- 1Department of Biology, Valparaiso University, Valparaiso, IN, United States
- 2Department of Physiology and Biophysics, Case Western Reserve University School of Medicine, Cleveland, OH, United States
- 3Auckland Bioengineering Institute, University of Auckland, Auckland, NZ, United States
This Hypothesis & Theory contribution accompanies the research paper by Bouyer et al. (Frontiers in Physiology 2024), the first to employ out-of-equilibrium (OOE) CO2/HCO3− solutions to examine systematically the intracellular pH (pHi) effects of extracellular (o) metabolic acidosis (MAc) and its components: an isolated decrease in pHo (pure acidosis, pAc) and an isolated decrease in [HCO3−]o (pure metabolic/down, pMet↓). In this study, after reviewing various types of acid–base disturbances and the use of OOE solutions, we discuss pHi “state” (ΔpHi, in response to a single acid–base challenge) and “behavior” (the ΔpHi transition observed between two successive challenges), along with approaches for quantifying state and behavior. We then discuss the molecular basis of how individual extracellular acid–base disturbances influence pHi via effects on—and interactions among—acid–base transporters, acid–base sensors, and cellular constitution. Next, we examine the determinants of states and behaviors, their impact on the buffering of extracellular acid loads, and how variability in state and behavior might arise. We conclude with a consideration of how mathematical models—despite their inherent limitations—might assist in the interpretation of experiments and qualitative models presented in this study. Among the themes that emerge are (1) hippocampal neurons must have distinct sensors for pHo and [HCO3−]o; (2) these pHo- and [HCO3−]o-driven signal transduction pathways produce additive pHi effects in naïve neurons (those not previously challenged by an acid–base disturbance); and (3) these pathways produce highly non-additive pHi effects in neurons previously challenged by MAc.
Introduction
Virtually, all biological processes—including those of the central nervous system—are sensitive to changes in pH. Mammals regulate the pH of the blood and extracellular fluid by adjusting the ratio of the two members of the key buffer pair: CO2 and HCO3−. The lungs control [CO2] by altering ventilation. The kidneys control [HCO3−] by altering the rate at which they secrete H+ into the tubule fluid and simultaneously move HCO3− into the blood.
The acid–base status of the blood and extracellular fluid has a major influence on the pH inside the cells. Thus, we expect factors that disturb the extracellular [CO2]/[HCO3−] ratio to influence the acid–base status of cells, as discussed by Bouyer et al. (2024). Conversely, as cells attempt to stabilize their own pH, they move acid–base equivalents across the plasma membrane, thereby disrupting the acid–base status of the extracellular fluid.
A recent paper by Bouyer et al. (2024) explored how one particular extracellular acid–base disturbance, termed metabolic acidosis, affects the pH of rat hippocampal (HC) neurons in primary culture. As discussed in the following sections, metabolic acidosis involves a decrease in both extracellular pH and [HCO3−]. Bouyer and his colleagues used techniques to independently lower each of these parameters and, in the process, made several interesting—and in one case, startling—observations about how single and successive bouts of metabolic acidosis (or its components) affect neuronal pHi.
The purposes of this Hypothesis and Theory contribution are twofold: (1) to provide the readers of the Bouyer paper with some background for understanding the reported findings and (2) to offer potential explanations for the sometimes unexpected observations. Note that the general principles introduced in this study—for single rat HC neurons in the Bouyer paper—ought to apply to any individual eukaryotic cell, including those that are part of more complex systems such as neuron–glial co-cultures, brain slices, intact brains, diverse epithelia, and even more complex tissues like the renal cortex and blood–brain barrier. Each cell type (including different types of neurons) may require a unique set of parameters to account for their pHi homeostatic mechanisms. Moreover, complex structures likely call for unique forms of cell–cell communication and, thus, control over transporters and sensors.
Acid–base disturbances
Metabolic acidosis (MAc) is a common and potentially life-threatening acid–base disorder in mammals, including humans. It is caused by a depletion of extracellular (o) HCO3−, which leads to a decrease in both [HCO3−]o and pHo. In a living animal, MAc generally triggers a compensatory increase in ventilation, which lowers [CO2]o and thereby mitigates the decrease in pHo. Under these conditions, all three fundamental CO2/HCO3− acid–base parameters underwent changes, making it difficult to attribute the effects of compensated MAc to decreased [HCO3−]o, decreased pHo, or decreased [CO2]o—or some combination of the three.
In vitro, we can equilibrate artificial solutions with a known partial pressure of CO2, thereby preventing changes in [CO2]o. Even under these conditions, however, MAc is usually associated with two altered parameters—a decrease in [HCO3−]o and a decrease in pHo—therefore, it is still difficult to know whether the effects of MAc are due to the reduction in [HCO3−]o per se or pHo per se.
In addition to MAc, the three other fundamental acid–base disturbances (see Boron, 2017) are metabolic alkalosis (MAlk), in which an increase in [HCO3−]o causes pHo to increase; respiratory acidosis (RAc), in which an increase in [CO2]o causes pHo to decrease; and respiratory alkalosis (RAlk), in which a decrease in [CO2]o causes pHo to increase. In all of these cases, the disturbance in an intact animal leads to changes in all three acid–base parameters, along the lines discussed in the first paragraph. In the laboratory, it is possible—under equilibrium conditions—to change two at a time.
A breakthrough occurred in 1995 with the development of a rapid-mixing approach for generating out-of-equilibrium (OOE) CO2/HCO3− solutions (Zhao et al., 1995), which—over a wide range of pH values—can have any combination of [CO2]o, [HCO3−]o, and pHo.
The use of OOE solutions offers a promising approacht o determining the extent to which individual acid–base parameters contribute to the physiological effects of MAc. The first such study was by Zhao et al. (2003), who found—on a background of a normal CO2/HCO3− solution—that the isolated removal of basolateral (BL; i.e., blood-side) HCO3− from isolated, perfused proximal tubules (PTs)—leaving [CO2]BL and pHBL unchanged—caused the rate of transepithelial HCO3− reabsorption (JHCO3), measured over ∼20 min, to increase. Thus, this challenge—the most extreme possible example of MAc but without acidosis—produced the appropriate compensatory response.
Extending the work of Zhao and her coworkers, Zhou et al. (2005) used OOE solutions in a study in which they systematically varied [CO2]BL between 0% and 20% (leaving [HCO3−]BL and pHBL fixed), varied [HCO−3]BL from 0 mM to 44 mM (leaving [CO2]BL and pHBL fixed), or varied pHBL from 6.8 to 8.0 (leaving [CO2]BL and [HCO−3]BL fixed). Surprisingly, they found that acute1 changes in pHBL had no effect on JHCO3 over the ∼20-min duration of the challenges. However, starting at conditions that mimicked the composition of normal arterial blood—[CO2]o = 5%, [HCO3−]o = 22 mM; pHo = 7.40—isolated changes in [CO2]o or [HCO3−]o produced the appropriate compensatory effects:
(1) Isolated decrease in [HCO3−]o ([CO2]o and pHo constant). Bouyer et al. (2024) named this disturbance “pure metabolic/down (pMet↓).” It is the metabolic part of MAc but without acidosis. Both Zhao et al. (2003) and Zhou et al. (2005) found that pMet↓ caused JHCO3 to increase, which would tend to compensate for MAc.
(2) Isolated increase in [HCO3−]o ([CO2]o and pHo constant). Bouyer et al. (2024) introduced the term “pure metabolic/up (pMet↑)” in their nomenclature to describe this disturbance. It is the metabolic part of MAlk but without alkalosis. Zhou et al. (2005) found that pMet↑ caused JHCO3 to decrease, which would tend to compensate for MAlk.
(3) Isolated increase in [CO2]o ([HCO3−]o and pHo constant). Bouyer et al. (2024) did not propose a name for this disturbance, but we suggest “pure respiratory/up (pResp↑),” where we understand the arrow as pertaining to [CO2]o. It is the respiratory part of RAc but without acidosis. Zhou et al. (2005) found that pResp↑ caused JHCO3 to increase, which would tend to compensate for RAc.
(4) Isolated decrease in [CO2]o ([HCO3−]o and pHo constant). Bouyer et al. (2024) did not propose a name for this disturbance, but we suggest “pure respiratory/down (pResp↓),” where we again understand the arrow as pertaining to [CO2]o. It is the respiratory part of RAlk but without alkalosis. Both Zhao et al. (2003) and Zhou et al. (2005) found that pResp↓ caused JHCO3 to decrease, which would tend to compensate for RAlk.
In a somewhat different protocol, Bouyer et al. (2003) started with a rabbit PT exposed on both the apical (i.e., lumen) and basolateral sides to a CO2/HCO3−-free solution. Adding equilibrated CO2/HCO3− to the basolateral side caused a rapid increase in [Ca2+]i, whereas adding CO2/HCO3− to the lumen had no effect on [Ca2+]i. Switching to an OOE basolateral solution that contained physiological CO2 but not HCO3− (“pure CO2”) replicated the increase in [Ca2+]i, whereas switching to an OOE basolateral solution that contained physiological HCO3− but not CO2 (“pure HCO3−”) had little effect on [Ca2+]i. Thus, it may be that it is basolateral CO2—in part acting through Ca2+—that triggers an increase in JHCO3 in PTs. With our current knowledge of receptor protein tyrosine phosphatase γ (RPTPγ), we would now hypothesize that—if we started with equilibrated CO2/HCO3− in the luminal and basolateral solutions—an isolated decrease in [HCO3−]o would have the same effect on [Ca2+]i as would increasing [CO2]o.
The results of Zhao et al. (2003), Bouyer et al. (2003), and Zhou et al. (2005) were the first to unequivocally demonstrate that, independent of pH, each of the two components of the major blood buffer—CO2 and HCO3−—can act as acute, potent modulators of a biological function.
Neuronal pHi homeostasis in the face of metabolic acidosis
In an earlier study of cultured rat neurons, Bouyer and colleagues (2004) examined the effects of all four fundamental acid–base disturbances on the pHi of both medullary-raphé (MR) neurons and HC neurons. For MAlk, RAc, and RAlk (but not MAc), both MR and HC neurons exhibited fully reversible pHi changes, with ΔpHi/ΔpHo ratios of ∼60%. For MAc, the responses were more intriguing. Although most MR neurons and some HC neurons exhibited a ΔpHi/ΔpHo of ∼65%, some MR neurons and most HC neurons exhibited a ΔpHi/ΔpHo of only ∼9% (Bouyer et al., 2004). Later, Salameh and colleagues (2014) coined the terms “MAc-sensitive” and “MAc-resistant” to describe cells like those reported by Bouyer in response to a single acid–base challenge. Interestingly, and apropos of the most recent paper by Bouyer et al. (2024), Bouyer’s 2004 neurons that we would now term MAc-resistant, when switched from a MAc solution to a control solution, they often exhibited a pHi rebound to a value above the initial baseline pHi. A theoretical analysis led Bouyer et al. (2004) to hypothesize that the MAc-resistant neurons have a sensor for extracellular HCO3− and that a decrease in [HCO3−]o triggers an immediate stimulation of neuronal acid–base transporters that minimizes the MAc-induced decrease in pHi.
Salameh et al. (2014), based on observed MAc-induced pHi changes in 10 cell types, proposed that the demarcation between MAc-resistant and MAc-sensitive is a (ΔpHi)/(ΔpHo) of 40%. They pointed out that any such quantitative criterion is somewhat arbitrary.
Salameh et al. (2014) also extended the protocol of Bouyer et al. (2004) by including two successive MAc challenges, MAc1 and MAc2, separated by a period of recovery in a control CO2/HCO3− solution. Comparing the pHi induced by MAc2 vs. MAc1, they categorized neurons as “adapting” to the MAc challenge when ΔpHi during MAc2—(ΔpHi)2/MAc—was sufficiently smaller in magnitude than (ΔpHi)1/MAc, being “consistent” if the two ΔpHi values were reasonably close and “decompensating” if the magnitude of (ΔpHi)2/MAc was sufficiently greater than that of (ΔpHi)1/MAc.
In their recent paper, Bouyer et al. (2024) expanded upon previous work by examining substitutions of pAc or pMet↓ for MAc in HC rat neurons in primary culture. They referred to resistance and sensitivity as two relative “states” of neurons, defined for single challenges (e.g., MAc1 and MAc2). They also referred to adaptation, consistency, and decompensation, defined for the transition from the first to the second challenge, as three “behaviors.”
In her PhD dissertation, Taki (2024) examined the twin challenges of MAc and RAc in murine co-cultures of HC neurons and astrocytes. Analyzing their data along the lines of Bouyer et al. (2024), Taki et al. found that the global knockout of RPTPζ, a candidate sensor of [CO2]o and [HCO3−]o expressed mainly in the central nervous system (CNS), led to much larger acidifications than those observed in cells from WT mice.
In the following sections2, we provide a more formal presentation of state and behavior, along with methods for assessing them.
Out-of-equilibrium solutions
“The basics”
In the paper by Bouyer et al. (2024), the major contribution is the use of OOE solutions to dissect the contributions of the two components of MAc: the decreased pHo per se and the decreased [HCO3−]o per se. The key to understanding OOE technology is the fact that the interconversion between CO2 and H2O, on one hand, and H+ and HCO3−, on the other hand, involves two reactions, one of which is very slow and the other is very fast. The OOE approach separates chemical species on opposite sides of the slow reaction in the following sequence:
Although we can independently control [CO2]o, [H+]o (i.e., pHo), and [HCO3−]o per se, we have less influence over other chemical species that depend directly or indirectly on any of the entities in the preceding two-step reaction. An important example is [CO3=]o, which depends on both [H+]o and [HCO3−]o:
Moreover, the concentration of the NaCO3− ion pair depends on both [Na+]o and [CO3=]o (as in Equation 3):
If we assume for a moment that the second reaction in Equation 1 is infinitely slow—and if the reaction sequence in Equation 1 represents the only significant pathway between CO2/H2O and H+/HCO3−—then it is easy to observe how we could control [CO2] independently of [H+] and [HCO3−] and vice versa. The “slow” reaction in Equation 1 is slow enough that we can exploit it to make OOE solutions. The principle behind the OOE approach is to mix, with sufficient speed, two dissimilar CO2/HCO3−/pH solutions.
Other reactions and considerations
In addition to the reactions shown in Equation 1, which is typically the pathway shown in textbooks (see Boron, 2017), a parallel mechanism also converts CO2 to HCO3−:
The concentration values below the braces approximately correspond to a physiological partial pressure of CO2 (PCO2) and a pH value of 7.5 at 25°C. Multiplying these concentration values by the forward rate constant yields a reaction velocity of
In the case of the first reaction in Equation 1,
the forward rate constant predicts a reaction rate of
H2CO3, the product of the “CO2 + H2O” reaction in Equation 6, would rapidly break down to form H+ and HCO3−. Thus, it is reasonable to compare the velocity (Equation 7) of the “CO2 + H2O″ reaction in Equation 6 with that of the “CO2 + OH−” reaction in Equation 4, which is only ∼3.5% as fast (Equation 5). This is why the OH−reaction in Equation 4 is generally ignored at physiological blood pH. However, the OH−pathway in Equation 4 is strikingly pH-sensitive because as pH increases, [OH−] increases exponentially. Thus, at a pH value of 9.0, the velocity of the “CO2 + OH−” reaction in Equation 4 is already 11% greater than that of the “CO2 + H2O” reaction in Equation 6. At pH 10.0, it is 11-fold faster and so on.
The pH sensitivity of the OH−reaction has important implications for generating OOE solutions. Imagine that you wanted to generate a “pure CO2 solution,” one with a physiological [CO2] but almost no HCO3− at pH 7.4. You might be inclined to mix a CO2 solution at low pH (e.g., 5.4, where most of the carbon would be in the form of CO2) with a CO2/HCO3−-free solution at a very high pH (e.g., 10). However, you will find that your final [CO2] will be much lower than expected, whereas your final [HCO3−] will be much higher. The reason, it seems, is that the macroscopic mixing process described in Figure 1 initially generates microdomains that contain unmixed versions of the acidic/high-CO2 solution and the very alkaline solution. At the interface, the reaction CO2 + OH− → HCO3− unexpectedly consumes your CO2 and disrupts your anticipated near-HCO3−-free state.
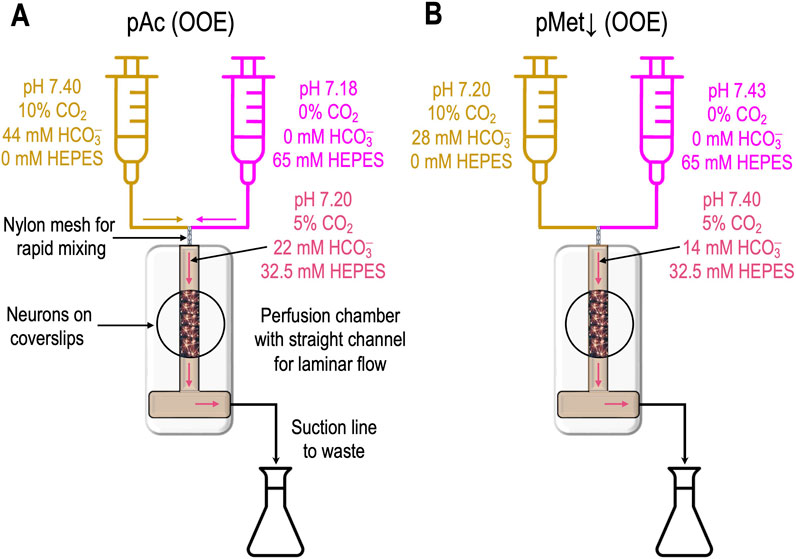
Figure 1. Generation of out-of-equilibrium solutions (A). A heavy-duty dual syringe pump drives (at identical rates) two syringes, the acid–base contents of which are summarized by the gold-colored and magenta labels. The blended gold/magenta labels indicate the composition of the solution at the instant of mixing. The continuously generated OOE solution flows past the neurons in the chamber, before being discarded into a waste receptacle and then suctioned into an external waste container. (B) Generation of a pure metabolic/down (pMet↓) solution. The approach is the same as in panel A except for the contents of the two syringes.
