- 1Department of Child Health, University of Arizona College of Medicine-Phoenix, Phoenix, AZ, United States
- 2A.T. Still University Kirksville College of Osteopathic Medicine, Kirksville, MO, United States
- 3Translational Neurotrauma and Neurochemistry, Barrow Neurological Institute at Phoenix Children’s Hospital, Phoenix, AZ, United States
- 4West Virginia University School of Medicine, Rockefeller Neuroscience Institute, Morgantown, WV, United States
- 5Phoenix VA Healthcare System, Phoenix, AZ, United States
Traumatic brain injury (TBI) is associated with diffuse axonal injury (DAI), a primary pathology linked to progressive neurodegeneration and neuroinflammation, including chronic astrogliosis, which influences long-term post-TBI recovery and morbidity. Sex-based differences in blood-brain barrier (BBB) permeability increases the risk of accelerated brain aging and early-onset neurodegeneration. However, few studies have evaluated chronic time course of astrocytic responses around cerebrovascular in the context of aging after TBI and sex dependence. We observed increased glial fibrillary acidic protein (GFAP)-labeled accessory processes branching near and connecting with GFAP-ensheathed cortical vessels, suggesting a critical nuance in astrocyte-vessel interactions after TBI. To quantify this observation, male and female Sprague Dawley rats (∼3 months old, n = 5–6/group) underwent either sham surgery or midline fluid percussion injury. Using immunohistochemical analysis, we quantified GFAP-labeled astrocyte primary and accessory processes that contacted GFAP-ensheathed vessels in the somatosensory barrel cortex at 7, 56, and 168 days post-injury (DPI). TBI significantly increased GFAP-positive primary processes at 7 DPI (P < 0.01) in both sexes. At 56 DPI, these vessel-process interactions remained significantly increased exclusively in males (P < 0.05). At 168 DPI, both sexes showed a significant reduction in vessel-process interactions compared to 7 DPI (P < 0.05); however, a modest but significant injury effect reemerged in females (P < 0.05). A similar sex-dependent pattern in the number of accessory processes provides novel evidence of long-term temporal changes in astrocyte-vessel interactions. TBI-induced changes in astrocyte-vessel interactions may indicate chronic BBB vulnerability and processes responsible for early onset vascular and neurodegenerative pathology.
1 Introduction
Traumatic brain injury (TBI) presents one of the most complex neurological insults. Each year, millions of individuals suffer from TBI, raising significant concern about the long-term health effects (Mayer et al., 2017). Chronic consequences of TBI accelerate brain aging, where predicted brain age differences were 5 years greater than controls (Dennis et al., 2024). TBI is also associated with an increased long-term risk of early-onset cardiovascular diseases, stroke, dementia, and neurodegenerative diseases (Kiraly and Kiraly, 2007; Stewart et al., 2022; Schneider et al., 2023). Diffuse axonal injury (DAI) is one of the most common and potentially most insidious pathological features due to its involvement in chronic pathogenesis, where the magnitude of DAI links with symptom severity in clinical and preclinical studies (Johnson et al., 2013; Smith et al., 2013; Blennow et al., 2016). Traumatic DAI initiates chronic neurodegeneration, neuroinflammation, and oxidative stress, leading to chronic blood-brain barrier (BBB) dysfunction and maladaptive vascular remodeling, all of which are implicated in accelerating brain aging (Faden and Loane, 2015; Cash and Theus, 2020; Lin et al., 2022; Lu et al., 2023). These TBI-induced changes accelerate brain aging, resulting in cerebrovascular impairment, an early indicator of cognitive decline and a hallmark of age-related neurodegenerative diseases (Senatorov et al., 2019; Barisano et al., 2022; Knox et al., 2022; Sulimai et al., 2023).
Astrocytes are major glial cells that establish direct structural and functional contact with vasculature to regulate BBB permeability, blood flow, energy uptake, and waste clearance (Koehler et al., 2009; Mathiisen et al., 2010; Baldwin et al., 2023). Astrocyte endfeet directly interact with the cerebral vessels, particularly endothelial cells, the basement membrane, and pericytes, and are critical for the formation and maintenance of the BBB (Cabezas et al., 2014). In pathological conditions, astrocyte reactivity increases the expression of glial fibrillary acidic protein (GFAP), which is associated with hypertrophy, proliferation, and changes in function, making it a standard and widely utilized marker for studying the response to TBI and the influence of interventions. It is important to note that astrocyte pathophysiology is an emerging field with a growing number of phenotypes and classifications (Verkhratsky et al., 2023). This manuscript will use the term “reactive astrocytes” as a generalized descriptor for increased GFAP-expressing astrocytes. Chronic astrocyte reactivity after TBI, reported in clinical and preclinical studies, presents a diversity of phenotypes that can contribute directly to vascular pathology (George et al., 2022). Astrocyte endfeet ensheath the vasculature, and disruptions to this dynamic interaction affect global neurological function (Mills et al., 2022). Astrocyte reactivity after TBI results in morphological and functional changes that may be either beneficial (supporting homeostasis and BBB repair) or detrimental (promoting BBB permeability and impaired blood flow) (Burda et al., 2016; Munoz-Ballester and Robel, 2023). Chronic astrocytic reactivity is a key player in pathology progression, contributing to vascular damage and the development of inflammatory cascades (Kempuraj et al., 2021). Morphological remodeling of reactive astrocytes leads to loss of gliovascular interaction, which can influence the barrier properties after TBI (Villapol et al., 2014). Additionally, vascular aging disrupts astrocyte associations with blood vessels, further increasing barrier permeability (Dunn et al., 2021). These processes are implicated in long-term recovery and morbidity, significantly affecting BBB permeability and increasing the risk of accelerated brain aging and neurodegenerative diseases.
Sex differences in healthy and disease-associated cerebrovascular aging, astrocyte reactivity, and vascular cognitive impairment have been well-documented in the literature (Robison et al., 2019). TBI accelerates brain aging partly due to BBB dysfunction, with astrocytes playing a crucial role in cerebrovascular pathology. Notable sex differences exist in astrocytic responses to TBI, yet astrocyte-vascular interactions and how they differ by sex over time have not been fully evaluated, highlighting the importance of sex-specific research to address distinct impacts on vascular health and neurodegenerative risks (Chisholm and Sohrabji, 2016; Honarpisheh and McCullough, 2019; Hubbard et al., 2022; Cantone et al., 2023; Zhang et al., 2023). This gap underscores the need to evaluate astrocytic responses over time, particularly their interactions with blood vessels, as several studies have shown their implications in both the short- and long-term effects of TBI (Mira et al., 2021). However, the findings are often inconsistent due to variations in injury types, evaluation time points, inclusion of female subjects, and outcome measures (Burda et al., 2016; Munoz-Ballester and Robel, 2023; Verkhratsky et al., 2023). Most existing profiles are limited to evaluations under 2 months post-injury, focus predominantly on males, and often involve penetrating injuries associated with glial scar formation (Villapol et al., 2014). The use of diverse outcome measures and differences in the duration of astrocyte reactivity and molecular profiles add to the complexity, making interpretation across different preclinical models and time points challenging. To date, few studies have extended temporal assessments of astrocyte-vessel interactions to 6 months post-injury with sex as a biological variable using a highly reproducible DAI model without cavitation. The few supporting reports confirm unique astrocyte phenotypes associated with chronic BBB dysfunction (George et al., 2022). Temporal profiles of astrocyte-vessel interactions after DAI are needed to address these gaps for a comprehensive understanding of astrocytic roles in TBI and their contributions to accelerated brain aging (Badaut and Bix, 2014; Díaz-Castro et al., 2023).
