
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 25 June 2024
Sec. Gastrointestinal Sciences
Volume 15 - 2024 | https://doi.org/10.3389/fphys.2024.1394030
This article is part of the Research Topic Gastrointestinal Damage and Metabolic Disorders View all 10 articles
Background: Fibroblast growth factor 21 (FGF21) is a stress-inducible hormone that regulates nutrient and metabolic homeostasis. Inflammatory state is one of the stimulators of FGF21 secretion. The aim of the study was to assess correlations between serum FGF21 level and inflammatory markers as well as nutritional status indicators in patients with inflammatory bowel disease (IBD).
Methods: Fasting serum FGF21 level was measured using ELISA test in 105 IBD patients and 17 healthy controls. There were 31 subjects with active ulcerative colitis (UC), 16 with inactive UC, 36 with active Crohn’s disease (CD), and 22 with inactive CD. Clinical and endoscopic activity of IBD was evaluated based on validated scales and indices. Fecal calprotectin, serum CRP, and selected parameters of nutritional status were tested in all patients.
Results: Serum FGF21 level was characterized by fluctuations depending on the IBD activity. FGF21 level was significantly higher in both active UC and CD compared to inactive phases of the diseases and to the controls. A correlation between FGF21 and fecal calprotectin levels was also found in UC and CD. Additionally, in CD, FGF21 level positively correlated with CRP level. In both UC and CD, a negative correlation was noted between FGF21 level and nutritional status parameters including cholesterol, protein, albumin levels, and BMI.
Conclusion: The intensity of intestinal inflammation is related to FGF21 level, which correlates negatively with nutritional status indicators in IBD. The disturbances in FGF21 secretion may contribute to the multifactorial pathogenesis of malnutrition and weight loss in IBD patients.
The family of fibroblast growth factors (FGF) consists of 22 structurally related peptides with a diverse range of cellular functions (Beenken and Mohammadi, 2009; Degirolamo et al., 2016). Among them, there is a group of three factors with endocrine properties – FGF19, FGF21, and FGF23 (Łukawska and Mulak, 2022). These endocrine FGFs are released into the bloodstream and exert their effects on distant tissues regulating multiple metabolic processes (Beenken and Mohammadi, 2009; Degirolamo et al., 2016; Łukawska and Mulak, 2022). The function of endocrine FGFs depends on the presence of their receptors and co-receptors α-Klotho or β-Klotho. The co-receptors expression in target organs determines the tissue-specific action of the FGFs (Degirolamo et al., 2016).
FGF21 is a protein produced mainly in the liver, adipose tissue, muscles, and the pancreas. FGF21 requires the presence of β-Klotho to activate appropriate receptors in target tissues. FGF21 is involved in the metabolism of lipids, carbohydrates, and proteins. It also participates in energy expenditure and body weight regulation (Dolegowska et al., 2019). The main inducers of the FGF21 expression include fasting state, overfeeding, inflammation, and physical activity (Martínez-Garza et al., 2019). The mode of FGF21 action is considered not only endocrine but also paracrine and autocrine (Martínez-Garza et al., 2019). One of the main target organs of FGF21 is white adipose tissue (WAT). FGF21 can both inhibit and stimulate lipolysis in WAT, after meals and during fasting, respectively (Xie et al., 2020). Additionally, during fasting, FGF21 stimulates gluconeogenesis, ketogenesis, and fatty acid oxidation (Gadaleta et al., 2011). Interestingly, FGF21 may cross the blood-brain barrier and exert effect at the central nervous system related to glucose homeostasis and body weight regulation (Lan et al., 2017). Moreover, FGF21 induces the production of corticotropin-releasing hormone activating the hypothalamic-pituitary-adrenal axis and increasing gluconeogenesis in the liver during prolonged starvation (BonDurant and Potthoff, 2018). The results of previous experimental and clinical studies have shown that inflammatory stimuli may also induce the FGF21 expression (Feingold et al., 2012; Gariani et al., 2013).
Inflammatory bowel disease (IBD) is a chronic recurrent immune-mediated disorder of the gastrointestinal tract that encompass ulcerative colitis (UC) and Crohn’s disease (CD) (Zhang and Li, 2014). IBD is characterized by a wide spectrum of intestinal and extra-intestinal symptoms as well as systemic complications. The disease progresses with periods of flares and remissions (Torres et al., 2017; Ungaro et al., 2017). Due to intestinal inflammation, diarrhea, malabsorption, dietary limitations, and anorexia, patients with IBD are at increased risk of malnutrition, the prevalence of which among that group of patients ranges from 20% to 85% (Balestrieri et al., 2020).
