
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 20 March 2024
Sec. Exercise Physiology
Volume 15 - 2024 | https://doi.org/10.3389/fphys.2024.1371638
Introduction: The purpose of this study was to discuss the causal relationship between physical activity and platelet traits.
Methods: A dataset from a large-scale European physical activity and platelet traits was collected by using Mendelian randomization of the study. For the analysis, the inverse variance weighting method, weighted median and MR-Egger were used to estimate causal effects. The sensitivity analyses were also performed using Cochran’s Q test, funnel plots and Leave-one-out analysis.
Results: Light DIY, other exercises, strenuous sports, walking for pleasure were significantly associated with a decrease in platelet crit. But none of the heavy /light DIY was associated with increase in platelet crit. Other exercises and strenuous sports were associated with decrease in platelet count.
Conclusion: Some types of physical activity have a causal relationship with platelet crit and platelet count. However, the types of physical activity we studied have not supported a causal relationship with mean platelet volume and platelet distribution width.
Platelets are produced by the bone marrow and play a crucial role in maintaining vascular integrity and preventing physiological and pathological processes such as hemorrhage (Salles et al., 2008), inflammation, tumor metastasis (Schiffer et al., 2018; Pogorzelska et al., 2020), in which functional characteristics are emerging and have important genetic implications (Bunimov et al., 2013). However, some of the traits of platelets may be altered due to several diseases, thereby increasing the likelihood of developing platelet-related disorders. By way of example, platelet counts are significantly lower in critically ill COVID-19 patients (Jiang et al., 2020; Lippi et al., 2020), and 58%–95% of critically ill patients suffer from mild thrombocytopenia (Fu et al., 2020; Levi et al., 2020; Thachil, 2020). Moreover, many diseases can affect platelet traits. The platelet count level is higher, and the mean platelet volume (MPV) is lower in patients with active disease (Zareifar et al., 2014). Reactive thrombocytosis and higher platelet specific volume and platelet distribution width (PDW) are frequent in tuberculosis disease, with a relationship between thrombocytosis and inflammatory response (Sahin et al., 2012). Therefore, improving platelet function and treating platelet-related disorders might be beneficial if platelet traits or platelet indices can be improved.
Many studies have been conducted to point out that physical activity, exercise, or other types of sports can have different effects on platelet counts and MPV. The average platelet volume decreases in hematological parameters measured after exercise (Boyali et al., 2018; Durmuş et al., 2021). Some studies have also shown increased MPV after exercise (Bachero-Mena et al., 2017; erdoğan, 2020). However, some findings have also demonstrated no change in MPV after exercise (Lundberg Slingsby et al., 2017; Tóth-Zsámboki et al., 2017; Heber et al., 2020). Likewise, there are different conclusions about changes in platelet counts after exercise, however more studies have obtained the result that platelet counts do not change after exercise training (Coppola et al., 2004; Wang and Chow, 2004; Bachero-Mena et al., 2017; Lundberg Slingsby et al., 2017; Boyali et al., 2018; erdoğan, 2020; Heber et al., 2020) and relatively few studies have obtained a decrease in platelet counts (Burri et al., 1996) and an increase in platelet counts (Tóth-Zsámboki et al., 2017). Findings from another study on PDW, one of the platelet traits, showed different results in different populations with varying exercise modalities or other sports programs. The difference in PDW between athletes and sedentary college students was not significant (p > 0.05) (Kırbaş et al., 2015). Healthy people also showed no substantial changes in PDW after long-distance runs such as marathons (Kratz et al., 2006). However, results from another study that analyzed hematological parameters in male handball players before and after a match showed decreased PDW after the (Koc et al., 2012). There are fewer studies on the effect of physical activity or exercise, compared to the first three indices, etc., on the platelet-specific volume. The studies that have been published are mostly cross-sectional and have shown that there is still ambivalence regarding the effect of exercise modalities such as physical activity on platelet traits, and the causal relationship between physical activity and platelet traits remains unclear.
Since the possibility of reverse causality and confounding cannot be completely ruled out using observational studies because of the inherent shortcomings of traditional designs, the chance of biased associations and conclusions is highly probable (Sekula et al., 2016). While Mendelian randomization (MR) can assess possible causality between modifiable exposures and outcomes using the genetic variants associated with them and minimize the potential for bias due to confounding and reverse-direction causality (Zhuang et al., 2020; Skrivankova et al., 2021). Given the uncertainty of the causal relationship between physical activity and platelet shape, for this study, we have assessed the potential causal effect of physical activity on four platelet traits (MPV, platelet crit, PDW, and platelet count) using an MR design with data from a Large Genome-Wide Association Study (GWAS).
Our two-sample MR study relied on summary-level data from the published and available OpenGWAS. No additional ethical approvals were required because the data were reanalyzed at the abstract level. MR was analyzed using genetic instrumental variables (IVs) to assess the causal relationship between exposure and outcome. The MR analysis was based on the following three core assumptions (as shown in Figure 1): 1) The selected IVs must be significantly associated with exposure (PA) (Lawlor et al., 2008; Sekula et al., 2016). 2) It is independent of the complexity of the exposure-outcome association (Pierce et al., 2011). 3) IV affects outcome only through exposure (PA) and has no other pathway to affect outcome (Pierce et al., 2011). The MR should be able to infer causality without unmeasured confounding and reverse causality when these assumptions are being fulfilled (Pierce et al., 2011).