Another reaction can also wreak havoc with the creation of OOE solutions. Assume that you wanted to generate a “pure HCO3−” solution, one with a physiological [HCO3−] but almost no CO2 at pH 7.4. You might be inclined to mix an HCO3− solution at a high pH value (e.g., 9.4, where most of the carbon would be in the form of HCO3− and CO3=) with a CO2/HCO3−-free solution at a very low pH value (e.g., 5). However, you will find that your final [HCO3−] will be much lower than expected, whereas your final [CO2] will be much higher. The reason is that the hypothesized microdomains contain unmixed versions of the alkaline/high-HCO3− solution and the very acidic solution. At the interface, the reaction H+ + HCO3− → H2CO3 rapidly consumes HCO3− while increasing [H2CO3] to very high levels, whereupon the reaction H2CO3 → CO2 + H2O disrupts your anticipated near-CO2-free state.
The challenges described in the preceding two paragraphs are discussed in conjunction with figure 1 in Zhao et al. (2003).
“Pure acidosis”
Figure 1A shows how to generate a “pure acidosis” (pAc) solution by rapidly mixing “solution 5a” and “solution 5b,” as defined in table3 1 in the paper by Bouyer et al. (2024). At the instant the two solutions combine, the “mixture” comprises (except for pH, which is complicated by buffer reactions) ½ A and ½ B. By trial and error (and making small pH adjustments to solution 5b, which contains the non-HCO3− buffer HEPES), one can achieve the desired pHo (i.e., 7.20 in the case of pAc) and the desired [CO2]o of (10% + 0%)/2 = 5% and the target [HCO3−]o of (44 mM + 0 mM)/2 + 22 mM.
The abovementioned solution is out of equilibrium at the instant of mixing but gradually degrades to equilibrium as the solution approaches the experimental chamber over a period of ∼100 ms. The [CO2]o/[HCO3−]o ratio dictates a pH value of 7.4, although the actual pHo value is 7.20 (i.e., higher [H+]o). Because [H+]o is too high for the extant [CO2]o/[HCO3−]o ratio, the chemical reaction as decribed in Equation 8
proceeds (i.e., to consume excess H+ so that pHo will slowly increase) until both the CO2/HCO3− and HEPES buffer systems are simultaneously in equilibrium. We estimate that slight (∼1%) degradation occurs as the newly mixed solution approaches the chamber and that another 1% degradation may occur as the solution flows through the chamber for removal at the other end. Thus, this technology continuously generates the desired OOE solution “online”.
“Pure metabolic/down”
Figure 1B illustrates how to generate a “pure metabolic/down” (pMet↓)4 solution by mixing “solution 6a” and “solution 6b,” as defined in table 1 in Bouyer et al. (2024). The approach is similar to that outlined above for pAc, except that our titration targets a pHo value of 7.40 and a [HCO3−]o value of (28 mM + 0 mM)/2 + 14 mM. In this case, the [CO2]o/[HCO3−]o ratio of (5%)/(14 mM) dictates a pH value of 7.20, although the actual pHo value is 7.40 (i.e., lower [H+]o). Because [H+]o is too low for the extant [CO2]o/[HCO3−]o ratio, the chemical reactions as decribed in Equation 9
proceed (i.e., to generate H+ so that pHo will slowly decrease) until both the CO2/HCO3− and HEPES buffer systems are simultaneously in equilibrium.
Zhao and colleagues (2003) examined many of the technical details of employing OOE solutions, particularly in isolated, perfused renal PTs.
State and behavior
State
“State” describes the degree of pHi change—resistant vs. sensitive—as it applies to each challenge. The state is not a quantum value—like the distinct “on” and “off” positions of a light switch—but rather like the relative brightness of a light controlled by a dimmer mechanism. The distribution of pHi changes in response to MAc is more or less continuous, and the designation as resistant or sensitive is a semi-quantitative description.
In Figure 2A, we reproduce—for the reader’s convenience—three pHi recordings from Bouyer et al. (2024). The blue record represents one of the neurons in the MAc–MAc protocol of figure 3a of Bouyer et al. (2024). The red record is from the pAc. MAc protocol of figure 6a. The green record is from the MAc-pMet↓ protocol of figure 9a.
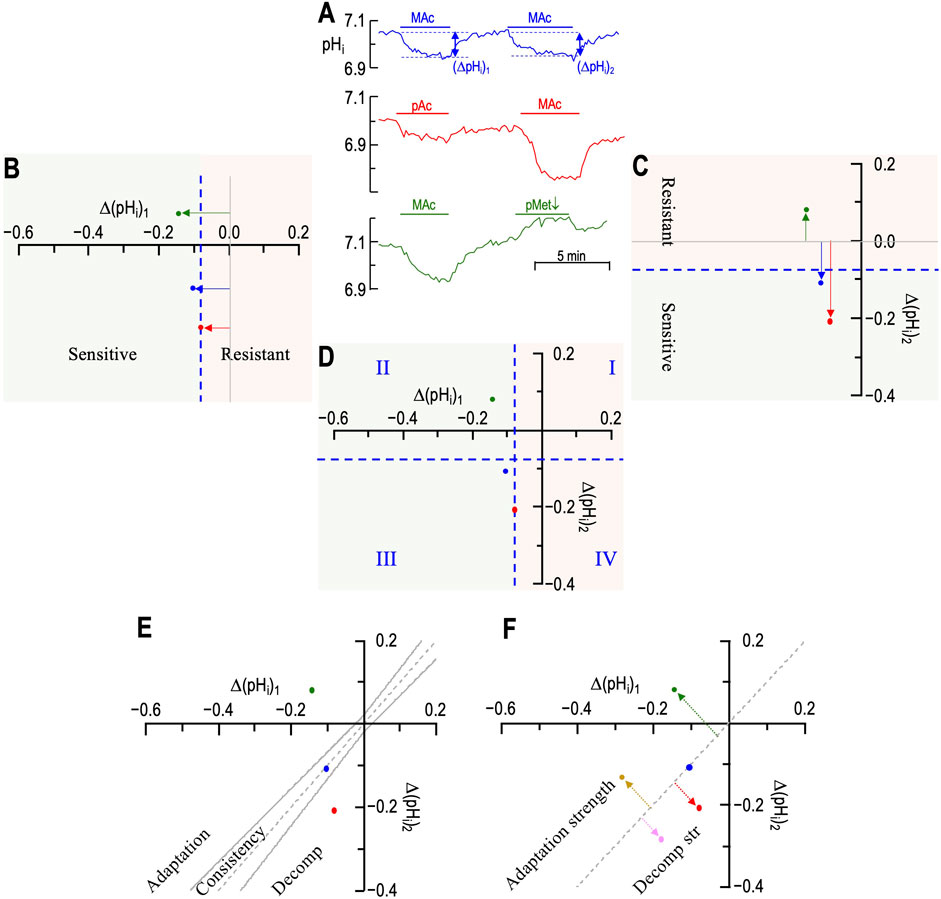
Figure 2. Plots of state, behavior, and behavior strength. (A) Three examples of experimental pHi recordings. The blue record is from figure 3a of Bouyer et al. (2024); red, from figure 6a and green from figure 9a. (B) Graphical plot of “states” during the first challenge. The three horizontal arrows, with their tails on (ΔpHi)1 = 0, indicate the magnitude and direction of the pHi change during challenge #1. According to the convention of Salameh et al., 2014, the vertical dashed blue line—again drawn at (ΔpHi)/(ΔpHo) = 40%—is the demarcation between the “resistant” and “sensitive” states. The green point in the positive territory indicates paradoxical alkalinization. (C) Graphical plot of “states” during the second challenge. The three vertical arrows, with their tails on (ΔpHi)2 = 0, indicate the magnitude and direction of the pHi change during challenge #2. According to the convention of Salameh et al., 2014, the horizontal dashed blue line—drawn at (ΔpHi)/(ΔpHo) = 40%—is the demarcation between the “resistant” and “sensitive” states. (D) “State” diagram for twin challenges. This panel is an overlay of the previous two. I–IV indicate the quadrants formed by the two dashed blue lines. For example, the blue point in QIII represents a neuron for which the state was sensitive for both challenges. (E) Hourglass plot for ‘behavior.’ The gray dashed line is the line of identity. For points lying on it (e.g., approximately true for blue point), (ΔpHi)2 = (ΔpHi)1. Points lying within the hourglass—formed by the upper and lower confidence limits defined by Salameh et al., 2014—define consistency of pHi changes between the two challenges. Points above the hourglass represent adaptation; points below the hourglass represent decompensation (decomp). (F) Behavior strength (d±, table 2 in Bouyer et al. (2024)). The arrows are orthogonal to the line of identity. Arrow length (units: pH) indicates adaptation strength (gold and green) or decompensation strength (pink and red points taken from figure 3 of Bouyer et al., 2024).
In Figure 2B, the single axis (i.e., x-axis) represents the (ΔpHi)1 for each of the three neurons in panel A. Following the “40%” definition of Salameh et al. (2014), the vertical dashed blue line represents the demarcation between “resistant” and “sensitive” neurons for (ΔpHi)1. Because ΔpHo was 0.2, this blue line is 40% × 0.2 = 0.08 pH units to the left of where the y-axis would be (represented by the vertical gray line). The 40% figure emanates from a study of multiple cell lines and represents a natural break in the data (see Salameh et al., 2014). Because this figure is somewhat arbitrary, one could imagine adjusting it to match the degree of MAc or the nature of a disturbance (e.g., MAc vs. MAlk vs. RAc). We have chosen to adhere to the original definition to facilitate data comparisons.
• The red point representing (ΔpHi)1/pAc lies just to the left of the vertical dashed blue line because pAc1 resulted in a pHi decrease of 0.084 (i.e., the point is 0.084 to the left of the vertical gray line).
• The blue point lies slightly more to the left because (ΔpHi)1/MAc was −0.105.
• The green point lies further to the left because (ΔpHi)1/MAc was −0.141.
All three neurons are in the green (ΔpHi)1 sensitive zone.
In Figure 2C, the single axis (i.e., y-axis) represents (ΔpHi)2 for each of the same three neurons in panel A. The horizontal dashed blue line represents the demarcation between “resistant” and “sensitive” neurons for (ΔpHi)2 and is 0.08 pH units below where the x-axis would be (represented by the horizontal gray line).
• The red point representing (ΔpHi)2/MAc lies well below the horizontal dashed blue line because MAc2 resulted in a pHi decrease of 0.208 (the point is 0.208 below the horizontal gray line).
• The blue point lies only slightly below the blue line because (ΔpHi)2/MAc was −0.108.
• The green point lies paradoxically above the horizontal gray line because (ΔpHi)2/pMet↓ was +0.085.
The blue and red neurons are both in the green (ΔpHi)2 sensitive zone, whereas the green neuron is in the peach-colored (ΔpHi)2 resistant zone (which also includes paradoxical alkalinizations).
Figure 2D shows an overlay of panels B and C. The intersecting blue dashed lines now define four quadrants (Q):
• I. Any neurons in QI are resistant for both (ΔpHi)1 and (ΔpHi)2.
• II. Sensitive during (ΔpHi)1 → resistant during (ΔpHi)2.
• III. Sensitive during both (ΔpHi)1 and (ΔpHi)2
• IV. Resistant during (ΔpHi)1 → sensitive during (ΔpHi)2
Behavior
“Behavior” describes the change in ΔpHi in the transition from the first to the second challenge. By definition, behavior has meaning only for two or more challenges. We term the graphical representation of behavior the “hourglass plot” (Figure 2E), which we build around the line of identity (LoI) that describes an experimental result, in which (ΔpHi)2 = (ΔpHi)1. This is the dashed gray line running from the lower left, through the origin, to the upper right. The curved parts of the hourglass represent confidence limits, as defined by Salameh et al. (2014) and described mathematically in equations 1 and 2 of the paper by Bouyer et al. (2024). Although the precise values of confidence limits are somewhat arbitrary, the hourglass provides an indication of the following behaviors:
• A “consistent” behavior is one in which the point representing the neuron lies within the hourglass, as typified by the blue neuron, which lies on the LoI.
• An “adaptive” behavior is one in which (ΔpHi)2 is sufficiently larger (in the algebraic sense) than (ΔpHi)1, that is, the point lies above the hourglass. The green neuron, although hardly typical, exhibits adaptation. A more typical example would fall between the x-axis and the upper bound of the hourglass.
• A “decompensating” behavior is one in which (ΔpHi)2 is sufficiently smaller (in the algebraic sense) than (ΔpHi)1, that is, the point lies below the hourglass, as typified by the red neuron.
Note that—as defined by Salameh et al. (2014)—a change in state does not necessarily produce an adaptive or decompensating behavior (the change in ΔpHi must be sufficiently large). Conversely, the behavior can be adaptive or decompensating, although the state does not change (e.g., a point can be above or below the hourglass in QI).
Behavior strength
The hourglass analysis provides a useful visual display. However, from a quantitative perspective, it categorizes a cell only in a ternary fashion (i.e., adaptive, consistent, and decompensating) and can categorize a population only by referring to fractions of cells with particular behaviors. Bouyer et al. (2024) introduced two variations in these concepts, in which one computes the distance of a point to the LoI. Figure 2F shows five points. Blue, red, and green represent the three neurons from panel A; the pink and gold points are two arbitrary examples from figure 3b of the recent Bouyer paper. The dashed line associated with each point represents the distance from the point to the LoI.
In one variation, the distance is unsigned (dAbsolute)—all values are positive distances—so that average dAbsolute describes the dispersion of the points from the LoI.
In the other variation, the distance is signed d±. Positive d± values (e.g., gold and green points)—represent points above/to the left of the LoI and thus describe the strength of adaptation. Negative values (e.g., pink and red points) represent points below/to the right of the LoI and thus describe the strength of decompensation. The blue point lies virtually on the LoI and thus has a d± value of ∼0. The mean d± value of a population describes the overall direction and “behavior strength”—a term coined in the dissertation by Taki (2024). An advantage of the d± approach is that one can perform statistical tests on populations of cells (e.g., wild-type vs. knockout).
Molecular basis of the effects of extracellular acid–base disturbances
We propose that the acute1 response (e.g., state and behavior/d±) of a cell to single or paired acid–base disturbances depends on a combination of three factors:
(1) near-instantaneous effects on the extracellular surface of acid–base transporters, both acid extruders (factor ‘1a’) and acid loaders (factor ‘1b’);
(2) extremely rapid effects on sensors (factor ‘2’) that detect changes in extracellular parameters and then rapidly modulate the transporters in factor ‘1’; and
(3) more slowly developing changes in cellular parameters that we will term “cellular constitution”—the collection of all ion-concentration, metabolic, and signaling properties that modulate factors ‘1’ and ‘2’ over the course of the challenge and that may persist to varying extents after the removal of the challenge. Note that the actions of factors ‘1’ and ‘2’ contribute to the constitution (factor ‘3’).
An important principle is that only factors ‘1’ and ‘2’ can influence pHi over the first few seconds of a challenge. Later, gradually developing changes comprising ‘3’ can contribute not only to the pHi time course during the challenge but also to the response to a subsequent challenge.
Before discussing factor ‘1’ through ‘3,’ we begin by considering the influences that cause pHi to change or remain stable.
Fundamental law of pHi regulation
Figure 3A illustrates the major acid-extrusion and acid-loading mechanisms in a cell such as a CNS neuron. Two reviews consider the detailed properties of these transporters, including sensitivity to acid–base challenges (Ruffin et al., 2025; Thornell et al., 2025).
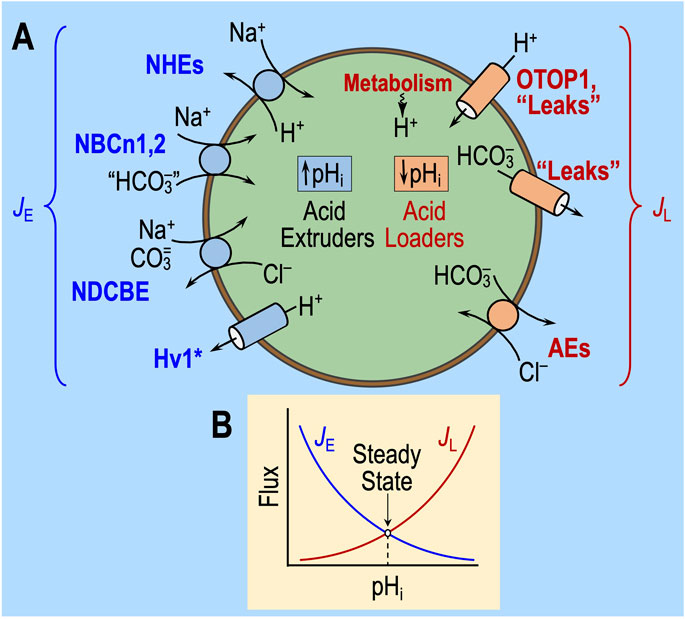
Figure 3. Regulation of intracellular pH. (A) Cell model of acid extruders (blue, on left) and acid loaders (red, on right). Acid extruders (some of which are shown in this figure) mediate the efflux of acid equivalents or the uptake of alkali equivalents. Acid loaders (some of which are shown in this figure, including cellular metabolism) mediate the uptake of acid equivalents or the efflux of alkali equivalents. Absent from this drawing are the electrogenic Na/HCO3 cotransporters, which seem not to make a major contribution in neurons but are extremely important in astrocytes. Some, if not all, of the Na+-coupled “HCO3−” transporters actually carry carbonate (CO3=) or the NaCO3− ion pair. H+/monocarboxylate cotransporters are not present in this diagram. MCT1 in astrocytes mediates the efflux of lactate and H+ and thus operates as an acid extruder. The closely related MCT2 mediates the uptake of this lactate in neurons, where it behaves as an acid loader. *The voltage-gated proton channel Hv1 opens only at depolarized voltages and exhibits outward rectification (i.e., it operates as an acid extruder). (B) Kinetic model of pHi regulation. The transmembrane flux is on the y-axis and pHi on the x-axis. The shapes of the curves are for illustration only. JE, rate of acid extrusion from all sources; JL, rate of acid loading from all sources. When JE = JL, pHi is stable. Surface/volume ratio and buffering power have no influence on steady-state pHi. Cl/HCO3 exchanger (AE); HV1, voltage-gated H+ channel; Na-H exchangers (NHE); electroneutral Na-HCO3 cotransporter (NBCn); Na-driven Cl/HCO3 exchanger (NDCBE); other H+ channels (OTOP1).
