
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol., 25 March 2025
Sec. Exercise Physiology
Volume 16 - 2025 | https://doi.org/10.3389/fphys.2025.1459717
Lactate accumulation will appear in athlete skeletal muscle after intense exercise. If the high lactate level maintains, athletes will sustain fatigue and athletic capacity decline due to internal environment and normal metabolism disruption. In order to enhance athlete physical recovery and exercise performance in high-intensity sport events, it is of great significance to explore the scientific intervention procedures based on quicker lactate clearance in skeletal muscle and blood after exercise. This article collects classic and novel literature in terms of lactate metabolism, lactate clearance and fatigue monitoring during exercise by searching PubMed database and then summarizes comprehensive insights into athlete physical recovery with corresponding figures and charts. We introduce the generation and transformation process of lactate, lactate clearance pathways and the fatigue monitoring methods for athletes in detail. The lactate clearance pathways involve biochemical pathways (oxygen inhalation, amino acids supplement, targeting free radical, alkaline reserve, targeting vasomotion, ribose supplement), physical activities (exercise-mediated activities, non-exercise activities) and training methods (interval training, altitude training) to accelerate lactate metabolism. The biochemical factors for monitoring athletic fatigue level involve blood, urine, sweat, saliva and exhaled gas. We hope this review can offer some significant and scientific assistance for athlete recovering after exercise and improving sport achievements based on quicker lactate clearance.
After long-time and high-intensity training and competition, the body of athletes will show fatigue due to excess carbohydrate consumption and oxidation function decline, followed by metabolic imbalance, cell membrane oxidative damage, serum lactate accumulation (Von Duvilard et al., 2004; Kaviani et al., 2020; Kruk et al., 2021). When the acidic metabolites generated during exercise reduce the acidity of body fluids to the threshold, the blood pH will decrease and the electrolyte balance will break, which will greatly impair the exercise performance of athletes (Kamińska et al., 2020; Zhu et al., 2020). At present, there is little effective ways to directly eliminate sport fatigue, and high-intensity training and competition require more effective physical recovery for athletes. Thus, it is necessary and significant for developing scientific recovery procedures with fewer side effects and no doping ingredients in athlete physical recovery.
During exercise, lactate is generated from anaerobic glycolysis in skeletal muscle and delivered to the body through blood circulation, taking a certain time to be metabolically cleared (Hall et al., 2016). Normally, lactate will be eliminated in a period of time after exercise. But for athletes, long-term intense exercise will induce the imbalance between lactate generation and clearance, lactate accumulation will eventually cause fatigue and prolong athlete physical recovery (Lee et al., 2023). Hence, serum lactate content is an important index to measure and judge the fatigue of athletes. To monitor the blood lactate level of athletes after exercise, and develop scientific recovery procedures for lactate clearance according to the principle of lactate metabolism will help to improve the physical recovery and exercise performance of athletes.
In this study, we search and download the classic and novel literature concerning lactate metabolism, lactate clearance and fatigue monitoring during exercise from PubMed database. The comprehensive understandings of athlete physical recovery have been summarized and the analysis results are shown in corresponding figures and charts. As follow, we introduce the generation and transformation process of lactate, lactate clearance pathways and the biochemical factors of monitoring fatigue for athletes in detail, hoping to provide a certain theoretic and practical basis for exploring the scientific intervention procedures based on quicker lactate clearance after exercise.
In human body, the energy supply pathways of carbohydrate metabolism mainly include aerobic oxidation and anaerobic glycolysis in skeletal muscle, which play an essential role in exercise maintaining. In different exercise patterns, the energy supply pathways of carbohydrate metabolism is different. The aerobic oxidation of carbohydrate is mainly applied in long-term endurance exercise or quiet state, while anaerobic glycolysis is mainly applied in short-time intense exercise (Figure 1).
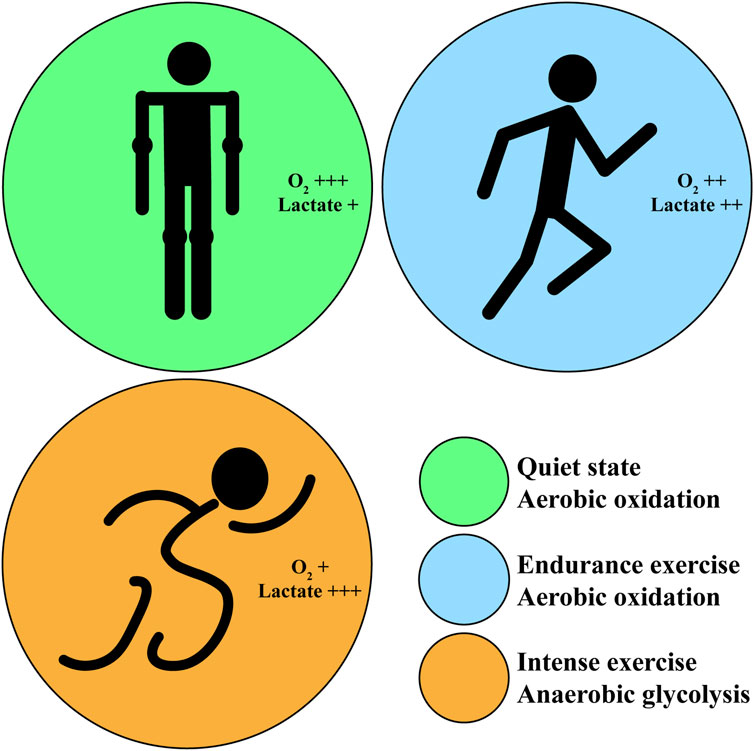
Figure 1. The oxygen consumption and lactate generation corresponding to different exercise intensity. Green circle: in quiet state, the body proceeds aerobic oxidation and generates little lactate with sufficient oxygen. Blue circle: in endurance exercise, the body proceeds aerobic oxidation and generates more lactate with decreasing oxygen; athletes feel fatigue after exercise. Orange circle: in intense exercise, the body proceeds anaerobic glycolysis and generates excess lactate with little oxygen; athletes feel very fatigue after exercise.
The main process of carbohydrate aerobic oxidation involves: in cytoplasm, glucose converts to pyruvate; in mitochondria, pyruvate converts to acetyl-CoA and enters tricarboxylic acid cycle (TCA) with oxaloacetate; ATP is generated from electron transfer chain and substrate level phosphorylation, accompanied by CO2 and H2O (Tran et al., 2019; Hsu et al., 2019). TCA cycle is a common pathway of energy metabolism and transformation in the body, and plays a key role in carbohydrate aerobic oxidation. Among them, the main catalytic enzymes of TCA cycle include citrate synthase, α-ketoglutarate dehydrogenase, succinic dehydrogenase and so on, which are highly expressed in skeletal muscle tissues. Oxaloacetate and acetyl-CoA converting into citrate requires the catalysis of citrate synthase, the oxidative decarboxylation of α-ketoglutarate converting into succinyl-CoA and CO2 requires α-ketoglutarate dehydrogenase, and succinic dehydrogenase plays a regulatory role in the oxidation of succinate converting into fumarate (Ren et al., 2020; Hansen and Gibson, 2022; Balnis et al., 2021). Their activities can be generally applied as an indicator to characterize the progress of TCA cycle and the balance between aerobic oxidation and anaerobic glycolysis during exercise (Figure 2).
