
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Physiol. , 09 April 2025
Sec. Exercise Physiology
Volume 16 - 2025 | https://doi.org/10.3389/fphys.2025.1558214
Background: Accumulating evidence suggested the potential role of exercise in alleviating rheumatoid arthritis (RA). However, whether exercise improves physical function (walk test, grip strength, muscle strength, joint assessments) and inflammatory biomarkers in patients with RA is unclear. This umbrella meta-analysis aimed to examine the effect of exercise in patients with RA.
Method: PubMed, Scopus, Web of Science, Embase, and Cochrane Central Library databases were systematically searched for meta-analyses of randomized control trials (RCTs) to retrieve relevant studies. The effect sizes were pooled using a random-effects model, with standardized or weighted mean differences (SMDs or WMDs) and 95% confidence intervals (CIs) as summary statistics.
Results: Seventeen studies were included. The improving effects of exercise on fatigue levels (SMD = −0.28, 95% CI: −0.44, −0.13), pain intensity (ES = −0.50, 95% CI: −0.87, −0.14), disease activity score in joints (DAS) (WMD = −0.54, 95% CI: −0.99, −0.09; and SMD = −0.47, 95% CI: −0.64, −0.30), and ESR (ES = −0.85, 95% CI: −1.66, −0.03) were significant. No significant impact on the hand grip, muscle strength, walk test, joints and inflammatory biomarkers was observed.
Conclusion: Exercise significantly reduces fatigue, pain, DAS, and ESR in RA but shows no impact on grip strength, muscle strength, walk test, joints, or other inflammatory biomarkers. This highlights its role in symptom management rather than broad physiological changes.
Rheumatoid arthritis (RA), and knee osteoarthritis (OA) are chronic autoimmune disorders affecting approximately 18 million individuals worldwide (Vos, 2020). It is characterized by persistent synovial inflammation, leading to joint pain, swelling, and stiffness, which can result in significant functional impairment and reduced quality of life (Jahid et al., 2023). A hallmark of RA is the loss of immune tolerance, where the immune system erroneously targets self-antigens in the synovium. This autoimmune response leads to the activation of CD4+ T cells, B cells, and macrophages, resulting in the production of pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 (IL-1) (Guo et al., 2018; Yasmeen et al., 2024). These cytokines perpetuate inflammation and promote the proliferation of synovial fibroblasts, contributing to pannus formation and subsequent joint destruction (Davis, 2003).
Effective RA management aims to control inflammation, prevent joint damage, and preserve functional capacity. While pharmacological agents, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and biological agents, including TNF inhibitors and IL-6 receptor antagonists, are widely used to control inflammation and slow disease progression, non-pharmacological strategies play a crucial role in complementing medical therapy (Singh et al., 2015). Among these, structured exercise programs are specifically recommended to improve disease outcomes by targeting muscle strength, joint mobility, and overall physical function in RA (Health Quality Ontario, 2018). Clinical guidelines recommend exercise therapy as a primary treatment for knee QA (Holden et al., 2023). In RA, exercise interventions designed to enhance muscle support around affected joints can mitigate pain and stiffness while potentially modulating inflammatory processes (Li and Wang, 2022). A structured exercise program for people with RA typically focuses on a combination of low-impact aerobics and stretching exercises to maintain joint range of motion, and muscle strengthening exercises, all designed to alleviate pain, improve mobility, and enhance overall function (Health Quality Ontario, 2018; Ghalamsiah and Nourshahi, 2023).
Emerging evidence suggests that exercise exerts its therapeutic effects through both systemic and localized molecular pathways. Regular physical activity modulates pro-inflammatory cytokines such as IL-6 and TNF-α, while increasing anti-inflammatory cytokines like interleukin-10 (IL-10) (El-Kader et al., 2013). Additionally, mechanical loading during resistance and aerobic exercise stimulates cartilage-resident chondrocytes to produce extracellular matrix components, enhancing cartilage resilience and joint health (Sun, 2010; Smith, 2020). Neural adaptations, including increased endorphin release, further contribute to pain relief, while improvements in mitochondrial function and cardiovascular efficiency alleviate fatigue (Schwarz and Kindermann, 1992). These mechanisms highlight the multifaceted benefits of exercise in RA, and knee OA management.
Despite the promising role of exercise (Smith, 2020; Mudano et al., 2019), inconsistencies in study findings (Wu et al., 2023; Wen and Chai, 2021) and substantial heterogeneity in methodologies present challenges in drawing definitive conclusions. Variations in exercise type, frequency, duration, and intensity, coupled with differences in participant characteristics and outcome measures, have led to mixed results (Smith, 2020; Mudano et al., 2019; Wu et al., 2023; Wen and Chai, 2021). This article aims to address these gaps by conducting an umbrella meta-analysis (a comprehensive synthesis that integrates findings from previous meta-analyses) to synthesize evidence from multiple meta-analyses on the effects of different exercise modalities on pain, fatigue, and hand grip strength in individuals with RA. By systematically evaluating subgroup effects, including variations in exercise type, duration, and participant demographics, this study seeks to provide a nuanced understanding of the role of exercise in RA, and knee OA management.
In this meta-analysis, we followed PRISMA guidelines for reporting systematic reviews and meta-analyses (PRISMA) (Takkouche and Norman, 2011). It has been registered with PROSPERO under the registration number CRD42024229245.
To identify relevant studies, a comprehensive search was conducted in the following databases: PubMed, Scopus, Web of Science, Embase, and Cochrane Central Library, up to November 2024. The search strategies for all databases can be found in Supplementary Material 1. To increase sensitivity, wildcard terms (e.g., “*”) were used. The search was restricted to English-language publications. Reference lists of all relevant studies were manually checked to ensure the inclusion of all eligible studies. The Kappa value obtained in this study was approximately 0.8, indicating a better inter-rater consistency compared to the Kappa values in other studies. This suggested that our evaluation criteria and methods were effective and ensured the reliability of the research results.
