- 1Department of Nephrology, Institute of Kidney Diseases, West China Hospital of Sichuan University, Chengdu, China
- 2Health Management Center, General Practice Medical Center, West China Hospital, Sichuan University, Chengdu, China
Peritoneal dialysis (PD) is currently one of the effective methods for treating end-stage renal disease (ESRD). However, long-term exposure to high concentration glucose in peritoneal dialysis environment could lead to peritoneal fibrosis (PF), impaired peritoneal filtration function, decreased peritoneal dialysis efficiency, and even withdrawal from peritoneal dialysis in patients. Considerable evidence suggests that peritoneal fibrosis after peritoneal dialysis is related to crucial factors such as mesothelial-to-mesenchymal transition (MMT), inflammatory response, and angiogenesis, etc. In our review, we summarize the pathophysiological mechanisms and further illustrate the future strategies against PF.
Introduction
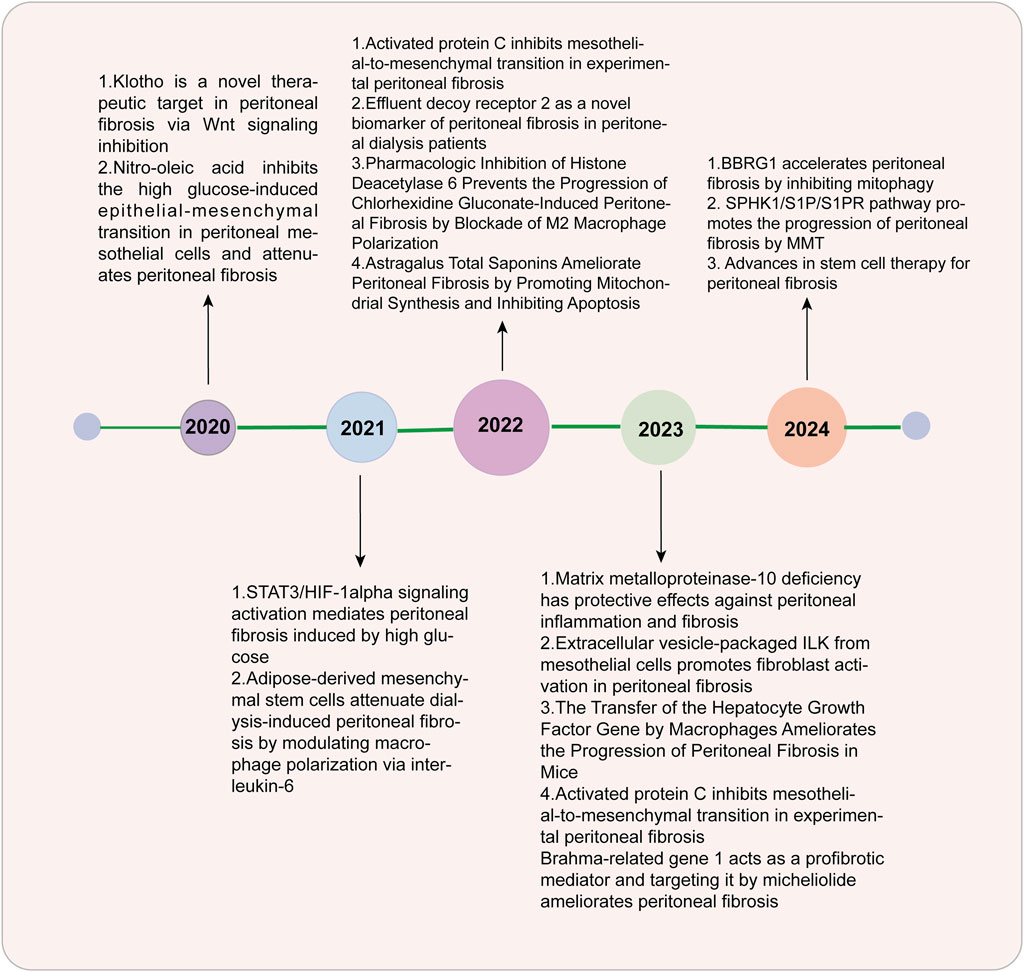
An increasing number of patients worldwide are relying on dialysis, an alternative treatment for patients with end-stage renal disease (ESRD) (Zhou et al., 2016). It is estimated that over 10% of these patients are expected to go through peritoneal dialysis (PD) (Zhou et al., 2016). Peritoneal dialysis has been widely used due to its convenience and high economic benefits, but the occurrence of fibrosis hinders its further development (Masola et al., 2022). The peritoneal membrane (PM) is semipermeable and is used for ultrafiltration and diffusion in PD patients (Masola et al., 2022). Many vital structures are involved in mesothelial monolayers and submesothelial dense areas, such as fibroblasts, macrophages, peritoneal lymphatic vessels, and peritoneal capillaries (Branco et al., 2023a). In approximately 50%–80% of patients receiving peritoneal dialysis treatment, fibrosis may be monitored in the first 1–2 years (Zhao et al., 2023a). In fibrosis development, mesothelial cells (MCs) go through the process named mesothelial-to-mesenchymal transition (MMT) and transform into fibroblasts, which can lead to peritoneal fibrosis (PF) through the excessive production of extracellular matrix (ECM) deposited mainly in submesothelial areas (Branco et al., 2023a; Zhao et al., 2023a; Krediet, 2018). Changes in the morphology and function of the peritoneum occurred during long-term peritoneal dialysis. Therefore, PF can damage the ultrafiltration function of the peritoneum, leading to the failure of filtering excess water and metabolic waste (Krediet, 2018; Branco et al., 2023b). There are three main characteristics in PF, including the thickening of the submesothelial layer, lack of MCs, and angiogenesis (Zhao et al., 2024; Fan et al., 2008; García-López et al., 2012; Krediet et al., 2000). PF is a main risk factor for PD patients who ultimately withdraw and transfer to hemodialysis (Branco et al., 2023a). The key process of PF is MMT. The function and structure of mesothelial cells are altered due to the presence of bioincompatible peritoneal dialysate, such as glucose and glycation end products (Fan et al., 2008; García-López et al., 2012), but the mechanisms underlying these processes are still largely unclear. In recent years, more research has been conducted on PF (Figure 1), and some signaling pathways related to PF have been explored and discovered. Medications targeting these mechanisms have been validated in animal models and in vitro experiments, there is a hope that they will be applied for clinical practice in future.
Pathophysiology of peritoneal fibrosis
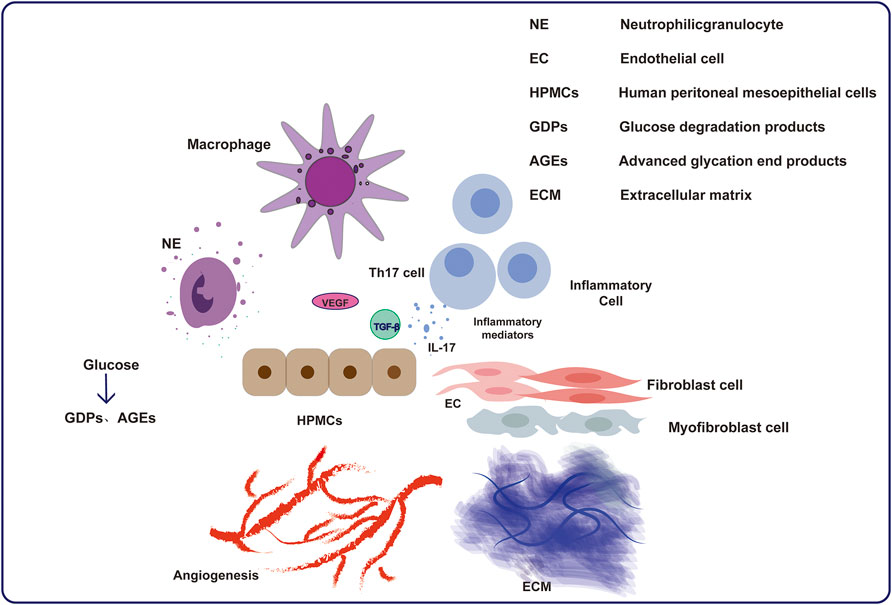
The decrease in peritoneal filtration function can be attributed to the bioincompatibility of peritoneal dialysis fluid and the accumulation of metabolic toxins in ESRD patients (Masola et al., 2022). There are three main parts during the development of the PF: MMT, angiogenesis and inflammation (Krediet et al., 2000; de Lima et al., 2013). Peritoneal inflammation is promoted by infection and the biological incompatibility of dialysates. The definition of MMT included not only the deprivation of MCs, and deposition of extracellular matrix in submesothelial zones but also the outcome of transformation from mesothelial cells into fibroblastoid cells (Yáñez-Mó et al., 2003). Structural changes in the peritoneum, including a decrease in mesothelial cells, an increase in fibers under the mesothelium, and neovascularization, are pathological results of inflammatory injury repair and reconstruction (Selgas et al., 2006). The extracellular matrix is deposited in submesothelial areas and produced by transformed MCs, leading to PF (Li et al., 2022a; Strippoli et al., 2016; López-Cabrera, 2014). Inflammation also induces neoangiogenesis, which increases the solute diffusion surface area and PF but also reduces water permeability (Aroeira et al., 2007; Aroeira et al., 2005). In PD dialysate, glucose and glucose degradation products are the predominant components responsible for changing MC function and structure. Transforming growth factor-β (TGF-β) and vascular endothelial growth factor (VEGF) are generated by MCs and immune cells (Terri et al., 2021; Aguilera et al., 2005). Furthermore, the MMT of peritoneal mesothelial cells altered solute transport and is associated with angiogenic process (Li et al., 2023a). All these factors jointly interact and lead to the progression of PF.
