
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol. , 06 August 2024
Sec. Exercise Physiology
Volume 15 - 2024 | https://doi.org/10.3389/fphys.2024.1430458
This article is part of the Research Topic New Perspectives and Insights on Heart Rate Variability in Exercise and Sports View all 7 articles
Objective: Heart rate variability (HRV) is an important non-invasive marker for the assessment of an organism’s autonomic physiological regulatory pathways. Lower HRV has been shown to correlate with increased mortality. HRV is influenced by various factors or diseases. The aim of this narrative review is to describe the current state of knowledge on factors influencing HRV and their significance for interpretation.
Methods: The narrative review only included reviews, meta-analyses, and cohort studies which were published until 2021. HRV confounders were grouped into four categories (non-influenceable physiological factors, diseases, influenceable lifestyle factors and external factors).
Results: The review found that HRV was decreased not only in non-influenceable physiological factors (e.g., age, gender, ethnicity) but also in connection with various number of acute and chronic diseases (e.g., psychiatric diseases, myocardial infarction, heart failure), influenceable lifestyle factors (e.g., alcohol abuse, overweight, physical activity), and external factors (e.g., heat, noise, shift work, harmful- and hazardous substances).
Conclusion: In order to improve the quality of HRV studies and to ensure accurate interpretation, it is recommended that confounders be taken into account in future diagnostic measurements or measurements in the workplace (e.g., as part of health promotion measures) in order to counteract data bias.
The measurement and analysis of heart rate variability (HRV), which is based on the variation between consecutive NN intervals, has become an established procedure over the past 2 decades since the publication of the first guideline (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). Not only have there been advances in recording technology (smaller, more portable, more accurate devices) (Koerber T et al., 2000), but NN intervals can now also be measured by small chest strap and pulse watch systems (Wallén et al., 2012). Technological developments have reduced the costs of recording and analysis and have facilitated outpatient applications. HRV is also becoming increasingly important in clinical medicine, in particular to supplement established diagnostic procedures or to monitor progress. This requires a basic understanding of recording and analysing HRV, for which reference can be made to the relevant guidelines (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996; Sassi et al., 2015; Sammito et al., 2024).
The variability of the successive differences between the NN intervals depends on sympathetic and parasympathetic influences. Mathematical algorithms can be used to calculate various HRV parameters from a time series of successive NN intervals. It is customary to make a distinction between so-called HRV parameters of the time domain and frequency domain and so-called non-linear HRV parameters (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996; Sassi et al., 2015; Sammito et al., 2024).
Increased HRV is generally defined as a state in which the variability of successive cardiac actions is increased or, in the case of reduced variability, HRV is said to be reduced. Depending on the respective HRV parameter, a higher variability (=increased HRV) can be accompanied by a higher value in the respective parameter and vice versa, but for some HRV parameters this is the other way round. In addition, some so-called non-linear HRV parameters are based on other mechanisms and are therefore in part less susceptible to external interfering factors.
A decrease in HRV has been shown to correlate with increased mortality, for example, after myocardial infarction (Buccelletti et al., 2009; Huikuri and Stein, 2013; Song et al., 2014), strokes (Yperzeele et al., 2015), bypass operations (Lakusic et al., 2013), heart failure (Sandercock and Brodie, 2006), or chronic obstructive pulmonary disease (Handa et al., 2012). An association could also be shown for manifestation of hypertension 3 years later if the HRV was decreased (Liao et al., 1996; Singh et al., 1998; Schroeder et al., 2003).
The HRV analysis can be performed based on both a short-term (5 min, sometimes shorter) and a long-term measurement (usually 24 h) (Sammito et al., 2024). Although the analysis windows are different, the reduction of HRV in underlying diseases is evident in both the short-term and long-term measurements. While intra-individual comparisons are usually uncomplicated, such confounders play a role when inter-individual comparisons are to be made between individuals or groups. In this case, it is important to know possible influencing factors and their effect on HRV.
The group of authors published a first review on factors influencing heart rate variability in 2016 (Sammito and Böckelmann, 2016). Since then, a number of new findings have been added, making an update of this work urgently necessary. Based on an updated narrative review the authors of this article have included known literature to this topic supplemented by information from national and international guidelines (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996; Sassi et al., 2015; Sammito et al., 2024), and presented the HRV confounders grouped in four categories (uncontrollable physiological factors, diseases, controllable lifestyle factors and external factors). Each search included the search terms “HRV” or “heart rate variability” and each confounder or superordinate term, e.g., heart disease. References primarily include meta-analyses and systematic reviews on the topic, supplemented by cohort studies. Published articles were considered if they were written in English or German and were published up to the end of 2021.
In addition to non-influenceable physiological parameters, a number of factors come from the lifestyle habits of the test persons, from the consequences of these habits and from external circumstances. A number of diseases are associated with a decrease in HRV, while the influence on the vegetative nervous system can be regarded more as a result of diseases and only rarely as a potential cause of these decrease.
Non-influenceable physiological factors include age, sex, pregnancy and circadian rhythm.
A person’s HRV first increases sharply until they reach the age of 1 year and continues to increase considerably until they reach the age of 15 years, while the resting heart rate decreases (Eyre et al., 2014). The HRV is highest in young adulthood and decreases nonlinearly with age (Umetani et al., 1998; Fagard et al., 1999; Kuo et al., 1999; Fukusaki et al., 2000; Fagard, 2001; Ferrari, 2002; Felber Dietrich et al., 2006; Britton et al., 2007; Zhang, 2007; Barantke et al., 2008; Greiser et al., 2009; Stein et al., 2009; Voss et al., 2009; Shiogai et al., 2010; Haerting et al., 2012; Voss et al., 2012; Abhishekh et al., 2013; Alvares et al., 2016).
Furthermore, there is a difference between women and men, with most studies showing higher parasympathetic activity shown in most studies in women compared to men (Tsuji et al., 1996; Jensen-Urstad et al., 1997; Agelink et al., 2001; Snieder et al., 2007; Barantke et al., 2008; Sookan and McKune, 2012; Abhishekh et al., 2013; Koenig and Thayer, 2016), which however showed a smaller difference after the age of 50 years (Fagard et al., 1999; Kuo et al., 1999; Fagard, 2001). This circumstance seems to be related to the postmenopausal change in the hormonal situation in women (Huikuri et al., 1996; Fagard, 2001). Some of the studies showed a higher baseline sympathetic activity in women (Ramaekers et al., 1998; Umetani et al., 1998; Felber Dietrich et al., 2006; Huang et al., 2012). In summary, it can be assumed that there is a difference in HRV between men and women up to the age of 50 years and therefore gender must be considering as a confounding factor when interpreting HRV.
HRV, like a number of other physiological parameters, is subject not only to age and gender, but also to a circadian rhythm (Sammito et al., 2016). HRV increases during the night and decreases considerably during the morning hours. This must be taken into account, particularly for short-term measurements of a few minutes to a few hours, since intra- and interpersonal comparisons of short-term measurements can only be meaningful if the same time of day is taken into account.
While a genetic disposition of the HRV has been discussed in twin studies (Riese et al., 2007), Riese et al. (2014) found no association between eight key genes for the presence of acetylcholine receptors as part of the autonomic nervous system and the HRV level in an analysis of several cohort studies involving a total of 6,470 test persons. In contrast, ethnicicity seems to have an influence on HRV. In a meta-analysis based on a systematic reference survey involving 17 studies and a total of 11,162 test persons, Hill et al. (2015) established a significantly higher short-term resting HRV in African-American test persons than in American subjects of European origin.
The effects of various diseases on HRV have been examined in many studies. HRV is consistently lower in patients with these diseases than in healthy test persons. What´s certain is that a low HRV can be found in patients with cardiovascular diseases like cardiac insufficiency (Scalvini et al., 1998; Biswas et al., 2000; Guzzetti et al., 2001; Davies et al., 2002; Lasisi et al., 2012), hypertension (Silvetti et al., 2001; Carthy, 2014), coronary heart disease (CHD) with and without angina pectoris and after myocardial infarction (Huikuri and Mäkikallio, 2001; Huikuri and Stein, 2013).
