- Great Lakes Bioenergy Research Center, University of Wisconsin-Madison, Madison, WI, USA
Perennials have a number of traits important for profitability and sustainability of a biofuel crop. Perennialism is generally defined as the ability to grow and reproduce in multiple years. In temperate climates, many perennial plants enter dormancy during winter and recycle nutrients, such as nitrogen, to below ground structures for the next growing season. Nitrogen is expensive to produce and application of nitrogen increases the potent greenhouse gas NOx. Perennial bioenergy crops have been evaluated for biomass yields with nitrogen fertilization, location, year, and genotype as variables. Flowering time and dormancy are closely related to the N recycling program. Substantial variation for flowering time and dormancy has been identified in the switchgrass (Panicum virgatum L.) species, which provides a source to identify the genetic components of N recycling, and for use in breeding programs. Some studies have addressed recycling specifically, but flowering time and developmental differences were largely ignored, complicating interpretation of the results. Future studies on recycling need to appreciate plant developmental stage to allow comparison between experiments. A perennial/annual model(s) and more environmentally controlled experiments would be useful to determine the genetic components of nitrogen recycling. Increasing biomass yield per unit of nitrogen by maximizing recycling might mean the difference for profitability of a biofuel crop and has the added benefit of minimizing negative environmental effects from agriculture.
Perennialism
Providing sufficient biomass to replace a significant portion of fossil fuel use is a major challenge for the bioenergy industry. Bioenergy crops need to be profitable for the grower and environmentally sustainable, while not competing with food crops. Therefore, low productivity land with marginal soils have been targeted as a primary location for growing bioenergy crops.
Certain traits of perennial plants can contribute to the sustainability of a bioenergy industry. Perennials are generally defined as plants that live for many years and reproduce in multiple years (iteroparity), compared to annuals that reproduce once and then die (semelparity). Perennials retain shoots that do not flower at the end of the season, and instead develop and flower the following season (in grasses and other herbaceous temperate perennials these shoots are at the crown of the plant or in underground stems). Perenniality is likely ancestral to the annual growth habit, since flowering plants originated in warmer eras, which permit continuous growth. Dormancy and the annual growth habit are two adaptations that plants have evolved to survive as they adapted to cooler climates over geologic time.
Perennials have certain advantages over annuals as bioenergy crops. Perennials do not require the energy inputs for planting every season. Growing perennials greatly reduces the capacity for erosion and in fact typically increases soil carbon. The increased soil carbon is due to the deep and extensive root systems of certain perennials; such root systems also permit growth in drier regions. Perennials typically require less fertilizer than annual crops. The reduced requirement for fertilizer is particularly apparent in perennials that have evolved a yearly nutrient recycling and shoot “die-back” program as an adaptation to growth in temperate climates. The recycling saves nutrients such as N and P for next seasons growth and results in senescing shoots with lower N and P content, which facilitates biomass processing. One Miscanthus plot has been harvested continually for 14 years with no inputs and no decrease in yield (Christian et al., 2008).
Nutrient Recycling in Perennial Bioenergy Crops
In preparation for the following season, perennials cease vegetative growth and initiate flowering mid season. In environments that require a period of dormancy during the year (drought/winter), perennials recycle a portion of their nutrients to below ground structures for growth once the dormant period has passed (McKendrick et al., 1975; Clark, 1977; Hayes, 1985; Beale and Long, 1997; Lemus et al., 2008). How recycling is initiated and the factors regulating recycling remain unknown, but optimizing this trait could result in a significant increase in yields while at the same time reducing inputs. Furthermore, nutrient recycling and storage allows for perennial species to initiate growth immediately in the spring outcompeting annuals that need to emerge from seeds and send out roots to acquire nutrients.
One way to increase perennial biomass production is to extend the vegetative phase by delaying flowering and dormancy, but this may also have a negative effect on end of the season recycling. Harvest date has a large effect on biomass quality and stand longevity; later harvest dates increase quality and longevity by allowing the recycling program to be completed (Sanderson et al., 1999; Reynolds et al., 2000; Muir et al., 2001; Mulkey et al., 2006). In practice, it is best to harvest after a killing frost, since any recycling would cease at that point. When biomass was harvested green, N content exceeded 1.5% compared to less than 0.5% if harvested in the winter, and delaying harvest until at least late summer is advantageous for long-term sustainable biomass production (Casler and Boe, 2003; Adler et al., 2006; Heaton et al., 2009).
In most environments, nitrogen and precipitation are the limiting factors for plant growth, and the primary energy input for crops is usually nitrogen fertilizer (Biermann et al., 1999; Monti and Venturi, 2003; Boehmel et al., 2008). Nitrogen is energy intensive to produce and requires energy for application. Applied agricultural nitrogen is also a primary source of NOx greenhouse gases. One study estimated that the NOx produced from fertilizing bioenergy crops would mitigate any potential carbon dioxide decrease, since NOx species are more potent greenhouse gases than CO2 (Crutzen et al., 2008).
