- 1Department of Anesthesia, First Medical Centre of Chinese PLA General Hospital, Beijing, China
- 2National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital, Beijing, China
Objective: The ideal intra-operative inspired oxygen concentration remains controversial. We aimed to investigate the association between the intraoperative fraction of inspired oxygen (FiO2) and the incidence of postoperative pulmonary complications (PPCs) in patients undergoing non-cardiothoracic surgery.
Methods: This was a retrospective cohort study of elderly patients who underwent non-cardiothoracic surgery between April 2020 and January 2022. According to intraoperative FiO2, patients were divided into low (≤60%) and high (>60%) FiO2 groups. The primary outcome was the incidence of a composite of pulmonary complications (PPCs) within the first seven postoperative days. Propensity score matching (PSM) and inverse probability treatment weighting (IPTW) were conducted to adjust for baseline characteristic differences between the two groups. Multivariate logistic regression analysis was used to calculate the odds ratios (OR) for FiO2 and PPCs.
Results: Among the 3,515 included patients with a median age of 70 years (interquartile range: 68–74), 492 (14%) experienced PPCs within the first 7 postoperative days. Elevated FiO2 was associated with an increased risk of PPCs in all the logistic regression models. The OR of the FiO2 > 60% group was 1.252 (95%CI, 1.015–1.551, P = 0.038) in the univariate analysis. In the multivariate logistic regression models, the ORs of the FiO2 > 60% group were 1.259 (Model 2), 1.314 (Model 3), and 1.32 (model 4). A balanced covariate distribution between the two groups was created using PSM or IPTW. The correlation between elevated FiO2 and an increased risk of PPCs remained statistically significant with PSM analysis (OR, 1.393; 95% CI, 1.077–1.804; P = 0.012) and IPTW analysis (OR, 1.266; 95% CI, 1.086–1.476; P = 0.003).
Conclusion: High intraoperative FiO2 (>60%) was associated with the postoperative occurrence of pulmonary complications, independent of predefined risk factors, in elderly non-cardiothoracic surgery patients. High intraoperative FiO2 should be applied cautiously in surgical patients vulnerable to PPCs.
1 Introduction
Postoperative pulmonary complications (PPCs), such as respiratory infection, atelectasis, and hypoxemia, occurred in 10%–59% of patients undergoing surgery, especially in elderly patients with physiological dysfunction. The occurrence of PPCs is associated with an increased risk of morbidity and mortality in hospitalized patients (Pearse et al., 2012; Canet et al., 2010; Fernandez-Bustamante et al., 2017).
Several risk factors are closely related to PPCs, including patient factors, surgical types and timing, and anesthesia management (Sun et al., 2023). Among these factors, the benefits and risks of a high fraction of inspired oxygen (FiO2) during anesthesia remain debated in the scientific literature. The World Health Organization (WHO) recommends applying high FiO2 to reduce the risk of postoperative surgical site infections in patients undergoing general anesthesia (Leaper and Edmiston, 2017). Chinese anesthesiologists have become accustomed to administering higher FiO2 levels throughout the course of intraoperative mechanical ventilation management (Wang et al., 2022). On one hand, high intraoperative FiO2 could increase the safety margin in cases of intraoperative emergencies, such as desaturation in cases of airway loss or ventilation failure (Weenink et al., 2020). However, it may accelerate atelectasis formation during mechanical ventilation, promote coronary vasoconstriction, increase peripheral vascular resistance, and decreases cardiac output (Martin and Grocott, 2015).
Although some recent studies have analyzed the correlation between high FiO2 and PPCs, the recruited samples were not large enough to be representative. In addition, no articles have specifically analyzed elderly patients undergoing non-cardiothoracic surgery. Therefore, we conducted a retrospective study to examine the association between the level of FiO2 and the incidence of PPCs in patients undergoing non-cardiothoracic surgery.
2 Materials and methods
The methods used in this study were modified based on our team’s previous publication (Zhang et al., 2021). The study protocol was reviewed and approved by the Institutional Ethics Committee of the Chinese PLA General Hospital (No. S2021-135-01). The study protocol complied with the Declaration of Helsinki, and the need for informed consent was waived because the study was retrospective. This manuscript adhered to the applicable guidelines as presented in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
2.1 Study subjects
We identified patients who had undergone non-cardiothoracic surgery between April 2020 and January 2022 at the First Medical Center of the Chinese PLA General Hospital, a tertiary academic hospital in Beijing, China.
The inclusion criteria were as follows: age 65 years or older; BMI >18 kg/m2 and <35 kg/m2; and duration of general anesthesia >60 min. Patients who presented with an American Society of Anesthesiologists (ASA) classification ≥ IV or had missing clinical data of >50% were excluded. Among the patients who underwent multiple surgeries during the study period, only the first eligible surgery was considered. A flow diagram of the patient selection process is shown in Figure 1.
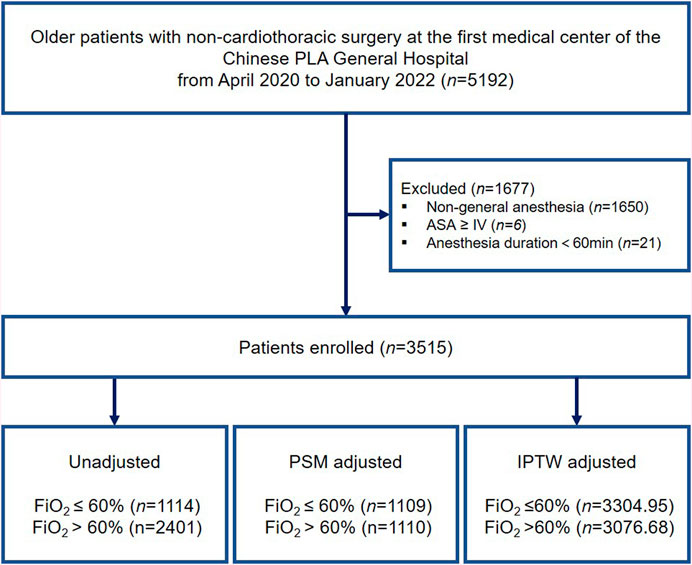
Figure 1. Study flow diagram. ASA, American Society of Anesthesiologists; PSM, propensity score matching; IPTW, inverse probability treatment weighting.