Also, patients with metabolic disorders also show reduced HRV. Metabolic syndrome often leads to a reduction of the HRV (Liao et al., 1998; Hemingway et al., 2005; Stein et al., 2007; Min et al., 2008; Gehi et al., 2009; Koskinen et al., 2009; Assoumou et al., 2010; Chang et al., 2012), especially in women (Stuckey et al., 2014). HRV is also reduced in manifest diabetes mellitus (Tsuji et al., 1996; Singh et al., 2000; Karayannis et al., 2012; Kuehl and Stevens, 2012; Benichou et al., 2018), although a correlation between disease duration and HRV reduction is only found in very poorly controlled diabetes mellitus (Stein et al., 2007). This is mainly due to peripheral neuropathy caused by microcirculatory disturbances (Barrett et al., 2017).
Reduced HRV is also evident in numerous psychiatric disorders. Patients with anorexia nervosa (Chalmers et al., 2014), anxiety disorders (Aasman et al., 1987; Friedman, 2007; Chalmers et al., 2014; Alvares et al., 2016; Paniccia et al., 2017), bipolar disorder (Alvares et al., 2016; Bassett, 2016; Faurholt-Jepsen et al., 2017; Carr et al., 2018), borderline personality disorder (Koenig et al., 2016b), bulimia nervosa (Peschel et al., 2016) (major) depression (Birkhofer et al., 2005; Kemp et al., 2010; Kapfhammer, 2011; Stapelberg et al., 2012; Alvares et al., 2016; Bassett, 2016; Brown et al., 2018), epilepsy (Lotufo et al., 2012), panic attacks (Aasman et al., 1987; Friedman and Thayer, 1998), posttraumatic stress disorder (Sammito et al., 2015) and schizophrenia (Clamor et al., 2016) have typically shown reduced HRV. In the case of substance addiction (Alvares et al., 2016), the HRV is also usually reduced.
There is also evidence for several other diseases that the HRV is reduced in patients with this diagnosis, such as chronic obstructive pulmonary disease (COPD) (Roque et al., 2014; Mohammed et al., 2015), chronic kidney failure (Zhang and Wang, 2014), in the early stages of Duchenne muscular dystrophy and in manifest disease (da Silva et al., 2018), regular headaches (Barloese, 2016; Koenig et al., 2016c), chronic pain (Koenig et al., 2016a; Tracy et al., 2016), and long-/post-covid (Suh et al., 2023). There is also scientific evidence that HRV is reduced in burnout symptoms (Thielmann et al., 2021; Wekenborg et al., 2022).
However, it is important to remember that for some diseases there is no scientific evidence for a reduced HRV. So, the influence of breast cancer on HRV is unclear (Arab et al., 2016) and based on a systematic literature search, HRV does not currently appear to be changed in the presence of rheumatoid arthritis (Adlan et al., 2014). A reduction in HRV in the presence of sleep disorders is currently not supported, too, by the scientific literature (Dodds et al., 2017). Something similar can be found in untreated obstructive sleep apnea syndrome.
In the scientific literature, there is a basically consistent picture of the modifiable lifestyle factors: positively associated lifestyle factors, which go hand in hand with a healthy lifestyle, increase the HRV, while negatively associated lifestyle factors reduce it. Thus, the HRV is usually reduced in situations of acute alcohol consumption (Ralevski et al., 2019). A low, constant alcohol consumption with an alcohol content of one standard drink for women or two standard drinks for men usually leads to a short-term but no long-term change in HRV or an increased HRV, while chronic alcohol abuse leads to a reduction of HRV (Karpyak et al., 2014; Ralevski et al., 2019). Increased body mass index (BMI) and increased mass of body fat often cause a fall in the HRV (Fraley et al., 2005). In regard to physical activity, initially, there is a decrease in the HRV due to increased activity of the sympathetic system (Bernardi and Piepoli, 2001), but regular physical activity leads to an increase in the parasympathetic activity which in turn causes a rise in HRV (Bernardi and Piepoli, 2001; Braith and Edwards, 2003; Rennie et al., 2003; Felber Dietrich et al., 2006; Hottenrott et al., 2006; Grässler et al., 2021). Endurance training normally increases the HRV (Aubert et al., 2003; Sandercock et al., 2005; Hottenrott et al., 2006; Routledge et al., 2010; Bellenger et al., 2016; Grässler et al., 2021). Endurance, coordinative, and multimodal training increase HRV in older adults but not resistance training (Grässler et al., 2021). These effects can be also seen in patients with myocardial infarction and patients with heart failure (Routledge et al., 2010) or diabetes mellitus II (Bhati et al., 2018). Similar effects could be observed in individuals who perform high-intensity interval training (HIIT) which generally increases HRV and has been shown to be particularly effective in healthy subjects (Grässler et al., 2021) and patients with metabolic syndrome (Abreu et al., 2019). In contrast, high-intensity training and competition series, on the other hand, can lead to reduced HRV (Aubert et al., 2003; Hottenrott et al., 2006). During strength training, there is usually no change in HRV in healthy individuals, while strength training is usually associated with an increase in HRV in subjects with chronic illnesses (Bhati et al., 2019).
Other lifestyle habits such as smoking can lead to a dose-dependent decrease in HRV (Felber Dietrich et al., 2007; Dinas et al., 2013). Even in non-smokers, passive smoking, e.g., at home or at work leads to a reduction in the HRV (Felber Dietrich et al., 2007; Wilson et al., 2010; Dinas et al., 2013). Stress (e.g., mental, work-related) generally leads to decreased parasympathetic activity and thus to a reduction in the HRV (Dishman et al., 2000; Lehrer, 2003a; Chandola et al., 2008; Chandola et al., 2010; Looser et al., 2010; Clays et al., 2011; Järvelin-Pasanen et al., 2018).
HRV have been used for biofeedback in cases of stress recovery and recently also in the treatment of posttraumatic stress disorder, e.g., for an objective view on the effects of stress relaxation (Lehrer et al., 2003; Lehrer et al., 2003; Del Pozo et al., 2004; Lehrer et al., 2006; Peira et al., 2013). However, until now, only short-term effects of such interventions have been observed. It has not yet been possible to demonstrate a long-term effect (Peira et al., 2013). Nevertheless, biofeedback should be considered as a possible confounder.
The effects of respiration on HRV are reflected in the form of respiratory sinus arrhythmia (RSA) and is seen in the HF band. On the whole, the HRV parameter, Root Mean Square of Successive Differences (RMSSD), does not seem to be affected by respiration (Hill and Siebenbrock, 2009). For the rest of the parameters, the present state of knowledge is not conclusive (Jennings and Mack, 1984; Kanters et al., 1997; Schaffer et al., 2014).
In addition to climatic conditions and work-related parameters, several harmful substances and medications also have a direct or indirect influence on HRV. Climatic factors lead to changes in HRV due to the physiological response of the vegetative nervous system. Heat increases the activity of the sympathetic nervous system activity, which reduces the HRV (Ren et al., 2011; Wu et al., 2013). Long-term exposure to cold (e.g., at work or during the winter months) was found to have no effect on HRV (Harinath et al., 2005; Bortkiewicz et al., 2006; Ren et al., 2011) due to adaptation effects, e.g., after 60 days. Hypobaric hypoxia usually leads to short-term sympathetic activation (Bhaumik et al., 2013) and long-term to a reduction in HRV (Dhar et al., 2014). Noise exposure also decrease HRV by increasing sympathetic nervous system activity (Lee et al., 2010; Kraus et al., 2013; Schnell et al., 2013; Veternik et al., 2018).
