- 1Henan University of Chinese Medicine, Zhengzhou, Henan, China
- 2Key Laboratory of Chinese Internal Medicine of Ministry of Education and Beijing, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 3The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, Henan, China
Objective: This study aims to systematically review the risk factors for major adverse cardiovascular events (MACE) in patients with coronary heart disease who have undergone percutaneous coronary intervention (PCI).
Design: Systematic review and meta-analysis.
Data sources: The Cochrane Library, PubMed, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang Database, and VIP Database for Chinese Technical Periodicals (VIP) were screened until December 2024.
Eligibility criteria for selecting studies: Case-control studies or cohort studies on the risk factors for MACE in patients with coronary heart disease who underwent PCI. Data extraction and synthesis: The literature review, data extraction, and quality evaluation were conducted by two independent researchers, and the meta-analysis was performed using RevMan 5.4 software.
Main outcomes: The main outcome was that MACE occurred during the follow-up period.
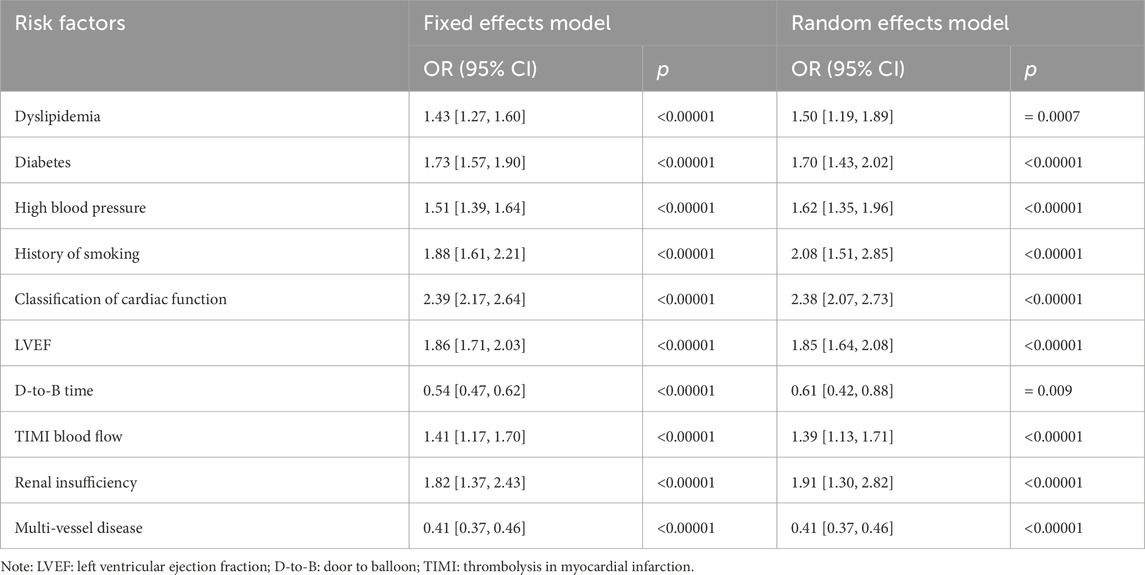
Results: A total of 40 articles were included. The meta-analysis erevealed that dyslipidemia (OR = 1.50; 95% CI [1.19, 1.89], p = 0.0007), diabetes mellitus (OR = 1.70; 95% CI [1.43, 2.02], p < 0.00001), hypertension (OR = 1.62; 95% CI [1.35, 1.96], p < 0.0001), history of smoking (OR = 2.08; 95% CI [1.51, 2.85], p < 0.0001), poorer ventricular function (OR = 2.39; 95% CI [2.17–2.64], p < 0.0001), impaired left ventricular ejection fraction (LVEF) (OR = 1.86; 95% CI [1.71–2.03], p < 0.0001), door to balloon (D-to-B) time (OR = 0.61; 95% CI [0.42–0.88]; p = 0.009), thrombolysis in myocardial infarction (TIMI) (OR = 1.41; 95% CI [1.17, 1.70], p = 0.0004), renal dysfunction (OR = 1.82; 95% CI [1.37, 2.43], p < 0.0001), and multi-vessel coronary artery disease (OR = 0.41; 95% CI [0.37, 0.46], p < 0.0001) were significantly associated with MACE after PCI.
Conclusion: The independent risk factors of MACE after PCI are dyslipidemia, hypertension, diabetes mellitus, smoking history, Killip class > II, LVEF ≤40%, D-to-B time >90 min, TIMI flow grade ≤ II, renal insufficiency, and multivessel disease.
1 Introduction
Coronary heart disease (CHD) is one of the leading causes of death in the global population (Ananth et al., 2023). Current therapies for CHD include traditional drug therapy, percutaneous coronary intervention (PCI), and coronary artery bypass graft surgery (Baydoun et al., 2019). PCI is currently recommended as the primary revascularization strategy for CHD patients. After PCI, the incidence of major adverse cardiovascular events (MACE) contributes significantly to morbidity and mortality rates (Holm et al., 2023; Liu et al., 2024). A number of studies have shown that within 30 days after PCI, the rehospitalization rates, and the disability rates were very high, which seriously affected the quality of life of patients (Iqbal et al., 2024; Qanitha et al., 2018).
Clinical studies have shown that multiple factors can affect the occurrence of PCI related MACE. The main factors include the following three aspects: 1) Factors related to patients, such as multi vessel disease, atherosclerosis of whole blood vessels, age over 65, low left ventricular ejection fraction (<40%), previous history of myocardial infarction, previous coronary artery bypass grafting, type 2 diabetes, hyperlipidemia, hypertension, chronic or end-stage renal disease and renal failure, anemia, preoperative troponin increase, unstable angina pectoris, and preoperative low-density lipoprotein cholesterol level increase (Madhavan et al., 2020); 2) Factors related to lesions, such as greater saphenous vein bridge vascular disease, eccentric lesions, large plaques and platelet thrombotic burden, plaque rupture; 3) Risk factors related to surgery, such as number of implanted stents, total length of stents, total time of balloon dilation, and total number of balloon dilations (Lin et al., 2023; Nasir et al., 2020; Wang et al., 2020b; Zhang et al., 2024).
Although PCI can effectively reduce the mortality rate, a variety of adverse cardiac events, such as acute heart failure and malignant arrhythmia, may occur after PCI, leading to a poorer long-term prognosis (Kim et al., 2018). The ultimate goal of percutaneous coronary intervention is not only to prolong the patient’s survival but also to improve the patient’s prognosis and quality of life (Head et al., 2018). Multiple clinical studies have demonstrated that MACE occurring within the first year following PCI significantly impact the long-term prognosis of CHD patients (Farshidi et al., 2018; Lu et al., 2023). Current evidence suggests that MACE incidence is associated not only with modifiable lifestyle factors including dietary habits and daily behaviors, but also with established cardiovascular risk factors (Kolkailah et al., 2018). Furthermore, accumulated clinical data indicate that persistent exposure to CHD risk factors continues to influence patient outcomes during the post-PCI recovery period (Khamis et al., 2012). These findings advocate for a paradigm shift toward personalized, multifactorial risk stratification and management in post-PCI care, particularly targeting modifiable factors such as glycemic control and smoking cessation. However, the evaluation indexes are complex, and the results are different. Their reliability and clinical significance need further study. By searching the published literature, this study aims to analyze and explore the risk factors for MACE in patients with CHD after PCI, so as to provide references for the prevention and treatment of MACE after PCI.
2 Materials and methods
2.1 Search strategy
The meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Page et al., 2021). The Cochrane Library, PubMed, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang Database, and VIP Database for Chinese Technical Periodicals (VIP) were searched from January 2000 to December 2024. The keywords were combined with free words and retrieved manually from foreign medical information resources such as Key Words Platform, the Chinese Journal of Cardiovascular Disease, and the Chinese Journal of Nursing. The Chinese keywords were “coronary angiography, coronary intervention, percutaneous coronary intervention, coronary intervention, and coronary heart disease intervention”. The English keywords were “acute coronary syndrome, coronary atherosclerotic heart disease, coronary heart disease, cardiovascular disease, percutaneous coronary intervention, percutaneous coronary revascularization, coronary angiography, coronary angiographies, percutaneous coronary intervention, percutaneous coronary revascularization,” “risk factors, factor, risk, population at risk, forecasting factor, relative risk, risks,” and “major adverse cardiovascular events, cardiac death, non-fatal myocardial infarction, target-vessel revascularization, stent thrombosis”. Specific search strategies such as PubMed database are as follows: (“Percutaneous Coronary Intervention” [MeSH] OR PCI OR “coronary angioplasty” OR “coronary angiographies” OR “percutaneous coronary intervention” OR “percutaneous coronary” OR “percutaneous coronary revascularization”) AND (“Major Adverse Cardiovascular Events” [MeSH] OR MACE OR “myocardial infarction” OR “cardiac death” OR “stent thrombosis”) AND (“Risk Factors” [MeSH] OR “predictors” OR “prognostic factors”) AND (“humans”{ [Filter]) AND (“2000/01/01” [Date - Publication]: “2023/6/30” [Date - Publication]) AND (English [Language] OR Chinese [Language]).
2.2 Inclusion and exclusion criteria of literature
Inclusion criteria were as follows: 1) Case-control studies (CCS) or cohort studies (CS); 2) Publicly published research in Chinese or English; 3) The research subjects were coronary heart disease patients who underwent PCI treatment; 4) The Newcastle Ottawa scale (NOS) scores were ≥7; and 5) The outcome indicators included MACE.
Exclusion criteria were as follows: 1) Repeated publications of literature; 2) Literature published in languages other than Chinese and English; 3) Research without full text, incomplete basic data, or inability to extract data; 4) Literature reviews; 5) The data of odds ratio (OR), 95% confidence interval (CI), and standard error (SE) were not provided, and the data provided could not be converted into OR value, 95% CI and SE; 6) The definition of risk factors not aligned with the European Society of Cardiology (ESC) or the American College of Cardiology (ACC) guidelines; and 7) Patients who had undergone coronary artery bypass grafting.
