- 1Center for Transplantation Sciences, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States
- 2Graduate Program in Physical Education, Catholic University of Brasilia, Brasília, Brazil
- 3Graduate Program in Genomic Sciences and Biotechnology, Catholic University of Brasilia, Brasília, Brazil
- 4Department of Medicine, Catholic University of Brasilia, Brasília, Brazil
- 5Hospital de Clínicas de Porto Alegre, Porto Alegre, Rio Grande do Sul, RS – Brasil
Introduction: Exercise is widely recognized for its benefits to chronic kidney disease (CKD) patients. However, the specific impact of different exercise modalities on CKD-related outcomes remains unclear. This study sought to summarize the effects of different exercise modalities on the main outcomes impacted by CKD.
Methods: We searched for systematic review with meta-analysis in PubMed, Embase, Web of Science, Scopus, and Cochrane databases. We evaluated the methodological quality of included studies by AMSTAR2 tool and by individually evaluating the heterogeneity, sample power, and statistical significances from meta-analyses.
Results: We included 44 meta-analyses, encompassing 35,432 CKD patients in pre-dialysis and dialysis stages (peritoneal and hemodialysis). Data from meta-analyses with highly suggestive or strong evidence grading suggests that aerobic and combined training were most effective in improving cardiorespiratory fitness (main effect: 2.1, 95% CI: 0.8–3.4, and main effect: 3.4; 95% CI: 2.4–4.6, respectively). Combined training showed a consistent benefit in psychosocial domains (main effect: −7.3; 95% CI: −9.31 to −53). All exercise modalities significantly improve functional performance, except isometric training, which impacted just fistula maturation (main effect: 0.84; 95% CI: 0.5–1.2).
Conclusion: Exercise emerges as a potential non-pharmacological therapy for CKD patients. Tailoring exercise to specific outcomes appears to be crucial, as different exercise modalities exhibit varying effectiveness.
1 Introduction
Chronic kidney disease (CKD) poses a growing global health challenge, impacting individuals and healthcare systems worldwide (Kovesdy, 2022; August, 2023). More than 800 million subjects are estimated to have CKD, reflecting the widespread prevalence of this condition (Jha et al., 2023; KDIGO, 2020, 2020). The progressive decline in kidney function associated with CKD leads to a range of complications, from diminished quality of life to major cardiovascular events (Jha et al., 2023; Tsai et al., 2015). To mitigate these deleterious effects, CKD patients often require several pharmacological interventions (de Boer et al., 2022; Navaneethan et al., 2023; Dong et al., 2022; Fan et al., 2022). While pharmaceutical treatments are essential to CKD management, they are frequently complemented by non-pharmacological approaches to address the nature of this disease (Rovin et al., 2021; Bishop et al., 2023; Johansen and Painter, 2012). Exercise has emerged as a promising non-pharmacological intervention for CKD, as it appears to improve outcomes of CKD patients, without reported side effects (Sprick et al., 2022; Corrêa et al., 2021a; Beetham et al., 2022; de Araújo et al., 2023; Gadelha et al., 2021; Corrêa et al., 2021b; Deus et al., 2022).
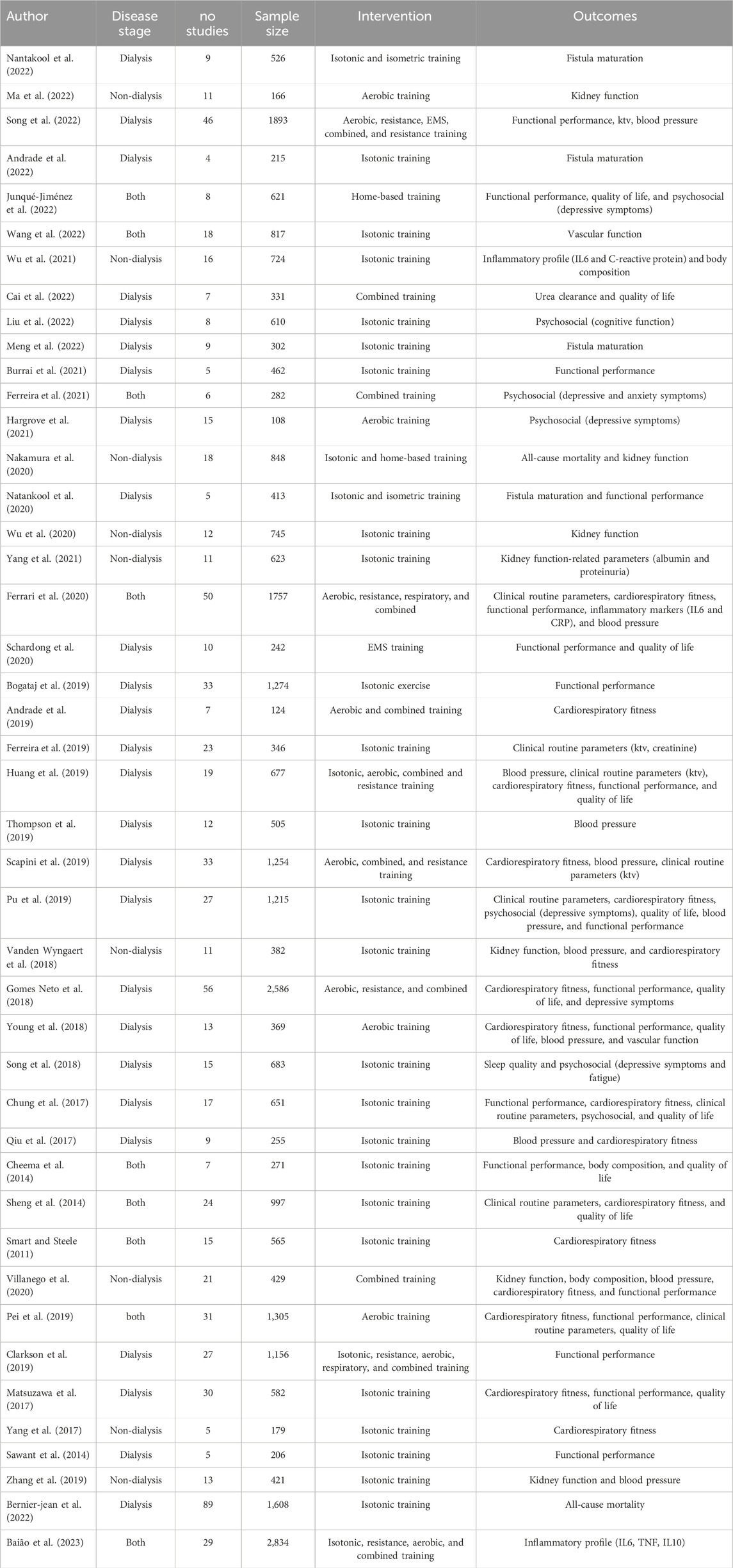
Recent studies have showcased its potential to enhance both physical and psychological health parameters (Deus et al., 2021). Many systematic reviews and meta-analyses have consolidated the role of exercise in improving CKD-related outcomes (Andrade et al., 2022; Andrade et al., 2019; Baião et al., 2023; Bernier-Jean et al., 2022; Bogataj et al., 2019; Burrai et al., 2021; Cai et al., 2022; Cheema et al., 2014; Chung et al., 2017; Clarkson et al., 2019; Ferrari et al., 2020; Ferreira et al., 2019; Ferreira et al., 2021; Gomes Neto et al., 2018; Hargrove et al., 2021; Huang et al., 2019; Junqué-Jiménez et al., 2022; Liu et al., 2022; Ma et al., 2022; Matsuzawa et al., 2017; Meng et al., 2022; Nakamura et al., 2020; Nantakool et al., 2022; Nantakool et al., 2020; Pei et al., 2019; Pu et al., 2019; Qiu et al., 2017; Sawant et al., 2014; Scapini et al., 2019; Schardong et al., 2020; Sheng et al., 2014; Smart and Steele, 2011; Song et al., 2022; Song et al., 2018; Thompson et al., 2019; Vanden Wyngaert et al., 2018; Villanego et al., 2020; Wang et al., 2022; Wu et al., 2021; Wu et al., 2020; Yang et al., 2017; Yang et al., 2020; Young et al., 2018; Zhang et al., 2019), leading major international kidney disease organizations to endorse its inclusion in CKD management (Milam, 2016). While two umbrella reviews have demonstrated the comprehensive positive impact of exercise (Zhang et al., 2022) and resistance training (Perez-Dominguez et al., 2023) on variables such as blood pressure, muscle strength, body composition, dialysis-related symptoms, and quality of life, a comprehensive understanding of the collective influence of different exercise modalities on CKD-related outcomes remains unclear. Prior reviews often focus on specific modalities or isolated outcomes, leaving gaps in the broader understanding of how different exercise approaches impact a range of clinically relevant outcomes. The variability in study designs and populations across reviews poses challenges in consolidating findings into actionable insights. Covering a wide range of outcomes, including blood pressure, body composition, functional performance, and quality of life is relevant for understanding the impact of exercise on CKD (Zhang et al., 2022; Perez-Dominguez et al., 2023).
A deeper understanding of how specific exercise modalities influence CKD-related outcomes is critical for refining clinical guidelines (Rovin et al., 2021; Bishop et al., 2023; Johansen and Painter, 2012; Sprick et al., 2022). Tailored exercise prescriptions may optimize benefits such as improving cardiorespiratory fitness, mitigating inflammation, and enhancing quality of life (Corrêa et al., 2021a; Gadelha et al., 2021; Ferrari et al., 2020; Pei et al., 2019). Aerobic training has demonstrated significant improvements in inflammatory markers and cardiorespiratory fitness, contributing to better cardiovascular health (Ferrari et al., 2020; Pei et al., 2019; Diniz et al., 2021). Resistance training is essential for enhancing muscle strength and functional performance, addressing the prevalent issue of sarcopenia in CKD patients (Corrêa et al., 2021a; Gadelha et al., 2021; Schoenfeld, 2010). Combined training may provide synergistic benefits by targeting both cardiorespiratory and musculoskeletal outcomes (Burrai et al., 2021; Chung et al., 2017).
