- 1International Tourism Institute, Hainan College of Economics and Business, Haikou, China
- 2Institute of Processing & Design of Agroproducts, Hainan Academy of Agricultural Science, Haikou, China
The leaves of the common melon are approximately round and uncut. A natural mutant of melon named bm7, which has palmately-lobed leaves. We found that bm7 plants were more conducive to increasing planting density and achieving higher yields, and showed better disease resistance. Therefore, the germplasm resources of muskmelon split leaves have the potential of application. Previous study showed that the palmately-lobed leaf trait was controlled by a single recessive gene, pll (numbered MELO3C010784). By cloning and sequencing the pll genes of Jiashi (wide-type) and bm7 (mutant) plants, we noticed that there was no mutation in this gene, but its expression level in bm7 was far below than that in Jiashi. The silencing of pll gene with virus induced gene silencing (VIGS) confers palmately-lobed leaf trait of Jiashi plants. We found three mutations in the pll gene promoter of bm7 plants, located at 373, 493, 506 bp upstream the translation initiation codon. Then, we had found the pll gene promoter activity of bm7 was significantly lower than that of Jiashi. In addition, the expression level and promoter activity of pll gene in Jiashi plants were not affected by environmental factors, but in bm7 plants, the expression level and promoter activity of pll gene, all decreased with the increase in light intensity and/or temperature. All the results indicated that the mutations convert the pll gene promoter from constitutive to inducible, and results in significantly reduced expression of pll gene, conferring the palmately-lobed leaf trait of melon.
1 Introduction
Leaf, an important organ associated with photosynthesis of plants, can convert solar energy into bioenergy, providing oxygen, food, fiber, biomass and other materials for human survival. In addition, plant leaf also has the ability to sense environmental changes, receive and transmit environmental signals (Edgerton, 2009). Changes in leaf morphology may affect plant physiological functions such as photosynthesis, transpiration and stress resistance, and eventually influencing plant growth and development. Therefore, leaf development has always been one of the major focuses of plant science (Tsukaya, 2013; Avramova et al., 2015). Research on leaf development at the genetic level began in the 1990s (Smith et al., 1992), and has revealed many genes associated with leaf morphogenesis. The leaves of common melons are approximately round and not distinctly lobed. However, we found a natural mutant of melon, named bm7, which has palmately lobed leaves.
Melon, watermelon, pumpkin, etc. are important cash crops of the cucurbitaceae family, and they all involve split leaf phase Genetic analysis showed that the split leaf traits were controlled by a single gene, and there were recessive differences, but there was no gene function report. The leaves of most watermelon varieties are deeply pinnatifid, however, a natural mutant with nonlobed leaf, Black Diamond, was first reported by Mohr in 1953 (Mohr, 1953), and this study showed that the nonlobed leaf trait was controlled by an incompletely-dominant gene nl. Another watermelon with nonlobed leaf, Banye No. 1, was discovered in 1983 (Chai and Wang, 1983), and it was reported that its leaf trait was controlled by a recessive gene. Huang (1988) developed a new watermelon cultivar with nonlobed leaf, Zhongkai No.1. Cui et al. further proved that this leaf trait was controlled by a recessive gene (Cui et al., 1996). Then, a tetraploid watermelon cultivar with nonlobed leaf QB-3 was derived from an introduced diploid cultivar in 1993 (Zhou, 1993). Another watermelon cultivar with nonlobed leaf Kaifeng No. 25 was generated in 2008 (Li et al., 2008). Two lobed leaf-linked genes lo-1 and lo-2 have been reported in pumpkin (Dyutin, 1980; Herrington and Brown, 2013). In melons, only Ganesan et al. reported that the schizoleaf trait of Maine Rock was controlled by a dominant single gene and named it L (Ganesan and Sambandam, 1985).
Arabidopsis thaliana plants with mutations in the genes that regulate gibberellin (GA) synthesis have lobed leaf margins. The lobed leaf trait in tomato plant disappears when exogenous gibberellin is applied (Hay et al., 2002). Decreasing GA signal or GA synthesis promotes the formation of lobed leaf margins, while application of exogenous GA or enhanced GA signal inhibits KNOX gene expression, resulting in decreased depth and number of leaf lobes (Byrne et al., 2000). The study of Zhang et al. suggested that low expression of BrLOM2 gene may be the main reason causing lobed leaf trait in Chinese cabbage (Zhang et al., 2016). However, our previous work showed that the application of exogenous GA has no effect on leaf shape of the mutant bm7.
Our earlier studies have shown that the palmately lobed leaf trait of bm7 is governed by a recessive gene pll. Then, using map-based cloning and SSR markers, this gene was located to a region of approximately 9 000 bp, which contains only one candidate gene (Gao et al., 2014). In melonomic database, pll gene is numbered cm010784, spans over 3 392 bp, and consists of seven exons and six introns. In addition, we also found that bm7 plants had different leaf traits under different light intensity and temperature, which is different from the stable traits of most mutants. In this study, using a melon cultivar with nonlobed leaf Jiashi as a control, pll gene was cloned and sequenced, and its expression in both Jiashi and bm7 plants grown under different light and temperature conditions was quantified and compared. We also found that the silencing of pll gene via virus-induced gene silencing (VIGS) conferred the palmately lobed leaf trait of Jiashi plants. After measuring pll gene promoter activity in both Jiashi and bm7 plants, we found that the promoter activity and expression level of pll gene and leaf lobe depth of bm7 were all influenced by light intensity and air temperature. These results provide a theoretical basis for further revealing the formation of palmately-lobed leaf trait in plants.
2 Materials and methods
2.1 Plant material and treatments
Jiashi and bm7 plants were provided by the College of Life Science and Technology, Xinjiang University, Urumqi, China. Melon seeds were sown in a greenhouse at 30°C, and DNA sample used for gene cloning and vector construction was extracted from true leaves. RNA was extracted from 2-, 5-, 8-, 11-, 14- and 17-day-old true leaves for fluorescence quantitative PCR. Tobacco plants (Nicotiana NC89.) were used for transient expression analysis of transgenes.
2.2 Gene and promoter cloning and sequencing
Genomic DNA was extracted from plants with the Hi-DNAsecure Plant Kit (TIANGEN, China). The fragment (3 451 bp) including pll gene (3 392 bp) was amplified by polymerase chain reaction (PCR) using Long Taq DNA polymerase (TIANGEN, China), the forward primer 5’-TTTCATTACCACAAAATTCTTTCAAT-3’ and the reverse primer 5’-ATTGGGTTCTTGCAGCTAGGAAAAGA-3’, and then, the gene DNA was cloned into the pEASY-T5 Zero Cloning vector (TRANS, China) for sequencing.
Based on the genomic sequence of pll (LOC-Cm010784), a 1 531-bp regulatory region upstream of the translation initiation codon was identified in the melonomics database. The region of the putative pll promoter was PCR amplified with the forward primer 5’-TTGCCTGCAGGACTGTTTCATACCCCACCCC-3’ and the reverse primer 5’-CGCGGATCCCAAACCTCTTGGCTCCTCTTA-3’. The cloned fragment was inserted into pEASY-T5 Zero Cloning vector (TRANS, China) for sequencing. ‘TTGCCTGCAGG’ is the restriction enzyme and protected base of Sbf I, ‘CGCGGATCC’ is the restriction enzyme and protected base of BamHI. The two restriction enzyme sequences were introduced into promoter fragment for the following experiment of pBI121 vector construction.