2.2 Study outcome
The primary outcome was the incidence of a composite of pulmonary complications within the first seven postoperative days. PPCs were defined using the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) study criteria, including postoperative respiratory infection, respiratory failure, bronchospasm, atelectasis, pulmonary embolism, pleural effusion, aspiration pneumonia, pulmonary edema, and pneumothorax (Canet et al., 2010; Mazo et al., 2014).
2.3 Definition of variables and data collection
After descriptive analysis of the retrieved data, we found that a large number of physicians in our department prefer to apply a high intraoperative FiO2, while others tend to use a FiO2 of less than 60%. Only 2.22% of the patients received a FiO2 of 21%–50% intraoperatively, 29.47% received a FiO2 of 50%–60%, 7.1% received a FiO2 of 60%–90% and 61.22% received a FiO2 of 100%. The first quartile (Q1) of the distribution was at an FiO2 of 60%. Therefore, according to the distribution of FiO2 in the retrieved data, the threshold of FiO2 in this study was set to 60% for the prediction of PPCs. Consequently, the FiO2 was stratified into low (≤60%) and high (>60%) for subsequent analyses.
The electronic medical record system was used to collect demographic, preoperative and postoperative data including medication, lab results and radiology reports, etc. Preoperative covariates of interest, such as age, sex, body mass index (BMI), ASA classification, hypertension, diabetes mellitus, smoking history, drinking history, and tumor characteristics (benign or malignant), were noted. The indices derived from the preoperative laboratory data, including hemoglobin, leukocyte, creatinine, and glucose levels, were defined as the most recent counts measured within 3 days prior to surgery. In addition, surgical and anesthesia information was extracted from the anesthesia information management system, including tidal volume, respiratory rate, surgery type (laparoscopic or open surgery), duration of anesthesia, blood loss, fluid loss, infusion volume, crystalloid or colloid infusion volume, blood transfusion volume, intraoperative antibiotics, and opioid use. All related data were extracted from the database to calibrate the results of PPCs. Data were manually revised and reviewed according to the original medical and anesthesia records, if abnormal, such as missing, duplicate and outlier, were found during the data cleansing process.
2.4 Statistical analysis
The baseline characteristics and outcomes of the patients were summarized using frequencies and descriptive statistics. Continuous variables are expressed as mean (SD) or median (interquartile range), and categorical variables are presented as n (%). In the analysis of baseline data, the chi-square test was used for classified data, and Fisher’s test was used if the frequency was <5. If the continuous data were normal, variance analysis was used. If continuous data were not normal, a rank sum test was used.
Multivariate logistic regression was performed to control for potential confounding effects and evaluate the relationship between FiO2 and PPCs. In the logistic regression analysis, multiple models were constructed with different covariates to calculate the odds ratios (OR) of FiO2 and PPCs.
In subsequent analyses, propensity score (PS) analysis was conducted, including propensity score matching (PSM) and inverse probability treatment weighting (IPTW), to examine the PPCs associated with FiO2. To adjust for between-group differences, PSs were developed to reflect the application of FiO2 in each patient during surgery. PS, a composite score, was derived from synthesized baseline characteristics. Clinically relevant covariates (the aforementioned 25 covariates) were included in the multivariate logistic regression model to yield PS. In PSM, matching between the two groups was randomly conducted with PS at a 1:2 ratio using the greedy nearest-neighbor approach, with a caliper width of 0.2. Symmetric trimming was performed in IPTW to minimize the adverse effects of extreme PS outliers. Patients with an estimated PS beyond the range (10%–90%) were excluded. After obtaining matched or weighted data, kernel density plots and standardized mean difference (SMD) were applied to assess the balance of covariates between the two groups. An SMD <0.1 was deemed as acceptable deviations for the particular covariate. The association between FIO2 and PPCs was estimated using multivariate logistic regression analysis to calculate the adjusted OR.
In several previous meta-analyses or studies, advanced age, duration of anesthesia, laparoscopic surgery, smoking history, and BMI were associated with shortened PPCs. Therefore, subgroup analyses were performed according to the aforementioned parameters to explore the potential interactions. Multivariate logistic regression analyses were performed separately for each subgroup to calculate the adjusted OR. A two-sided P < 0.05 was considered statistically significant. Statistical analyses were performed using R (version 4.0.5; R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Patient characteristics
A total of 5,192 elderly patients who underwent non-cardiothoracic surgery between April 2020 and January 2022 were enrolled in this study. Figure 1 illustrates a flow diagram of patient selection. After applying the inclusion and exclusion criteria, 3,515 eligible patients remained in the analysis, with a median age of 70 years (IQR: 68–74 years), of whom 1,618 (46.0%) were women. Among the patients, 1896 (53.94%) underwent laparoscopic surgery and 303 (0.086%) received blood transfusions. Of the entire patient cohort, 492 (14.00%) patients experienced PPCs after surgery.
