- 1Fédération ENAC ISAE-SUPAERO ONERA, Toulouse, France
- 2Spaceship FR, CNES, Toulouse, France
- 3MEDES, Institute for Space Physiology and Medicine, Toulouse, France
- 4Neurology Department, University Hospital of Toulouse, Toulouse, France
In the past decade, there has been a surge in interest in space exploration studies, particularly due to the prospect of exploring distant planets such as Mars. However, long-duration space missions may pose cognitive challenges resulting from spaceflight-induced perceptual and motor changes, prolonged cephalic fluid shifts, and high cognitive load. One method for monitoring cognitive activity is functional near-infrared spectroscopy (fNIRS), a technique not yet tested under prolonged microgravity conditions beyond parabolic flight periods. Since fNIRS relies on cerebral oxygenation levels, should we adjust it for the fluid shift? To address this, the study explores the impact of simulated microgravity on cerebral oxygenation measures using fNIRS during a cognitive task, employing head-down tilt at different inclination levels and the Toulouse N-back Task (assessing memory and mental calculation) with varying difficulty levels. Eighteen subjects participated in the experiment. The results indicated that increasing difficulty levels of the cognitive task led to decreased accuracy, longer response times, and higher perceived difficulty scores. The inclination levels did not affect task performance. Increased difficulty was also concomitant with increasing HbO and decreasing HbR concentrations unaffected by the head-down tilt angle variations. These promising findings suggest that fNIRS measures could be used under microgravity conditions to measure cognitive load without correction for fluid shift.
1 Introduction
During the last decade, humanity witnessed an increased interest in space exploration accompanied by the democratization of access to and beyond Karman’s line: a new space station assembly, docking of a module to the International Space Station, increasing commercial spaceflight, development of new crewed vehicles, Starship tests, etc. In a few years, professional astronauts may walk on the Moon and undertake a voyage to Mars, while undertrained space tourists rush on the low orbit with short hops, in-orbit sojourns, or even extra-vehicular walks. However, humans are not designed to function in space and many aspects relative to cognitive functioning in this environment remain challenging. Spaceflight is known to induce perceptual and motor deficits (Clément and Reschke, 2010; Kuldavletova et al., 2023; Tays et al., 2021), and cognitive load is an important factor that can further affect motor performance (Burcal et al., 2019) and be increased by vestibular deficits (Clément et al., 2023; Bigelow and Agrawal, 2015; Hanes and McCollum, 2006). Although the literature does not suggest clear evidence of the deleterious impact of spaceflight on cognitive performance (Strangman et al., 2014; Casler and Cook, 1999), exposure to radiation during deep space missions could negatively impact cognitive function (Cacao and Cucinotta, 2019; Rabin et al., 2014; Acharya et al., 2019). Hence, it is crucial to monitor the cognitive effort of astronauts both to continue sustaining the high-level cognitive performance of current operations and prevent possible deficits associated with deep space exploration.
Cognitive load can be estimated using self-report measures (Naismith et al., 2015), objective monitoring of the body activity (heart rate, respiration rate, eye movements, brain activity) (Ayres et al., 2021), secondary-task performance (Park and Brünken, 2015; Greenberg and Zheng, 2022), or observer ratings. Most of these measures present disadvantages for online monitoring: self-report and observer ratings are subjective and might not reflect the true cognitive load; the secondary-task method interrupts the operator and is not suitable in operational settings; cardiac and respiratory measures are more sensitive to the stress and arousal rather than workload; eye movements might reflect the cognitive load but mostly reveal the visual attention distribution. Brain activity measurement techniques such as electroencephalography (EEG, Chikhi et al., 2022; Ghani et al., 2020; Antonenko et al., 2010) or functional near-infrared spectroscopy (fNIRS, Ferrari and Quaresima, 2012; Aghajani et al., 2017; Herff et al., 2014) are good candidates for measuring cognitive load.
EEG is a technique that was already successfully used for scientific experiments aboard the International Space Station for many years (Fiedler et al., 2023; Van Ombergen et al., 2017). fNIRS measurement is a more recent technique that has been successfully used to measure cognitive load in numerous studies, consistently demonstrating increased activity in prefrontal regions as task difficulty rises (Causse et al., 2017; Ayaz et al., 2012; Mandrick et al., 2016). This increase reflects heightened activation in areas responsible for critical cognitive processes, such as rational reasoning (Donoso et al., 2014), working memory (Funahashi, 2017), planning (Tanji and Hoshi, 2001), and more. During effortful tasks, the increased demands on executive function are reflected by heightened prefrontal cortex activity (Shenhav et al., 2017), a region shown to exhibit a linear increase in activity with working memory (WM) load (Braver et al., 1997). These studies collectively suggest that the prefrontal cortex region, in particular, dorsolateral ones can serve as a reliable proxy measure of mental workload.