2.3 RNA extraction and real-time quantitative RT-PCR expression analysis
Total RNA samples were extracted from leaf material with the EasyPure Plant RNA Kit (TRANS, China) following the manufacturer’s protocol. CDNA of interest was amplified by PCR using the forward primer 5’-ATAACAATAATAGTTGGTT-3’ and the reverse primer 5’-GAGTGATGATGAAGAGAGG-3’, synthesized with the EasyScript First-Strand cDNA Synthesis SuperMix (TRANS China), to be used as the template for RT-PCR (Zienkiewicz et al., 2018). Quantitative RT-PCR reactions were performed in Bio-RAD MyiQTM Real-time PCR Detection System (Bio-Rad, USA) using the TransStart Top Green qPCR SuperMix (TRANS, China) according to the manufacturer’s instructions, and the CmActin gene (MELO3C011913) was employed as the internal control for RT-PCR analysis. The primers of CmActin gene: the forward as 5’-CCAAAGGCTGCAAGAATAGC-3’ and the reverse as 5’- TTTGACCTTTGGGTGGGTAG-3’. Amplification was carried out through initial denaturation at 94°C for 2 min, followed by 38 cycles of denaturation at 94°C for 3 min, annealing at 57°C for 30 s, and elongation at 72°C for 2 min. The PCR products from each amplification reaction were separated on 2.5% (w/v) agarose gels (Bi et al., 2017).
2.4 VIGS vector construction and gene silencing
Single-stranded cDNA was prepared from 1 mg of total RNA. A fragment of 405 bp from PLL corresponding to nucleotides 355 to 759 of the gene and including a region that was conserved in this family, the fragment was amplified by PCR using the following primers: 5’-CTCAAGCTTACCCCCAAATTCTCTGCTTT-3’(Hind III site was introduced) as forward and 5’-CTGAAGCTTCAATGCCTGAAGTGTCCTCA-3’(BamHI site was introduced) as reverse. The resulting PCR products were cloned into pEASY-T5 Zero Cloning vector (TRANS, China). The cDNAs of gene were excised from pEASY-T5 Zero by digestion and cloned into TRV2 cut with Hind III and BamHI (Roche Diagnostics) to obtain TRV: PLL. Agrobacterium strain containing TRV vectors was grown at 28°C in YEB medium containing 10 mmol/L MES and 20 mmol/L acetosyringone with the appropriate antibiotics. After 24 h, Agrobacterium cells were harvested and resuspended in Agrobacterium infiltration buffer (10 mmol/L MgCl2, 10 mmol/L MES, pH 5.6, 150 mmol/L acetosyringone). Then the Agrobacterium suspension was injected into the stem of the plant with a syringe (Lentz et al., 2018; Yan et al., 2018).
2.5 Promoter vector construction
After sequencing the promoter fragment, the cloned fragment was inserted upstream of the GUS gene in the vector pBI121 using BamHI and SbfI (Avilés-Arnaut and Dé lano-Frier, 2012; Spangler et al., 2019).
2.6 Leaf disc vacuum infiltration
The Agrobacterium transformed to express the genes of interest were cultured in LB medium containing kanamycin (50mg/mL), and rifampicin (100mg/mL) overnight at 28°C by shaking at 180 r/min. Bacterial concentrations were determined by measuring optical density (OD) at 600 nm.
Leaf discs were cut from Nicotiana NC89 leaves approximately 7-8 weeks after germination using a cork borer (8.5 mm). Poured the Agrobacterium suspension and leaf disc into a 20 ml syringe, pulled the plunger to create a small vacuum in the syringe and shook vigorously then released plunger rapidly. After infiltration, the well-infiltrated leaf discs were selected and incubated upper side down in Petri dishes with MS medium containing 0.8% agar under lighting programs (16 h of light: 8 h of dark) at 23°C (Matsuo et al., 2016).
2.7 Histochemical GUS assay
For histochemical staining, the Agrobacterium infiltrated leaf discs were washed twice with 50 mM Tris-HCl buffer (pH 7.5) containing carbenicillin (500 mg/mL) and then twice with distilled water. The washed leaf discs were prefixed with chilled 90% (v/v) aqueous acetone and then washed with chilled water. They were then immersed in GUS staining solution consisting of 50 mM phosphate buffer (pH 7.2), 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 0.2%(v/v) Triton X-100, and 2 mM X-Gluc salt. They were then incubated overnight at 37°C. Stained leaf discs were successively submerged in 25%, 50%, 70%, and 95% (v/v) ethanol to remove chlorophyll (Dai et al., 2017).
2.8 Quantitative GUS activity assay
The leaf blade to be tested was placed in a mortar, ground to a fine powder by adding liquid nitrogen, transferred to a sterile centrifuge tube. After extraction buffer was added, the sample was centrifuged at 12 000 r/min and 4°C for 10 min to collect the supernatant (GUS protein extract). Then, 50 μl of the GUS protein extract was added to 450 μl of 4-MUG (2 mmol/L), incubated at 37°C for 30 min, before fluorescence emission was measured, at 365 nm excitation and 455 nm emission. GUS activity was calculated according to the relative change in the product per unit time. Three biological replicates were used in this experiment (Dai et al., 2017).
2.9 Statistical analysis
The data were analyzed using the Statistical Analysis System (version 9.1, SAS Institute, Cary, NC, USA). Significant changes in gene expression were determined using Fisher’s least significant differences (P < 0.05).
3 Results
3.1 No mutation sites were found in the pll gene of bm7 plants, but there was a significant difference in pll gene expression between Jiashi and bm7
Sequencing analysis showed that both the pll gene fragments cloned from Jiashi and bm7 have the same sequences, which are consistent with the reference gene of melon. Since changes in gene expression can result in changes in phenotypical traits, we examined the expression of pll gene in both Jiashi and bm7. As shown in Figure 1, there was a significant difference in pll gene expression between Jiashi and bm7. In detail, the expression level of pll gene in Jiashi was approximately equal to that of CmActin gene, but was 25 times higher than pll gene expression level of in bm7.