Patients were then grouped into low (≤60%, n = 1,114, 31.69%) and high (>60%, n = 2,401, 68.31%) FiO2 groups. The differences in baseline characteristics between the groups are shown in Table 1. Compared with patients with FiO2 ≤ 60%, those with FiO2 > 60% had a higher incidence of PPCs.
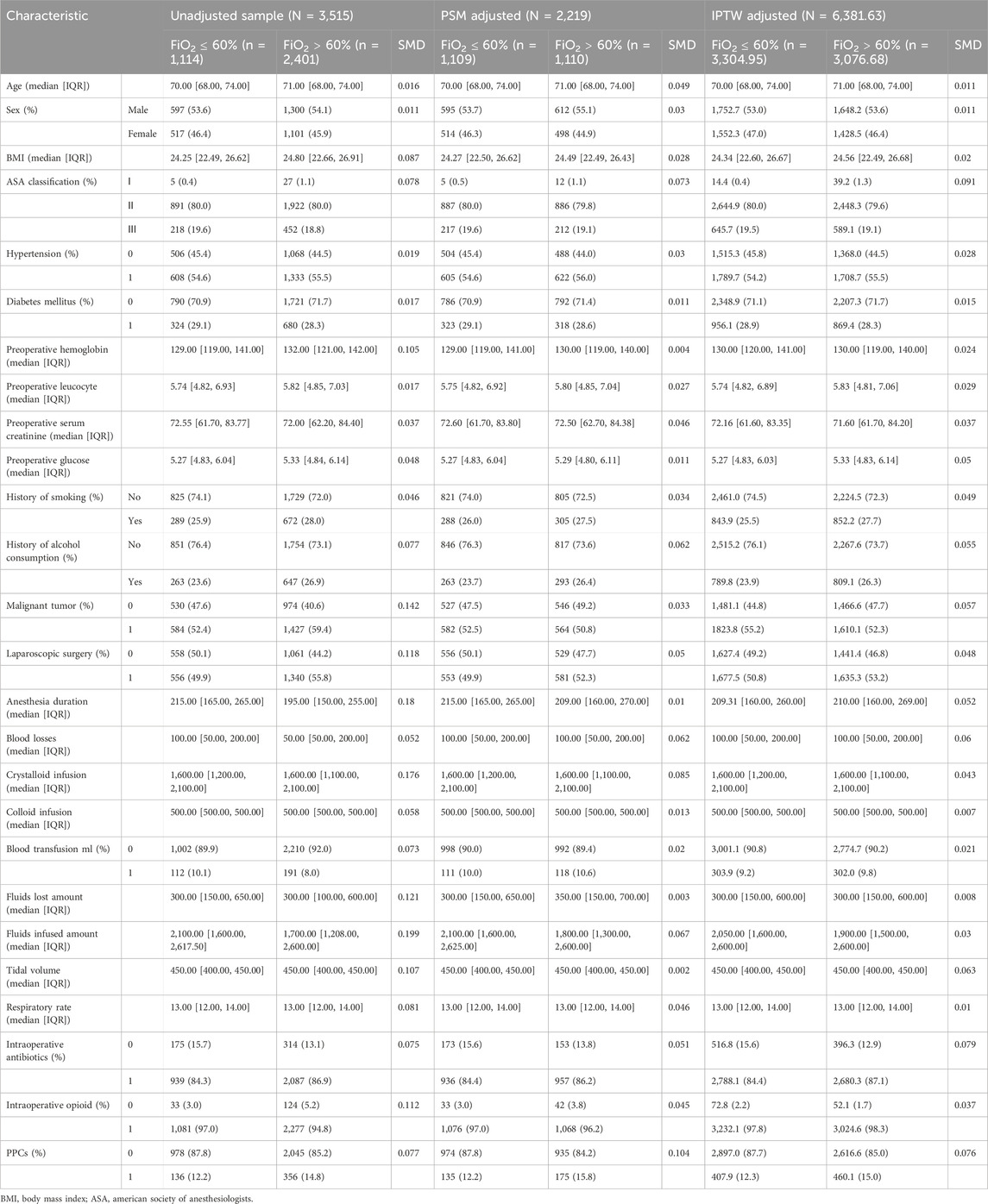
Table 1. Baseline characteristics unadjusted sample, propensity score-matched sample, and inverse probability of treatment-weighted sample.
3.2 Correlation between FiO2 and PPCs
Three logistic regression models were used to investigate the correlation between FiO2 and PPCs. Elevated FiO2 was associated with an increased risk of PPCs in all models. The OR of the FiO2 > 60% group was 1.252 (95%CI:1.015–1.551, P = 0.038) in the univariate analysis. In the multivariate logistic regression models, the ORs of the FiO2 > 60% group were 1.259 (model 2), 1.314 (model 3), and 1.32 (model 4), respectively. The P values were <0.05 for all models (Table 2). The overall univariate and multivariate logistic regression results are presented in Table 2.

Table 2. Association between intraoperative FiO2 and occurrence of PPCs using the logistic regression model and propensity score analysis.
3.3 PSM and IPTW analysis and adjustment
PSM and IPTW analyses were performed as planned. Seven variables (BMI, preoperative hemoglobin level, anesthesia duration, malignant tumor, tidal volume, respiratory rate, and intraoperative opioid use) were matched in constructing the PSM cohort. Eight variables (BMI, sex, preoperative hemoglobin, anesthesia duration, malignant tumor, tidal volume, respiratory rate, and intraoperative opioid use) were matched to construct the IPTW cohort. A total of 1,114 patients in the FiO2 ≤ 60% group and 2,401 patients in the FiO2 > 60% group were matched. The distribution of propensity scores of the patients before and after PSM and IPTW is displayed in Figures 2, 3, respectively. The baseline characteristics and variables were balanced between the two groups (SMD <0.1) (Table 1). In the logistic regression analysis performed after PSM, FiO2 > 60% was still an independent predictor of PPCs, with an OR of 1.393 (95% CI: 1.077–1.804, P = 0.012). Similarly, in the logistic regression performed after IPTW, FiO2>60% was an independent predictor of PPCs, with an OR of 1.266 (95% CI: 1.086–1.476, P = 0.003) (Table 2).
