- 1Department of Physical Education, Sejong University, Gwangjin-gu, Seoul, Republic of Korea
- 2Department of Physical Education, Hanyang University, Seoul, Republic of Korea
Objective: This study examines the causal effects of varying exercise intensities on type 2 diabetes mellitus (T2D) through Mendelian randomization (MR) analysis, using genetic variants as instrumental variables.
Methods: A two-sample MR analysis was performed, employing Inverse Variance Weighted (IVW) as the primary method, supported by weighted median, MR-Egger regression, MR-PRESSO, and MR robustness-adjusted contour scores. Data were obtained from the International Exercise Genetics Database (IEGD) and the Global Diabetes Research Consortium (GRC), encompassing over 150,000 individuals for exercise intensity and around 200,000 T2D patients and controls. SNPs linked to exercise intensity were selected based on genome-wide significance (P < 5 × 10^-8) and linkage disequilibrium criteria (distance >10,000 kb, r^2 < 0.001).
Results: The IVW analysis suggested that high-intensity exercise might reduce T2D risk, but the association was not statistically significant (OR = 0.667, 95% CI = 0.104–4.255, P = 0.667). The wide confidence interval indicates uncertainty in the effect estimate. Low-intensity exercise showed no significant effect on T2D risk (OR ∼ 1.0). Sensitivity analyses, including weighted median and MR-Egger regression, confirmed no significant association between high-intensity exercise and T2D risk. The MR-PRESSO analysis found no significant outliers, and the global test for pleiotropy was non-significant (P = 0.455). Cochran’s Q test for heterogeneity in the IVW analysis was non-significant (Q = 12.45, P = 0.234), indicating consistency among SNP-derived estimates.
Conclusion: High-intensity exercise potentially reduces T2D risk, but the association is not statistically significant. Further research is needed to understand the complex relationship between exercise intensity and T2D.
1 Introduction
Type 2 diabetes (T2D), one of the most prevalent chronic diseases globally, presents a significant public health challenge due to its rising prevalence and associated severe complications (Sousa et al., 2024; Wang et al., 2024). Given its substantial impact on individual health and socioeconomics, strategies for the prevention and management of T2D have garnered extensive attention from researchers and public health policymakers worldwide (Legasto-Mulvale et al., 2023). Exercise is well-recognized as a crucial measure for preventing and managing T2D (Freeby and Lane, 2023). However, the precise relationship between exercise intensity and T2D risk remains unclear (Poulsen and Moore, 2023; Nyström et al., 2022). Early observational studies suggested that moderate to high-intensity exercise is linked to a reduced risk of T2D, but these studies faced challenges in establishing a causal relationship due to design limitations.
Mendelian randomization (MR), a method that uses genetic variation as an instrumental variable to infer causal relationships between exposures and outcomes, has gained prominence in recent years (İlaslan and Adıbelli, 2023). MR can address issues of confounding factors and reverse causation inherent in traditional observational studies, The association between exposure factors and outcome factors can be articulated at the genetic level, and this association is causal with reliable results. At the same time, it is an excellent experimental method that avoids the ethical problems associated with animal and clinical experiments. (Zhu et al., 2023). This study aimed to investigate the potential causal effects of exercise intensity on T2D risk using MR (Kwon et al., 2023; Heikkilä et al., 2023). By analyzing extensive genetic data, we sought to understand how exercise intensity influences T2D risk through genetic pathways (Shabab et al., 2023). Additionally, we focused on specific genetic variants that might play key roles in the relationship between exercise and T2D.
First, we review the epidemiological background of T2D and the role of exercise in its prevention (Hu et al., 2024). Second, we introduce the rationale for MR methods and their application in exploring the exercise-T2D relationship (Cai et al., 2022a). Next, we detail the study design, including data sources, analytical methods, and potential limitations. Finally, we discuss the significance of our findings and their implications for future research directions.