Shift work with a night shift usually results in an activation of the sympathetic nervous system (SNS) and a reduction of the parasympathetic nervous system (PNS) and thus a reduction in HRV, whereby there is a correlation between the duration of shift work in years and the reduction of HRV (Ha et al., 2001; Chung et al., 2009; Lindholm et al., 2012; Wehrens et al., 2012; Järvelin-Pasanen et al., 2013; Amirian et al., 2014; Jensen et al., 2016).
Some harmful substances (including acute diesel and biodiesel inhalation (Brito et al., 2010), chronic exposure to lead (Murata et al., 1995; Böckelmann et al., 2002), acute exposure to cadmium (Feng et al., 2015), carbon disulfide (Bortkiewicz et al., 1997; Jhun et al., 2003), however, not in the case of long-term low-dose exposure (Reinhardt et al., 1997); long-term mercury exposure (Grandjean et al., 2004), especially as a fetal mercury exposure (Grandjean et al., 2004) and neurotoxic styrene (Murata et al., 1991a; Murata et al., 1991b), and some medications (e.g., beta-blockers, ACE inhibitors, antiarrhythmics and psychotropic drugs) have been found to have a direct or indirect influence on HRV.
Figure 1 provides a summary of the results referring to the factors and covers the four main categories, i.e., non-influenceable physiological factors, diseases, influenceable lifestyle factors, and external factors.
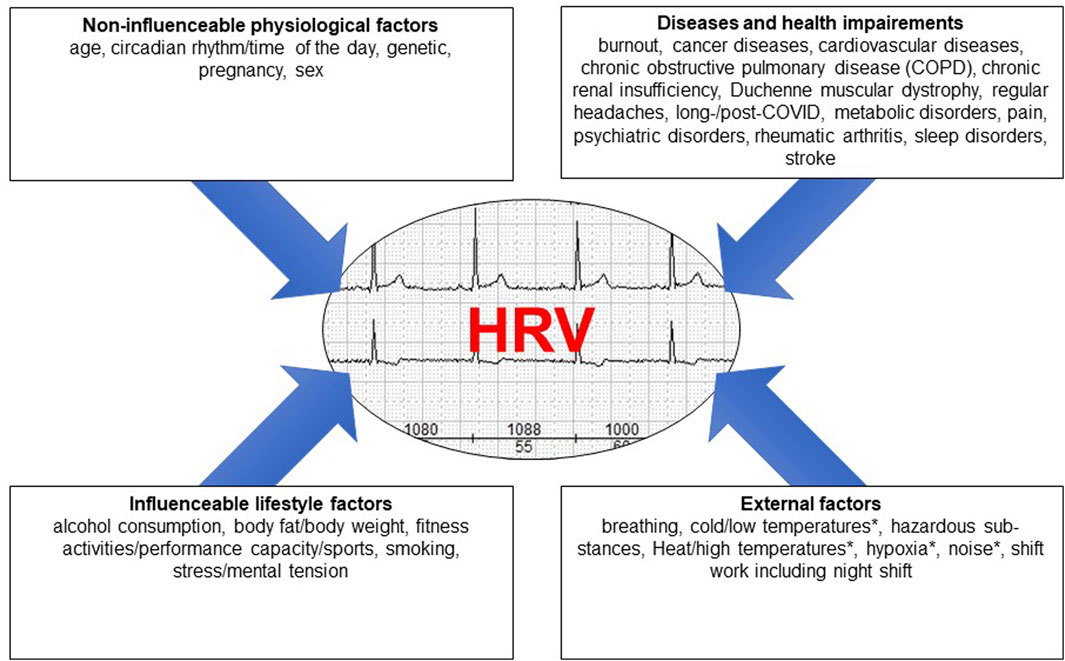
Figure 1. The different factors influencing HRV grouped into four main areas, * = HRV decrease as a result of a physiological reaction to a physical stimulus.
A decrease in HRV has been observed not only in association with non-influenceable physiological factors such as age, gender, and ethnicity, but also in association with a variety of acute and chronic diseases. Numerous lifestyle factors have both a positive and a negative effects on HRV. There are also physical influences that affect HRV. These should be recognized when analyzing HRV in intra- and interpersonal comparisons. Although not all of the factors on the list have yet been fully researched, awareness of the many factors is of crucial importance in the measurement of HRV (both under laboratory conditions and during medical practice), its analysis and its assessment.
SS: Conceptualization, Methodology, Writing–original draft, Writing–review and editing. BT: Methodology, Writing–review and editing. IB: Supervision, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
SS an active Bundeswehr officer and works for the German Federal Ministry of Defense. All authors declared that the research was conducted in the absence of any commercial, financial, or non-financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aasman J., Mulder G., Mulder L. J. (1987). Operator effort and the measurement of heart-rate variability. Hum. Factors 29, 161–170. doi:10.1177/001872088702900204
Abhishekh H. A., Nisarga P., Kisan R., Meghana A., Chandran S., Trichur R., et al. (2013). Influence of age and gender on autonomic regulation of heart. J. Clin. Monit. Comput. 27, 259–264. doi:10.1007/s10877-012-9424-3
Abreu R. M. de, Rehder-Santos P., Simões R. P., Catai A. M. (2019). Can high-intensity interval training change cardiac autonomic control? A systematic review. Braz. J. Phys. Ther. 23, 279–289. doi:10.1016/j.bjpt.2018.09.010
Adlan A. M., Lip G. Y. H., Paton J. F. R., Kitas G. D., Fisher J. P. (2014). Autonomic function and rheumatoid arthritis: a systematic review. Semin. Arthritis. Rheum. 44, 283–304. doi:10.1016/j.semarthrit.2014.06.003
Agelink M. W., Malessa R., Baumann B., Majewski T., Akila F., Zeit T., et al. (2001). Standardized tests of heart rate variability: normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clin. Aut. Res. 11, 99–108. doi:10.1007/BF02322053
Alvares G. A., Quintana D. S., Hickie I. B., Guastella A. J. (2016). Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: a systematic review and meta-analysis. J. Psychiatry. Neurosci. 41, 89–104. doi:10.1503/jpn.140217
Amirian I., Toftegård Andersen L., Rosenberg J., Gögenur I. (2014). Decreased heart rate variability in surgeons during night shifts. Can. J. Surg. 57, 300–304. doi:10.1503/cjs.028813
Arab C., Dias D. P. M., Barbosa R. T. d. A., Carvalho T. D. de, Valenti V. E., Crocetta T. B., et al. (2016). Heart rate variability measure in breast cancer patients and survivors: a systematic review. Psychoneuroendocrinology 68, 57–68. doi:10.1016/j.psyneuen.2016.02.018
Assoumou H. G. N., Pichot V., Barthelemy J. C., Dauphinot V., Celle S., Gosse P., et al. (2010). Metabolic syndrome and short-term and long-term heart rate variability in elderly free of clinical cardiovascular disease: the PROOF study. Rejuvenation Res. 13, 653–663. doi:10.1089/rej.2010.1019
Aubert A. E., Seps B., Beckers F. (2003). Heart rate variability in athletes. Sports Med. Auckl. N.Z. 33, 889–919. doi:10.2165/00007256-200333120-00003
Barantke M., Krauss T., Ortak J., Lieb W., Reppel M., Burgdorf C., et al. (2008). Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J. Cardiovasc. Electrophysiol. 19, 1296–1303. doi:10.1111/j.1540-8167.2008.01257.x
Barloese M. C. J. (2016). A review of cardiovascular autonomic control in cluster headache. Headache 56, 225–239. doi:10.1111/head.12730
Barrett E. J., Liu Z., Khamaisi M., King G. L., Klein R., Klein B. E. K., et al. (2017). Diabetic microvascular disease: an endocrine society scientific statement. J. Clin. Endocrinol. Metab. 102, 4343–4410. doi:10.1210/jc.2017-01922
Bassett D. (2016). A literature review of heart rate variability in depressive and bipolar disorders. Aust. N. Z. J. Psychiatry 50, 511–519. doi:10.1177/0004867415622689
Bellenger C. R., Fuller J. T., Thomson R. L., Davison K., Robertson E. Y., Buckley J. D. (2016). Monitoring athletic training status through autonomic heart rate regulation: a systematic review and meta-analysis. Sports Med. Auckl. N.Z. 46, 1461–1486. doi:10.1007/s40279-016-0484-2
Benichou T., Pereira B., Mermillod M., Tauveron I., Pfabigan D., Maqdasy S., et al. (2018). Heart rate variability in type 2 diabetes mellitus: a systematic review and meta-analysis. PloS one 13, e0195166. doi:10.1371/journal.pone.0195166
Bernardi L., Piepoli M. F. (2001). Autonomic nervous system adaptation during physical exercise. Italian heart J. 2, 831–839.
