- College of Life Sciences, Zhejiang Normal University, Jinhua, Zhejiang, China
Dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) anion channel by genetic mutations causes the inherited disease cystic fibrosis (CF). CF lung disease that involves multiple disorders of epithelial function likely results from loss of CFTR function as an anion channel conducting chloride and bicarbonate ions and its function as a cellular regulator modulating the activity of membrane and cytosol proteins. In the absence of CFTR activity, abundant mucus accumulation, bacterial infection and inflammation characterize CF airways, in which inflammation-associated tissue remodeling and damage gradually destroys the lung. Deciphering the link between CFTR dysfunction and bacterial infection in CF airways may reveal the pathogenesis of CF lung disease and guide the development of new treatments. Research efforts towards this goal, including high salt, low volume, airway surface liquid acidosis and abnormal mucus hypotheses are critically reviewed.
1 Introduction
Cystic fibrosis (CF) is an inherited autosomal recessive disease caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) (Riordan et al., 1989), an epithelial anion channel that primarily conducts Cl− and HCO3− (Anderson et al., 1991; Poulsen et al., 1994). CF mutations impair CFTR function mainly by severely disrupting protein expression and channel function in the cell membrane (Amaral and Farinha, 2013), leading to abnormal water absorption (Matsui et al., 1998), mucus secretion (Joo et al., 2002), pH regulation (Pezzulo et al., 2012), bacterial infection (Reece et al., 2021) and inflammation (Khan et al., 1995) in many epithelia-lined organs, including the lungs, intestine, liver, pancreas, sweat glands and reproductive tract (Shteinberg et al., 2021). Thus, CF is well known as a multiple-organ disease (Shteinberg et al., 2021).
Nowadays, more than 2000 CFTR mutations have been identified (Shteinberg et al., 2021), and at least 719 mutations that cause CF are reported (https://cftr2.org/) (Sosnay et al., 2013). Currently, lung failure due to chronic and recurrent bacterial infection is the major cause of death in people with CF (Ciofu et al., 2013). Bacteria Staphylococcus aureus (Durfey et al., 2021) and Haemophilus influenza (Green and Jones, 2022) are commonly observed in the airways of both young and adult people with CF. Moreover, Pseudomonas aeruginosa is the bacteria particularly noted and observed in CF airways with chronic lung infections (Lund-Palau et al., 2016; Durfey et al., 2021). Therefore, understanding how CFTR dysfunction impairs the defense mechanisms of airway epithelia against bacterial infection is critical for exploring an effective treatment for CF.
CFTR involves two major physiological functions in epithelial cells. First, CFTR is well characterized as a Cl− channel (Anderson et al., 1991); it also partially conducts HCO3− (Poulsen et al., 1994), but little Na+ (Tabcharani et al., 1997). In CF, the patient who carries mutant CFTR producing the least amount of cAMP-stimulated Cl− current displays the worst exocrine pancreatic deficiency (Sheppard et al., 1993; Sheppard et al., 1995), suggesting that defective Cl− transport would be a possible cause of CF disease. In CF epithelia, lack of CFTR-mediated Cl− conductance may hinder the apical Na+ absorption, so that NaCl may be retained in the airway surface liquid (ASL) resulting in the “high salt” condition, which impairs bacterial killing in ASL (Smith et al., 1996). In addition, the lack of HCO3− transport in genetically modified CFTR−/− newborn pigs decreases ASL pH resulting in reduced bacterial killing upon airway epithelia, suggesting that “ASL acidosis” is a major defect leading to CF lung disease (Pezzulo et al., 2012).
It is of interest that CFTR proteins are abundantly expressed in forkhead box I1 (FOXI1)-positive pulmonary ionocytes (Montoro et al., 2018; Plasschaert et al., 2018), while airway epithelia of FOXI1-knockout mice display increased ciliary beat frequency and mucus viscosity (Montoro et al., 2018). Lei et al. (Lei et al., 2023) demonstrated that apical membrane CFTR Cl− channels collaborate with basolateral membrane barttin/ClC-K Cl− channels in ionocytes for transepithelial Cl− absorption, leading to fluid absorption. Moreover, FOXI1-knockout and CF ferrets both display reduced ASL volume and impaired mucociliary clearance due to ASL abnormalities, including slow fluid absorption but also absent fluid secretion, lack of CFTR-mediated ASL alkalization and increased mucus viscosity (Yuan et al., 2023). Therefore, albeit less than about 1% of total epithelial cells in airways (Montoro et al., 2018; Plasschaert et al., 2018; Lei et al., 2023), ionocytes regulate ASL homeostasis. However, whether the dysfunction of ionocytes contributes to the pathogenesis of CF lung diseases needs to be further explored.
Second, CFTR regulates the activity of several membrane and cytosol proteins (Li and Naren, 2010; Lim et al., 2017). Normally, CFTR-mediated transepithelial Cl− transport together with the epithelial Na+ channel (ENaC)-mediated Na+ transport control the salt and water absorption of epithelia (Matalon et al., 2015). Early studies (Kunzelmann et al., 1995; Stutts et al., 1995; Ismailov et al., 1996; Matsui et al., 1998; Tarran et al., 2005) proposed a popular mechanism by which the loss of CFTR function in CF epithelia might enhance ENaC activity, resulting in excess NaCl and water absorption, followed by reduced ASL height that impairs both the mucociliary clearance mechanism and bacterial eradication from the airway surface. In addition to this “low volume” hypothesis, CFTR acting as a regulator also interacts with and stimulates the Cl−/HCO3− exchanger (Lee et al., 1999), but inhibits Na+/H+ exchanger (NHE) (Ahn et al., 2001). Moreover, CFTR directly binds to many intracellular proteins via its regulatory (R) domain (Bozoky et al., 2013) and N- (Naren et al., 1997) and C-termini (Hall et al., 1998; Li and Naren, 2010). Thus, CF mutations may disrupt CFTR function as an anion channel and regulator in epithelial cells. However, which defective CFTR function primarily leads to CF lung diseases remains in debate.
A strategy to address this question is to explore which type of CFTR function, once impaired, causes recurrent airway bacterial infection, a key step that elicits excess inflammatory responses and consequent tissue damage in the CF lung (Cabrini et al., 2020; Ribeiro et al., 2023). The physicochemical properties and movement of mucus is another key factor altered upon CF epithelia (Matsui et al., 1998; Hoegger et al., 2014; Esther et al., 2019; Keith et al., 2022). It is noted that accumulated and abnormal mucus disrupts the mucociliary clearance mechanism and impairs bacterial eradication from the airway surface (Matsui et al., 1998; Boucher, 2007). Moreover, bacterial killing on the apical side of epithelia is attenuated in CF (Smith et al., 1996; Pezzulo et al., 2012). Therefore, both mucus accumulation and weakened bactericidal activity in ASL may prevent CF airway epithelia from removing airborne bacteria and lead to bacterial colonization (Stoltz et al., 2015; Ribeiro et al., 2023).
However, the link between CFTR dysfunction and bacterial colonization in CF airways remains incompletely understood. This review will first describe the improvement of CF lung function by the restoration of CFTR activity in the epithelial cells, secondly discuss the epithelial defense mechanisms that remove bacteria from the surface of airways, and then update current understanding of how CFTR dysfunction disrupts these defense mechanisms, leading to bacterial colonization on CF epithelia.
2 The cocktail drug therapy of CFTR potentiators and correctors
One way to understand how CFTR dysfunction causes loss of bacterial eradication from the airways is to examine the improvement in lung function when CFTR function is restored pharmaceutically by potentiators that enhance its channel activity and correctors that increase CFTR protein expression at the plasma membrane (Ribeiro et al., 2023; Thornton and Parkins, 2023). Recently FDA-approved triple therapy with elexacaftor/tezacaftor/ivacaftor (ETI) (Keating et al., 2018; Heijerman et al., 2019; Middleton et al., 2019) has been used to treat people with cystic fibrosis carrying at least one ΔF508 allele (Tummler, 2023), in which ivacaftor (VX-770) acts as a potentiator to enhance the channel activity of the ΔF508 or G551D mutants (Van Goor et al., 2009); tezacaftor (VX-661) (Rowe et al., 2017; Taylor-Cousar et al., 2017) and elexacaftor (VX-445) (Keating et al., 2018; Laselva et al., 2021) work as correctors but elexacaftor also partially acts as a potentiator (Laselva et al., 2021). The promising outcomes show that the combination treatment substantially recovers CF lung function by reducing pulmonary exacerbations and hospitalizations per patient per year (Bower et al., 2023), improving percent predicted forced expiratory volume in 1 s (ppFEV1) up to 10% (Bower et al., 2023; Atteih et al., 2024), diminishing the release of inflammatory mediators, such as the B cell activating factors, IL-6, IL-8 and IL-22, C-reactive protein and soluble TNF (Casey et al., 2023; Schaupp et al., 2023; Atteih et al., 2024), advancing sputum viscoelastic properties (Schaupp et al., 2023), lowering the sweat Cl− concentration and lung clearance index2.5 (Goralski et al., 2023), and decreasing the detection and diversity of bacteria like S. aureus and P. aeruginosa in CF microbiological samples (Dittrich et al., 2024) and sputum (Nichols et al., 2023; Schaupp et al., 2023). Notably, ETI treatment is safe for use in children aged 2–5 years (Goralski et al., 2023) and pregnant women (Cimino et al., 2024).
However, ETI only shows moderate increases in ppFEV1 in subjects who were ineligible for enrollment in registration studies and those with severe airway obstruction (ppFEV1 < 40) (Fila et al., 2023). Although ETI treatment reduces positive bacterial cultures in patients (Bower et al., 2023), bacteria not in the CF pathogen genera persist in the sputum of patients and are not changed by ETI treatment (Martin et al., 2023; Nichols et al., 2023). These findings may reflect the complex responses of microbiota to ETI treatment. Details about this topic can be found in previous excellent reviews (Ribeiro et al., 2023; Thornton and Parkins, 2023).