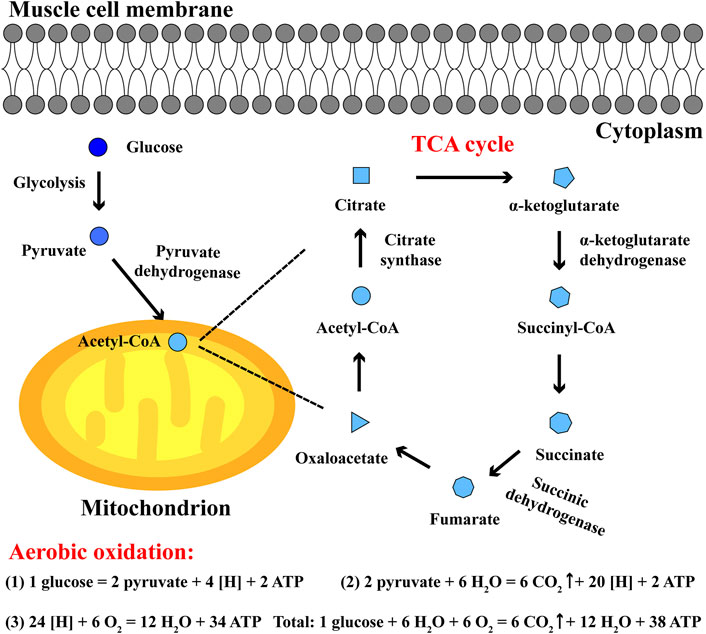
Figure 2. The aerobic oxidation and TCA cycle in skeletal muscle cells. In cytoplasm, glucose converts to pyruvate; in mitochondria, pyruvate converts to acetyl-CoA and enters TCA cycle. In aerobic oxidation, one glucose eventually generates 6 CO2, 12 H2O and 38 ATP during endurance exercise.
The chemical formula of lactate is C3H6O3 and its molecular weight is 90.08. In aqueous solution, the carboxyl group releases a proton to produce the lactate ion CH3CH(OH)COO−. Generally, lactate level rises when tissue energy cannot be satisfied through aerobic oxidation. Skeletal muscle cannot obtain enough oxygen, or it cannot process oxygen quickly enough. In this case, pyruvate dehydrogenase is unable to convert pyruvate into acetyl-CoA in time, and pyruvate begins to accumulate. In mammals, there are three main subtypes of lactate dehydrogenase (LDH): LDHA, LDHB and LDHC (Deme and Telekes, 2017). During exercise, LDH mainly regulates the metabolic balance of lactate and pyruvate in skeletal muscle, where LDHA and LDHB are abundant. If pyruvate cannot be completely metabolized by aerobic oxidation, LDHA will mediate the transformation of pyruvate into lactate; in contrast, LDHB mediates the transformation of lactate into pyruvate when pyruvate is significantly reduced (Figure 3A) (Read et al., 2001; Gupta, 2012). During high-intensity exercise, LDHA plays a dominant role in order to improve the anaerobic glycolysis efficiency of skeletal muscle.
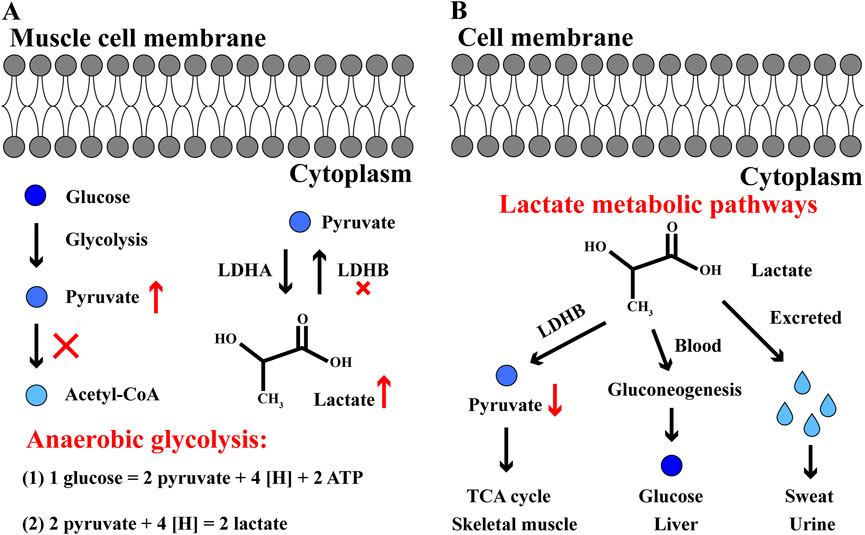
Figure 3. The anaerobic glycolysis and lactate metabolic pathways in skeletal muscle cells (A) In cytoplasm, glucose converts to pyruvate, but pyruvate cannot convert to acetyl-CoA and enter TCA cycle. In anaerobic glycolysis, one glucose eventually generates two lactate and 2 ATP during intense exercise. (B) Three pathways of lactate metabolism. Firstly, lactate converts to pyruvate and enter TCA cycle in skeletal muscle; secondly, lactate converts to glucose through gluconeogenesis in liver; thirdly, lactate is excreted in sweat and urine. The red and up arrow indicates increasing effect, the red and down arrow indicates reducing effect, the red cross indicates inhibitory effect.
The lactate generated by skeletal muscle during exercise mainly metabolizes into three pathways after exercise: firstly, in skeletal muscle, lactate oxidizes into CO2 and H2O; secondly, lactate enters into liver with blood circulation and converts to glucose/glycogen through gluconeogenesis; thirdly, it is excreted in sweat and urine (Figure 3B) (Hui et al., 2017; Chen et al., 2021; Wang et al., 2022). Among these three metabolic pathways, oxidation is the main destination for lactate metabolism after exercise. With LDH catalytic reaction, lactate is dehydrogenated and oxidized to pyruvate, which is completely oxidized and decomposed through TCA cycle (Park et al., 2022). Thus, the serum LDH content and activity obviously increase and the maximum appear after exercise. Meanwhile, lactate accumulation after exercise will increase the blood lactate level, and in the long run, pathologically elevated lactate levels can induce protein lactation in the body, eventually improve the development of several diseases such as sepsis, tumor, Alzheimer’s disease (Huang et al., 2023). Hence, it is necessary and significant for developing scientific recovery procedures in athlete physical recovery based on quicker lactate clearance.
