
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Physiol., 20 May 2024
Sec. Respiratory Physiology and Pathophysiology
Volume 15 - 2024 | https://doi.org/10.3389/fphys.2024.1394865
Introduction: Fibromyalgia (FM) is a common condition in patients with obstructive sleep apnea-hypopnea syndrome (OSAHS). This meta-analysis aimed to evaluate differences in sleep monitoring indicators between patients with OSAHS and positive FM and patients with OSAHS and negative FM and to determine the incidence of FM in patients with OSAHS.
Methods: An exhaustive literature review was conducted to analyze the incidence of FM in patients with OSAHS, using online databases, including PubMed, EMBASE, Web of Science, CNKI, and Wanfang, both in English and Chinese. The quality of the included studies was assessed by two researchers using the Newcastle−Ottawa Scale scores. The acquired data were analyzed using Stata 11.0 software. Continuous variables were combined and analyzed using the weighted mean difference as the effect size. Conjoint analyses were performed using random-effects (I2 > 50%) or fixed-effect (I2 ≤ 50%) models based on I2 values.
Results: Fourteen studies met the inclusion criteria. This study showed that 21% of patients with OSAHS experienced FM. Subgroup analyses were performed based on race, age, sex, body mass index, and diagnostic criteria for patients with OSAHS. These findings indicate that obese patients with OSAHS have a higher risk of FM, similar to females with OSAHS. Regarding most sleep monitoring indicators, there were no discernible differences between patients with OSAHS with positive FM and those with negative FM. However, patients with positive FM had marginally lower minimum arterial oxygen saturation levels than those with negative FM. The current literature suggests that patients with OSAHS have a high incidence of FM (21%), and FM has little effect on polysomnographic indicators of OSAHS.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024510786, identifier CRD42024510786
Fibromyalgia (FM) is a rheumatic disorder characterized by non-articular rheumatic pain syndrome, atopic myalgia, and hyperalgesia, the diagnosis and treatment of which are poorly understood (Passos et al., 2020). In addition to chronic pain, joint tenderness, muscle fatigue, sleep problems, cognitive impairment, and depression may occur (Tesio et al., 2018). FM affects 2–4% of the population, 80–90% of whom are females, and the age of onset is between 30 and 50 years (Gardoki-Souto et al., 2022). Although the exact cause of FM is unknown, it is known to be due to increased pain sensitivity due to dysfunction of the central nervous system (Kiso et al., 2018). Genetic and environmental factors are associated with the development of FM. Studies have shown that individuals who may suffer from FM may be at increased risk if their first-degree relatives are affected (D'Agnelli et al., 2019; Altınbilek et al., 2019). Certain family environmental factors, including acquired coping mechanisms for life challenges, have been implicated as inherent components in the development of FM (Bergman, 2005).
Obstructive sleep apnea-hypopnea syndrome (OSAHS) is a disorder characterized by apnea, decreased respiration, and decreased oxygen saturation level due to collapse of the upper airway, manifested by daytime sleepiness, fatigue, and inattention (Pei and Gui, 2021). The signs and symptoms of OSAHS and FM are similar. Treatment of FM is ineffective, and patients with FM often experience sleep problems, fatigue, and pain (Oka et al., 2020; Pei and Gui, 2021). Moldofsky et al. originally defined FM syndrome as musculoskeletal pain, fatigue, and sleep disturbances (Moldofsky et al., 1975). Most studies have examined the sleep quality of patients with FM, as there is no discernible difference in sleep duration between patients with FM and healthy controls (Moldofsky, 1989; Shaver et al., 1997). Similar sleep patterns, feelings of restlessness, and daytime sleepiness were observed in both the OSAHS and FM groups, suggesting a possible correlation between the two disorders. Mutlu et al. (Mutlu et al., 2020) evaluated female patients with FM using polysomnography (PSG) based on the results of a daytime sleepiness questionnaire and found that 65.9% of the patients had concurrent OSAHS. Sepici et al. (Sepici et al., 2007) performed PSG in a 55-year-old woman with FM for up to 10 years after complaining of waking up in the morning with fatigue, restless sleep, and daytime sleepiness, indicating severe OSAHS. These findings suggest that sleep monitoring in patients with FM is highly warranted; consequently, the incidence of FM in patients with OSAHS warrants further investigation.
Meresh et al. (Meresh et al., 2019) conducted a retrospective single-center study and identified a potential relationship between OSAHS and FM. However, this study did not systematically analyze the incidence of FM in patients with OSAHS. Furthermore, the incidence of FM may vary between OSAHS subgroups. Therefore, to statistically evaluate the incidence of FM in OSAHS, generalization and meta-analysis of all available data are critical. We investigated and analyzed the pathophysiology of FM in patients with OSAHS, as well as the link between the two conditions. We compared the differences in sleep monitoring indicators between patients with OSAHS and positive FM and between those with OSAHS and negative FM.
Our meta-analysis was registered in the Prospective Register of Systematic Reviews (PROSPERO, https://www.crd.york.ac.uk/PROSPERO/, ID: CRD42024510786). We conducted an exhaustive search of databases, including PubMed, Web of Science, Wanfang, CNKI, and EMBASE, for research on the start of FM in patients with OSAHS. The time restriction for the search was established from the inception of the database until 1 March 2024. The keywords and subject phrases included “fibromyalgia” or “fibromyalgia syndrome” and “obstructive sleep apnea-hypopnea syndrome” or “obstructive sleep apnea” or “OSA” or “OSAHS” or “OSAS.”
The conditions for eligibility are outlined below.
1) Cohort studies, cross-sectional studies, case-control studies, or randomized controlled trials.
2) FM diagnostic criteria: Participants must have a clinical diagnosis that satisfies the diagnostic criteria of FM established by the American College of Rheumatology (ACR) in 1990, 2010, and 2016 (Wolfe et al., 1990; Wolfe et al., 2010; Wolfe et al., 2016). There were no age restrictions for participation.
3) Based on PSG, subjects met OSAHS diagnostic criteria (adults: apnea-hypopnea index [AHI] score ≥5/h, child: AHI score ≥1/h) (Li and He, 2021).
Traditional criteria (adults: AHI score <5, normal; AHI score 5–14, mild OSAHS; AHI score 15–29, moderate OSAHS; and AHI score ≥30, severe OSAHS, child: AHI score <1, normal; AHI score 1–5, mild OSAHS; AHI score 5–9, moderate OSAHS; and AHI score ≥10, severe OSAHS) were used to determine the severity of OSAHS (Baker et al., 2017).
The following were the exclusion criteria:
1) Letters, reviews, editorials, case reports, and other types of literature review.
2) Unable to obtain sufficient information from the original article or contact the corresponding author for additional information.
3) Studies without human participants.
4) Studies involving patients with OSAHS who had cancer, endocrine disorders, cerebrovascular accidents, chronic heart failure, or chronic respiratory diseases in the past.
5) Overlaps between studies and data from the same author.
Two researchers conducted separate searches of the aforementioned databases for pertinent articles, following the aforementioned criteria. They screened potentially eligible articles by reviewing their titles and abstracts, procured complete texts, and meticulously reviewed the full texts for reevaluation. A third researcher examined the disagreement between the two researchers about the inclusion or exclusion of an article and consulted the data to decide on its inclusion or exclusion.
One author assessed the quality of the studies using the Newcastle−Ottawa Scale (NOS) (Stang, 2010). Higher and equal to six “stars” were considered of great quality; between three and five “stars” were considered of moderate quality; and less than or equal to two “stars” were considered of low quality.