In addition to these well-studied approaches, other modalities such as isometric, home-based, and respiratory exercises have emerged as practical alternatives that may overcome barriers to exercise adherence, particularly for patients with limited mobility or access to structured programs (Nantakool et al., 2022; Liu-Ambrose et al., 2019). Electromyostimulation (EMS) offers a novel avenue for improving muscle strength in patients unable to engage in traditional exercise, while respiratory training may target specific deficits in lung function and overall endurance (Ferrari et al., 2020; Song et al., 2022). These modalities, though less explored, hold promise for expanding the scope of CKD management and addressing unique patient needs. Exploring these approaches collectively is crucial for bridging gaps in current evidence and tailoring interventions for clinical practice.
To fill this gap, this umbrella review sought to summarize the impact of different exercise modalities on specific CKD-related outcomes: spanning blood pressure, body composition, cardiorespiratory fitness, clinical routine parameters, functional performance, inflammatory markers, kidney function, psychosocial domains, quality of life, sleep quality, vascular function, and mortality. Within this framework, we explore the ramifications of aerobic, resistance, combined, eletromyostimulation (EMS), isometric, home-based, and respiratory training in CKD. This exploration reveals that each modality may exhibit varying degrees of effectiveness for specific outcomes, underscoring the importance of customizing exercise regimens for this population.
2 Methods
2.1 Protocol and registration
We performed an umbrella-review according to the Cochrane Handbook recommendations (Higgins and Green, 2008) and reported the results according to the preferred reporting items for overviews of reviews (PRIOR) statement (Gates et al., 2022). The protocol for this review was registered in the International Prospective Register of Systematic Reviews https://www.crd.york.ac.uk/prospero/#recordDetails with registration number: CRD42022381825.
2.2 Criteria for considering studies for this review
Eligible studies for this umbrella review were systematic reviews with meta-analyses published in peer-reviewed journals. The population of interest included adults diagnosed with CKD at any stage. Studies were considered if they evaluated chronic exercise interventions, including aerobic, resistance, combined, isometric, EMS, home-based, or respiratory training, compared to usual care, no intervention, or other exercise modalities. Eligible outcomes included physiological parameters (e.g., blood pressure, kidney function), physical performance (e.g., muscle strength, cardiorespiratory fitness), and psychosocial factors (e.g., quality of life, sleep quality). There were no restrictions on language, age, sex, or disease stage. Exercise training regimens were categorized by modality where specified, and studies with unspecified regimens were classified as “isotonic” training for consistency. Studies were excluded if they did not include a meta-analytical component or if the population was non-CKD or pediatric.
2.3 Search and selection strategy
Two authors (TM and RL) independently reviewed published meta-analytic systematic reviews by searching PubMed, Scopus, Web of Science, and the Cochrane Library from their inception to December 2023. All searches were adapted from the MEDLINE search strategy as reported: (“Exercise training” [MeSH]) AND (“Chronic Kidney Disease” [MeSH). We reviewed the trials’ bibliographies, identifying and contacting some of the authors in the field to clarify trial eligibility or to identify additional published or unpublished data. Next, two review authors (TM and RL) independently checked the references identified by the search strategy. The full texts of all potentially relevant studies were obtained for independent assessment. Disagreements were solved through discussion, and a third review author (HLC) acted as arbitrator where necessary. All citations were downloaded into EndNote X9®, duplicates were removed, and an identification number was assigned to each article.
2.4 Data extraction
The same authors collected data in sufficient detail to better extract properties including studies based on PICO: Population: CKD patients; Intervention: exercise training; Comparator, no exercise group; Outcome: CKD-related outcomes. We also extracted Statistical aspects such as P-value, heterogeneity (I2), main effect, and largest study effect were also extracted. Data was collected from text, tables, and figures. After extracting the data, two authors (TM and RL) graded the risk of bias in the included trials. Disagreements were resolved through discussion and a third reviewer (HLC) acted as moderator where necessary. Authors of primary studies did not extract data from their own studies. RL entered the data into the Excell (Microsoft Corporation) and HLC checked data entry. We calculated the corrected covered area (CCA) to assess the overlap of primary studies. The CCA accounts for the number of times primary studies are included across different reviews, providing a value that ranges from 0 (no overlap) to 1 (complete overlap). All graphs and tables in the present study were created using R and RStudio version 4.3.2.
2.5 Assess of risk of bias in included studies
The risk of bias for eligible studies was assessed using the AMSTAR 2 tool (Shea et al., 2017), which evaluates the methodological quality of systematic reviews and meta-analyses. The tool consists of 16 items, each scored as “yes,” “partial yes,” or “no.” Scoring percentages determined quality classification (high, medium, or low). The risk of bias assessment was conducted independently by two authors, with disagreements resolved by a third author. Two authors performed the risk of bias assessment independently using the AMSTAR 2 tool (Shea et al., 2017), which evaluates the methodological quality of systematic reviews and meta-analyses. This checklist contains 16 items, and each item was answered with a “yes” (1 point), “partial yes” (0.5 points) or “no” (0 points). The percentage score for each study was calculated using the total score as the numerator and the highest score of 16 points as the denominator. A meta-analysis scoring ≥80% was classified as high quality, 40%–79% as medium quality and those scoring <40% as low quality. Disagreements were solved by a third author (HLC).
2.6 Statistical methodology
This umbrella review synthesized evidence from included meta-analyses by evaluating methodological quality, heterogeneity, and consistency across studies. Heterogeneity was assessed using the I2 statistic, with thresholds of <25%, 25%–50%, and >50% indicating low, moderate, and high heterogeneity, respectively. Random-effects models were applied to account for variability in study populations and methodologies. The grading of evidence relied on effect size estimates, confidence intervals, and sample size, as defined in the grading framework. Statistical significance thresholds were based on predefined p-values, and heterogeneity levels informed the strength of evidence. Meta-analyses with inconsistent findings or significant heterogeneity were noted but not excluded, to provide a comprehensive synthesis of available evidence. No additional tests for publication bias or excessive significance bias were conducted. All graphs and tables in the present study were created using R and RStudio version 4.3.2.
2.7 Grading evidence
The grading of evidence for all included meta-analyses was established through an adapted framework, as previously described (Bellou et al., 2018; Papadimitriou et al., 2021; Botelho et al., 2022). This framework categorized evidence into four levels: strong, highly suggestive, suggestive, and weak. These levels were determined by thresholds for sample size, significance level, and heterogeneity. Strong evidence was attributed to meta-analyses with a sample size of more than 385 participants, a p-value ≤10−⁶, and low heterogeneity (I2 <50%). Highly suggestive evidence was assigned to meta-analyses with more than 385 participants, a random-effects p-value ≤10−⁶, and a significant effect reported in the largest contributing study. Suggestive evidence was applied to meta-analyses with more than 385 participants and a p-value ≤10−³. Weak evidence included meta-analyses that did not meet the above criteria. The threshold of 385 participants was chosen to ensure a 95% confidence level in estimates based on the global prevalence of CKD, which affects approximately 800 million individuals worldwide (Kovesdy, 2022; Kadam and Bhalerao, 2010).
3 Results
3.1 Characteristics of the included meta-analysis
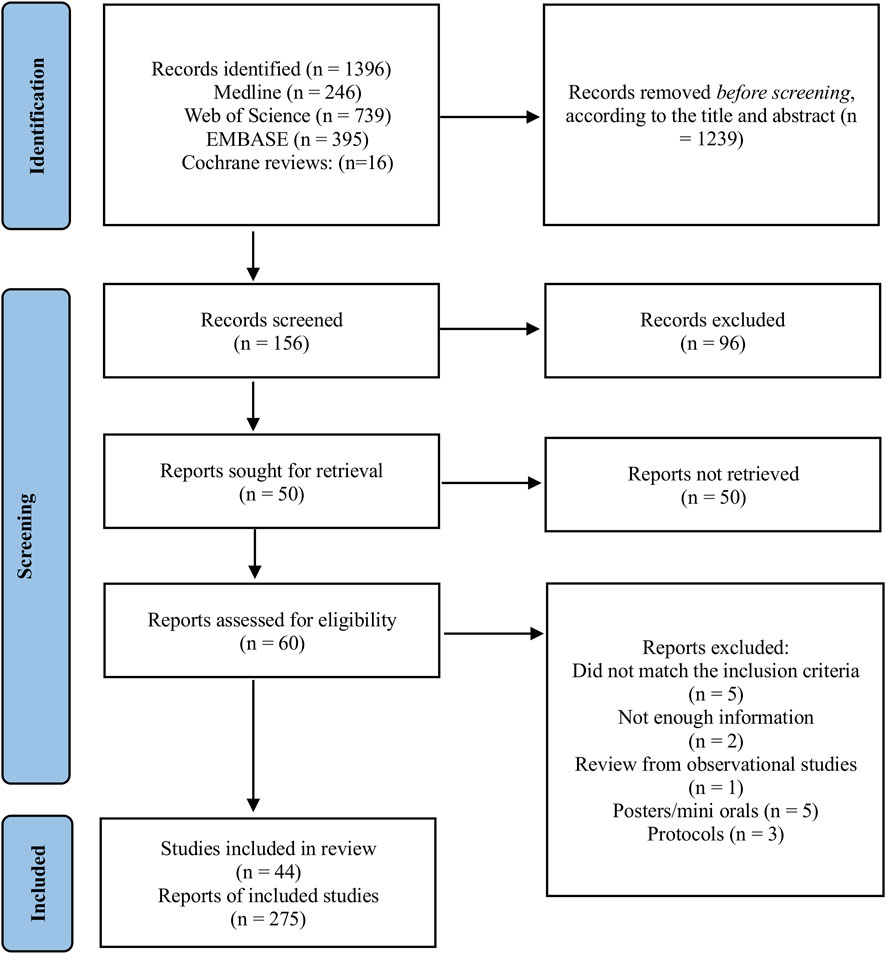
A total of 1,396 records were identified through database searches, including Medline (n = 246), Web of Science (n = 739), EMBASE (n = 395), and Cochrane Reviews (n = 16). After screening titles and abstracts, 1,239 records were excluded, leaving 156 records for further screening. Of these, 96 records were excluded based on inclusion and exclusion criteria applied during full-text screening. Following this, 60 reports were assessed for eligibility. Specific reasons for exclusions included reports that did not meet the inclusion criteria for study design or population (n = 5), lacked sufficient information for evaluation (n = 2), reviewed observational studies (n = 1), posters or mini-oral presentations without sufficient detail (n = 5), and protocols without completed analyses (n = 3). In total, we included 44 meta-analyses comprising data from 839 randomized controlled trials involving 35,431 patients with CKD, with patient numbers ranging from 124 to 3,846, encompassing both non-dialysis (stage 1–4) and dialysis patients. Each study was treated as an independent report according to the number of exercise modalities and interventions analyzed, resulting in a total of 275 reports (Figure 1). While duration of exercise interventions encompassed chronic exercise regimens (ranging from 4 to 24 weeks), it was not consistently reported between studies which limited detailed analysis on the impact of duration on our studied outcomes. The overall CCA for all exercise modalities was calculated as 0.46, reflecting a low to moderate overlap of primary studies. When stratifying intervention type the overlap varied. Isometric training and resistance training displayed a moderate overlap (0.5 and 0.6, respectively). Aerobic training presented a low overlap of primary studies (0.14). Combined and respiratory training presented a CCA >0.6 suggesting a moderate to high overlap.