We previously demonstrated that GFAP density was significantly increased in the primary somatosensory cortex (S1BF; Figure 1B) at 7- and 56-days post-injury (DPI) and that GFAP intensity increased in shams over 6 months post-surgery (Sabetta et al., 2023). GFAP positive (+) astrocyte processes ensheathed vessels were clearly distinguished by their larger diameter and perpendicular or lateral trajectory to the brain surface, cylindrical shape, and clear lumen, with apparent changes in astrocyte-vessel interactions (Figure 1A). At 100× magnification, we predominantly observed GFAP-primary processes with no obvious process branches proximal to the GFAP ensheathed vessels (black arrow Figure 1C). At a subacute time point post-injury, we detected an increased number of accessory processes branching proximal to the vessels and contacting the GFAP + ensheathment around the vessel (orange arrow in Figure 1C), indicating a novel nuance in astrocyte-vessel interactions. We sought to determine the changes in primary and accessory GFAP + processes that interact with vessels to assess if a single TBI chronically disrupts astrocyte-vessel interactions indicative of chronic BBB vulnerability in male and female rats at 7-, 56-, and 168-days post-injury in a rat model of diffuse TBI.
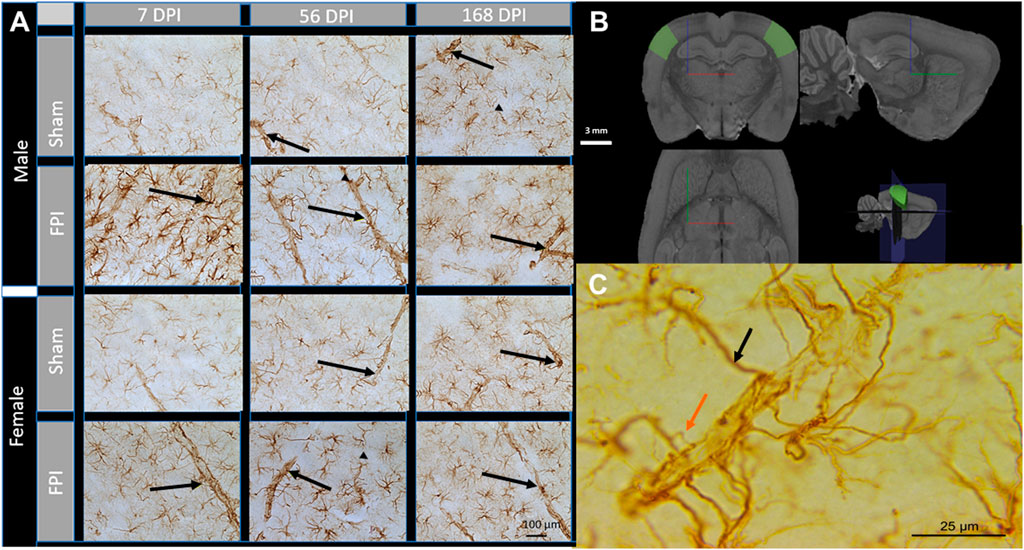
Figure 1. Astrocyte interaction with blood vessels after TBI. (A) Representative images of GFAP immunohistochemistry in the S1BF acquired at 40× magnification. Black arrows indicate GFAP-ensheathed vasculature. At 7 DPI, astrocytes displayed reactive morphology with an increased number of cells, larger cell bodies, and more pronounced processes. Scale bar = 100 (B) 3D schematic showing the S1BF, where images of vessels were captured within cortical layer IV (green areas). Brain region defined by the Waxholm Space Atlas of the Sprague Dawley rat brain (v4) (Papp et al., 2014). White scale bar = 3 mm; red, blue, and green lines represent x, y, and z directions, respectively. (C) A scaled-up image of GFAP + processes ensheathing a cortical vessel. The black arrow indicates a primary process directly from the astrocyte, and the orange arrow indicates an accessory process branching proximal to GFAP + ensheathment. Scale Bar = 25 µm.
2 Methods
2.1 Animals
A total of 64 young adult age-matched male and naturally cycling female Sprague-Dawley rats (3–4 months old; males 367 ± 3 g and females 235 ± 1.5 g; n = 5–6/group; Inotiv (formerly Envigo), Indianapolis, IN, United States of America) from the same study were used in these experiments. Rats were housed in temperature (68°F–79°F) and humidity-regulated 12:12 h light:dark cycle room with free access to food (Teklad 2918) and water (Innovive, San Diego, CA, United States of America) and acclimatized for at least 1 week before experiments. All procedures were conducted in compliance with ARRIVE guidelines and consistent with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals approved by the Institutional Animal Care and Use Committee (protocol #18-384) at the University of Arizona College of Medicine-Phoenix.
2.2 Surgeries
Midline fluid percussion injury (FPI) induces DAI without cavitation or contusion (Lifshitz et al., 2016). The surgery was performed similarly to our previous publications (Bromberg et al., 2020; Krishna et al., 2020; Sabetta et al., 2023). Cages of rats (2/cage) were randomized to either FPI or sham groups. Briefly, rats were anesthetized with isoflurane (5% in 100% oxygen for 5 min) prepared for aseptic surgery and placed into a stereotaxic frame (Kopf Instruments, Tujunga, CA) and anesthesia maintained at 2.5% for the procedure’s duration. A 4.8 mm circular craniectomy was centered on the sagittal suture midway between bregma and lambda, and the skull flap was removed carefully, ensuring the underlying dura and superior sagittal sinus remained intact. An injury hub was fixed directly over the craniectomy using cyanoacrylate gel and methyl-methacrylate (Hygenic Corp., Akron, OH) and filled with 0.9% sterile saline. The incision was then partially sutured closed on the anterior and posterior edges with 4.0 Ethilon sutures. Topical lidocaine and antibiotic ointment were applied. Rats were returned to a pre-warmed holding cage post-surgery and monitored until ambulatory.
2.3 Midline FPI
Two hours following surgical procedures and the return of ambulation, rats were re-anesthetized, the hub was filled with 0.9% sterile saline, and attached to the male end of a fluid percussion device (Custom Design and Fabrication, Richmond, VA). After the return of a pedal withdrawal response, an injury averaging 1.8–2.0 atmospheric pressure (atm) for males and 1.7–1.9 atm for females was administered by releasing the pendulum (from 16° for males and 15.5° for females) onto the fluid-filled cylinder. Shams were attached to the device, but the pendulum was not released after a positive pedal withdrawal response. Immediately after administration of the injury, the fencing response, apnea, seizures, and the return of righting reflex were recorded for brain-injured animals, and the hub was removed en bloc (McIntosh et al., 1987; Hosseini and Lifshitz, 2009). Inclusion criteria required that injured rats have a righting reflex time ranging from 6 to 10 min and a fencing response indicative of mild-to-moderate TBI. Rats were re-anesthetized, the surgery site inspected for herniation and dural integrity, the incision was closed, and topical lidocaine and antibiotic ointment were applied. Rats were then placed in a pre-warmed holding cage for recovery. Post-operative monitoring was performed for 5 days by physical evaluation. Rats were pair-housed in the same room according to injury status and sex throughout the study. All cohorts were time post-injury matched, with age- and sex-matched shams at each time point.