The results of previous studies in animal models have suggested that FGF21 as a metabolic regulator, secreted during inflammation, may take part in the pathogenesis of IBD (Liu et al., 2017; Liu et al., 2023). It has been shown that dextran sulfate sodium-induced colitis resulted in increased expression of FGF21, while the absence of FGF21 alleviated colitis symptoms, reduced adipose tissue lipolysis and prevented weight loss (Liu et al., 2017; Liu et al., 2023). Furthermore, also in IBD patients the acute phase of the disease was found to be associated with a significant increase in serum FGF21 level (Tomasik et al., 2010; Liu et al., 2017). While low body weight in IBD has multifactorial pathogenesis, it may be hypothesized that higher FGF21 level could contribute to the state of malnutrition. Colitis-induced FGF21 expression may subsequently activate lipolysis in WAT and weight loss (Liu et al., 2017). However, the pathophysiological link between IBD and FGF21 remains to be unraveled.
The aim of the current study was to assess the correlation between serum FGF21 level and inflammatory markers such as CRP and fecal calprotectin as well as indicators of nutritional status in patients with IBD.
This cross-sectional study was performed at the Department of Gastroenterology and Hepatology of Wroclaw Medical University (Poland) between January 2021 and March 2023. All enrolled patients underwent a detailed clinical interview to assess their symptoms, associated disorders, and current treatment. The patients underwent also routine diagnostic assessments, including a physical examination and fasting blood laboratory tests. Additionally, they provided stool samples to determine calprotectin content. In patients with clinical indications, colonoscopy and/or enterography were also carried out.
Clinical and endoscopic activity of IBD was evaluated based on validated scales and indices. The Rachmilewitz index (Rachmilewitz, 1989) and the Mayo Endoscopic Score (Rubin et al., 2019) were applied to UC patients. The Crohn’s Disease Activity Index (CDAI) (Freeman, 2008) and the Simple Endoscopic Score for CD (SES-CD) (Daperno et al., 2004) were used in CD patients. Patients were categorized as being in either active or inactive phase of the disease based on the assessment of clinical, laboratory, and endoscopic criteria. Patients who fulfilled the criteria outlined as fecal calprotectin level lower than 200 μg/g, 0–4 points in the Rachmilewitz index and 0–2 points in the Mayo Endoscopic Score (for UC patients), the CDAI score lower than 200 points, and the SES-CD score lower than 7 points (for CD patients) were categorized as being in remission. All other subjects were assigned to the active phase group. CD patients with active changes in enterography were automatically included in the active phase group.
Among 105 IBD patients there were 31 patients with active UC, 16 patients with inactive UC, 36 patients with active CD, and 22 patients with inactive CD. The control group consisted of 17 healthy volunteers without gastroenterological symptoms. To exclude undiagnosed IBD or other intestinal inflammation, fecal calprotectin test was performed in all controls.
The exclusion criteria were as follows: pancreatitis, chronic liver diseases (except single cysts and steatosis), diabetes, body mass index (BMI) ≥ 30 kg/m2, treated hyperlipidemia, ischemic heart disease, chronic kidney diseases (except single cysts and kidney stones), malignancies, alcohol dependence syndrome, history of abdominal surgical procedures (except appendectomy and procedures related to IBD).
The fasting blood and stool samples provided by participants were stored at −80°C until the analysis. The quantitative evaluation of serum FGF21 [pg/ml] and fecal calprotectin [μg/g] levels were performed by immunoenzymatic methods: Human FGF-21 ELISA (BioVendor, Laboratorni medicina a.s., Czech Republic) and EK-CAL (Bühlmann Laboratories, Switzerland), respectively.
The obtained individual results are presented as numbers, percentage, mean values with standard deviation (±SD), or medians with the lower and upper quartiles (Q1-Q3). The normality of data was determined using the Shapiro-Wilk test. To compare quantitative variables with a normal distribution we assessed the homogeneity of variances using the Levene test, and then conducted a Student’s t-test (no significant variance difference) or a t-test with independent variances (significant variance heterogeneity), respectively. The Mann-Whitney U test compared quantitative variables with abnormal distribution. To compare categorical variables, the assumption of expected frequencies was checked – values less than 5 in a maximum of 20% of cell fields for the chi-square test, and proceed with Pearson’s chi-square test of independence or the Fisher exact test, respectively. The Spearman rank correlation coefficient and Kendall Tau correlations were calculated to test associations between variables. The statistical significance level was set at p < 0.05.