Initially, two researchers retrieved and assessed the data separately from the literature in the following manner: (Passos et al., 2020): basic information about the article: first author, publication date, and country; (Tesio et al., 2018); number and incidence of patients with positive FM and OSAHS; (Gardoki-Souto et al., 2022); baseline parameters for comparison in the study population, such as body mass index (BMI), AHI scores, mean oxygen saturation level, minimum oxygen saturation level, total sleep time, and sleep monitoring indicators; (Kiso et al., 2018); measurements of OSAHS and their study types; and (D'Agnelli et al., 2019) study quality. The researcher contacted the authors by phone or email at least twice to determine if the included literature lacked the necessary information and if they agreed to provide the missing data.
Stata software (version 11.0; StataCorp LLC, College Station, TX, United States) was used to compile and analyze the retrieved data. With a 95% confidence interval (CI), we normalized and represented continuous variables as weighted mean differences (WMD). The statistical heterogeneity of the included studies was determined using the I2 statistic in the heterogeneity test. A fixed effects model was used for the analysis and p ≥ 0.10 and I2 < 50% indicated that there was no statistical heterogeneity between the studies. The random-effects model was used for analysis when p < 0.10 or I2 > 50%, suggesting that there was statistical heterogeneity between studies. Subgroup and meta-regression analyses were performed to explore the sources of heterogeneity. The entire population was divided into groups according to mean age, mean BMI, sex, diagnostic criteria, and ethnicity for the subgroup analysis. For the sensitivity analysis, which investigated how each study affected the overall effect size, one study was eliminated at a time. The publication bias of the included literature (≥10 articles) was examined using Begg’s and Egger’s tests on Stata 11.0.
A total of 187 relevant research articles were retrieved from the database. After screening the abstracts and titles, 139 duplicate studies were excluded and 48 were included. Twenty-six articles were excluded because they were irrelevant to the topic title. Eight of the 22 articles were eliminated after a comprehensive analysis of their entire texts and downloads, as well as a review of the inclusion and exclusion criteria. Four of these articles were reviews in which two were letters to the editor, one lacked pertinent data, and the other was an animal study. Therefore, these articles were excluded. Finally, 14 articles were included in our meta-analysis (Figure 1). The prevalence of FM in patients with OSAHS was reported in a total of 14 articles (Meresh et al., 2019; Dahan et al., 2006; O'Donovan et al., 2006; Wahner-Roedler et al., 2007; Chung et al., 2014; Rosenfeld et al., 2015; Terzi and Yılmaz, 2017; Yildirim and Alp, 2017; Köseoğlu et al., 2017; Johnson et al., 2020; Sanders et al., 2020; Wickwire et al., 2020; Altıntop Geçkil and Aydoğan Baykara, 2022; Cigdem Karacay et al., 2023). Six articles (Rosenfeld et al., 2015; Köseoğlu et al., 2017; Terzi and Yılmaz, 2017; Yildirim and Alp, 2017; Altıntop Geçkil and Aydoğan Baykara, 2022; Cigdem Karacay et al., 2023) compared sleep monitoring metrics in patients with positive FM and OSAHS to those with negative FM. The AHI scores, minimum SaO2 level, mean saturation oxygen level, total sleep duration, efficiency, latency, rapid eye movement (REM), N1, N2, N3, and Epworth sleepiness scale scores were among the markers used for sleep monitoring. Basic information on the included studies is presented in Table 1. Detailed sleep metrics are shown in Supplementary Tables S1, S2.
A meta-analysis of 14 publications from 16 studies was performed to examine the frequency of FM in patients with OSAHS (Table 1). A total of 23,473 patients were included in this study. Among these patients, 4,308 were tested positive and 19,169 negative for FM. The meta-analysis showed that the collective incidence of FM positivity was 21% (95% CI = 0.16–0.26, p < 0.001, I2 = 98.5%) after merging the effect values (Figure 2).
Males had an overall incidence of 3% of patients with OSAHS and positive FM (95% CI = 0.01–0.04, p = 0.002, I2 = 0%), meanwhile, females had an overall incidence of 23% (95% CI = 0.11–0.36, p < 0.001, I2 = 90.5%), according to a sex-based subgroup analysis (Table 2).
According to racial-based subgroup analysis, the total incidence of patients in the Caucasian population with positive FM and OSAHS was 21% (95% CI = 0.14–0.27, p < 0.001, I2 = 96.2%) (Table 2); only one article (Chung et al., 2014) reported an overall incidence of 30% in Chinese.
BMI-based subgroup analysis revealed a 25% incidence of OSAHS and positive FM among patients with a mean BMI ≥30 (95% CI = 0.13–0.38, p < 0.001, I2 = 96.2%). Patients with a mean BMI <30 had an incidence of 18% OSAHS and positive FM (95% CI = 0.12–0.25, p < 0.001, I2 = 99.0%) (Table 2).
Only one publication (Wickwire et al., 2020) revealed a 25% incidence of OSAHS with positive FM among patients aged ≥65; OSAHS with positive FM was observed in 20% of patients aged <65 years (95% CI = 0.15–0.26, p < 0.001, I2 = 98.4%) (Table 2). Age was not specified sufficiently for classification in one study (Dahan et al., 2006).
Based on the criteria established in 1990, the proportion of patients with OSAHS and positive FM was 15% (95% CI = 0.03–0.27, p = 0.014, I2 = 92.0%); this figure increased to 32% (95% CI, 0.28–0.36, p < 0.001, I2 = 66.0%) and it reached 20% (95% CI = 0.15–0.26, p < 0.001, I2 = 97.0%) according to the 2016 criteria (Table 2).
The incidence of FM in patients with OSAHS was shown to be very heterogeneous (I2 = 98.4%) in the combined analysis. Therefore, a meta-regression analysis was performed to determine the sources of heterogeneity. The results of the analysis indicated that the p-values for race, BMI, age, and sex were 0.078, 0.093, 0.114, and 0.063, respectively. These values indicate that the characteristics described above did not significantly influence heterogeneity. A sensitivity analysis was performed to remove 16 studies. The outcomes of the meta-analysis of the remaining articles were compared with those obtained before the initial exclusion. Eliminating individual studies did not have a significant impact on overall results. The sensitivity analysis of the incidence meta-analysis is shown in Supplementary Figure S1A. The incorporation of research into continuous variables for sleep monitoring indicators is limited. The study did not conduct a subgroup analysis or meta-regression.
The meta-analysis of the incidence of FM did not show any indication of publication bias in the conjoint analysis using Egger’s (p = 0.191) and Begg’s (p = 0.152) methods (Supplementary Figure S1B).
Data on AHI scores of patients with positive FM compared to those with negative FM were obtained from six studies. The findings indicated that the AHI scores between the positive FM and negative FM groups were similar (WMD = −1.26, 95% CI = −3.62–1.10, p = 0.296, I2 = 0.0%) (Figure 3A).
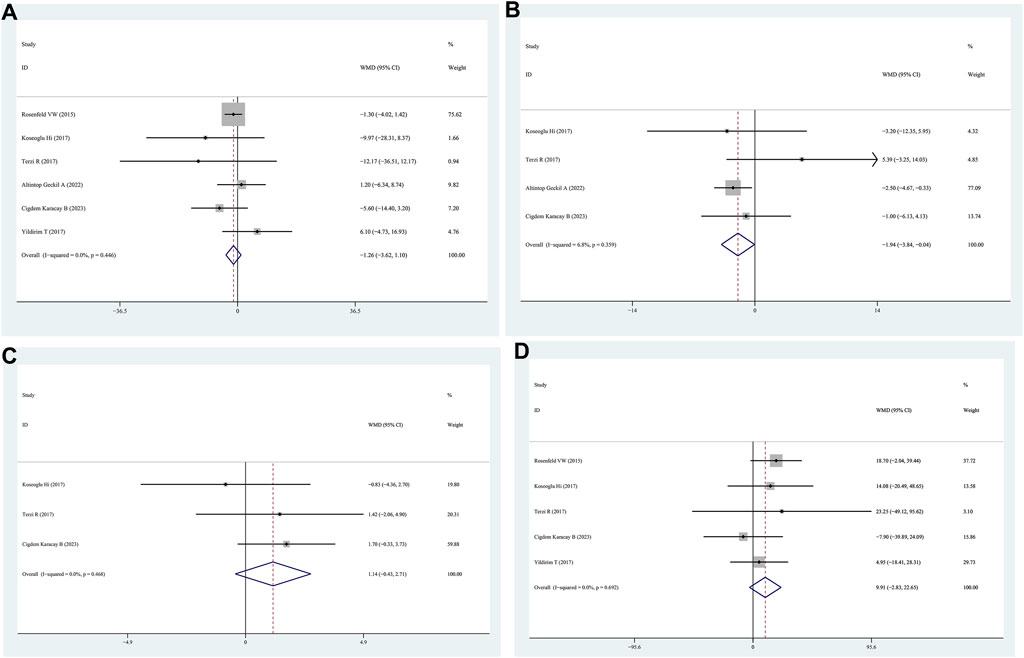
Figure 3. WMD forest plot and its 95%CI for AHI, Minimum SaO2, Mean SaO2, and total sleep time in FM + OSAHS group and FM- OSAHS group. (A) AHI; (B) Minimum SaO2; (C) Mean SaO2; (D) total sleep time.
