
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci., 14 July 2015
Sec. Plant Physiology
Volume 6 - 2015 | https://doi.org/10.3389/fpls.2015.00523
This article is part of the Research TopicPlant cell wall integrity in plant developmentView all 12 articles
Plant cells do not, in general, migrate. They maintain a fixed position relative to their neighbors, intimately linked through growth and differentiation. The mediator of this connection, the pectin-rich middle lamella, is deposited during cell division and maintained throughout the cell’s life to protect tissue integrity. The maintenance of adhesion requires cell wall modification and is dependent on the actin cytoskeleton. There are developmental processes that require cell separation, such as organ abscission, dehiscence, and ripening. In these instances, the pectin-rich middle lamella must be actively altered to allow cell separation, a process which also requires cell wall modification. In this review, we will focus on the role of pectin and its modification in cell adhesion and separation. Recent insights gained in pectin gel mechanics will be discussed in relation to existing knowledge of pectin chemistry as it relates to cell adhesion. As a whole, we hope to begin defining the physical mechanisms behind a cells’ ability to hang on, and how it lets go.
Most plant cells maintain a fixed position during development, attached to their neighbors by a shared cell wall interface. Since plant development relies on the harmonious combination of cell division, cell expansion and cell differentiation, it is essential that individual cells coordinate their development with that of their neighbors- with precise maintenance of cell adhesion or permission of cell separation when required. Within this review we will paint a picture of the interconnected roles of the cell wall and the cytoskeleton in cell adhesion, and in its release, by summarizing data from across decades and species.
In order to understand how the cell wall mediates cell–cell adhesion, we must first examine its composition and organization, focusing on the primary cell wall. Polysaccharides (mainly cellulose, hemi-cellulose, and pectin) represent about 90% of the cell wall mass with the remaining 10% comprising structural and polysaccharide-modifying proteins (Albersheim et al., 1996). Cell wall polysaccharides are synthesized at the level of the plasma membrane or delivered via the cytoskeleton and the secretory pathway (Moore and Staehelin, 1988; Lerouxel et al., 2006; Toyooka et al., 2009; Kang et al., 2011; Worden et al., 2012; Kim and Brandizzi, 2014). Modifying proteins are also delivered by cytoskeletal routes, and as such these materials and their delivery are key to understanding cell adhesion and separation. Furthermore, we must understand how the cell wall interface between two cells is formed, organized, and maintained.
In Arabidopsis leaves, roughly 50% of the cell wall is pectin and it comprises the matrix in which the cellulosic elements are embedded (Zablackis et al., 1995; Harholt et al., 2010). Pectin polysaccharides are galacturonic acid polymers and are represented by three major types: homogalacturonan (HG), rhamnogalacturonan-I (RG-I), and rhamnogalacturonan-II (RG-II) (Atmodjo et al., 2013). Pectic polysaccharides are synthesized in the golgi and delivered to the cell wall by secretory vesicles moving primarily along the actin cytoskeleton (Toyooka et al., 2009; Kim and Brandizzi, 2014), although there is recent evidence for kinesin-dependent pectin delivery via microtubules (Zhu et al., 2015).
The cell wall is formed during cell division when a cell plate is formed between two new cells, resulting from a massive directed exocytosis, and possible contributions from endocytosis, of HG-pectin-containing vesicles (Dhonukshe et al., 2006; Reichardt et al., 2007; Miart et al., 2014; Drakakaki, 2015). Soon afterward, cellulose synthases arrive, hemicellulose delivery commences, and a new wall is generated for each cell with a pectin-rich area, the middle lamella, between them (Figure 1). Callose is also deposited at the cell plate during cytokinesis, but after cell division ends it is restricted to the plasmodesmata in the primary walls of growing cells (Northcote et al., 1989; Scherp et al., 2001). As such, the pectin-rich middle lamella is the major physical mediator of cell adhesion and separation. For the bulk of this review we will focus on the role of pectin, and its modifiers, in the middle lamella, and on their role in maintaining cell adhesion or permitting cell separation.
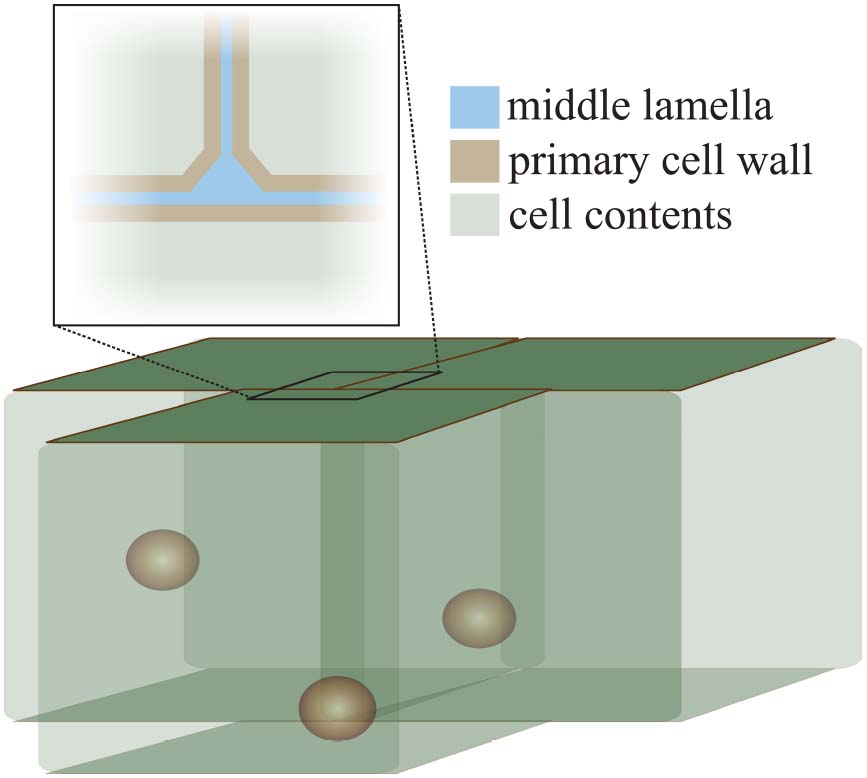
Figure 1. The structure of the cell wall at the cell–cell interface. This diagram illustrates the position of the middle lamella (pectin-rich, blue) and the primary cell walls (pectin-hemicellulose-cellulose, brown) at the junction of three cells. The characteristic “tri-junction” is evident. Spheres inside cells represent cell nuclei for illustration.