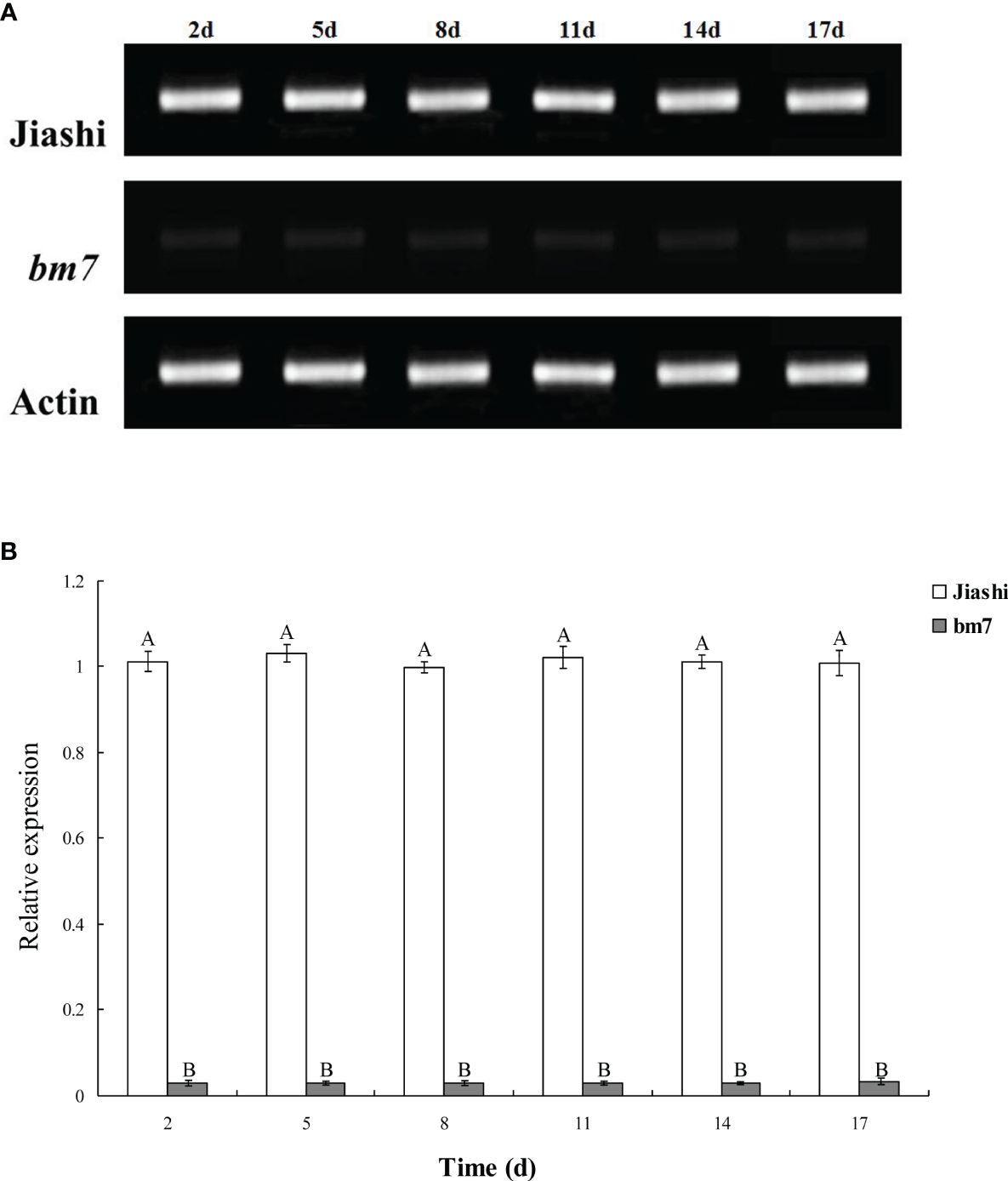
Figure 1 Expression of pll gene in Jiashi and bm7. Relative expression levels of pll gene in 2-, 5-, 8-, 11-, 14- and 17-day-old leaves of Jiashi and bm7. The macro comparison of pll gene expression was shown in (A), and the quantitative expression analysis was shown in (B). Diferent letters for each species denote signifcant diferences (p ≤ 0.05).
3.2 Low expression of pll gene conferred the palmately lobed leaf character of melon
Although there was a significant difference in the expression of pll gene between Jiashi and bm7, it was still uncertain that the low expression of pll gene is the cause of palmately lobed leaf character. So, a 405-bp domain in the first exon of the pll gene of Jiashi plant was silenced by VIGS to observe the changes in leaf shape. The results showed that the leaves became palmately lobed after pll gene was silenced (Figures 2A, B), proving that low expression of pll gene in Jiashi plants is the cause of palmately lobed leaves.
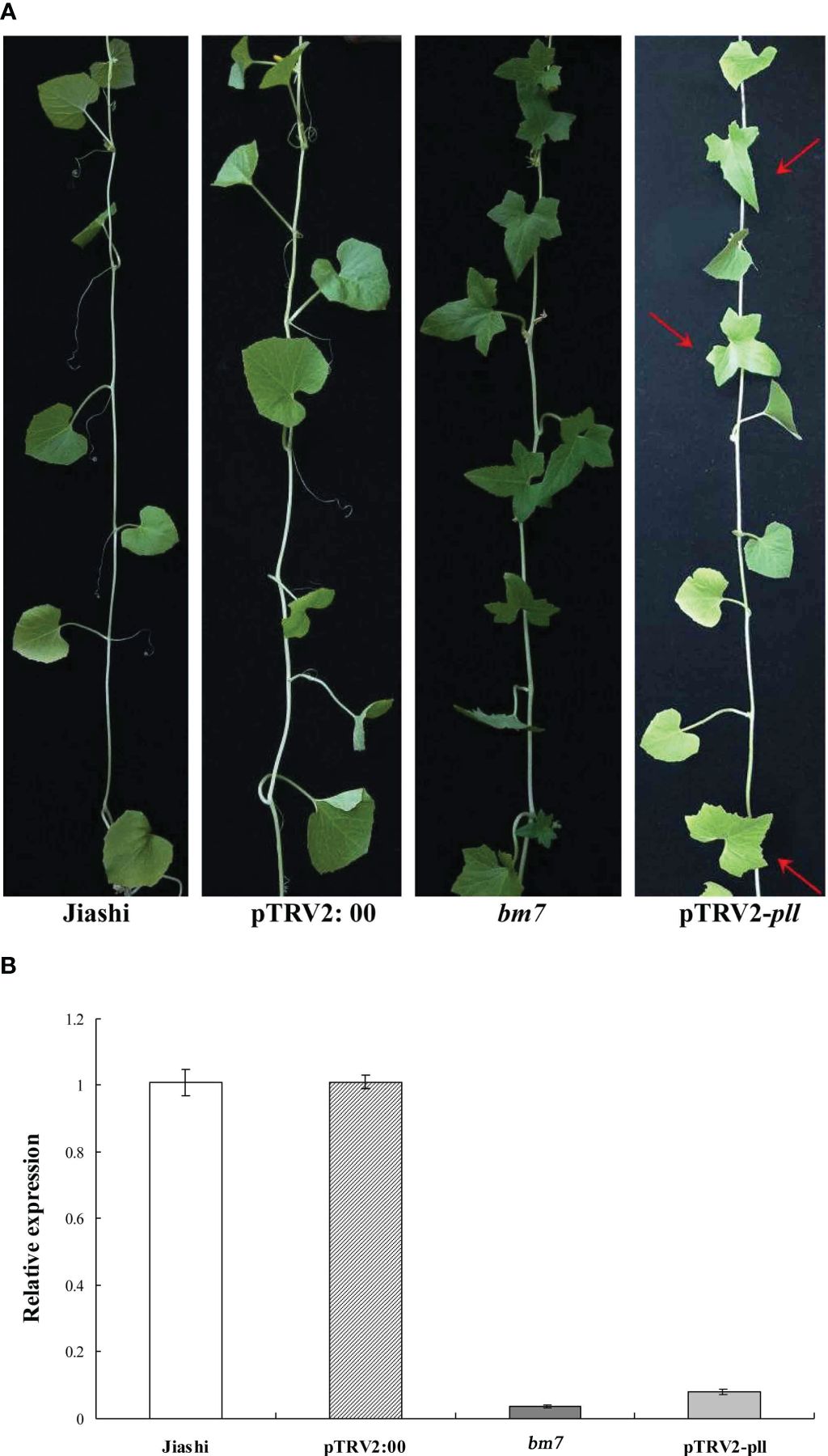
Figure 2 The pll gene of Jiashi was silenced by VIGS. (A) The leaf phenotype of Jiashi, pTRV2:00(negative control of empty vector), bm7 and pTRV2-pll (pll-silencing) plants. (B) Relative expression of pll gene in Jiashi, pTRV2:00, bm7 and pll-silencing Jiashi plants. The red arrow points to a leaf with a split shape, indicating that the pll gene had been successfully silenced.