With sufficient precipitation, Miscanthus giganteus out produces most other bioenergy crops (Heaton et al., 2009), but the clonal nature and lack of natural variation could be detrimental when challenged with biotic stresses or drought. Native warm-season prairie grasses, such as switchgrass and big bluestem, are two species being developed for sustainable biomass production. These species are native to large regions of North America, and are adapted to the regions where dedicated bioenergy will be grown, and thus there are substantial genetic variations for breeding programs.
Several studies have demonstrated movement of nitrogen from shoots to below ground structures in the later part of the growing season in both switchgrass and big bluestem, sometimes over 50% (Hayes, 1985; Tufekcioglu et al., 2003; Lemus et al., 2008; Yang et al., 2009; Garten et al., 2010). Thus these species have a valuable trait for a dedicated bioenergy crop-robust end of the season N recycling. Additionally, switchgrass, big bluestem, and several other C4 warm-season perennial grasses can recycle of up to 30% of shoot nitrogen during drought, presumably as a protective measure for plant survival (Hayes, 1985; Heckathorn and DeLucia, 1994, 1996). Determining the signals leading to drought induced recycling would enable a comparison to end-of-season recycling.
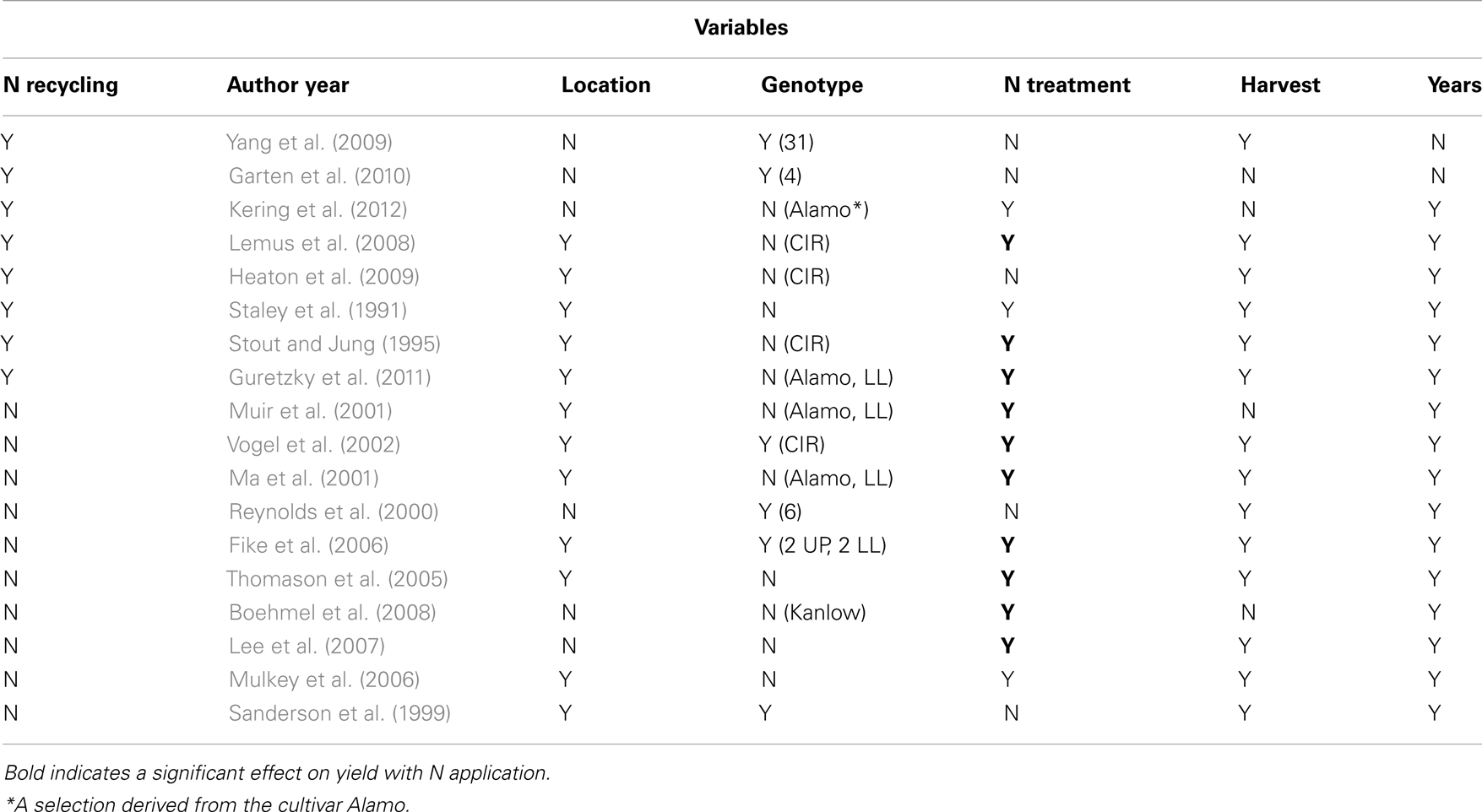
A number of publications address N application and yield, and a subset are listed in Table 1. Variables in most of the studies include years, N application rate, harvest regime, location, and/or cultivar. While the specific techniques differ, nearly every study observed a decrease in total N in above ground tissue during the second half of the growing season, and in some cases it was directly demonstrated that below ground N content increased (Lemus et al., 2008). At ground level or below ground biomass can be 84% of total plant biomass and consists of the crown, rhizomes (underground stems), and roots, providing a large sink for N storage (Frank et al., 2004). In two studies, it was shown that half of the aboveground N was translocated to rhizomes and roots by the time the plants became dormant (Garten et al., 2010; Kering et al., 2012).