The middle lamella between two cells is rich in pectin; its levels and chemical modification are key to regulating adhesion. Modification of pectin affects its ability to gel and act as glue between cells. HG pectin is gelled by calcium-mediated crosslinking. Newly delivered HG-pectin is highly methyl-esterified which makes it more fluid. The activity of a wall-modifying protein, pectin methyl-esterase (PME), removes the methyl groups of HG. De-esterified HG is readily cross-linked by calcium leading to a stiffer material and altering the mechanical properties of the cell wall (Micheli, 2001; Willats et al., 2001; Peaucelle et al., 2011; Braybrook et al., 2012). PME activity can be counteracted by the activity of another family of cell wall proteins, pectin methyl-esterase inhibitors (PMEIs) and as such the balance of these two proteins and their activities have effects on the mechanical properties of the middle lamella.
Homogalacturonan pectin, in its de-esterified or low esterified form, is found in the middle lamella and in the corners of cell junctions (Figure 2; Bush et al., 2001; Parker et al., 2001; McCartney and Knox, 2002; Guillemin et al., 2005). Since de-esterified HG tends to form Ca2+ gels readily it is also important to note that calcium ions are enriched in the middle lamella (Figure 2; Rihouey et al., 1995; Huxham et al., 1999; Bush et al., 2001). The role of HG-Ca2+ gels in cell adhesion is underscored by the effects of treatment with calcium chelators such as EDTA (ethylenediaminetetraacetic acid), HMP (sodium hexametaphosphate) and CDTA (1,2-Diaminocyclohexanetetraacetic) which result in cell separation in various plants (Letham, 1960; Ng et al., 2000; McCartney and Knox, 2002). Arabidopsis pme3 mutants, as well as lines overexpressing the PME inhibitors AtPMEI-1 and AtPMEI-2, display an increased efficiency in protoplast isolation from leaf mesophyll tissue, which indicates that cells were less adhesive and more easily separated from each other (Lionetti et al., 2015).
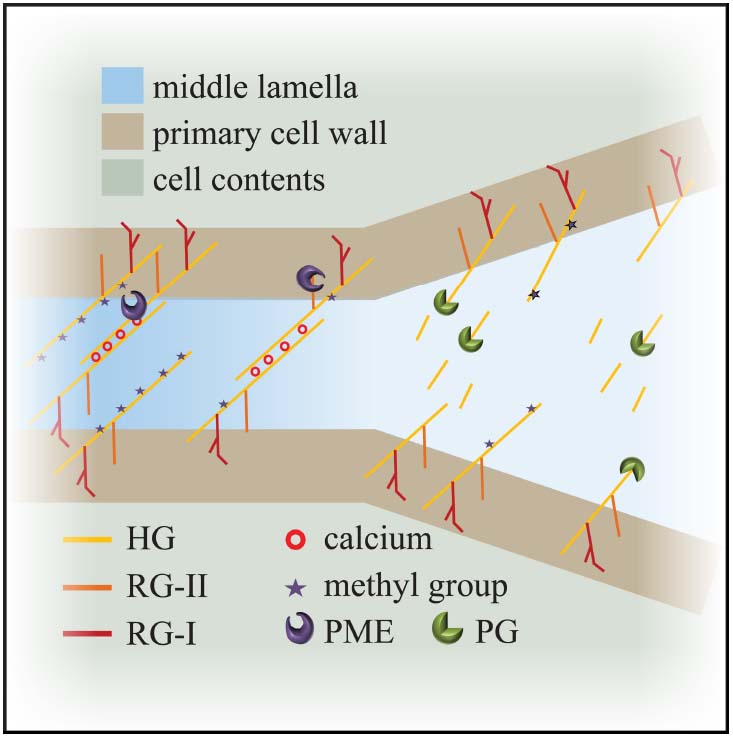
Figure 2. Model for cell adhesion and cell separation. Cross linking of the de-esterified pectin polymers maintains cell adhesion at the level of the middle lamella. Degradation of the de-esterified pectins by enzymes like polygalacturonases weakens connections and leads to cell separation. HG: homogalacturonan; RG: rhamanogalacturonan; PME: pectin methyl-esterase; PG: polygalacturonase.
The effect of PME alteration is not Arabidopsis specific, implying a wide role for PMEs in cell adhesion across species; anti-sense-mediated down-regulation of PME in tomato fruit led to a loss of fruit integrity and a change in the ionic composition of the fruit (Tieman and Handa, 1994). The importance of pectin in adhesion even extends beyond land plants; the calcium cross-linked HG-rich extracellular matrix of the green algae Penium margaritaceum has been shown to be crucial for cell adhesion (Domozych et al., 2014). Together, these data position pectin de-esterification and calcium-mediated gelling as a key positive regulator of cell wall adhesion.
Given the role of HG methyl-esterification in pectin gelling, it follows that the methyl transferases which act during pectin biogenesis are key for adhesion as well. Localized in the golgi, they transfer methyl groups onto newly synthesized HG-pectin. Mutations in putative methyl-transferases in Arabidopsis have severe effects on growth and cell adhesion. The qua2 mutant shows a 50% reduction in HG and severe cell adhesion defects (Mouille et al., 2007). The qua2-allelic tumorous shoot development (tsd2) mutant shows cell adhesion defects in the shoot apex, leaves and hypocotyl (Frank et al., 2002; Krupková et al., 2007). Interestingly, neither qua2 nor tsd2 show a difference from wild-type in their relative pectin esterification levels, evidence which indicates that while relative amounts of esterification may not be important absolute levels may be. Additionally, mutants in, or over expression of, the closely related methyl-transferase QUA3 show no change in cell adhesion (Miao et al., 2011), indicating that the roles of different methyl-transferases in cell adhesion are likely highly specific, or alternatively highly redundant, in a QUA family containing 29 genes in Arabidopsis (Atmodjo et al., 2013).
