
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 21 March 2025
Sec. Avian Physiology
Volume 16 - 2025 | https://doi.org/10.3389/fphys.2025.1534385
This article is part of the Research Topic Rising Stars in Avian Physiology: 2024 View all 9 articles
 Kari L. Harding1
Kari L. Harding1 Emmillie Boot1
Emmillie Boot1 Jackson O. Evans2
Jackson O. Evans2 Sanjay B. Shah2
Sanjay B. Shah2 Ramon D. Malheiros1
Ramon D. Malheiros1 Kenneth E. Anderson1*
Kenneth E. Anderson1*The poultry industry faces a major impediment in dealing with highly pathogenic avian influenza (HPAI). Large outbreaks have resulted in depletion of available resources needed for desired depopulation methods, leading to the need for alternative methods. This study was conducted to explore alternative ventilation shutdown procedures and how they affect laying hens throughout the process. Three treatments evaluated were ventilation shutdown plus heat (VSDH), ventilation shutdown plus heat and relative humidity (VSDHRh), and ventilation shutdown plus carbon dioxide (VSDCO2). There were two phases used: one phase was used to study treatment effects on the hens’ EEG responses from beginning to time of death and how laying hens behaved. Phase 2 examined how these treatments affected hen blood chemistry and HSP70 during the process. VSDCO2 had a significantly quicker time of death (P = 0.0003), and VSDH and VSDHRh were not different. There were no differences in pre- or post-corticosterone levels in Phase 1; however, there was a trend (P = 0.07) toward significance in the post corticosterone levels. Heat shock protein 70 (HSP70) levels were higher (P = 0.0001) in the VSDCO2 treatment, which could be due to the protein upregulation to prevent apoptosis. In Phase 2, VSDH corticosterone had a significantly greater treatment effect compared to VSDHRh and VSDCO2. corticosterone levels were significantly greater than those of VSDHRh. There were no significant treatment effects in Phase 2 for HSP70 expression; however, the sequence was significant, with the HSP70 being significantly greater at 75% to the average time of death than at 100% to the average time of death. Overall, VSDHRh could be a good alternative for the industry to use to rapidly depopulate laying hen facilities. However, more research on this treatment and more in-depth stress parameters measured needs to be conducted to fully determine how it affects laying hens.
Highly pathogenic avian influenza (HPAI) is an infectious disease that has become a major concern for the poultry industry. Beginning in 2014 and carrying over into 2015, the poultry industry experienced a major outbreak of HPAI. This outbreak resulted in the loss of roughly 45 million birds, mostly laying hens or pullets (Greene, 2015). Currently an outbreak of HPAI that was first reported in 2022 and is ongoing has led to 96.91 million birds being depopulated. Of these flocks, 496 have been commercial and 655 have been backyard flocks (USDA, 2024). According to the American Veterinary Medical Association (AVMA), depopulation is defined as “methods by which large numbers of animals must be destroyed quickly and efficiently with as much consideration given to the welfare of animals as practicable, given extenuating circumstances” (AVMA, 2019). Current preferred depopulation methods for floor-reared poultry include use of water-based expanding foam, captive bolt guns, and water-based foam nozzles. Cage-housed laying hens have permitted methods such as CO2 kill carts, CO2 injection throughout the entire house, and partial-house gassing. The injection of CO2 for whole-house or partial-house is a preferred method for floor-reared poultry as well. Under constrained circumstances, some of the following methods are permitted for floor-reared poultry: (VSD) plus (hyperthermia), controlled demolition, and exsanguination, and for caged-house poultry, use of compressed air foam, captive bolt guns, and VSD+ are some of the permitted methods. While all of these methods are successful, the preferred methods are most often quicker methods of depopulation compared to the permitted methods for both housing systems. Ventilation shutdown alone is not permitted for either of these poultry housing types, and therefore was not used for this study. The challenge that was identified from the outbreaks was that multi-tiered housing systems such as conventional or colony cages and aviaries, along with high-rise houses, provided hindrances to some of the preferred depopulation methods. In both outbreaks, supplies, namely, CO2, were rapidly depleted or not available, resulting in an increase in the suffering of affected birds and leading to the potential for a greater spread of the virus. This led to the need for development of alternative depopulation methods to decrease the potential for a greater spread of the disease and to mitigate bird suffering and biosecurity risks. Eberle-Krish et al. (2018) evaluated ventilation shutdown plus CO2 (VSDCO2) and ventilation shutdown plus heat (VSDH) vs ventilation shutdown (VSD). They reported that VSDCO2 was more rapid than all other treatments, followed by VSDH, both with 100% mortality compared to VSD alone, which did not meet the 100% mortality standard. Other research studies have found that including high relative humidity levels with heat reduces the time it takes for total depopulation (Zhao et al., 2019). Another study looking at the addition of steam showed that all treatments using steam were significantly faster to observe the first hen death and complete mortality compared to ventilation shutdown plus heat alone (Mendoza and Williams, 2024). There is very limited research evaluating how laying hen electroencephalograms (EEGs) change throughout any of the VSD processes. One study evaluated the EEGs of laying hens during application of whole-house CO2. The results in individual birds indicated the changes between normal, transitional, suppressed, and isoelectric EEG phases. The results reported that out of ten laying hens, nine exhibited a decrease in EEG amplitude at each of the phases (Mckeegan et al., 2011). Another trial utilized EEGs to determine time to unconsciousness and brain death in broilers, layers, turkeys, and ducks. It was reported that turkeys reached time to brain death faster when water-based foam was used, compared to layers and ducks in which they reach brain death sooner with use of CO2 gas (Benson et al., 2012). While these studies have observed what one method looks like for laying hens, there are no known studies that have compared EEG signals of laying hens across treatments. There is currently no research comparing the different ventilation shutdown plus methods for their effects on laying hen blood physiology and behaviors either.
All research procedures involving animals in these trials were approved by the North Carolina State University Institutional Animal Care and Use Committee (IACUC # 21-310). This study was conducted in the Bird Wing of the Prestage Department of Poultry Science at North Carolina State University. Throughout the study, all animals were monitored by veterinarians and animal welfare specialists employed by the university.