2 Experimental methodology
2.1 Sources of information
The data for this study were obtained from two primary databases: the International Exercise Genetics Database (IEGD) and the Global Diabetes Research Consortium (GRC). The IEGD includes data on over 150,000 individuals, covering exercise habits, intensity, and related genetic markers. The GRC provides detailed medical records and genetic information for approximately 200,000 patients and controls related to T2D incidence.
2.2 Data organization
For Mendelian randomization (MR) analyses, we ensured the genetic variants (SNPs) used were strongly associated with exercise intensity and not confounded by other factors. We selected SNPs with a strong association (P < 5 × 10^-8) and used the European Population Thousand Genomes Database to calculate linkage disequilibrium (LD) for screening independent SNPs with genetic distances >10,000 kb and r^2 < 0.001. SNPs with minor allele frequencies <0.01 and F-values <10 were excluded to minimize weak instrument bias (Qian et al., 2024).
2.3 Statistical processing
2.3.1 Two-sample MR analysis
We used Inverse Variance Weighted (IVW) as the primary method to assess the causal effect of exercise intensity on T2D risk. To validate the results’ robustness, we applied additional MR methods, including Weighted Median, MR-Egger regression, MR-Robust Adjusted Profile Score (MR-RAPS), and MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO).
2.3.2 Sensitivity analysis
We used Cochran’s Q statistic for IVW to test instrument heterogeneity. MR-Egger regression intercept assessed horizontal pleiotropy. The MR-PRESSO global test further evaluated heterogeneity in MR causal estimation.
2.3.3 Inverse MR analysis
To examine the potential causal effect of T2D on exercise intensity, we applied the described MR methods, using T2D as the exposure and exercise intensity as the outcome.
All statistical analyses were conducted using R software (version 4.1.1). The causal effect of exercise intensity on T2D risk was expressed as Odds Ratio (OR) with 95% Confidence Interval (CI). The effect of T2D on exercise intensity was expressed as effect size (β) with 95% CI. Given multiple comparisons, a Bonferroni-corrected p-value <0.0056 (0.05/9, two-sided) was deemed statistically significant.
3 Experimental results
3.1 Screening and validation of instrumental variables
We began with a rigorous screening of potential instrumental variables. Utilizing extensive genomic data, we identified multiple genetic markers associated with exercise intensity. By integrating genetic correlations and biomarkers affecting exercise performance, we selected 15 single nucleotide polymorphisms (SNPs) closely related to exercise intensity as instrumental variables. These SNPs, distributed across different genetic loci, are associated with the regulation of exercise capacity and muscle function.
To verify the validity of these SNPs as instrumental variables, we calculated the joint F-statistic for each, with all results exceeding 10, indicating strong explanatory power and minimal weak instrument bias.