Detailed characteristics of the included studies are provided in Table 1. Furthermore, we present detailed information on the included studies, describing each variable and the measurements used in Supplementary Tables S1, S2, respectively.
3.2 Risk of bias assessment
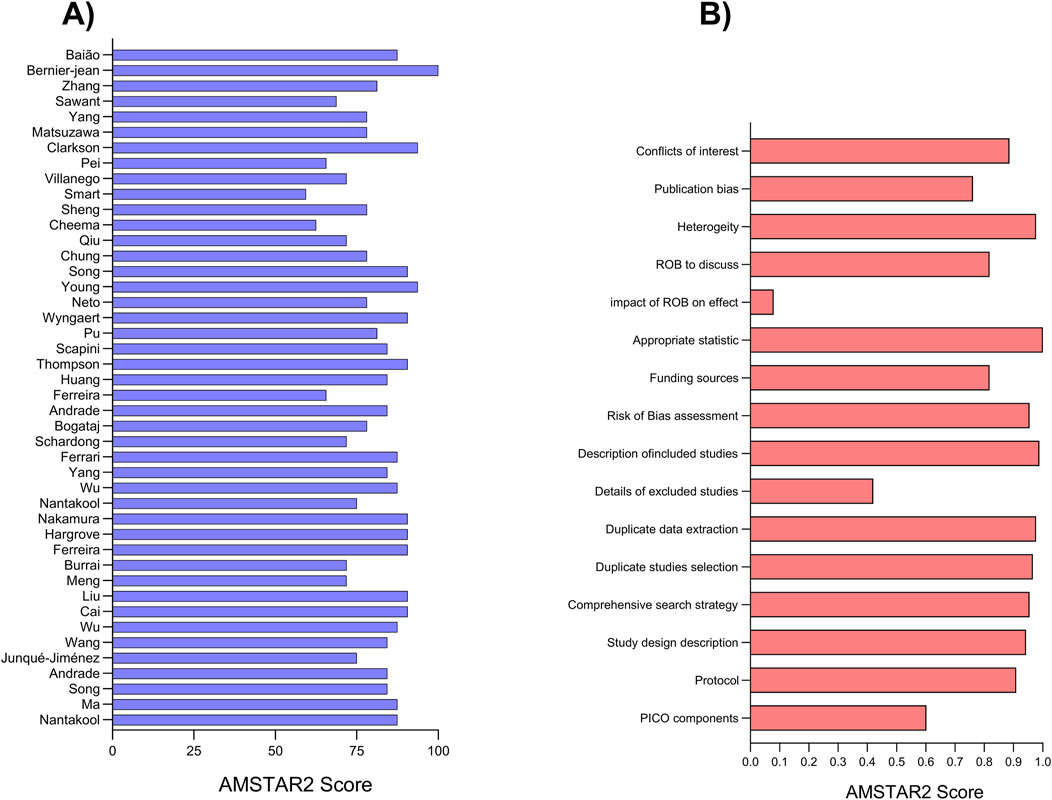
The mean risk of bias score across all studies was 13 (equivalent to 81.61%), with scores ranging from a minimum of 9.5 (59.38%) to a maximum of 16 (100%). Notably, most studies received scores above 13, indicating a low risk of bias, with over 80% of studies surpassing this threshold. Described in Figure 2.
3.3 Trends in publications over time
Over time, we observe a substantial growth in both RCTs, and systematic reviews dedicated to studying the impact of exercise on CKD (Figure 3A). Notably, while the number of RCTs significantly surpasses that of systematic reviews, both exhibit a similar upward trend in publication numbers (Figure 3B). The studies included in our analysis span the years 2011–2023, and an interesting pattern emerges. Early studies from 2011 to 2014 primarily explored the effects of exercise without differentiating between exercise modalities. However, a significant shift occurred in 2014, marked by the emergence of studies specifically investigating the effects of aerobic and combined training. Furthermore, from 2018 onwards, more studies have begun to explore a broader range of exercise modalities, encompassing EMS, home-based, isometric, resistance, and respiratory training (Supplementary Figure S1). The primary outcomes assessed in these studies include blood pressure, body composition, cardiorespiratory fitness, functional performance, and quality of life (Supplementary Figure S2). For a more comprehensive understanding of the number and proportion of studies per exercise modality and outcome, please refer to Supplementary Tables S3, S4.
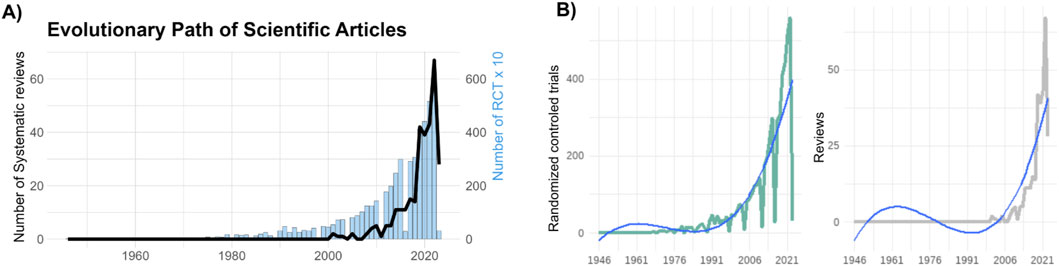
Figure 3. Evolutionary Path of Scientific Articles. (A) Temporal evolution of scientific articles in the field of exercise and chronic kidney disease. The graph showcases the growth of systematic reviews, represented by the black line and randomized controlled trials (RCTs), represented as blue bars over different time periods, revealing the dynamic trajectory of knowledge expansion. For a better visualization, we divided by 10 the number of RCTs to be fitted in the same figure. (B) Showcases a positive polynomial curve depicting the cumulative number of RCTs over time (left panel), and systematic reviews (right panel). The curve’s upward trajectory accentuates the growth of RCTs investigating the relationship between exercise and chronic kidney disease.
3.4 Statistical significance and efficacy of exercise modalities
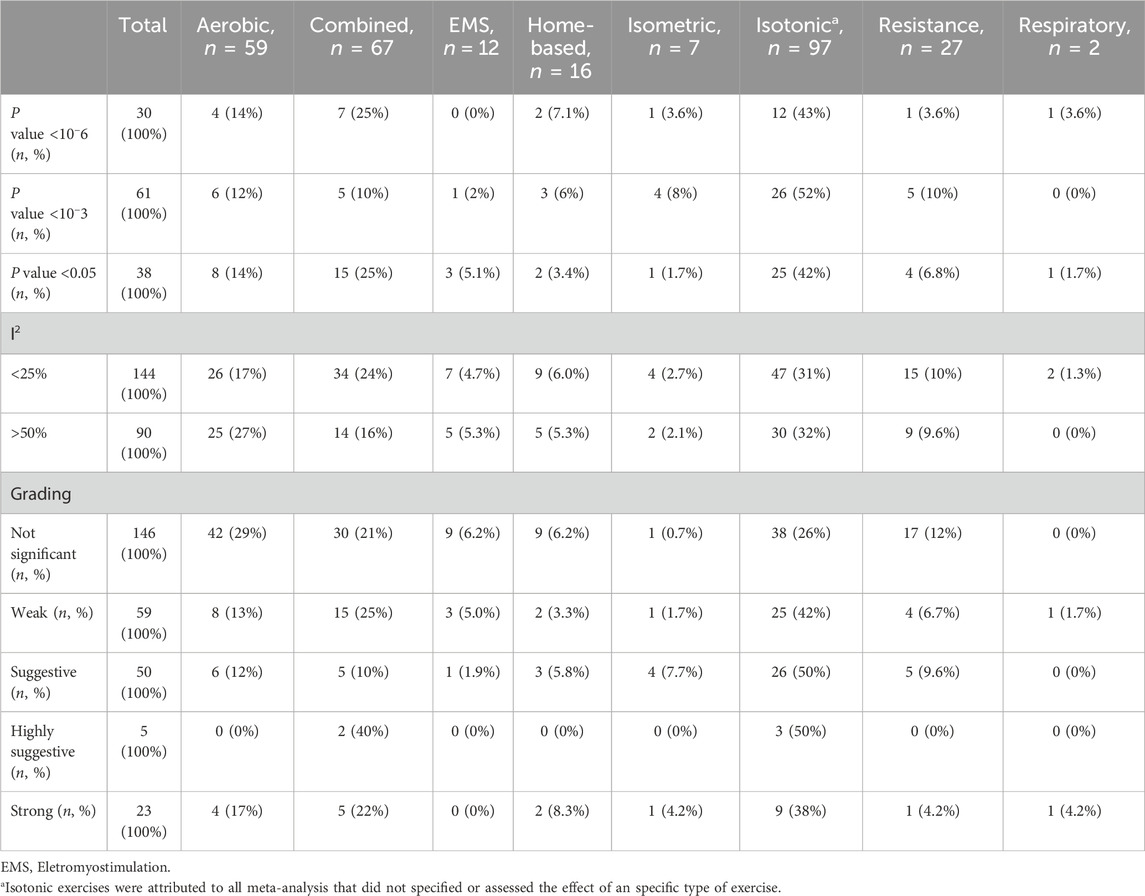
From 275 reports, 113 (41%) presented an overall P-value <0.05, 68 (24%) demonstrated a P < 0.01, 30 (11%) showed a P < 10−3, and 28 (10%) a P < 10−6. Notably, 138 (50%) of the reports did not exhibit a statistically significant effect (P > 0.05). We observed a higher number of significant meta-analyses (P < 0.05) in aerobic and combined training: 18 (15%) and 27 (24%) reports, respectively. Only 4 (3.5%) reports showed a statistically significant effect for EMS training. We found 7 (6.2%) significant reports for home-based training. There were 6 (5.3%) significant reports for isometric training, 10 (8.8%) for resistance training, and 2 (1.8%) for respiratory training. Moreover, all interventions seem to be effective in improving functional performance, except isometric training.