Angiogenesis and fibrosis, such as the inflammatory response and MMT process, seem to be closely related (Aguilera et al., 2005; Li et al., 2023a). The reduction in MCs and PF in PD patients can be attributed to the exposure to bioincompatible dialysates or peritonitis caused by various pathogenic microorganisms. The peritoneal immune response involves different cells, such as MC and macrophages, which further mobilize different inflammatory cells. During this process, MCs and those inflammatory cells can produce abundant inflammatory mediators to establish complex interactions, resulting in inflammation, further leading to changes in the structure and function of the peritoneum. Many of these inflammatory mediators play important roles in PF, possibly by stimulating fibroblast proliferation and inducing the MMT process, leading to increased ECM deposition and further increasing the severity of PF. The level of intraperitoneal interleukin-6 (IL-6) increases due to high glucose dialysates, which causes the subsequent development of PF (Yang et al., 2020). Chemokines can stimulate neutrophils from the bone marrow and promote their development. For example, chemokine ligand 5 (CCL5), which is synthesized by peritoneal fibroblasts, can attract mononuclear leukocytes for linking (Kawka et al., 2014). Inflammation is caused by MC lesions, and the aggregation of macrophages simultaneously exacerbates this process (Zhou et al., 2016). Myofibroblasts are involved in multiple pathological processes (Kawka et al., 2014). The overexpression of these cytokines stimulates related immune cells to produce inflammatory responses. Activated resident fibroblasts secrete excess extracellular matrix, which plays a crucial role in PF (Kendall and Feghali-Bostwick, 2014). Myofibroblasts are not only produced by resident fibroblasts, but also by mesothelial cells and fibrocytes (Kendall and Feghali-Bostwick, 2014).
The most similar and significant changes in the peritoneum of PD patients, which means that the development of PF is related to mesothelial cell transformation and angiogenesis of peritoneal mesothelium (Apte et al., 2019). Peritoneal inflammation causes angiogenesis of the peritoneum and long-term PF in the long run. Risk factors such as peritonitis, catheterization, uremia, advanced glycosylation end products can contribute to angiogenesis. Angiogenesis plays an important role in the progression of PF, as demonstrated by the correlation between the extent of vascularization and the area of fibrotic tissues. In addition, research has shown that interleukin-8 (IL-8), fibroblast growth factor 2 (FGF-2), and especially VEGF may lead to an increase in the number of peritoneal capillaries and may further increase vascular permeability (Simons et al., 2016), causing ultrafiltration failure. VEGF plays a dominant role in mediating the functions of ECs, such as their formation, migration, and interactions. The concentration of VEGF in PD patients’ effluent increases with PD duration. When patients switched from dialysate to glucose-free PDF, the level of VEGF decreased at the same time, indicating potential relevance (Branco et al., 2023a). As shown in Figure 2, understanding the mechanism of PF and its interaction with angiogenesis is crucial for preserving peritoneal ultrafiltration function and maintaining dialysis.
Signaling pathway of peritoneal fibrosis
The key fibrogenic factors trigger the downstream intracellular signaling pathways by interacting with their relevant receptors (Zhou et al., 2016). The main mechanism includes MMT, angiogenesis, and activation of inflammation.
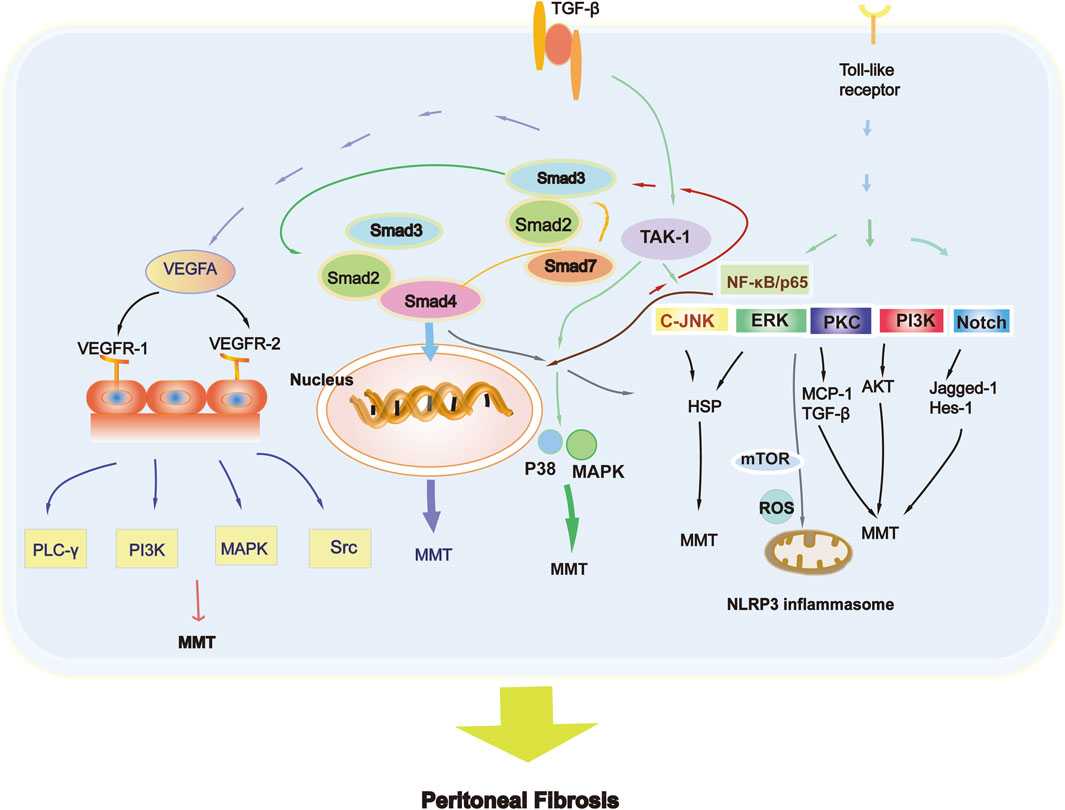
The system involved in the MMT is TGF-β, and the high production of VEGF is an obvious consequence (Shirai et al., 2022). VEGF and its corresponding receptors have recently been detailed described (Zhou et al., 2016). A high level of VEGF can cause vasodilation, accelerating solute transport and decreasing peritoneal transport, ultimately causing fibrosis (Zhou et al., 2016). Glucose degradation products (GDPs) can be produced in high-glucose dialysate (Boulanger et al., 2004). Moreover, the production of advanced glycation end products (AGEs) increased either, which may cause peritoneal inflammation and even fibrosis (Boulanger et al., 2004; Khan, 2023; Cho et al., 2014). The products can motivate peritoneal macrophages, further stimulating the synthesis of cytokines and interleukins by peritoneal mesothelial cells (Kitamura et al., 2012). Toll-like receptor ligand-mediated signaling pathways and the NOD-like receptor protein 3/IL-1β signaling pathway are the main mechanisms mediating inflammation in MMT (Zhang et al., 2017).
The TGF-β superfamily regulates cell growth and differentiation. These proteins play an important role in various physiological and pathological processes, including cell proliferation, differentiation, adhesion, migration, and regulation of immune responses. TGF-β is widely involved in the formation of fibrosis either (Zhou et al., 2016). In the development process of PF, the activation of TGF-β1 is an early marker of its pathogenesis. As the peritoneum remains in biocompatible PD fluid for a long time, glucose and glucose degradation products further promote PF (Zhang et al., 2017). The activation of TGF-β is complex, including two main ways: independent and dependent on Smads (Li et al., 2018). However, Smad2 and Smad3 have distinct effects on PF. Fibrosis and dysfunction of the peritoneum are aggravated when the Smad2 gene is knocked-out, while Smad3 gene deletion prevents PF (Patel et al., 2010), which is a critical blocking target. These findings suggest that Smad3 can exacerbate PF, while Smad2 has a protective effect (Duan et al., 2014; Sun et al., 2023). Many studies also have validated that the overexpression of Smad7 can prevent and reverse fibrosis (Nie et al., 2007). Peritoneal angiogenesis can be alleviated by the Smad7 gene, which reduces capillary vessel density and inhibits the production of VEGF (Zhou et al., 2016), decreasing the activation of p38 and nuclear factor-κB (NF-κB), indicating its powerful role in inhibiting neovascularization in the PF (Silva et al., 2019).
In some situations, the entire mechanism of PF cannot be explained. The most famous Smad-independent signaling pathways related to PF have also been widely studied (Lupinacci et al., 2019). Results found that TGF-β1 causes peritoneal injury not only through Smad but also through Smad-independent pathways. Numerous studies have also illustrated the link between multiple signaling pathways in peritoneal MCs and animal models, such as the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), c-Jun N-terminal kinase (JNK), and TGF-β/Smad3 pathways (Liu et al., 2012). TGF-β1 activates kinase 1 (TAK1) induced by TGF- β1 regulates the transcription of target genes and plays a vital role in TGF-β1 mediated peritoneal fibrosis, by activating JNK and p38 MAPK in the Smad independent signaling pathway (Zhao et al., 2019). The Smad and Smad-independent pathways are worthy of further exploration because they are both critical for MMT development (Mo et al., 2023). Notch and heat shock proteins also act as fibrogenic or antifibrogenic factors that participate in the process of PF. In mouse models of PF, the Notch signaling pathway is highly activated (Zhu et al., 2010), increasing the expression levels of Jagged-1 and Enhancer of split homolog-1 (HES-1). Heat shock protein 70 can protect rat peritoneal MCs from high sugar PD dialysate-induced PF through the extracellular signal-regulated kinase (ERK) and TGF-β/Smad pathways (Yang et al., 2015).
Angiogenesis is characterized in PD patients receiving long-term treatment, and its degree is also related to PF. VEGF is a large gene family with significant structural and functional similarities, including not only VEGFA, but also VEGFB, VEGFC, and VEGFD (Shibuya, 2011). VEGF plays a major role in the angiogenesis of the peritoneum (Shibuya, 2011; Zhu et al., 2021). VEGF production is correlated with bioincompatible PD dialysate, growth factors, and inflammatory cytokines (Shibuya, 2011).
The VEGFR-1 is associated with the production of vascular endothelial growth factors, while VEGFR-2 mediates the proliferation, migration and angiogenesis of ECs (Yang et al., 2015). VEGFA triggers the process of phosphorylation of Phospholipase C gamma (PLC-γ), PI3K, mitogen-activated protein kinase (MAPK), and the Src Kinase family when binding to VEGFR-2. VEGFC and VEGFD regulate angiogenesis mostly in lymphatic ECs by binding to VEGFR-3 (Simons et al., 2016). Based on these results, the Figure 3 depicts the main MMT signaling pathway involved in PF.
Therapeutic strategies against peritoneal fibrosis
Based on the MMT mechanism
TGF-β1 is well known for inducing the process of MMT and PF, and thrombospondin-1 (TSP-1) can activate TGF-β1 in vivo experiments and induce MMT via the TGF-β1/Smad3 signaling pathway (Shi et al., 2020; Jiang et al., 2020; Heo et al., 2021). Therefore, blocking the MMT process will become an effective method to inhibit PF.