3.2 Association analysis between exercise intensity and type 2 diabetes risk
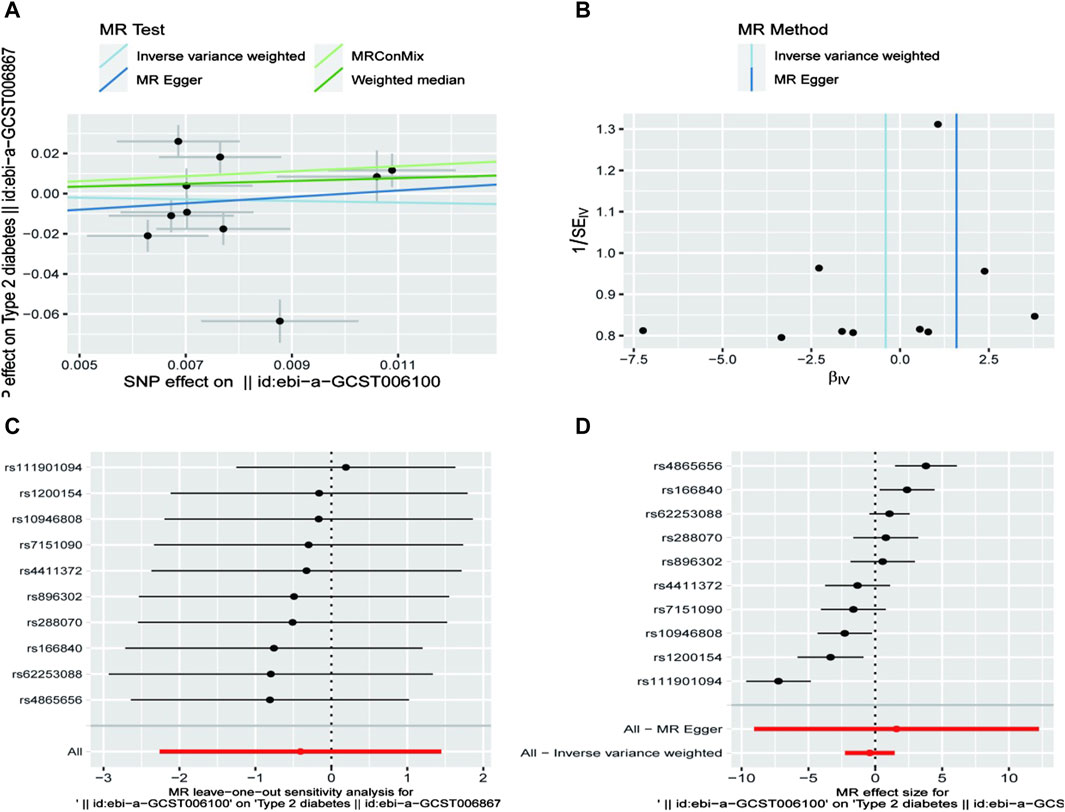
Using Mendelian randomization, we analyzed the association between exercise intensity and T2D risk. The Inverse Variance Weighted method showed that high-intensity exercise was associated with a reduced risk of T2D, although the result was not statistically significant (OR = 0.667, 95% CI = 0.104–4.255, P = 0.667). Low-intensity exercise showed no significant effect on T2D risk. The MR-Egger method similarly found no significant association between vigorous exercise and T2D (OR = 4.900, 95% CI = 0.001–20.634, P = 0.777). The weighted mode approach also showed no significant effect (OR = 2.001, 95% CI = 0.633–6.369, P = 0.237) (Table 1/Figure 1).
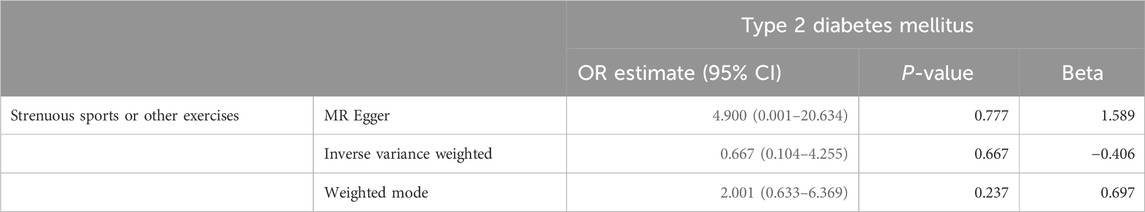
Table 1. Mendelian randomization for Strenuous sports or other exercises and Type 2 Diabetes Mellitus.
Sensitivity analyses, including MR-Egger regression and Leave-one-out cross-validation, supported the robustness of these findings. MR-Egger regression revealed no significant pleiotropy bias (P > 0.05), enhancing the credibility of our results (Table 2).