3.5 Dialysis vs. non-dialysis
Notably, there is a trend in the included meta-analyses to investigate more ESKD than non-dialysis patients for all interventions included. Regarding the outcomes, 19 (16%) reports with functional performance and cardiorespiratory fitness exhibited a significant P-value. Most outcomes reported in the included meta-analyses investigated ESKD patients, except for body composition (dialysis: n = 1/non-dialysis: n = 5) and all-cause mortality (dialysis: n = 1/non-dialysis: n = 2). However, a more comprehensive examination of the robustness of these effects is still required for a clearer understanding.
3.6 Analysis of evidence grading
Next, we conducted a comprehensive assessment of our study’s intervention, providing an overview of the statistical significance, heterogeneity, and evidence grading related to various exercise modalities (Table 2). Twenty-eight out of 275 (10%) meta-analyses presented strong or highly suggestive evidence grading for exercise modalities, and thirty-six for outcomes. Aerobic and combined training exhibited the highest number of strong meta-analyses (17% and 22%, respectively) (Table 2). More details about each intervention and outcome are described in Supplementary Table S3, respectively.
3.7 Strength of evidence for exercise modalities
After selection of the highly suggestive and strong studies, we performed an assessment of the strength of evidence between exercise modalities and outcomes. Specific findings from different exercise modalities emerge as follows: Aerobic training exhibits robust evidence for reducing inflammatory markers (main effect: −3.28; 95% CI: −4.68 to −1.88), enhancing functional performance (main effect: 64.98; 95% CI: 43.86–86.11), and improving cardiorespiratory fitness (main effect: 2.1; 95% CI: 0.8–3.4). Combined training significantly impacts psychosocial aspects (main effect: −7.3; 95% CI: −9.31 to −53), functional performance (main effect: 31.68; 95% CI: 16.91–46.46), and cardiorespiratory fitness (main effect: 3.4, 95% CI: 2.4–4.6). Resistance training, respiratory training, and home-based training robustly improve functional performance (main effect: 30.23; 95% CI: 24.55–35.92/main effect: 117.62; 95% CI: 67.26–167.99/main effect: 22.18; 95% CI: 15–29.36, respectively). Isometric training exhibits promise in enhancing fistula maturation (main effect: 0.84; 95% CI: 0.45–1.23). Notably, improvements in functional performance are a common thread across most of these interventions. Illustrated in Figure 4.
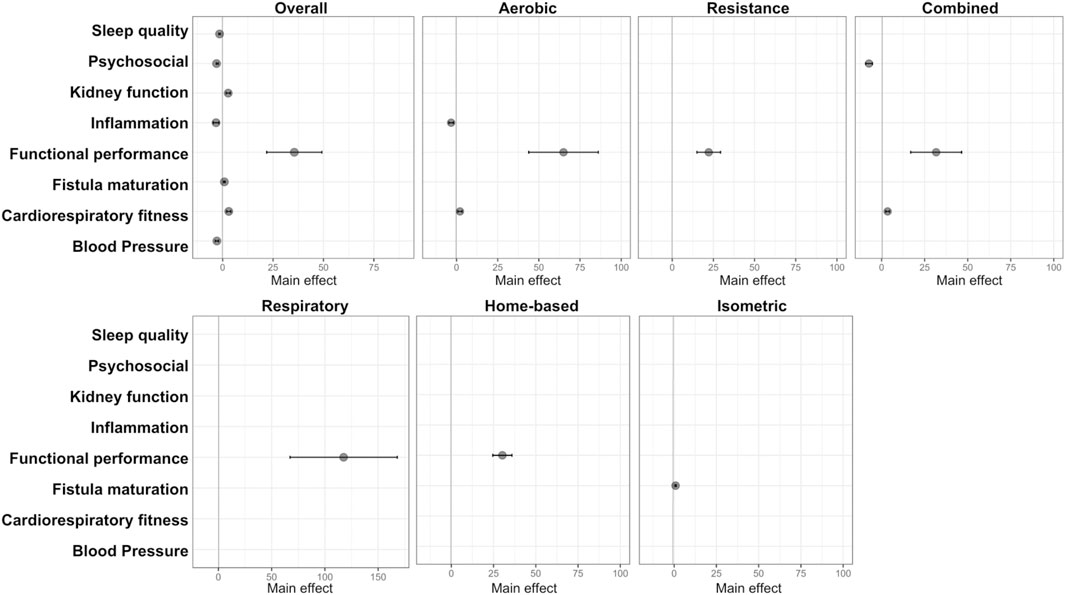
Figure 4. Forest plot displaying results with strong or highly suggestive evidence from the umbrella review on each exercise intervention and improved outcomes in CKD.
4 Discussion
This umbrella review depicted the potential benefits of exercise for CKD patients following an extensive summarization of systematic reviews with meta-analysis. The pooled 44 studies provided valuable findings in the specificity of exercise modalities in impacting different outcomes in this population (Andrade et al., 2022; Andrade et al., 2019; Baião et al., 2023; Bernier-Jean et al., 2022; Bogataj et al., 2019; Burrai et al., 2021; Cai et al., 2022; Cheema et al., 2014; Chung et al., 2017; Clarkson et al., 2019; Ferrari et al., 2020; Ferreira et al., 2019; Ferreira et al., 2021; Gomes Neto et al., 2018; Hargrove et al., 2021; Huang et al., 2019; Junqué-Jiménez et al., 2022; Liu et al., 2022; Ma et al., 2022; Matsuzawa et al., 2017; Meng et al., 2022; Nakamura et al., 2020; Nantakool et al., 2022; Nantakool et al., 2020; Pei et al., 2019; Pu et al., 2019; Qiu et al., 2017; Sawant et al., 2014; Scapini et al., 2019; Schardong et al., 2020; Sheng et al., 2014; Smart and Steele, 2011; Song et al., 2022; Song et al., 2018; Thompson et al., 2019; Vanden Wyngaert et al., 2018; Villanego et al., 2020; Wang et al., 2022; Wu et al., 2021; Wu et al., 2020; Yang et al., 2017; Yang et al., 2020; Young et al., 2018; Zhang et al., 2019). We found robust evidence on the effects of aerobic exercise in improving inflammatory markers, functional performance, and cardiorespiratory fitness (Ferrari et al., 2020; Pei et al., 2019). Combined training robustly improved psychosocial parameters (depression and anxiety symptoms), functional performance, and cardiorespiratory fitness (Ferrari et al., 2020; Gomes Neto et al., 2018; Huang et al., 2019; Villanego et al., 2020). Home-based, respiratory, and resistance training demonstrated strong evidence in improving functional performance (Ferrari et al., 2020; Gomes Neto et al., 2018; Junqué-Jiménez et al., 2022). Finally, isometric training seems to robustly improve fistula maturation (Nantakool et al., 2022). Although previous and ongoing randomized controlled trials and meta-analyses continue to investigate the impact of different exercise modalities in this population, addressing this in a single study can be impractical. Therefore, this review serves as a valuable resource for enhancing our understanding of the effect of exercise in CKD by summarizing the main results of recent systematic reviews with meta-analysis. This study may act as a guide for future research in this field, shedding some light on how each exercise modality may benefit CKD-related outcomes.
CKD patients often experience mitochondrial dysfunction, strongly associated with oxidative stress, impairing aging process, inflammatory profile, and functional performance (Bai et al., 2023; Rao et al., 2018). On the other hand, aerobic exercise has been shown to stimulate mitochondrial biogenesis, mainly through the activation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) (Memme et al., 2021; Alizadeh Pahlavani et al., 2022). This molecular pathway has been recognized as an important signaling pathway to improve oxidative metabolism and overall homeostasis (Bai et al., 2023; Rao et al., 2018; Alizadeh Pahlavani et al., 2022; Koch et al., 2017). Moreover, aerobic training has been found to have anti-inflammatory effects (Petersen and Pedersen, 2005). This is a relevant scenario in the field of nephrology as chronic inflammation is a hallmark of CKD, driven by the upregulation of pro-inflammatory cytokines, such as IL-6 and TNF-α (Ebert et al., 2021). Notably, aerobic exercise can downregulate the expression of these pro-inflammatory cytokines by inhibiting the activation of nuclear factor-kappa B (NF-κB), leading to a better inflammatory profile (Diniz et al., 2021; Guo et al., 2022). This inhibition is partly mediated through the activation of adenosine monophosphate-activated protein kinase (AMPK), a metabolic sensor that also has anti-inflammatory properties (Diniz et al., 2021; Xiang et al., 2019). These molecular mechanisms provide a basis for understanding the robust effects of aerobic exercise observed in inflammation, functional performance, and cardiorespiratory fitness in our review.
Resistance training has been widely used by clinicians and researchers to improve health-related parameters in CKD (Corrêa et al., 2021a; Gadelha et al., 2021; Corrêa et al., 2021b; Deus et al., 2022). This training modality is underpinned by several mechanisms related to the increase in muscle mass and strength, which, in turn, contribute to its robust effects presented by this study. One of the primary mechanisms driving the benefits of resistance training is the promotion of muscle hypertrophy. Resistance training involves subjecting muscles to external resistance, which leads to muscle damage at the microscopic level (Schoenfeld, 2010; Lim et al., 2022). This damage triggers a repair and growth process, primarily promoted by the activation of satellite cells, leading to muscle regeneration and growth (Fukada et al., 2022). In response to resistance training, these satellite cells become activated and fuse with existing muscle fibers, resulting in an increase in muscle size. This process is known as myogenesis.