As described previously (Roos and Boron, 1981; Boron, 2004; Bevensee and Boron, 2013; Occhipinti et al., 2020; Thornell et al., 2025), the fundamental law of pHi regulation is
Here, dpHi/dt is the time rate of change of pHi; ρ is the surface-to-volume ratio of the cell; β is total intracellular buffering power; JE is the sum of the rates of all individual acid-extrusion processes (the rates of which are JE1, JE2, etc.), such as those on the left side of Figure 3A; and JL is the sum of the rates of all individual acid-loading processes (the rates of which are JL1, JL2, etc.), such as those on the right side of Figure 3A.
As illustrated in Figure 3B, JE tends to increase as pHi decreases, whereas JL tends to have the opposite pHi dependence. In a steady state (i.e., when dpHi/dt = 0), pHi is stable because JE = JL. An acid–base challenge can initiate a change in pHi (i.e., displace dpHi/dt from 0) only by altering JE and/or JL, which, in turn, can occur only by producing near-instantaneous effects on transporters (factor ‘1,’ above) or sensors that rapidly regulate transporters (factor ‘2’). The subsequent time course of pHi depends on evolving changes in JE and JL, which, in turn, must reflect changes in cellular properties—for example, ΔpHi, Δ[HCO3−]i, Δ[CO3=], and other downstream parameters—that secondarily modulate the pHi dependence and other kinetic properties of transporters. Thus, the evolving pHi dependencies of JE and JL determine the new steady-state pHi, at which JE and JL come into balance during the challenge. These evolving changes could not only affect what we observe as the “state” during challenge #1, but they could also be sufficiently long-lasting to affect the “state” during challenge #2, thereby revealing themselves as “behavior.”
Note that changes in ρ or β cannot affect steady-state pHi and, thus, cannot underlie a resistant/sensitive phenotype (i.e., state) or an adaptive/consistent/decompensative phenotype (i.e., behavior).5
Factor ‘1’: effects on acid–base transporters
In the following analyses, the effects of acid–base challenges on transporters would be rapid-onset/rapid-offset but, as noted in the previous section, could evolve during the challenge.
“Acidosis” (Ac)
In the absence of CO2/HCO3−, the only major acid–base transporters operative would be Na-H exchangers (NHEs) and H+ channels (Figure 4), as well as MCT2 monocarboxylate cotransporters, which mediate the cotransport of H+ and lactate. Although the physiological role of MCT2 is to import into neurons lactate generated by astrocytes (Ransom, 2017), the solutions in the paper by Bouyer et al. (2024) contain no lactate. Thus, to the extent that it operates, MCT2 would mediate H+/lactate efflux and—like the Na-H exchangers—function as an acid extruder. Independent of any allosteric effects, lowering pHo would slow H+ efflux via both routes and thereby tend to lower pHi, as indeed Bouyer et al. (2024) observed during Ac1.
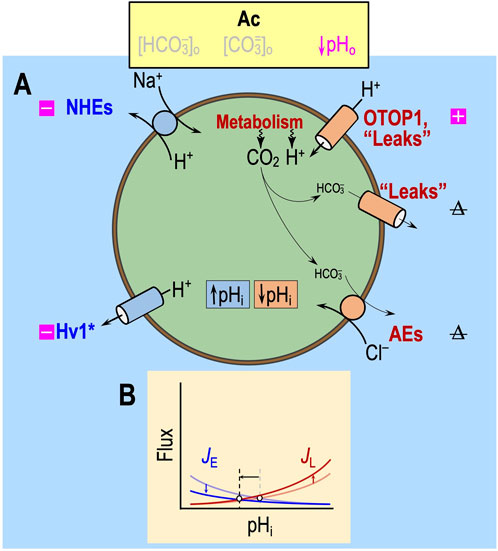
Figure 4. Effect of acidosis in the absence of CO2/HCO3− (Ac) on transporters. (A) Cell model. In the nominal absence of extracellular CO2/HCO3−, “HCO3−” transporters have a much-reduced effect on pHi homeostasis. The metabolic production of CO2, via the overall reaction CO2 + H2O → H+ + HCO3− (likely catalyzed by carbonic anhydrases), produces HCO3− but at levels that are most likely far lower than those observed under more physiological conditions. Thus, acid loading via HCO3− efflux is likely to be very low. The metabolically produced CO2 itself exits the cell passively, either via the lipid phase of the membrane or channels (see Michenkova et al., 2021), and has no direct effect on pHi. Not shown in this figure—the solutions used by Bouyer et al. (2024) did not contain lactate—is the H+/monocarboxylate cotransporter MCT2, which physiologically mediates lactate uptake into neurons and would likely be stimulated by acidosis. (B) Kinetic model. In this figure, with reduced “HCO3−” transport even under control conditions, we show markedly reduced JE (rate of acid loading from all sources) and JL (rate of acid extrusion from all sources), as indicated by the semi-transparent blue and red curves. The more deeply colored curves indicate a JE decrease and an JL increase due to the effects of Ac on the pathways in panel (A). The horizontal arrow represents the anticipated effect on steady-state pHi. Note that the removal of CO2/HCO3− may lower, have no effect on, or increase steady-state pHi, depending on the initial pHi and acid–base physiology of the cell. Boxes with “minus” symbols indicate inhibition, and magenta indicates a pHo effect. Boxes with “plus” symbols indicate the corresponding stimulation. The struck-out Δ indicates no change. In the marquee, we indicate nominally absent parameters in gray. HV1, voltage-gated H+ channel; JE, acid-extrusion rate; JL, acid-loading rate.
“Pure acidosis” or ↓pHo (pAc)
In the presence of CO2/HCO3− (Figures 5A, B), pAc would exhibit all the effects of Ac (↓ JE and ↑ JL), presumably tending to lower pHi. In addition, pAc would lead to a modest decrease in [CO3=]o, which (because the Na+-coupled HCO3− transporters appear to carry a form of CO3=; see Lee et al., 2023) would lead to a further (with respect to the one that we predict in Ac), albeit modest, decrease in JE and, thus, a decrease in pHi. Finally, it is possible that the decrease in pHo would have allosteric effects on various acid–base transporters, although we cannot infer the net direction without resorting to a more sophisticated quantitative approach (see Discussion).
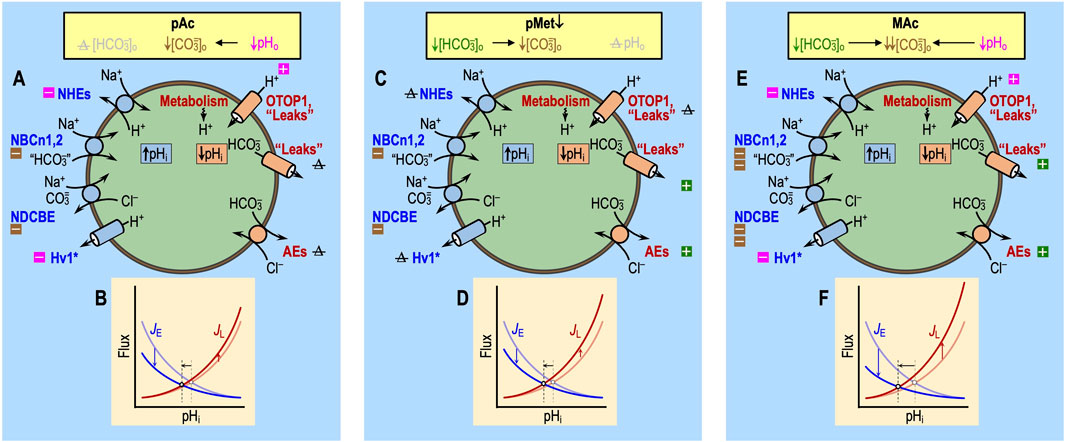
Figure 5. Effect of pAc, pMet↓, and MAc on transporters. Note that the only factors that can contribute to the initial (i.e., near-instantaneous) dpHi/dt induced by an extracellular challenge are those that immediately impact proteins facing the extracellular fluid: (1) acid–base transport pathways (including “leaks”), like those in the incomplete list shown here, and (2) rapidly responding extracellular sensors, like those shown in Figure 6. Later during the challenge, other pathways can come into play as cellular constitution changes and indirectly impacts acid–base transporters. (A) Pure acidosis: cellular model. The decrease in pHo per se (magenta symbols) will produce the direct inhibition of Na-H exchangers (NHEs) and the voltage-gated H+ channel Hv1 and direct stimulation of other H+ channels like OTOP1 and “leakage” (i.e., unidentified) pathways. Indirectly, the decreased pHo will lower [CO3=]o (brown symbols), which will slow Na+-driven HCO3− transporters—the Na+-driven Cl-HCO3 exchanger NDCBE and the electroneutral Na/HCO3 cotransporters NBCn1 and NBCn2—that are known or believed to carry some form of CO3=. We expect true HCO3− pathways to be unaffected by pAc because [HCO3−]o per se does not change. Note: pAc indicates an isolated pHo decrease in the presence of CO2 and HCO3− not to be confused with Ac in Figure 4, which indicates an isolated pHo decrease in the absence of CO2 and HCO3−. (B) Pure acidosis: kinetic model. The semi-transparent curves—blue for JE (rate of acid loading from all sources) and red for JL (rate of acid extrusion from all sources)—represent control conditions and are the same as in Figure 3B. The more deeply colored curves indicate a JE decrease and a JL increase, both are consequences of the effects of pAc on the pathways in panel (A). The horizontal black arrow represents the anticipated effect on steady-state pHi. (C) Pure metabolic/down: cell model. The decrease in [HCO3−]o per se (green symbols) is expected to produce the direct stimulation of HCO3− leakage pathways and Cl-HCO3 exchange via anion exchangers (AEs) and indirect inhibition of Na+-coupled HCO3− transporters, slowing down Na+-driven HCO3− transporters, which either are known or believed to carry some form of CO3=. We expect true H+ pathways to be unaffected by pMet↓ because pHo per se does not change. (D) Pure metabolic/down: kinetic model. The meanings of the curves and symbols are the same as in panel B, compared to which we expect smaller effects on JE but larger effects on JL. The horizontal arrow indicates that the decrease in pHi is approximately the same length as in panel (B). The data from Bouyer et al. (2024) indicate that this panel-D arrow should only be ∼40% as long as that in panel (B). We propose that the difference could be due to the stimulatory effect of an extracellular HCO3− sensor (see Figure 6) that would increase JE under the conditions of pMet↓. (E) Metabolic acidosis: cell model. Here, we superimpose the effects of panels A and C. (F) Metabolic acidosis: kinetic model. Here, we superimpose the effects of panels B and D, generating a larger decrease in steady-state pHi than each alone. The result of simply adding ΔpHi effects in panels B and D is greater in magnitude than ΔpHi actually observed by Bouyer et al. (2024). The reason, as suggested in the legend for panel D, may be that extracellular HCO3− sensors (see Figure 6) reduce the decrease in pHi caused by pMet↓ (see Figure 10). Boxes with “minus” symbols indicate inhibition; green indicates an effect of [HCO3−]o per se; magenta indicates a pHo effect; and brown indicates a [CO3=]o effect. Boxes with “plus” symbols indicate the corresponding stimulation. The struck-out Δ value indicates no change. In the marquee, we indicate the unchanged parameters in gray. JE, acid-extrusion rate; JL, acid-loading rate.
“Pure metabolic” or ↓[HCO3−]o (pMet↓)
Still considering events occurring in the presence of CO2/HCO3−, pMet↓ (Figures 5C, D) would have only one of the predicted effects of pAc: with pMet↓, the decrease in [HCO3−]o would lower [CO3=]o and thus modestly reduce JE. The decrease in [HCO3−]o would also accelerate the efflux of HCO3− via the Cl-HCO3 exchanger AE3, thereby increasing JL. Thus, the effects of pMet↓ on both JL and JE would tend to lower pHi.
Metabolic acidosis (MAc)
Finally, the impact of MAc (Figures 5E, F) strictly on acid–base transporters ought to be approximately the sum of the individual impacts of pAc and pMet↓, adjusted for the non-additive effects on [CO3=]o, as discussed by Bouyer et al. (2024).6
In this section, we have limited ourselves to the direct effects of challenges on acid–base transporters. In the next two sections, we will see that these are only the first part of the story: ΔpHo and Δ[HCO3−]o also have direct effects on sensors and indirect effects on cellular constitution, both of which are likely to modulate acid–base transporters and thus affect the pHi time course. Later, we will consider the combined effects of acid–base disturbances on all three factors, namely, transporters, sensors, and constitution.7 Moreover, Figure 11 illustrates the apparent additivity of pAc1 and pMet↓1. In conjunction with Figure 13, we will discuss the non-additivity of pAc2 and pMet↓2.
Factor ‘2’: effects on sensors of the extracellular acid–base status
The introduction of the paper by Bouyer et al. (2025), the review by Ruffin et al. (2025), and the work by Thornell et al. (2025) summarize several classes of acid–base sensors. GPR68 (OGR1) is one of at least four pHo-sensitive G-protein-coupled receptors (GPCRs) and is present in medulloblastoma tissue (Huang et al., 2008), rat HC neurons (Schneider et al., 2012), and rat anterior pituitary gland (Horiguchi et al., 2014). Figure 6 depicts GPR68, in particular, and the presumed effects of MAc. HC acid-sensing ion channels (ASICs) (Alvarez et al., 2003) could play a role as pHo-sensors. On the other hand, although the tandem pore domain acid-sensing K+ (TASK) channels are present in multiple brain regions (Lesage, 2003), they are not in the hippocampus. Finally, the putative extracellular CO2/HCO3− sensors, RPTPγ and RPTPζ, are widely distributed in the CNS (Müller et al., 2004; Hayashi et al., 2005; Lamprianou et al., 2006) and could potentially contribute to the pHi physiology in the study by Bouyer et al. (2024). Recent work by Taki et al. (2024) shows that murine HC neurons (but not astrocytes) express both RPTPγ and RPTPζ. Moreover, in her PhD dissertation, Taki (2024) showed that the global knockout of RPTPζ in mice greatly reduces the ability of HC neurons to resist the pHi decrease caused by MAc or RAc.
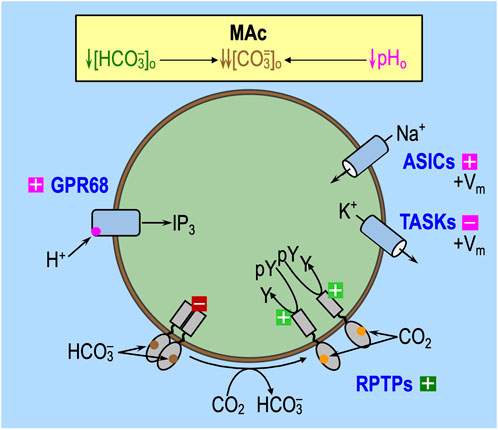
Figure 6. Effect of MAc on extracellular acid–base sensors. Note that the only factors that can contribute to the initial (i.e., near-instantaneous) dpHi/dt induced by an extracellular challenge are those that immediately impact proteins facing the extracellular fluid: (1) acid–base transport pathways (including “leaks”), like the ones in the incomplete list shown in Figure 5E, and (2) rapidly responding extracellular sensors, like the ones shown here. Later during the challenge, other pathways can come into play as cellular constitution changes and indirectly impacts acid–base transporters. GPR68 (OGR1) and at least three other G-protein-coupled receptors can sense H+ or a pHo− sensitive metabolite and lead to an increase in IP3/Ca2+. The ASICs and TASKs are families of pHo− sensitive channels. In both cases, decreases in pHo lead to depolarization of the membrane, which, in turn, could have other signaling effects. In cells (e.g., astrocytes) with substantial electrogenic Na/HCO3 cotransporter activity, MAc would lead to decreased Na+ and CO3= influx (or increased efflux), with the effect of augmenting depolarization. RPTPγ and RPTPζ have, in common, the presence of an extracellular carbonic-anhydrase–like domain (CALD), hypothesized to bind either HCO3− or CO2. In the monomeric state—hypothesized to be favored by low [HCO3−]o—the active tyrosine phosphatase dephosphorylates tyrosine residues. Extracellular boxes with “minus” symbols indicate inhibition, and magenta indicates an effect of low pHo per se. Extracellular boxes with “plus” symbols indicate stimulation by low pHo (magenta) or low [HCO3−]o (dark green). The intracellular dark-red box with a “minus” symbol indicates blockade of tyrosine phosphatase activity. The light-green box with a “plus” symbol indicates an active tyrosine phosphatase. IP3, inositol trisphosphate; pY, phosphotyrosine group; Y, tyrosine.
The activation of extracellular acid–base sensors, with a slight delay, could modulate the activity of acid–base transporters and thereby contribute to—or oppose—the initiation of pHi changes predicted in Figure 5 during an acid–base challenge. The continuing actions of these extracellular sensors—that is, their effects on transporters and cellular constitution—likely impact the evolution of the pHi change later during the challenge and produce longer lasting effects that influence “behavior” in the second of two challenges.
Effect of pAc on extracellular sensors
In the experiments of Bouyer et al. (2024), Ac (see Figure 4) and pAc (see Figures 5A, B) could act through pH-sensitive GPCRs and ion channels, which, in principle, could alter the (JE–JL) balance and thereby contribute to “state” (i.e., resistance vs. sensitivity). In Figure 6, the magenta “plus” and “minus” symbols indicate the anticipated effects of pAc on extracellular sensors.