3 Bacterial eradication by airway epithelia
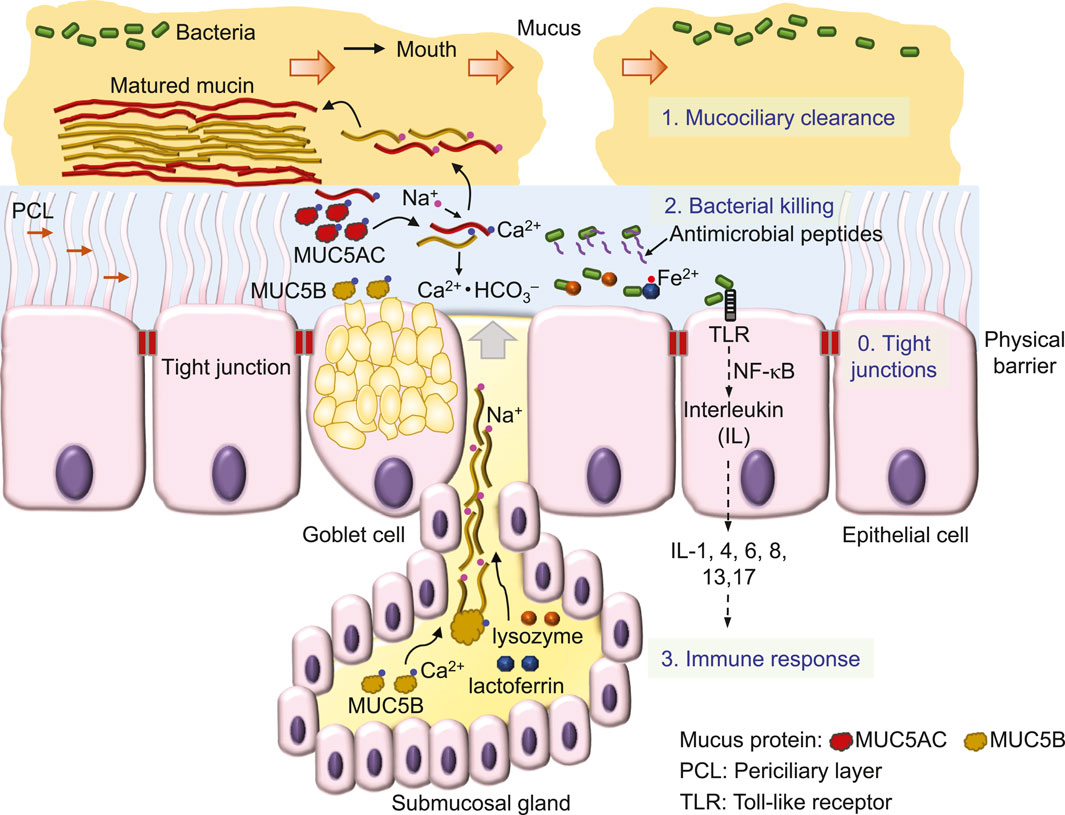
Inhaled air carries various particles, including debris, allergens and pathogens like viruses and bacteria (Costantini et al., 2022). To effectively remove inhaled substances, airway epithelia employ a 4-level defense scheme, known as the innate immune system (Myszor and Gudmundsson, 2023) (Figure 1). A single layer of epithelial cells, connected by tight junctions that firmly seal the apical side of cell membranes, preventing water and ions from passing through the paracellular pathway between cells, forms a physical barrier to serve as the fundamental defense mechanism of airway epithelia against bacteria invasion (Otani and Furuse, 2020; Myszor and Gudmundsson, 2023) (Figure 1). Additional defense mechanisms include 1) the mucociliary clearance mechanism that mechanically removes bacteria from the airway surface (Bustamante-Marin and Ostrowski, 2017), 2) bacterial killing by antimicrobial substances (Geitani et al., 2020) and 3) epithelial inflammatory responses (Yu and Kotsimbos, 2023).
First, the mucociliary clearance mechanism is constructed by viscoelastic and discontinued mucus (Rogers et al., 2022; Abrami et al., 2024) that flow on a watery and gel-like layer, the periciliary layer (PCL) (Widdicombe and Widdicombe, 1995; Button et al., 2012) (Figure 1). Sticky mucus traps inhaled bacteria, debris and other particles, while the cilia of epithelial cells in the underlying PCL propel the mucus and trapped substances (Figure 1) towards to the pharynx, where they form the sputum for expectoration (Bustamante-Marin and Ostrowski, 2017; Rogers et al., 2022).
The major component of this mucociliary clearance mechanism is the mucin protein, an O-linked glycoprotein containing a core protein of MUC5AC (5654 amino acids) secreted from Goblet cells or MUC5B (5762 amino acids) secreted mainly from submucosal glands (Hovenberg et al., 1996; Ermund et al., 2018; Thornton et al., 2018). By disulfide bonds formed between individual proteins, mucins are polymerized into a high-molecular weight glycoprotein of ∼2–50 mD (Thornton et al., 2018).
This large mucin molecule is stored in the vesicles of the cell, secreted upon cell stimulation by various signals and transformed from a granular shape into a flat and extended bundle during the journey to the surface of ASL (Ermund et al., 2018; Jaramillo et al., 2018) (Figure 1). Because of glycosylation, mucin contains lots of sugar groups for attracting water, physically contributing to the gel formation of the PCL (Button et al., 2012) and forming viscoelastic mucus (Puchelle et al., 1995). Interestingly, MUC5B secretion is upregulated by airway inflammation (Kim et al., 2004a) and required for airway defense (Roy et al., 2014). Acting as the first line to remove invading bacteria, mucociliary clearance is defective in CF (Matsui et al., 1998; Hoegger et al., 2014; Esther et al., 2019; Keith et al., 2022), a major reason that facilitates bacterial colonization in CF airways.
Second, those bacteria that escape mucociliary clearance are targeted by a large number of antimicrobial proteins, such as lysozyme and lactoferrin and peptides, including β-defensins, LL-37 and CCL20 (Myszor and Gudmundsson, 2023) (Figure 1) which are secreted from surface and submucosal gland epithelia (Bals et al., 1998; Singh et al., 1998). These antimicrobials destroy bacteria mainly by perforating the bacterial outer membrane and cell wall (Parker and Prince, 2011; Andersson et al., 2016) to cause osmotic damage to the cell (Ellison and Giehl, 1991). For example, lysozyme kills Gram-positive bacteria by degrading the surface peptidoglycan wall (Wadstrom and Hisatsune, 1970), whereas lactoferrin by binding and lowering available ferrous ion inhibits the growth of Gram-negative bacteria (Ellison and Giehl, 1991). The antimicrobial peptide magainin 2 amide forms pores in the lipid membrane of bacteria, leading to cell lysis (Wenk and Seelig, 1998), whereas other antimicrobial peptides are bacteriostatic by penetrating into bacteria and interfering with cellular metabolism (Andersson et al., 2016), such as β-defensins (Sugiarto and Yu, 2007) and indolicidin (Ghosh et al., 2014) that bind DNA duplexes and then inhibit DNA synthesis (Subbalakshmi and Sitaram, 1998). Interestingly, after binding to the receptor on the cell membrane, β-defensins (Yang et al., 1999) and LL-37 (De et al., 2000; Niyonsaba et al., 2002) are also chemotactic for immune cells, including mast cells, monocytes and T cells (Andersson et al., 2016; Myszor and Gudmundsson, 2023). Finally, chemical compounds, such as H2O2 and SCN−, are secreted by airway epithelial cells to kill bacteria (Fischer, 2009; Ashtiwi et al., 2021).
Third, bacteria colonization on the surface of epithelia induces the cellular immune response (Figure 1), followed by tissue inflammation and damage that eventually cause lung failure in people with CF [for details, see the review (Ribeiro et al., 2023)]. CFTR is also expressed in immune cells, such as neutrophils (Painter et al., 2006; Zhou et al., 2013), monocytes (Sorio et al., 2011; Zhang et al., 2022) and macrophages (Di et al., 2006), but it remains unclear whether CFTR dysfunction disrupts the immune response of these cells and whether this significantly contributes to the development of CF lung disease.
The following sections of this review focus on current research progress and perspectives about the mechanisms by which CFTR dysfunction causes defects in the 1st- and 2nd-levels of airway defense, mucociliary clearance and antimicrobial agents, respectively (Figure 2).
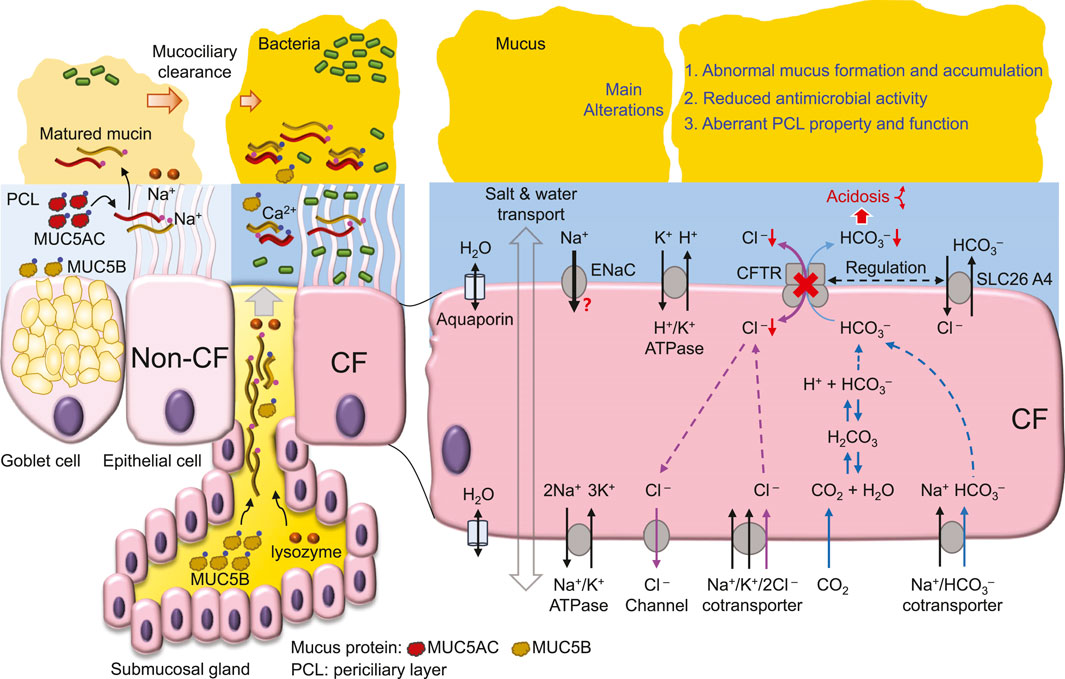
4 The Cl− transport defect and the high salt hypothesis
CF-associated mutations diminish CFTR function primarily by reducing 1) mRNA synthesis, 2) protein expression, 3) channel regulation and 4) channel conductance, or by increasing 5) protein degradation at the cell membrane (Amaral and Farinha, 2013). For example, the most prevalent CF mutation ΔF508 by introducing intrinsic structural flaws (Chen et al., 2019) blocks CFTR protein expression at the cell membrane (Cheng et al., 1990), whereas ΔF508, G551D and G1349D mutations greatly decrease channel activity (Dalemans et al., 1991; Cai and Sheppard, 2002; Cai et al., 2006; Bompadre et al., 2007; Chen et al., 2009; Chen et al., 2017) with further alterations in channel responses to gating potentiators (Hwang et al., 1997; Cai and Sheppard, 2002; Cai et al., 2006; Bompadre et al., 2007) and intracellular pH (Chen et al., 2009; Chen et al., 2017). Therefore, the current drug therapy for CF aims to elevate the protein expression and channel activity of mutant CFTRs by correctors and potentiators, respectively (Bose et al., 2020).