A recent study revealed that Canagliflozin could significantly ameliorate the hypoxia in human peritoneal mesothelial cells (HPMCs) (Wang et al., 2023), decrease hypoxia-inducible factor 1 alpha (HIF-1α) abundance, and ameliorate PF. These results could provide a new direction for clinical application. Empagliflozin has a clear effect on PD-induced fibrosis by inhibiting the TGF-β/Smad signaling pathway. Applying Empagliflozin treatment or downregulation of SGLT-2 expression greatly improved hypoxia-related pathological alterations of peritoneum (Shentu et al., 2021; Shi et al., 2022a). Endoglin suppresses PF by modulating the activation of the TGF-β/ALK/Smad signaling pathway (Huang et al., 2022). Inhibiting endoglin can improve PF, which will provide a new potential therapeutic for PF. Elevated dipeptidyl peptidase IV inhibitors (DPP4) activity is significantly associated with peritoneal dysfunction, and inhibiting DPP4 can protect PD patients from the PD failure (Li et al., 2021). A reduction in Nestin reportedly helps to relieve HIF1-α-induced PF, which indicates a novel mechanism of PF (Shentu et al., 2020).
Under high-glucose dialysate conditions, the expression of glucose transporter protein in the peritoneum increases, all of which are inhibited by Canagliflozin (Wang et al., 2023), a sodium-glucose cotransporter type 2 (SGLT2) inhibitor. Glucose absorption causes pseudohypoxia, and then myofibroblasts are induced by intracellular hypoxia, which leads to the upregulated expression of the glucose transporter 1 (GLUT-1), further increasing the production of profibrotic and angiogenetic factors (Krediet, 2021). The level of GLUT-1 secreted by myofibroblasts leads to a reduction in the osmotic gradient for ultrafiltration, ultimately leading to decreased peritoneal filtration function (Krediet, 2021; Feng et al., 2022).
Research has revealed a novel mechanism by which STAT3/HIF-1α signal transduction is associated with PF (Song et al., 2024). They demonstrated for the first time that inhibiting the transmission of STAT3 weakened high glucose mediated MMT and PF (Yang et al., 2021a). Peritoneal dialysis (PD) remains limited due to the destruction of ultrafiltration barriers caused by PF. In addition to traditional signaling pathways, there is another pathway involved. According to reports, an estrogen receptor 1 (ESR1) inhibitor, tamoxifen (TAM), affects fibrosis by improving the MMT of HPMC and increasing ultrafiltration rate (Zhao et al., 2023b). ESR1 significantly increases after long-term exposure to PD dialysate, TAM can reduce H19 levels by decreasing ESR1 transcription of H19 and suppressing the VEGFA (Zhao et al., 2023b). Thus, targeting ESR1/H19/VEGFA pathway indicating its prospective application in improving MMT.
Research has shown that overexpressed microRNA-26a and microRNA-200a could alleviate PF, while the decreased expression of microRNA-21a can reduce fibrosis (Si et al., 2019). These results showed that miRNAs could be potential treatment innovations (Wu et al., 2022). Activated protein C can inhibit PF, decreases the level of inflammatory mediators, reduces collagen deposition, and inhibits the process of MMT transition via the TGF-β1 and Smad3 pathways (Giri et al., 2023). Another research found that Peptide Hormones ELA and Apelin (APLN) have potential therapeutic effects on PF by suppressing MMT process (Xie et al., 2022).
Based on angiogenesis
Angiogenesis is also an important part of the development of PF related to peritoneal dialysis (Tawada et al., 2021). In peritoneal mesothelial cells, H19 transcribed by ESR1 binds to the transcription cofactor p300, further activating the VEGFA (Zhao et al., 2023b). Targeting the ESR1/H19/VEGFA pathway provides new treatment directions for long-term PD patients (Zhao et al., 2023b). Studies showed a significant increase in the expression of the enhancer of zest homolog 2 (EZH2) in the peritoneum, which was associated with high expression of vascular markers, suggesting a link with peritoneal angiogenesis. In vitro studies have demonstrated that inhibition of EZH2 by 3-DZNeP or EZH2 siRNA prevents peritoneal angiogenesis via two pathways (Shi et al., 2022b): the Wnt1/β-catenin pathway and the IL-6/STAT3 pathway. Furthermore, VEGFR2/ERK1/2/HIF-1α Axis participates in in vitro angiogenesis, and decreased expression of EZH2 can inhibit the activation of the angiogenesis pathway.
The mesothelial cell protein αB-crystallin, which is related to angiogenesis and fibrosis, was identified. Lithium chloride (LiCl) interacts with it, which means that it can serve as a cell protective PDF supplement and may provide a translatable therapeutic strategy to inhibit PF (Herzog et al., 2021).
Based on inflammation
Silymarin (SM) is a polyphenolic flavonoid, that is isolated from the milk thistle (Bai et al., 2023). It has a diverse pharmacological effects, such as antioxidant, anti-inflammatory, antiviral, and antifibrotic effects (Bai et al., 2023). SM can mitigate peritoneal dysfunction, and reduce the expression of fibrotic factors. The expression level of Smad7 increased, while the expression levels of TGF-β1, p-Smad2 and p-Smad3 decreased. SM may be an efficient and novel therapy for preventing the development of PF. Research also finds that selective inhibitor of type 5 of the PDE enzyme and 5-HT2B receptor may have therapeutic potential in suppressing PF by reducing inflammatory mediators (Chaturvedi et al., 2024).
Cell motility protein 1 (ELMO1) is a regulatory factor activated by Rac that regulates neutrophil chemotaxis, which suggests that the inhibition of ELMO1 could be a may be an effective target for treating peritoneal inflammation and fibrosis (Yu et al., 2023a). It has been found that PF can be alleviated by molecular hydrogen, which is conducive to eliminating intracellular ROS and inhibiting the PTEN/AKT/mTOR signaling pathway (Lu et al., 2020). Molecule hydrogen may be a may be a safe and effective curative option for PF.
Research has shown that histone deacetylase 6 (HDAC6) is closely related to PF induced by high glucose peritoneal dialysate (Shi et al., 2022c), (Shi et al., 2021a). Tubstatin A (TA) can significantly inhibit the development of PF by inhibiting HDAC6, so HDAC6 may be an innovative target for treating PF (Shi et al., 2022c; Shi et al., 2021a). Blocking HDAC6 can selectively inhibit the polarization of M2 macrophages through several key signaling pathways (Margetts, 2023). Additionally, MMPs (matrix metalloproteinase) have been reported in the context of peritoneal injury, and MMP-10 is associated with PF. Research has shown that the expression of MMP-10 is significantly increased in a mouse model of PF (Margetts, 2023; Ishimura et al., 2023). The inflammatory responses induced by the inhibition of HDAC6 significantly decreased the expression of MMP-2 and MMP-9 so it could be a potential treatment target for PF (Bontempi et al., 2022). These results indicate that histone deacetylase (HDAC) drug inhibitors may be a promising agent for treating fibrotic diseases and cancer. HDAC1-3 inhibitors induce the expression of TGFBRI mRNA-targeting miRNAs (Bontempi et al., 2022). The underlying mechanism may be summarized into HDAC1-WT1-miR-769-5p, and miR-769-5p silencing further increased the level of mesenchymal gene expression (Bontempi et al., 2022). Because HDAC1 inhibition relieves fibrosis, it may have a potential therapeutic effect aimed at PF. Results showed that miR-122-5p overexpression can cause PF by acting upon Smad5 via the Wnt/β-catenin/pathway (Liu et al., 2022a).
E-type prostaglandin receptor 4 (EP4) is significantly overexpressed in the PD patients, and researches have suggested that EP4 antagonists can alleviate the progression of PF (Luo et al., 2022). In addition, ONO-AE3-208, an EP4 receptor antagonist suppressed PF by weakening the NLR family pyrin domain containing 3 (NLRP3) inflammasome and increasing the phosphorylation of NF-κB (p-p65) (Babaev et al., 2008). Parthenolide (PTL) is an accepted inhibitor extracted from Tanacetum balsamita that can be inhibited by the NF- κB/TGF-β/Smad signaling axis, inhibits inflammation and reduces PF (Zhang et al., 2022). Chronic inflammation including peritonitis, can lead to PF, so inhibiting the activation of inflammasomes can become a therapeutic target (Zhao et al., 2023c; Arangia et al., 2023; Kadoya et al., 2023). A study has also found that a new type of antiplatelet drug has the effect of improving the inflammatory environment of the peritoneum and can alleviate PF (Liu et al., 2023).
Fatty acid oxidation (FAO) also plays a part in peritoneal fibrogenesis. Treatment of PD mice with the carnitine palmitoyltransferase 1A (CPT1A) activator C75 induces therapeutic benefits, while inhibition of FAO can lead to more severe fibrosis in PD mice (Su et al., 2023). These results demonstrated a latent therapeutic effect of inhibiting FAO. Apolipoprotein A-I (apoA-I) is the principal component of high-density lipoprotein (HDL) and has anti-inflammatory and antioxidant properties. ApoA-I and its peptide mimetics can regulate oxidative stress and the inflammatory response, reducing PF caused by peritoneal dialysis (Lu et al., 2023). Furthermore, study has also found that peritoneal dialysis increases lipid deposition in HPMC, while angiotensin II type 1 receptor (AT2) improves lipid metabolism and reduces PF by inhibiting oxidized-LDL receptor-1 (LOX-1) (Liu et al., 2022b).
HG stimulation leads to further renin-angiotensin system (RAS) activation, ultimately leading to PF. Researchers have shown that RAS-mediated ECM production is associated with lipid accumulation in HPMCs and plays a role in the low-density lipoprotein receptor (LDLr) pathway (Liu et al., 2021a). New finding suggests that the activation of free fatty acid receptor 4 could alleviate PF, which focuses on the MMT process (Zhang et al., 2024).
Lactobacillus casei Zhang (LCZ) has beneficial effects such as anti-inflammatory and antioxidative effects. One study revealed that it can modulate the gut microbiota, and ameliorate PF through the butyrate/PPAR-γ/NF-κB pathway, which is beneficial for preventing PD-induced PF (Wu et al., 2023).
Th17-mediated inflammation is a key element in PF. The underlying mechanism is the development of fibrosis accompanied by a slight decrease in regulatory T cells (Tregs), a kind of anti-inflammatory T cell (Raby et al., 2018). These T cells can regulate the number of inflammatory Th17 cells, which are found be involved in the development of PF. The data showed that their balance is regulated by the leukocyte antigen CD69 (Liappas et al., 2016).
Researchers have discovered Salvia miltiorrhiza and its active ingredients salvianolic acid A (Sal A) can reduce oxidative damage, alleviate peritoneal tissue inflammation and neovascularization by activating Nuclear Respiratory Factor 2 (NRF2) (Zhou et al., 2022). Many Chinese herbal ingredients have strong anti-inflammatory and antioxidant properties, which can be explored for application in PF.
