- 1Basic Medical Research Center, Shanxi Medical University, Taiyuan, Shanxi, China
- 2Department of Ophthalmology, Shanxi Medical University Second Affiliated Hospital, Taiyuan, Shanxi, China
The SNX-BAR subfamily is a component of the sorting nexins (SNXs) superfamily. Distinct from other SNXs, which feature a PX domain for phosphoinositide binding, the SNX-BAR subfamily includes a BAR domain that induces membrane curvature. Members of the SNX-BAR subfamily work together to recognize and select specific cargo, regulate receptor signaling, and manage cargo sorting both with and without the involvement of sorting complexes. They play a crucial role in maintaining cellular homeostasis by directing intracellular cargo to appropriate locations through endo-lysosomal, autophagolysosomal, and ubiquitin-proteasome pathways. This subfamily thus links various protein homeostasis pathways. This review examines the established and hypothesized functions of the SNX-BAR subfamily, its role in intracellular protein sorting and stability, and explores the potential involvement of subfamily dysfunction in the pathophysiology of cardiovascular and neurodegenerative diseases.
1 Introduction
Cells rely on a multitude of transmembrane proteins, along with their associated proteins and lipids (such as signaling receptors, ion channels, and polar markers), collectively referred to as “cargo,” to interact with their environment. Endosomes, crucial metabolic centers in eukaryotic cells, dictate the fate of endocytic cargo and play a key role in maintaining cellular homeostasis during material exchange (Antonescu et al., 2014; Gilleron et al., 2019) and information transfer (Naslavsky and Caplan, 2018). Once internalized, cargo is sorted through the endocytic network and typically follows one of two paths: it may be recycled to various organelles (Cullen and Steinberg, 2018a) or, in some cases, transported to the trans-Golgi network (TGN) or recycled endosomes and returned to the plasma membrane (PM) via the secretory pathway (Doherty and McMahon, 2009; Repnik et al., 2013). Alternatively, cargo labeled with ubiquitin is encapsulated in intraluminal vesicles (ILVs), which are then budded off from sorting endosomes and ultimately delivered to lysosomes for degradation (Schoneberg et al., 2017a; McCullough et al., 2013; Schoneberg et al., 2017b; Simonetti et al., 2023).
The SNX family, a highly conserved and diverse group of membrane-associated proteins, is vital for regulating the equilibrium of cargo circulation, retrograde transport, and degradation (Hanley and Cooper, 2020). Currently, 33 mammalian SNXs have been identified (Cullen and Korswagen, 2011) and classified into five subfamilies based on their domains: SNX-PX, SNX-BAR (Bin/Amphiphysin/Rvs), SNX-FERM (protein 4.1/ezrin/radixin/moesin), SNX-PXA-RGS-PXC, and other unique SNX subfamilies (Gallon and Cullen, 2015a; Zhang et al., 2018) (Figure 1). Among these, SNX-BARs are the most prevalent and crucial for recycling from endosomes to the TGN and plasma membrane (van Weering et al., 2012a; Wassmer et al., 2007). Studies have demonstrated that the involvement of SNX-BARs in endosomal recycling is dependent on the mammalian retromer complex [VPS26A (or VPS26B)/VPS35/VPS29], which is responsible for cargo recognition. SNX-BARs facilitate membrane remodeling and the formation of tubules and vesicles for cargo transport (Steinberg et al., 2013), thus assisting in the recirculation of endosomes to the PM. While often associated with Retromer-related SNX-BARs (Cullen and Steinberg, 2018b; Yong et al., 2020a), some studies indicate that SNX-BARs can also function independently of retromer in membrane remodeling and cargo sorting (Simonetti et al., 2017a; Kvainickas et al., 2017a; Seaman, 2021; Wang et al., 2018a; McNally and Cullen, 2018).
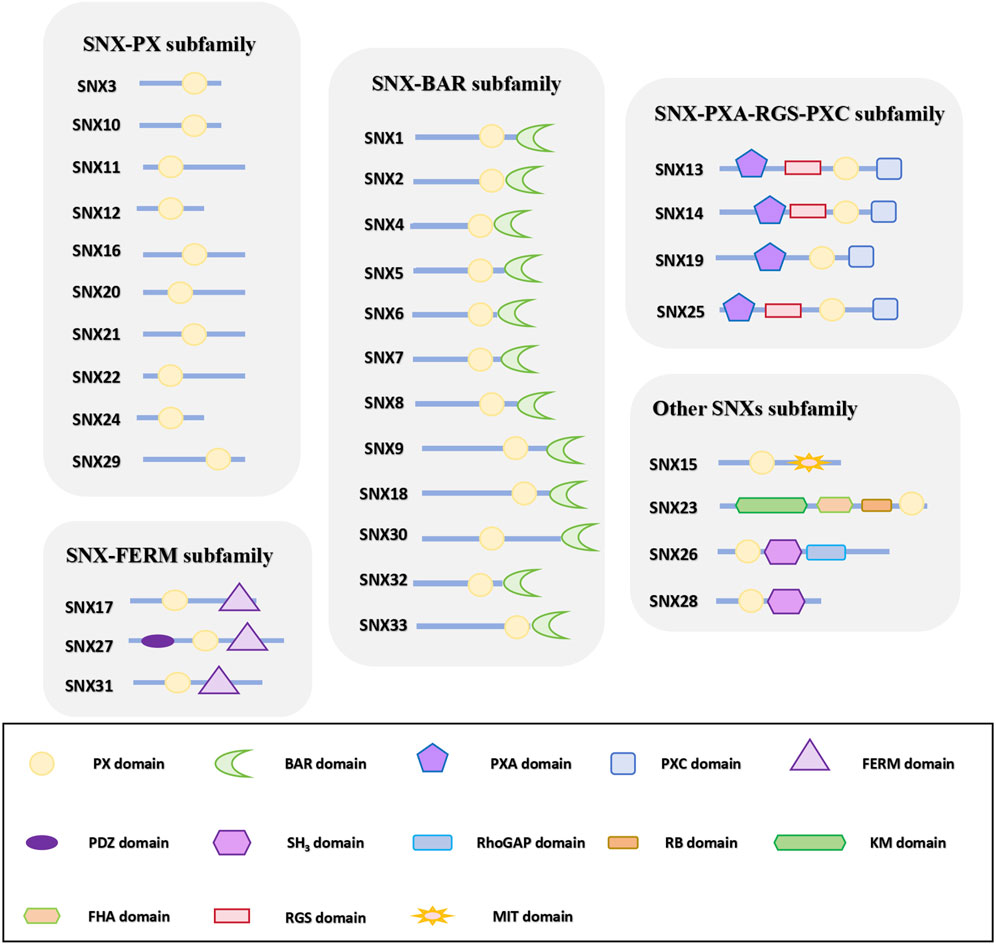
Figure 1. SNXs subfamily classification according to domain organization. The 33 identified members of the SNX family are classified into five subfamilies based on their domain architecture. Some members, featuring distinct domains that do not align with the other subfamilies, are assigned to a separate category. All SNX family members harbor a conserved PX domain, with additional domain variations observed across different subfamilies. PX domain: phagocyte oxidase (phox) homology domain; BAR domain: BinAmphiphysin/Rvs domain; PXA domain: PX-associated domain A; PXC domain: PX-associated domain C; FERM domain: protein 4.1/ezrin/radixin/moesin domain; PDZ domain: postsynaptic density 95/discs large/zonula occludens domain; SH3 domain: Src Homology 3 domain; KM domain: kinesin motor domain; RB domain: Rab5-binding domain; FHA domain: forkhead associated domain; MIT domain, microtubule interacting and trafficking domain; RGS: regulator of G-protein signaling domain.
Maintaining cellular homeostasis involves protein quality control mechanisms that eliminate misfolded, damaged, or redundant proteins and organelles through three primary pathways: the endosome-lysosome pathway, the autophagy-lysosome pathway (ALP), and the ubiquitin-proteasome pathway (UPS) (Chen et al., 2011; Wang and Le, 2019; Pohl and Dikic, 2019). This review will discuss how distinct members of the SNX-BAR subfamily regulate these three protein quality control pathways to maintain cellular homeostasis.
2 SNX-BAR subfamily domain and biochemical characteristics
Cell signaling relies on the aggregation of proteins within specific modular regions, which imparts distinct activities or functions to the cell (Mayer, 2015). The SNX-BAR subfamily, as illustrated in Figure 1, includes SNX1, SNX2, SNX4, SNX5, SNX6, SNX7, SNX8, SNX9, SNX18, SNX30, SNX32, and SNX33 (Yong et al., 2020b). In addition to the common PX and BAR domains, SNX9, SNX18, and SNX33 also possess SH3 domains.
2.1 PX domain (family characteristics)
The SNX family consists of peripheral membrane proteins involved in protein sorting and transport, all of which feature a shared PX (Phox) domain (Gallon and Cullen, 2015b; Cullen, 2008a). The PX domain was initially identified in the NADPH oxidase subunits p40Phox and p47Phox and has since been observed in various other proteins, including SNX1 (Ponting, 1996). This domain is characterized by three α-helical chains and three anti-parallel β-strands, comprising approximately 100–130 residues (Bravo et al., 2001). A conserved sequence within the PX domain forms a positively charged, proline-rich ring that binds to the negatively charged phosphate groups of phosphoinositides (PIPs) (Seet and Hong, 2006). This interaction provides recruitment signals and facilitates allosteric regulation of different peripheral membrane proteins. The PX domain can bind various phosphoinositides (Teasdale and Collins, 2012), with phosphatidylinositol 3-phosphate (PtdIns3P) being a primary lipid target for PX-domain proteins in mammals (Lucas et al., 2016a). Additionally, the PX domain serves as a protein interaction module. For example, the crystal structures of SNX3 in complex with the VPS26 and VPS35 subunits of the retromer complex demonstrate that the PX domain directly mediates these protein interactions (Lucas et al., 2016b). Furthermore, the PX domains of SNX-BAR subfamily members SNX5 and SNX6 have been shown to interact directly with proteins from the bacterial pathogen Chlamydia trachomatis (Aeberhard et al., 2015; Mirrashidi et al., 2015).