Early studies (Sheppard et al., 1993; Sheppard et al., 1995) demonstrate that CF-associated mutations that cause more severe pancreatic insufficiency in people with CF display little or no CFTR-mediated transmembrane Cl− transport. These data suggest that the deficit in transmembrane Cl− transport is correlated with the level of CF epithelial disease. Consistent with this idea, in cultured CF human bronchial epithelia (HBE), bacterial killing on the apical surface is defective (Smith et al., 1996), possibly due to reduced bactericidal activity of lysozyme and lactoferrin caused by the high NaCl concentration in ASL (Smith et al., 1996). Although reduced Cl− transport in CF epithelia may cause Cl− (Smith et al., 1996; Matsui et al., 1998; Zabner et al., 1998; Kozlova et al., 2006) and Na+ (Zabner et al., 1998; Kozlova et al., 2006) retention in ASL, some studies find no difference in ASL Na+ (Matsui et al., 1998; Pezzulo et al., 2012) and Cl− (Matsui et al., 1998) concentration between wild-type and CF epithelia. Of note, the reported ASL ion concentrations vary greatly among different studies, which employ distinct methods [e.g., (Verkman et al., 2003; Kozlova et al., 2006; Pezzulo et al., 2012), for details, see the review (Verkman et al., 2003)] to report higher (Smith et al., 1996) or unchanged (Matsui et al., 1998) concentrations in cultured CF epithelia compared to normal. Moreover, the sputum salt concentration is higher in people with CF than control subjects (Grandjean Lapierre et al., 2017). Therefore, a new and reliable method may be required to measure the salt concentration in ASL to resolve these controversial findings.
The high salt hypothesis is also challenged by evidence that aquaporin water channels are expressed on both the apical and basolateral membrane of airway epithelial cells (Kreda et al., 2001; Song et al., 2001). However, aquaporins have minor effects on airway humidification, ASL hydration and iso-osmolar fluid absorption (Song et al., 2001). Even if the osmolality is similar on either side of the apical membrane of airway epithelia, the salt concentration of ASL and cytosol may not be the same due to other factors such as extracellular mucins (Button et al., 2012; Henderson et al., 2014) and intracellular proteins that all contribute to the osmotic pressure affecting salt and water movement.
In addition, bacteria inoculation enhances epithelial fluid secretion in intact isolated swine tracheas (Luan et al., 2014). These data are consistent with the idea that CFTR-mediated Cl− and fluid secretion may modulate the flow of mucus upon airways (Puchelle et al., 1995) to regulate mucociliary clearance of invading bacteria (Caverly et al., 2022). Thus, defective Cl− transport of CF epithelia may impair bacterial eradication by mucociliary clearance, additionally to the high salt in ASL.
CFTR Cl− transport is important for myeloperoxidase-mediated bactericidal hypochlorous acid (HOCl) production in the phagosome of neutrophils (Wang and Nauseef, 2022). Consequently, mutant CFTR may attenuate bacterial killing by neutrophils and cause immunodeficiency with abundant neutrophil inflammation in CF airways (Wang and Nauseef, 2022). CFTR also transports SCN− (Linsdell, 2016). Compared to normal, the concentration of SCN− in CF ASL is reduced in mice (Gould et al., 2010) and pigs (Lorentzen et al., 2011) but not humans (Lorentzen et al., 2011). Interestingly, the SCN− concentration in ASL is about 30-fold higher than that in serum (Lorentzen et al., 2011), and people with CF showing higher ASL SCN− levels display better lung function (Lorentzen et al., 2011). The data support the findings that SCN− by reacting with H2O2 and HOCl acts as an antioxidant to neutralize peroxides and hence, protect epithelial cells from these oxidative injuries during neutrophil inflammation (Xu et al., 2009). Future work is required to assess the significance of peroxide-mediated injury in the pathogenesis of CF lung disease.
5 HCO3− transport and the ASL acidosis hypothesis
HCO3− conductance is about one-quarter that of Cl− conductance for wild-type CFTR (Poulsen et al., 1994) (Figure 2). CF-associated mutations that diminish CFTR Cl− transport likely also impair HCO3− secretion (Smith and Welsh, 1992), whereas the conductivity ratio of HCO3−/Cl− is somewhat variable among mutant CFTRs (Choi et al., 2001). Apical HCO3− efflux can be enhanced by reciprocal interactions and stimulations between CFTR and other HCO3− transporters, such as SLC26A3 (DRA) and SLC26A6 (Ko et al., 2002; Ko et al., 2004), whereas CF mutations may disrupt this cooperative HCO3− secretion (Choi et al., 2001; Ko et al., 2002). These findings suggest an important role for CFTR in regulating HCO3− secretion into ASL.
The importance of the HCO3− deficit in CF is evident from the positive correlation of pancreatic sufficient CF patients with HCO3− transport across the cell membrane (Choi et al., 2001). Moreover, HCO3− deficit-induced ASL acidosis in CFTR−/− newborn pig airways results in impaired bacterial killing (Pezzulo et al., 2012; Shah et al., 2016a) partly due to reduced antimicrobial activity of lysozyme, lactoferrin, β-defensin-3 and LL-37 in the airways (Pezzulo et al., 2012; Abou Alaiwa et al., 2014b). In CF airways, the acidic pH of ASL increases ASL viscosity (Tang et al., 2016), causing abnormal mucus movement (Hoegger et al., 2014). These data emphasize the pathological development of CF airway disease with acidic ASL disrupting airway bacterial killing and mucus movement, resulting in chronic bacterial colonization (Stoltz et al., 2015) (Figure 2).
A direct test of this hypothesis is exploring whether CFTR dysfunction acidifies the ASL of people with CF and the apical liquid of cultured epithelia or cells. Previous studies (Pezzulo et al., 2012; Shah et al., 2016a; Li et al., 2016; Tang et al., 2016) indicate that the ASL pH of cultured CF pig airway epithelia is more acidic than that of non-CF. Using a pH microelectrode, other studies demonstrate that the apical pH of cultured epithelia or the extracellular pH of epithelial cells from different human cell lines, such as CFBE41o− (Simonin et al., 2019), Calu-3 (Shan et al., 2012), IB3-1 vs. C38 (Valdivieso et al., 2019) and CuFi-1 vs. NuLi cells (Muraglia et al., 2019) or from human bronchial epithelial cells (Coakley et al., 2003; Haggie et al., 2016; Scudieri et al., 2018; Simonin et al., 2019; Gianotti et al., 2020) is consistently more acidic in CF than that in non-CF. The pH difference (∆pH) between CF and non-CF among these studies is about 0.2–0.65 (Coakley et al., 2003; Pezzulo et al., 2012; Shan et al., 2012; Haggie et al., 2016; Scudieri et al., 2018; Simonin et al., 2019; Gianotti et al., 2020), whereas well-differentiated epithelia with better epithelial polarization and more CFTR expressed in the apical membrane than those of less differentiated epithelia (Sheppard et al., 1994), exhibit larger ∆pH (∼0.5) decreases in CF than non-CF epithelia (Scudieri et al., 2018; Gianotti et al., 2020). These data are consistent with the idea that the loss of CFTR-mediated HCO3− secretion in CF airways leads to ASL acidosis (Pezzulo et al., 2012; Stoltz et al., 2015). Moreover, transgenic mice overexpressing the proton pump, non-gastric H+/K+ ATPase (ATP12A) in the apical membrane of airway epithelia have reduced ASL pH and develop CF-like lung disease (Shah et al., 2016b), further supporting this ASL acidosis hypothesis.
However, direct measurements of ASL pH in animals or human subjects with or without CF reveal complex mechanisms that participate in ASL pH regulation. When compared to that of wild-type animals, the ASL pH is more acidic in CF newborn pigs (Pezzulo et al., 2012; Shah et al., 2016b) and 1–6 month-old rats (Birket et al., 2018) but not in adult mice (Jayaraman et al., 2001). In humans, CF neonates (<1 month old) exhibit ASL pH lower than healthy controls (Abou Alaiwa et al., 2014a; Abou Alaiwa et al., 2018) and CF submucosal gland secretion from nasal biopsies is also more acidic than that from non-CF (Song et al., 2006). However, no difference in ASL pH between CF and non-CF is found in either children (McShane et al., 2003; Abou Alaiwa et al., 2014a; Schultz et al., 2017; Abou Alaiwa et al., 2018) or adults (McShane et al., 2003). A study using primary cultures of human airway epithelial cells observed no difference in the pH of the apical fluid between CF and non-CF (Schultz et al., 2017). Conversely, with HCO3−-free culture medium, cultured CF epithelia still show ASL pH lower than non-CF (Scudieri et al., 2018). Thus, in addition to CFTR-mediated HCO3− secretion, age-associated adaptions (Abou Alaiwa et al., 2018) and other regulatory mechanisms, such as ATP/histamine-stimulated proton secretion (Song et al., 2006), pendrin (SLC26A4, a Cl−/anion exchanger) (Haggie et al., 2016; Simonin et al., 2019) (Figure 2) and H+/K+-ATPase (ATP12A) (Scudieri et al., 2018; Simonin et al., 2019) may all contribute to ASL pH regulation.
For instance, pendrin containing 870 amino acids is an electroneutral transporter exchanging Cl− for HCO3−, I−, NO3−, SCN− or HCO2− across the cell membrane (Haggie et al., 2016; Tamma and Dossena, 2022). In airway epithelia, pendrin is found abundantly on the apical membrane of ciliated epithelial cells but little in submucosal glands (Kim et al., 2019) and its gene expression is not significantly increased in CFTR-rich ionocytes (Rehman et al., 2020). Cl−/HCO3− exchange by pendrin in airway epithelial cells is upregulated by the cytokines IL-13 to reduce ASL depth (Haggie et al., 2016), by IL-17A (Adams et al., 2014), by IL-17 together with TNF-α to alkalinize ASL pH (Rehman et al., 2020) and by IL-4 to stimulate pendrin-mediated HCO3− secretion and CFTR-mediated electrogenic Cl− efflux (Kim et al., 2019). These data suggest that pendrin promotes Cl−/HCO3− transport during inflammation (Haggie et al., 2016; Kim et al., 2019; Rehman et al., 2020), but whether functional interplay between CFTR and pendrin (Rehman et al., 2020; Tamma and Dossena, 2022) is defective in CF airway leading to loss of HCO3− secretion remains to be explored in future work.
As most studies demonstrate that the apical solution of cultured epithelia is more acidic in CF than non-CF, the data suggest that CF epithelia have defective ASL pH regulation. Although CFTR-mediated HCO3− secretion is defective in CF epithelia (Smith and Welsh, 1992; Choi et al., 2001) and plays a role in ASL acidosis of neonates with CF (Abou Alaiwa et al., 2014a; Abou Alaiwa et al., 2018), it remains unclear whether abnormalities resulting from ASL acidosis in CF neonates would be the major factor causing later progressive deteriorations in the lung function of children and adult patients. Future investigation of the relationship between ASL pH, salt concentration, antimicrobial activity and the physicochemical properties of mucus may reveal the major underlying mechanism of CF lung disease caused by defective CFTR ion transport.