Minimum SaO2 levels in patients with positive FM were compared to those in patients with negative FM in four studies. The patients in the positive FM group had a lower minimum SaO2 than those in the negative FM group, according to the findings (WMD = −1.94, 95% CI = −3.84–0.04, p = 0.045, I2 = 6.8%) (Figure 3B).
Four studies reported lower SaO2 levels in patients with positive FM than in those with negative FM. The mean saturation oxygen levels of the positive FM and negative FM groups did not differ according to the data (WMD = 1.14, 95% CI = −0.43–2.71, p = 0.154, I2 = 0.0%) (Figure 3C).
Patients with positive FM were compared to those with negative FM in five studies that provided data on total sleep time. The findings indicated that there was no statistically significant distinction in the overall sleep time between patients in the positive FM group and those in the negative FM group (WMD = 9.91, 95% CI = −2.83–22.65, p = 0.128, I2 = 0%) (Figure 3D).
Sleep latency data were obtained from three studies comparing patients with positive and negative FM. The sleep latency values for the positive FM and negative FM groups did not differ according to the findings (WMD = −0.55, 95% CI = −7.53–6.43, p = 0.878, I2 = 62.0%) (Figure 4A).
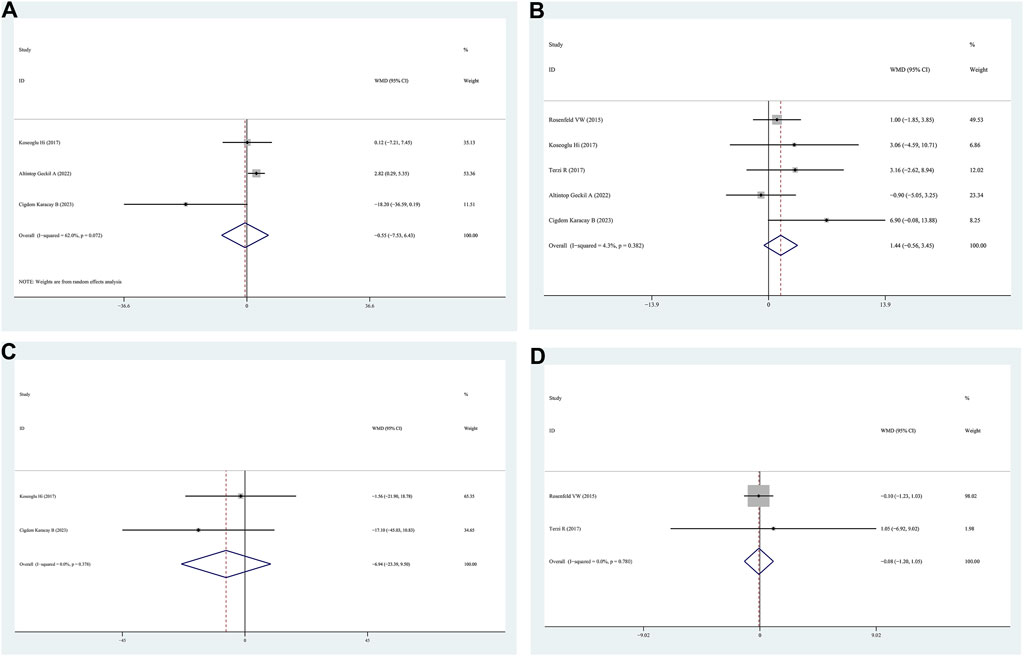
Figure 4. WMD forest plot and its 95%CI for Sleep latency, Sleep efficiency, REM, and Epworth Sleepiness Scale in FM + OSAHS group and FM- OSAHS group. (A) Sleep latency; (B) Sleep efficiency; (C) REM; (D) Epworth Sleepiness Scale. REM: rapid eye movement.
Sleep efficiency data were obtained from five studies that compared patients with positive and negative FM. The findings indicated that the sleep efficiency values of the positive FM and negative FM groups were similar (WMD = 1.44, 95% CI = −0.56–3.45, p = 0.154, I2 = 4.3%) (Figure 4B).
REM data were obtained from two studies that compared patients with positive and negative FM. The findings (WMD = −6.94, 95% CI = −23.39–9.50, p = 0.408, I2 = 0.0%) indicated that there were no differences in REM between the positive FM and negative FM groups (Figure 4C).
Data from two studies that compared patients with positive FM with those with negative FM on the Epworth sleepiness scale were presented. The findings indicated that no significant differences were observed between the positive FM and negative FM groups on the Epworth sleepiness scale (WMD = −0.08, 95% CI = −1.20–1.05, p = 0.893, I2 = 56.2%) (Figure 4D).
N1/N2/N3 data for patients with positive FM compared to those with negative FM were provided in three studies. No significant differences were observed in N1/N2/N3 between the positive FM and negative FM groups, according to the findings (WMD = −3.19, 95% CI = −7.16–0.78, p = 0.054, I2 = 56.2%; WMD = −0.64, 95% CI = −3.96–2.67, p = 0.704, I2 = 0.0%; N3: WMD = 0.35, 95% CI = −3.14–3.85, p = 0.844, I2 = 0.0%) (Figures 5A–C).
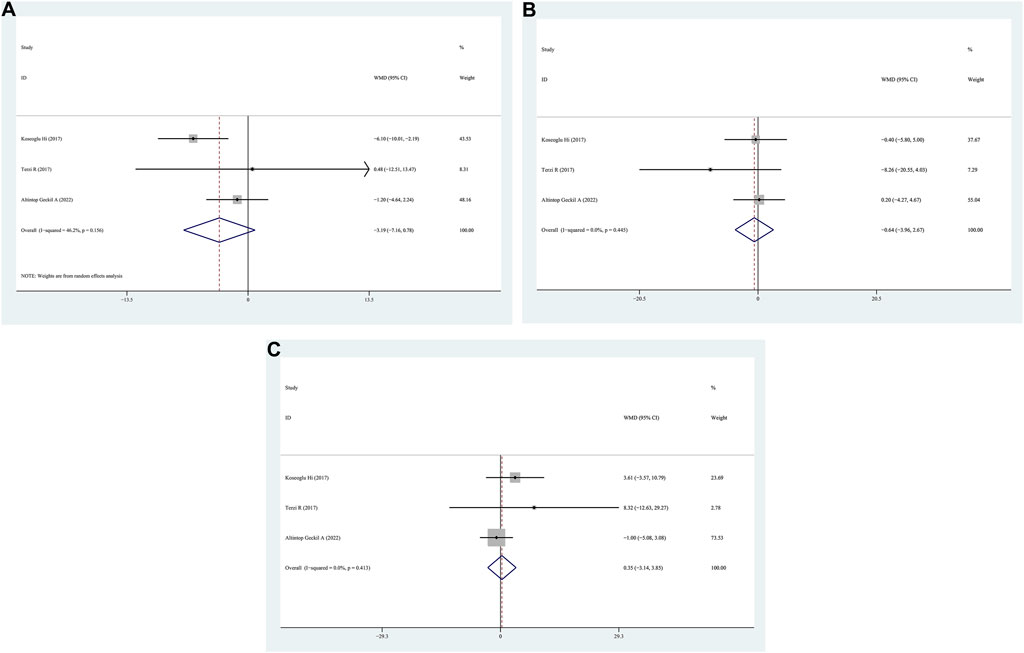
Figure 5. WMD forest plot and its 95%CI for N1/N2/N3 in FM + OSAHS group and FM- OSAHS group. (A) N1; (B) N2; (C) N3.