Effect of pMet↓ on extracellular sensors
With pMet↓ (see Figures 5C, D), the decreased [HCO3−]o would trigger HCO3− sensors (Figure 6). In the experiments of Bouyer et al. (2024), pMet↓1 is unique among acid–base challenges in producing only about half the acidification of the other challenges (i.e., MAc1, Ac1, and pAc1). pMet↓ could promote monomerization of RPTPγ (see Figure 6), as suggested by preliminary data (Moss et al., 2018), and thereby increase the tyrosine phosphatase activity. In renal proximal tubules, it appears that this action would increase JE. Following this logic, pMet↓—acting through RPTPγ (and possibly also RPTPζ)—could promote a resistant state and, if persistent, could promote adaptation behavior in a later challenge. In Figure 6, the dark-green extracellular “plus” symbol indicates the anticipated effects of pAc on extracellular sensors.
Effect of MAc on extracellular sensors
The most straightforward hypothesis might be that the integrated “sensor” effects of pAc and MAc, described above (Figure 6), would summate to produce the integrated “sensor” effects of MAc (see extracellular “plus” and minus symbols in Figure 6). This may or may not be true in naïve neurons, as shown in Figure 8. However, for neurons previously exposed to MAc1, the integrated “sensor” effects of pAc2 and pMet↓2 may interfere with one another, as hypothesized in the discussion of Figure 14.
Factor ‘3’: effects on cellular constitution
The effects of acid–base disturbances on transporters (see factor ‘1,’ just above) and extracellular sensors (see factor ‘2,’ above) could begin instantaneously or nearly so and continue throughout the challenge (Figure 7). Although, upon the removal of the challenge, the effects on transporters and sensors per se may cease just as instantaneously as they had commenced, the more slowly developing consequences of altered transporter activity on intracellular solute concentrations (e.g., pHi, [HCO3−]i, [CO3=]i, [Na+]i, and [Cl−]i), membrane potential (Vm), and of altered sensor activation on downstream signaling pathways (e.g., phosphorylation state and protein trafficking) could evolve during the acid–base challenge and also persist for some time.8 In addition to constitutional changes produced directly by acid–base transporters (‘1’) and extracellular sensors (‘2’), indirect influences could include myriad effects. For example, Vm changes could affect voltage-sensitive channels and transporters and thereby affect neuronal firing and such parameters as [Ca2+]i. Alterations in ion concentrations would impact transporters and channels other than those depicted in Figure 3. For example, increased [Na+]i would stimulate the Na–K pump, which would tend to lower [Na+]i, increase [K+]i, and hyperpolarize the cell. Changes in pHi could directly impact pHi sensors (reviewed by Thornell et al., 2025) and—because [HCO3−]i changes in the same direction as pHi—could secondarily impact the soluble adenylyl cyclase sAC (Chen et al., 2000), present in some HC axon terminals (Chen et al., 2013). In locus coeruleus chemosensitive neurons, the activation of sAC increases L-type Ca2+-currents and limits the hypercapnia-induced increase in the firing rate (Imber et al., 2014).
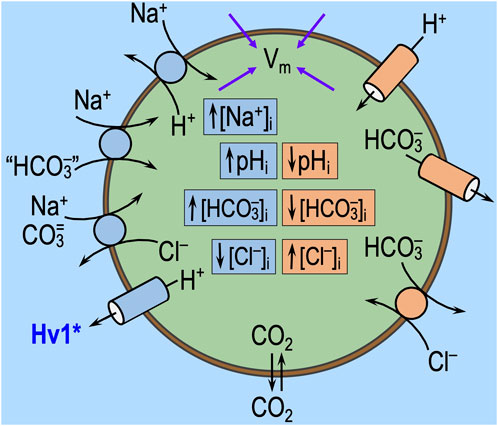
Figure 7. Direct effects of acid–base transport on Vm and intracellular ion concentrations. In this figure, we show the acid–base transport pathways from Figure 3, with blue and peach-colored boxes indicating the normal effects of these pathways on intracellular pH (pHi) and ion concentrations. We also show membrane potential (Vm), which is determined by intracellular solute concentrations and the state of ion channels and electrogenic transporters. Extracellular acid–base disturbances, like those shown in Figure 5, trigger direct changes in transport activity. Extracellular acid–base sensors (see Figure 6) may modulate this transporter activity. If these transport pathways undergo net stimulation (or inhibition), the concentration changes shown in this figure will be accentuated (or attenuated). The arrows leading to Vm indicate that the rapid extracellular challenges or slower intracellular concentration changes can alter Vm. In addition to the “direct” effect of changes in transport on the cellular constitution and the “secondary” effects of the extracellular sensors, we expect more complex changes to evolve over time. These complex changes could affect a myriad of membrane proteins and metabolic/signaling pathways, thereby altering the activity of the acid–base transport pathways in ways that influence “state” and “behavior.”
The above mentioned effects could produce changes in the number of acid–base transporter proteins in the plasma membrane (due to trafficking, protein degradation, and eventually protein synthesis) and changes in their unitary or “per-molecule” activities (due to alterations in intracellular ionic and post-translational modifications). Thus, the “functional activity” of transporters (i.e., protein number × unitary activity) underlying many JE1, JE2, … and JL1, JL2, … terms introduced in our introduction of Equation 10 may change over the evolution of the acid–base disturbance and then persist for some time.
Taki (2024) suggests that in a MAc–MAc protocol, progressively lower and lower pre-MAc2 pHi values correlate with an increase in the degree of adaptation behavior. Because higher pHi values just before MAc2 translate to higher [HCO−3]i values just before MAc2 (assuming that CO2 has equilibrated across the cell membrane), it is possible that sAC (which senses cytoplasmic HCO3−) could participate in neuronal state and/or behavior. Other pHi-sensitive processes could respond during a challenge, and the extracellular sensors could affect these or vice versa.
In acutely dissociated HC CA1 neurons, Brett et al. (2002) have shown that the inhibition of protein kinase A (PKA) inhibits Cl-HCO3 exchange but stimulates Na+-dependent Cl-HCO3 exchange, thereby increasing pHi in low-pHi neurons. In high-pHi neurons, the effects are the opposite. The stimulation of PKA has the opposite set of effects. In the protocols of Bouyer et al. (2024), decreases in pHo could have activated pHo− sensitive GPCRs that elevate [cAMP]i (Radu et al., 2005) and thereby contributed to state and behavior.
Determinants of neuronal state and behavior
Even before the work of Bouyer et al. (2024), Bouyer et al. (2004) had shown that some HC and medullary raphé neurons exhibit smaller pHi decreases than other neurons—what Salameh et al. (2014) would later term MAc resistance vs. sensitivity. Salameh et al. (2014) later showed that resistance/sensitivity and adaptation, consistency and decompensation phenotypes occur in multiple cell types other than HC neurons and astrocytes.
We hypothesize that state—resistance vs. sensitivity—depends both on the pre-existing status of the three factors discussed above and how constitutional changes evolve during the challenge. The pre-existing status, which could reflect the previous history of acid–base and other challenges, comprises the kinetic properties of each acid–base transporter and all factors (e.g., the impact of extra- and intracellular sensors) that influence these kinetic parameters.
We hypothesize that behavior—adaptation vs. consistency vs. decompensation—depends on all of the elements that determine the state during the first of two challenges and the persistence of all changes in cellular parameters from the first challenge to the next. Presumably, these parameter changes eventually extinguish with time. However, to the extent that the changes persist, they represent a sort of memory of the previous challenge that influences how a cell responds to a future challenge. Examples of persistent changes could include alterations in the numbers of various acid–base transporters and sensors that are resident in the plasma membrane, their post-translational states, and cellular constitution.
Although it was outside the scope of the study by Bouyer et al. (2024), it would be illuminating to examine the challenges opposite to those in that study (i.e., metabolic alkalosis or MAlk, pure alkalosis or pAlk, and pure metabolic/upward or pMet↑), as well as respiratory acidosis (RAc) and alkalosis (RAlk), pure respiratory/up (an isolated increase in [CO2]o or pR↑), and pure respiratory/down (pR↓). Note, however, that in the study by Bouyer et al. (2004), it was MAc—not RAc, MAlk, or RAlk—that seemed to generate pHi responses that were the most idiosyncratic.
State: resistance vs. sensitivity
Salameh et al. (2014) defined MAc-resistant cells as those for which pHi decreases by <40% of ΔpHo. Regardless of where one draws the dashed blue lines in Figures 2B, D, some cells will be more resistant/sensitive than others. Bouyer et al. (2024) observed a continuum of ΔpHi values that presumably depend on the factors noted in the previous section:9 rapid effects on ‘1’ acid–base transporters, ‘2’ extracellular acid–base sensors, and ‘3’ more slowly developing effects on cellular constitution. Figure 8A summarizes the interdependence of factors ‘1’–‘3’ for a naïve cell with an “average10” pHi decrease during MAc1. The initial (i) steady-state pHi (i.e., pHi prevailing just before MAc) is described by the intersections of the semi-transparent blue and red curves. We now discuss the impact of MAc1 on cells in four different states—sensitive and resistant plus “average” and “paradoxical” (an extreme variant of resistant)—and then raise the issue of how pAc1 and pMet↓1 contribute to MAc1.
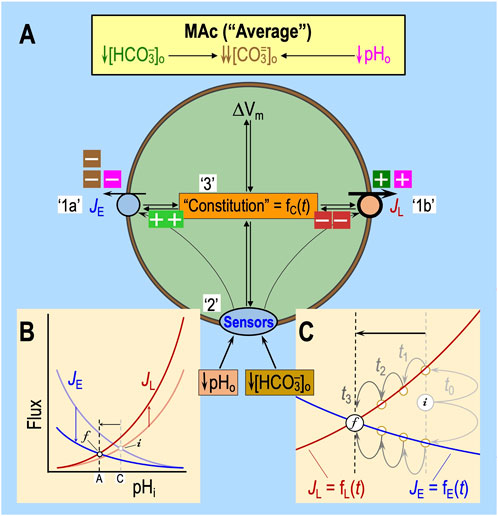
Figure 8. Model of average “state” during MAc. (A) Cellular model of the effects of MAc. In this figure, we suppose a state response to MAc that is on the border of resistant and sensitive—that is, “average.” The blue JE symbol represents the total flux mediated by all mechanisms of acid extrusion (factor ‘1a’), whereas the red JL symbol includes fluxes of all mechanisms of acid loading (factor ‘1b’ in white box). The oval “Sensors” symbol includes all sensors that respond to changes in [HCO3−]o or pHo (factor ‘2’). RPTPγ and RPTPζ presumably also respond to changes in [CO2]o, which did not occur in the experiments conducted by Bouyer et al. (2024). The extracellular dark-green, brown, and magenta “plus” and “minus” symbols have the same meaning as detailed in the previous figures (i.e., indicating which aspect of the MAc challenge produces the stimulation or inhibition, as shown in Figure 5E). The intracellular light-green “plus” and dark-red “minus” symbols (emanating from “Sensors” and Constitution) indicate enhancement or depression. Although we show equal numbers of intracellular light-green “plus” symbols and red “minus” symbols, it is really some combination of the two that reflects the relative degrees of transporter stimulation/inhibition by “Sensors” and/or “Constitution.” The black double arrows indicate that JE influences cellular constitution (factor ‘3’) and vice versa. The same holds true for ΔVm, JL, and the hypothesized sensors (see Figure 6) for extracellular H+ (e.g., GPR68) and HCO3− (e.g., RPTPγ and ζ). Note that we hypothesize that constitution is a function of time. (B) Kinetic model. This panel is a reproduction of the material shown in Figure 5F. (C) Higher magnification view of the kinetic model shown in panel (B). As illustrated in panel B, MAc instantly causes the JE curve to shift downward and the JL curve to shift upward, as indicated by the more deeply colored blue and red curves, respectively. In this figure, in panel C, we reproduce, at higher magnification, the newly shifted JE (blue) and JL (red) curves, the two vertical dashed lines, the horizontal arrow, and the points that we label “i” (initial) and “f” (final). Before MAc, the semi-transparent blue and red curves (shown in panel B but not C) passed through point “i.” Upon the imposition of MAc, at time “t0,” the JE value instantaneously jumps upward to meet the more deeply colored red curve, as indicated by the upper light gray arrow, and the JL value instantly jumps downward to meet the more deeply colored blue curve. Because JL > JE, that is, Δ(JE–JL) is negative, pHi begins to decrease at its maximal rate for this experiment. As pHi decreases (moving leftward on red and blue curves), JL decreases and JE increases. After time t1, Δ(JE–JL) is still negative but to lesser extent than at time t0. Thus, pHi decreases more slowly, eventually reaching time t3, where JE and JL come back into balance—that is, Δ(JE–JL) = 0—so that pHi is in a new, lower steady state at point “f” than during control conditions at point “i.” Because cellular constitution changes during the MAc challenge, JE and JL are both functions of time.
“Average” cells
Viewed in the context of Equation 10, for all but a small fraction of cells with paradoxical responses (discussed below11), the imposition of MAc temporarily shifts the difference (JE–JL) in the negative direction (see Figure 8B), initiating a decrease in pHi that plays out over several minutes. At the instant of the switch to MAc (see Figure 8C, t0), pHi has not yet changed. Nevertheless, JE jumps to the new JE vs. pHi curve (bright blue), which we presume to be below the original one. Simultaneously, JL jumps to the new JL vs. pHi curve, which we presume to be above the original one.12 As a result, JL exceeds JE at t0, and pHi begins to decrease at a rate determined by ρ, β, and Δ(JE–JL) in Equation 10. As pHi declines, JE increases gradually (t1, t2, and t3) and JL decreases. At t3, JE and JL have once more attained a balance at the final (f) steady state. Although Figure 8C depicts the JE and JL curves as being static (i.e., having fixed shapes and positions in the two-dimensional space of the chart), the shapes and positions of JE vs. pHi and JL vs. pHi could evolve over time, in response to changes in the extracellular sensors and cellular constitution, both of which potentially impact JE and JL.
Sensitive cells
For cells that respond to MAc with a relatively large pHi decrease, the net effect of MAc on factors ‘1’–‘3’ must be to produce a highly negative Δ(JE–JL) over the period of the MAc challenge. Some neurons are unusually sensitive to MAc. For example, examination of figures 3b, 5b, 7b, and 9b in Bouyer et al. (2024) reveals that, during MAc1, some HC neurons (a total of 35 out of 230 or ∼15.2%) exhibit a decrease in pHi that is even greater in magnitude than the decrease in pHo during MAc; in other words, (ΔpHi)1/MAc < −0.20. In these neurons, MAc1 must have produced a sufficiently large negative shift in Δ(JE–JL), integrated over the period of the challenge, to produce an unusually large intracellular acidification. In the cell model of Figure 9A, we imagine that MAc causes a large decrease in JE and a large increase in JL. In Figure 9B, we imagine a large downward shift (or a shallower slope) in the JE curve and a large upward shift (or steeper slope) in the JL relationship. Either a sufficiently large JE downshift or JL upshift could produce a highly negative Δ(JE–JL) and thus a highly MAc-sensitive state.
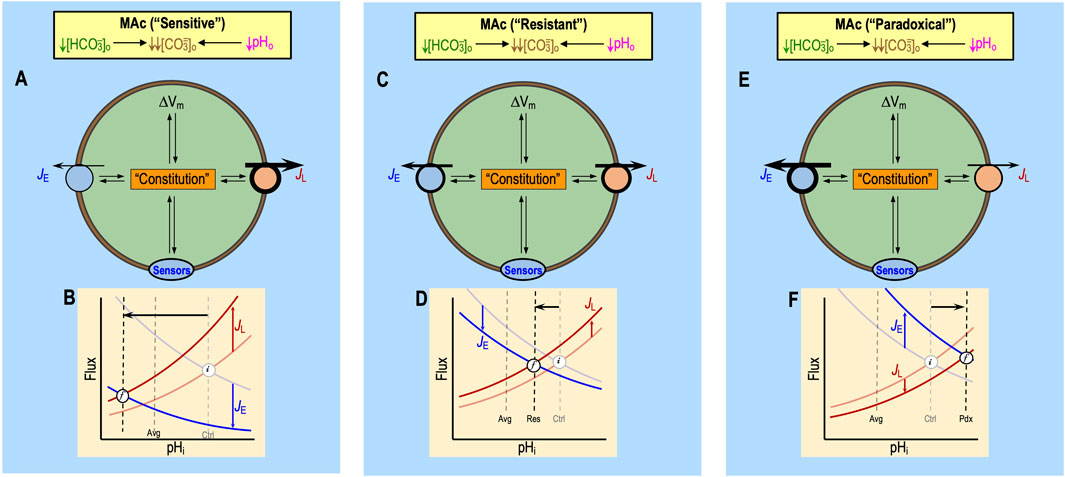
Figure 9. Models of sensitive, resistant, and paradoxical states during MAc. We hypothesize that MAc produces the usual initial percent inhibition (extracellular “minus” symbols) or stimulation (extracellular “plus” symbols) of each transporter (see Figure 5E) and sensor (see Figure 6), regardless of the subsequent pHi response that is indicative of state—sensitive, resistant, or paradoxical (exaggerated version of resistant). Instead, differences in the state would reflect differences in (1) transporter numbers, (2) sensor numbers, and (3) cellular constitution (which would influence the intrinsic transporter and sensor activity). In the cellular model panels (A, C, E), the thicknesses of arrows for JE (rate of acid loading from all sources) and JL (rate of acid extrusion from all sources) reflect functional activities (i.e., product of the protein number and intrinsic activity per protein). In the kinetic model panels (B, D, F), the semi-transparent curves (blue for JE and red for JL) are the same as the curves shown in Figure 3B; their intersections reflect initial (i) pHi values. The more deeply colored curves indicate the hypothetical JE and JL curves that prevail in each of the three states, and their intersections reflect final (F) pHi values. The horizontal black arrows represent the anticipated effect on steady-state pHi (i.e., i → (F). (A) MAc-sensitive neuron: cellular model. The sensitive state, reflecting the status of sensors and cellular constitution, results in some combination of depressed acid extrusion and elevated acid loading. (B) MAc-sensitive neuron: kinetic model. The deeply colored curves indicate a large JE decrease and a large JL increase due to the effects of MAc on the pathways in panel A for this neuron with a sensitive state. The result is a large decrease in steady-state pHi. Note: these two curves are the most exaggerated JE and JL curves, compared to the “average” cell shown in Figure 8, “resistant” cell shown in Figure 9C, and “paradoxical” cell shown in Figure 9E. (C) MAc-resistant neuron: cellular model. The resistant state, reflecting the status of sensors and cellular constitution, results in some combination of a modest JE decrease and a modest JL decrease, both of which are in opposite directions compared to the “sensitive” neuron shown in Figure 9A, “average” shown in Figure 8, and “paradoxical” shown in Figure 9E. (D) MAc-resistant neuron: kinetic model. The deeply colored curves indicate only a modest JE decrease vs. the larger one in panel (B) and a modest JL increase vs. the larger one in panel (B) due to the effects of MAc on the pathways in panel C for this neuron with a resistant state. The result is only a modest decrease in steady-state pHi. (E) paradoxical response to MAc: cellular model. The paradoxical response is an extreme variant of the resistant state and reflects that the status of sensors and cellular constitution results in some combination of a robust increase in JE and a modest decrease in JL. Note that the directions of these changes are opposite those of the “sensitive” neuron shown in Figure 9A, the “average” neuron shown in Figure 8, and the “resistant” neuron shown in Figure 9C. We propose a possible cellular mechanism of the paradoxical responses to MAc1 shown in Figure 10. (F) Paradoxical response to MAc: kinetic model. The deeply colored curves indicate some combination of a robust JE increase (vs. the decreases in the other examples) and a modest JL decrease (vs. the increases in the other panels) due to the effects of MAc on the pathways in panel E for this paradoxical neuron. The result is an increase in steady-state pHi.