2.4 Histology
Brains were collected at 7-, 56-, and 168 days post-injury (DPI), rinsed with ice-cold phosphate-buffered saline (PBS), hemisected, and one hemisphere post-fixed in 4% paraformaldehyde for 24 h, transferred to fresh PBS with sodium azide, and shipped to Neuroscience Associates Inc. (Knoxville, TN). Brains were coded and randomized into two gelatin blocks (MultiBrain® Technology, NeuroScience Associates, Knoxville, TN) to be batch-processed for simultaneous histological and immunohistochemical staining. 40 μm thick sections were taken in the coronal plane, stained with glial fibrillary acidic protein (GFAP); primary Ab: Dako, Z0334, 1:75,000; secondary Ab: Vector, BA-1000) using the free-floating technique and visualized using 3,3′-Diaminobenzidine (DAB).
2.5 Imaging and analysis
Image capture and analysis was completed by investigators blinded to injury status, days post-injury, and sex. Images were acquired on an upright Zeiss Axio Imager 2 equipped with a Hamamatsu ORCA-flash 4.0 digital camera (catalog #C13440). An oil-immersion 100×/1.2 C-Apochromat lens was used to capture one image in the S1BF localized to layer 4 per 3 adjacent sections per animal using Paxinos and Watson atlas (Figure 1B) (Paxinos and Watson, 2007). A total of 192 images were taken for GFAP-positive stained astrocytes connected to the GFAP-ensheathed cerebral vasculature using Neurolucida software (MBF) and exported to ImageJ (National Institutes of Health) software. The number of primary astrocyte processes connecting to GFAP-ensheathed vessels and accessory branches near the vessels were quantified to assess astrocyte contributions to the BBB (Figure 1C). Vessel length was measured using the line tool in ImageJ software scaled in micrometers to normalize process counts to the vessel length as a quantitative metric for comparison (Abramoff et al., 2004). The average vessel length was similar between all groups, with an overall average of 132.5 ± 1.54 µm. Measurements from the 3 adjacent sections were averaged (per rat) for statistical analysis. Image analysis was carried out by two investigators. Data were analyzed to address the following questions for each sex: (1) Effect of FPI: Was there an impact of FPI? (2) Effect of time post-FPI: Did the timing post-FPI indicate aging with injury, and if so, when? (3) Effect in shams over time: Were there changes in sham groups over time (3 months at time of injury, 9 months at 168 DPI)? If so, when? (4) Sex-related effects or interactions: Was there evidence of any potential effects or interactions related to sex?
2.6 Statistics
Group sizes were determined from previous publications, primarily using male rats at 28 DPI (Hoffman et al., 2017; Thomas et al., 2018; Beitchman et al., 2019; Bromberg et al., 2020), where an n = 5 per group was shown to provide >90% power to detect a significant increase in GFAP intensity after FPI with a representative effect size of d = 3.4 (Thomas et al., 2018). However, due to the exploratory nature of this study, including chronic time points and the inclusion of females, data were analyzed separately for each sex to ensure appropriate power. A two-way ANOVA was initially conducted with factors of injury (FPI vs. sham) and days post-injury (DPI; 7 vs. 56 vs. 168). To explore potential sex differences, a subsequent three-way ANOVA was performed with sex (male vs. female) as an additional factor. If significant effects or interactions involving sex were identified (at P < 0.05), they are reported in the results section. Normality was assessed using the Kolmogorov-Smirnov test, and homogeneity of variances was evaluated using the Brown-Forsythe test. When these assumptions were violated, data were log-transformed to meet the criteria. Reported statistics include results from transformed data, though raw data are presented in all graphs for clarity. Tukey’s post hoc tests were performed to clarify significant main effects and interactions identified by ANOVA, (P < 0.05). Data are presented as mean + SEM. Outliers were identified using ROUT analysis (Q = 1%), and 1 outlier was detected (male 7 days sham). Shams were compared across MultiBrain® blocks and time points to detect potential block or cohort effects, and no significant differences were detected. All statistical analyses were performed using GraphPad Prism (version 10.2.2).
3 Results
3.1 DAI caused an increased number of GFAP-positive primary processes per vessel length at 7 DPI with sex-dependent long-term trajectories
Representative 100× photomicrographs of GFAP-positive processes ensheath vessels in the S1BF (Figure 2A). As shown in Figure 2B, two-way ANOVA revealed a significant effect of FPI (F1,25 = 11.87, P = 0.002) and DPI (F2,25 = 5.34, P = 0.012) in males. Post-hoc analysis reveals significant differences between FPI and sham at 7 DPI (P < 0.01) and 56 DPI (P < 0.05), with the number of processes in FPI animals returning to sham levels by 168 DPI. In females, primary process numbers varied as a function of FPI (F1, 26 = 23.96, P < 0.0001) and FPI × DPI interaction (F2, 26 = 4.16, P = 0.027). The follow-up comparisons indicated that FPI increased the primary processes at 7 DPI compared to shams (P < 0.0001). While processes significantly decreased between 7 DPI and 168 DPI (P < 0.05), a small but significant effect was present between sham and FPI at 168 DPI (Figure 2C). We measured no changes in sham rats as a function of time. A three-way ANOVA indicated no sex differences or sex interactions.
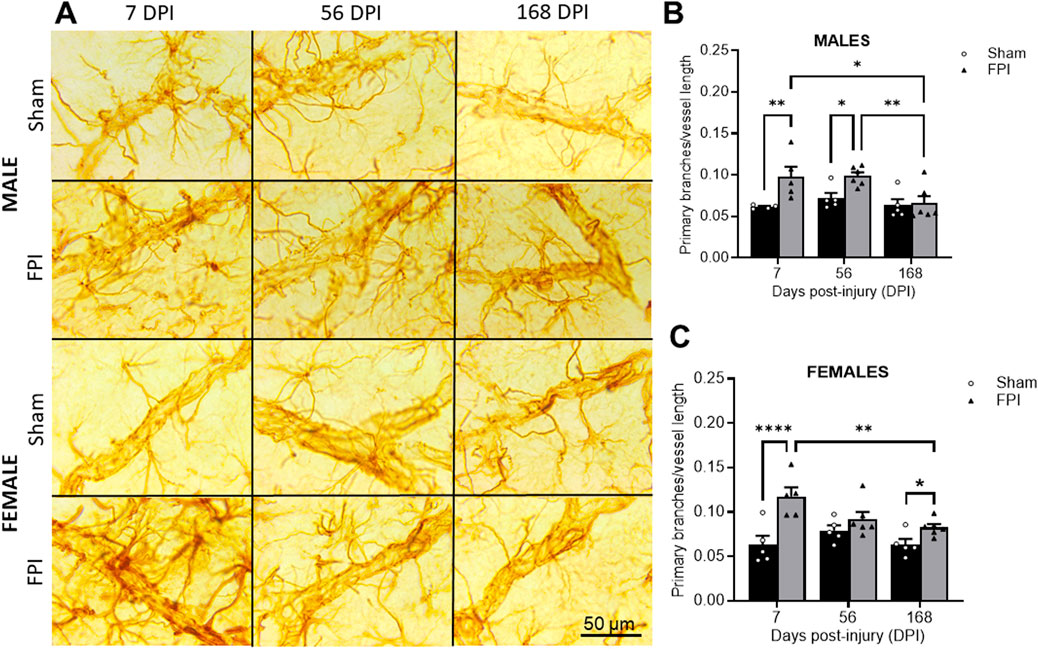
Figure 2. FPI caused an increased number of GFAP + primary processes per vessel length at 7 DPI that remained elevated longer in males. (A) Representative 100× GFAP immunostained images within layer IV of the S1BF region of sham and FPI rats at 7, 56, and 168 DPI of both sexes. (B) In males, an injury effect was detected at 7 and 56 DPI compared to age- and sex-matched shams. The number of processes decreased in FPI rats to sham levels by 168 days. (C) In females, FPI increased primary processes at 7 DPI compared to sham. Process numbers significantly declined between 7 and 168 DPI, where an effect of FPI was still present at 168 DPI N=5–6/sex. Data were analyzed by two-way ANOVA followed by Tukey’s multiple comparison tests. *P < 0.05, **P < 0.01, and ****P < 0.0001. Error bars indicate + SEM. Scale bar = 50 µm.