2.3 Literature screening and data extraction
The quality of the literature was evaluated independently by two researchers. Duplicate articles were eliminated, and the title and abstract were read individually according to the inclusion and exclusion criteria. The full texts of the articles were further read for screening. In case of any disagreement, a decision was made through a discussion or consultation with a third party. The data, including the first author, the year of publication, the place of study, the period of study, the type of study, the source of samples, the number of cases in the case group and the control group, and the related risk factors, were extracted. The main outcome was that MACE occurred during the follow-up period.
2.4 Methodological quality assessment
The qualities of the chosen literature were evaluated using the NOS. The total score of NOS for CCS or CS was 9 points, where more than 7 points were classified as high-quality literature.
2.5 Statistical methods
Review Manager software (version 5.4; the Cochrane Collaboration, Copenhagen, Denmark) was used for meta-analysis. Firstly, heterogeneity was analyzed. If p ≥ 0.1 or I2 ≤ 50%, indicating homogeneity between studies, a fixed-effects model was used for pooled analysis. If p < 0.1 or I2 ≥ 50%, indicating heterogeneity between studies, a random-effects model was used. Furthermore, sensitivity analysis was used to identify sources of heterogeneity. Subgroup analyses were performed when necessary. If there was homogeneity (p ≥ 0.1, I2 ≤ 50%) within and between subgroups, the fixed-effects model was used. The random-effects model was used if there was heterogeneity (p ≤ 0.1, I2 > 50%). The results with a p-value lower than 0.05 (p < 0.05) were considered statistically significant. Publication bias was assessed by funnel plot visual inspection. Sensitivity analysis was used to evaluate the stability of the results by changing the combined model.
3 Results
3.1 Results of literature search
A total of 10,887 articles were retrieved, including 1,675 from the Cochrane Library, 3,714 from PubMed, 1,528 from Web of Science, 472 from CNKI, 1,553 from Wanfang, and 1,963 from the VIP database. After removing duplicates and applying the inclusion and exclusion criteria, 40 studies were finally chosen, including 11 in Chinese and 29 in English (Figure 1) (Al-Fiadh et al., 2008; Barthelemy et al., 2015; Batchelor et al., 2020; Birkemeyer et al., 2014; Bufe et al., 2010; Chandrasekhar et al., 2017; Chen, 2016; Elkoustaf et al., 2006; Fan, 2015; Fath-Ordoubadi et al., 2012; Gan, 2017; Gao, 2017; Ghauharali-Imami et al., 2015; Gunnarsson et al., 2024; He, 2016; Hirakawa et al., 2006; Idris et al., 2017; Jackson et al., 2011; Jarrah et al., 2017; Kumbhani et al., 2012; Li et al., 2021; Liao et al., 2015; Lin and Lin, 2024; Ma, 2014; Meller et al., 2013; Motovska et al., 2008; Murphy et al., 2023; Numasawa et al., 2015; Otten et al., 2013; Pendyala et al., 2013; Pu et al., 2011; Toyota et al., 2013; Velders et al., 2013; Wang N. et al., 2020; Wijnbergen et al., 2013; Xu et al., 2022; Yao and Li, 2022; Zanchi et al., 2009; Zhang, 2023; Zimmermann et al., 2009).
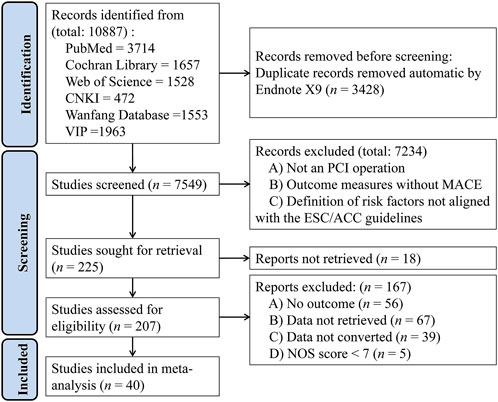
Figure 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flow diagram for studies included in and excluded from the meta-analysis.
The basic characteristics and quality evaluation results of the included literature are shown in Table 1. The total sample size was 117,127. Ten risk factors, each featured in more than three research articles, were included in the final study. All included articles were of high quality with an NOS score of ≥7 (Table 2).
3.2 Results of meta-analysis
3.2.1 Dyslipidemia
Fourteen studies reported the effect of dyslipidemia on the risk of developing MACE after PCI, and there was no statistical heterogeneity among the studies (p < 0.0001, I2 = 68%). Thus, a random-effects model was used for the meta-analysis. Patients with dyslipidemia exhibited a significantly elevated risk of post-PCI MACE (OR = 1.50; 95% CI [1.19–1.89]; p = 0.0007) (Figure 2A).
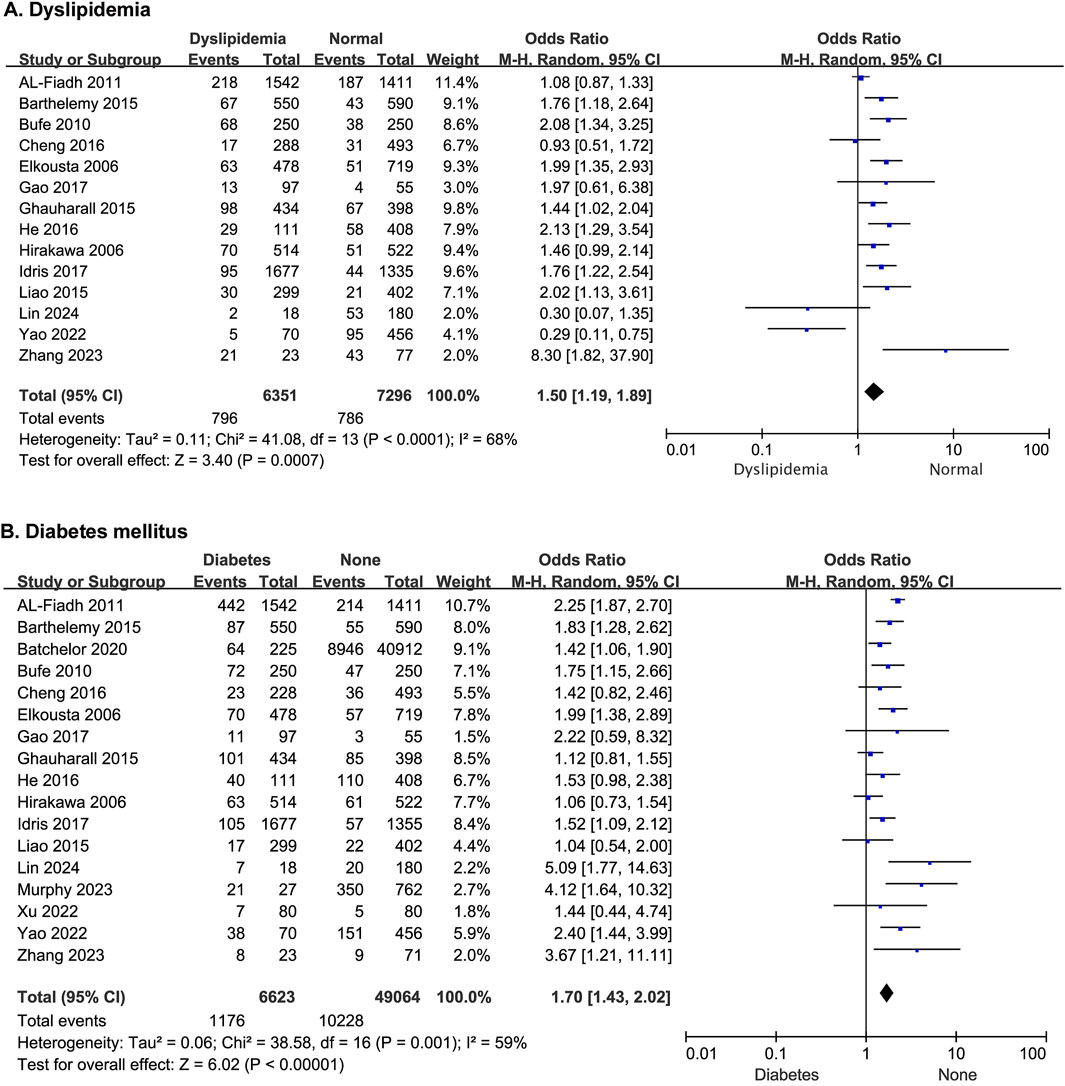
Figure 2. The forest plot depicting the effects of dyslipidemia and diabetes mellitus on MACE after PCI. (A) Dyslipidemia (B) Diabetes mellitus.
3.2.2 Diabetes mellitus
Seventeen studies reported the effect of diabetes mellitus on the risk of developing MACE after PCI, with statistical heterogeneity among the studies (p = 0.001, I2 = 59%), but the heterogeneity did not change significantly after a sensitivity analysis. A random-effects model was thus used for the meta-analysis. The results showed that patients with diabetes had a higher risk of MACE after PCI (OR = 1.70; 95% CI [1.43–2.02]; p < 0.00001) (Figure 2B).
3.2.3 Hypertension
Eighteen studies reported the effect of hypertension on the risk of developing MACE after PCI, with statistical heterogeneity among the studies (p < 0.00001, I2 = 72%), which did not change significantly after a sensitivity analysis. A random-effects model was therefore used for the meta-analysis. The results showed that patients with hypertension had a higher risk of MACE after PCI (OR = 1.62; 95% CI [1.35–1.96]; p < 0.00001) (Figure 3A).