According to the lactate threshold, serum lactate in 2–4 mM represents aerobic metabolism and low-intensity exercise; 4–12 mM represents aerobic metabolism transforming to anaerobic metabolism and moderate exercise; over 12 mM represents anaerobic metabolism and high-intensity exercise (Sliwowski et al., 2013). The serum lactate level can be applied to monitor the exercise intensity and fatigue level of athletes. And the key to effectively clear lactate is to select proper methods according to the understanding of the essence of lactate metabolism. In this section, we introduce scientific recovery procedures to clear lactate in athletes after exercise, including biochemical pathways, physical activities and training methods to accelerate lactate metabolism (Table 1).
The body still maintains high metabolic level and high oxygen consumption after exercise, suffering oxygen starvation for a period of time. Then lactate cannot convert into CO2 and H2O via oxygenolysis as soon. The uptake of high concentration oxygen can quickly elevate the blood oxygen saturation to a higher level, which accelerates lactate oxygenolysis and restores internal pH; Meanwhile the improvement of hypoxic environment can enhance the antioxidant system to eliminate peroxide and accelerate physical recovery (Hauser et al., 2014; Xiao and Li, 2006).
Oxygenolysis is the main metabolic pathway of lactate after exercise, including converting to pyruvate and TCA cycle. Pyruvate is converted to acetyl-CoA with pyruvate dehydrogenase catalytic reaction, and acetyl-CoA plus oxaloacetate generate citrate to initiate TCA cycle, thereby the oxidation rate of lactate is associated with the TCA cycle initiator oxaloacetate (Figure 2) (Chhimpa et al., 2023). Meanwhile aspartic acid and glutamic acid can be converted into oxaloacetate (Holeček, 2023; Hertz and Hertz, 2003). Thus supplement with aspartic acid and glutamic acid after exercise will promote the synthesis of oxaloacetate and enhance the oxidation of lactate in TCA cycle, so as to quickly clear lactate in muscle and accelerate the recovery of blood lactate level.
High-intensity exercise dramatically increases free radical generation in skeletal muscle due to the aggravated oxidative stress, and the free radical-mediated cellular damage will induce aerobic and anaerobic metabolism disorder which impairs the lactate clearance after exercise to a certain extent (Parker et al., 2014). Thus, targeting free radical is an important strategy for athletes decreasing blood lactate level and recovering after exercise. Some studies indicated that the polyphenols in fruits and tea and vitamins showed great antioxidant capacity to protect against exercise-induced free radical, consequently improving lactate clearance and sport performance (Parenteau et al., 2023; Zhao et al., 2022; Peng et al., 2009). Hence the supplement of polyphenols and vitamins are recommended for athletes in daily training.
There are buffer solutions such as bicarbonate and hydrophosphate in the blood to maintain body acid-base balance, among which bicarbonate buffer pair has a strong buffer capacity, and the physically dissolved CO2 in the blood can maintain the dynamic stability of H2CO3 (McKay et al., 2020; Pilch et al., 2020). When lactate enters the blood from skeletal muscle, it combines with carbonate to generate H2CO3, which can antagonize the changes in blood acidity caused by lactate accumulation. However, the self-reserve of alkaline in athletes cannot afford to neutralize excess lactate. Before training and competition, athletes are recommended to properly supplement alkaline food and alkaline drinks, so that the improvement of alkaline electrolyte storage can enhance the athletic ability to neutralize blood lactate, delay the decline of exercise performance and reduce exercise fatigue (Baranauskas et al., 2020; Newbury et al., 2021).
During exercise, skeletal muscle contraction causes mechanical compression of vascular endothelial cells, and the reduced blood flow aggravates hypoxia process. After exercise, the contraction of skeletal muscle relieve slowly and the breathing frequency of athletes reduces, leading to the slow vasomotion and lactate oxygenolysis. Recent years, herbal medicine like ginkgo biloba extract has been applied to promote endothelial cell relaxation, increase erythrocyte deformability and reduce blood viscosity, so that the vasomotion after exercise liberates blood flow and enhance lactate oxygenolysis with normal oxygen saturation (Kim et al., 2010).
In addition to the glycolic and oxidative pathways, pentose phosphate pathway is another complex energy supply mode of carbohydrate oxidation and ATP production (Icard and Lincet, 2012; Ghergurovich et al., 2021). In high-intensity exercise, if we can enhance the sport effects of pentose phosphate pathway supplying ATP in a synergistic manner, the elevating proportion of prophosphate energy supply and the decline of glycolysis will reduce lactate generation and improve athletic lactate metabolism. A randomized controlled trial showed that ribose supplement is recommended to effectively clear lactate after exercise based on alternative energy supply mode (Cao et al., 2020).
The traditional idea of sport training believes that intensity is the core factor to improve athletic ability, but excess exercise will cause sport injuries. With the change of the focus of modern sport training concept, more scholars and coaches realize that the improvement of athlete competitive level depends more on the accumulation of sport effects. Besides exercise intensity, athletes should pay attention to fatigue recovery in order to obtain better sport achievement. With the deeper understanding of the field of exercise fatigue recovery, the importance of controlling exercise intensity and recovery to training effect has become increasingly prominent. Therefore, exercise recovery is an essential way to improve training effect and sport performance, and special emphasis is placed on the positive effect of physical activities accelerating lactate metabolism.
After competition or training, the body of athletes still maintain in a high metabolic level, the sudden stop of exercise will reduce blood flow and oxygen, blocking the blood lactate dispersion with blood circulation. The lactate accumulation is not conducive to lactate clearance. Nowadays, in terms of lactate clearance, physical activities of exercise recovery has evolved into many forms, which can be divided into two broad categories: exercise-mediated activities and non-exercise activities. Both of them play a vital role in relieving muscle tension, improving blood circulation, replenishing oxygen, regulating endocrine system and accelerating lactate metabolism. The exercise-mediated activities involve: jog (Losnegard et al., 2015), stretch (Yui et al., 2021), bicycle ergometer (De Ridder et al., 2022) the non-exercise activities involve: sleep (Rempe and Wisor, 2015), muscle massage (Ferreira et al., 2023), foam roller (Adamczyk et al., 2020), cold and warm immersion (Adamczyk et al., 2016; Sautillet et al., 2024), acupuncture (Rosa et al., 2023), foot reflexology (Cai et al., 2022), light irradiation (Baroni et al., 2010), magnetic treatment (Lefaucheur et al., 2017), electric nerve stimulation (Neric et al., 2009) and so on.
With the increase of exercise intensity, the blood lactate concentration of athletes will sharply rise at a certain node, called lactate threshold, and the corresponding exercise intensity is lactate threshold intensity (Faude et al., 2009). The emergence of lactate threshold represents the energy supply of athletes transforming from aerobic oxidation to anaerobic glycolysis. In terms of innate factor and acquired training, the changes of blood lactate level and lactate threshold of athletes are different, which can objectively reflect the exercise intensity, aerobic and anaerobic metabolic capacity of athletes (Löllgen and Leyk, 2018). Thus lactate threshold is applied as a reference by coaches to schedule training and control training load, meanwhile applied as a training method to improve athlete endurance, including interval training and altitude training.
