- Sports Rehabilitation Research Center, China Institute of Sport Science, Beijing, China
Background: The Fibromyalgia Syndrome (FMS) is a multifaceted chronic pain disorder that exerts a substantial impact on the overall state of health and quality of life of patients.
Purpose: Investigate the effects of exercise therapy and adherence to the American College of Sports Medicine (ACSM) guidelines on treatment outcomes in FMS patients.
Methods: The literature search, which concluded in October 2023, encompassed studies investigating the impact of exercise interventions on patients diagnosed with FMS and providing adequate data for calculating standardized mean difference (SMD). The primary outcome measures encompassed the Fibromyalgia Impact Questionnaire (FIQ) and Health Assessment Questionnaire (HAQ), while secondary outcome measures comprised pain levels, sleep quality, fatigue, and mental health.
Results: Among 4,008 records, 19 studies (patients = 857) were eligible for qualitative synthesis. The meta-analysis revealed that the SMD for overall state of health impact was −0.94 (95%CI −1.26, −0.63), and the pooled SMD for the subgroup with high adherence to ACSM guidelines was −1.17 (95%CI −1.65, −0.69). The SMD for the subgroup with low or uncertain adherence was −0.73 (95%CI −1.12, −0.34). The overall effects included a −1.21 (95%CI −1.62, −0.79) SMD for pain relief, with high adherence achieving a −1.32 (95%CI −2.00, −0.64) SMD and low adherence a −1.06 (95%CI −1.55, −0.57) SMD. Mental health improvements showed a −0.95 (95%CI −1.32, −0.57) overall SMD, with high and low adherence subgroups at −0.96 (95%CI −1.62, −0.30) and −0.94 (95%CI −1.29, −0.60), respectively. Sleep quality impact was −1.59 (95%CI −2.31, −0.87) overall, with high adherence at −1.71 (95%CI −2.58, −0.83) and low adherence at −1.11 (95%CI −1.88, −0.33). Fatigue impact had a −1.55 (95%CI −2.26, −0.85) overall SMD, with −1.77 (95%CI −3.18, −0.36) for high adherence and −1.35 (95%CI −2.03, −0.66) for low adherence.
Conclusion: Exercise therapy can improve the overall state of health, pain, sleep, and fatigue of FMS patients, particularly when adhering to ACSM guidelines. However, adherence levels do not affect mental health gains, indicating a need for future research on psychological impact.
Systematic Review Registration:: https://inplasy.com/inplasy-2024-3-0106/, identifier INPLASY202430106.
1 Introduction
The Fibromyalgia Syndrome (FMS) is a chronic condition characterized by widespread, diffuse pain throughout the body, often accompanied by sleep disturbances, mood fluctuations, and cognitive changes (Bannwarth, et al., 2009; Branco, et al., 2010; Chinn, et al., 2016; Garrido-Ardila, et al., 2021). The FMS arises from a multitude of physiological mechanisms, such as central sensitization, peripheral mechanisms, and aberrant neural pain processing (Rodríguez-Almagro, et al., 2023). Central sensitization refers to an augmented responsiveness of the central nervous system to pain signals, resulting in heightened pain perception among patients (Staud, et al., 2007; Goubert, et al., 2017; Oliva, et al., 2022). Peripheral pain mechanisms involve aberrant signaling within muscles and soft tissues (Giesecke, et al., 2004; González-Roldán, et al., 2016). Furthermore, FMS patients frequently exhibit neurological abnormalities characterized by increased sympathetic activity and metabolic dysregulation in the hippocampus (Wood, et al., 2009). Initial studies (Wolfe, et al., 1995; Zhu, 2021) indicated a general population prevalence ranging from approximately 2%–4%, recent research highlights significant geographical variations and the influence of regional healthcare differences. For instance, Sarzi-Puttini et al. report higher prevalence rates in Western countries (Sarzi-Puttini, et al., 2020), contrasting sharply with much lower rates in China (with an outpatient diagnosis rate of 0.25%) (Zeng, et al., 2010) as noted by Zeng et al., cultural and systemic factors may affect the identification of the disease. Furthermore, factors such as increased societal stressors, accelerated pace of modern life, significant reduction in physical activity levels and unhealthy dietary habits have led to a significant rise in FMS incidence rates (Giorgi, et al., 2023; Lee, et al., 2024). Particularly noteworthy is a recent epidemiological study (Althobaiti, et al., 2022) conducted in Saudi Arabia which reported an alarming prevalence rate of 7.6%. This underscores both the high occurrence of this disease in specific regions and its substantial impact on patients’ quality of life and socioeconomic status while emphasizing the urgent need for exploring more effective treatment approaches.
In the realm of treatment strategies, drug therapy offers limited efficacy due to the diverse pathogenesis involved in FMS as well as concerns about potential side effects (Zhang, et al., 2022). However, exercise therapy has garnered significant attention due to its practicality, cost-effectiveness, and potential for long-term benefits (Macfarlane, et al., 2017; Maffei, 2020). Numerous studies have shown that exercise can relieve FMS symptoms through various mechanisms. Firstly, it enhances muscle strength and reduces muscle fatigue to alleviate pain (Estévez-López, et al., 2021). Secondly, exercise stimulates the production of endogenous opioids and β-endorphins which activate descending pain inhibition mechanisms, thus mitigating pain perception (Tan, et al., 2022). Thirdly, exercise promotes nerve growth, metabolite regulation, and improves central nervous system function, reducing fatigue levels while enhancing cognitive function (Valim, et al., 2013). Recent studies (Busch, et al., 2011) have consistently demonstrated that exercise therapy holds promise in alleviating symptoms such as pain, sleep disturbances, and depression among patients diagnosed with FMS, while also offering notable economic and safety advantages. For instance, a study (Larsson, et al., 2015) demonstrated significant enhancements in Fibromyalgia Impact Questionnaire (FIQ) scores and pain levels following a 15-week intervention incorporating a resistance exercise program. Similarly, the research team led by Ana Assumpção has validated the beneficial therapeutic effects of flexibility and resistance exercises on individuals with FMS (Assumpção, et al., 2018).
The recent systematic review and meta-analysis (Rodríguez-Almagro, et al., 2023) conducted to assess the effects of Physical exercise-based therapy (PEBT) on patients with FMS revealed statistically significant outcomes in terms of pain relief standardized mean differences (SMD) −0.62 (95%CI −0.78, −0.46) and improvement in mental health [SMD = 0.48 (95%CI 0.29, 0.67)]. Furthermore, it was concluded that an optimal PEBT dosage would consist of 21–40 sessions, administered three times weekly for a duration ranging from 31 to 60 mins. PEBT includes a large variety of aerobic, resistance, strength, balance, and proprioceptive exercises. Consequently, the study encompassed a diverse range of exercise patterns, exhibiting significant heterogeneity within groups. Moreover, there was considerable variation in the number of control groups incorporated in the studies due to limited availability for each type of control therapy. This potential scarcity may impact the overall quality of evidence derived from this study. In contrast, the American College of Sports Medicine (ACSM) provides comprehensive guidelines based on an extensive body of scientific research that is more systematic and standardized (Savin, et al., 2023; Xijuan et al., 2023; Zhian, et al., 2023). This guideline recommends standard forms of exercise that include aerobic, flexibility, and resistance exercises, with well-defined intensity levels for disease management (Richards, et al., 2003; Álvarez-Gallardo, et al., 2019; Thompson, et al., 2020; Yapeng, et al., 2021). However, some studies, such as the systematic review conducted by Busch and Alvarez-Gallardo, have demonstrated that exercise therapy recommended by the ACSM effectively alleviates symptoms in patients with FMS(Álvarez-Gallardo, et al., 2019; Busch, et al., 2007). Specifically, Alvarez-Gallardo’s study highlighted that the exercise intervention program recommended by the ninth edition ACSM exhibited reduced efficacy in treating FMS. This observation was attributed to incomplete descriptions of exercise intervention protocols and adherence in randomized controlled trials included within the review. Moreover, the latest ACSM guidelines exhibit certain modifications in comparison to the ninth edition, including a reduction in the recommended frequency of aerobic exercise from 2–4 times per week to 1–3 times per week, as well as a change in the suggested frequency of flexibility exercises from 1–3 times per week progressing to 5 times per week, to now being advised at 2–3 times per week. Consequently, despite the broad rationale (Thompson, et al., 2013; Thompson, et al., 2020), empirical research on the actual effectiveness of the most recent ACSM guidelines for FMS management remains relatively limited, particularly regarding the impact of exercise intervention adherence on treatment outcomes.
Hence, the objective of this study is to conduct a comprehensive assessment of the impact that adherence to the latest exercise guidelines recommended by ACSM has on treatment outcomes for patients with FMS. This will be accomplished through a systematic review and meta-analysis of existing literature. The ultimate aim is to establish a basis for developing more precise and personalized exercise prescriptions for clinical management.
2 Materials and methods
2.1 Search strategy
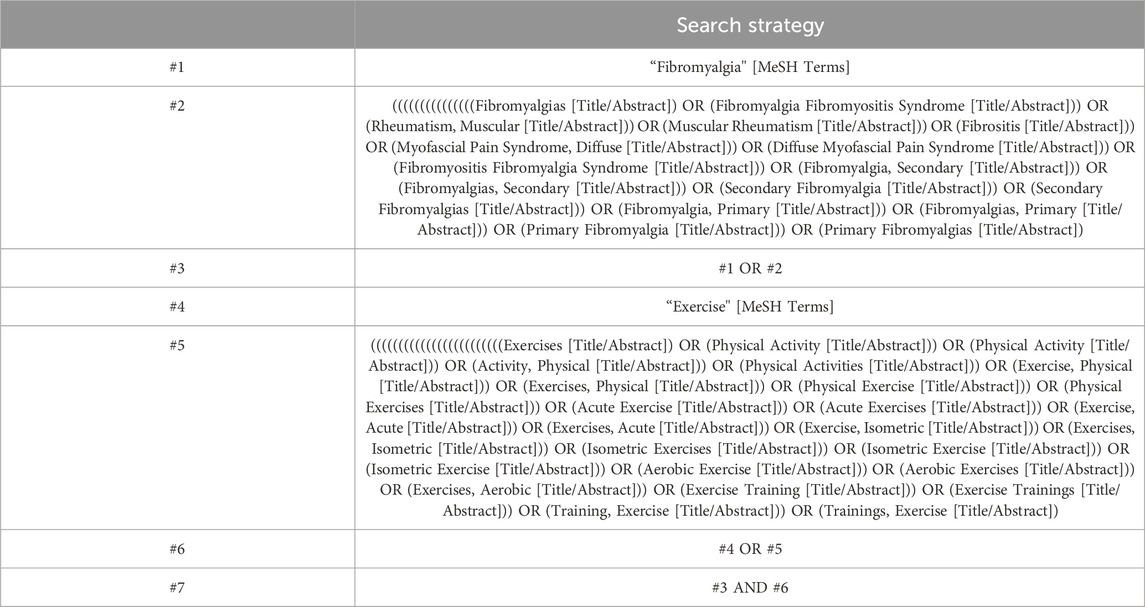
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards were followed for conducting this meta-analysis. The protocol for this review has been registered with the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) under the registration number INPLASY202430106. The registration details can be accessed at https://inplasy.com/inplasy-2024-3-0106/. Literature searches were independently conducted by two researchers from the establishment of the database up until October 2023. Computer searches were performed in various databases including PubMed, Embase, Cochrane Library and Web of Science. Our search strategy adhered to the PICOS principles with a focus on study subjects’ interventions and research methodologies. The detailed search strategy is presented in Table 1.