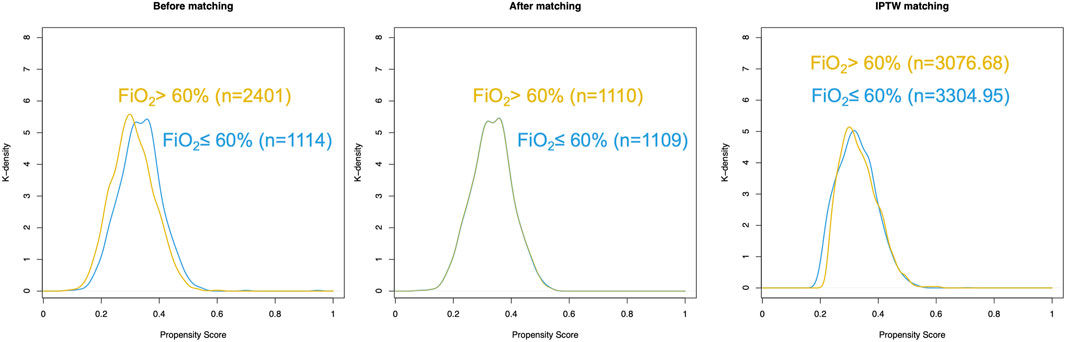
Figure 2. Distribution of propensity scores in patients with postoperative pulmonary complications (low and high FiO2 groups). (A) Before matching. (B) After matching. (C) IPTW matching.
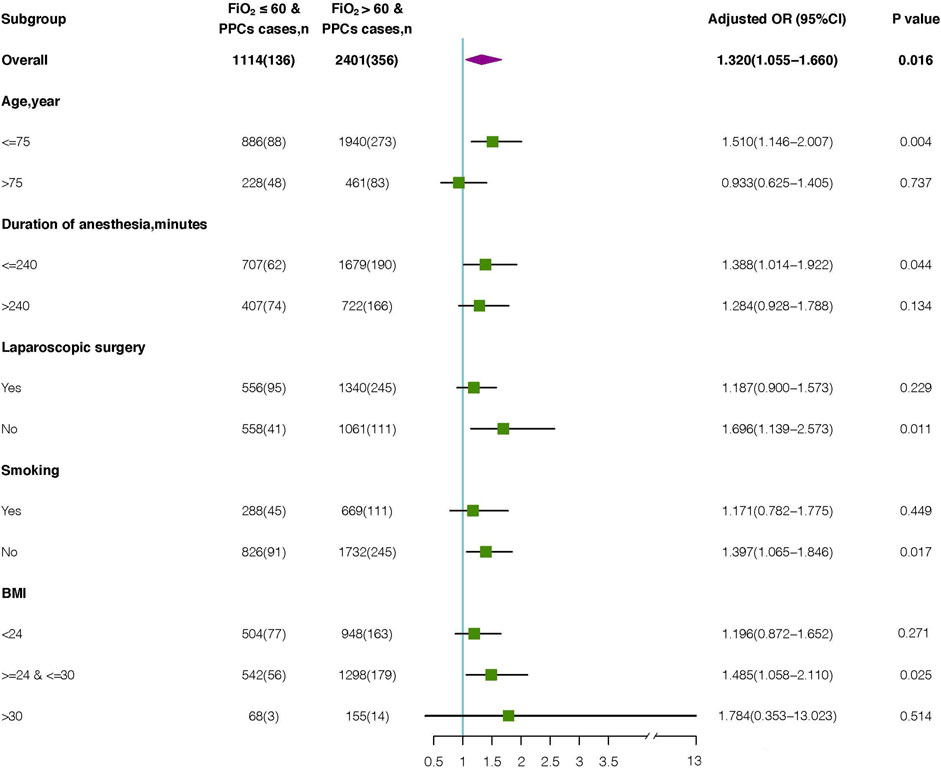
Figure 3. Subgroup analysis of the association between intraoperative FiO2 and occurrence of PPCs. OR, odds ratio.
3.4 Subgroup analyses
Among the 2,401 elderly patients with FiO2 > 60%, 461 (19.2%) were aged >75 years, 722 (30.1%) had a duration of anesthesia >240 min, 1,340 (55.8%) underwent laparoscopic surgery, 669 (27.9%) had a history of smoking, and 948 (39.5%) had 18<BMI<24 kg/m2. The BMI of 155 (6.5%) patients was 30–35 kg/m2.