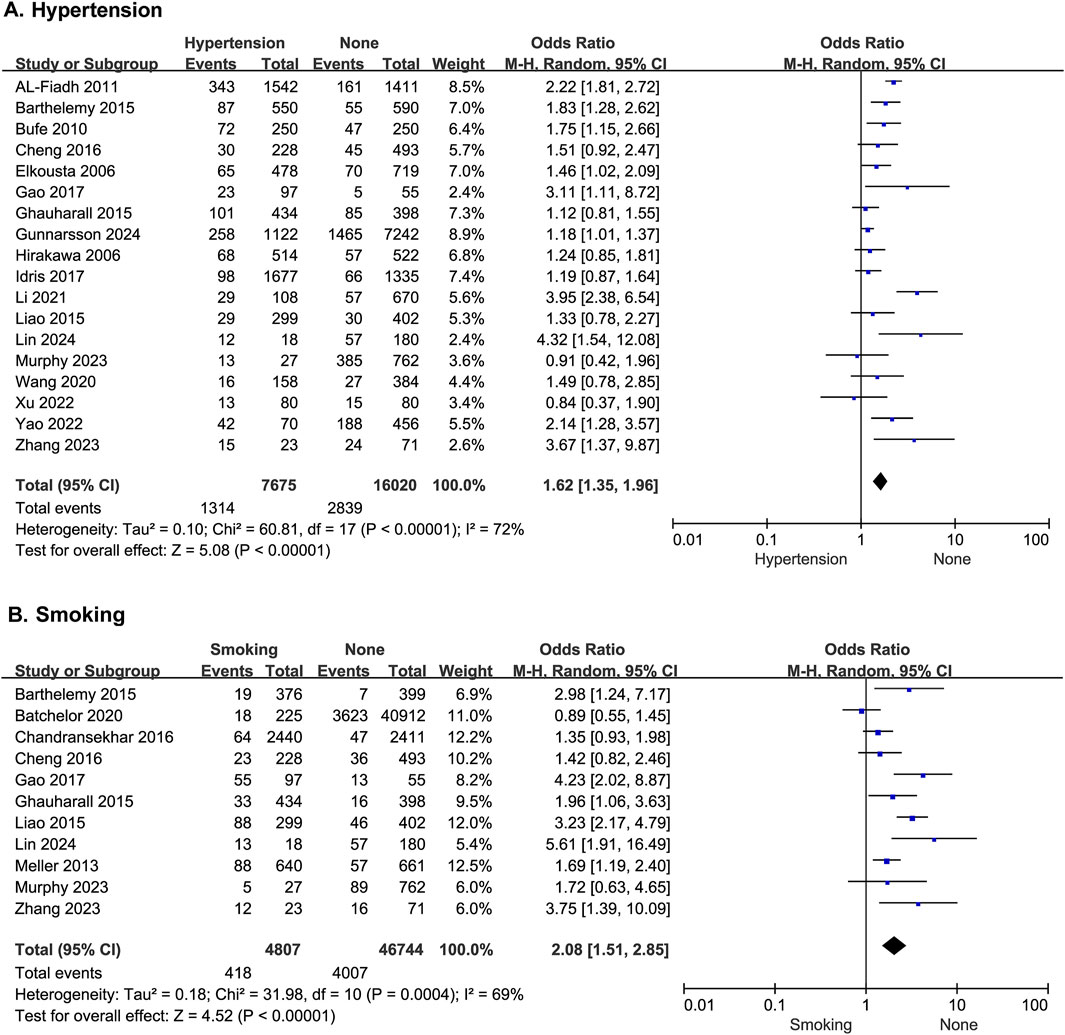
Figure 3. The forest plot depicting the effects of hypertension and history of smoking on MACE after PCI. (A) Hypertension (B) Smoking.
3.2.4 History of smoking
Eleven studies reported the effect of history of smoking on the risk of developing MACE after PCI, with statistical heterogeneity among the studies (p = 0.0004, I2 = 69%), but the heterogeneity did not change significantly after a sensitivity analysis. A random-effects model was therefore used for the meta-analysis. The results showed that patients with a history of smoking had a higher risk of MACE after PCI (OR = 2.08; 95% CI [1.51–2.85]; p < 0.0001) (Figure 3B).
3.2.5 Classification of heart function
Five studies reported the effect of heart function on the odds of developing MACE after PCI, with no statistical heterogeneity among the studies (p = 0.15, I2 = 41%). Thus, a fixed-effects model was used for the meta-analysis. The results showed that patients with Killip heart function > II had a higher risk of MACE than those with Killip heart function ≤ II (OR = 2.39; 95% CI [2.17–2.64]; p < 0.0001) (Figure 4A).
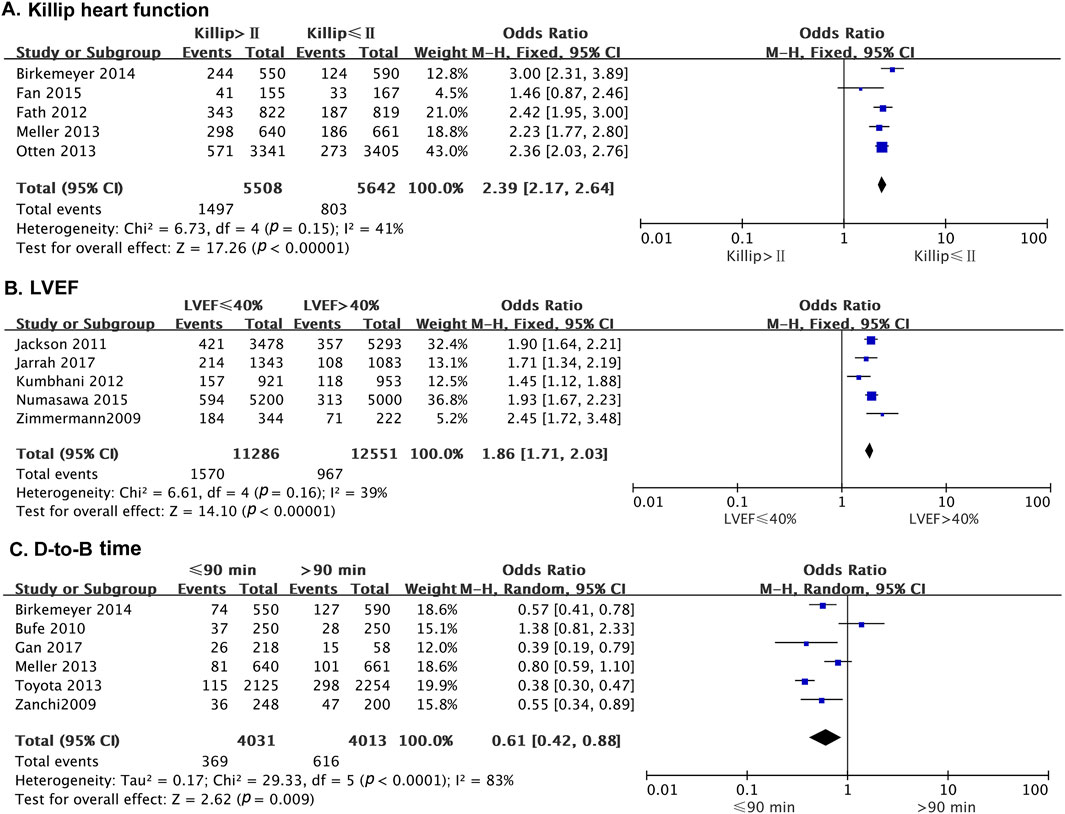
Figure 4. The forest plot depicting the effects of Killip’s cardiac function, LVEF, and D-to-B on MACE after PCI. (A) Killip heart function (B) LVEF (C) D-to-B time.
3.2.6 Left ventricular ejection fraction
Five studies reported the effect of left ventricular ejection fraction (LVEF) on the risk of MACE in patients after PCI. There was no statistical heterogeneity among the studies (p = 0.16, I2 = 39%). A fixed-effects model was thus used for the meta-analysis. The results indicated that the risk of MACE was significantly higher in patients with LVEF ≤40% than in those with LVEF >40% (OR = 1.86; 95% CI [1.71–2.03]; p < 0.0001) (Figure 4B).
3.2.7 Door to balloon time
Six studies reported the effect of door to balloon (D-to-B) time on the occurrence of MACE in patients after PCI. There was no statistical heterogeneity among the studies (p = 0.37, I2 = 7%). Therefore, a fixed-effect model was used for the meta-analysis. The results showed that patients with D-to-B time duration >90 min had a higher risk of MACE than those with D-to-B time duration ≤90 min (OR = 0.61; 95% CI [0.42–0.88]; p = 0.009) (Figure 4C).
3.2.8 Thrombolysis in myocardial infarction blood flow
Five studies reported the risk of MACE in patients after PCI with subsequent slow flow and no-reflow. There was no statistical heterogeneity among the studies (p = 0.37, I2 = 7%). A fixed-effect model was therefore used for the meta-analysis. The results showed that thrombolysis in myocardial infarction (TIMI) blood flow ≤ II was significantly higher than TIMI flow > II (OR = 1.41; 95% CI [1.17–1.70]; p = 0.0004) (Figure 5A).