Based on apoptosis
The intrinsic antifibrotic mechanism has rarely been explored. JNK-related leucine zipper protein (JLP) has recently been found to have an antagonistic effect on TGF-β induced fibrosis process (Tian et al., 2022). JLP deficiency exacerbates PF in mice models. Knocking down JLP leads to an increased profibrotic response of human peritoneal mesothelial cell line (HMrSV5) cells to high-glucose peritoneal dialysis solution (HGPDS) stimulation, which is associated with epithelial mesenchymal transition, increased autophagy, cell apoptosis, and enhanced TGF-β1/Smad signal activation. These findings provide a new direction for novel therapeutics for PF. As mentioned earlier, autophagy may participate in the pathological mechanism of PF (Shi et al., 2021b). A recent study suggests that inhibiting the mTOR signaling pathway can activate autophagy during PD and inhibit PF (Jia et al., 2022). These results indicate that autophagy may be a potential method for preventing and treating PF.
Astragalus as a traditional Chinese medicine, which has been found to have significant anti-fibrotic effects and can be used for PF (Gong et al., 2022). One study suggested that ATS treatment reduces the thickness of peritoneal tissue in PF mouse models and increases the survival ability of peritoneal mesothelial cells (PMCs) (Li et al., 2022b). Therefore, it can inhibit PF through Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) mediated cell apoptosis and is an effective therapeutic agent (Ruan et al., 2024). Mesothelial cell pyroptosis stimulates downstream inflammatory responses via caspase-3 and Gasdermin E (GSDME), to activate macrophages additionally (Ruan et al., 2024). GSDME deficient mice are immune to PD induced PF and ultrafiltration failure (Ruan et al., 2024). Therefore, melatonin can alleviate mesothelial cell pyroptosis and reduce PF.
Other novel therapeutic strategies
Stem cells
Research has shown that adipose derived mesenchymal stem cells (ADSCs) have immunomodulatory and antifibrotic effects on PF (Yang et al., 2021b). In vitro experiments have also shown that mesenchymal stem cells have a positive effect on improving PF and can serve as one of the targets (Yu et al., 2023b; Nagasaki et al., 2021). In PD-related PF, mesenchymal stem cells are in an inflammatory filled state, such as TGF-β1, to polarize macrophages into M2 phenotype by secreting IL-6 (Shao et al., 2023; Zhou et al., 2023).
Gene therapy
Brahma related gene 1 (BRG1) is a key factor in organ fibrosis, and micheliolide (MCL) has been found the ability to inhibit PF in mice (Li et al., 2023b). A recent study revealed that BRG1 may be a mediator of PF and MCL targeting the asparagine (N1540) residue of BRG1 may be a new therapeutic strategy for PF (Li et al., 2023b).
Hepatocyte growth factor (HGF) is a classical gene that plays a part in antifibrotic role (Obata et al., 2023). Research has shown that sonoporation-based hHGF transfection plays a significant role in early PF (Nishimura et al., 2021). Moreover, the transplantation of HGF-M can inhibit the development of PF and may have a potential effect on alleviating PF (Yoshimine et al., 2021).
MicroRNAs (miRNAs)
MiRNAs have been shown to be associated with various diseases and have the potential to serve as disease biomarkers and therapeutic targets (Brown and Naldini, 2009). Restoration of miR-15a-5p restrained the inflammation and fibrosis of HPMCs, and the miR-15a-5p/VEGFA pathway may be potential targets for preventing PF (Shang et al., 2019). MiR-199a-5p and miR-214-3p play important role in PF by targeting claudin-2 and E-cadherin. Overexpression of miR-30a can reduce the increase of Snai1 induced by TGF-β1 and inhibit the occurrence of MMT (Che et al., 2017). miR-129-5p pathway has significant roles in EMT via targeting SIP-1 and SOX4 by inhibiting EMT process (Xiao et al., 2015). miR-30b, miR-145 and miR-200 family are all involved in the occurrence of MMT (Liu et al., 2014; Wu et al., 2019; Guo et al., 2018; Chu et al., 2019). These findings illustrated that numerous miRNAs are involved in PF that they may be served as novel therapeutic targets for PF.
Remaining drugs and therapeutic mechanisms
Nintedanib, a multiple tyrosine kinase inhibitor, can inhibit MMT and attenuate PF (Liu et al., 2021b). It also has an effect on reducing inflammation and angiogenesis. It has therapeutic ability in the prevention and treatment of PF (Cui et al., 2022).
Saikosaponin D (SSD), a monomeric substance extracted from the Bupleurum chinense, has been discovered to slow down PF and have anti-inflammatory and anti-fibrotic effects (Ruiqi et al., 2021). The silent information regulator sirtuin 1 (SIRT1) ameliorated PF via TGF-β signaling by inhibiting the expression of protein matrix in both in vivo and in vitro experiments (Guo et al., 2021).
Peritoneal EVs regulate the mutation of mesothelial cells and fibroblasts in PF (Szebeni et al., 2024). EVs produced by mesothelial cells are rich in integrin-linked kinase (ILK), which can activate fibroblasts via the p38-mitogen-activated protein kinase (MAPK) signaling pathway (Huang et al., 2023a). Extracellular vesicles also have an impact on the process of PF by acting on TGF-β (Szebeni et al., 2024; Huang et al., 2023a). In the future, targeting EVs or ILK may provide new therapeutic directions for PF.
1,25-dihydroxyvitamin D3 [1,25- (OH) 2D3], also known as active vitamin D3, is involved in various physiological metabolic process in the body (Da et al., 2020). In vitro and in vivo experiments revealed that the progression of PF can be delayed by regulating the expression levels of heat shock protein 47 (HSP47) and connective tissue growth factor (CTGF) (Da et al., 2020). Therefore, these results demonstrated that blockade of 1,25-(OH)2D3 can ameliorate peritoneal thickness and has a good effect on PF.
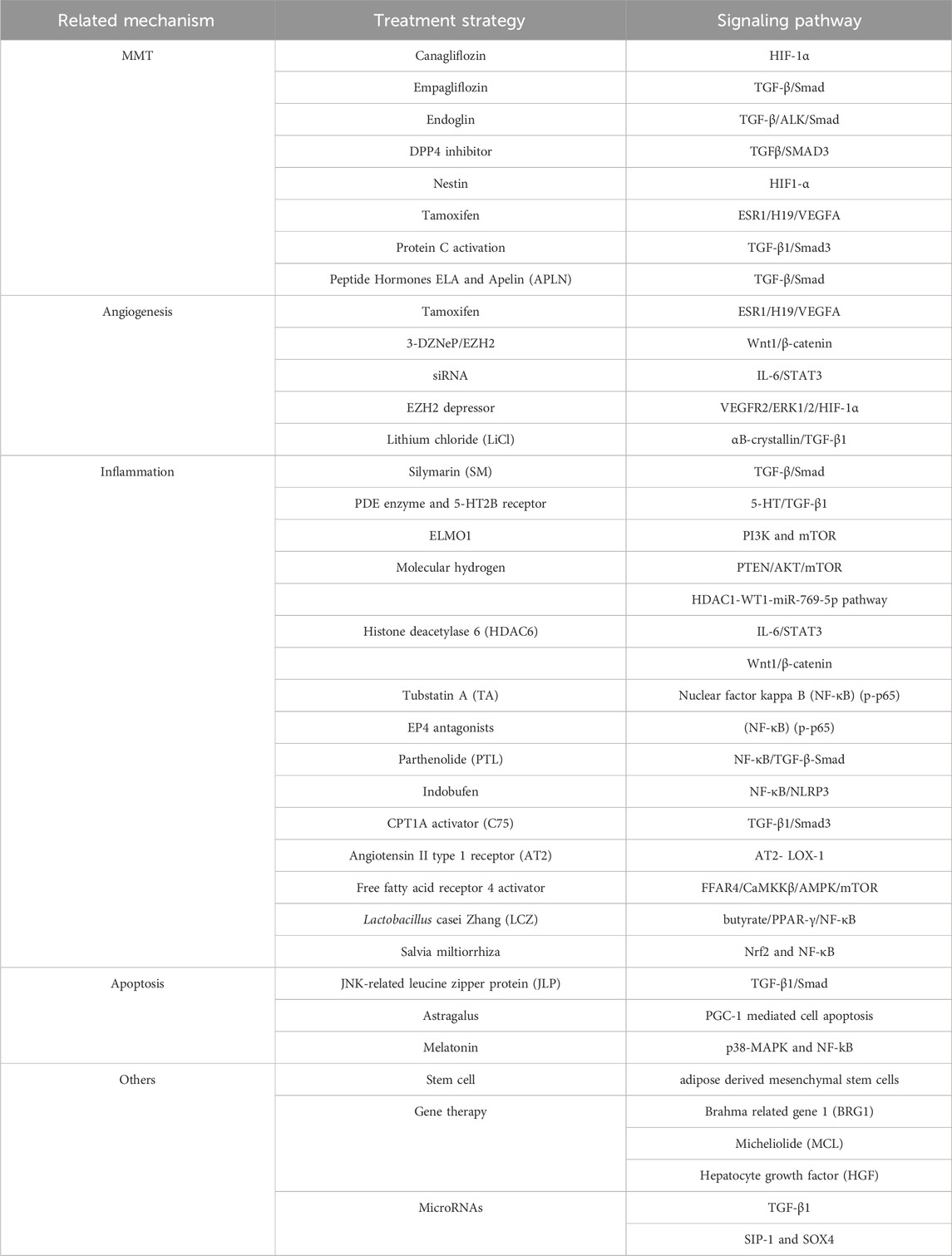
Simultaneously changing the composition of dialysis fluid may ameliorate PF, and lactate-based peritoneal dialysate is implicated in the development of peritoneal structural and functional changes (Masola et al., 2021). The study confirmed that steviol glycosides (SG) exhibit better biocompatibility as a penetrating agent than glucose (Kopytina et al., 2022). Therefore, changing the gradient of the dialysate, and using more biocompatible osmotic agents will be the direction for preventing PF (Pap et al., 2023). Biocompatible low glucose degradation products have shown superiority compared to traditional PD dialysate (Elphick et al., 2018; Moinuddin et al., 2024). Table 1 roughly summarizes the treatment strategies for improving peritoneal fibrosis based on different mechanisms in current research.