6 The role of epithelial Na+ transport in bacterial eradication in CF airways
Comprehensive reviews about the Na+ hyperabsorption or low volume hypothesis can be found in previous literatures (Tarran et al., 2006; Lazarowski and Boucher, 2021). The main concept of this hypothesis is that CFTR dysfunction in CF may result in hyperactivity of ENaC (Stutts et al., 1995; Matsui et al., 1998), leading to transepithelial hyperabsorption of NaCl and water (Matsui et al., 1998). Consequently, the height of ASL is reduced, causing cilia bending and deformation, impairing mucociliary clearance (Matsui et al., 1998) and preventing bacterial eradication (Tarran et al., 2006). This hypothesis is also supported by transgenic mice overexpressing the ENaC-β subunit in airway epithelia, which generates CF-like lung inflammation and disease (Mall et al., 2004).
Two ideas to explain CFTR-mediated regulation of ENaC activity are the modulation of channel activity by the intracellular Cl− concentration and direct interactions between the two channels (Berdiev et al., 2009). However, the basis of the low volume hypothesis, including ENaC hyperactivity by loss of CFTR function and Na+ hyperabsorption in CF epithelia is not supported by several experimental approaches, such as patch-clamp studies of ENaC channel activity (Nagel et al., 2005), the measurement of transepithelial Na+ absorption (Chen et al., 2010; Itani et al., 2011; Willumsen and Boucher, 1991a, b) and amiloride-sensitive changes in short-circuit currents of cultured pig airway epithelia (Chen et al., 2010). Similarly, treatment of CF airway epithelia with hypertonic solution that likely facilitates epithelial water secretion against Na+ and fluid hyperabsorption achieves only a moderate, short-term improvement of lung function in people with CF (Wark and McDonald, 2018). Therefore, deeper insight into the interaction of CFTR and ENaC is required to further investigate this hypothesis.
7 CFTR-mediated regulation of membrane transporters and intracellular proteins
CFTR interacts with many membrane transporters to regulate epithelial function (Iazzi et al., 2023). For example, CFTR modulates the activity of the Na+/H+ exchanger (NHE) (Ahn et al., 2001) and Cl−/HCO3− exchanger (Lee et al., 1999; Ko et al., 2004) to adjust extra- and intracellular pH, implicating the function of these transporters in ASL pH regulation. CFTR through its N-terminal peptide interacts with filamin A (Smith et al., 2010) and the SNARE proteins syntaxin 1A (Naren et al., 1997) and SNAP-23 (Cormet-Boyaka et al., 2002). Moreover, CFTR uses the type I PDZ-binding motif (D/E)T(R/K)L in its C-terminal peptide to interact with Na+/H+ exchange regulatory cofactor 1 (NHERF1) (Hall et al., 1998; Martin et al., 2020), NHERF2 (Sun et al., 2000), NHERF3 (also known as PDZK1, CAP70 or NaPi-Cap1) (Wang et al., 2000), NHERF4 (IKEPP) (Hegedus et al., 2003), CFTR-associated ligand (CAL) (Cheng et al., 2002) and Shank2 (Kim et al., 2004b). Finally, the R domain of CFTR interacts with the antisigma factor antagonist (STAS) domain of SLC26 transporters (Ko et al., 2004), calmodulin (Bozoky et al., 2017) and other intracellular proteins (Bozoky et al., 2013). However, it remains unclear whether defects in these CFTR-mediated interactions or regulation play a significant role in CF lung pathogenesis.
8 The association of CFTR dysfunction with mucus accumulation in CF
A major hallmark of CF disease is the accumulation of mucus in the lumen of different ducts, such as the airways, gastrointestinal tract, bile duct and reproductive systems (Hansson, 2019). In CF airways, thick and sticky mucus detain airborne bacteria, resulting in bacterial colonization, biofilm formation and later bacterial invasion of the epithelium, inducing severe and recurrent inflammation (Reece et al., 2021; Shteinberg et al., 2021). Therefore, the removal of airway surface mucus facilitates bacterial eradication in CF (Belli et al., 2021).
Airway mucus is secreted by Goblet cells in the surface epithelium and submucosal glands (Morrison et al., 2019) and is matured in ASL after transformation from a core structure to a hydrated and extended mucin molecule (Abdullah et al., 2017) (Figure 1). In this maturation process, Ca2+ in mucin bundles is replaced by Na+ in ASL, followed by mucin hydration and extension (Verdugo, 1990; Kesimer et al., 2010; Morrison et al., 2019) (Figure 1). In CF airways (Figure 2), mucus secretion is exaggerated due to bacterial contact with the epithelium (Ben Mohamed et al., 2012), cytokine secretion, such as IL-1 (Balazs and Mall, 2019), IL-8 (Ben Mohamed et al., 2012), and IL-13 (Zhu et al., 1999), and at a later stage, the hyperplasia of Goblet cells and glands (Morrison et al., 2019). In addition, other factors such as ASL acidosis (Ambort et al., 2012; Tang et al., 2016), reduced HCO3− secretion (Saint-Criq et al., 2022) and airway surface dehydration (Abdullah et al., 2017) may alter the viscosity (Tang et al., 2016), maturation (Abdullah et al., 2017) and movement (Matsui et al., 1998; Hoegger et al., 2014) of mucus, likely impairing the mucociliary clearance mechanism (Figure 2). Thus, the dehydration (Abdullah et al., 2017) and deformation (Tang et al., 2016) of mucus that impairs bacterial eradication have been the focus of much recent attention in CF airway research.
CFTR dysfunction facilitates airway bacterial colonization and infection, leading to excess inflammation (Ribeiro et al., 2023) that further causes tissue remodeling and damage, such as epithelial-mesenchymal transition (Quaresma et al., 2022). These epithelial disorders may form a positive but detrimental feedback loop, where inflammation-induced Goblet cell hyperplasia (Morrison et al., 2019) continuously stimulates mucus secretion and then mucus accumulation in CF airways further traps more bacteria, hyperactivating the inflammatory responses of epithelia. In addition, sterile inflammation triggered by mucus plugging via the interleukin-1 signaling pathway (Balazs and Mall, 2019) amplifies this vicious cycle. Indeed, an urgent task in future research is to effectively interrupt this cycle to develop important new treatments for CF lung disease.
9 Closing remarks
Over 3 decades of CF research has demonstrated that lack of CFTR-mediated HCO3− secretion and subsequent ASL acidosis provides a convincing mechanism that leads to abnormal mucus and bacterial eradication (Stoltz et al., 2015) at least in the CF airways of neonates (Abou Alaiwa et al., 2014a; Abou Alaiwa et al., 2018). Deficits of CFTR-mediated Cl− transport and regulation of other proteins may also contribute to the pathogenesis of CF. Clarifying the role of each CFTR functional defect in the development of CF lung disease may reveal the major routes responsible for the cycles of bacterial infection, inflammation and mucus accumulation in CF airways (Ribeiro et al., 2023). New treatments that prohibit airway bacterial colonization or abnormal mucus accumulation would be promising approaches to treat CF lung disease and perhaps also other infectious lung diseases.
Author contributions
MW: Writing–original draft, Writing–review and editing, Funding acquisition. J-HC: Conceptualization, Funding acquisition, Validation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This manuscript was supported by the Zhejiang Provincial Natural Science Foundation of China, No. LY20C050001 to J-HC and No. LQ23C110001 to MW, and Science and Technology Program of Jinhua City, No. 2022-3-143 to J-HC and No. 2023-3-079 to MW.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdullah L. H., Evans J. R., Wang T. T., Ford A. A., Makhov A. M., Nguyen K., et al. (2017). Defective postsecretory maturation of MUC5B mucin in cystic fibrosis airways. JCI Insight 2, e89752. doi:10.1172/jci.insight.89752
Abou Alaiwa M. H., Beer A. M., Pezzulo A. A., Launspach J. L., Horan R. A., Stoltz D. A., et al. (2014a). Neonates with cystic fibrosis have a reduced nasal liquid pH; a small pilot study. J. Cyst. Fibros. 13, 373–377. doi:10.1016/j.jcf.2013.12.006
Abou Alaiwa M. H., Launspach J. L., Grogan B., Carter S., Zabner J., Stoltz D. A., et al. (2018). Ivacaftor-induced sweat chloride reductions correlate with increases in airway surface liquid pH in cystic fibrosis. JCI Insight 3, e121468. doi:10.1172/jci.insight.121468
Abou Alaiwa M. H., Reznikov L. R., Gansemer N. D., Sheets K. A., Horswill A. R., Stoltz D. A., et al. (2014b). pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc. Natl. Acad. Sci. U. S. A. 111, 18703–18708. doi:10.1073/pnas.1422091112
Abrami M., Biasin A., Tescione F., Tierno D., Dapas B., Carbone A., et al. (2024). Mucus structure, viscoelastic properties, and composition in chronic respiratory diseases. Int. J. Mol. Sci. 25, 1933. doi:10.3390/ijms25031933
Adams K. M., Abraham V., Spielman D., Kolls J. K., Rubenstein R. C., Conner G. E., et al. (2014). IL-17A induces pendrin expression and chloride-bicarbonate exchange in human bronchial epithelial cells. PLoS One 9, e103263. doi:10.1371/journal.pone.0103263
Ahn W., Kim K. H., Lee J. A., Kim J. Y., Choi J. Y., Moe O. W., et al. (2001). Regulatory interaction between the cystic fibrosis transmembrane conductance regulator and HCO3- salvage mechanisms in model systems and the mouse pancreatic duct. J. Biol. Chem. 276, 17236–17243. doi:10.1074/jbc.M011763200
Amaral M. D., Farinha C. M. (2013). Rescuing mutant CFTR: a multi-task approach to a better outcome in treating cystic fibrosis. Curr. Pharm. Des. 19, 3497–3508. doi:10.2174/13816128113199990318