From an agronomic point of view, robust recycling will allow for a lower rate of N application, while generating good yields of biomass. Many studies show a positive correlation of increased biomass with increasing N application (Table 1, Bold). These studies also establish that higher rates of N application lead to increases of N in harvested tissue and a decrease in the amount of biomass produced per gram of N applied (Staley et al., 1991; Muir et al., 2001; Vogel et al., 2002; Lewandowski et al., 2003; Mulkey et al., 2006; Lemus et al., 2008; Guretzky et al., 2011). For example, in Guretzky et al. (2011), application of 225 kg Nha-1 increased switchgrass biomass by 85%, yet N content of harvested biomass increased by 182%, compared to no N applied. Thus robust N recycling will produce biomass with less N, mitigating some of the environmental damage caused by NOx resulting from N application.
One complication of interpreting N use studies is the age and developmental stage of the plants, because results will be affected by both plant size and developmental stage and the impact of developmental stages on physiological/biochemical analyses can be larger than genetic differences in herbaceous annual crops. Using a developmental index, such as that developed by Moore et al. (1991), allows for comparison across genotypes and studies. Recently, a standardization protocol for switchgrass sample collection has also been developed (Hardin et al., 2013). Determining the maximum and minimum N content in the plant is key to determining resorption efficiency. The maximum value is likely to be at the initiation of reproductive structures, presumably before whole plant senescence begins. In addition, there are overall mass decreases at the end of the season, due to carbohydrate depletion and translocation, thus corrections need to be made to get an accurate N recycling estimate (Van Heerwaarden et al., 2003; Heaton et al., 2009).
Many studies have also evaluated natural genetic variation for N recycling in switchgrass. However, large environmental effects often masked possible genetic contributions, or too few genotypes were included for a robust analysis. Yang et al. (2009) looked at 31 accessions, but the plants were at different developmental stages when harvested complicating interpretation of the results. Experiments designed to specifically address genotype differences for N recycling have focused on differences between upland and lowland cultivars, the two major switchgrass cytotypes, with lowland accessions appearing to have greater N recycling (Porter, 1966; Yang et al., 2009). Recent work has shown that gene flow does occur between upland and lowland cultivars, despite differences in flowering time (3–4 weeks), which provides a mechanism for generating allelic variability (Zhang et al., 2011).
Flowering Time and Dormancy in Switchgrass
Determining the factors that affect flowering time and dormancy in switchgrass is likely to be difficult, due to the extensive natural variation and genetic complexity within the species. Environmental factors to be considered include temperature and precipitation, which are variable from year to year, and photoperiod. Common garden experiments show that ecotypes of switchgrass are locally adapted, and the timing of reproductive development is correlated to the length of the local growing season (Cornelius and Johnston, 1941; Eberhart and Newell, 1959; McMillan, 1959, 1965; Hopkins et al., 1995; Sanderson and Wolf, 1995; Casler et al., 2004, 2007b; Berdahl et al., 2005; Casler, 2005). Variation also exists for leaf appearance rate, and end-of-season dormancy (Figure 1), all of which influences the length of active growth and biomass accumulation (McMillan, 1959; Van Esbroeck et al., 1997, 2004).
Spring emergence begins the growing season and is mostly a function of temperature (Sanderson and Wolf, 1995). McCarty (1986) showed that in native fields there was more annual variation in spring emergence than anthesis. However, there is a genetic component since the Alamo cultivar is the first to emerge in the spring in common garden experiments, and northern ecotypes are delayed compared to southern ecotypes (Hsu et al., 1985; Parrish and Fike, 2005).
Vegetative growth creates the greatest biomass accumulation and ends with initiation of floral development. Vegetative growth is also influenced by temperature, with lower temperatures increasing the duration of the vegetative stage (Benedict, 1940; Sanderson and Wolf, 1995). There is also variability among switchgrass cultivars for photoperiod sensitivity in the vegetative stage; in some cultivars flowering is inhibited by short days. For example, Alamo, a southern lowland, had twice the length of vegetative growth compared to CIR (Cave-In-Rock), an upland variety from Illinois (Sanderson et al., 1996; Van Esbroeck et al., 2003). The photoperiod effect also explains the cessation of vegetative growth observed in northern cultivars in early summer when grown in southern locations (Sanderson et al., 1996).