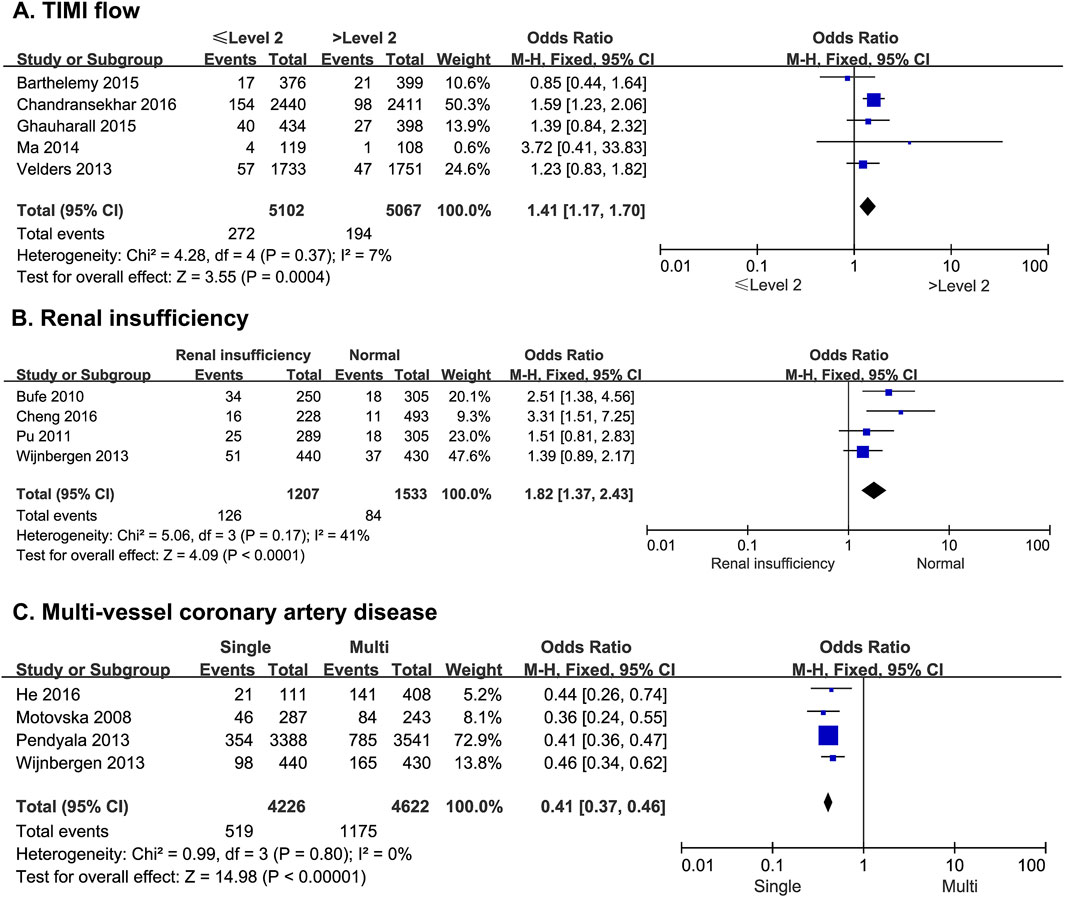
Figure 5. The forest plot depicting the effects of TIMI flow, renal insufficiency, and multi-vessel coronary artery disease on MACE after PCI. (A) TIMI flow (B) renal insufficiency (C) multi-vessel coronary artery disease.
3.2.9 Renal insufficiency
Four studies reported the risk of MACE due to renal insufficiency in patients undergoing PCI. There was no statistical heterogeneity among the studies (p = 0.17, I2 = 41%). A fixed-effect model was thus used for the meta-analysis. The results showed that the risk of MACE was significantly higher in patients with renal dysfunction than in patients with normal renal function (OR = 1.82; 95% CI [1.37–2.43]; p < 0.0001) (Figure 5B).
3.2.10 Multi-vessel coronary artery disease
Four studies reported the risk of MACE in patients with single-vessel or multi-vessel coronary artery disease after PCI. There was no statistical heterogeneity among the studies (p = 0.80, I2 = 0%). Thus, a fixed-effect model was used for the meta-analysis. The results showed that the risk of MACE was significantly higher in patients with multi-vessel disease than in those with single-vessel disease (OR = 0.41; 95% CI [0.37–0.46]; p < 0.0001) (Figure 5C).
3.3 Publication bias
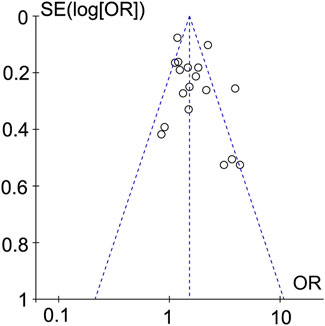
Studies that set dyslipidemia as a risk factor were plotted in a funnel plot and subjected to a publication bias test. The results did not show significant asymmetry, suggesting that there was less likelihood of publication bias (Figure 6).
3.4 Sensitivity analysis
The results showed that there was no significant difference between the two models, suggesting that the results of this study are stable and reliable (Table 3).
4 Discussion
This meta-analysis of 40 high-quality studies (NOS ≥7) identified dyslipidemia, hypertension, diabetes mellitus, smoking history, Killip class > II, LVEF ≤40%, D-to-B time >90 min, TIMI flow grade ≤ II, renal insufficiency, and multi-vessel disease as independent predictors of post-PCI MACE. The homogeneity of baseline characteristics across studies minimized confounding bias and enhanced the validity of the results.
Metabolic syndromes, characterized by central obesity, hypertension, and dyslipidemia, constitute a cluster of traditional risk factors that synergistically exacerbate coronary artery disease progression. Among them, impaired fasting glucose and hypertension were verified to be associated with a higher risk of MACE after PCI in patients with acute coronary syndrome (Hosseini et al., 2024). Previous studies have demonstrated that the high triglyceride-glucose (TyG) index was associated with an elevated risk of MACE in patients with acute or ST-elevation myocardial infarction undergoing PCI (Köktürk et al., 2024; Luo et al., 2019).
In this study, we also found that dyslipidemia is an independent risk factor for PCI prognosis. Dyslipidemia is characterized by increased levels of triglycerides or low-density lipoprotein cholesterol (LDL-C) and decreased levels of high-density lipoprotein cholesterol (HDL-C), is closely linked to atherogenesis. The LDL-C/HDL-C ratio serves as a reliable marker for assessing coronary artery disease severity in acute coronary syndrome (ACS) patients (Yuan et al., 2024). Elevated LDL-C/HDL-C ratios correlate with higher MACE risk in CHD patients after PCI (Ren and Wang, 2023). The atherogenic index of plasma (AIP), calculated as log (TG/HDL-C), reflects the atherogenic potential of lipoprotein profiles and has been independently associated with MACE (Frohlich and Dobiásová, 2003; Özen et al., 2023). A meta-analysis of 1,055,309 patients confirmed that elevated serum total cholesterol and LDL-C levels increase cardiovascular mortality, whereas higher HDL-C levels exert a protective effect (Jung et al., 2022). Emerging evidence underscores the prognostic value of the ApoB/ApoA-I ratio, which independently predicts 1-year MACE in post-PCI cohorts, potentially reflecting atherogenic lipoprotein imbalances (Zhang et al., 2025). Mechanistically, dyslipidemia drives plaque instability via oxidative modification of small dense LDL particles, which activate NF-κB-mediated endothelial inflammation and foam cell formation (Hasheminasabgorji and Jha, 2021; Higashi, 2023).
Diabetes mellitus is a well-established independent risk factor for MACE following PCI (Latif et al., 2022). Admission glycosylated hemoglobin (HbA1c) levels significantly predict MACE occurrence in diabetic patients undergoing PCI (Bagheri et al., 2024). A large single-center study of 10,724 PCI patients reported significantly higher MACE rates in diabetic compared to non-diabetic individuals (Wang et al., 2018). After PCI of chronic total occlusions of coronary arteries, significant endothelial and smooth muscle dysfunction were present in the distal segments of the successfully recanalized chronic total coronary occlusions (Brugaletta et al., 2012). Higher stress hyperglycemia, a transient elevation of blood glucose, was also reported as a risk factor of MACE (Huang et al., 2022). Diabetes mellitus conferred a 1.7-fold MACE risk (OR = 1.70), primarily attributable to endothelial injury from sustained hyperglycemia (Kaur et al., 2018; Li et al., 2024). Mechanistically, mitochondrial ROS overproduction via NOX4 activation and advanced glycation end product (AGE) accumulation impair nitric oxide bioavailability, while elevated IL-6/TNF-α levels exacerbate vascular inflammation (Knapp et al., 2019; Mordi et al., 2022). These pathways are corroborated by HbA1c’s prognostic value in predicting post-PCI outcomes (Bagheri et al., 2024).
Hypertension significantly contributes to long-term MACE risk post-PCI (OR = 1.62), though its association with in-hospital mortality remains debated (Qi et al., 2023; Saluveer et al., 2017). Prolonged hypertension may promote vascular fibrosis via angiotensin II/angiotensin II type I receptor (Ang II/AT1R) pathway, leading to vascular fibrosis, luminal stenosis, and ventricular remodeling. Long-term elevated blood pressure leads to abnormal vascular wall shear stress, activates NF-κ B pathway, and promotes the release of inflammatory factors (such as IL-6 and TNF- α) (Cao et al., 2022; Elmarasi et al., 2024). The acute blood pressure control during PCI could mitigate immediate risks, explaining the null in-hospital mortality difference observed in some cohorts (Qi et al., 2023).
Smoking history doubled MACE risk (OR = 2.08) in this analysis, consistent with its well-documented cardiovascular toxicity. However, a study revealed that the risk of heart disease is essentially the same as that of non-smokers, after 15 years of smoking cessation (Ahmed et al., 2015). Other paradoxical studies report lower mortality in active smokers post-PCI—a phenomenon attributed to younger smoker demographics or attenuated inflammatory responses (Kumar et al., 2023). Nevertheless, smoking cessation remains critical, as its net cardiovascular harm outweighs transient protective effects.
The Killip classification of cardiac function in patients with acute myocardial infarction (AMI) is considered to be an important index of risk stratification in patients with AMI. Those whose cardiac function is above Killip II grade are considered high-risk. Killip classification ≥ III was an independent predictor of new-onset atrial fibrillation (Shiba et al., 2019). Killip class > I was an independent predictor of MACE in STEMI patients after PCI (Luo et al., 2019). In the present study, we showed that Killip class > II was also an independent risk factor of MACE after PCI. Studies have found that the increased BNP level in Killip II patients reflects the increased ventricular wall tension, activates the cGMP PKG pathway, leads to Ca2+ overload of cardiomyocytes and the conversion of energy metabolism from fatty acid oxidation to glycolysis, and exacerbates myocardial stunning (Teragawa et al., 2024).