Ambort D., Johansson M. E., Gustafsson J. K., Nilsson H. E., Ermund A., Johansson B. R., et al. (2012). Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc. Natl. Acad. Sci. U. S. A. 109, 5645–5650. doi:10.1073/pnas.1120269109
Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., et al. (1991). Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 253, 202–205. doi:10.1126/science.1712984
Andersson D. I., Hughes D., Kubicek-Sutherland J. Z. (2016). Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug resist. updat. 26, 43–57. doi:10.1016/j.drup.2016.04.002
Ashtiwi N. M., Sarr D., Rada B. (2021). DUOX1 in mammalian disease pathophysiology. J. Mol. Med. Berl. 99, 743–754. doi:10.1007/s00109-021-02058-2
Atteih S. E., Armbruster C. R., Hilliam Y., Rapsinski G. J., Bhusal J. K., Krainz L. L., et al. (2024). Effects of highly effective modulator therapy on the dynamics of the respiratory mucosal environment and inflammatory response in cystic fibrosis. Pediatr. Pulmonol. Online ahead of print. doi:10.1002/ppul.26898
Balazs A., Mall M. A. (2019). Mucus obstruction and inflammation in early cystic fibrosis lung disease: emerging role of the IL-1 signaling pathway. Pediatr. Pulmonol. 54 (Suppl. 3), S5-S12. doi:10.1002/ppul.24462
Bals R., Wang X., Zasloff M., Wilson J. M. (1998). The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. U. S. A. 95, 9541–9546. doi:10.1073/pnas.95.16.9541
Belli S., Prince I., Savio G., Paracchini E., Cattaneo D., Bianchi M., et al. (2021). Airway clearance techniques: the right choice for the right patient. Front. Med. (Lausanne) 8, 544826. doi:10.3389/fmed.2021.544826
Ben Mohamed F., Garcia-Verdugo I., Medina M., Balloy V., Chignard M., Ramphal R., et al. (2012). A crucial role of Flagellin in the induction of airway mucus production by Pseudomonas aeruginosa. PLoS One 7, e39888. doi:10.1371/journal.pone.0039888
Berdiev B. K., Qadri Y. J., Benos D. J. (2009). Assessment of the CFTR and ENaC association. Mol. Biosyst. 5, 123–127. doi:10.1039/b810471a
Birket S. E., Davis J. M., Fernandez C. M., Tuggle K. L., Oden A. M., Chu K. K., et al. (2018). Development of an airway mucus defect in the cystic fibrosis rat. JCI Insight 3, e97199. doi:10.1172/jci.insight.97199
Bompadre S. G., Sohma Y., Li M., Hwang T. C. (2007). G551D and G1349D, two CF-associated mutations in the signature sequences of CFTR, exhibit distinct gating defects. J. Gen. Physiol. 129, 285–298. doi:10.1085/jgp.200609667
Bose S. J., Krainer G., Ng D. R. S., Schenkel M., Shishido H., Yoon J. S., et al. (2020). Towards next generation therapies for cystic fibrosis: folding, function and pharmacology of CFTR. J. Cyst. Fibros. 19 (Suppl. 1), S25-S32. doi:10.1016/j.jcf.2019.12.009
Boucher R. C. (2007). Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu. Rev. Med. 58, 157–170. doi:10.1146/annurev.med.58.071905.105316
Bower J. K., Volkova N., Ahluwalia N., Sahota G., Xuan F., Chin A., et al. (2023). Real-world safety and effectiveness of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis: interim results of a long-term registry-based study. J. Cyst. Fibros. 22, 730–737. doi:10.1016/j.jcf.2023.03.002
Bozoky Z., Ahmadi S., Milman T., Kim T. H., Du K., Di Paola M., et al. (2017). Synergy of cAMP and calcium signaling pathways in CFTR regulation. Proc. Natl. Acad. Sci. U. S. A. 114, E2086-E2095. doi:10.1073/pnas.1613546114
Bozoky Z., Krzeminski M., Muhandiram R., Birtley J. R., Al-Zahrani A., Thomas P. J., et al. (2013). Regulatory R region of the CFTR chloride channel is a dynamic integrator of phospho-dependent intra- and intermolecular interactions. Proc. Natl. Acad. Sci. U. S. A. 110, E4427–E4436. doi:10.1073/pnas.1315104110
Bustamante-Marin X. M., Ostrowski L. E. (2017). Cilia and mucociliary clearance. Cold Spring Harb. Perspect. Biol. 9, a028241. doi:10.1101/cshperspect.a028241
Button B., Cai L. H., Ehre C., Kesimer M., Hill D. B., Sheehan J. K., et al. (2012). A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337, 937–941. doi:10.1126/science.1223012
Cabrini G., Rimessi A., Borgatti M., Lampronti I., Finotti A., Pinton P., et al. (2020). Role of cystic fibrosis bronchial epithelium in neutrophil chemotaxis. Front. Immunol. 11, 1438. doi:10.3389/fimmu.2020.01438
Cai Z., Sheppard D. N. (2002). Phloxine B interacts with the cystic fibrosis transmembrane conductance regulator at multiple sites to modulate channel activity. J. Biol. Chem. 277, 19546–19553. doi:10.1074/jbc.M108023200
Cai Z., Taddei A., Sheppard D. N. (2006). Differential sensitivity of the cystic fibrosis (CF)-associated mutants G551D and G1349D to potentiators of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel. J. Biol. Chem. 281, 1970–1977. doi:10.1074/jbc.M510576200
Casey M., Gabillard-Lefort C., McElvaney O. F., McElvaney O. J., Carroll T., Heeney R. C., et al. (2023). Effect of elexacaftor/tezacaftor/ivacaftor on airway and systemic inflammation in cystic fibrosis. Thorax 78, 835–839. doi:10.1136/thorax-2022-219943
Caverly L. J., Riquelme S. A., Hisert K. B. (2022). The impact of highly effective modulator therapy on cystic fibrosis microbiology and inflammation. Clin. Chest. Med. 43, 647–665. doi:10.1016/j.ccm.2022.06.007
Chen J. H., Cai Z., Sheppard D. N. (2009). Direct sensing of intracellular pH by the cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel. J. Biol. Chem. 284, 35495–35506. doi:10.1074/jbc.M109.072678
Chen J. H., Stoltz D. A., Karp P. H., Ernst S. E., Pezzulo A. A., Moninger T. O., et al. (2010). Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell. 143, 911–923. doi:10.1016/j.cell.2010.11.029
Chen J. H., Xu W., Sheppard D. N. (2017). Altering intracellular pH reveals the kinetic basis of intraburst gating in the CFTR Cl− channel. J. Physiol. 595, 1059–1076. doi:10.1113/JP273205
Chen X., Zhu S., Zhenin M., Xu W., Bose S. J., Wong M. P., et al. (2019). A defective flexible loop contributes to the processing and gating defects of the predominant cystic fibrosis-causing mutation. FASEB J. 33, 5126–5142. doi:10.1096/fj.201801218RR
Cheng J., Moyer B. D., Milewski M., Loffing J., Ikeda M., Mickle J. E., et al. (2002). A Golgi-associated PDZ domain protein modulates cystic fibrosis transmembrane regulator plasma membrane expression. J. Biol. Chem. 277, 3520–3529. doi:10.1074/jbc.M110177200
Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., et al. (1990). Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 63, 827–834. doi:10.1016/0092-8674(90)90148-8
Choi J. Y., Muallem D., Kiselyov K., Lee M. G., Thomas P. J., Muallem S. (2001). Aberrant CFTR-dependent HCO3- transport in mutations associated with cystic fibrosis. Nature 410, 94–97. doi:10.1038/35065099
Cimino G., Sorrenti S., Murciano M., Galoppi P., Ascenzioni F., Botta B., et al. (2024). Use of elexacaftor/tezacaftor/ivacaftor combination in pregnancy. Arch. Gynecol. Obstet. 309, 9–15. doi:10.1007/s00404-023-06962-5
Ciofu O., Hansen C. R., Hoiby N. (2013). Respiratory bacterial infections in cystic fibrosis. Curr. Opin. Pulm. Med. 19, 251–258. doi:10.1097/MCP.0b013e32835f1afc
Coakley R. D., Grubb B. R., Paradiso A. M., Gatzy J. T., Johnson L. G., Kreda S. M., et al. (2003). Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc. Natl. Acad. Sci. U. S. A. 100, 16083–16088. doi:10.1073/pnas.2634339100
Cormet-Boyaka E., Di A., Chang S. Y., Naren A. P., Tousson A., Nelson D. J., et al. (2002). CFTR chloride channels are regulated by a SNAP-23/syntaxin 1A complex. Proc. Natl. Acad. Sci. U. S. A. 99, 12477–12482. doi:10.1073/pnas.192203899
Costantini C., Nunzi E., Romani L. (2022). From the nose to the lungs: the intricate journey of airborne pathogens amid commensal bacteria. Am. J. Physiol. Cell. Physiol. 323, C1036–C1043. doi:10.1152/ajpcell.00287.2022
Dalemans W., Barbry P., Champigny G., Jallat S., Dott K., Dreyer D., et al. (1991). Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature 354, 526–528. doi:10.1038/354526a0
De Y., Chen Q., Schmidt A. P., Anderson G. M., Wang J. M., Wooters J., et al. (2000). LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192, 1069–1074. doi:10.1084/jem.192.7.1069
Di A., Brown M. E., Deriy L. V., Li C., Szeto F. L., Chen Y., et al. (2006). CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat. Cell. Biol. 8, 933–944. doi:10.1038/ncb1456
Dittrich A. M., Sieber S., Naehrlich L., Burkhart M., Hafkemeyer S., Tummler B., et al. (2024). Use of elexacaftor/tezacaftor/ivacaftor leads to changes in detection frequencies of Staphylococcus aureus and Pseudomonas aeruginosa dependent on age and lung function in people with cystic fibrosis. Int. J. Infect. Dis. 139, 124–131. doi:10.1016/j.ijid.2023.11.013
Durfey S. L., Pipavath S., Li A., Vo A. T., Ratjen A., Carter S., et al. (2021). Combining ivacaftor and intensive antibiotics achieves limited clearance of cystic fibrosis infections. mBio 12, e0314821. doi:10.1128/mbio.03148-21
Ellison R. T., Giehl T. J. (1991). Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Investig. 88, 1080–1091. doi:10.1172/JCI115407
Ermund A., Trillo-Muyo S., Hansson G. C. (2018). Assembly, release, and transport of airway mucins in pigs and humans. Ann. Am. Thorac. Soc. 15, S159-S163. doi:10.1513/AnnalsATS.201804-238AW
Esther C. R., Muhlebach M. S., Ehre C., Hill D. B., Wolfgang M. C., Kesimer M., et al. (2019). Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci. Transl. Med. 11, eaav3488. doi:10.1126/scitranslmed.aav3488
Fila L., Grandcourtova A., Bilkova A., Drevinek P. (2023). Elexacaftor-tezacaftor-ivacaftor in patients with cystic fibrosis ineligible for clinical trials: a 24-week observational study. Front. Pharmacol. 14, 1178009. doi:10.3389/fphar.2023.1178009
