- 1Department of Cell Biology and Molecular Genetics, University of Maryland, College Park, MD, USA
- 2Laboratory of Functional Plant Biology, Department of Physiology, Ghent University, Ghent, Belgium
Ethylene is a simple two carbon atom molecule with profound effects on plants. There are quite a few review papers covering all aspects of ethylene biology in plants, including its biosynthesis, signaling and physiology. This is merely a logical consequence of the fascinating and pleiotropic nature of this gaseous plant hormone. Its biochemical precursor, 1-aminocyclopropane-1-carboxylic acid (ACC) is also a fairly simple molecule, but perhaps its role in plant biology is seriously underestimated. This triangularly shaped amino acid has many more features than just being the precursor of the lead-role player ethylene. For example, ACC can be conjugated to three different derivatives, but their biological role remains vague. ACC can also be metabolized by bacteria using ACC-deaminase, favoring plant growth and lowering stress susceptibility. ACC is also subjected to a sophisticated transport mechanism to ensure local and long-distance ethylene responses. Last but not least, there are now a few exciting studies where ACC has been reported to function as a signal itself, independently from ethylene. This review puts ACC in the spotlight, not to give it the lead-role, but to create a picture of the stunning co-production of the hormone and its precursor.
The Discovery of ACC
The discovery of ethylene as a plant growth regulator can be attributed to the work of the Russian scientist Neljubov (1901). He reported that dark-grown pea seedlings showed a reduced hypocotyl growth in combination with an exaggerated hypocotyl bending when exposed to illumination gas (Neljubov, 1901). Neljubov (1901) could pinpoint ethylene gas as the active component that caused dark-grown pea seedlings to bend, by flowing the illumination gas over several filters prior to exposing the seedlings. This typical ethylene response of dark-grown seedlings was later defined as the triple response: (1) shortening of the hypocotyl and roots, (2) radial swelling of the hypocotyl, and (3) the exaggeration of the apical hook (Knight et al., 1910). In 1934, conclusive evidence that ethylene is a natural product from plants, was presented by the English scientist (Gane, 1934). It took another 30 years before the primary steps of the ethylene biosynthesis pathway were elucidated (see Figure 1). Lieberman and Mapson (1964) first reported that ethylene could be produced from the amino acid methionine, taking advantage of the high rates of ethylene production from apples for their experimental work (Figure 1). 13 years later, Adams and Yang (1977) made tremendous progress in understanding the biosynthesis pathway of ethylene, when they discovered that S-adenosyl-L-methionine (SAM) was an intermediate between methionine and ethylene. Yang and co-workers also showed that 5′-methylthioadenosine (MTA) was formed as a by-product from SAM and that MTA could be recycled back to methionine (Murr and Yang, 1975). The elaboration of the different reaction steps of the methionine cycle in plants, now often referred to as the Yang-cycle, was mainly inspired by the biochemical similarities between the plant pathway and the methionine salvage cycle which was already known for prokaryotes, yeast, and mammalians. An-up-to-date overview of the methionine and SAM metabolism in plants is given by Sauter et al. (2013). The major discovery that made the methionine cycle in plants unique from all other organisms, was the characterization of 1-aminocyclopropane-1-carboxylic acid (ACC) as the intermediate between SAM and ethylene (Adams and Yang, 1979). Adams and Yang (1979) were able to identify ACC as the precursor for ethylene by feeding experiments on apple tissue, using radio-labeled methionine. Upon incubation of apple disks, they observed a shift from ethylene production in air, toward an unknown compound that was retained in the tissue when treated with nitrogen (lack of oxygen inhibits oxidation of ACC toward ethylene). By using a pH-dependent ion mobility assay, they could characterize this unknown component as an amino acid. Subsequently, the component was identified as ACC, using co-migration of synthetic ACC for both paper-chromatography and paper-electrophoresis (Adams and Yang, 1979). They further showed that the conversion of radioactively labeled methionine toward ethylene decreased when unlabeled ACC was supplemented, yet the conversion of labeled ACC to ethylene was almost not affected when unlabeled methionine was supplemented, suggesting that externally supplied ACC is in fact used to produce ethylene. Additional evidence for ACC being the intermediate precursor between SAM and ethylene was obtained by treating apple tissue with [S]-trans-2-amino-4(2′-aminoethoxy)trans-3-butenoic acid, also known as AVG (2-amino-ethoxy-vinylglycine), a pyridoxal-5′-phosphate (PLP or vitamin B6) dependent enzyme inhibitor, which was later known to inhibit the enzymatic conversion of SAM toward ACC. The identification of ACC as the precursor of ethylene was a major breakthrough in the understanding of the ethylene biosynthesis pathway in plants, and was part of the foundation for many new discoveries in the field of ethylene biology.
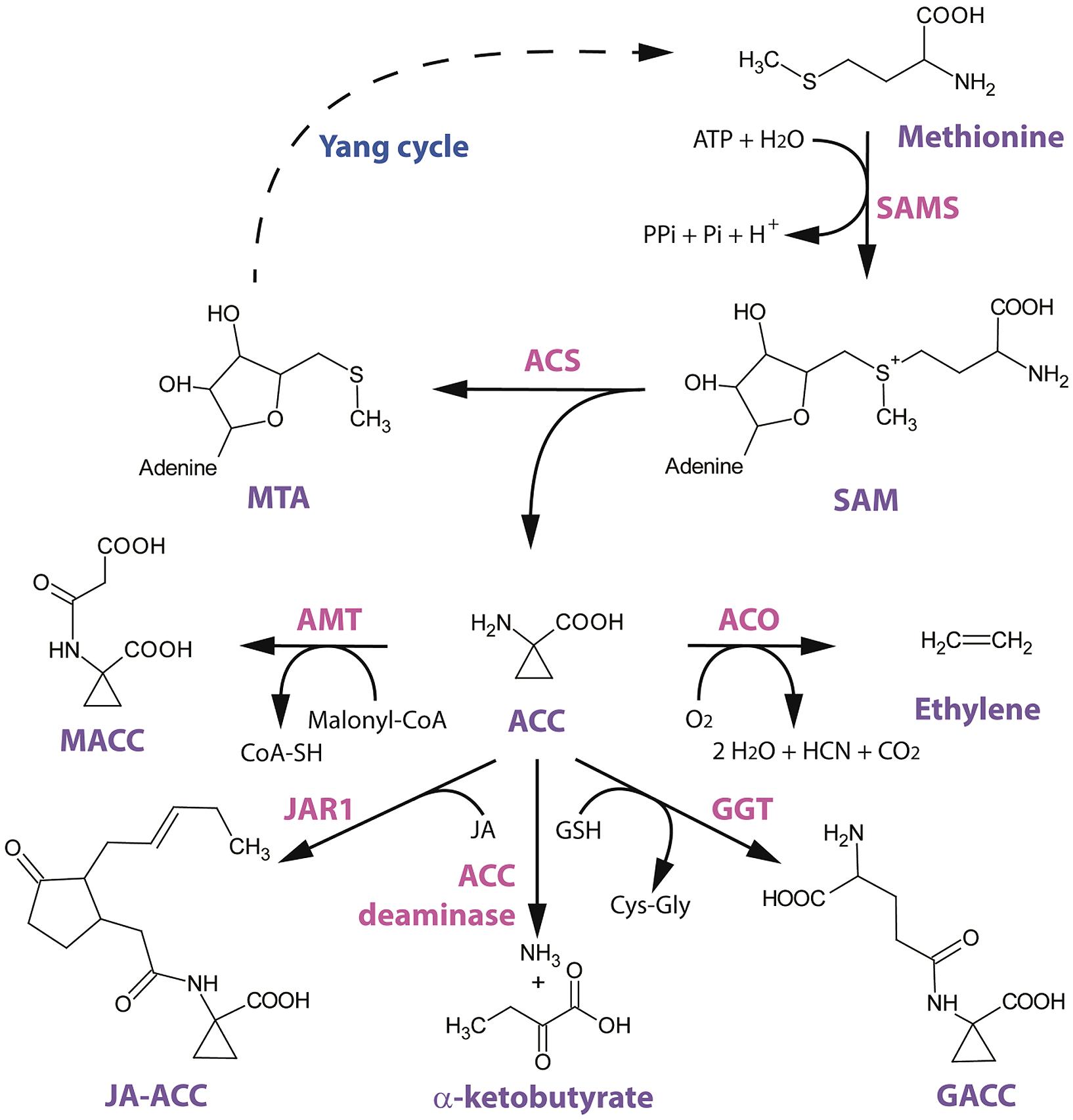
FIGURE 1. Structural scheme of ethylene biosynthesis and 1-aminocyclopropane-1-carboxylic acid (ACC) conjugation/metabolism. The amino acid methionine is converted to S-adenosyl-L-methionine (SAM) by SAM-synthetase (SAMS) with the requirement of ATP. The general precursor SAM is then converted to ACC by ACC-synthase (ACS). This reaction also involves the cleavage of 5′-methylthioadenosine (MTA), which is recycled back to methionine by the Yang cycle (dotted line indicates multiple enzymatic steps). ACC can be converted to ethylene by ACC-oxidase (ACO) in the presence of oxygen. ACC can also be converted to its major conjugate 1-malonyl-ACC (MACC) by the yet uncharacterized ACC-N-malonyl transferase (AMT) with the requirement of malonyl-Coenzyme-A. A second derivate of ACC is γ-glutamyl-ACC (GACC) which is formed by γ-glutamyl-transpeptidase (GGT) with the requirement of glutathione (GSH). Another novel derivate of ACC is jasmonyl-ACC (JA-ACC), which is formed by jasmonic acid resistance 1 (JAR1). ACC can also be metabolized by the bacterial (and plant) ACC deaminase into ammonium and α-ketobutyrate.