Benedict (1940) showed that a single ecotype of switchgrass flowered under short-day conditions (10 h), but not in long-day conditions (18 h), thus classifying switchgrass as a short-day plant. In a common garden experiment, McMillan (1959) demonstrated substantial variation for floral initiation within and between eight switchgrass populations from different locations. In a native field experiment over 15 years, anthesis was largely controlled by photoperiod, with little year to year variation (McCarty, 1986). Hopkins et al. (1995) demonstrated the strong photoperiod effect with Midwestern accession having nearly identical heading dates for 2 years at three locations with similar latitude. However, within population variability also exists since natural populations contain plants entering anthesis over a 3 week period (Jones and Brown, 1951).
Development and flowering time in switchgrass has been recorded in a number of studies, which reveal latitudinal adaptation reflected by higher survival rates among local populations in reciprocal transplant experiments (Sanderson et al., 1999; Casler et al., 2004). Thus, flowering time or maturity is highly variable among switchgrass varieties, with photoperiodic differences along a north–south gradient (McMillan, 1959; Casler et al., 2004, 2007b; Casler, 2005). There also appears to be variability in the length of the flowering period with northern clones having 1 week between inflorescence exsertion and initial anthesis, while southern clones took 4–6 weeks (McMillan, 1959). One study (Van Esbroeck et al., 2003) showed that “photoperiod did not appear to affect the initiation of reproductive development but rather the period of panicle exsertion.”
The evidence from multiple studies indicates that the two major cytotypes of switchgrass, upland, and lowland, have differences in photoperiod sensitivity. For example, CIR, an upland northern cultivar, showed the largest response to an artificially extended photoperiod (18 h) in a greenhouse with an increased yield of 129 and 98% in two trials, while Alamo showed no change (Van Esbroeck et al., 2003). In general in common garden experiments, lowland ecotypes have a heading date 2–4 weeks later than upland types (Casler et al., 2004; Cortese et al., 2010). In work from Taliaferro (2002), heading date was recorded for 113 switchgrass germplasms with variable flowering time [167–257 Days Of Year (DOY) (Taliaferro, 2002)]. Grouping accessions according to cytotype and ploidy generates three significantly different groups (Lowland4×, Upland4×, Upland8×) when evaluating heading data (P = 0.0002), however, most variation is within each class (Figure 2). Using additional descriptive parameters for Cluster analysis, such as morphological differences, generates nine core groups, and the ANOVA in Figure 2 shows the extensive natural variability of heading dates for eight of the groups (one group, DOY248 and DOY257, is excluded due to sample size n = 2). On average the photoperiod effect results in 0.8 day earlier heading for each degree north a switchgrass population is moved (Casler et al., 2007b).
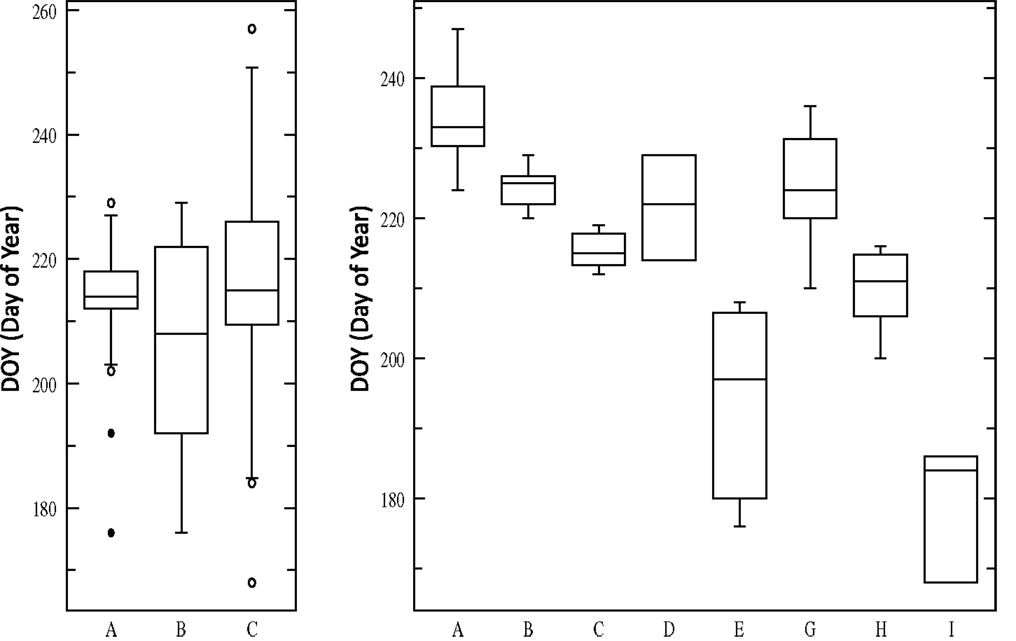
Figure 2. ANOVA (Analysis of Variance) of heading date for 113 switchgrass germplasms. Left: three groups based upon cytotype and ploidy (Lowland4×, Upland4×, Upland8×). Right: eight groups based upon cytotype, ploidy, and morphological characteristics.