Fischer H. (2009). Mechanisms and function of DUOX in epithelia of the lung. Antioxid. Redox Signal. 11, 2453–2465. doi:10.1089/ars.2009.2558
Geitani R., Moubareck C. A., Xu Z., Karam Sarkis D., Touqui L. (2020). Expression and roles of antimicrobial peptides in innate defense of airway mucosa: potential implication in cystic fibrosis. Front. Immunol. 11, 1198. doi:10.3389/fimmu.2020.01198
Ghosh A., Kar R. K., Jana J., Saha A., Jana B., Krishnamoorthy J., et al. (2014). Indolicidin targets duplex DNA: structural and mechanistic insight through a combination of spectroscopy and microscopy. ChemMedChem 9, 2052–2058. doi:10.1002/cmdc.201402215
Gianotti A., Capurro V., Delpiano L., Mielczarek M., Garcia-Valverde M., Carreira-Barral I., et al. (2020). Small molecule anion carriers correct abnormal airway surface liquid properties in cystic fibrosis airway epithelia. Int. J. Mol. Sci. 21, 1488. doi:10.3390/ijms21041488
Goralski J. L., Hoppe J. E., Mall M. A., McColley S. A., McKone E., Ramsey B., et al. (2023). Phase 3 open-label clinical trial of elexacaftor/tezacaftor/ivacaftor in children aged 2-5 years with cystic fibrosis and at least one F508del allele. Am. J. Respir. Crit. Care Med. 208, 59–67. doi:10.1164/rccm.202301-0084OC
Gould N. S., Gauthier S., Kariya C. T., Min E., Huang J., Brian D. J. (2010). Hypertonic saline increases lung epithelial lining fluid glutathione and thiocyanate: two protective CFTR-dependent thiols against oxidative injury. Respir. Res. 11, 119. doi:10.1186/1465-9921-11-119
Grandjean Lapierre S., Phelippeau M., Hakimi C., Didier Q., Reynaud-Gaubert M., Dubus J. C., et al. (2017). Cystic fibrosis respiratory tract salt concentration: an exploratory cohort study. Med. Baltim. 96, e8423. doi:10.1097/MD.0000000000008423
Green H. D., Jones A. M. (2022). Managing pulmonary infection in adults with cystic fibrosis: adult cystic fibrosis series. Chest 162, 66–75. doi:10.1016/j.chest.2022.02.007
Haggie P. M., Phuan P. W., Tan J. A., Zlock L., Finkbeiner W. E., Verkman A. S. (2016). Inhibitors of pendrin anion exchange identified in a small molecule screen increase airway surface liquid volume in cystic fibrosis. FASEB J. 30, 2187–2197. doi:10.1096/fj.201600223R
Hall R. A., Ostedgaard L. S., Premont R. T., Blitzer J. T., Rahman N., Welsh M. J., et al. (1998). A C-terminal motif found in the β2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc. Natl. Acad. Sci. U. S. A. 95, 8496–8501. doi:10.1073/pnas.95.15.8496
Hansson G. C. (2019). Mucus and mucins in diseases of the intestinal and respiratory tracts. J. Intern. Med. 285, 479–490. doi:10.1111/joim.12910
Hegedus T., Sessler T., Scott R., Thelin W., Bakos E., Varadi A., et al. (2003). C-terminal phosphorylation of MRP2 modulates its interaction with PDZ proteins. Biochem. Biophys. Res. Commun. 302, 454–461. doi:10.1016/s0006-291x(03)00196-7
Heijerman H. G. M., McKone E. F., Downey D. G., Van Braeckel E., Rowe S. M., Tullis E., et al. (2019). Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 394, 1940–1948. doi:10.1016/S0140-6736(19)32597-8
Henderson A. G., Ehre C., Button B., Abdullah L. H., Cai L. H., Leigh M. W., et al. (2014). Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J. Clin. Investig. 124, 3047–3060. doi:10.1172/JCI73469
Hoegger M. J., Fischer A. J., McMenimen J. D., Ostedgaard L. S., Tucker A. J., Awadalla M. A., et al. (2014). Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 345, 818–822. doi:10.1126/science.1255825
Hovenberg H. W., Davies J. R., Herrmann A., Linden C. J., Carlstedt I. (1996). MUC5AC, but not MUC2, is a prominent mucin in respiratory secretions. Glycoconj. J. 13, 839–847. doi:10.1007/BF00702348
Hwang T. C., Wang F., Yang I. C., Reenstra W. W. (1997). Genistein potentiates wild-type and delta F508-CFTR channel activity. Am. J. Physiol. 273, C988–C998. doi:10.1152/ajpcell.1997.273.3.C988
Iazzi M., Sadeghi S., Gupta G. D. (2023). A proteomic survey of the cystic fibrosis transmembrane conductance regulator surfaceome. Int. J. Mol. Sci. 24, 11457. doi:10.3390/ijms241411457
Ismailov I. I., Awayda M. S., Jovov B., Berdiev B. K., Fuller C. M., Dedman J. R., et al. (1996). Regulation of epithelial sodium channels by the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 271, 4725–4732. doi:10.1074/jbc.271.9.4725
Itani O. A., Chen J. H., Karp P. H., Ernst S., Keshavjee S., Parekh K., et al. (2011). Human cystic fibrosis airway epithelia have reduced Cl- conductance but not increased Na+ conductance. Proc. Natl. Acad. Sci. U. S. A. 108, 10260–10265. doi:10.1073/pnas.1106695108
Jaramillo A. M., Azzegagh Z., Tuvim M. J., Dickey B. F. (2018). Airway mucin secretion. Ann. Am. Thorac. Soc. 15, S164-S170. doi:10.1513/AnnalsATS.201806-371AW
Jayaraman S., Song Y., Vetrivel L., Shankar L., Verkman A. S. (2001). Noninvasive in vivo fluorescence measurement of airway-surface liquid depth, salt concentration, and pH. J. Clin. Investig. 107, 317–324. doi:10.1172/JCI11154
Joo N. S., Irokawa T., Wu J. V., Robbins R. C., Whyte R. I., Wine J. J. (2002). Absent secretion to vasoactive intestinal peptide in cystic fibrosis airway glands. J. Biol. Chem. 277, 50710–50715. doi:10.1074/jbc.M208826200
Keating D., Marigowda G., Burr L., Daines C., Mall M. A., McKone E. F., et al. (2018). VX-445-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N. Engl. J. Med. 379, 1612–1620. doi:10.1056/NEJMoa1807120
Keith J. D., Henderson A. G., Fernandez-Petty C. M., Davis J. M., Oden A. M., Birket S. E. (2022). Muc5b contributes to mucus abnormality in rat models of cystic fibrosis. Front. Physiol. 13, 884166. doi:10.3389/fphys.2022.884166
Kesimer M., Makhov A. M., Griffith J. D., Verdugo P., Sheehan J. K. (2010). Unpacking a gel-forming mucin: a view of MUC5B organization after granular release. Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L15–L22. doi:10.1152/ajplung.00194.2009
Khan T. Z., Wagener J. S., Bost T., Martinez J., Accurso F. J., Riches D. W. (1995). Early pulmonary inflammation in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 151, 1075–1082. doi:10.1164/ajrccm/151.4.1075
Kim D., Huang J., Billet A., Abu-Arish A., Goepp J., Matthes E., et al. (2019). Pendrin mediates bicarbonate secretion and enhances cystic fibrosis transmembrane conductance regulator function in airway surface epithelia. Am. J. Respir. Cell. Mol. Biol. 60, 705–716. doi:10.1165/rcmb.2018-0158OC
Kim D. H., Chu H. S., Lee J. Y., Hwang S. J., Lee S. H., Lee H. M. (2004a). Up-regulation of MUC5AC and MUC5B mucin genes in chronic rhinosinusitis. Arch. Otolaryngol. Head. Neck Surg. 130, 747–752. doi:10.1001/archotol.130.6.747
Kim J. Y., Han W., Namkung W., Lee J. H., Kim K. H., Shin H., et al. (2004b). Inhibitory regulation of cystic fibrosis transmembrane conductance regulator anion-transporting activities by Shank2. J. Biol. Chem. 279, 10389–10396. doi:10.1074/jbc.M312871200
Ko S. B., Shcheynikov N., Choi J. Y., Luo X., Ishibashi K., Thomas P. J., et al. (2002). A molecular mechanism for aberrant CFTR-dependent HCO3- transport in cystic fibrosis. EMBO J. 21, 5662–5672. doi:10.1093/emboj/cdf580
Ko S. B., Zeng W., Dorwart M. R., Luo X., Kim K. H., Millen L., et al. (2004). Gating of CFTR by the STAS domain of SLC26 transporters. Nat. Cell. Biol. 6, 343–350. doi:10.1038/ncb1115
Kozlova I., Vanthanouvong V., Johannesson M., Roomans G. M. (2006). Composition of airway surface liquid determined by X-ray microanalysis. Ups. J. Med. Sci. 111, 137–153. doi:10.3109/2000-1967-016
Kreda S. M., Gynn M. C., Fenstermacher D. A., Boucher R. C., Gabriel S. E. (2001). Expression and localization of epithelial aquaporins in the adult human lung. Am. J. Respir. Cell. Mol. Biol. 24, 224–234. doi:10.1165/ajrcmb.24.3.4367
Kunzelmann K., Kathofer S., Greger R. (1995). Na+ and Cl- conductances in airway epithelial cells: increased Na+ conductance in cystic fibrosis. Pflugers Arch. 431, 1–9. doi:10.1007/BF00374371
Laselva O., Bartlett C., Gunawardena T. N. A., Ouyang H., Eckford P. D. W., Moraes T. J., et al. (2021). Rescue of multiple class II CFTR mutations by elexacaftor+tezacaftor+ivacaftor mediated in part by the dual activities of elexacaftor as both corrector and potentiator. Eur. Respir. J. 57, 2002774. doi:10.1183/13993003.02774-2020
Lazarowski E. R., Boucher R. C. (2021). Purinergic receptors in airway hydration. Biochem. Pharmacol. 187, 114387. doi:10.1016/j.bcp.2020.114387
Lee M. G., Choi J. Y., Luo X., Strickland E., Thomas P. J., Muallem S. (1999). Cystic fibrosis transmembrane conductance regulator regulates luminal Cl-/HCO3- exchange in mouse submandibular and pancreatic ducts. J. Biol. Chem. 274, 14670–14677. doi:10.1074/jbc.274.21.14670
Lei L., Traore S., Romano Ibarra G. S., Karp P. H., Rehman T., Meyerholz D. K., et al. (2023). CFTR-rich ionocytes mediate chloride absorption across airway epithelia. J. Clin. Investig. 133, e171268. doi:10.1172/JCI171268
Li C., Naren A. P. (2010). CFTR chloride channel in the apical compartments: spatiotemporal coupling to its interacting partners. Integr. Biol. (Camb) 2, 161–177. doi:10.1039/b924455g
Li X., Tang X. X., Vargas Buonfiglio L. G., Comellas A. P., Thornell I. M., Ramachandran S., et al. (2016). Electrolyte transport properties in distal small airways from cystic fibrosis pigs with implications for host defense. Am. J. Physiol. Lung Cell. Mol. Physiol. 310, L670–L679. doi:10.1152/ajplung.00422.2015