2.2 BAR domain (special)
The SNX-BAR subfamily features a unique Bin/amphiphysin/Rvs (BAR) domain at the carboxyl terminus. This BAR domain, located adjacent to the PX domain (Carlton et al., 2004a), enables the SNX-BAR subfamily to recognize and interact with various membrane properties—such as curvature, lipid composition, and cargo density—allowing them to traverse between cytoplasmic and endocytic network membranes (Carlton et al., 2004b; van Weering et al., 2012b; Pylypenko et al., 2007). The BAR domain plays a crucial role in membrane morphodynamics, acting as a membrane-binding domain that senses membrane curvature and promotes membrane tabulation (Takei et al., 1999). It binds to membrane surfaces with specific curvatures (Peter et al., 2004) and facilitates dimerization of SNX-BAR proteins through internal interaction sites (Dislich et al., 2011). Hydrophobic and charged interactions among dimers restrict the formation of functional SNX-BAR homodimers or heterodimers (Sierecki et al., 2014). For instance, homodimers of SNX9, SNX18, and SNX33 are associated with the plasma membrane, while SNX8 homodimers localize to endosomes (van Weering et al., 2012c). Additionally, ESCPE-1, composed of SNX1/SNX2 and SNX5/SNX6/SNX32 heterodimers, is involved in endosome-to-Golgi (TGN) recovery and endosome-to-plasma membrane recycling (van Weering et al., 2012d). Conversely, heterodimers of SNX4:SNX7 and SNX4:SNX30 are implicated in autophagy biogenesis (Anton et al., 2020a).
2.3 SH3 domain (non-major domain)
The BAR domain is rarely found in isolation; it is most commonly associated with the SH3 (Src Homology 3) domain, aside from the PX domain (Carman and Dominguez, 2018). The SH3 domain, comprising 60 amino acids, is a prominent protein interaction region found in signaling proteins (Dionne et al., 2022). It regulates and participates in various cellular processes, including intercellular signaling, protein transport, and degradation (Kaneko et al., 2008; Tatarova et al., 2012). The SH3 domain often contributes to protein complex formation through interactions with other protein regions or through isomeric regulation, thereby stabilizing interactions mediated by other domains of the host protein (Dionne et al., 2021).
3 SNX-BAR subfamily and endosome-lysosome pathway
The endosome-lysosome pathway refers to the process by which cells degrade waste materials and damaged proteins through the fusion of endosomes with lysosomes. Research indicates that in mammalian cells, approximately 50%–180% of the plasma membrane surface area cycles through endocytosis and exocytosis every hour (Steinman et al., 1983). Cellular contents and membranes are delivered to early endosomes (EEs), also known as sorting endosomes (SEs), in the peripheral cytoplasm via primary endocytic vesicles. EEs accumulate cargo and either recycle directly to the plasma membrane or through circulating endosomes in the perinuclear region. During this process, EEs mature into late endosomes (LEs), which then fuse with acid hydrolase-rich transport vesicles from the Golgi complex to form a transient organelle, the endolysosome. Under the influence of a proton pump, lysosomes mature and actively degrade their contents for cellular reutilization. This process is essential for protein quality control and maintaining cellular homeostasis (Huotari and Helenius, 2011).
The BAR domain of the SNX-BAR subfamily plays a crucial role in forming and stabilizing the tubular subdomains of endosome-mediated cargo recovery (Cullen, 2008b). Additionally, it is involved in regulating protein degradation, endosome-Golgi repair, and endosomal system recycling (Wang et al., 2018b). Besides targeting ubiquitin-labeled goods for lysosomal degradation via the endosomal sorting complex required for transport (ESCRT) (Cullen and Steinberg, 2018c), other internalized materials can be directed to the Golgi apparatus or plasma membrane through reverse transport or recycling complexes, assisted by SNXs and the actin-remodeling WASH complex (Chen K. E. et al., 2019). Within the SNX-BAR subfamily, SNX5 facilitates the retrograde transport of monoamine transporters (VMAT) from endosomes to the Golgi reticulum, where they are assembled into dense core vesicles (Xu et al., 2022) via adapter protein 3 (AP-3) (Figure 2D). SNX9 and SNX18 are involved in plasma membrane endocytosis pathways, including clathrin-mediated protein aggregation, and can functionally compensate for each other (Figure 2A) (Park et al., 2010). SNX32 binds to the immunoglobulin superfamily member Basigin (BSG) via its PX domain, promoting its transport to the cell surface to maintain glial homeostasis (Figure 2D) (Sugatha et al., 2023). Abnormal processing of amyloid precursor protein (APP) results in amyloid beta peptide (Aβ), a pathological marker in Alzheimer’s disease (Zhang et al., 2019). BACE1, involved in APP hydrolysis, is regulated by SNX6, which modulates its retrograde transport (Figure 2D) (Okada et al., 2010), while SNX33 affects APP endocytosis (Takada-Takatori et al., 2019) and α-secretase cleavage of APP (Figure 2A) (Schobel et al., 2008). Furthermore, studies have shown that the binding of SNX4 with SNX7 or SNX30 complexes, or with the retromer complex, facilitates the transport and recycling of autophagy-related protein 9A (ATG9A) and transferrin receptor (TfR) from the plasma membrane to endosomes. (Figure 2C) (Anton et al., 2020a) (Cullen, 2008c). Recent studies also highlight an independent role of SNX-BAR proteins in autophagy, detailed in Section 3.
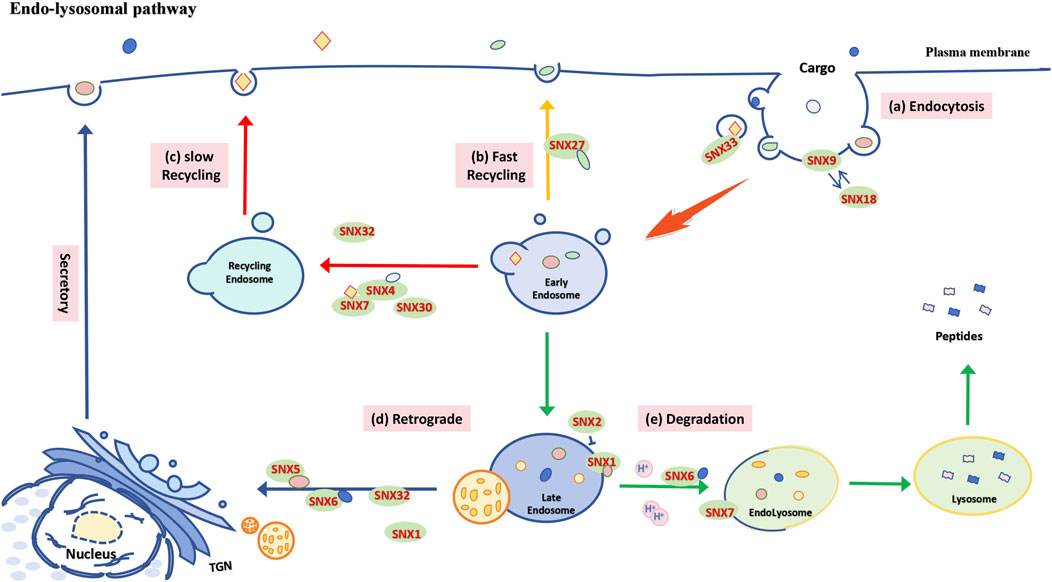
Figure 2. Functional schematic of the SNX-BAR subfamily in the endosome-lysosome pathway. (A) Plasma membrane endocytosis: SNX9 and SNX18 regulate cargo internalization through clathrin, while SNX33 is involved in the endocytosis of APP. (B) Fast recycling pathway: After cargo is internalized into cells via primary endocytic vesicles, it is delivered to early endosomes (EE) and then recycled back to the plasma membrane through a recycling endosome pathway, which may be mediated by different SNXs in either direct or perinuclear regions. In mammalian cells, the SNX27-mediated rapid endosomal recycling pathway promotes the swift return of cargo to the cell surface. (C) Slow recycling pathway: SNX4, through heterodimerization with SNX7 and SNX30, facilitates the transport and recycling of ATG9A and TfR from the plasma membrane to the endosome. (D) Retrograde transport: Following cargo accumulation, EE converts into late endosomes (LE), and some internalized cargo is transported to the Golgi apparatus or plasma membrane via retromer complexes or recycling complexes, with assistance from specific SNXs (e.g., SNX1, SNX5, SNX6, SNX32). (E) Endolysosomal degradation: A portion of the cargo is sorted by SNXs (such as SNX1, SNX6, SNX7) and fuses with transport vesicles originating from the Golgi complex, which are rich in acidic hydrolases, to form endolysosomes for degradation. Notably, SNX1 and SNX2 exhibit antagonistic roles in regulating the sorting of PAR1 to lysosomes.
In mammalian cells, cargo can be recycled to the cell surface via SNX4 (Figure 2C) and SNX27-mediated (Figure 2B) endocytic pathways, while other SNX-BAR family members assist in sorting cargo to lysosomes for degradation (Mallet and Maxfield, 1999; Goldenring, 2015). For example, SNX1 mediates the sorting of protease-activated receptor 1 (PAR1) to lysosomes independently of the reverse transport complex, whereas SNX2 may indirectly regulate PAR1 sorting by affecting SNX1 localization (Figure 2D) (Gullapalli et al., 2006). SNX1 also binds to the epidermal growth factor receptor (EGFR), enhancing its lysosomal degradation (Figure 2E) (Kurten et al., 1996). Furthermore, SNX6-mediated endolysosomal degradation of the tumor suppressor p27 (Kip1) contributes to cell cycle progression (Figure 2E) (Fuster et al., 2010a). SNX4 inhibits BACE1 transport to lysosomes, leading to Aβ accumulation (Figure 2C) (Muller et al., 2017), while overexpression of SNX7 improves APP lysosomal degradation and reduces Aβ production (Figure 2E) (Xu et al., 2018).