Bhati P., Moiz J. A., Menon G. R., Hussain M. E. (2019). Does resistance training modulate cardiac autonomic control? A systematic review and meta-analysis. Clin. Aut. Res. 29, 75–103. doi:10.1007/s10286-018-0558-3
Bhati P., Shenoy S., Hussain M. E. (2018). Exercise training and cardiac autonomic function in type 2 diabetes mellitus: a systematic review. Diabetes Metab. Syndr. 12 (1), 69–78. doi:10.1016/j.dsx.2017.08.015
Bhaumik G., Dass D., Bhattacharyya D., Sharma Y. K., Singh S. B. (2013). Heart rate variabilty changes during first week of acclimatization to 3500 m altitude in Indian military personnel. Indian. J. Physiol. Pharmacol. 57, 16–22.
Birkhofer A., Schmidt G., Förstl H. (2005). Heart and brain -- the influence of psychiatric disorders and their therapy on the heart rate variability. Fortschr. Neurol. Psychiatr. 73, 192–205. doi:10.1055/s-2004-830109
Biswas P. K., Basu S., Mitra K. K., Chowdhury S. P., Chatterjee B. P., Das Biswas A., et al. (2000). Heart rate variability in dilated cardiomyopathy. Indian. Heart. J. 52, 187–191.
Böckelmann I., Pfister E. A., McGauran N., Robra B.-P. (2002). Assessing the suitability of crosssectional and longitudinal cardiac rhythm tests with regard to identifying effects of occupational chronic lead exposure. J. Occup. Environ. Med. 44, 59–65. doi:10.1097/00043764-200201000-00010
Bortkiewicz A., Gadzicka E., Szymczak W. (1997). Heart rate variability in workers exposed to carbon disulfide. J. Aut. Nerv. Syst. 66, 62–68. doi:10.1016/s0165-1838(97)00045-3
Bortkiewicz A., Gadzicka E., Szymczak W., Szyjkowska A., Koszada-Włodarczyk W., Makowiec-Dabrowska T. (2006). Physiological reaction to work in cold microclimate. Int. J. Occup. Med. Environ. Health 19, 123–131. doi:10.2478/v10001-006-0020-y
Braith R. W., Edwards D. G. (2003). Neurohormonal abnormalities in heart failure: impact of exercise training. Congest. Heart. Fail. 9, 70–76. doi:10.1111/j.1527-5299.2003.00277.x
Brito J. M., Belotti L., Toledo A. C., Antonangelo L., Silva F. S., Alvim D. S., et al. (2010). Acute cardiovascular and inflammatory toxicity induced by inhalation of diesel and biodiesel exhaust particles. J. Soc. Toxicol. 116, 67–78. doi:10.1093/toxsci/kfq107
Britton A., Shipley M., Malik M., Hnatkova K., Hemingway H., Marmot M. (2007). Changes in heart rate and heart rate variability over time in middle-aged men and women in the general population (from the Whitehall II Cohort Study). Am. J. Cardiol. 100, 524–527. doi:10.1016/j.amjcard.2007.03.056
Brown L., Karmakar C., Gray R., Jindal R., Lim T., Bryant C. (2018). Heart rate variability alterations in late life depression: a meta-analysis. J. Affect. Disord. 235, 456–466. doi:10.1016/j.jad.2018.04.071
Buccelletti E., Gilardi E., Scaini E., Galiuto L., Persiani R., Biondi A., et al. (2009). Heart rate variability and myocardial infarction: systematic literature review and metanalysis. Eur. Rev. Med. Pharmacol. Sci. 13 (4), 299–307.
Carr O., Vos M. de, Saunders K. E. A. (2018). Heart rate variability in bipolar disorder and borderline personality disorder: a clinical review. Evid. Based. Ment. Health 21, 23–30. doi:10.1136/eb-2017-102760
Carthy E. R. (2014). Autonomic dysfunction in essential hypertension: a systematic review. Ann. Med. Surg. 3 (1), 2–7. doi:10.1016/j.amsu.2013.11.002
Chalmers J. A., Quintana D. S., Abbott M. J.-A., Kemp A. H. (2014). Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front. Psychiatry 5, 80. doi:10.3389/fpsyt.2014.00080
Chandola T., Britton A., Brunner E., Hemingway H., Malik M., Kumari M., et al. (2008). Work stress and coronary heart disease: what are the mechanisms? Eur. Heart J. 29, 640–648. doi:10.1093/eurheartj/ehm584
Chandola T., Heraclides A., Kumari M. (2010). Psychophysiological biomarkers of workplace stressors. Neurosci. Biobehav. Rev. 35, 51–57. doi:10.1016/j.neubiorev.2009.11.005
Chang Y.-W., Lin J.-D., Chen W.-L., Yen C.-F., Loh C.-H., Fang W.-H., et al. (2012). Metabolic syndrome and short-term heart rate variability in adults with intellectual disabilities. Res. Dev. Disabil. 33, 1701–1707. doi:10.1016/j.ridd.2012.04.005
Chung M.-H., Kuo T. B. J., Hsu N., Chu H., Chou K.-R., Yang C. C. H. (2009). Sleep and autonomic nervous system changes - enhanced cardiac sympathetic modulations during sleep in permanent night shift nurses. Scand. J. Work, Environ. Health 35, 180–187. doi:10.5271/sjweh.1324
Clamor A., Lincoln T. M., Thayer J. F., Koenig J. (2016). Resting vagal activity in schizophrenia: meta-analysis of heart rate variability as a potential endophenotype. Br. J. Psychiatry 208, 9–16. doi:10.1192/bjp.bp.114.160762
Clays E., Bacquer D. de, Crasset V., Kittel F., Smet P. de, Kornitzer M., et al. (2011). The perception of work stressors is related to reduced parasympathetic activity. Int. Arch. Occup. Environ. Health 84, 185–191. doi:10.1007/s00420-010-0537-z
da Silva T. D., Massetti T., Crocetta T. B., Mello Monteiro C. B. de, Carll A., Vanderlei L. C. M., et al. (2018). Heart rate variability and cardiopulmonary dysfunction in patients with Duchenne muscular dystrophy: a systematic review. A Syst. Rev. Pediatr. Cardiol. 39, 869–883. doi:10.1007/s00246-018-1881-0
Davies L. C., Colhoun H., Coats A. J. S., Piepoli M., Francis D. P. (2002). A noninvasive measure of baroreflex sensitivity without blood pressure measurement. Am. Heart J. 143, 441–447. doi:10.1067/mhj.2002.121263
Del Pozo J. M., Gevirtz R. N., Scher B., Guarneri E. (2004). Biofeedback treatment increases heart rate variability in patients with known coronary artery disease. Am. Heart J. 147, E11. doi:10.1016/j.ahj.2003.08.013
Dhar P., Sharma V. K., Hota K. B., Das S. K., Hota S. K., Srivastava R. B., et al. (2014). Autonomic cardiovascular responses in acclimatized lowlanders on prolonged stay at high altitude: a longitudinal follow up study. PloS one 9, e84274. doi:10.1371/journal.pone.0084274
Dinas P. C., Koutedakis Y., Flouris A. D. (2013). Effects of active and passive tobacco cigarette smoking on heart rate variability. Int. J. Cardiol. 163, 109–115. doi:10.1016/j.ijcard.2011.10.140
Dishman R. K., Nakamura Y., Garcia M. E., Thompson R. W., Dunn A. L., Blair S. N. (2000). Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. Int. J. Psychophysiol. 37, 121–133. doi:10.1016/s0167-8760(00)00085-4
Dodds K. L., Miller C. B., Kyle S. D., Marshall N. S., Gordon C. J. (2017). Heart rate variability in insomnia patients: a critical review of the literature. Sleep. Med. Rev. 33, 88–100. doi:10.1016/j.smrv.2016.06.004
Eyre E. L. J., Duncan M. J., Birch S. L., Fisher J. P. (2014). The influence of age and weight status on cardiac autonomic control in healthy children: a review. Auton. Neurosci. 186, 8–21. doi:10.1016/j.autneu.2014.09.019
Fagard R. H. (2001). A population-based study on the determinants of heart rate and heart rate variability in the frequency domain. Verh. - K. Acad. Geneeskd. Belg. 63, 57–91.