Generally, fNIRS has a good signal-to-noise ratio and is less sensitive to electrical noise compared to EEG (Hasan et al., 2020; Ghafoor et al., 2021), as it measures hemodynamic changes (oxygenated/deoxygenated hemoglobin) in response to brain activity. EEG can be affected by artifacts from muscles, movement, breathing, or external electrical interference, including radio communications. However, advanced filtering methods are available for EEG, and the setup of both techniques involves similar constraints in terms of electrode placement and comfort, especially considering the recent generalization of dry EEG electrodes.
fNIRS has already been tested in microgravity during parabolic flights (Zhang et al., 2011; Schneider et al., 2013) or real flight of light aircraft (Dehais et al., 2018; Gateau et al., 2018). However, compared to only a few seconds of short microgravity periods or short aircraft tests, longer stays in microgravity associated with human spaceflight could be very challenging for fNIRS measurements. fNIRS is an optical imaging method that detects changes induced by brain activity in oxygenated (oxyHb) and deoxygenated (deoxyHb) hemoglobin blood levels (León-Carrión and León-Domínguez, 2012). In microgravity, the redistribution of fluid towards the brain also alters the cerebral hemodynamics (Kawai et al., 2003; Du et al., 2021; Lee et al., 2021). Hence, to monitor online cognitive load using fNIRS, we need to understand whether the measurements of cognitive load levels by this technique are affected by microgravity-induced fluid shift. In particular, we used head-down tilt simulated microgravity with different inclination levels (pre-0°, −10°, −20°, post-0°) and a cognitive task with multiple levels of difficulty (Toulouse N-back Task with n = 0, 1, and 2). While the tilted participants performed the task, their prefrontal cortex oxygenation levels were monitored using a simple two-channel fNIRS system. An increase in task difficulty should result in an increase in blood oxygenation in the prefrontal area, regardless of the level of tilt, provided that tilt will not affect the measurements.
2 Materials and methods
2.1 Subjects
Eighteen healthy volunteers, 9 men and 9 women (age 24.28
The protocol was conducted following the Declaration of Helsinki and approved by the Ethics Committee (CPP 23.01719.000252) and the French Health Authorities. The study was carried out and promoted by the Institute for Space Medicine and Physiology (MEDES, Toulouse France) and sponsored by the French Space Agency (CNES, Spaceship FR project).
All volunteers signed a written consent form before the experiment and were aware of their right to withdraw from the experiment without prejudice at any time. The volunteers performed two experimental sessions: one described in the present study; and another one with a different set of sensors used by another research team and beyond the scope of the present paper.
2.2 Head-down tilt setup
Head-down tilt (HDT) is a relevant experimental setup for simulating the effects of microgravity on the human body (Watenpaugh, 2016), particularly fluid-shift (London et al., 1983; Watenpaugh, 2016). During the experiment, the volunteers were placed in a supine position on a bed that could be tilted to different angles according to head-to-toe axis. It allows the head to be lower than the lower extremities and induces fluid shift. Other axes such as lateral (lying on one side) or dorsoventral (lying face up vs. down) could have slight effects on local tissue perfusion, but these changes are much smaller in scale compared to fluid shifts along the head-to-toe axis.
The researchers often use multiples of 6° (Marshall-Goebel et al., 2017) or multiples 10° (Kermorgant et al., 2022; Cooke et al., 2003) as possible tilt angle values. We chose to use multiples of 10° to maximize possible differences between conditions. The tilt angle was verified using both analog and digital inclinometer. The cognitive task was displayed on a laptop computer screen placed above the participants’ chests mounted on a hospital table with adjustable height and inclination. The responses were given using a CedrusBox response box placed on the participants’ abdomen at a comfortable length reachable by both hands. The response box had two buttons: green for “yes” and red for “no”.
2.3 Experimental design
The participants were head-down tilted for 20 min at each position at 0°, −10°, −20°, and back to 0°. This protocol enabled a controlled and progressive fluid shift from 0° to −20°. However, returning to a baseline position [e.g., seated posture, as in Chouchou et al. (2020)] was not feasible due to time constraints. Such a transition would have extended the protocol by over an hour without offering substantial scientific value. Existing research indicates that a resting tilt duration of approximately 6 min is sufficient to elicit a cardiovascular hemodynamic response (Whittle et al., 2022), while around 15 min is enough to induce cerebral hemodynamic changes (Kato et al., 2024).
In the analysis, we refer to it as the HDT angle factor with four levels: Pre-0°, −10°, −20°, and Post-0°. The total experimental time was 80 min. After 15 min of tilt in a given position, volunteers completed the cognitive task (Toulouse N-back Task), followed by the NASA-TLX and Perceived n-back difficulty questionnaires. Figure 1 shows the order of the tilt levels.