The characteristics of the studied groups of patients are presented in Table 1. The whole group of IBD patients included 68 males (65%) and 37 females (35%), at median age of 33 (27–41) years. There were no significant differences between the patient groups with respect to gender and age. The median duration of the disease was 63 (13–132) months. Of note, subjects with active UC were characterized by the shortest median disease duration amounting to 18 months and 39% of those patients were diagnosed with IBD within 1 year preceding the study. The control group (n = 17) included 9 males and 8 females, at median age of 28 (27–30) years and mean BMI of 22.4 ± 2.8 kg/m2.
The results of the evaluation of clinical and endoscopic IBD activity based on the applied scales and indices are presented in Table 1.
In most cases, the patients in an active phase of IBD were administered steroids. Three patients with inactive UC and 3 with inactive CD were still on steroids while tapering their dose. They were admitted to the hospital for check-ups after an exacerbation of the disease which had occurred 2–3 months earlier.
The results of blood and stool tests in IBD patients including the evaluation of serum CRP and fecal calprotectin are presented in Table 2. Mean fecal calprotectin in the control group amounted to 15.3 ± 10.3 μg/g.
Analyzing the serum FGF21 level in IBD patients, a clear tendency for higher values in active IBD phase was observed. The median serum FGF21 level was higher in active UC than in inactive UC, as well as in the control group. However, there was no significant difference in FGF21 level between patients with inactive UC and the controls. The same fluctuation depending on disease activity was observed in the group of CD patients (Figure 1). An elevated concentration of FGF21 (>300 pg/mL) was detected in 39 patients that accounted for 37% of all IBD patients. Most of those subjects (32/39, 82%) were in the active phase. The increased FGF21 level was found in 48% of patients with active UC and 47% of patients with active CD. FGF21 values below the normal range were not detected in any of the studied IBD patients.
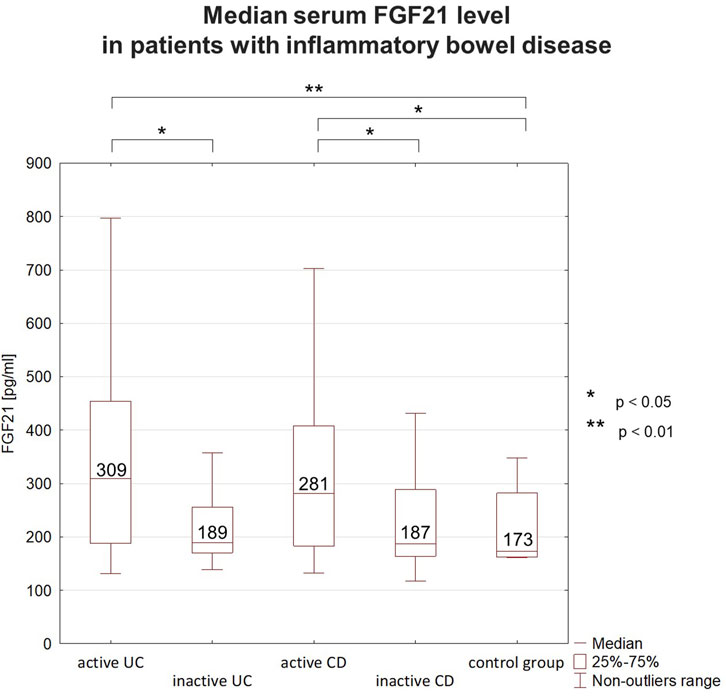
Figure 1. Median serum FGF21 levels in patients with inflammatory bowel disease (IBD). In IBD patients FGF21 level fluctuated depending on the disease activity. Higher concentrations were observed during flares than during remission. FGF21 concentrations during remission were comparable to those in the control group. FGF21 - fibroblast growth factor 21, UC–ulcerative colitis, CD–Crohn’s disease.
Evaluating the nutritional status, underweight (BMI <18.5 kg/m2) was diagnosed in 17% of the IBD patients. Weight loss exceeding 5% of body mass within 1 month was documented in 21% of the subjects. Selected nutrient deficiencies detected in the studied patients are presented in Table 3. Almost 30% of subjects with active UC and active CD had low cholesterol level. Almost 40% of patients with active UC and 17% of patients with active CD had hypoproteinemia. More than 50% of patients with active UC and almost 30% of patients with active CD had hypoalbuminemia. Iron deficiency and anemia was also commonly observed. Hemoglobin levels lower than 11.9 g/dL in women and lower than 12.9 g/dL in men were reported in 81% of patients with active UC, 25% with inactive UC, 44% with active CD, and 32% with inactive CD. Vitamin D deficiency was confirmed in 64% of the patients, with a deep deficiency (<10 ng/mL) identified in 14% of them. Nutritional deficiencies were more often observed in patients with active UC than those with inactive UC, except for low vitamin D levels. In contrast, these deficiencies occurred at similar rates for patients with CD regardless of whether the disease was active or inactive, except for low albumin levels, which were more prevalent in active CD.