Supporting a hypothesis for a pectin-level-effect on cell adhesion (and resultant effect on the de-methyl-esterified pectin level), several glycosyl transferase mutants display cell adhesion defects; glycosyl transferases are responsible for pectin synthesis in the golgi. The quasimodo-1 (qua1) mutant in Arabidopsis displays reduced HG content, a decreased esterification level and cell adhesion problems (Bouton et al., 2002; Leboeuf et al., 2005). Note that qua1 is also defective in xylan biosynthesis (Orfila et al., 2005). The ectopically parting cells 1 (epc1) mutant affecting a glycosyl transferase displays reduced cell adhesion in the cotyledons and hypocotyl (Singh et al., 2005). When these data are taken into account it becomes clear that while the balance between esterified and de-esterified pectin is important, so is the overall level of HG pectin.
As previously introduced, there are two other pectins to consider as well—RG-I and RG-II, although their roles in cell adhesion are more complex and less well studied. In tobacco, the nolac-H18 mutant has reduced RG-II pectin and exhibits crumbled shoots and abnormal meristem cell adhesion indicating a role in adhesion (Iwai et al., 2002). On the other hand, in the Arabidopsis echidna (ech) mutant RG-I and xyloglucan are low but cell adhesion is wild-type (Gendre et al., 2011, 2013). These discrepancies indicate specificities in pectin-mediated cell adhesion that extend beyond a simple story where pectin-equals-glue. They hint at a complex story for pectin within the middle lamella and its influences on cell adhesion.
The delivery of pectin and its modifying proteins occurs mainly via the actin cytoskeleton. It is therefore unsurprising that defects in actin filament organization affect cell adhesion. The Actin-related protein2/3 complex (Arp2/3) is highly conserved and is the key component in regulating branching and nucleation of actin filaments (Higgs and Pollard, 2001). Mutants in ARP2/3 complex subunits have been characterized in Arabidopsis where they are associated with disorganization of the actin cytoskeleton, defects in cell shape, and ectopic cell separation in hypocotyls (Le et al., 2003; Li et al., 2003; Mathur et al., 2003a,b; El-Assal et al., 2004; Saedler et al., 2004). Mutants in up-stream regulators of the Arp2/3 complex also display defects in cell adhesion as seen in the spike1 mutant (Qiu et al., 2002). Interestingly, no difference in cell wall composition between wild-type and the arp2 mutant has been observed. The only observed difference was an abnormal thickening at the three-way wall junction of the mutant, possibly indicating altered composition at the middle lamella (Dyachok et al., 2008). We still have only a basic understanding of how actin structure might ultimately affect cell adhesion, and we cannot exclude effects on wall components beyond pectin; but the evidence presented here points toward the delivery of components and pectin-modifying proteins to the cell wall.
It is perhaps not just delivery of components and modifying proteins to the cell wall that affect adhesion but also their recycling. Actin is a key player in endocytosis in plants, yeast and animals (Moreau et al., 1997; Roszak and Rambour, 1997; Schaerer-Brodbeck and Riezman, 2000; Insall et al., 2001; Merrifield et al., 2004; Benesch et al., 2005; Kaksonen et al., 2006). Given the adhesion defects described above, when actin is disrupted, it is plausible that actin-mediated endocytosis might also be involved in maintaining cell wall integrity. Recycling of cell wall components has been demonstrated in germinating Arabidopsis seeds and maize root tip cells (Baluška et al., 2002; Pagnussat et al., 2012). Cell wall modifying proteins may also be recycled, as seen in the case of PMEI endocytosis in growing pollen tubes (Röckel et al., 2008). These data suggest that recycling from the cell wall by endocytosis may be necessary to maintain cell wall integrity and cell adhesion, but this area needs to be further explored.
During some developmental processes cell adhesion is purposefully dissolved leading to cell separation. For example, natural phenomena that require cell separation are observed in leaf abscission, fruit dehiscence, fruit ripening, tetraspore separation, pollen release and root cap cell sloughing. The study of these processes gives us an insight into the mechanisms controlling cell adhesion and separation in plants. Next we will examine how pectin (and its regulation) contributes to the phenomenon of cell separation.
Unsurprisingly, given its role in adhesion, there are several examples of pectin alterations which block regulated cell separation. Inhibition of PME activity prevents separation of root border cells in pea (Wen et al., 1999). In Arabidopsis, the mutants quartet1 (qrt1), a PME, and quartet3 (qrt3), a polygalacturonase (PG), result in the failure of tetraspores separation (Rhee et al., 2003; Francis et al., 2006). This implies that both PME and PG activity are necessary to separate the tetrads: PME removes the methyl groups from HG and subsequently PG breaks down the pectins, releasing the individual pollen grains (Figure 2). With respect to cell separation, it is worth considering the interplay between PME and PG in some more detail.
Polygalacturonases are enzymes that cleave de-esterified HG backbones via hydrolysis; as such they depend on PME activity. They are represented by a large gene family in Arabidopsis with diverse expression profiles (Kim et al., 2006; González-Carranza et al., 2007). PGs have been implicated as positive regulators of cell separation, fruit ripening, abscission, cell growth and dehiscence (Jenkins et al., 1999; Sander et al., 2001; Atkinson et al., 2002, 2012; González-Carranza et al., 2007; Xiao et al., 2014). Mutations in the PG coding genes QRT2 and QRT3 lead to problems in organ dehiscence, abscission and tetraspore separation in Arabidopsis (Rhee et al., 2003; Ogawa et al., 2009). PGs are involved in silique dehiscence in Arabidopsis and Brassica (Jenkins et al., 1999; Roberts and McCann, 2000; Sander et al., 2001) and silique development is also accompanied by an increase in PME activity which reinforces the interconnected roles of PME and PG (Louvet et al., 2011). These analyses indicate that PG-mediated, PME-dependent, pectin degradation is a key event in cell separation during development.