GWAS summary statistics for exposure were obtained from the OpenGWAS database, which included 460,376 individuals of European ancestry (222,470 cases and 237,906 controls) (Table 1). GWAS data for platelet traits were obtained from a GWAS conducted on UK Biobank participants, which included 350,474 participants.
In this MR study, single nucleotide diversity (SNPs) of genome-wide significance were extracted from the GWAS database as IV. The correlation between instrumental variables and physical activity was assessed using broader thresholds (p < 1 × 10−5). The specific process of screening was as follows: 1) SNPs capable of being analyzed were extracted from the pooled data of different physical activity types over the past 4 weeks using the TwoSampleMR extension package of the R software. 2) SNPs with LDr2<0.01, kb = 5,000 were selected to avoid potential bias from solid linkage disequilibrium. 3) Remove the echo sequence. 4) Remaining SNPs were queried based on GWAS data for platelet traits. 5) The assessment of the strength of each genetic tool was materialized by calculating the F statistic, which was calculated as F = β exposure 2/SEexposure 2 (Yu et al., 2023). If F > 10, the selected IV has a solid potential for predicting platelet traits. The screening results and F-values are shown in Supplementary Material Excel S1–S6.
The primary analysis in our study was the inverse-variance weighting (IVW) method, which supposes that all instruments are valid (Burgess et al., 2013). Meanwhile, the weighted median (WM) and MR-Egger methods were used as supplementary analytical methods. According to the assumptions of the IVW method, the estimation of the total effect was realized based on the weighted average of the inverse variance of the instrumental variable effect estimates. IVW has maximum efficacy without horizontal polytomous bias (Burgess et al., 2013). Based on this, IVW can provide the most accurate assessment (Sekula et al., 2016). The WM method studies the bias caused by invalid IVs. If there are 50% invalid IVs, then WM can produce a robust estimate (Bowden et al., 2016). Although the MR-Egger method can study bias due to horizontal pleiotropy (Sanderson, 2021), it is possible that its estimates may not be accurate enough due to the effect of outlier genetic variation (Bowden et al., 2016). The method has the lowest statistical efficacy regarding detection effect (Bowden et al., 2015).
We also have analyzed the sensitivity analysis. Sensitivity analysis is an essential method for assessing potential heterogeneity and horizontal pleiotropy. Cochran’s Q statistic is a widely used tool for evaluating heterogeneity between studies in a meta-analysis (COCHRAN, 1950). This tool can also be used in MR analysis (Greco et al., 2015; Bowden et al., 2019). Meanwhile, leave-one-out analyses were performed to calculate the effect of eliminating one of the individual SNPs on the overall results. All statistical analyses were performed using RStudio version 4.3.1 with the R packages TwoSampleMR version 0.5.6 and Mendelian Randomization version 0.6.0.
First, SNPs were selected separately in the exposure selection for genes of different physical activity types, and the six sets of SNPs were picked, as shown in Table 1. The above-selected SNPs were harmonized with effector alleles from the platelet profile database (platelet count, platelet crit, MPV, and PDW), and six sets of genetic tools were selected to elucidate the genetic causality between PA and platelet profiles. Second, when horizontal diversity was present (p < 0.05) if it was significant for moderate horizontal diversity, then some SNPs were excluded from the outlier test process (p < 0.05) and reanalyzed. If heterogeneity was still substantial after reanalysis, all outliers were excluded. Third, the leave-one-out method was used to analyze whether SNPs still existed, which affected the stability of the results. A final and reliable conclusion could be drawn if SNPs that affected the results were not detected. This study was conducted according to the guidelines of the STROBE-MR statement.
As shown in Figure 2, IVW’s analysis showed that for the types of physical activity in the past 4 weeks, Light DIY (e.g., pruning, watering the lawn) [odds ratio (OR) = 0.9200,5% confidence interval (CI):0.8489-0.9971, p = 0.0421] was associated with a reduction in platelet crit in platelet profile.
Neither heavy DIY (e.g., weeding, lawn mowing, carpentry, digging) nor light DIY [OR = 1.6349, 95% CI: 1.3085-2.0427, p = 0.0000] was associated with increased platelet crit in the platelet profile. This result was the same as the analysis of WM (OR = 1.6002, 95% CI: 1.6562-2.1970, p = 0.0032).
Other exercises (e.g., swimming, cycling, keeping fit, bowling) (OR = 0.8555,95%CI:0.7898-0.9267, p = 0.0001) were associated with a decrease in platelet count and the same analyses were obtained with WM (OR = 0.8545,95% CI: 0.7641–0.855). And (OR = 0.7984,95%CI: 0.7384-0.8633, p = 0.0000) was also associated with a decrease in platelet crit, with the same results shown by WM (OR = 0.8093,95%CI:0.7294-0.8908, p = 0.0001).
Strenuous sports (OR = 0.7814,95%CI:0.6412-0.9521, p = 0.0144) were associated with decreased platelet count. Associated also with reduced platelet crit (OR = 0.6870,95CI:0.5691-0.8295, p = 0.0000), as analyzed by WM (OR = 0.7171,95%CI:0.5484-0.9377, p = 0.0121).