As a grouped training, interval training contains exercise intensity from low to high and recovery time, which emphasizes multiple groups of repetition and is characterized by short time and high energy consumption (Seiler et al., 2013; Haugen et al., 2021). The recovery time among training can improve the lactate release from skeletal muscle to blood and lactate clearance, compared with continuous training, interval training allows athletes experience more intense training by synergizing the recovery time and training intensity, which can effectively improve the endurance and competitive level of athletes. An animal study showed that after running intervention of interval training, the endurance performance of rats was improved more than continuous training, with the enhancing AMPK-PGC-1α signaling pathway, a key factor of mitochondria metabolism regulation (Pengam et al., 2023).
In the altitude with low oxygen, athletes suffer double hypoxia stimulation during high-intensity training, so that the energy supply of anaerobic glycolysis of skeletal muscle stands greater test than that in the plain with rich oxygen, which improves the generation, tolerance, transformation of lactate and the anaerobic metabolic capacity of athletes (Millet et al., 2010). In the training of same intensity, the level of blood lactate after altitude training is higher than that of plain training, indicating that the elevated anaerobic glycolysis promotes the generation and transformation of blood lactate. Hence, altitude training can elevate lactate threshold and the ability of oxidative clearance of blood lactate, showing a positive enhancing effect on the physiological performance and athletic capacity of athletes. A current observational study found that moderate altitude significantly increased the lactate threshold velocity and heart rate and upper body muscle mass of elite Chinese cross-country skiers after over 4 weeks of training (Yu et al., 2022).
Sports load is increasing with the continuous elevation of modern athletic level. Moderate exercise fatigue with scientific recovery procedures can promote the exercise performance of athletes, but excessive fatigue is unfavorable to improve athletic capacity, causes sport injuries and damages athlete physical health. Thus it is of great theoretical and practical significance to accurately monitor sport fatigue and define fatigue degree for making scientific training plan, improving training effect and avoiding injuries caused by excess fatigue. In this section, we introduce several biochemical factors and the detection methods for monitoring athletic fatigue level, including blood, urine, sweat, saliva and exhaled gas (Table 2).
The monitoring of blood components can provide abundant information reflecting the changes of athlete body functions, their biochemical markers such as lactate, hemoglobin, testosterone, cortisol and urea have been widely applied in the human movement science investigation (Krishnan et al., 2022; Kloner et al., 2016; Powell et al., 2013; Batiha et al., 2022). Intense exercise consumes excess energy, so that skeletal muscle utilizes lactate metabolism to maintain the energy supply in a short time. Large amount of lactate is generated from LDH oxidation and released into the blood circulation, making athletes feel more fatigue. Thus the lactate and LDH levels in the blood after exercise are valuable indicators to monitor athletic fatigue, which can be measured by common methods of colorimetric (Meng et al., 2023) and sandwich-enzyme-linked immunosorbent assay (ELISA) kits (Li et al., 2020). After high-intensity exercise, the aggravated oxidative stress in the skeletal muscle of athletes increases the free radical level to impair erythrocyte membrane, leading to the release of intracellular components in blood like hemoglobin, metabolism disorder and endurance loss (Higgins et al., 2020). Thus measuring hemoglobin release and reactive oxygen species (ROS) level can reflect the extent of athletic fatigue, with sandwich-ELISA (Dönmez Türkmen et al., 2022) and fluorescent probe (Xiao et al., 2023) methods, respectively. Fu Q et al. develops a portable and rapid smartphone-based hemoglobin point-of-care testing platform with azide-hemiglobin method which might be employed for athletes monitoring hemoglobin level in the blood after exercise, especially the advantages of low price and field test in playground (Fu et al., 2022). However, blood drawing will cause minor trauma of athletes and it take time to separate serum from blood samples. In recent years, non-invasive detection has been continuously improved in urine, sweat, saliva and exhaled gas, showing important application value in monitoring athletic fatigue.
Athletes consume vast nutrients during high-intensity exercise, and the dramatic changes in urine composition can be employed to monitor the degree of fatigue of athletes. For urine protein, the secretion of epinephrine and norepinephrine during exercise will lead to its elevation, and the common detection methods include Coomassie brilliant blue (colormetric), biuret (colormetric) and benzethonium chloride (turbidimetric) (Aoki et al., 2023; Hokazono et al., 2021; Özcan et al., 2024). For creatinine, the metabolite of creatine and indicator of muscle fatigue, its metabolism can be measured by colormetric assay kit (Zhang et al., 2021) and mass spectrometry (Niesser et al., 2012).
Perspiration is a manifestation of exercise metabolism and carries plentiful metabolic messages. Measuring the sweat pH by colormetric reagents can indirectly reflect the dehydration of athletes and the degree of athletic fatigue (Hamouti et al., 2011). Lactate is another important indicator of exercise fatigue in sweat and the detection method is the same with blood lactate (colormetric) (Meng et al., 2023). Using flexible substrate and electrode amperometric lactate biosensor, Imani S et al. invents a wearable chemical biosensing system to monitor lactate level on the skin, which might be employed for athletes monitoring sweat lactate level in real time during exercise (Imani et al., 2016).
During intense exercise, the decline of adrenoreceptor of athlete salivary glands reduces the salivary immunoglobulin level (sIgA) (Matsubara et al., 2010). Testosterone is a steroid hormone that promotes protein synthesis to enhance muscle strength and erythrocyte growth, closely related to athletic capacity and fatigue (Heather, 2022). The salivary immunoglobulin and testosterone levels are important indicators of athletic function monitoring, their dynamic changes can be measured by sandwich- (Paixão et al., 2021) and competitive-ELISA (Xie et al., 2021), respectively.
Using mass spectrometry, more compounds have been identified in exhaled gas, which provides a non-invasive and fast check to monitor athletic fatigue. The exhaled gas reflects athletic cardio-pulmonary function, the glucose, amino acid, urea and other exercise metabolites can be employed to monitor athletic fatigue with mass spectrometry (Chen et al., 2007).
The improvement of athletic level is the result of moderate fatigue caused by training and the body function adapting to new level after recovery. Thus fatigue and recovery are essential parts for athletes training and competition. However, if training overloads and the body can not recover in time, fatigue will gradually accumulate. When the limit of body tolerance is exceeded, it will cause great harm to athletic health. Therefore, the elimination and real-time monitoring of fatigue after exercise have important theoretical and practical significance to arrange scientific training plans, improve training effects, create excellent score, and avoid injuries caused by excess fatigue. For this purpose, we introduce and summarize the generation and transformation process of lactate metabolism, the biochemical pathways, physical activities and training methods to clear lactate, the biochemical factors for monitoring athlete fatigue level in detail in this study.