In summary, members of the SNX-BAR subfamily play crucial roles in the endocytosis of various cargo, both fast and slow recycling, retrograde transport, and endolysosomal degradation, thereby contributing significantly to cellular homeostasis and protein quality control.
4 SNX-BAR subfamily and the autophagy-lysosomal pathway (ALP)
The autophagy-lysosome pathway refers to the process by which cells transport long-lived, highly conserved proteins, dysfunctional or redundant organelles, and protein aggregates into the bilayer membrane of the autophagosome, which are then delivered to the lysosome for degradation. This process efficiently supplies energy and raw materials, thereby sustaining cellular homeostasis (Klionsky, 2007; Lamb et al., 2013). Autophagy is triggered in eukaryotic cells by various external factors (e.g., nutrient deprivation, hypoxia, ischemia) and internal factors (e.g., organelle aging, protein misfolding, DNA damage) that disrupt intracellular homeostasis. It is a critical adaptive mechanism that helps cells manage stress and maintain homeostasis (Dikic, 2017a; Galluzzi et al., 2017). Notably, SNX-BAR proteins operate independently of the reverse transport complex during autophagy (Simonetti et al., 2017b; Kvainickas et al., 2017b). In response to physiological stress, SNXs reposition to participate in macroautophagy. Cargo is encapsulated by autophagosomes through selective or non-selective mechanisms, with membrane extension occurring via contributions from various sources, including the plasma membrane and Golgi apparatus (Guo et al., 2012).
The PX domain of SNX family members binds to different phosphoinositides (PIPs), including PI(3)P, which associates with ATG8 (LC3) to initiate autophagosome membrane formation (Dooley et al., 2014). Members of the SNX-BAR subfamily possess a BAR domain that induces membrane curvature, facilitating the recruitment of autophagy-related proteins and aiding in the extension and closure of the autophagosome membrane (Suarez et al., 2014; Blood and Voth, 2006). Extensive research indicates that PI(3)P-binding BAR domain proteins are closely linked to autophagy biogenesis (Feng et al., 2020; Rodgers et al., 2022). In yeast, the autophagosome assembly site is known as the phage assembly site (PAS) (Hollenstein and Kraft, 2020). Loss of SNX4 impairs PAS formation and delays the autophagy response (Popelka et al., 2017). Recent phosphoproteomic studies in yeast have identified SNX4 as a direct substrate of Atg1/ULK1 (Hu et al., 2019), suggesting that SNX4 phosphorylation may shift its role from endosomal sorting to autophagy induction (Figure 3E). SNX18, a positive autophagy regulator, facilitates the transport of ATG9A and ATG16L1 from cycling endosomes to the autophagosome assembly site by recruiting Dynamin-2 to promote membrane elongation (Soreng et al., 2018). However, phosphorylation of SNX18 at S233 negatively regulates its autophagy function (Figure 3C) (Knaevelsrud et al., 2013).
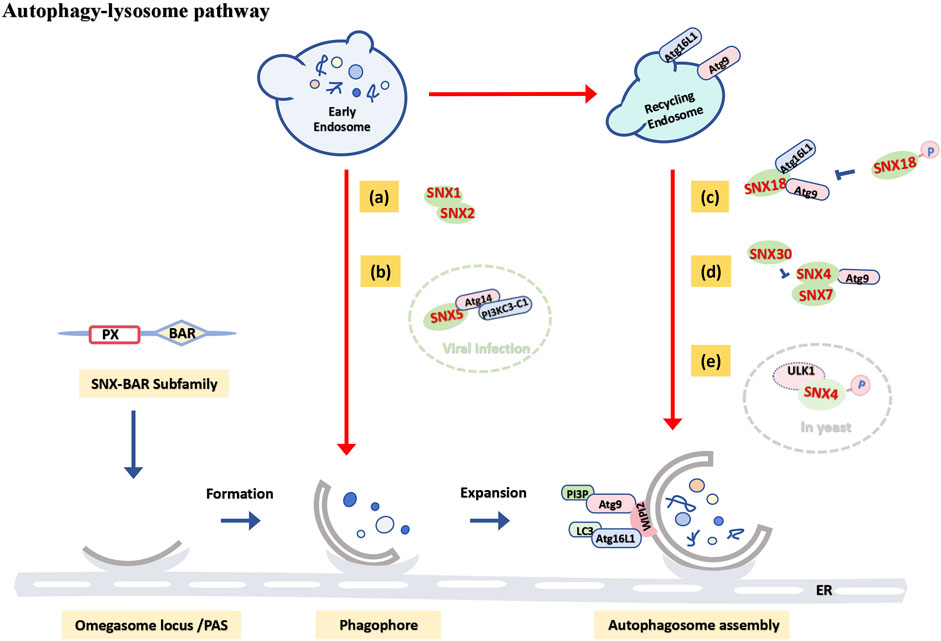
Figure 3. Functional schematic of the SNX-BAR subfamily in the autophagy-lysosome pathway. When cargo is positioned in early endosomes or delivered to recycling endosomes: (A) SNX1 and SNX2 cooperate to regulate endosomal tubule formation, participating in starvation-induced autophagy. (B) In virus-induced autophagy, SNX5 interacts with the PI3KC3-C1 complex to initiate autophagosome formation (green dashed circle). (C) SNX18 positively regulates autophagy by promoting the transport of ATG9A and ATG16L1 to autophagosome assembly sites, though phosphorylation at the S233 site negatively regulates its autophagic function. (D) SNX4-SNX7 and SNX4-SNX30 heterodimers jointly regulate autophagosome assembly by controlling ATG9 transport. (E) In yeast models, SNX4 may be phosphorylated by ULK1, diverting it from its endosomal sorting function and inducing autophagy (gray dashed circle).
Autophagy induced by viral infection represents a specialized type of autophagy. Genome-wide RNA interference screenings have revealed that SNX5 is essential exclusively for virus-induced autophagy. Following viral infection, SNX5 interacts with Beclin1 and the Class III phosphatidylinositol 3-kinase (PI3KC3) complex 1 (PI3KC3-C1), which includes ATG14, at early endosomes containing virions. This interaction increases PI3KC3-C1 kinase activity and recruits PI3P and WIPI2 to these endosomes, Initiates the first stage of autophagosome formation, which is the formation of the isolation membrane. Deletion of SNX5 increases cell susceptibility and mortality to viral infection in vitro (Figure 3B) (Dong et al., 2021), indicating that enhancing SNX5 expression and autophagy induction may be crucial for the immune response to viral infections.
Members of the SNX-BAR subfamily can function both independently and in heterodimer complexes. For instance, Studies have shown that SNX1 and SNX2 cooperatively induce and regulate the involvement of endosomal tubules in the formation of the isolation membrane (Figure 3A) (Da et al., 2023), with a strong association with starvation-induced autophagy (Da and Morel, 2023). SNX4 is necessary for effective lipidation of LC3 and autophagosome assembly in mammalian cells, while SNX4-SNX7 heterodimers regulate autophagosome assembly by controlling ATG9 transport (Figure 3D) (Anton et al., 2020b). Future research may explore how variations in the balance between heterodimers like SNX4-SNX7 and SNX4-SNX30 affect early autophagy stages.
In summary, the SNX-BAR subfamily can maintain cellular homeostasis primarily through involvement in the biogenesis of autophagosomes, influencing the autophagy-lysosome pathway, independent of retromer transport complexes.
5 SNX-BAR subfamily and ubiquitin-proteasome pathway (UPS)
The ubiquitin-proteasome pathway is the primary proteolytic route for misfolded, damaged, and short-lived proteins. Ubiquitin-tagged proteins are typically targeted for degradation by the proteasome or lysosome, thereby regulating protein quality control and maintaining cellular homeostasis (Budenholzer et al., 2017; Bochtler et al., 1999). Ubiquitin (Ub) is a 76-amino acid protein with a molecular weight of approximately 8.5 kDa, ubiquitously present in eukaryotic cells. It forms a covalent bond between the carboxyl group (-COOH) of its C-terminal glycine and the amino group (-NH2) of substrate lysine (Sun and Chen, 2004). Ubiquitination is a common post-translational modification where E1 ubiquitin-activating enzyme, using ATP energy, forms a UB-E1 complex with ubiquitin. This complex then transfers ubiquitin to E2 ubiquitin-conjugating enzyme, forming the UB-E2 complex through transesterification. E3 ubiquitin ligase subsequently attaches the UB-E2 complex to specific target proteins. The type of linkage and the length of the ubiquitin chains confer various biological functions (Akutsu et al., 2016; Dikic, 2017b).
Numerous studies have shown that sorting nexins (SNXs) influence proteasome activity and substrate degradation through several mechanisms. This discussion focuses on the mechanisms involving the SNX-BAR subfamily. These mechanisms include inhibiting the ubiquitination of protein substrates and modulating ubiquitin-specific factors. For example, FBW7, an E3 ubiquitin ligase, interacts with SNX5 to reduce the ubiquitination and degradation of cancer-associated proteins such as c-Myc, NOTCH1, and Cyclin E1, thereby promoting the progression of head and neck squamous cell carcinoma (HNSCC) (Figure 4A) (Cai et al., 2019). Similarly, SNX6 downregulates Cullin3-mediated ubiquitination and subsequent degradation of programmed death ligand 1 (PD-L1) by binding to Cullin3, thus enhancing cancer cells’ ability to evade immune surveillance (Figure 4B) (Ghosh et al., 2021). SNX8 interacts directly with fatty acid synthase (FASN) to promote its ubiquitination and proteasomal degradation by recruiting TRIM28, an E3 ubiquitin ligase, and enhancing TRIM28-FASN interactions (Figure 4C) (Hu et al., 2021). Additionally, Mib1, an E3 ubiquitin ligase, regulates SNX18 recruitment of guanosine triphosphate (GTP) - dynamin 2 in a ubiquitin ligase-dependent manner, facilitating the endocytosis of Delta-like 1 (Dll1) and enabling effective Notch signaling for normal development and tissue homeostasis (Figure 4D) (Okano et al., 2016).