There was no significant correlation of serum FGF21 level with the clinical and endoscopic disease activity indices (Table 4).
A significant positive correlation between FGF21 and fecal calprotectin levels were identified in both UC and CD, along with a correlation between FGF21 and CRP levels in CD patients, but not in UC patients (Table 4).
Negative correlations were observed between FGF21 level and total cholesterol, protein, and albumin levels in both UC and CD patients. Moreover, in UC patients, negative correlations of FGF21 level with hemoglobin level and BMI were noticed, and a positive correlation between FGF21 and triglyceride levels. In CD patients negative correlations of FGF21 level with iron and vitamin D levels were detected (Table 4).
In this study, we have confirmed that serum FGF21 levels are higher in patients with active IBD, both UC and CD, compared to patients with inactive IBD and healthy controls. The available data on FGF21 level fluctuations in adult IBD patients are scarce. In one study higher plasma FGF21 levels in IBD patients compared to healthy controls were reported; however, the investigated group of patients was not divided into subjects with active and inactive disease (Liu et al., 2017). In another study conducted in children with IBD, serum FGF21 level was higher in the disease flare with a subsequent significant decrease after treatment (Tomasik et al., 2010). Additional compelling evidence regarding the role of FGF21 in intestinal inflammation comes from studies in animal models which show that chemically induced colitis is one of the factors stimulating the secretion of FGF21 (Liu et al., 2017; Liu et al., 2023; Al-Aqil et al., 2018). In an experimental IBD model, Liu et al. (2023) demonstrated that endogenous FGF21 was increased in dextran sulfate sodium-induced colitis, which contributed to the progression of the disease and a significant loss of body weight. The abovementioned study has also shed light on one of the potential mechanisms contributing to the FGF21 action. In FGF21 knockout mice, FGF21 depletion attenuated the severity of chemically induced acute colitis by enhancing the activation of the interleukin 22-STAT3 signaling pathway in intestinal epithelial cells (Liu et al., 2023).
The identification of FGF receptors and β-Klotho co-receptors within intestinal tissues supports the hypothesis that FGF21 plays a role in intestinal pathophysiology of IBD (Danopoulos et al., 2017; Aaldijk et al., 2023). Indeed, an immunohistochemistry analysis revealed that in the intestines of UC patients there is increased FGF21 expression located in the extra-epithelial compartment, while increased β-Klotho co-receptor expression is observed mainly on the surface of the intestinal epithelium (Muise et al., 2008; Rydén, 2009).
The current results not only confirm previous observations, but further indicate that FGF21 level correlates with the intensity of intestinal inflammation reflected by fecal calprotectin level. Moreover, the association between FGF21 and CRP levels is also present in CD patients. The fact that no correlation was found between FGF21 and CRP levels in UC patients may be related to quite wide range of CRP values in that group, particularly in active phase of disease. Additionally, it has been suggested that in UC, fecal calprotectin level has a better significance for detecting colitis compared to CRP (Anindita et al., 2023). Noteworthy, other researchers (Gariani et al., 2013) demonstrated a correlation between FGF21 level and CRP level in patients with systemic inflammatory response syndrome and had even proposed FGF21 as a non-specific marker for systemic inflammation.
A novel aspect of the current research is related to the evaluation of potential correlation between serum FGF21 level and validated clinical and endoscopic disease activity scales and indices in IBD. However, despite the observed fluctuations in FGF21 level in active versus inactive IBD phases, as well as the presence of correlation between FGF21 and inflammatory markers, no significant association of FGF21 level with validated disease activity scales and indices were found. Nevertheless, FGF21 as an inflammatory marker doesn’t have to correlate directly with more complex scales and indices of clinical or endoscopic activity of the diseases.