The lactate accumulation caused by intense exercise will reduce the pH of body fluid to block energy supply by glycometabolism, suppress the sensitivity of muscle fibers to calcium ions and muscle contraction, thereby the impair internal environment and disrupt normal metabolism generate fatigue and damage athletic capacity for athlete body (Cairns, 2006). Hence, the quicker lactate clearance in muscle and blood after exercise is beneficial to eliminate fatigue and enhance exercise performance in high-intensity sport events. In this study, we introduce some scientific pathways without doping violations to clear lactate in athletes after exercise, including biochemical pathways to accelerate lactate metabolism, physical activities to accelerate lactate metabolism and training to raise lactate threshold (Table 1). Particularly, the biochemical pathways to accelerate lactate metabolism involve oxygen inhalation, amino acids supplement, targeting free radical, alkaline reserve, targeting vasomotion and ribose supplement, concerning exercise-mediated hypoxia, main lactate metabolic pathway (aerobic metabolism/TCA cycle), exercise-mediated oxidative stress, lactate-mediated blood acidity, muscle tension-mediated blood obstruction and other energy supply mode instead of anaerobic glycolysis. There are many factors affecting the lactate clearance, including gender and age of athletes, self-athletic ability, sport event features, exercise load and environment, lactate clearance methods and time. A correct understanding of the factors affecting the lactate clearance and a targeted selection of lactate clearance methods are the important prerequisite for the rapid lactate clearance after exercise. Also, the nutrients and medicines applied to clear lactate should be accessible, portable and nontoxic for athletes training and competition. However, only studying the micro-changes of athletic metabolism can effectively clear lactate. Thus, multi-omics analyses such as transcriptome, proteome and metabolome are recommended for further exploring the micro-changes and molecular mechanism of athletic metabolism during exercise and physical recovery based on lactate clearance. Meanwhile, the applicability of each lactate clearance pathway and corresponding sport events necessitates sufficient tests for verification.
According to the different assessment criteria, the methods of exercise fatigue monitoring are mainly involved subjective evaluation, physiological monitoring and biochemical monitoring. The subjective evaluation is a convenient and non-invasive method that athletes fill in sport science questionnaires according to their training conditions, but the results are not objective and accurate, thereby serving as an auxiliary method for fatigue monitoring (Royer et al., 2023). The physiological monitoring can be used to evaluate the athletic status by providing objective data of dynamic changes in brain cell current, heart rate, blood pressure, muscle tension, body weight and other physiological indexes, but the results are interfered by individual differences, low accuracy and other various factors (Burkow-Heikkinen, 2011; Saw et al., 2016; Nocera et al., 2023; Lundstrom et al., 2023). Biochemical monitoring for exercise fatigue has been developed rapidly in recent years, since exercise is closely related to the material and energy metabolism of athletic body, such as carbohydrate, protein, lipid metabolism, and the metabolites are eliminated through circulatory system. The biochemical monitoring methods for athlete fatigue we introduce are based on the metabolites in blood, urine, sweat, saliva and exhaled gas after exercise, indicating the athlete metabolic status. Biochemical monitoring is advanced in providing objective results in athletic rapid, field and non-invasive detection, showing a good application prospect in monitoring athletic fatigue and elevating sport performance. Among them, athletic fatigue is induced and characterized by lactate accumulation, so it’s a key concern to monitor lactate level after exercise. The data from biochemical monitoring will help to monitor lactate level in time after exercise, and effectively clear lactate and elevate sport performance. However, the proper methods applied to monitor lactate level depend on the sport event features, as well as reagents and instruments in lab. And the sensitivity, accuracy and repeatability of monitoring methods in corresponding sport events also necessitate sufficient tests for verification.
Collectively, our review summarizes the generation and transformation process of lactate metabolism, the biochemical pathways, physical activities and training methods to clear lactate, the biochemical factors for monitoring athlete fatigue level in detail from literature, which shows positive significance in improving lactate clearance of sport players, and provides a certain theoretic and practical basis for guiding sport practice. We will cooperate with the athlete teams of Beijing Sport University and conduct sufficient tests in the corresponding sport events to verify the lactate clearance pathways and fatigue monitoring methods we introduce in this study, in order to offer some help for improving training effects, physical recovery and sport performance of athletes.
TH: Conceptualization, Investigation, Software, Writing–original draft, Writing–review and editing. ZL: Investigation, Software, Writing–original draft. KW: Investigation, Software, Writing–original draft. XM: Conceptualization, Supervision, Writing–review and editing. LZ: Conceptualization, Supervision, Funding acquisition, Writing–review and editing.
The author(s) declare that financial support was received for the research, and/or publication of this article. This work was supported by the grants from Dynamic Analysis of Player Position Topology in 3×3 Basketball in International Competition and the Influence on Tactical Success Rate (24QN024).
The authors declare no conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adamczyk J. G., Gryko K., Boguszewski D. (2020). Does the type of foam roller influence the recovery rate, thermal response and DOMS prevention? PLoS One 15, e0235195. doi:10.1371/journal.pone.0235195
Adamczyk J. G., Krasowska I., Boguszewski D., Reaburn P. (2016). The use of thermal imaging to assess the effectiveness of ice massage and cold-water immersion as methods for supporting post-exercise recovery. J. Therm. Biol. 60, 20–25. doi:10.1016/j.jtherbio.2016.05.006