3.3 The pll gene promoter of bm7 had mutations at three sites, and the activity of pll gene promoter of Jishi and bm7 was also significantly different
Promoters are important elements in the regulation of gene expression. Therefore, we sequenced and detected the activity of pll gene promoters of Jiashi and bm7. Sequencing of the cloned fragments revealed that there were three mutation sites at the 373 (T to C), 493 (C to T) and 506 bp (A to C) upstream the translation initiation codon of the pll gene in bm7 plant (Figure 3A). The GUS activity was detected by Agrobacterium-mediated transient gene expression, and the GUS activity of Jiashi was significantly higher than that of bm7 (Figure 3B). As shown in, the pll gene promoter activity of bm7 was significantly lower than that of Jiashi (Figure 3C).
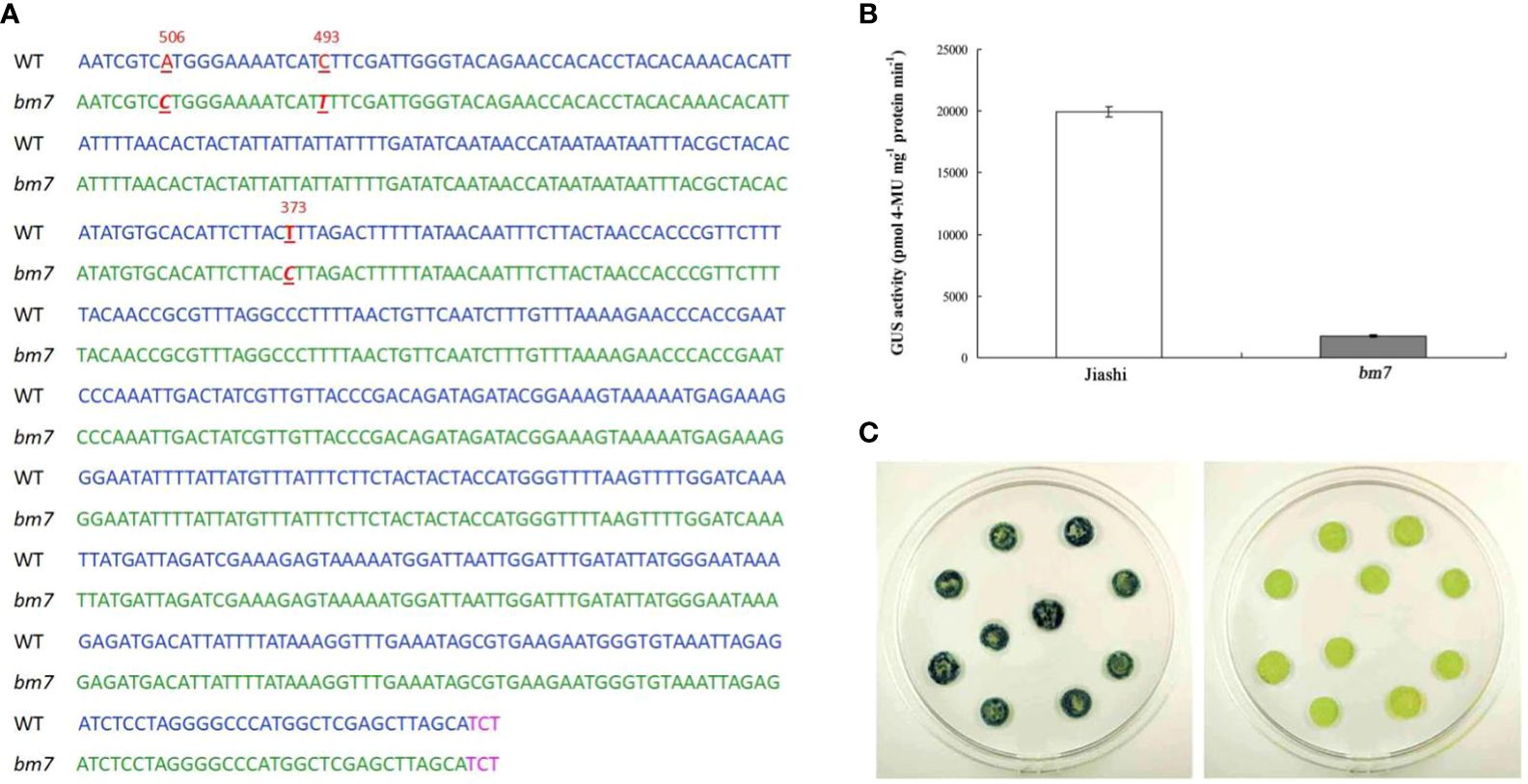
Figure 3 Detection of pll gene promoter activity of Jiashi and bm7. (A) Mutation sites of pll gene promoters of bm7, the blue letters are Jiashi and the green letters are bm7, and TCT is the translation initiation codon of pll gene. (B) GUS activity in NC89 tobacco leaf discs. (C) GUS staining of transformed leaf discs.