The following PICOS criteria were applied for study selection: Population (P): Adults aged ≥18 years with RA, and knee OA: Intervention (I): exercises; Comparison (C): Placebo or control group; Outcomes (O): Walk test, pain, muscle strength, joints (joint count, joint tenderness, swollen joints, tender joint count), grip strength of both hands, fatigue, disease activity score in joints (DAS), c-reactive protein (CRP), erythrocyte sedimentation rate (ESR), interleukin 6 (IL-6), and tumour necrosis factor-alpha (TNF-α) (S): Systematic review and meta-analysis studies, providing effect sizes and corresponding confidence intervals (CI) for each outcome. Studies in vitro or in vivo, controlled clinical trials, observational studies, case reports, and quasi-experimental studies lacking adequate data for effect size calculations were excluded.
A checklist based on the Measurement Tool to Assess Systematic Reviews (AMSTAR) 2 was used by two independent reviewers to assess the methodological quality of the included studies (Shea, 2017). Discrepancies were resolved through discussion or consultation with a senior author. Studies scoring ≥7 were included in the analysis.
Two independent reviewers screened the titles and abstracts and then conducted a full-text evaluation of the studies that met the eligibility criteria. Extracted data included: study characteristics (author, publication year, location), participant details (sample size and age), intervention specifics (physical exercise type, and duration), and outcomes (effect sizes (ESs) with 95% confidence intervals [CIs] for walk test, pain, muscle strength, joints, grip strength, fatigue, DAS, ESR, IL-6, TNF-α, and CRP. Disagreements were resolved through discussion with a third reviewer.
Effect sizes [weighted mean difference (WMD) or standardized mean difference (SMD)] and corresponding CIs were pooled using random-effects models when heterogeneity was significant (I2 > 50%, p < 0.1). Heterogeneity was assessed using the Cochran’s Q test and I2 statistic. Subgroup analyses were performed to explore heterogeneity based on variables such as mean age, frequency, intervention duration, and type of ESs. Sensitivity analysis evaluates the reliability of the findings by removing individual studies from the analysis. Publication bias was evaluated using funnel plots (for markers with >10 included studies) and statistical tests [Begg’s (Begg and Mazumdar, 1994) and Egger’s tests (Egger et al., 1997)]. If bias was detected, a trim-and-fill method was applied. All statistical analyses were conducted using STATA software version 16.0 (Stata Corp, College Station, TX, United States), with a significance threshold of p < 0.05.
Of the initial 3,510 records retrieved from the electronic databases, 679 studies were identified as duplicates and excluded, while 2,831 publications were ruled out after reviewing titles and abstracts. The remaining 36 studies underwent full-text screening, during which 19 were excluded based on criteria specified in the PRISMA flowchart (Figure 1). Ultimately, 17 meta-analyses met the eligibility criteria and were included in the umbrella meta-analysis. The basic characteristics of the included articles are shown in Table 1. The publication years of these studies ranged from 2004 to 2023. The exercise duration varies from 15 to 50 min, and the frequency of exercise sessions ranges from one to four. The number of participants ranged from 206 to 2,405, with ages varying between 47 and 58 years. Most studies have used aerobic exercise, and some have combined aerobic with resistance exercise.
Regarding the quality of studies based on the AMSTAR-2 criteria, six studies were rated as high quality, and 11 as moderate. The quality of assessment is shown in Table 2.
Our findings revealed that exercise did not significantly improve hand grip (ES = 0.13, 95% CI: −0.19, 0.44; P = 0.431; I2 = 82.5%, p < 0.001) (Figure 2A) and muscle strength (SMD = 0.06, 95% CI: −0.58, 0.70; P = 0.858; I2 = 46.2%, p = 0.156) (Figure 2B). The non-significant outcomes from Begg’s tests confirm the reliability of the results from the meta-analyses (P > 0.05).
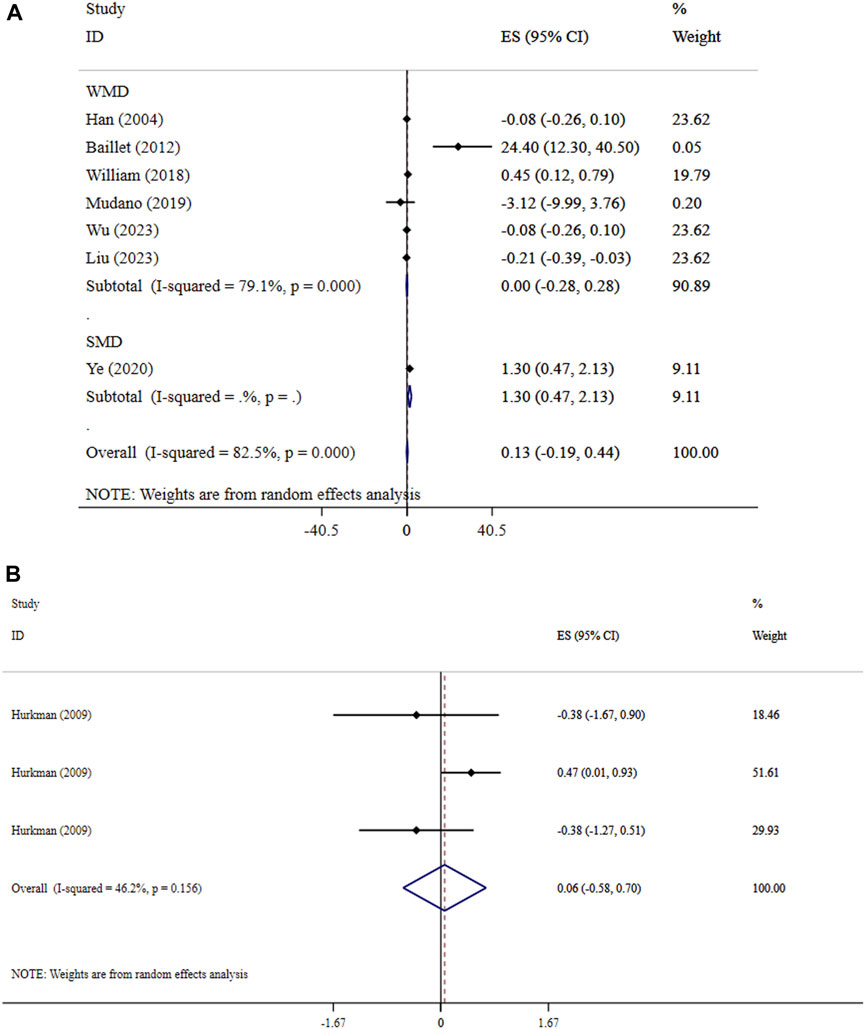
Figure 2. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of exercise on hand grip and (A), and muscle strength (B).