Figure 4. Functional schematic of the SNX-BAR subfamily in the ubiquitin-proteasome pathway. E3 ligases recognize specific substrates and catalyze the covalent attachment of ubiquitin molecules to mark substrates for proteasomal degradation. In this process: (A) SNX5 interacts with FBW7 (E3), reducing the ubiquitination and subsequent proteasomal degradation of downstream oncogenic proteins mediated by FBW7. (B) SNX6 interacts with Cullin3 (E3) to downregulate Cullin3-mediated ubiquitination and degradation of PD-L1. (C) SNX8 directly binds FASN and recruits TRIM28 (E3), thereby promoting the ubiquitination and degradation of FASN. (D) Mib1 (E3) regulates SNX18 recruitment of dynamin 2 in a ubiquitin ligase activity-dependent manner, promoting the endocytosis of Dll1. (E) Itch (E3) interacts with SNX9 through its PRD domain, mediating the ubiquitination and degradation of SNX9. (F) p27 is dual-regulated by both the ubiquitin-proteasome pathway (red arrow) and the SNX6-mediated endolysosomal pathway (green arrow).
In addition to their roles in UPS regulation, SNXs themselves are regulated by UPS mechanisms. Deubiquitinating enzymes (DUBs) modulate substrate activity and abundance by removing ubiquitin-bound proteins (Sowa et al., 2009). For instance, DUBs have been reported to increase stability by interacting with SNX3 (Boulkroun et al., 2008a) and SNX27 (Stangl et al., 2019). The E3 ubiquitin ligase Itch, a member of the NEDD4 family, regulates intracellular levels of SNX9 through interaction with a proline-rich domain (PRD), mediating SNX9 ubiquitination and degradation (Figure 4E) (Baumann et al., 2010). Although reports on the regulation of SNX-BAR subfamily members by UPS and the activities of E1 and E2 enzymes are limited, they support the hypothesis of mutual interaction between SNX-BAR subfamily members and the UPS.
Notably, selective proteolysis is primarily mediated by both the UPS and the autophagy-lysosomal pathway (ALP), and recent research has highlighted their functional interrelation (Korolchuk et al., 2010; Ji and Kwon, 2017). Ubiquitin plays a key role in targeting proteins for degradation via polyvesicles (Pelham, 2002a), and selective recognition of autophagic substrates in mammalian cells depends on ubiquitin (Pelham, 2002b). Thus, the shared requirement for targeted substrate degradation by ubiquitin integrates the UPS and ALP into a cohesive degradation system (Zientara-Rytter and Subramani, 2019; Chen R. H. et al., 2019). For example, growth suppressor p27 is regulated by both proteasomal degradation (Borriello et al., 2007; Abbastabar et al., 2018) and the SNX6-mediated endolysosomal pathway (Figure 4F) (Fuster et al., 2010b). Similarly, following TORC1 inhibition, riboproteasomes in yeast are degraded by SNX4-Atg20 and SNX4-ATG41-mediated autophagy (Waite et al., 2016). Although the precise mechanisms remain unclear, these findings suggest that SNX-BAR subfamily members may further modulate UPS activity through their influence on autophagy.
In summary, the SNX-BAR subfamily primarily participates in the ubiquitin-proteasome pathway through modulation by, or interaction with, E3 ubiquitin ligases. Additionally, the SNX-BAR family is a key player in linking the UPS and ALP pathways to form a coordinated degradation system.
6 The role of the SNX-BAR subfamily in cytopathological activity and disease
Cargo sorting is essential for maintaining cellular homeostasis, and its dysfunction underlies various diseases, including cardiovascular conditions, neurodegenerative disorders, and cancer (Waite et al., 2016). SNXs, by regulating protein sorting and transport, play crucial roles in membrane transport, organelle movement, cell signaling, and entosis. Dysfunction within the SNX-BAR subfamily can lead to receptor malfunctions and disrupted homeostasis, contributing to disease development (Cullen, 2008d).
SNXs influence cell membrane composition, impacting neuronal excitability, signaling, cognitive responses, and drug resistance (Harashima et al., 2012). Disorders in the SNX-BAR subfamily are linked to several neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and Down syndrome (Kiani et al., 2003). The role of SNX-BAR in Alzheimer’s disease is detailed in Part II. Notably, SNX5 has been shown to facilitate luteal ptosis in Parkinson’s disease, offering new insights into potential pharmacological targets (Huang et al., 2022). Reduced SNX6 expression impairs synaptic function and spatial memory in CA1 pyramidal neurons (Niu et al., 2017), while SNX8 exacerbates abnormal cholesterol levels and acts as a β-toxic enhancer in Alzheimer’s disease (Muirhead and Dev, 2014). SNX32 is associated with a higher risk of Alzheimer’s disease (Kibinge et al., 2020; Ou et al., 2021), and increased SNX33 expression reduces endocytosis of amyloid precursor protein (Takada-Takatori, 2021).
Recent research highlights the pivotal role of SNXs in cardiovascular disease (Yang et al., 2019), suggesting they could be promising therapeutic targets (Yarmohammadi et al., 2022). SNXs affect blood pressure maintenance through the regulation of G protein-coupled receptors, lipid metabolism, and inflammation (Boulkroun et al., 2008b; Singh et al., 2015). For instance, knockout of SNX1, SNX5, and SNX19 impacts hypertension in animal models (Villar et al., 2013a; Villar et al., 2013b). Lower levels of SNX1 are linked to elevated triglycerides and cholesterol (Burden et al., 2004), while reduced SNX5 expression leads to decreased sodium excretion and increased glucosin and glucose levels, contributing to insulin resistance—a key marker of type 2 diabetes and heart failure (Li et al., 2015; Valera et al., 2003). Such changes heighten cardiovascular disease risk.
Additionally, SNX1 is proposed as a potential tumor suppressor and prognostic marker for gastric cancer (Zhan et al., 2018). SNX2 may serve as a marker for active thyroid cells in both normal and hyperactive thyroid conditions (Kanzawa et al., 2014). Recent findings suggest targeting SNX9 could prevent T cell exhaustion and enhance anti-tumor immunity (Trefny et al., 2023). Overexpression of SNX18 has been linked to increased bacterial internalization and represents a new target for virulence proteins like SopB (Liebl et al., 2017). SNX7 is emerging as a biomarker for diagnosing, prognosticating, and predicting responses to chemotherapy and immunotherapy in liver cancer (Chen et al., 2023). Furthermore, genetic variants reducing SNX7 expression are associated with cognitive dysfunction in psychosis and bipolar disorder (Erhardt et al., 2017).
Given the SNX-BAR subfamily’s critical role in maintaining cellular homeostasis, future research is likely to further elucidate their relationships with neurodegenerative, cardiovascular, and other diseases.
7 Conclusion and prospects
This review focuses on the interactions between the SNX-BAR subfamily and the three major pathways involved in maintaining protein homeostasis. This review examines the role of the SNX-BAR subfamily in maintaining protein homeostasis. Like other sorting nexin (SNX) subfamilies, the SNX-BAR subfamily is anticipated to have distinct roles in regulating complex signal transduction and cargo transport, thus enabling precise cellular function regulation. As research on the SNX-BAR subfamily’s involvement in cellular homeostasis advances, our understanding of these proteins continues to expand.
Currently, we have elucidated their fundamental role in protein transport and sorting within the endocytosis pathway, leading to new insights into their contribution to cellular protein quality control. In response to various external stress signals, the SNX-BAR subfamily utilizes conserved evolutionary sequences to direct substrates to specific destinations, either for recycling or degradation. This regulation of intracellular transport by the SNX-BAR subfamily is precisely timed and spatially controlled, highlighting its active role in cellular responses (The summary is shown in Table 1).
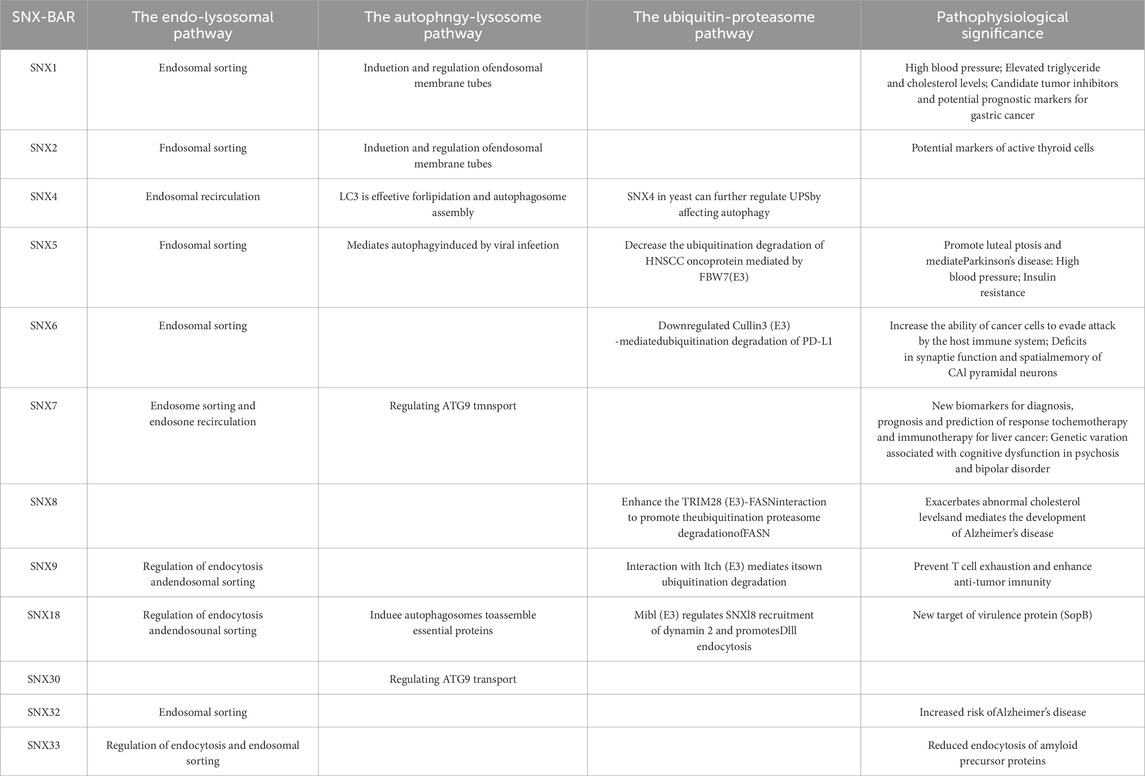
Table 1. SNX-BAR subfamily members involved in three cellular homeostasis pathways. Detailed involvement of 12 SNX-BAR subfamily members in these pathways and their significance in disease and pathophysiology are listed.