Both Phase 1 and Phase 2 experiments described below were conducted in four chambers that were housed in a windowless, temperature-controlled room. The chambers were made with Plexiglass® with fittings that allowed for CO2 sampling using external meters to collect data and for the injection of gas or humidity. The temperature and relative humidity for each chamber was recorded using Aranet sensors (Aranet, Riga, Latvia; model: SKU: TDSPT8U2; accuracy: ±0.3°C and ±2%). These sensors recorded every minute with the base placed approximately 4 cm (0.04 m) from the top of each chamber and the sensor reaching bird eye level when standing. Three sides, top, and bottom were covered with 2.5 cm of the closed cell insulation board, with only the front panel uncovered to all the hens to be observed. The chamber size was 1.68 ft. (0.51 m) × 1.68 ft. (0.51 m) × 1.68 ft. (0.51 m) equating to the space volume per hen in a typical caged layer facility. The room containing the chambers was maintained at 26°C to prevent heat loss to the environment. In each chamber, a 100-W incandescent light bulb built into the chamber was used as the heat source for the heat treatments described in Table 1. This allowed for temperatures to reach 40°C, which was chosen based on the Department of Environment, Food, and Rural Affairs (DEFRA, 2009). All chambers were equipped with raised 1 in. × 2 in. (0.305 m × 0.610 m) welded wire floors to mimic cages floors and to allow the hens to maintain posture. A total of three treatments were analyzed in both Phase 1 and Phase 2, and they included ventilation shutdown plus heat (VSDH), ventilation shutdown plus heat and relative humidity (VSDHRh), and ventilation shutdown plus CO2 (VSDCO2). Phase 1 had four replicates per treatment, totaling 12 white commercial laying hens that were approximately 69 weeks of age. These hens were maintained in the Bird Wing at North Carolina State University and were randomly assigned to the treatments. These hens were housed individually, in conventional cages with no additional treatments or procedures for at least 1 week prior to trial initiation. Treatment hens were removed, and blood was obtained from the brachial vein to obtain a baseline level of blood chemistry and corticosterone levels prior to treatment. Core body temperatures for each hen were evaluated using a SuperMeter® (Stamford, CT, United States; Model: HHM290) before and after treatment. The brain electrical output was measured in millivolts (mV) by using an electroencephalogram (EEG). Individual electrodes were insulated, except at the tips, and were attached to the pre-amplifier (AD Instruments, Colorado Springs, CO, United States), which transfers the EEG signals to a laptop-based recording that was continuously monitored. Prior to electrode placement, the birds were hobbled, which allowed them to stand and walk, but prevented them from scratching their heads and pulling out the electrodes. Hobbling was conducted using a rubber band that was placed on the shanks and above the dewclaws. The electrode tips were 32-gauge needles attached to the insulated wire and were inserted subcutaneously. Electrode colors are of three types: red, black, and green. The red electrode was placed on one side of the hen’s cranium, and the black electrode was placed on the other side. The green electrode, also known as the ground, was inserted between the wattles in the lower mandible near the neck. Each electrode tip was secured in its place on the head by using surgical adhesive by employing the catheter taping method. These three leads were then taped together and wound under the wing scapular joint, reducing the probability of the bird getting tangled up and pulling the leads out. Once electrodes were placed, the bird was placed into a chamber with the included treatment.
The start time was determined at the point the chamber was sealed, and the EEG was plugged into the pre-amplifier. The EEG data were recorded at a standard frequency band of 10–50 Hertz (Hz) and sampled at 100 Hz/channel. The EEG was recorded until time of death was called by a veterinarian. Each hen’s behaviors were monitored throughout the treatment and recorded in real time every 2 min by a trained observer. To keep up with these 2-min checks, timers were utilized. Behaviors that were observed and their descriptors are described in Table 2 based on definitions by Hurnik et al. (1995). The CO2 concentrations were measured with two 0%–100% CO2 monitors (CO2 Meter, Inc., Ormond Beach, FL; model: CM-0003; accuracy: ±70 ppm ± 5% of measured value). These were also recorded on minute intervals as well. Once time of death (TOD) was called for each hen, they were removed from the treatment chamber, with blood being collected by severing the hepatic artery and collecting blood samples from the body cavity within 5 min of TOD. Blood was collected in BD Vacutainer tubes containing lithium heparin to prevent clotting. Approximately 0.1 mL of heparinized blood was then placed on the i-STAT® diagnostic system using CG8+ cartridges (Abbott Park, IL, United States; Model: 03P8825) for blood chemistry analysis. Birds were then necropsied with the brain being collected and placed into RNAlater® (Thermo Fisher Scientific, Waltham, MA: Catalog #AM7021) solution. This tube was placed in a refrigerator at 4°C for 24 h and afterward moved to a −20° freezer.
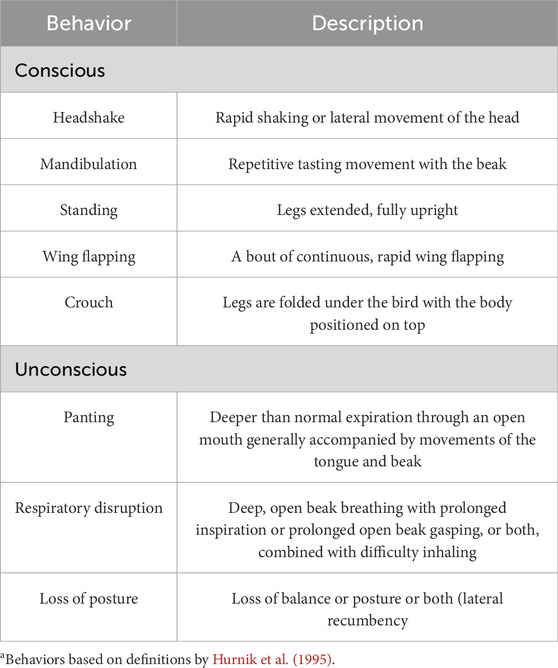
Table 2. Description of the behaviorsa analyzed and reported every 2 min as hens underwent treatment.
Phase 2 of this trial specifically compared the three treatments with respect to physiological changes (blood chemistry and gene expression) over four time points. Ten ∼69-week-old hens were used for each treatment with two replications per treatment, totaling 30 hens. The TOD for the birds in Phase 1 was averaged for each treatment. The average TOD was then quartered, giving a total of four-time intervals, plus one baseline bird that never entered the chamber. The calculated time points to remove the birds are shown in Table 3. This allowed for the evaluation of the hens at the physiological progression over time. At each calculated removal time point, the hen was removed from the chamber, and her blood was collected for blood chemistry and corticosterone analyses within 60 s. The hen was restrained on its side and allowed to bleed via the brachial vein. Between 1.5 and 2 mL of the sample blood was collected using a 3-mL syringe and then transferred to a BD Vacutainer tube with lithium heparin. Then, a 0.1 mL sample of the heparinized blood was placed in the i-STAT® diagnostic system using CG8+ cartridges for blood chemistry analysis. The remaining blood was gently shaken to prevent clotting. The blood was then centrifuged and the plasma supernatant collected and frozen at −20°C for future corticosterone analysis. The hen was then euthanized by a trained individual via cervical dislocation, and the brain tissue was collected and placed in RNAlater® and refrigerated at 4°C for 24 h then moved to a −20°C freezer for later gene expression analysis.
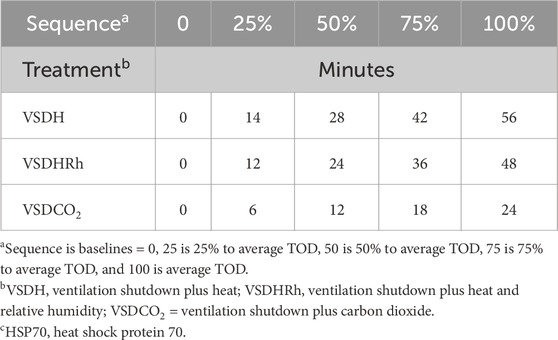
Table 3. Calculated sampling times in minutes of removal times to determine changes in blood chemistry and HSP70c over time.