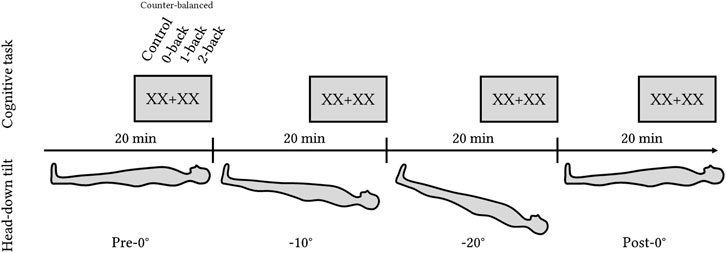
Figure 1. Experimental design with four levels of HDT angles: pre-0°, −10°, −20°, and post-0°. Each position lasted for 20 min with the cognitive task (Toulouse N-back Task) administered during the last 5 min of each level.
2.4 Toulouse N-back task
At the end of each tilt, the participants performed the cognitive task called the Toulouse N-back Task (TNT, Mandrick et al., 2013; Causse et al., 2017). TNT is an adaptation of a classical n-back task where the participants perform the n-back task on the results of arithmetical operations. The arithmetic operations consisted of addition/subtraction of multiples of 10 between 10 and 90 (for example, 10 + 40 or 90–30).
The task had three levels of difficulty (0-, 1-, and 2-back) and a control reaction time task to control for motor response where the participants had to press any button as soon as the stimulus (either “00 + 00″ or “00–00″) appeared on the screen. In the 0-back condition, the participants had to compare the result of the operation with 50. In the 1-back condition, the participants had to compare the result of the operation with the result of the previous one. In the 2-back, they had to compare the results with the result obtained two operations before. Figure 2 illustrates the four conditions.
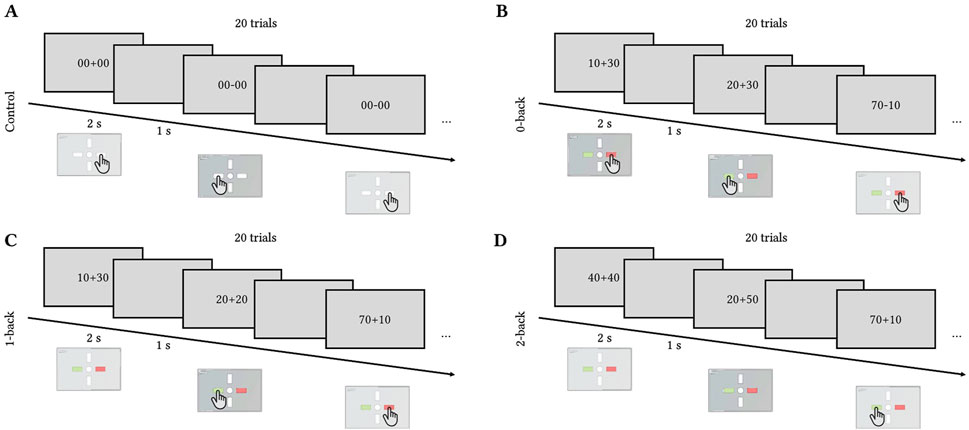
Figure 2. Toulouse N-back Task timecourse. Each trial lasted for 2 s with 1 s inter-trial interval. Each block of difficulty included 20 trials. (A) Control (press left or right button), (B) 0-back (compare the result to 50), (C) 1-back (compare the result to the previous one), (D) 2-back (compare the result to the one obtained two trials ago).
The three difficulty levels were counterbalanced across tilt levels and participants. Each trial lasted for 2 s with 1 s of inter-stimulus interval. Each block contained 20 trials ((2 + 1)
2.5 Data acquisition
During the cognitive tasks, the response times and accuracy were recorded using a Cedrus response box. At the end of each tilt condition, once the cognitive task was completed, the participants filled out the NASA-TLX questionnaire evaluating the mental, physical, and temporal demands, performance, effort, and frustration levels associated with a given tilt. After NASA-TLX completion, the participants were asked to rate the difficulty of each n-back level (0-, 1-, or 2-back) on a scale from 1 (very easy) to 7 (very hard).
The hemodynamic responses (fNIRS signal) were recorded using the low-cost fNIRS device by biosignalplux (PLUX, Lisbon, Portugal). The fNIRS device was equipped with two sensors comprising each an infrared emitter with a peak at 860 nm, a red emitter with a peak at 660 nm, and a detector placed at 20 mm. fNIRS measures are limited to the outer cortex of the brain, roughly 5–8 mm of the brain's surface (Santosa et al., 2018), and cannot record activity in the subcortical area. The fNIRS data were recorded at 10 Hz and a resolution of 8 bits. Two sensors were maintained on participants’ foreheads using a flexible headband roughly at the Fp1 and Fp2 positions of the international 10/20 placement system, corresponding to the dorsolateral prefrontal cortex region. The fNIRS data was transmitted to the computer via Bluetooth using OpenSignals software, sent to Lab Streaming Layer, and recorded using Lab Recorder software.