Walking for recreation (not as a means of transportation) was associated with decreased platelet crit (OR = 0.8363,95%CI:0.7599-0.9205, p = 0.0003).
The weighted median method for some of the results needs to be consistent with the primary analysis of the IVW method. A null hypothesis for half of the IVs weakens statistical efficacy. It can result in weak statistical effects and potentially false negatives. As another estimation method, the MR-Egger method may have led to poorer precision of the results because of its wider implementation interval. Moreover, the effect of outlier genetic variation has likely led to the estimation efficacy inconsistent with the IVW method. Based on these two points, the MR-Egger method was mainly used to assess horizontal pleiotropy during the study (The results of heterogeneity detection are shown in Figures 3, 4).
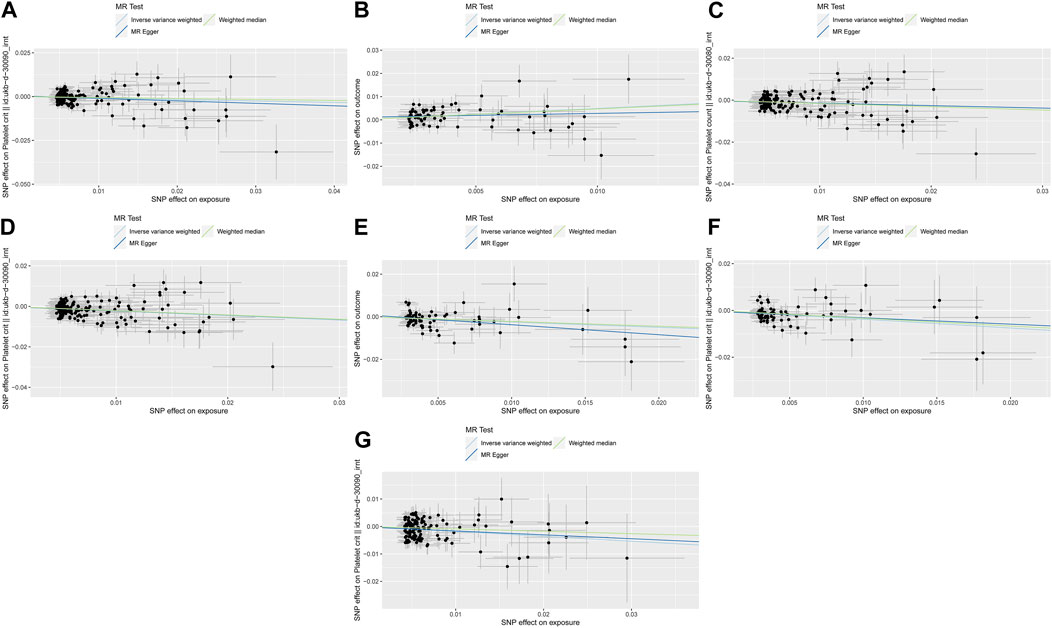
Figure 3. The three lines represent the estimated effect size. Light DIY and Platelet crit (A); None of the Heavy /Light DIY and Platelet crit (B); Other exercises and Platelet count (C) and Platelet crit (D); Strenuous sports and Platelet count (E) and Platelet crit (F); Walking for pleasure and Platelet crit (G).
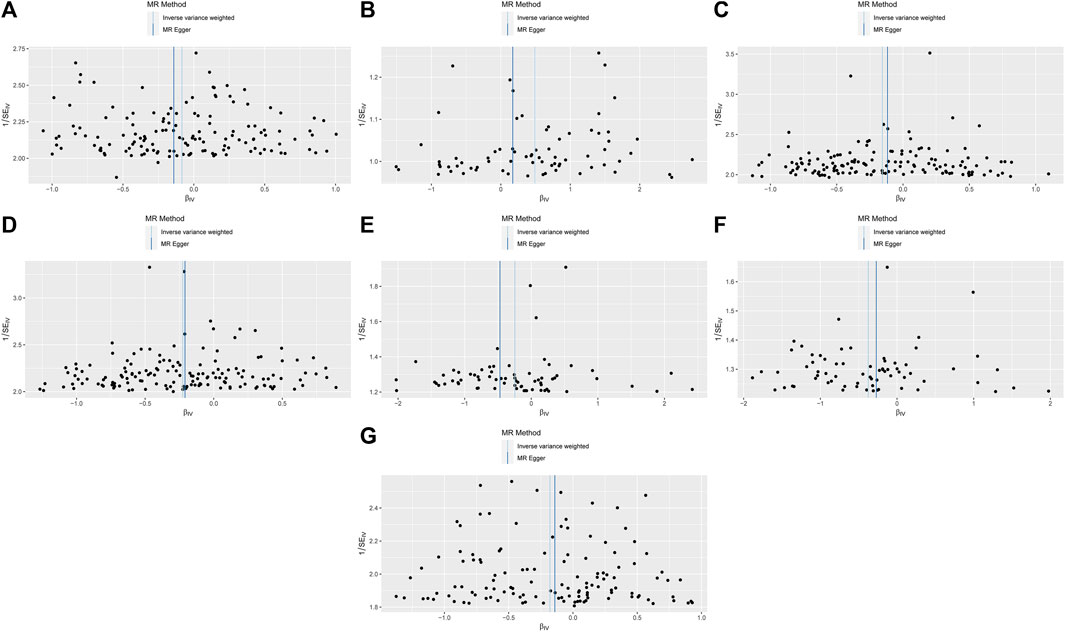
Figure 4. MR funnel-plot.Light DIY and Platelet crit (A); None of the Heavy /Light DIY and Platelet crit (B); Other exercises and Platelet count (C) and Platelet crit (D); Strenuous sports and Platelet count (E) and Platelet crit (F); Walking for pleasure and Platelet crit (G).