Advanced age, duration of anesthesia, laparoscopic surgery, smoking history, and BMI were associated with shortened PPCs. To explore potential interactions, we performed a subgroup analysis according to the parameters above. (Figure 3). Multivariate logistic regression analyses were conducted for each subgroup to calculate the adjusted OR. The OR of FiO2 was significant for age subgroups [≥75 years: OR (95%CI): 0.933 (0.625–1.405), P = 0.737; <75 years: OR (95%CI): 1.510 (1.146–2.007), P = 0.004]. Additionally, an increased risk of PPCs was observed for both duration of anesthesia ≤240 min (odds ratio [OR], 1.388; 95% CI: 1.014–1.922; P = 0.044) and duration of anesthesia >240 min (OR: 1.284; 95% CI: 0.928–1.788; P = 0.134). In patients who underwent laparoscopic surgery, high FiO2 was significantly correlated with PPCs (OR, 1.187; 95%CI: 0.900–1.573; P = 0.229). This increased risk was also significant in those who underwent non-laparoscopic surgery (OR, 1.696; 95% CI: 1.139–2.573; P = 0.011). The correlation between Intraoperative FiO2 and PPCs was significant in individuals with (OR:1.171; 95%CI: 0.782–1.775; P = 0.449) and without (OR: 1.397; 95%CI: 1.065–1.846; P = 0.017) history of smoking. Additionally, in each BMI group, high FiO2 was significantly associated with an increased risk of PPCs (18<BMI<24 group: OR: 1.196; 95% CI: 0.872–1.652; P = 0.271) (24≤BMI≤30 group: OR: 1.148; 95% CI: 1.058–2.110; P = 0.025) (30<BMI<35 group: OR: 1.784; 95% CI: 0.353–13.023; P = 0.514).
4 Discussion
In this retrospective analysis of surgical patients undergoing intraoperative mechanical ventilation for elderly non-cardiothoracic surgery, we found that a high intraoperative FiO2 (>60%) was significantly associated with a composite outcome of PPCs. We conducted propensity score matching (PSM) and inverse probability treatment weighting (IPTW) to adjust for baseline characteristic differences between intraoperative high FiO2 and low FiO2 patients, and the findings remained consistent. However, this correlation existed only in non-laparoscopic surgery and was not significant in laparoscopic surgery in our subgroup analysis.
4.1 Controversy over FiO2
Although the World Health Organization (WHO) in 2018 and the Centers for Disease Control and Prevention (CDC) in 2017 recommended the application of high perioperative FiO2 (Berríos-Torres et al., 2017; World Health Organization, 2018), this recommendation and related meta-analyses used to support them have been widely criticized (de Jonge et al., 2019; Myles et al., 2019; Hedenstierna et al., 2019). The ideal FiO2 setting during intraoperative mechanical ventilation is a topic of ongoing debate among anesthesiologists. Advocates argue that high-inspired FiO2 reduces surgical site infection (SSI) (Hovaguimian et al., 2013; Kuh et al., 2023) and postoperative nausea and vomiting (PONV) (Greif et al., 1999) and extends the safety margin in cases of acute intraoperative emergencies. Conversely, opponents contend that high-inspired FiO2 does not reduce SSI and further increases atelectasis formation (Park et al., 2021), oxidative stress during surgery (Oldman et al., 2021), and exacerbates cancer effects (Meyhoff et al., 2012).
At present, studies on the effects of high intraoperative FiO2 on PPCs are conflicting. We found a significant association between high intraoperative FiO2 and PPCs, which is consistent with a large, single-center retrospective database study including 73,922 cases. High intraoperative FiO2 was associated with major respiratory complications in a dose-dependent manner (Staehr-Rye et al., 2017). Moreover, a multicenter observational cohort study showed that patients at the 75th percentile for the area under the curve of the FiO2 had 14% greater odds of lung injury (12%–16%) than patients at the 25th centile (McIlroy et al., 2022). However, in a randomized trial of 251 patients who underwent abdominal surgery with lung-protective ventilation, the incidence of PPCs was similar in patients who received FiO2 of 30% versus 80%, although the severity of PPCs was reduced by low FiO2 (Li et al., 2020). Likewise, in a post-hoc analysis of a prospective single-center alternating cohort trial including nearly 5,000 surgical patients who received 30% or 80% FiO2 during surgery, the incidence of PPCs was similar between the groups (Cohen et al., 2019). Similarly, Ferrando et al. found that the occurrence rate of PPCs did not differ between patients ventilated with FiO2 of 30% and 80%, despite the application of a protocolized ventilation strategy (Ferrando et al., 2020). Notably, the outcome measurements, evaluative dimensions, and ventilation strategies were not entirely the same among these studies and had a lower power of testing as a secondary outcome.
4.2 Underlying mechanisms of lung injury
Several mechanisms have been reported to contribute to lung injury after application of high FiO2 (Horncastle and Lumb, 2019). Higher FiO2 maintained after intubation promoted atelectasis in 90% of patients and was related to an increase in the low ventilation perfusion ratio (Östberg et al., 2017).-Atelectasis is likely to be the focus of infection and may contribute to additional pulmonary complications. One of the main reasons for the development of PPCs is hyperoxia-induced absorptive atelectasis, which is common during general anesthesia and can persist for several days after surgery (Hedenstierna and Edmark, 2010). Other plausible mechanisms include the generation of reactive oxygen species (ROS) and pro-inflammatory factors and the impairment of gas exchange (Nagato et al., 2012; Romagnoli et al., 2015; Gore et al., 2010). Considering that hyperoxia in cardiopulmonary bypass (CPB) did not result in any increase in respiratory complications because of non-ventilated lung during CPB (Abou-Arab et al., 2019), oxygen toxicity to the lung may occur directly through the endotracheal tube to the lung.