2.6 Data processing
2.6.1 Behavior
For each participant and each Toulouse N-back Task session, we computed the number of correct responses and the correct response times (in
2.6.2 fNIRS
First, we converted the raw intensities for each wavelength to optical density using the formula
The data was filtered using a moving average filter with a window size of 50 samples (Pinti et al., 2019). The HbO and HbR signals were then averaged over 60-second periods for each task level (control, 0-, 1-, and 2-back) for each HDT angle value. As a baseline procedure, we used subtraction of the averaged HbO and HbR signals during the 10 s preceding the cognitive task at each HDT level. The data were averaged between two channels. Both channels were excessively noisy for five participants, and these participants were removed from the HbO and HbR analyses. All data processing was performed using MATLAB custom-made scripts.
2.6.3 Statistical analyses
Statistical analyses were carried out using JASP software. We performed repeated measures analyses of variance (rm-ANOVAs) for accuracy, response times, task load index, perceived n-back difficulty, and HbO and HbR concentration changes. The factor levels are explicited in the following section for each result. Greenhouse-Geisser correction was applied for p-values when necessary.
3 Results
3.1 Behavior
3.1.1 Accuracy
Two-way repeated measures ANOVA (0-back, 1-back, and 2-back

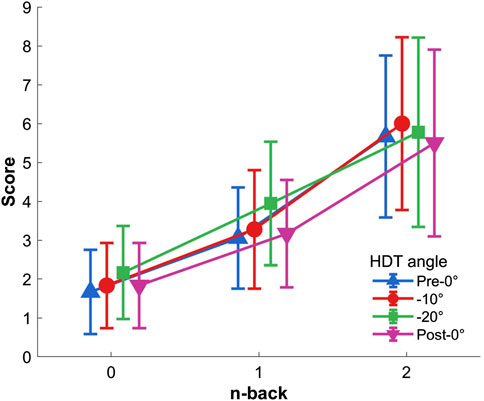
Figure 3. Response accuracy to the Toulouse N-back Task according to each level of difficulty and HDT angle. Vertical bars represent standard deviation.
3.1.2 Response times
3.1.2.1 Control reaction task included
Two-way repeated measures ANOVA (Control, 0-back, 1-back, 2-back
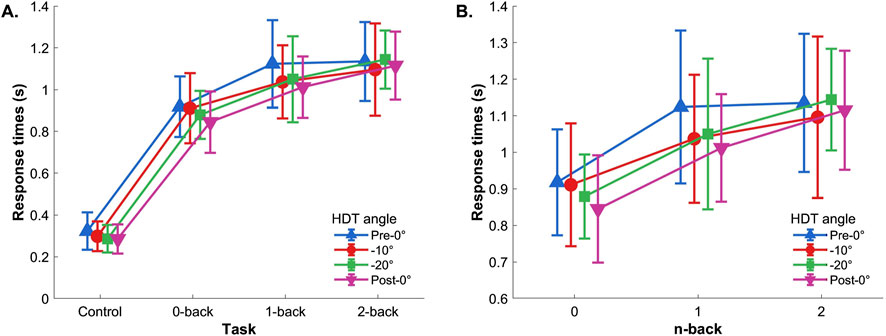
Figure 4. Reaction time. (A) With control task and (B) without control task. Vertical bars represent standard deviation.
3.1.2.2 Control reaction task excluded
If the control task was excluded from the analysis, the two-way repeated-measures ANOVA (0-back, 1-back, and 2-back
3.1.3 Subjective measures (task load index and perceived n-back difficulty)
The two-way repeated-measures ANOVA (Mental demand, Physical demand, Temporal demand, Performance, Effort, Frustration
The two-way repeated-measures ANOVA (0-back, 1-back, and 2-back
3.2 fNIRS measures
3.2.1 HbO concentration changes
The two-way repeated measures ANOVA (Control, 0-back, 1-back, and 2-back
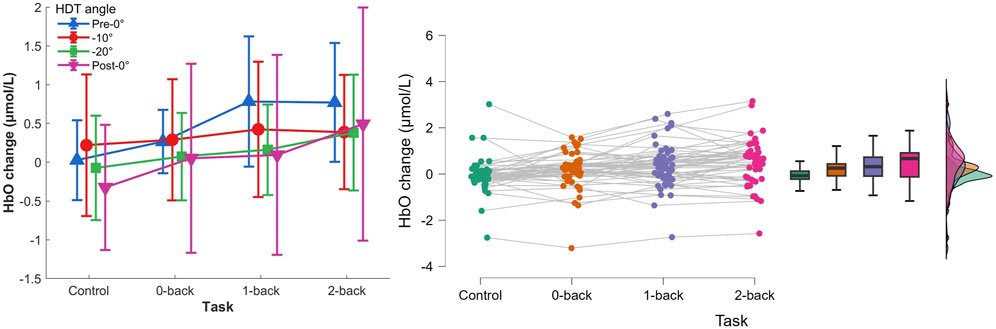
Figure 7. Left) HbO concentration changes per HDT angle and task conditions. Vertical bars represent standard deviation. Right) Raincloud plot of HbO concentration changes per task condition.