2.2 Eligibility criteria
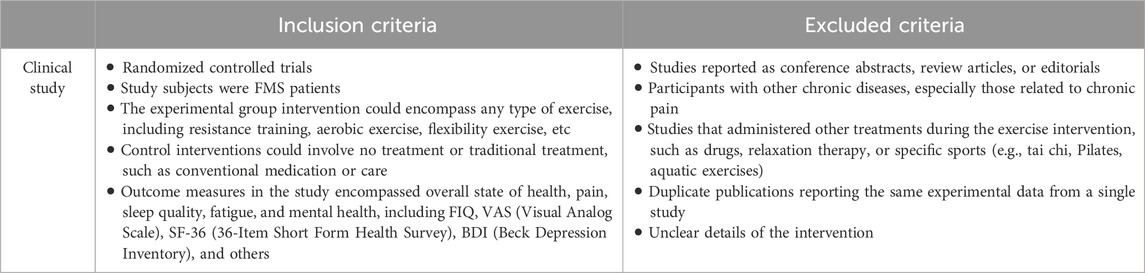
The inclusion and exclusion criteria are shown in Table 2.
2.3 Study selection
The titles and abstracts of the retrieved literature were independently assessed by two reviewers to determine inclusion criteria. If either reviewer considered a study potentially meeting the criteria, the full text of the article was obtained. Subsequently, both reviewers independently evaluated whether the full text met inclusion criteria. In cases of disagreement, a third reviewer facilitated final decision-making through discussion until consensus was reached. There were no restrictions on participant age, gender, body mass index, publication date or language.
2.4 Quality assessment
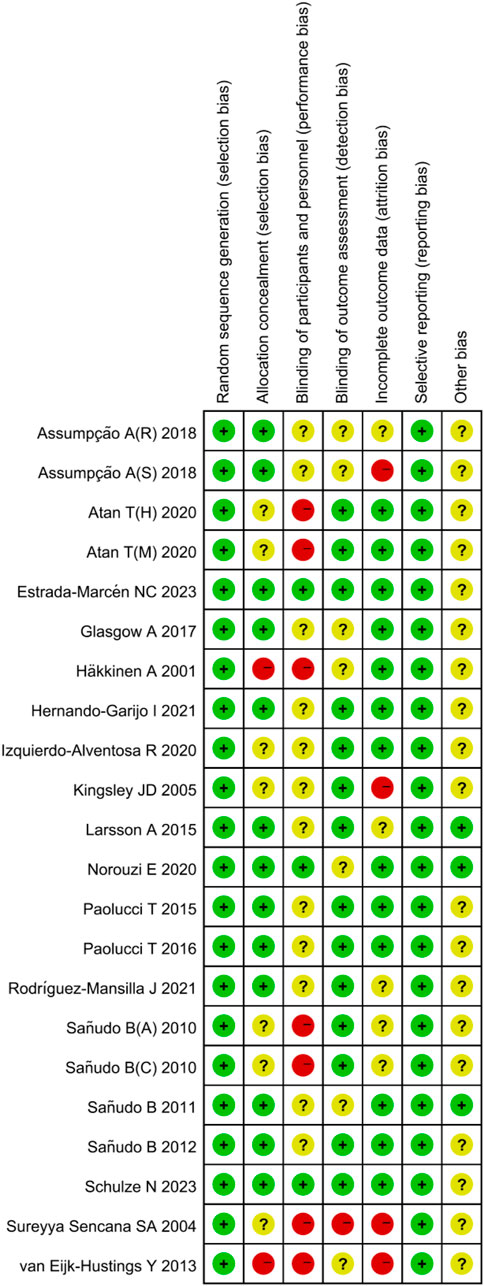
The quality of the included studies was assessed by two pairs of authors using the Cochrane Collaboration’s recommended quality evaluation standard for randomized controlled trials (Review Manager 5.4). The assessment encompassed random sequence generation, allocation concealment, blinding of participants and personal, blinding of outcome assessment, incomplete outcome data, selective reporting, other bias. Reviewers scored individual studies based on the Cochrane Handbook’s guidelines, categorizing the risk of bias in each domain as “low risk,” “some concerns,” or “high risk.” If all domains were deemed low risk, the overall risk of bias was considered low; if some domains raised “some concerns” and none were rated as high risk, the overall risk of bias was labelled as “some concerns”; and if any single domain was rated as “high risk,” the overall risk of bias was deemed “high risk."
2.5 Data extraction and analysis
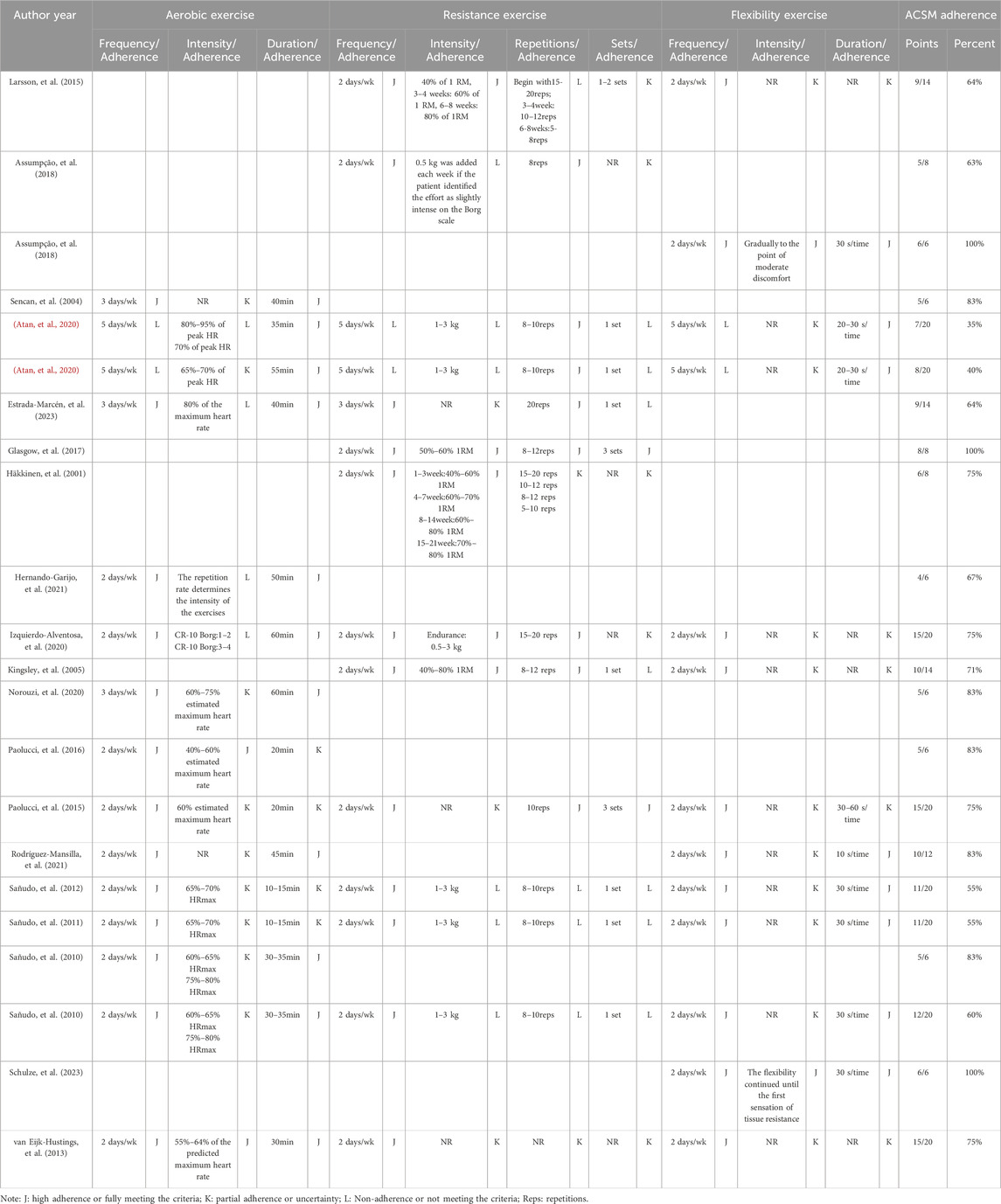
The data extraction process was independently conducted by two authors. In this study, primary outcomes included FIQ and Health Assessment Questionnaire (HAQ), which reflect the overall state of health, while secondary outcomes comprised pain, sleep quality, fatigue, and mental health. An Excel spreadsheet was prepared in advance to extract relevant data, covering publication characteristics (author names, publication year, and country), methodological characteristics (number of study groups, group designs, interventions, and sample sizes), participant characteristics (age, sex ratio), exercise details (intervention frequency, intensity, duration, repetition numbers, and set numbers), as well as risk assessment and outcome features. The Webplotdigitizer software was used to extract data when outcome data were presented graphically without clear textual descriptions. The exercise dosage and adherence were subsequently evaluated according to the ACSM recommendations for patients with FMS. Each aspect of the exercise intervention in each study was independently scored by two authors according to the criteria defined by the ACSM (the 11th Edition) (Liguori, et al., 2021) recommended dosage, in order to assess exercise intervention adherence (see Table 3). The scoring ranged from 0 to 2 points for each exercise indicator, with 2 points indicating high adherence or fully meeting the criteria, 1 point indicating partial adherence or uncertainty, and 0 points indicating non-adherence or not meeting the criteria. In cases of disagreement, a discussion was held with a third author to reach a consensus. Using this scoring system, the proportion of exercise dosage adherence in each study according to the ACSM recommended dosage was calculated. A proportion of ≥75% was classified as high adherence with ACSM recommendations, while a proportion of <75% was classified as low or uncertain adherence.
We conducted this meta-analysis using Stata 15.0 to compare the outcomes of the selected studies. These studies were divided into two groups based on their adherence to the ACSM guidelines, categorized as high, low, or indeterminate adherence. To assess the heterogeneity within each subgroup, we employed the Higgins I2 statistic following the guidelines outlined in the Cochrane Handbook. A fixed-effects model was applied when the I2 value was 50% or less, while a random-effects model was utilized for I2 values exceeding 50%. The effect size was expressed as the SMD along with its corresponding 95% Confidence Interval (95% CI). We examined publication bias by generating a funnel plot to explore the relationship between each study’s effect size and its standard error. To assess funnel plot asymmetry, we conducted sensitivity analysis using Stata 15.0, progressively excluding studies to assess the robustness of our results.