ACC and Ethylene Biosynthesis
As mentioned above, ACC is produced from SAM, releasing MTA. This reaction is catalyzed by the enzyme ACC-synthase (ACS; Boller et al., 1979). ACS is a member of the PLP-dependent enzymes, which use vitamin B6 as a co-factor for its enzymatic function. ACS was localized in the cytosol by activity assays on extracts retrieved after differential centrifugation (Boller et al., 1979). ACS genes were first characterized in zucchini by Sato and Theologis (1989) and in tomato by Van Der Straeten et al. (1990). ACS is encoded by a multigene family of 12 members in Arabidopsis, eight of which encode functional ACC synthases [ACS2 (named ACS1 in Van Der Straeten et al., 1992), ACS4-9, ACS11]. In addition, there is one inactive isoform (AtACS1) and one pseudogene (AtACS3; Yamagami et al., 2003). ACS was found to form functional dimers of which the 3D structure was determined by Capitani et al. (1999). The formation of heterodimers increases the structural and functional complexity of the ACS protein family (Tsuchisaka et al., 2009). The large ACS gene family displays a tissue-specific and differential expression pattern in Arabidopsis (Tsuchisaka and Theologis, 2004). Using single and multiple acs knock-out mutants, it was demonstrated that there are specific developmental and physiological roles for individual members of the ACS gene-family, but also that there is a complex combinatorial interplay amongst them (Tsuchisaka et al., 2009). A diverse group of internal and external signals modulate the level of ethylene biosynthesis in numerous plant species, acting at the level of ACS gene expression. These inducers include auxin, cytokinin, brassinosteroids, ethylene, copper, mechano-stimuli, ozone, pathogens and wounding (Van Der Straeten et al., 1992; Rodrigues-Pousada et al., 1993; Botella et al., 1995; Cary et al., 1995; Liang et al., 1996; Vahala et al., 1998; Woeste et al., 1999).
Three types of ACS proteins are recognized, based on their C-terminal structure. Type I ACS proteins contain in their C-terminal domain one putative calcium-dependent protein kinase (CDPK) phosphorylation target site and three mitogen-activated protein kinase (MAPK) phosphorylation sites (Yoon and Kieber, 2013). Type II ACS proteins only contain the MAPK phosphorylation sites, while type III ACS do not contain any phosphorylation sites (Yoon and Kieber, 2013). These post-translational phosphorylation sites play an important role in the stability of the ACS protein (Chae and Kieber, 2005). Both in Arabidopsis (Chae et al., 2003; Kim et al., 2003; Liu and Zhang, 2004; Wang et al., 2004; Yoshida et al., 2005, 2006; Joo et al., 2008; Christians et al., 2009; Lyzenga et al., 2012) and in tomato (Tatsuki and Mori, 2001; Kamiyoshihara et al., 2010) it was shown that differential phosphorylation of certain ACS members directed the protein for proteasomal degradation. Protein stability of certain ACS members is further regulated by the protein phosphatase 2A (PP2A; Skottke et al., 2011) and PP2C (Ludwikow et al., 2014), demonstrating a complex balance between phosphorylation and dephosphorylation to secure protein activity and stability.
The second ethylene biosynthesis protein is ACC-oxidase (ACO), which converts ACC to ethylene in the presence of oxygen. It took a long time before ACO activity could be demonstrated in vitro. The key aspect in isolating ACO was the addition of ascorbic acid (vitamin C) to the extraction media, as was first reported by Ververidis and John (1991) who isolated ACO from melon tissue and quantified in vitro ACO activity. Although the exact role of ascorbic acid for protein stability/activity remained uncertain for a long time (Rocklin et al., 2004), it was recently clarified that ascorbic acid participates in the ring opening of ACC, by providing a single-electron to the active site (Murphy et al., 2014). This catalytic reaction releases ethylene and a cyanoformate ion [NCCO2]-, which is subsequently decomposed into CO2 and CN- (Murphy et al., 2014). The reactive cyanide (CN-) is subsequently detoxified by β-cyanoalanine synthase to produce β-cyanoalanine (Miller and Conn, 1980). ACO belongs to the superfamily of dioxygenases that require iron (Fe2+) as co-factor and bicarbonate as activator (Dong et al., 1992; Zhang et al., 2004). The subcellular localization of ACO remains vague, as some studies localize ACO in the cytosol (Reinhardt et al., 1994; Chung et al., 2002; Hudgins et al., 2006), while other localize ACO at the plasma membrane (Rombaldi et al., 1994; Ramassamy et al., 1998). Although the ACO protein sequence does not contain any predicted transmembrane domains, it is still possible that the protein associates with the plasma membrane via (in)direct interactions. ACO is also encoded by a multigene family of five members in Arabidopsis [ACO1, 2, 4, At1g12010 (AOC3) and At1g77330 (ACO5)]. Expression of different members of the tomato ACO family in Escherichia coli showed that each isoform had a specific in vitro enzyme activity (Bidonde et al., 1998).
It is well accepted that ACS is the rate limiting step of ethylene biosynthesis in plants (Yang and Hoffman, 1984) although there are examples where ACO is the rate limiting step, e.g., during post-climacteric ripening of tomato fruit (Van de Poel et al., 2012). Three ACO genes are also auto-regulated by ethylene in Arabidopsis (De Paepe et al., 2004). This might suggest that the regulation of ACO expression and/or activity is more complex than anticipated. There are some hints for a putative post-transcriptional and/or post-translational regulatory mechanisms of ACO as suggested by Dilley et al. (2013) and as investigated through mathematical modeling by Van de Poel et al. (2014a).
Both ACS and ACO are two well-studied enzymes that exclusively participate in the ethylene biosynthesis pathway. Both proteins are well characterized, but many more questions remain unanswered. While the post-translational regulation of ACS has been revealed, the biochemical and mechanistic details of this protein modification are still unclear. Transcriptional and functional characterization of the different ACS gene-family members has shone light on the combinatorial interplay, nevertheless, much more work is needed to elucidate the exact role of each isoform and how they interact with each other. Much less is known about the post-translational regulation and combinatorial interplay of ACO. A lack of genetic studies focusing on ACO, raises the question whether or not ACO shares a similar structural, biochemical and post-translational complexity as ACS.
ACC as a Pivotal Molecule: ACC Conjugates and the Control of Ethylene Biosynthesis
1-Aminocyclopropane-1-carboxylic acid is best known as the direct precursor of ethylene in the ethylene biosynthesis pathway. However, there also exist three different conjugates of ACC, suggesting that the biochemical regulation of the available ACC pool is more complex than anticipated, which in turn can possibly affect the eventual levels of the plant hormone ethylene.
Shortly after the identification of ACC as the intermediate between SAM and ethylene in the ethylene biosynthesis pathway, a first conjugated form of ACC, called malonyl-ACC (MACC), was discovered by Amrhein et al. (1981) in buckwheat seedlings and by Hoffman et al. (1982) in wheat leaves. MACC is formed by ACC-N-malonyl transferase (AMT) which was purified from tomato extracts (Martin and Saftner, 1995), although not structurally characterized. It was shown that the conjugation of ACC into MACC was stimulated by ethylene in preclimacteric tomatoes (Liu et al., 1985a), grapefruit flavedo (outer peel; Liu et al., 1985b) and tobacco leaves (Philosoph-Hadas et al., 1985), indicative for a feedback control of ethylene biosynthesis. Martin and Saftner (1995) also showed that the activity of AMT was ethylene inducible and that its activity correlated with the increase in ethylene production during climacteric ripening of tomato (Martin and Saftner, 1995). The exact amino acid and gene sequences of AMT are not yet known, and no putative AMT gene is annotated in the Arabidopsis genome, limiting more in-depth genetic and molecular studies. Because MACC does not participate in any other known biological conversions, MACC formation might be a mechanism to control the available ACC pool. This hypothesis was further strengthened by the observation that MACC could be translocated from the cytosol into the vacuole (and back) by ATP-dependent tonoplast carriers (Bouzayen et al., 1988, 1989; Tophof et al., 1989). Nonetheless, the reconversion of MACC toward ACC by an unknown MACC-hydrolase was reported twice in literature (Jiao et al., 1986; Hanley et al., 1989). The ability to hydrolyze MACC back into ACC and the ability to ‘store’ MACC in the vacuole is an interesting mechanism to regulate the cellular availability of MACC. Moreover, because MACC has no other biochemical role besides being an ACC conjugate, the regulation of MACC levels can also affect the available pool of ACC and possibly ethylene production levels. This hypothesis was investigated by in silico mathematical modeling, showing that the reconversion of MACC to ACC could have a potential stimulating effect on ethylene production during climacteric fruit ripening of tomato (Van de Poel et al., 2014a).
A second important derivative of ACC is γ-glutamyl-ACC (GACC), which was discovered in crude tomato extracts of ACC-N-malonyltransferase (Martin et al., 1995). These crude protein extracts were able to form a new ACC derivative, which could be identified as GACC (Martin et al., 1995). GACC is formed by the reaction of ACC with the tripeptide glutathione (GSH) by a γ-glutamyl-transpeptidase (also called γ-glutamyl-transferase, GGT; Martin et al., 1995; Martin and Slovin, 2000). While a first report, based on in vitro studies, stated that GACC was the most abundant ACC derivative in tomato (Martin and Saftner, 1995; Martin et al., 1995), another study found that MACC was the most abundant ACC derivative in vivo in tomato fruit during climacteric ripening (Peiser and Yang, 1998). The Arabidopsis genome contains four genes (GGT1-4), of which only GGT1 and GGT2 are catalytically active (Martin et al., 2007). Both GGT1 and GGT2 are co-expressed predominantly in rapidly growing tissue, and are localized extracellularly, which raised the question about the role of extracellular GACC (Martin et al., 2007). A knock-out mutant of GGT1 shows rapid senescence, while GGT3 knock-outs have a reduced rosette size and silique number (Martin et al., 2007). The effect of GACC formation by GGT on ACC availability and possibly ethylene biosynthesis remains to be investigated.
A third derivative of ACC is jasmonyl-ACC (JA-ACC). This molecule was discovered by screening for amino acid conjugates of JA, using GC-MS (Staswick and Tiryaki, 2004). Four amino acid conjugates of JA (JA-Ile, JA-Val, JA-Leu, and JA-Phe) were quantified in Arabidopsis tissue (Staswick and Tiryaki, 2004). Interestingly, the same authors also demonstrated that JA forms a conjugate with ACC (JA-ACC) in Arabidopsis leaves (Staswick and Tiryaki, 2004). Recombinant JAR1 enzyme was found to be able to form JA-ACC in vitro. Strangely, levels of JA-ACC were higher in the leaves of jar1 mutants compared to wild-type plants. It was also shown that JA-ACC inhibits root growth in Arabidopsis. Elegant genetic experiments with JA signaling mutants (coi1-35) showed that the JA-ACC-induced root inhibition was independent of JA signaling. Furthermore, the ethylene signaling mutant etr1-1 and the double mutant etr1-1 jar1-1 were insensitive to the JA-ACC treatment and displayed no inhibition of root growth, indicating that JA-ACC acts via the ethylene signaling pathway (Staswick and Tiryaki, 2004). Most likely the ACC moiety of JA-ACC is responsible for an increase in ethylene production which results in the root growth inhibition response. These experiments suggest that JA-ACC might serve as a pivotal molecule which can function as a modulator of the hormonal cross-talk between the ethylene and jasmonic acid pathway, although the exact molecular and biochemical mechanism of JA-ACC function remains unclear.