Fagard R. H., Pardaens K., Staessen J. A. (1999). Influence of demographic, anthropometric and lifestyle characteristics on heart rate and its variability in the population. J. Hypertens. 17, 1589–1599. doi:10.1097/00004872-199917110-00013
Faurholt-Jepsen M., Kessing L. V., Munkholm K. (2017). Heart rate variability in bipolar disorder: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 73, 68–80. doi:10.1016/j.neubiorev.2016.12.007
Felber Dietrich D., Schindler C., Schwartz J., Barthélémy J.-C., Tschopp J.-M., Roche F., et al. (2006). Heart rate variability in an ageing population and its association with lifestyle and cardiovascular risk factors: results of the SAPALDIA study. Europace 8, 521–529. doi:10.1093/europace/eul063
Felber Dietrich D., Schwartz J., Schindler C., Gaspoz J.-M., Barthélémy J.-C., Tschopp J.-M., et al. (2007). Effects of passive smoking on heart rate variability, heart rate and blood pressure: an observational study. Int. J. Epidemiol. 36, 834–840. doi:10.1093/ije/dym031
Feng W., He X., Chen M., Deng S., Qiu G., Li X., et al. (2015). Urinary metals and heart rate variability: a cross-sectional study of urban adults in Wuhan, China. Environ. Health. Perspect. 123, 217–222. doi:10.1289/ehp.1307563
Ferrari A. U. (2002). Modifications of the cardiovascular system with aging. Am. J. Geriatr. Cardiol. 11, 30–33. doi:10.1111/1467-8446.00044-i1
Fraley M. A., Birchem J. A., Senkottaiyan N., Alpert M. A. (2005). Obesity and the electrocardiogram. Obes. Rev. 6, 275–281. doi:10.1111/j.1467-789X.2005.00199.x
Friedman B. H. (2007). An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol. Psychol. 74, 185–199. doi:10.1016/j.biopsycho.2005.08.009
Friedman B. H., Thayer J. F. (1998). Autonomic balance revisited: panic anxiety and heart rate variability. J. Psychosom. Res. 44, 133–151. doi:10.1016/s0022-3999(97)00202-x
Fukusaki C., Kawakubo K., Yamamoto Y. (2000). Assessment of the primary effect of aging on heart rate variability in humans. Clin. Auton. Res. 10, 123–130. doi:10.1007/BF02278016
Gehi A. K., Lampert R., Veledar E., Lee F., Goldberg J., Jones L., et al. (2009). A twin study of metabolic syndrome and autonomic tone. J. Cardiovasc. Electrophysiol. 20, 422–428. doi:10.1111/j.1540-8167.2008.01363.x
Grandjean P., Murata K., Budtz-Jørgensen E., Weihe P. (2004). Cardiac autonomic activity in methylmercury neurotoxicity: 14-year follow-up of a Faroese birth cohort. J. Pediatr. 144, 169–176. doi:10.1016/j.jpeds.2003.10.058
Grässler B., Thielmann B., Böckelmann I., Hökelmann A. (2021). Effects of different exercise interventions on heart rate variability and cardiovascular health factors in older adults: a systematic review. Eur. Rev. Aging. Phys. Act. 18, 24. doi:10.1186/s11556-021-00278-6
Greiser K. H., Kluttig A., Schumann B., Swenne C. A., Kors J. A., Kuss O., et al. (2009). Cardiovascular diseases, risk factors and short-term heart rate variability in an elderly general population: the CARLA study 2002-2006. Eur. J. Epidemiol. 24, 123–142. doi:10.1007/s10654-009-9317-z
Guzzetti S., Magatelli R., Borroni E., Mezzetti S. (2001). Heart rate variability in chronic heart failure. Auton. Neurosci. 90, 102–105. doi:10.1016/S1566-0702(01)00274-0
Ha M., Kim J., Park J., Chung H. K. (2001). Blood pressure and heart rate variability in workers of 8-hour shifts. J. Hum. Ergol. 30, 229–233.
Haerting J., Kluttig A., Greiser K. H., Nuding S., Werdan K. (2012). A cohort study investigating risk factors for cardiovascular disease in an urban elderly East-German population (CARLA study). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 55, 795–800. doi:10.1007/s00103-012-1493-4
Handa R., Poanta L., Rusu D., Albu A. (2012). The role of heart rate variability in assessing the evolution of patients with chronic obstructive pulmonary disease. Rom. J. Intern. Med. 50, 83–88.
Harinath K., Malhotra A. S., Pal K., Prasad R., Kumar R., Sawhney R. C. (2005). Autonomic nervous system and adrenal response to cold in man at Antarctica. Wilderness Environ. Med. 16, 81–91. doi:10.1580/pr30-04.1
Hemingway H., Shipley M., Brunner E., Britton A., Malik M., Marmot M. (2005). Does autonomic function link social position to coronary risk? The White-hall II study. Circulation 111, 3071–3077. doi:10.1161/CIRCULATIONAHA.104.497347
Hill L. K., Hu D. D., Koenig J., Sollers J. J., Kapuku G., Wang X., et al. (2015). Ethnic differences in resting heart rate variability: a systematic review and metaanalysis. Psychosom. Med. 77, 16–25. doi:10.1097/PSY.0000000000000133
Hill L. K., Siebenbrock A. (2009). Are all measures created equal? Heart rate variability and respiration - biomed 2009. Biomed. Sci. Instrum. 45, 71–76.