Given the role of FGF21 as an endocrine metabolic regulator that is expressed in many metabolically active tissues such as the liver and WAT as well as its concomitant involvement in intestinal inflammation, one of the aims of the current study was to assess the relation between FGF21 level and nutritional status parameters in IBD patients. Among the studied IBD patients, 17% of subjects were underweight. A significant percentage of patients were characterized by hypocholesterolemia, hypoproteinemia, hypoalbuminemia, iron deficiency anemia and vitamin D deficiency. The observed deficiencies in our study were more prevalent compared to some previous reports (Casanova et al., 2017; Prieto et al., 2021). Particularly frequently nutritional disturbances were detected in patients with active UC. To some extend it could be related to the relatively short duration of the disease (many patients in that group were only recently diagnosed). In fact, according to the available data, malnutrition is more prevalent among patients with a recently diagnosed IBD (Gold et al., 2023). Furthermore, patients with UC are more prone to develop nutritional deficiencies during active phase of the disease, whereas subjects with CD typically develop features of malnutrition gradually over an extended period of time (Balestrieri et al., 2020). Regarding a high rate of vitamin D deficiency in the studied population (amounting to 64% of the subjects), it was comparable to the results of previous studies performed in IBD patients in Poland (Krela-Kaźmierczak et al., 2015; Tulewicz-Marti et al., 2022). Importantly, vitamin D3 deficiency affected a large percentage of IBD patients in remission.
While analyzing the associations between FGF21 level and selected nutritional status parameters, we found that FGF21 level negatively correlated with BMI, total cholesterol, total protein, albumin, iron, hemoglobin and vitamin D levels. A positive correlation of FGF21 and triglyceride levels may be associated with increased lipolysis activity.
The spectrum of nutritional disturbances in IBD patients is wide. On one hand, malnutrition is a major complication of IBD and it is primarily responsible for chronic weight loss (Scaldaferri et al., 2017). On the other hand, numerous recent studies have linked IBD to metabolic syndrome, which includes diabetes, obesity, and dyslipidemia, as they share some common pathophysiological links including inflammation, adipose tissue dysregulation, and gut dysbiosis (Michalak et al., 2016; Szilagyi, 2020; Verdugo-Meza et al., 2020). Experimental and clinical evidence supports parallels between metabolic nature of gut inflammation in IBD and the inflammatory state in metabolic diseases (Adolph et al., 2022). Specifically, the role of adipose tissue in the development of metabolic syndrome and IBD has been extensively studied (Choe et al., 2016). Noteworthy, in various metabolic disorders such as obesity, hyperlipidemia, diabetes, and metabolic dysfunction-associated steatotic liver disease, elevated serum FGF21 level were also recorded (Yang et al., 2023). However, in these conditions the FGF21 resistance seems to occur (Aaldijk et al., 2023).
A recently published meta-analysis of clinical trials demonstrated that treatment with FGF21 analogues significantly reduces total cholesterol level and contributes to weight loss (Carbonetti et al., 2023). Additionally, several other studies propose FGF21 as potential therapeutic agent with anti-inflammatory effect (Feingold et al., 2012; Singhal et al., 2016; Wang et al., 2018). Contrary, an increase in FGF21 level during the active phase of IBD exerts negative metabolic effects contributing to malnutrition and weight loss and exacerbating inflammation. This discrepancy may be explained by the possible dual action of FGF21. In fact, FGF21 has the capability to either suppress or promote lipolysis in WAT, depending on whether the person is during fasting or in the postprandial state, respectively (Xie et al., 2020). Similarly, pro- or anti-inflammatory action of FGF21 may depend on the given pathophysiological state such as acute versus chronic inflammation. In experimental studies, it has been shown that overexpression of FGF21 may reduce hepatic cholesterol production (Huang et al., 2017), increase the liver production of bile salts from cholesterol (Al-Aqil et al., 2018), induce the lipolysis of adipose tissue (Liu et al., 2017), reduce muscle mass and strength (Oost et al., 2019). Therefore, modulation of FGF21 signaling pathway could emerge as a target in IBD and related metabolic disorders. However, the effects of exogenous FGF21 treatment on acute and chronic colitis and colitis recovery have not been adequately examined so far.
Among limitations of the study is its cross-sectional character. Furthermore, the effects of used medications may constitute confounding factors affecting the results. For example, steroid therapy leads to enhanced FGF21 expression in the liver (Vispute et al., 2017; Al-Aqil et al., 2018). However, since many studies have confirmed that inflammatory stimuli are inducers of FGF21 expression, the use of steroids most likely only contributes to the FGF21 level increase, but is not solely responsible for the effect. Additionally, to further explore the association between FGF21 and nutritional status, it would be useful to perform more detailed analysis including the evaluation of fat mass index, fat-free mass index or muscle strength. It might be important given that some IBD patients with sarcopenia have normal BMI values (Scaldaferri et al., 2017; Balestrieri et al., 2020). The novelty of the study is related to the pioneer report on FGF21 level fluctuations in adult IBD patients in active and inactive phases of the disease as well as on the correlation of FGF21 level with inflammatory markers and nutritional status parameters.