Moreover, resistance training stimulates the synthesis of muscle proteins, especially myofibrillar proteins (Schoenfeld, 2010; Schiaffino et al., 2013). The mechanistic target of rapamycin (mTOR) pathway plays a central role in this process. mTOR is a signaling pathway that regulates protein synthesis, cell growth, and muscle hypertrophy (Schiaffino et al., 2013). Resistance training activates mTOR, leading to increased protein synthesis and muscle growth. Additionally, resistance training is known to enhance muscle strength in CKD patients (Corrêa et al., 2021a; Gadelha et al., 2021; Corrêa et al., 2021b). This is partly achieved through neural adaptations. The mechanistic insights mentioned above provide a basis for understanding why resistance training robustly improves functional performance. Furthermore, the strong associations between higher muscle mass and a better disease prognosis and survival are rooted in the benefits of resistance training (Gadelha et al., 2021; de Luca Corrêa et al., 2023). Increased muscle mass is associated with improved metabolic health, better glucose control, and a reduced risk of insulin resistance, which might be a key tool to improve the health status from individuals with CKD (Gadelha et al., 2021; Deus et al., 2022; Deus et al., 2021; de Luca Corrêa et al., 2023).
Taken together it is rational to combine aerobic and resistance training to achieve an optimized benefit in this population, as has been explored in previous studies (Andrade et al., 2019; Cai et al., 2022; Ferrari et al., 2020; Ferreira et al., 2021; Gomes Neto et al., 2018; Nakamura et al., 2020; Scapini et al., 2019; Song et al., 2022; Villanego et al., 2020). The synergy of these two modalities, known as combined training, is more likely to induce a comprehensive range of beneficial effects, including those related to psychosocial wellbeing (Ferreira et al., 2021). The mechanisms underlying the effects of combined training may be attributed to the additive or synergistic effects of aerobic and resistance training (Dor-Haim et al., 2018). As discussed earlier, resistance exercises in combined training promote muscle hypertrophy and the synthesis of muscle proteins. This leads to an increase in muscle mass and strength, which are associated with enhanced physical performance and overall health. Aerobic exercises, on the other hand, improve cardiorespiratory fitness and metabolic parameters (Diniz et al., 2021; Guo et al., 2022; Fleg, 2012; Seals et al., 2019). Furthermore, the combination of aerobic and resistance training can have a positive impact on psychological wellbeing.
One of the mechanisms for this effect is the release of endorphins during exercise (Harber and Sutton, 1984; Mikkelsen et al., 2017), playing a key role in improving mood, symptoms of depression, and enhancing overall psychosocial wellbeing (Harber and Sutton, 1984; Mikkelsen et al., 2017). Another mechanism is the modulation of neurotransmitters and hormones, including serotonin and brain-derived neurotrophic factor (BDNF) (Deus et al., 2021). Both aerobic and resistance training have been shown to influence these neurochemicals, which are associated with mood regulation and cognitive function. The approach of combined training, by addressing both physical and psychological aspects, contributes to its consistent effects on psychosocial factors, including reduced depression and anxiety symptoms.
Isometric training involves static muscle contractions in which the length of the muscle remains unchanged (Nantakool et al., 2022; Nantakool et al., 2020). Unlike dynamic exercises such as aerobic or resistance training, isometric exercises focus on maintaining a specific muscle position or tension without movement. One of the key mechanisms through which isometric training can significantly impact fistula maturation is related to its effects on vascular function (Nantakool et al., 2022; Nantakool et al., 2020). Isometric exercises can generate sustained muscle tension and increase intra-arterial pressure in the exercising limb. This elevation in intra-arterial pressure is thought to stimulate adaptive responses in the blood vessels, including the vascular endothelium (Souza et al., 2018). Moreover, isometric training has been shown to enhance endothelial function by promoting the release of nitric oxide, a potent vasodilator molecule (Souza et al., 2018). This vasodilatory effect can improve blood flow, leading to vascular benefits. These mechanisms highlight the relevance of isometric training in improving vascular access and overall CKD management, especially in patients undergoing hemodialysis.
Despite the beneficial effects of exercise, adherence to exercise programs remains a challenge in this field (Clyne and Anding-Rost, 2021). In this context, modalities such as home-based training offer the potential to encourage more participants to continue their exercise routines. Our findings reveal robust evidence supporting the effectiveness of home-based training in improving functional performance. Home-based approaches can accommodate different exercise modalities, tailored to meet individual patient needs (de Araújo et al., 2023; Liu-Ambrose et al., 2019). However, it is essential to note that patients receiving home-based training may be physically distant from the clinical support typically available in hospitals and clinics. As a result, strict adherence to established procedures for monitoring vital signs and assessing adverse events, as outlined in the primary exercise guidelines, is crucial to ensure safety and efficacy. Exercise training should adopt a more conservative approach, and any progressive increase in intensity or volume should be approached with caution. Similarly, respiratory interventions are designed to address specific physiological needs. While its impacts may be less pronounced compared to other modalities (Corrêa et al., 2021a; de Araújo et al., 2023; Corrêa et al., 2021b), it offers valuable benefits, such as enhancing lung capacity, addressing compromised lung function in CKD patients, and contributing to overall mobility and functionality (Slee and Reid, 2022; Wilund et al., 2021). By understanding the contributions of each exercise modality, clinicians and researchers can design targeted exercise interventions that address the diverse needs of CKD patients (Evans et al., 2022). This personalized approach holds the potential to optimize outcomes and enhance the quality of life for individuals grappling with the challenges of CKD.
To attain optimized benefits from physical conditioning through exercise, CKD patients require appropriate care and interventions. These measures are applicable from the early stages of CKD but become crucial for patients in advanced stages (3B and higher) and to those undergoing dialysis treatments. Of utmost importance are the nutritional aspects that require a healthy diet with adequate protein and calorie intake, low sodium content, and often fluid restriction (Ikizler et al., 2020). Additionally, effective control and treatment of diabetes mellitus, blood pressure and metabolic acidosis may also be necessary (Navaneethan et al., 2023; Flythe et al., 2020). The management of bone mineral disorders, especially secondary hyperparathyroidism, is also important and includes the use of phosphate binders and calcimimetics as needed (Ketteler et al., 2018). Anemia, a nearly universal finding in the advanced stages of CKD, is managed through iron replacement and erythropoiesis-stimulating agents. Correcting anemia to recommended hemoglobin target levels is crucial not only for exercise endurance but also for a variety of other benefits (Babitt et al., 2021). Ultimately, having adequate vascular access, preferably through an arteriovenous fistula, is essential for achieving proper dialysis dosing. Without it, none of the aforementioned interventions will be successful (Baker et al., 2022).
This study presents some limitations that should be mentioned. First, the findings from this study rely on published meta-analyses, so we should not assume which exercise modality is the best to improve one outcome of another. However, this study was the first to highlight the main effects of each exercise modality on different outcomes available in the literature. Second, the heterogeneity of individuals with CKD may impact the main findings of this study, and we encourage new meta-analysis addressing specific subpopulations of this conditions, including, sex differences, dialysis modality, and dialysis technique, which were not factors addressed by the included studies. An important limitation of this study is that the included systematic reviews and meta-analyses did not provide specific details about the impact of training features according to age, and years in dialysis vintage, or specific CKD stage. For the latter, studies have broadly classified as either dialysis and non-dialysis groups, which included mostly CKD stages 3–5. Additionally, while all protocols included in this study were chronic (lasting more than 4 weeks), the specific duration of different exercise modalities and the impact on cardio-respiratory fitness and other measured parameters were not consistently reported. Future studies and systematic reviews should consider the inclusion, duration of modality and analysis of these subgroups to provide more detailed and personalized insights into how different exercise modalities may benefit patients at different stages of the disease, age groups, and dialysis durations. The insights garnered from this umbrella review have significant implications for the management of CKD. This study’s findings provide clinicians, researchers, and policymakers with evidence-based guidance for implementing exercise interventions within CKD management protocols. Furthermore, the comprehensive synthesis serves as a steppingstone for future research endeavors, inviting exploration of mechanistic underpinnings of exercise’s impact on CKD. Our study has far-reaching implications for CKD management, influencing clinical practice, research priorities, and patient-centered care. Finally, investigating optimal exercise intensity, frequency, and duration for specific outcomes and CKD stages can refine intervention protocols.
5 Conclusion
This umbrella review explored different exercise interventions in CKD, offering insights into the application of exercise as a complementary therapeutic tool for this population. The interplay between exercise modalities and outcomes underscored the potential of exercise in improving CKD patients. In sum, exercise training is a potent non-pharmacological tool to improve CKD-related outcomes in non-dialysis and dialysis patients.