Hottenrott K., Hoos O., Esperer H. D. (2006). Heart rate variability and physical exercise. Current status. Herz 31, 544–552. doi:10.1007/s00059-006-2855-1
Huang W., Zhu T., Pan X., Hu M., Lu S.-E., Lin Y., et al. (2012). Air pollution and autonomic and vascular dysfunction in patients with cardiovascular disease: interactions of systemic inflammation, overweight, and gender. Am. J. Epidemiol. 176, 117–126. doi:10.1093/aje/kwr511
Huikuri H. V., Mäkikallio T. H. (2001). Heart rate variability in ischemic heart disease. Aut. Neurosci. 90, 95–101. doi:10.1016/S1566-0702(01)00273-9
Huikuri H. V., Pikkujämsä S. M., Airaksinen K. E., Ikäheimo M. J., Rantala A. O., Kauma H., et al. (1996). Sex-related differences in autonomic modulation of heart rate in middle-aged subjects. Circulation 94, 122–125. doi:10.1161/01.cir.94.2.122
Huikuri H. V., Stein P. K. (2013). Heart rate variability in risk stratification of cardiac patients. Prog. Cardiovasc. Dis. 56, 153–159. doi:10.1016/j.pcad.2013.07.003
Järvelin-Pasanen S., Ropponen A., Tarvainen M. P., Karjalainen P. A., Louhevaara V. (2013). Differences in heart rate variability of female nurses between and within normal and extended work shifts. Ind. Health 51, 154–164. doi:10.2486/indhealth.ms1368
Järvelin-Pasanen S., Sinikallio S., Tarvainen M. P. (2018). Heart rate variability and occupational stress-systematic review. Ind. Health 56, 500–511. doi:10.2486/indhealth.2017-0190
Jennings J. R., Mack M. E. (1984). Does aging differentially reduce heart rate variability related to respiration? Exp. Aging. Res. 10, 19–23. doi:10.1080/03610738408258536
Jensen M. A., Garde A. H., Kristiansen J., Nabe-Nielsen K., Hansen Å. M. (2016). The effect of the number of consecutive night shifts on diurnal rhythms in cortisol, melatonin and heart rate variability (HRV): a systematic review of field studies. Int. Arch. Occup. Environ. Health. 89, 531–545. doi:10.1007/s00420-015-1093-3
Jensen-Urstad K., Storck N., Bouvier F., Ericson M., Lindblad L. E., Jensen-Urstad M. (1997). Heart rate variability in healthy subjects is related to age and gender. Acta. Physiol. Scand. 160, 235–241. doi:10.1046/j.1365-201X.1997.00142.x
Jhun H.-J., Yim S.-H., Kim R., Paek D. (2003). Heartrate variability of carbon disulfide-poisoned subjects in Korea. Int. Arch. Occup. Environ. Health 76, 156–160. doi:10.1007/s00420-002-0391-8
Kanters J. K., Højgaard M. V., Agner E., Holstein-Rathlou N. H. (1997). Influence of forced respiration on nonlinear dynamics in heart rate variability. Am. J. Physiol. 272, R1149–R1154. doi:10.1152/ajpregu.1997.272.4.R1149
Kapfhammer H.-P. (2011). The relationship between depression, anxiety and heart disease - a psychosomatic challenge. Psychiatr. Danub. 23, 412–424.
Karayannis G., Giamouzis G., Cokkinos D. V., Skoularigis J., Triposkiadis F. (2012). Diabetic cardiovascular autonomic neuropathy: clinical implications. Expert Rev. Cardiovasc. Ther. 10, 747–765. doi:10.1586/erc.12.53
Karpyak V. M., Romanowicz M., Schmidt J. E., Lewis K. A., Bostwick J. M. (2014). Characteristics of heart rate variability in alcohol-dependent subjects and nondependent chronic alcohol users. Alcohol. Clin. Exp. Res. 38, 9–26. doi:10.1111/acer.12270
Kemp A. H., Quintana D. S., Gray M. A., Felmingham K. L., Brown K., Gatt J. M. (2010). Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol. Psychiatry 67, 1067–1074. doi:10.1016/j.biopsych.2009.12.012
Koenig J., Falvay D., Clamor A., Wagner J., Jarczok M. N., Ellis R. J., et al. (2016a). Pneumogastric (vagus) nerve activity indexed by heart rate variability in chronic pain patients compared to healthy controls: a systematic review and meta-analysis. Pain Physician 19, E55–E78. doi:10.36076/ppj/2016.19.e55
Koenig J., Kemp A. H., Feeling N. R., Thayer J. F., Kaess M. (2016b). Resting state vagal tone in borderline personality disorder: a meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 64, 18–26. doi:10.1016/j.pnpbp.2015.07.002
Koenig J., Thayer J. F. (2016). Sex differences in healthy human heart rate variability: a meta-analysis. Neurosci. Biobehav. Rev. 64, 288–310. doi:10.1016/j.neubiorev.2016.03.007
Koenig J., Williams D. P., Kemp A. H., Thayer J. F. (2016c). Vagally mediated heart rate variability in headache patients - a systematic review and meta-analysis. Cephalalgia 36, 265–278. doi:10.1177/0333102415583989
Koerber T., Ismer B., von Knorre G. H. (2000). Influences of Holter recording technology on time domain heart rate variability – laboratory investigations. Herzschr. Elektrophys. 11, 56–65.
Koskinen T., Kähönen M., Jula A., Mattsson N., Laitinen T., Keltikangas-Järvinen L., et al. (2009). Metabolic syndrome and short-term heart rate variability in young adults. The cardiovascular risk in young Finns study. Diabet. Med. 26, 354–361. doi:10.1111/j.1464-5491.2009.02686.x
Kraus U., Schneider A., Breitner S., Hampel R., Rückerl R., Pitz M., et al. (2013). Individual daytime noise exposure during routine activities and heart rate variability in adults: a repeated measures study. Environ. Health Perspect. 121, 607–612. doi:10.1289/ehp.1205606
Kuehl M., Stevens M. J. (2012). Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat. Rev. Endocrinol. 8, 405–416. doi:10.1038/nrendo.2012.21
Kuo T. B., Lin T., Yang C. C., Li C. L., Chen C. F., Chou P. (1999). Effect of aging on gender differences in neural control of heart rate. Am. J. Physiology 277, H2233–H2239. doi:10.1152/ajpheart.1999.277.6.H2233
Lakusic N., Mahovic D., Sonicki Z., Slivnjak V., Baborski F. (2013). Outcome of patients with normal and decreased heart rate variability after coronary artery bypass grafting surgery. Int. J. Cardiol. 166, 516–518. doi:10.1016/j.ijcard.2012.04.040
Lasisi G. T., Adebola A. P., Ogah O. S., Daniel F. A. (2012). Prevalence of ventricular arrhythmias and heart rate variability pattern in chronic heart failure. Niger. Postgrad. Med. J. 19, 157–162. doi:10.4103/1117-1936.169663
Lee G.-S., Chen M.-L., Wang G.-Y. (2010). Evoked response of heart rate variability using short-duration white noise. Aut. Neurosci. 155, 94–97. doi:10.1016/j.autneu.2009.12.008
Lehrer P. (2003a). Applied psychophysiology: beyond the boundaries of biofeedback (mending a wall, a brief history of our field, and applications to control of the muscles and cardiorespiratory systems). Appl. Psychophysiol. Biofeedback 28, 291–304. doi:10.1023/a:1027330909265
Lehrer P., Vaschillo E., Lu S.-E., Eckberg D., Vaschillo B., Scardella A., et al. (2006). Heart rate variability biofeedback: effects of age on heart rate variability, baroreflex gain, and asthma. Chest 129, 278–284. doi:10.1378/chest.129.2.278
Lehrer P. M., Vaschillo E., Vaschillo B., Lu S.-E., Eckberg D. L., Edelberg R., et al. (2003). Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosom. Med. 65, 796–805. doi:10.1097/01.psy.0000089200.81962.19
Liao D., Cai J., Barnes R. W., Tyroler H. A., Rautaharju P., Holme I., et al. (1996). Association of cardiac autonomic function and the development of hypertension: the ARIC study. Am. J. Hypertens. 9, 1147–1156. doi:10.1016/s0895-7061(96)00249-x