In conclusion, our results show that FGF21 level correlates directly with the intensity of intestinal inflammation and inversely with nutritional status of IBD patients. Therefore, the multifactorial pathogenesis of malnutrition and weight loss in IBD patients may be related to disturbances in FGF21 level. Further studies are warranted to clarify the exact mechanism of complex action of FGF21 within the gut-liver axis to unravel potential new therapeutic targets in IBD and related metabolic disturbances.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee at the Wroclaw Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
AL: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing–original draft. AM: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Validation, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by a research grant from Wroclaw Medical University, Poland: SUBZ.C130.23.016.
The authors would like to express their gratitude to Dr. Maria Jasińska for her expert technical assistance. We wish to thank also Mrs. Łucja Janek for statistical analyses. The content of this manuscript has been presented in part at the 18th Congress of ECCO. Copenhagen, Denmark, March 1–4, 2023. J Crohns Colitis 2023:17; Suppl.1, i191, P022.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aaldijk A. S., Verzijl C. R. C., Jonker J. W., Struik D. (2023). Biological and pharmacological functions of the FGF19- and FGF21-coreceptor beta klotho. Front. Endocrinol. (Lausanne) 14, 1150222. doi:10.3389/fendo.2023.1150222
Adolph T. E., Meyer M., Schwärzler J., Mayr L., Grabherr F., Tilg H. (2022). The metabolic nature of inflammatory bowel diseases. Nat. Rev. Gastroenterol. Hepatol. 19 (12), 753–767. doi:10.1038/S41575-022-00658-y
Al-Aqil F. A., Monte M. J., Peleteiro-Vigil A., Briz O., Rosales R., González R., et al. (2018). Interaction of glucocorticoids with FXR/FGF19/FGF21-mediated ileum-liver crosstalk. Biochim. Biophys. Acta. Mol. Basis Dis. 1864, 2927–2937. doi:10.1016/j.bbadis.2018.06.003
Anindita B., Sugihartono T., Miftahussurur M., Maimunah U., Nusi I. A., Setiawan P. B., et al. (2023). High levels of fecal calprotectin and C-reactive protein in patients with colitis. J. Med. Life. 16 (1), 48–51. doi:10.25122/jml-2021-0311
Balestrieri P., Ribolsi M., Guarino M. P. L., Emerenziani S., Altomare A., Cicala M. (2020). Nutritional aspects in inflammatory bowel diseases. Nutrients 12 (2), 372. doi:10.3390/nu12020372
Beenken A., Mohammadi M. (2009). The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug. Discov. 8 (3), 235–253. doi:10.1038/nrd2792
BonDurant L. D., Potthoff M. J. (2018). Fibroblast growth factor 21: a versatile regulator of metabolic homeostasis. Annu. Rev. Nutr. 38, 173–196. doi:10.1146/annurev-nutr-071816-064800
Carbonetti M. P., Almeida-Oliveira F., Majerowicz D. (2023). Use of FGF21 analogs for the treatment of metabolic disorders: a systematic review and meta-analysis. Arch. Endocrinol. Metab. 68, e220493. doi:10.20945/2359-4292-2022-0493
Casanova M. J., Chaparro M., Molina B., Merino O., Batanero R., Dueñas-Sadornil C., et al. (2017). Prevalence of malnutrition and nutritional characteristics of patients with inflammatory bowel disease. J. Crohns Colitis 11 (12), 1430–1439. doi:10.1093/ecco-jcc/jjx102
Choe S. S., Huh J. Y., Hwang I. J., Kim J. I., Kim J. B. (2016). Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front. Endocrinol. (Lausanne) 7, 30. doi:10.3389/fendo.2016.00030
Danopoulos S., Schlieve C. R., Grikscheit T. C., Alam D.Al (2017). Fibroblast growth factors in the gastrointestinal tract: twists and turns. Dev. Dyn. 246 (4), 344–352. doi:10.1002/dvdy.24491
Daperno M., D’Haens G., Van Assche G., Baert F., Bulois P., Maunoury V., et al. (2004). Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest. Endosc. 60 (4), 505–512. doi:10.1016/s0016-5107(04)01878-4