Conclusion and future exploration
Fibrosis is presented as increased fiber tissue and reduced mesothelial cells, which can occur in any tissue or organ and can lead to organ destruction and malfunction (Lai et al., 2024). PF has long been the main complication in PD patients, leading to the failure of peritoneal dialysis and increasing the burden of medical expenses. Just as renal fibrosis involves multiple mechanisms (Huang et al., 2023b), there is an urgent need to discover more biomarkers for early identification and intervention in PF. Peritoneal inflammation, endothelial mesothelium transformation, and angiogenesis are the three main mechanisms of PF. Extensive researches have been conducted on the mechanisms of MMT, inflammation, angiogenesis and apoptosis, which are closely associated with the process of PF. Numerous studies have also illustrated the new development of PF in MicroRNAs, gene therapy and stem cells. Many targeted drugs and new treatment methods have been found to produce marked effects via different mechanisms (Hu et al., 2023). Some medications have also been applied in clinical practice. In addition, upgrading the component of peritoneal dialysis fluid will also be a promising option for improving PF. Although further trials are needed, the current results illustrate that there will be additional novel approaches for the treatment of PF, that can maintain peritoneal function and improve the current status of peritoneal dialysis treatment.
Author contributions
YK: Conceptualization, Data curation, Methodology, Writing–original draft, Writing–review and editing. YL: Data curation, Writing–review and editing. PF: Methodology, Supervision, Writing–review and editing. LM: Conceptualization, Project administration, Resources, Supervision, Writing–review and editing, Methodology.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by a grant from the National Natural Science Foundation of China (82370737) and 1.3.5 project for disciplines of excellence from West China Hospital of Sichuan University (ZYGD23015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aguilera A., Yáñez-Mo M., Selgas R., Sánchez-Madrid F., López-Cabrera M. (2005). Epithelial to mesenchymal transition as a triggering factor of peritoneal membrane fibrosis and angiogenesis in peritoneal dialysis patients. Curr. Opin. Investig. Drugs 6 (3), 262–268.
Apte R. S., Chen D. S., Ferrara N. (2019). VEGF in signaling and disease: beyond discovery and development. Cell. 176 (6), 1248–1264. doi:10.1016/j.cell.2019.01.021
Arangia A., Marino Y., Fusco R., Siracusa R., Cordaro M., D'Amico R., et al. (2023). Fisetin, a natural polyphenol, ameliorates endometriosis modulating mast cells derived NLRP-3 inflammasome pathway and oxidative stress. Int. J. Mol. Sci. 24 (6), 5076. doi:10.3390/ijms24065076
Aroeira L. S., Aguilera A., Sánchez-Tomero J. A., Bajo M. A., del Peso G., Jiménez-Heffernan J. A., et al. (2007). Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J. Am. Soc. Nephrol. 18 (7), 2004–2013. doi:10.1681/ASN.2006111292
Aroeira L. S., Aguilera A., Selgas R., Ramírez-Huesca M., Pérez-Lozano M. L., Cirugeda A., et al. (2005). Mesenchymal conversion of mesothelial cells as a mechanism responsible for high solute transport rate in peritoneal dialysis: role of vascular endothelial growth factor. Am. J. kidney Dis. 46 (5), 938–948. doi:10.1053/j.ajkd.2005.08.011
Babaev V. R., Chew J. D., Ding L., Davis S., Breyer M. D., Breyer R. M., et al. (2008). Macrophage EP4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell. metab. 8 (6), 492–501. doi:10.1016/j.cmet.2008.09.005
Bai Y., Wang L., Wang L., Ge W. (2023). Silymarin ameliorates peritoneal fibrosis by inhibiting the TGF-β/Smad signaling pathway. Naunyn-Schmiedeberg's archives Pharmacol. 396 (10), 2379–2391. doi:10.1007/s00210-023-02450-4
Bontempi G., Terri M., Garbo S., Montaldo C., Mariotti D., Bordoni V., et al. (2022). Restoration of WT1/miR-769-5p axis by HDAC1 inhibition promotes MMT reversal in mesenchymal-like mesothelial cells. Cell. death Dis. 13 (11), 965. doi:10.1038/s41419-022-05398-0
Boulanger E., Wautier M. P., Gane P., Mariette C., Devuyst O., Wautier J. L. (2004). The triggering of human peritoneal mesothelial cell apoptosis and oncosis by glucose and glycoxydation products. Nephrol. dialysis, Transplant 19 (9), 2208–2216. doi:10.1093/ndt/gfh277
Branco P., Calça R., Martins A. R., Mateus C., Jervis M. J., Gomes D. P., et al. (2023a). Fibrosis of peritoneal membrane, molecular indicators of aging and frailty unveil vulnerable patients in long-term peritoneal dialysis. Int. J. Mol. Sci. 24 (5), 5020. doi:10.3390/ijms24055020
Branco P., Martins A. R., Calça R., Mateus C., Jervis M. J., Rodrigues A., et al. (2023b). Alpha-klotho and peritoneal membrane status: a hypothesis generating study. Eur. J. Clin. investigation 53 (3), e13903. doi:10.1111/eci.13903
Brown B. D., Naldini L. (2009). Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat. Rev. Genet. 10 (8), 578–585. doi:10.1038/nrg2628
Chaturvedi S., Singh H., Agarwal V., Jaiswal A., Prasad N. (2024). Unravelling the role of Sildenafil and SB204741 in suppressing fibrotic potential of peritoneal fibroblasts obtained from PD patients. Front. Pharmacol. 14, 1279330. doi:10.3389/fphar.2023.1279330
Che M., Shi T., Feng S., Li H., Zhang X., Feng N., et al. (2017). The MicroRNA-199a/214 cluster targets E-cadherin and claudin-2 and promotes high glucose-induced peritoneal fibrosis. J. Am. Soc. Nephrol. 28 (8), 2459–2471. doi:10.1681/ASN.2016060663
Cho Y., Johnson D. W., Craig J. C., Strippoli G. F., Badve S. V., Wiggins K. J. (2014). Biocompatible dialysis fluids for peritoneal dialysis. Cochrane database Syst. Rev. (3), CD007554. doi:10.1002/14651858.CD007554.pub2
Chu J. Y. S., Chau M. K. M., Chan C. C. Y., Tai A. C. P., Cheung K. F., Chan T. M., et al. (2019). miR-200c prevents TGF-β1-induced epithelial-to-mesenchymal transition and fibrogenesis in mesothelial cells by targeting ZEB2 and Notch1. Mol. Ther. Nucleic acids 17, 78–91. doi:10.1016/j.omtn.2019.05.008
Cui B., Yu C., Zhang S., Hou X., Wang Y., Wang J., et al. (2022). Delayed administration of nintedanib ameliorates fibrosis progression in CG-induced peritoneal fibrosis mouse model. Kidney Dis. 8 (4), 319–333. doi:10.1159/000523852
Da J., Yang Y., Dong R., Shen Y., Zha Y. (2020). Therapeutic effect of 1,25(OH)2-VitaminD3 on fibrosis and angiogenesis of peritoneum induced by chlorhexidine. Biomed. Pharmacother. 129, 110431. doi:10.1016/j.biopha.2020.110431
de Lima S. M., Otoni A., Sabino A. deP., Dusse L. M., Gomes K. B., Pinto S. W., et al. (2013). Inflammation, neoangiogenesis and fibrosis in peritoneal dialysis. Clin. Chim. acta 421, 46–50. doi:10.1016/j.cca.2013.02.027
Duan W. J., Yu X., Huang X. R., Yu J. W., Lan H. Y. (2014). Opposing roles for Smad2 and Smad3 in peritoneal fibrosis in vivo and in vitro. Am. J. pathology 184 (8), 2275–2284. doi:10.1016/j.ajpath.2014.04.014
Elphick E. H., Teece L., Chess J. A., Do J. Y., Kim Y. L., Lee H. B., et al. (2018). Biocompatible solutions and long-term changes in peritoneal solute transport. Clin. J. Am. Soc. Nephrol. 13 (10), 1526–1533. doi:10.2215/CJN.02380218
Fan S. L., Pile T., Punzalan S., Raftery M. J., Yaqoob M. M. (2008). Randomized controlled study of biocompatible peritoneal dialysis solutions: effect on residual renal function. Kidney Int. 73 (2), 200–206. doi:10.1038/sj.ki.5002574
Feng J., Lu M., Li W., Li J., Meng P., Li Z., et al. (2022). PPARγ alleviates peritoneal fibrosis progression along with promoting GLUT1 expression and suppressing peritoneal mesothelial cell proliferation. Mol. Cell. Biochem. 477 (7), 1959–1971. doi:10.1007/s11010-022-04419-y
García-López E., Lindholm B., Davies S. (2012). An update on peritoneal dialysis solutions. Nat. Rev. Nephrol. 8 (4), 224–233. doi:10.1038/nrneph.2012.13
Giri H., Biswas I., Rezaie A. R. (2023). Activated protein C inhibits mesothelial-to-mesenchymal transition in experimental peritoneal fibrosis. J. thrombosis haemostasis 21 (1), 133–144. doi:10.1016/j.jtha.2022.10.012
Gong F., Qu R., Li Y., Lv Y., Dai J. (2022). Astragalus Mongholicus: a review of its anti-fibrosis properties. Front. Pharmacol. 13, 976561. doi:10.3389/fphar.2022.976561
Guo R., Hao G., Bao Y., Xiao J., Zhan X., Shi X., et al. (2018). MiR-200a negatively regulates TGF-β1-induced epithelial-mesenchymal transition of peritoneal mesothelial cells by targeting ZEB1/2 expression. Am. J. physiology. Ren. physiology 314 (6), F1087-F1095–F1095. doi:10.1152/ajprenal.00566.2016