The post hoc analyses showed that HbO concentration changes were significantly higher during 1-back and 2-back tasks (
3.2.2 HbR concentration changes
The two-way repeated measures ANOVA (0-back, 1-back, and 2-back
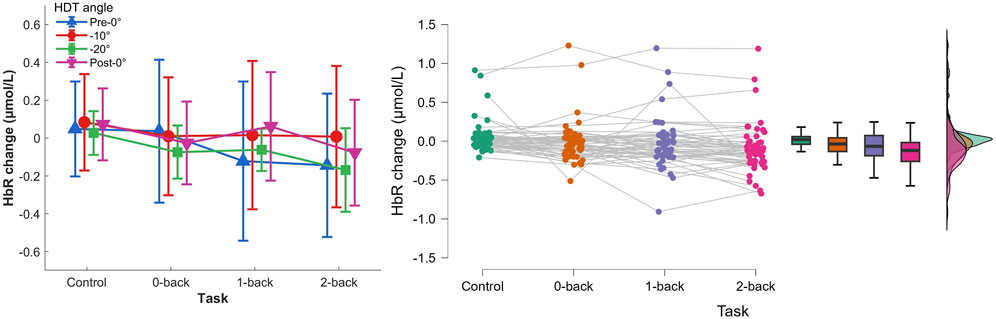
Figure 8. Left) HbR concentration changes per HDT angle and task conditions. Vertical bars represent standard deviation. Right) Raincloud plot of HbR concentration changes per task condition.
4 Discussion and conclusion
Microgravity, along with its simulations like head-down tilt, leads to cephalic fluid shift, potentially influencing the assessment of cerebral oxygenation changes. We investigated the effects of simulated microgravity on cerebral oxygenation measures using functional near-infrared spectroscopy (fNIRS) during a cognitive task. The study employed head-down tilt at various inclination levels and the Toulouse N-back Task with different difficulty levels. To record brain activity, we used a simple two-channel portable fNIRS sensor and a basic data processing pipeline, considering future applications for cognitive load monitoring in spaceflight conditions. The emphasis was on simplicity in hardware and data processing for optimal functionality. The objectives of the study were to demonstrate that the cognitive load induced by different difficulty levels yielded significantly different signal amplitudes (HbO and HbR concentration changes), and to quantify the impact of simulated microgravity on these amplitudes.
First, the behavioral results showed that the different levels of difficulty of the Toulouse N-back Task efficiently generated different cognitive loads: with increasing
Second, the results showed significant variations in oxygenated (HbO) and deoxygenated hemoglobin (HbR) levels as a function of the cognitive load. The HbO increase accompanied by the HbR decrease, in line with the literature, indicated a higher cerebral activity with higher task difficulty. The results were not affected by the head-down tilt angles. It suggests that the simple two-channel fNIRS equipment and simple data processing pipeline can be used for monitoring cognitive load without being impacted by short-term microgravity conditions. It suggests that the previous results on fNIRS measures of cognitive load obtained in normogravity conditions can be applied during spaceflight without specific correction for the fluid shift.
An experiment of longer duration could help strengthen our results and confirm that long space missions would be fully compatible with fNIRS measurements. Also, while the results of this study are promising, the fNIRS signal can present numerous artifacts induced by sensor displacement, facial grimace, or sweating. It forced us to exclude five participants from the analyses as both channels were excessively noisy and consider only one channel for analyses for seven more participants. Hence, a more sophisticated system with an increased number of channels can add redundancy and improve the chances of obtaining a clear signal. These additional channels could be placed near the locations used in this study (above the dorsolateral prefrontal cortex), but could also be added to other parts of the central executive network, a broader network activated by effortful tasks, of which the prefrontal cortex is a part. For example, a study could target the parietal cortex, another critical region of the central executive network (Causse et al., 2022). However, as stated previously, a lower number of fNIRS channels increases the likelihood of the system being accepted, as it is less intrusive.