Author contributions
HC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. TR: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. RS: Data curation, Formal Analysis, Methodology, Writing–original draft, Writing–review and editing. VM: Data curation, Investigation, Methodology, Writing–original draft, Writing–review and editing. TSA: Data curation, Investigation, Methodology, Writing–original draft, Writing–review and editing. WS: Data curation, Investigation, Methodology, Writing–original draft, Writing–review and editing. RN: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing–original draft, Writing–review and editing. LD: Methodology, Project administration, Resources, Validation, Writing–original draft, Writing–review and editing. AR: Investigation, Methodology, Project administration, Writing–original draft, Writing–review and editing. JB: Investigation, Methodology, Project administration, Writing–original draft, Writing–review and editing. TBA: Investigation, Methodology, Project administration, Writing–original draft, Writing–review and editing. RV: Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft, Writing–review and editing. KY: Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft, Writing–review and editing. DM: Formal Analysis, Investigation, Project administration, Writing–original draft, Writing–review and editing. RM: Data curation, Investigation, Methodology, Writing–original draft, Writing–review and editing. TB: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing–original draft, Writing–review and editing. LR: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. TR, HC, LD, JM, RS, and TA were supported by a grant provided by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brazil–Finance Code 001. This study was financed in part by the Conselho Nacional de Desenvolvimento Científico e Tecnológico and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CNPq)—Brasil—Finance Code 001. This work was funded by the Fundação de Apoio à Pesquisa do Distrito Federal with grants from demanda espontânea–Edital 09/2022. TR is a recipient of the level 2 Productivity and Quality Fellowship from CNPq.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2024.1444976/full#supplementary-material
References
Alizadeh Pahlavani H., Laher I., Knechtle B., Zouhal H. (2022). Exercise and mitochondrial mechanisms in patients with sarcopenia. Front. physiology 13, 1040381. doi:10.3389/fphys.2022.1040381
Andrade F. P., Benvenutti H., da Silva K. C., Rovedder P. M. E. (2022). Effects of upper limb exercise programs on the arteriovenous fistula in patients on hemodialysis: a systematic review and meta-analysis. J. Vasc. access 23 (5), 770–777. doi:10.1177/11297298211001166
Andrade F. P., Rezende P. S., Ferreira T. S., Borba G. C., Müller A. M., Rovedder P. M. E. (2019). Effects of intradialytic exercise on cardiopulmonary capacity in chronic kidney disease: systematic review and meta-analysis of randomized clinical trials. Sci. Rep. 9 (1), 18470. doi:10.1038/s41598-019-54953-x
August P. (2023). Chronic kidney disease - another step forward. N. Engl. J. Med. 388 (2), 179–180. doi:10.1056/NEJMe2215286
Babitt J. L., Eisenga M. F., Haase V. H., Kshirsagar A. V., Levin A., Locatelli F., et al. (2021). Controversies in optimal anemia management: conclusions from a kidney disease: improving global outcomes (KDIGO) conference. Kidney Int. 99 (6), 1280–1295. doi:10.1016/j.kint.2021.03.020
Bai M., Wu M., Jiang M., He J., Deng X., Xu S., et al. (2023). LONP1 targets HMGCS2 to protect mitochondrial function and attenuate chronic kidney disease. EMBO Mol. Med. 15 (2), e16581. doi:10.15252/emmm.202216581
Baião V. M., Cunha V. A., Duarte M. P., Andrade F. P., Ferreira A. P., Nóbrega O. T., et al. (2023). Effects of exercise on inflammatory markers in individuals with chronic kidney disease: a systematic review and meta-analysis. Metabolites 13 (7), 795. doi:10.3390/metabo13070795
Baker L. A., March D. S., Wilkinson T. J., Billany R. E., Bishop N. C., Castle E. M., et al. (2022). Clinical practice guideline exercise and lifestyle in chronic kidney disease. BMC Nephrol. 23 (1), 75. doi:10.1186/s12882-021-02618-1
Beetham K. S., Krishnasamy R., Stanton T., Sacre J. W., Douglas B., Isbel N. M., et al. (2022). Effect of a 3-year lifestyle intervention in patients with chronic kidney disease: a randomized clinical trial. J. Am. Soc. Nephrol. JASN 33 (2), 431–441. doi:10.1681/ASN.2021050668
Bellou V., Belbasis L., Tzoulaki I., Evangelou E. (2018). Risk factors for type 2 diabetes mellitus: an exposure-wide umbrella review of meta-analyses. PloS one 13 (3), e0194127. doi:10.1371/journal.pone.0194127
Bernier-Jean A., Beruni N. A., Bondonno N. P., Williams G., Teixeira-Pinto A., Craig J. C., et al. (2022). Exercise training for adults undergoing maintenance dialysis. Cochrane database Syst. Rev. 1 (1), Cd014653. doi:10.1002/14651858.CD014653
Bishop N. C., Burton J. O., Graham-Brown M. P. M., Stensel D. J., Viana J. L., Watson E. L. (2023). Exercise and chronic kidney disease: potential mechanisms underlying the physiological benefits. Nat. Rev. Nephrol. 19 (4), 244–256. doi:10.1038/s41581-022-00675-9
Bogataj Š., Pajek M., Pajek J., Buturović Ponikvar J., Paravlic A. (2019). Exercise-based interventions in hemodialysis patients: a systematic review with a meta-analysis of randomized controlled trials. J. Clin. Med. 9 (1), 43. doi:10.3390/jcm9010043
Botelho J., Mascarenhas P., Viana J., Proença L., Orlandi M., Leira Y., et al. (2022). An umbrella review of the evidence linking oral health and systemic noncommunicable diseases. Nat. Commun. 13 (1), 7614. doi:10.1038/s41467-022-35337-8
Burrai F., Brioni E., Iodice M., Apuzzo L., Burrai D. F. (2021). Gli effetti degli esercizi fisici sulla resistenza cardiovascolare e capacità funzionale nei pazienti in emodialisi: una revisione sistematica e meta-analisi. G. Ital. Nefrol.
Cai X., Zeng D., Deng J. (2022). A systematic review and meta-analysis of the efficacy of aerobic exercise combined with resistance training on maintenance hemodialysis patients. Ann. Palliat. Med. 11 (4), 1360–1368. doi:10.21037/apm-22-226
Cheema B. S., Chan D., Fahey P., Atlantis E. (2014). Effect of progressive resistance training on measures of skeletal muscle hypertrophy, muscular strength and health-related quality of life in patients with chronic kidney disease: a systematic review and meta-analysis. Sports Med. 44 (8), 1125–1138. doi:10.1007/s40279-014-0176-8
Chung Y. C., Yeh M. L., Liu Y. M. (2017). Effects of intradialytic exercise on the physical function, depression and quality of life for haemodialysis patients: a systematic review and meta-analysis of randomised controlled trials. J. Clin. Nurs. 26 (13-14), 1801–1813. doi:10.1111/jocn.13514
Clarkson M. J., Bennett P. N., Fraser S. F., Warmington S. A. (2019). Exercise interventions for improving objective physical function in patients with end-stage kidney disease on dialysis: a systematic review and meta-analysis. Am. J. physiology Ren. physiology 316 (5), F856–F872. doi:10.1152/ajprenal.00317.2018
Clyne N., Anding-Rost K. (2021). Exercise training in chronic kidney disease-effects, expectations and adherence. Clin. kidney J. 14 (Suppl. 2), ii3–ii14. doi:10.1093/ckj/sfab012
Corrêa H. L., Neves R. V. P., Deus L. A., Maia B. C. H., Maya A. T., Tzanno-Martins C., et al. (2021b). Low-load resistance training with blood flow restriction prevent renal function decline: the role of the redox balance, angiotensin 1-7 and vasopressin(✰,✰✰). Physiology and Behav. 230, 113295. doi:10.1016/j.physbeh.2020.113295
Corrêa H. L., Neves R. V. P., Deus L. A., Souza M. K., Haro A. S., Costa F., et al. (2021a). Blood flow restriction training blunts chronic kidney disease progression in humans. Med. Sci. sports Exerc. 53 (2), 249–257. doi:10.1249/MSS.0000000000002465
de Araújo T. B., de Luca Corrêa H., de Deus L. A., Neves R. V. P., Reis A. L., Honorato F. S., et al. (2023). The effects of home-based progressive resistance training in chronic kidney disease patients. Exp. Gerontol. 171, 112030. doi:10.1016/j.exger.2022.112030
de Boer I. H., Khunti K., Sadusky T., Tuttle K. R., Neumiller J. J., Rhee C. M., et al. (2022). Diabetes management in chronic kidney disease: a consensus report by the American diabetes association (ADA) and kidney disease: improving global outcomes (KDIGO). Diabetes care 45 (12), 3075–3090. doi:10.2337/dci22-0027
de Luca Corrêa H., Gadelha A. B., Vainshelboim B., Dutra M. T., Ferreira-Júnior J. B., Deus L. A., et al. (2023). Could sarcopenia-related mortality in end-stage renal disease be underpinned by the number of hospitalizations and cardiovascular diseases? Int. urology Nephrol. 55 (1), 157–163. doi:10.1007/s11255-022-03291-5
Deus L. A., Corrêa H. L., Neves R. V. P., Reis A. L., Honorato F. S., Araújo T. B., et al. (2022). Metabolic and hormonal responses to chronic blood-flow restricted resistance training in chronic kidney disease: a randomized trial. Appl. physiology, Nutr. metabolism = Physiologie appliquee, Nutr. metabolisme 47 (2), 183–194. doi:10.1139/apnm-2021-0409
Deus L. A., Corrêa H. L., Neves R. V. P., Reis A. L., Honorato F. S., Silva V. L., et al. (2021). Are resistance training-induced BDNF in hemodialysis patients associated with depressive symptoms, quality of life, antioxidant capacity, and muscle strength? An insight for the muscle-brain-renal Axis. Int. J. Environ. Res. public health 18 (21), 11299. doi:10.3390/ijerph182111299
Diniz T. A., de Lima Junior E. A., Teixeira A. A., Biondo L. A., da Rocha L. A. F., Valadão I. C., et al. (2021). Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-α signaling in obese mice. Life Sci. 266, 118868. doi:10.1016/j.lfs.2020.118868
Dong L., Xu W., Deng Y., Tan J., Qin W. (2022). Efficacy and safety of potassium binders in the treatment of patients with chronic kidney disease and hyperkalemia. Eur. J. Pharmacol. 931, 175174. doi:10.1016/j.ejphar.2022.175174
Dor-Haim H., Barak S., Horowitz M., Yaakobi E., Katzburg S., Swissa M., et al. (2018). Improvement in cardiac dysfunction with a novel circuit training method combining simultaneous aerobic-resistance exercises. A randomized trial. PloS one 13 (1), e0188551. doi:10.1371/journal.pone.0188551
Ebert T., Neytchev O., Witasp A., Kublickiene K., Stenvinkel P., Shiels P. G. (2021). Inflammation and oxidative stress in chronic kidney disease and dialysis patients. Antioxidants and redox Signal. 35 (17), 1426–1448. doi:10.1089/ars.2020.8184
Evans M., Lewis R. D., Morgan A. R., Whyte M. B., Hanif W., Bain S. C., et al. (2022). A narrative review of chronic kidney disease in clinical practice: current challenges and future perspectives. Adv. Ther. 39 (1), 33–43. doi:10.1007/s12325-021-01927-z
Fan Z., Chen J., Yang Q., He J. (2022). Network pharmacology and experimental validation to reveal the pharmacological mechanisms of chongcaoyishen decoction against chronic kidney disease. Front. Mol. Biosci. 9, 847812. doi:10.3389/fmolb.2022.847812
Ferrari F., Helal L., Dipp T., Soares D., Soldatelli Â., Mills A. L., et al. (2020). Intradialytic training in patients with end-stage renal disease: a systematic review and meta-analysis of randomized clinical trials assessing the effects of five different training interventions. J. Nephrol. 33 (2), 251–266. doi:10.1007/s40620-019-00687-y
Ferreira G. D., Bohlke M., Correa C. M., Dias E. C., Orcy R. B. (2019). Does intradialytic exercise improve removal of solutes by hemodialysis? A systematic review and meta-analysis. Archives Phys. Med. rehabilitation 100 (12), 2371–2380. doi:10.1016/j.apmr.2019.02.009
Ferreira T. L., Ribeiro H. S., Ribeiro A. L. A., Bonini-Rocha A. C., Lucena J. M. S., de Oliveira P. A., et al. (2021). Exercise interventions improve depression and anxiety in chronic kidney disease patients: a systematic review and meta-analysis. Int. urology Nephrol. 53 (5), 925–933. doi:10.1007/s11255-020-02612-w
Fleg J. L. (2012). Aerobic exercise in the elderly: a key to successful aging. Discov. Med. 13 (70), 223–228.