Consistent with previous reports (Huang et al., 2023b), LVEF was established as an independent risk factor of MACE after PCI in this study. LVEF is an important index for evaluating cardiac ejection function, influencing prognosis and cardiac function. Studies have shown that patients with LVEF <50% have an increased risk of ST (Hu, 2019). Patients with LVEF <50% were independent predictors of 30-day and longer-term mortality for PCI (Mamas et al., 2014). A lower LVEF indicates decreased cardiac output and coronary flow velocity and increased platelet-to-collagen contact, thus increasing the rate of thrombosis.
Prolonged D-to-B time (>90 min) exacerbates ischemic burden by promoting thrombus propagation and microvascular obstruction, thereby attenuating the benefits of timely reperfusion. Thus, the prognosis for patients is often poor (Tran et al., 2017). Shortening the D-to-B time is the key factor in reducing adverse reactions after PCI. After myocardial ischemia for more than 90 min, ATP depletion leads to dysfunction of Na+/K+ pump, triggering intracellular Na+ overload and reverse Ca2+ influx, activating mitochondrial permeability transition pore opening, and inducing cardiomyocyte apoptosis (Mastoor et al., 2025).
The TIMI flow grade is the main indicator of myocardial blood perfusion and velocity. A lower TIMI grade is associated with slower blood flow velocity and poorer myocardial perfusion. Tissue microcirculation disturbance is prevalent in patients with low TIMI preoperatively. Even after PCI thrombectomy and vascular dilation, the cardiac blood flow is difficult to recover, which may have adverse effects on the cardiac supply of blood. TIMI ≤ grade 2 indicates coronary microvascular embolism (CME), which is associated with increased platelet neutrophil complex formation and vWF multimer release. The glycocalyx on the surface of endothelial cells in CME area was destroyed, resulting in no reflow phenomenon and expanding the infarct size (Arce et al., 2021). Moreover, elevated levels of hemoglobin and decreased levels of mean platelet volume had a significant association with an advanced grade of TIMI flow in patients who underwent PCI (Parsa et al., 2022). TIMI flow grade I–III is associated with better in-hospital and 1-year outcomes, specifically significantly lower cardiovascular mortality compared to patients who had TIMI flow grade 0 at initial angiography (Shaaban et al., 2022).
Many studies have shown that chronic kidney disease is not only an independent risk factor for cardiovascular morbidity but also significantly influences the prognosis of CHD (Limpijankit et al., 2022; Okina et al., 2021; Polanska-Skrzypczyk et al., 2013). Worsening renal function is an important predictor of mortality in patients with acute myocardial infarction undergoing primary PCI. With the deterioration of the estimated glomerular filtration rate, the short-term and long-term prognosis of patients decreased significantly (Limpijankit et al., 2022; Okina et al., 2021; Polanska-Skrzypczyk et al., 2013). Declining renal function activates NLRP3 inflammasome and promotes IL-18 secretion (Huang et al., 2023a; Thomas et al., 2022). Meanwhile, uremic toxins (such as indole sulfate) induced vascular smooth muscle cell dysfunction and accelerate atherosclerosis (Karbowska et al., 2017; Yu et al., 2022).
Multi-vessel coronary artery disease mainly refers to diffuse lesions involving more than two vessels, which easily cause diffuse myocardial injury, and has become the focus and difficulty in the treatment of CHD. Multi-vessel disease is frequently encountered in primary PCI for myocardial infarction (Akbari and Al-Lamee, 2022; Cho and Nam, 2018). It is still controversial whether complete revascularization is necessary for patients with myocardial infarction and what strategy should be used for the same. Current guidelines for high myocardial infarction recommend that only the infarct-related vessel be addressed during primary PCI unless shock is concurrent (Akbari and Al-Lamee, 2022; Nozoe et al., 2014). Among patients undergoing primary PCI, multi-vessel disease directly indicates a significant increase in postoperative complications, mortality, morbidity, and length of hospitalization (Batra, et al., 2018; Toma et al., 2017). Contrary to expectations, multi-vessel disease was inversely associated with MACE in this study. This paradoxical finding may stem from selection bias, as patients with multi-vessel involvement often receive more intensive surveillance and adjunctive therapies.
This study expands traditional risk stratification models by incorporating dynamic variables such as D-to-B time and TIMI flow, which are rarely included in prior frameworks. Methodological rigor—including dual-blind screening and exclusion of low-quality studies (NOS <6)—enhanced result reliability compared to earlier meta-analyses. The limitations of this study were as follows: 1) The inclusion of only Chinese and English studies may introduce geographic and publication bias, limiting the generalizability of findings to other populations. 2) The number of studies on some risk factors was insufficient to be included, resulting in a lack of data on them in the included studies. 3) The original study included case-control and cohort studies, which lacked high-quality prospective studies and had low demonstration strength.
Based on the above findings, this study proposes the following clinical practice enlightenment to optimize the postoperative management of PCI: firstly, for high-risk patients (such as diabetes mellitus, D-to-B time ≥90 min or multi vessel lesions), it is recommended to dynamically evaluate plaque stability and vascular remodeling through inflammatory markers and coronary imaging 3–6 months after PCI, so as to early identify the risk of restenosis or microcirculation disorders. Secondly, antithrombotic therapy needs to be individualized. Referring to the dual antiplatelet therapy (DAPT) evidence in 2022, the course of treatment for patients with high bleeding risk can be shortened to 6 months, or replaced with ticagrelor monotherapy, so as to balance the risk of ischemia and bleeding. In addition, comprehensive intervention should focus on multiple risk factors: strict control of LDL-C, optimization of blood glucose management, and strengthening smoking cessation support. In the future, a dynamic risk assessment system can be built by combining new markers and artificial intelligence models to promote precise hierarchical management.
5 Conclusion
The independent risk factors of MACE after PCI are dyslipidemia, history of hypertension, history of diabetes, history of smoking, Killip class > II, LVEF ≤40%, D-to-B time >90 min, TIMI blood flow ≤ II, renal insufficiency, and multi-vessel disease. During the period of clinical treatment, we should strengthen the control of various risk factors, reduce their influence, and achieve effective control through regular follow-up after discharge. This will significantly improve the prognosis of patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YZ: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review and editing. HS: Supervision, Writing – review and editing. YL: Data curation, Writing – original draft, Writing – review and editing. NZ: Methodology, Writing – original draft. JZ: Methodology, Writing – original draft. SW: Visualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the National Natural Science Foundation of China (No. 82205081), the 16th batch of Special Support of China Postdoctoral Science Foundation (In Station) (No. 2023T160069), the Fundamental Research Funds for the Central Universities (No. 2024-JYB-KYPT-01), and the 2024 Henan Provincial Higher Education Institutions Young Backbone Teachers Training Program (No. 2024GGJS071).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed A. A., Patel K., Nyaku M. A., Kheirbek R. E., Bittner V., Fonarow G. C., et al. (2015). Risk of heart failure and death after prolonged smoking cessation: role of amount and duration of prior smoking. Circ. Heart Fail. 8 (4), 694–701. doi:10.1161/circheartfailure.114.001885
Akbari T., Al-Lamee R. (2022). Percutaneous coronary intervention in multi-vessel disease. Cardiovasc Revasc Med. 44, 80–91. doi:10.1016/j.carrev.2022.06.254
Al-Fiadh A., Proimos G., Andrianopoulos N., Duffy S., Farouque O., Tongyoo S., et al. (2008). Contemporary outcomes in female patients undergoing percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS). Heart Lung Circ. 17 (Suppl. S3), S162. doi:10.1016/j.hlc.2008.05.386
Ananth C. V., Rutherford C., Rosenfeld E. B., Brandt J. S., Graham H., Kostis W. J., et al. (2023). Epidemiologic trends and risk factors associated with the decline in mortality from coronary heart disease in the United States, 1990-2019. Am. Heart J. 263, 46–55. doi:10.1016/j.ahj.2023.05.006
Arce N. A., Cao W., Brown A. K., Legan E. R., Wilson M. S., Xu E. R., et al. (2021). Activation of von Willebrand factor via mechanical unfolding of its discontinuous autoinhibitory module. Nat. Commun. 12 (1), 2360. doi:10.1038/s41467-021-22634-x
Bagheri B., Jalalian R., Mousavi F. S., Azizi S., Alipour A., Mousavi F., et al. (2024). The role of hemoglobin A1c as a predictor of major adverse cardiovascular events in patients with type 2 diabetes mellitus after percutaneous coronary intervention: a case-cohort study. BMC Cardiovasc Disord. 24 (1), 583. doi:10.1186/s12872-024-04267-2
Barthelemy O., Degrell P., Berman E., Kerneis M., Petroni T., Silvain J., et al. (2015). Sex-related differences after contemporary primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Arch. Cardiovasc Dis. 108 (8-9), 428–436. doi:10.1016/j.acvd.2015.03.002
Batchelor R., Dinh D., Brennan A., Lefkovits J., Reid C., Duffy S. J., et al. (2020). Incidence, predictors and clinical outcomes of stent thrombosis following percutaneous coronary intervention in contemporary practice. Heart Lung Circ. 29 (10), 1433–1439. doi:10.1016/j.hlc.2019.10.009
Batra M. K., Rasool S. I., Solangi B. A., Khan N., Karim M., Hassan Rizvi S. N. (2018). Multivessel disease as A prognostic marker in patients presenting for primary percutaneous coronary intervention. J. Ayub Med. Coll. Abbottabad 30 (4), 534–538.