Exercise did not lead to significant effects on joint count (ES = −0.61, 95% CI: −1.73, 0.51; P = 0.286; I2 = 84.4%, p = 0.002), tender joints (ES = −0.06, 95% CI: −0.35, 0.22; P = 0.671; I2 = 0.0%, P = 0.872), swollen joints (ES = 0.92, 95% CI: −0.83, 2.67; P = 0.301; I2 = 47.8%, p = 0.125), and walk test (ES = −0.48, 95% CI: −1.19, 0.23; p = 0.189; I2 = 61.9%, p = 0.033) in patients with RA (Figures 3A, B). The sensitivity analysis results supported the outcome’s validity (p < 0.05). The results of Begg’s tests did not detect publication bias (P > 0.05).
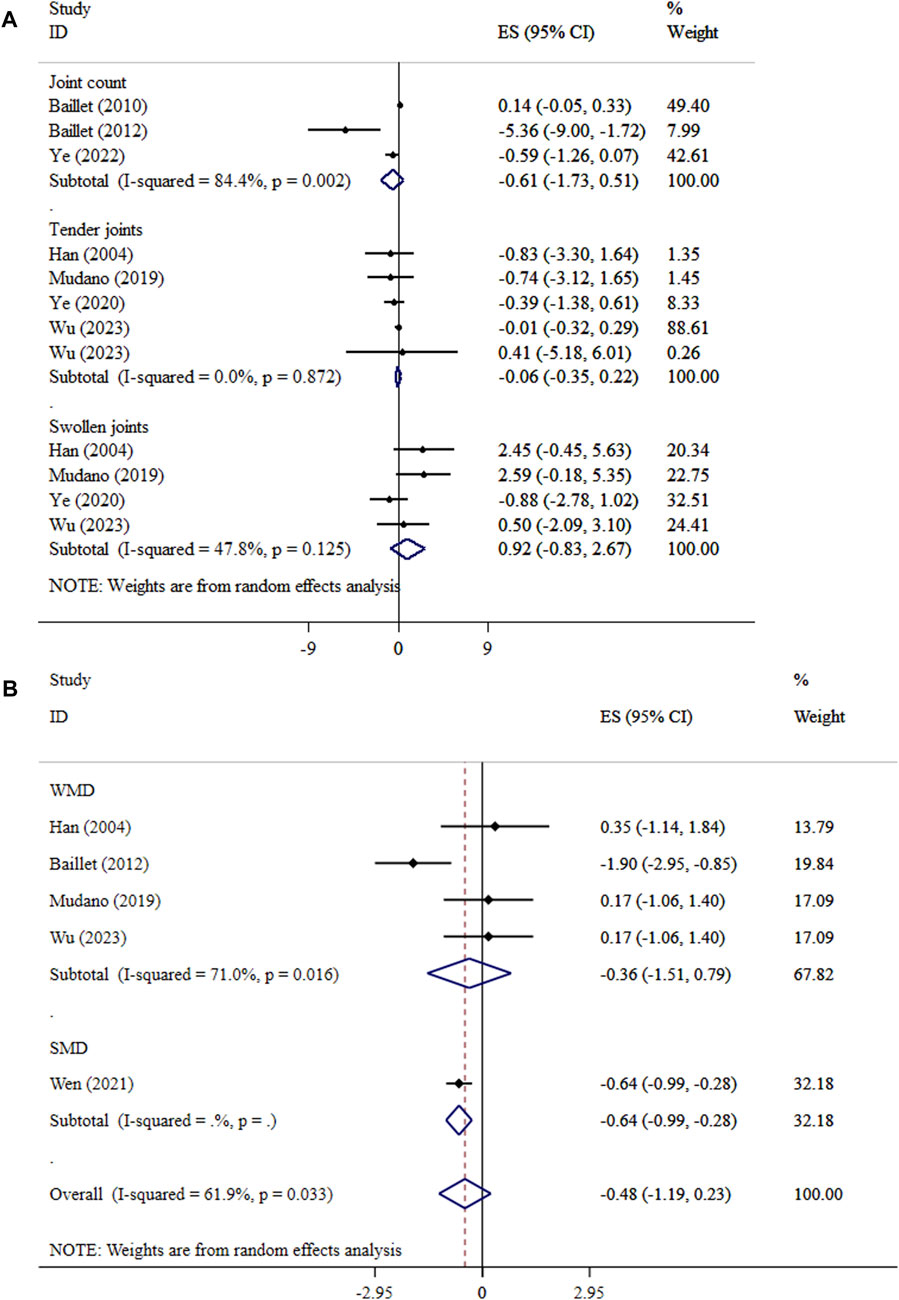
Figure 3. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of exercise on joints and (A), and walk test (B).
The improving effect of exercise on fatigue levels was significant (SMD = −0.28, 95% CI: −0.44, −0.13; p < 0.001; I2 = 55.1%, p = 0.108) (Figure 4A). The results of the SMD analysis and the sensitivity testing were consistent (P < 0.05). Subgroup analyses showed that the largest decreases in fatigue were observed in aerobic with resistance exercise (Table 3). Begg’s tests showed no statistical evidence of publication bias (P > 0.05).