Aoki H., Miyazaki R., Ohama M., Murata M., Asai K., Ogata G., et al. (2023). Urine protein quantification in human urine on boron-doped diamond electrodes based on the electrochemical reaction of Coomassie brilliant blue. Analyst 148, 4396–4405. doi:10.1039/d3an01000g
Balnis J., Drake L. A., Vincent C. E., Korponay T. C., Singer D. V., Lacomis D., et al. (2021). SDH subunit C regulates muscle oxygen consumption and fatigability in an animal model of pulmonary emphysema. Am. J. Respir. Cell Mol. Biol. 65, 259–271. doi:10.1165/rcmb.2020-0551OC
Baranauskas M., Jablonskienė V., Abaravičius J. A., Samsonienė L., Stukas R. (2020). Dietary acid-base balance in high-performance athletes. Int. J. Environ. Res. Public Health 17, 5332. doi:10.3390/ijerph17155332
Baroni B. M., Leal Junior E. C., Geremia J. M., Diefenthaeler F., Vaz M. A. (2010). Effect of light-emitting diodes therapy (LEDT) on knee extensor muscle fatigue. Photomed. Laser Surg. 28, 653–658. doi:10.1089/pho.2009.2688
Batiha G. E., Al-Kuraishy H. M., Al-Gareeb A. I., Welson N. N. (2022). Pathophysiology of Post-COVID syndromes: a new perspective. Virol. J. 19, 158. doi:10.1186/s12985-022-01891-2
Burkow-Heikkinen L. (2011). Non-invasive physiological monitoring of exercise and fitness. Neurol. Res. 33, 3–17. doi:10.1179/1743132810Y.0000000014
Cai D. C., Chen C. Y., Lo T. Y. (2022). Foot reflexology: recent research trends and prospects. Healthc. (Basel) 11, 9. doi:10.3390/healthcare11010009
Cairns S. P. (2006). Lactic acid and exercise performance: culprit or friend? Sports Med. 36, 279–291. doi:10.2165/00007256-200636040-00001
Cao W., Qiu J., Cai T., Yi L., Benardot D., Zou M. (2020). Effect of D-ribose supplementation on delayed onset muscle soreness induced by plyometric exercise in college students. J. Int. Soc. Sports Nutr. 17, 42. doi:10.1186/s12970-020-00371-8
Chen A. N., Luo Y., Yang Y. H., Fu J. T., Geng X. M., Shi J. P., et al. (2021). Lactylation, a novel metabolic reprogramming code: current status and prospects. Front. Immunol. 12, 688910. doi:10.3389/fimmu.2021.688910
Chen H., Wortmann A., Zhang W., Zenobi R. (2007). Rapid in vivo fingerprinting of nonvolatile compounds in breath by extractive electrospray ionization quadrupole time-of-flight mass spectrometry. Angew. Chem. Int. Ed. Engl. 46, 580–583. doi:10.1002/anie.200602942
Chhimpa N., Singh N., Puri N., Kayath H. P. (2023). The novel role of mitochondrial citrate synthase and citrate in the pathophysiology of alzheimer's disease. J. Alzheimers Dis. 94, S453–S472. doi:10.3233/JAD-220514
Deme D., Telekes A. (2017). Prognostic importance of lactate dehydrogenase (LDH) in oncology. Orv. Hetil. 158, 1977–1988. doi:10.1556/650.2017.30890
De Ridder F., Ledeganck K. J., De Winter B., Braspenning R., Delbeke D., Renard E., et al. (2022). Trends of glucose, lactate and ketones during anaerobic and aerobic exercise in subjects with type 1 diabetes: the ACTION-1 study. Diabetes Metab. Res. Rev. 38, e3537. doi:10.1002/dmrr.3537
Dönmez Türkmen A., Ünlü G., Musayeva G., Akkuş E., Özen A. G., Önal P., et al. (2022). Can the prognosis of COVID-19 disease Be determined by fecal markers and cytokines? J. Interferon Cytokine Res. 42, 542–549. doi:10.1089/jir.2022.0098
Faude O., Kindermann W., Meyer T. (2009). Lactate threshold concepts: how valid are they? Sports Med. 39, 469–490. doi:10.2165/00007256-200939060-00003
Ferreira R. M., Silva R., Vigário P., Martins P. N., Casanova F., Fernandes R. J., et al. (2023). The effects of massage guns on performance and recovery: a systematic review. J. Funct. Morphol. Kinesiol. 8, 138. doi:10.3390/jfmk8030138
Fu Q., Qi T., Wu Z., He Y., Guan S., Luo S., et al. (2022). A portable smartphone-based hemoglobin point-of-care testing platform for accurate anemia diagnostics. Biosens. Bioelectron. 217, 114711. doi:10.1016/j.bios.2022.114711
Ghergurovich J. M., Lang J. D., Levin M. K., Briones N., Facista S. J., Mueller C., et al. (2021). Local production of lactate, ribose phosphate, and amino acids within human triple-negative breast cancer. Med 2, 736–754. doi:10.1016/j.medj.2021.03.009
Gupta G. S. (2012). LDH-C4: a target with therapeutic potential for cancer and contraception. Mol. Cell Biochem. 371, 115–127. doi:10.1007/s11010-012-1428-2
Hall M. M., Rajasekaran S., Thomsen T. W., Peterson A. R. (2016). Lactate: friend or foe. PM R. 8, S8–S15. doi:10.1016/j.pmrj.2015.10.018
Hamouti N., Del Coso J., Ortega J. F., Mora-Rodriguez R. (2011). Sweat sodium concentration during exercise in the heat in aerobically trained and untrained humans. Eur. J. Appl. Physiol. 111, 2873–2881. doi:10.1007/s00421-011-1911-6
Hansen G. E., Gibson G. E. (2022). The α-ketoglutarate dehydrogenase complex as a hub of plasticity in neurodegeneration and regeneration. Int. J. Mol. Sci. 23, 12403. doi:10.3390/ijms232012403
Haugen T., Sandbakk Ø., Enoksen E., Seiler S., Tønnessen E. (2021). Crossing the golden training divide: the science and practice of training world-class 800- and 1500-m runners. Sports Med. 51, 1835–1854. doi:10.1007/s40279-021-01481-2
Hauser A., Zinner C., Born D. P., Wehrlin J. P., Sperlich B. (2014). Does hyperoxic recovery during cross-country skiing team sprints enhance performance? Med. Sci. Sports Exerc. 6, 787–794. doi:10.1249/MSS.0000000000000157
Heather A. K. (2022). Transwoman elite athletes: their extra percentage relative to female Physiology. Int. J. Environ. Res. Public Health 19, 9103. doi:10.3390/ijerph19159103
Hertz L., Hertz E. (2003). Cataplerotic TCA cycle flux determined as glutamate-sustained oxygen consumption in primary cultures of astrocytes. Neurochem. Int. 43, 355–361. doi:10.1016/s0197-0186(03)00022-6
Higgins M. R., Izadi A., Kaviani M. (2020). Antioxidants and exercise performance: with a focus on vitamin E and C supplementation. Int. J. Environ. Res. Public Health 17, 8452. doi:10.3390/ijerph17228452
Hokazono E., Ota E., Goto T., Fukumoto S., Kayamori Y., Uchiumi T., et al. (2021). Development of a protein assay with copper chelator chromeazurol B, based on the biuret reaction. Anal. Biochem. 630, 114320. doi:10.1016/j.ab.2021.114320
Holeček M. (2023). Roles of malate and aspartate in gluconeogenesis in various physiological and pathological states. Metabolism 145, 155614. doi:10.1016/j.metabol.2023.155614
Hsu C. C., Tsai H. H., Fu T. C., Wang J. S. (2019). Exercise training enhances platelet mitochondrial bioenergetics in stroke patients: a randomized controlled trial. J. Clin. Med. 8, 2186. doi:10.3390/jcm8122186