Baydoun H., Jabbar A., Nakhle A., Irimpen A., Patel T., Ward C. (2019). Revascularization of left main coronary artery. Cardiovasc Revasc Med. 20 (11), 1014–1019. doi:10.1016/j.carrev.2018.11.001
Birkemeyer R., Schneider H., Rillig A., Ebeling J., Akin I., Kische S., et al. (2014). Do gender differences in primary PCI mortality represent a different adherence to guideline recommended therapy? a multicenter observation. BMC Cardiovasc Disord. 14, 71. doi:10.1186/1471-2261-14-71
Brugaletta S., Martin-Yuste V., Padro T., Alvarez-Contreras L., Gomez-Lara J., Garcia-Garcia H. M., et al. (2012). Endothelial and smooth muscle cells dysfunction distal to recanalized chronic total coronary occlusions and the relationship with the collateral connection grade. JACC Cardiovasc Interv. 5 (2), 170–178. doi:10.1016/j.jcin.2011.10.012
Bufe A., Wolfertz J., Dinh W., Bansemir L., Koehler T., Haltern G., et al. (2010). Gender-based differences in long-term outcome after ST-elevation myocardial infarction in patients treated with percutaneous coronary intervention. J. Womens Health (Larchmt) 19 (3), 471–475. doi:10.1089/jwh.2009.1371
Cao G., Xuan X., Hu J., Zhang R., Jin H., Dong H. (2022). How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun. Signal. CCS 20 (1), 180. doi:10.1186/s12964-022-00993-2
Chandrasekhar J., Baber U., Sartori S., Faggioni M., Aquino M., Kini A., et al. (2017). Sex-related differences in outcomes among men and women under 55 years of age with acute coronary syndrome undergoing percutaneous coronary intervention: results from the PROMETHEUS study. Catheter Cardiovasc Interv. 89 (4), 629–637. doi:10.1002/ccd.26606
Chen P. (2016). Clinical characteristics and in-hospital prognosis of patients with mild to moderate chronic renal insufficiency STEMI. Jilin: Jilin University.
Cho Y. K., Nam C. W. (2018). Percutaneous coronary intervention in patients with multi-vessel coronary artery disease: a focus on physiology. Korean J. Intern Med. 33 (5), 851–859. doi:10.3904/kjim.2018.006
Elkoustaf R. A., Mamkin I., Mather J. F., Murphy D., Hirst J. A., Kiernan F. J., et al. (2006). Comparison of results of percutaneous coronary intervention for non–ST-elevation acute myocardial infarction or unstable angina pectoris in men versus women. Am. J. Cardiol. 98 (2), 182–186. doi:10.1016/j.amjcard.2006.01.071
Elmarasi M., Elmakaty I., Elsayed B., Elsayed A., Zein J. A., Boudaka A., et al. (2024). Phenotypic switching of vascular smooth muscle cells in atherosclerosis, hypertension, and aortic dissection. J. Cell. physiology 239 (4), e31200. doi:10.1002/jcp.31200
Fan X. (2015). Investigation and analysis of disease factors and health management strategies in patients with coronary heart disease undergoing PCI. Xi’an: Fourth Military Medical University of PLA.
Farshidi H., Abdi A., Madani A., Moshiri S., Ghasemi A., Hakimian R. (2018). Major adverse cardiovascular event (MACE) after percutaneous coronary intervention in one-year follow-up study. Electron. physician 10 (2), 6383–6389. doi:10.19082/6383
Fath-Ordoubadi F., Barac Y., Abergel E., Danzi G. B., Kerner A., Nikolsky E., et al. (2012). Gender impact on prognosis of acute coronary syndrome patients treated with drug-eluting stents. Am. J. Cardiol. 110 (5), 636–642. doi:10.1016/j.amjcard.2012.04.039
Frohlich J., Dobiásová M. (2003). Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin. Chem. 49 (11), 1873–1880. doi:10.1373/clinchem.2003.022558
Gan K. (2017). Analysis of risk factors and prognosis in patients with PCI-related myocardial infarction. Nanning: Guangxi Medical University.
Gao Y. (2017). Study on the change of quality of life and its influencing factors in patients with coronary heart disease at 1 year after PCI. Dalian: Dalian Medical University.
Ghauharali-Imami S., Bax M., Haasdijk A., Schotborgh C., Oemrawsingh P., Bech J., et al. (2015). The impact of gender on long-term mortality in patients with multivessel disease after primary percutaneous coronary intervention. Neth Heart J. 23 (12), 592–599. doi:10.1007/s12471-015-0754-x
Gunnarsson O. S., Pihlsgård M., Handmark M., Sarno G., Gonçalves I., Timpka S. (2024). Major adverse cardiovascular events following coronary artery stenting by history of hypertensive disorder of pregnancy. J. Am. Heart Assoc. 13 (20), e035448. doi:10.1161/jaha.124.035448
Hasheminasabgorji E., Jha J. C. (2021). Dyslipidemia, diabetes and atherosclerosis: role of inflammation and ROS-redox-sensitive factors. Biomedicines 9 (11), 1602. doi:10.3390/biomedicines9111602
He L. (2016). Clinical study on the predictive factors of complications of emergency PCI bleeding and its influence on prognosis. Suzhou: Suzhou University.
Head S. J., Milojevic M., Daemen J., Ahn J. M., Boersma E., Christiansen E. H., et al. (2018). Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet 391 (10124), 939–948. doi:10.1016/S0140-6736(18)30423-9
Higashi Y. (2023). Endothelial function in dyslipidemia: roles of LDL-cholesterol, HDL-cholesterol and triglycerides. Cells 12 (9), 1293. doi:10.3390/cells12091293
Hirakawa Y., Masuda Y., Uemura K., Kuzuya M., Kimata T., Iguchi A. (2006). Differences in in-hospital mortality between men and women with acute myocardial infarction undergoing percutaneous coronary intervention in Japan: tokai Acute Myocardial Infarction Study (TAMIS). Am. Heart J. 151 (6), 1271–1275. doi:10.1016/j.ahj.2005.07.007
Holm N. R., Andreasen L. N., Neghabat O., Laanmets P., Kumsars I., Bennett J., et al. (2023). OCT or angiography guidance for PCI in complex bifurcation lesions. N. Engl. J. Med. 389 (16), 1477–1487. doi:10.1056/NEJMoa2307770
Hosseini K., Khalaji A., Behnoush A. H., Soleimani H., Mehrban S., Amirsardari Z., et al. (2024). The association between metabolic syndrome and major adverse cardiac and cerebrovascular events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Sci. Rep. 14 (1), 697. doi:10.1038/s41598-024-51157-w
Hu Z. (2019). The effect of nicorandil combined with Rosuvastatin on LVEF and serum CK-MB levels in patients with coronary heart disease after PCI. J. Med. Forum 40 (3), 152–153.
Huang G., Zhang Y., Zhang Y., Ma Y. (2023a). Chronic kidney disease and NLRP3 inflammasome: pathogenesis, development and targeted therapeutic strategies. Biochem. biophysics Rep. 33, 101417. doi:10.1016/j.bbrep.2022.101417
Huang L., Zhang J., Huang Q., Cui R., Chen J. (2023b). In-hospital major adverse cardiovascular events after primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction: a retrospective study under the China chest pain center (standard center) treatment system. BMC Cardiovasc Disord. 23 (1), 198. doi:10.1186/s12872-023-03214-x
Huang Y. W., An Y. H., Yin X. S., Li Z. P. (2022). Association of the stress hyperglycemia ratio and clinical outcomes in patients with cardiovascular diseases: a systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 26 (24), 9258–9269. doi:10.26355/eurrev_202212_30679
Idris H., French J. K., Shugman I. M., Hopkins A. P., Juergens C. P., Thomas L. (2017). Influence of age and gender on clinical outcomes following percutaneous coronary intervention for acute coronary syndromes. Heart, Lung Circulation 26 (6), 554–565. doi:10.1016/j.hlc.2016.10.021
Iqbal Z., Mengal M. N., Ashraf T., Salongi B. A., Kumar R., Bhatti K. I., et al. (2024). Assessment of coronary collaterals among patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention and its impact on in-hospital and 30-day mortality: a prospective observational study. J. Saudi Heart Assoc. 36 (4), 352–359. doi:10.37616/2212-5043.1403
Jackson E. A., Moscucci M., Smith D. E., Share D., Dixon S., Greenbaum A., et al. (2011). The association of sex with outcomes among patients undergoing primary percutaneous coronary intervention for ST elevation myocardial infarction in the contemporary era: insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). Am. Heart J. 161 (1), 106–112. doi:10.1016/j.ahj.2010.09.030
Jarrah M. I., Hammoudeh A. J., Al-Natour D. B., Khader Y. S., Tabbalat R. A., Alhaddad I. A., et al. (2017). Gender differences in risk profile and outcome of Middle Eastern patients undergoing percutaneous coronary intervention. Saudi Med. J. 38 (2), 149–155. doi:10.15537/smj.2017.2.16301
Jung E., Kong S. Y., Ro Y. S., Ryu H. H., Shin S. D. (2022). Serum cholesterol levels and risk of cardiovascular death: a systematic review and a dose-response meta-analysis of prospective cohort studies. Int. J. Environ. Res. public health 19 (14), 8272. doi:10.3390/ijerph19148272
Karbowska M., Kaminski T. W., Marcinczyk N., Misztal T., Rusak T., Smyk L., et al. (2017). The uremic toxin indoxyl sulfate accelerates thrombotic response after vascular injury in animal models. Toxins 9 (7), 229. doi:10.3390/toxins9070229
Kaur R., Kaur M., Singh J. (2018). Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 17 (1), 121. doi:10.1186/s12933-018-0763-3
Khamis S. S., Ashraf D. G., Ahmad H. A., El-Saeed G. K., Essa E. S. (2012). Study of urine neutrophil gelatinase associated lipocalin (NGAL) in post percutaneous coronary intervention contrast induced acute kidney injury. J. Am. Sci. 8 (6), 615–623.