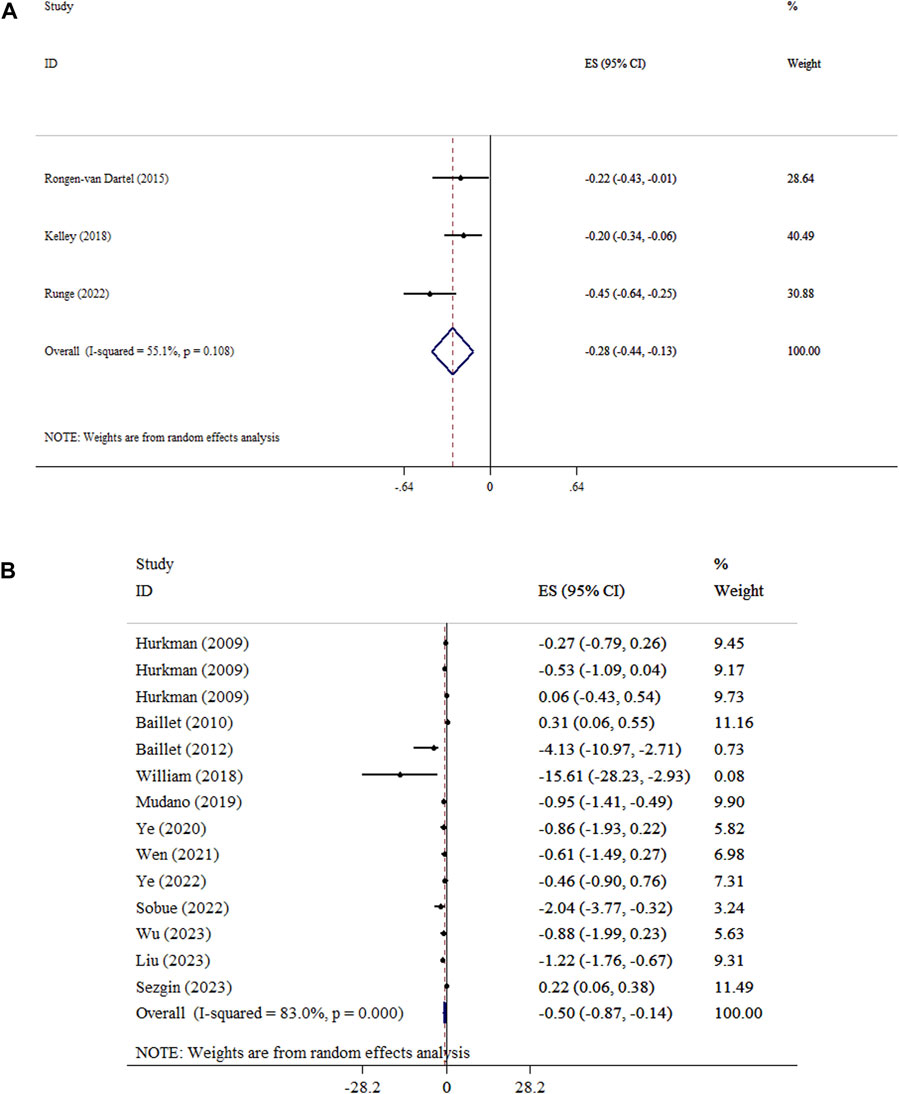
Figure 4. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of exercise on fatigue and (A), and pain (B).
Our results showed that exercise reduced pain intensity in patients with RA (ES = −0.50, 95% CI: −0.87, −0.14; P=0.007), with high heterogeneity (I2 = 83.0%, P<0.001) (Figure 4B), and in patients with knee OA (SMD = −0.49, 95% CI: −0.61, −0.37; P<0.001). The conclusions remained stable, as verified by the sensitivity analysis. Subgroup analysis showed that exercise led to the greatest reduction in pain intensity in duration <30 min, and ES type (WMD analysis) (Table 3). Findings from Egger’s and Begg’s tests and visual inspection of the funnel plot confirm the absence of significant publication bias (P > 0.05) (Supplementary Figure 1).
Exercise significantly decreased DAS levels in both WMD and SMD analyses. The WMD analysis demonstrated an overall effect size of −0.54 (95% CI: −0.99, −0.09; P = 0.018) with low heterogeneity (I2 = 0.0%, P = 0.949). Similarly, the SMD analysis showed a significant decrease (SMD = −0.47, 95% CI: −0.64, −0.30; P < 0.001; I2 = 0.0%, P = 0.508) (Figure 5A). Sensitivity analysis approved the robustness of findings in both WMD and SMD metrics. Publication bias was not detected using Begg’s test. (P > 0.05).
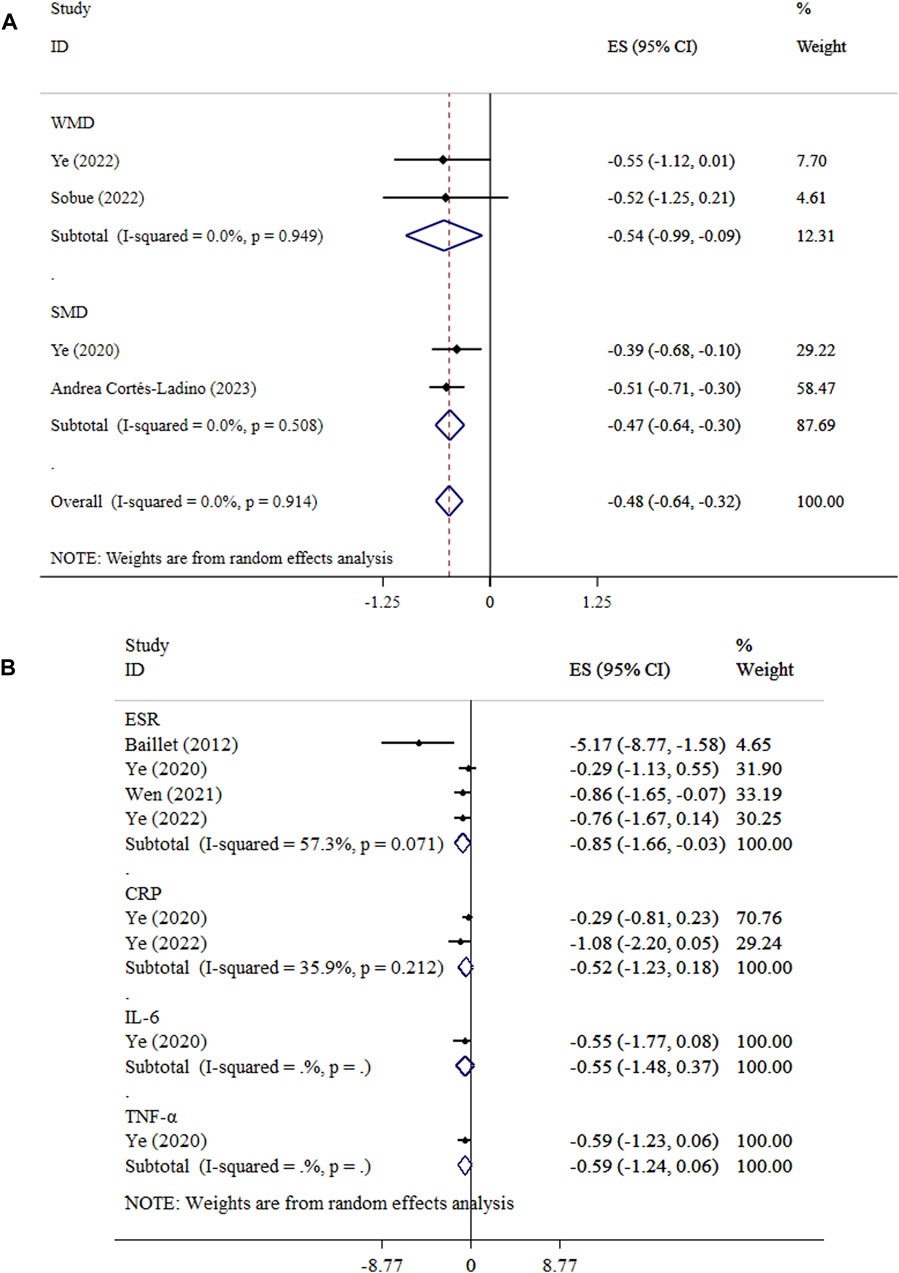
Figure 5. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of exercise on DAS and (A), and inflammatory biomarkers (B).