It eliminated the outliers that made the results unstable due to anomalies until a stable result was obtained. The sensitivity test results are shown in Figure 5. After reanalysis, based on the results of the heterogeneity and pleiotropy tests, as shown in Table 2, it is unlikely that there is heterogeneity and horizontal pleiotropy (p > 0.05). It also showed that the study’s results were almost unlikely to be affected by the effects of heterogeneity bias. The analysis of reverse MR showed no indication of an inverse relationship.
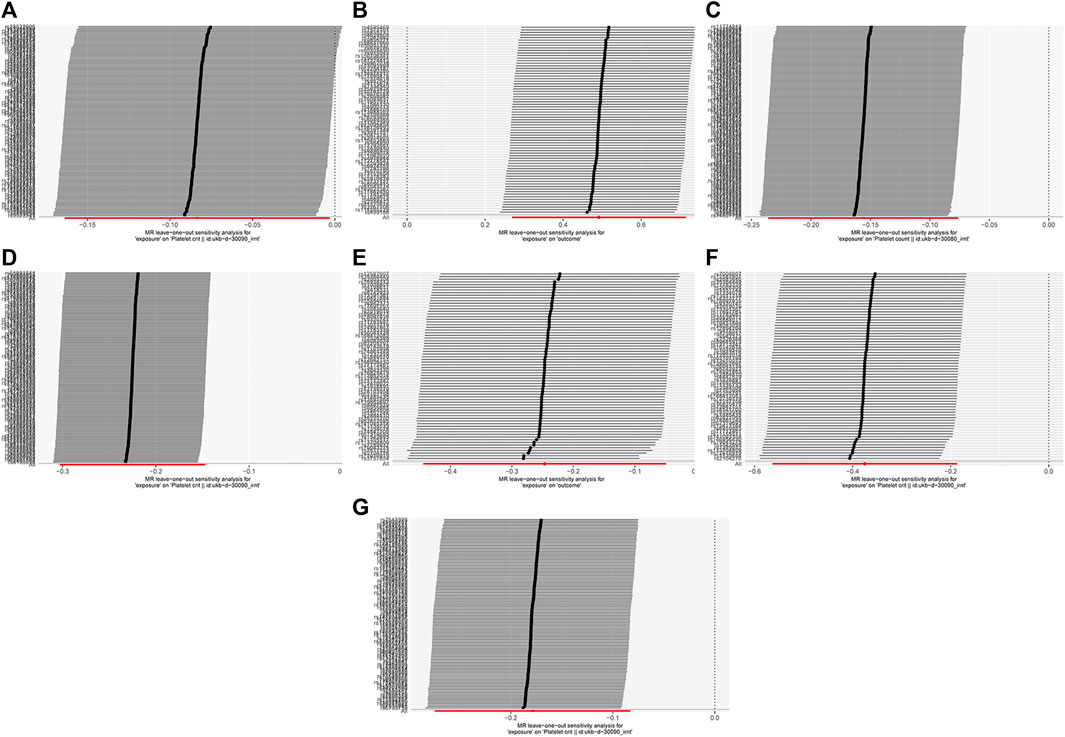
Figure 5. MR leave-one-out sensitivity analysis. Light DIY and Platelet crit (A); None of the Heavy /Light DIY and Platelet crit (B); Other exercises and Platelet count (C) and Platelet crit (D); Strenuous sports and Platelet count (E) and Platelet crit (F); Walking for pleasure and Platelet crit (G).