Resistant cells
For cells that respond to MAc with a relatively small pHi decrease, the net effect of MAc on factors ‘1’–‘3’ must be to produce a negative Δ(JE–JL) that is relatively small in magnitude. In the cell model of Figure 9C, we assume that MAc causes a modest decrease in JE and a modest increase in JL. Figure 9D represents these JE/JL changes as a more modest JE downshift and JL upshift, although either effect could dominate to produce a modestly negative Δ(JE–JL) and thus a highly MAc-resistant state.
Salameh et al. (2017) revealed an interesting mechanism by which HC neurons mitigate the decrease in pHi during MAc, a process that depends on changes in cellular composition. In HC neurons cultured from WT mice, MAc tends to induce a pHi decrease that is initially rapid but limited in magnitude (Salameh et al., 2017). However, in HC neurons cultured from mice genetically deficient in the Cl-HCO3 exchanger AE3 (an acid loader), MAc induces a relatively slow initial decrease in pHi (reflecting the absence of AE3 and thus a smaller, initial MAc-induced negative shift in JL) that continues for some time. The result is a slow but large decrease in pHi. Salameh et al argued that, in WT neurons, the robust activity of AE3 loads the cell with Cl−, which, in turn, increases JE by stimulating both the Na+-driven Cl-HCO3 exchanger and NHEs, which often have a positive dependence on [Cl−]i (see Parker, 1983; Davis et al., 1994; Rajendran et al., 1995, 1999; Hogan et al., 1997; Bevensee et al., 1999). We interpret this hypothesized increase in [Cl−]i as a gradual change in cellular constitution that progressively increases JE over time and thereby tends to bring JE and JL into balance at a relatively high pHi—that is, the WT neurons appear to be relatively resistant to MAc. Thus, we would expect that neurons with relatively high functional activities of AE3, NDCBE, or NHE would tend to be more MAc-resistant, whereas neurons with lower functional activities would tend to be more MAc-sensitive.
Paradoxical responses
Returning to the paper by Bouyer et al. (2024), an examination of their figures 3b, 5b, 7b, and 9b—all of which have MAc as challenge #1—reveals that, during MAc1, a small fraction of HC neurons (a total of 22 out of 230 or ∼9.6%) exhibit a paradoxical alkalinization. In other words, for these 22 neurons, (ΔpHi)1/MAc > 0, so the points representing each lie to the right of the y-axis in a state diagram like that in Figure 2D. The net effect of MAc1 in these 22 paradoxical neurons must have been to produce an immediate and sustained positive shift in Δ(JE–JL), as illustrated in Figures 9E, F.
Analogous to the 22 paradoxical pHi increases discussed above is a non-physiological case that results from exposing naïve neurons to pMet↓. As summarized in figure 8b of Bouyer et al. (2024), 20 of 52 neurons (38%) alkalinized in response to pMet↓1.
We are unaware of any mechanism through which MAc1 (see Figures 5E, F) or pMet↓1 (see Figures 5C, D) could act directly on transporters to produce such an immediately positive, paradoxical pHi increase. Rather, it is more likely that, in a subset of neurons, extracellular sensors detect the decrease in [HCO3−]o (in MAc1 or pMet↓1) and/or pHo (in MAc1) and respond by producing a marked and extremely rapid increase in (JE–JL) that overwhelms the more typical acidifying effects of MAc1 (Figures 8, 9A–F) and pMet↓1.
Given that (1) an isolated decrease in basolateral [HCO3−]o (delivered via an OOE solution) acutely increases JE in renal PTs (Zhou et al., 2005), (2) PTs are insensitive to acute, isolated decreases in basolateral pHo (OOE solution) during this time frame (Zhou et al., 2005), (3) the PT response requires RPTPγ (Zhou et al., 2016), and (4) RPTPγ and RPTPζ are present in virtually every mouse HC neuron (Taki et al., 2024), we propose the following mechanism (Figure 10) by which the ∼10% of naïve HC neurons subjected to MAc1 and the nearly 40% subjected to pMet↓ exhibit a paradoxical pHi increase: the decrease in [HCO3−]o triggers the monomerization of RPTPγ or RPTPζ (see Figure 6), leading to the dephosphorylation of certain phosphotyrosines and, as a consequence, the rapid stimulation of acid extruders and/or inhibition of acid loaders.
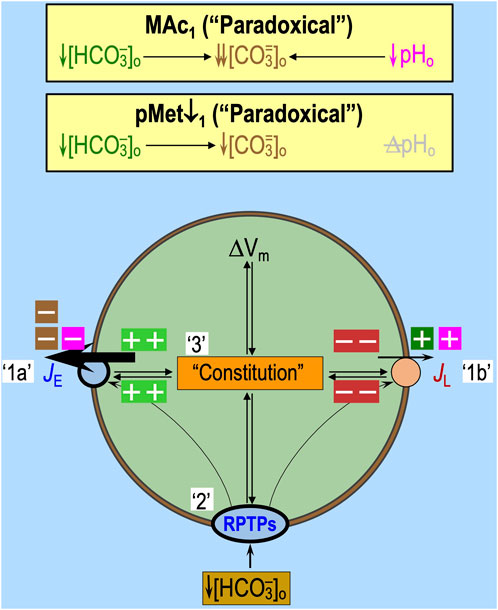
Figure 10. Hypothesized mechanistic model, for a naïve HC neuron, of paradoxical pHi increases induced by MAc or pMet↓. We hypothesize that MAc1 produces the usual initial percent inhibition (extracellular brown or magenta “minus” symbols) or stimulation (extracellular dark-green or magenta “plus” symbols) of each transporter (see Figure 5E) and sensor (see Figure 6), regardless of the subsequent pHi response indicative of state. pMet↓1 would produce only the effects indicated by extracellular dark-green and brown “minus” and “plus” symbols (i.e., not magenta symbols). Compared to other naïve neurons, the paradoxical responses to MAc1 or pMet↓1 (state) would reflect differences in (1) transporter numbers, (2) sensor numbers, and (3) cellular constitution (which would influence intrinsic transporter and sensor activity). The thicknesses of the arrows for JE (rate of acid loading from all sources) and JL (rate of acid extrusion from all sources) and the RPTPs (oval) reflect functional activities (i.e., product of the protein number and intrinsic activity per protein). The intracellular light-green “plus” symbols and red “minus” symbols indicate the relative effects of cellular constitution (including signaling from RPTPs) on JE and JL. Although we show equal numbers of intracellular light-green “plus” symbols and red “minus” symbols, it is really some combination of the two that reflects the relative degrees of transporter stimulation/inhibition by “Sensors” and/or “Constitution.” We predict that pMet↓1, lacking the pHo effects of MAc1, would have relatively more light-green “plus” symbols and fewer red “minus” symbols, thus indicating a greater net increase in Δ(JE–JL) and a greater paradoxical pHi increase than MAc1.
Additivity of pAc1 and pMet↓1
The data from Bouyer et al. (2024) show that, in naïve neurons, the average ΔpHi elicited by pAc1 and the average ΔpHi elicited by pMet↓1 approximately summate to the average ΔpHi elicited by MAc1 in a population of rat HC neurons. The reported contributions were ∼70% for pAc1 and ∼30% for pMet↓. Figure 11 illustrates how this additivity could occur in a single “average” neuron. Considering only the direct effects of acid–base disturbances on transporters—that is, without the effect of the hypothesized extracellular H+ and HCO3− sensors—we predicted that the ΔpHi effect of pMet↓1 would have been similar to that of pAc1, so the two would have summed to a ΔpHi value greater than that produced by MAc1. Thus, we hypothesize that in naïve HC neurons, the effect of decreased pHo on extracellular-H+ sensors produces a relatively weak stimulation of acid extrusion overloading (i.e., weak opposition to the pHi decrease), whereas the effect of decreased [HCO3−]o produces a relatively strong stimulation (i.e., strong opposition to the pHi decrease).
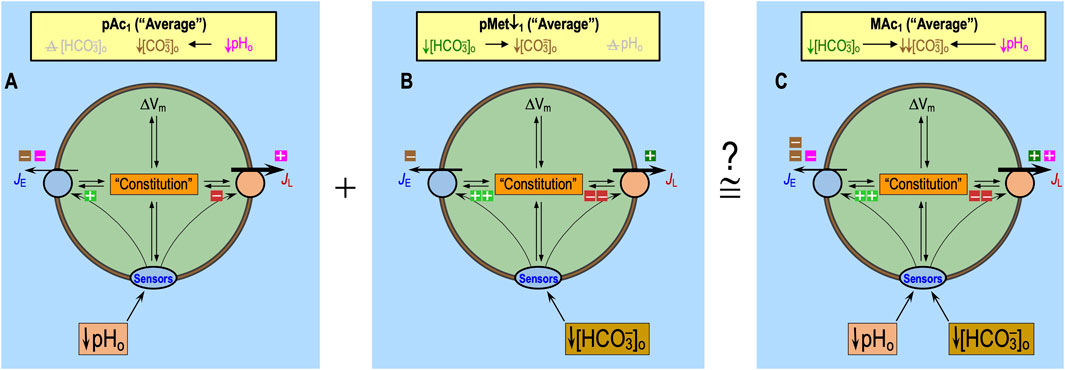
Figure 11. Hypothesized cellular mechanism, for naïve neurons, of additivity: pAc1 + pMet↓1 ≅ MAc1. (A) Effect of pure acidosis on an “average” HC neuron. We hypothesize that the decrease in pHo stimulates only one class of sensors (e.g., GPR68) with relatively weak functional activity. (B) Effect of pure metabolic/down on an “average” HC neuron. We hypothesize that the decrease in [HCO3−]o stimulates only one class of sensors (e.g., RPTPγ/RPTPζ) with relatively strong functional activity. (C) Effect of metabolic acidosis on an “average” HC neuron. We hypothesize that the simultaneous decreases in both pHo and [HCO3−]o stimulate both classes of sensors. The symbols have the same meanings as in previous figures. The thickness of the arrows representing JE (rate of acid loading from all sources) and JL (rate of acid extrusion from all sources) and the thickness of the lines surrounding “Sensors” reflect the relative functional activities. The numbers of intracellular light-green “plus” symbols and red “minus” symbols (some of which are shown as halves) reflect the degree of stimulation or inhibition by the Sensors and/or Constitution. Although we show equal numbers of intracellular light-green “plus” symbols and red “minus” symbols, it is really some combination of the two that reflects the relative degrees of transporter stimulation/inhibition by “Sensors” and/or “Constitution.”
Summary
At the population level, the “state” revealed by MAc1 in naïve neurons seems to be the sum of the effects of pAc1 and pMet↓1. The degree of resistance (or sensitivity) to MAc depends on how, integrated over the period of the challenge, MAc affects the (JE–JL) balance (see Equation 10). In turn, this balance depends on the cell’s complement of acid–base transporters and extracellular acid–base sensors, initial cellular constitution, and how the cell modulates these factors over the course of the MAc challenge.
Behavior: adaptation vs. consistency vs. decompensation
The three types of behavior must reflect persistent effects (or lack thereof) on the three factors introduced above13 to produce, during MAc2, a state that is the same, more resistant, or more sensitive than during the preceding MAc1.
In the next three subsections, we (1) present hypotheses of how behaviors arise, (2) explore insights from the non-additivity of pAc2 and pMet↓2 [a conclusion reached in equations 6 & 7 of Bouyer et al. (2024)], and (3) consider parameters that could affect behavior.
Models of behaviors
Figure 12 presents cellular models of adaptation, consistency, and decompensation. In each case, the intracellular bright green “plus” boxes indicate stimulation of acid extrusion via some combination of the three factors: an increase in the number of transporters at the cell surface, an increase in the functional activity of extracellular sensors to increase JE, and changes in the cellular constitution that increase the unitary activity of acid extruders. The red “minus” boxes indicate the opposite for acid loading. Note that, in our cellular models, increases in JE and decreases in JL are interchangeable because they could produce similar changes in Δ(JE–JL). For simplicity, we show equal numbers of “plus” and “minus” boxes. In Figure 12, panels A1, A2, and A3 are identical.
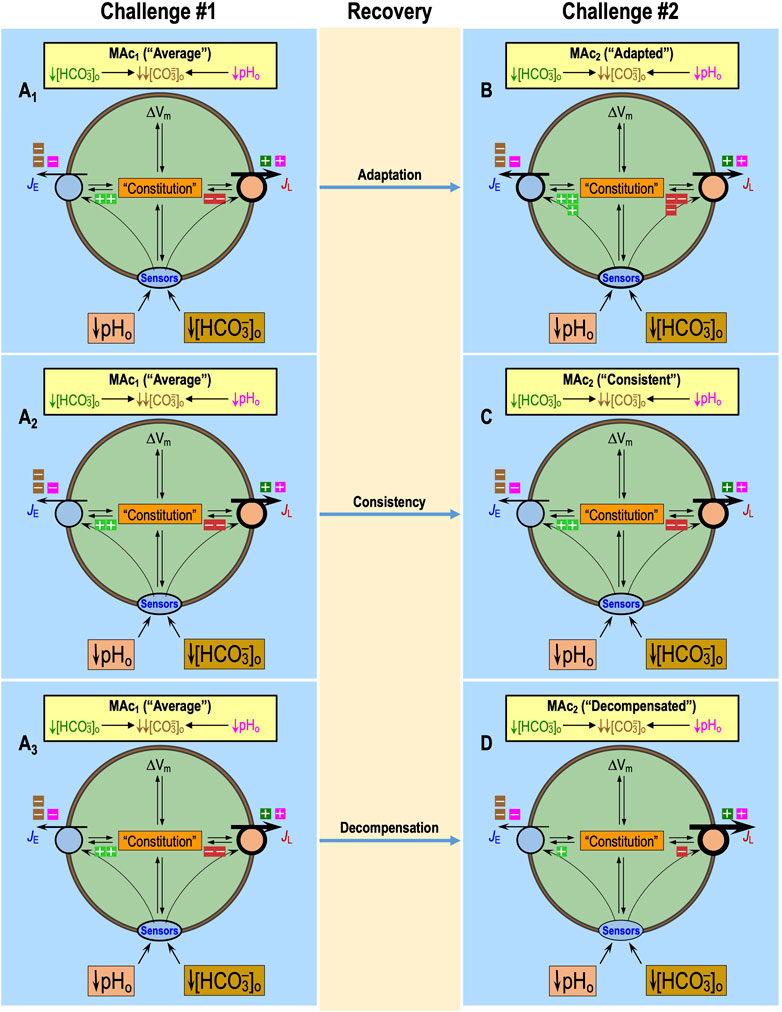
Figure 12. Model of the mechanisms of behavior. (A1–A3) Cell state during challenge #1. (A1–A3) These are identical and reflect the state of an “average” naïve HC neuron during the first challenge with MAc. After this MAc1 challenge, the neuron spends several minutes in a recovery period, exposed to a control CO2/HCO3− solution. The thicknesses of arrows for JE (rate of acid loading from all sources) and JL (rate of acid extrusion from all sources) reflect functional activities (i.e., product of the protein number and intrinsic activity per protein). (B) State of an adapted neuron during a second MAc challenge. We hypothesize that during MAc2, individual transporters, individual sensors, and cellular constitution have changed in such a way as to make (JE–JL) more positive than during MAc1 and thereby reduce the magnitude of (ΔpHi)2 compared to (ΔpHi)1. (C) State of a consistent neuron during a second MAc challenge. We hypothesize that during MAc2, the net effect on (JE–JL) is the same as during MAc1 in panel A, although individual transporters, individual sensors, and cellular constitution may have changed. (D) State of a decompensated neuron during a second MAc challenge. We hypothesize that during MAc2, individual transporters, individual sensors, and cellular constitution have changed in such a way as to make (JE–JL) more negative than during MAc1 and thereby increase the magnitude of (ΔpHi)2 compared to (ΔpHi)1. The symbols have the same meanings, as detailed in the previous figures. The thickness of the arrows representing JE and JL and the thickness of the lines surrounding “Sensors” reflect the relative functional activities. The numbers of intracellular light-green “plus” symbols and red “minus” symbols reflect the degree of stimulation or inhibition by the Sensors and/or Constitution. Although we show equal numbers of intracellular light-green “plus” symbols and red “minus” symbols, it is really some combination of the two that reflects the relative degrees of transporter stimulation/inhibition by “Sensors” and/or “Constitution.”