In summary, the findings demonstrated that increasing difficulty levels in the cognitive task resulted in reduced accuracy, prolonged response times, and increased perceived difficulty scores. Task performance remained unaffected by the inclination levels. Additionally, the results revealed an increase in oxygenated hemoglobin (HbO) and a decrease in deoxygenated hemoglobin (HbR) concentrations with task difficulty, and these trends were not influenced by the head-down tilt angles. These encouraging results suggest that fNIRS measures can serve as a reliable indicator of cognitive load in microgravity conditions without the need for correction due to cephalic fluid shift.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by CPP 23.01719.000252 (Sud-Méditerranée III, France). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
VP: Conceptualization, Data curation, Formal Analysis, Investigation, Writing–original draft, Writing–review and editing. TK: Data curation, Formal Analysis, Investigation, Project administration, Writing–original draft. LM: Conceptualization, Methodology, Project administration, Writing–review and editing. LB: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing–review and editing. MC: Conceptualization, Methodology, Supervision, Writing–review and editing. AP: Writing–review and editing, Funding acquisition, Project administration. AP-L: Conceptualization, Investigation, Methodology, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was carried out and promoted by the Institute for Space Medicine and Physiology (MEDES, Toulouse France) and sponsored by the French Space Agency (CNES, Spaceship FR project).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acharya M. M., Baulch J. E., Klein P. M., Al Anoud D. B., Apodaca L. A., Kramár E. A., et al. (2019). New concerns for neurocognitive function during deep space exposures to chronic, low dose-rate, neutron radiation. Eneuro 6, 0094. doi:10.1523/ENEURO.0094-19.2019
Aghajani H., Garbey M., Omurtag A. (2017). Measuring mental workload with eeg+ fnirs. Front. Hum. Neurosci. 11, 359. doi:10.3389/fnhum.2017.00359
Antonenko P., Paas F., Grabner R., Van Gog T. (2010). Using electroencephalography to measure cognitive load. Educ. Psychol. Rev. 22, 425–438. doi:10.1007/s10648-010-9130-y
Ayaz H., Shewokis P. A., Bunce S., Izzetoglu K., Willems B., Onaral B. (2012). Optical brain monitoring for operator training and mental workload assessment. Neuroimage 59, 36–47. doi:10.1016/j.neuroimage.2011.06.023
Ayres P., Lee J. Y., Paas F., van Merriënboer J. J. (2021). The validity of physiological measures to identify differences in intrinsic cognitive load. Front. Psychol. 12, 702538. doi:10.3389/fpsyg.2021.702538
Bigelow R. T., Agrawal Y. (2015). Vestibular involvement in cognition: visuospatial ability, attention, executive function, and memory. J. Vestib. Res. 25, 73–89. doi:10.3233/VES-150544
Braver T. S., Cohen J. D., Nystrom L. E., Jonides J., Smith E. E., Noll D. C. (1997). A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5, 49–62. doi:10.1006/nimg.1996.0247
Burcal C. J., Needle A. R., Custer L., Rosen A. B. (2019). The effects of cognitive loading on motor behavior in injured individuals: a systematic review. Sports Med. 49, 1233–1253. doi:10.1007/s40279-019-01116-7
Cacao E., Cucinotta F. A. (2019). Meta-analysis of cognitive performance by novel object recognition after proton and heavy ion exposures. Radiat. Res. 192, 463–472. doi:10.1667/RR15419.1
Casler J. G., Cook J. R. (1999). Cognitive performance in space and analogous environments. Int. J. cognitive ergonomics 3, 351–372. doi:10.1207/s15327566ijce0304_5
Causse M., Chua Z., Peysakhovich V., Del Campo N., Matton N. (2017). Mental workload and neural efficiency quantified in the prefrontal cortex using fnirs. Sci. Rep. 7, 5222. doi:10.1038/s41598-017-05378-x
Causse M., Lepron E., Mandrick K., Peysakhovich V., Berry I., Callan D., et al. (2022). Facing successfully high mental workload and stressors: an fmri study. Hum. Brain Mapp. 43, 1011–1031. doi:10.1002/hbm.25703
Chikhi S., Matton N., Blanchet S. (2022). Eeg power spectral measures of cognitive workload: a meta-analysis. Psychophysiology 59, e14009. doi:10.1111/psyp.14009
Chouchou F., Pichot V., Costes F., Guillot M., Barthelemy J.-C., Bertoletti L., et al. (2020). Autonomic cardiovascular adaptations to acute head-out water immersion, head-down tilt and supine position. Eur. J. Appl. Physiology 120, 337–347. doi:10.1007/s00421-019-04278-4
Clément G., Kuldavletova O., Macaulay T. R., Wood S. J., Morales D. C. N., Toupet M., et al. (2023). Cognitive and balance functions of astronauts after spaceflight are comparable to those of individuals with bilateral vestibulopathy. Front. neurology 14, 1284029. doi:10.3389/fneur.2023.1284029
Cooke W. H., Pellegrini G. L., Kovalenko O. A. (2003). Dynamic cerebral autoregulation is preserved during acute head-down tilt. J. Appl. Physiology 95, 1439–1445. doi:10.1152/japplphysiol.00524.2003
Dehais F., Dupres A., Di Flumeri G., Verdiere K., Borghini G., Babiloni F., et al. (2018). “Monitoring pilot’s cognitive fatigue with engagement features in simulated and actual flight conditions using an hybrid fnirs-eeg passive bci,” in 2018 IEEE international conference on systems, man, and cybernetics (SMC), USA, 7-10 Oct. 2018 (IEEE), 544–549.