This systematic evaluation and meta-analysis provide unique information on the clinical correlates of FM in patients with OSAHS. This study describes the incidence of FM in patients with OSAHS and the effects of race, BMI, age, and sex on the incidence of FM. Furthermore, this study compared differences in sleep monitoring indicators between the OSAHS with positive FM and OSAHS groups.
In general, the incidence of FM is high in patients with OSAHS (21%). Cigdem et al. (Cigdem Karacay et al., 2023) found that headache, afternoon fatigue, and morning fatigue were considerably more common in individuals with OSAHS and FM. In addition, patients with OSAHS and FM have low pain thresholds and high pain intensities. Patients with OSAHS may have more pressure points and a longer duration of chronic pain (Lahaye et al., 2023). Similarly, Terzi et al. (Terzi and Yılmaz, 2017) discovered that patients with OSAHS and FM had lower total myalgic and control scores than patients with only OSAHS. Intermittent hypoxia increases pain sensitivity by altering the pain transmission pathway in patients with OSAHS (Wu et al., 2015). Therefore, patients with OSAHS have a higher incidence of FM. In particular, a higher percentage of female patients with OSAHS had comorbid FM than male patients with OSAHS. Different diagnostic criteria affected the incidence of FM, which was supported by subgroup analysis based on diagnostic criteria in this study. An epidemiological survey based on the 1990 ACR of rheumatology diagnostic criteria showed a male-to-female ratio of 9:1 for FM, which was attributed to the fact that a significant number of male patients with peripheral pain had unremarkable somatic tenderness (Jiang et al., 2020). In contrast, the diagnostic criteria for FM revised by the ACR in 2010 and 2016 do not require a diagnosis based on the number of pressure points, revealing more male patients with FM and a 3:1 female-to-male ratio (Queiroz, 2013). However, its incidence remains higher in women than in men. Most previous studies have shown that women experience more pressure points than men (Jiao et al., 2021). Females have lower pain thresholds and show a higher sensitivity to noxious stimuli from mechanical stress and electrical, thermal, ischemic, and cold stimuli, which may be related to differences in sex hormones, endogenous analgesic systems, brain structure, function, sex chromosomes, and psychosocial factors between women and men (Fillingim et al., 2009; Mogil, 2012; Bartley and Fillingim, 2013; Packiasabapathy and Sadhasivam, 2018; Meyer-Frießem et al., 2020; Sauch et al., 2022).
In addition, the incidence of FM in patients with OSAHS varies by race, which could be attributed to variations in genetic polymorphisms, lifestyle habits, and body size in populations from different regions (Zorina-Lichtenwalter et al., 2024). Furthermore, the incidence of FM was slightly higher in obese patients with OSAHS. There is a bidirectional association between OSAHS and obesity (He et al., 2022), which is a critical factor that influences FM (D'Onghia et al., 2021). One study suggested that obese females with FM are more likely to experience chronic pain than non-obese females with FM (Koçyiğit and Okyay, 2018). Obesity leads to a higher prevalence of OSAHS and FM, probably due to joint pain caused by joint loading due to high BMI and generalized pain due to thick subcutaneous fat (Costa et al., 2023; Mathkhor and Ibraheem, 2023). Furthermore, obesity aggravates the severity of OSAHS, and shortening of sleep duration and poor sleep quality promote pain sensitivity (Larsen et al., 2022).
This study found a 22% incidence of FM in patients aged <65 years. Wickwire et al. (Wickwire et al., 2020) reported that the overall incidence of FM in older patients with OSAHS and positive FM was 25%. A previous study showed that the duration of FM symptoms increases with age (Cronan et al., 2002). Sarzi-Puttini (Sarzi-Puttini et al., 2020) reported that FM is the third most common musculoskeletal disorder, and its incidence increases with age. This could be explained by the fact that the organism is more susceptible to pain and fatigue with age, which are closely related to aging, and older people may suffer from other age-related health problems that exacerbate their symptoms of FM.
In particular, the patient had depression, anxiety, restless legs syndrome, elevated autoimmune titers, migraine, and possible vulvodynia or vaginismus as comorbidities of FM (Treves and Qazi, 2022). Stehlik et al. (Stehlik et al., 2009) found that 64% of a group of female patients diagnosed with FM also concurrently suffered from restless legs syndrome. Compared to patients who suffer only from FM, patients with both FM and restless legs syndrome experience sleep disturbances and pronounced daytime sleepiness more frequently. Civelek et al. (Civelek et al., 2014) also reported that the prevalence of restless legs syndrome was higher in the FM group than in the normal population, and the quality of sleep and quality of life were worse in patients with restless legs syndrome. We contend that patients with OSAHS and restless leg syndrome may be more prone to FM. Consistent with these findings, in the study by Altıntop et al., periodic limb movements in sleep (PLMS) was found to be significantly higher in the OSAHS with positive FM group than in the OSAHS with negative FM group (Altıntop Geçkil and Aydoğan Baykara, 2022). One study (Tayag-Kier et al., 2000) suggested that juvenile FM subjects exhibit excessive movement activity during sleep. A total of 38% of patients with FM had an abnormally elevated PLMS index (>5/h), indicating the presence of PLMS in these subjects (Tayag-Kier et al., 2000). The abovementioned studies imply that restless legs syndrome and PLMS might be confounding factors when evaluating the incidence of FM in patients with OSAHS. Unfortunately, the included studies lacked data on possible confounding factors, such as the number of individuals with restless legs syndrome and PLMS; therefore, we were unable to adjust for these factors. We hope that in the future, more studies will focus on the detection of restless legs syndrome and PLMS in these populations (FM plus OSAHS).
It is characterized by chronic generalized discomfort, fatigue, and sleep difficulties (López-Medina and Moltó, 2020). FM is associated with comorbidities such as rheumatism, mental disorders, gastrointestinal disorders, cardiovascular disorders, and peripheral neuropathy (Muzammil and Cooper, 2011; Duan et al., 2019; Wu et al., 2021). Sleep difficulties are common in patients with FM (Lee et al., 2016). Patients with FM have a short duration of sleep, resulting in poor sleep, poorer sleep quality, and impaired sleep efficiency (Martinsen et al., 2014). Rizzi et al. (Rizzi et al., 2017) showed that sleep had the same effects as stress in patients with FM. A vicious circle is created during sleep: pain increases sympathetic cardiovascular activation and reduces sleep efficiency, causing lighter sleep, higher cyclic alternating pattern rate, more arousal, higher PLMS index, and an increased occurrence of periodic breathing, leading to abnormal cardiovascular neural control and exaggerated pain sensitivity. Aberrant autonomic nervous system responses are biological markers of FM (Kang et al., 2016). Patients with FM show sympathetic and parasympathetic hypoactivity in autonomic function (Martínez-Lavín et al., 1998; Raj et al., 2000; Cohen et al., 2001). In FM, sympathetic vascular modulation is reduced, cardiac vagal withdrawal is impaired, and orthostatic tolerance is reduced (Furlan et al., 2005). Autonomic dysfunction is characterized by sustained sympathetic hyperactivity and hyperreactivity to stress in autonomic dysfunction (Di Franco et al., 2010). One study reported that the blunted heart rate response during exercise observed in patients with FM could be associated with the desensitization of cardiac β1 receptors through a heightened sympathetic activity similar to heart failure (Lauer, 2004). Therefore, autonomic cardiovascular dysfunction may be involved in the complex etiopathogenesis of FM syndrome and increase the risk of cardiac events.