3.2 FPI increases in GFAP-positive accessory processes parallel FPI-induced increases in primary processes
Representative photomicrographs of GFAP-positive accessory processes ending contributing to GFAP-ensheathed vessels in the S1BF (Figure 3A). In males, an effect of FPI was measured (F1, 25 = 7.76; P = 0.010), where a post hoc analysis identified significance between FPI and sham at 56 DPI (P < 0.05; see Figure 3B). As shown in Figure 3C, in females, an effect of FPI was also measured (F1, 26 = 6.95; P = 0.014), where a post hoc analysis identified significance between FPI and sham at 7 DPI (P < 0.05). Sham rats did not change as a function of time. A three-way ANOVA indicated no sex differences or interactions.
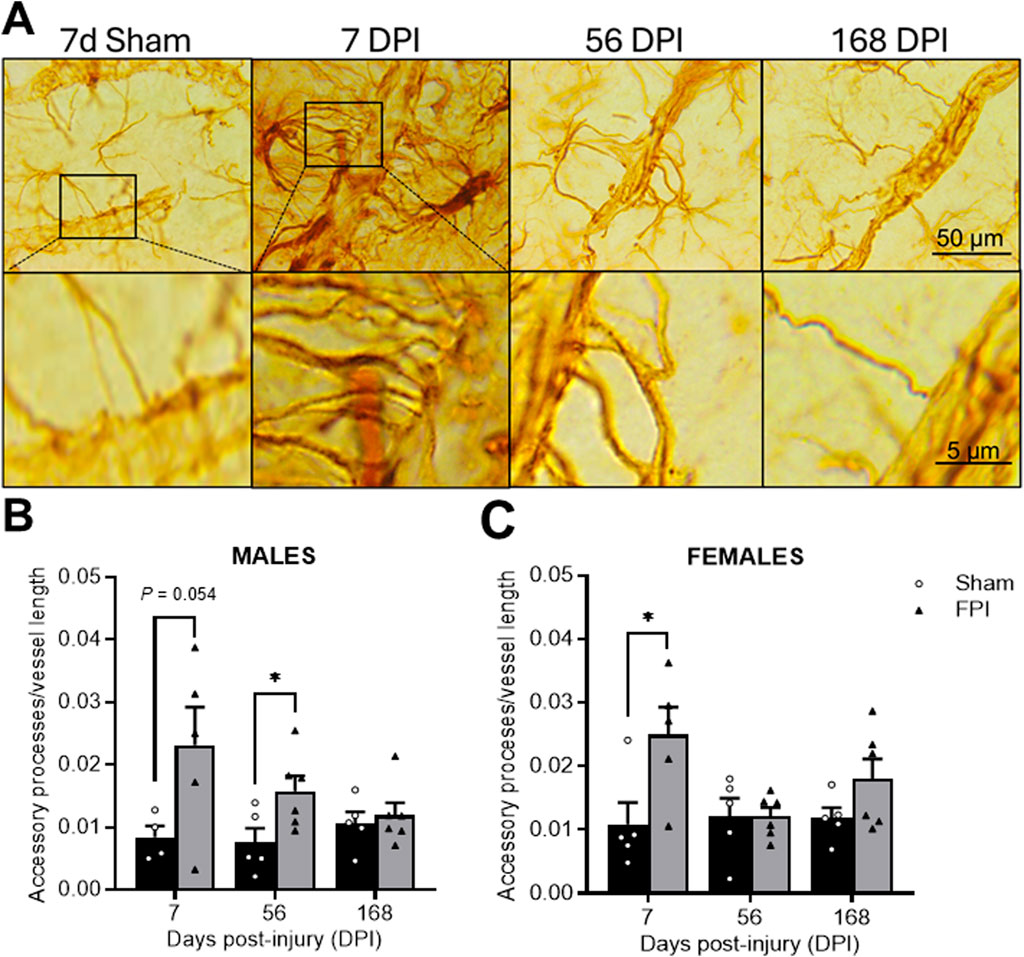
Figure 3. TBI promotes time-dependent changes in the number of GFAP-labeled accessory processes. (A) Representative GFAP immunostained images within the S1BF region of sham and FPI rats at 7, 56, and 168 DPI from both sexes to highlight changes in accessory processes over time. The top row are images captured at 100×. The bottom row images magnify the accessory process interactions with the vessel. (B) In males, an FPI increased accessory processes at 56 DPI compared to age- and sex-matched shams. (C) In females, FPI increased primary processes at 7 DPI compared to sham N=5–6/sex. Data were analyzed by a two-way ANOVA followed by Tukey’s multiple comparison tests. *P < 0.05 and **P < 0.01. Error bars indicate + SEM. Scale bar = 50 µm.
4 Discussion
We sought to evaluate temporal changes in astrocytes interacting with the vasculature as a function of time post-injury and sex. Our results demonstrate chronically increased number of primary processes in contact with the GFAP + ensheathment around blood vessels with proximal accessory branches tending to co-occur at 7 DPI (both sexes), 56 DPI (in males), and 168 DPI (in females). Despite measuring overall GFAP reactivity increased in sham controls (Sabetta et al., 2023), the increase in proximal accessory branches was unique to brain-injured rats. While temporal profiles of the sexes have subtle differences, a posteriori three-way ANOVAs did not detect significant effects of sex.
The S1BF was strategically chosen for this study due to its large anatomical representation of the whisker barrel circuit, along with dense and well-organized vascular network and distinct cortical layers, offering greater reproducibility in measuring interactions across animals. Larger diameter cortical vessels with a perpendicular or lateral trajectory are characteristics of intracortical arterioles that are the gateway of blood supply to the deeper brain structures. Cortical arterioles are known to be compromised during aging and neurodegenerative diseases, playing a critical role in maintaining cerebral perfusion, with their dysfunction linked to cognitive decline and dementia (Kress et al., 2014). Post-TBI vascular dysfunction may be more prominent at 6 months post-injury in the cortex, where fluid percussion injury induces spreading depolarization, acute hypoperfusion, and changes in neurovascular volume (Ziebell et al., 2016; Balanca et al., 2017). Astrocytes in the S1BF show increased GFAP immunodensity up to 2 months post-injury, where the vast majority (>90%) are in direct vascular contact (Hösli et al., 2022; Sabetta et al., 2023), potentially contributing to vascular dysfunction. In this DAI model, the somatosensory cortex is known for its highly reproducible late-onset and persistent hypersensitivity to whisker stimulation, with several known glial, neuronal, and functional changes over time (McNamara et al., 2010; Thomas et al., 2012; Lafrenaye et al., 2014; Thomas et al., 2017; Thomas et al., 2018; Krishna et al., 2020). Together, this information can be expanded in future studies to comprehensively evaluate the chronic impact of altered astrocyte-vessel interactions within behaviorally relevant nuclei and white matter tracts, focusing on how these changes affect vascular and circuit function in the development of behavioral deficits or recovery post-TBI.