Plasma corticosterone levels for Phase 1 and Phase 2 were collected by using an ELISA corticosterone kit following the manufacturer’s guidelines. Corticosterone concentrations were determined by a standard curve as nanograms of corticosterone per milliliter of plasma with each sample run in duplicates. The kit was validated using known standards for each plate run, plotting the values, and adding a best fit line. Values were deemed acceptable with an R2 value of ≥0.90 (Cayman Chemical Company, Item: 501320, Ann Arbor MI, United States). For brain samples from Phase 1 and Phase 2, an approximately 1-cm sample of the brain was taken to isolate RNA. These samples were placed in homogenization vials with screw caps, and 1 mL of TRI Reagent™ solution was added to each sample. Each sample was then homogenized using a bead-beater for 20 s and then allowed to incubate at room temperature for 5 min. Then, 200 µL of chloroform was administered to each sample and then vortexed for approximately 10 s. The sample was allowed to sit at room temperature for 2 min and then centrifuged for 20 min at 13,000 × g. Completion of the RNA isolation was performed using an RNeasy kit (QIAGEN, Hilden Germany) according to the manufacturer’s guidelines. RNA levels were quantified using the NanoDrop 2000 (Thermo Fisher Scientific-Waltham, MA) to ensure the correct RNA dilutions were obtained. The ideal RNA concentration was between 100 and 1,000 ng/μL, and these were utilized due to ease of pipetting in downstream applications. The RNA was then transcribed into cDNA for qPCR by utilizing a high-capacity cDNA synthesis kit (Applied Biosystems Waltham, Massachusetts, United States). The qPCR was completed on each sample in triplicate. All wells contained 2.5 ng of samples, 500 nM of gene specific forward and reverse primers (Table 4), and 2X power SYBR green master mix (Applied Biosystems Waltham, Massachusetts, United States), and RNase-Free H2O was added to finalize the volume to 20 µL. The qPCR was performed on the Applied Biosystems StepOnePlus real-time PCR system. Results were normalized to the expression of the basic housekeeping gene, beta actin. The reciprocal was taken for ease of interpretation. The formula used for this is as follows: 1/(target gene cycle threshold/beta actin cycle threshold). All data were then converted to the reciprocal cycle thresholds or CT−1. ΔΔCT was then calculated by first averaging each treatment CT−1, and VSDH was set to be the control. The average for VSDH was then subtracted from each CT−1 to obtain the ΔCT. Because VSDH was used as the control, the ΔΔCT values are the same as the ΔCT values. Each of these values were put into the following equation: 2−ΔΔCT.
A one-way ANOVA was used to evaluate the treatment differences in Phase 1, and a two-way ANOVA was used to evaluate if there were any treatment, sequence, or interaction effects between the two in Phase 2. Significant differences were accepted with α ≤ 0.05, and if there were any differences observed, a Tukey’s HSD was utilized for pairwise comparisons. The EEG data were transformed by taking the absolute value of the integral, allowing for the mitigation of the baseline noise, which was relative to the baseline at each 10-s interval, which was conducted using the following equation:
A hyperbolic arcsine function was used on each value to emphasize the lower millivolt (mV) readings. These transformed EEG data were then analyzed with GLM with full factorial effects of CO2, heat, and heat and humidity fit to each of several response variables. The transformed EEG data used the integral area under the curve that was calculated using the trapezoid method using an NPARM analysis. Behavior data were summarized as a frequency of behaviors that the hens performed as conscious (voluntary) or unconscious (involuntary) behaviors, overlaid with summarized EEG brain activity over the same time intervals. The correlation analysis examined the relationship between the VSDH, VSDHRh, VSDCO2, laying hen EEGs and the behavior profiles. Electroencephalogram activity and conscious and unconscious behaviors was analyzed using Pearson Linear Correlation Analysis in SAS JMP-PRO® 12.2.0 (SAS Institute, 1989).
Table 5 shows the starting and ending chamber temperatures along with relative humidity for each treatment as well as start and end CO2 levels. There were no significant differences between starting relative humidity or starting CO2 values of the chamber. There was a significant difference in the start chamber temperature, with the VSDHRh being significantly higher than the starting chamber temperature in the VSDCO2 due to the heating of the outside room for both VSDH and VSDHRh and not the VSDCO2 treatment. There was a significantly lower end chamber temperature for VSDCO2 compared to the other two treatments. This is because this is the only treatment that does not have heat supplemented. The ending relative humidity percentage was significantly greater in the VSDHRh compared to the other two treatments due to addition of humidity. The ending CO2 levels were significantly greater in VSDCO2, whereas the other two treatments were not different from one another. Table 6 depicts that while there were no significant differences in the pre-core body temperature of hens among the treatments, there was a significantly lower post-core body temperature in the VSDCO2. This again, is due to there being no additional heat being supplemented to this treatment, and the mode of death was hypoxia rather than hyperthermia.
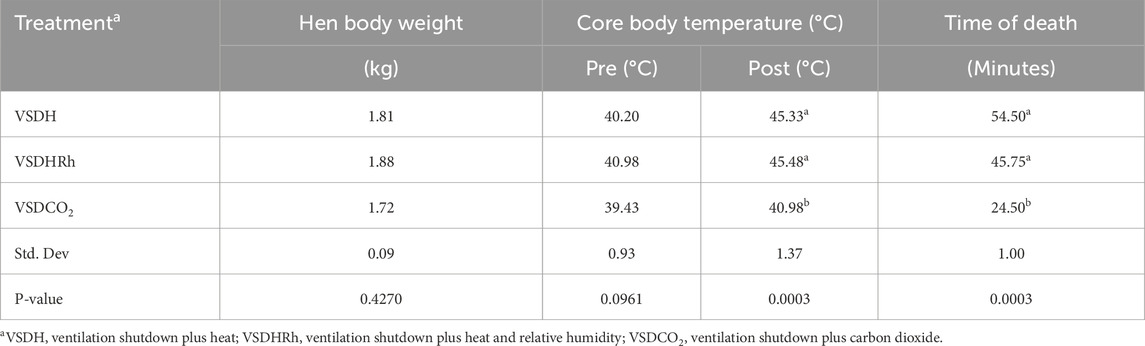
Table 6. Pre- and post-core body temperatures and time of death of hens subjected to each treatment.