Lim S. H., Legere E. A., Snider J., Stagljar I. (2017). Recent progress in CFTR interactome mapping and its importance for cystic fibrosis. Front. Pharmacol. 8, 997. doi:10.3389/fphar.2017.00997
Linsdell P. (2016). Anion conductance selectivity mechanism of the CFTR chloride channel. Biochim. Biophys. Acta 1858, 740–747. doi:10.1016/j.bbamem.2016.01.009
Lorentzen D., Durairaj L., Pezzulo A. A., Nakano Y., Launspach J., Stoltz D. A., et al. (2011). Concentration of the antibacterial precursor thiocyanate in cystic fibrosis airway secretions. Free Radic. Biol. Med. 50, 1144–1150. doi:10.1016/j.freeradbiomed.2011.02.013
Luan X., Campanucci V. A., Nair M., Yilmaz O., Belev G., Machen T. E., et al. (2014). Pseudomonas aeruginosa triggers CFTR-mediated airway surface liquid secretion in swine trachea. Proc. Natl. Acad. Sci. U. S. A. 111, 12930–12935. doi:10.1073/pnas.1406414111
Lund-Palau H., Turnbull A. R., Bush A., Bardin E., Cameron L., Soren O., et al. (2016). Pseudomonas aeruginosa infection in cystic fibrosis: pathophysiological mechanisms and therapeutic approaches. Expert Rev. Respir. Med. 10, 685–697. doi:10.1080/17476348.2016.1177460
Mall M., Grubb B. R., Harkema J. R., O'Neal W. K., Boucher R. C. (2004). Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat. Med. 10, 487–493. doi:10.1038/nm1028
Martin C., Guzior D. V., Gonzalez C. T., Okros M., Mielke J., Padillo L., et al. (2023). Longitudinal microbial and molecular dynamics in the cystic fibrosis lung after elexacaftor-tezacaftor-ivacaftor therapy. Respir. Res. 24, 317. doi:10.1186/s12931-023-02630-z
Martin E. R., Barbieri A., Ford R. C., Robinson R. C. (2020). In vivo crystals reveal critical features of the interaction between cystic fibrosis transmembrane conductance regulator (CFTR) and the PDZ2 domain of Na+/H+ exchange cofactor NHERF1. J. Biol. Chem. 295, 4464–4476. doi:10.1074/jbc.RA119.012015
Matalon S., Bartoszewski R., Collawn J. F. (2015). Role of epithelial sodium channels in the regulation of lung fluid homeostasis. Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L1229–L1238. doi:10.1152/ajplung.00319.2015
Matsui H., Grubb B. R., Tarran R., Randell S. H., Gatzy J. T., Davis C. W., et al. (1998). Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 95, 1005–1015. doi:10.1016/s0092-8674(00)81724-9
McShane D., Davies J. C., Davies M. G., Bush A., Geddes D. M., Alton E. W. (2003). Airway surface pH in subjects with cystic fibrosis. Eur. Respir. J. 21, 37–42. doi:10.1183/09031936.03.00027603
Middleton P. G., Mall M. A., Drevinek P., Lands L. C., McKone E. F., Polineni D., et al. (2019). Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N. Engl. J. Med. 381, 1809–1819. doi:10.1056/NEJMoa1908639
Montoro D. T., Haber A. L., Biton M., Vinarsky V., Lin B., Birket S. E., et al. (2018). A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560, 319–324. doi:10.1038/s41586-018-0393-7
Morrison C. B., Markovetz M. R., Ehre C. (2019). Mucus, mucins, and cystic fibrosis. Pediatr. Pulmonol. 54 (Suppl. 3), S84-S96. doi:10.1002/ppul.24530
Muraglia K. A., Chorghade R. S., Kim B. R., Tang X. X., Shah V. S., Grillo A. S., et al. (2019). Small-molecule ion channels increase host defences in cystic fibrosis airway epithelia. Nature 567, 405–408. doi:10.1038/s41586-019-1018-5
Myszor I. T., Gudmundsson G. H. (2023). Modulation of innate immunity in airway epithelium for host-directed therapy. Front. Immunol. 14, 1197908. doi:10.3389/fimmu.2023.1197908
Nagel G., Barbry P., Chabot H., Brochiero E., Hartung K., Grygorczyk R. (2005). CFTR fails to inhibit the epithelial sodium channel ENaC expressed in Xenopus laevis oocytes. J. Physiol. 564, 671–682. doi:10.1113/jphysiol.2004.079046
Naren A. P., Nelson D. J., Xie W., Jovov B., Pevsner J., Bennett M. K., et al. (1997). Regulation of CFTR chloride channels by syntaxin and Munc18 isoforms. Nature 390, 302–305. doi:10.1038/36882
Nichols D. P., Morgan S. J., Skalland M., Vo A. T., Van Dalfsen J. M., Singh S. B., et al. (2023). Pharmacologic improvement of CFTR function rapidly decreases sputum pathogen density, but lung infections generally persist. J. Clin. Investig. 133, e167957. doi:10.1172/JCI167957
Niyonsaba F., Iwabuchi K., Someya A., Hirata M., Matsuda H., Ogawa H., et al. (2002). A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology 106, 20–26. doi:10.1046/j.1365-2567.2002.01398.x
Otani T., Furuse M. (2020). Tight junction structure and function revisited. Trends Cell. Biol. 30, 805–817. doi:10.1016/j.tcb.2020.08.004
Painter R. G., Valentine V. G., Lanson N. A., Leidal K., Zhang Q., Lombard G., et al. (2006). CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry 45, 10260–10269. doi:10.1021/bi060490t
Parker D., Prince A. (2011). Innate immunity in the respiratory epithelium. Am. J. Respir. Cell. Mol. Biol. 45, 189–201. doi:10.1165/rcmb.2011-0011RT
Pezzulo A. A., Tang X. X., Hoegger M. J., Abou Alaiwa M. H., Ramachandran S., Moninger T. O., et al. (2012). Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487, 109–113. doi:10.1038/nature11130
Plasschaert L. W., Zilionis R., Choo-Wing R., Savova V., Knehr J., Roma G., et al. (2018). A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560, 377–381. doi:10.1038/s41586-018-0394-6
Poulsen J. H., Fischer H., Illek B., Machen T. E. (1994). Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc. Natl. Acad. Sci. U. S. A. 91, 5340–5344. doi:10.1073/pnas.91.12.5340
Puchelle E., de Bentzmann S., Zahm J. M. (1995). Physical and functional properties of airway secretions in cystic fibrosis-therapeutic approaches. Respiration 62 (Suppl. 1), 2–12. doi:10.1159/000196486
Quaresma M. C., Botelho H. M., Pankonien I., Rodrigues C. S., Pinto M. C., Costa P. R., et al. (2022). Exploring YAP1-centered networks linking dysfunctional CFTR to epithelial-mesenchymal transition. Life Sci. Alliance 5, e202101326. doi:10.26508/lsa.202101326
Reece E., Bettio P. H. A., Renwick J. (2021). Polymicrobial interactions in the cystic fibrosis airway microbiome impact the antimicrobial susceptibility of Pseudomonas aeruginosa. Antibiot. (Basel) 10, 827. doi:10.3390/antibiotics10070827
Rehman T., Thornell I. M., Pezzulo A. A., Thurman A. L., Romano Ibarra G. S., Karp P. H., et al. (2020). TNFalpha and IL-17 alkalinize airway surface liquid through CFTR and pendrin. Am. J. Physiol. Cell. Physiol. 319, C331–C344. doi:10.1152/ajpcell.00112.2020
Ribeiro C. M. P., Higgs M. G., Muhlebach M. S., Wolfgang M. C., Borgatti M., Lampronti I., et al. (2023). Revisiting host-pathogen interactions in cystic fibrosis lungs in the era of CFTR modulators. Int. J. Mol. Sci. 24, 5010. doi:10.3390/ijms24055010
Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., et al. (1989). Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245, 1066–1073. doi:10.1126/science.2475911
Rogers T. D., Button B., Kelada S. N. P., Ostrowski L. E., Livraghi-Butrico A., Gutay M. I., et al. (2022). Regional differences in mucociliary clearance in the upper and lower airways. Front. Physiol. 13, 842592. doi:10.3389/fphys.2022.842592
Rowe S. M., Daines C., Ringshausen F. C., Kerem E., Wilson J., Tullis E., et al. (2017). Tezacaftor-ivacaftor in residual-function heterozygotes with cystic fibrosis. N. Engl. J. Med. 377, 2024–2035. doi:10.1056/NEJMoa1709847
Roy M. G., Livraghi-Butrico A., Fletcher A. A., McElwee M. M., Evans S. E., Boerner R. M., et al. (2014). Muc5b is required for airway defence. Nature 505, 412–416. doi:10.1038/nature12807
Saint-Criq V., Guequen A., Philp A. R., Villanueva S., Apablaza T., Fernandez-Moncada I., et al. (2022). Inhibition of the sodium-dependent HCO3- transporter SLC4A4, produces a cystic fibrosis-like airway disease phenotype. Elife 11, e75871. doi:10.7554/eLife.75871
Schaupp L., Addante A., Voller M., Fentker K., Kuppe A., Bardua M., et al. (2023). Longitudinal effects of elexacaftor/tezacaftor/ivacaftor on sputum viscoelastic properties, airway infection and inflammation in patients with cystic fibrosis. Eur. Respir. J. 62, 2202153. doi:10.1183/13993003.02153-2022
Schultz A., Puvvadi R., Borisov S. M., Shaw N. C., Klimant I., Berry L. J., et al. (2017). Airway surface liquid pH is not acidic in children with cystic fibrosis. Nat. Commun. 8, 1409. doi:10.1038/s41467-017-00532-5
Scudieri P., Musante I., Caci E., Venturini A., Morelli P., Walter C., et al. (2018). Increased expression of ATP12A proton pump in cystic fibrosis airways. JCI Insight 3, e123616. doi:10.1172/jci.insight.123616
Shah V. S., Ernst S., Tang X. X., Karp P. H., Parker C. P., Ostedgaard L. S., et al. (2016a). Relationships among CFTR expression, HCO3- secretion, and host defense may inform gene- and cell-based cystic fibrosis therapies. Proc. Natl. Acad. Sci. U. S. A. 113, 5382–5387. doi:10.1073/pnas.1604905113
Shah V. S., Meyerholz D. K., Tang X. X., Reznikov L., Abou Alaiwa M., Ernst S. E., et al. (2016b). Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science 351, 503–507. doi:10.1126/science.aad5589
Shan J., Liao J., Huang J., Robert R., Palmer M. L., Fahrenkrug S. C., et al. (2012). Bicarbonate-dependent chloride transport drives fluid secretion by the human airway epithelial cell line Calu-3. J. Physiol. 590, 5273–5297. doi:10.1113/jphysiol.2012.236893
Sheppard D. N., Carson M. R., Ostedgaard L. S., Denning G. M., Welsh M. J. (1994). Expression of cystic fibrosis transmembrane conductance regulator in a model epithelium. Am. J. Physiol. 266, L405–L413. doi:10.1152/ajplung.1994.266.4.L405
Sheppard D. N., Ostedgaard L. S., Winter M. C., Welsh M. J. (1995). Mechanism of dysfunction of two nucleotide binding domain mutations in cystic fibrosis transmembrane conductance regulator that are associated with pancreatic sufficiency. EMBO J. 14, 876–883. doi:10.1002/j.1460-2075.1995.tb07069.x