Our information surrounding the role of PG in promoting cell separation goes well beyond Arabidopsis, again highlighting an ancient role for pectin in cell connectivity: overexpression of a PG1 subunit, OsBURP16, in rice decreased cell adhesion and overexpression of PG in apple caused premature leaf shedding due to reduced adhesion in the abscission zone (Atkinson et al., 2012; Liu et al., 2014). Conversely, down-regulation of PG in apples increased fruit firmness and cell adhesion (Atkinson et al., 2012). This correlates well with findings in strawberry where the down-regulation of PG reduced fruit softening (Quesada et al., 2009). Interestingly, the effect of PG alteration in tomato is incongruent with all other evidence. Down-regulation of a fruit-ripening-specific PG in tomato only slightly reduced fruit softening (Kramer et al., 1992; Langley et al., 1994). In line with this phenotype, the down-regulation of PG only yielded a slight reduction in pectin de-polymerisation in fruits (Brummell and Labavitch, 1997). Lastly, overexpression of PG in tomato could restore ripening in a ripening and softening inhibited mutant (rin) but not softening (Giovannoni et al., 1989). While these data indicate that PG has only a minor role in tomato fruit ripening (in contrast to strawberry and apple, as above), PG activity was much higher in tomato fruit homogenates compared to the intact tissue indicating that PG mediated softening in tomato may be regulated less by the quantity of the enzyme, and more by activity through the biochemical environment (Kramer et al., 1992). Overall, there is a strong trend for the importance of PG in mediating cell separation, further underlining the role of pectin in the process as well.
Throughout this review, we have seen several instances of contradictory evidence surrounding the role of pectin in cell adhesion and separation. For example, in one tissue PME activity promotes adhesion and in another separation: high esterification level reduces cell adhesion in the mesophyll and the pericarp (Tieman and Handa, 1994; Lionetti et al., 2015), but simultaneously causes increased cell adhesion and blocks cell separation in tetraspores and root border cells (Wen et al., 1999; Rhee et al., 2003). It is likely that this difference is due to a complex mix of other modifying proteins and a complex biochemical environment; as an illustration, the presence of PG in ripening fruit would increase the likelihood that de-esterified pectin would be depolymerised, not cross-linked with Ca2+. This does not negate the importance of pectin and the middle lamella, but instead highlights the complexity of cell adhesion and separation.
The activity of PMEs is also highly diverse. The Arabidopsis genome contains 66 PME-related genes (Tian et al., 2006) and what little we know about their activity indicates they are highly regulated. Solution pH has been shown to affect the activity of PMEs in persimmon and apple (Alonso et al., 1997; Denès et al., 2000) and PME activity is also salt dependent (reviewed in, Jolie et al., 2010). To make the situation more complex, it is important to recall that PME activity can be counteracted by PMEI proteins, and interestingly some of the predicted PMEs also contain inhibitor domains (Tian et al., 2006). We still have very little information on how most of the PMEs are specifically regulated and very little idea about their developmental specificity. Again, we have more evidence that adhesion and separation are complex processes worthy of dissection.
While the middle lamella is mostly pectin, it also contains some hemicellulose. As such, it is not unexpected that xyloglucans have been implicated in fruit softening (Rose and Bennett, 1999; Vicente et al., 2007). Immuno-labelling of hemicellulose (LM15 antibody) in unripe fruits of tomato showed signal in the wall at points of cell adhesion, which was lost in ripe fruit. As in the case of pectins, it is not only altered levels of hemicellulose that affect cell adhesion, but also the modification of existing hemicellulose and its effect on cell wall structure as a whole. The wall loosening protein expansin modifies the connection between hemicellulose and cellulose; EXPANSIN1 (Expa1) is involved in tomato fruit ripening and its down-regulation reduced the amount of pectin de-polymerisation (Brummell et al., 1999). This data simply reinforces the complex nature of the cell wall and cell adhesion.
In the end, we can make some well-founded conclusions about the role of pectin in cell adhesion and separation. The physical position of the middle lamella, its pectin-rich nature and its accumulation of calcium all point to a crucial role for pectin in these processes. As with any cell wall-mediated process the effect of transgenic and mutational analyses is complicated by redundancy and compensation, and so our current understanding is limited. Experimental evidence also tells us that the tissue specific context involving other modifying proteins, their deposition and recycling and the biochemical environment are also critical. In spite of these difficulties, it is clear that pectin and calcium are required for proper cell adhesion and that pectin modification and degradation are strictly required for cell separation in its various developmental contexts. The details of which enzyme performs which task in which tissue, how altering delivery of such modifying proteins or pectin itself might regulate connectivity, the role of other wall components and the cytoskeleton remain to be ironed out. With current advances in experimental techniques and interests in adhesion and separation growing, understanding these processes is an achievable and exciting goal.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The writing of this review was carried out with the help of grant BB-L002884-1 (BBSRC, UK). We wish to thank the other members of our research team and colleagues at The Sainsbury Laboratory for their support and friendship.