The TOD in minutes is reported in Table 6, and the VSDCO2 treatment has a significantly shorter time of death at 24.50 min than the other two treatments analyzed. This was expected based on previous studies (Eberle-Krish et al., 2018). Results from the composite EEG for all treatments are shown in Figure 1. These results indicate that hens did go unconscious in the later stages of these methods, with sporadic spikes in the EEG mV intensity. This agrees with those results found by Mckeegan et al. (2011) which observed relatively consistent changes over time within the laying hen EEGs. This study also reported that the EEG signal was heavily affected soon after CO2 was injected, which was observed in this study. Figure 2 depicts the integral area under the electroencephalographic (EEG) composite graph for VSDH, VSDHRh, and VSDCO2 to TOD using the trapezoidal method. The area under the graph of these transformed EEGs was not different among the three treatments. This was unexpected because the TOD for the VSDCO2 treatment was significantly shorter. These results could be because the brainwave activity was variable and relatively high throughout the initial time for all the methods. Figure 3 depicts the frequency of voluntary and involuntary behavioral responses from beginning to time of death. There were no significant differences between the behaviors of the birds undergoing their respective treatments. There were no significant differences in the strength of the EEGs compared to all other treatments, as shown in Table 7. This indicates that even though the EEGs shown in Figure 1 had different patterns of mV strength, there was no difference in the percentages of the mV strengths between the methods. This is supported by the area under the EEG graphs shown in Figure 2, which indicates there are no differences between the depopulation methods compared in this work. There were significant shifts in conscious and unconscious behavior observed, as shown in Figure 3. At the midpoint of each depopulation method, the shift from conscious to unconscious behaviors was dramatic and consistent. This appears to correspond with Wang et al. (2016) observations, where a decline in neuron function in hyperthermia conditions above normal core body temperature was observed. Table 8 illustrates that based on the behavior observations as they relate to the EEG wave strength, there is a poor correlation between hen behaviors and EEG wave strength. VSDH was significant for both conscious and unconscious behaviors, as shown in Table 8. Figure 4 depicts the slope of the transformed EEG reading for each treatment. There was no significant difference between them, showing that there was no difference in magnitude over the course of each treatment when compared to one another.
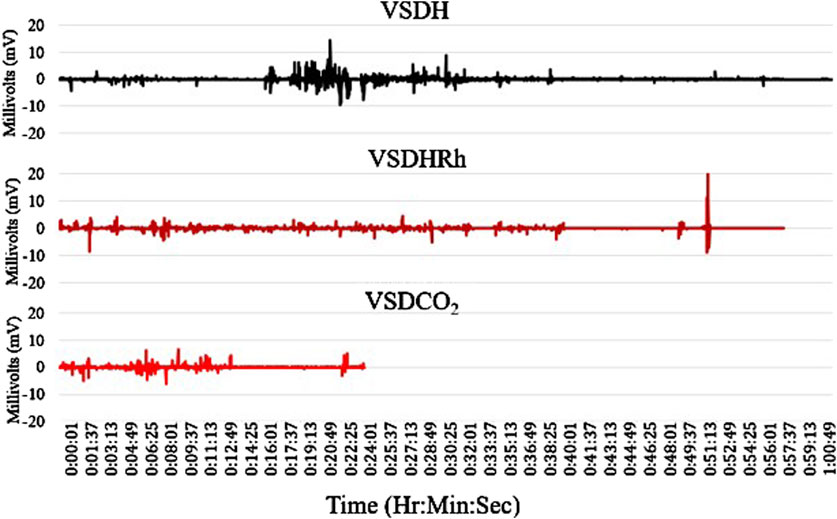
Figure 1. Composite electroencephalograms (EEGs) of the four birds per each treatment for VSDH, VSDHRh, and VSDCO2 from initiation to time of death (TOD). VSDH, ventilation shutdown plus heat; VSDHRh, ventilation shutdown plus heat and relative humidity; VSDCO2, ventilation shutdown plus carbon dioxide.
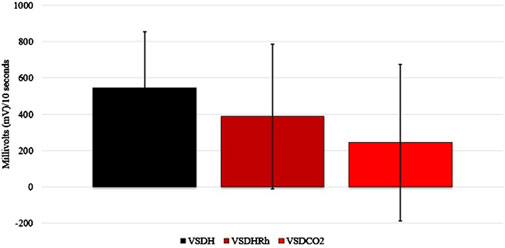
Figure 2. Integral area under the electroencephalogram (EEG) graph calculated of the transformed composite EEGs for VSDH, VSDHRh, and VSDCO2 through TOD using the trapezoid method. VSDH, ventilation shutdown plus heat; VSDHRh, ventilation shutdown plus heat and relative humidity; VSDCO2, ventilation shutdown plus carbon dioxide.
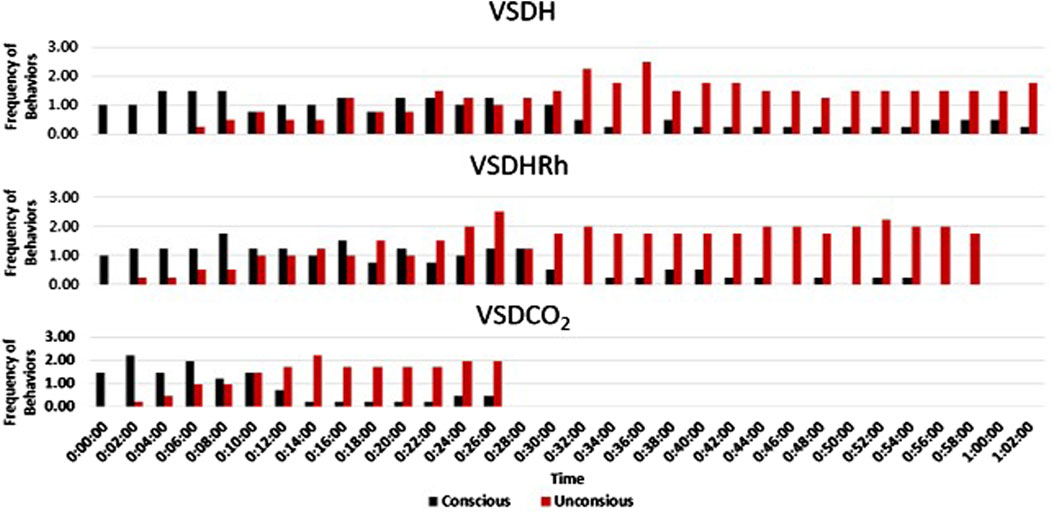
Figure 3. Frequency of conscious (voluntary) and unconscious (involuntary) behavioral responses for VSDH, VSDHRh, and VSDCO2 through to time of death. VSDH, ventilation shutdown plus heat; VSDHRh, ventilation shutdown plus heat and relative humidity; VSDCO2, ventilation shutdown plus carbon dioxide. Unconscious behaviors were determined when the electroencephalogram (EEG) readings were below 0.01 millivolts.

Table 7. Effect of ventilation shutdown treatment groups on strength of the electroencephalographic waves (mV).