3 Results
3.1 Study selection
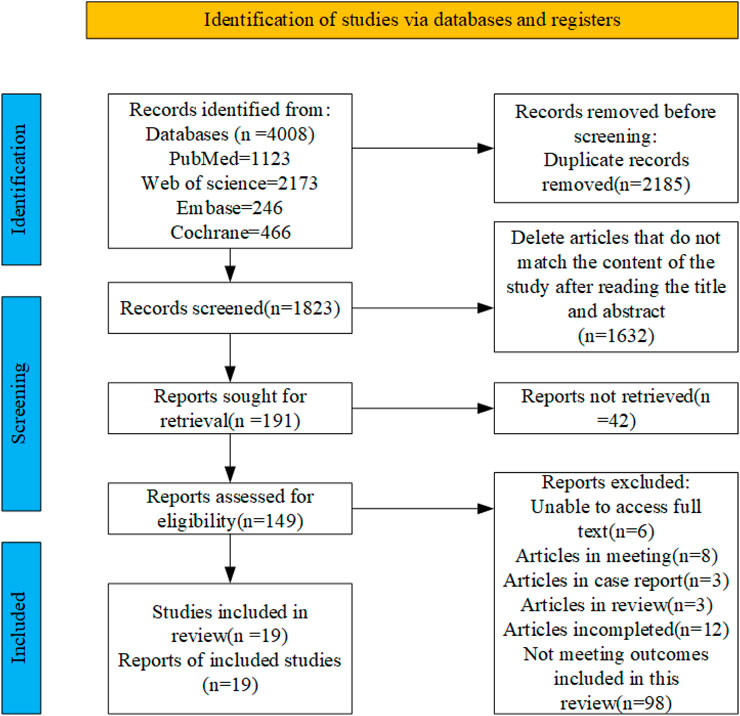
A total of 4,008 articles were retrieved from four databases: PubMed (n = 1,123), Embase (n = 246), Web of Science (n = 2,173), and Cochrane (n = 466). After removing duplicates, 1823 records remained. Subsequently, following a thorough review of the titles and abstracts, 149 articles were identified as potential candidates for inclusion. Finally, after a comprehensive examination of the full texts, 19 relevant articles were included, the flowchart illustrating the literature selection process is presented in Figure 1.
3.2 Study characteristics
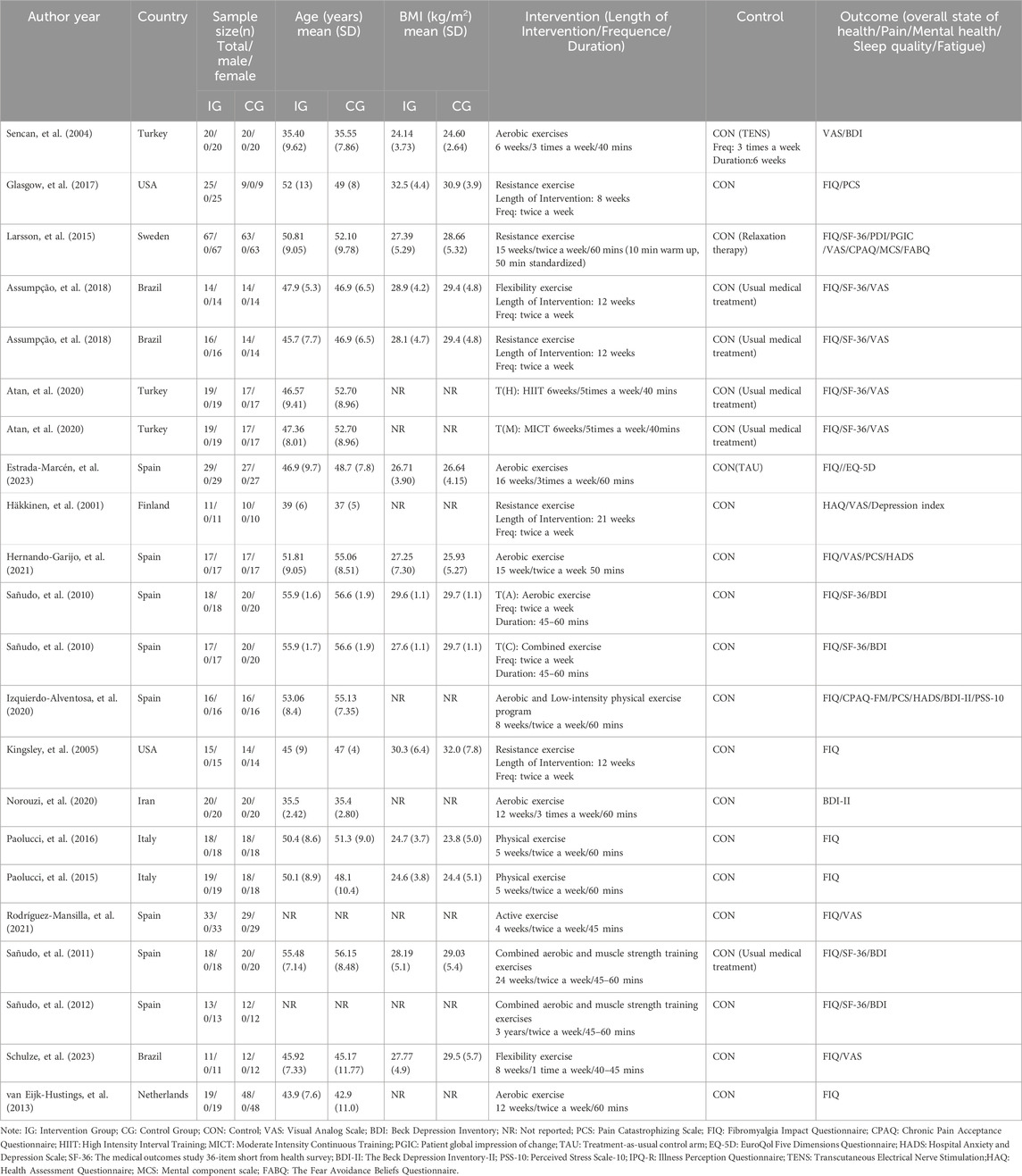
The 19 included articles encompassed 22 comparative research studies, with 3 articles reporting on two exercise intervention groups. In total, 857 participants were involved across the 22 studies, with 421 participants in the intervention group and 436 participants in the control group. Geographically, seven studies originated from Spain, three from Turkey and Brazil each, and two each from Italy and the USA, while Finland, the Netherlands, Iran, Sweden, and Northern Ireland each contributed one study. The duration of interventions across the 22 studies ranged from 5 weeks to 18 months. Regarding exercise dosage based on ACSM recommendations, 15 studies addressed aerobic exercise, 14 studies focused on resistance exercise, and 13 studies involved flexibility exercise. The characteristics of the included studies are outlined in Table 4.
3.3 Risk of bias
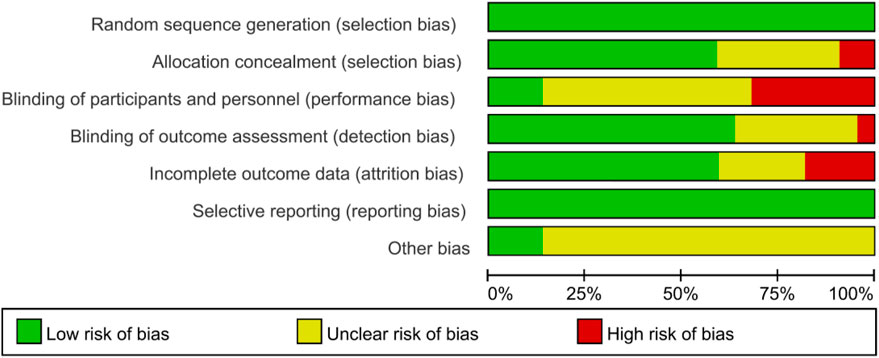
All studies indicated a low risk of bias in random sequence generation. Out of the 19 included studies, 12 were deemed to have a low risk of allocation concealment bias, while five did not report the allocation method, leading to an uncertain risk. Two studies were identified as having a high risk of allocation concealment bias. Blinding of both researchers and participants posed challenges due to the difficulty of implementing exercise interventions in a double-blind manner, resulting in a relatively high overall risk of bias in this category. Regarding outcome assessment blinding, 12 studies utilized blinded assessors, indicating a low risk, while six studies did not specify the outcome assessment method, raising some concerns, and one study lacked outcome blinding, hence classified as having a high risk. Among the 15 studies with incomplete outcome reporting, consistency in the number of subjects post-intervention compared to baseline was observed in most, leading to a low risk assessment. In 12 studies, the dropout rate was small (≤4 individuals), raising some concerns, while four studies experienced significant differences in subject numbers pre and post-intervention (≥7 individuals), resulting in a high risk assessment. The risk of selective reporting bias was low across the studies, and there were no high risks of other biases identified (Figures 2, 3).
3.4 Meta-analyses
Adherence with the ACSM recommendations was observed in 12 out of 22 results (3 studies were divided into two results), achieving a adherence rate of ≥75%. In the remaining 10 results, adherence with ACSM was less than 75%. The primary reasons for low adherence included discrepancies between the exercise intervention dosage and the ACSM recommendations, as well as insufficient information on exercise prescription for proper evaluation. When considering outcome measures, the proportions of adherence were analyzed as follows: For studies with the overall state of health as the outcome measure, 10 results demonstrated high adherence to ACSM guidelines, while 10 studies exhibited low or uncertain adherence. For studies measuring pain outcomes, eight showed high adherence to ACSM guidelines, while 6 had low or uncertain adherence. For studies focusing on mental health outcomes, 8 displayed high adherence to ACSM guidelines, while seven had low or uncertain adherence. Regarding sleep quality outcomes, four achieved high adherence to ACSM guidelines compared to only one study demonstrating low or uncertain adherence. As for fatigue outcomes, there were four studies that adhered well to ACSM guidelines and an equal number that had low or uncertain adherence. The detailed results are shown in Table 5.
3.4.1 Overall state of health
In our analysis of 20 studies involving 777 participants, we investigated the impact of exercise interventions on the overall state of health and pain levels of patients with FMS. Upon conducting a heterogeneity test, we observed significant variability among the studies (I2 = 75%, p < 0.00001). Therefore, we employed a random effects model for statistical analysis. The analysis revealed a total combined SMD of −0.94 (95%CI −1.26, −0.63), indicating a beneficial effect of exercise intervention on the overall state of health of FMS patients. To further explore the differences in outcomes based on adherence with the ACSM recommendations, we performed subgroup analysis, categorizing the studies into two groups: high ACSM adherence and low or uncertain ACSM adherence. The combined SMD for the subgroup with high adherence was −1.17 (95%CI −1.65, −0.69), while for the subgroup with low or uncertain adherence, the combined SMD was −0.73 (95%CI −1.12, −0.34). Subgroup difference analysis demonstrated a distinction between exercise interventions with high ACSM adherence and those with low or uncertain ACSM adherence (Figure 4). From these results, we can conclude that exercise interventions with high ACSM adherence have better therapeutic effects for FMS patients compared to those with low or uncertain ACSM adherence. In terms of heterogeneity within the subgroups, we observed individual study heterogeneity of 76% in the high ACSM adherence subgroup and 71.6% in the low or uncertain ACSM adherence subgroup. Visual inspection of the funnel plot (Figure 9A) indicated approximate symmetry on both sides, suggesting the absence of publication bias. Sensitivity analysis (Figure 10A) demonstrated that no single study significantly influenced the overall results, confirming the robustness of our findings.