Sleep plays an important role in the etiology and treatment of FM. Alpha-wave intrusion during deep sleep is a common sleep disturbance associated with FM (Roizenblatt et al., 2011). Sleep-disordered breathing has also been associated with FM in some studies (Shah et al., 2006). Women with FM experience disordered breathing (Shah et al., 2006). FM may be a marker of occult sleep apnea in males (May et al., 1993). This is a particularly notable correlation to explore because care for patients with FM is often scattered due to comorbidities (Garcia-Campayo et al., 2008; Munipalli et al., 2022). As patients with OSAHS and FM commonly suffer from psychiatric comorbidities, they are often treated by psychiatric and pain clinics that prescribe benzodiazepines and opioids (Thorpe et al., 2018; Wang et al., 2019; Cutrufello et al., 2020). Opioids combined with benzodiazepines can worsen OSA outcomes and patients may become dependent on opioid painkillers (Fitzcharles et al., 2011; Cunningham et al., 2016). The mechanisms by which the brain modulates pain involve complex pathways, the neural circuits underlying which are poorly understood. Hyperalgesia is associated with a loss of 4 h of REM sleep (Roehrs et al., 2006). Rosenfeld et al. (Rosenfeld et al., 2015) reported a unique electroencephalography in patients with FM and reported that the incidence of complications associated with OSAHS was 45%. They also found a low delta/alpha ratio during non-REM sleep in patients with FM. Some studies have shown increased sympathetic activity during sleep in both OSAHS and FM (Kang et al., 2016; Tang et al., 2022), and that the pathophysiological mechanisms of both disorders involve central sensitization and serotonin deficiency (Altıntop Geçkil and Aydoğan Baykara, 2022). In addition, some clinical manifestations of FM, such as fatigue, reduced exercise capacity, and cold intolerance, can be explained by growth hormone deficiency. Moreover, the levels decrease further as FM progresses (Jones et al., 2007). Approximately 70% of the daily release of growth hormone occurs during the N3 and REM sleep phases (Van Cauter et al., 1998). OSAHS is a significant factor that affects the GH release of growth hormone (Francisco et al., 2023). Therefore, FM and OSAHS share common pathological mechanisms and the two conditions mutually influence each other, creating a vicious cycle.
FM can be managed more effectively using a multidisciplinary strategy that includes rheumatologists, physiotherapists, and psychiatrists. Some studies have recommended polysomnographic monitoring, particularly in patients with FM with severe daytime drowsiness (Mutlu et al., 2020). Patients with FM can benefit from OSAHS evaluation and patients with OSAHS may benefit from the FM assessment (Cappelleri et al., 2009; Köseoğlu et al., 2017). In this study, most of the sleep monitoring indicators did not differ substantially between patients with OSAHS with positive FM and those with OSAHS with negative FM. However, when patients with OSAHS experience chronic pain, such as FM, it is crucial to monitor changes in pain thresholds. Furthermore, the effects of nocturnal hypoxemia and sleep disturbances on pain thresholds were evaluated. Recent findings contradict those of previous studies on the association between OSAHS severity and FM coexistence. Koseoglu et al. (Köseoğlu et al., 2017) demonstrated that FM and OSAHS did not affect polysomnographic indicators. However, this meta-analysis showed that patients with OSAHS and FM had a lower minimum oxygen saturation, suggesting muscle dysfunction due to increased tissue hypoxia, and this may explain the increased pain complaints in patients with FM. However, in terms of several sleep monitoring metrics, including AHI score, sleep latency, mean saturation oxygen level, sleep efficiency, total sleep duration, N1/N2/N3, REM, and the Epworth sleepiness scale score, the OSAHS with positive FM group did not differ significantly from the OSAHS with negative FM group. However, a link between AHI and FM severity has previously been documented previously (Mutlu et al., 2020). Altıntop et al. (Altıntop Geçkil and Aydoğan Baykara, 2022) reported longer sleep latency in patients with OSAHS and FM. These findings indicate a lack of consensus on the association between sleep monitoring indices in OSAHS and FM, which could be attributed to the small sample size of a single study. Second, different environments and equipment for sleep monitoring affected the data; therefore, larger clinical studies are needed. In summary, this study suggests that patients with OSAHS have a high incidence of FM, which may help clinicians assess FM in patients with OSAHS and implement proactive interventions. Continuous positive airway pressure (CPAP) therapy is the main treatment for OSAHS (Gagnadoux et al., 2011). In a study that evaluated 14 patients with OSAHS, there was a significant improvement in FM symptoms after 3 weeks of CPAP therapy (Gold et al., 2004). Furthermore, opioids and benzodiazepines used to treat FM can exacerbate OSAHS and increase pain (Cunningham et al., 2016). Therefore, determining the incidence and performing early treatments such as CPAP in patients with OSAHS and FM can prevent unnecessary FM treatment.
This study has several limitations. First, the heterogeneity may have been caused by differences in the definition of FM between studies, subject characteristics, and diagnostic thresholds. Therefore, interpreting meta-analysis data is difficult. Second, due to the lack of data, this review was unable to establish a causal link between OSAHS and FM. Third, these studies lack concrete evidence to highlight the clinical impact of FM on healthcare expenditure, an issue that future research should examine. Fourth, differences in severity between FM and pain measurement standards can restrict the therapeutic applicability of the data. The difficulty in diagnosing FM and the successive definitions of this pathology over time certainly limit the scope of the results (Qureshi et al., 2021). The latest definition is more rigorous and the literature published before 2015 should be treated with caution.
The incidence of FM is high among patients with OSAHS. In clinical practice, patients with OSAHS undergo FM screening. Additionally, most of the sleep monitoring indicators in patients with OSAHS and FM were not significantly different from those in patients with OSAHS but without FM.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
JH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. MC: Methodology, Software, Writing–original draft. NH: Data curation, Investigation, Software, Conceptualization, Formal Analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. BW: Conceptualization, Data curation, Formal Analysis, Project administration, Writing–original draft, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Priming Scientific Research Foundation for the Introduced Talents of The First Affiliated Hospital of Chengdu Medical College (CYFY-GQ59). Natural Science Foundation of Sichuan Province (Grant No. 2022NSFSC0725).