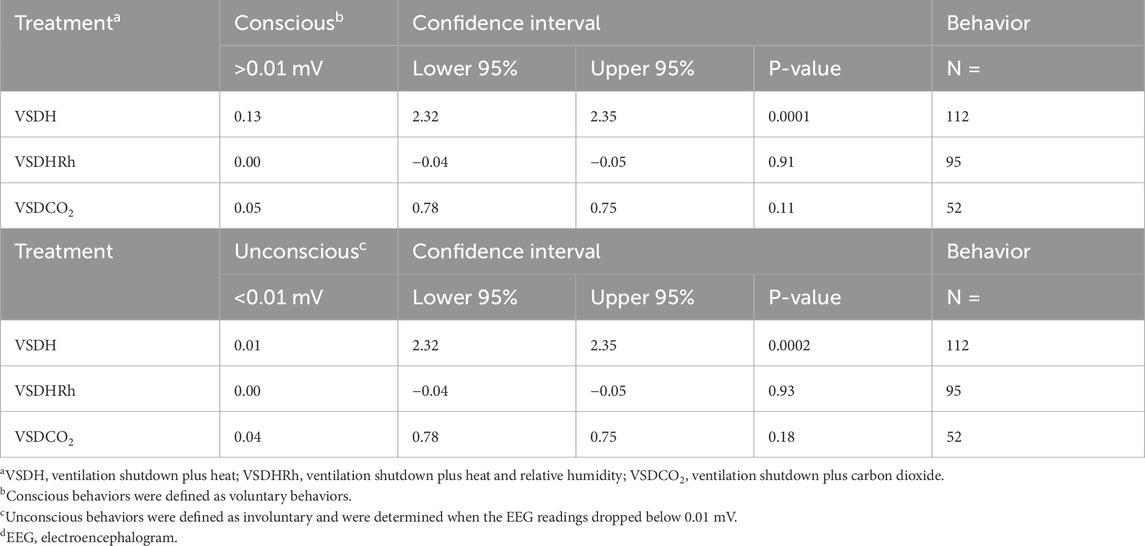
Table 8. Pearson linear correlation coefficient associated with behavior observations as they relate to the EEGd strength.
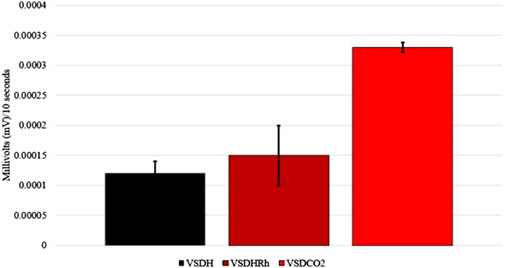
Figure 4. Slopes of the transformed electroencephalogram (EEG) readings of laying hens undergoing different methods of ventilation shutdown. VSDH, ventilation shutdown plus heat; VSDHRh, ventilation shutdown plus heat and relative humidity; VSDCO2, ventilation shutdown plus carbon dioxide.
Heat shock protein 70 (HSP70) levels were significantly upregulated in the VSDCO2 treatment compared to the other two treatments (Figure 5). HSP70 ensures correct protein folding and prevents apoptosis. This gene is heavily upregulated during stress but can be upregulated to make adjustments biologically (Hassan et al., 2019). The elevated levels observed in the VSDCO2 treatment could have just been a biological adjustment or could have been a response to the reaction to the CO2 injection, which is an irritant that could cause bird stress. However, more research should be conducted on this to pinpoint exactly why this occurred. Table 9 shows the baseline blood chemistry parameters for each treatment with only significant differences in sodium, potassium, and ionized calcium. This could be due to the stress of handling and new environment. No blood chemistry parameters in Phase 1 were significantly different among the treatments, as shown in Table 10. Table 11 depicts the corticosterone levels of hens subjected to different treatments before they entered the chamber, and after TOD was called and they were removed from the chamber. There were no significant differences observed between treatments for either pre or post treatment; however, there was a trend (P = 0.07) for laying hen corticosterone after chamber removal; therefore, there could have potentially been differences observed with a larger sample size.
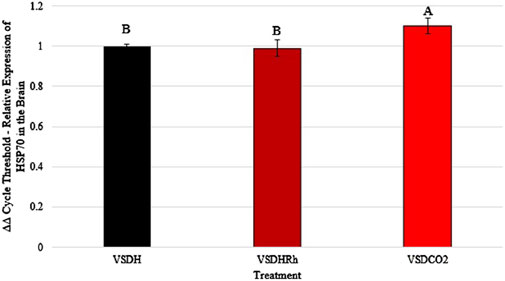
Figure 5. *Heat shock protein 70 (HSP70) levels of laying hens exposed to different methods of ventilation shutdown. VSDH, ventilation shutdown plus heat; VSDHRh, ventilation shutdown plus heat and relative humidity; VSDCO2, ventilation shutdown plus carbon dioxide. A, B — different uppercase letters denote significant differences P < 0.05. *Depicts parameters with a power of analysis of >0.80.
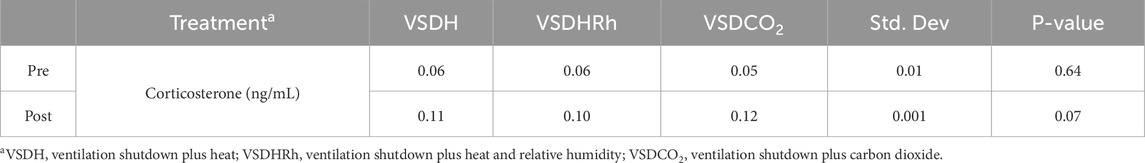
Table 11. Changes in laying hen corticosterone by treatment before using VSDH, VSDHRh, or VSDCO2 and after.
Phase 2 looked at how these treatments affected the hens over time by removing the hens at specific time points. Due to the small sample size in this phase, a power of analysis was conducted with parameters with a power greater than 0.80 being labeled. There were no significant treatment or interaction effects with the HSP70 levels for Phase 2 as shown in Table 12. There was, however, a significant sequence effect. The 75% to average TOD was significantly greater than that of the 100% to average TOD. This was thought to be due to the HSP70 being overwhelmed with the misfolded proteins and apoptosis, which resulted in the downregulation of the HSP70 gene at the 100% average time of death. Table 13 depicts the treatment, sequence, and interaction of each on laying hen corticosterone before and after the trial. There were no treatment, sequence, or interaction effects on the pre-corticosterone levels. There also were no sequence or interaction effects on the post-corticosterone levels. A significant treatment effect was observed with VSDH having greater levels at 0.16 ng/mL than all treatments, with VSDCO2 at 0.12 ng/mL being significantly greater than VSDHRh at 0.06 ng/mL. The higher levels of corticosterone in VSDH align with higher levels that are observed in heat stress when compared to hens that are not (Li et al., 2020). Tables 14, 15 depict the baseline blood chemistry for Phase 2 laying hens before they were subjected to their respective treatments. The effects of treatment, sequence, and the interaction as it pertains to blood chemistry parameters from Phase 2 are shown in Tables 16, 17. There is currently no research on how ventilation shutdown plus (hyperthermia) or VSDCO2 affects blood chemistry. There were no treatment or sequence effects observed for pH, pCO2, pO2, BEecf, HCO3, TCO2, Na, K, iCa, and Hct. Some heat stress studies have reported no changes in blood pH (Barret et al., 2019). Other studies reported that they did see increases in blood pH (Koelkebeck and Odom, 1994). There were, however, sequence effects for sO2, which dropped significantly at 25% to average TOD; however, they recovered and did not drop again. This could be due to potential increased respiration due to a new environment and the noises at the beginning of the trial. Glucose had a significant sequence effect as well, with both 75% and 100% to average TOD calculated were significantly greater compared to that of the baseline. There were no differences between the other two time points. Koelkebeck and Odom, 1995 also reported there was no change in plasma glucose when laying hens were subjected to heat stress and high CO2 exposure. Other studies reported that when increasing the environmental temperature for chicks to 40°C for 2 h resulted in no significant impact on blood glucose levels (Bogin et al., 1981). This agrees with our findings of no significant changes in the blood glucose levels when analyzing treatment effects. The significant increase in glucose for the final two points in the sequence could be due to the body increasing energy availability during the treatments overall. There were no significant interaction effects in either Na, glucose, Hct, sO2, pO2, HCO3, or TCO2. There was a significant interaction in pH with VSDCO2 becoming significantly lower with the 100% TOD having a pH of 7.04. This was expected due to the acidic environment inhalation of CO2 creates in blood. In turn, this resulted in a significantly higher pCO2 level in the blood stream at 88.90, which was higher than both 75% and 100% to average TOD in VSDHRh and higher than 75% average TOD in VSDH. The BEecf parameter was significantly lower in the 100% average TOD for VSDCO2 compared to the final two sequence points for both VSDH and VSDHRh, most likely due to the increased acidic environment caused by the CO2. There was a significantly greater amount of K in the VSDCO2 treatment at 50% and 100% to average TOD compared to all other treatments. This could possibly be due to the K trying to balance the body’s acidic environment. There was also an interaction for iCa with VSDHRh having significantly lower levels at 100% average TOD than all other treatment sequences, except for VSDHRh 75% average TOD. This potentially could be due to increased panting by the birds, leading to water loss.
