8:81. doi: 10.1186/1471-2229-8-81
Wang, S., Li, E., Porth, I., Chen, J. G., Mansfield, S. D., and Douglas, C. J. (2014). Regulation of secondary cell wall biosynthesis by poplar R2R3 MYB transcription factor PtrMYB152 in Arabidopsis. Sci. Rep. 4:5054. doi: 10.1038/srep05054
Wang, X., Wang, X., Hu, Q., Dai, X., Tian, H., Zheng, K., et al. (2015). Characterization of an activation-tagged mutant uncovers a role of GLABRA2 in anthocyanin biosynthesis in Arabidopsis. Plant J. 83, 300–311. doi: 10.1111/tpj.12887
Zhu, Z. X., Liu, Y., Liu, S. J., Mao, C. Z., Wu, Y. R., and Wu, P. (2011). A gain-of-function mutation in OsIAA11 affects lateral root development in rice. Mol. Plant 5, 154–161. doi: 10.1093/mp/ssr074
Keywords: OsIAA9, auxin signaling, gravitropism, lateral root formation, Arabidopsis thaliana, Oryza sativa
Citation: Luo S, Li Q, Liu S, Pinas NM, Tian H and Wang S (2015) Constitutive Expression of OsIAA9 Affects Starch Granules Accumulation and Root Gravitropic Response in Arabidopsis. Front. Plant Sci. 6:1156. doi: 10.3389/fpls.2015.01156
Received: 08 September 2015; Accepted: 04 December 2015;
Published: 22 December 2015.
Edited by:
Frantisek Baluska, University of Bonn, GermanyReviewed by:
Xuelin Wu, Harvey Mudd College, USACopyright © 2015 Luo, Li, Liu, Pinas, Tian and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shucai Wang, d2FuZ3NjNTUwQG5lbnUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.