Flythe J. E., Chang T. I., Gallagher M. P., Lindley E., Madero M., Sarafidis P. A., et al. (2020). Blood pressure and volume management in dialysis: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 97 (5), 861–876. doi:10.1016/j.kint.2020.01.046
Fukada S. I., Higashimoto T., Kaneshige A. (2022). Differences in muscle satellite cell dynamics during muscle hypertrophy and regeneration. Skelet. muscle 12 (1), 17. doi:10.1186/s13395-022-00300-0
Gadelha A. B., Cesari M., Corrêa H. L., Neves R. V. P., Sousa C. V., Deus L. A., et al. (2021). Effects of pre-dialysis resistance training on sarcopenia, inflammatory profile, and anemia biomarkers in older community-dwelling patients with chronic kidney disease: a randomized controlled trial. Int. urology Nephrol. 53 (10), 2137–2147. doi:10.1007/s11255-021-02799-6
Gates M., Gates A., Pieper D., Fernandes R. M., Tricco A. C., Moher D., et al. (2022). Reporting guideline for overviews of reviews of healthcare interventions: development of the PRIOR statement. BMJ (Clin. Res. ed) 378, e070849. doi:10.1136/bmj-2022-070849
Gomes Neto M., de Lacerda F. F. R., Lopes A. A., Martinez B. P., Saquetto M. B. (2018). Intradialytic exercise training modalities on physical functioning and health-related quality of life in patients undergoing maintenance hemodialysis: systematic review and meta-analysis. Clin. Rehabil. 32 (9), 1189–1202. doi:10.1177/0269215518760380
Guo D., Tong Y., Jiang X., Meng Y., Jiang H., Du L., et al. (2022). Aerobic glycolysis promotes tumor immune evasion by hexokinase2-mediated phosphorylation of IκBα. Cell metab. 34 (9), 1312–1324.e6. doi:10.1016/j.cmet.2022.08.002
Harber V. J., Sutton J. R. (1984). Endorphins and exercise. Sports Med. (Auckl., NZ) 1 (2), 154–171. doi:10.2165/00007256-198401020-00004
Hargrove N., El Tobgy N., Zhou O., Pinder M., Plant B., Askin N., et al. (2021). Effect of aerobic exercise on dialysis-related symptoms in individuals undergoing maintenance hemodialysis: a systematic review and meta-analysis of clinical trials. Clin. J. Am. Soc. Nephrol. CJASN 16 (4), 560–574. doi:10.2215/CJN.15080920
Huang M., Lv A., Wang J., Xu N., Ma G., Zhai Z., et al. (2019). Exercise training and outcomes in hemodialysis patients: systematic review and meta-analysis. Am. J. Nephrol. 50 (4), 240–254. doi:10.1159/000502447
Ikizler T. A., Burrowes J. D., Byham-Gray L. D., Campbell K. L., Carrero J. J., Chan W., et al. (2020). KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am. J. kidney Dis. official J. Natl. Kidney Found. 76 (3 Suppl. 1), S1–s107. doi:10.1053/j.ajkd.2020.05.006
Jha V., Al-Ghamdi S. M. G., Li G., Wu M. S., Stafylas P., Retat L., et al. (2023). Global economic burden associated with chronic kidney disease: a pragmatic review of medical costs for the inside CKD research programme. Adv. Ther. 40, 4405–4420. doi:10.1007/s12325-023-02608-9
Johansen K. L., Painter P. (2012). Exercise in individuals with CKD. Am. J. kidney Dis. official J. Natl. Kidney Found. 59 (1), 126–134. doi:10.1053/j.ajkd.2011.10.008
Junqué-Jiménez A., Morera-Mas A., Pérez-Ventana-Ortiz C., Andreu-Periz L., Segura-Ortí E. (2022). Home-based exercise programs in patients with chronic kidney disease: a systematic review and META-analysis. Worldviews evidence-based Nurs. 19 (4), 322–337. doi:10.1111/wvn.12579
Kadam P., Bhalerao S. (2010). Sample size calculation. Int. J. Ayurveda Res. 1 (1), 55–57. doi:10.4103/0974-7788.59946
KDIGO 2020 (2020). KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 98 (4s), S1–s115. doi:10.1016/j.kint.2020.06.019
Ketteler M., Block G. A., Evenepoel P., Fukagawa M., Herzog C. A., McCann L., et al. (2018). Diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder: synopsis of the kidney disease: improving global outcomes 2017 clinical practice guideline update. Ann. Intern. Med. 168 (6), 422–430. doi:10.7326/M17-2640
Koch R. E., Josefson C. C., Hill G. E. (2017). Mitochondrial function, ornamentation, and immunocompetence. Biol. Rev. Camb. Philosophical Soc. 92 (3), 1459–1474. doi:10.1111/brv.12291
Kovesdy C. P. (2022). Epidemiology of chronic kidney disease: an update 2022. Kidney Int. Suppl. 12 (1), 7–11. doi:10.1016/j.kisu.2021.11.003
Lim C., Nunes E. A., Currier B. S., McLeod J. C., Thomas A. C. Q., Phillips S. M. (2022). An evidence-based narrative review of mechanisms of resistance exercise-induced human skeletal muscle hypertrophy. Med. Sci. sports Exerc. 54 (9), 1546–1559. doi:10.1249/MSS.0000000000002929
Liu H., Song Y., Zhao D., Zhan M. (2022). Effect of exercise on cognitive impairment in patients undergoing haemodialyses: a systematic review and meta-analysis of randomised controlled trials. J. Ren. care 48 (4), 243–252. doi:10.1111/jorc.12420
Liu-Ambrose T., Davis J. C., Best J. R., Dian L., Madden K., Cook W., et al. (2019). Effect of a home-based exercise program on subsequent falls among community-dwelling high-risk older adults after a fall: a randomized clinical trial. Jama 321 (21), 2092–2100. doi:10.1001/jama.2019.5795
Ma Q., Gao Y., Lu J., Liu X., Wang R., Shi Y., et al. (2022). The effect of regular aerobic exercise on renal function in patients with CKD: a systematic review and meta-analysis. Front. physiology 13, 901164. doi:10.3389/fphys.2022.901164
Matsuzawa R., Hoshi K., Yoneki K., Harada M., Watanabe T., Shimoda T., et al. (2017). Effectiveness of exercise training on exercise tolerance, physical function, and quality of life in elderly people undergoing hemodialysis: a systematic review and meta-analysis. Kidney Int. Rep.
Memme J. M., Erlich A. T., Phukan G., Hood D. A. (2021). Exercise and mitochondrial health. J. physiology 599 (3), 803–817. doi:10.1113/JP278853
Meng L., Zhang T., Ho P. (2022). Effect of exercises on the maturation of newly created arteriovenous fistulas over distal and proximal upper limb: a systematic review and meta-analysis. J. Vasc. access, 11297298221100446.