The effect of exercise on CRP (ES = −0.52, 95% CI: −1.23, 0.18; P = 0.147; I2 = 35.9%, P = 0.212), IL-6 (ES = −0.55, 95% CI: −1.48, 0.37; P = 0.244), and TNF-α (ES = −0.59, 95% CI: −1.24, 0.06; P = 0.073) levels did not reach statistical significance (Figure 5B). Begg’s tests indicated no evidence of publication bias (P > 0.05). Exercise led to a significant reduction in ESR (ES = −0.85, 95% CI: −1.66, −0.03; P = 0.042; I2 = 5.3%, P = 0.071).
This study evaluated the impact of exercise interventions on various health outcomes in patients with RA. The analysis revealed that exercise did not significantly improve hand grip strength or overall muscle strength, as well as joint count metrics, including total joint count, tender joints, and swollen joints. Performance in walk tests also did not show significant enhancement. Conversely, exercise in both aerobic and resistance type interventions led to a significant reduction in fatigue levels. WMD analysis showed that pain intensity decreased significantly following exercise, particularly in interventions lasting less than 30 min per session in patients with RA, and knee OA. However, results on pain must be interpreted with precaution due to high heterogeneity, as well as the non-significant effects in other subgroups. Regarding disease activity, exercise significantly lowered DAS levels. Inflammatory biomarkers such as CRP, IL-6, and TNF-α did not show significant changes post-exercise. However, ESR demonstrated a significant reduction. The significant results on pain, fatigue, and DAS were not clinically important (Ward et al., 2015). Defining an exact clinically important difference for ESR is challenging due to its variability and sensitivity to numerous factors beyond RA activity (Ward, 2004). Consequently, ESR changes are often interpreted in conjunction with other clinical assessments. Therefore, exercise can only be considered as an adjutant therapy approach or preventive strategy in managing RA patients (Tahira, 2023; Faraziani and Eken, 2024).
The findings suggest that while exercise interventions may not significantly enhance hand grip strength, overall muscle strength, joint counts, or walk test performance in patients with RA, they are effective in reducing fatigue, pain intensity, and disease activity. The lack of improvement in muscle strength and joint metrics could be attributed to the heterogeneity of exercise protocols, variations in disease severity among participants, and the relatively short duration of some interventions.
The observed small but significant reduction in fatigue with aerobic exercise may be attributed to its positive effects on cardiovascular fitness (Patel et al., 2017), muscle endurance (Markov et al., 2022), and overall systemic inflammation (Al-Jiffri and Abd El-Kader, 2021), all of which are key contributors to fatigue in individuals with RA. Aerobic exercise enhances oxygen delivery to tissues (Joyner and Casey, 2015), reduces lactate buildup (Spurway, 1992), and improves mitochondrial function (Porter et al., 2015), which collectively increase energy efficiency and reduce perceived fatigue. Additionally, regular aerobic activity may modulate inflammatory cytokines, potentially alleviating the fatigue associated with systemic inflammation in RA (Al-Jiffri and Abd El-Kader, 2021). Aerobic exercise training is more effective than resistance exercise in modulation of inflammatory cytokines (Al-Jiffri and Abd El-Kader, 2021). Engaging in resistance training can enhance proteins that are involved in muscle remodeling and angiogenesis, leading to relieving fatigue (Englund et al., 2022). However, resistance training, while beneficial for building muscle strength and functional capacity, may not directly target the physiological mechanisms underlying fatigue to the same extent as aerobic exercise (Schroeder et al., 2019). Some metabolites including thromboxane, prostaglandins, bradykinin and purinergic type 2X receptors, and ion channels in response to resistance exercise may be involved in fatigue induced by resistance training (Zając et al., 2015). However, combining aerobic and resistance exercises could not dilute the specific benefits of aerobic on fatigue.
Duration of exercise sessions emerged as an important modifier of pain outcomes. Interventions with sessions lasting less than 30 min were associated with a stronger reduction in pain, possibly due to better adherence or reduced risk of exacerbating joint pain and fatigue. However, the high heterogeneity of pooled analysis indicates variability in responses, underscoring the need for personalized exercise programs tailored to individual characteristics. Exercise stimulates the production of endorphins, as the natural painkillers and mood elevators (Lima et al., 2017). Moreover, engaging in exercise can lead to a temporary reduction in pain sensitivity, known as exercise-induced hypoalgesia (EIH) (Rice et al., 2019). In addition, exercise influences pain processing pathways, potentially altering nociceptive, neuropathic, and psychosocial pain mechanisms (Chimenti et al., 2018). However, the analgesic effects of exercise can vary, especially in individuals with chronic pain, where central analgesic systems may respond differently (Nijs et al., 2012).