Flowering time has a large influence on biomass yield, with southern (lowland) varieties producing 2–3× more phytomers (root and shoot meristems) compared to northern (upland) varieties when grown in their native locations, partially related to a longer growing season (Casler, 2012). The strong adaptation gradient results in some lowland varieties never flowering at northern locations and lacking cold tolerance, and upland varieties being heat intolerant and flowering too early for maximal biomass in southern locations (Sanderson et al., 1999; Casler et al., 2004, 2007a). In fact most switchgrass varieties are well adapted to their local environment and cannot be moved more than one hardiness zone without adverse affects on productivity and survival (Casler et al., 2004). The genetic parameters that control flowering are unknown and investigation into the endogenous and exogenous factors influencing flowering time will be valuable for developing region-specific cultivars. Very few of the studies in Table 1 acknowledge flowering time differences; thus, to relate the data from different studies, samples should be collected at a uniform stage of development, and flowering time data on cultivars and locations are needed to identify the genetic components controlling N recycling.
End-of-season dormancy is especially important for N recycling. Among C4 species, McMillan (1965) noted that “early flowering switchgrass and big bluestem from the northern USA exhibited earlier dormancy than ecotypes originating in southern USA.” In photoperiod experiments with multiple accessions, 12.5 h of light affected the two earliest flowering ecotypes differently, with clones from Minnesota going dormant while clones from Colorado continuing vegetative growth. Castro et al. (2011) determined that the photoperiod at emergence was key for the timing of dormancy, and growing upland cytotypes in 24 h of light prevented dormancy. Thus there is natural variation for photoperiodic-induced dormancy with northern ecotypes having a greater response to photoperiod, likely as a mechanism to avoid freeze damage (Benedict, 1940; Van Esbroeck et al., 2004).
Photoperiod sensitivity has an influence on nearly every stage of development, and there is substantial natural variation that can be utilized for breeding and identification of the molecular components controlling photoperiod sensitivity. Together, these studies show that photoperiod sensitivity not only varies among cultivars, but also varies with developmental phase. Increasing photoperiod insensitivity could increase yield in northern ecotypes provided proper nutrient recycling can be maintained.
Current and Future Directions
The effects of a nitrogen gradient on root architecture were evaluated in two Brachypodium accessions where heritable differences in root system architecture were dependent on N concentration (Ingram et al., 2012). However, evaluating growth of eight divergent Brachypodium accessions grown under eight different N concentrations failed to identify accession differences, and final N content was mostly influenced by flowering time, with later flowering accessions producing more leaves and thus having more total N (Schwartz and Amasino, unpublished). Since Brachypodium is an annual plant, both of these experiments more likely address N uptake, and not internal N recycling.
Investigating N recycling would be greatly facilitated by identifying a high-throughput and genetically amenable system to study that has robust end-of-season recycling. A perennial model system would enable the study of N recycling to the crown and roots. Hopefully such a model could undergo the yearly life cycle in a highly controlled environment (greenhouse) or in common garden experiments, thus reducing the environmental variation for identification of genetic differences. Reciprocal transplant experiments are another method to identify genetic variability. To thoroughly investigate perennialism and manipulate perennial traits, however will likely require multiple systems to investigate due to general variability for this trait (i.e., the temperate perennial life history probably arose independently multiple times).
Determining what cues initiate N recycling in perennials and how the recycling rate is controlled will be key to manipulate N recycling. Both annuals and perennials have the ability to recycle nitrogen for growth throughout the season, but temperate perennials differ by having two sinks for translocating N at the end of the season, the seeds (acropetal) and the crown/root system (basipetal). Thus some exogenous or endogenous factor promotes translocation downward in perennials in the second half of the growing season. The trigger could be the initiation of flowering, and/or changes in photoperiod, or simply robust growth in the crown and roots, which creates a sink. Determining how the crown and roots become a sink for nutrients is imperative to tailoring N recycling for specific crops and environments.
To make a substantial contribution to the bioenergy field, it will be important to identify the genetic basis of N dynamics. One study using a cross between perennial and annual rice discovered a transcription factor (Rhz3) required for rhizome growth (Hu et al., 2003). Determining the effects and manipulating expression of this gene in annual and perennial species may provide insight into the role of rhizomes in N recycling. Genetic differences might also be identified by tissue-specific expression studies. For example, a developmental profile of rhizomes over a season might help assess their role in N recycling, and how that sink is activated mid season.