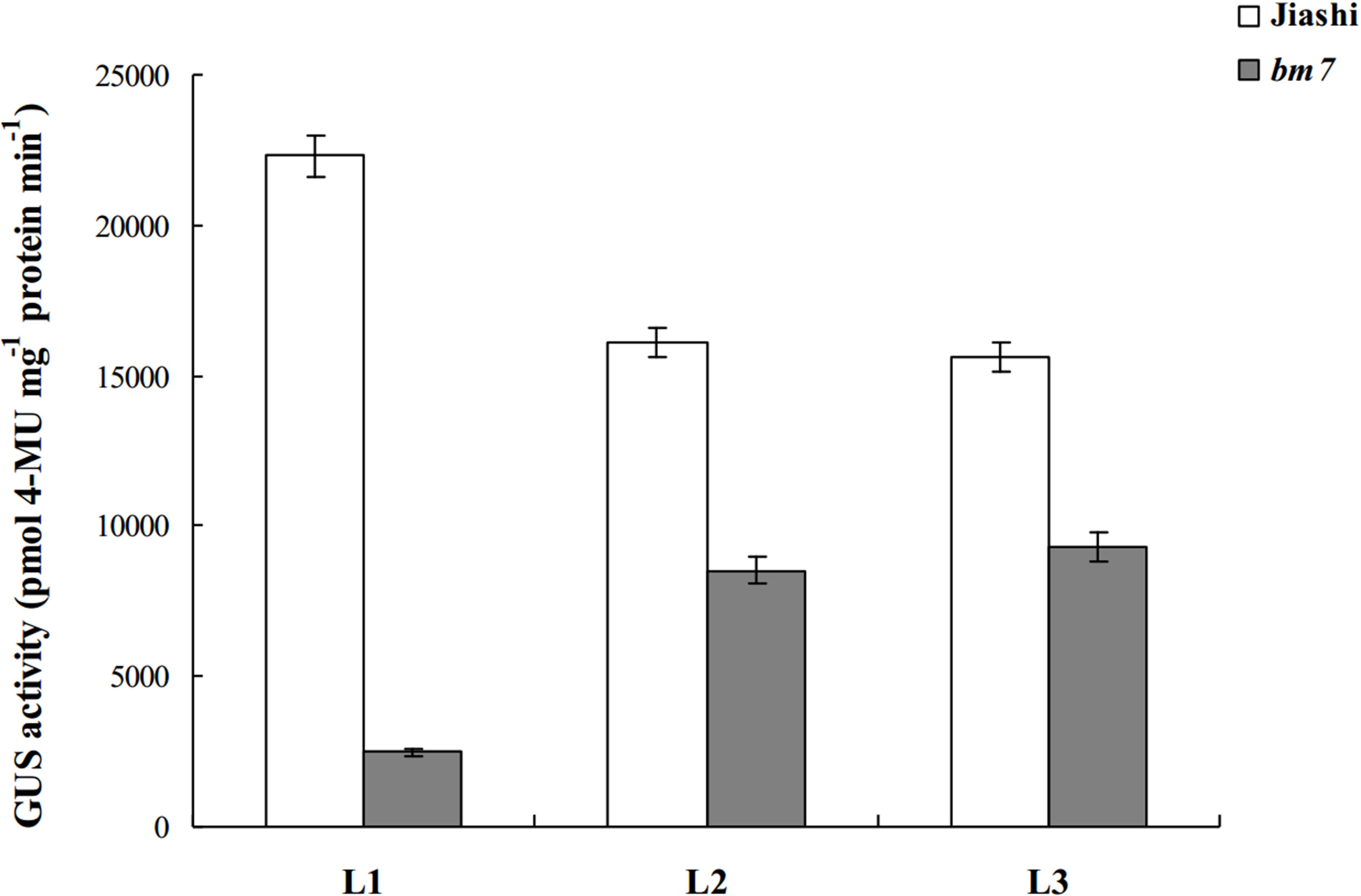
3.4 Mutation sites -373 and -506 influence promoter activity greatly
We constructed PBI121 transient expression vectors carrying each of the following three full-length promoter fragments: L1 (containing mutation sites -373, -493 and -506), L2 (containing mutation sites -493 and -506) and L3 (containing mutation site -506), to analyze the effect of the three mutation sites on the activity of pll gene promoter. The results are shown in Figure 4. In bm7, L2 and L1 promoter fragments exhibited significant difference in activity, while L2 and L3 promoter fragments did not, indicating that the mutation site -373 has a greater influence than the mutation site -493 on promoter activity. In addition, the activity of L3 promoter fragment showed significant difference between Jiashi and bm7, indicating the mutation site -506 also has a great effect on promoter activity. Moreover, L1 fragment showed more significant difference in activity between Jiashi and bm7 than the other two fragments, indicating that the three mutation points have an additive effect on promoter activity, so simultaneous mutation of the three sites is able to further reduce activity of pll promoter.
3.5 Expression of pll gene and leaf shape of bm7 plants were influenced by light and temperature
We found that the pll gene expression and leaf shape of Jiashi did not change in different environments (Figures 5A, C). However, pll gene expression and leaf lobe depth of bm7 varied significantly among different environments (Figures 5B, D). The expression level of pll gene in bm7 plants exposed to intense sunlight and high temperature in outdoor environment was about two times lower than that in indoor environment, while pll gene in Jiashi plants showed no significant differences between the two environments (Figure 5E).
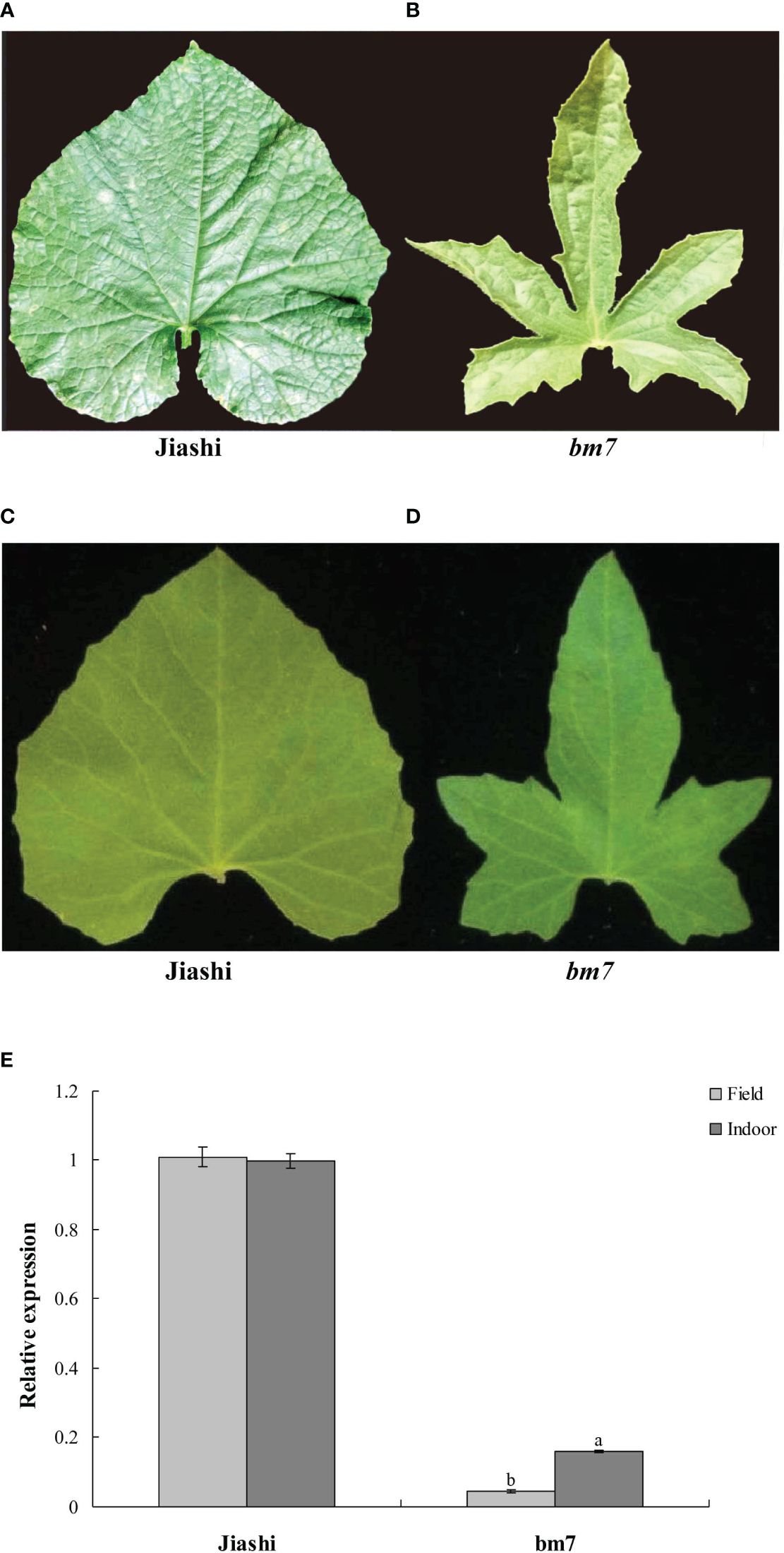
Figure 5 pll gene expression and leaf shape of Jiashi and bm7 in outdoor and indoor environments. Leaf shape of Jiashi (A) and bm7 (B) in outdoor environment; (C) Leaf shape of Jiashi (C) and bm7 (D) in indoor environment; Expression of pll gene in Jiashi and bm7 in outdoor and indoor environments (E). The letters "a, b" are symbols that indicate a significant difference between the two.