4.3 Subgroup analyses
In subgroup analysis, we found that in patients aged ≤75 years, duration of anesthesia ≤240 min, non-laparoscopic surgery, non-smoking, and 24≤ BMI ≤30 subgroup, high FiO2 was associated with an increase in PPCs. A systematic review showed that when compared with patients <50 years old, patients aged 70–79 years had odds ratios (OR) of 3.90 (CI: 2.70–5.65) of developing PPCs (Smetana et al., 2006). Therefore, the results showed that only patients in younger older (aged ≤75 years) subgroup, high FiO2 was associated with an increase in PPCs. In a meta-analysis of 107 cohort and case-control studies, preoperative smoking was associated with an increased risk for PPCs (RR 1.73, 95% CI: 1.35–2.23) (Gore et al., 2010). Likewise, only in non-smoking subgroup, high FiO2 was associated with an increase in PPCs. In a retrospective study (n = 141,802), PPCs were no more common among obese adults (BMI >30 kg/m2) than among those with a healthy weight (BMI 18.5–24.9 kg/m2) (Sood et al., 2015). Interestingly, underweight patients sustained more PPCs, which may be due to the experience of frailty. A recent study found that frailty was significantly associated with PPCs in elderly patients who underwent cardiac surgery (Fan et al., 2023; Chen et al., 2022). It is plausible that only in 24≤ BMI ≤30 subgroup, high FiO2 was associated with an increase in PPCs. Surgical procedures lasting more than 3–4 h are associated with a higher risk of pulmonary complications (McAlister et al., 2003). Similarly, only in duration of anesthesia ≤240 min subgroup, high FiO2 was associated with an increase in PPCs. In patients aged >75 years, duration of anesthesia >240 min, current smoking, BMI <24 kg/m2, and BMI >30 kg/m2 subgroups, high FiO2 was not associated with PPCs. It might be due to the fact that compared to advanced age, duration of surgery, smoking, and BMI, FiO2 had weaker impact on PPCs. In the laparoscopic surgery subgroup, patients who underwent laparoscopic surgery were more likely to develop atelectasis, and thus, PPCs were more common. Considering the differential power of the occurrence of PPCs, it is plausible that high FiO2 was not associated with PPCs in the laparoscopic surgery subgroup.
4.4 Strengths and limitations
The analyses in this study were based on a large dataset that largely reflects routine clinical practice. Data were retrieved from accurate prospective recordings of intraoperative management and postoperative complications. Before initiating the data analysis, we discussed and finalized the protocol, including definitions of risk factors and outcomes, statistical methods, and quality control. This observational design allowed us to collect a large number of 3,515 elderly surgical patients, which can provide sufficient power to detect differences in relatively infrequent complications. Meanwhile, a variety of potential confounding factors were included, such as preoperative and intraoperative data, which allowed for precise effect size evaluation. Additionally, sensitivity analyses, such as PSM or IPTW and subgroup analyses, were successfully performed to further validate the robustness of our findings. Finally, to the best of our knowledge, this is the first study to explore the association between intraoperative FiO2 and the incidence of PPCs in elderly non-cardiothoracic surgical patients.
However, this study has some important limitations must be mentioned. First, to reduce the risk of confounding factors, we adjusted for a large number of different risk factors and performed several sensitivity analyses, including propensity scoring. Second, residual and unmeasured potential confounding factors cannot be completely ruled out in observational studies. Third, patients with chronic lung diseases or underwent chest surgery were not included due to the potential influence on the outcomes of our study and the nature of the retrospective study. In addition, the results were derived from a single-center study; thus, the generalizability of our findings may be limited to other centers. Future randomized controlled trials are needed to confirm our results.
5 Conclusion
In this analysis of administrative data, a high intraoperative FiO2 (>60%) was associated with the postoperative occurrence of pulmonary complications independent of predefined risk factors in elderly non-cardiothoracic surgery patients. This finding was robust in a series of sensitivity analyses, including PSM and IPTW.
The preprint of this manuscript entitled Higher fraction of inspired oxygen during anesthesia increase the risk of postoperative pulmonary complications in patients undergoing non-cardiothoracic surgery: A retrospective cohort study is available at doi. org/10.21203/rs.3.rs-4286848/v1.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The datasets generated and/or analysed during the current study are not publicly available as individual privacy could be compromised but are available from the corresponding authors on reasonable request. Requests to access these datasets should be directed to CZ, cG93ZXJ6Y3NAMTI2LmNvbQ==.
Ethics statement
The studies involving humans were approved by the Institutional Ethics Committee of the Chinese PLA General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’; legal guardians/next of kin because the need for informed consent was waived because the study was retrospective.
Author contributions
TW: Writing–original draft, Writing–review and editing. WZ: Writing–original draft, Writing–review and editing. LM: Data curation, Writing–review and editing. JW: Data curation, Writing–review and editing. XM: Data curation, Writing–review and editing. LL: Data curation, Writing–review and editing. JC: Data curation, Writing–review and editing. JL: Writing–review and editing. WM: Writing–review and editing. CZ: Writing–original draft, Writing–review and editing, Conceptualization, Data curation, Investigation, Methodology, Project administration.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. This research received no external funding.