Sheppard D. N., Rich D. P., Ostedgaard L. S., Gregory R. J., Smith A. E., Welsh M. J. (1993). Mutations in CFTR associated with mild-disease-form Cl- channels with altered pore properties. Nature 362, 160–164. doi:10.1038/362160a0
Shteinberg M., Haq I. J., Polineni D., Davies J. C. (2021). Cystic fibrosis. Lancet 397, 2195–2211. doi:10.1016/s0140-6736(20)32542-3
Simonin J., Bille E., Crambert G., Noel S., Dreano E., Edwards A., et al. (2019). Airway surface liquid acidification initiates host defense abnormalities in Cystic Fibrosis. Sci. Rep. 9, 6516. doi:10.1038/s41598-019-42751-4
Singh P. K., Jia H. P., Wiles K., Hesselberth J., Liu L., Conway B. A., et al. (1998). Production of beta-defensins by human airway epithelia. Proc. Natl. Acad. Sci. U. S. A. 95, 14961–14966. doi:10.1073/pnas.95.25.14961
Smith J. J., Travis S. M., Greenberg E. P., Welsh M. J. (1996). Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 85, 229–236. doi:10.1016/s0092-8674(00)81099-5
Smith J. J., Welsh M. J. (1992). cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J. Clin. Investig. 89, 1148–1153. doi:10.1172/JCI115696
Smith L., Page R. C., Xu Z., Kohli E., Litman P., Nix J. C., et al. (2010). Biochemical basis of the interaction between cystic fibrosis transmembrane conductance regulator and immunoglobulin-like repeats of filamin. J. Biol. Chem. 285, 17166–17176. doi:10.1074/jbc.M109.080911
Song Y., Jayaraman S., Yang B., Matthay M. A., Verkman A. S. (2001). Role of aquaporin water channels in airway fluid transport, humidification, and surface liquid hydration. J. Gen. Physiol. 117, 573–582. doi:10.1085/jgp.117.6.573
Song Y., Salinas D., Nielson D. W., Verkman A. S. (2006). Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am. J. Physiol. Cell. Physiol. 290, C741–C749. doi:10.1152/ajpcell.00379.2005
Sorio C., Buffelli M., Angiari C., Ettorre M., Johansson J., Vezzalini M., et al. (2011). Defective CFTR expression and function are detectable in blood monocytes: development of a new blood test for cystic fibrosis. PLoS One 6, e22212. doi:10.1371/journal.pone.0022212
Sosnay P. R., Siklosi K. R., Van Goor F., Kaniecki K., Yu H., Sharma N., et al. (2013). Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat. Genet. 45, 1160–1167. doi:10.1038/ng.2745
Stoltz D. A., Meyerholz D. K., Welsh M. J. (2015). Origins of cystic fibrosis lung disease. N. Engl. J. Med. 372, 351–362. doi:10.1056/NEJMra1300109
Stutts M. J., Canessa C. M., Olsen J. C., Hamrick M., Cohn J. A., Rossier B. C., et al. (1995). CFTR as a cAMP-dependent regulator of sodium channels. Science 269, 847–850. doi:10.1126/science.7543698
Subbalakshmi C., Sitaram N. (1998). Mechanism of antimicrobial action of indolicidin. FEMS Microbiol. Lett. 160, 91–96. doi:10.1111/j.1574-6968.1998.tb12896.x
Sugiarto H., Yu P. L. (2007). Mechanisms of action of ostrich beta-defensins against Escherichia coli. FEMS Microbiol. Lett. 270, 195–200. doi:10.1111/j.1574-6968.2007.00642.x
Sun F., Hug M. J., Lewarchik C. M., Yun C. H., Bradbury N. A., Frizzell R. A. (2000). E3KARP mediates the association of ezrin and protein kinase A with the cystic fibrosis transmembrane conductance regulator in airway cells. J. Biol. Chem. 275, 29539–29546. doi:10.1074/jbc.M004961200
Tabcharani J. A., Linsdell P., Hanrahan J. W. (1997). Halide permeation in wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels. J. Gen. Physiol. 110, 341–354. doi:10.1085/jgp.110.4.341
Tamma G., Dossena S. (2022). Functional interplay between CFTR and pendrin: physiological and pathophysiological relevance. Front. Biosci. Landmark Ed. 27, 75. doi:10.31083/j.fbl2702075
Tang X. X., Ostedgaard L. S., Hoegger M. J., Moninger T. O., Karp P. H., McMenimen J. D., et al. (2016). Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J. Clin. Investig. 126, 879–891. doi:10.1172/JCI83922
Tarran R., Button B., Boucher R. C. (2006). Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu. Rev. Physiol. 68, 543–561. doi:10.1146/annurev.physiol.68.072304.112754
Tarran R., Button B., Picher M., Paradiso A. M., Ribeiro C. M., Lazarowski E. R., et al. (2005). Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J. Biol. Chem. 280, 35751–35759. doi:10.1074/jbc.M505832200
Taylor-Cousar J. L., Munck A., McKone E. F., van der Ent C. K., Moeller A., Simard C., et al. (2017). Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N. Engl. J. Med. 377, 2013–2023. doi:10.1056/NEJMoa1709846
Thornton C. S., Parkins M. D. (2023). Microbial epidemiology of the cystic fibrosis airways: past, present, and future. Semin. Respir. Crit. Care Med. 44, 269–286. doi:10.1055/s-0042-1758732
Thornton D. J., Sharpe C., Ridley C. (2018). Intracellular processing of human secreted polymeric airway mucins. Ann. Am. Thorac. Soc. 15, S154-S158. doi:10.1513/AnnalsATS.201802-143AW
Tummler B. (2023). Post-approval studies with the CFTR modulators elexacaftor-tezacaftor-ivacaftor. Front. Pharmacol. 14, 1158207. doi:10.3389/fphar.2023.1158207
Valdivieso A. G., Clauzure M., Massip-Copiz M. M., Cancio C. E., Asensio C. J. A., Mori C., et al. (2019). Impairment of CFTR activity in cultured epithelial cells upregulates the expression and activity of LDH resulting in lactic acid hypersecretion. Cell. Mol. Life Sci. 76, 1579–1593. doi:10.1007/s00018-018-3001-y
Van Goor F., Hadida S., Grootenhuis P. D., Burton B., Cao D., Neuberger T., et al. (2009). Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. U. S. A. 106, 18825–18830. doi:10.1073/pnas.0904709106
Verdugo P. (1990). Goblet cells secretion and mucogenesis. Annu. Rev. Physiol. 52, 157–176. doi:10.1146/annurev.ph.52.030190.001105
Verkman A. S., Song Y., Thiagarajah J. R. (2003). Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am. J. Physiol. Cell. Physiol. 284, C2–C15. doi:10.1152/ajpcell.00417.2002
Wadstrom T., Hisatsune K. (1970). Bacteriolytic enzymes from Staphylococcus aureus. Specificity of ction of endo-beta-N-acetylglucosaminidase. Biochem. J. 120, 735–744. doi:10.1042/bj1200735
Wang G., Nauseef W. M. (2022). Neutrophil dysfunction in the pathogenesis of cystic fibrosis. Blood 139, 2622–2631. doi:10.1182/blood.2021014699
Wang S., Yue H., Derin R. B., Guggino W. B., Li M. (2000). Accessory protein facilitated CFTR-CFTR interaction, a molecular mechanism to potentiate the chloride channel activity. Cell. 103, 169–179. doi:10.1016/s0092-8674(00)00096-9
Wark P., McDonald V. M. (2018). Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst. Rev. 9, CD001506. doi:10.1002/14651858.CD001506.pub4
Wenk M. R., Seelig J. (1998). Magainin 2 amide interaction with lipid membranes: calorimetric detection of peptide binding and pore formation. Biochemistry 37, 3909–3916. doi:10.1021/bi972615n
Widdicombe J. H., Widdicombe J. G. (1995). Regulation of human airway surface liquid. Respir. Physiol. 99, 3–12. doi:10.1016/0034-5687(94)00095-h
Willumsen N. J., Boucher R. C. (1991a). Sodium transport and intracellular sodium activity in cultured human nasal epithelium. Am. J. Physiol. 261, C319–C331. doi:10.1152/ajpcell.1991.261.2.C319
Willumsen N. J., Boucher R. C. (1991b). Transcellular sodium transport in cultured cystic fibrosis human nasal epithelium. Am. J. Physiol. 261, C332–C341. doi:10.1152/ajpcell.1991.261.2.C332
Xu Y., Szep S., Lu Z. (2009). The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc. Natl. Acad. Sci. U. S. A. 106, 20515–20519. doi:10.1073/pnas.0911412106
Yang D., Chertov O., Bykovskaia S. N., Chen Q., Buffo M. J., Shogan J., et al. (1999). Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286, 525–528. doi:10.1126/science.286.5439.525
Yu C., Kotsimbos T. (2023). Respiratory infection and inflammation in cystic fibrosis: a dynamic interplay among the host, microbes, and environment for the ages. Int. J. Mol. Sci. 24, 4052. doi:10.3390/ijms24044052
Yuan F., Gasser G. N., Lemire E., Montoro D. T., Jagadeesh K., Zhang Y., et al. (2023). Transgenic ferret models define pulmonary ionocyte diversity and function. Nature 621, 857–867. doi:10.1038/s41586-023-06549-9
Zabner J., Smith J. J., Karp P. H., Widdicombe J. H., Welsh M. J. (1998). Loss of CFTR chloride channels alters salt absorption by cystic fibrosis airway epithelia in vitro. Mol. Cell. 2, 397–403. doi:10.1016/s1097-2765(00)80284-1
Zhang X., Moore C. M., Harmacek L. D., Domenico J., Rangaraj V. R., Ideozu J. E., et al. (2022). CFTR-mediated monocyte/macrophage dysfunction revealed by cystic fibrosis proband-parent comparisons. JCI Insight 7, e152186. doi:10.1172/jci.insight.152186
Zhou Y., Song K., Painter R. G., Aiken M., Reiser J., Stanton B. A., et al. (2013). Cystic fibrosis transmembrane conductance regulator recruitment to phagosomes in neutrophils. J. Innate Immun. 5, 219–230. doi:10.1159/000346568
Keywords: cystic fibrosis, CFTR, bacterial infection, airway epithelia, mucus, bicarbonate
Citation: Wu M and Chen J-H (2024) CFTR dysfunction leads to defective bacterial eradication on cystic fibrosis airways. Front. Physiol. 15:1385661. doi: 10.3389/fphys.2024.1385661
Received: 16 February 2024; Accepted: 04 April 2024;
Published: 18 April 2024.
Edited by:
Ian Michael Thornell, The University of Iowa, United StatesReviewed by:
Deborah M. Cholon, University of North Carolina at Chapel Hill, United StatesCarlos A. Flores, Centro de Estudios Científicos, Chile
Copyright © 2024 Wu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeng-Haur Chen, amVuZy1oYXVyLWNoZW5AempudS5lZHUuY24=
 Min Wu
Min Wu Jeng-Haur Chen
Jeng-Haur Chen