Guo Y., Wang L., Gou R., Wang Y., Shi X., Zhang Y., et al. (2021). Ameliorative role of SIRT1 in peritoneal fibrosis: an in vivo and in vitro study. Cell. Biosci. 11 (1), 79. doi:10.1186/s13578-021-00591-8
Heo J. Y., Do J. Y., Lho Y., Kim A. Y., Kim S. W., Kang S. H. (2021). TGF-β1 receptor inhibitor SB525334 attenuates the epithelial to mesenchymal transition of peritoneal mesothelial cells via the TGF-β1 signaling pathway. Biomedicines 9 (7), 839. doi:10.3390/biomedicines9070839
Herzog R., Sacnun J. M., González-Mateo G., Bartosova M., Bialas K., Wagner A., et al. (2021). Lithium preserves peritoneal membrane integrity by suppressing mesothelial cell αB-crystallin. Sci. Transl. Med. 13 (608), eaaz9705. doi:10.1126/scitranslmed.aaz9705
Hu W., Li G., Dong W., He P., Liu W., Wu Y., et al. (2023). Single-cell sequencing reveals peritoneal environment and insights into fibrosis in CAPD patients. iScience 26 (4), 106336. doi:10.1016/j.isci.2023.106336
Huang Q., Sun Y., Peng L., Sun J., Sha Z., Lin H., et al. (2023a). Extracellular vesicle-packaged ILK from mesothelial cells promotes fibroblast activation in peritoneal fibrosis. J. Extracell. vesicles 12 (7), e12334. doi:10.1002/jev2.12334
Huang Q., Xiao R., Lu J., Zhang Y., Xu L., Gao J., et al. (2022). Endoglin aggravates peritoneal fibrosis by regulating the activation of TGF-β/ALK/Smads signaling. Front. Pharmacol. 13, 973182. doi:10.3389/fphar.2022.973182
Huang R., Fu P., Ma L. (2023b). Kidney fibrosis: from mechanisms to therapeutic medicines. Signal Transduct. Target. Ther. 8 (1), 129. doi:10.1038/s41392-023-01379-7
Ishimura T., Ishii A., Yamada H., Osaki K., Toda N., Mori K. P., et al. (2023). Matrix metalloproteinase-10 deficiency has protective effects against peritoneal inflammation and fibrosis via transcription factor NFκΒ pathway inhibition. Kidney Int. 104 (5), 929–942. doi:10.1016/j.kint.2023.08.010
Jia M., Qiu H., Lin L., Zhang S., Li D., Jin D. (2022). Inhibition of PI3K/AKT/mTOR signalling pathway activates autophagy and suppresses peritoneal fibrosis in the process of peritoneal dialysis. Front. Physiol. 13, 778479. doi:10.3389/fphys.2022.778479
Jiang N., Zhang Z., Shao X., Jing R., Wang C., Fang W., et al. (2020). Blockade of thrombospondin-1 ameliorates high glucose-induced peritoneal fibrosis through downregulation of TGF-β1/Smad3 signaling pathway. J. Cell. Physiol. 235 (1), 364–379. doi:10.1002/jcp.28976
Kadoya H., Hirano A., Umeno R., Kajimoto E., Iwakura T., Kondo M., et al. (2023). Activation of the inflammasome drives peritoneal deterioration in a mouse model of peritoneal fibrosis. FASEB J. 37 (9), e23129. doi:10.1096/fj.202201777RRR
Kawka E., Witowski J., Fouqet N., Tayama H., Bender T. O., Catar R., et al. (2014). Regulation of chemokine CCL5 synthesis in human peritoneal fibroblasts: a key role of IFN-γ. Mediat. Inflamm. 2014, 590654. doi:10.1155/2014/590654
Kendall R. T., Feghali-Bostwick C. A. (2014). Fibroblasts in fibrosis: novel roles and mediators. Front. Pharmacol. 5, 123. doi:10.3389/fphar.2014.00123
Khan S. F. (2023). Updates on infectious and other complications in peritoneal dialysis: core curriculum 2023. Am. J. kidney Dis. official J. Natl. Kidney Found. 82 (4), 481–490. doi:10.1053/j.ajkd.2023.03.011
Kitamura M., Nishino T., Obata Y., Furusu A., Hishikawa Y., Koji T., et al. (2012). Epigallocatechin gallate suppresses peritoneal fibrosis in mice. Chemico-biological Interact. 195 (1), 95–104. doi:10.1016/j.cbi.2011.11.002
Kopytina V., Pascual-Antón L., Toggweiler N., Arriero-País E. M., Strahl L., Albar-Vizcaíno P., et al. (2022). Steviol glycosides as an alternative osmotic agent for peritoneal dialysis fluid. Front. Pharmacol. 13, 868374. doi:10.3389/fphar.2022.868374
Krediet R. T. (2018). Ultrafiltration failure is a reflection of peritoneal alterations in patients treated with peritoneal dialysis. Front. Physiol. 9, 1815. doi:10.3389/fphys.2018.01815
Krediet R. T. (2021). Acquired decline in ultrafiltration in peritoneal dialysis: the role of glucose. J. Am. Soc. Nephrol. 32 (10), 2408–2415. doi:10.1681/ASN.2021010080
Krediet R. T., Zweers M. M., van der Wal A. C., Struijk D. G. (2000). Neoangiogenesis in the peritoneal membrane. Perit. dialysis Int. 20 (Suppl. 2), S19–S25. doi:10.1177/089686080002002s05
Lai W., Wang B., Huang R., Zhang C., Fu P., Ma L. (2024). Ferroptosis in organ fibrosis: from mechanisms to therapeutic medicines. J. Transl. Intern. Med. 12 (1), 22–34. doi:10.2478/jtim-2023-0137
Li F., Wang Y., Tian J., Zhou Z., Yin W., Qin X., et al. (2022a). Inhibition of calpain9 attenuates peritoneal dialysis-related peritoneal fibrosis. Front. Pharmacol. 13, 962770. doi:10.3389/fphar.2022.962770
Li J., Liu Y., Liu J. (2023a). A review of research progress on mechanisms of peritoneal fibrosis related to peritoneal dialysis. Front. Physiol. 14, 1220450. doi:10.3389/fphys.2023.1220450
Li L., Shen N., Wang N., Wang W., Tang Q., Du X., et al. (2018). Inhibiting core fucosylation attenuates glucose-induced peritoneal fibrosis in rats. Kidney Int. 93 (6), 1384–1396. doi:10.1016/j.kint.2017.12.023
Li S., Luo C., Chen S., Zhuang Y., Ji Y., Zeng Y., et al. (2023b). Brahma-related gene 1 acts as a profibrotic mediator and targeting it by micheliolide ameliorates peritoneal fibrosis. J. Transl. Med. 21 (1), 639. doi:10.1186/s12967-023-04469-w
Li Y. C., Sung P. H., Yang Y. H., Chiang J. Y., Yip H. K., Yang C. C. (2021). Dipeptidyl peptidase 4 promotes peritoneal fibrosis and its inhibitions prevent failure of peritoneal dialysis. Commun. Biol. 4 (1), 144. doi:10.1038/s42003-021-01652-x
Li Z. H., Xu R., Shi J., Yu M. S., Zhong Y., He W. M., et al. (2022b). Astragalus total saponins ameliorate peritoneal fibrosis by promoting mitochondrial synthesis and inhibiting apoptosis. Am. J. Chin. Med. 50 (1), 261–274. doi:10.1142/S0192415X22500094
Liappas G., González-Mateo G. T., Sánchez-Díaz R., Lazcano J. J., Lasarte S., Matesanz-Marín A., et al. (2016). Immune-regulatory molecule CD69 controls peritoneal fibrosis. J. Am. Soc. Nephrol. 27 (12), 3561–3576. doi:10.1681/ASN.2015080909
Liu F., Yu C., Qin H., Zhang S., Fang L., Wang Y., et al. (2021b). Nintedanib attenuates peritoneal fibrosis by inhibiting mesothelial-to-mesenchymal transition, inflammation and angiogenesis. J. Cell. Mol. Med. 25 (13), 6103–6114. doi:10.1111/jcmm.16518
Liu F., Zhang H., Wu H., Yang S., Liu J., Wang J. (2023). The effects of indobufen on micro-inflammation and peritoneal transport function in patients undergoing continuous ambulate peritoneal dialysis: a prospective randomized controlled study. J. Pharmacol. Exp. Ther. 384 (2), 296–305. doi:10.1124/jpet.122.001138
Liu H., Zhang N., Tian D. (2014). MiR-30b is involved in methylglyoxal-induced epithelial-mesenchymal transition of peritoneal mesothelial cells in rats. Cell. Mol. Biol. Lett. 19 (2), 315–329. doi:10.2478/s11658-014-0199-z
Liu J., Feng Y., Li N., Shao Q. Y., Zhang Q. Y., Sun C., et al. (2021a). Activation of the RAS contributes to peritoneal fibrosis via dysregulation of low-density lipoprotein receptor. Am. J. physiology. Ren. physiology 320 (3), F273–F284. doi:10.1152/ajprenal.00149.2020
Liu J., Jin B., Lu J., Feng Y., Li N., Wan C., et al. (2022b). Angiotensin II type 2 receptor prevents extracellular matrix accumulation in human peritoneal mesothelial cell by ameliorating lipid disorder via LOX-1 suppression. Ren. Fail. 44 (1), 1687–1697. doi:10.1080/0886022X.2022.2133729
Liu Q., Zhang Y., Mao H., Chen W., Luo N., Zhou Q., et al. (2012). A crosstalk between the Smad and JNK signaling in the TGF-β-induced epithelial-mesenchymal transition in rat peritoneal mesothelial cells. PLoS One 7 (2), e32009. doi:10.1371/journal.pone.0032009
Liu Y., Ma Z., Huang Z., Zou D., Li J., Feng P. (2022a). MiR-122-5p promotes peritoneal fibrosis in a rat model of peritoneal dialysis by targeting Smad5 to activate Wnt/β-catenin pathway. Ren. Fail 44 (1), 191–203. doi:10.1080/0886022X.2022.2030360
López-Cabrera M. (2014). Mesenchymal conversion of mesothelial cells is a key event in the pathophysiology of the peritoneum during peritoneal dialysis. Adv. Med. 2014, 473134. doi:10.1155/2014/473134
Lu H., Chen W., Liu W., Si Y., Zhao T., Lai X., et al. (2020). Molecular hydrogen regulates PTEN-AKT-mTOR signaling via ROS to alleviate peritoneal dialysis-related peritoneal fibrosis. FASEB J. 34 (3), 4134–4146. doi:10.1096/fj.201901981R
Lu J., Gao J., Sun J., Wang H., Sun H., Huang Q., et al. (2023). Apolipoprotein A-I attenuates peritoneal fibrosis associated with peritoneal dialysis by inhibiting oxidative stress and inflammation. Front. Pharmacol. 14, 1106339. doi:10.3389/fphar.2023.1106339