Albersheim, P., Darvill, A., O’Neill, M., Schols, H., and Voragen, A. (1996). An hypothesis: the same six polysaccharides are components of the primary cell walls of all higher plants. Pectins Pectinases 14, 47–53. doi: 10.1016/S0921-0423(96)80245-0
Alonso, J., Howell, N., and Canet, W. (1997). Purification and characterisation of two pectinmethylesterase from persimmon (Diospyros kaki). J. Sci. Food Agric. 75, 352–358. doi: 10.1002/(SICI)1097-0010(199711)75:3<352::AID-JSFA885>3.0.CO;2-G
Atkinson, R. G., Schröder, R., Hallett, I. C., Cohen, D., and MacRae, E. A. (2002). Overexpression of polygalacturonase in transgenic apple trees leads to a range of novel phenotypes involving changes in cell adhesion. Plant Physiol. 129, 122–133. doi: 10.1104/pp.010986
Atkinson, R., Sutherland, P., Johnston, S., Gunaseelan, K., Hallett, I., Mitra, D., et al. (2012). Down-regulation of POLYGALACTURONASE1 alters firmness, tensile strength and water loss in apple (Malus × domestica) fruit. BMC Plant Biol. 12:129. doi: 10.1186/1471-2229-12-129
Atmodjo, M. A., Hao, Z., and Mohnen, D. (2013). Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 64, 747–779. doi: 10.1146/annurev-arplant-042811-105534
Baluška, F., Hlavacka, A., Šamaj, J., Palme, K., Robinson, D. G., Matoh, T., et al. (2002). F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol. 130, 422–431. doi: 10.1104/pp.007526
Benesch, S., Polo, S., Lai, F. P. L., Anderson, K. I., Stradal, T. E. B., Wehland, J., et al. (2005). N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits. J. Cell Sci. 118, 3103–3115. doi: 10.1242/jcs.02444
Bouton, S., Leboeuf, E., Mouille, G., Leydecker, M.-T., Talbotec, J., Granier, F., et al. (2002). QUASIMODO1 encodes a putative membrane-bound glycosyltransferase required for normal pectin synthesis and cell adhesion in Arabidopsis. Plant Cell Online 14, 2577–2590. doi: 10.1105/tpc.004259
Braybrook, S. A., Hofte, H., and Peaucelle, A. (2012). Probing the mechanical contributions of the pectin matrix: insights for cell growth. Plant Signal. Behav. 7, 1037–1041. doi: 10.4161/psb.20768
Brummell, D. A., Harpster, M. H., Civello, P. M., Palys, J. M., Bennett, A. B., and Dunsmuir, P. (1999). Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell Online 11, 2203–2216. doi: 10.1105/tpc.11.11.2203
Brummell, D. A., and Labavitch, J. M. (1997). Effect of antisense suppression of endopolygalacturonase activity on polyuronide molecular weight in ripening tomato fruit and in fruit homogenates. Plant Physiol. 115, 717–725.
Bush, M. S., Marry, M., Huxham, M. I., Jarvis, M. C., and McCann, M. C. (2001). Developmental regulation of pectic epitopes during potato tuberisation. Planta 213, 869–880. doi: 10.1007/s004250100570
Denès, J.-M., Baron, A., Renard, C. M. G. C., Péan, C., and Drilleau, J.-F. (2000). Different action patterns for apple pectin methylesterase at pH 7.0 and 4.5. Carbohydr. Res. 327, 385–393. doi: 10.1016/S0008-6215(00)00070-7
Dhonukshe, P., Baluška, F., Schlicht, M., Hlavacka, A., Šamaj, J., Friml, J., et al. (2006). Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev. Cell 10, 137–150. doi: 10.1016/j.devcel.2005.11.015
Domozych, D. S., Sørensen, I., Popper, Z. A., Ochs, J., Andreas, A., Fangel, J. U., et al. (2014). Pectin metabolism and assembly in the cell wall of the charophyte green alga Penium margaritaceum. Plant Physiol. 165, 105–118. doi: 10.1104/pp.114.236257
Drakakaki, G. (2015). Polysaccharide deposition during cytokinesis: challenges and future perspectives. Plant Sci. 236, 177–184. doi: 10.1016/j.plantsci.2015.03.018
Dyachok, J., Shao, M.-R., Vaughn, K., Bowling, A., Facette, M., Djakovic, S., et al. (2008). Plasma membrane-associated SCAR complex subunits promote cortical F-actin accumulation and normal growth characteristics in Arabidopsis roots. Mol. Plant 1, 990–1006. doi: 10.1093/mp/ssn059
El-Assal, S. E.-D., Le, J., Basu, D., Mallery, E. L., and Szymanski, D. B. (2004). DISTORTED2 encodes an ARPC2 subunit of the putative Arabidopsis ARP2/3 complex. Plant J. 38, 526–538. doi: 10.1111/j.1365-313X.2004.02065.x
Francis, K. E., Lam, S. Y., and Copenhaver, G. P. (2006). Separation of Arabidopsis pollen tetrads is regulated by QUARTET1, a Pectin Methylesterase Gene. Plant Physiol. 142, 1004–1013. doi: 10.1104/pp.106.085274
Frank, M., Guivarc’h, A., Krupková, E., Lorenz-Meyer, I., Chriqui, D., and Schmülling, T. (2002). Tumorous Shoot Development (TSD) genes are required for co-ordinated plant shoot development. Plant J. 29, 73–85. doi: 10.1046/j.1365-313x.2002.01197.x
Gendre, D., McFarlane, H. E., Johnson, E., Mouille, G., Sjödin, A., Oh, J., et al. (2013). Trans-golgi network localized ECHIDNA/Ypt interacting protein complex is required for the secretion of cell wall polysaccharides in Arabidopsis. Plant Cell Online 25, 2633–2646. doi: 10.1105/tpc.113.112482
Gendre, D., Oh, J., Boutté, Y., Best, J. G., Samuels, L., Nilsson, R., et al. (2011). Conserved Arabidopsis ECHIDNA protein mediates trans–Golgi-network trafficking and cell elongation. Proc. Natl. Acad. Sci. U.S.A. 108, 8048–8053. doi: 10.1073/pnas.1018371108