We thank Bullet Edits Limited for linguistic editing and proofreading of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2024.1394865/full#supplementary-material
Altınbilek T., Terzi R., Başaran A., Tolu S., Küçüksaraç S. (2019). Evaluation of the effects of neural therapy in patients diagnosed with fibromyalgia. Turk J. Phys. Med. Rehabil. 65, 1–8. doi:10.5606/tftrd.2019.1931
Altıntop Geçkil A., Aydoğan Baykara R. (2022). Coexistence of obstructive sleep apnea syndrome and fibromyalgia. Tuberk. Toraks 70, 37–43. doi:10.5578/tt.20229905
Baker M., Scott B., Johnson R. F., Mitchell R. B. (2017). Predictors of obstructive sleep apnea severity in adolescents. JAMA Otolaryngol. Head. Neck Surg. 143, 494–499. doi:10.1001/jamaoto.2016.4130
Bartley E. J., Fillingim R. B. (2013). Sex differences in pain: a brief review of clinical and experimental findings. Br. J. Anaesth. 111, 52–58. doi:10.1093/bja/aet127
Bergman S. (2005). Psychosocial aspects of chronic widespread pain and fibromyalgia. Disabil. Rehabil. 27, 675–683. doi:10.1080/09638280400009030
Cappelleri J. C., Bushmakin A. G., McDermott A. M., Dukes E., Sadosky A., Petrie C. D., et al. (2009). Measurement properties of the medical outcomes study sleep scale in patients with fibromyalgia. Sleep. Med. 10, 766–770. doi:10.1016/j.sleep.2008.09.004
Chung S. D., Lin C. C., Liu S. P., Lin H. C. (2014). Obstructive sleep apnea increases the risk of bladder pain syndrome/interstitial cystitis: a population-based matched-cohort study. Neurourol. Urodyn. 33, 278–282. doi:10.1002/nau.22401
Cigdem Karacay B., Sahbaz T., Zerman N., Tuncay F. (2023). The impact of fibromyalgia syndrome on obstructive sleep apnea syndrome in terms of pain threshold, daytime symptoms, anxiety, depression, disease severity, and sleep quality: a polysomnographic study. Sleep. Breath. 27, 1473–1479. doi:10.1007/s11325-023-02831-2
Civelek G. M., Ciftkaya P. O., Karatas M. (2014). Evaluation of restless legs syndrome in fibromyalgia syndrome: an analysis of quality of sleep and life. J. Back Musculoskelet. Rehabil. 27, 537–544. doi:10.3233/bmr-140478
Cohen H., Neumann L., Alhosshle A., Kotler M., Abu-Shakra M., Buskila D. (2001). Abnormal sympathovagal balance in men with fibromyalgia. J. Rheumatol. 28, 581–589.
Costa V., Gianlorenço A. C., Daibes M., Queiroz F., Lacerda G., Martinez-Magallanes D., et al. (2023). Physical conditioning, obesity and fibromyalgia: causal relationship or confounding? Princ. Pract. Clin. Res. 9, 63–68. doi:10.21801/ppcrj.2023.93.2
Cronan T. A., Serber E. R., Walen H. R., Jaffe M. (2002). The influence of age on fibromyalgia symptoms. J. Aging Health 14, 370–384. doi:10.1177/08964302014003004
Cunningham J. L., Evans M. M., King S. M., Gehin J. M., Loukianova L. L. (2016). Opioid tapering in fibromyalgia patients: experience from an interdisciplinary pain rehabilitation program. Pain Med. 17, 1676–1685. doi:10.1093/pm/pnv079
Cutrufello N. J., Ianus V. D., Rowley J. A. (2020). Opioids and sleep. Curr. Opin. Pulm. Med. 26, 634–641. doi:10.1097/mcp.0000000000000733
D'Agnelli S., Arendt-Nielsen L., Gerra M. C., Zatorri K., Boggiani L., Baciarello M., et al. (2019). Fibromyalgia: genetics and epigenetics insights may provide the basis for the development of diagnostic biomarkers. Mol. Pain 15, 1744806918819944. doi:10.1177/1744806918819944
Dahan V., Kimoff R. J., Petrof B. J., Benedetti A., Diorio D., Trojan D. A. (2006). Sleep-disordered breathing in fatigued postpoliomyelitis clinic patients. Arch. Phys. Med. Rehabil. 87, 1352–1356. doi:10.1016/j.apmr.2006.07.256
Di Franco M., Iannuccelli C., Valesini G. (2010). Neuroendocrine immunology of fibromyalgia. Ann. N. Y. Acad. Sci. 1193, 84–90. doi:10.1111/j.1749-6632.2009.05344.x
D'Onghia M., Ciaffi J., Lisi L., Mancarella L., Ricci S., Stefanelli N., et al. (2021). Fibromyalgia and obesity: a comprehensive systematic review and meta-analysis. Semin. Arthritis Rheum. 51, 409–424. doi:10.1016/j.semarthrit.2021.02.007
Duan Y., Zhou X., Su H., Jiang K., Wu W., Pan X., et al. (2019). Balloon angioplasty or stent implantation for pulmonary vein stenosis caused by fibrosing mediastinitis: a systematic review. Cardiovasc Diagn Ther. 9, 520–528. doi:10.21037/cdt.2019.09.14
Fillingim R. B., King C. D., Ribeiro-Dasilva M. C., Rahim-Williams B., Riley J. L. (2009). Sex, gender, and pain: a review of recent clinical and experimental findings. J. Pain 10, 447–485. doi:10.1016/j.jpain.2008.12.001
Fitzcharles M. A., Ste-Marie P. A., Gamsa A., Ware M. A., Shir Y. (2011). Opioid use, misuse, and abuse in patients labeled as fibromyalgia. Am. J. Med. 124, 955–960. doi:10.1016/j.amjmed.2011.05.031
Francisco G. R., Simões J. L. B., de Carvalho B. G., Guerra P. H., Bagatini M. D. (2023). The outcomes of growth hormone therapy in the obstructive sleep apnea parameters of Prader-Willi syndrome patients: a systematic review. Eur. Arch. Otorhinolaryngol. 281, 2235–2242. doi:10.1007/s00405-023-08406-x
Furlan R., Colombo S., Perego F., Atzeni F., Diana A., Barbic F., et al. (2005). Abnormalities of cardiovascular neural control and reduced orthostatic tolerance in patients with primary fibromyalgia. J. Rheumatol. 32, 1787–1793.
Gagnadoux F., Le Vaillant M., Goupil F., Pigeanne T., Chollet S., Masson P., et al. (2011). Influence of marital status and employment status on long-term adherence with continuous positive airway pressure in sleep apnea patients. PLoS One 6, e22503. doi:10.1371/journal.pone.0022503
Garcia-Campayo J., Magdalena J., Magallón R., Fernández-García E., Salas M., Andrés E. (2008). A meta-analysis of the efficacy of fibromyalgia treatment according to level of care. Arthritis Res. Ther. 10, R81. doi:10.1186/ar2455
Gardoki-Souto I., Redolar-Ripoll D., Fontana M., Hogg B., Castro M. J., Blanch J. M., et al. (2022). Prevalence and characterization of psychological trauma in patients with fibromyalgia: a cross-sectional study. Pain Res. Manag. 2022, 2114451. doi:10.1155/2022/2114451
Gold A. R., Dipalo F., Gold M. S., Broderick J. (2004). Inspiratory airflow dynamics during sleep in women with fibromyalgia. Sleep 27, 459–466. doi:10.1093/sleep/27.3.459
He J., Li X., Yu M. (2022). The correlation of serum/plasma IGF-1 concentrations with obstructive sleep apnea hypopnea syndrome: a meta-analysis and meta-regression. Front. Endocrinol. (Lausanne) 13, 922229. doi:10.3389/fendo.2022.922229
Jiang L., D'Souza R. S., Oh T., Vincent A., Mohabbat A. B., Ashmore Z., et al. (2020). Sex-related differences in symptoms and psychosocial outcomes in patients with fibromyalgia: a prospective questionnaire study. Mayo Clin. Proc. Innov. Qual. Outcomes 4, 767–774. doi:10.1016/j.mayocpiqo.2020.06.009
Jiao J., Cheng Z., Wang W., Zhao Y., Jiang Q. (2021). Demographic characteristics and clinical features of fibromyalgia in China: a cross-sectional study. Rheumatol. Ther. 8, 817–831. doi:10.1007/s40744-021-00303-1