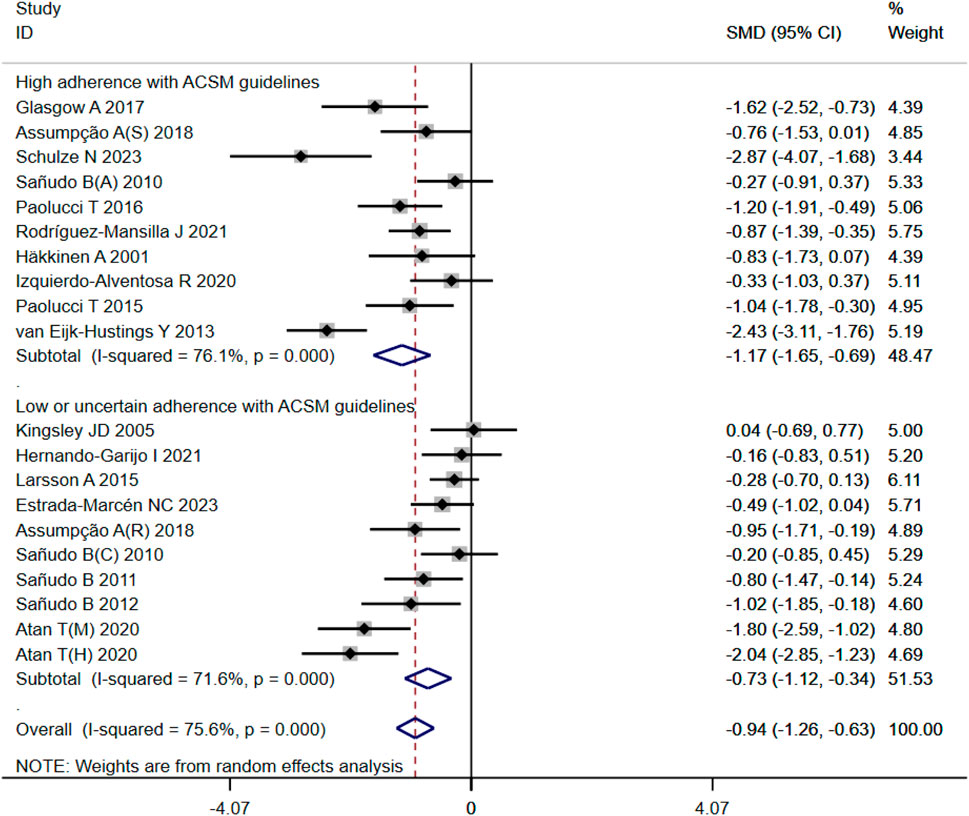
Figure 4. Forest plot of meta-analysis on the effect of exercise intervention and ACSM adherence on overall state of health in FMS patients.
3.4.2 Pain
In the 14 studies with 564 participants that focused on pain as the outcome measure, we encountered a similar heterogeneity issue (I2 = 79%, p < 0.00001), prompting us to use a random effects model for statistical analysis. The analysis revealed a total combined SMD of −1.21 (95% CI −1.62, −0.79), indicating a beneficial effect of exercise intervention on pain relief in FMS patients. Subgroup analysis based on adherence with ACSM recommendations revealed a combined SMD of −1.32 (95% CI −2.00, −0.64) for the high ACSM adherence subgroup and −1.06 (95% CI −1.55, −0.57) for the low or uncertain ACSM adherence subgroup. Subgroup difference analysis (Figure 5) demonstrated better therapeutic effects on pain relief for high ACSM adherence. Within the high ACSM adherence subgroup, the individual study heterogeneity for pain was 84%, while in the low or uncertain ACSM adherence subgroup, it was 69.2%. The funnel plot analysis (Figure 9B) indicated approximate symmetry on both sides, suggesting no obvious publication bias. Sensitivity analysis (Figure 10B) revealed that no single study significantly impacted the overall results, further supporting the robustness of our findings.
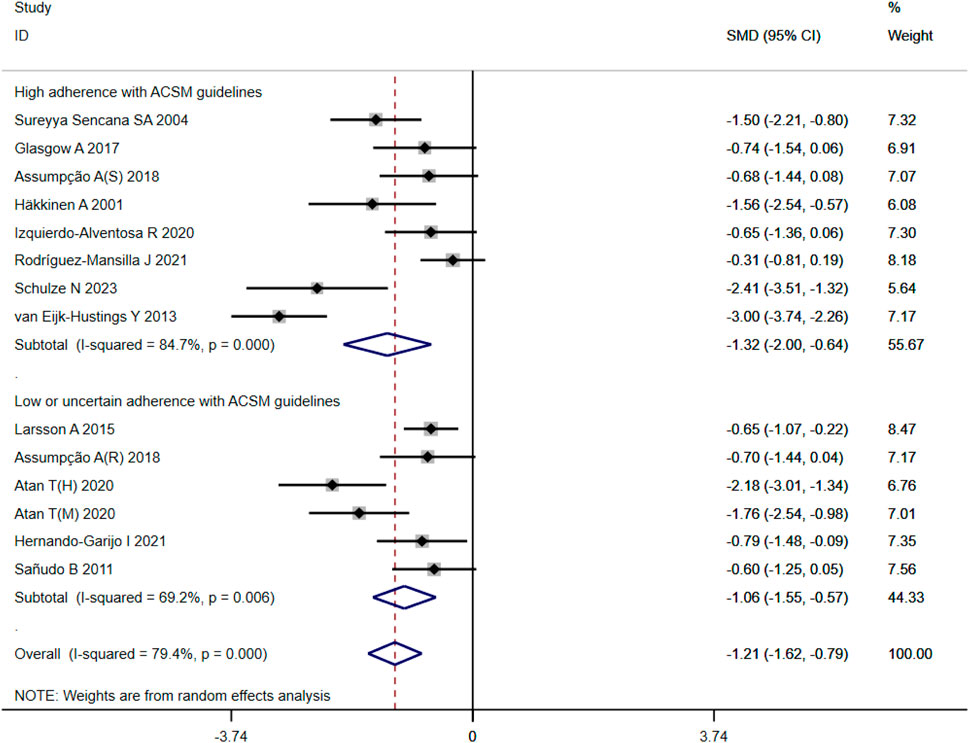
Figure 5. Forest plot of meta-analysis on the effect of exercise intervention and ACSM adherence on pain in FMS patients.
3.4.3 Mental health
A total of 525 participants from 15 studies were included in our analysis of the effects of exercise intervention on mental health in FMS patients. A heterogeneity test was conducted, revealing I2 to be greater than 50% (I2 = 74%, p < 0.00001). Consequently, a random effects model was employed for statistical analysis. Our findings indicated a significant beneficial effect of exercise intervention on mental health in FMS patients, with a total combined SMD of −0.95 (95%CI −1.32, −0.57). Subgroup analysis was conducted based on the level of adherence with ACSM recommendations. The combined SMD for the subgroup with high ACSM adherence was −0.96 (95%CI −1.62, −0.30), while for the subgroup with low or uncertain ACSM adherence, the combined SMD was −0.94 (95%CI −1.29, −0.60). Subgroup difference analysis demonstrated no significant difference between exercise interventions with high ACSM adherence and those with low or uncertain ACSM adherence (Figure 6). Therefore, we conclude that exercise interventions with high ACSM adherence do not yield better therapeutic effects on mental health in FMS patients compared to those with low or uncertain ACSM adherence. In the subgroup with high adherence, there was a heterogeneity of 84% among the individual studies measuring mental health. In the low or uncertain adherence subgroup, heterogeneity was 37.5%. Funnel plot inspection (Figure 9C) showed approximate symmetry on both sides, indicating no obvious publication bias. Sensitivity analysis (Figure 10C) indicated that no single study significantly influenced the overall results, thus confirming the robustness of our findings.
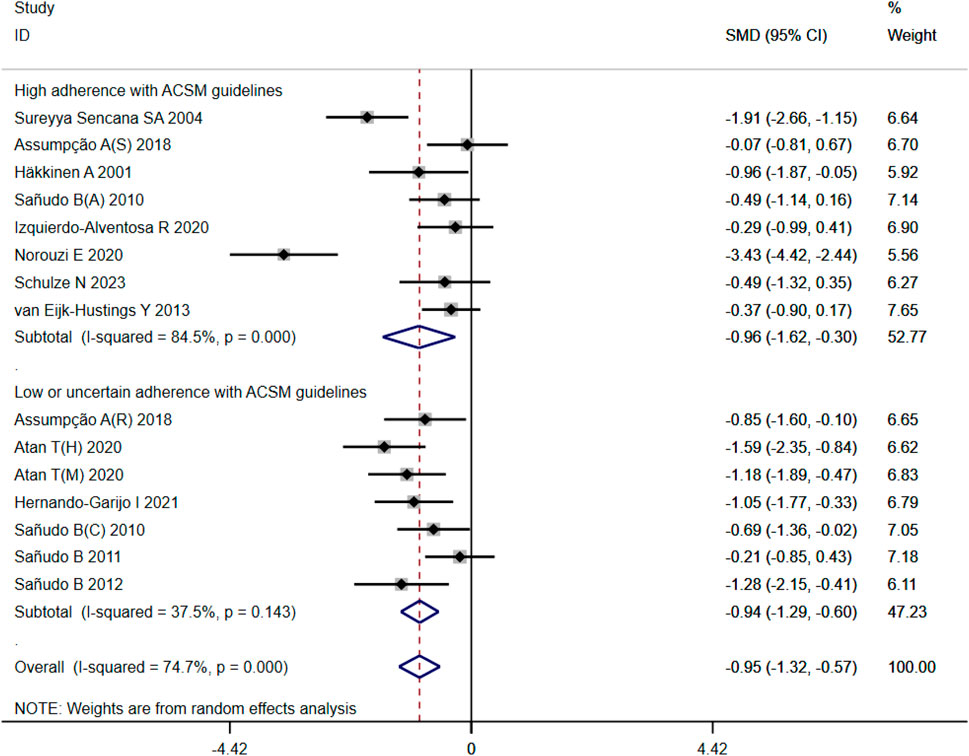
Figure 6. Forest plot of meta-analysis on the effect of exercise intervention and ACSM adherence on mental health in FMS patients.
3.4.4 Sleep quality
When analyzing the outcome of sleep quality in 169 participants from five studies, we observed significant heterogeneity (I2 = 74%, p = 0.004) during heterogeneity testing. Therefore, we utilized a random effects model for statistical analysis. Our analysis revealed a total combined SMD of −1.59 (95% CI −2.31, −0.87), indicating a beneficial effect of exercise intervention on the sleep quality of FMS patients. To further explore the data, we conducted subgroup analysis based on the proportion of adherence with ACSM recommendations. In the subgroup with high ACSM adherence, the combined SMD was −1.71 (95% CI −2.58, −0.83), while in the subgroup with low or uncertain ACSM adherence, the SMD was −1.11 (95% CI −1.88, −0.33). Subgroup difference analysis indicated a significant distinction between exercise interventions with high ACSM adherence and those with low or uncertain ACSM adherence (Figure 7). Therefore, we conclude that exercise interventions with high ACSM adherence have superior therapeutic effects on sleep quality scores in FMS patients compared to those with low or uncertain ACSM adherence. Within the subgroup with high ACSM adherence, the heterogeneity was 77%. Examination of the funnel plot (Figure 9D) displayed approximate symmetry on both sides, suggesting no evident publication bias. Sensitivity analysis (Figure 10D) indicated that no single study significantly influenced the overall results, affirming the robustness of our findings.