Members of the SNX-BAR subfamily exhibit diverse cellular localizations and functions, depending on their transport routes. However, research on the mechanisms governing their combined or individual roles in intracellular transport remains limited. Understanding the interactions among SNX family members is an important area for future investigation.
Increasing evidence underscores the significant role of the SNX-BAR subfamily in the progression of various diseases and suggests that specific subfamily members could serve as potential pharmacological targets. Currently, the molecular mechanisms by which the SNX-BAR subfamily influences cardiovascular and neurodegenerative diseases are still in the early stages of exploration and require further development to become effective targets for clinical interventions.
Author contributions
YL: Project administration, Visualization, Writing–original draft. YLi: Investigation, Project administration, Writing–original draft. JX: Funding acquisition, Investigation, Writing–original draft. WG: Data curation, Visualization, Writing–original draft. MM: Funding acquisition, Investigation, Writing–original draft. XW: Conceptualization, Investigation, Writing–review and editing. LW: Investigation, Writing–review and editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant Nos 31871177 and 82271523), the Basic Research Project of the Shanxi Science and Technology Department (Grant Nos 202303021221134 and 202303021222133), Shanxi Province Higher Education “Billion Project” Science and Technology Guidance Project (Grant No. BYJL034).
Acknowledgments
We are especially grateful to Dr. Hao Haihu of Bethune Hospital of Shanxi Province for his guidance on the topic selection, ideas, views and arguments of this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbastabar M., Kheyrollah M., Azizian K., Bagherlou N., Tehrani S. S., Maniati M., et al. (2018). Multiple functions of p27 in cell cycle, apoptosis, epigenetic modification and transcriptional regulation for the control of cell growth: a double-edged sword protein. DNA Repair (Amst) 69, 63–72. doi:10.1016/j.dnarep.2018.07.008
Aeberhard L., Banhart S., Fischer M., Jehmlich N., Rose L., Koch S., et al. (2015). The proteome of the isolated Chlamydia trachomatis containing vacuole reveals a complex trafficking platform enriched for retromer components. PLoS Pathog. 11 (6), e1004883. doi:10.1371/journal.ppat.1004883
Akutsu M., Dikic I., Bremm A. (2016). Ubiquitin chain diversity at a glance. J. Cell. Sci. 129 (5), 875–880. doi:10.1242/jcs.183954
Anton Z., Betin V. M. S., Simonetti B., Traer C. J., Attar N., Cullen P. J., et al. (2020a). A heterodimeric SNX4--SNX7 SNX-BAR autophagy complex coordinates ATG9A trafficking for efficient autophagosome assembly. J. Cell. Sci. 133 (14), jcs246306. doi:10.1242/jcs.246306
Anton Z., Betin V. M. S., Simonetti B., Traer C. J., Attar N., Cullen P. J., et al. (2020b). A heterodimeric SNX4--SNX7 SNX-BAR autophagy complex coordinates ATG9A trafficking for efficient autophagosome assembly. J. Cell. Sci. 133 (14), jcs246306. doi:10.1242/jcs.246306
Antonescu C. N., McGraw T. E., Klip A. (2014). Reciprocal regulation of endocytosis and metabolism. Cold Spring Harb. Perspect. Biol. 6 (7), a016964. doi:10.1101/cshperspect.a016964
Baumann C., Lindholm C. K., Rimoldi D., Lévy F. (2010). The E3 ubiquitin ligase Itch regulates sorting nexin 9 through an unconventional substrate recognition domain. FEBS J. 277 (13), 2803–2814. doi:10.1111/j.1742-4658.2010.07698.x
Blood P. D., Voth G. A. (2006). Direct observation of Bin/amphiphysin/Rvs (BAR) domain-induced membrane curvature by means of molecular dynamics simulations. Proc. Natl. Acad. Sci. U. S. A. 103 (41), 15068–15072. doi:10.1073/pnas.0603917103
Bochtler M., Ditzel L., Groll M., Hartmann C., Huber R. (1999). The proteasome. Annu. Rev. Biophys. Biomol. Struct. 28, 295–317. doi:10.1146/annurev.biophys.28.1.295
Borriello A., Cucciolla V., Oliva A., Zappia V., Della Ragione F. (2007). p27Kip1 metabolism: a fascinating labyrinth. Cell. Cycle 6 (9), 1053–1061. doi:10.4161/cc.6.9.4142
Boulkroun S., Ruffieux-Daidié D., Vitagliano J. J., Poirot O., Charles R. P., Lagnaz D., et al. (2008a). Vasopressin-inducible ubiquitin-specific protease 10 increases ENaC cell surface expression by deubiquitylating and stabilizing sorting nexin 3. Am. J. Physiol. Ren. Physiol. 295 (4), F889–F900. doi:10.1152/ajprenal.00001.2008
Boulkroun S., Ruffieux-Daidié D., Vitagliano J. J., Poirot O., Charles R. P., Lagnaz D., et al. (2008b). Vasopressin-inducible ubiquitin-specific protease 10 increases ENaC cell surface expression by deubiquitylating and stabilizing sorting nexin 3. Am. J. Physiol. Ren. Physiol. 295 (4), F889–F900. doi:10.1152/ajprenal.00001.2008
Bravo J., Karathanassis D., Pacold C. M., Pacold M. E., Ellson C. D., Anderson K. E., et al. (2001). The crystal structure of the PX domain from p40(phox) bound to phosphatidylinositol 3-phosphate. Mol. Cell. 8 (4), 829–839. doi:10.1016/s1097-2765(01)00372-0
Budenholzer L., Cheng C. L., Li Y., Hochstrasser M. (2017). Proteasome structure and assembly. J. Mol. Biol. 429 (22), 3500–3524. doi:10.1016/j.jmb.2017.05.027
Burden J. J., Sun X. M., García A. B. G., Soutar A. K. (2004). Sorting motifs in the intracellular domain of the low density lipoprotein receptor interact with a novel domain of sorting nexin-17. J. Biol. Chem. 279 (16), 16237–16245. doi:10.1074/jbc.M313689200
Cai J., Sun M., Hu B., Windle B., Ge X., Li G., et al. (2019). Sorting nexin 5 controls head and neck squamous cell carcinoma progression by modulating FBW7. J. Cancer 10 (13), 2942–2952. doi:10.7150/jca.31055
Carlton J., Bujny M., Peter B. J., Oorschot V. M. J., Rutherford A., Mellor H., et al. (2004a). Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr. Biol. 14 (20), 1791–1800. doi:10.1016/j.cub.2004.09.077
Carlton J., Bujny M., Peter B. J., Oorschot V. M. J., Rutherford A., Mellor H., et al. (2004b). Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr. Biol. 14 (20), 1791–1800. doi:10.1016/j.cub.2004.09.077
Carman P. J., Dominguez R. (2018). BAR domain proteins-a linkage between cellular membranes, signaling pathways, and the actin cytoskeleton. Biophys. Rev. 10 (6), 1587–1604. doi:10.1007/s12551-018-0467-7
Chen B., Retzlaff M., Roos T., Frydman J. (2011). Cellular strategies of protein quality control. Cold Spring Harb. Perspect. Biol. 3 (8), a004374. doi:10.1101/cshperspect.a004374
Chen J., Gao G., Zhang Y., Dai P., Huang Y. (2023). Comprehensive analysis and validation of SNX7 as a novel biomarker for the diagnosis, prognosis, and prediction of chemotherapy and immunotherapy response in hepatocellular carcinoma. BMC Cancer 23 (1), 899. doi:10.1186/s12885-023-11405-0
Chen K. E., Healy M. D., Collins B. M. (2019a). Towards a molecular understanding of endosomal trafficking by Retromer and Retriever. Traffic 20 (7), 465–478. doi:10.1111/tra.12649
Chen R. H., Chen Y. H., Huang T. Y. (2019b). Ubiquitin-mediated regulation of autophagy. J. Biomed. Sci. 26 (1), 80. doi:10.1186/s12929-019-0569-y
Cullen P. J. (2008a). Endosomal sorting and signalling: an emerging role for sorting nexins. Nat. Rev. Mol. Cell. Biol. 9 (7), 574–582. doi:10.1038/nrm2427
Cullen P. J. (2008b). Endosomal sorting and signalling: an emerging role for sorting nexins. Nat. Rev. Mol. Cell. Biol. 9 (7), 574–582. doi:10.1038/nrm2427
Cullen P. J. (2008c). Endosomal sorting and signalling: an emerging role for sorting nexins. Nat. Rev. Mol. Cell. Biol. 9 (7), 574–582. doi:10.1038/nrm2427
Cullen P. J. (2008d). Endosomal sorting and signalling: an emerging role for sorting nexins. Nat. Rev. Mol. Cell. Biol. 9 (7), 574–582. doi:10.1038/nrm2427
Cullen P. J., Korswagen H. C. (2011). Sorting nexins provide diversity for retromer-dependent trafficking events. Nat. Cell. Biol. 14 (1), 29–37. doi:10.1038/ncb2374