Acknowledgments
We thank Tongyan Sun from Hangzhou Le9 Healthcare Technology Co., Ltd. for her help with the statistical analysis of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abou-Arab O., Huette P., Martineau L., Beauvalot C., Beyls C., Josse E., et al. (2019). Hyperoxia during cardiopulmonary bypass does not decrease cardiovascular complications following cardiac surgery: the CARDIOX randomized clinical trial. Intensive Care Med. 45, 1413–1421. doi:10.1007/s00134-019-05761-4
Berríos-Torres S. I., Umscheid C. A., Bratzler D. W., Leas B., Stone E. C., Kelz R. R., et al. (2017). Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 152, 784–791. doi:10.1001/jamasurg.2017.0904
Canet J., Gallart L., Gomar C., Paluzie G., Vallès J., Castillo J., et al. (2010). Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 113, 1338–1350. doi:10.1097/ALN.0b013e3181fc6e0a
Chen D., Ding Y., Zhu W., Fang T., Dong N., Yuan F., et al. (2022). Frailty is an independent risk factor for postoperative pulmonary complications in elderly patients undergoing video-assisted thoracoscopic pulmonary resections. Aging Clin. Exp. Res. 34, 819–826. doi:10.1007/s40520-021-01988-8
Cohen B., Ruetzler K., Kurz A., Leung S., Rivas E., Ezell J., et al. (2019). Intra-operative high inspired oxygen fraction does not increase the risk of postoperative respiratory complications: alternating intervention clinical trial. Eur. J. Anaesthesiol. 36, 320–326. doi:10.1097/eja.0000000000000980
de Jonge S., Egger M., Latif A., Loke Y. K., Berenholtz S., Boermeester M., et al. (2019). Effectiveness of 80% vs 30-35% fraction of inspired oxygen in patients undergoing surgery: an updated systematic review and meta-analysis. Br. J. Anaesth. 122, 325–334. doi:10.1016/j.bja.2018.11.024
Fan G., Fu S., Zheng M., Xu W., Ma G., Zhang F., et al. (2023). Association of preoperative frailty with pulmonary complications after cardiac surgery in elderly individuals: a prospective cohort study. Aging Clin. Exp. Res. 35, 2453–2462. doi:10.1007/s40520-023-02527-3
Fernandez-Bustamante A., Frendl G., Sprung J., Kor D. J., Subramaniam B., Martinez Ruiz R., et al. (2017). Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators. JAMA Surg. 152, 157–166. doi:10.1001/jamasurg.2016.4065
Ferrando C., Aldecoa C., Unzueta C., Belda F. J., Librero J., Tusman G., et al. (2020). Effects of oxygen on post-surgical infections during an individualised perioperative open-lung ventilatory strategy: a randomised controlled trial. Br. J. Anaesth. 124, 110–120. doi:10.1016/j.bja.2019.10.009
Gore A., Muralidhar M., Espey M. G., Degenhardt K., Mantell L. L. (2010). Hyperoxia sensing: from molecular mechanisms to significance in disease. J. Immunotoxicol. 7, 239–254. doi:10.3109/1547691x.2010.492254
Greif R., Laciny S., Rapf B., Hickle R. S., Sessler D. I. (1999). Supplemental oxygen reduces the incidence of postoperative nausea and vomiting. Anesthesiology 91, 1246–1252. doi:10.1097/00000542-199911000-00014
Hedenstierna G., Edmark L. (2010). Mechanisms of atelectasis in the perioperative period. Best. Pract. Res. Clin. Anaesthesiol. 24, 157–169. doi:10.1016/j.bpa.2009.12.002
Hedenstierna G., Meyhoff C. S., Perchiazzi G., Larsson A., Wetterslev J., Rasmussen L. S. (2019). Modification of the World Health organization global guidelines for prevention of surgical site infection is needed. Anesthesiology 131, 765–768. doi:10.1097/aln.0000000000002848
Horncastle E., Lumb A. B. (2019). Hyperoxia in anaesthesia and intensive care. BJA Educ. 19, 176–182. doi:10.1016/j.bjae.2019.02.005
Hovaguimian F., Lysakowski C., Elia N., Tramèr M. R. (2013). Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary function: systematic review and meta-analysis of randomized controlled trials. Anesthesiology 119, 303–316. doi:10.1097/ALN.0b013e31829aaff4
Kuh J. H., Jung W. S., Lim L., Yoo H. K., Ju J. W., Lee H. J., et al. (2023). The effect of high perioperative inspiratory oxygen fraction for abdominal surgery on surgical site infection: a systematic review and meta-analysis. Sci. Rep. 13, 15599. doi:10.1038/s41598-023-41300-4
Leaper D. J., Edmiston C. E. (2017). World Health Organization: global guidelines for the prevention of surgical site infection. J. Hosp. Infect. 95, 135–136. doi:10.1016/j.jhin.2016.12.016
Li X. F., Jiang D., Jiang Y. L., Yu H., Zhang M. Q., Jiang J. L., et al. (2020). Comparison of low and high inspiratory oxygen fraction added to lung-protective ventilation on postoperative pulmonary complications after abdominal surgery: a randomized controlled trial. J. Clin. Anesth. 67, 110009. doi:10.1016/j.jclinane.2020.110009
Martin D., Grocott M. (2015). Oxygen therapy and anaesthesia: too much of a good thing? Anaesthesia 70, 522–527. doi:10.1111/anae.13081
Mazo V., Sabaté S., Canet J., Gallart L., de Abreu M. G., Belda J., et al. (2014). Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology 121, 219–231. doi:10.1097/aln.0000000000000334
McAlister F. A., Khan N. A., Straus S. E., Papaioakim M., Fisher B. W., Majumdar S. R., et al. (2003). Accuracy of the preoperative assessment in predicting pulmonary risk after nonthoracic surgery. Am. J. Respir. Crit. Care Med. 167, 741–744. doi:10.1164/rccm.200209-985BC