Kim Y., Jeong M. H., Ahn Y., Kim J. H., Hong Y. J., Sim D. S., et al. (2018). Results of a 10-year experience in korea using drug-eluting stents during percutaneous coronary intervention for acute myocardial infarction (from the korea acute myocardial infarction registry). Am. J. Cardiol. 122 (3), 365–373. doi:10.1016/j.amjcard.2018.04.026
Knapp M., Tu X., Wu R. (2019). Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharmacol. Sin. 40 (1), 1–8. doi:10.1038/s41401-018-0042-6
Köktürk U., Onalan O., Somuncu M. U., Uygur B., Erbay İ., Çakan F., et al. (2024). The prognostic value of the triglyceride-glucose index in forecasting ten-year major adverse cardiovascular events in non-diabetic patients with acute myocardial infarction undergoing percutaneous coronary intervention. Turk Kardiyol. Dernegi arsivi Turk Kardiyol. Derneginin yayin organidir 52 (4), 253–259. doi:10.5543/tkda.2023.58554
Kolkailah A. A., Alreshq R. S., Muhammed A. M., Zahran M. E., Anas El-Wegoud M., Nabhan A. F. (2018). Transradial versus transfemoral approach for diagnostic coronary angiography and percutaneous coronary intervention in people with coronary artery disease. Cochrane Database Syst. Rev. 4 (4), CD012318. doi:10.1002/14651858.CD012318.pub2
Kumar R., Shaikh A. H., Ahmed R., Siddiqui M. N., Rahooja K., Chachar K., et al. (2023). Unfolding the reality of the smoking paradox in a South Asian cohort of patients presenting with ST-elevation acute coronary syndrome undergoing primary percutaneous coronary intervention. SAGE Open Med. 11, 20503121231206932. doi:10.1177/20503121231206932
Kumbhani D. J., Shishehbor M. H., Willis J. M., Karim S., Singh D., Bavry A. A., et al. (2012). Influence of gender on long-term mortality in patients presenting with non-ST-elevation acute coronary syndromes undergoing percutaneous coronary intervention. Am. J. Cardiol. 109 (8), 1087–1091. doi:10.1016/j.amjcard.2011.11.044
Latif A., Ahsan M. J., Kabach A., Kapoor V., Mirza M., Ahsan M. Z., et al. (2022). Impact of diabetes mellitus on outcomes of percutaneous coronary intervention in chronic total occlusions: a systematic review and meta-analysis. Cardiovasc Revasc Med. 37, 68–75. doi:10.1016/j.carrev.2021.06.017
Li L., Wang W., Li T., Sun Y., Gao Y., Wang L., et al. (2021). Gender-related difference in D-dimer level predicts in-hospital heart failure after primary PCI for ST-segment elevation myocardial infarction. Dis. Markers 2021, 7641138. doi:10.1155/2021/7641138
Li X., Zou J., Lin A., Chi J., Hao H., Chen H., et al. (2024). Oxidative stress, endothelial dysfunction, and N-acetylcysteine in type 2 diabetes mellitus. Antioxidants & redox Signal. 40 (16-18), 968–989. doi:10.1089/ars.2023.0524
Liao X., Wang R., You T. (2015). Analysis of nosocomial influence factors of patients with acute ST-segment elevation myocardial infarction after percutaneous coronary intervention. J. Qiqihar Univ. Med. 36 (14), 2087–2089.
Limpijankit T., Chandavimol M., Srimahachota S., Kanoksilp A., Jianmongkol P., Siriyotha S., et al. (2022). Dose-dependent effect of impaired renal function on all-cause mortality in patients following percutaneous coronary intervention. Clin. Cardiol. 45 (8), 882–891. doi:10.1002/clc.23877
Lin L., Lin Y. (2024). Analysis of the influencing factors of major adverse cardiovascular events in elderly patients with coronary heart disease after percutaneous coronary intervention. Chin. J. Mod. Drug Appl. 18 (11), 48–51. doi:10.14164/j.cnki.cn11-5581/r.2024.11.012
Lin L., Lu W., Wang X., Pan L., Wang X., Zheng X., et al. (2023). Short-term outcomes of drug-coated balloon versus drug-eluting stent for de novo saphenous vein graft lesions in coronary heart disease. Front. Cardiovasc. Med. 10, 982880. doi:10.3389/fcvm.2023.982880
Liu Z., Cheng J., Zhou S., Li X., Yang M., Zhang Y. (2024). Prediction of major adverse cardiovascular events following acute myocardial infarction using electrocardiogram DETERMINE score. BMC Cardiovasc Disord. 24 (1), 705. doi:10.1186/s12872-024-04409-6
Lu Y., Wang Y., Zhou B. (2023). Predicting long-term prognosis after percutaneous coronary intervention in patients with acute coronary syndromes: a prospective nested case-control analysis for county-level health services. Front. Cardiovasc. Med. 10, 1297527. doi:10.3389/fcvm.2023.1297527
Luo E., Wang D., Yan G., Qiao Y., Liu B., Hou J., et al. (2019). High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. 18 (1), 150. doi:10.1186/s12933-019-0957-3
Ma J. (2014). Analysis of influencing factors of perioperative heart failure in patients with acute STEMI during PCI. Changchun: Jilin University.
Madhavan M. V., Kirtane A. J., Redfors B., Généreux P., Ben-Yehuda O., Palmerini T., et al. (2020). Stent-related adverse events >1 Year after percutaneous coronary intervention. J. Am. Coll. Cardiol. 75 (6), 590–604. doi:10.1016/j.jacc.2019.11.058
Mamas M. A., Anderson S. G., O'Kane P. D., Keavney B., Nolan J., Oldroyd K. G., et al. (2014). Impact of left ventricular function in relation to procedural outcomes following percutaneous coronary intervention: insights from the British Cardiovascular Intervention Society. Eur. Heart J. 35 (43), 3004–12a. doi:10.1093/eurheartj/ehu303
Mastoor Y., Murphy E., Roman B. (2025). Mechanisms of postischemic cardiac death and protection following myocardial injury. J. Clin. investigation 135 (1), e184134. doi:10.1172/jci184134
Meller S. M., Lansky A. J., Costa R. A., Soffler M., Costantini C. O., Brodie B. R., et al. (2013). Implications of myocardial reperfusion on survival in women versus men with acute myocardial infarction undergoing primary coronary intervention. Am. J. Cardiol. 112 (8), 1087–1092. doi:10.1016/j.amjcard.2013.05.052
Mordi I. R., Trucco E., Syed M. G., MacGillivray T., Nar A., Huang Y., et al. (2022). Prediction of major adverse cardiovascular events from retinal, clinical, and genomic data in individuals with type 2 diabetes: a population cohort study. Diabetes care 45 (3), 710–716. doi:10.2337/dc21-1124
Motovska Z., Widimsky P., Aschermann M.PRAGUE Study Group Investigators (2008). The impact of gender on outcomes of patients with ST elevation myocardial infarction transported for percutaneous coronary intervention: analysis of the PRAGUE-1 and 2 studies. Heart 94 (3), e5. doi:10.1136/hrt.2006.110866
Murphy A., Vogel B., Sartori S., Spirito A., Chen S., Baber U., et al. (2023). Predictors of major adverse cardiovascular events in Young patients undergoing percutaneous coronary intervention. Heart, Lung Circulation 32, S445–S446. doi:10.1016/j.hlc.2023.06.681
Nasir M., Shafique H. M., Hussain S., Tuyyab F., Aziz S., Khadim R. (2020). Percutaneous coronary intervention for left main coronary artery bifurcation lesions: two-stent versus one-stent strategy for comparison of 6-month MACE. J. Coll. Physicians Surgeons--Pakistan JCPSP 30 (9), 894–899. doi:10.29271/jcpsp.2020.09.894
Nozoe M., Sakamoto T., Taguchi E., Miyamoto S., Fukunaga T., Nakao K. (2014). Clinical manifestation of early phase left ventricular rupture complicating acute myocardial infarction in the primary PCI era. J. Cardiol. 63 (1), 14–18. doi:10.1016/j.jjcc.2013.06.012
Numasawa Y., Kohsaka S., Miyata H., Noma S., Suzuki M., Ishikawa S., et al. (2015). Gender differences in in-hospital clinical outcomes after percutaneous coronary interventions: an insight from a Japanese multicenter registry. PloS one 10 (1), e0116496. doi:10.1371/journal.pone.0116496
Okina Y., Miura T., Senda K., Taki M., Kobayashi M., Kanai M., et al. (2021). Prognostic ability of mid-term worsening renal function after percutaneous coronary intervention: findings from the SHINANO registry. Heart Vessels 36 (10), 1496–1505. doi:10.1007/s00380-021-01837-8
Otten A. M., Maas A. H., Ottervanger J. P., Kloosterman A., van 't Hof A. W., Dambrink J. H., et al. (2013). Is the difference in outcome between men and women treated by primary percutaneous coronary intervention age dependent? Gender difference in STEMI stratified on age. Eur. Heart J. Acute Cardiovasc Care 2 (4), 334–341. doi:10.1177/2048872612475270
Özen Y., Bilal Özbay M., Yakut I., Kanal Y., Abdelmottelaeb W., Nriagu B. N., et al. (2023). Atherogenic index of plasma and triglyceride-glucose index to predict more advanced coronary artery diseases in patients with the first diagnosis of acute coronary syndrome. Eur. Rev. Med. Pharmacol. Sci. 27 (9), 3993–4005. doi:10.26355/eurrev_202305_32305
Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Parsa S. A., Nourian S., Safi M., Namazi M. H., Saadat H., Vakili H., et al. (2022). The association between hematologic indices with TIMI flow in STEMI patients who undergo primary percutaneous coronary intervention. Cardiovasc Hematol. Disord. Drug Targets 22, 162–167. doi:10.2174/1871529X22666220913122046