Cullen P. J., Steinberg F. (2018a). To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell. Biol. 19 (11), 679–696. doi:10.1038/s41580-018-0053-7
Cullen P. J., Steinberg F. (2018b). To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell. Biol. 19 (11), 679–696. doi:10.1038/s41580-018-0053-7
Cullen P. J., Steinberg F. (2018c). To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell. Biol. 19 (11), 679–696. doi:10.1038/s41580-018-0053-7
Da G. J., Charles J., Djebar M., Alvarez-Valadez K., Botti J., Morel E. (2023). A SNX1-SNX2-VAPB partnership regulates endosomal membrane rewiring in response to nutritional stress. Life Sci. Alliance 6 (3), e202201652. doi:10.26508/lsa.202201652
Da G. J., Morel E. (2023). Canonical and non-canonical roles of SNX1 and SNX2 in endosomal membrane dynamics. Contact (Thousand Oaks). 6:25152564231217867. doi:10.1177/25152564231217867
Dikic I. (2017a). Proteasomal and autophagic degradation systems. Annu. Rev. Biochem. 86, 193–224. doi:10.1146/annurev-biochem-061516-044908
Dikic I. (2017b). Proteasomal and autophagic degradation systems. Annu. Rev. Biochem. 86, 193–224. doi:10.1146/annurev-biochem-061516-044908
Dionne U., Bourgault É., Dubé A. K., Bradley D., Chartier F. J. M., Dandage R., et al. (2021). Protein context shapes the specificity of SH3 domain-mediated interactions in vivo. Nat. Commun. 12 (1), 1597. doi:10.1038/s41467-021-21873-2
Dionne U., Percival L. J., Chartier F. J. M., Landry C. R., Bisson N. (2022). SRC homology 3 domains: multifaceted binding modules. Trends Biochem. Sci. 47 (9), 772–784. doi:10.1016/j.tibs.2022.04.005
Dislich B., Than M. E., Lichtenthaler S. F. (2011). Specific amino acids in the BAR domain allow homodimerization and prevent heterodimerization of sorting nexin 33. Biochem. J. 433 (1), 75–83. doi:10.1042/BJ20100709
Doherty G. J., McMahon H. T. (2009). Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902. doi:10.1146/annurev.biochem.78.081307.110540
Dong X., Yang Y., Zou Z., Zhao Y., Ci B., Zhong L., et al. (2021). Sorting nexin 5 mediates virus-induced autophagy and immunity. Nature 589 (7842), 456–461. doi:10.1038/s41586-020-03056-z
Dooley H. C., Razi M., Polson H. E. J., Girardin S. E., Wilson M. I., Tooze S. A. (2014). WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol. Cell. 55 (2), 238–252. doi:10.1016/j.molcel.2014.05.021
Erhardt S., Schwieler L., Imbeault S., Engberg G. (2017). The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 112 (Pt B), 297–306. doi:10.1016/j.neuropharm.2016.05.020
Feng Z., Kovalev N., Nagy P. D. (2020). Key interplay between the co-opted sorting nexin-BAR proteins and PI3P phosphoinositide in the formation of the tombusvirus replicase. PLoS Pathog. 16 (12), e1009120. doi:10.1371/journal.ppat.1009120
Fuster J. J., González J. M., Edo M. D., Viana R., Boya P., Cervera J., et al. (2010a). Tumor suppressor p27(Kip1) undergoes endolysosomal degradation through its interaction with sorting nexin 6. FASEB J. 24 (8), 2998–3009. doi:10.1096/fj.09-138255
Fuster J. J., González J. M., Edo M. D., Viana R., Boya P., Cervera J., et al. (2010b). Tumor suppressor p27(Kip1) undergoes endolysosomal degradation through its interaction with sorting nexin 6. FASEB J. 24 (8), 2998–3009. doi:10.1096/fj.09-138255
Gallon M., Cullen P. J. (2015a). Retromer and sorting nexins in endosomal sorting. Biochem. Soc. Trans. 43 (1), 33–47. doi:10.1042/BST20140290
Gallon M., Cullen P. J. (2015b). Retromer and sorting nexins in endosomal sorting. Biochem. Soc. Trans. 43 (1), 33–47. doi:10.1042/BST20140290
Galluzzi L., Baehrecke E. H., Ballabio A., Boya P., Bravo-San Pedro J. M., Cecconi F., et al. (2017). Molecular definitions of autophagy and related processes. EMBO J. 36 (13), 1811–1836. doi:10.15252/embj.201796697
Ghosh C., Xing Y., Li S., Hoyle R. G., Sun M., Li J., et al. (2021). Sorting nexin 6 interacts with Cullin3 and regulates programmed death ligand 1 expression. FEBS Lett. 595 (20), 2558–2569. doi:10.1002/1873-3468.14191
Gilleron J., Gerdes J. M., Zeigerer A. (2019). Metabolic regulation through the endosomal system. Traffic 20 (8), 552–570. doi:10.1111/tra.12670
Goldenring J. R. (2015). Recycling endosomes. Curr. Opin. Cell. Biol. 35, 117–122. doi:10.1016/j.ceb.2015.04.018
Gullapalli A., Wolfe B. L., Griffin C. T., Magnuson T., Trejo J. (2006). An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1: evidence for retromer-Hrs-and Tsg101-independent functions of sorting nexins. Mol. Biol. Cell. 17 (3), 1228–1238. doi:10.1091/mbc.e05-09-0899
Guo Y., Chang C., Huang R., Liu B., Bao L., Liu W. (2012). AP1 is essential for generation of autophagosomes from the trans-Golgi network. J. Cell. Sci. 125 (Pt 7), 1706–1715. doi:10.1242/jcs.093203
Hanley S. E., Cooper K. F. (2020). Sorting nexins in protein homeostasis. Cells 10 (1), 17. doi:10.3390/cells10010017
Harashima S., Horiuchi T., Wang Y., Notkins A. L., Seino Y., Inagaki N. (2012). Sorting nexin 19 regulates the number of dense core vesicles in pancreatic β-cells. J. Diabetes Investig. 3 (1), 52–61. doi:10.1111/j.2040-1124.2011.00138.x
Hollenstein D. M., Kraft C. (2020). Autophagosomes are formed at a distinct cellular structure. Curr. Opin. Cell. Biol. 65, 50–57. doi:10.1016/j.ceb.2020.02.012
Hu Y., He W., Huang Y., Xiang H., Guo J., Che Y., et al. (2021). Fatty acid synthase-suppressor screening identifies sorting nexin 8 as a therapeutic target for NAFLD. Hepatology 74 (5), 2508–2525. doi:10.1002/hep.32045
Hu Z., Raucci S., Jaquenoud M., Hatakeyama R., Stumpe M., Rohr R., et al. (2019). Multilayered control of protein turnover by TORC1 and Atg1. Cell. Rep. 28 (13), 3486–3496. doi:10.1016/j.celrep.2019.08.069
Huang Z., Han J., Wu P., Wu C., Fan Y., Zhao L., et al. (2022). Sorting nexin 5 plays an important role in promoting ferroptosis in Parkinson's disease. Oxid. Med. Cell. Longev. 2022, 5463134. doi:10.1155/2022/5463134
Huotari J., Helenius A. (2011). Endosome maturation. EMBO J. 30 (17), 3481–3500. doi:10.1038/emboj.2011.286
Ji C. H., Kwon Y. T. (2017). Crosstalk and interplay between the ubiquitin-proteasome system and autophagy. Mol. Cells 40 (7), 441–449. doi:10.14348/molcells.2017.0115
Kaneko T., Li L., Li S. S. (2008). The SH3 domain--a family of versatile peptide- and protein-recognition module. Front. Biosci. 13, 4938–4952. doi:10.2741/3053
Kanzawa M., Hara S., Semba S., Yokozaki H., Hirokawa M., Itoh T. (2014). Sorting Nexin 2 (SNX2): a potential marker of active thyrocytes in normal and hyperfunctioning thyroid disorders. Appl. Immunohistochem. Mol. Morphol. 22 (4), 302–307. doi:10.1097/PAI.0b013e31828badd3
Kiani B., Magro C. M., Ross P. (2003). Endobronchial presentation of Hodgkin lymphoma: a review of the literature. Ann. Thorac. Surg. 76 (3), 967–972. doi:10.1016/s0003-4975(03)00140-1
Kibinge N. K., Relton C. L., Gaunt T. R., Richardson T. G. (2020). Characterizing the causal pathway for genetic variants associated with neurological phenotypes using human brain-derived proteome Data. Am. J. Hum. Genet. 106 (6), 885–892. doi:10.1016/j.ajhg.2020.04.007
Klionsky D. J. (2007). Autophagy: from phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell. Biol. 8 (11), 931–937. doi:10.1038/nrm2245
Knaevelsrud H., Søreng K., Raiborg C., Håberg K., Rasmuson F., Brech A., et al. (2013). Membrane remodeling by the PX-BAR protein SNX18 promotes autophagosome formation. J. Cell. Biol. 202 (2), 331–349. doi:10.1083/jcb.201205129
Korolchuk V. I., Menzies F. M., Rubinsztein D. C. (2010). Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett. 584 (7), 1393–1398. doi:10.1016/j.febslet.2009.12.047
Kurten R. C., Cadena D. L., Gill G. N. (1996). Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science 272 (5264), 1008–1010. doi:10.1126/science.272.5264.1008
Kvainickas A., Jimenez-Orgaz A., Nägele H., Hu Z., Dengjel J., Steinberg F. (2017a). Cargo-selective SNX-BAR proteins mediate retromer trimer independent retrograde transport. J. Cell. Biol. 216 (11), 3677–3693. doi:10.1083/jcb.201702137