Donoso M., Collins A. G., Koechlin E. (2014). Human cognition. Foundations of human reasoning in the prefrontal cortex. Science 344, 1481–1486. doi:10.1126/science.1252254
Du J., Cui J., Yang J., Wang P., Zhang L., Luo B., et al. (2021). Alterations in cerebral hemodynamics during microgravity: a literature review. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 27 (e928108–1), e928108. doi:10.12659/MSM.928108
Ferrari M., Quaresima V. (2012). A brief review on the history of human functional near-infrared spectroscopy (fnirs) development and fields of application. Neuroimage 63, 921–935. doi:10.1016/j.neuroimage.2012.03.049
Fiedler P., Haueisen J., Alvarez A. M. C., Cheron G., Cuesta P., Maestú F., et al. (2023). Noise characteristics in spaceflight multichannel eeg. Plos one 18, e0280822. doi:10.1371/journal.pone.0280822
Funahashi S. (2017). Working memory in the prefrontal cortex. Brain Sci. 7, 49. doi:10.3390/brainsci7050049
Gateau T., Ayaz H., Dehais F. (2018). In silico vs. over the clouds: on-the-fly mental state estimation of aircraft pilots, using a functional near infrared spectroscopy based passive-bci. Front. Hum. Neurosci. 12, 187. doi:10.3389/fnhum.2018.00187
Ghafoor U., Yang D., Hong K.-S. (2021). Neuromodulatory effects of hd-tacs/tdcs on the prefrontal cortex: a resting-state fnirs-eeg study. IEEE J. Biomed. Health Inf. 26, 2192–2203. doi:10.1109/jbhi.2021.3127080
Ghani U., Signal N., Niazi I. K., Taylor D. (2020). Erp based measures of cognitive workload: a review. Neurosci. and Biobehav. Rev. 118, 18–26. doi:10.1016/j.neubiorev.2020.07.020
Greenberg K., Zheng R. (2022). Cognitive load theory and its measurement: a study of secondary tasks in relation to working memory. J. Cognitive Psychol. 34, 497–515. doi:10.1080/20445911.2022.2026052
Hanes D. A., McCollum G. (2006). Cognitive-vestibular interactions: a review of patient difficulties and possible mechanisms. J. Vestib. Res. 16, 75–91. doi:10.3233/ves-2006-16301
Hasan M. A., Khan M. U., Mishra D. (2020). A computationally efficient method for hybrid eeg-fnirs bci based on the pearson correlation. BioMed Res. Int. 2020, 1838140. doi:10.1155/2020/1838140
Herff C., Heger D., Fortmann O., Hennrich J., Putze F., Schultz T. (2014). Mental workload during n-back task-quantified in the prefrontal cortex using fNIRS. Front. Hum. Neurosci. 7, 935. doi:10.3389/fnhum.2013.00935
Kato T., Ogawa Y., Iwasaki K. I. (2024). Effects of the angle of head-down tilt on dynamic cerebral autoregulation during combined exposure to cephalad fluid shift and mild hypercapnia. Exp. Physiol. doi:10.1113/EP091807
Kawai Y., Setogawa A., Shimoyama R., Ueda K., Asai Y., Tatebayashi K., et al. (2003). Effects of microgravity on cerebral hemodynamics. Yonago Acta Medica 46, 1–8.
Kermorgant M., Labrunée M., Despas F., Hélissen O., Geeraerts T., Lambert E., et al. (2022). How does head position induced intracranial pressure changes impact sympathetic activity and cerebral blood flow? Aut. Neurosci. 243, 103036. doi:10.1016/j.autneu.2022.103036
Kuldavletova O., Navarro Morales D. C., Quarck G., Denise P., Clément G. (2023). Spaceflight alters reaction time and duration judgment of astronauts. Front. Physiology 14, 1141078. doi:10.3389/fphys.2023.1141078
Lee J. K., Koppelmans V., Pasternak O., Beltran N. E., Kofman I. S., De Dios Y. E., et al. (2021). Effects of spaceflight stressors on brain volume, microstructure, and intracranial fluid distribution. Cereb. Cortex Commun. 2, tgab022. doi:10.1093/texcom/tgab022
León-Carrión J., León-Domínguez U. (2012). Functional near-infrared spectroscopy (fnirs): principles and neuroscientific applications. Neuroimaging methods, 48–74. doi:10.5772/23146
London G., Levenson J., Safar M., Simon A., Guerin A., Payen D. (1983). Hemodynamic effects of head-down tilt in normal subjects and sustained hypertensive patients. Am. J. Physiology-Heart Circulatory Physiology 245, H194–H202. doi:10.1152/ajpheart.1983.245.2.H194
Mandrick K., Derosiere G., Dray G., Coulon D., Micallef J.-P., Perrey S. (2013). Prefrontal cortex activity during motor tasks with additional mental load requiring attentional demand: a near-infrared spectroscopy study. Neurosci. Res. 76, 156–162. doi:10.1016/j.neures.2013.04.006
Mandrick K., Peysakhovich V., Rémy F., Lepron E., Causse M. (2016). Neural and psychophysiological correlates of human performance under stress and high mental workload. Biol. Psychol. 121, 62–73. doi:10.1016/j.biopsycho.2016.10.002