The three above-mentioned derivatives of ACC (MACC, GACC, and JA-ACC) are perhaps not the only ones. Future metabolic studies might reveal additional conjugates of ACC. Nonetheless, these three derivatives can potentially play an important role in the regulation of the pool of available ACC, which in turn can affect eventual ethylene production levels, with physiological and developmental consequences. Genetic perturbations of the formation of ACC derivatives could be a useful tool to unravel their exact roles. In addition, a more detailed structural and biochemical characterization of the enzymes involved in the formation of ACC derivatives is essential.
ACC Deaminase and Plants
As mentioned above, plants possess several mechanisms to control their pool of ACC, for example by converting it to ethylene or to conjugates like MACC, GACC, or JA-ACC. Another unique way to metabolize ACC, is the deamination of ACC. ACC deaminase was first discovered in bacteria. Some plant growth-promoting bacteria are capable of processing the plant-borne ACC by converting it into ammonia and α-ketobutyrate using the enzyme ACC deaminase (Honma and Shimomura, 1978). ACC deaminase was retrieved in for example, Pseudomonas sp. strain ACP (Honma and Shimomura, 1978), Pseudomonas chloroaphis 6G5 (Klee et al., 1991), Pseudomonas putida GR12-2 (Jacobson et al., 1994) and Pseudomonas putida UW4 (Hontzeas et al., 2004).
The bacterial ACC deaminase is a PLP-dependent enzyme with a rather low affinity for ACC (the reported Km value is 1.5–15 mM; Glick et al., 2007). Nonetheless, relatively low concentrations of ACC (100 nM) can already induce the expression of the ACC deaminase gene (acdS), but so do other amino acids like L-alanine, DL-alanine, and DL-valine (Jacobson et al., 1994). acdS expression is also under the regulation of the nitrogen fixation (nif) promotor of some Rhizobia, linking ACC deaminase with nodule formation (Nukui et al., 2006; Nascimento et al., 2012).
Plant growth-promoting bacteria that harbor ACC deaminase must interact with the root environment in order to access plant-produced ACC. It was shown that root exudates contain certain amounts of ACC, which might attract ACC deaminase containing bacteria and establish the rhizosphere interaction (Penrose et al., 2001). It has been proposed that bacterial ACC deaminase can reduce the endogenous ethylene levels of plant roots by limiting the amount of available ACC, which will in turn prevent ethylene-induced root growth inhibition, and thus promote plant growth (Glick et al., 1998, 2007; Glick, 2014). Another model proposes that plant growth-promoting bacteria produce IAA which can be taken up by the plant, and can induce the expression of ACS, resulting in an increase in ACC production, providing a nitrogen supply for the bacteria (Glick et al., 1998, 2007; Glick, 2014).
There are many beneficial effects of ACC deaminase containing bacteria on plant growth, particularly in relation to stress tolerance. For instance, ACC deaminase containing bacteria can reduce stress susceptibility of plants during flooding (Barnawal et al., 2012; Li et al., 2013), drought (Mayak et al., 2004a), salinity (Mayak et al., 2004b; Nadeem et al., 2007, 2010), flower senescence (Nayani et al., 1998; Ali et al., 2012), metal pollution (Glick, 2010), organic pollution (Gurska et al., 2009) and pathogens (Glick, 2014 and references therein). In addition, it has been reported that the presence of ACC deaminase can increase the symbiotic performance of Rhizobial strains (Ma et al., 2003).
Hence, bacterial ACC deaminase is also used as a biotechnological tool to control endogenous ACC levels and consequently lower ethylene production in plants. Transgenic plants overexpressing bacterial ACC deaminase were shown to be more resistant to growth inhibition when confronted with fungal pathogens (Lund et al., 1998; Robison et al., 2001), salt stress (Sergeeva et al., 2006), and metals (Grichko et al., 2000; Nie et al., 2002).
Plants themselves also contain a homolog of the bacterial ACC deaminase. In Arabidopsis, it was demonstrated that the previously known enzyme D-cysteine desulfydrase also possesses ACC deaminase activity (McDonnell et al., 2009). Antisense lines showed a decreased ACC deaminase activity, an increased sensitivity to ACC and produced more ethylene (McDonnell et al., 2009). These results indicate that the plant-specific ACC deaminase might be another metabolic shunt regulating ACC levels and ethylene production in plants.
ACC Transport and its Role During Root Stress
Besides the biosynthesis, conjugation, and catabolism of a hormone, short or long range transport is another important aspect to regulate proper dosage of a hormonal signal within an organism. Often, hormones are synthesized at one site and transported to another site for their action. Hormonal transport from one cell to another is an advanced process, that facilitates tissue specific or long-distance physiological processes or stress responses. Because ethylene is a gaseous molecule, it can freely diffuse from one cell to a neighboring cell, evoking mainly local responses. The presence of aerenchyma or large intercellular voids facilitates rapid long-distance transport of ethylene gas in plant organs. But long-distance ethylene responses can also be achieved by transport of its precursor ACC. Often, but not always, ACC is transported from the roots to the shoot, when the roots are exposed to stress (McManus, 2012). Yet, local ACC transport between cells of the same tissue type and intracellular transport is also possible, illustrating the molecular complexity of ACC transport.
One of the best characterized ACC transport systems is the translocation of ACC from the roots to the shoots of tomato plants suffering from flooding or root hypoxia. A lack of oxygen in the rhizosphere will induce the expression of ACS in the roots (Olson et al., 1995; Shiu et al., 1998) resulting in an increased ACS activity (Bradford et al., 1982; Wang and Arteca, 1992). The excess of ACC in the roots is not converted to ethylene due to a lack of oxygen and the absence of ACO in the roots. Rather, ACC is loaded into the xylem and transported to the shoots (Bradford and Yang, 1980). Once arrived at the shoots, ACC is converted into ethylene by ACO, which is already present in the leaves (English et al., 1995). In tomato, root hypoxia will result in an epinastic response of leaves due to an increased ethylene production (Figure 2A; Doubt, 1917; Jackson and Campbell, 1976). Differential expression of both ACS and ACO during hypoxia was also observed in Arabidopsis (Peng et al., 2005), sunflower seedlings (Finlayson et al., 1991), maize (Atwell et al., 2006; Geisler-Lee et al., 2010), and rice (Zarembinski and Theologis, 1993, 1997; Van Der Straeten et al., 1997, 2001; Zhou et al., 2001). Interestingly, long-distance transport of ACC has also been suggested to occur during other root stress conditions as for example drought (Davies et al., 2000; Sobeih et al., 2004; Skirycz et al., 2011), rehydration after drought (Tudela et al., 1992), nutrient stress (Lynch and Brown, 1997) and salinity (Feng and Barker, 1992; Ghanem et al., 2008).
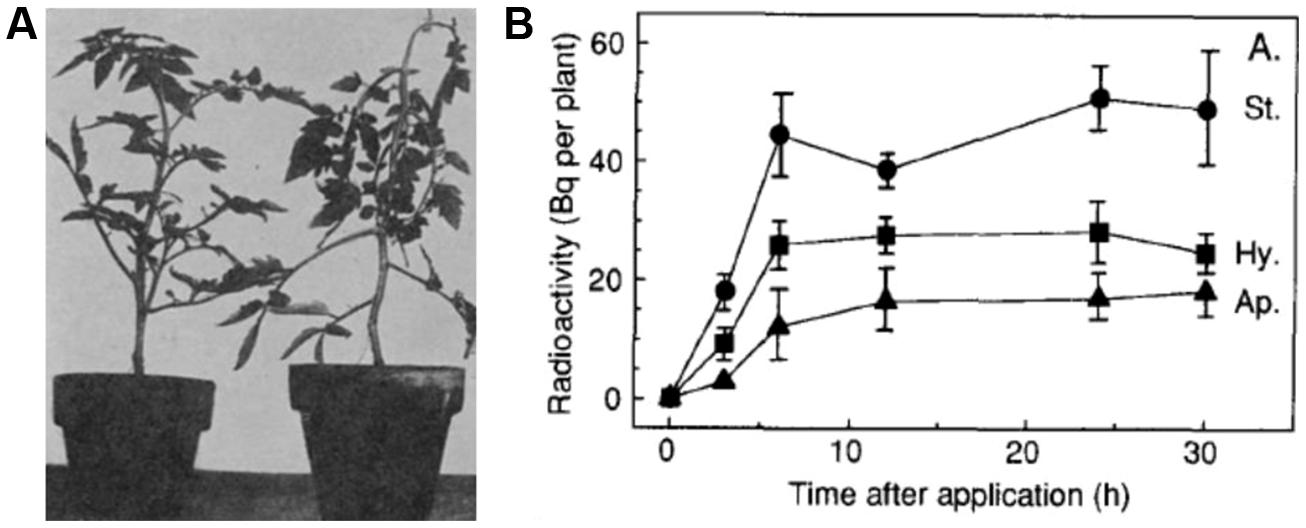
FIGURE 2. 1-Aminocyclopropane-1-carboxylic acid transport in plants. (A) The epinastic response of tomato plants treated with ethylene gas as first observed by Doubt (1917). Figure reproduced from Doubt (1917). (B) ACC translocation via the phloem was demonstrated by measuring total radioactivity over time in the stem (St.), hypocotyl (Hy.), and apex (Ap.) after the foliar application of radioactive ACC to the oldest leaf (leaf 1) of 21 day old cotton plants. Figure reproduced from Morris and Larcombe (1995).
Another long-distance ACC transport system is achieved via the phloem. Foliar applied radioactive ACC was found to be transported via the phloem to other aerial parts in tomato (Amrhein et al., 1982) and in cotton plants (Morris and Larcombe, 1995; Figure 2B). It should be noted that the foliar applied ACC was also rapidly converted into MACC, which was not found to be transported via the phloem (Morris and Larcombe, 1995). This immobility of MACC is in accordance with the earlier observations that MACC is actively transported from the cytosol into the vacuoles, where it could be subsequently stored (Bouzayen et al., 1988, 1989; Tophof et al., 1989). Interestingly, there could be a link between phloem transport of ACC and the Yang cycle. Pommerrenig et al. (2011) showed that Yang cycle genes were specifically expressed in phloem, indicating that recycling of MTA toward SAM is preferentially carried out in this tissue. Perhaps MTA recycling is stimulated by high rates of ACC synthesis in phloem cells, or the recycled SAM forms a pool for phloem-specific ACC production. In roots, spatiotemporal gene expression profiling demonstrated that different ACS isoforms are expressed (but not exclusively) in the vascular tissue (Brady et al., 2007; Dugardeyn et al., 2008). Moreover, the loading of ACC to this tissue could affect the homeostasis of SAM and consequently, polyamines (Pommerrenig et al., 2011).