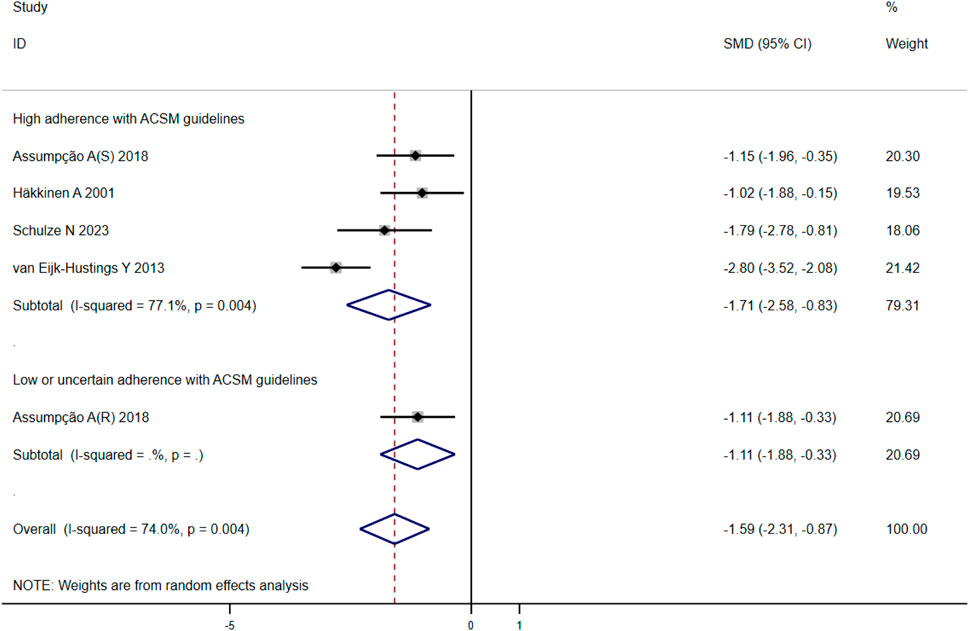
Figure 7. Forest plot of meta-analysis on the effect of exercise intervention and ACSM adherence on sleep quality in FMS patients.
3.4.5 Fatigue
After analyzing the outcome of fatigue in 279 participants from eight studies, we observed significant heterogeneity during heterogeneity testing (I2 = 84.0%, p < 0.001), so we utilized a random effects model for statistical analysis. Our analysis revealed a total combined SMD of −1.55 (95% CI −2.26, −0.85), indicating a beneficial effect of exercise intervention on the fatigue of FMS patients. To further explore the data, we conducted subgroup analysis based on the proportion of adherence with ACSM recommendations. In the subgroup with high ACSM adherence, the combined SMD was −1.77 (95% CI −3.18, −0.36), while in the subgroup with low or uncertain ACSM adherence, the combined SMD was −1.35 (95% CI −2.03, −0.66) (Figure 8). Subgroup difference analysis indicated a distinction between exercise interventions with high ACSM adherence and those with low or uncertain ACSM adherence. In conclusion, the findings suggest that adherence to ACSM recommendations for exercise intervention may help relieve fatigue in patients with FMS. The results of this analysis indicate that adhering to ACSM recommendations for exercise interventions can have a superior therapeutic effect on relieving fatigue compared to low or uncertain adherence to these recommendations. Examination of the funnel plot (Figure 9E) and sensitivity analysis (Figure 10E) support the robustness of these findings.
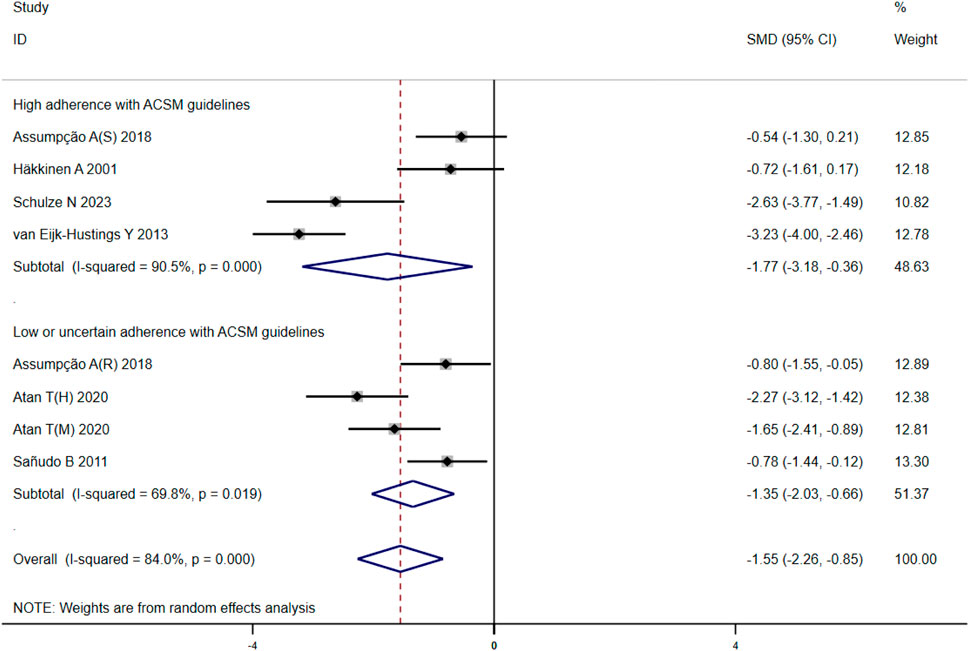
Figure 8. Forest plot of meta-analysis on the effect of exercise intervention and ACSM adherence on fatigue in FMS patients.

Figure 9. Funnel plot of meta-analysis on the effect of exercise intervention and ACSM adherence on the overall state of health (A), pain (B), mental health (C), sleep quality (D), and fatigue (E) in FMS patients.
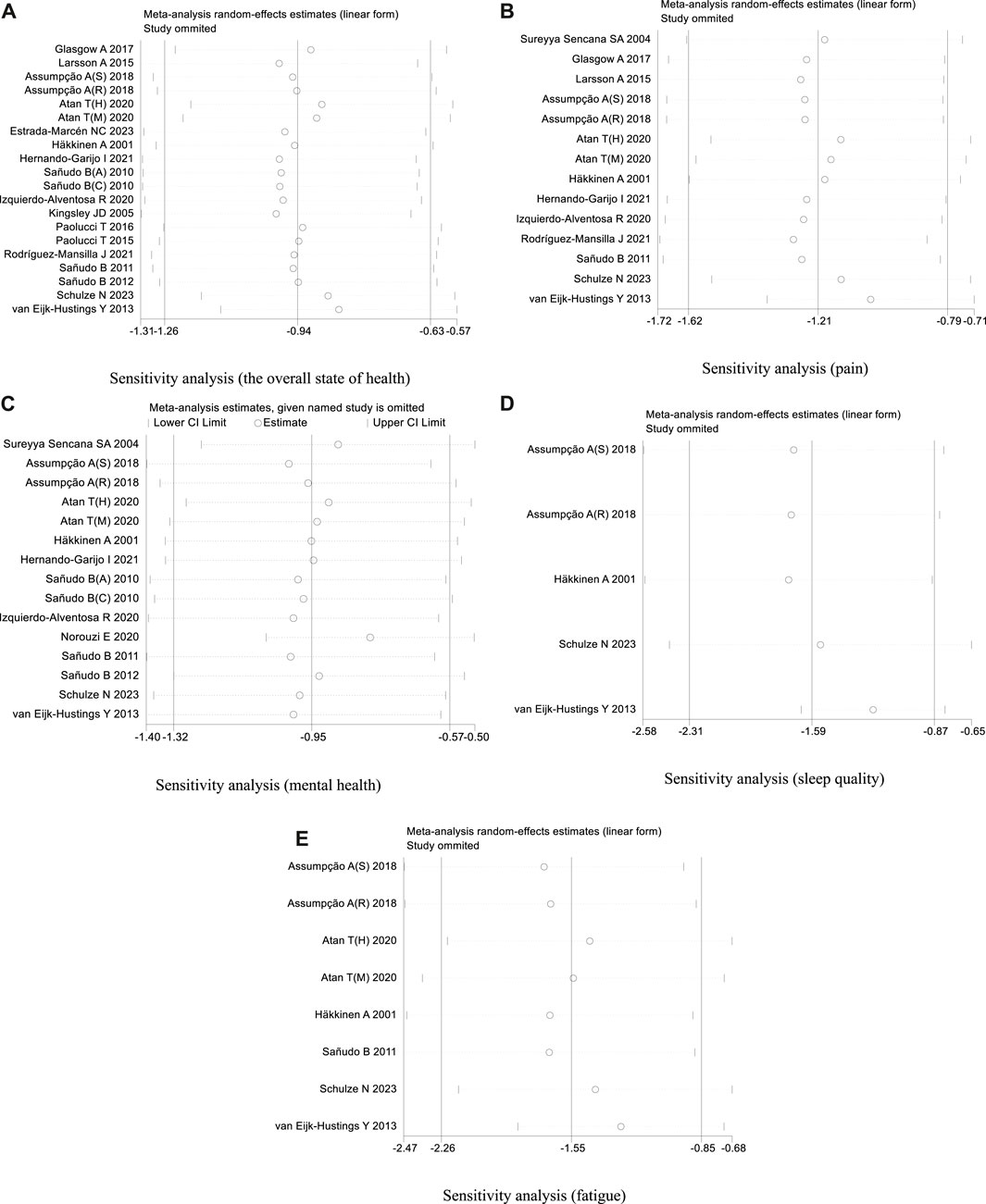
Figure 10. (A) Sensitivity analysis (the overall state of health). (B) Sensitivity analysis (pain). (C) Sensitivity analysis (mental health). (D) Sensitivity analysis (sleep quality). (E) Sensitivity analysis (fatigue).
4 Discussion
The ACSM is widely recognized as a leading authority in sports medicine, playing a crucial role in managing various chronic diseases through its evidence-based exercise guidelines (Cui, et al., 2023; Giorgi, et al., 2023; Xijuan, et al., 2023; Yamei, et al., 2023). In 2017, Moseng et al. conducted research demonstrating that patients who adhered closely to the recommended exercise intensities outlined by ACSM experienced more significant pain relief from hip osteoarthritis compared to those with lower adherence (Moseng, et al., 2017). This finding reinforces the importance of maintaining exercise intensity in accordance with ACSM guidelines for effective management of chronic motion diseases.