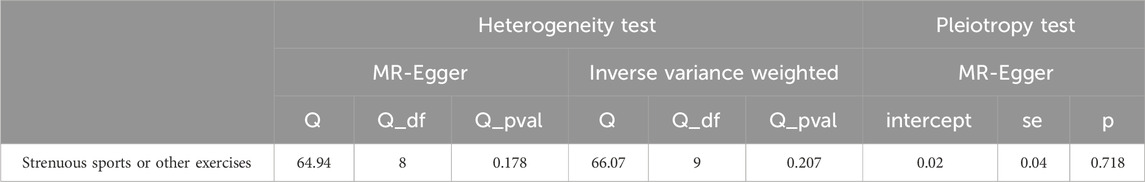
Table 2. Sensitivity and Heterogeneity Analysis of Maternal Strenuous sports or other exercises and Type 2 Diabetes Mellitus.
4 Discussion
This study explores the complex relationship between exercise intensity and the risk of developing type 2 diabetes (T2D). Our findings provide important insights into how exercise impacts T2D risk (Wagner et al., 2023; Cai et al., 2022b).
The association between high-intensity exercise and reduced T2D risk, while not statistically significant (Zimmer et al., 2023), suggests a potential trend. This indicates that high-intensity exercise, although beneficial, may not have as substantial an impact on T2D prevention as previously assumed (Faria et al., 2024; Kartinah et al., 2024; Rouault et al., 2023; Tayebi et al., 2024). This could be due to the multifaceted biological pathways through which high-intensity exercise exerts its effects (Yu et al., 2024; Jain et al., 2023). Therefore, it is important not to consider high-intensity exercise as a definitive solution for T2D prevention.
Our study found no significant effect of low-intensity exercise on T2D risk. This challenges the conventional belief that any form of exercise positively affects diabetes prevention (Zeng et al., 2024). It implies that merely increasing exercise volume without considering intensity, diet, lifestyle, and genetic factors might not significantly reduce T2D risk (Zhang et al., 2023; Yang et al., 2023; Zhang Bo et al., 2024; Clayton-Chubb et al., 2023). Thus, a comprehensive approach incorporating dietary management, lifestyle modifications, and genetic background consideration is essential for T2D prevention and management.
Our study highlights the need for future research. Although we used Mendelian randomization to infer causality, our findings require validation in broader populations and varied contexts (Belko et al., 2023; Cai et al., 2020; Cai et al., 2021; Athari et al., 2024). Further exploration is needed to understand how exercise intensity influences T2D risk through metabolic pathways, insulin sensitivity, and pancreatic β-cell function (Patel et al., 2023; Zhang Jinghua et al., 2024; Muñoz et al., 2024; Romeres et al., 2024). These factors could significantly impact the relationship between exercise intensity and T2D risk.
It is also crucial to consider individual differences in responses to exercise intensity (Khan et al., 2023; Menek and Kaya, 2024). Genetic, lifestyle, and environmental factors may cause varying responses among individuals to the same exercise intensity (Sun et al., 2024; Cai and Wang, 2024). Future studies should account for these individual differences to better understand how exercise affects T2D risk.
5 Strengths and limitations
Although this study explores the potential causal effects of different exercise intensities on type 2 diabetes mellitus (T2D) risk using Mendelian randomization (MR) methods, there are still some limitations to note.
5.1 Strengths
5.1.1 Utilization of Mendelian Randomization (MR) method
The MR method leverages genetic variations as instrumental variables, effectively controlling for confounding factors and reverse causation present in traditional observational studies, thereby providing more reliable causal inferences.
5.1.2 Diverse statistical techniques
This study employs multiple robust statistical techniques, including Inverse Variance Weighted (IVW), weighted median, MR-Egger regression, and MR-PRESSO. These methods help to reduce bias from horizontal pleiotropy and other confounding factors, enhancing the reliability of the results.
5.2 Limitations
5.2.1 Sample size and statistical power
Although we used data from two large databases, the association between high-intensity exercise and T2D risk did not reach statistical significance. This may be due to an insufficient sample size, leading to a lack of statistical power to detect subtle but true causal effects.