Huang W., Su J., Chen X., Li Y., Xing Z., Guo L., et al. (2023). High-intensity interval training induces protein lactylation in different tissues of mice with specificity and time dependence. Metabolites 13, 647. doi:10.3390/metabo13050647
Hui S., Ghergurovich J. M., Morscher R. J., Jang C., Teng X., Lu W., et al. (2017). Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118. doi:10.1038/nature24057
Icard P., Lincet H. (2012). A global view of the biochemical pathways involved in the regulation of the metabolism of cancer cells. Biochim. Biophys. Acta 1826, 423–433. doi:10.1016/j.bbcan.2012.07.001
Imani S., Bandodkar A. J., Mohan A. M., Kumar R., Yu S., Wang J., et al. (2016). A wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 7, 11650. doi:10.1038/ncomms11650
Kamińska J., Podgórski T., Kryściak J., Pawlak M. (2020). Effect of simulated matches on post-exercise biochemical parameters in women's indoor and beach handball. Int. J. Environ. Res. Public Health 17, 5046. doi:10.3390/ijerph17145046
Kaviani M., Chilibeck P. D., Gall S., Jochim J., Zello G. A. (2020). The effects of low- and high-glycemic index sport nutrition bars on metabolism and performance in recreational soccer players. Nutrients 12, 982. doi:10.3390/nu12040982
Kim B. H., Kim K. P., Lim K. S., Kim J. R., Yoon S. H., Cho J. Y., et al. (2010). Influence of Ginkgo biloba extract on the pharmacodynamic effects and pharmacokinetic properties of ticlopidine: an open-label, randomized, two-period, two-treatment, two-sequence, single-dose crossover study in healthy Korean male volunteers. Clin. Ther. 32, 380–390. doi:10.1016/j.clinthera.2010.01.027
Kloner R. A., Carson C., Dobs A., Kopecky S., Mohler E. R. (2016). Testosterone and cardiovascular disease. J. Am. Coll. Cardiol. 67, 545–557. doi:10.1016/j.jacc.2015.12.005
Krishnan S., Sarda S., Kunzweiler C., Wu M., Sundaresan S., Huynh L., et al. (2022). Literature review of fatigue scales and association with clinically meaningful improvements in outcomes among patients with and without paroxysmal nocturnal hemoglobinuria. Adv. Ther. 39, 1959–1975. doi:10.1007/s12325-022-02111-7
Kruk J., Aboul-Enein B. H., Duchnik E. (2021). Exercise-induced oxidative stress and melatonin supplementation: current evidence. J. Physiol. Sci. 71, 27. doi:10.1186/s12576-021-00812-2
Lee S., Choi Y., Jeong E., Park J., Kim J., Tanaka M., et al. (2023). Physiological significance of elevated levels of lactate by exercise training in the brain and body. J. Biosci. Bioeng. 135, 167–175. doi:10.1016/j.jbiosc.2022.12.001
Lefaucheur J. P., Chalah M. A., Mhalla A., Palm U., Ayache S. S., Mylius V. (2017). The treatment of fatigue by non-invasive brain stimulation. Neurophysiol. Clin. 47, 173–184. doi:10.1016/j.neucli.2017.03.003
Li H. M., Guo H. L., Xu C., Liu L., Hu S. Y., Hu Z. H., et al. (2020). Inhibition of glycolysis by targeting lactate dehydrogenase A facilitates hyaluronan synthase 2 synthesis in synovial fibroblasts of temporomandibular joint osteoarthritis. Bone 141, 115584. doi:10.1016/j.bone.2020.115584
Löllgen H., Leyk D. (2018). Exercise testing in sports medicine. Dtsch. Arztebl. Int. 115, 409–416. doi:10.3238/arztebl.2018.0409
Losnegard T., Andersen M., Spencer M., Hallén J. (2015). Effects of active versus passive recovery in sprint cross-country skiing. Int. J. Sports Physiol. Perform. 10, 630–635. doi:10.1123/ijspp.2014-0218
Lundstrom C. J., Foreman N. A., Biltz G. (2023). Practices and applications of heart rate variability monitoring in endurance athletes. Int. J. Sports Med. 44, 9–19. doi:10.1055/a-1864-9726
Matsubara Y., Shimizu K., Tanimura Y., Miyamoto T., Akimoto T., Kono I. (2010). Effect of acupuncture on salivary immunoglobulin A after a bout of intense exercise. Acupunct. Med. 28, 28–32. doi:10.1136/aim.2009.001677
McKay A. K. A., Peeling P., Binnie M. J., Goods P. S. R., Sim M., Cross R., et al. (2020). Topical sodium bicarbonate: No improvement in blood buffering capacity or exercise performance. Int. J. Sports Physiol. Perform. 15, 1005–1011. doi:10.1123/ijspp.2019-0345
Meng J. J., Shen J. W., Li G., Ouyang C. J., Hu J. X., Li Z. S., et al. (2023). Light modulates glucose metabolism by a retina-hypothalamus-brown adipose tissue axis. Cell 186, 398–412.e17. doi:10.1016/j.cell.2022.12.024
Millet G. P., Roels B., Schmitt L., Woorons X., Richalet J. P. (2010). Combining hypoxic methods for peak performance. Sports Med. 40, 1–25. doi:10.2165/11317920-000000000-00000
Neric F. B., Beam W. C., Brown L. E., Wiersma L. D. (2009). Comparison of swim recovery and muscle stimulation on lactate removal after sprint swimming. J. Strength Cond. Res. 23, 2560–2567. doi:10.1519/JSC.0b013e3181bc1b7a
Newbury J. W., Cole M., Kelly A. L., Chessor R. J., Sparks S. A., McNaughton L. R., et al. (2021). The time to peak blood bicarbonate (HCO3-), pH, and the strong ion difference (SID) following sodium bicarbonate (NaHCO3) ingestion in highly trained adolescent swimmers. PLoS One 16, e0248456. doi:10.1371/journal.pone.0248456
Niesser M., Koletzko B., Peissner W. (2012). Determination of creatinine in human urine with flow injection tandem mass spectrometry. Ann. Nutr. Metab. 61, 314–321. doi:10.1159/000342774
Nocera A., Sbrollini A., Romagnoli S., Morettini M., Gambi E., Burattini L. (2023). Physiological and biomechanical monitoring in American football players: a scoping review. Sensors (Basel) 23, 3538. doi:10.3390/s23073538
Özcan Ö., van Wijk J. A., Bosch A. M., den Elzen W. P., Heijboer A. C., Fischer J. C. (2024). Case report: skin protective barrier cream interference in benzethonium chloride method for urine protein measurement in a 6-month-old girl. Ann. Clin. Biochem. 61, 150–153. doi:10.1177/00045632231218833
Paixão V., Almeida E. B., Amaral J. B., Roseira T., Monteiro F. R., Foster R., et al. (2021). Elderly subjects supplemented with L-glutamine shows an improvement of mucosal immunity in the upper airways in response to influenza virus vaccination. Vaccines (Basel) 9, 107. doi:10.3390/vaccines9020107
Parenteau F., Puglia V. F., Roberts M., Comtois A. S., Bergdahl A. (2023). Cranberry supplementation improves physiological markers of performance in trained runners. Phys. Act. Nutr. 27, 8–14. doi:10.20463/pan.2023.0032
Park J. S., Saeed K., Jo M. H., Kim M. W., Lee H. J., Park C. B., et al. (2022). LDHB deficiency promotes mitochondrial dysfunction mediated oxidative stress and neurodegeneration in adult mouse brain. Antioxidants (Basel) 11, 261. doi:10.3390/antiox11020261