Pendyala L. K., Torguson R., Loh J. P., Kitabata H., Minha S. A., Badr S., et al. (2013). Comparison of adverse outcomes after contemporary percutaneous coronary intervention in women versus men with acute coronary syndrome. Am. J. Cardiol. 111 (8), 1092–1098. doi:10.1016/j.amjcard.2012.12.040
Polanska-Skrzypczyk M., Karcz M., Bekta P., Kepka C., Przyluski J., Kruk M., et al. (2013). Prognostic value of renal function in STEMI patients treated with primary PCI: ANIN Registry. Br. J. Cardiol. 2013 (20), 65. doi:10.5837/bjc.2013.17
Pu J., Shan P., Ding S., Qiao Z., Jiang L., Song W., et al. (2011). Gender differences in epicardial and tissue-level reperfusion in patients undergoing primary angioplasty for acute myocardial infarction. Atherosclerosis 215 (1), 203–208. doi:10.1016/j.atherosclerosis.2010.11.019
Qanitha A., Uiterwaal C., Henriques J. P. S., Alkatiri A. H., Mappangara I., Mappahya A. A., et al. (2018). Characteristics and the average 30-day and 6-month clinical outcomes of patients hospitalised with coronary artery disease in a poor South-East Asian setting: the first cohort from Makassar Cardiac Center, Indonesia. BMJ Open 8 (6), e021996. doi:10.1136/bmjopen-2018-021996
Qi S., Zhan Y., Chen Y., Xu T. (2023). Effect of antecedent hypertension on mortality after acute coronary syndromes in the coronary intervention era: a meta-analysis. Heart Lung Circ. 32 (10), 1189–1197. doi:10.1016/j.hlc.2023.08.007
Ren X., Wang X. (2023). Association of the low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and major adverse cardiac and cerebrovascular events in patients with coronary heart disease undergoing percutaneous coronary intervention: a cohort study. Curr. Med. Res. Opin. 39 (9), 1175–1181. doi:10.1080/03007995.2023.2246889
Saluveer O., Redfors B., Angerås O., Dworeck C., Haraldsson I., Ljungman C., et al. (2017). Hypertension is associated with increased mortality in patients with ischaemic heart disease after revascularization with percutaneous coronary intervention - a report from SCAAR. Blood Press. 26 (3), 166–173. doi:10.1080/08037051.2016.1270162
Shaaban R., El Etriby A., Kamal D., Mostafa A. E. (2022). Prognostic impact of pre-interventional culprit artery thrombolysis in myocardial infarction (TIMI) flow in patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention. Egypt Heart J. 74 (1), 52. doi:10.1186/s43044-022-00289-3
Shiba T., Kondo Y., Senoo K., Nakano M., Okubo K., Ishio N., et al. (2019). Proximal occlusion in the right coronary artery involving the atrial branch as a strong predictor of new-onset atrial fibrillation in acute myocardial infarction. Int. Heart J. 60 (6), 1308–1314. doi:10.1536/ihj.18-713
Teragawa H., Uchimura Y., Oshita C., Hashimoto Y., Nomura S. (2024). Clinical characteristics and major adverse cardiovascular events in diabetic and non-diabetic patients with vasospastic angina. Diabetes, metabolic syndrome Obes. targets Ther. 17, 2135–2146. doi:10.2147/dmso.S462234
Thomas J. M., Huuskes B. M., Sobey C. G., Drummond G. R., Vinh A. (2022). The IL-18/IL-18R1 signalling axis: diagnostic and therapeutic potential in hypertension and chronic kidney disease. Pharmacol. & Ther. 239, 108191. doi:10.1016/j.pharmthera.2022.108191
Toma A., Stahli B. E., Gick M., Gebhard C., Nuhrenberg T., Mashayekhi K., et al. (2017). Impact of multi-vessel versus single-vessel disease on outcomes after percutaneous coronary interventions for chronic total occlusions. Clin. Res. Cardiol. 106 (6), 428–435. doi:10.1007/s00392-016-1072-z
Toyota T., Furukawa Y., Ehara N., Funakoshi S., Morimoto T., Kaji S., et al. (2013). Sex-based differences in clinical practice and outcomes for Japanese patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ. J. 77 (6), 1508–1517. doi:10.1253/circj.cj-12-1161
Tran D. T., Welsh R. C., Ohinmaa A., Thanh N. X., Kaul P. (2017). Temporal trends of reperfusion strategies and hospital mortality for patients with STEMI in percutaneous coronary intervention-capable hospitals. Can. J. Cardiol. 33 (4), 485–492. doi:10.1016/j.cjca.2016.12.002
Velders M. A., Boden H., van Boven A. J., van der Hoeven B. L., Heestermans A. A., Cannegieter S. C., et al. (2013). Influence of gender on ischemic times and outcomes after ST-elevation myocardial infarction. Am. J. Cardiol. 111 (3), 312–318. doi:10.1016/j.amjcard.2012.10.007
Wang H., Gao Z., Song Y., Tang X., Xu J., Jiang P., et al. (2018). Impact of diabetes mellitus on percutaneous coronary intervention in Chinese patients: a large single-center data. Angiology 69 (6), 540–547. doi:10.1177/0003319717735226
Wang N., Zuo Y., Li Z., Wang S. (2020a). Risk factors and prognosis of heart failure with preserved ejection fraction in patients with acute myocardial infarction after emergency PCI. Chin. J. Evidence-Bases Cardiovasc. Med. 12 (9), 1122–1124. doi:10.3969/j.issn.1674-4055.2020.09.25
Wang P., Qiao H., Wang R., Hou R., Guo J. (2020b). The characteristics and risk factors of in-stent restenosis in patients with percutaneous coronary intervention: what can we do. BMC Cardiovasc Disord. 20 (1), 510. doi:10.1186/s12872-020-01798-2
Wijnbergen I., Tijssen J., van 't Veer M., Michels R., Pijls N. H. (2013). Gender differences in long-term outcome after primary percutaneous intervention for ST-segment elevation myocardial infarction. Catheter Cardiovasc Interv. 82 (3), 379–384. doi:10.1002/ccd.24800
Xu S., Gu F., Tao W. (2022). Risk of heart failure following direct PCI and the predictive value of NT-proBNP and inflammatory factors in patients with acute ST-segment el-evation myocardial infarction. China Mod. Dr. 60 (11), 52–55.
Yao W., Li J. (2022). Risk factors and prediction nomogram model for 1-year readmission for major adverse cardiovascular events in patients with STEMI after PCI. Clin. Appl. thrombosis/hemostasis official J. Int. Acad. Clin. Appl. Thrombosis/Hemostasis. 28, 10760296221137847. doi:10.1177/10760296221137847
Yu H., Zhou C., Hu D., Li C., Wang Q., Xue W., et al. (2022). Uremic toxin indoxyl sulfate induces dysfunction of vascular smooth muscle cells via integrin-β1/ERK signaling pathway. Clin. Exp. Nephrol. 26 (7), 640–648. doi:10.1007/s10157-022-02195-z
Yuan S., Li L., Pu T., Fan X., Wang Z., Xie P., et al. (2024). The relationship between NLR, LDL-C/HDL-C, NHR and coronary artery disease. PLoS One 19 (7), e0290805. doi:10.1371/journal.pone.0290805
Zanchi J., Miric D., Giunio L., Vukovic I., Markovic B., Duplancic D., et al. (2009). Gender differences in in-hospital mortality and angiographic findings of patients with acute ST-segment elevation myocardial infarction (STEMI) undergoing percutaneous coronary intervention (PCI). Coll. Antropol. 33 (4), 1359–1362. doi:10.1161/circulationaha.106.664979
Zhang D., Yan R., Wang H. Y., Zhang R., Zhao Z., Gao G., et al. (2024). Technological advances are associated with better clinical outcomes of percutaneous coronary intervention in patients with unprotected left main disease. J. Am. Heart Assoc. 13 (16), e033929. doi:10.1161/jaha.123.033929
Zhang J., Liu M., Gao J., Tian X., Song Y., Zhang H., et al. (2025). ApoB/ApoA-Ι is associated with major cardiovascular events and readmission risk of patients after percutaneous coronary intervention in one year. Sci. Rep. 15 (1), 996. doi:10.1038/s41598-024-84092-x
Zhang T. (2023). The influencing factors of cardiovascular adverse events in patients with coronary heart disease after percutaneous coronary intervention. Med. J. Chin. People's Health 35 (21), 5–7+11. doi:10.3969/j.issn.1672-0369.2023.21.002
Keywords: coronary heart disease, percutaneous coronary intervention, risk factors, major cardiovascular adverse events, meta-analysis
Citation: Zhai Y, Shang H, Li Y, Zhang N, Zhang J and Wu S (2025) A Systematic Review of risk factors for major adverse cardiovascular events in patients with coronary heart disease who underwent percutaneous coronary intervention. Front. Physiol. 16:1514585. doi: 10.3389/fphys.2025.1514585
Received: 21 October 2024; Accepted: 28 March 2025;
Published: 09 April 2025.
Edited by:
German Ebensperger, University of Chile, ChileReviewed by:
David Wu, The University of Chicago, United StatesHan Yu, Qilu Hospital, Shandong University, China
Pengning Chen, Tokyo Medical and Dental University, Japan
Copyright © 2025 Zhai, Shang, Li, Zhang, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: You Zhai, emhhaXlvdUBoYWN0Y20uZWR1LmNu; Hongcai Shang, c2hhbmdob25nY2FpQGZveG1haWwuY29t
†ORCID: You Zhai, orcid.org/0000-0003-1803-5191; Hongcai Shang, orcid.org/0000-0001-6628-354X
 You Zhai
You Zhai Hongcai Shang
Hongcai Shang Yan Li1
Yan Li1 Shangwen Wu
Shangwen Wu