Adaptation
Figures 12A1, B illustrate our model for how the three factors conspire to produce Δ(JE–JL) that is less negative during MAc2 than during the earlier MAc1. As implied by the presentation in the previous paragraph, a cell could achieve adaptation by increasing JE from MAc1 to MAc2 without any change (or even a smaller decrease) in JL, or a decrease in JL without any change (or even a smaller increase) in JE. In any case, MAc2 elicits a smaller pHi decrease than MAc1—adaptation.
Consistency
Figures 12A2, C illustrate our model, showing how overall (JE–JL)—regardless of changes in individual components of JE and JL—remains approximately the same during MAc2, as during MAc1 so that (ΔpHi)2 ≅ (ΔpHi)1,—consistency.
Decompensation
Figures 12A3, D illustrate our model, showing how overall (JE–JL)—regardless of changes in individual components of JE and JL—decreases during MAc2 compared to MAc1. As a result, MAc2 elicits a larger pHi decrease than MAc1,—decompensation.
pAc2 and pMet↓2
In our presentation of Figure 11, we observed that, in a population of naïve neurons, the sum of ΔpHi values in pAc1 and pMet↓1 is approximately equal to MAc1. Figure 13 illustrates an analogous analysis of pAc and pMet↓ but for neurons previously challenged in period #1 with MAc.
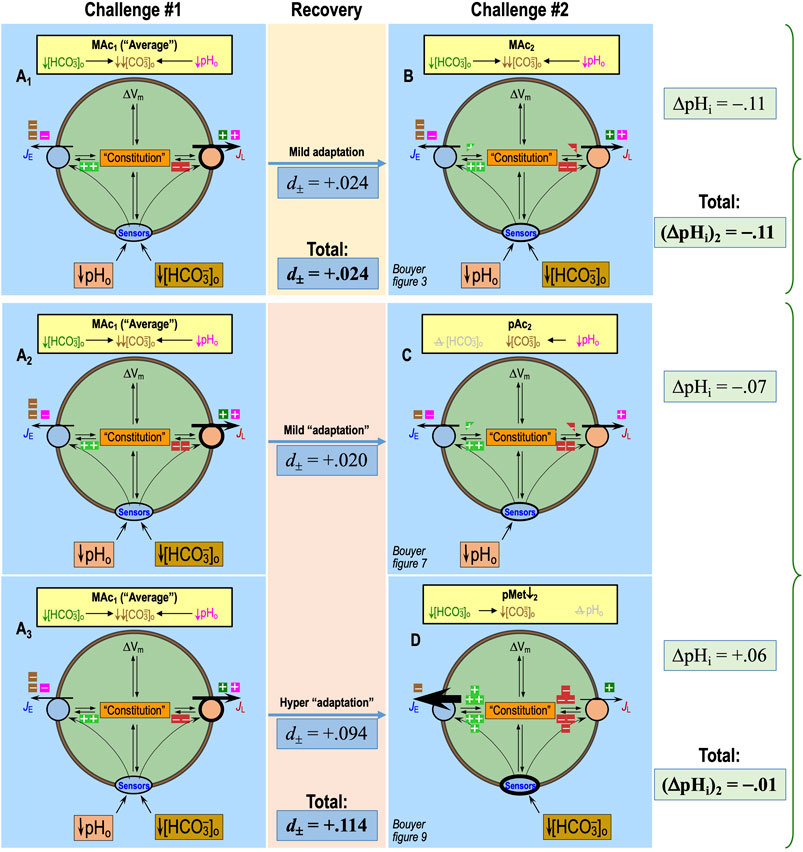
Figure 13. Model of the mechanism of non-additivity in MAc1-treated neurons: pAc2 + pMet↓2 >> MAc2. We hypothesize that MAc1 produces the usual initial percent inhibition (extracellular brown or magenta “minus” symbols) or stimulation (extracellular dark-green or magenta “plus” symbols) of each transporter (see Figure 5E) and sensor (see Figure 6) and generates an average-sized pHi decrease (see Figure 8). After the recovery period, we expose the cell either to MAc2 panels (A1–B), pAc2 panels (A2–C), or pMet↓2 panels (A3–D). The different behavior responses during challenge #2 would reflect differences in (1) transporter numbers, (2) sensor numbers, and (3) cellular constitution (which would influence intrinsic transporter and sensor activity). The symbols have the same meanings as detailed in the previous figures. The thickness of the arrows representing JE (rate of acid loading from all sources) and JL (rate of acid extrusion from all sources) and the thickness of the lines surrounding “Sensors” reflect the relative functional activities (i.e., product of the protein number and intrinsic activity per protein). Although we show equal numbers of intracellular light-green “plus” symbols and red “minus” symbols, it is really a combination of the two that reflects the relative degrees of transporter stimulation/inhibition by “Sensors” and/or “Constitution.” The d± values are from table 2 of Bouyer et al. (2024). The “ΔpHi” values in light-green boxes refer to challenge #2 and are obtained from the figure 3 panel (B), figure 7 panel (C), and figure 9 panel (D) of the same paper. The bolded “Total” values—either d± or (ΔpHi)2—are either the same as for the single respective values in panel B or the sum of the two respective values for panels C and D. A1–A3, cell state during challenge #1. A1, A2, and A3 are identical. MAc1 produces a negative shift in Δ(JE–JL) and, therefore, a pHi decrease, which is greater in magnitude than in panels B, C, or D. (B) Average response to MAc2. We hypothesize that some combination of increased JE and decreased JL produces a modestly negative Δ(JE–JL) that is smaller in magnitude than during MAc1. As a result, Δ(pHi)2/MAc has a smaller magnitude than (ΔpHi)1/MAc—a mild adaptation. (C) Average response to pAc2. We hypothesize that some combination of increased JE and decreased JL produces a modestly negative Δ(JE–JL) that—as is the case in panel B—is smaller in magnitude than during MAc1. As a result, Δ(pHi)2/pAc has a smaller magnitude than (ΔpHi)1/MAc—a mild “adaptation.” (D) Average response to pMet↓2. We hypothesize that some combination of increased JE and decreased JL produces a massively positive Δ(JE–JL). As a result, Δ(pHi)2/pMet↓ is frankly positive—a “hyper adaptation.”
Non-additivity of pAc2 and pMet↓2 during MAc–MAc
Figures 13A1, B show cellular models of the average MAc–MAc data in figure 3 of Bouyer et al. (2024), who reported a mild decrease in ΔpHi from MAc1 (−0.14) to MAc2 (−0.11) in concert with a modestly positive d± (+0.024). In Figure 13B, we model this mild adaptation by adding a “half-plus” for JE and a “half-minus” for JL. Many combinations of JE and JL changes—produced by changes in the three factors13—could have elicited the required modest increase in (JE–JL).
Figures 13A2, C show models of the acidosis part of that MAc2. Because in figure 7 of Bouyer et al. (2024), the (ΔpHi)1/MAc value was smaller (−0.11) than that in their figure 3 (−0.14), we interpret (ΔpHi)2/pAc (−0.07) as representing the 0MAc-pAc equivalent of mild adaptation. The d± value of figure 7 (+0.020) was similar to that of figure 3 (+0.024). Therefore, we model pAc2 in Figure 13C similarly to MAc2 in Figure 13B, with an addition of a half-plus to JE and half-minus to JL. Our interpretation of Bouyer et al. (2024) data is that the stimulation of pHo sensors by pAc2 provides all the impetus necessary to produce the usual changes observed during MAc2 of a MAc–MAc protocol; in other words, no input from HCO3− sensors is necessary to account for (ΔpHi)2/MAc.
Figures 13A3, D show models of the ↓[HCO3−]o part of that MAc2. A note of caution: figure 9 of Bouyer et al. (2024) reports that (ΔpHi)1/MAc was smaller (−0.07) than both the population average (−0.11 in their figure 1) and the value reported in figure 3 (−0.14). Nevertheless, (ΔpHi)2 during pMet↓2 is notably striking: an average pHi increase of +0.06 with ∼87% of all neurons exhibiting a net pHi increase during pMet↓2 of the MAc-pMet↓ protocol. Thus, we are dealing with a strong phenotype. Furthermore, we recall that in naïve neurons, pMet↓1 produced the smallest recorded average pHi decrease (−0.04) and nearly 40% of the neurons underwent a frank pHi increase (see their figure 8). Returning to Bouyer’s figure 9, d± is also strikingly large (0.094). Thus, in evaluating the data underlying through CD, we reach similar conclusions whether we sum population ΔpHi values (−0.07 + +0.06 = −0.01) or population d± values (+0.020 + +0.094 = +0.114): in other words, the sum of the parts in panels C and D is far greater than the overall result in panel B ((ΔpHi)2 = −0.11, d± = +0.024). How is this discrepancy possible?
We propose that HC neurons, during MAc2 of a physiological MAc–MAc challenge, normally engage in coincidence detection involving two sets of acid–base sensors—one for extracellular H+ and another for extracellular HCO3−. When the two challenges arrive with approximate simultaneity, their respective signal transduction cascades have the effect of muting one another, especially muting the strong actions of decreased [HCO3−]o during MAc2.
Extreme paradoxical behavior pMet↓2
In Figure 10, we proposed—knowing the pHi responses to pMet↓1 and pMet↓2—that a small subset (∼10%) of naïve rat HC neurons respond to MAc1 with an unbalanced activation of RPTPγ or RPTPζ, resulting in a paradoxical pHi increase.
We propose that, also in naïve neurons, pMet↓1—without accompanying muting contributed by decreased pHo in MAc1—produces an even more unbalanced net stimulation of acid extrusion. In nearly 40% of the population, this results in a paradoxical pHi increase.
Finally, in HC neurons primed with an MAc1 challenge and then allowed to recover, the subsequent exposure to pMet↓2 produces the greatest unbalanced net increase in (JE–JL) such that ∼87% of Bouyer’s HC neurons exhibited a paradoxical pHi increase. During/after MAc1, the neuron does not “know” what the experimenter intends for the second challenge. MAc1 and the subsequent recovery period must set the stage for the truly remarkable alkalinizing response (i.e., ↑pHi) to pMet↓2 in Bouyer’s figure 9. Bouyer likened this phenomenon to placing 100 glasses of room-temperature water into a functioning refrigerator, only to find that, after their removal, 87 glasses had warmed up (!).
MAc2 in a normal MAc–MAc protocol
During MAc–MAc, one can view MAc2—in a neuron that has already been preconditioned by MAc1—as being pMet↓2 (see Figure 13D) supplemented with pAc2. We propose that the pAc2 component—acting via ↓pHo sensors like GPR68, ASICs, and TASKs (see Figure 6)—nullifies most of the alkalinizing effects of pMet↓2 (Figure 14), an example of antagonism. Such H+-induced nullification may also occur to some extent in naïve neurons, and its variability could underlie some of the “state” variability observed in naïve neurons, as well as in those first challenged with MAc1 and later subjected to MAc2. See the legend of Figure 14 for a consideration of how our cartoon model is an oversimplification of the complexities of physiology. Refer to the section on mathematical modeling14 for suggestions on how to address these complexities.
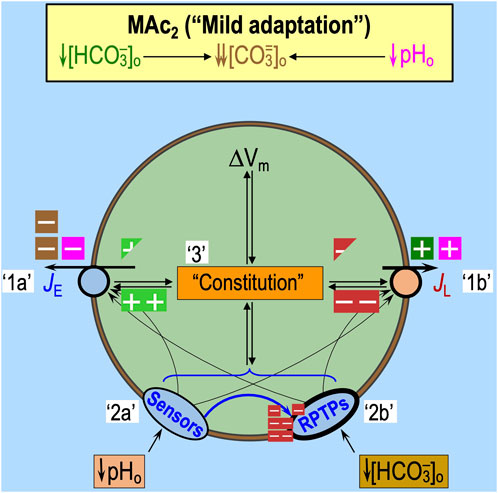
Figure 14. Revised mechanistic model of mild adaptation during MAc2: coincidence detection. Inspired by the unique predictions in Figure 13D, we present this general model. We envisage that the response to MAc2 represents more than the simple additivity of low-pHo and low-[HCO3−]o stimuli, as depicted in Figure 13B. In this figure, we split the generic ↓pHo/↓[HCO3−]o “Sensors” icon into separate sensors for ↓pHo (‘2a’) and ↓[HCO3−]o (‘2b’). Although we retain the ability of the now-separate sensors—acting in parallel—to stimulate acid extrusion (‘1a’) and inhibit acid loading (‘1b’) and interact with “Constitution” (‘3’), we now introduce a new concept: the ↓pHo sensors (‘2a’) must normally act during MAc2 to antagonize the ↓[HCO3−]o sensor (‘2b’). Thus, we envisage that the sensors act in three ways: (1) pre-conditioned by MAc1, the ↓[HCO3−]o sensors (e.g., RPTPγ and/or RPTPζ) are poised to produce—via actions of JE, JL, and Constitution—a massive increase in (JE–JL), which, by itself, would produce a paradoxical pHi increase, as modeled in Figure 13D. Perhaps also pre-conditioned by MAc1, the ↓pHo sensors (e.g., GPR68) have two effects. (2) Parallel increase—via actions of JE, JL, and Constitution—in (JE–JL). (3) Massive inhibition of the response of the RPTPs to the low-[HCO3−]o stimulus. This model is a great oversimplification. The JE (rate of acid loading from all sources) and JL (rate of acid extrusion from all sources) icons represent a multitude of individual transporters. The sensors, although split into separate detectors of ↓pHo and ↓[HCO3−]o, could represent multiple examples of each (see Figure 6). Constitution we defined as “the collection of all ion-concentration, metabolic, and signaling properties.” We envisage all of the individual effects to be time-dependent, both in terms of activation and deactivation (e.g., persistence). Dependencies of transporters, sensors, and enzymes on the concentrations of their relevant substrates/ligands are almost certainly nonlinear. When two arrows point at a target, the effects could be sub-additive, simply additive, or supra-additive (i.e., synergistic). The proposed inhibitory effect of the ↓pHo on the ↓[HCO3−]o sensors is an example of antagonism. Assembling all of these complexities into a useful model is a task for mathematical modeling. Although we show equal numbers of intracellular light-green “plus” symbols and red “minus” symbols, it is really some combination of the two that reflects the relative degrees of transporter stimulation/inhibition by “Sensors” and/or “Constitution.”
Parameters’ potentially governing behavior
Not addressed in the studies of Salameh et al. (2014) and Bouyer et al. (2024) are several important unresolved questions regarding the duration of events required for establishing behaviors:
• Challenge #1: How does the development of a behavior depend on challenge #1, particularly its:
o nature (e.g., MAc1 vs. pMet↓1)?
o intensity (e.g., degree of lowering pHo or [HCO3−]o)?
o duration?
• Recovery-period duration: How does the development of a particular behavior depend on the duration of the interlude period between challenges #1 and #2? The answer could depend on
o Preceding challenge #1 (nature, intensity, and duration)
o Challenge #2 (nature and intensity)
• Extinguishment: Over what duration of recovery period would the behavior-inducing effects of challenge #1 extinguish? The answer could depend on
o Preceding challenge #1 (duration, nature, and intensity)
o Challenge #2 (nature and intensity)
We suggest that a fruitful initial approach for addressing the abovementioned questions could be to use the MAc-pMet↓ protocol as a test case because it produces the most reproducible and remarkable responses. Recall that figure 9b in the study of Bouyer et al. (2024) shows that ∼87% of neurons subjected to this protocol exhibit a paradoxical pHi increase.
It is of interest that, in the study of Bouyer et al. (2024) and the PhD dissertation of Taki (2024), the initial MAc1-induced pHi decrease (indicative of a negatively shifted [JE–JL]) was not followed by a delayed pHi increase (reminiscent of adaptation) or pHi decrease (reminiscent of decompensation) during MAc1. Thus, we can conclude that either (1) the duration of challenge #1 (e.g., 7 min in the Taki study) was too brief for the development of a secondary change in (JE–JL) or (2) the removal of challenge #1 is necessary for the development of the behavior observed during challenge #2.
Although we have primarily focused on acid–base parameters as potential modulators of behavior, other environmental factors—metabotropic signaling molecules and the ionic milieu (e.g., [K+]o)—also could also be in play.
Summary
At the population level, the behavior evidenced during MAc2 is quite different from the simple sum of pAc2 and pMet↓2. Behavior could depend on the nature, intensity, and duration of challenge #1, as well as the duration of the recovery period. The response to pMet↓2, perhaps mediated by RPTPγ/ζ, is extremely powerful, capable of producing rather consistent paradoxical increases in pHi.
Impact on extracellular buffering
Resistant state and adaptation
Both a relatively resistant state and an adaptive behavior could be the appropriate “selfish” response of neurons, for which a relatively large acidic pHi shift would have a negative impact on the physiological role of analogous neurons in an intact brain. Such hypothetical neurons—those especially critical under a particular set of circumstances—may be programmed to reduce the magnitude of acidic shifts using the strategies outlined above. The price to pay for such selfishness is that the cell’s small negative (JE–JL) necessarily results in the extrusion of acid into the extracellular space (see Figure 9C and Figure 12B), which lays an extra low-pHo burden on neighboring cells.
Average state and consistency
Both an average10 state and a consistent behavior could be the appropriate “unselfish” response of neurons, for which an acidic pHi shift would have a limited impact on the physiological role of an analogous neuron in an intact brain. By allowing themselves to acidify moderately during MAc1 and/or to acidify no more during MAc2 than during MAc1, such neurons perform an important function by buffering extracellular acid and reducing extracellular acid loads experienced by neighboring cells.
Sensitive state and decompensation
Both a relatively sensitive state and a decompensating behavior could be the appropriate “altruistic” response of neurons, for which a relatively severe acidic pHi shift would have limited impact on the physiological role of an analogous neuron in an intact brain. By allowing themselves to acidify to a relatively large degree during MAc1, and/or acidifying more during MAc2 than during MAc1, these altruistic neurons buffer disproportionately greater fractions of extracellular acid loads and thereby spare their neighboring cells.