Johnson K. G., Johnson D. C., Thomas R. J., Rastegar V., Visintainer P. (2020). Cardiovascular and somatic comorbidities and sleep measures using three hypopnea criteria in mild obstructive sleep-disordered breathing: sex, age, and body mass index differences in a retrospective sleep clinic cohort. J. Clin. Sleep. Med. 16, 1683–1691. doi:10.5664/jcsm.8644
Jones K. D., Deodhar P., Lorentzen A., Bennett R. M., Deodhar A. A. (2007). Growth hormone perturbations in fibromyalgia: a review. Semin. Arthritis Rheum. 36, 357–379. doi:10.1016/j.semarthrit.2006.09.006
Kang J. H., Kim J. K., Hong S. H., Lee C. H., Choi B. Y. (2016). Heart rate variability for quantification of autonomic dysfunction in fibromyalgia. Ann. Rehabil. Med. 40, 301–309. doi:10.5535/arm.2016.40.2.301
Kiso T., Sekizawa T., Uchino H., Tsukamoto M., Kakimoto S. (2018). Analgesic effects of ASP3662, a novel 11β-hydroxysteroid dehydrogenase 1 inhibitor, in rat models of neuropathic and dysfunctional pain. Br. J. Pharmacol. 175, 3784–3796. doi:10.1111/bph.14448
Koçyiğit B. F., Okyay R. A. (2018). The relationship between body mass index and pain, disease activity, depression and anxiety in women with fibromyalgia. PeerJ 6, e4917. doi:10.7717/peerj.4917
Köseoğlu H., İnanır A., Kanbay A., Okan S., Demir O., Çeçen O., et al. (2017). Is there a link between obstructive sleep apnea syndrome and fibromyalgia syndrome? Turk Thorac. J. 18, 40–46. doi:10.5152/TurkThoracJ.2017.16036
Lahaye C., Miolanne M., FaVira B., Dubray C., Beudin P., et al. (2023). Enhanced pain sensitivity in obese patients with obstructive sleep apnoea syndrome is partially reverted by treatment: an exploratory study. Eur. J. Pain 27, 624–635. doi:10.1002/ejp.2085
Larsen D. B., Bendix L., Abeler K., Petersen K. K., Sprehn M., Bruun K. D., et al. (2022). Obstructive sleep apnea is common in patients with high-impact chronic pain - an exploratory study from an interdisciplinary pain center. Scand. J. Pain 22, 106–117. doi:10.1515/sjpain-2021-0112
Lauer M. S. (2004). Chronotropic incompetence: ready for prime time. J. Am. Coll. Cardiol. 44, 431–432. doi:10.1016/j.jacc.2004.05.001
Lee L. K., Ebata N., Hlavacek P., DiBonaventura M., Cappelleri J. C., Sadosky A. (2016). Humanistic and economic burden of fibromyalgia in Japan. J. Pain Res. 9, 967–978. doi:10.2147/jpr.S110707
Li X., He J. (2021). The association between serum/plasma leptin levels and obstructive sleep apnea syndrome: a meta-analysis and meta-regression. Front. Endocrinol. (Lausanne) 12, 696418. doi:10.3389/fendo.2021.696418
López-Medina C., Moltó A. (2020). Comorbid pain in axial spondyloarthritis, including fibromyalgia. Ther. Adv. Musculoskelet. Dis. 12, 1759720x20966123. doi:10.1177/1759720x20966123
Martínez-Lavín M., Hermosillo A. G., Rosas M., Soto M. E. (1998). Circadian studies of autonomic nervous balance in patients with fibromyalgia: a heart rate variability analysis. Arthritis Rheum. 41, 1966–1971. doi:10.1002/1529-0131(199811)41:11<1966::Aid-art11>3.0.Co;2-o
Martinsen S., Flodin P., Berrebi J., Löfgren M., Bileviciute-Ljungar I., Ingvar M., et al. (2014). Fibromyalgia patients had normal distraction related pain inhibition but cognitive impairment reflected in caudate nucleus and hippocampus during the Stroop Color Word Test. PLoS One 9, e108637. doi:10.1371/journal.pone.0108637
Mathkhor A. J., Ibraheem N. M. (2023). Prevalence and Impact of obesity on fibromyalgia syndrome and its allied symptoms. J. Fam. Med. Prim. Care 12, 123–127. doi:10.4103/jfmpc.jfmpc_2052_22
May K. P., West S. G., Baker M. R., Everett D. W. (1993). Sleep apnea in male patients with the fibromyalgia syndrome. Am. J. Med. 94, 505–508. doi:10.1016/0002-9343(93)90085-4
Meresh E. S., Artin H., Joyce C., Birch S., Daniels D., Owens J. H., et al. (2019). Obstructive sleep apnea co-morbidity in patients with fibromyalgia: a single-center retrospective analysis and literature review. Open Access Rheumatol. 11, 103–109. doi:10.2147/oarrr.S196576
Meyer-Frießem C. H., Attal N., Baron R., Bouhassira D., Finnerup N. B., Freynhagen R., et al. (2020). Pain thresholds and intensities of CRPS type I and neuropathic pain in respect to sex. Eur. J. Pain 24, 1058–1071. doi:10.1002/ejp.1550
Mogil J. S. (2012). Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 13, 859–866. doi:10.1038/nrn3360
Moldofsky H. (1989). Sleep and fibrositis syndrome. Rheum. Dis. Clin. North Am. 15, 91–103. doi:10.1016/s0889-857x(21)00035-1
Moldofsky H., Scarisbrick P., England R., Smythe H. (1975). Musculosketal symptoms and non-REM sleep disturbance in patients with "fibrositis syndrome" and healthy subjects. Psychosom. Med. 37, 341–351. doi:10.1097/00006842-197507000-00008
Munipalli B., Allman M. E., Chauhan M., Niazi S. K., Rivera F., Abril A., et al. (2022). Depression: a modifiable risk factor for poor outcomes in fibromyalgia. J. Prim. Care Community Health 13, 21501319221120738. doi:10.1177/21501319221120738
Mutlu P., Zateri C., Zohra A., Ozerdogan O., Mirici A. N. (2020). Prevalence of obstructive sleep apnea in female patients with fibromyalgia. Saudi Med. J. 41, 740–745. doi:10.15537/smj.2020.7.25165
Muzammil S., Cooper H. C. (2011). Acute pancreatitis and fibromyalgia: cytokine link. N. Am. J. Med. Sci. 3, 206–208. doi:10.4297/najms.2011.3205
O'Donovan C. A., Rissmiller R., Rinn A., Fleming S., White J. R., McCall W. V., et al. (2006). Outcome of patients with sleep complaints and normal polysomnograms. J. Clin. Sleep. Med. 2, 325–327. doi:10.5664/jcsm.26594
Oka H., Miki K., Kishita I., Kong D. F., Uchida T. (2020). A multicenter, prospective, randomized, placebo-controlled, double-blind study of a novel pain management device, AT-02, in patients with fibromyalgia. Pain Med. 21, 326–332. doi:10.1093/pm/pnz064
Packiasabapathy S., Sadhasivam S. (2018). Gender, genetics, and analgesia: understanding the differences in response to pain relief. J. Pain Res. 11, 2729–2739. doi:10.2147/jpr.S94650
Passos J. O. S., Dos Santos A. M. V., Morais C. L. M., Martin F. L., Cavalcante A. F., Lemos T., et al. (2020). Spectrochemical analysis in blood plasma combined with subsequent chemometrics for fibromyalgia detection. Sci. Rep. 10, 11769. doi:10.1038/s41598-020-68781-x
Pei C., Gui S. (2021). Effect of arterial blood bicarbonate (HCO(3)(-)) concentration on the accuracy of STOP-Bang questionnaire screening for obstructive sleep apnea. BMC Pulm. Med. 21, 366. doi:10.1186/s12890-021-01720-2
Queiroz L. P. (2013). Worldwide epidemiology of fibromyalgia. Curr. Pain Headache Rep. 17, 356. doi:10.1007/s11916-013-0356-5
Qureshi A. G., Jha S. K., Iskander J., Avanthika C., Jhaveri S., Patel V. H., et al. (2021). Diagnostic challenges and management of fibromyalgia. Cureus 13, e18692. doi:10.7759/cureus.18692
Raj S. R., Brouillard D., Simpson C. S., Hopman W. M., Abdollah H. (2000). Dysautonomia among patients with fibromyalgia: a noninvasive assessment. J. Rheumatol. 27, 2660–2665.