We determined the expression of pll gene in the leaves of Jiashi and bm7 under three different light intensity and temperature conditions. At 30°C, the expression of pll gene of bm7 plants gradually declined while the leaf lobe depth gradually increased, with light intensity increasing from 20 to 180 LUX (Figures 6A, B). At a light intensity of 50 LUX, the expression of pll gene of bm7 plants gradually declined while the leaf lobe depth gradually increased, with temperature increasing from 20 to 40°C (Figures 6C, D). These results indicated that the expression of pll gene in bm7 was negatively regulated by temperature and/or light intensity, and the leaf lobe depth increased with the increase of light intensity and/or temperature. However, the pll gene expression and leaf shape of Jiashi plants were not affected by light and/or temperature.
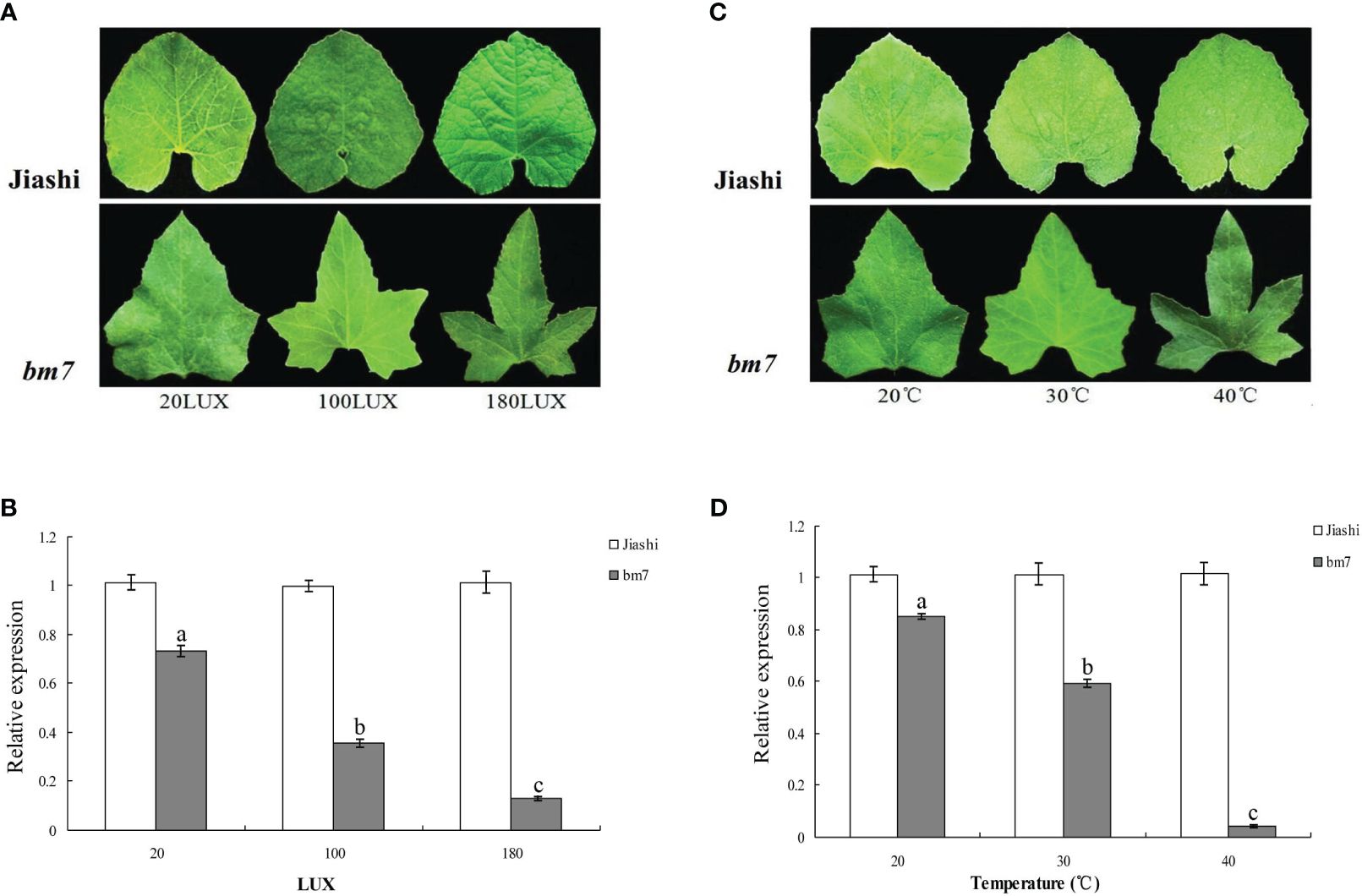
Figure 6 Expression of pll gene and leaf shape of Jiashi and bm7 plants under different light and temperature conditions. (A) The leaf blade shape of Jiashi and bm7 at light intensity of 20, 100 and 180 LUX and temperature of 30°C; (B) The pll gene expression of Jiashi and bm7 at light intensity of 20, 100 and 180 LUX and temperature of 30°C; (C) the leaf blade shape of Jiashi and bm7 at light intensity of 50 LUX and temperature of 20, 30, and 40°C; (D) The pll gene expression of Jiashi and bm7 at light intensity of 50 LUX and temperature of 20, 30, and 40°C. The letters "a–c" are symbols that indicate a significant difference between the three.
3.6 The pll gene promoter activity of bm7 was affected by light and temperature
We measured the pll gene promoter activity under different light intensity and temperature conditions. GUS staining showed that similar to pll gene expression, the promoter activity of this gene in Jiashi plant was not affected by light intensity or temperature, but that in bm7 plants gradually decreased with light intensity or temperature increasing (Figures 7A–D).

Figure 7 pll gene promoter activity of Jiashi and bm7 plants under different light and temperature conditions. (A) GUS activity of tobacco NC89 leaf discs at 20, 100 and 180 LUX; (B) GUS staining of transformed tobacco leaf discs after 20 days of culture at 20, 100 and 180 LUX; (C) GUS activity of tobacco NC89 leaf discs at 20, 30 and 40°C; (D) GUS staining of transformed tobacco leaf discs after 20 days of culture at 20, 30 and 40°C. The letters "a–c" are symbols that indicate a significant difference between the three.