The exact molecular mechanism by which ACC is loaded into the xylem and/or the phloem and subsequently transported throughout the plant is still unknown, but is an important element in our understanding of long-distance ethylene signaling via ACC and how plants deal with different root and leaf stress conditions.
Long- or medium–long-distance ACC transport was also observed (or speculated) during different developmental processes. Tissue specific gene expression profiling of maize root cells showed that there are differences between ACS and ACO expression patterns (Gallie et al., 2009). ACO was predominantly expressed in the protophloem sieve elements and the companion cells, while ACS was expressed only in the root cortex. This discrepancy led the authors to hypothesize that ACC could be transported from the site of synthesis to the site of consumption, in order to ensure the ethylene production levels observed (Gallie et al., 2009). Differences in ACS and ACO expression patterns predicted in silico in Arabidopsis roots support the same hypothesis (Dugardeyn et al., 2008). A similar reasoning was made by Jones and Woodson (1997, 1999), who observed differences in ACO and ACS transcripts in different cell-types of carnation flower. They also postulated that ACC transport from sites with a high ACS expression (in e.g., petals and styles) secured the ability of ACO to produce ethylene in cells with a high ACO expression (for example the ovaries; Jones and Woodson, 1997, 1999).
Of course one should take into account that gene expression levels not always reflect actual protein levels, and that post-translational modifications can play an important role in protein stability and activity. A targeted metabolomics and proteomics study by Van de Poel et al. (2014b) investigated the tissue specificity of the ethylene biosynthesis pathway in tomato fruit. They observed that the pericarp tissue produced the highest amount of ethylene (and high ACO activity), while the pericarp had the lowest ACS activity and ACC content compared to other tissues. Perhaps ACC is transported from neighboring tissues with a high ACS activity or ACC content such as the locular gel, toward the pericarp in order to secure high rates of ethylene production during climacteric ripening of tomato (Van de Poel et al., 2014b).
Besides long-distance, short-distance intracellular transport of ACC was also observed in barley and wheat mesophyll cells (Tophof et al., 1989) and maize mesophyll cells (Saftner and Martin, 1993). ACC is transported across the tonoplast by carriers that rely on an electrochemical potential gradient of protons, and which are stimulated by the supplementation of ATP (Saftner and Martin, 1993). This intracellular compartmentalization of ACC allows the plant to precisely regulate the cellular pool of ACC, possibly also affecting ethylene biosynthesis.
Clearly, more research is needed to further unravel the exact molecular and biochemical mechanisms which assure intracellular, inter- and intra-tissue and long-distance ACC transport in plants, and their corresponding physiological effects.
ACC as a Signaling Molecule
1-Aminocyclopropane-1-carboxylic acid holds a key position in many physiological processes as it is the direct precursor in the biosynthesis of ethylene. A balanced supply and consumption of ACC is essential to achieve the necessary production level of ethylene within a given spatial and temporal context. As shown above, the pool of ACC is regulated by a complex interaction of production, consumption, modification, and transport. Interestingly, recent findings have suggested a perhaps even more important role for ACC, as a signaling molecule independent from ethylene (Yoon and Kieber, 2013).
A first report by Xu et al. (2008) investigated the role of ACC signaling in relation with FEI1 and FEI2, which are leucine-rich repeat receptor-like kinases. The fei1 fei2 mutant displays a severe defect in anisotropic root growth due to a reduced cellulose microfiber content in the cell wall at the root tip (Figure 3). The fei1 fei2 phenotype can be reversed by the application of ethylene biosynthesis inhibitors, but not by ethylene signaling inhibitors. The application of both aminooxy-acetic acid (AOA) or α-aminoisobutyric acid (AIB) specifically inhibits ethylene biosynthesis and can reverse the phenotype of the fei1 fei2 mutant (Figure 3). AOA is an inhibitor of PLP-dependent enzymes, and will affect the activity of ACS resulting in a reduced ethylene production. AIB on the other hand is a structural analog of ACC and acts as a competitive inhibitor of ACC preventing ethylene production at the level of ACO. Ethylene signaling inhibitors such as 1-methylcyclopropane (1-MCP) and silver ions, did not affect the fei1 fei2 phenotype (Figure 3). Similarly, genetics showed that the fei1 fei2 mutant crossed with etr1-3 (a mutation in the ethylene receptor causing severe ethylene insensitivity), nor ein2-50 (a central regulator of ethylene signaling causing ethylene insensitivity) could reverse the phenotype. All together this study showed that the typical fei1 fei2 phenotype was not affected by ethylene signaling, but could be reversed by ethylene biosynthesis inhibitors. This suggests that the signal reversing the fei1 fei2 phenotype originated independent from ethylene signaling, involved ACS and is possibly ACC itself (Xu et al., 2008).
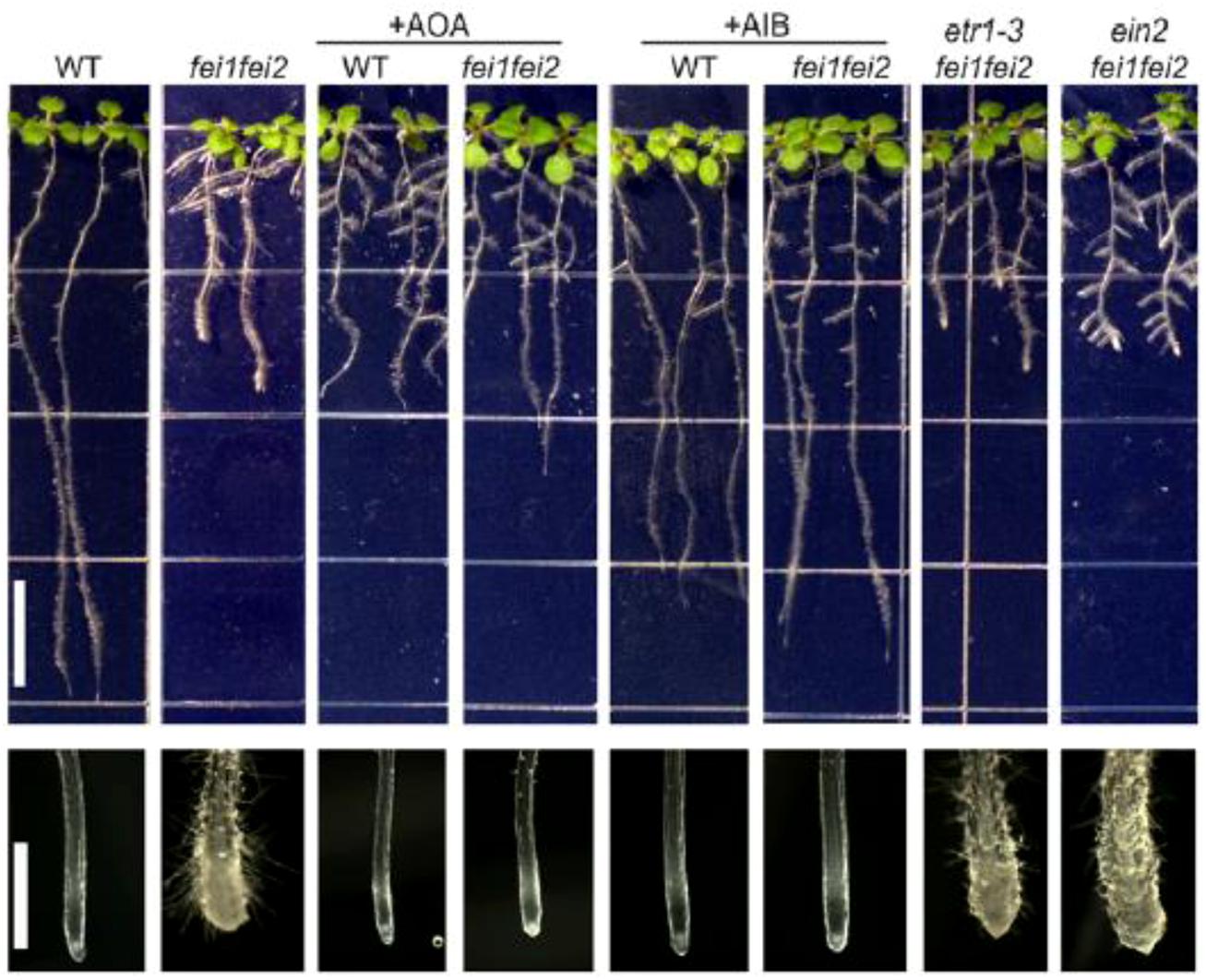
FIGURE 3. Role of ACC/ethylene on the fei phenotype. Root phenotypes of seedlings grown on MS medium containing 0% sucrose for 4 days and then transferred to MS medium containing 4.5% sucrose, or additionally supplemented with AOA (0.375 mM) or AIB (1 mM) as indicated. Note that the distribution of lateral roots in the fei1 fei2 mutants in the presence of high sucrose is variable; the architecture of the fei1 fei2 ein2 triple mutant is not substantially different from that of the fei1 fei2 parent. The close-ups of the root tips clearly show the typical swelling of the fei1 fei2 mutant. (Bar = 1 cm). Figure reproduced from Xu et al. (2008).
A second report by Tsang et al. (2011) linked ACC signaling with cell elongation and cell wall composition of roots. Specific ethylene biosynthesis inhibitors [AVG, AOA, and 2-anilino-7-(4-methoxyphenyl)-7,8-dihydro-5(6H)-quinazolinone (7303)] could reverse the inhibition of root cell expansion which was induced by an isoxaben treatment (a cellulose biosynthesis inhibitor causing cell wall stress). Similarly, as observed by Xu et al. (2008), an ethylene signaling inhibitor (silver ions) could not reverse the isoxaben-induced reduction in root cell elongation. Also, the ein3 eil1 ethylene insensitive mutant responded to ACC and isoxaben, providing genetic evidence of an ACC signaling mechanism independent of ethylene signaling. They also showed that the application of ACC without isoxaben, inhibited root cell elongation and was partially ethylene-dependent and partially ethylene-independent. Altogether, their results demonstrate that monitoring of cell wall integrity requires an ACC sensing/signaling mechanism, which can result in a reduction of root cell elongation, when disrupted. In addition, Tsang et al. (2011) showed that this inhibition of root cell elongation required auxin and reactive oxygen species (ROS) signaling, downstream of ACC signaling.