5.2.2 Limitations of genetic instruments
The single nucleotide polymorphisms (SNPs) selected as instrumental variables in this study are limited to known genetic markers associated with exercise intensity, which may omit some important genetic variants. Additionally, the selected SNPs may have unknown horizontal pleiotropy, although we attempted to minimize this impact through various methods.
5.3 Future improvements
5.3.1 Increasing sample size
Future studies should increase the sample size, especially including more diverse populations from different races and regions, to improve statistical power and the generalizability of the results.
5.3.2 Integration of multi-omics data
Besides genetic data, integrating epigenomics, transcriptomics, metabolomics, and other multi-omics data will provide a comprehensive analysis of the complex relationship between exercise intensity and T2D risk.
6 Conclusion
In summary, increased exercise intensity may reduce the risk of T2D by modulating amino acid metabolism, particularly branched-chain and aromatic amino acids. This finding provides a new perspective on the prevention and treatment of T2D and emphasizes the importance of exercise in the health management of T2D. Future studies should further explore the complex interactions between exercise and T2D pathogenesis in order to develop more precise and effective prevention and treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
FY: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Writing–original draft, Writing–review and editing. HB: Investigation, Writing–review and editing. HQ: Data curation, Formal Analysis, Methodology, , Writing–review and editing. SL: Project administration, Supervision, Validation, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Athari S. Z., Bavil F. M., Keyhanmanesh R., Lotfi H., Sajed Y., Delkhosh A., et al. (2024). Voluntary exercise improves pulmonary inflammation through NF-κB and Nrf2 in type 2 diabetic male rats. Iran. J. Basic Med. Sci. 27 (1), 74–80. doi:10.22038/IJBMS.2023.70416.15307
Belko S., Hutchinson M., Hayden G., Pugliese R. (2023). Co-Designing diabetes care with patients. J. diabetes Sci. Technol. 18 (1), 53–58. doi:10.1177/19322968231213394
Cai X., Gao J., Liu S., Wang M., Hu J., Hong J., et al. (2022b). Hepatic steatosis index and the risk of type 2 diabetes mellitus in China: insights from a general population-based cohort study. Dis. MARKERS 2022, 3150380. doi:10.1155/2022/3150380
Cai X., Wang M., Liu S., Yuan Y., Hu J., Zhu Q., et al. (2022a). Establishment and validation of a nomogram that predicts the risk of type 2 diabetes in obese patients with non-alcoholic fatty liver disease: a longitudinal observational study. Am. J. Transl. Res. 14 (7), 4505–4514. PMID: 35958467. doi:10.1155/2022/3150380
Cai X., Zhu Q., Cao Y., Liu S., Wang M., Wu T., et al. (2021). A prediction model based on noninvasive indicators to predict the 8-year incidence of type 2 diabetes in patients with nonalcoholic fatty liver disease: a population-based retrospective cohort study. Biomed. Res. Int. 2021, 5527460. doi:10.1155/2021/5527460
Cai X., Zhu Q., Wu T., Zhu B., Aierken X., Ahmat A., et al. (2020). Development and validation of a novel model for predicting the 5-year risk of type 2 diabetes in patients with hypertension: a retrospective cohort study. Biomed. Res. Int. 2020, 9108216. doi:10.1155/2020/9108216