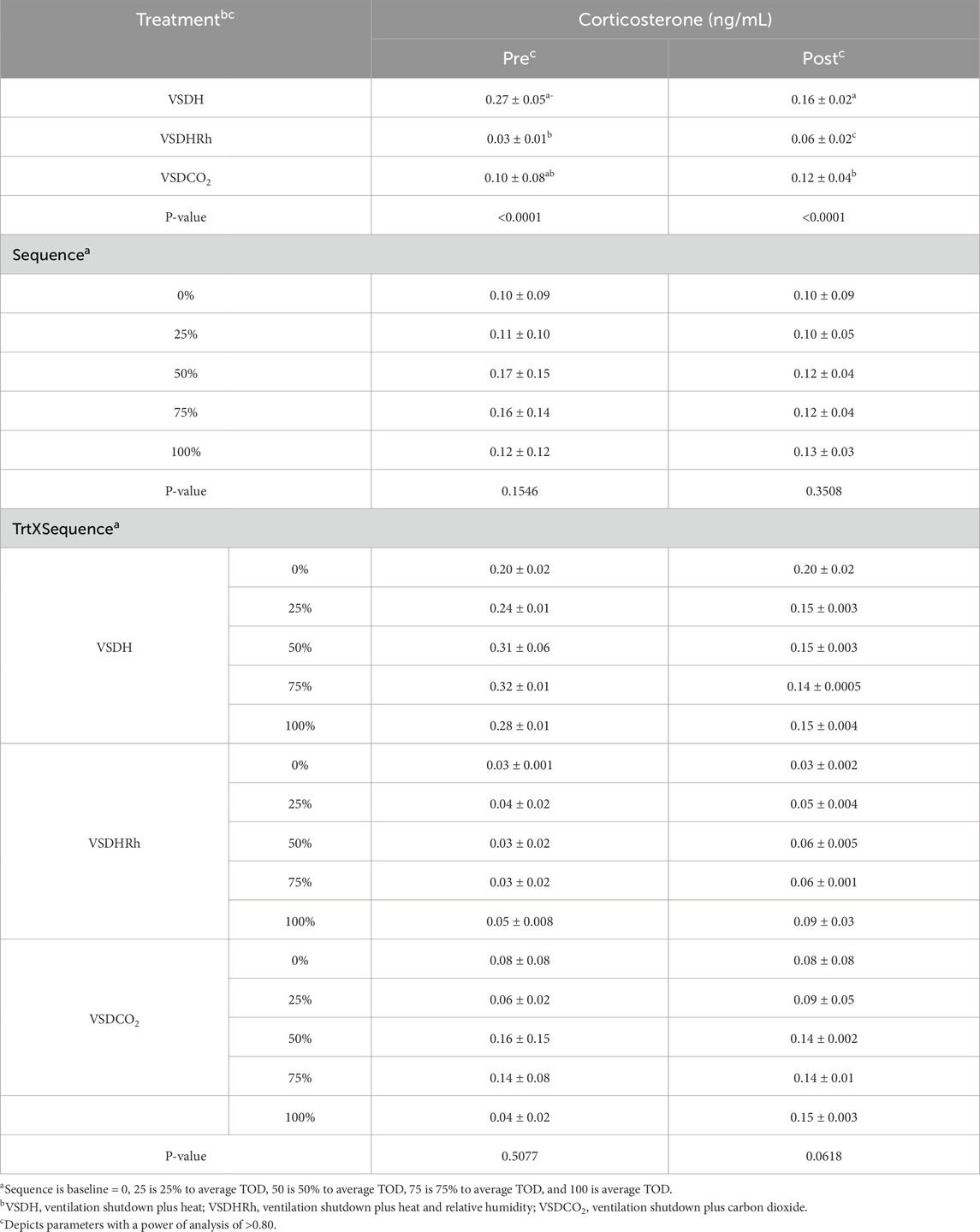
Table 13. Effects of treatment, sequence, and their interaction on laying hen corticosterone pre and post treatment.