In a third report (Tsuchisaka et al., 2009) an octuple acs mutant was made to study the interplay between different ACS isoforms. The octuple line was created by introduction of two amiRNA lines (ACS8 and ACS11) into the hexuple mutant acs2,4,5,6,7,9 creating an octuple mutant line with complete or severe inhibition of ACS function. The lines that showed a very strong silencing of ACS8 and ACS11, displayed embryo lethality. This suggests that ethylene biosynthesis (or ACC biosynthesis) is essential for Arabidopsis viability, while this is not the case for the single (ctr1 and ein2) and double (ctr1 ein2) ethylene signaling mutants (Kieber et al., 1993; Roman et al., 1995; Alonso et al., 1999). This phenotypic discrepancy between ethylene biosynthesis and signaling once more suggests that ACC can acts as a signaling molecule itself, independent from ethylene, at least during embryo development and Arabidopsis viability. In addition, Tsuchisaka et al. (2009) characterized a wide variety of physiological and developmental processes of their single and multiple acs knock-out lines, and observed many phenotypes which were similar as for previously described ethylene signaling mutants. But interestingly they also observed several phenotypes like reduced branching, which were not observed in ethylene-insensitive mutants. These discrepancies could again be caused by ACC acting as a signaling molecule. However, it is also possible that individual ACS members have unique roles in developmental processes, and that knocking-out multiple ACS members might result in pleiotropic effects irrelevant to ACC or ethylene metabolism. Finally, it must be noted that such a severe genetic interference in ACC metabolism might also affect upstream SAM or MTA levels or the pool of downstream ACC conjugates, which in turn could signal themselves and affect cell physiology to contribute to the phenotypic differences observed.
All together these reports suggest a role for ACC as a signaling molecule to regulate plant development and growth, independent from ethylene. The exact molecular mechanism by which ACC signaling operates, and whether or not there is an ACC receptor and downstream signaling components, remains to be investigated. Future biochemical studies with specific ethylene biosynthesis and signaling inhibitors, in combination with genetics to create higher order ethylene biosynthesis/signaling mutants (like etr1ers1etr2ein4ers2ctr1ein2 or multiple aco knock-outs), could shed light on the role of ACC as a signaling molecule. It also still needs to be elucidated whether this unique title of “signaling molecule” is to be awarded to ACC, or rather to one if its (unknown) downstream derivatives.
Conclusion
Over the years, a lot of work has been done on ACC since its discovery in 1979, and it has become clear that ACC is more than just the precursor of ethylene. Its role in ethylene biosynthesis is well characterized, although there are still many questions concerning the two unique proteins that are associated with ACC in ethylene biosynthesis: ACS and ACO. Pioneering work on the characterization of post-translational modifications and the combinatorial interplay of ACS isoforms, has opened our eyes to the complex regulation of ethylene biosynthesis at the protein level. More mechanistic details are to be uncovered, probably including a complex post-translational control of ACO. Furthermore, ACC is conjugated into MACC, GACC, and JA-ACC. These derivatives are an elegant biochemical shunt to regulate the pool of ACC available for ethylene production, although it remains rather speculative what the exact biological roles are for these ACC conjugates. A better characterization of the participating enzymes is necessary to further elucidate the importunateness of ACC derivatives. Furthermore, ACC can also be used by bacterial (and plant) ACC-deaminase, adding another layer of metabolic complexity to the regulation of ACC levels. It is also well established that ACC can be easily transported over short (intracellular and intra-tissue) and long-distances (via the xylem and phloem), providing the plant with an elaborate system to control local and remote ethylene responses. Last but not least, ACC has been identified as a potential signaling molecule, independent of ethylene. This property of ACC is perhaps the most exciting, opening new avenues in ACC research, with potentially profound effects on plant physiology. The molecular mechanism by which ACC is signaling and the identity of other putative signaling components in such an ‘ACC pathway’ remain to be discovered.
Author Contributions
Bram Van de Poel and Dominique Van Der Straeten conceived the topic and wrote the manuscript
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a Belgian American Educational Foundation Fellowship, a CBMG/CMNS Merit Postdoctoral Fellowship (University of Maryland) and a BOF Postdoctoral Fellowship (Ghent University) to Bram Van de Poel. Dominique Van Der Straeten gratefully acknowledges support from the Research Foundation Flanders (FWO) and Ghent University.
References
Adams, D. O., and Yang, S. F. (1977). Methionine metabolism in apple tissue – implication of S-adenosylmethionine as an intermediate in conversion of methionine to ethylene. Plant Physiol. 60, 892–896. doi: 10.1104/pp.60.6.892
Adams, D. O., and Yang, S. F. (1979). Ethylene biosynthesis – identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc. Natl. Acad. Sci. U.S.A. 76, 170–174. doi: 10.1073/pnas.76.1.170
Ali, S., Charles, T. C., and Glick, B. R. (2012). Delay of carnation flower senescence by bacterial endo- phytes expressing ACC deaminase. J. Appl. Microbiol. 113, 1139–1144. doi: 10.1111/j.1365-2672.2012.05409.x
Alonso, J. M., Hirayama, T., Roman, G., Nourizadeh, S., and Ecker, J. R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284, 2148–2152. doi: 10.1126/science.284.5423.2148
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Amrhein, A., Schneebeck, D., Skorupka, H., Stockigt, J., and Tophof, S. (1981). Identification of a major metabolite of the ethylene precursor 1-aminocyclopropane-l-carboxylic acid in higher Plants. Naturwissenschaften 68, 619–620. doi: 10.1007/BF00398617
Amrhein, N., Breuing, F., Eberle, J., Skorupka, H., and Tophof, S. (1982). “The metabolism of 1-aminocyclopropane-1-carboxylic acid,” in Plant Growth Substances, ed. P. H. Wareigng (London: Academic Press), 249–258
Atwell, B. J., Drew, M. C., and Jackson, M. B. (2006). The influence of oxygen deficiency on ethylene synthesis, 1-aminocyclopropane-1-carboxylic acid levels and aerenchyma formation in roots of Zea mays. Physiol. Plant. 71, 15–22.
Barnawal, D., Bharti, N., Maji, D., Chanotiya, C. S., and Kalra, A. (2012). 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation. Plant Physiol. Biochem. 58, 227–235. doi: 10.1016/j.plaphy.2012.07.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bidonde, S., Ferrer, M. A., Zegzouti, H., Ramassamy, S., Latche, A., Pech, J. C.,et al. (1998). Expression and characterization of three tomato 1-aminocyclopropane-1-carboxylate oxidase cDNAs in yeast. Eur. J. Biochem. 253, 20–26. doi: 10.1046/j.1432-1327.1998.2530020.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Boller, T., Herner, R. C., and Kende, H. (1979). Assay for and enzymatic formation of an ethylene precursor, 1-aminocyclopropane-l-carboxylic acid. Planta 145, 293–303. doi: 10.1007/BF00454455
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Botella, J. R., Arteca, R. N., and Frangos, J. (1995). A mechanical strain-induced 1-aminocyclopropane-1-carboxylic acid synthase gene. Proc. Natl. Acad. Sci. U.S.A. 92, 1595–1598. doi: 10.1073/pnas.92.5.1595
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bouzayen, M., Latche, A., Alibert, G., and Pech, J. C. (1988). Intracellular sites of synthesis and storage of 1-(malonylamino)cyclopropane-1-carboxylic acid in acer-pseudoplatanus cells. Plant Physiol. 88, 613–617. doi: 10.1104/pp.88.3.613
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bouzayen, M., Latche, A., Pech, J. C., and Marigo, G. (1989). Carrier-mediated uptake of 1-(malonylamino)cyclopropane-1-carboxylic acid in vacuoles isolated from catharanthus-roseus cells. Plant Physiol. 91, 1317–1322. doi: 10.1104/pp.91.4.1317
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bradford, K. J., Hsiao, T. C., and Yang, S. F. (1982). Inhibition of ethylene synthesis in tomato plants subjected to anaerobic root stress. Plant Physiol. 70, 1503–1507. doi: 10.1104/pp.70.5.1503
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bradford, K. J., and Yang, S. F. (1980). Xylem transport of 1-aminocyclopropane-1-carboxylic acid, an ethylene precursor, in waterlogged tomato plants. Plant Physiol. 65, 322–326. doi: 10.1104/pp.65.2.322
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brady, S. M., Orlando, D. A., Lee, J.-Y., Koch, J., Dinney, J. R., Mace, D.,et al. (2007). A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318, 801–806. doi: 10.1126/science.1146265
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Capitani, G., Hohenester, E., Feng, L., Storici, P., Kirsch, J. F., and Jansonius, J. N. (1999). Structure of 1-aminocyclopropane-1-carboxylate synthase, a key enzyme in the biosynthesis of the plant hormone ethylene. J. Mol. Biol. 294, 745–756. doi: 10.1006/jmbi.1999.3255
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cary, A. J., Liu, W., and Howell, S. H. (1995). Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elonagion. Plant Physiol. 107, 1075–1082. doi: 10.1104/pp.107.4.1075
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chae, H. S., Faure, F., and Kieber, J. J. (2003). The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell 15, 545–559. doi: 10.1105/tpc.006882
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chae, H. S., and Kieber, J. J. (2005). Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci. 10, 291–296. doi: 10.1016/j.tplants.2005.04.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Christians, M. J., Gingerich, D. J., Hansen, M., Binder, B. M., Kieber, J. J., and Vierstra, R. D. (2009). The BTB ubiquitin ligases ETO1, EOL1, and EOL2 act collectively to regulate ethylene biosynthesis in Arabidopsis by controlling type-2 ACC synthase levels. Plant J. 57, 332–345. doi: 10.1111/j.1365-313X.2008.03693.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chung, M. C., Chou, S. J., Kuang, L. Y., Charng, Y. Y., and Yang, S. F. (2002). Subcellular localization of 1-aminocyclopropane-1-carboxylic acid oxidase in apple fruit. Plant Cell Physiol. 43, 549–554. doi: 10.1093/pcp/pcf067
Davies, W. J., Bacon, M. A., Thompson, D. S., Sobeih, W., and Rodriguez, L. G. (2000). Regulation of leaf and fruit growth in plants growing in drying soil: exploitation of the plants’ chemical signalling system and hydraulic architecture to increase the efficiency of water use in agriculture. J. Exp. Bot. 51, 1617–1626. doi: 10.1093/jexbot/51.350.1617
De Paepe, A., Vuylsteke, M., Van Hummelen, P., Zabeau, M., and Van Der Staeten, D. (2004). Transcriptional profiling by cDNA-AFLP and microarray analysis reveals novel insights into the early response to ethylene in Arabidopsis. Plant J. 39, 537–559. doi: 10.1111/j.1365-313X.2004.02156.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dilley, D. R., Wang, W., Kadirjan-Kalbach, D., Ververidis, F., Beaudry, R., and Padmanabhan, K. (2013). 1-Aminocyclopropane-1-carboxylic acid oxidase reaction mechanism and putative post-translational activities of the ACCO protein. AoB Plants 5:plt031. doi: 10.1093/aobpla/plt031
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dong, J. G., Fernandezmaculet, J. C., and Yang, S. F. (1992). Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proc. Natl. Acad. Sci. U.S.A. 89, 9789–9793. doi: 10.1073/pnas.89.20.9789
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Doubt, S. L. (1917). The response of plants to illuminating gas. Bot. Gaz. 63, 209–224. doi: 10.1086/332006
Dugardeyn, J., Vandenbussche, F., and Van Der Straeten, D. (2008). To grow or not to grow: what can we learn on ethylene-gibberellin cross-talk by in silico gene expression analysis. J. Exp. Bot. 59, 1–16. doi: 10.1093/jxb/erm349
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
English, P. J., Lycett, G. W., Roberts, J. A., and Jackson, M. B. (1995). Increased 1-aminocyclopropane-1-carboxylic acid oxidase activity in shoots of flooded tomato plants raises ethylene production to physiologically active levels. Plant Physiol. 109, 1435–1440.