Kvainickas A., Jimenez-Orgaz A., Nägele H., Hu Z., Dengjel J., Steinberg F. (2017b). Cargo-selective SNX-BAR proteins mediate retromer trimer independent retrograde transport. J. Cell. Biol. 216 (11), 3677–3693. doi:10.1083/jcb.201702137
Lamb C. A., Yoshimori T., Tooze S. A. (2013). The autophagosome: origins unknown, biogenesis complex. Nat. Rev. Mol. Cell. Biol. 14 (12), 759–774. doi:10.1038/nrm3696
Li F., Yang J., Jones J. E., Villar V. A. M., Yu P., Armando I., et al. (2015). Sorting nexin 5 and dopamine d1 receptor regulate the expression of the insulin receptor in human renal proximal tubule cells. Endocrinology 156 (6), 2211–2221. doi:10.1210/en.2014-1638
Liebl D., Qi X., Zhe Y., Barnett T. C., Teasdale R. D. (2017). SopB-mediated recruitment of SNX18 facilitates Salmonella typhimurium internalization by the host cell. Front. Cell. Infect. Microbiol. 7, 257. doi:10.3389/fcimb.2017.00257
Lucas M., Gershlick D. C., Vidaurrazaga A., Rojas A. L., Bonifacino J. S., Hierro A. (2016a). Structural mechanism for cargo recognition by the retromer complex. Cell. 167 (6), 1623–1635. doi:10.1016/j.cell.2016.10.056
Lucas M., Gershlick D. C., Vidaurrazaga A., Rojas A. L., Bonifacino J. S., Hierro A. (2016b). Structural mechanism for cargo recognition by the retromer complex. Cell. 167 (6), 1623–1635. doi:10.1016/j.cell.2016.10.056
Mallet W. G., Maxfield F. R. (1999). Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J. Cell. Biol. 146 (2), 345–359. doi:10.1083/jcb.146.2.345
Mayer B. J. (2015). The discovery of modular binding domains: building blocks of cell signalling. Nat. Rev. Mol. Cell. Biol. 16 (11), 691–698. doi:10.1038/nrm4068
McCullough J., Colf L. A., Sundquist W. I. (2013). Membrane fission reactions of the mammalian ESCRT pathway. Annu. Rev. Biochem. 82, 663–692. doi:10.1146/annurev-biochem-072909-101058
McNally K. E., Cullen P. J. (2018). Endosomal retrieval of cargo: retromer is not alone. Trends Cell. Biol. 28 (10), 807–822. doi:10.1016/j.tcb.2018.06.005
Mirrashidi K. M., Elwell C. A., Verschueren E., Johnson J. R., Frando A., Von Dollen J., et al. (2015). Global mapping of the inc-human interactome reveals that retromer restricts Chlamydia infection. Cell. Host Microbe 18 (1), 109–121. doi:10.1016/j.chom.2015.06.004
Muirhead G., Dev K. K. (2014). The expression of neuronal sorting nexin 8 (SNX8) exacerbates abnormal cholesterol levels. J. Mol. Neurosci. 53 (1), 125–134. doi:10.1007/s12031-013-0209-z
Muller U. C., Deller T., Korte M. (2017). Not just amyloid: physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 18 (5), 281–298. doi:10.1038/nrn.2017.29
Naslavsky N., Caplan S. (2018). The enigmatic endosome - sorting the ins and outs of endocytic trafficking. J. Cell. Sci. 131 (13), jcs216499. doi:10.1242/jcs.216499
Niu Y., Dai Z., Liu W., Zhang C., Yang Y., Guo Z., et al. (2017). Ablation of SNX6 leads to defects in synaptic function of CA1 pyramidal neurons and spatial memory. Elife 6, e20991. doi:10.7554/eLife.20991
Okada H., Zhang W., Peterhoff C., Hwang J. C., Nixon R. A., Ryu S. H., et al. (2010). Proteomic identification of sorting nexin 6 as a negative regulator of BACE1-mediated APP processing. FASEB J. 24 (8), 2783–2794. doi:10.1096/fj.09-146357
Okano M., Matsuo H., Nishimura Y., Hozumi K., Yoshioka S., Tonoki A., et al. (2016). Mib1 modulates dynamin 2 recruitment via Snx18 to promote Dll1 endocytosis for efficient Notch signaling. Genes. cells. 21 (5), 425–441. doi:10.1111/gtc.12350
Ou Y. N., Yang Y. X., Deng Y. T., Zhang C., Hu H., Wu B. S., et al. (2021). Identification of novel drug targets for Alzheimer's disease by integrating genetics and proteomes from brain and blood. Mol. Psychiatry 26 (10), 6065–6073. doi:10.1038/s41380-021-01251-6
Park J., Kim Y., Lee S., Park J. J., Park Z. Y., Sun W., et al. (2010). SNX18 shares a redundant role with SNX9 and modulates endocytic trafficking at the plasma membrane. J. Cell. Sci. 123 (Pt 10), 1742–1750. doi:10.1242/jcs.064170
Pelham H. R. (2002a). Insights from yeast endosomes. Curr. Opin. Cell. Biol. 14 (4), 454–462. doi:10.1016/s0955-0674(02)00352-6
Pelham H. R. (2002b). Insights from yeast endosomes. Curr. Opin. Cell. Biol. 14 (4), 454–462. doi:10.1016/s0955-0674(02)00352-6
Peter B. J., Kent H. M., Mills I. G., Vallis Y., Butler P. J. G., Evans P. R., et al. (2004). BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303 (5657), 495–499. doi:10.1126/science.1092586
Pohl C., Dikic I. (2019). Cellular quality control by the ubiquitin-proteasome system and autophagy. Science 366 (6467), 818–822. doi:10.1126/science.aax3769
Ponting C. P. (1996). Novel domains in NADPH oxidase subunits, sorting nexins, and PtdIns 3-kinases: binding partners of SH3 domains? Protein Sci. 5 (11), 2353–2357. doi:10.1002/pro.5560051122
Popelka H., Damasio A., Hinshaw J. E., Klionsky D. J., Ragusa M. J. (2017). Structure and function of yeast Atg20, a sorting nexin that facilitates autophagy induction. Proc. Natl. Acad. Sci. U. S. A. 114 (47), E10112-E10121–E10121. doi:10.1073/pnas.1708367114
Pylypenko O., Lundmark R., Rasmuson E., Carlsson S. R., Rak A. (2007). The PX-BAR membrane-remodeling unit of sorting nexin 9. EMBO J. 26 (22), 4788–4800. doi:10.1038/sj.emboj.7601889
Repnik U., Cesen M. H., Turk B. (2013). The endolysosomal system in cell death and survival. Cold Spring Harb. Perspect. Biol. 5 (1), a008755. doi:10.1101/cshperspect.a008755
Rodgers S. J., Jones E. I., Arumugam S., Hamila S. A., Danne J., Gurung R., et al. (2022). Endosome maturation links PI3Kα signaling to lysosome repopulation during basal autophagy. EMBO J. 41 (19), e110398. doi:10.15252/embj.2021110398
Schobel S., Neumann S., Hertweck M., Dislich B., Kuhn P. H., Kremmer E., et al. (2008). A novel sorting nexin modulates endocytic trafficking and alpha-secretase cleavage of the amyloid precursor protein. J. Biol. Chem. 283 (21), 14257–14268. doi:10.1074/jbc.M801531200
Schoneberg J., Lee I. H., Iwasa J. H., Hurley J. H. (2017a). Reverse-topology membrane scission by the ESCRT proteins. Nat. Rev. Mol. Cell. Biol. 18 (1), 5–17. doi:10.1038/nrm.2016.121
Schoneberg J., Lee I. H., Iwasa J. H., Hurley J. H. (2017b). Reverse-topology membrane scission by the ESCRT proteins. Nat. Rev. Mol. Cell. Biol. 18 (1), 5–17. doi:10.1038/nrm.2016.121
Seaman M. (2021). The retromer complex: from genesis to revelations. Trends Biochem. Sci. 46 (7), 608–620. doi:10.1016/j.tibs.2020.12.009
Seet L. F., Hong W. (2006). The Phox (PX) domain proteins and membrane traffic. Biochim. Biophys. Acta 1761 (8), 878–896. doi:10.1016/j.bbalip.2006.04.011
Sierecki E., Stevers L. M., Giles N., Polinkovsky M. E., Moustaqil M., Mureev S., et al. (2014). Rapid mapping of interactions between Human SNX-BAR proteins measured in vitro by AlphaScreen and single-molecule spectroscopy. Mol. Cell. Proteomics 13 (9), 2233–2245. doi:10.1074/mcp.M113.037275
Simonetti B., Daly J. L., Cullen P. J. (2023). Out of the ESCPE room: emerging roles of endosomal SNX-BARs in receptor transport and host-pathogen interaction. Traffic 24 (6), 234–250. doi:10.1111/tra.12885
Simonetti B., Danson C. M., Heesom K. J., Cullen P. J. (2017a). Sequence-dependent cargo recognition by SNX-BARs mediates retromer-independent transport of CI-MPR. J. Cell. Biol. 216 (11), 3695–3712. doi:10.1083/jcb.201703015
Simonetti B., Danson C. M., Heesom K. J., Cullen P. J. (2017b). Sequence-dependent cargo recognition by SNX-BARs mediates retromer-independent transport of CI-MPR. J. Cell. Biol. 216 (11), 3695–3712. doi:10.1083/jcb.201703015
Singh V., Yang J., Cha B., Chen T. e., Sarker R., Yin J., et al. (2015). Sorting nexin 27 regulates basal and stimulated brush border trafficking of NHE3. Mol. Biol. Cell. 26 (11), 2030–2043. doi:10.1091/mbc.E14-12-1597