In this review, comprehensive assessment tools, including the FIQ, were utilized to evaluate multiple health dimensions. These dimensions encompassed the overall state of health and secondary outcomes such as pain intensity, mental wellbeing, sleep quality, and fatigue levels, which exert a significant influence on the overall quality of life experienced by individuals with FMS. Our findings suggest that higher adherence levels to ACSM guidelines resulted in superior pain relief outcomes compared to lower adherence levels. We consider that this improvement in pain relief can be attributed to movement-based interventions which enhance muscle strength and mitigate muscle loss (Estévez-López, et al., 2021). Muscle gain is an effective strategy for pain management in patients with FMS (Gavi, et al., 2014). Furthermore, exercise stimulates the production of endogenous opioids and β-endorphins, which activate descending nociceptive inhibitory mechanisms and result in hypoalgesia (Tan, et al., 2022). Additionally, we observed some research such as Atan T’s study (Atan, et al., 2020) shows a high negative effect, demonstrated higher negative effects, SMD = −2.18 (95%CI −3.01, −1.34) in high-intensity interval training (HIIT) and [SMD = −1.76 (95%CI −2.54, −0.98)] in moderate intensity continuous training (MICT), suggesting that the low-adherence group may also experience improved pain relief in specific cases; however, overall outcomes were not as favorable as those observed in the high-adherence group. Similarly, this phenomenon was reflected in fatigue and mental health outcomes (Figure 6; Figure 8). Their interventions all included aerobic, resistance, and flexibility exercises, but their higher frequency (5 days per week) and intensity (80%–95% of peak HR) resulted in a low ACSM adherence group (Table 5). Sensitivity analysis showed that the results of Atan T were stable (Figure 10B). We considered that a combination of exercise modalities might be more effective in improving the symptoms of FMS in patients with low adherence to ACSM guidelines. This may be due to the combined effect of these exercise modalities, which jointly improve the physical condition of patients through different mechanisms, thus achieving better symptom relief. However, subgroup analyses were not performed because of the limited number of included studies. In terms of fatigue relief, the ACSM high adherence group also showed some advantages. Physiologically, a wide range of central nervous system aberrations have been documented in FMS(Wood, et al., 2009; González-Roldán, et al., 2016), including metabolic and structural abnormalities in the hippocampus, dysfunction of the hypothalamic-pituitary-adrenal axis, and sympathetic hyperactivity (Martinez-Lavin, 2007). These alterations contribute to the elevated levels of fatigue observed in patients with FMS. Exercise has demonstrated potential to reverse these abnormalities by modulating metabolite levels and promoting angiogenesis, neurogenesis, and hippocampal connectivity, thereby potentially alleviating fatigue (Valim, et al., 2013). Furthermore, our meta-analysis reveals that exercise exerts a favorable effect on sleep quality among patients with FMS. This improvement may be attributed to reduced sympathetic tone and increased parasympathetic activity induced by exercise, leading to muscle and nerve relaxation. Consequently, stress is reduced and sleep quality is enhanced (Petzke, et al., 2000; Sarzi-Puttini, et al., 2006). It should be noted that chronic pain is directly associated with poor sleep quality which impacts psychological states such as depression and anxiety (Stults-Kolehmainen, et al., 2014). A cross-sectional study involving 233 patients with chronic pain reported that 36% experienced depression while 66.1% had poor sleep quality (Alhalal, et al., 2021). Therefore, this emphasizes once again the diversity and relevance of physiological mechanisms underlying FMS.
Interestingly, no significant differences were observed between the high and low adherence groups in terms of mental health outcomes. This unexpected finding may suggest that while exercise generally has a positive impact on mental health by reducing symptoms of depression and anxiety, it remains unclear how strict adherence to the ACSM’s exercise recommendations specifically influences these outcomes (Williams, et al., 2011; Krupa, et al., 2024). This complexity could be attributed to the multifactorial nature of mental health problems, which extend beyond exercise adherence (Lima, et al., 2017; Zabihiyeganeh, et al., 2023). For instance, Daniel Rodriguez-Almagro’s study demonstrated that PEBT was most effective in improving SF-36 psychological domain scores when conducted between 21 and 40 sessions [SMD = 0.51 (95%CI 0.28, 0.73)] (Rodríguez-Almagro, et al., 2023). These findings imply that the overall duration of exercise therapy may also influence various outcome measures related to FMS. Similarly, Atan T’s study revealed a favorable effect on mental health after completing five sessions per week for up to 6 weeks (Atan, et al., 2020). However, the Sanudo B’s study implemented twice a week intervention for a duration of up to 6 months (Sañudo, et al., 2012), while the Hernando-Garijo I’s study encompassed 30 sessions (Hernando-Garijo, et al., 2021) and the Izquierdo-Alventosa R’s study involved 16 sessions (Izquierdo-Alventosa, et al., 2020). Studies with different durations of intervention have yielded different results, Sanudo B 2012 for [SMD = −1.28 (95%CI −2.15, −0.41)], Hernando-Garijo I for [SMD = −1.05 (95%CI −1.77, −0.33)], Izquierdo Alventosa R for [SMD −0.29 (95%CI −0.99, 0.41)]. It is plausible that the duration of intervention may have influenced the final results, as shorter sessions (e.g., lasting only for16 sessions) might not fully harness the potential benefits of exercise interventions, leading to smaller effects sizes. How long the improvement in mental health and other measures becomes stable after the intervention is a direction for future research. This may explain the lack of significant differences in mental health outcomes between the high-adherence and low-adherence groups. Currently, a multidisciplinary treatment team utilizing cognitive behavioral therapy (CBT) combined with physical therapy is the most effective way to enhance the mental health of patients with FMS(Monticone, et al., 2015; Ploutarchou, et al., 2023). While exercise may have beneficial effects on anxiety and depression, it may be an added advantage to overall symptom improvement (Bennie, et al., 2020; Juan, et al., 2020). In summary, these results demonstrate further diversity and relevance of FMS in physiological mechanisms and provide clinical evidence for understanding potential mechanisms of exercise in human physiological function regulation and chronic disease management. Long-term treatment courses combining multiple modalities are suggested for improving mental health and other symptoms in FMS patients.
Previous randomized controlled trials have demonstrated several advantages of exercise therapy for FMS patients (Zis, et al., 2017; Marcuzzi, et al., 2022). These include improved cardiovascular fitness, increased muscle strength, enhanced flexibility, and better overall physical functioning (Juan, 2019; Ye et al., 2021). Aerobic exercises contribute significantly to cardiovascular health, resistance exercises enhance muscle strength, and flexibility exercises improve range of motion (Haack, et al., 2020; El-Sawy, et al., 2024). Together, these improvements help in managing FMS more effectively, aligning well with the ACSM recommendations (Siracusa, et al., 2021). However, exercise therapy is not without its challenges. For instance, potential confounders such as participants’ baseline health status, mental health, or lifestyle choices could still have an impact on the results. These factors may influence both adherences to exercise regimens and the measured outcomes. For instance, participants with better baseline health or more positive psychological states might find it easier to adhere to exercise guidelines and experience greater benefits. Conversely, those with poorer baseline health or higher levels of psychological distress might struggle more with adherence, thereby affecting their outcomes. Patients with severe limitations or comorbidities may find it difficult to adhere to rigorous exercise regimens (Yiru, et al., 2019; Liu et al., 2020). Additionally, the intensity and frequency required to achieve significant benefits might not be feasible for all types of diseases and patients, raising concerns about safety and practicality. For instance, overexertion can potentially lead to flare-ups in pain and fatigue, counteracting the benefits of exercise (Busch, et al., 2008). Our study categorized ACSM adherence into high, uncertainty, and low levels based on participants’ adherence to prescribed intensity and frequency. High adherence was associated with better physical health outcomes, demonstrating the importance of following structured exercise guidelines. This classification helps in evaluating the effectiveness of exercise dosages tailored to individual capabilities and limitations. To provide a more integrated view of exercise therapy in FMS management, it is essential to consider the therapeutic benefits of different exercise modalities in relation to ACSM guidelines. We suggest adherence to these guidelines ensures that the diverse effects of aerobic, resistance, and flexibility exercises are maximized, leading to comprehensive improvements in health, pain relief, sleep, and fatigue management.
5 Limitations
Several limitations were encountered in this study. Firstly, the inclusion of reference papers may introduce selective bias, potentially affecting the generalizability of the study results. Moreover, certain articles retrieved during literature search provided unclear or incorrect definitions of HIIT. Additionally, some studies reported relevant data that were not recorded (NR), which may have introduced errors in our assessment and may have amplified errors in subsequent analyses. To address these issues, we strictly adhered to inclusion and exclusion criteria that specified particular forms of exercise, exercise frequency, and exercise duration. The aggregation of results was performed using a random-effects model to account for interstudy variation. Furthermore, a sensitivity analysis was conducted to identify significant biases in the studies and assess their impact on the overall findings. It is important to note that the choice of adherence threshold can influence the results. To balance methodological rigor with an adequate sample size, we selected a 75% adherence threshold. Setting a threshold of 100% would limit the number of included studies and reduce statistical power and generalizability; however, it could provide valuable insights if achieved under perfect adherence conditions. Conversely, setting a threshold of 50% may include less rigorous studies and dilute observed effects. By choosing a 75% adherence threshold, we included a larger pool of studies that enhanced statistical robustness and methodologic rigor in our findings. Despite the efforts made to address the aforementioned limitations, readers should interpret our results cautiously given the limited availability of research on intervention measures for FMS. Future studies are warranted to further investigate the specific efficacy of different types and intensities of exercise for FMS, as well as determine optimal exercise protocols.
6 Conclusion
This meta-analysis demonstrates that adherence to the exercise guidelines of the ACSM significantly enhances the overall state of health, pain, sleep quality, and fatigue management in patients diagnosed with FMS. Importantly, a high level of adherence to ACSM guidelines leads to superior improvements in these domains compared to low or uncertain adherence. These findings suggest that structured exercise programs based on ACSM recommendations can effectively optimize multiple dimensions of health in FMS patients. Furthermore, while exercise positively impacts mental health, our analysis indicates that strict adherence to exercise guidelines does not significantly influence mental health outcomes compared to low adherence, thereby highlighting the intricate role of psychological factors in FMS. The future treatment of patients with FMS should consider incorporating movement-based interventions in combination with other therapeutic modalities to optimize the management of FMS and enhance mental health in patients.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
TH: Conceptualization, Data curation, Formal analysis, Supervision, Visualization, Writing–original draft, Writing–review and editing. RX: Data curation, Formal analysis, Writing–review and editing. JW: Validation, Writing–review and editing. HY: Data curation, Writing–review and editing. LL: Writing–review and editing.