The BBB integrity depends on astrocyte coverage of the vascular surface, with evidence indicating that the alterations in gliovascular interactions are more prominent in neurological disorders and age-related neurodegenerative diseases (Alvarez et al., 2013). Disruptions of astrocyte-vascular interaction could lead to impaired hemodynamic responses and loss of neurovascular coupling, perpetuating pathology progression (Zlokovic, 2011). The primary GFAP-positive processes are major long branches that emanate directly from the soma and continue to envelop vasculature (Khakh and Sofroniew, 2015). Our previous work showed increased GFAP immunoreactivity at 7 and 56 DPI in the sensory cortex, which was similar between males and females after experimental TBI (Sabetta et al., 2023). These data indicate chronic changes in astrocyte vessel interactions with accessory processes following a similar temporal profile as the TBI-induced increase in primary processes, clearly indicating that an increase in accessory processes is injury-mediated and an associated pathology with increased astrocyte-vessel interactions. Considering our previous outcomes, these data indicate that the distribution of GFAP may differ between males and females over the measured times post-injury. Further, this observation may indicate that females have a differentially regulated astrocytic response due to altered protective mechanisms, hormonal regulation, or inflammatory responses. Estrogens can exert a protective action on vascular surfaces by promoting recovery of endothelial cell loss after vascular damage (Krasinski et al., 1997); however, well-powered studies and more detailed examination of alternate outcomes are needed to support this speculation.
Given that astrocytes undergo acute, subacute, and chronic morphological and functional remodeling after TBI, this observation is consistent with previous studies after lateral FPI demonstrating increased arborization in A1 (GFAP+/C3+) astrocytes at 7 DPI (Clark et al., 2019), indicating a pathological astrocyte phenotype; albeit these were ipsilateral to a focal injury. Relatedly, astroglial reactivity with the appearance of longer processes was also observed at 1, 7, and 30 DPI in the somatosensory region of mice; however, these changes were not associated with vascular interactions (Clément et al., 2020). Alternatively, increased astrocyte processes observed at 7 DPI after the diffuse TBI may indicate ongoing adaptive changes to facilitate vascular repair by promoting BBB integrity, providing metabolic support, synaptic activity, and controlling blood flow (Attwell et al., 2010). Further investigation is needed to determine whether these responses are beneficial or detrimental in the case of DAI. Astrocytes extend highly branched processes that split into secondary and tertiary accessory processes, which could be indicative of increased endfeet in contact with blood vessels (Baldwin et al., 2023). Experimental TBI studies have reported time-course changes in cortical vascularization with the appearance of revascularization at 7 DPI (Jullienne et al., 2018), which may explain the increase in astrocyte processes. Aging is known to induce astrocytic gliosis, swelling of endfeet, and loss of interaction with the vasculature, which may be linked to neurodegeneration (Duncombe et al., 2017; Bors et al., 2018). Differences in outcomes may be related to our 6-month post-injury time point being in a relatively young rat (9 months old). Evaluating the effects at 18+ months post-injury could provide a more comprehensive understanding of the chronic consequences of DAI on astrocyte-vessel interactions.
In terms of mechanisms, it was previously reported that acute neurodegeneration may precede reactive astrogliosis, promoting neurovascular reformation (Villapol et al., 2014). Our previous publications indicate ongoing neurodegeneration in both male and female rats, persisting up to at least 2 months in the S1BF and 6 months in deeper nuclei after DAI (Lifshitz and Lisembee, 2012; Thomas et al., 2018; Sabetta et al., 2023). This neurodegeneration can lead to prolonged phagocytosis driven by activated microglia/macrophages, which are in bidirectional communication with endothelial cells and release pro-inflammatory factors (Dudvarski Stankovic et al., 2016). The crosstalk between astrocytes and microglia/macrophages can alter the astrocytic phenotype, influencing astrocyte-vessel interactions that may promote BBB permeability, neurovascular remodeling, neurovascular uncoupling, metabolic alterations, and changes in astrocytic endfeet-enriched proteins (Abbott et al., 2006; Yi and Hazell, 2006; Salehi et al., 2017; Matejuk and Ransohoff, 2020; George et al., 2022). The culmination of these events could create a feedback loop that exacerbates both neurodegeneration and vascular dysfunction and thereby changes in astrocyte morphology. However, it is also important to consider the potential for protective mechanisms, such as the ongoing clearance of debris, release of neurotrophic factors, and induction of anti-inflammatory states, which can promote neuronal survival, tissue repair, regenerative neuroplasticity, and BBB integrity (Simon et al., 2017; Mira et al., 2021). The chronic presentation of accessory processes could indicate enhanced coverage of the BBB and be protective of functional impairment in the vascular microenvironment. The load of neurodegeneration or promotion of adaptive processes may shift the balance between chronic pathophysiology and repair, determining whether the system leans towards exacerbating or mitigating vascular dysfunction (Thomas et al., 2015). Further investigation into the genotype and phenotype of interacting astrocytes may elucidate their functional roles, where these interactions may serve as biomarkers to monitor traumatically induced vascular damage, recovery, and response to interventions, particularly during the subacute and chronic post-injury periods.
With the role of chronic astrocyte reactivity becoming increasingly recognized as a contributor to vascular pathology following TBI, this study is, to the best of our knowledge, the first to highlight novel sub-acute and chronic astrocyte-vessel interactions that may have significant implications for sex-dependent trajectories following diffuse TBI (George et al., 2022; Díaz-Castro et al., 2023). While these findings present intriguing biomarkers for future research, there are limitations to be considered. Results were limited to the evaluation of GFAP + processes, where additional markers could help identify arterioles from venules to indicate if accessory processes are associated with one or the other. Additional markers for endothelial cells, pericytes, or the blood-brain barrier would provide more detail about interactions with specific vascular components. Incorporating markers of glymphatic clearance, hypoxia, and vascular integrity may indicate functional impact. Results are not directly correlated with behavior, however, the somatosensory cortex is the highly integrated cortical relay responsible for the development of the reproducible late-onset and persistent hypersensitivity to whisker stimulation previously reported from our lab and others (McNamara et al., 2010; Thomas et al., 2012; Lafrenaye et al., 2014; Thomas et al., 2017; Thomas et al., 2018; Krishna et al., 2020). Behavioral assessments were intentionally excluded from the experimental design due to indications of chronic HPA axis dysregulation where perceived stressors could potentially influence astrocyte morphology and confound data interpretation (Rowe et al., 2016; Hoffman et al., 2017; Beitchman et al., 2019; Bromberg et al., 2020; Rowe et al., 2020; Fulop et al., 2023; Valenza et al., 2024). Additionally, examining the modulation of ovarian hormones could further elucidate the observed sex-dependent differences.