Marshall-Goebel K., Mulder E., Bershad E., Laing C., Eklund A., Malm J., et al. (2017). Intracranial and intraocular pressure during various degrees of head-down tilt. Aerosp. Med. Hum. Perform. 88, 10–16. doi:10.3357/AMHP.4653.2017
Naismith L. M., Cheung J. J., Ringsted C., Cavalcanti R. B. (2015). Limitations of subjective cognitive load measures in simulation-based procedural training. Med. Educ. 49, 805–814. doi:10.1111/medu.12732
Park B., Brünken R. (2015). The rhythm method: a new method for measuring cognitive load—an experimental dual-task study. Appl. Cogn. Psychol. 29, 232–243. doi:10.1002/acp.3100
Pinti P., Scholkmann F., Hamilton A., Burgess P., Tachtsidis I. (2019). Current status and issues regarding pre-processing of fnirs neuroimaging data: an investigation of diverse signal filtering methods within a general linear model framework. Front. Hum. Neurosci. 12, 505. doi:10.3389/fnhum.2018.00505
Rabin B. M., Shukitt-Hale B., Gomes S., Carrihill-Knoll K. L. (2014). Cognitive effects of partial and whole-body exposures to 16o particles. J. Radiat. Res. 55, i100–i101. doi:10.1093/jrr/rrt187
Santosa H., Zhai X., Fishburn F., Huppert T. (2018). The nirs brain analyzir toolbox. Algorithms 11, 73. doi:10.3390/a11050073
Schneider S., Abeln V., Askew C. D., Vogt T., Hoffmann U., Denise P., et al. (2013). Changes in cerebral oxygenation during parabolic flight. Eur. J. Appl. physiology 113, 1617–1623. doi:10.1007/s00421-013-2588-9
Shenhav A., Musslick S., Lieder F., Kool W., Griffiths T. L., Cohen J. D., et al. (2017). Toward a rational and mechanistic account of mental effort. Annu. Rev. Neurosci. 40, 99–124. doi:10.1146/annurev-neuro-072116-031526
Strangman G. E., Sipes W., Beven G. (2014). Human cognitive performance in spaceflight and analogue environments. Aviat. space, Environ. Med. 85, 1033–1048. doi:10.3357/ASEM.3961.2014
Tanji J., Hoshi E. (2001). Behavioral planning in the prefrontal cortex. Curr. Opin. Neurobiol. 11, 164–170. doi:10.1016/s0959-4388(00)00192-6
Tays G. D., Hupfeld K. E., McGregor H. R., Salazar A. P., De Dios Y. E., Beltran N. E., et al. (2021). The effects of long duration spaceflight on sensorimotor control and cognition. Front. neural circuits 15, 723504. doi:10.3389/fncir.2021.723504
Van Ombergen A., Demertzi A., Tomilovskaya E., Jeurissen B., Sijbers J., Kozlovskaya I. B., et al. (2017). The effect of spaceflight and microgravity on the human brain. J. neurology 264, 18–22. doi:10.1007/s00415-017-8427-x
Watenpaugh D. E. (2016). Analogs of microgravity: head-down tilt and water immersion. J. Appl. Physiology 120, 904–914. doi:10.1152/japplphysiol.00986.2015
Whittle R. S., Keller N., Hall E. A., Vellore H. S., Stapleton L. M., Findlay K. H., et al. (2022). Gravitational dose-response curves for acute cardiovascular hemodynamics and autonomic responses in a tilt paradigm. J. Am. Heart Assoc. 11, e024175. doi:10.1161/JAHA.121.024175
Wray S., Cope M., Delpy D. T., Wyatt J. S., Reynolds E. O. R. (1988). Characterization of the near infrared absorption spectra of cytochrome aa3 and haemoglobin for the non-invasive monitoring of cerebral oxygenation. Biochimica Biophysica Acta (BBA)-Bioenergetics 933, 184–192. doi:10.1016/0005-2728(88)90069-2
Keywords: fNIRS, cognitive load, cerebral oxygenation, head-down tilt, n-back
Citation: Peysakhovich V, Kiehl T, Martinez LV, Boyer L, Causse M, Paillet A and Pavy-Le Traon A (2025) Short-term microgravity effects simulation does not affect fNIRS measures of cerebral oxygenation changes induced by cognitive load. Front. Physiol. 16:1425302. doi: 10.3389/fphys.2025.1425302
Received: 29 April 2024; Accepted: 24 January 2025;
Published: 19 February 2025.
Edited by:
Patricia A. Shewokis, Drexel University, United StatesReviewed by:
Ajitkumar Mulavara, KBR, Inc., United StatesTakehito Ouchi, Tokyo Dental College, Japan
Copyright © 2025 Peysakhovich, Kiehl, Martinez, Boyer, Causse, Paillet and Pavy-Le Traon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vsevolod Peysakhovich, dnNldm9sb2QucGV5c2FraG92aWNoQGlzYWUuZnImI3gwMjAwYTs=; Anne Pavy-Le Traon, cGF2eS1sZXRyYW9uLmFAY2h1LXRvdWxvdXNlLmZy
 Vsevolod Peysakhovich
Vsevolod Peysakhovich Thibault Kiehl1,2
Thibault Kiehl1,2 Mickaël Causse
Mickaël Causse Anne Pavy-Le Traon
Anne Pavy-Le Traon