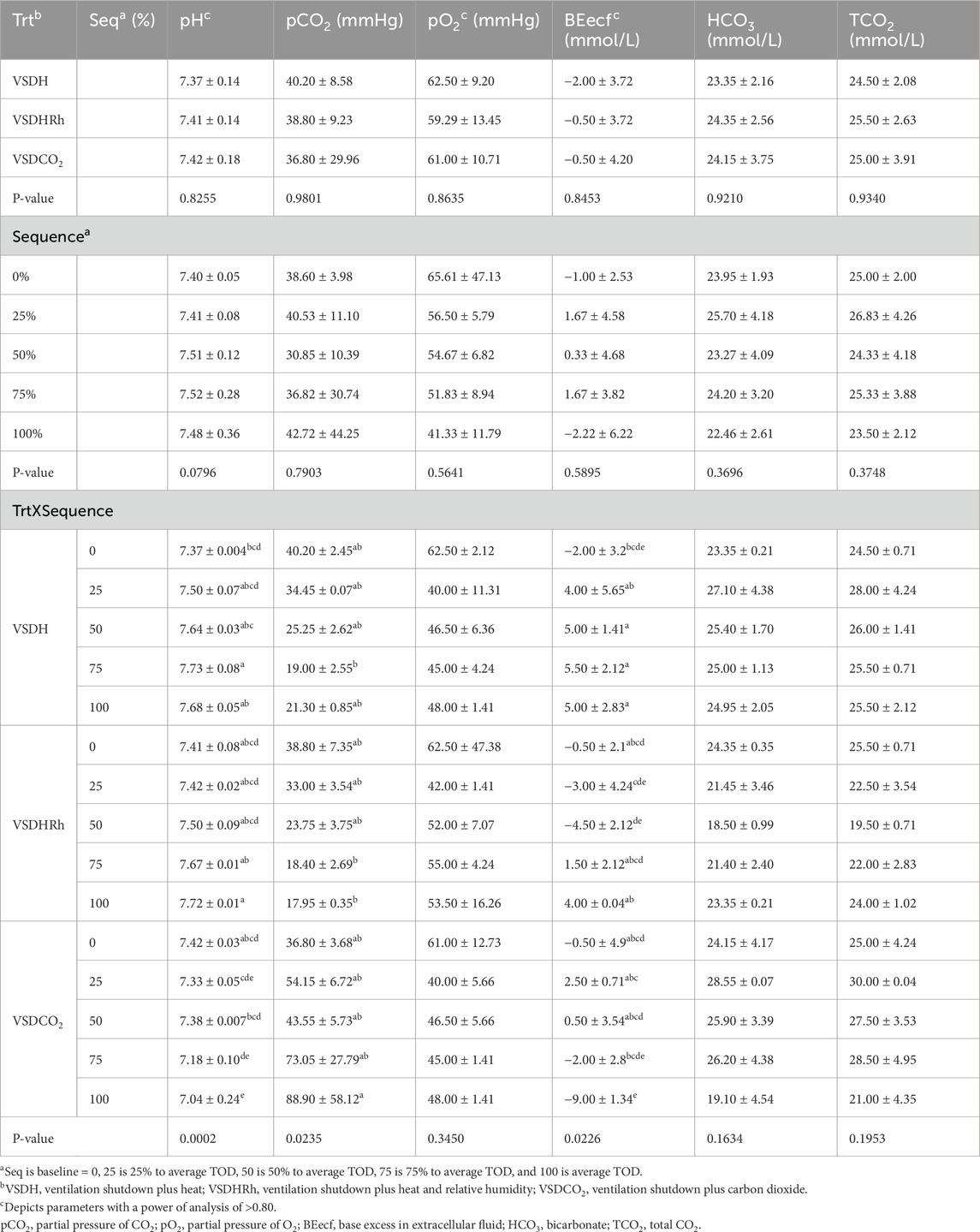
Table 16. Changes in the blood chemistry of laying hens overtime when depopulated using VSDH (hyperthemic method), VSDHRh (hyperthermic method), or VSDCO2.
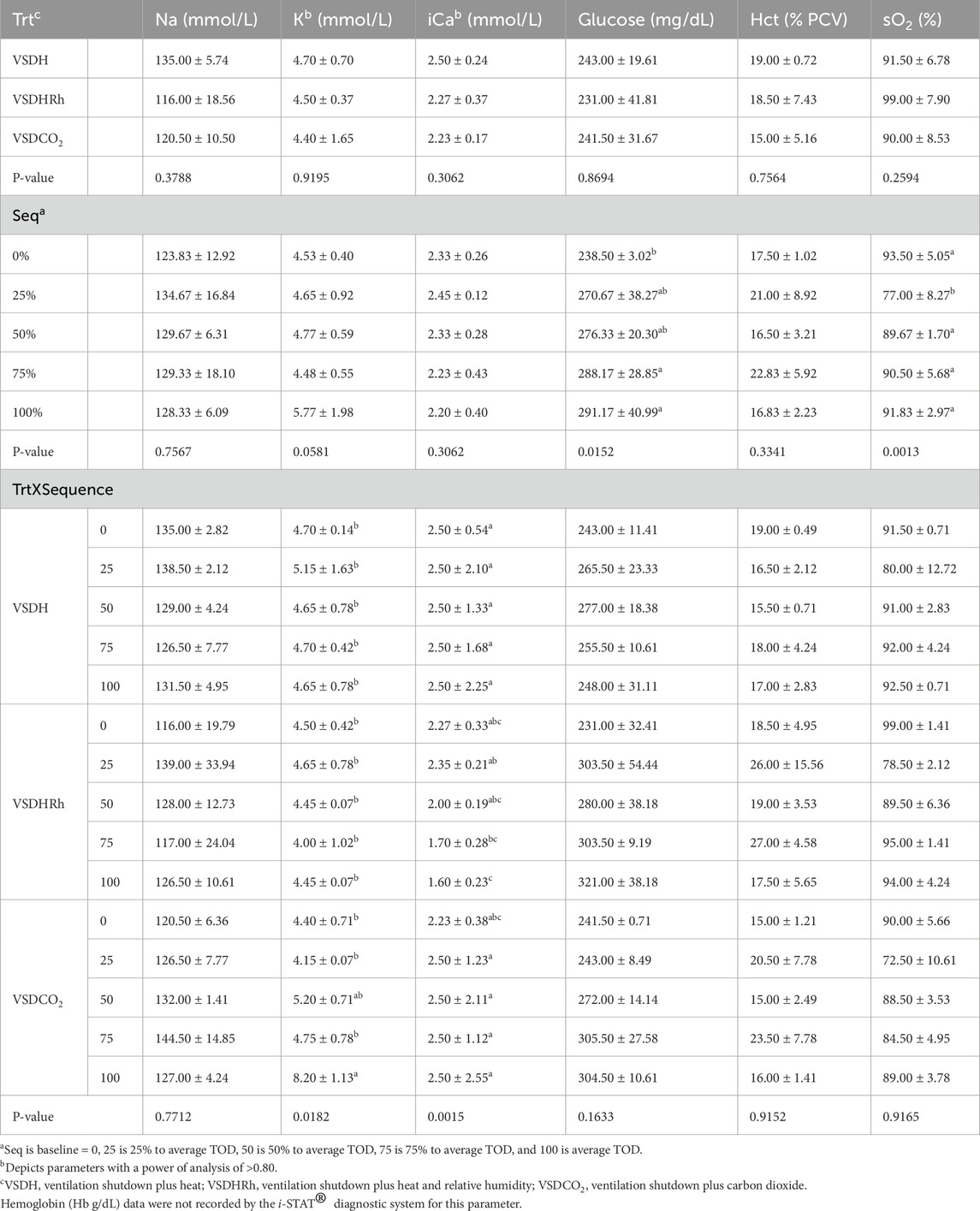
Table 17. Blood chemistry change of laying hens overtime when depopulated using VSDH (hyperthemic method), VSDHRh (hyperthermic method), or VSDCO2.
This study was conducted to try to get an understanding of what birds are experiencing throughout different depopulation methods. Due to the nature of this study, the smallest possible sample size that could get approved was utilized. This was to try to get a better understanding of the blood physiology, behaviors, and TOD for laying hens undergoing these treatments. Bird variability could have played a large factor in significance and non-significance. A larger sample size is necessary to allow for a stronger understanding of what exactly is occurring in the laying hen. It would also be beneficial to conduct this study analyzing different parameters to fully understand the stress that the laying hens are undergoing.