5 Conclusion
Our work provides novel evidence of sex-dependent temporal changes in astrocyte-vessel interactions post-TBI, highlighting significant differences in how males and females respond to brain injury over time. The observed increase in accessory astrocyte processes and their connection with cortical vessels underscores the critical role of astrocytes in maintaining barrier integrity and the potential implications for chronic BBB disruption. These findings suggest that changes in astrocyte morphology may serve as valuable biomarkers for assessing the impact of TBI on vascular function. Understanding nuanced interactions offers a promising avenue for developing targeted treatments, where early interventions and chronological assessment of physiological and behavioral outcomes could provide insight into the functional roles associated with increased interactions. Future research should continue to explore the molecular mechanisms driving these changes and the potential for sex-specific therapeutic strategies, ultimately contributing to improved outcomes for TBI patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee at the University of Arizona (protocol #18-384). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ZS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Visualization, Writing–original draft, Writing–review and editing. GK: Formal Analysis, Methodology, Project administration, Supervision, Validation, Writing–original draft, Writing–review and editing, Data curation, Investigation. TC: Writing–review and editing. ML: Data curation, Formal Analysis, Writing–review and editing. PA: Writing–review and editing. TT: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Institutes of Health (Grant reference number R01NS100793), Arizona Biomedical Research Commission through Arizona Department of Health Services (ADHS14-00003606), Phoenix Children’s Hospital Mission Support to TT. This work is solely the authors’ responsibility and does not necessarily represent the official views of the funding agencies. The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Acknowledgments
We thank Jim Baun and his team at Neuroscience Associates for assistance in generating the histology archive.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbott N. J., Rönnbäck L., Hansson E. (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53. doi:10.1038/nrn1824
Alvarez J. I., Katayama T., Prat A. (2013). Glial influence on the blood brain barrier. Glia 61, 1939–1958. doi:10.1002/glia.22575
Attwell D., Buchan A. M., Charpak S., Lauritzen M., Macvicar B. A., Newman E. A. (2010). Glial and neuronal control of brain blood flow. Nature 468, 232–243. doi:10.1038/nature09613
Badaut J., Bix G. J. (2014). Vascular neural network phenotypic transformation after traumatic injury: potential role in long-term sequelae. Transl. Stroke Res. 5, 394–406. doi:10.1007/s12975-013-0304-z
Balanca B., Meiller A., Bezin L., Dreier J. P., Marinesco S., Lieutaud T. (2017). Altered hypermetabolic response to cortical spreading depolarizations after traumatic brain injury in rats. J. Cereb. Blood Flow. Metab. 37, 1670–1686. doi:10.1177/0271678X16657571
Baldwin K. T., Murai K. K., Khakh B. S. (2023). Astrocyte morphology. Trends Cell Biol. 34, 547–565. doi:10.1016/j.tcb.2023.09.006
Barisano G., Montagne A., Kisler K., Schneider J. A., Wardlaw J. M., Zlokovic B. V. (2022). Blood-brain barrier link to human cognitive impairment and Alzheimer's Disease. Nat. Cardiovasc Res. 1, 108–115. doi:10.1038/s44161-021-00014-4
Beitchman J. A., Griffiths D. R., Hur Y., Ogle S. B., Bromberg C. E., Morrison H. W., et al. (2019). Experimental traumatic brain injury induces chronic glutamatergic dysfunction in amygdala circuitry known to regulate anxiety-like behavior. Front. Neurosci. 13, 1434. doi:10.3389/fnins.2019.01434
Blennow K., Brody D. L., Kochanek P. M., Levin H., Mckee A., Ribbers G. M., et al. (2016). Traumatic brain injuries. Nat. Rev. Dis. Prim. 2, 16084. doi:10.1038/nrdp.2016.84
Bors L., Tóth K., Tóth E. Z., Bajza Á., Csorba A., Szigeti K., et al. (2018). Age-dependent changes at the blood-brain barrier. A Comparative structural and functional study in young adult and middle aged rats. Brain Res. Bull. 139, 269–277. doi:10.1016/j.brainresbull.2018.03.001
Bromberg C. E., Condon A. M., Ridgway S. W., Krishna G., Garcia-Filion P. C., Adelson P. D., et al. (2020). Sex-dependent pathology in the HPA Axis at a sub-acute period after experimental traumatic brain injury. Front. Neurol. 11, 946. doi:10.3389/fneur.2020.00946
Burda J. E., Bernstein A. M., Sofroniew M. V. (2016). Astrocyte roles in traumatic brain injury. Exp. Neurol. 275, 305–315. doi:10.1016/j.expneurol.2015.03.020
Cabezas R., Avila M., Gonzalez J., El-Bacha R. S., Baez E., Garcia-Segura L. M., et al. (2014). Astrocytic modulation of blood brain barrier: perspectives on Parkinson's disease. Front. Cell Neurosci. 8, 211. doi:10.3389/fncel.2014.00211
Cantone M., Fisicaro F., Ferri R., Bella R., Pennisi G., Lanza G., et al. (2023). Sex differences in mild vascular cognitive impairment: a multimodal transcranial magnetic stimulation study. PLoS One 18, e0282751. doi:10.1371/journal.pone.0282751
Cash A., Theus M. H. (2020). Mechanisms of blood-brain barrier dysfunction in traumatic brain injury. Int. J. Mol. Sci. 21, 3344. doi:10.3390/ijms21093344
Chisholm N. C., Sohrabji F. (2016). Astrocytic response to cerebral ischemia is influenced by sex differences and impaired by aging. Neurobiol. Dis. 85, 245–253. doi:10.1016/j.nbd.2015.03.028
Clark D. P. Q., Perreau V. M., Shultz S. R., Brady R. D., Lei E., Dixit S., et al. (2019). Inflammation in traumatic brain injury: roles for toxic A1 astrocytes and microglial-astrocytic crosstalk. Neurochem. Res. 44, 1410–1424. doi:10.1007/s11064-019-02721-8
Clément T., Lee J. B., Ichkova A., Rodriguez-Grande B., Fournier M. L., Aussudre J., et al. (2020). Juvenile mild traumatic brain injury elicits distinct spatiotemporal astrocyte responses. Glia 68, 528–542. doi:10.1002/glia.23736
Dennis E. L., Vervoordt S., Adamson M. M., Houshang A., Bigler E. D., Caeyenberghs K., et al. (2024). Accelerated aging after traumatic brain injury: an ENIGMA multi-cohort mega-analysis. Ann. Neurol. 96, 365–377. doi:10.1002/ana.26952
Díaz-Castro B., Robel S., Mishra A. (2023). Astrocyte endfeet in brain function and pathology: open questions. Annu. Rev. Neurosci. 46, 101–121. doi:10.1146/annurev-neuro-091922-031205
Dudvarski Stankovic N., Teodorczyk M., Ploen R., Zipp F., Schmidt M. H. H. (2016). Microglia-blood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathol. 131, 347–363. doi:10.1007/s00401-015-1524-y
Duncombe J., Lennen R. J., Jansen M. A., Marshall I., Wardlaw J. M., Horsburgh K. (2017). Ageing causes prominent neurovascular dysfunction associated with loss of astrocytic contacts and gliosis. Neuropathology Appl. Neurobiol. 43, 477–491. doi:10.1111/nan.12375
Dunn C., Sturdivant N., Venier S., Ali S., Wolchok J., Balachandran K. (2021). Blood–brain barrier breakdown and astrocyte reactivity evident in the absence of behavioral changes after repeated traumatic brain injury. Neurotrauma Rep. 2, 399–410. doi:10.1089/neur.2021.0017
Faden A. I., Loane D. J. (2015). Chronic neurodegeneration after traumatic brain injury: alzheimer disease, chronic traumatic encephalopathy, or persistent neuroinflammation? Neurotherapeutics 12, 143–150. doi:10.1007/s13311-014-0319-5