Funding
The authors declare that financial support was received for the research, authorship, and publication of this article. This research was part of Project 17-40 supported by the Fundamental Research Funds for the China Institute of Sport Science.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alhalal E. A., Alhalal I. A., Alaida A. M., Alhweity S. M., Alshojaa A. Y., Alfaori A. T. (2021). Effects of chronic pain on sleep quality and depression: a cross-sectional study. Saudi Med. J. 42 (3), 315–323. doi:10.15537/smj.42.3.20200768
Althobaiti N. K., Amin B. A., Alhamyani A. D., Alzahrani S. M., Alamri A. M., Alhomayani F. K. H. (2022). Prevalence of fibromyalgia syndrome in taif city, Saudi Arabia. Cureus 14 (12), e32489. doi:10.7759/cureus.32489
Álvarez-Gallardo I. C., Bidonde J., Busch A., Westby M., Kenny G. P., Delgado-Fernández M., et al. (2019). Therapeutic validity of exercise interventions in the management of fibromyalgia. J. Sports Med. Phys. Fit. 59 (5), 828–838. doi:10.23736/s0022-4707.18.08897-7
Assumpção A., Matsutani L. A., Yuan S. L., Santo A. S., Sauer J., Mango P., et al. (2018). Muscle stretching exercises and resistance training in fibromyalgia: which is better? A three-arm randomized controlled trial. Eur. J. Phys. Rehabil. Med. 54 (5), 663–670. doi:10.23736/s1973-9087.17.04876-6
Atan T., Karavelioğlu Y. (2020). Effectiveness of high-intensity interval training vs moderate-intensity continuous training in patients with fibromyalgia: a pilot randomized controlled trial. Arch. Phys. Med. Rehabil. 101 (11), 1865–1876. doi:10.1016/j.apmr.2020.05.022
Bannwarth B., Blotman F., Roué-Le Lay K., Caubère J. P., André E., Taïeb C. (2009). Fibromyalgia syndrome in the general population of France: a prevalence study. Jt. Bone Spine 76 (2), 184–187. doi:10.1016/j.jbspin.2008.06.002
Bennie J. A., De Cocker K., Biddle S. J. H., Teychenne M. J. (2020). Joint and dose-dependent associations between aerobic and muscle-strengthening activity with depression: a cross-sectional study of 1.48 million adults between 2011 and 2017. Depress Anxiety 37 (2), 166–178. doi:10.1002/da.22986
Branco J. C., Bannwarth B., Failde I., Abello Carbonell J., Blotman F., Spaeth M., et al. (2010). Prevalence of fibromyalgia: a survey in five European countries. Semin. Arthritis Rheum. 39 (6), 448–453. doi:10.1016/j.semarthrit.2008.12.003
Busch A. J., Barber K. A., Overend T. J., Peloso P. M., Schachter C. L. (2007). Exercise for treating fibromyalgia syndrome. Cochrane Database Syst. Rev. (4), Cd003786. doi:10.1002/14651858.CD003786.pub2
Busch A. J., Schachter C. L., Overend T. J., Peloso P. M., Barber K. A. (2008). Exercise for fibromyalgia: a systematic review. J. Rheumatol. 35 (6), 1130–1144. doi:10.1002/14651858.CD003786.pub2
Busch A. J., Webber S. C., Brachaniec M., Bidonde J., Bello-Haas V. D., Danyliw A. D., et al. (2011). Exercise therapy for fibromyalgia. Curr. Pain Headache Rep. 15 (5), 358–367. doi:10.1007/s11916-011-0214-2
Chinn S., Caldwell W., Gritsenko K. (2016). Fibromyalgia pathogenesis and treatment options update. Curr. Pain Headache Rep. 20 (4), 25. doi:10.1007/s11916-016-0556-x
Cui W., Li D., Jiang Y., Gao Y. (2023). Effects of exercise based on ACSM recommendations on bone mineral density in individuals with osteoporosis: a systematic review and meta-analyses of randomized controlled trials. Front. Physiol. 14, 1181327. doi:10.3389/fphys.2023.1181327
El-Sawy E. A., Abdul Hakim M. M., El-Zohiery A., Salama S. M. (2024). Significance of inflammatory markers in primary Fibromyalgia syndrome and their relation in assessing the disease severity. Egypt J. Immunol. 31 (1), 67–74. doi:10.55133/eji.310108
Estévez-López F., Maestre-Cascales C., Russell D., Álvarez-Gallardo I. C., Rodriguez-Ayllon M., Hughes C. M., et al. (2021). Effectiveness of exercise on fatigue and sleep quality in fibromyalgia: a systematic review and meta-analysis of randomized trials. Arch. Phys. Med. Rehabil. 102 (4), 752–761. doi:10.1016/j.apmr.2020.06.019
Estrada-Marcén N. C., Casterad-Seral J., Montero-Marin J., Serrano-Ostáriz E. (2023). Can an aerobic exercise programme improve the response of the growth hormone in fibromyalgia patients? A randomised controlled trial. Int. J. Environ. Res. Public Health 20 (3), 2261. doi:10.3390/ijerph20032261
Garrido-Ardila E. M., González-López-Arza M. V., Jiménez-Palomares M., García-Nogales A., Rodríguez-Mansilla J. (2021). Effects of physiotherapy vs. Acupuncture in quality of life, pain, stiffness, difficulty to work and depression of women with fibromyalgia: a randomized controlled trial. J. Clin. Med. 10 (17), 3765. doi:10.3390/jcm10173765
Gavi M. B., Vassalo D. V., Amaral F. T., Macedo D. C., Gava P. L., Dantas E. M., et al. (2014). Strengthening exercises improve symptoms and quality of life but do not change autonomic modulation in fibromyalgia: a randomized clinical trial. PLoS One 9 (3), e90767. doi:10.1371/journal.pone.0090767
Giesecke T., Gracely R. H., Grant M. A., Nachemson A., Petzke F., Williams D. A., et al. (2004). Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 50 (2), 613–623. doi:10.1002/art.20063
Giorgi V., Bazzichi L., Batticciotto A., Pellegrino G., Di Franco M., Sirotti S., et al. (2023). Fibromyalgia: one year in review 2023. Clin. Exp. Rheumatol. 41 (6), 1205–1213. doi:10.55563/clinexprheumatol/257e99
Glasgow A., Stone T. M., Kingsley J. D. (2017). Resistance exercise training on disease impact, pain catastrophizing and autonomic modulation in women with fibromyalgia. Int. J. Exerc Sci. 10 (8), 1184–1195.
González-Roldán A. M., Bomba I. C., Diesch E., Montoya P., Flor H., Kamping S. (2016). Controllability and hippocampal activation during pain expectation in fibromyalgia syndrome. Biol. Psychol. 121 (Pt A), 39–48. doi:10.1016/j.biopsycho.2016.09.007
Goubert D., De Pauw R., Meeus M., Willems T., Cagnie B., Schouppe S., et al. (2017). Lumbar muscle structure and function in chronic versus recurrent low back pain: a cross-sectional study. Spine J. 17 (9), 1285–1296. doi:10.1016/j.spinee.2017.04.025
Haack M., Simpson N., Sethna N., Kaur S., Mullington J. (2020). Sleep deficiency and chronic pain: potential underlying mechanisms and clinical implications. Neuropsychopharmacology 45 (1), 205–216. doi:10.1038/s41386-019-0439-z
Häkkinen A., Häkkinen K., Hannonen P., Alen M. (2001). Strength training induced adaptations in neuromuscular function of premenopausal women with fibromyalgia: comparison with healthy women. Ann. Rheum. Dis. 60 (1), 21–26. doi:10.1136/ard.60.1.21
Hernando-Garijo I., Ceballos-Laita L., Mingo-Gómez M. T., Medrano-de-la-Fuente R., Estébanez-de-Miguel E., Martínez-Pérez M. N., et al. (2021). Immediate effects of a telerehabilitation program based on aerobic exercise in women with fibromyalgia. Int. J. Environ. Res. Public Health 18 (4), 2075. doi:10.3390/ijerph18042075
Izquierdo-Alventosa R., Inglés M., Cortés-Amador S., Gimeno-Mallench L., Chirivella-Garrido J., Kropotov J., et al. (2020). Low-intensity physical exercise improves pain catastrophizing and other psychological and physical aspects in women with fibromyalgia: a randomized controlled trial. Int. J. Environ. Res. Public Health 17 (10), 3634. doi:10.3390/ijerph17103634
Juan J. (2019). Progress in diagnosis and treatment of fibromyalgia syndrome. Clin. Focus 34 (04), 293–298. doi:10.3969/j.issn.1004-583X.200019.04.00
Juan W., Rui L., Wei-Wen Z. (2020). Chronic neck pain and depression: the mediating role of sleep quality and exercise. Psychol. Health Med. 25 (8), 1029–1035. doi:10.1080/13548506.2020.1724308
Kingsley J. D., Panton L. B., Toole T., Sirithienthad P., Mathis R., McMillan V. (2005). The effects of a 12-week strength-training program on strength and functionality in women with fibromyalgia. Arch. Phys. Med. Rehabil. 86 (9), 1713–1721. doi:10.1016/j.apmr.2005.04.014
Krupa A. J., Chrobak A. A., Sołtys Z., Korkosz M., Nowakowski J., Dudek D., et al. (2024). Psychopathological symptoms in fibromyalgia and their associations with resistance to pharmacotherapy with SNRI. Psychiatr. Pol., 1–18. doi:10.12740/PP/OnlineFirst/176000
Larsson A., Palstam A., Löfgren M., Ernberg M., Bjersing J., Bileviciute-Ljungar I., et al. (2015). Resistance exercise improves muscle strength, health status and pain intensity in fibromyalgia--a randomized controlled trial. Arthritis Res. Ther. 17 (1), 161. doi:10.1186/s13075-015-0679-1
Lee L. R., Zhang L. B., Li Y., Yuqiao Z., Ziran Y., wish X., et al. (2024). Analysis of current research status of traditional Chinese medicine in fibromyalgia syndrome based on CiteSpace. Acad. J. Shanghai Univ. Traditional Chin. Med. 38 (01), 75–81. doi:10.16306/j.1008-861x.2024.01.011
Liguori G., Feito Y., Fountaine C., Roy B. A. (2021). ACSM'S guidelines for exercise testing and prescription eleventh edition. Philadelphia: Wolters Kluwer.