4 Discussion
As a unique germplasm resource, the mutant bm7, which has palmately lobed leaves, has the following potentials: (I) The leaves of bm7 are less overlapped with each other and occupy less space than those of other cultivars, so it is more tolerant to high-density planting to improve melon production per unit area. (II) Lobed leaves can improve plant’s resistance to direct sunlight exposure (Vogel, 2009). Cotton cultivars that have moderately lobed leaves exhibit high photosynthetic efficiency (Barber, 2009). (III) Lobed leaves can also improve melon’s resistance to disease to some extent. Field trials showed that lobed leaf mutant is less susceptible to powdery mildew than its wild-type sibling lines (without lobed leaf trait). (IV) Lobed leaf trait can be used as an indicator to identify the purity of F1 generation seeds in hybrid-seed production.
By comparing with the published melon genome data, we found that the similarity between pll gene and ANT transcription factor is up to 89.3%. Studies have shown that ANT is involved in the early development of all tissues except roots, and maintains the ability of cells to proliferate continuously (Klucher et al., 1996; Elliott et al., 1996; Yamaguchi et al., 2015). Bandupriya et al. reported that ANT gene is involved in the formation of serrated leaf margins, and Arabidopsis thaliana plants that overexpress the ANT gene cloned from coconut have serrated leaf margins (Bandupriya and Dunwell, 2012), which is the only report that ANT is associated with split leaf development.
Gene mutation can cause changes in plant phenotypes. Mutations in the genes associated with gibberellin biosynthesis in Arabidopsis can lead to the formation of foliage leaf lobes, and leaf polymorphism in tomato can be eliminated by the use of exogenous gibberellins (Hay et al., 2002). However, in this study, we found no mutation in the coding sequence of the pll gene in mutant bm7.
Changes in gene expression levels can also lead to changes in plant phenotypes. By cloning leaf morphology- related gene BrLOM2 using RNA-Seq technology, Zhang et al. confirmed that the low expression of this gene might be the main reason for the formation leaf lobes (Zhang et al., 2016). Li et al. mapped the major QTL loci controlling the lobe depth at leaf margin of Brassica napus L., and found that the expression level of BrGA20OX3 in the leaves of the parental material Yellow Sarson C634 (early flowering stage, Indian rape) was only 1/6 of the normal level, suggesting that leaf lobes of the parent C634 might be caused by the low expression of BrGA20OX3 gene (Li et al., 2009). Therefore, we speculated that palmate lobes in leaf was probably a result of gene expression regulation. By measuring the expression of pll gene in Jiashi and bm7, we found that the expression level of pll gene in bm7 was only about 1/25 of that in Jiashi.
Although the expression of pll gene in mutants is significantly lower than that in wide-type plants, but there may be multiple genes being expressed differently between plants. It was still unclear whether the low expression of pll gene is the cause of palmately-lobed leaves. So, in this study, pll gene was silenced by VIGS, an effective method for verifying gene function. The results showed that palmately lobed leaves were observed after pll gene was silenced in Jiashi plants, which was similar to the leaf trait of the mutant. The results confirmed that the low expression of pll gene is the cause of palmately lobed leaves of melon plants.
Promoter, an important component in the regulation of gene expression, can control the timing and level of gene expression. Constitutive promoters are not regulated by external environmental factors, and can drive gene expression continuously (Sun et al., 2018). Our results showed that environment had no significant effect on the expression of pll gene in Jiashi. Transient expression analysis suggested that the promoter of pll gene in Jiashi was constitutive, as its activity was not affected by light and temperature.
Inducible promoters can significantly increase or decrease the transcription level of a gene under certain physical or chemical signal stimulation. The expression of GUS gene in the roots and leaves of transgenic rice was greatly regulated via the treatment with MeJA and Xanthomonas oryzae (Li et al., 2018), indicating that MeJA and X. oryzae can induce the PRJA140 promoter. In addition, mutations in promoter or changes in DNA conformation can also affect gene expression and plant phenotypes. Alexandr Muterko et al. found that allelic variations at VRN-A1 and VRN-B1 could be identified based on DNA curvature and flexibility in the promoter region, and single-nucleotide mutations within VRN-box change DNA conformation, and influence the vernalization requirement and growth habit of wheat (Muterko et al., 2016). In the present study we found that there are three mutations in pll gene promoter of bm7, and this gene is highly expressed at high light intensity and/or low temperature. The transient expression analysis in tobacco indicated its promoter activity decreases with light intensity and/or temperature increasing, suggesting that pll gene promoter of bm7 plants is a typical inducible promoter. Therefore, we can speculate that the mutations change the pll gene promoter from constitutive to inducible, resulting in the changes in pll gene expression, which ultimately leads to the formation of palmately-lobed leaf trait of melon.
A promoter is a region of DNA that initiates transcription of a particular gene, and it may contains a number of response elements. Mutations may cause changes in these response elements, or changes in the spatial structure of the DNA fragment. All these variations may affect the characteristics of the promoter. Therefore, the molecular mechanism by which the pll gene promoter is converted from constitutive to inducible still needs to be further studied.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
LY: Methodology, Software, Writing – review & editing. WD: Methodology, Writing – review & editing. XZ: Formal analysis, Software, Writing – review & editing. QZ: Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Hainan College of Economics and Business high-level talent introduction project (hnjmk2022303).
Acknowledgments
We thank Mr. Fan Wen Lin (College of Life Science and Technology, Xinjiang University) for Jiashi and bm7 melon seeds.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Avilés-Arnaut H., Délano-Frier J. P. (2012). Characterization of the tomato prosystemin promoter: organ-specific expression, hormone specificity and methyl jasmonate responsiveness by deletion analysis in transgenic tobacco plants. J. Integr. Plant Biol. 54, 15–32. doi: 10.1111/j.1744-7909.2011.01084.x
Avramova V., Sprangers K., Beemster. G. T. S. (2015). The maize leaf: another perspective on growth regulation. Trends Plant science. 20, 787–797. doi: 10.1016/j.tplants.2015.09.002
Bandupriya H. D. D., Dunwell J. M. (2012). Overexpression of CnANT, coconut BABYBOOM homologue alters plant growth and morphology in transgenic Arabidopsis plants. Trop. Agric. Res. 23, 349–387. doi: 10.4038/tar.v23i3.4662
Barber J. (2009). Photosynthetic energy conversion: natural and artificial. Chem. Soc. Rev. 38, 185–196. doi: 10.1039/B802262N
Bi C., Ma Y., Wang X. F., Zhang D. P. (2017). Overexpression of the transcription factor NF-YC9 confers abscisic acid hypersensitivity in Arabidopsis. Plant Mol. Biol. 95, 425–439. doi: 10.1007/s11103-017-0661-1
Byrne M. E., Barley R., Curtis M., Arroyo J. M., Dunham M., Hudson A., et al. (2000). Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature. 408, 967–971. doi: 10.1038/35050091
Chai X. R., Wang Y. H. (1983). Inheritance and utilization of the entire leaf type of watermelon. China Fruits. 1, 8–10. doi: 10.16626/j.cnki.issn1000-8047.1983.01.011
Cui D. X., Fan E. P., Huang W., Wu Y. Y., Yang S. J., Huang S. J. (1996). The breeding and genetical analysis of the new water melon CV.XINKAI with entire leaf. Southwest Chain J. Agric. Sci. 9, 125–127. doi: 10.16213/j.cnki.scjas.1996.01.022
Dai X. H., Liu Z. H., Qiao M., Li J., Li S., Xiang F. N. (2017). ARR12 promotes de novo shoot regeneration in Arabidopsis thaliana via activation of WUSCHEL expression. Journal of Integrative. Plant Biol. 59, 747–758. doi: 10.1111/jipb.12567
Dyutin K. E. (1980). Spontaneous mutant of Cucurbita maxima Duch. with lobed leaves. Genetika Ussr. 16, 176–178.