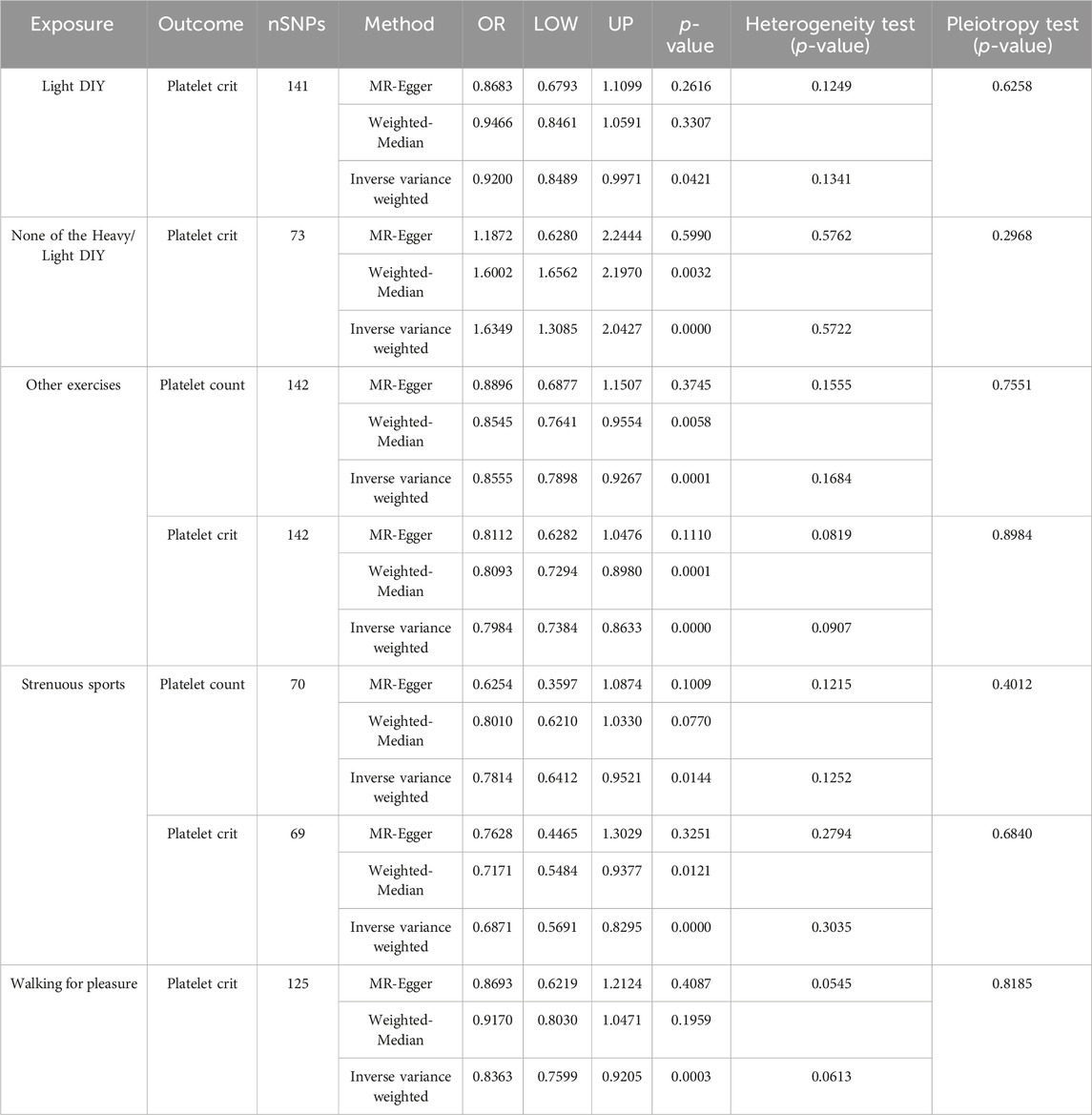
Table 2. Causal relationship and heterogeneity detection between physical activity and platelet traits.
This study showed that light DIY, other exercises, strenuous sports, and walking for pleasure were associated with decreased platelet crit (p < 0.05) in different physical activities in the past 4 weeks. Neither heavy nor light DIY was associated with an increase in Platelet crit (p < 0.05). Other exercises and strenuous sports were associated with decreased Platelet count (p < 0.05).
The other exercises and strenuous sports in our study were associated with a decrease in platelet count. A decrease in platelet count is generally defined as a decrease in the number of platelets per unit volume of blood. It was indicated in previous studies that acute exercise leads to a transient increase in platelet count. The mechanism of this increase may be due to the concentration of blood concentration and the release of platelets from the liver, lungs, and spleen (Chamberlain et al., 1990). However, swimming, cycling, keeping fit, bowling and strenuous sports have a different mode and intensity of exercise than the acute mode of exercise, which may be the result of the appearance of a decrease in platelet count rather than the underlying reasons for the increase. In addition, platelet mass in healthy subjects is kept constant by close adjustment, and MPV is negatively correlated with platelet counts (Wiwanitkit, 2004; Adibi et al., 2007; Margetic, 2012). There is substantial evidence that the major determinant of platelet counts is genetics (Daly, 2011), which means that platelet counts have a high genetic heritability (Buckley et al., 2000; Biino et al., 2011). Meanwhile, other acquisitional factors, such as physical activity, smoking status, and alcohol consumption, have been shown to change the platelet counts (Lippi et al., 2014; Hong et al., 2015).
Another characteristic is light DIY, other exercises, strenuous sports, walking for pleasure and None of the Heavy/Light DIY were respectively associated with a decrease and an increase in platelet crit. Platelet crit refers to the volume of platelets in blood volume occupied by platelets in the blood (Budak et al., 2016) and its results are influenced by both PLT and MPV (Giacomini et al., 2001; Chandrashekar, 2013). Thus, the amount of platelets in the blood is maintained in a state of equilibrium by regeneration and elimination under physiological conditions. The decrease in platelet crit due to physical activity or forms of exercise such as pruning, watering the lawn, swimming, walking for pleasure, etc., may be due to an isolated decrease in platelet count or MPV, or a simultaneous decrease in platelet crit. Neither heavy nor light physical activity implies that moderate physical activity may increase platelet crit. Based on the physiological mechanism of acute exercise-induced platelet count increase, it is hypothesized that appropriate physical activity, which shrinks blood concentration, results in an increase in the product of platelet count and MPV.