Intraspecific crosses between upland and lowland cultivars show hybrid vigor (heterosis) for many traits, including biomass, indicating a rich source of genetic variation in switchgrass (Casler, 2012). Analyses of segregating populations derived from such wide crosses may be one strategy to make progress in understanding biomass traits at the molecular level.
The N dynamics of a given species in a given environment may be a critical factor in biofuel profitability. Future genetic and biochemical studies of N recycling and the control of the initiation of flowering and dormancy have great potential to increase the yield and sustainability of bioenergy crops.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Laura Smith, Mike Casler, Randy Jackson, John Sedbrook, Tom Ream, and Daniel Woods for helpful discussions. This work was funded by the US Department of Energy Great Lakes Bioenergy Research Center (http://www.greatlakesbioenergy.org/).
References
Adler, P. R., Sanderson, M. A., Boateng, A. A., Weimer, P. J., and Jung, H.-J. G. (2006). Biomass yield and biofuel quality of switchgrass harvested in fall or spring. Agron. J. 98, 1518–1525.
Beale, C. V., and Long, S. P. (1997). Seasonal dynamics of nutrient accumulation and partitioning in the perennial C4-grasses Miscanthus × giganteus and Spartina cynosuroides. Biomass Bioenergy 12, 419–428.
Benedict, H. M. (1940). Effect of day length and temperature on the flowering and growth of four species of grasses. J. Agric. Res. 61, 661–672.
Berdahl, J. D., Frank, A. B., Krupinsky, J. M., Carr, P. M., Hanson, J. D., and Johnson, H. A. (2005). Biomass yield, phenology, and survival of diverse switchgrass cultivars and experimental strains in western North Dakota. Agron. J. 97, 549–555.
Biermann, S., Rathke, G.-W., Huïsbergen, K.-J., and Diepenbrock, W. (1999). Energy Recovery by Crops in Dependence on the Input of Mineral Fertilizer. Brussels: Martin-Luther-University.
Boehmel, C., Lewandowski, I., and Claupein, W. (2008). Comparing annual and perennial energy cropping systems with different management intensities. Agric. Syst. 96, 224–236.
Casler, M. (2012). “Switchgrass breeding, genetics, and genomics,” in Switchgrass, ed. A. Monti (New York: Springer), 29–54.
Casler, M. D. (2005). Ecotypic variation among switchgrass populations from the northern USA. Crop Sci. 45, 388–398.
Casler, M. D., and Boe, A. R. (2003). Cultivar × environment interactions in switchgrass. Crop Sci. 43, 2226–2233.
Casler, M. D., Stendal, C. A., Kapich, L., and Vogel, K. P. (2007a). Genetic diversity, plant adaptation regions, and gene pools for switchgrass. Crop Sci. 47, 2261–2273.
Casler, M. D., Vogel, K. P., Taliaferro, C. M., Ehlke, N. J., Berdahl, J. D., Brummer, E. C., et al. (2007b). Latitudinal and longitudinal adaptation of switchgrass populations. Crop Sci. 47, 2249–2260.
Casler, M. D., Vogel, K. P., Taliaferro, C. M., and Wynia, R. L. (2004). Latitudinal adaptation of switchgrass populations. Crop Sci. 44, 293–303.
Castro, J. C., Boe, A., and Lee, D. K. (2011). A simple system for promoting flowering of upland switchgrass in the greenhouse. Crop Sci. 51, 2607–2614.
Christian, D. G., Riche, A. B., and Yates, N. E. (2008). Growth, yield and mineral content of Miscanthus × giganteus grown as a biofuel for 14 successive harvests. Ind. Crops Prod. 28, 320–327.
Cornelius, D. R., and Johnston, C. O. (1941). Differences in plant type and reaction to rust among several collections of Panicum virgatum L. Agron. J. 33, 115–124.
Cortese, L., Honig, J., Miller, C., and Bonos, S. (2010). Genetic diversity of twelve switchgrass populations using molecular and morphological markers. Bioenergy Res. 3, 262–271.
Crutzen, P. J., Mosier, A. R., Smith, K. A., and Winiwarter, W. (2008). N2O release from agro-biofuel production negates global warming reduction by replacing fossil fuels. Atmos. Chem. Phys. 8, 389–395.
Eberhart, S. A., and Newell, L. C. (1959). Variation in domestic collections of switchgrass, Panicum virgatum L1. Agron. J. 51, 613–616.
Fike, J. H., Parrish, D. J., Wolf, D. D., Balasko, J. A., Green, J. T. Jr, Rasnake, M., et al. (2006). Switchgrass production for the upper southeastern USA: influence of cultivar and cutting frequency on biomass yields. Biomass and Bioenergy 30, 207–213.
Frank, A. B., Berdahl, J. D., Hanson, J. D., Liebig, M. A., and Johnson, H. A. (2004). Biomass and carbon partitioning in switchgrass. Crop Sci. 44, 1391–1396.
