
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol., 07 February 2024
Sec. Cell Physiology
Volume 15 - 2024 | https://doi.org/10.3389/fphys.2024.1362987
This article is part of the Research TopicDirect Modulation of Ion Channels by G-proteinsView all 6 articles
GPCR-Gi protein pathways are involved in the regulation of vagus muscarinic pathway under physiological conditions and are closely associated with the regulation of internal visceral organs. The muscarinic receptor-operated cationic channel is important in GPCR-Gi protein signal transduction as it decreases heart rate and increases GI rhythm frequency. In the SA node of the heart, acetylcholine binds to the M2 receptor and the released Gβγ activates GIRK (I(K,ACh)) channel, inducing a negative chronotropic action. In gastric smooth muscle, there are two muscarinic acetylcholine receptor (mAChR) subtypes, M2 and M3. M2 receptor activates the muscarinic receptor-operated nonselective cationic current (mIcat, NSCC(ACh)) and induces positive chronotropic effect. Meanwhile, M3 receptor induces hydrolysis of PIP2 and releases DAG and IP3. This IP3 increases intracellular Ca2+ and then leads to contraction of GI smooth muscles. The activation of mIcat is inhibited by anti-Gi/o protein antibodies in GI smooth muscle, indicating the involvement of Gαi/o protein in the activation of mIcat. TRPC4 channel is a molecular candidate for mIcat and can be directly activated by constitutively active GαiQL proteins. TRPC4 and TRPC5 belong to the same subfamily and both are activated by Gi/o proteins. Initial studies suggested that the binding sites for G protein exist at the rib helix or the CIRB domain of TRPC4/5 channels. However, recent cryo-EM structure showed that IYY58-60 amino acids at ARD of TRPC5 binds with Gi3 protein. Considering the expression of TRPC4/5 in the brain, the direct G protein activation on TRPC4/5 is important in terms of neurophysiology. TRPC4/5 channels are also suggested as a coincidence detector for Gi and Gq pathway as Gq pathway increases intracellular Ca2+ and the increased Ca2+ facilitates the activation of TRPC4/5 channels. More complicated situation would occur when GIRK, KCNQ2/3 (IM) and TRPC4/5 channels are co-activated by stimulation of muscarinic receptors at the acetylcholine-releasing nerve terminals. This review highlights the effects of GPCR-Gi protein pathway, including dopamine, μ-opioid, serotonin, glutamate, GABA, on various oragns, and it emphasizes the importance of considering TRPC4/5 channels as crucial players in the field of neuroscience.
GPCR-Gi protein pathways are involved in the regulation of vagus muscarinic pathway under physiological conditions and are closely associated with the regulation of internal visceral organs. The muscarinic receptor-operated cationic channel is important in GPCR-Gi protein signal transduction as it decreases heart rate and increases gastrointestinal (GI) rhythm frequency. Among five muscarinic acetylcholine receptors (mAChRs)—M1 to M5—, M2 and M4 receptors primarily utilize Gi/o signaling. The M2 receptor, in particular, mediate the effects of parasympathetic stimulation on the heart and GI organs. The most significant involvement among receptor-operated ion channels in this Gi-related process is definitely that of TRPC channels, especially TRPC4/5 channels.
To begin with, the TRP channel superfamily, comprising 28 mammalian cation channels across seven subfamilies—TRPC, TRPV, TRPA, TRPM, TRPP, TRPN and TRPML (Minke et al., 1975; Zhang et al., 2003; Zhang et al., 2023). Within the subfamilies, TRPC is known to be activated by PLC signaling pathways that lead to membrane depolarization and the elevation in cytosolic Ca2+ concentration. Among the various kinds of PLC signaling pathways, the Gq/11-PLCβ and receptor tyrosine kinase (RTK)-PLCγ pathways are the most commonly known. TRPC ion channels are non-selective cation channels with variable ion selectivity and Ca2+ permeability. These receptor-operated channels affects membrane potential and Ca2+ signaling in different ways to regulate the physiological conditions (Jeon et al., 2020a). In addition to activation by PLC signaling, direct activation by Gαi is known only for TRPC4/5 channels. Previous studies have shown that Gαi2 prefers to bind with TRPC4 whereas Gαi3 prefers TRPC5 (Jeon et al., 2008; Jeon et al., 2012). Recently, the dual activation of TRPC4 by both Gi and Gq signaling pathways has been recognized significant in brain (Yang et al., 2015; Jeon et al., 2020b; Tian et al., 2022). TRPC4 activation requires coincident Gi/o stimulation as well as PLC activity (Thakur et al., 2016). Neurons encode distinct messages that reflect the activation of two ion channels, TRPC4 and GIRK, through coincident Gq/11 and Gi/o signaling, transmitting the messages to downstream neurons (Tian et al., 2022).