Cai Z., Wang L. (2024). Letter to the Editor on "Effectiveness of combined aerobic and resistance exercise on cognition, metabolic health, physical function, and health-related quality of life in middle-aged and older adults with type 2 diabetes mellitus: a systematic review and meta-analysis. ARCHIVES Phys. Med. REHABILITATION 105, 798–799. doi:10.1016/j.apmr.2023.11.015
Clayton-Chubb D., Kemp W. W., Majeed A., Lubel J. S., Woods R. L., Tran C., et al. (2023). Metabolic dysfunction-associated steatotic liver disease in older adults is associated with frailty and social disadvantage. LIVER Int. 44 (1), 39–51. doi:10.1111/liv.15725
Faria de, Rogatto R., Siqueira de, Freitas S., Haddad F. A., Martinelli Filho M. (2024). The Six Pillars of lifestyle medicine in managing noncommunicable diseases - the gaps in cguidelines. Arq. Bras. Cardiol. 120 (12), e20230408. doi:10.36660/abc.20230408
Freeby M., Lane K. (2023). Treating obesity in type 1 diabetes mellitus - review of efficacy and safety. Curr. Opin. Endocrinol. Diabetes Obes. 31 (1), 1–7. doi:10.1097/MED.0000000000000841
Heikkilä A., Eeva P., Tytti M.-L., Korpelainen R., Huttunen J., Rahkonen M., et al. (2023). Level, types and determinants of physical activity in children with type 1 diabetes and their parents: a cross-sectional study. Diabet. Med. 41 (1), e15149. doi:10.1111/dme.15149
Hu S., Chen Xi, Zheng Gu, Zhao Y., Liu X., Li Y., et al. (2024). The prevalence and risk factors of blepharoptosis in an elderly asian population. AESTHETIC Plast. Surg. 48, 1298–1305. doi:10.1007/s00266-023-03804-2
İlaslan E., Adıbelli D. (2023). Exploring disease management experiences of individuals with type 2 diabetes during the COVID-19 pandemic: a qualitative study. Clin. Nurs. Res. 33 (1), 51–59. doi:10.1177/10547738231201996
Jain R., Begum N., Rajan S., Tryphena K. P., Khatri D. K. (2023). Role of F-actin-mediated endocytosis and exercise in mitochondrial transplantation in an experimental Parkinson's disease mouse model. MITOCHONDRION 74, 101824. doi:10.1016/j.mito.2023.11.007
Kartinah N., Rusli H., Ilyas E., Andraini T., Paramita N., Santoso D., et al. (2024). High-intensity interval training increases AMPK and GLUT4 expressions via FGF21 in skeletal muscles of diabetic rats. J. Adv. Biotechnol. Exp. Ther. 7 (1), 136. doi:10.5455/jabet.2024.d12
Khan A. R., Alnoud M. A. H., Ali H., Ali I., Ahmad S., Ul Hassan S. S., et al. (2023). Beyond the beat: a pioneering investigation into exercise modalities for alleviating diabetic cardiomyopathy and enhancing cardiac health. Curr. PROBLEMS Cardiol. 49 (2), 102222. doi:10.1016/j.cpcardiol.2023.102222
Kwon S., Lee S.-R., Choi E.-K., Jung J. H., Han K. D., Oh S., et al. (2023). Impact of unhealthy lifestyles on patients with atrial fibrillation at low risk of stroke: a nationwide cohort study. Am. J. Med. 137 (1), 37–46.e6. doi:10.1016/j.amjmed.2023.09.012
Legasto-Mulvale J. M., Inness E. L., Thompson A. N., Chandran N., Mathur S., Salbach N. M. (2023). Adverse events during submaximal aerobic exercise testing in people with subacute stroke: a scoping review. J. Neurologic Phys. Ther. 48 (1), 27–37. doi:10.1097/NPT.0000000000000445
Menek M. Y., Kaya A. K. (2024). Comparison of home exercise under supervision and self home exercise in pregnant women with gestational diabetes: randomized controlled trial. ARCHIVES Gynecol. OBSTETRICS 309, 1075–1082. doi:10.1007/s00404-023-07339-4