Giovannoni, J. J., DellaPenna, D., Bennett, A. B., and Fischer, R. L. (1989). Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell Online 1, 53–63. doi: 10.1105/tpc.1.1.53
González-Carranza, Z. H., Elliott, K. A., and Roberts, J. A. (2007). Expression of polygalacturonases and evidence to support their role during cell separation processes in Arabidopsis thaliana. J. Exp. Bot. 58, 3719–3730. doi: 10.1093/jxb/erm222
Guillemin, F., Guillon, F., Bonnin, E., Devaux, M.-F., Chevalier, T., Knox, P., et al. (2005). Distribution of pectic epitopes in cell walls of the sugar beet root. Planta 222, 355–371. doi: 10.1007/s00425-005-1535-3
Harholt, J., Suttangkakul, A., and Vibe Scheller, H. (2010). Biosynthesis of pectin. Plant Physiol. 153, 384–395. doi: 10.1104/pp.110.156588
Higgs, H. N., and Pollard, T. D. (2001). Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 70, 649–676. doi: 10.1146/annurev.biochem.70.1.649
Huxham, I. M., Jarvis, M. C., Shakespeare, L., Dover, C. J., Johnson, D., Knox, J. P., et al. (1999). Electron-energy-loss spectroscopic imaging of calcium and nitrogen in the cell walls of apple fruits. Planta 208, 438–443. doi: 10.1007/s004250050580
Insall, R., Müller-Taubenberger, A., Machesky, L., Köhler, J., Simmeth, E., Atkinson, S. J., et al. (2001). Dynamics of the Dictyostelium Arp2/3 complex in endocytosis, cytokinesis, and chemotaxis. Cell Motil. Cytoskeleton 50, 115–128. doi: 10.1002/cm.10005
Iwai, H., Masaoka, N., Ishii, T., and Satoh, S. (2002). A pectin glucuronyltransferase gene is essential for intercellular attachment in the plant meristem. Proc. Natl. Acad. Sci. U.S.A. 99, 16319–16324. doi: 10.1073/pnas.252530499
Jenkins, E. S., Paul, W., Craze, M., Whitelaw, C. A., Weigand, A., and Roberts, J. A. (1999). Dehiscence-related expression of an Arabidopsis thaliana gene encoding a polygalacturonase in transgenic plants of Brassica napus. Plant Cell Environ. 22, 159–167. doi: 10.1046/j.1365-3040.1999.00372.x
Jolie, R. P., Duvetter, T., Van Loey, A. M., and Hendrickx, M. E. (2010). Pectin methylesterase and its proteinaceous inhibitor: a review. Carbohydr. Res. 345, 2583–2595. doi: 10.1016/j.carres.2010.10.002
Kaksonen, M., Toret, C. P., and Drubin, D. G. (2006). Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 7, 404–414. doi: 10.1038/nrm1940
Kang, B.-H., Nielsen, E., Preuss, M. L., Mastronarde, D., and Staehelin, L. A. (2011). Electron tomography of RabA4b- and PI-4Kβ1-labeled trans golgi network compartments in Arabidopsis. Traffic 12, 313–329. doi: 10.1111/j.1600-0854.2010.01146.x
Kim, J., Shiu, S.-H., Thoma, S., Li, W.-H., and Patterson, S. (2006). Patterns of expansion and expression divergence in the plant polygalacturonase gene family. Genome Biol. 7:R87. doi: 10.1186/gb-2006-7-9-r87
Kim, S.-J., and Brandizzi, F. (2014). The plant secretory pathway: an essential factory for building the plant cell wall. Plant Cell Physiol. 55, 687–693. doi: 10.1093/pcp/pct197
Kramer, M., Sanders, R., Bolkan, H., Waters, C., Sheeny, R. E., and Hiatt, W. R. (1992). Postharvest evaluation of transgenic tomatoes with reduced levels of polygalacturonase: processing, firmness and disease resistance. Postharvest Biol. Technol. 1, 241–255. doi: 10.1016/0925-5214(92)90007-C
Krupková, E., Immerzeel, P., Pauly, M., and Schmülling, T. (2007). The TUMOROUS SHOOT DEVELOPMENT2 gene of Arabidopsis encoding a putative methyltransferase is required for cell adhesion and co-ordinated plant development. Plant J. 50, 735–750. doi: 10.1111/j.1365-313X.2007.03123.x
Langley, K. R., Martin, A., Stenning, R., Murray, A. J., Hobson, G. E., Schuch, W. W., et al. (1994). Mechanical and optical assessment of the ripening of tomato fruit with reduced polygalacturonase activity. J. Sci. Food Agric. 66, 547–554. doi: 10.1002/jsfa.2740660420
Le, J., El-Assal, S. E.-D., Basu, D., Saad, M. E., and Szymanski, D. B. (2003). Requirements for Arabidopsis ATARP2 and ATARP3 during epidermal development. Curr. Biol. 13, 1341–1347. doi: 10.1016/S0960-9822(03)00493-7
Leboeuf, E., Guillon, F., Thoiron, S., and Lahaye, M. (2005). Biochemical and immunohistochemical analysis of pectic polysaccharides in the cell walls of Arabidopsis mutant QUASIMODO 1 suspension-cultured cells: implications for cell adhesion. J. Exp. Bot. 56, 3171–3182. doi: 10.1093/jxb/eri314
Lerouxel, O., Cavalier, D. M., Liepman, A. H., and Keegstra, K. (2006). Biosynthesis of plant cell wall polysaccharides—a complex process. Curr. Opin. Plant Biol. 9, 621–630. doi: 10.1016/j.pbi.2006.09.009
Letham, D. S. (1960). The separation of plant cells with ethylenediaminetetraacetic acid. Exp. Cell Res. 21, 353–360. doi: 10.1016/0014-4827(60)90267-6
Li, S., Blanchoin, L., Yang, Z., and Lord, E. M. (2003). The putative Arabidopsis Arp2/3 complex controls leaf cell morphogenesis. Plant Physiol. 132, 2034–2044. doi: 10.1104/pp.103.028563
Lionetti, V., Cervone, F., and De Lorenzo, G. (2015). A lower content of de-methylesterified homogalacturonan improves enzymatic cell separation and isolation of mesophyll protoplasts in Arabidopsis. Phytochemistry 112, 188–194. doi: 10.1016/j.phytochem.2014.07.025