The physiologic impact of physical activity on platelet traits is a complex and multifactorial process. On the whole, the physiologic significance of moderate physical activity on platelet traits is to maintain moderate platelet numbers and function and to ensure a normal coagulation response. As a general rule, platelets have a lifespan of approximately 7–10 days (Allan et al., 2023). Physical activity helps stimulate the bone marrow to produce new platelets, which has a positive impact on the availability of sufficient numbers of new platelets to enter the circulatory system at the end of the platelet’s life span. The traits of the newly produced platelets are usually maintained within normal physiologic parameters. And moderate physical activity helps to promote good circulation (Duncker and Bache, 2008; De Ciuceis et al., 2023), which ensures that the newborn platelets can quickly get to where they are needed in the body, which is critical for maintaining proper platelet counts and maintaining coagulation. In addition, moderate physical activity induces the release of anticoagulant substances such as nitric oxide from the body, which helps to maintain the thinning of the blood and reduces the potential for platelet adhesion and aggregation within the blood vessels (Barale et al., 2023). It is essential to note that excessive physical activity may hurt platelet longevity and function, for example, platelet index and MPV, among others, can serve as markers of platelet degranulation/activation (Yetkin, 2008) when the release of platelets is not normal, which may be indicative of platelet overactivation. Transitional activation of platelets is more likely to be cleared or consumed more rapidly in the circulation due to aberrant activation, resulting in a reduced lifespan. Therefore, moderate and sustained physical activity may be more conducive to maintaining good platelet traits and platelet physiology.
This is the first MR study to examine the causal relationship between physical activity and platelet shape. The main strengths of this study are as follows: First, we have adopted the MR analysis method, which can effectively avoid the interference of confounding factors and reverse causality. Second, we rigorously screened SNPs to assure the independence of IVs and included tools with more powerful genetic functions. Third, most MR analyses reinforce the requirement for consistent β-direction in MR methods, and such consistency was ensured in our study.
Nevertheless, our study has some limitations. First, our study was conducted on a European group, and no differences between genders were distinguished, so it cannot be immediately generalized to other ethnic groups with different cultural backgrounds. Second, DNA methylation, RNA editing, and inactive transposons remain unavoidable problems for MR analysis.
Our findings support a causal relationship between partial physical activity type and partial platelet traits. For future studies, if more effective methods can be found to reduce bias, more extensive GWAS pooled data are needed to validate our results using MR analysis again. Furthermore, by conducting relevant studies in different ethnic populations from different cultural backgrounds, implementing dynamic monitoring of platelet traits, and further exploring the physiological mechanism and biological mechanisms by which physical activity affects their changes, it can be better applied to clinical research.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
MJ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. ZW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. FH: Investigation, Supervision, Validation, Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2024.1371638/full#supplementary-material
Adibi P., Faghih Imani E., Talaei M., Ghanei M. (2007). Population-based platelet reference values for an Iranian population. Int. J. Lab. Hematol. 29 (3), 195–199. doi:10.1111/j.1751-553X.2006.00843.x
Allan H. E., Vadgama A., Armstrong P. C., Warner T. D. (2023). Platelet ageing: a review. Thromb. Res. 231, 214–222. doi:10.1016/j.thromres.2022.12.004
Bachero-Mena B., Pareja-Blanco F., González-Badillo J. J. (2017). Enhanced strength and sprint levels, and changes in blood parameters during a complete athletics season in 800 m high-level athletes. Front. Physiol. 8, 637. doi:10.3389/fphys.2017.00637
Barale C., Melchionda E., Tempesta G., Morotti A., Russo I. (2023). Impact of physical exercise on platelets: focus on its effects in metabolic chronic diseases. Antioxidants (Basel) 12 (8), 1609. doi:10.3390/antiox12081609
Biino G., Balduini C. L., Casula L., Cavallo P., Vaccargiu S., Parracciani D., et al. (2011). Analysis of 12,517 inhabitants of a Sardinian geographic isolate reveals that predispositions to thrombocytopenia and thrombocytosis are inherited traits. Haematologica 96 (1), 96–101. doi:10.3324/haematol.2010.029934
Bowden J., Davey Smith G., Burgess S. (2015). Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44 (2), 512–525. doi:10.1093/ije/dyv080
Bowden J., Davey Smith G., Haycock P. C., Burgess S. (2016). Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40 (4), 304–314. doi:10.1002/gepi.21965