Garten, C. T., Smith, J. L., Tyler, D. D., Amonette, J. E., Bailey, V. L., Brice, D. J., et al. (2010). Intra-annual changes in biomass, carbon, and nitrogen dynamics at 4-year old switchgrass field trials in west Tennessee, USA. Agric. Ecosyst. Environ. 136, 177–184.
Guretzky, J., Biermacher, J., Cook, B., Kering, M., and Mosali, J. (2011). Switchgrass for forage and bioenergy: harvest and nitrogen rate effects on biomass yields and nutrient composition. Plant Soil 339, 69–81.
Hardin, C. F., Fu, C., Xiao, X., Shen, H., Stewart, C. N., Parrot, W., et al. (2013). Standardization of switchgrass sample collection for cell wall and biomass trait analysis. Bioenergy Res. doi:10.1007/s12155-012-9292-1
Hayes, D. C. (1985). Seasonal nitrogen translocation in big bluestem during drought conditions. J. Range Manag. 38, 406–410.
Heaton, E. A., Dohleman, F. G., and Long, S. P. (2009). Seasonal nitrogen dynamics of Miscanthus × giganteus and Panicum virgatum. Glob. Change Biol. Bioenergy 1, 297–307.
Heckathorn, S. A., and DeLucia, E. H. (1994). Drought-induced nitrogen retranslocation in perennial C4 grasses of tallgrass prairie. Ecology 75, 1877–1886.
Heckathorn, S. A., and DeLucia, E. H. (1996). Retranslocation of shoot nitrogen to rhizomes and roots in prairie grasses may limit loss of N to grazing and fire during drought. Funct. Ecol. 10, 369–400.
Hopkins, A. A., Vogel, K. P., Moore, K. J., Johnson, K. D., and Carlson, I. T. (1995). Genotic variability and genotype × environment interactions among switchgrass accessions from the midwestern USA. Crop Sci. 35, 565–571.
Hsu, F. H., Nelson, C. J., and Matches, A. G. (1985). Temperature effects on germination of perennial warm-season forage grasses. Crop Sci. 25, 215–220.
Hu, F. Y., Tao, D. Y., Sacks, E., Fu, B. Y., Xu, P., Li, J., et al. (2003). Convergent evolution of perenniality in rice and sorghum. Proc. Natl. Acad. Sci. U.S.A. 100, 4050–4054.
Ingram, P. A., Zhu, J., Davis, I. W., Benfey, P. N., and Elich, T. (2012). High-throughput imaging and analysis of root system architecture in Brachypodium distachyon under differential nutrient availability. Crop Sci. 51, 2607–2614.
Jones, M. D., and Brown, J. G. (1951). Pollination cycles of some grasses in Oklahoma. Agron. J. 43, 218–222.
Kering, M. K., Butler, T. J., Biermacher, J. T., and Guretzky, J. A. (2012). Biomass yield and nutrient removal rates of perennial grasses under nitrogen fertilization. Bioenergy Res. 5, 61–70.
Lee, D. K., Owens, V. N., and Doolittle, J. J. (2007). Switchgrass and soil carbon sequestration response to ammonium nitrate, manure, and harvest frequency on conservation reserve program land. Agron. J. 99, 462–468.
Lemus, R., Parrish, D., and Abaye, O. (2008). Nitrogen-use dynamics in switchgrass grown for biomass. Bioenergy Res. 1, 153–162.
Lewandowski, I., Scurlock, J. M. O., Lindvall, E., and Christou, M. (2003). The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 25, 335–361.
Ma, Z., Wood, C. W., and Bransby, D. I. (2001). Impact of row spacing, nitrogen rate, and time on carbon partitioning of switchgrass. Biomass and Bioenergy 20, 413–419.
McCarty, M. K. (1986). A fifteen-year phenological record of pasture plants near lincoln, Nebraska. Weed Sci. 34, 218–224.
McKendrick, J. D., Owensby, C. E., and Hyde, R. M. (1975). Big bluestem and indiangrass vegetative reproduction and annual reserve carbohydrate and nitrogen cycles. Agro-Ecosystems 2, 75–93.
McMillan, C. (1959). The role of ecotypic variation in the distribution of the central grassland of North America. Ecol. Monogr. 29, 285–308.
McMillan, C. (1965). Ecotypic differences with four North American prairie grasses: II. Behavioral variation with transplanted community fractions. Am. J. Bot. 52, 55–65.
Monti, A., and Venturi, G. (2003). Comparison of the energy performance of fibre sorghum, sweet sorghum and wheat monocultures in northern Italy. Eur. J. Agron. 19, 35–43.
Moore, K. J., Moser, L. E., Vogel, K. P., Waller, S. S., Johnson, B. E., and Pedersen, J. F. (1991). Describing and quantifying growth stages of perennial forage grasses. Agron. J. 83, 1073–1077.
Muir, J. P., Sanderson, M. A., Ocumpaugh, W. R., Jones, R. M., and Reed, R. L. (2001). Biomass production of ‘Alamo’ switchgrass in response to nitrogen, phosphorus, and row spacing. Agron. J. 93, 896–901.