The discrepancy in the significance of exercise effects on pain between WMD and SMD analyses can be attributed to the different ways these effect size metrics handle variability in the outcome measures. WMD directly uses the raw mean differences in pain scores between intervention and control groups, maintaining the original scale of measurement (Andrade, 2020). This makes WMD particularly sensitive to absolute changes in pain levels, especially in studies using the same or similar pain scales. In this case, WMD might have captured a consistent reduction in pain across studies that used comparable measurement tools, leading to a significant result. On the other hand, SMD standardizes the effect size by dividing the mean difference by the pooled standard deviation, allowing comparison across studies that use different pain scales. This standardization reduces the impact of absolute changes and emphasizes the relative magnitude of the effect in the context of variability within and between studies. If there is substantial heterogeneity in study designs, population characteristics, or baseline pain levels, SMD can dilute the observed effect, leading to a non-significant result (Andrade, 2020).
The reduction in DAS signifies that exercise contributes to overall disease activity attenuation, possibly by modulating inflammatory processes and improving physical function. The non-significant changes in CRP, IL-6, and TNF-α levels suggest that while exercise improves clinical outcomes, its impact on systemic inflammation markers may be limited or require longer intervention periods to become evident. The significant decrease in ESR indicates a potential reduction in inflammation, as ESR is a general marker of inflammatory activity (Alende-Castro et al., 2019). Moreover, the limited measurement of inflammatory biomarkers (CRP in only two studies, IL-6 and TNF-α in just one) may have introduced bias, potentially contributing to the observed lack of significant results, thereby affecting the robustness and generalizability of the findings. Exercise potentially improves pain and fatigue through a reduction in systemic inflammation, mediated by decreased levels of pro-inflammatory cytokines such as IL-6, TNF-α, and CRP (Andrade, 2020). Increased production of anti-inflammatory cytokines like IL-10 may further contribute to symptom relief (Małkowska and Sawczuk, 2023). In the context of joint health, mechanical loading during resistance and aerobic exercise may stimulate chondrocytes to produce extracellular matrix components, thereby improving cartilage resilience (Leong and Sun, 2014). Emerging evidence suggests that exercise may also influence epigenetic regulation of inflammatory genes, thereby exerting long-term anti-inflammatory effects (Plaza-Diaz et al., 2022).
This umbrella meta-analysis has several limitations that must be considered. First, the high degree of heterogeneity (I2 > 60% in most subgroups) across studies, particularly in terms of exercise duration and type, as well as age, limits the generalizability of the findings. Variability in reporting standards and the use of different effect size metrics, such as WMD and SMD, further complicates comparisons. Additionally, subgroup analyses were constrained by the availability of data, resulting in small sample sizes for specific exercise types. Limited short intervention periods of included studies limits the generalizability of the findings. Another notable limitation is the reliance on self-reported outcomes, such as pain and fatigue, which are subjective and susceptible to bias. However, the strengths of this study include its comprehensive scope, the integration of multiple meta-analyses, and the systematic exploration of key moderators such as age, and exercise frequency. By synthesizing evidence from diverse populations, this review provides a more holistic understanding of exercise interventions in RA management.
Future research should focus on standardizing methodologies in exercise trials to reduce heterogeneity and enhance comparability across studies. Investigations into novel exercise regimens, such as high-intensity interval training or tailored interventions for specific subpopulations, are warranted. Longitudinal studies with longer follow-up periods are needed to assess the sustainability of benefits. Moreover, future trials should include objective biomarkers to complement subjective measures, offering a more precise evaluation of exercise effects. Incorporating molecular and mechanistic analyses in these trials could elucidate the pathways through which exercise modulates RA outcomes, paving the way for personalized exercise prescriptions. Moreover, the combination of dietary factors and nutritional approaches with physical activity in future studies can provide innovative strategies for managing RA. This integration can synergize the anti-inflammatory and antioxidant effects of a healthy diet with the benefits of improved muscle strength, flexibility, and pain reduction achieved through exercise.
This umbrella meta-analysis underscores the beneficial role of exercise in managing RA, and knee OA, particularly in reducing pain, fatigue, disease activity, and ESR. Future research should focus on personalized approaches, novel regimens, and objective biomarkers to refine non-pharmacological strategies for RA, and knee OA management.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
XF: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Writing–original draft, Writing–review and editing. LZ: Investigation, Project administration, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. CW: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing–original draft, Writing–review and editing. JY: Conceptualization, Data curation, Formal Analysis, Investigation, Project administration, Writing–original draft, Writing–review and editing. HZ: Investigation, Project administration, Resources, Supervision, Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. The Zhejiang Provincial Medical and Health Science and Technology Plan. 2024KY1377.
I would like to thank my family and hospital leaders for their support and help in completing my dissertation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1558214/full#supplementary-material
Alende-Castro V., Alonso-Sampedro M., Vazquez-Temprano N., Tuñez C., Rey D., García-Iglesias C., et al. (2019). Factors influencing erythrocyte sedimentation rate in adults: new evidence for an old test. Med. Baltim. 98 (34), e16816. doi:10.1097/MD.0000000000016816
Al-Jiffri O. H., Abd El-Kader S. M. (2021). Aerobic versus resistance exercises on systemic inflammation and sleep parameters in obese subjects with chronic insomnia syndrome. Afr. Health Sci. 21 (3), 1214–1222. doi:10.4314/ahs.v21i3.30
Andrade C. (2020). Mean difference, standardized mean difference (SMD), and their use in meta-analysis: as simple as it gets. J. Clin. Psychiatry 81 (5), 20f13681. doi:10.4088/JCP.20f13681
Andrea Cortés-Ladino C., Augusto Arias-Ortiz W., Porras-Ramírez A. (2023). Effectiveness of Yoga and Acupuncture in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat. Med. (1), 9098442.
Baillet A., Vaillant M., Guinot M., Juvin R., Gaudin P. (2012). Efficacy of resistance exercises in rheumatoid arthritis: meta-analysis of randomized controlled trials. Rheumatology, 51 (3), 519–527. doi:10.1093/rheumatology/ker330
Baillet A., Zeboulon N., Gossec L., Combescure C., Bodin L. A., Juvin R., et al. (2010). Efficacy of cardiorespiratory aerobic exercise in rheumatoid arthritis: meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken), 62 (7) 984–992.