Bowden J., Del Greco M. F., Minelli C., Zhao Q., Lawlor D. A., Sheehan N. A., et al. (2019). Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int. J. Epidemiol. 48 (3), 728–742. doi:10.1093/ije/dyy258
Boyali E., Sevindi T., Yüksel M., Demir H. (2018). The effects of preparation period exercises on the hematological parameters of the taekwondo athletes. Phys. Educ. students 23, 9–15. doi:10.15561/20755279.2019.0102
Buckley M., James J., Brown D., Whyte G., Dean M., Chesterman C., et al. (2000). A novel approach to the assessment of variations in the human platelet count. Thrombosis haemostasis 83, 480–484. doi:10.1055/s-0037-1613840
Budak Y. U., Polat M., Huysal K. (2016). The use of platelet indices, plateletcrit, mean platelet volume and platelet distribution width in emergency non-traumatic abdominal surgery: a systematic review. Biochem. Med. Zagreb. 26 (2), 178–193. doi:10.11613/bm.2016.020
Bunimov N., Fuller N., Hayward C. P. (2013). Genetic loci associated with platelet traits and platelet disorders. Semin. Thromb. Hemost. 39 (3), 291–305. doi:10.1055/s-0033-1334466
Burgess S., Butterworth A., Thompson S. G. (2013). Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37 (7), 658–665. doi:10.1002/gepi.21758
Burri B. J., Van Loan M., Keim N. L. (1996). Moderate exercise training and low-energy diets are associated with small changes in indices of platelet aggregation and blood coagulation in overweight women. Nutr. Res. 16 (9), 1451–1458. doi:10.1016/0271-5317(96)00157-1
Chamberlain K. G., Tong M., Penington D. G. (1990). Properties of the exchangeable splenic platelets released into the circulation during exercise-induced thrombocytosis. Am. J. Hematol. 34 (3), 161–168. doi:10.1002/ajh.2830340302
Chandrashekar V. (2013). Plateletcrit as a screening tool for detection of platelet quantitative disorders. J. Hematol. 2, 22–26. doi:10.4021/jh70w
Cochran W. G. (1950). The comparison of percentages in matched samples. Biometrika 37 (3-4), 256–266. doi:10.1093/biomet/37.3-4.256
Coppola L., Grassia A., Coppola A., Tondi G., Peluso G., Mordente S., et al. (2004). Effects of a moderate-intensity aerobic program on blood viscosity, platelet aggregation and fibrinolytic balance in young and middle-aged sedentary subjects. Blood Coagul. Fibrinolysis 15 (1), 31–37. doi:10.1097/00001721-200401000-00006
Daly M. E. (2011). Determinants of platelet count in humans. Haematologica 96 (1), 10–13. doi:10.3324/haematol.2010.035287
De Ciuceis C., Rizzoni D., Palatini P. (2023). Microcirculation and physical exercise in hypertension. Hypertension 80 (4), 730–739. doi:10.1161/HYPERTENSIONAHA.122.19465
Duncker D. J., Bache R. J. (2008). Regulation of coronary blood flow during exercise. Physiol. Rev. 88 (3), 1009–1086. doi:10.1152/physrev.00045.2006
Durmuş İ., Kalaycıoğlu E., Çetin M., Şahin H. B., Kırış T. (2021). Exercise-based cardiac rehabilitation has a strong relationship with mean platelet volume reduction. Arq. Bras. Cardiol. 116 (3), 434–440. doi:10.36660/abc.20190514
erdoğan R. (2020). Effects of endurance workouts on thyroid hormone metabolism and biochemical markers in athletes. Brain Broad Res. Artif. Intell. Neurosci. 11, 136–146. doi:10.18662/brain/11.3/114
Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T., et al. (2020). Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J. Infect. 80 (6), 656–665. doi:10.1016/j.jinf.2020.03.041
Giacomini A., Legovini P., Gessoni G., Antico F., Valverde S., Salvadego M. M., et al. (2001). Platelet count and parameters determined by the Bayer ADVIA 120 in reference subjects and patients. Clin. Lab. Haematol. 23 (3), 181–186. doi:10.1046/j.1365-2257.2001.00391.x
Greco M. F., Minelli C., Sheehan N. A., Thompson J. R. (2015). Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med. 34 (21), 2926–2940. doi:10.1002/sim.6522
Heber S., Fischer B., Sallaberger-Lehner M., Hausharter M., Ocenasek H., Gleiss A., et al. (2020). Effects of high-intensity interval training on platelet function in cardiac rehabilitation: a randomised controlled trial. Heart 106 (1), 69–79. doi:10.1136/heartjnl-2019-315130
Hong J., Min Z., Bai-shen P., Jie Z., Ming-ting P., Xian-zhang H., et al. (2015). Investigation on reference intervals and regional differences of platelet indices in healthy Chinese Han adults. J. Clin. Lab. Anal. 29 (1), 21–27. doi:10.1002/jcla.21721
Jiang S. Q., Huang Q. F., Xie W. M., Lv C., Quan X. Q. (2020). The association between severe COVID-19 and low platelet count: evidence from 31 observational studies involving 7613 participants. Br. J. Haematol. 190 (1), e29–e33. doi:10.1111/bjh.16817
Kırbaş Ş., Tetik Dündar S., Aaykora E., Duran B. (2015). An examination of the impact of regular exercise participation on blood platelet parameters. World J. Med. Sci. 12, 79–82. doi:10.5829/idosi.wjms.2015.12.2.9330
Koc H., Tekin A., Ozturk A., Saraymen R., Gokdemir K., Eliöz M. (2012). The effect of acute exercises on blood hematological parameters in handball players. Afr. J. Microbiol. Res. 6, 2027–2032. doi:10.5897/AJMR-11-1247