Luo Q., Liu M., Tan Y., Chen J., Zhang W., Zhong S., et al. (2022). Blockade of prostaglandin E2 receptor 4 ameliorates peritoneal dialysis-associated peritoneal fibrosis. Front. Pharmacol. 13, 1004619. doi:10.3389/fphar.2022.1004619
Lupinacci S., Perri A., Toteda G., Vizza D., Puoci F., Parisi O. I., et al. (2019). Olive leaf extract counteracts epithelial to mesenchymal transition process induced by peritoneal dialysis, through the inhibition of TGFβ1 signaling. Cell. Biol. Toxicol. 35 (2), 95–109. doi:10.1007/s10565-018-9438-9
Margetts P. J. (2023). Matrix metalloproteinase 10 and the slow demise of the peritoneal membrane. Kidney Int. 104 (5), 880–882. doi:10.1016/j.kint.2023.09.009
Masola V., Bonomini M., Borrelli S., Di Liberato L., Vecchi L., Onisto M., et al. (2022). Fibrosis of peritoneal membrane as target of new therapies in peritoneal dialysis. Int. J. Mol. Sci. 23 (9), 4831. doi:10.3390/ijms23094831
Masola V., Bonomini M., Onisto M., Ferraro P. M., Arduini A., Gambaro G. (2021). Biological effects of XyloCore, a glucose sparing PD solution, on mesothelial cells: focus on mesothelial-mesenchymal transition, inflammation and angiogenesis. Nutrients 13 (7), 2282. doi:10.3390/nu13072282
Mo M., Zeng Y., Zeng Y., Li S., He X., Chen X., et al. (2023). N-methylpiperazine-diepoxyovatodiolide ameliorates peritoneal fibrosis via suppressing TGF-β/Smad and JAK/STAT signaling pathway. Chemico-biological Interact. 382, 110589. doi:10.1016/j.cbi.2023.110589
Moinuddin Z., Wang K., Fullwood C., Wiredu E., Hutchison A., Vardhan A., et al. (2024). Renal hyperparathyroidism-a risk factor in the development of encapsulating peritoneal sclerosis. Front. Endocrinol. 15, 1282925. doi:10.3389/fendo.2024.1282925
Nagasaki K., Nakashima A., Tamura R., Ishiuchi N., Honda K., Ueno T., et al. (2021). Mesenchymal stem cells cultured in serum-free medium ameliorate experimental peritoneal fibrosis. Stem Cell. Res. Ther. 12 (1), 203. doi:10.1186/s13287-021-02273-1
Nie J., Dou X., Hao W., Wang X., Peng W., Jia Z., et al. (2007). Smad7 gene transfer inhibits peritoneal fibrosis. Kidney Int. 72 (11), 1336–1344. doi:10.1038/sj.ki.5002533
Nishimura K., Ogawa K., Kawaguchi M., Fumoto S., Mukai H., Kawakami S. (2021). Suppression of peritoneal fibrosis by sonoporation of hepatocyte growth factor gene-encoding plasmid DNA in mice. Pharmaceutics 13 (1), 115. doi:10.3390/pharmaceutics13010115
Obata Y., Abe K., Miyazaki M., Koji T., Tabata Y., Nishino T. (2023). The transfer of the hepatocyte growth factor gene by macrophages ameliorates the progression of peritoneal fibrosis in mice. Int. J. Mol. Sci. 24 (8), 6951. doi:10.3390/ijms24086951
Pap D., Pajtók C., Veres-Székely A., Szebeni B., Szász C., Bokrossy P., et al. (2023). High salt promotes inflammatory and fibrotic response in peritoneal cells. Int. J. Mol. Sci. 24 (18), 13765. doi:10.3390/ijms241813765
Patel P., Sekiguchi Y., Oh K. H., Patterson S. E., Kolb M. R., Margetts P. J. (2010). Smad3-dependent and -independent pathways are involved in peritoneal membrane injury. Kidney Int. 77 (4), 319–328. doi:10.1038/ki.2009.436
Raby A. C., González-Mateo G. T., Williams A., Topley N., Fraser D., López-Cabrera M., et al. (2018). Targeting Toll-like receptors with soluble Toll-like receptor 2 prevents peritoneal dialysis solution-induced fibrosis. Kidney Int. 94 (2), 346–362. doi:10.1016/j.kint.2018.03.014
Ruan H., Li X., Zhou L., Zheng Z., Hua R., Wang X., et al. (2024). Melatonin decreases GSDME mediated mesothelial cell pyroptosis and prevents peritoneal fibrosis and ultrafiltration failure. Sci. China. Life Sci. 67 (2), 360–378. doi:10.1007/s11427-022-2365-1
Ruiqi L., Ming P., Qihang S., Yangyang L., Junli C., Wei L., et al. (2021). Saikosaponin D inhibits peritoneal fibrosis in rats with renal failure by regulation of TGFβ1/BMP7/Gremlin1/Smad pathway. Front. Pharmacol. 12, 628671. doi:10.3389/fphar.2021.628671
Selgas R., Bajo A., Jiménez-Heffernan J. A., Sánchez-Tomero J. A., Del Peso G., Aguilera A., et al. (2006). Epithelial-to-mesenchymal transition of the mesothelial cell-its role in the response of the peritoneum to dialysis. Nephrol. Dial. Transpl. 21 (Suppl. 2), ii2–ii7. doi:10.1093/ndt/gfl183
Shang J., He Q., Chen Y., Yu D., Sun L., Cheng G., et al. (2019). miR-15a-5p suppresses inflammation and fibrosis of peritoneal mesothelial cells induced by peritoneal dialysis via targeting VEGFA. J. Cell. Physiol. 234 (6), 9746–9755. doi:10.1002/jcp.27660
Shao Q., Jiang C., Zhang Q., Liu J., Jin B., Zhao M., et al. (2023). Knockdown of AK142426 suppresses M2 macrophage polarization and inflammation in peritoneal fibrosis via binding to c-Jun. J. gene Med. 25 (9), e3524. doi:10.1002/jgm.3524
Shentu Y., Jiang H., Liu X., Chen H., Yang D., Zhang J., et al. (2020). Nestin promotes peritoneal fibrosis by protecting HIF1-α from proteasomal degradation. Front. Physiol. 11, 517912. doi:10.3389/fphys.2020.517912
Shentu Y., Li Y., Xie S., Jiang H., Sun S., Lin R., et al. (2021). Empagliflozin, a sodium glucose cotransporter-2 inhibitor, ameliorates peritoneal fibrosis via suppressing TGF-β/Smad signaling. Int. Immunopharmacol. 93, 107374. doi:10.1016/j.intimp.2021.107374
Shi P., Zhan Z., Ye X., Lu Y., Song K., Sheng F., et al. (2022a). The antioxidative effects of empagliflozin on high glucose-induced epithelial-mesenchymal transition in peritoneal mesothelial cells via the Nrf2/HO-1 signaling. Ren. Fail. 44 (1), 1528–1542. doi:10.1080/0886022X.2022.2118066
Shi Y., Hu Y., Wang Y., Ma X., Tang L., Tao M., et al. (2021b). Blockade of autophagy prevents the development and progression of peritoneal fibrosis. Front. Pharmacol. 12, 724141. doi:10.3389/fphar.2021.724141
Shi Y., Li J., Chen H., Hu Y., Tang L., Wang Y., et al. (2022b). Inhibition of EZH2 suppresses peritoneal angiogenesis by targeting a VEGFR2/ERK1/2/HIF-1α-dependent signaling pathway. J. Pathol. 258 (2), 164–178. doi:10.1002/path.5987
Shi Y., Li J., Chen H., Hu Y., Tang L., Zhou X., et al. (2022c). Pharmacologic inhibition of histone deacetylase 6 prevents the progression of chlorhexidine gluconate-induced peritoneal fibrosis by blockade of M2 macrophage polarization. Front. Immunol. 13, 899140. doi:10.3389/fimmu.2022.899140
Shi Y., Ni J., Tao M., Ma X., Wang Y., Zang X., et al. (2020). Elevated expression of HDAC6 in clinical peritoneal dialysis patients and its pathogenic role on peritoneal angiogenesis. Ren. Fail. 42 (1), 890–901. doi:10.1080/0886022X.2020.1811119
Shi Y., Tao M., Ni J., Tang L., Liu F., Chen H., et al. (2021a). Requirement of histone deacetylase 6 for interleukin-6 induced epithelial-mesenchymal transition, proliferation, and migration of peritoneal mesothelial cells. Front. Pharmacol. 12, 722638. doi:10.3389/fphar.2021.722638
Shibuya M. (2011). Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes. cancer 2 (12), 1097–1105. doi:10.1177/1947601911423031
Shirai Y., Miura K., Ike T., Sasaki K., Ishizuka K., Horita S., et al. (2022). Cumulative dialytic glucose exposure is a risk factor for peritoneal fibrosis and angiogenesis in pediatric patients undergoing peritoneal dialysis using neutral-pH fluids. Kidney Int. Rep. 7 (11), 2431–2445. doi:10.1016/j.ekir.2022.08.013
Si M., Wang Q., Li Y., Lin H., Luo D., Zhao W., et al. (2019). Inhibition of hyperglycolysis in mesothelial cells prevents peritoneal fibrosis. Sci. Transl. Med. 11 (495), eaav5341. doi:10.1126/scitranslmed.aav5341
Silva F. M. O., Costalonga E. C., Silva C., Carreira A. C. O., Gomes S. A., Sogayar M. C., et al. (2019). Tamoxifen and bone morphogenic protein-7 modulate fibrosis and inflammation in the peritoneal fibrosis model developed in uremic rats. Mol. Med. Camb. Mass. 25 (1), 41. doi:10.1186/s10020-019-0110-5
Simons M., Gordon E., Claesson-Welsh L. (2016). Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell. Biol. 17 (10), 611–625. doi:10.1038/nrm.2016.87
Song Q., Wang P., Wang H., Pan M., Li X., Yao Z., et al. (2024). Integrative analysis of chromatin accessibility and transcriptome landscapes in the induction of peritoneal fibrosis by high glucose. J. Transl. Med. 22 (1), 243. doi:10.1186/s12967-024-05037-6
Strippoli R., Moreno-Vicente R., Battistelli C., Cicchini C., Noce V., Amicone L., et al. (2016). Molecular mechanisms underlying peritoneal EMT and fibrosis. Stem cells Int. 2016, 3543678. doi:10.1155/2016/3543678
Su W., Hu Z., Zhong X., Cong A., Zhang Y., Zhou Z., et al. (2023). Restoration of CPT1A-mediated fatty acid oxidation in mesothelial cells protects against peritoneal fibrosis. Theranostics 13 (13), 4482–4496. doi:10.7150/thno.84921