Soreng K., Munson M. J., Lamb C. A., Bjørndal G. T., Pankiv S., Carlsson S. R., et al. (2018). SNX18 regulates ATG9A trafficking from recycling endosomes by recruiting Dynamin-2. EMBO Rep. 19 (4), e44837. doi:10.15252/embr.201744837
Sowa M. E., Bennett E. J., Gygi S. P., Harper J. W. (2009). Defining the human deubiquitinating enzyme interaction landscape. Cell. 138 (2), 389–403. doi:10.1016/j.cell.2009.04.042
Stangl A., Elliott P. R., Pinto-Fernandez A., Bonham S., Harrison L., Schaub A., et al. (2019). Regulation of the endosomal SNX27-retromer by OTULIN. Nat. Commun. 10 (1), 4320. doi:10.1038/s41467-019-12309-z
Steinberg F., Gallon M., Winfield M., Thomas E. C., Bell A. J., Heesom K. J., et al. (2013). A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat. Cell. Biol. 15 (5), 461–471. doi:10.1038/ncb2721
Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. (1983). Endocytosis and the recycling of plasma membrane. J. Cell. Biol. 96 (1), 1–27. doi:10.1083/jcb.96.1.1
Suarez A., Ueno T., Huebner R., McCaffery J. M., Inoue T. (2014). Bin/Amphiphysin/Rvs (BAR) family members bend membranes in cells. Sci. Rep. 4, 4693. doi:10.1038/srep04693
Sugatha J., Priya A., Raj P., Jaimon E., Swaminathan U., Jose A., et al. (2023). Insights into cargo sorting by SNX32 and its role in neurite outgrowth. Elife 12, e84396. doi:10.7554/eLife.84396
Sun L., Chen Z. J. (2004). The novel functions of ubiquitination in signaling. Curr. Opin. Cell. Biol. 16 (2), 119–126. doi:10.1016/j.ceb.2004.02.005
Takada-Takatori Y. (2021). Donepezil reduces amyloid precursor protein endocytosis by resulting from increase in the expression of sorting nexin protein 33. Yakugaku Zasshi 141 (6), 851–856. doi:10.1248/yakushi.20-00251-6
Takada-Takatori Y., Nakagawa S., Kimata R., Nao Y., Mizukawa Y., Urushidani T., et al. (2019). Donepezil modulates amyloid precursor protein endocytosis and reduction by up-regulation of SNX33 expression in primary cortical neurons. Sci. Rep. 9 (1), 11922. doi:10.1038/s41598-019-47462-4
Takei K., Slepnev V. I., Haucke V., De Camilli P. (1999). Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell. Biol. 1 (1), 33–39. doi:10.1038/9004
Tatarova Z., Brábek J., Rösel D., Novotný M. (2012). SH3 domain tyrosine phosphorylation--sites, role and evolution. PLoS One 7 (5), e36310. doi:10.1371/journal.pone.0036310
Teasdale R. D., Collins B. M. (2012). Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: structures, functions and roles in disease. Biochem. J. 441 (1), 39–59. doi:10.1042/BJ20111226
Trefny M. P., Kirchhammer N., Auf der Maur P., Natoli M., Schmid D., Germann M., et al. (2023). Deletion of SNX9 alleviates CD8 T cell exhaustion for effective cellular cancer immunotherapy. Nat. Commun. 14 (1), 86. doi:10.1038/s41467-022-35583-w
Valera M. M., Scarfone A., Calvani M., Greco A. V., Mingrone G. (2003). Insulin clearance in obesity. J. Am. Coll. Nutr. 22 (6), 487–493. doi:10.1080/07315724.2003.10719326
van Weering J. R., Sessions R. B., Traer C. J., Kloer D. P., Bhatia V. K., Stamou D., et al. (2012a). Molecular basis for SNX-BAR-mediated assembly of distinct endosomal sorting tubules. EMBO J. 31 (23), 4466–4480. doi:10.1038/emboj.2012.283
van Weering J. R., Sessions R. B., Traer C. J., Kloer D. P., Bhatia V. K., Stamou D., et al. (2012c). Molecular basis for SNX-BAR-mediated assembly of distinct endosomal sorting tubules. EMBO J. 31 (23), 4466–4480. doi:10.1038/emboj.2012.283
van Weering J. R., Sessions R. B., Traer C. J., Kloer D. P., Bhatia V. K., Stamou D., et al. (2012d). Molecular basis for SNX-BAR-mediated assembly of distinct endosomal sorting tubules. EMBO J. 31 (23), 4466–4480. doi:10.1038/emboj.2012.283
van Weering J. R., Verkade P., Cullen P. J. (2012b). SNX-BAR-mediated endosome tubulation is co-ordinated with endosome maturation. Traffic 13 (1), 94–107. doi:10.1111/j.1600-0854.2011.01297.x
Villar V. A., Armando I., Sanada H., Frazer L. C., Russo C. M., Notario P. M., et al. (2013a). Novel role of sorting nexin 5 in renal D(1) dopamine receptor trafficking and function: implications for hypertension. FASEB J. 27 (5), 1808–1819. doi:10.1096/fj.12-208439
Villar V. A., Jones J. E., Armando I., Asico L. D., Escano C. S., Lee H., et al. (2013b). Sorting nexin 1 loss results in D5 dopamine receptor dysfunction in human renal proximal tubule cells and hypertension in mice. J. Biol. Chem. 288 (1), 152–163. doi:10.1074/jbc.M112.428458
Waite K. A., De-La Mota-Peynado A., Vontz G., Roelofs J. (2016). Starvation induces proteasome autophagy with different pathways for core and regulatory particles. J. Biol. Chem. 291 (7), 3239–3253. doi:10.1074/jbc.M115.699124
Wang J., Fedoseienko A., Chen B., Burstein E., Jia D., Billadeau D. D. (2018a). Endosomal receptor trafficking: retromer and beyond. Traffic 19 (8), 578–590. doi:10.1111/tra.12574
Wang J., Fedoseienko A., Chen B., Burstein E., Jia D., Billadeau D. D. (2018b). Endosomal receptor trafficking: retromer and beyond. Traffic 19 (8), 578–590. doi:10.1111/tra.12574
Wang Y., Le W. D. (2019). Autophagy and ubiquitin-proteasome system. Adv. Exp. Med. Biol. 1206, 527–550. doi:10.1007/978-981-15-0602-4_25
Wassmer T., Attar N., Bujny M. V., Oakley J., Traer C. J., Cullen P. J. (2007). A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J. Cell. Sci. 120 (Pt 1), 45–54. doi:10.1242/jcs.03302
Xu H., Chang F., Jain S., Heller B. A., Han X., Liu Y., et al. (2022). SNX5 targets a monoamine transporter to the TGN for assembly into dense core vesicles by AP-3. J. Cell. Biol. 221 (5), e202106083. doi:10.1083/jcb.202106083
Xu S., Zhang L., Brodin L. (2018). Overexpression of SNX7 reduces Aβ production by enhancing lysosomal degradation of APP. Biochem. Biophys. Res. Commun. 495 (1), 12–19. doi:10.1016/j.bbrc.2017.10.127
Yang J., Villar V. A. M., Rozyyev S., Jose P. A., Zeng C. (2019). The emerging role of sorting nexins in cardiovascular diseases. Clin. Sci. (Lond) 133 (5), 723–737. doi:10.1042/CS20190034
Yarmohammadi F., Hayes A. W., Karimi G. (2022). Sorting nexins as a promising therapeutic target for cardiovascular disorders: an updated overview. Exp. Cell. Res. 419 (1), 113304. doi:10.1016/j.yexcr.2022.113304
Yong X., Zhao L., Deng W., Sun H., Zhou X., Mao L., et al. (2020a). Mechanism of cargo recognition by retromer-linked SNX-BAR proteins. PLoS Biol. 18 (3), e3000631. doi:10.1371/journal.pbio.3000631
Yong X., Zhao L., Deng W., Sun H., Zhou X., Mao L., et al. (2020b). Mechanism of cargo recognition by retromer-linked SNX-BAR proteins. PLoS Biol. 18 (3), e3000631. doi:10.1371/journal.pbio.3000631
Zhan X. Y., Zhang Y., Zhai E., Zhu Q. Y., He Y. (2018). Sorting nexin-1 is a candidate tumor suppressor and potential prognostic marker in gastric cancer. PeerJ 6, e4829. doi:10.7717/peerj.4829
Zhang H., Huang T., Hong Y., Yang W., Zhang X., Luo H., et al. (2018). The retromer complex and sorting nexins in neurodegenerative diseases. Front. Aging Neurosci. 10, 79. doi:10.3389/fnagi.2018.00079
Zhang P., Kishimoto Y., Grammatikakis I., Gottimukkala K., Cutler R. G., Zhang S., et al. (2019). Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nat. Neurosci. 22 (5), 719–728. doi:10.1038/s41593-019-0372-9
Keywords: SNX-BAR subfamily, signal transmission, cargo sorting, endosome, autophagy, ubiquitin
Citation: Long Y, Li Y, Xue J, Geng W, Ma M, Wang X and Wang L (2025) Mechanisms by which SNX-BAR subfamily controls the fate of SNXs’ cargo. Front. Physiol. 16:1559313. doi: 10.3389/fphys.2025.1559313
Received: 12 January 2025; Accepted: 20 February 2025;
Published: 12 March 2025.
Edited by:
Amilcare Barca, University of Salento, ItalyReviewed by:
Volodymyr Tsvilovskyy, Heidelberg University, GermanyValeria Rivarola, National Council for Scientific and Technical Research (CONICET), Argentina
Copyright © 2025 Long, Li, Xue, Geng, Ma, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Wang, bWlycm9yMDExN0AxMjYuY29t
 Yaolin Long
Yaolin Long Yang Li1
Yang Li1 Xiaohui Wang
Xiaohui Wang Li Wang
Li Wang