Liu, H., Ma, Y. A. N., Chen, N. A., Guo, S., Liu, H., Guo, X., et al. (2014). Overexpression of stress-inducible OsBURP16, the β subunit of polygalacturonase 1, decreases pectin content and cell adhesion and increases abiotic stress sensitivity in rice. Plant Cell Environ. 37, 1144–1158. doi: 10.1111/pce.12223
Louvet, R., Rayon, C., Domon, J.-M., Rusterucci, C., Fournet, F., Leaustic, A., et al. (2011). Major changes in the cell wall during silique development in Arabidopsis thaliana. Phytochemistry 72, 59–67. doi: 10.1016/j.phytochem.2010.10.008
Mathur, J., Mathur, N., Kernebeck, B., and Hülskamp, M. (2003a). Mutations in Actin-related proteins 2 and 3 affect cell shape development in Arabidopsis. Plant Cell Online 15, 1632–1645. doi: 10.1105/tpc.011676
Mathur, J., Mathur, N., Kirik, V., Kernebeck, B., Srinivas, B. P., and Hülskamp, M. (2003b). Arabidopsis CROOKED encodes for the smallest subunit of the ARP2/3 complex and controls cell shape by region specific fine F-actin formation. Development 130, 3137–3146. doi: 10.1242/dev.00549
McCartney, L., and Knox, P. (2002). Regulation of pectic polysaccharide domains in relation to cell development and cell properties in the pea testa. J. Exp. Bot. 53, 707–713. doi: 10.1093/jexbot/53.369.707
Merrifield, C. J., Qualmann, B., Kessels, M. M., and Almers, W. (2004). Neural Wiskott Aldrich Syndrome Protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts. Eur. J. Cell Biol. 83, 13–18. doi: 10.1078/0171-9335-00356
Miao, Y., Li, H.-Y., Shen, J., Wang, J., and Jiang, L. (2011). QUASIMODO 3 (QUA3) is a putative homogalacturonan methyltransferase regulating cell wall biosynthesis in Arabidopsis suspension-cultured cells. J. Exp. Bot. 62, 5063–5078. doi: 10.1093/jxb/err211
Miart, F., Desprez, T., Biot, E., Morin, H., Belcram, K., Höfte, H., et al. (2014). Spatio-temporal analysis of cellulose synthesis during cell plate formation in Arabidopsis. Plant J. 77, 71–84. doi: 10.1111/tpj.12362
Micheli, F. (2001). Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 6, 414–419. doi: 10.1016/S1360-1385(01)02045-3
Moore, P., and Staehelin, L. A. (1988). Immunogold localization of the cell-wall-matrix polysaccharides rhamnogalacturonan I and xyloglucan during cell expansion and cytokinesis in Trifolium pratense L., implication for secretory pathways. Planta 174, 433–445. doi: 10.1007/BF00634471
Moreau, V., Galan, J. M., Devilliers, G., Haguenauer-Tsapis, R., and Winsor, B. (1997). The yeast actin-related protein Arp2p is required for the internalization step of endocytosis. Mol. Biol. Cell 8, 1361–1375. doi: 10.1091/mbc.8.7.1361
Mouille, G., Ralet, M.-C., Cavelier, C., Eland, C., Effroy, D., Hématy, K., et al. (2007). Homogalacturonan synthesis in Arabidopsis thaliana requires a Golgi-localized protein with a putative methyltransferase domain. Plant J. 50, 605–614. doi: 10.1111/j.1365-313X.2007.03086.x
Ng, A., Parker, M. L., Parr, A. J., Saunders, P. K., Smith, A. C., and Waldron, K. W. (2000). Physicochemical characteristics of onion (Allium cepa L.) tissues. J. Agric. Food Chem. 48, 5612–5617. doi: 10.1021/jf991206q
Northcote, D. H., Davey, R., and Lay, J. (1989). Use of antisera to localize callose, xylan and arabinogalactan in the cell-plate, primary and secondary walls of plant cells. Planta 178, 353–366. doi: 10.1007/BF00391863
Ogawa, M., Kay, P., Wilson, S., and Swain, S. M. (2009). ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 21, 216–233. doi: 10.1105/tpc.108.063768
Orfila, C., Sørensen, S., Harholt, J., Geshi, N., Crombie, H., Truong, H.-N., et al. (2005). QUASIMODO1 is expressed in vascular tissue of Arabidopsis thaliana inflorescence stems, and affects homogalacturonan and xylan biosynthesis. Planta 222, 613–622. doi: 10.1007/s00425-005-0008-z
Pagnussat, L., Burbach, C., Baluška, F., and de la Canal, L. (2012). Rapid endocytosis is triggered upon imbibition in Arabidopsis seeds. Plant Signal. Behav. 7, 416–421. doi: 10.4161/psb.19669
Parker, C. C., Parker, M. L., Smith, A. C., and Waldron, K. W. (2001). Pectin distribution at the surface of potato parenchyma cells in relation to cell-cell adhesion. J. Agric. Food Chem. 49, 4364–4371. doi: 10.1021/jf0104228
Peaucelle, A., Braybrook, S. A., Le Guillou, L., Bron, E., Kuhlemeier, C., and Höfte, H. (2011). Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr. Biol. 21, 1720–1726. doi: 10.1016/j.cub.2011.08.057
Qiu, J.-L., Jilk, R., Marks, M. D., and Szymanski, D. B. (2002). The Arabidopsis SPIKE1 gene is required for normal cell shape control and tissue development. Plant Cell Online 14, 101–118. doi: 10.1105/tpc.010346
Quesada, M. A., Blanco-Portales, R., Posé, S., García-Gago, J. A., Jiménez-Bermúdez, S., Muñoz-Serrano, A., et al. (2009). Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiol. 150, 1022–1032. doi: 10.1104/pp.109.138297
Reichardt, I., Stierhof, Y.-D., Mayer, U., Richter, S., Schwarz, H., Schumacher, K., et al. (2007). Plant cytokinesis requires de novo secretory trafficking but not endocytosis. Curr. Biol. 17, 2047–2053. doi: 10.1016/j.cub.2007.10.040
Rhee, S. Y., Osborne, E., Poindexter, P. D., and Somerville, C. R. (2003). Microspore separation in the quartet 3 mutants of Arabidopsis is impaired by a defect in a developmentally regulated polygalacturonase required for pollen mother cell wall degradation. Plant Physiol. 133, 1170–1180. doi: 10.1104/pp.103.028266