Lima L. V., Abner T. S. S., Sluka K. A. (2017). Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. J. Physiol. 595 (13), 4141–4150. doi:10.1113/jp273355
Liu J., Yuan X., Ye P., Sun Q., Zhibo Yu, Zhongcheng Li, et al. (2020). Depressive symptoms, sleep quality and cognitive characteristics of female patients with fibromyalgia. Hainan Med. J. 31 (09), 1128–1131. doi:10.3969/j.issn.1003-6350.2020.09.012
Macfarlane G. J., Kronisch C., Dean L. E., Atzeni F., Häuser W., Fluß E., et al. (2017). EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 76 (2), 318–328. doi:10.1136/annrheumdis-2016-209724
Maffei M. E. (2020). Fibromyalgia: recent advances in diagnosis, classification, pharmacotherapy and alternative remedies. Int. J. Mol. Sci. 21 (21), 7877. doi:10.3390/ijms21217877
Marcuzzi A., Skarpsno E. S., Nilsen T. I. L., Mork P. J. (2022). The interplay between multisite pain and insomnia on the risk of anxiety and depression: the HUNT study. BMC Psychiatry 22 (1), 124. doi:10.1186/s12888-022-03762-0
Martinez-Lavin M. (2007). Biology and therapy of fibromyalgia. Stress, the stress response system, and fibromyalgia. Arthritis Res. Ther. 9 (4), 216. doi:10.1186/ar2146
Monticone M., Cedraschi C., Ambrosini E., Rocca B., Fiorentini R., Restelli M., et al. (2015). Cognitive-behavioural treatment for subacute and chronic neck pain. Cochrane Database Syst. Rev. 2015 (5), Cd010664. doi:10.1002/14651858.CD010664.pub2
Moseng T., Dagfinrud H., Smedslund G., Østerås N. (2017). The importance of dose in land-based supervised exercise for people with hip osteoarthritis. A systematic review and meta-analysis. Osteoarthr. Cartil. 25 (10), 1563–1576. doi:10.1016/j.joca.2017.06.004
Norouzi E., Hosseini F., Vaezmosavi M., Gerber M., Pühse U., Brand S. (2020). Zumba dancing and aerobic exercise can improve working memory, motor function, and depressive symptoms in female patients with Fibromyalgia. Eur. J. Sport Sci. 20 (7), 981–991. doi:10.1080/17461391.2019.1683610
Oliva V., Gregory R., Brooks J. C. W., Pickering A. E. (2022). Central pain modulatory mechanisms of attentional analgesia are preserved in fibromyalgia. Pain 163 (1), 125–136. doi:10.1097/j.pain.0000000000002319
Paolucci T., Baldari C., Di Franco M., Didona D., Reis V., Vetrano M., et al. (2016). A new rehabilitation tool in fibromyalgia: the effects of perceptive rehabilitation on pain and function in a clinical randomized controlled trial. Evid. Based Complement. Altern. Med. 2016, 7574589. doi:10.1155/2016/7574589
Paolucci T., Vetrano M., Zangrando F., Vulpiani M. C., Grasso M. R., Trifoglio D., et al. (2015). MMPI-2 profiles and illness perception in fibromyalgia syndrome: the role of therapeutic exercise as adapted physical activity. J. Back Musculoskelet. Rehabil. 28 (1), 101–109. doi:10.3233/bmr-140497
Petzke F., Clauw D. J. (2000). Sympathetic nervous system function in fibromyalgia. Curr. Rheumatol. Rep. 2 (2), 116–123. doi:10.1007/s11926-000-0051-5
Ploutarchou G., Savva C., Karagiannis C., Pavlou K., O'Sullivan K., Korakakis V. (2023). The effectiveness of cognitive behavioural therapy in chronic neck pain: a systematic review with meta-analysis. Cogn. Behav. Ther. 52 (5), 523–563. doi:10.1080/16506073.2023.2236296
Richards S. C. M., Scott D. L., XiaoBin W. (2003). Prescribed exercise in people with fibromyalgia parallel group randomised controlled trial. Chin. Ed. BMJ (02), 126. doi:10.1136/bmj.325.7357.185
Rodríguez-Almagro D., Del Moral-García M., López-Ruiz M. D. C., Cortés-Pérez I., Obrero-Gaitán E., Lomas-Vega R. (2023). Optimal dose and type of exercise to reduce pain, anxiety and increase quality of life in patients with fibromyalgia. A systematic review with meta-analysis. Front. Physiol. 14, 1170621. doi:10.3389/fphys.2023.1170621
Rodríguez-Mansilla J., Mejías-Gil A., Garrido-Ardila E. M., Jiménez-Palomares M., Montanero-Fernández J., González-López-Arza M. V. (2021). Effects of non-pharmacological treatment on pain, flexibility, balance and quality of life in women with fibromyalgia: a randomised clinical trial. J. Clin. Med. 10 (17), 3826. doi:10.3390/jcm10173826
Sañudo B., Carrasco L., de Hoyo M., McVeigh J. G. (2012). Effects of exercise training and detraining in patients with fibromyalgia syndrome: a 3-yr longitudinal study. Am. J. Phys. Med. Rehabil. 91 (7), 561–569. doi:10.1097/PHM.0b013e31824faa03
Sañudo B., Galiano D., Carrasco L., Blagojevic M., de Hoyo M., Saxton J. (2010). Aerobic exercise versus combined exercise therapy in women with fibromyalgia syndrome: a randomized controlled trial. Arch. Phys. Med. Rehabil. 91 (12), 1838–1843. doi:10.1016/j.apmr.2010.09.006
Sañudo B., Galiano D., Carrasco L., de Hoyo M., McVeigh J. G. (2011). Effects of a prolonged exercise program on key health outcomes in women with fibromyalgia: a randomized controlled trial. J. Rehabil. Med. 43 (6), 521–526. doi:10.2340/16501977-0814
Sarzi-Puttini P., Atzeni F., Diana A., Doria A., Furlan R. (2006). Increased neural sympathetic activation in fibromyalgia syndrome. Ann. N. Y. Acad. Sci. 1069, 109–117. doi:10.1196/annals.1351.009
Sarzi-Puttini P., Giorgi V., Marotto D., Atzeni F. (2020). Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 16 (11), 645–660. doi:10.1038/s41584-020-00506-w
Savin E., Rosenn G., Tsur A. M., Hen O., Ehrenberg S., Gendelman O., et al. (2023). The possible onset of fibromyalgia following acute COVID-19 infection. PLoS One 18 (2), e0281593. doi:10.1371/journal.pone.0281593
Schulze N. B. B., Barreto T., Alencar G. G., da Silva T. A., Duarte A., Ranzolin A., et al. (2023). The effect of myofascial release of the physiological chains on the pain and health status in patients with fibromyalgia, compared to passive muscle stretching and a control group: a randomized controlled clinical trial. Disabil. Rehabil., 1–14. doi:10.1080/09638288.2023.2255130
Sencan S., Ak S., Karan A., Muslumanoglu L., Ozcan E. E., Berker E. (2004). A study to compare the therapeutic efficacy of aerobic exercise and paroxetine in fibromyalgia syndrome. J. Back Musculoskelet. Rehabilitation 57 (2), 213. doi:10.3233/ch-141858
Siracusa R., Paola R. D., Cuzzocrea S., Impellizzeri D. (2021). Fibromyalgia: pathogenesis, mechanisms, diagnosis and treatment options update. Int. J. Mol. Sci. 22 (8), 3891. doi:10.3390/ijms22083891
Staud R., Koo E., Robinson M. E., Price D. D. (2007). Spatial summation of mechanically evoked muscle pain and painful aftersensations in normal subjects and fibromyalgia patients. Pain 130 (1-2), 177–187. doi:10.1016/j.pain.2007.03.015
Stults-Kolehmainen M. A., Sinha R. (2014). The effects of stress on physical activity and exercise. Sports Med. 44 (1), 81–121. doi:10.1007/s40279-013-0090-5
Tan L., Cicuttini F. M., Fairley J., Romero L., Estee M., Hussain S. M., et al. (2022). Does aerobic exercise effect pain sensitisation in individuals with musculoskeletal pain? A systematic review. BMC Musculoskelet. Disord. 23 (1), 113. doi:10.1186/s12891-022-05047-9
Thompson P. D., Arena R., Riebe D., Pescatello L. S.American College of Sports Medicine (2013). ACSM's new preparticipation health screening recommendations from ACSM's guidelines for exercise testing and prescription, ninth edition. Curr. Sports Med. Rep. 12 (4), 215–217. doi:10.1249/JSR.0b013e31829a68cf
Thompson W. R., Sallis R., Joy E., Jaworski C. A., Stuhr R. M., Trilk J. L. (2020). Exercise is medicine. Am. J. Lifestyle Med. 14 (5), 511–523. doi:10.1177/1559827620912192
Valim V., Natour J., Xiao Y., Pereira A. F., Lopes B. B., Pollak D. F., et al. (2013). Effects of physical exercise on serum levels of serotonin and its metabolite in fibromyalgia: a randomized pilot study. Rev. Bras. Reumatol. 53 (6), 538–541. doi:10.1016/j.rbr.2013.02.001
van Eijk-Hustings Y., Kroese M., Tan F., Boonen A., Bessems-Beks M., Landewé R. (2013). Challenges in demonstrating the effectiveness of multidisciplinary treatment on quality of life, participation and health care utilisation in patients with fibromyalgia: a randomised controlled trial. Clin. Rheumatol. 32 (2), 199–209. doi:10.1007/s10067-012-2100-7
Williams D. A., Arnold L. M. (2011). Measures of fibromyalgia: fibromyalgia impact Questionnaire (FIQ), brief pain inventory (BPI), multidimensional fatigue inventory (MFI-20), medical outcomes study (MOS) sleep scale, and multiple ability self-report Questionnaire (MASQ). Arthritis Care Res. Hob. 63 (11), S86–S97. doi:10.1002/acr.20531
Wolfe F., Ross K., Anderson J., Russell I. J., Hebert L. (1995). The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 38 (1), 19–28. doi:10.1002/art.1780380104
Wood P. B., Ledbetter C. R., Glabus M. F., Broadwell L. K., Patterson J. C. (2009). Hippocampal metabolite abnormalities in fibromyalgia: correlation with clinical features. J. Pain 10 (1), 47–52. doi:10.1016/j.jpain.2008.07.003
Xijuan L., Xin Li, Zhang X., Gao R., Bowen Li, Wang J., et al. (2023). New progress in exercise prescription for chronic diseases: an update of ACSM Exercise Testing and Exercise Prescription (11th edition). Chin. J. Sports Med. 42 (08), 658–664. doi:10.16038/j.1000-6710.2023.08.005
Yamei W., Ho-jun Y., Jing L. (2023). Research progress on the mechanism of fibromyalgia. Chin. J. Pain Med. 29 (09), 697–701+705. doi:10.3969/j.issn.1006-9852.2023.09.009
Yapeng Z., BaoYong L., Ruixue H., ShiBing L., Jianping L. (2021). Qigong for fibromyalgia syndrome: a systematic review and meta-analysis of randomized clinical trials. World Chin. Med. 16 (24), 3615–3623. doi:10.3969/j.issn.1673-7202.2021.24.009
Ye C., Dongfeng L., Mengyu L., Lidong Hu, Han Y., Hou J., et al. (2021). Practice guideline of exercise intervention for patients with fibromyalgia (2021). Acad. J. Chin. PLA Med. Sch. 42 (08), 790–797. doi:10.3969/j.issn.2095-5227.2021.08.002
Yiru Y., Jian W. (2019). Effect of acupuncture on the sleep and life quality in the fibromyalgia syndrome patients. World J. Sleep Med. 6 (08), 1054–1056. doi:10.3969/j.issn.2095-7130.2019.01.019
Zabihiyeganeh M., Afshar S. V., Kadijani A. A., Janbozorgi M., Akbari A., Yahyazadeh H., et al. (2023). How durable are the effects of cognitive-behavioural therapy in controlling fibromyalgia symptoms? A prospective cohort study. Musculoskelet. Care 21 (3), 890–894. doi:10.1002/msc.1766
Zeng Q. Y., Zang C. H., Lin L., Chen S. B., Li X. F., Xiao Z. Y., et al. (2010). Epidemiologic study of soft tissue rheumatism in Shantou and Taiyuan, China. Chin. Med. J. Engl. 123 (15), 2058–2062. doi:10.3760/cma.j.issn.0366-6999.2010.15.019
Zhang K. D., Wang L. Y., Zhang Z. H., Zhang D. X., Lin X. W., Meng T., et al. (2022). Effect of exercise interventions on health-related quality of life in patients with fibromyalgia syndrome: a systematic review and network meta-analysis. J. Pain Res. 15, 3639–3656. doi:10.2147/jpr.S384215
Zhian Z., Shuwei B., Yang L., Ming L., Aixia M., Wenhui Z. (2023). Effects of general practice mode combined with duloxetine on psychological status, sleep quality and serum cytokines in patients with fibromyalgia syndrome. J. Pract. Med. 39 (21), 2822–2826. doi:10.3969/j.issn.1006-5725.2023.21.022
Zhu Q. (2021). Fibromyalgia China expert consensus clinical diagnosis and treatment. Chin. J. pain Med. 27 (10), 721–727. doi:10.3969/j.issn.1006-9852.2021.10.001
Keywords: Fibromyalgia, exercise dosage, ACSM, meta-analysis, treatment outcomes
Citation: Han T, Xi R, Wang J, Yan H and Li L (2024) Adherence to ACSM exercise guidelines and its influence on Fibromyalgia treatment outcomes: a meta-analysis of randomized controlled trials. Front. Physiol. 15:1413038. doi: 10.3389/fphys.2024.1413038
Received: 06 April 2024; Accepted: 24 June 2024;
Published: 19 July 2024.
Edited by:
Ahmad Alkhatib, Birmingham City University, United KingdomReviewed by:
Paulo Sergio Chagas Gomes, Universidade do Estado do Rio de Janeiro, BrazilJosé Alexandre Bachur, University of Franca, Brazil
Cui Wenlai, Capital Institute of Physical Education and Sports, China
Xuanhui Guo, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2024 Han, Xi, Wang, Yan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianran Han, aGFudGlhbnJhbkBjaXNzLmNu
 Tianran Han
Tianran Han Rui Xi
Rui Xi Jialin Wang
Jialin Wang Huiqian Yan
Huiqian Yan Linhua Li
Linhua Li