Degirolamo C., Sabbà C., Moschetta A. (2016). Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat. Rev. Drug. Discov. 15 (1), 51–69. doi:10.1038/nrd.2015.9
Dolegowska K., Marchelek-Mysliwiec M., Nowosiad-Magda M., Slawinski M., Dolegowska B. (2019). FGF19 subfamily members: FGF19 and FGF21. J. Physiol. Biochem. 75 (2), 229–240. doi:10.1007/s13105-019-00675-7
Feingold K. R., Grunfeld C., Heuer J. G., Gupta A., Cramer M., Zhang T., et al. (2012). FGF21 is increased by inflammatory stimuli and protects leptin-deficient ob/ob mice from the toxicity of sepsis. Endocrinology 153 (6), 2689–2700. doi:10.1210/en.2011-1496
Freeman H. J. (2008). Use of the Crohn’s disease activity index in clinical trials of biological agents. World J. Gastroenterol. 14 (26), 4127–4130. doi:10.3748/wjg.14.4127
Gadaleta R. M., van Erpecum K. J., Oldenburg B., Willemsen E. C. J., Renooij W., Murzilli S., et al. (2011). Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60 (4), 463–472. doi:10.1136/gut.2010.212159
Gariani K., Drifte G., Dunn-Siegrist I., Pugin J., Jornayvaz F. R. (2013). Increased FGF21 plasma levels in humans with sepsis and SIRS. Endocr. Connect. 2 (3), 146–153. doi:10.1530/EC-13-0040
Gold S. L., Rabinowitz L. G., Manning L., Keefer L., Rivera-Carrero W., Stanley S., et al. (2023). High prevalence of malnutrition and micronutrient deficiencies in patients with inflammatory bowel disease early in disease course. Inflamm. Bowel Dis. 29 (3), 423–429. doi:10.1093/ibd/izac102
Huang Z., Xu A., Cheung B. M. Y. (2017). The potential role of fibroblast growth factor 21 in lipid metabolism and hypertension. Curr. Hypertens. Rep. 19 (4), 28. doi:10.1007/s11906-017-0730-5
Krela-Kaźmierczak I., Szymczak A., Tomczak M., Łykowska-Szuber L., Linke K., Eder P. (2015). Calcium and phosphate metabolism in patients with inflammatory bowel diseases. Pol. Arch. Med. Wewn. 125 (7-8), 588–590. doi:10.20452/pamw.2981
Lan T., Morgan D. A., Rahmouni K., Sonoda J., Fu X., Burgess S. C., et al. (2017). FGF19, FGF21, and an FGFR1/β-klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metab. 26 (5), 709–718. doi:10.1016/j.cmet.2017.09.005
Liu L., Li F., Shao T., Zhang L., Lee J., Dryden G., et al. (2023). FGF21 depletion attenuates colitis through intestinal epithelial IL-22-STAT3 activation in mice. Nutrients 15 (9), 2086. doi:10.3390/nu15092086
Liu L., Zhao C., Yang Y., Kong X., Shao T., Ren L., et al. (2017). Fibroblast growth factor 21 deficiency attenuates experimental colitis-induced adipose tissue lipolysis. Gastroenterol. Res. Pract. 2017, 3089378. doi:10.1155/2017/3089378
Łukawska A., Mulak A. (2022). Physiological and pathophysiological role of endocrine fibroblast growth factors. Postep. Hig. Med. Dosw. 76 (1), 39–53. doi:10.2478/ahem-2022-0045
Martínez-Garza Ú., Torres-Oteros D., Yarritu-Gallego A., Marrero P. F., Haro D., Relat J. (2019). Fibroblast growth factor 21 and the adaptive response to nutritional challenges. Int. J. Mol. Sci. 20 (19), 4692. doi:10.3390/ijms20194692
Michalak A., Mosińska P., Fichna J. (2016). Common links between metabolic syndrome and inflammatory bowel disease: current overview and future perspectives. Pharmacol. Rep. 68 (4), 837–846. doi:10.1016/j.pharep.2016.04.016
Muise E. S., Azzolina B., Kuo D. W., El-Sherbeini M., Tan Y., Yuan X., et al. (2008). Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol. Pharmacol. 74 (2), 403–412. doi:10.1124/mol.108.044826
Oost L. J., Kustermann M., Armani A., Blaauw B., Romanello V. (2019). Fibroblast growth factor 21 controls mitophagy and muscle mass. J. Cachexia Sarcopenia Muscle 10 (3), 630–642. doi:10.1002/jcsm.12409
Prieto J. M. I., Andrade A. R., Magro D. O., Imbrizi M., Nishitokukado I., Ortiz-Agostinho C. L., et al. (2021). Nutritional global status and its impact in Crohn’s disease. J. Can. Assoc. Gastroenterol. 4 (6), 290–295. doi:10.1093/jcag/gwab006