Begg C. B., Mazumdar M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. doi:10.2307/2533446
Chimenti R. L., Frey-Law L. A., Sluka K. A. (2018). A mechanism-based approach to physical therapist management of pain. Phys. Ther. 98 (5), 302–314. doi:10.1093/ptj/pzy030
Davis L. S. (2003). A question of transformation: the synovial fibroblast in rheumatoid arthritis. Am. J. Pathol. 162 (5), 1399–1402. doi:10.1016/S0002-9440(10)64272-1
Egger M., Davey Smith G., Schneider M., Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. bmj 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
El-Kader A., Gari A., Salah El-Den A. (2013). Impact of moderate versus mild aerobic exercise training on inflammatory cytokines in obese type 2 diabetic patients: a randomized clinical trial. Afr. Health Sci. 13 (4), 857–863. doi:10.4314/ahs.v13i4.1
Englund S., Piehl F., Kierkegaard M. (2022). High-intensity resistance training in people with multiple sclerosis experiencing fatigue: a randomised controlled trial. Multiple Scler. Relat. Disord. 68, 104106. doi:10.1016/j.msard.2022.104106
Faraziani F., Eken Ö. (2024). Enhancing cognitive abilities and delaying cognitive decline in the elderly through exercise-based health management systems. Int. J. Sport Stud. Health 7 (2), 13–22. doi:10.61838/kman.intjssh.7.2.2
Ghalamsiah N., Nourshahi M. (2023). Acute effects of high-intensity functional training with or without whole-body electromyostimulation on serum irisin and brain-derived neurotrophic factor in overweight individuals. Sci. and Sports 38 (8), 799–806. doi:10.1016/j.scispo.2022.08.004
Guo Q., Wang Y., Xu D., Nossent J., Pavlos N. J., Xu J. (2018). Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 6, 15. doi:10.1038/s41413-018-0016-9
Han A., Robinson V., Judd M., Taixiang W., Wells G., Tugwell P. (2004). Tai chi for treating rheumatoid arthritis. Cochrane Database Syst Rev. (3), Cd004849. doi:10.1002/14651858.Cd004849
Health Quality Ontario (2018). Structured education and neuromuscular exercise program for hip and/or knee osteoarthritis: a health Technology assessment. Ont. Health Technol. Assess. Ser. 18 (8), 1–110.
Holden M. A., Hattle M., Runhaar J., Riley R. D., Healey E. L., Quicke J., et al. (2023). Moderators of the effect of therapeutic exercise for knee and hip osteoarthritis: a systematic review and individual participant data meta-analysis. The Lancet Rheumatology, 5(7),e386–e400.
Hurkmans E., van der Giesen F. J., Vlieland T. P. V., Schoones J., Van den Ende E. C. (2009). Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database Syst Rev. (4).
Jahid M., Khan K. U., Rehan-Ul-Haq , Ahmed R. S. (2023). Overview of rheumatoid arthritis and scientific understanding of the disease. Mediterr. J. Rheumatol. 34 (3), 284–291. doi:10.31138/mjr.20230801.oo
Joyner M. J., Casey D. P. (2015). Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol. Rev. 95 (2), 549–601. doi:10.1152/physrev.00035.2013
Kelley G. A., Kelley K. S., Callahan L. F.(2018). Aerobic exercise and fatigue in rheumatoid arthritis participants: a meta-analysis using the minimal important difference approach. Arthritis Care Res. 70 (12), 1735–1739.
Leong D. J., Sun H. B. (2014). Mechanical loading: potential preventive and therapeutic strategy for osteoarthritis. J. Am. Acad. Orthop. Surg. 22 (7), 465–466. doi:10.5435/JAAOS-22-07-465
Li Z., Wang X. Q. (2022). Clinical effect and biological mechanism of exercise for rheumatoid arthritis: a mini review. Front. Immunol. 13, 1089621. doi:10.3389/fimmu.2022.1089621
Liu T., Huang C., Zhou L., Zong R., Zhou X. (2023). Effects of routine drug therapy in combination with health exercise training on pain and joint function in patients with rheumatoid arthritis: A meta analysis. Trop. J. Pharm. Res. 22 (3), 633–645.
Lima L. V., Abner T. S. S., Sluka K. A. (2017). Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. J. Physiol. 595 (13), 4141–4150. doi:10.1113/JP273355
Małkowska P., Sawczuk M. (2023). Cytokines as biomarkers for evaluating physical exercise in trained and non-trained individuals: a narrative review. Int. J. Mol. Sci. 24 (13), 11156. doi:10.3390/ijms241311156
Markov A., Chaabene H., Hauser L., Behm S., Bloch W., Puta C., et al. (2022). Acute effects of aerobic exercise on muscle strength and power in trained male individuals: a systematic review with meta-analysis. Sports Med. 52 (6), 1385–1398. doi:10.1007/s40279-021-01615-6
Mudano A. S., Tugwell P., Wells G. A., Singh J. A. (2019). Tai Chi for rheumatoid arthritis. Cochrane Database Syst. Rev. 9 (9), Cd004849. doi:10.1002/14651858.CD004849.pub2
Nijs J., Kosek E., Van Oosterwijck J., Meeus M. (2012). Dysfunctional endogenous analgesia during exercise in patients with chronic pain: to exercise or not to exercise? Pain Physician 15 (3 Suppl. l), Es205–13.
Patel H., Alkhawam H., Madanieh R., Shah N., Kosmas C. E., Vittorio T. J. (2017). Aerobic vs anaerobic exercise training effects on the cardiovascular system. World J. Cardiol. 9 (2), 134–138. doi:10.4330/wjc.v9.i2.134
Plaza-Diaz J., Izquierdo D., Torres-Martos Á., Baig A. T., Aguilera C. M., Ruiz-Ojeda F. J. (2022). Impact of physical activity and exercise on the epigenome in skeletal muscle and effects on systemic metabolism. Biomedicines 10 (1), 126. doi:10.3390/biomedicines10010126
Porter C., Reidy P. T., Bhattarai N., Sidossis L. S., Rasmussen B. B. (2015). Resistance exercise training alters mitochondrial function in human skeletal muscle. Med. Sci. Sports Exerc 47 (9), 1922–1931. doi:10.1249/MSS.0000000000000605
Rice D., Nijs J., Kosek E., Wideman T., Hasenbring M. I., Koltyn K., et al. (2019). Exercise-induced hypoalgesia in pain-free and chronic pain populations: state of the art and future directions. J. Pain 20 (11), 1249–1266. doi:10.1016/j.jpain.2019.03.005
Rongen-van Dartel S., Repping-Wuts H., Flendrie M., Bleijenberg G., Metsios G., van Den Hout W., et al. (2015). Effect of aerobic exercise training on fatigue in rheumatoid arthritis: a meta-analysis. Arthritis Care Res. 67 (8), 1054–1062.