Mulkey, V. R., Owens, V. N., and Lee, D. K. (2006). Management of switchgrass-dominated conservation reserve program lands for biomass production in south Dakota. Crop Sci. 46, 712–720.
Parrish, D. J., and Fike, J. H. (2005). The biology and agronomy of switchgrass for biofuels. Crit. Rev. Plant Sci. 24, 423–459.
Porter, C. L. (1966). An analysis of variation between upland and lowland switchgrass, Panicum virgatum L, in central Oklahoma. Ecology 47, 980–992.
Reynolds, J. H., Walker, C. L., and Kirchner, M. J. (2000). Nitrogen removal in switchgrass biomass under two harvest systems. Biomass Bioenergy 19, 281–286.
Sanderson, M. A., Reed, R. L., McLaughlin, S. B., Wullschleger, S. D., Conger, B. V., Parrish, D. J., et al. (1996). Switchgrass as a sustainable bioenergy crop. Bioresour. Technol. 56, 83–93.
Sanderson, M. A., Reed, R. L., Ocumpaugh, W. R., Hussey, M. A., Van Esbroeck, G., Read, J. C., et al. (1999). Switchgrass cultivars and germplasm for biomass feedstock production in Texas. Bioresour. Technol. 67, 209–219.
Sanderson, M. A., and Wolf, D. D. (1995). Morphological development of switchgrass in diverse environments. Agron. J. 87, 908–915.
Staley, T. E., Stout, W. L., and Jung, G. A. (1991). Nitrogen use by tall fescue and switchgrass on acidic soils of varying water holding capacity. Agron. J. 83, 732–738.
Stout, W. L., and Jung, G. A. (1995). Biomass and nitrogen accumulation in switchgrass: effects of soil and environment. Agron. J. 87, 663–669.
Taliaferro, C. M. (2002). Breeding and Selection of New Switchgrass Varieties for Increased Biomass Production. US Department of Energy Office of Biomass, Project Summary, Oak Ridge National Laboratory, Oak Ridge.
Thomason, W. E., Raun, W. R., Johnson, G. V., Taliaferro, C. M., Freeman, K. W., Wynn, K. J., et al. (2005). Switchgrass response to harvest frequency and time and rate of applied nitrogen. J. Plant Nutr. 27, 1199–1226.
Tufekcioglu, A., Raich, J. W., Isenhart, T. M., and Schultz, R. C. (2003). Biomass, carbon and nitrogen dynamics of multi-species riparian buffers with an agricultural watershed in Iowa, USA. Agrofor. Syst. 57, 187–198.
Van Esbroeck, G. A., Hussey, M. A., and Sanderson, M. A. (1997). Leaf appearance rate and final leaf number of switchgrass cultivars. Crop Sci. 37, 864–870.
Van Esbroeck, G. A., Hussey, M. A., and Sanderson, M. A. (2003). Variation between Alamo and cave-in-rock switchgrass in response to photoperiod extension. Crop Sci. 43, 639–643.
Van Esbroeck, G. A., Hussey, M. A., and Sanderson, M. A. (2004). Reversal of dormancy in switchgrass with low-light photoperiod extension. Bioresour. Technol. 91, 141–144.
Van Heerwaarden, L. M., Toet, S., and Aerts, R. (2003). Current measures of nutrient resorption efficiency lead to a substantial underestimation of real resorption efficiency: facts and solutions. Oikos 101, 664–669.
Vogel, K. P., Brejda, J. J., Walters, D. T., and Buxton, D. R. (2002). Switchgrass biomass production in the midwest USA. Agron. J. 94, 413–420.
Yang, J., Worley, E., Wang, M., Lahner, B., Salt, D., Saha, M., et al. (2009). Natural variation for nutrient use and remobilization efficiencies in switchgrass. Bioenergy Res. 2, 257–266.
Keywords: nitrogen recycling, perennialism, switchgrass, flowering time, dormancy, bioenergy crops
Citation: Schwartz C and Amasino R (2013) Nitrogen recycling and flowering time in perennial bioenergy crops. Front. Plant Sci. 4:76. doi: 10.3389/fpls.2013.00076
Received: 30 December 2012; Accepted: 17 March 2013;
Published online: 22 April 2013.
Edited by:
Samuel P. Hazen, University of Massachusetts, USAReviewed by:
Zeng-Yu Wang, The Samuel Roberts Noble Foundation, USAJiading Yang, The Samuel Roberts Noble Foundation, USA
Copyright: © 2013 Schwartz and Amasino. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and subject to any copyright notices concerning any third-party graphics etc.
*Correspondence: Richard Amasino, Department of Biochemistry, University of Wisconsin-Madison, 433 Babcock Drive, Madison, WI 53706, USA. e-mail:YW1hc2lub0BiaW9jaGVtLndpc2MuZWR1