Edgerton M. D. (2009). Increasing crop productivity to meet global needs for feed, food, and fuel. Plant Physiol. 149, 7–13. doi: 10.1104/pp.108.130195
Elliott R. C., Betzner A. S., Huttner E., Oakes M. P., Tucker W. Q., Gerentes D., et al. (1996). AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell. 8, 155–168. doi: 10.1105/tpc.8.2.155
Ganesan J., Sambandam C. N. (1985). nheritance of leaf shape in muskmelon (Cucumis melo L.). A qualitative approach. Annamalai. Univ. Agric. Res. Ann. 12, 53–58.
Gao X., Ning X., Wang. Y. (2014). Fine mapping of a gene that confers palmately lobed leaf(pll)in melon (Cucumis melo L.). Euphytica. 200, 337–347. doi: 10.1007/s10681-014-1151-z
Hay A., Kaur H., Phillips A., Hedden P., Hake S., Tsiantis M. (2002). The gibberellin pathway mediates KNOTTED1-type homeobox function in plants with different body plans. Curr. Biol. 12, 1557–1565. doi: 10.1016/S0960-9822(02)01125-9
Herrington M. E., Brown P. J. (2013). Inheritance of leaf and fruit characteristics in Cucurbita maxima Duch. cv. Queensland Blue X Cucurbita Ecuadorensis Cutler and Whitaker. Queensland J. Agric. Anim. Sci. 45, 45–48. doi: 10.21273/HORTSCI.23.3.603
Huang S. J. (1988). Breeding and utilization of a new watermelon variety “Zhongkai No.1”. China watermelon melon. 1, 15–19. doi: 10.16861/j.cnki.zggc.1988.01.006
Klucher K. M., Chow H., Reiser L., Fischer. R. L. (1996). The AINTEGUMENTA gene of arabidopsis required for ovule and female gametophyte development is related to the floral homeotic gene APETALA2. Plant Cell. 8, 137–153. doi: 10.1105/tpc.8.2.137
Lentz E. M., Kuon J., Alder A., Mangel N., Zainuddin I. M. (2018). Cassava geminivirus agroclones for virus-induced gene silencing in cassava leaves and roots. Plant Methods 14, 1–9. doi: 10.1186/s13007-018-0340-5
Li X. T., Huo Z. B., Wang J. L., Li W. (2008). An early maturing watermelon F1 hybrid-Jucheng 20 Zao. China Cucurbits vegetables. 4, 19–22. doi: 10.16861/j.cnki.zggc.2008.04.006
Li F., Kitashiba H., Inaba K., Nishio T. (2009). A brassica rapa linkage map of EST-based SNP markers for identification of candidate genes controlling flowering time and leaf morphological traits. DNA Res. 16, 311. doi: 10.1093/dnares/dsp020
Li T. T., Li L. L., Chen L. H., Gao L. F. (2018). Cloning and functional identification of meJA and bacterial blight-induced promoter in rice. Mol. Plant Breeding. 16, 689–695. doi: 10.13271/j.mpb.016.000689
Matsuo K., Fukuzawa N., Matsumura T. (2016). A simple agroinfiltration method for transient gene expression in plant leaf discs. J. Bioscience Bioengineering. 122, 351–356. doi: 10.1016/j.jbiosc.2016.02.001
Muterko A., Kalendar R., Salina E. (2016). Novel alleles of the VERNALIZATION1 genes in wheat are associated with modulation of DNA curvature and flexibility in the promoter region. BMC Plant Biol. 16, 66–81. doi: 10.1186/s12870-015-0691-2
Smith L. G., Greene B., Veit. B. (1992). A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Dev. (Cambridge England). 116, 21–30. doi: 10.1242/dev.116.1.21
Spangler J. R., Caruana J. C., Phillips D. A., Walper S. A. (2019). Broad range shuttle vector construction and promoter evaluation for the use of Lactobacillus plantarum WCFS1 as a microbial engineering platform. Synth Biol. (Oxf). 23, ysz012. doi: 10.1093/synbio/ysz012
Sun Q. X., Yuan C. L., Wang X. Z., Tang Y. Y., Wu Q., Wang Z. W., et al. (2018). Reviews of progress on peanut promoters. Chin. J. Oil Crop Sci. 40, 452–458. doi: 10.7505/j.issn.1007-9084.2018.03.020
Vogel S. (2009). Leaves in the lowest and highest winds: temperature, force and shape. New phytologist. 183, 13–26. doi: 10.1111/j.1469-8137.2009.02854.x
Yamaguchi N., Jeong C. W., Nolewilson S., Krizek B. A., Wagner D. (2015). AINTEGUMENTA and AINTEGUMENTA-LIKE6/PLETHORA3 induce LEAFY expression in response to auxin to promote the onset of flower formation in Arabidopsis. Plant Physiol. 102, 069902. doi: 10.1104/pp.15.00969
Yan H. J., Shi S. C., Ma N., Cao X. Q., Zhang H., Qiu X. Q., et al. (2018). Graft-accelerated virus-induced gene silencing facilitates functional genomics in rose flowers. Plant Biol. J. Integrative. 60, 34–44. doi: 10.1111/jipb.v60.1
Zhang S.-J., Yan F.-F., Yu Z.-J., Zhang M.-K., Hui M.-X. (2016). Cloning and expression analysis of brLOM2, a gene involved in lobed leaf development in pakchoi. Acta Hortic. Sinica. 43, 2029–2038. doi: 10.16420/j.issn.0513-353x.2016-0252
Zhou Q. (1993). Preliminary report on breeding of whole leaf tetraploid QB-3 watermelon. China watermelon melon. 2, 628. doi: 10.16861/j.cnki.zggc.1993.02.003
Keywords: muskmelon, lobed leaf trait, PLL gene, promoter, transient expression
Citation: Yong L, Dai W, Zhang X and Zhang Q (2024) Mutations alter pll gene promoter from constitutive to inducible, coffering palmately lobed leaf trait of melon. Front. Plant Physiol. 2:1372307. doi: 10.3389/fphgy.2024.1372307
Received: 17 January 2024; Accepted: 03 April 2024;
Published: 29 April 2024.
Edited by:
Yasuyuki Kawaharada, Iwate University, JapanCopyright © 2024 Yong, Dai, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Zhang, ODIxODYxMTI3QHFxLmNvbQ==
†These authors share first authorship
 LiangMin Yong1†
LiangMin Yong1† Qiang Zhang
Qiang Zhang