McIlroy D. R., Shotwell M. S., Lopez M. G., Vaughn M. T., Olsen J. S., Hennessy C., et al. (2022). Oxygen administration during surgery and postoperative organ injury: observational cohort study. Bmj 379, e070941. doi:10.1136/bmj-2022-070941
Meyhoff C. S., Jorgensen L. N., Wetterslev J., Christensen K. B., Rasmussen L. S.PROXI Trial Group (2012). Increased long-term mortality after a high perioperative inspiratory oxygen fraction during abdominal surgery: follow-up of a randomized clinical trial. Anesth. Analg. 115, 849–854. doi:10.1213/ANE.0b013e3182652a51
Myles P. S., Carlisle J. B., Scarr B. (2019). Evidence for compromised data integrity in studies of liberal peri-operative inspired oxygen. Anaesthesia 74, 573–584. doi:10.1111/anae.14584
Nagato A. C., Bezerra F. S., Lanzetti M., Lopes A. A., Silva M. A. S., Porto L. C., et al. (2012). Time course of inflammation, oxidative stress and tissue damage induced by hyperoxia in mouse lungs. Int. J. Exp. Pathol. 93, 269–278. doi:10.1111/j.1365-2613.2012.00823.x
Oldman A. H., Martin D. S., Feelisch M., Grocott M. P. W., Cumpstey A. F. (2021). Effects of perioperative oxygen concentration on oxidative stress in adult surgical patients: a systematic review. Br. J. Anaesth. 126, 622–632. doi:10.1016/j.bja.2020.09.050
Östberg E., Auner U., Enlund M., Zetterström H., Edmark L. (2017). Minimizing atelectasis formation during general anaesthesia-oxygen washout is a non-essential supplement to PEEP. Ups. J. Med. Sci. 122, 92–98. doi:10.1080/03009734.2017.1294635
Park M., Jung K., Sim W. S., Kim D. K., Chung I. S., Choi J. W., et al. (2021). Perioperative high inspired oxygen fraction induces atelectasis in patients undergoing abdominal surgery: a randomized controlled trial. J. Clin. Anesth. 72, 110285. doi:10.1016/j.jclinane.2021.110285
Pearse R. M., Moreno R. P., Bauer P., Pelosi P., Metnitz P., Spies C., et al. (2012). Mortality after surgery in Europe: a 7 day cohort study. Lancet 380, 1059–1065. doi:10.1016/s0140-6736(12)61148-9
Romagnoli S., Becatti M., Bonicolini E., Fiorillo C., Zagli G. (2015). Protective ventilation with low fraction of inspired oxygen and radicals of oxygen production during general anaesthesia. Br. J. Anaesth. 115, 143–144. doi:10.1093/bja/aev180
Smetana G. W., Lawrence V. A., Cornell J. E.American College of Physicians (2006). Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann. Intern Med. 144, 581–595. doi:10.7326/0003-4819-144-8-200604180-00009
Sood A., Abdollah F., Sammon J. D., Majumder K., Schmid M., Peabody J. O., et al. (2015). The effect of body mass index on perioperative outcomes after major surgery: results from the national surgical quality improvement program (ACS-NSQIP) 2005-2011. World J. Surg. 39, 2376–2385. doi:10.1007/s00268-015-3112-7
Staehr-Rye A. K., Meyhoff C. S., Scheffenbichler F. T., Vidal Melo M. F., Gätke M. R., Walsh J. L., et al. (2017). High intraoperative inspiratory oxygen fraction and risk of major respiratory complications. Br. J. Anaesth. 119, 140–149. doi:10.1093/bja/aex128
Sun Q., Zhang T., Liu J., Cui Y., Tan W. (2023). A 20-year bibliometric analysis of postoperative pulmonary complications: 2003-2022. Heliyon 9, e20580. doi:10.1016/j.heliyon.2023.e20580
Wang J., Cao X., Qi T. (2022). Effect of different inhaled oxygen concentrations on postoperative pulmonary complications in elderly patients undergoing laparoscopic radical surgery for colon cancer. Pract. Geriatr. 36, 69–72. doi:10.3969/j.issn.1003-9198.2022.01.018
Weenink R., de Jonge S. W., van Hulst R. A., Wingelaar T. T., van Ooij P. J. A. M., Immink R. V., et al. (2020). Perioperative hyperoxyphobia: justified or not? Benefits and harms of hyperoxia during surgery. J. Clin. Med. 9, 642. doi:10.3390/jcm9030642
World Health Organization (2018). WHO guidelines approved by the guidelines review committee. Geneva: World Health Organization.
Zhang F., Ma Y., Yu Y., Sun M., Li H., Lou J., et al. (2021). Type 2 diabetes increases risk of unfavorable survival outcome for postoperative ischemic stroke in patients who underwent non-cardiac surgery: a retrospective cohort study. Front. Aging Neurosci. 13, 810050. doi:10.3389/fnagi.2021.810050
Keywords: lung-protective ventilation, fraction of inspired oxygen, postoperative pulmonary complications, elderly, non-cardiothoracic surgery
Citation: Wang T, Zhao W, Ma L, Wu J, Ma X, Liu L, Cao J, Lou J, Mi W and Zhang C (2024) Higher fraction of inspired oxygen during anesthesia increase the risk of postoperative pulmonary complications in patients undergoing non-cardiothoracic surgery: a retrospective cohort study. Front. Physiol. 15:1471454. doi: 10.3389/fphys.2024.1471454
Received: 27 July 2024; Accepted: 30 September 2024;
Published: 18 October 2024.
Edited by:
Qinghe Meng, Upstate Medical University, United StatesReviewed by:
Geresu Gebeyehu, Addis Ababa University, EthiopiaMehrdad Behnia, University Of Central Florida, United States
Copyright © 2024 Wang, Zhao, Ma, Wu, Ma, Liu, Cao, Lou, Mi and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changsheng Zhang, cG93ZXJ6Y3NAMTI2LmNvbQ==; Jingsheng Lou, bG91amluZ3NoZW5nQDE2My5jb20=; Weidong Mi, d3dkZDE5NjJAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Tianzhu Wang1†
Tianzhu Wang1† Weixing Zhao
Weixing Zhao Xiaojing Ma
Xiaojing Ma Jiangbei Cao
Jiangbei Cao Jingsheng Lou
Jingsheng Lou Changsheng Zhang
Changsheng Zhang