Rihouey, C., Morvan, C., Borissova, I., Jauneau, A., Demarty, M., and Jarvis, M. (1995). Structural features of CDTA-soluble pectins from flax hypocotyls. Carbohydr. Polymers 28, 159–166. doi: 10.1016/0144-8617(95)00094-1
Roberts, K., and McCann, M. C. (2000). Xylogenesis: the birth of a corpse. Curr. Opin. Plant Biol. 3, 517–522. doi: 10.1016/S1369-5266(00)00122-9
Röckel, N., Wolf, S., Kost, B., Rausch, T., and Greiner, S. (2008). Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J. 53, 133–143. doi: 10.1111/j.1365-313X.2007.03325.x
Rose, J. K. C., and Bennett, A. B. (1999). Cooperative disassembly of the cellulose–xyloglucan network of plant cell walls: parallels between cell expansion and fruit ripening. Trends Plant Sci. 4, 176–183. doi: 10.1016/S1360-1385(99)01405-3
Roszak, R., and Rambour, S. (1997). Uptake of Lucifer Yellow by plant cells in the presence of endocytotic inhibitors. Protoplasma 199, 198–207. doi: 10.1007/BF01294506
Saedler, R., Zimmermann, I., Mutondo, M., and Hülskamp, M. (2004). The Arabidopsis KLUNKER gene controls cell shape changes and encodes the AtSRA1 homolog. Plant Mol. Biol. 56, 775–782. doi: 10.1007/s11103-004-4951-z
Sander, L., Child, R., Ulvskov, P., Albrechtsen, M., and Borkhardt, B. (2001). Analysis of a dehiscence zone endo-polygalacturonase in oilseed rape (Brassica napus) and Arabidopsis thaliana: evidence for roles in cell separation in dehiscence and abscission zones, and in stylar tissues during pollen tube growth. Plant Mol. Biol. 46, 469–479. doi: 10.1023/A:1010619002833
Schaerer-Brodbeck, C., and Riezman, H. (2000). Functional Interactions between the p35 Subunit of the Arp2/3 Complex and Calmodulin in Yeast. Mol. Biol. Cell 11, 1113–1127. doi: 10.1091/mbc.11.4.1113
Scherp, P., Grotha, R., and Kutschera, U. (2001). Occurrence and phylogenetic significance of cytokinesis-related callose in green algae, bryophytes, ferns and seed plants. Plant Cell Rep. 20, 143–149. doi: 10.1007/s002990000301
Singh, S. K., Eland, C., Harholt, J., Scheller, H. V., and Marchant, A. (2005). Cell adhesion in Arabidopsis thaliana is mediated by ECTOPICALLY PARTING CELLS 1—a glycosyltransferase (GT64) related to the animal exostosins. Plant J. 43, 384–397. doi: 10.1111/j.1365-313X.2005.02455.x
Tian, G.-W., Chen, M.-H., Zaltsman, A., and Citovsky, V. (2006). Pollen-specific pectin methylesterase involved in pollen tube growth. Dev. Biol. 294, 83–91. doi: 10.1016/j.ydbio.2006.02.026
Tieman, D. M., and Handa, A. K. (1994). Reduction in pectin methylesterase activity modifies tissue integrity and cation levels in ripening tomato (Lycopersicon esculentum Mill.) fruits. Plant Physiol. 106, 429–436.
Toyooka, K., Goto, Y., Asatsuma, S., Koizumi, M., Mitsui, T., and Matsuoka, K. (2009). A mobile secretory vesicle cluster involved in mass transport from the golgi to the plant cell exterior. Plant Cell Online 21, 1212–1229. doi: 10.1105/tpc.108.058933
Vicente, A. R., Saladié, M., Rose, J. K. C., and Labavitch, J. M. (2007). The linkage between cell wall metabolism and fruit softening: looking to the future. J. Sci. Food Agric. 87, 1435–1448. doi: 10.1002/jsfa.2837
Wen, F., Zhu, Y., and Hawes, M. C. (1999). Effect of pectin methylesterase gene expression on pea root development. Plant Cell Online 11, 1129–1140. doi: 10.1105/tpc.11.6.1129
Willats, W. G. T., Orfila, C., Limberg, G., Buchholt, H. C., van Alebeek, G.-J. W. M., Voragen, A. G. J., et al. (2001). Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls: implications for pectin methyl esterase action, matrix properties, and cell adhesion. J. Biol. Chem. 276, 19404–19413. doi: 10.1074/jbc.M011242200
Worden, N., Park, E., and Drakakaki, G. (2012). Trans-golgi network—An intersection of trafficking cell wall components. J. Integr. Plant Biol. 54, 875–886. doi: 10.1111/j.1744-7909.2012.01179.x
Xiao, C., Somerville, C., and Anderson, C. T. (2014). POLYGALACTURONASE INVOLVED IN EXPANSION1 functions in cell elongation and flower development in Arabidopsis. Plant Cell Online 26, 1018–1035. doi: 10.1105/tpc.114.123968
Zablackis, E., Huang, J., Muller, B., Darvill, A. G., and Albersheim, P. (1995). Characterization of the cell-wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol. 107, 1129–1138. doi: 10.1104/pp.107.4.1129
Keywords: cell adhesion, pectin, polygalacturonase, pectin methylesterase, cell separation
Citation: Bou Daher F and Braybrook SA (2015) How to let go: pectin and plant cell adhesion. Front. Plant Sci. 6:523. doi: 10.3389/fpls.2015.00523
Received: 17 April 2015; Accepted: 29 June 2015;
Published: 14 July 2015.
Edited by:
Takumi Higaki, The University of Tokyo, JapanReviewed by:
Thomas S. Nuhse, The University of Manchester, UKCopyright © 2015 Bou Daher and Braybrook. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siobhan A. Braybrook, The Sainsbury Laboratory, University of Cambridge, Bateman Street, Cambridge CB2 1LR, UK,c2lvYmhhbi5icmF5YnJvb2tAc2xjdS5jYW0uYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.