Feng, J. N., and Barker, A. V. (1992). Ethylene evolution and ammonium accumulation by tomato plants under water and salinity stresses. J. Plant Nutr. 15, 2471–2490. doi: 10.1080/01904169209364488
Finlayson, S. A., Foster, K. R., and Reid, D. M. (1991). Transport and metabolism of 1-aminocyclopropane- 1-carboxylic acid in sunflower (Helianthus annuus L.) Seedlings. Plant Physiol. 96, 1360–1367. doi: 10.1104/pp.96.4.1360
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gallie, D. R., Geisler-Lee, J., Chen, J., and Jolley, B. (2009). Tissue-specific expression of the ethylene biosynthetic machinery regulates root growth in maize. Plant Mol. Biol. 69, 195–211. doi: 10.1007/s11103-008-9418-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gane, R. (1934). Production of ethylene by some ripening fruits. Nature 134, 1008–1008. doi: 10.1038/1341008a0
Geisler-Lee, J., Caldwell, C., and Gallie, D. R. (2010). Expression of the ethylene biosynthetic machinery in maize roots is regulated in response to hypoxia. J. Exp. Bot. 61, 857–871. doi: 10.1093/jxb/erp362
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ghanem, M. E., Albacete, A., Martinez-Andujar, C., Acosta, M., Romero-Aranda, R., Dodd, I. C.,et al. (2008). Hormonal changes during salinity-induced leaf senescence in tomato (Solanum lycopersicum L.). J. Exp. Bot. 59, 3039–3050. doi: 10.1093/jxb/ern153
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Glick, B. R. (2010). Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 28, 367–374. doi: 10.1016/j.biotechadv.2010.02.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Glick, B. R. (2014). Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 169, 30–39. doi: 10.1016/j.micres.2013.09.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Glick, B. R., Cheng, Z., Czarny, J., and Duan, J. (2007). Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur. J. Plant Pathol. 119, 329–339. doi: 10.1007/s10658-007-9162-4
Glick, B. R., Penrose, D. M., and Li, J. (1998). A model for the lowering of plant ethylene concentrations by growth-promoting rhizobacteria. J. Theor. Biol. 190, 63–68. doi: 10.1006/jtbi.1997.0532
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Grichko, V. P., Filby, B., and Glick, B. R. (2000). Increased ability of transgenic plants expressing the bacterial enzyme ACC deaminase to accumulate Cd, Co, Cu, Ni, Pb, and Zn. J. Biotechnol. 81, 45–53. doi: 10.1016/S0168-1656(00)00270-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gurska, J., Wang, W., Gerhardt, K. E., Khalid, A. M., Isherwood, D. M., Huang, X.-D.,et al. (2009). Field test of a multi-process phytoremediation system at a petroleum sludge contam-inated land farm. Environ. Sci. Technol. 43, 4472–4479. doi: 10.1021/es801540h
Hanley, K. M., Meir, S., and Bramlage, W. J. (1989). Activity of aging carnation flower parts and the effects of 1-(malonylamino)cyclopropane-1-carboxylic acid-induced ethylene. Plant Physiol. 91, 1126–1130. doi: 10.1104/pp.91.3.1126
Hoffman, N. E., Yang, S. F., and Mckeon, T. (1982). Identification of 1-(malonylamino)cyclopropane-1-carboxylic acid as a major conjugate of 1-aminocyclopropane-1-carboxylic acid, an ethylene precursor in higher-plants. Biochem. Biophys. Res. Commun. 104, 765–770. doi: 10.1016/0006-291X(82)90703-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Honma, M., and Shimomura, T. (1978). Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric. Biol. Chem. 43, 1825–1831. doi: 10.1271/bbb1961.42.1825
Hontzeas, N., Zoidakis, J., Glick, B. R., and Abu-Omar, M. M. (2004). Expression and characterization of 1-aminocyclopropane-1-carboxylate deaminase from the rhizobacterium Pseudomonas putida UW4: a key enzyme in bacterial plant growth promotion. Biochim. Biophys. Acta 1703, 11–19. doi: 10.1016/j.bbapap.2004.09.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hudgins, J. W., Ralph, S. G., Franceschi, V. R., and Bohlmann, J. (2006). Ethylene in induced conifer defense: cDNA cloning, protein expression, and cellular and subcellular localization of 1-aminocyclopropane-1-carboxylate oxidase in resin duct and phenolic parenchyma cells. Planta 224, 865–877. doi: 10.1007/s00425-006-0274-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jackson, M. B., and Campbell, D. J. (1976). Waterlogging and petiole epinasty in tomato – role of ethylene and low oxygen. New Phytol. 76, 21–29. doi: 10.1111/j.1469-8137.1976.tb01434.x
Jacobson, C. B., Pasternak, J. J., and Glick, B. R. (1994). Partial purification and characterization of ACC deaminase from the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2. Can. J. Microbiol. 40, 1019–1025. doi: 10.1139/m94-162
Jiao, X. Z., Philosoph-Hadas, S., Su, L. Y., and Yang, S. F. (1986). The conversion of 1-(malonylamino)cyclopropane-1-carboxylic acid to 1-aminocyclo propane- 1-carboxylic acid in plant tissues. Plant Physiol. 81, 637–641. doi: 10.1104/pp.81.2.637
Jones, M., and Woodson, W. (1999). Differential expression of three members of the 1-aminocyclopropane-1-carboxylate synthase gene family in carnation. Plant Physiol. 119, 755–764. doi: 10.1104/pp.119.2.755
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jones, M. L., and Woodson, W. R. (1997). Pollination-induced ethylene incarnation. Role of stylar ethylene in corolla senescence. Plant Physiol. 115, 205–212.
Joo, S., Liu, Y., Lueth, A., and Zhang, S. (2008). MAPK phosphorylation-induced stabilization of ACS6 protein is mediated by the non-catalytic C-terminal domain, which also contains the cis-determinant for rapid degradation by the 26S proteasome pathway. Plant J. 54, 129–140. doi: 10.1111/j.1365-313X.2008.03404.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kamiyoshihara, Y., Iwata, M., Fukaya, T., Tatsuki, M., and Mori, H. (2010). Turnover of LeACS2, a wound-inducible 1-aminocyclopropane-1-carboxylic acid synthase in tomato, is regulated by phosphorylation/dephosphorylation. Plant J. 64, 140–150. doi: 10.1111/j.1365-313X.2010.04316.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kieber, J. J., Rothenberg, M., Roman, G., Feldmann, K. A., and Ecker, J. R. (1993). CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72, 427–441. doi: 10.1016/0092-8674(93)90119-B
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kim, C. Y., Liu, Y., Thorne, E. T., Yang, H., Fukushige, H., Gassmann, W.,et al. (2003). Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant Cell 15, 2707–2718. doi: 10.1105/tpc.011411
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Klee, H. J., Hayford, M. B., Kretzmer, K. A., Barry, G. F., and Kishmore, G. M. (1991). Control of ethylene synthesis by expression of a bacterial enzyme in transgenic tomato plants. Plant Cell 3, 1187–1193. doi: 10.1105/tpc.3.11.1187
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Knight, L. I., Rose, R. C., and Crocker, W. (1910). Effects of various gases and vapors upon etiolated seedlings of the sweet pea. Science 31, 635–636.
Li, J., McConkey, B. J., Cheng, Z., Guo, S., and Glick, B. R. (2013). Identification of plant growth-promoting bacteria-responsive proteins in cucumber roots under hypoxic stress using a proteomic approach. J. Proteomics 84, 119–131. doi: 10.1016/j.jprot.2013.03.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Liang, X., Shen, N. F., and Theologis, A. (1996). Li+-regulated 1-aminocyclopropane-1-carboxylate synthase gene expression in Arabidopsis thaliana. Plant J. 10, 1027–1036. doi: 10.1046/j.1365-313X.1996.10061027.x
Lieberman, M., and Mapson, L. W. (1964). Genesis and biogenesis of ethylene. Nature 204, 343–345. doi: 10.1038/204343a0
Liu, Y., Su, L. Y., and Yang, S. F. (1985a). Ethylene promotes the capability to malonylate 1-aminocyclopropane-1-carboxylic acid and D-amino acids in preclimacteric tomato fruits. Plant Physiol. 77, 891–895. doi: 10.1104/pp.77.4.891
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Liu, Y., Hoffman, N. E., and Yang, S. F. (1985b). Ethylene-promoted malonylation of 1-aminocyclopropane-1-carboxylic acid participated in autoinhibition of ethylene synthesis in grapefruit flavedo disks. Planta 164, 565–568. doi: 10.1007/BF00395976
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Liu, Y., and Zhang, S. (2004). Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16, 3386–3399. doi: 10.1105/tpc.104.026609
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ludwikow, A., Ciesla, A., Kasprowicz-Maluski, A., Mitula, F., Tajdel, M., Galganski, L.,et al. (2014). Arabidopsis protein phosphatase 2C ABI1 interacts with type I ACC synthases and is involved in the regulation of ozone-induced ethylene biosynthesis. Mol. Plant 7, 960–967. doi: 10.1093/mp/ssu025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lund, S. T., Stall, R. E., and Klee, H. J. (1998). Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell 10, 371–382. doi: 10.1105/tpc.10.3.371
Lynch, J., and Brown, K. M. (1997). Ethylene and plant responses to nutritional stress. Physiol. Plant. 100, 613–619. doi: 10.1111/j.1399-3054.1997.tb03067.x
Lyzenga, W. J., Booth, J. K., and Stone, S. L. (2012). The Arabidopsis RING-type E3 ligase XBAT32 mediates the proteasomal degradation of the ethylene biosynthetic enzyme, 1-aminocyclopropane-1-carboxylate synthase 7. Plant J. 71, 23–34. doi: 10.1111/j.1365-313X.2012.04965.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ma, W., Guinel, F. C., and Glick, B. R. (2003). Rhizobium leguminosarum biovar viciae 1-aminocyclopropane-1-carboxylate deaminase promotes nodulation of pea plants. Appl. Environ. Microbiol. 69, 4396–4402. doi: 10.1128/AEM.69.8.4396-4402.2003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martin, M. N., Cohen, J. D., and Saftner, R. A. (1995). A new 1-aminocyclopropane-1-carboxylic acid-conjugating activity in tomato fruit. Plant Physiol. 109, 917–926. doi: 10.1104/pp.109.3.917
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martin, M. N., and Saftner, R. A. (1995). Purification and characterization of 1-aminocyclopropane-1-carboxylic acid N-malonyltransferase from tomato fruit. Plant Physiol. 108, 1241–1249.