Kratz A., Wood M. J., Siegel A. J., Hiers J. R., Van Cott E. M. (2006). Effects of marathon running on platelet activation markers: direct evidence for in vivo platelet activation. Am. J. Clin. Pathol. 125 (2), 296–300. doi:10.1309/prf5-n7p2-xm6e-243h
Lawlor D. A., Harbord R. M., Sterne J. A., Timpson N., Davey Smith G. (2008). Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27 (8), 1133–1163. doi:10.1002/sim.3034
Levi M., Thachil J., Iba T., Levy J. H. (2020). Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 7 (6), e438–e440. doi:10.1016/s2352-3026(20)30145-9
Lippi G., Plebani M., Henry B. M. (2020). Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta 506, 145–148. doi:10.1016/j.cca.2020.03.022
Lippi G., Salvagno G. L., Danese E., Skafidas S., Tarperi C., Guidi G. C., et al. (2014). Mean platelet volume (MPV) predicts middle distance running performance. PLoS One 9 (11), e112892. doi:10.1371/journal.pone.0112892
Lundberg Slingsby M. H., Nyberg M., Egelund J., Mandrup C. M., Frikke-Schmidt R., Kirkby N. S., et al. (2017). Aerobic exercise training lowers platelet reactivity and improves platelet sensitivity to prostacyclin in pre- and postmenopausal women. J. Thromb. Haemost. 15 (12), 2419–2431. doi:10.1111/jth.13866
Margetic S. (2012). Inflammation and hemostasis. Biochem. medica 22 (1), 49–62. doi:10.11613/BM.2012.006
Pierce B. L., Ahsan H., Vanderweele T. J. (2011). Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 40 (3), 740–752. doi:10.1093/ije/dyq151
Pogorzelska K., Krętowska A., Krawczuk-Rybak M., Sawicka-Żukowska M. (2020). Characteristics of platelet indices and their prognostic significance in selected medical condition - a systematic review. Adv. Med. Sci. 65 (2), 310–315. doi:10.1016/j.advms.2020.05.002
Sahin F., Yazar E., Yıldız P. (2012). Prominent features of platelet count, plateletcrit, mean platelet volume and platelet distribution width in pulmonary tuberculosis. Multidiscip. Respir. Med. 7 (1), 38. doi:10.1186/2049-6958-7-38
Salles I. I., Feys H. B., Iserbyt B. F., De Meyer S. F., Vanhoorelbeke K., Deckmyn H. (2008). Inherited traits affecting platelet function. Blood Rev. 22 (3), 155–172. doi:10.1016/j.blre.2007.11.002
Sanderson E. (2021). Multivariable mendelian randomization and mediation. Cold Spring Harb. Perspect. Med. 11 (2), a038984. doi:10.1101/cshperspect.a038984
Schiffer C. A., Bohlke K., Delaney M., Hume H., Magdalinski A. J., McCullough J. J., et al. (2018). Platelet transfusion for patients with cancer: American society of clinical oncology clinical practice guideline update. J. Clin. Oncol. 36 (3), 283–299. doi:10.1200/jco.2017.76.1734
Sekula P., Del Greco M. F., Pattaro C., Köttgen A. (2016). Mendelian randomization as an approach to assess causality using observational data. J. Am. Soc. Nephrol. 27 (11), 3253–3265. doi:10.1681/asn.2016010098
Skrivankova V. W., Richmond R. C., Woolf B. A. R., Yarmolinsky J., Davies N. M., Swanson S. A., et al. (2021). Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. Jama 326 (16), 1614–1621. doi:10.1001/jama.2021.18236
Thachil J. (2020). What do monitoring platelet counts in COVID-19 teach us? J. Thromb. Haemost. 18 (8), 2071–2072. doi:10.1111/jth.14879
Tóth-Zsámboki E., Horváth Z., Hajtman L., Leé S., Pállinger É., Kuklis E., et al. (2017). Cardiac rehabilitation programme as a non-pharmacological platelet inhibitory tool in acute coronary syndrome survivors. Eur. J. Prev. Cardiol. 24 (11), 1148–1156. doi:10.1177/2047487317704937
Wang J. S., Chow S. E. (2004). Effects of exercise training and detraining on oxidized low-density lipoprotein-potentiated platelet function in men. Arch. Phys. Med. Rehabil. 85 (9), 1531–1537. doi:10.1016/j.apmr.2003.08.112
Wiwanitkit V. (2004). Plateletcrit, mean platelet volume, platelet distribution width: its expected values and correlation with parallel red blood cell parameters. Clin. Appl. Thromb. Hemost. 10 (2), 175–178. doi:10.1177/107602960401000208
Yetkin E. (2008). Mean platelet volume not so far from being a routine diagnostic and prognostic measurement. Thromb. Haemost. 100 (1), 3–4. doi:10.1160/th08-05-0336
Yu H., Wan X., Yang M., Xie J., Xu K., Wang J., et al. (2023). A large-scale causal analysis of gut microbiota and delirium: a Mendelian randomization study. J. Affect Disord. 329, 64–71. doi:10.1016/j.jad.2023.02.078
Zareifar S., Farahmand Far M. R., Golfeshan F., Cohan N. (2014). Changes in platelet count and mean platelet volume during infectious and inflammatory disease and their correlation with ESR and CRP. J. Clin. Lab. Anal. 28 (3), 245–248. doi:10.1002/jcla.21673
Keywords: physical activity, platelet traits, Mendelian randomization, causal relation, physiological indices
Citation: Jia M, Wang Z and Hu F (2024) Causal relationship between physical activity and platelet traits: a Mendelian randomization study. Front. Physiol. 15:1371638. doi: 10.3389/fphys.2024.1371638
Received: 16 January 2024; Accepted: 28 February 2024;
Published: 20 March 2024.
Edited by:
Diego Fernández Lázaro, University of Valladolid, SpainReviewed by:
Desheng Zhu, Shanghai Jiao Tong University, ChinaCopyright © 2024 Jia, Wang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyong Wang, d3p5MzYxMEAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.