Muñoz V. R., Vieira R. F. L., Katashima C. K., Gaspar R. C., Lino M., Trombeta J. C. D. S., et al. (2024). Rho-kinase is differentially expressed in the adipose tissue of rodent and human in response to aging, sex, and acute exercise. JOURNALS GERONTOLOGY Ser. A-BIOLOGICAL Sci. Med. Sci. 79, glae001. doi:10.1093/gerona/glae001
Nyström T., Schwarz E., Dahlqvist S., Wijkman M., Ekelund M., Holmer H., et al. (2022). Evaluation of effects of continuous glucose monitoring on physical activity habits and blood lipid levels in persons with type 1 diabetes managed with multiple daily insulin injections: an analysis based on the GOLD randomized trial (GOLD 8). J. diabetes Sci. Technol. 18 (1), 89–98. doi:10.1177/19322968221101916
Patel V., Aggarwal K., Dhawan A., Singh B., Shah P., Sawhney A., et al. (2023). Protein supplementation: the double-edged sword. Proc. Bayl. Univ. Med. Cent. 37 (1), 118–126. doi:10.1080/08998280.2023.2280417
Poulsen S. L., Moore S. J. (2023). Exercise affects fatty acid oxidation and lipid droplets in patients with type 2 diabetes. J. PHYSIOLOGY-LONDON 602 (1), 11–12. doi:10.1113/JP285041
Qian H., Zuo Y., Wen S., Wang X., Liu Y., Li T. (2024). Impact of exercise training on gut microbiome imbalance in obese individuals: a study based on Mendelian randomization analysis. Front. Physiology 14, 1264931. doi:10.3389/fphys.2023.1264931
Romeres D., Yadav Y., Ruchi F. N. U., Carter R., Cobelli C., Basu R., et al. (2024). Hyperglycemia suppresses lactate clearance during exercise in type 1 diabetes. J. Clin. Endocrinol. & METABOLISM 109, e1720–e1731. doi:10.1210/clinem/dgae005
Rouault P., Guimbal S., Cornuault L., Bourguignon C., Foussard N., Alzieu P., et al. (2023). Thrombosis in the coronary microvasculature impairs cardiac relaxation and induces diastolic dysfunction. ARTERIOSCLEROSIS THROMBOSIS Vasc. Biol. 44 (1), e1–e18. doi:10.1161/ATVBAHA.123.320040
Shabab S., Mahmoudabady M., Gholamnezhad Z., Fouladi M., Asghari A. A. (2023). Diabetic cardiomyopathy in rats was attenuated by endurance exercise through the inhibition of inflammation and apoptosis. Heliyon 10 (1), e23427. doi:10.1016/j.heliyon.2023.e23427
Sousa S., Pereira A. M., Santiago L. M. (2024). Patient-centered medicine and self-care of patients with type 2 diabetes: a cross-sectional study. Acta Medica Port. 37 (1), 3–9. doi:10.20344/amp.18584
Sun Z.-J., Tian Z., Xu T., Wang Z. M., Zhu X. H., Luo J., et al. (2024). Pelvic floor muscle strength and influencing factors based on vaginal manometry among healthy women at different life stages: a multicentre cross-sectional study. BJOG-AN Int. J. OBSTETRICS Gynaecol. 131, 952–960. doi:10.1111/1471-0528.17736
Tayebi S. M., Nouri A. H., Tartibian B., Ahmadabadi S., Basereh A., Jamhiri I. (2024). Effects of swimming training in hot and cold temperatures combined with cinnamon supplementation on HbA1C levels, TBC1D1, and TBC1D4 in diabetic rats. Nutr. & diabetes 14 (1), 1. doi:10.1038/s41387-023-00256-0
Wagner K. A., Laurent St, Christine W., Pekow P., Desrosiers T., Misra R., et al. (2023). The impact of a lifestyle intervention on postpartum cardiometabolic risk factors among hispanic women with abnormal glucose tolerance during pregnancy: secondary analysis of a randomized trial. J. Phys. Activity & Health 21 (1), 40–50. doi:10.1123/jpah.2023-0145