Martin, M. N., Saladores, P. H., Lambert, E., Hudson, A. O., and Leustek, T. (2007). Localization of members of the gamma-glutamyl transpeptidase family identifies sites of glutathione and glutathione S-conjugate hydrolysis. Plant Physiol. 144, 1715–1732. doi: 10.1104/pp.106.094409
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martin, M. N., and Slovin, J. P. (2000). Purified γ-glutamyl transpeptidases from tomato exhibit high affinity for glutathione and glutathione S-conjugates. Plant Physiol. 122, 1417–1426. doi: 10.1104/pp.122.4.1417
Mayak, S., Tirosh, T., and Glick, B. R. (2004a). Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 166, 525–530. doi: 10.1016/j.plantsci.2003.10.025
Mayak, S., Tirosh, T., and Glick, B. R. (2004b). Plant growth-promoting bacteria that confer resistance in tomato to salt stress. Plant Physiol. Biochem. 42, 565–572. doi: 10.1016/j.plaphy.2004.05.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
McDonnell, L., Plett, J. M., Andersson-Gunneras, S., Kozela, C., Dugardeyn, J., Van Der Straeten, D.,et al. (2009). Ethylene levels are regulated by a plant encoded 1-aminocyclopropane-1-carboxylic acid deaminase. Physiol. Plant. 136, 94–109. doi: 10.1111/j.1399-3054.2009.01208.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
McManus, M. T. (2012). The Plant Hormone Ethylene – Annual Plant Reviews. Hoboken, NJ: Wiley-Blackwell. doi: 10.1002/9781118223086
Miller, J. M., and Conn, E. E. (1980). Metabolism of hydrogen cyanide by higher plants. Plant Physiol. 65, 1199–1202. doi: 10.1104/pp.65.6.1199
Morris, D., and Larcombe, N. J. (1995). Phloem transport and conjugation of foliar-applied 1-aminoc clopropane-1-carboxylic acid in cotton (Gossypium hirsutum L.). J. Plant Physiol. 146, 429–436. doi: 10.1016/S0176-1617(11)82004-3
Murphy, L. J., Robertson, K. N., Harroun, S. G., Brosseau, C. L., Werner-Zwanziger, U., Moilanen, J.,et al. (2014). A simple complex on the verge of breakdown: isolation of the elusive cyanoformate ion. Science 344, 75–78. doi: 10.1126/science.1250808
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Murr, D. P., and Yang, S. F. (1975). Conversion of 5′-methylthioadenosine to methionine by apple tissue. Phytochemistry 14, 1291–1292. doi: 10.1016/S0031-9422(00)98613-8
Nadeem, S. M., Zahair, Z. A., Naveed, M., and Arshad, M. (2007). Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Can. J. Microbiol. 53, 1141–1149. doi: 10.1139/W07-081
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nadeem, S. M., Zahair, Z. A., Naveed, M., Asghar, H. N., and Asghar, M. (2010). Rhizobacteria capable of producing ACC-deaminase may mitigate salt stress in wheat. Soil Sci. Soc. Am. J. 74, 533–542. doi: 10.2136/sssaj2008.0240
Nascimento, F. X., Brígido, C., Glick, B. R., Oliveira, S., and Alho, L. (2012). Mesorhizobium ciceri LMS-1 expressing an exogenous 1-aminocyclopropane-1-carboxylate (ACC) deaminase increases its nodulation abilities and chickpea plant resistance to soil constraints. Lett. Appl. Microbiol. 55, 15–21. doi: 10.1111/j.1472-765X.2012.03251.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nayani, S., Mayak, S., and Glick, B. R. (1998). The effect of plant growth promoting rhizobacteria on the senescence of flower petals. Ind. J. Exp. Biol. 36, 836–839.
Neljubov, D. (1901). Uber die horizontale nutation der Stengel von Pisum sativum und einiger anderer Pflanzen. Beih. Bot. Centralb. 10, 128–139.
Nie, L., Shah, S., Burd, G. I., Dixon, D. G., and Glick, B. R. (2002). Phytoremediation of arsenate contam-inated soil by transgenic canola and the plant growth-promoting bacterium Enterobacter cloacae CAL2. Plant Physiol. Biochem. 40, 355–361. doi: 10.1016/S0981-9428(02)01375-X
Nukui, N., Minamisawa, K., Ayabe, S.-I., and Aoki, T. (2006). Expression of the 1-aminocyclopropane-1-carboxylic acid deaminase gene requires symbiotic nitrogen-fixing regulator gene nifA2 in Mesorhizobium loti MAFF303099. Appl. Environ. Microbiol. 72, 4964–4969. doi: 10.1128/AEM.02745-05
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Olson, D. C., Oetiker, J. H., and Yang, S. F. (1995). Analysis of LE-ACS3, a 1-aminocyclopropane-1-carboxylic acid synthase gene expressed during flooding in the roots of tomato plants. J. Biol. Chem. 270, 14056–14061. doi: 10.1074/jbc.270.23.14056
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Peiser, G., and Yang, S. F. (1998). Evidence for 1-(malonylamino)cyclopropane-1-carboxylic acid being the major conjugate of aminocyclopropane-1-carboxylic acid in tomato fruit. Plant Physiol. 116, 1527–1532. doi: 10.1104/pp.116.4.1527
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Peng, H.-P., Lin, T.-Y., Wang, N.-N., and Shih, M. C. (2005). Differential expression of genes encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis during hypoxia. Plant Mol. Biol. 58, 15–25. doi: 10.1007/s11103-005-3573-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Penrose, D. M., Moffatt, B. A., and Glick, B. R. (2001). Determination of 1-aminocyclopropane-1-carboxylic acid (ACC) to assess the effects of ACC deaminase-containing bacteria on roots of canola seedlings. Can. J. Microbiol. 47, 77–80. doi: 10.1139/w00-128
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Philosoph-Hadas, S., Meir, S., and Aharoni, N. (1985). Autoinhibition of ethylene production in tobacco leaf-disks – enhancement of 1-aminocyclopropane-1-carboxylic acid conjugation. Physiol. Plant. 63, 431–437. doi: 10.1111/j.1399-3054.1985.tb02322.x
Pommerrenig, B., Feussner, K., Zierer, W., Rabinovych, V., Klebl, F., Feussner, I.,et al. (2011). Phloem-specific expression of Yang cycle genes and identification of novel Yang cycle enzymes in Plantago and Arabidopsis. Plant Cell 23, 1904–1919. doi: 10.1105/tpc.110.079657
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ramassamy, S., Olmos, E., Bouzayen, M., Pech, J. C., and Latche, A. (1998). 1-aminocyclopropane-1-carboxylate oxidase of apple fruit is periplasmic. J. Exp. Bot. 49, 1909–1915. doi: 10.1093/jexbot/49.329.1909
Reinhardt, D., Kende, H., and Boller, T. (1994). Subcellular-localization of 1-aminocyclopropane-1-carboxylate oxidase in tomato cells. Planta 195, 142–146. doi: 10.1007/BF00206302
Robison, M. M., Shah, S., Tamot, B., Pauls, K. P., Moffatt, B. A., and Glick, B. R. (2001). Reduced symptoms of Verticillium wilt in transgenic tomato expressing a bacterial ACC deaminase. Mol. Plant Pathol. 2, 135–145. doi: 10.1046/j.1364-3703.2001.00060.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rocklin, A. M., Kato, K., Liu, H. W., Que, L., and Lipscomb, J. D. (2004). Mechanistic studies of 1-aminocyclopropane-1-carboxylic acid oxidase: single turnover reaction. J. Biol. Inorg. Chem. 9, 171–182. doi: 10.1007/s00775-003-0510-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rodrigues-Pousada, R. A., De Rycke, R., Dedonder, A., Van Caeneghem, W., Engler, G., Van Montagu, M.,et al. (1993). The Arabidopsis 1-amioncyclopropane-1-carboxylate synthase gene 1 is expressed during early development. Plant Cell 5, 897–891. doi: 10.1105/tpc.5.8.897
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Roman, G., Lubarsky, B., Kieber, J. J., Rothenberg, M., and Ecker, J. R. (1995). Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics 139, 1393–1409. doi: 10.1186/1471-2164-9-44
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rombaldi, C., Lelievre, J. M., Latche, A., Petitprez, M., Bouzayen, M., and Pech, J. C. (1994). Immunocytolocalization of 1-aminocyclopropane-1-carboxylic acid oxidase in tomato and apple fruit. Planta 192, 453–460. doi: 10.1007/BF00203582
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Saftner, R. A., and Martin, M. N. (1993). Transport of 1-aminocyclopropane-1-carboxylic acid into isolated maize mesophyll vacuoles. Physiol. Plant. 87, 535–543. doi: 10.1111/j.1399-3054.1993.tb02504.x
Sato, T., and Theologis, A. (1989). Cloning the mRNA encoding 1-aminocycloproane-1-carboxylate synthase, the key enzyme for ethylene biosynthesis in plants. Proc. Natl. Acad. Sci. U.S.A. 86, 6621–6625. doi: 10.1073/pnas.86.17.6621
Sauter, M., Moffatt, B. M., Saechao, M. C., Hell, R., and Wirtz, M. (2013). Methionine salvage and S-adenosylmethionine: essential links between sulfur, ethylene and polyamine biosynthesis. Biochem. J. 451, 145–154. doi: 10.1042/BJ20121744
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sergeeva, E., Shah, S., and Glick, B. R. (2006). Tolerance of transgenic canola expressing a bacterial ACC deaminase gene to high concentrations of salt. World J. Microbiol. Biotechnol. 22, 277–282. doi: 10.1007/s11274-005-9032-1
Shiu, O. Y., Oetiker, J. H., Yip, W. K., and Yang, S. F. (1998). The promoter of LE-ACS 7, an early flooding-induced 1-aminocyclopropane-1-carboxylate synthase gene of the tomato, is tagged by a Sol 3 transposon. Proc. Natl. Acad. Sci. U.S.A. 95, 10334–10339. doi: 10.1073/pnas.95.17.10334
Skirycz, A., Claes, H., De Bodt, S., Oikawa, A., Shinoda, S., Andriankaja, M.,et al. (2011). Pause-and-stop: the effects of osmotic stress on cell proliferation during early leaf development in Arabidopsis and a role for ethylene signaling in cell cycle arrest. Plant Cell 23, 1876–1888. doi: 10.1105/tpc.111.084160