Sun J., Tang L., Shan Y., Yu M., Sheng L., Huang L., et al. (2023). TMT quantitative proteomics and network pharmacology reveal the mechanism by which asiaticoside regulates the JAK2/STAT3 signaling pathway to inhibit peritoneal fibrosis. J. Ethnopharmacol. 309, 116343. doi:10.1016/j.jep.2023.116343
Szebeni B., Veres-Székely A., Pap D., Bokrossy P., Varga Z., Gaál A., et al. (2024). Extracellular vesicles of patients on peritoneal dialysis inhibit the TGF-β- and PDGF-B-mediated fibrotic processes. Cells 13 (7), 605. doi:10.3390/cells13070605
Tawada M., Ito Y., Banshodani M., Yamashita M., Shintaku S., Sun T., et al. (2021). Vasculopathy plays an important role during the development and relapse of encapsulating peritoneal sclerosis with conventional peritoneal dialysis solutions. Transplantation 36 (8), 1519–1526. doi:10.1093/ndt/gfaa073
Terri M., Trionfetti F., Montaldo C., Cordani M., Tripodi M., Lopez-Cabrera M., et al. (2021). Mechanisms of peritoneal fibrosis: focus on immune cells-peritoneal stroma interactions. Front. Immunol. 12, 607204. doi:10.3389/fimmu.2021.607204
Tian M., Zhang L., Wang Y., Deng M., Peng C., Liang W., et al. (2022). Loss of JNK-associated leucine zipper protein promotes peritoneal dialysis-related peritoneal fibrosis. Kidney Dis. 8 (2), 168–179. doi:10.1159/000521564
Wang J., Lv X., A-Ni-Wan A. S., Tian S. S., Wang J. M., Liu H. Y., et al. (2023). Canagliflozin alleviates high glucose-induced peritoneal fibrosis via HIF-1α inhibition. Front. Pharmacol. 14, 1152611. doi:10.3389/fphar.2023.1152611
Wu J., Huang Q., Li P., Wang Y., Zheng C., Lei X., et al. (2019). MicroRNA-145 promotes the epithelial-mesenchymal transition in peritoneal dialysis-associated fibrosis by suppressing fibroblast growth factor 10. J. Biol. Chem. 294 (41), 15052–15067. doi:10.1074/jbc.RA119.007404
Wu K. L., Chou C. Y., Chang H. Y., Wu C. H., Li A. L., Chen C. L., et al. (2022). Peritoneal effluent MicroRNA profile for detection of encapsulating peritoneal sclerosis. Clin. Chimica Acta. 536, 45–55. doi:10.1016/j.cca.2022.09.007
Wu Z., Zuo X., Wang X., Shi M., Zhu H., Cao C., et al. (2023). The probiotic Lactobacillus casei Zhang-mediated correction of gut dysbiosis ameliorates peritoneal fibrosis by suppressing macrophage-related inflammation via the butyrate/PPAR-γ/NF-κB pathway. Food Funct. 14 (15), 6840–6852. doi:10.1039/d3fo01518a
Xiao L., Zhou X., Liu F., Hu C., Zhu X., Luo Y., et al. (2015). MicroRNA-129-5p modulates epithelial-to-mesenchymal transition by targeting SIP1 and SOX4 during peritoneal dialysis. Lab. Invest. 95 (7), 817–832. doi:10.1038/labinvest.2015.57
Xie S., Xu F., Lu Y., Zhang Y., Li X., Yu M., et al. (2022). Elabela attenuates the TGF-β1-induced epithelial-mesenchymal transition of peritoneal mesothelial cells in patients receiving peritoneal dialysis. Front. Pharmacol. 13, 890881. doi:10.3389/fphar.2022.890881
Yáñez-Mó M., Lara-Pezzi E., Selgas R., Ramírez-Huesca M., Domínguez-Jiménez C., Jiménez-Heffernan J. A., et al. (2003). Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N. Engl. J. Med. 348 (5), 403–413. doi:10.1056/NEJMoa020809
Yang C. Y., Chang P. Y., Chen J. Y., Wu B. S., Yang A. H., Lee O. K. (2021b). Adipose-derived mesenchymal stem cells attenuate dialysis-induced peritoneal fibrosis by modulating macrophage polarization via interleukin-6. Stem Cell. Res. and Ther. 12 (1), 193. doi:10.1186/s13287-021-02270-4
Yang J., Zhu T., Liu X., Zhang L., Yang Y., Zhang J., et al. (2015). Heat shock protein 70 protects rat peritoneal mesothelial cells from advanced glycation end-products-induced epithelial-to-mesenchymal transition through mitogen‑activated protein kinases/extracellular signal-regulated kinases and transforming growth factor-β/Smad pathways. Mol. Med. Rep. 11 (6), 4473–4481. doi:10.3892/mmr.2015.3271
Yang X., Bao M., Fang Y., Yu X., Ji J., Ding X. (2021a). STAT3/HIF-1α signaling activation mediates peritoneal fibrosis induced by high glucose. J. Transl. Med. 19 (1), 283. doi:10.1186/s12967-021-02946-8
Yang X., Yan H., Jiang N., Yu Z., Yuan J., Ni Z., et al. (2020). IL-6 trans-signaling drives a STAT3-dependent pathway that leads to structural alterations of the peritoneal membrane. Am. J. physiology. Ren. physiology 318 (2), F338-F353–F353. doi:10.1152/ajprenal.00319.2019
Yoshimine H., Tanoue S., Ibi Y., Minami M., Nakahara M., Tokunaga K., et al. (2021). Hepatocyte growth factor ameliorates methylglyoxal-induced peritoneal inflammation and fibrosis in mouse model. Clin. Exp. Nephrol. 25 (9), 935–943. doi:10.1007/s10157-021-02067-y
Yu F., Yang J., Chen J., Wang X., Cai Q., He Y., et al. (2023b). Bone marrow mesenchymal stem cell-derived exosomes alleviate peritoneal dialysis-associated peritoneal injury. Stem cells Dev. 32 (7-8), 197–211. doi:10.1089/scd.2022.0244
Yu S., Geng X., Liu H., Zhang Y., Cao X., Li B., et al. (2023a). ELMO1 deficiency reduces neutrophil chemotaxis in murine peritonitis. Int. J. Mol. Sci. 24 (9), 8103. doi:10.3390/ijms24098103
Zhang J., Li H., Zhong H., Chen X., Hu Z. X. (2024). Omega-3 polyunsaturated fatty acids protect peritoneal mesothelial cells from hyperglycolysis and mesothelial-mesenchymal transition through the FFAR4/CaMKKβ/AMPK/mTOR signaling pathway. Int. Immunopharmacol. 128, 111561. doi:10.1016/j.intimp.2024.111561
Zhang Y., Feng W., Peng X., Zhu L., Wang Z., Shen H., et al. (2022). Parthenolide alleviates peritoneal fibrosis by inhibiting inflammation via the NF-κB/TGF-β/Smad signaling axis. Lab. Invest. 102 (12), 1346–1354. doi:10.1038/s41374-022-00834-3
Zhang Z., Jiang N., Ni Z. (2017). Strategies for preventing peritoneal fibrosis in peritoneal dialysis patients: new insights based on peritoneal inflammation and angiogenesis. Front. Med. 11 (3), 349–358. doi:10.1007/s11684-017-0571-2
Zhao H., Zhang H. L., Jia L. (2023a). High glucose dialysate-induced peritoneal fibrosis: pathophysiology, underlying mechanisms and potential therapeutic strategies. Biomed. Pharmacother. 165, 115246. doi:10.1016/j.biopha.2023.115246
Zhao J., Liu H., Hong Z., Luo W., Mu W., Hou X., et al. (2023c). Tanshinone I specifically suppresses NLRP3 inflammasome activation by disrupting the association of NLRP3 and ASC. Mol. Med. Camb. Mass. 29 (1), 84. doi:10.1186/s10020-023-00671-0
Zhao J. L., Guo M. Z., Zhu J. J., Zhang T., Min D. Y. (2019). Curcumin suppresses epithelial-to-mesenchymal transition of peritoneal mesothelial cells (HMrSV5) through regulation of transforming growth factor-activated kinase 1 (TAK1). Cell. Mol. Biol. Lett. 24, 32. doi:10.1186/s11658-019-0157-x
Zhao T., Ding T., Sun Z., Shao X., Li S., Lu H., et al. (2024). SPHK1/S1P/S1PR pathway promotes the progression of peritoneal fibrosis by mesothelial-mesenchymal transition. FASEB J. 38 (2), e23417. doi:10.1096/fj.202301323R
Zhao T., Sun Z., Lai X., Lu H., Liu L., Li S., et al. (2023b). Tamoxifen exerts anti-peritoneal fibrosis effects by inhibiting H19-activated VEGFA transcription. J. Transl. Med. 21 (1), 614. doi:10.1186/s12967-023-04470-3
Zhou F., Yao L., Lu X., Li Y., Han X., Wang P. (2022). Therapeutic targeting of gsk3β-regulated Nrf2 and NFκB signaling pathways by salvianolic acid A ameliorates peritoneal fibrosis. Front. Med. 9, 804899. doi:10.3389/fmed.2022.804899
Zhou Q., Bajo M. A., Del Peso G., Yu X., Selgas R. (2016). Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney Int. 90 (3), 515–524. doi:10.1016/j.kint.2016.03.040
Zhou X., Chen H., Shi Y., Li J., Ma X., Du L., et al. (2023). Histone deacetylase 8 inhibition prevents the progression of peritoneal fibrosis by counteracting the epithelial-mesenchymal transition and blockade of M2 macrophage polarization. Front. Immunol. 14, 1137332. doi:10.3389/fimmu.2023.1137332
Zhu F., Li T., Qiu F., Fan J., Zhou Q., Ding X., et al. (2010). Preventive effect of Notch signaling inhibition by a gamma-secretase inhibitor on peritoneal dialysis fluid-induced peritoneal fibrosis in rats. Am. J. Pathol. 176 (2), 650–659. doi:10.2353/ajpath.2010.090447
Keywords: peritoneal fibrosis, pathophysiology, therapeutics, peritoneal dialysis, mechanism
Citation: Kang Y, Liu Y, Fu P and Ma L (2024) Peritoneal fibrosis: from pathophysiological mechanism to medicine. Front. Physiol. 15:1438952. doi: 10.3389/fphys.2024.1438952
Received: 27 May 2024; Accepted: 21 August 2024;
Published: 04 September 2024.
Edited by:
Xiaoyan Zhang, East China Normal University, ChinaCopyright © 2024 Kang, Liu, Fu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Fu, ZnVwaW5naHhAc2N1LmVkdS5jbg==; Liang Ma, bGlhbmdfbUBzY3UuZWR1LmNu
†These authors have contributed equally to this work
 Yingxi Kang1†
Yingxi Kang1† Ping Fu
Ping Fu Liang Ma
Liang Ma