Rachmilewitz D. (1989). Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ 298 (6666), 82–86. doi:10.1136/bmj.298.6666.82
Rubin D. T., Ananthakrishnan A. N., Siegel C. A., Sauer B. G., Long M. D. (2019). ACG clinical guideline: ulcerative colitis in adults. Am. J. Gastroenterol. 114 (3), 384–413. doi:10.14309/ajg.0000000000000152
Rydén M. (2009). Fibroblast growth factor 21: an overview from a clinical perspective. Cell. Mol. Life Sci. 66 (13), 2067–2073. doi:10.1007/s00018-009-0003-9
Scaldaferri F., Pizzoferrato M., Lopetuso L. R., Musca T., Ingravalle F., Sicignano L. L., et al. (2017). Nutrition and IBD: malnutrition and/or sarcopenia? A practical guide. Gastroenterol. Res. Pract. 2017, 8646495. doi:10.1155/2017/8646495
Singhal G., Fisher F. M., Chee M. J., Tan T. G., El Ouaamari A., Adams A. C., et al. (2016). Fibroblast growth factor 21 (FGF21) protects against high fat diet induced inflammation and islet hyperplasia in pancreas. PLoS One 11 (2), e0148252. doi:10.1371/journal.pone.0148252
Szilagyi A. (2020). Relationship(s) between obesity and inflammatory bowel diseases: possible intertwined pathogenic mechanisms. Clin. J. Gastroenterol. 13 (2), 139–152. doi:10.1007/s12328-019-01037-y
Tomasik P. J., Pieczarkowski S. H., Fyderek K., Sztefko K. (2010). Fgf-21 in inflammatory bowel diseases. Pediatr. Res. 68, 523. doi:10.1203/00006450-201011001-01052
Torres J., Mehandru S., Colombel J., Peyrin-Biroulet L. (2017). Crohn’s disease. Lancet 389 (10080), 1741–1755. doi:10.1016/S0140-6736(16)31711-1
Tulewicz-Marti E. M., Lewandowski K., Rydzewska G. (2022). Bone metabolism alteration in patients with inflammatory bowel disease. J. Clin. Med. 11 (4), 4138. doi:10.3390/jcm11144138
Ungaro R., Mehandru S., Allen P. B., Peyrin-Biroulet L., Colombel J. F. (2017). Ulcerative colitis. Lancet 389 (10080), 1756–1770. doi:10.1016/S0140-6736(16)32126-2
Verdugo-Meza A., Ye J., Dadlani H., Ghosh S., Gibson D. L. (2020). Connecting the dots between inflammatory bowel disease and metabolic syndrome: a focus on gut-derived metabolites. Nutrients 12 (5), 1434. doi:10.3390/nu12051434
Vispute S. G., Bu P., Le Y., Cheng X. (2017). Activation of GR but not PXR by dexamethasone attenuated acetaminophen hepatotoxicities via Fgf21 induction. Toxicology 378, 95–106. doi:10.1016/j.tox.2017.01.009
Wang Q., Yuan J., Yu Z., Lin L., Jiang Y., Cao Z., et al. (2018). FGF21 attenuates high-fat diet-induced cognitive impairment via metabolic regulation and anti-inflammation of obese mice. Mol. Neurobiol. 55 (6), 4702–4717. doi:10.1007/s12035-017-0663-7
Xie Y., Su N., Yang J., Tan Q., Huang S., Jin M., et al. (2020). FGF/FGFR signaling in health and disease. Signal. Transduct. Target. Ther. 5 (1), 181. doi:10.1038/s41392-020-00222-7
Yang M., Liu C., Jiang N., Liu Y., Luo S., Li C., et al. (2023). Fibroblast growth factor 21 in metabolic syndrome. Front. Endocrinol. (Lausanne) 14, 1220426. doi:10.3389/fendo.2023.1220426
Keywords: inflammatory bowel disease, fibroblast growth factors, disease activity, fecal calprotectin, malnutrition
Citation: Łukawska A and Mulak A (2024) A correlation of serum fibroblast growth factor 21 level with inflammatory markers and indicators of nutritional status in patients with inflammatory bowel disease. Front. Physiol. 15:1394030. doi: 10.3389/fphys.2024.1394030
Received: 29 February 2024; Accepted: 05 June 2024;
Published: 25 June 2024.
Edited by:
Daniele Maria-Ferreira, Instituto de Pesquisa Pelé Pequeno Príncipe, BrazilReviewed by:
Beata Kasztelan-Szczerbinska, Medical University of Lublin, PolandCopyright © 2024 Łukawska and Mulak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Agata Łukawska, YWdhdGEubHVrYXdza2E1NUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.