Rizzi M., Radovanovic D., Santus P., Airoldi A., Frassanito F., Vanni S., et al. (2017). Influence of autonomic nervous system dysfunction in the genesis of sleep disorders in fibromyalgia patients. Clin. Exp. Rheumatol. 35 (Suppl. 105), 74–80. doi:10.1136/annrheumdis-2017-eular.3365
Roehrs T., Hyde M., Blaisdell B., Greenwald M., Roth T. (2006). Sleep loss and REM sleep loss are hyperalgesic. Sleep 29, 145–151. doi:10.1093/sleep/29.2.145
Roizenblatt S., Neto N. S., Tufik S. (2011). Sleep disorders and fibromyalgia. Curr. Pain Headache Rep. 15, 347–357. doi:10.1007/s11916-011-0213-3
Rosenfeld V. W., Rutledge D. N., Stern J. M. (2015). Polysomnography with quantitative EEG in patients with and without fibromyalgia. J. Clin. Neurophysiol. 32, 164–170. doi:10.1097/wnp.0000000000000134
Sanders A. E., Greenspan J. D., Fillingim R. B., Rathnayaka N., Ohrbach R., Slade G. D. (2020). Associations of sleep disturbance, atopy, and other health measures with chronic overlapping pain conditions. J. Oral Facial Pain Headache 34, s73–s84. doi:10.11607/ofph.2577
Sarzi-Puttini P., Giorgi V., Marotto D., Atzeni F. (2020). Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 16, 645–660. doi:10.1038/s41584-020-00506-w
Sauch V. G., Miró Catalina Q., Vidal-Alaball J. (2022). Prevalence and incidence of patients with fibromyalgia in catalonia between 2010 and 2017: a descriptive observational study. J. Prim. Care Community Health 13, 21501319221094169. doi:10.1177/21501319221094169
Sepici V., Tosun A., Köktürk O. (2007). Obstructive sleep apnea syndrome as an uncommon cause of fibromyalgia: a case report. Rheumatol. Int. 28, 69–71. doi:10.1007/s00296-007-0375-9
Shah M. A., Feinberg S., Krishnan E. (2006). Sleep-disordered breathing among women with fibromyalgia syndrome. J. Clin. Rheumatol. 12, 277–281. doi:10.1097/01.rhu.0000249771.97221.36
Shaver J. L., Lentz M., Landis C. A., Heitkemper M. M., Buchwald D. S., Woods N. F. (1997). Sleep, psychological distress, and stress arousal in women with fibromyalgia. Res. Nurs. Health 20, 247–257. doi:10.1002/(sici)1098-240x(199706)20:3<247::aid-nur7>3.0.co;2-i
Stang A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. doi:10.1007/s10654-010-9491-z
Stehlik R., Arvidsson L., Ulfberg J. (2009). Restless legs syndrome is common among female patients with fibromyalgia. Eur. Neurol. 61, 107–111. doi:10.1159/000180313
Tang S. S., Liang C. H., Liu Y. L., Wei W., Deng X. R., Shi X. Y., et al. (2022). Intermittent hypoxia is involved in gut microbial dysbiosis in type 2 diabetes mellitus and obstructive sleep apnea-hypopnea syndrome. World J. Gastroenterol. 28, 2320–2333. doi:10.3748/wjg.v28.i21.2320
Tayag-Kier C. E., Keenan G. F., Scalzi L. V., Schultz B., Elliott J., Zhao R. H., et al. (2000). Sleep and periodic limb movement in sleep in juvenile fibromyalgia. Pediatrics 106, E70. doi:10.1542/peds.106.5.e70
Terzi R., Yılmaz Z. (2017). Evaluation of pain sensitivity by tender point counts and myalgic score in patients with and without obstructive sleep apnea syndrome. Int. J. Rheum. Dis. 20, 340–345. doi:10.1111/1756-185x.12629
Tesio V., Di Tella M., Ghiggia A., Romeo A., Colonna F., Fusaro E., et al. (2018). Alexithymia and depression affect quality of life in patients with chronic pain: a study on 205 patients with fibromyalgia. Front. Psychol. 9, 442. doi:10.3389/fpsyg.2018.00442
Thorpe J., Shum B., Moore R. A., Wiffen P. J., Gilron I. (2018). Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database Syst. Rev. 2, Cd010585. doi:10.1002/14651858.CD010585.pub2
Treves G., Qazi M. (2022). Extensive workup on early-onset fibromyalgia: a case report. Cureus 14, e21714. doi:10.7759/cureus.21714
Van Cauter E., Plat L., Copinschi G. (1998). Interrelations between sleep and the somatotropic axis. Sleep 21, 553–566.
Wahner-Roedler D. L., Olson E. J., Narayanan S., Sood R., Hanson A. C., Loehrer L. L., et al. (2007). Gender-specific differences in a patient population with obstructive sleep apnea-hypopnea syndrome. Gend. Med. 4, 329–338. doi:10.1016/s1550-8579(07)80062-3
Wang D., Yee B. J., Grunstein R. R. (2019). Does sleep apnea worsen the adverse effects of opioids and benzodiazepines on chronic obstructive pulmonary disease? Ann. Am. Thorac. Soc. 16, 1237–1238. doi:10.1513/AnnalsATS.201907-504ED
Wickwire E. M., Jobe S. L., Oldstone L. M., Scharf S. M., Johnson A. M., Albrecht J. S. (2020). Lower socioeconomic status and co-morbid conditions are associated with reduced continuous positive airway pressure adherence among older adult medicare beneficiaries with obstructive sleep apnea. Sleep 43, zsaa122. doi:10.1093/sleep/zsaa122
Wolfe F., Clauw D. J., Fitzcharles M. A., Goldenberg D. L., Häuser W., Katz R. L., et al. (2016). 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 46, 319–329. doi:10.1016/j.semarthrit.2016.08.012
Wolfe F., Clauw D. J., Fitzcharles M. A., Goldenberg D. L., Katz R. S., Mease P., et al. (2010). The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. Hob. 62, 600–610. doi:10.1002/acr.20140
Wolfe F., Smythe H. A., Yunus M. B., Bennett R. M., Bombardier C., Goldenberg D. L., et al. (1990). The American College of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 33, 160–172. doi:10.1002/art.1780330203
Wu J., Li P., Wu X., Chen W. (2015). Chronic intermittent hypoxia decreases pain sensitivity and increases the expression of HIF1α and opioid receptors in experimental rats. Sleep. Breath. 19, 561–568. doi:10.1007/s11325-014-1047-0
Wu Q., Xu C., Wang L. (2021). A patient with focal myositis and primary cutaneous diffuse large B-cell lymphoma: a case report. Front. Oncol. 11, 658907. doi:10.3389/fonc.2021.658907
Yildirim T., Alp R. (2017). The role of oxidative stress in the relation between fibromyalgia and obstructive sleep apnea syndrome. Eur. Rev. Med. Pharmacol. Sci. 21, 20–29.
Keywords: fibromyalgia, obstructive sleep apnea-hypopnea syndrome, meta-analysis, incidence, sleep indices
Citation: He J, Chen M, Huang N and Wang B (2024) Fibromyalgia in obstructive sleep apnea-hypopnea syndrome: a systematic review and meta-analysis. Front. Physiol. 15:1394865. doi: 10.3389/fphys.2024.1394865
Received: 02 March 2024; Accepted: 06 May 2024;
Published: 20 May 2024.
Edited by:
Yasumasa Okada, Murayama Medical Center (NHO), JapanReviewed by:
Frederic Roche, Université Jean Monnet, FranceCopyright © 2024 He, Chen, Huang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Wang, MTk5NjE3MTczMzFAMTYzLmNvbQ==; Jie He, MTM1NDAyNDY5NzRAMTYzLmNvbSYjeDAyMDBhOw==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.