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Skottke, K. R., Yoon, G. M., Kieber, J. J., and DeLong, A. (2011). Protein phosphatase 2A controls ethylene biosynthesis by differentially regulating the turnover of ACC synthase isoforms. PLoS Genet. 7:e1001370. doi: 10.1371/journal.pgen.1001370.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sobeih, W. Y., Dodd, I. C., Bacon, M. A., Grierson, D., and Davies, W. J. (2004). Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. J. Exp. Bot. 55, 2353–2363. doi: 10.1093/jxb/erh204
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Staswick, P. E., and Tiryaki, I. (2004). The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16, 2117–2127. doi: 10.1105/tpc.104.023549
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tatsuki, M., and Mori, H. (2001). Phosphorylation of tomato 1-aminocyclopropane-1-carboxylic acid synthase, LE-ACS2, at the C-terminal region. J. Biol. Chem. 276, 28051–28057. doi: 10.1074/jbc.M101543200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tophof, S., Martinoia, E., Kaiser, G., Hartung, W., and Amrhein, N. (1989). Compartmentation and transport of 1-aminocyclopropane-1-carboxylic acid and N-malonyl-1-aminocyclopropane-1-carboxylic acid in barley and wheat mesophyll-cells and protoplasts. Physiol. Plant. 75, 333–339. doi: 10.1111/j.1399-3054.1989.tb04635.x
Tsang, D. L., Edmond, C., Harrington, J. L., and Nühse, T. S. (2011). Cell wall integrity controls root elongation via a general 1-aminocyclopropane-1-carboxylic acid-dependent, ethylene-independent pathway. Plant Physiol. 156, 596–604. doi: 10.1104/pp.111.175372
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tsuchisaka, A., and Theologis, A. (2004). Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol. 136, 2982–3000. doi: 10.1104/pp.104.049999
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tsuchisaka, A., Yu, G., Jin, H., Alonso, J. M., Ecker, J. R., Zhang, X.,et al. (2009). A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics 183, 979–1003. doi: 10.1534/genetics.109.107102
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tudela, D., Primo-millo, E., Citricultura, D., De Valencia, I., and Horta, M. D. (1992). 1-aminocyclopropane-1-carboxylic acid transported from roots to shoots promotes leaf abscission in Cleopatra Mandarin (Citrus reshni Hort. ex Tan.) seedlings rehydrated after water stress. Plant Physiol. 100, 131–137. doi: 10.1104/pp.100.1.131
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vahala, J., Schlagnhaufer, C. D., and Pell, E. J. (1998). Induciton of an ACC synthase cDNA by ozone in light-grown Arabidopsis thaliana leaves. Physiol. Plant. 103, 45–50. doi: 10.1034/j.1399-3054.1998.1030106.x
Van de Poel, B., Bulens, I., Hertog, M. L. A. T. M., Nicolai, B., and Geeraerd, A. (2014a). A transcriptomics-based kinetic model for ethylene biosynthesis in tomato (Solanum lycopersicum) fruit: development, validation and exploration of novel regulatory mechanisms. New Phytol. 202, 952–963. doi: 10.1111/nph.12685
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Van de Poel, B., Bulens, I., Markoula, A., Hertog, M. L. A. T., Dreesen, R., Wirtz, M.,et al. (2012). Targeted systems biology profiling of tomato fruit reveals coordination of the Yang cycle and a distinct regulation of ethylene biosynthesis during postclimacteric ripening. Plant Physiol. 160, 1498–1514. doi: 10.1104/pp.112.206086
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Van de Poel, B., Vandenzavel, N., Smet, C., Nicolay, T., Bulens, I., Mellidou, I.,et al. (2014b). Tissue specific analysis reveals a differential organization and regulation of both ethylene biosynthesis and E8 during climacteric ripening of tomato. BMC Plant Biol. 14:11. doi: 10.1186/1471-2229-14-11
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Van Der Straeten, D., Anuntalabhochai, S., Van Caeneghem, W., Zhou, Z., Gielen, J., and Van Montagu, M. (1997). Expression of three members of the ACC synthase gene family in deepwater rice by submergence, wounding and hormonal treatments. Plant Sci. 124, 79–87. doi: 10.1016/S0168-9452(97)04609-8
Van Der Straeten, D., Rodrigues-Pousada, R. A., Villarroel, R., Hnaley, S., Goodman, H. M., and Van Montagu, M. (1992). Cloning, genetic mapping, and expression analysis of an Arabidopsis thaliana gene that encodes 1-aminocycloropane-1-carboxlylate synthase. Proc. Natl. Acad. Sci. U.S.A. 89, 9969–9973. doi: 10.1073/pnas.89.20.9969
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Van Der Straeten, D., Van Wiemeersch, L., Goodman, H. M., and Van Montagu, M. (1990). Cloning and sequence of two different cDNA’s encoding 1-aminocyclopropane-1-carboxylate synthase in tomato. Proc. Natl. Acad. Sci. U.S.A. 87, 4859–4863. doi: 10.1073/pnas.87.12.4859
Van Der Straeten, D., Zhou, Z., Prinsen, E., Van Onckelen, H. A., and Van Montagu, M. C. (2001). A comparative molecular-physiological study of submergence response in lowland and deepwater rice. Plant Physiol. 125, 955–968. doi: 10.1104/pp.125.2.955
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ververidis, P., and John, P. (1991). Complete recovery in vitro of ethylene-forming enzyme-activity. Phytochemistry 30, 725–727. doi: 10.1016/0031-9422(91)85241-Q
Wang, K. L.-C., Yoshida, H., Lurin, C., and Ecker, J. R. (2004). Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature 428, 945–950. doi: 10.1038/nature02516
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wang, T., and Arteca, R. N. (1992). Effects of low root stress on ethylene biosynthesis in tomato plants (Lycopersicon esculentum Mill cv Heinz 1350). Plant Physiol. 98, 97–100. doi: 10.1104/pp.98.1.97
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Woeste, K. E., Vogel, J. P., and Kieber, J. J. (1999). Factors regulating ethylene biosynthesis in etiolated Arabidopsis thaliana seedlings. Physiol. Plant. 105, 478–484. doi: 10.1034/j.1399-3054.1999.105312.x
Xu, S.-L., Rahman, A., Baskin, T. I., and Kieber, J. J. (2008). Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell 20, 3065–3079. doi: 10.1105/tpc.108.063354
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yamagami, T., Tsuchisaka, A., Yamada, K., Haddon, W. F., Harden, L. E., and Theologis, A. (2003). Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. J. Biol. Chem. 278, 49102–49112. doi: 10.1074/jbc.M308297200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yang, S. F., and Hoffman, N. E. (1984). Ethylene biosynthesis and its regulation in higher-plants. Annu. Rev. Plant Physiol. Mol. Biol. 35, 155–189. doi: 10.1146/annurev.pp.35.060184.001103
Yoon, G. M., and Kieber, J. J. (2013). 1-Aminocyclopropane-1-carboxylic acid as a signalling molecule in plants. AoB Plants 5:plt017. doi: 10.1093/aobpla/plt017
Yoshida, H., Nagata, M., Saito, K., Wang, K. L. C., and Ecker, J. R. (2005). Arabidopsis ETO1 specifically interacts with and negatively regulates type 2 1-aminocyclopropane-1-carboxylate synthases. BMC Plant Biol. 5:14. doi: 10.1186/1471-2229-5-14
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yoshida, H., Wang, K. L., Chang, C. M., Mori, K., Uchida, E., and Ecker, J. R. (2006). The ACC synthase TOE sequence is required for interaction with ETO1 family proteins and destabilization of target proteins. Plant Mol. Biol. 62, 427–437. doi: 10.1007/s11103-006-9029-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zarembinski, T. I., and Theologis, A. (1993). Anaerobiosis and plant growth hormones induce two genes encoding 1-aminocyclopropane-1-carboxylate synthase in rice (Oryza sativa L.). Mol. Biol. Cell 4, 363–373. doi: 10.1091/mbc.4.4.363
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zarembinski, T. I., and Theologis, A. (1997). Expression characteristics of OS-ACS1 and OS-ACS2, two members of the 1-aminocyclopropane-1-carboxylate synthase gene family in rice (Oryza sativa L. cv. Habiganj Aman II) during partial submergence. Plant Mol. Biol. 33, 71–77. doi: 10.1023/B:PLAN.0000009693.26740.c3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhang, Z. H., Ren, J. S., Clifton, I. J., and Schofield, C. J. (2004). Crystal structure and mechanistic implications of 1-aminocyclopropane-1-carboxylic acid oxidase – The ethylene-forming enzyme. Chem. Biol. 11, 1383–1394. doi: 10.1016/j.chembiol.2004.08.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: 1-aminocyclopropane-1-carboxylic acid (ACC), ethylene, conjugation, deaminase, transport, signaling
Citation: Van de Poel B and Van Der Straeten D (2014) 1-aminocyclopropane-1-carboxylic acid (ACC) in plants: more than just the precursor of ethylene!. Front. Plant Sci. 5:640. doi: 10.3389/fpls.2014.00640
Received: 10 October 2014; Accepted: 28 October 2014;
Published online: 11 November 2014.
Edited by:
Domenico De Martinis, ENEA Italian National Agency for New Technologies, Energy and Sustainable Economic Development, ItalyReviewed by:
Brad Binder, University of Tennessee-Knoxville, USAChi-Kuang Wen, Chinese Academy of Sciences, China
Copyright © 2014 Van de Poel and Van Der Straeten. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dominique Van Der Straeten, Laboratory of Functional Plant Biology, Department of Physiology, Ghent University, K.L. Ledeganckstraat 35, 9000 Ghent, Belgium e-mail:ZG9taW5pcXVlLnZhbmRlcnN0cmFldGVuQHVnZW50LmJl
 Bram Van de Poel
Bram Van de Poel Dominique Van Der Straeten
Dominique Van Der Straeten