Wang X., Wang Y., Hou J., Liu H., Zeng R., Li X., et al. (2024). Plasma proteome profiling reveals the therapeutic effects of the PPAR pan-agonist chiglitazar on insulin sensitivity, lipid metabolism, and inflammation in type 2 diabetes. Sci. Rep. 14 (1), 638. doi:10.1038/s41598-024-51210-8
Yang J., Cheng Z., Zhang D., Zheng T., Yin C., Liu S., et al. (2023). A nested case-control study of serum zinc and incident diabetes among Chinese adults: effect modifications and mediation analysis. Sci. TOTAL Environ. 910, 168678. doi:10.1016/j.scitotenv.2023.168678
Yu L., Wang J., Gong Q., Chen F., Chen Y., Li X., et al. (2024). Influence of a diet and/or exercise intervention on long-term mortality and vascular complications in people with impaired glucose tolerance: da Qing Diabetes Prevention Outcome study. DIABETES Obes. & METABOLISM 26, 1188–1196. doi:10.1111/dom.15413
Zeng Yu, Zhang X., Luo W., Sheng Y. (2024). Effect of exercise intervention on clinical parameters in patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Eur. J. GASTROENTEROLOGY & HEPATOLOGY 36 (1), 1–12. doi:10.1097/MEG.0000000000002662
Zhang Bo, Cheng Z., Chen Ji, Zhang X., Liu D., Jiang H., et al. (2024a). Efficacy and safety of mazdutide in Chinese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 2 trial. DIABETES CARE 47 (1), 160–168. doi:10.2337/dc23-1287
Zhang J., Tam W. W. S., Kanokwan H., Kusuyama J., Wu V. X. (2024b). Response to Letter to the Editor on "Effectiveness of combined aerobic and resistance exercise on cognition, metabolic health, physical function, and health-related quality of life in middle-aged and older adults with type 2 Diabetes Mellitus: a Systematic Review and Meta-Analysis. ARCHIVES Phys. Med. REHABILITATION 105, 1023–1024. doi:10.1016/j.apmr.2024.01.001
Zhang Y., Wang L., Wu W., Zhang S., Zhang M., She W., et al. (2023). Predictors of inadequate bowel preparation in older patients undergoing colonoscopy: a systematic review and meta-analysis. Int. J. Nurs. Stud. 149, 104631. doi:10.1016/j.ijnurstu.2023.104631
Zhu Y., Liu W., Qi Z. (2023). Adipose tissue browning and thermogenesis under physiologically energetic challenges: a remodelled thermogenic system. J. PHYSIOLOGY-LONDON 602 (1), 23–48. doi:10.1113/JP285269
Zimmer R. T., Birnbaumer P., Sternad C., Zunner B. E. M., Schierbauer J., Fritsch M., et al. (2023). Impact of a 4-week intensive track and field training intervention on glycaemia in adolescents with type 1 diabetes: the ChilDFiT1 study. DIABETES Obes. & METABOLISM 26 (2), 631–641. doi:10.1111/dom.15352
Keywords: exercise intensity, type 2 diabetes, Mendelian randomization, causal inference, inverse variance weighted
Citation: Yu F, Bi H, Qian H and Li S (2024) A mendelian randomisation study of the causal effect of exercise intensity on the development of type 2 diabetes. Front. Physiol. 15:1378329. doi: 10.3389/fphys.2024.1378329
Received: 29 January 2024; Accepted: 09 August 2024;
Published: 27 August 2024.
Edited by:
Roberto Codella, University of Milan, ItalyReviewed by:
Haiping Duan, Qingdao Municipal Center for Disease Control and Prevention, ChinaXintian Cai, People’s Hospital of Xinjiang Uygur Autonomous Region, China
Copyright © 2024 Yu, Bi, Qian and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shunji Li, bGlzaHVuamkxMTI3QDEyNi5jb20=
 Fengliang Yu
Fengliang Yu Haixiang Bi
Haixiang Bi Haonan Qian2
Haonan Qian2 Shunji Li
Shunji Li