Astrocytes vs. neurons in the CNS
Although we have focused on neurons, it is interesting to recall that Salameh et al. (2014) found that, during a MAc–MAc protocol, ΔpHi/ΔpHo is consistently greater (by nearly 50%) for astrocytes than for neurons, both in cultures from the hippocampus and medullary raphé and both for MAc1 and MAc2. On the other hand, intrinsic intracellular buffering power (βI; see Thornell et al. (2025) has the opposite pattern for the two cell types. For cultured astrocytes from rat HC, βI is only ∼10 mM/pH at pHi 7.0 in one study (Bevensee et al., 1997), whereas βI of neurons acutely isolated from rat HC is much higher, ∼15 mM/pH at pHi 7.0 in another study (Bevensee et al., 1996). Thus, if we consider only βI, although their ΔpHi may be 50% greater, HC astrocytes would take up about the same amount of acid per unit volume of cytosol as neurons. On the other hand, total intracellular buffering power (βT) is the sum of βI and the open-system CO2/HCO3− buffering power (βopen), with the latter increasing exponentially15 with pHi and being the same in all cells. Thus, at relatively low pHi values, βT would be modestly lower in astrocytes than in neurons, and astrocytes (with a 50% greater ΔpHi) would buffer modestly more acid than neurons. At relatively high pHi values, βopen would overwhelm βI, and thus, βT would be rather similar in the two cell types; in this case, astrocytes would buffer much more acid than neurons. Because astrocytes can undergo rather large pHi changes and buffer more acid than neurons on average, we could view them as being altruistic compared to neurons.
Variability among neuronal responses
pH sensitivity of neurons
Changes in pHo or pHi can affect a wide range of electrophysiological properties because of the pHi/pHo sensitivity of virtually every neuronal component—including channels, receptors, transporters, enzymes (including those involved in neurotransmitter metabolism), and cytoskeletal elements. Thus, one would expect that inappropriate pHi changes could lead to CNS pathology. It is generally believed that high neuronal pHi is pro-epileptogenic (Hentschke et al., 2006; Jacobs et al., 2008; Sinning et al., 2011). Chesler and Kraig (1987), Chesler and Kraig (1989) and Ransom (2000) proposed a negative-feedback model, which was discussed and extended by Salameh et al. (2017). In this model, neuronal activity leads to an increase in [K+]o, causing depolarization-induced alkalinization (DIA) in astrocytes (Siebens and Boron, 1989a; Siebens and Boron, 1989b), that in turn would cause a fall in pHo—a local MAc—and, thus, a generalized reduction in neuronal excitability. We would expect that local MAc would lower neuronal pHi. Induced epileptiform activity lowers neuronal pHi, which recovers after the epileptiform activity ceases (Xiong et al., 2000; Raimondo et al., 2012). Although high pHo is also considered to be pro-epileptogenic, chronic low-grade metabolic acidosis may contribute to the development of chronic epilepsy (Yuen, 2006).
Based on the above discussion, one might have expected the distribution of neuronal pHi values to be within a relatively narrow range. However, a striking characteristic of mammalian HC neurons, freshly dissociated or cultured, is an unusually wide range of resting pHi values (Schwiening and Boron, 1994; Baxter and Church, 1996; Bevensee et al., 1996; Smith et al., 1998) compared to other cell types. Moreover, our laboratory has identified a wide range of ΔpHi responses to MAc (Bouyer et al., 2004; Salameh et al., 2014; 2017) and twin MAc–MAc challenges (Salameh et al., 2014; 2017). Bouyer et al. (2024) study confirms this diversity of responses to MAc (state) and MAc–MAc (behavior) and extends both aspects of diversity to the artificial acid–base disturbances pAc and pMet↓, alone and in combination with MAc.
Origin of pHi diversity in neurons
We have already presented hypotheses to address the molecular mechanisms underlying the diversity of state and behavior. We now ask, at a higher level, what is responsible for the aforementioned diversity? We offer four possibilities that are not mutually exclusive.
(1) Some of the diversity is unphysiological. For example, the above-cited studies show that the broad range of initial pHi values is greater in the absence than in the presence of CO2/HCO3− (which would presumably enable the full complement of pHi-regulatory mechanisms). It is possible that the range would be narrower still if we were to study the neurons in vivo, where they would be under the potential influence of metabotropic signaling and other influences from neighboring cells in a three-dimensional arrangement.
(2) Some of the acid–base diversity represents a diversity of neuronal subtypes, with each subtype, as studied in primary culture, having its own range of expressions for each relevant protein.
(3) Some of the diversity is intrinsic to neuronal physiology (nature), at least in primary culture, reflecting apparently stochastic differences in the numbers and localization of various proteins.
(4) Some of the diversity depends on the history of individual neurons (nurture), including differences in the acid–base microenvironment, patterns of neuronal activity, and other environmental parameters (e.g., cell–cell contacts) for these cells in primary culture.
Thus, each neuron in culture could have a set of properties that depends on neuronal subtype, stochastic variations in protein numbers/localization within that subtype (nature), and differences in neuronal history (nurture). Together, these factors could establish a constitution that determines how a particular neuron responds to one acid–base challenge (state) or a sequence of them (behavior).
Impact on cell function
A teleological question that arises is why should such diversity exist? One advantage of diversity could be to increase the probability that enough neurons in a circuit can withstand periodic acid–base challenges of various types. Another could be that neurons with different electrophysiological properties (and the underlying cohort of ion channels and other proteins, each with its own pHi sensitivity) could be more electrically stable with a resistant vs. sensitive state or with an adaptive vs. consistent vs. decompensatory behavior.
Discussion
Major conclusions
Regarding the experiments with OOE solutions, we believe that the main conclusions of the paper by Bouyer et al. (2024) can be summarized as follows.
“State” during challenge #1
In a population of naïve rat HC neurons, the effects of pAc1 and pMet↓1 on pHi—assessed as (ΔpHi)1—are approximately additive. In other words, in naïve HC neurons, whole MAc1 is approximately the sum of its parts (see Figure 11).
Acid–base sensors
The abovementioned results lead to the conclusion that rat HC neurons have separate sensors that detect (1) a decrease in pHo and (2) a decrease in [HCO3−]o (see Figure 6).
“Behavior” when challenge #1 is MAc1
In a population of neurons that has already experienced MAc1 followed by a recovery period, the subsequent effects of pAc2 and pMet↓2—as assessed by either (ΔpHi)2 or d±—are decidedly not additive (see Figure 13).
Coincidence detection
The abovementioned result leads us to conclude that—for this protocol, which spans MAc1 and a recovery period—pAc2 and pMet↓2 challenges must arrive at approximately the same time to reproduce the physiological effects of MAc2 (see Figure 14).
Molecular mechanism
Mouse HC neurons express both RPTPγ and RPTPζ (Lorenzetto et al., 2014; Taki et al., 2024). Based on the PhD dissertation of Taki (2024), who examined the effect of knocking out RPTPζ in MAc–MAc and RAc–RAc protocols on mouse HC neurons, and the work of Zhou et al. (2016), who examined the effect of knocking out RPTPγ in renal proximal tubules, we propose that the most likely HCO3− sensor(s) in the experiments of Bouyer et al. (2024) are some combination of RPTPγ and RPTPζ.
In addition, we urge additional experiments that further probe the molecular mechanisms underlying state and behavior and suggest an extension of the studies to include (1) the duration and intensity of the first challenge, (2) the duration of the recovery period, and (3) additional acid–base challenges that involve both equilibrated solutions (i.e., RAc, MAlk, and RAlk) and OOE solutions (pAlk, pMet↑, pResp↓, and pResp↑).
Mathematical modeling
Aside from a call for more wet-laboratory data, we urge the development of mathematical models—the counterparts of the qualitative models described with words and cartoons in the present paper—that could assist in the interpretation of experiments like those in the research paper by Bouyer et al. (2024). Boron and De Weer (1976) developed the first mathematical model of pHi regulation, a compartmental model that embodies the principles of the fundamental law of pHi regulation in Equation 10. Occhipinti et al. (2020), as part of the Physiome journal (which is part of the broader Physiome Project), wrote a retrospective of the BDW model that includes clarifications and updates, access to online implementations, and a summary of several post-BDW models of pHi regulation.
The set of two ordinary differential equations in the BDW model includes only one component of JL (an H+ pump, the rate of which varies with [H+]i—and thus time—according to a fixed rate constant) and a single component of JE (an HCO3− leak, the rate of which varies with [HCO3−]i—and thus pHi and time—according to a fixed HCO3− permeability). Although it would be straightforward to incorporate additional components of JE (i.e., JE1, JE2, … ) and JL (i.e., JL1, JL2, … ), imbuing these components (e.g., variants of NBCn1) with realistic estimates of sensitivity to pHi and pHo, as well as their respective substrates, would require major—but valuable—investments from funding agencies. The same applies to acid–base sensors and the broader issue of “cellular constitution,” which would describe diverse influences ranging from ion concentrations to signal transduction pathways.
Figure 14 is a cartoon model of how interactions among (1) transporters, (2) sensors, and (3) cellular constitution could account for the results of the recent study by Bouyer et al. (2024). As noted in the figure legend, the cartoon is greatly oversimplified: each transporter icon represents a multitude of individual proteins. The ↓pHo and ↓[HCO3−]o sensors could represent multiple examples of each (see Figure 6). We defined constitution as “the collection of all ion-concentration, metabolic, and signaling properties.” All of the interconnected components vary with time during—and after—and acid–base challenge. Their dependencies on concentrations of their relevant substrates/ligands are almost certainly nonlinear. Effects could have varying degrees of additivity or antagonism.
A goal of programs such as The Physiome is to develop modular mathematical components for each transporter and signaling pathway, assemble the components into various model cells, and inform the models from experiments like those reported in the research paper by Bouyer et al. (2024). We envision the development of such sophisticated models—the quantitative versions of those qualitative models in the present paper—and using them to interpret the research paper by Bouyer et al. (2024) and design future experiments.
Limitations to the model(s)
We begin by acknowledging the principle that “all models are wrong, but some are useful”—the first part of which is articulated by the British statistician Box (1976), who also emphasized the concept of “useful.”
The word and cartoon models presented in this paper are based on the fundamental law of pHi regulation (see Equation 10), which is mathematically expressed as follows:
This equation is analogous to the principle of continuity in physiology16 or fluid mechanics, which, in turn, is based on the conservation of mass. Thus, the basic model must be correct, at least at the integrative level of classical physics and chemistry.
The theoretical cartoon models presented in this paper could be tested by employing more sophisticated mathematical modeling approaches than that explained in Equation 10. These approaches could resort to “compartmental” models, treating the cell and the extracellular fluid as uniform compartments with instantaneous mixing (i.e., ignoring a detailed spatial description of the cell geometry and its effects on solute diffusion). More complex approaches could resort to 3D (“distributed”) reaction-diffusion models, in which one accounts for the diffusion (or transport) of solutes in 3D space/time, as well as the chemical reactions that occur in parallel 3D space/time. For example, such models can attempt to account for unconvected layers that surround a cell and how geometry impacts the time courses of solute concentrations. In either case, the modeler formulates the problem using differential equations and solves these using various numerical methods. Assumptions—and opportunities for error—abound at each conceptual step.
Limitations also arise in the complexity of the biological system and the oversimplifications by which we estimate individual terms, even in the relatively simplest of approaches (e.g., a compartmental system):
Surface-to-volume ratio (ρ)
Although distributed mathematical models can describe complex cell geometries explicitly, they face increasing computational challenges when solving numerically the resulting (partial) differential equations. Generally, whenever possible, modelers overcome this challenge by simplifying cellular geometry, assuming that a cell has a simple geometric shape (e.g., a sphere or a cylinder). In the case of the oocyte models of Somersalo et al. (2012) and Occhipinti et al. (2014), the authors took advantage of the oocyte’s being a spherical cell and further simplified the model by assuming spherical radial symmetry (i.e., only the distance from the cell center influences solute transients). Occhipinti et al. (2014) incorporated an amplification of the surface area to accommodate microvilli. Even so, the volumes and surface areas of living cells are not precisely known, and they can change with time.
Buffering power (β)
Modelers might break buffering into two components: open-system buffering power (due to a solute like CO2 or NH3 that can equilibrate across the cell membrane) and intrinsic buffering power (Boron, 1977). As discussed by Thornell et al. (2025), the intrinsic buffering power (βI) of the cytosol comprises chemical buffering (due to classic acid–base equilibria), biochemical buffering (due to other reactions that consume/generate H+), and organellar buffering (due to the movement of H+ equivalents across organellar membranes). Although it may be reasonably straightforward to account for open-system buffering, βI is, in principle, extraordinarily complicated because myriad components contribute to it, and this could change with time and metabolic state. In addition, intrinsic buffering power is pHi-sensitive—although it is possible to measure this, as first done by Boron (1977). The modeler might assume a constant/fixed βI, a constant pHi-dependent βI, or—as done by Somersalo et al. (2012) and Occhipinti et al. (2014)—represent βI with a single chemical buffer pair (HA
Acid extrusion (JE) and acid loading (JL)
As noted in our discussion during the introduction of Equation 10, the overall JE and JL each comprise a multitude of different transporters (see Figure 3), each with a distinct set of kinetic parameters. To the best of our knowledge, not even one acid–base transporter is fully described kinetically. Thus, modelers are left to estimate the parameter values—a further limitation to quantitative models. The numbers and activities of the individual JE/JL components are likely to change with time and acid–base challenges like those discussed in the present paper—further limiting models.
Extracellular acid–base sensors
In Figure 6, we introduced several classes of extracellular acid–base sensors, of which we know of several pHo-sensitive GPCRs (for review, see Thornell et al., 2025), pH-sensitive ion channels like ASICs and TASKs, and two CO2/HCO3−-sensitive RPTPs, each with several variants. GPCRs and RPTPs could each modulate individual acid–base transporters, probably as the result of complex signal transduction cascades. GPCRs and RPTPs could also modulate pHo-sensitive channels like ASICs and TASKs and a myriad of other cellular processes that constitute cellular constitution. We do not fully understand the role of any one of the above in modulating pHi homeostasis. All of the above uncertainty contributes to the limitations to models.
Cellular constitution
We defined cellular constitution as “the collection of all [intracellular] ion-concentration, metabolic, and signaling properties” that can directly impact (1) the transporters directly responsible for JE and JL and (2) extracellular acid–base sensors. This catch-all grouping of constantly changing (1) small inorganic and organic molecules and (2) peptides and other polymers (including proteins and nucleic acids) will be a major challenge to characterize. Liquid–liquid phase separations may be the loci of many important biochemical processes. The extensive uncertainty about all of the above processes contributes to model limitations.
Although the preceding discussion may seem discouraging, we are optimistic that—over the decades—a continuous effort by cellular physiologists will enable them to develop and inform models that—although “wrong”—become increasingly more “useful” in interpreting data and formulating further hypotheses (to be tested experimentally).
Data availability statement
Publicly available datasets were analyzed in this study. These data can be found here: in the companion paper.
Author contributions
PB: conceptualization, methodology, validation, writing–original draft, and writing–review and editing. RO: conceptualization, validation, writing–review and editing. ST: conceptualization and writing–review and editing. FM: conceptualization and writing–review and editing. WB: conceptualization, formal analysis, funding acquisition, methodology, project administration, writing–original draft, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by NIH grants NS 18400 and DK128315 and program project grant P01-HD32573 (PI: Gabriel Haddad). WB’s and FM’s contributions to this work were supported in part by the Department of Defense, Air Force Research Laboratory 711th Human Performance Wing, Studies and Analysis funding 22-012.
Acknowledgments
WB gratefully acknowledges the support of the Myers/Scarpa endowed chair. PB thanks the Valparaiso Summer Research Fellowship for its support in the elaboration of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1By acute, we loosely mean a few seconds to minutes. Over a period of hours, genomic responses to acid–base disturbances could involve other signaling pathways.
2See the section titled “State & Behavior.”
3We refer to figures, tables, equations, and named solutions in the 2024 Bouyer paper in italics, using all lowercase letters.
4Bouyer et al. (2024) chose this pMet↓ nomenclature to make room for a future “pMet↑” solution, in which [HCO3−]o would increase at a fixed [CO2]o and pHo.
5As embodied in Equation, ρ and β influence the rate at which pHi changes during extracellular acid–base disturbances but not the direction of the pHi change or even steady-state pHi.
6In the Discussion of that paper, see the section titled “Effects of acid–base challenges on [CO3=]o.”
7See “Determinants of neuronal state and behavior.”
8Here, we consider acute effects with a duration of minutes. If the challenges were to persist long enough, changes in protein synthesis could affect the quantity and identity of expressed proteins.
9See “Molecular basis … ”).
10We will use “average cell” in two closely related ways to denote a cell with a ΔpHi, that is (1) the mean value for the population and (2) near the boundary between “resistant” and “sensitive” states, as shown in Figure 8.
11See inline heading “Paradoxical responses.”
12Note that we make no statement about how the challenge affects the magnitudes or even the directions of the shifts in JE vs. pHi or JL vs. pHi (see Figures 8B, C) or the time courses of these shifts. We only require that the negative Δ(JE–JL), integrated over the period of the MAc challenge, produces (in this case) a “moderate” net intracellular acid load, resulting in a moderate decrease in pHi. For example, one could imagine a situation in which MAc caused JL to paradoxically increase but caused JE to increase even more. Because Δ(JE–JL) < 0, pHi would still decrease.
13(1) Identity and numbers of acid–base transporters in the plasma membrane, (2) extracellular acid–base sensors, and (3) cellular constitution. See “Molecular basis of effects of extracellular acid–base disturbances.”
14See “Discussion” > “Mathematical modeling.”
15βopen = 2.3 × [HCO3−]i = 2.3 × [HCO3−]o × 10(pHi–pHo).
16For example, the rate at which the volume of the chamber changes is determined by the difference between the blood inflow and blood outflow. If chamber volume is constant, then inflow must equal outflow.
References
Alvarez de la R., Krueger S. R., Kolar A., Shao D., Fitzsimonds R. M., Canessa C. M. (2003). Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J. Physiology 546, 77–87. doi:10.1113/jphysiol.2002.030692
Baxter K. A., Church J. (1996). Characterization of acid extrusion mechanisms in cultured fetal rat hippocampal neurones. J. Physiol. (Lond.) 493 (Pt 2), 457–470. doi:10.1113/jphysiol.1996.sp021396
Bevensee M. O., Bashi E., Schlue W. R., Boyarsky G., Boron W. F. (1999). Shrinkage-induced activation of Na+/H+ exchange in rat renal mesangial cells. Am. J. Physiol. 276, C674–C683. doi:10.1152/ajpcell.1999.276.3.C674
Bevensee M. O., Boron W. F. (2013). “Control of intracellular pH,” in Seldin and giebisch’s the kidney: physiology and pathophysiology (Academic Press), 1773–1835.