Liao D., Sloan R. P., Cascio W. E., Folsom A. R., Liese A. D., Evans G. W., et al. (1998). Multiple metabolic syndrome is associated with lower heart rate variability. The Atherosclerosis Risk in Communities Study. Diabetes care 21, 2116–2122. doi:10.2337/diacare.21.12.2116
Lindholm H., Sinisalo J., Ahlberg J., Hirvonen A., Hublin C., Partinen M., et al. (2012). Attenuation of vagal recovery during sleep and reduction of cortisol/melatonin ratio in late afternoon associate with prolonged daytime sleepiness among media workers with irregular shift work. Am. J. Ind. Med. 55, 643–649. doi:10.1002/ajim.22042
Looser R. R., Metzenthin P., Helfricht S., Kudielka B. M., Loerbroks A., Thayer J. F., et al. (2010). Cortisol is significantly correlated with cardiovascular responses during high levels of stress in critical care personnel. Psychosom. Med. 72, 281–289. doi:10.1097/PSY.0b013e3181d35065
Lotufo P. A., Valiengo L., Benseñor I. M., Brunoni A. R. (2012). A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia 53, 272–282. doi:10.1111/j.1528-1167.2011.03361.x
Min K.-B., Min J.-Y., Paek D., Cho S.-I. (2008). The impact of the components of metabolic syndrome on heart rate variability: using the NCEP-ATP III and IDF definitions. Pacing Clin. Electrophysiol. 31, 584–591. doi:10.1111/j.1540-8159.2008.01045.x
Mohammed J., Meeus M., Derom E., Da Silva H., Calders P. (2015). Evidence for autonomic function and its influencing factors in subjects with COPD: a systematic review. Respir. Care 60, 1841–1851. doi:10.4187/respcare.04174
Murata K., Araki S., Yokoyama K. (1991a). Assessment of the peripheral, central, and autonomic nervous system function in styrene workers. Am. J. Ind. Med. 20, 775–784. doi:10.1002/ajim.4700200609
Murata K., Araki S., Yokoyama K., Maeda K. (1991b). Autonomic and peripheral nervous system dysfunction in workers exposed to mixed organic solvents. Int. Arch. Occup. Environ. Health. 63, 335–340. doi:10.1007/BF00381584
Murata K., Araki S., Yokoyama K., Nomiyama K., Nomiyama H., Tao Y. X., et al. (1995). Autonomic and central nervous system effects of lead in female glass workers in China. Am. J. Ind. Med. 28, 233–244. doi:10.1002/ajim.4700280208
Paniccia M., Paniccia D., Thomas S., Taha T., Reed N. (2017). Clinical and non-clinical depression and anxiety in young people: a scoping review on heart rate variability. Aut. Neurosci. 208, 1–14. doi:10.1016/j.autneu.2017.08.008
Peira N., Pourtois G., Fredrikson M. (2013). Learned cardiac control with heart rate biofeedback transfers to emotional reactions. PLoS ONE 8, e70004. doi:10.1371/journal.pone.0070004
Peschel S. K. V., Feeling N. R., Vögele C., Kaess M., Thayer J. F., Koenig J. (2016). A meta-analysis on resting state high-frequency heart rate variability in bulimia nervosa. Eur. Eat. Disord. Rev. 24, 355–365. doi:10.1002/erv.2454
Ralevski E., Petrakis I., Altemus M. (2019). Heart rate variability in alcohol use: a review. Pharmacol. Biochem. Behav. 176, 83–92. doi:10.1016/j.pbb.2018.12.003
Ramaekers D., Ector H., Demyttenaere K., Rubens A., van de Werf F. (1998). Association between cardiac autonomic function and coping style in healthy subjects. Pacing Clin. Electrophysiol. 21, 1546–1552. doi:10.1111/j.1540-8159.1998.tb00241.x
Reinhardt F., Drexler H., Bickel A., Claus D., Ulm K., Angerer J., et al. (1997). Electrophysiological investigation of central, peripheral and autonomic nerve function in workers with long-term low-level exposure to carbon disulphide in the viscose industry. Int. Arch. Occup. Environ. Health 70, 249–256. doi:10.1007/s004200050215
Ren C., O'Neill M. S., Park S. K., Sparrow D., Vokonas P., Schwartz J. (2011). Ambient temperature, air pollution, and heart rate variability in an aging population. Am. J. Epidemiol. 173, 1013–1021. doi:10.1093/aje/kwq477
Rennie K. L., Hemingway H., Kumari M., Brunner E., Malik M., Marmot M. (2003). Effects of moderate and vigorous physical activity on heart rate variability in a British study of civil servants. Am. J. Epidemiol. 158, 135–143. doi:10.1093/aje/kwg120
Riese H., Muñoz L. M., Hartman C. A., Ding X., Su S., Oldehinkel A. J., et al. (2014). Identifying genetic variants for heart rate variability in the acetylcholine pathway. PloS one 9, e112476. doi:10.1371/journal.pone.0112476
Riese H., Rosmalen J. G. M., Ormel J., van Roon A. M., Oldehinkel A. J., Rijsdijk F. V. (2007). The genetic relationship between neuroticism and autonomic function in female twins. Psychol. Med. 37, 257–267. doi:10.1017/S0033291706009160
Roque A. L., Valenti V. E., Massetti T., da Silva T. D., Monteiro C. B. d. M., Oliveira F. R., et al. (2014). Chronic obstructive pulmonary disease and heart rate variability: a literature update. Int. Arch. Med. 7, 43. doi:10.1186/1755-7682-7-43
Routledge F. S., Campbell T. S., McFetridge-Durdle J. A., Bacon S. L. (2010). Improvements in heart rate variability with exercise therapy. Can. J. Cardiol. 26, 303–312. doi:10.1016/s0828-282x(10)70395-0
Sammito S., Böckelmann I. (2016). Factors influencing heart rate variability. Int. J. Cardiol. Forum 6, 18–22. doi:10.17987/icfj.v6i0.242
Sammito S., Sammito W., Böckelmann I. (2016). The circadian rhythm of heart rate variability. Biol. Rhythm Res. 47, 717–730. doi:10.1080/09291016.2016.1183887
Sammito S., Thielmann B., Klussmann A., Deußen A., Braumann K.-M., Böckelmann I. (2024). Guideline for the application of heart rate and heart rate variability in occupational medicine and occupational health science. J. Occup. Med. Tox. 19, 15. doi:10.1186/s12995-024-00414-9
Sammito S., Thielmann B., Zimmermann P., Böckelmann I. (2015). Influence of post-traumatic stress disorder on heart rate variability as marker of the autonomic nervous system - a systematic review. Fortschr. Neurol. Psychiatr. 83, 30–37. doi:10.1055/s-0034-1398779
Sandercock G. R. H., Brodie D. A. (2006). The role of heart rate variability in prognosis for different modes of death in chronic heart failure. Pacing Clin. Electrophysiol. 29, 892–904. doi:10.1111/j.1540-8159.2006.00457.x
Sandercock G. R. H., Bromley P. D., Brodie D. A. (2005). Effects of exercise on heart rate variability: inferences from meta-analysis. Med. Sci. Sports Exerc. 37, 433–439. doi:10.1249/01.mss.0000155388.39002.9d
Sassi R., Cerutti S., Lombardi F., Malik M., Huikuri H. V., Peng C.-K., et al. (2015). Advances in heart rate variability signal analysis: joint position statement by the eCardiology ESC working group and the European heart rhythm association coendorsed by the asia pacific heart rhythm society. Europace 17, 1341–1353. doi:10.1093/europace/euv015
Scalvini S., Volterrani M., Zanelli E., Pagani M., Mazzuero G., Coats A. J., et al. (1998). Is heart rate variability a reliable method to assess autonomic modulation in left ventricular dysfunction and heart failure? Assessment of autonomic modulation with heart rate variability. Int. J. Cardiol. 67, 9–17. doi:10.1016/s0167-5273(98)00252-6
Schaffer T., Hensel B., Weigand C., Schüttler J., Jeleazcov C. (2014). Evaluation of techniques for estimating the power spectral density of RR-intervals under paced respiration conditions. J. Clin. Monit. Comput. 28, 481–486. doi:10.1007/s10877-013-9447-4