As HPAI continues to be a major concern for the poultry industry, it is important to know the methods used for emergency depopulation and how they affect the laying hen. This study examined how three treatments, VSDH, VSDHRh, and VSDCO2, were compared with respect to laying hen TOD, blood physiology, and behaviors. VSDCO2 had a significantly shorter TOD than the other two treatments. While the VSDH and VSDHRh treatments were not significantly different, VSDHRh had a time reduction of 16%. There also were no significant differences associated with the hens’ EEGs nor their behaviors. There were significantly greater levels of HSP70 production in VSDCO2 compared to the other two treatments. Overall, this study demonstrated that while VSDCO2 may have significantly quicker TOD, in the event that there are limited supplies, both VSDH and VSDHRh may be available as alternatives to depopulate laying hen houses. Having multiple alternative methods that have met the guidelines as defined in the Red Book (USDA-APHIS, 2017), like VSDH and VSDHRh, may allow for the reduction in the spread of HPAI. The approval for use comes from the AVMA Depopulation Guidelines; however, the USDA makes the final decision on which methods will be administered depending on different factors such as bird type and housing type. Future research with these treatments may potentially provide a better understanding of laying hen stress and could measure respiratory rate, internal body temperature measurements, thyroid hormones like T3 and T4, and adrenocorticotropic hormone (ACTH) levels.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The animal study was approved by the North Carolina State University Institutional Animal Care and Use Committee (IACUC # 21-310). The study was conducted in accordance with the local legislation and institutional requirements.
KH: conceptualization, data curation, investigation, methodology, writing–original draft, and writing–review and editing. EB: data curation, investigation, methodology, and writing–review and editing. JE: data curation, investigation, methodology, software, and writing–review and editing. SS: conceptualization, data curation, funding acquisition, investigation, methodology, software, supervision, and writing–review and editing. RM: conceptualization, data curation, funding acquisition, investigation, methodology, supervision, and writing–review and editing. KA: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, supervision, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors would like to thank U.S. Poultry & Egg Association for funding this project (Project F99).
The authors would like the thank staff at Prestage Department of Poultry Science-North Carolina State University for all of their help throughout the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
HPAI, highly pathogenic avian influenza; VSD+, ventilation shutdown plus; VSDH, ventilation shutdown plus heat; VSDHRh, ventilation shutdown plus relative humidity; VSDCO2 ventilation shutdown plus carbon dioxide; CO2, carbon dioxide; mV, millivolts; EEG, electroencephalographic; Hz, hertz; TOD, time of death; HSP70, heat shock protein 70; Rh, relative humidity; min, minutes.
American Veterinary Medical Association (2019). AVMA guidelines for the depopulation of animals: 2019 edition. Available at: https://www.avma.org/resources-tools/avma-policies/avma-guidelines-depopulation-animals (Accessed June 17, 2024).
Barret N. W., Rowland K., Schmidt C. J., Lamont S. J., Rothschild M. F., Ashwell C. M., et al. (2019). Effects of acute and chronic heat stress on the performance, egg quality, body temperature, and blood gas parameters of laying hens. Poult. Sci. 98, 6684–6692. doi:10.3382/ps/pez541
Benson E. R., Aplhin R. L., Rankin M. K., Caputo M. P. (2012). Mass emergency water-based foam depopulation of poultry. BioOne Compl 56, 891–896. doi:10.1637/10160-040912-Reg.1
Bogin E., Weisman Y., Friedman Y. (1981). The effect of heat stress on the levels of certain blood constituents in chickens. Refuah Vet. 38, 98–104.
Department of Environment, Food, and Rural Affairs (DEFRA). (2009). Guidelines for killing poultry using ventilation shutdown (VSD).
Eberle-Krish K. N., Martin M. P., Malheiros R. D., Shah S. B., Livingston K. A., Anderson K. E. (2018). Evaluation of ventilation shutdown in a multi-level caged system. J. App. Poult. Res. 27, 555–563. doi:10.3382/japr/pfy036
Greene J. L. (2015). Congressional research service, update on the highly-pathogenic avian influenza outbreak of 2014-2015. Available at: http://sgp.fas.org/crs/misc/R44114 (Accessed June 17, 2024).
Hassan F., Nawaz A., Rehman M. S., Ali M. A., Dilshad S. M. R., Yang C. (2019). Prospects of HSP70 as a genetic marker for thermo-tolerance and immuno-modulation in animals under climate change scenario. Anim. Nutr. 5, 340–350. doi:10.1016/j.aninu.2019.06.005
Hurnik J. F., Webster A. B., Siegal P. B. (1995). Dictionary of farm animal behavior. Second Edition. Ames: Iowa State University Press.
Koelkebeck K. W., Odom T. W. (1994). Laying hen responses to acute heat stress and carbon dioxide supplementation: I. Blood gas changes and plasma lactate accumulation. Comp. Biochem. Physiol. 107A, 603–606. doi:10.1016/0300-9629(94)90358-1
Koelkebeck K. W., Odom T. W. (1995). Laying hen responses to acute heat stress and carbon dioxide supplementation: II. Changes in plasma enzymes, metabolites, and electrolytes. Comp. Biochem. Physiol. 112A, 119–122. doi:10.1016/0300-9629(95)00081-H
Li G. M., Liu L. P., Yin B., Liu Y. Y., Dong W. W., Gong S., et al. (2020). Heat stress decreases egg production of laying hens by inducing apoptosis of follicular cells via activating the FasL/fas and TNF-α systems. Poult. Sci. 99, 6084–6093. doi:10.1016/j.psj.2020.07.024
Mckeegan D. E. F., Sparks N. H. C., Sandilands V., Demmers T. G. M., Boulcott P., Wathers C. M. (2011). Physiological responses of laying hens during whole-house killing with carbon dioxide. Br. Poult. Sci. 52, 645–657. doi:10.1080/00071668.2011.640307
Mendoza A. V., Williams Z. (2024). Can steam be usable as a “plus” for ventilation shutdown? J. Appl. Poult. Res. 33, 100381. doi:10.1016/j.japr.2023.100381
United States Department of Agriculture (2024). Confirmations of highly pathogenic avian influenza in commercial and backyard flocks. Available at: https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/commercial-backyard-flocks (Accessed June 17, 2024).
United States Department of Agriculture Animal and Plant Health Inspection Service (2017). Foreign animal disease preparedness and response plan: highly pathogenic avian influenza response plan. Red Book. Available at: https://www.aphis.usda.gov/animal_health/emergency_management/downloads/hpai_response_plan.pdf (Accessed June 18, 2024).
Wang H., Kim M., Normoyle K. P., Llano D. (2016). Thermal regulation of the brain – an anatomical and physiological review for clinical neuroscientists. Front. Neurosci. 9, 528. doi:10.3389/fnins.2015.00528
Keywords: depopulation, physiology, relative humidity, highly pathogenic avian influenza, laying hen
Citation: Harding KL, Boot E, Evans JO, Shah SB, Malheiros RD and Anderson KE (2025) Determining how different ventilation shutdown plus methods change the electroencephalography, blood chemistry, corticosterone, and heat shock protein 70 of laying hens. Front. Physiol. 16:1534385. doi: 10.3389/fphys.2025.1534385
Received: 25 November 2024; Accepted: 18 February 2025;
Published: 21 March 2025.
Edited by:
Sandra G. Velleman, The Ohio State University, United StatesReviewed by:
Mahmoud Madkour, National Research Centre, EgyptCopyright © 2025 Harding, Boot, Evans, Shah, Malheiros and Anderson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenneth E. Anderson, a2FuZGVyc29AbmNzdS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.