Bevensee M. O., Cummins T. R., Haddad G. G., Boron W. F., Boyarsky G. (1996). pH regulation in single CA1 neurons acutely isolated from the hippocampi of immature and mature rats. J. Physiol. (Lond) 494, 315–328. doi:10.1113/jphysiol.1996.sp021494
Bevensee M. O., Weed R. A., Boron W. F. (1997). Intracellular pH regulation in cultured astrocytes from rat hippocampus. I. Role of HCO₃−. J. Gen. Physiol. 110, 453–465. doi:10.1085/jgp.110.4.453
Boron W. F. (1977). Intracellular pH transients in giant barnacle muscle fibers. Am. J. Physiol. 233, C61–C73. doi:10.1152/ajpcell.1977.233.3.C61
Boron W. F. (1985). Intracellular pH-regulating mechanism of the squid axon. Relation between the external Na+ and HCO3− dependences. J. Gen. Physiol. 85, 325–345. doi:10.1085/jgp.85.3.325
Boron W. F. (2004). Regulation of intracellular pH. Adv. Physiol. Educ. 28, 160–179. doi:10.1152/advan.00045.2004
Boron W. F. (2017). “Acid-base physiology,” in Medical physiology: a cellular and molecular approach. Editors W. F. Boron, and E. L. Boulpaep (Philadelphia, PA: Saunders Elsevier), 628–646.
Boron W. F., Boulpaep E. L. (1983). Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO₃− transport. J. Gen. Physiol. 81, 53–94. doi:10.1085/jgp.81.1.53
Boron W. F., De Weer P. (1976). Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J. Gen. Physiol. 67, 91–112. doi:10.1085/jgp.67.1.91
Boron W. F., Knakal R. C. (1989). Intracellular pH-regulating mechanism of the squid axon. Interaction between DNDS and extracellular Na+ and HCO₃−. J. Gen. Physiol. 93, 123–150. doi:10.1085/jgp.93.1.123
Boron W. F., Knakal R. C. (1992). Na+-dependent Cl−-HCO₃− exchange in the squid axon. Dependence on extracellular pH. J. Gen. Physiol. 99, 817–837. doi:10.1085/jgp.99.5.817
Boron W. F., Russell J. M. (1983). Stoichiometry and ion dependencies of the intracellular-pH-regulating mechanism in squid giant axons. J. Gen. Physiol. 81, 373–399. doi:10.1085/jgp.81.3.373
Bouyer P., Bradley S. R., Zhao J., Wang W., Richerson G. B., Boron W. F. (2004). Effect of extracellular acid-base disturbances on the intracellular pH of neurones cultured from rat medullary raphe or hippocampus. J. Physiol. (Lond.) 559, 85–101. doi:10.1113/jphysiol.2004.067793
Bouyer P., Zhou Y., Boron W. F. (2003). An increase in intracellular calcium concentration that is induced by basolateral CO2 in rabbit renal proximal tubule. Am. J. Physiol. Ren. Physiol. 285, F674–F687. doi:10.1152/ajprenal.00107.2003
Bouyer P. G., Salameh A. I., Zhou Y., Kolba T. N., Boron W. F. (2024). Effects of extracellular metabolic acidosis and out-of-equilibrium CO₂/HCO₃− solutions on intracellular pH in cultured rat hippocampal neurons. Front. Physiol. 15, 1434359. doi:10.3389/fphys.2024.1434359
Box G. E. P. (1976). Science and statistics. J. Am. Stat. Assoc. 71, 791–799. doi:10.1080/01621459.1976.10480949
Brett C. L., Kelly T., Sheldon C., Church J. (2002). Regulation of Cl−/HCO3− exchangers by cAMP-dependent protein kinase in adult rat hippocampal CA1 neurons. J. Physiol. (Lond.) 545, 837–853. doi:10.1113/jphysiol.2002.027235
Chen J., Martinez J., Milner T. A., Buck J., Levin L. R. (2013). Neuronal expression of soluble adenylyl cyclase in the mammalian brain. Brain Res. 1518, 1–8. doi:10.1016/j.brainres.2013.04.027
Chen Y., Cann M. J., Litvin T. N., Iourgenko V., Sinclair M. L., Levin L. R., et al. (2000). Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289, 625–628. doi:10.1126/science.289.5479.625
Chesler M., Kraig R. P. (1987). Intracellular pH of astrocytes increases rapidly with cortical stimulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 253, R666–R670. doi:10.1152/ajpregu.1987.253.4.R666
Chesler M., Kraig R. P. (1989). Intracellular pH transients of mammalian astrocytes. J. Neurosci. 9, 2011–2019. doi:10.1523/JNEUROSCI.09-06-02011.1989
Davis B. A., Hogan E. M., Boron W. F. (1994). Shrinkage-induced activation of Na+-H+ exchange in barnacle muscle fibers. Am. J. Physiology - Cell Physiology 266, C1744–C1753. doi:10.1152/ajpcell.1994.266.6.C1744
Hayashi N., Oohira A., Miyata S. (2005). Synaptic localization of receptor-type protein tyrosine phosphatase zeta/beta in the cerebral and hippocampal neurons of adult rats. Brain Res. 1050, 163–169. doi:10.1016/j.brainres.2005.05.047
Hentschke M., Wiemann M., Hentschke S., Kurth I., Hermans-Borgmeyer I., Seidenbecher T., et al. (2006). Mice with a targeted disruption of the Cl−/HCO3− exchanger AE3 display a reduced seizure threshold. Mol. Cell. Biol. 26, 182–191. doi:10.1128/MCB.26.1.182-191.2006
Hogan E. M., Davis B. A., Boron W. F. (1997). Intracellular Cl− dependence of Na-H exchange in barnacle muscle fibers under normotonic and hypertonic conditions. J. Gen. Physiol. 110, 629–639. doi:10.1085/jgp.110.5.629
Horiguchi K., Higuchi M., Yoshida S., Nakakura T., Tateno K., Hasegawa R., et al. (2014). Proton receptor GPR68 expression in dendritic-cell-like S100β-positive cells of rat anterior pituitary gland: GPR68 induces interleukin-6 gene expression in extracellular acidification. Cell Tissue Res. 358, 515–525. doi:10.1007/s00441-014-1958-x
Huang W. C., Swietach P., Vaughan-Jones R. D., Ansorge O., Glitsch M. D. (2008). Extracellular acidification elicits spatially and temporally distinct Ca2+ signals. Curr. Biol. 18, 781–785. doi:10.1016/j.cub.2008.04.049
Imber A. N., Santin J. M., Graham C. D., Putnam R. W. (2014). A HCO₃−-dependent mechanism involving soluble adenylyl cyclase for the activation of Ca2+ currents in locus coeruleus neurons. Biochim. Biophys. Acta 1842, 2569–2578. doi:10.1016/j.bbadis.2014.07.027
Jacobs S., Ruusuvuori E., Sipilä S. T., Haapanen A., Damkier H. H., Kurth I., et al. (2008). Mice with targeted Slc4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proc. Natl. Acad. Sci. U.S.A. 105, 311–316. doi:10.1073/pnas.0705487105
Lamprianou S., Vacaresse N., Suzuki Y., Meziane H., Buxbaum J. D., Schlessinger J., et al. (2006). Receptor protein tyrosine phosphatase γ is a marker for pyramidal cells and sensory neurons in the nervous system and is not necessary for normal development. Mol. Cell. Biol. 26, 5106–5119. doi:10.1128/MCB.00101-06
Lee S.-K., Occhipinti R., Moss F. J., Parker M. D., Grichtchenko I. I., Boron W. F. (2023). Distinguishing among HCO₃−, CO₃=, and H+ as substrates of proteins that appear to be “bicarbonate” transporters. J. Am. Soc. Nephrol. 34, 40–54. doi:10.1681/ASN.2022030289
Lesage F. (2003). Pharmacology of neuronal background potassium channels. Neuropharmacology 44, 1–7. doi:10.1016/s0028-3908(02)00339-8
Lorenzetto E., Moratti E., Vezzalini M., Harroch S., Sorio C., Buffelli M. (2014). Distribution of different isoforms of receptor protein tyrosine phosphatase γ (Ptprg-RPTP γ) in adult mouse brain: upregulation during neuroinflammation. Brain Struct. Funct. 219, 875–890. doi:10.1007/s00429-013-0541-7
Michenkova M., Taki S., Blosser M. C., Hwang H. J., Kowatz T., Moss F. J., et al. (2021). Carbon dioxide transport across membranes. Interface Focus 11, 20200090. doi:10.1098/rsfs.2020.0090
Moss F. J., Wass A. B., Watterson S. J., Boron W. F. (2018). Sensing and transduction of acid-base disturbances by receptor protein tyrosine phosphatase γ. FASEB J. 32, 864–865. doi:10.1096/fasebj.2018.32.1_supplement.864.5
Müller S., Lamszus K., Nikolich K., Westphal M. (2004). Receptor protein tyrosine phosphatase ζ as a therapeutic target for glioblastoma therapy. Expert Opin. Ther. Targets 8, 211–220. doi:10.1517/14728222.8.3.211
Occhipinti R., Musa-Aziz R., Boron W. F. (2014). Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO₂ fluxes across Xenopus oocytes plasma membranes. Am. J. Physiol. Cell Physiol. 307, C841–C858. doi:10.1152/ajpcell.00049.2014
Occhipinti R., Safaei S., Hunter P., Boron W. F. (2020). The Boron and De Weer model of intracellular pH regulation. Physiome. doi:10.36903/physiome.12871022
Parker J. C. (1983). Volume-responsive sodium movements in dog red blood cells. Am. J. Physiol. 244, C324–C330. doi:10.1152/ajpcell.1983.244.5.C324
Radu C. G., Nijagal A., McLaughlin J., Wang L., Witte O. N. (2005). Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc. Natl. Acad. Sci. U. S. A. 102, 1632–1637. doi:10.1073/pnas.0409415102
Raimondo J. V., Irkle A., Wefelmeyer W., Newey S. E., Akerman C. J. (2012). Genetically encoded proton sensors reveal activity-dependent pH changes in neurons. Front. Mol. Neurosci. 5, 68. doi:10.3389/fnmol.2012.00068
Rajendran V. M., Geibel J., Binder H. J. (1995). Chloride-dependent Na-H exchange. A novel mechanism of sodium transport in colonic crypts. J. Biol. Chem. 270, 11051–11054. doi:10.1074/jbc.270.19.11051
Rajendran V. M., Geibel J., Binder H. J. (1999). Role of Cl channels in Cl-dependent Na/H exchange. Am. J. Physiology - Gastrointest. Liver Physiology 276, G73–G78. doi:10.1152/ajpgi.1999.276.1.G73
Ransom B. R. (2000). Glial modulation of neural excitability mediated by extracellular pH: a hypothesis revisited. Prog. Brain Res. 125, 217–228. doi:10.1016/S0079-6123(00)25012-7
Ransom B. R. (2017). “The neuronal microenvironment,” in Medical physiology: a cellular and molecular approach. Editors W. F. Boron, and E. L. Boulpaep (Philadelphia, PA: Saunders Elsevier), 275–294.
Roos A., Boron W. F. (1981). Intracellular pH. Physiol. Rev. 61, 296–434. doi:10.1152/physrev.1981.61.2.296
Ruffin V. A., Taki S., Boron W. F., Moss F. J. (2025). Novel RPTPγ and RPTPζ splice variants from mixed neuron–astrocyte hippocampal cultures as well as from the hippocampi of newborn and adult mice. Front. Physiol. 15. doi:10.3389/fphys.2024.1406448
Salameh A. I., Hübner C. A., Boron W. F. (2017). Role of Cl−-HCO₃− exchanger AE3 in intracellular pH homeostasis in cultured murine hippocampal neurons, and in crosstalk to adjacent astrocytes. J. Physiol. (Lond.) 595, 93–124. doi:10.1113/JP272470
Salameh A. I., Ruffin V. A., Boron W. F. (2014). Effects of metabolic acidosis on intracellular pH responses in multiple cell types. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R1413–R1427. doi:10.1152/ajpregu.00154.2014
Schneider J. W., Goetsch S. C., Leng X., Ludwig S. M., Russell J. L., Yang C.-P., et al. (2012). Coupling hippocampal neurogenesis to brain pH through proneurogenic small molecules that regulate proton sensing G protein-coupled receptors. ACS Chem. Neurosci. 3, 557–568. doi:10.1021/cn300025a
Schwiening C. J., Boron W. F. (1994). Regulation of intracellular pH in pyramidal neurones from the rat hippocampus by Na+-dependent Cl−-HCO₃− exchange. J. Physiol. (Lond) 475, 59–67. doi:10.1113/jphysiol.1994.sp020049
Siebens A. W., Boron W. F. (1989a). Depolarization-induced alkalinization in proximal tubules. I. Characteristics and dependence on Na+. Am. J. Physiol. 256, F342–F353. doi:10.1152/ajprenal.1989.256.2.F342
Siebens A. W., Boron W. F. (1989b). Depolarization-induced alkalinization in proximal tubules. II. Effects of lactate and SITS. Am. J. Physiol. 256, F354–F365. doi:10.1152/ajprenal.1989.256.2.F354
Sinning A., Liebmann L., Kougioumtzes A., Westermann M., Bruehl C., Hübner C. A. (2011). Synaptic glutamate release is modulated by the Na+-driven Cl−/HCO3− exchanger Slc4a8. J. Neurosci. 31, 7300–7311. doi:10.1523/JNEUROSCI.0269-11.2011
Smith G. A., Brett C. L., Church J. (1998). Effects of noradrenaline on intracellular pH in acutely dissociated adult rat hippocampal CA1 neurones. J. Physiol. (Lond.) 512 (Pt 2), 487–505. doi:10.1111/j.1469-7793.1998.487be.x
Somersalo E., Occhipinti R., Boron W. F., Calvetti D. (2012). A reaction-diffusion model of CO2 influx into an oocyte. J. Theor. Biol. 309, 185–203. doi:10.1016/j.jtbi.2012.06.016
Taki S. (2024). Identification of novel murine RPTPγ and RPTPζ splice variants and the role of RPTPζ in modulating the neuronal and astrocytic intracellular pH response to metabolic or respiratory acidosis. Cleveland, OH: Case Western Reserve University Health Sciences. Available at: https://etd.ohiolink.edu/.
Taki S., Boron W. F., Moss F. J. (2024). Novel RPTPγ and RPTPζ splice variants from mixed neuron-astrocyte hippocampal cultures as well as from the hippocampi of newborn and adult mice. Front. Physiol. 15, 1406448. doi:10.3389/fphys.2024.1406448
Thornell I. M., Michenkova M., Taki S., Bevensee M. O., Boron W. F. (2025). “Intracellular pH homeostasis,” in Seldin and giebisch’s the kidney: physiology and pathophysiology. Editors R. J. Alpern, M. J. Caplan, O. W. Moe, and S. E. Quaggin (London: Elsevier/Academic Press). Available at: https://www.barnesandnoble.com/w/seldin-and-giebischs-the-kidney-robert-j-alpern/1138252983 (Accessed August 29, 2023).
Xiong Z. Q., Saggau P., Stringer J. L. (2000). Activity-dependent intracellular acidification correlates with the duration of seizure activity. J. Neurosci. 20, 1290–1296. doi:10.1523/JNEUROSCI.20-04-01290.2000
Yuen A. W. C. (2006). Low-grade chronic metabolic acidosis is a contributory mechanism in the development of chronic epilepsy. Epilepsy Behav. 8, 347–349. doi:10.1016/j.yebeh.2005.11.012
Zhao J., Hogan E. M., Bevensee M. O., Boron W. F. (1995). Out-of-equilibrium CO₂/HCO₃−solutions and their use in characterizing a new K+/HCO₃− cotransporter. Nature 374, 636–639. doi:10.1038/374636a0
Zhao J., Zhou Y., Boron W. F. (2003). Effect of isolated removal of either basolateral HCO3− or basolateral CO2 on HCO3− reabsorption by rabbit S2 proximal tubule. Am. J. Physiol. Ren. Physiol. 285, F359–F369. doi:10.1152/ajprenal.00013.2003
Zhou Y., Skelton L. A., Xu L., Chandler M. P., Berthiaume J. M., Boron W. F. (2016). Role of receptor protein tyrosine phosphatase γ in sensing extracellular CO₂ and HCO₃−. J. Am. Soc. Nephrol. 27, 2616–2621. doi:10.1681/ASN.2015040439
Keywords: CO2/HCO3− out-of-equilibrium solutions, pH regulation, pHo sensor, HCO3− sensor, neurons
Citation: Bouyer PG, Occhipinti R, Taki S, Moss FJ and Boron WF (2025) Effects of extracellular metabolic acidosis on the homeostasis of intracellular pH in hippocampal neurons. Front. Physiol. 15:1494956. doi: 10.3389/fphys.2024.1494956
Received: 11 September 2024; Accepted: 27 November 2024;
Published: 14 March 2025.
Edited by:
Michael L. Jennings, University of Arkansas for Medical Sciences, United StatesReviewed by:
Ira Kurtz, University of California, Los Angeles, United StatesFrancisco Suárez, Rey Juan Carlos University, Spain
Copyright © 2025 Bouyer, Occhipinti, Taki, Moss and Boron. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrice G. Bouyer, cGF0cmljZS5ib3V5ZXJAdmFscG8uZWR1; Walter F. Boron, d2FsdGVyLmJvcm9uQGNhc2UuZWR1
 Patrice G. Bouyer
Patrice G. Bouyer Rossana Occhipinti
Rossana Occhipinti Sara Taki
Sara Taki Fraser J. Moss
Fraser J. Moss Walter F. Boron
Walter F. Boron