Runge N., Arribas-Romano A., Labie C., Maîresse O., Goossens Z., Nijs J., et al. (2023). The effectiveness of exercise and physical activity programs on fatigue and sleep in people with arthritis–A systematic review with meta-analysis. Sleep Med. Rev. 71, 101832.
Schroeder E. C., Franke W. D., Sharp R. L., Lee D. C. (2019). Comparative effectiveness of aerobic, resistance, and combined training on cardiovascular disease risk factors: a randomized controlled trial. PLoS One 14 (1), e0210292. doi:10.1371/journal.pone.0210292
Sezgin M. G., Bektas H. (2023). The Effect of Coaching Programs on Physical Activity and Pain in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Pain Manag. Nurs. 24 (5), 549–557. doi:10.1371/journal.pone.0210292
Schwarz L., Kindermann W. (1992). Changes in beta-endorphin levels in response to aerobic and anaerobic exercise. Sports Med. 13 (1), 25–36. doi:10.2165/00007256-199213010-00003
Shea B. J. (2017). AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. bmj, 358. doi:10.1136/bmj.j4008
Singh J. A., Saag K. G., Bridges S. L., Akl E. A., Bannuru R. R., Sullivan M. C., et al. (2015). 2015 American College of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. Hob. 68 (1), 1–25. doi:10.1002/acr.22783
Smith J. K. (2020). Exercise as an adjuvant to cartilage regeneration therapy. Int. J. Mol. Sci. 21 (24), 9471. doi:10.3390/ijms21249471
Sobue Y., Kojima T., Ito H., Nishida K., Matsushita I., Kaneko Y., et al. (2022). Does exercise therapy improve patient-reported outcomes in rheumatoid arthritis? A systematic review and meta-analysis for the update of the 2020 JCR guidelines for the management of rheumatoid arthritis. Mod Rheumatol, 32 (1), 96–104. doi:10.1080/14397595.2021.1886653
Spurway N. C. (1992). Aerobic exercise, anaerobic exercise and the lactate threshold. Br. Med. Bull. 48 (3), 569–591. doi:10.1093/oxfordjournals.bmb.a072564
Sun H. B. (2010). Mechanical loading, cartilage degradation, and arthritis. Ann. N. Y. Acad. Sci. 1211, 37–50. doi:10.1111/j.1749-6632.2010.05808.x
Tahira S. (2023). The association between physical activity and cancer prevention, recovery, and recurrence: a narrative review. Health Nexus 1 (4), 54–66. doi:10.61838/kman.hn.1.4.7
Takkouche B., Norman G. (2011). PRISMA statement. Epidemiology 22 (1), 128. doi:10.1097/ede.0b013e3181fe7999
Vos T. (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. lancet 396 (10258), 1204–1222. doi:10.1016/S0140-6736(20)30925-9
Ward M. M. (2004). Relative sensitivity to change of the erythrocyte sedimentation rate and serum C-reactive protein concentration in rheumatoid arthritis. J. Rheumatol. 31 (5), 884–895.
Ward M. M., Guthrie L. C., Alba M. I. (2015). Clinically important changes in individual and composite measures of rheumatoid arthritis activity: thresholds applicable in clinical trials. Ann. Rheumatic Dis. 74 (9), 1691–1696. doi:10.1136/annrheumdis-2013-205079
Wen Z., Chai Y. (2021). Effectiveness of resistance exercises in the treatment of rheumatoid arthritis: a meta-analysis. A meta-analysis. Med. Baltim. 100 (13), e25019. doi:10.1097/MD.0000000000025019
Williams M. A., Srikesavan C., Heine P. J., Bruce J., Brosseau L., Hoxey-Thomas N., Lamb S. E. (2018). Exercise for rheumatoid arthritis of the hand. Cochrane Database Syst Rev. 2018 (7), CD003832.
Wu H., Wang Q., Wen G., Wu J., Wang Y. (2023). The effects of Tai Chi on physical function and safety in patients with rheumatoid arthritis: a systematic review and meta-analysis. Front. Physiol. 14, 1079841. doi:10.3389/fphys.2023.1079841
Yasmeen F., Pirzada R. H., Ahmad B., Choi B., Choi S. (2024). Understanding autoimmunity: mechanisms, predisposing factors, and cytokine therapies. Int. J. Mol. Sci. 25 (14), 7666. doi:10.3390/ijms25147666
Ye H., Weng H., Xu Y., Wang L., Wang Q., Xu G. (2022). Effectiveness and safety of aerobic exercise for rheumatoid arthritis: a systematic review and meta-analysis of randomized controlled trials. BMC Sports Sci Med Rehabil. 14 (1), 117.
Ye X., Chen Z., Shen Z., Chen G., Xu X. (2020). Yoga for treating rheumatoid arthritis: a systematic review and meta-analysis. Front. Med. 7 586665.
Keywords: exercise, walk, physical activity, inflamma-tion, meta, analysis
Citation: Fu X, Zhang L, Wang C, Yue J and Zhu H (2025) The effect of exercise therapy on pain, fatigue, bone function and inflammatory biomarkers individuals with rheumatoid arthritis and knee osteoarthritis: a meta-research review of randomized controlled trials. Front. Physiol. 16:1558214. doi: 10.3389/fphys.2025.1558214
Received: 09 January 2025; Accepted: 03 March 2025;
Published: 09 April 2025.
Edited by:
Antonino Patti, University of Palermo, ItalyReviewed by:
Khadijeh Irandoust, Imam Khomeini International University, IranCopyright © 2025 Fu, Zhang, Wang, Yue and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Zhang, WmhhbmdsaWFuZzcyM0AxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.