Fulop B., Hunyady A., Bencze N., Kormos V., Szentes N., Denes A., et al. (2023). IL-1 mediates chronic stress-induced hyperalgesia accompanied by microglia and astroglia morphological changes in pain-related brain regions in mice. Int. J. Mol. Sci. 24, 5479. doi:10.3390/ijms24065479
George K. K., Heithoff B. P., Shandra O., Robel S. (2022). Mild traumatic brain injury/concussion initiates an atypical astrocyte response caused by blood-brain barrier dysfunction. J. Neurotrauma 39, 211–226. doi:10.1089/neu.2021.0204
Hoffman A. N., Paode P. R., May H. G., Ortiz J. B., Kemmou S., Lifshitz J., et al. (2017). Early and persistent dendritic hypertrophy in the basolateral amygdala following experimental diffuse traumatic brain injury. J. Neurotrauma 34, 213–219. doi:10.1089/neu.2015.4339
Honarpisheh P., Mccullough L. D. (2019). Sex as a biological variable in the pathology and pharmacology of neurodegenerative and neurovascular diseases. Br. J. Pharmacol. 176, 4173–4192. doi:10.1111/bph.14675
Hösli L., Zuend M., Bredell G., Zanker H. S., De Oliveira C. E. P., Saab A. S., et al. (2022). Direct vascular contact is a hallmark of cerebral astrocytes. Cell Rep. 39, 110599. doi:10.1016/j.celrep.2022.110599
Hosseini A. H., Lifshitz J. (2009). Brain injury forces of moderate magnitude elicit the fencing response. Med. Sci. Sports Exerc 41, 1687–1697. doi:10.1249/MSS.0b013e31819fcd1b
Hubbard W. B., Velmurugan G. V., Brown E. P., Sullivan P. G. (2022). Resilience of females to acute blood-brain barrier damage and anxiety behavior following mild blast traumatic brain injury. Acta Neuropathol. Commun. 10, 93. doi:10.1186/s40478-022-01395-8
Johnson V. E., Stewart W., Smith D. H. (2013). Axonal pathology in traumatic brain injury. Exp. Neurol. 246, 35–43. doi:10.1016/j.expneurol.2012.01.013
Jullienne A., Salehi A., Affeldt B., Baghchechi M., Haddad E., Avitua A., et al. (2018). Male and female mice exhibit divergent responses of the cortical vasculature to traumatic brain injury. J. neurotrauma 35, 1646–1658. doi:10.1089/neu.2017.5547
Kempuraj D., Ahmed M. E., Selvakumar G. P., Thangavel R., Raikwar S. P., Zaheer S. A., et al. (2021). Acute traumatic brain injury-induced neuroinflammatory response and neurovascular disorders in the brain. Neurotox. Res. 39, 359–368. doi:10.1007/s12640-020-00288-9
Khakh B. S., Sofroniew M. V. (2015). Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18, 942–952. doi:10.1038/nn.4043
Kiraly M., Kiraly S. J. (2007). Traumatic brain injury and delayed sequelae: a review--traumatic brain injury and mild traumatic brain injury (concussion) are precursors to later-onset brain disorders, including early-onset dementia. ScientificWorldJournal 7, 1768–1776. doi:10.1100/tsw.2007.269
Knox E. G., Aburto M. R., Clarke G., Cryan J. F., O'driscoll C. M. (2022). The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 27, 2659–2673. doi:10.1038/s41380-022-01511-z
Koehler R. C., Roman R. J., Harder D. R. (2009). Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 32, 160–169. doi:10.1016/j.tins.2008.11.005
Krasinski K., Spyridopoulos I., Asahara T., Van Der Zee R., Isner J. M., Losordo D. W. (1997). Estradiol accelerates functional endothelial recovery after arterial injury. Circulation 95, 1768–1772. doi:10.1161/01.cir.95.7.1768
Kress B. T., Iliff J. J., Xia M., Wang M., Wei H. S., Zeppenfeld D., et al. (2014). Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76, 845–861. doi:10.1002/ana.24271
Krishna G., Bromberg C., Connell E. C., Mian E., Hu C., Lifshitz J., et al. (2020). Traumatic brain injury-induced sex-dependent changes in late-onset sensory hypersensitivity and glutamate neurotransmission. Front. Neurol. 11, 749. doi:10.3389/fneur.2020.00749
Lafrenaye A. D., Krahe T. E., Povlishock J. T. (2014). Moderately elevated intracranial pressure after diffuse traumatic brain injury is associated with exacerbated neuronal pathology and behavioral morbidity in the rat. J. Cereb. Blood Flow. Metab. 34, 1628–1636. doi:10.1038/jcbfm.2014.122
Lifshitz J., Lisembee A. M. (2012). Neurodegeneration in the somatosensory cortex after experimental diffuse brain injury. Brain Struct. Funct. 217, 49–61. doi:10.1007/s00429-011-0323-z
Lifshitz J., Rowe R. K., Griffiths D. R., Evilsizor M. N., Thomas T. C., Adelson P. D., et al. (2016). Clinical relevance of midline fluid percussion brain injury: acute deficits, chronic morbidities and the utility of biomarkers. Brain Inj. 30, 1293–1301. doi:10.1080/02699052.2016.1193628
Lin X., Chen L., Jullienne A., Zhang H., Salehi A., Hamer M., et al. (2022). Longitudinal dynamics of microvascular recovery after acquired cortical injury. Acta Neuropathol. Commun. 10, 59. doi:10.1186/s40478-022-01361-4
Lu Y., Jarrahi A., Moore N., Bartoli M., Brann D. W., Baban B., et al. (2023). Inflammaging, cellular senescence, and cognitive aging after traumatic brain injury. Neurobiol. Dis. 180, 106090. doi:10.1016/j.nbd.2023.106090
Matejuk A., Ransohoff R. M. (2020). Crosstalk between astrocytes and microglia: an overview. Front. Immunol. 11, 1416. doi:10.3389/fimmu.2020.01416
Mathiisen T. M., Lehre K. P., Danbolt N. C., Ottersen O. P. (2010). The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58, 1094–1103. doi:10.1002/glia.20990
Mayer A. R., Quinn D. K., Master C. L. (2017). The spectrum of mild traumatic brain injury: a review. Neurology 89, 623–632. doi:10.1212/WNL.0000000000004214
Mcintosh T. K., Noble L., Andrews B., Faden A. I. (1987). Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent. Nerv. Syst. Trauma 4, 119–134. doi:10.1089/cns.1987.4.119
Mcnamara K. C., Lisembee A. M., Lifshitz J. (2010). The whisker nuisance task identifies a late-onset, persistent sensory sensitivity in diffuse brain-injured rats. J. Neurotrauma 27, 695–706. doi:10.1089/neu.2009.1237
Mills W. A., Woo A. M., Jiang S., Martin J., Surendran D., Bergstresser M., et al. (2022). Astrocyte plasticity in mice ensures continued endfoot coverage of cerebral blood vessels following injury and declines with age. Nat. Commun. 13, 1794. doi:10.1038/s41467-022-29475-2
Mira R. G., Lira M., Cerpa W. (2021). Traumatic brain injury: mechanisms of glial response. Front. Physiol. 12, 740939. doi:10.3389/fphys.2021.740939
Munoz-Ballester C., Robel S. (2023). Astrocyte-mediated mechanisms contribute to traumatic brain injury pathology. WIREs Mech. Dis. 15, e1622. doi:10.1002/wsbm.1622
Papp E. A., Leergaard T. B., Calabrese E., Johnson G. A., Bjaalie J. G. (2014). Waxholm Space atlas of the Sprague Dawley rat brain. Neuroimage 97, 374–386. doi:10.1016/j.neuroimage.2014.04.001
Robison L. S., Gannon O. J., Salinero A. E., Zuloaga K. L. (2019). Contributions of sex to cerebrovascular function and pathology. Brain Res. 1710, 43–60. doi:10.1016/j.brainres.2018.12.030
Rowe R. K., Ortiz J. B., Thomas T. C. (2020). Mild and moderate traumatic brain injury and repeated stress affect corticosterone in the rat. Neurotrauma Rep. 1, 113–124. doi:10.1089/neur.2020.0019
Rowe R. K., Rumney B. M., May H. G., Permana P., Adelson P. D., Harman S. M., et al. (2016). Diffuse traumatic brain injury affects chronic corticosterone function in the rat. Endocr. Connect. 5, 152–166. doi:10.1530/EC-16-0031
Sabetta Z., Krishna G., Curry T., Adelson P. D., Thomas T. C. (2023). Aging with TBI vs. Aging: 6-month temporal profiles for neuropathology and astrocyte activation converge in behaviorally relevant thalamocortical circuitry of male and female rats. bioRxiv, 2023.2002.2006.527058.