Parker L., McGuckin T. A., Leicht A. S. (2014). Influence of exercise intensity on systemic oxidative stress and antioxidant capacity. Clin. Physiol. Funct. Imaging 34, 377–383. doi:10.1111/cpf.12108
Peng H., Chen Q., Tan Y. (2009). Frequent ejaculation associated free radical and lactic acid accumulation cause noninfectious inflammation and muscle dysfunction: a potential mechanism for symptoms in Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Med. Hypotheses 73, 372–373. doi:10.1016/j.mehy.2009.03.044
Pengam M., Goanvec C., Moisan C., Simon B., Albacète G., Féray A., et al. (2023). Moderate intensity continuous versus high intensity interval training: metabolic responses of slow and fast skeletal muscles in rat. PLoS One 18, e0292225. doi:10.1371/journal.pone.0292225
Pilch W., Kita B., Piotrowska A., Tota Ł., Maciejczyk M., Czerwińska-Ledwig O., et al. (2020). The effect of vitamin D supplementation on the muscle damage after eccentric exercise in young men: a randomized, control trial. J. Int. Soc. Sports Nutr. 17, 53. doi:10.1186/s12970-020-00386-1
Powell D. J., Liossi C., Moss-Morris R., Schlotz W. (2013). Unstimulated cortisol secretory activity in everyday life and its relationship with fatigue and chronic fatigue syndrome: a systematic review and subset meta-analysis. Psychoneuroendocrinology 38, 2405–2422. doi:10.1016/j.psyneuen.2013.07.004
Read J. A., Winter V. J., Eszes C. M., Sessions R. B., Brady R. L. (2001). Structural basis for altered activity of M- and H-isozyme forms of human lactate dehydrogenase. Proteins 43, 175–185. doi:10.1002/1097-0134(20010501)43:2<175::aid-prot1029>3.0.co;2-#
Rempe M. J., Wisor J. P. (2015). Cerebral lactate dynamics across sleep/wake cycles. Front. Comput. Neurosci. 8, 174. doi:10.3389/fncom.2014.00174
Ren M., Yang X., Bie J., Wang Z., Liu M., Li Y., et al. (2020). Citrate synthase desuccinylation by SIRT5 promotes colon cancer cell proliferation and migration. Biol. Chem. 401, 1031–1039. doi:10.1515/hsz-2020-0118
Rosa V. B. B., Dos Santos I. F. C., Souto L. G., de Paiva Porto E., Pizzigatti D., Cholfe B. F., et al. (2023). Effect of acupuncture on hematologic, muscular biomarkers, fibrinogen and serum lactate parameters in training rodeo bulls. Res. Vet. Sci. 158, 76–83. doi:10.1016/j.rvsc.2023.02.009
Royer N., Brownstein C. G., Kennouche D., Espeit L., Teston A., Boutet C., et al. (2023). A comprehensive evaluation of multiple sclerosis-related fatigue with a special focus on fatigability. Med. Sci. Sports Exerc. 55, 2002–2013. doi:10.1249/MSS.0000000000003233
Sautillet B., Bourdillon N., Millet G. P., Lemaître F., Cozette M., Delanaud S., et al. (2024). Hot water immersion: maintaining core body temperature above 38.5°C mitigates muscle fatigue. Scand. J. Med. Sci. Sports 34, e14503. doi:10.1111/sms.14503
Saw A. E., Main L. C., Gastin P. B. (2016). Monitoring the athlete training response: subjective self-reported measures trump commonly used objective measures: a systematic review. Br. J. Sports Med. 50, 281–291. doi:10.1136/bjsports-2015-094758
Seiler S., Jøranson K., Olesen B. V., Hetlelid K. J. (2013). Adaptations to aerobic interval training: interactive effects of exercise intensity and total work duration. Scand. J. Med. Sci. Sports 23, 74–83. doi:10.1111/j.1600-0838.2011.01351.x
Sliwowski R., Andrzejewski M., Wieczorek A., Barinow-Wojewódzki A., Jadczak L., Adrian S., et al. (2013). Changes in the anaerobic threshold in an annual cycle of sport training of young soccer players. Biol. Sport 30, 137–143. doi:10.5604/20831862.1044459
Tran K. N., Niu S., Ichiye T. (2019). Reduction potential calculations of the Fe-S clusters in Thermus thermophilus respiratory complex I. J. Comput. Chem. 40, 1248–1256. doi:10.1002/jcc.25785
Von Duvillard S. P., Braun W. A., Markofski M., Beneke R., Leithäuser R. (2004). Fluids and hydration in prolonged endurance performance. Nutrition 20, 651–656. doi:10.1016/j.nut.2004.04.011
Wang Z., Bai H., Yu W., Gao Z., Chen W., Yang Z., et al. (2022). Flexible bioelectronic device fabricated by conductive polymer-based living material. Sc.i Adv. 8, eabo1458. doi:10.1126/sciadv.abo1458
Xiao G. Q., Li H. C. (2006). Effects of inhalation of oxygen on free radical metabolism and oxidative, antioxidative capabilities of the erythrocyte after intensive exercise. Res. Sports Med. 14, 107–115. doi:10.1080/15438620600651355
Xiao S., Zhang Y., Liu Z., Li A., Tong W., Xiong X., et al. (2023). Alpinetin inhibits neuroinflammation and neuronal apoptosis via targeting the JAK2/STAT3 signaling pathway in spinal cord injury. CNS Neurosci. Ther. 29, 1094–1108. doi:10.1111/cns.14085
Xie F., Zhang J., Zhai M., Liu Y., Hu H., Yu Z., et al. (2021). Melatonin ameliorates ovarian dysfunction by regulating autophagy in PCOS via the PI3K-Akt pathway. Reproduction 162, 73–82. doi:10.1530/REP-20-0643
Yu Y., Wang R., Li D., Lu Y. (2022). Monitoring physiological performance over 4 Weeks moderate altitude training in elite Chinese cross-country skiers: an observational study. Int. J. Environ. Res. Public Health 20, 266. doi:10.3390/ijerph20010266
Yui J., Okano S., Nishizawa H. (2021). Relationship between skeletal muscle mass and blood lactate level reduction after short squat jumps in healthy adult non-athletes. J. Phys. Ther. Sci. 33, 717–721. doi:10.1589/jpts.33.717
Zhang X., He C., Chen Y., Chen C., Yan R., Fan T., et al. (2021). Cyclic reactions-mediated self-supply of H2O2 and O2 for cooperative chemodynamic/starvation cancer therapy. Biomaterials 275, 120987. doi:10.1016/j.biomaterials.2021.120987
Zhao X., Shi X., Liu Q., Li X. (2022). Tea polyphenols alleviates acetochlor-induced apoptosis and necroptosis via ROS/MAPK/NF-κB signaling in Ctenopharyngodon idellus kidney cells. Aquat. Toxicol. 246, 106153. doi:10.1016/j.aquatox.2022.106153
Keywords: athlete, physical recovery, lactate metabolism, lactate clearance, fatigue monitoring
Citation: Huang T, Liang Z, Wang K, Miao X and Zheng L (2025) Novel insights into athlete physical recovery concerning lactate metabolism, lactate clearance and fatigue monitoring: A comprehensive review. Front. Physiol. 16:1459717. doi: 10.3389/fphys.2025.1459717
Received: 05 July 2024; Accepted: 07 March 2025;
Published: 25 March 2025.
Edited by:
Daniel Rojas-Valverde, National University of Costa Rica, Costa RicaReviewed by:
Nemanja Lakicevic, University of Palermo, ItalyCopyright © 2025 Huang, Liang, Wang, Miao and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zheng, MjEzMUBic3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.