Schnell I., Potchter O., Epstein Y., Yaakov Y., Hermesh H., Brenner S., et al. (2013). The effects of exposure to environmental factors on Heart Rate Variability: an ecological perspective. Environ. Pollut. (Barking, Essex 1987) 183, 7–13. doi:10.1016/j.envpol.2013.02.005
Schroeder E. B., Liao D., Chambless L. E., Prineas R. J., Evans G. W., Heiss G. (2003). Hypertension, blood pressure, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Hypertension 42 (42), 1106–1111. doi:10.1161/01.HYP.0000100444.71069.73
Shiogai Y., Stefanovska A., McClintock P. V. E. (2010). Nonlinear dynamics of cardiovascular ageing. Phys. Rep. 488, 51–110. doi:10.1016/j.physrep.2009.12.003
Silvetti M. S., Drago F., Ragonese P. (2001). Heart rate variability in healthy children and adolescents is partially related to age and gender. Int. J. Cardiol. 81, 169–174. doi:10.1016/s0167-5273(01)00537-x
Singh J. P., Larson M. G., O'Donnell C. J., Wilson P. F., Tsuji H., Lloyd-Jones D. M., et al. (2000). Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). Am. J. Cardiol. 86, 309–312. doi:10.1016/s0002-9149(00)00920-6
Singh J. P., Larson M. G., Tsuji H., Evans J. C., O'Donnell C. J., Levy D. (1998). Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension 1979 (32), 293–297. doi:10.1161/01.hyp.32.2.293
Snieder H., van D., Lorenz J. P., Boomsma D. I., Thayer J. F. (2007). Sex differences and heritability of two indices of heart rate dynamics: a twin study. Twin Res. Hum. Genet. 10, 364–372. doi:10.1375/twin.10.2.364
Song T., Qu X. F., Zhang Y. T., Cao W., Han B. H., Li Y., et al. (2014). Usefulness of the heart-rate variability complex for predicting cardiac mortality after acute myocardial infarction. BMC Cardiovasc. Disord. 14, 59. doi:10.1186/1471-2261-14-59
Sookan T., McKune A. J. (2012). Heart rate variability in physically active individuals: reliability and gender characteristics. Cardiovasc. J. Afr. 23, 67–72. doi:10.5830/CVJA-2011.108
Stapelberg N. J., Hamilton-Craig I., Neumann D. L., Shum D. H. K., McConnell H. (2012). Mind and heart: heart rate variability in major depressive disorder and coronary heart disease - a review and recommendations. Aust. N. Z. J. Psychiatry 46, 946–957. doi:10.1177/0004867412444624
Stein P. K., Barzilay J. I., Chaves P. H. M., Domitrovich P. P., Gottdiener J. S. (2009). Heart rate variability and its changes over 5 years in older adults. Age Ageing 38, 212–218. doi:10.1093/ageing/afn292
Stein P. K., Barzilay J. I., Domitrovich P. P., Chaves P. M., Gottdiener J. S., Heckbert S. R., et al. (2007). The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: the Cardiovascular Health Study. Diabet. Med. 24, 855–863. doi:10.1111/j.1464-5491.2007.02163.x
Stuckey M. I., Tulppo M. P., Kiviniemi A. M., Petrella R. J. (2014). Heart rate variability and the metabolic syndrome: a systematic review of the literature. Diabetes Metab. Res. Rev. 30, 784–793. doi:10.1002/dmrr.2555
Suh H.-W., Kwon C.-Y., Lee B. (2023). Long-term impact of COVID-19 on heart rate variability: a systematic review of observational studies. Healthcare (Basel) 11, 1095. doi:10.3390/healthcare11081095
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, Bigger J. T., Camm A. J., Kleiger R. E., Malliani A., Moss A. J., et al. (1996). Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 17, 354–381. doi:10.1093/oxfordjournals.eurheartj.a014868
Thielmann B., Karlsen H. R., Darius S., Böckelmann I. (2021). Heart rate variability in different levels of burnout-cross-sectional study of different occupational groups heart rate variability and burnout. J. Occup. Environ. Med. 63, e622–e630. doi:10.1097/JOM.0000000000002307
Tracy L. M., Ioannou L., Baker K. S., Gibson S. J., Georgiou-Karistianis N., Giummarra M. J. (2016). Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain 157, 7–29. doi:10.1097/j.pain.0000000000000360
Tsuji H., Venditti F. J., Manders E. S., Evans J. C., Larson M. G., Feldman C. L., et al. (1996). Determinants of heart rate variability. J. Am. Coll. Cardiol. 28, 1539–1546. doi:10.1016/s0735-1097(96)00342-7
Umetani K., Singer D. H., McCraty R., Atkinson M. (1998). Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J. Am. Coll. Cardiol. 31, 593–601. doi:10.1016/s0735-1097(97)00554-8
Veternik M., Tonhajzerova I., Misek J., Jakusova V., Hudeckova H., Jakus J. (2018). The impact of sound exposure on heart rate variability in adolescent students. Physiol. Res. 67, 695–702. doi:10.33549/physiolres.933882
Voss A., Heitmann A., Schroeder R., Peters A., Perz S. (2012). Short-term heart rate variability--age dependence in healthy subjects. Physiol. Meas. 33, 1289–1311. doi:10.1088/0967-3334/33/8/1289
Voss A., Schulz S., Schroeder R., Baumert M., Caminal P. (2009). Methods derived from nonlinear dynamics for analysing heart rate variability. Philos. Trans. A. Math. Phys. Eng. Sci. 367, 277–296. doi:10.1098/rsta.2008.0232
Wallén M. B., Hasson D., Theorell T., Canlon B., Osika W. (2012). Possibilities and limitations of the Polar RS800 in measuring heart rate variability at rest. Eur. J. Appl. Physiol. 112, 1153–1165. doi:10.1007/s00421-011-2079-9
Wehrens S. M. T., Hampton S. M., Skene D. J. (2012). Heart rate variability and endothelial function after sleep deprivation and recovery sleep among male shift and non-shift workers. Work Environ. Health 38, 171–181. doi:10.5271/sjweh.3197
Wekenborg M. K., Hill L. K., Grabbe P., Thayer J. F., Kirschbaum C., Lindenlaub S., et al. (2022). Associations between burnout symptoms and social behaviour: exploring the role of acute stress and vagal function. BMC Public Health 22, 892. doi:10.1186/s12889-022-13333-3
Wilson M. D., McGlothlin J. D., Rosenthal F. S., Black D. R., Zimmerman N. J., Bridges C. D. (2010). Ergonomics. The effect of occupational exposure to environmental tobacco smoke on the heart rate variability of bar and restaurant workers. J. Occup. Environ. Hyg. 7, D44–D49. doi:10.1080/15459624.2010.483980
Wu S., Deng F., Liu Y., Shima M., Niu J., Huang Q., et al. (2013). Temperature, trafficrelated air pollution, and heart rate variability in a panel of healthy adults. Environ. Res. 120, 82–89. doi:10.1016/j.envres.2012.08.008
Yperzeele L., van Hooff R.-J., Nagels G., Smedt A. de, Keyser J. de, Brouns R. (2015). Heart rate variability and baroreceptor sensitivity in acute stroke: a systematic review. Int. J. Stroke 10, 796–800. doi:10.1111/ijs.12573
Zhang J. (2007). Effect of age and sex on heart rate variability in healthy subjects. J. Manipulative. Physiol. Ther. 30, 374–379. doi:10.1016/j.jmpt.2007.04.001
Keywords: autonomic nervous system, heart rate, analysis, sympathetic, parasympathicus
Citation: Sammito S, Thielmann B and Böckelmann I (2024) Update: factors influencing heart rate variability–a narrative review. Front. Physiol. 15:1430458. doi: 10.3389/fphys.2024.1430458
Received: 09 May 2024; Accepted: 22 July 2024;
Published: 06 August 2024.
Edited by:
Leonardo Alexandre Peyré-Tartaruga, University of Pavia, ItalyReviewed by:
Phyllis Kravet Stein, Washington University in St. Louis, United StatesCopyright © 2024 Sammito, Thielmann and Böckelmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Sammito, U3RlZmFuU2FtbWl0b0BidW5kZXN3ZWhyLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.