Salehi A., Zhang J. H., Obenaus A. (2017). Response of the cerebral vasculature following traumatic brain injury. J. Cereb. Blood Flow and Metabolism 37, 2320–2339. doi:10.1177/0271678X17701460
Schneider A. L. C., Peltz C. B., Li Y., Bahorik A., Gardner R. C., Yaffe K. (2023). Traumatic brain injury and long-term risk of stroke among US military veterans. Stroke 54, 2059–2068. doi:10.1161/STROKEAHA.123.042360
Senatorov V. V., Friedman A. R., Milikovsky D. Z., Ofer J., Saar-Ashkenazy R., Charbash A., et al. (2019). Blood-brain barrier dysfunction in aging induces hyperactivation of TGFβ signaling and chronic yet reversible neural dysfunction. Sci. Transl. Med. 11, eaaw8283. doi:10.1126/scitranslmed.aaw8283
Simon D. W., Mcgeachy M. J., Bayir H., Clark R. S., Loane D. J., Kochanek P. M. (2017). The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 13, 171–191. doi:10.1038/nrneurol.2017.13
Smith D. H., Hicks R., Povlishock J. T. (2013). Therapy development for diffuse axonal injury. J. Neurotrauma 30, 307–323. doi:10.1089/neu.2012.2825
Stewart I. J., Amuan M. E., Wang C. P., Kennedy E., Kenney K., Werner J. K., et al. (2022). Association between traumatic brain injury and subsequent cardiovascular disease among post-9/11-era veterans. JAMA Neurol. 79, 1122–1129. doi:10.1001/jamaneurol.2022.2682
Sulimai N., Brown J., Lominadze D. (2023). Vascular effects on cerebrovascular permeability and neurodegeneration. Biomolecules 13, 648. doi:10.3390/biom13040648
Thomas T. C., Colburn T. A., Korp K., Khodadad A., Lifshitz J. (2015). “Translational considerations for behavioral impairment and rehabilitation strategies after diffuse traumatic brain injury,” in Brain neurotrauma: molecular, neuropsychological, and rehabilitation aspects. Editor F. H. Kobeissy (Boca Raton (FL)).
Thomas T. C., Hinzman J. M., Gerhardt G. A., Lifshitz J. (2012). Hypersensitive glutamate signaling correlates with the development of late-onset behavioral morbidity in diffuse brain-injured circuitry. J. neurotrauma 29, 187–200. doi:10.1089/neu.2011.2091
Thomas T. C., Ogle S. B., Rumney B. M., May H. G., Adelson P. D., Lifshitz J. (2018). Does time heal all wounds? Experimental diffuse traumatic brain injury results in persisting histopathology in the thalamus. Behav. Brain Res. 340, 137–146. doi:10.1016/j.bbr.2016.12.038
Thomas T. C., Stockhausen E. M., Law L. M., Khodadad A., Lifshitz J. (2017). Rehabilitation modality and onset differentially influence whisker sensory hypersensitivity after diffuse traumatic brain injury in the rat. Restor. Neurol. Neurosci. 35, 611–629. doi:10.3233/RNN-170753
Valenza M., Facchinetti R., Torazza C., Ciarla C., Bronzuoli M. R., Balbi M., et al. (2024). Molecular signatures of astrocytes and microglia maladaptive responses to acute stress are rescued by a single administration of ketamine in a rodent model of PTSD. Transl. Psychiatry 14, 209. doi:10.1038/s41398-024-02928-6
Verkhratsky A., Butt A., Li B., Illes P., Zorec R., Semyanov A., et al. (2023). Astrocytes in human central nervous system diseases: a frontier for new therapies. Signal Transduct. Target Ther. 8, 396. doi:10.1038/s41392-023-01628-9
Villapol S., Byrnes K. R., Symes A. J. (2014). Temporal dynamics of cerebral blood flow, cortical damage, apoptosis, astrocyte-vasculature interaction and astrogliosis in the pericontusional region after traumatic brain injury. Front. Neurol. 5, 82. doi:10.3389/fneur.2014.00082
Yi J.-H., Hazell A. S. (2006). Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem. Int. 48, 394–403. doi:10.1016/j.neuint.2005.12.001
Zhang J., Li H., Xu Z., Lu J., Cao C., Shen H., et al. (2023). Oestrogen ameliorates blood-brain barrier damage after experimental subarachnoid haemorrhage via the SHH pathway in male rats. Stroke Vasc. Neurol. 8, 217–228. doi:10.1136/svn-2022-001907
Ziebell J. M., Rowe R. K., Harrison J. L., Eakin K. C., Colburn T., Willyerd F. A., et al. (2016). Experimental diffuse brain injury results in regional alteration of gross vascular morphology independent of neuropathology. Brain Inj. 30, 217–224. doi:10.3109/02699052.2015.1090012
Keywords: diffuse axonal injury, cerebrovascular astrocytes, blood-brain barrier, glial fibrillary acidic protein, aging with TBI, chronic neurodegeneration, perivascular astrocytes
Citation: Sabetta Z, Krishna G, Curry-Koski T, Lopez M, Adelson PD and Thomas TC (2024) Sex-dependent temporal changes in astrocyte-vessel interactions following diffuse traumatic brain injury in rats. Front. Physiol. 15:1469073. doi: 10.3389/fphys.2024.1469073
Received: 23 July 2024; Accepted: 11 September 2024;
Published: 25 September 2024.
Edited by:
Roshanak Rahimian, The University of the Pacific, United StatesReviewed by:
Mihika Gangolli, Henry M. Jackson Foundation for the Advancement of Military Medicine (HJF), United StatesFrancesc Jiménez-Altayó, Autonomous University of Barcelona, Spain
Copyright © 2024 Sabetta, Krishna, Curry-Koski, Lopez, Adelson and Thomas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Theresa Currier Thomas, dGhlcmVzYXRob21hc0Bhcml6b25hLmVkdQ==
†These authors share first authorship
 Zackary Sabetta1,2†
Zackary Sabetta1,2† Gokul Krishna
Gokul Krishna Theresa Currier Thomas
Theresa Currier Thomas