Mikkelsen K., Stojanovska L., Polenakovic M., Bosevski M., Apostolopoulos V. (2017). Exercise and mental health. Maturitas 106, 48–56. doi:10.1016/j.maturitas.2017.09.003
Milam R. H. (2016). Exercise guidelines for chronic kidney disease patients. J. Ren. Nutr. official J. Counc. Ren. Nutr. Natl. Kidney Found. 26 (4), e23–e25. doi:10.1053/j.jrn.2016.03.001
Nakamura K., Sasaki T., Yamamoto S., Hayashi H., Ako S., Tanaka Y. (2020). Effects of exercise on kidney and physical function in patients with non-dialysis chronic kidney disease: a systematic review and meta-analysis. Sci. Rep. 10 (1), 18195. doi:10.1038/s41598-020-75405-x
Nantakool S., Reanpang T., Prasannarong M., Pongtam S., Rerkasem K. (2022). Upper limb exercise for arteriovenous fistula maturation in people requiring permanent haemodialysis access. Cochrane database Syst. Rev. 10 (10), Cd013327. doi:10.1002/14651858.CD013327.pub2
Nantakool S., Rerkasem K., Reanpang T., Worraphan S., Prasannarong M. (2020). A systematic review with meta-analysis of the effects of arm exercise training programs on arteriovenous fistula maturation among people with chronic kidney disease. Hemodial. Int. Int. Symposium Home Hemodial. 24 (4), 439–453. doi:10.1111/hdi.12875
Navaneethan S. D., Zoungas S., Caramori M. L., Chan J. C. N., Heerspink H. J. L., Hurst C., et al. (2023). Diabetes management in chronic kidney disease: synopsis of the KDIGO 2022 clinical practice guideline update. Ann. Intern. Med. 176 (3), 381–387. doi:10.7326/M22-2904
Papadimitriou N., Markozannes G., Kanellopoulou A., Critselis E., Alhardan S., Karafousia V., et al. (2021). An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat. Commun. 12 (1), 4579. doi:10.1038/s41467-021-24861-8
Pei G., Tang Y., Tan L., Tan J., Ge L., Qin W. (2019). Aerobic exercise in adults with chronic kidney disease (CKD): a meta-analysis. Int. urology Nephrol. 51 (10), 1787–1795. doi:10.1007/s11255-019-02234-x
Perez-Dominguez B., Suso-Marti L., Dominguez-Navarro F., Perpiña-Martinez S., Calatayud J., Casaña J. (2023). Effects of resistance training on patients with End-Stage Renal Disease: an umbrella review with meta-analysis of the pooled findings. J. Nephrol. 36, 1805–1839. doi:10.1007/s40620-023-01635-7
Petersen A. M., Pedersen B. K. (2005). The anti-inflammatory effect of exercise. J. Appl. physiology 98 (4), 1154–1162. doi:10.1152/japplphysiol.00164.2004
Pu J., Jiang Z., Wu W., Li L., Zhang L., Li Y., et al. (2019). Efficacy and safety of intradialytic exercise in haemodialysis patients: a systematic review and meta-analysis. BMJ open 9 (1), e020633. doi:10.1136/bmjopen-2017-020633
Qiu Z., Zheng K., Zhang H., Feng J., Wang L., Zhou H. (2017). Physical exercise and patients with chronic renal failure: a meta-analysis. BioMed Res. Int. 2017, 7191826. doi:10.1155/2017/7191826
Rao M., Jaber B. L., Balakrishnan V. S. (2018). Chronic kidney disease and acquired mitochondrial myopathy. Curr. Opin. Nephrol. Hypertens. 27 (2), 113–120. doi:10.1097/MNH.0000000000000393
Rovin B. H., Adler S. G., Barratt J., Bridoux F., Burdge K. A., Chan T. M., et al. (2021). Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int. 100 (4), 753–779. doi:10.1016/j.kint.2021.05.015
Sawant A., House A. A., Overend T. J. (2014). Anabolic effect of exercise training in people with end-stage renal disease on hemodialysis: a systematic review with meta-analysis. Physiother. Can. Physiother. Can. 66 (1), 44–53. doi:10.3138/ptc.2012-59
Scapini K. B., Bohlke M., Moraes O. A., Rodrigues C. G., Inácio J. F., Sbruzzi G., et al. (2019). Combined training is the most effective training modality to improve aerobic capacity and blood pressure control in people requiring haemodialysis for end-stage renal disease: systematic review and network meta-analysis. J. Physiother. 65 (1), 4–15. doi:10.1016/j.jphys.2018.11.008
Schardong J., Stein C., Della Méa Plentz R. (2020). Neuromuscular electrical stimulation in chronic kidney failure: a systematic review and meta-analysis. Archives Phys. Med. rehabilitation 101 (4), 700–711. doi:10.1016/j.apmr.2019.11.008
Schiaffino S., Dyar K. A., Ciciliot S., Blaauw B., Sandri M. (2013). Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 280 (17), 4294–4314. doi:10.1111/febs.12253
Schoenfeld B. J. (2010). The mechanisms of muscle hypertrophy and their application to resistance training. J. strength Cond. Res. 24 (10), 2857–2872. doi:10.1519/JSC.0b013e3181e840f3
Seals D. R., Nagy E. E., Moreau K. L. (2019). Aerobic exercise training and vascular function with ageing in healthy men and women. J. physiology 597 (19), 4901–4914. doi:10.1113/JP277764
Shea B. J., Reeves B. C., Wells G., Thuku M., Hamel C., Moran J., et al. (2017). AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clin. Res. ed) 358, j4008. doi:10.1136/bmj.j4008
Sheng K., Zhang P., Chen L., Cheng J., Wu C., Chen J. (2014). Intradialytic exercise in hemodialysis patients: a systematic review and meta-analysis. Am. J. Nephrol. 40 (5), 478–490. doi:10.1159/000368722
Slee A., Reid J. (2022). Disease-related malnutrition in chronic kidney disease. Curr. Opin. Clin. Nutr. metabolic care 25 (3), 136–141. doi:10.1097/MCO.0000000000000830
Smart N., Steele M. (2011). Exercise training in haemodialysis patients: a systematic review and meta-analysis. Nephrol. Carlt. Vic. 16 (7), 626–632. doi:10.1111/j.1440-1797.2011.01471.x
Song Y., Chen L., Wang M., He Q., Xue J., Jiang H. (2022). The optimal exercise modality and intensity for hemodialysis patients incorporating Bayesian network meta-analysis and systematic review. Front. physiology 13, 945465. doi:10.3389/fphys.2022.945465
Song Y. Y., Hu R. J., Diao Y. S., Chen L., Jiang X. L. (2018). Effects of exercise training on restless legs syndrome, depression, sleep quality, and fatigue among hemodialysis patients: a systematic review and meta-analysis. J. pain symptom Manag. 55 (4), 1184–1195. doi:10.1016/j.jpainsymman.2017.12.472
Souza L. R., Vicente J. B., Melo G. R., Moraes V. C., Olher R. R., Sousa I. C., et al. (2018). Acute hypotension after moderate-intensity handgrip exercise in hypertensive elderly people. J. strength Cond. Res. 32 (10), 2971–2977. doi:10.1519/JSC.0000000000002460
Sprick J. D., Mammino K., Jeong J., DaCosta D. R., Hu Y., Morison D. G., et al. (2022). Aerobic exercise training improves endothelial function and attenuates blood pressure reactivity during maximal exercise in chronic kidney disease. J. Appl. physiology (1985) 132 (3), 785–793. doi:10.1152/japplphysiol.00808.2021
Thompson S., Wiebe N., Padwal R. S., Gyenes G., Headley S. A. E., Radhakrishnan J., et al. (2019). The effect of exercise on blood pressure in chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. PloS one 14 (2), e0211032. doi:10.1371/journal.pone.0211032
Tsai Y. C., Chiu Y. W., Tsai J. C., Kuo H. T., Hung C. C., Hwang S. J., et al. (2015). Association of fluid overload with cardiovascular morbidity and all-cause mortality in stages 4 and 5 CKD. Clin. J. Am. Soc. Nephrol. CJASN 10 (1), 39–46. doi:10.2215/CJN.03610414
Vanden Wyngaert K., Van Craenenbroeck A. H., Van Biesen W., Dhondt A., Tanghe A., Van Ginckel A., et al. (2018). The effects of aerobic exercise on eGFR, blood pressure and VO2peak in patients with chronic kidney disease stages 3-4: a systematic review and meta-analysis. PloS one 13 (9), e0203662. doi:10.1371/journal.pone.0203662
Villanego F., Naranjo J., Vigara L. A., Cazorla J. M., Montero M. E., García T., et al. (2020). Impact of physical exercise in patients with chronic kidney disease: sistematic review and meta-analysis. Nefrologia 40 (3), 237–252. doi:10.1016/j.nefro.2020.01.002
Wang H., Xie D., Wu L., Zhao L. (2022). Association of exercise with vascular function in patients with CKD: a meta-analysis of randomized controlled trials. Front. Med. 9, 904299. doi:10.3389/fmed.2022.904299
Wilund K. R., Thompson S., Viana J. L., Wang A. Y. (2021). Physical activity and health in chronic kidney disease. Contributions Nephrol. 199, 43–55. doi:10.1159/000517696
Wu L., Liu Y., Wu L., Yang J., Jiang T., Li M. (2021). Effects of exercise on markers of inflammation and indicators of nutrition in patients with chronic kidney disease: a systematic review and meta-analysis. Int. urology Nephrol. 54 (4), 815–826. doi:10.1007/s11255-021-02949-w
Wu X., Yang L., Wang Y., Wang C., Hu R., Wu Y. (2020). Effects of combined aerobic and resistance exercise on renal function in adult patients with chronic kidney disease: a systematic review and meta-analysis. Clin. Rehabil. 34 (7), 851–865. doi:10.1177/0269215520924459
Xiang H. C., Lin L. X., Hu X. F., Zhu H., Li H. P., Zhang R. Y., et al. (2019). AMPK activation attenuates inflammatory pain through inhibiting NF-κB activation and IL-1β expression. J. neuroinflammation 16 (1), 34. doi:10.1186/s12974-019-1411-x
Yang H., Wu X., Wang M. (2017). Exercise affects cardiopulmonary function in patients with chronic kidney disease: a meta-analysis. BioMed Res. Int. 2017, 6405797. doi:10.1155/2017/6405797
Yang L., Wu X., Wang Y., Wang C., Hu R., Wu Y. (2020). Effects of exercise training on proteinuria in adult patients with chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol. 21 (1), 172. doi:10.1186/s12882-020-01816-7
Young H. M. L., March D. S., Graham-Brown M. P. M., Jones A. W., Curtis F., Grantham C. S., et al. (2018). Effects of intradialytic cycling exercise on exercise capacity, quality of life, physical function and cardiovascular measures in adult haemodialysis patients: a systematic review and meta-analysis. Nephrol. Dial. Transplant. 33 (8), 1436–1445. doi:10.1093/ndt/gfy045
Zhang F., Bai Y., Zhao X., Huang L., Wang W., Zhou W., et al. (2022). Therapeutic effects of exercise interventions for patients with chronic kidney disease: an umbrella review of systematic reviews and meta-analyses. BMJ open 12 (9), e054887. doi:10.1136/bmjopen-2021-054887
Keywords: chronic kidney disease, exercise modalities, aerobic exercise, resistance training, combined training, eletromyostimulation, home-based exercise, meta-analysis
Citation: Correa HL, Rosa TS, Santos RL, Mestrinho VM, Aquino TS, Santos WO, Neves RP, Deus LA, Reis AL, Barbosa JM, Araujo TB, Verhoeff R, Yatim K, Mendes D, Manfro RC, Borges TJ and Riella LV (2025) The impact of different exercise modalities on chronic kidney disease: an umbrella review of meta-analyses. Front. Physiol. 15:1444976. doi: 10.3389/fphys.2024.1444976
Received: 06 June 2024; Accepted: 11 December 2024;
Published: 06 January 2025.
Edited by:
Alejandro Santos-Lozano, Miguel de Cervantes European University, SpainReviewed by:
Marios Papasotiriou, University of Patras, GreeceI-Shiang Tzeng, National Taipei University, Taiwan
Copyright © 2025 Correa, Rosa, Santos, Mestrinho, Aquino, Santos, Neves, Deus, Reis, Barbosa, Araujo, Verhoeff, Yatim, Mendes, Manfro, Borges and Riella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leonardo V. Riella, bHJpZWxsYUBtZ2guaGFydmFyZC5lZHU=
†These authors have contributed equally to this work and share first authorship
 Hugo L. Correa
Hugo L. Correa Thiago S. Rosa
Thiago S. Rosa Rafael L. Santos
Rafael L. Santos Vitoria M. Mestrinho4
Vitoria M. Mestrinho4 Rodrigo P. Neves
Rodrigo P. Neves Lysleine A. Deus
Lysleine A. Deus Andrea L. Reis
Andrea L. Reis Thais B. Araujo
Thais B. Araujo Daniel Mendes
Daniel Mendes Roberto C. Manfro
Roberto C. Manfro Thiago J. Borges
Thiago J. Borges Leonardo V. Riella
Leonardo V. Riella