As cryo-EM structure of TRPC4 and TRPC5 ion channels have been revealed, both channels came out to have similar binding sites−TRPC4/5 activators and inhibitors such as Pico145, Riluzole, HC-070, clemizole, PIP2, etc (see also Figure 6)− as their structure significantly overlaps (Duan et al., 2018; Duan et al., 2019; Won et al., 2023). Moreover, several features in intracellular regions of TRPC channels are conserved: the pre-S1 elbow is situated in the N-terminal domain, and the connecting helix runs parallel to the membrane bilayer. However, the binding interface with Gαi protein was conserved only in the N-terminal ankyrin repeat domain (ARD) of TRPC4 and TRPC5 channels, which means both channels may be the only direct modulators for Gα proteins in TRP subfamily (Won et al., 2023). The binding interface of Gα protein with its effector molecules was also found to be conserved in the Gαi-bound TRPC5 cryo-EM structure (Lyon et al., 2013; Won et al., 2023). In conjunction with the electrophysiological result demonstrating that Gαi3 increases the sensitivity of TRPC5 to phosphatidylinositol 4,5-bisphosphate (PIP2), this structural discovery provides evidence that ion channel activity can be directly regulated by Gα protein following GPCR activation. This finding may offer a structural framework for unraveling the crosstalk between two major classes of transmembrane proteins: GPCRs and ion channels. In this review, we specify the G protein related pathway and the direct relationship with TRPC ion channels in various internal organs. Also, possible drug development and disease control studies are introduced by targeting the GPCR-Gi-TRPC4/5 pathway.
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. The binding and hydrolysis of GTP to GDP, facilitated by specific regulatory factors, govern the activity of these molecules. When in the GTP-bound state, the switch turns on, and, when in the GDP-bound state, the switch turns off. The shutdown of the G protein cascade is possible due to the intrinsic GTPase activity of G proteins, as they belong to the larger group of enzymes called GTPases.
There are two classes of G proteins. The first class functions as monomeric small GTPases (small G proteins), while the second class functions as heterotrimeric G protein complexes. Small G proteins (also known as small GTPases, small GTP binding proteins and Ras protein superfamily) form an independent superfamily within the larger class of regulatory GTP hydrolases. This superfamily is made up of a diverse range of molecules that control a vast number of important processes and possess a common, structurally preserved GTP-binding domain (Agretti et al., 2007). The small G protein superfamily consists of Ras, Rho Rab, Rac, Sarl/Arf and Ran homologs. Within the family of small G proteins, Ras proteins are identified as the best-characterized members. Rasd1 belongs to the Ras superfamily of small GTPase, which is expressed in the brain, heart, liver, kidney, pancreas, skeletal muscle, and placenta (Tu and Wu, 1999; Bernal and Crespo, 2006; Bernal et al., 2021). Activation of Gαi subunits by Rasd1 is known to be the primary mechanism for activating TRPC4 (Wie et al., 2015). Another small G protein that may be a novel target for TRPC5, Rac1, is known to mediate podocyte injury in focal segmental glomerulosclerosis. Studies showed Rac1-activating mutations are responsible for inherited cases of focal segmental glomerulosclerosis, leading to the stimulation of TRPC5 ion channel activity and cytoskeletal remodeling in podocytes (Zhou et al., 2017).
The larger type of G protein, heterotrimeric G proteins are the most commonly found signal transducers in eukaryotic cells, and they mediate the effects of many pharmaceutical products. Heterotrimeric G proteins are the molecular switches that turn on intracellular signaling cascades in response to the activation of GPCRs by extracellular stimuli. GPCRs belong to the largest family of transmembrane receptors and act as the most fundamental signals that are involved in the regulation of internal visceral organs (Kim et al., 2012). Therefore, G proteins have a crucial role in defining the specificity and temporal characteristics of the cellular response (Oldham and Hamm, 2008). The activation of GPCRs promotes an alpha subunit (Gα) of a heterotrimeric G protein to exchange a nucleotide from GDP to GTP inside its pocket, thereby triggering the dissociation of a heterotrimeric G protein (Gαβγ) into Gα and Gβγ. Once activated, Gα proteins amplify the initial signal from the switch by activating effector molecules such as adenylyl cyclase, phospholipase C (PLC), and protein kinases (Liu et al., 2021). Due to the comparable density of ion channels in the plasma membrane (Clapham, 1994), various lines of evidence suggest that not only membrane-bound enzymes but also ion channels could serve as direct effectors of Gα and Gβγ proteins. There is a possibility that ion channels and GPCRs may coexist in close proximity, forming a signaling cluster within a specific region of the plasma membrane (Neves et al., 2002). The recent cryo-EM structure demonstrated that Gαi3 could directly activate the TRPC5 channels, and the channel requires both Ca2+ and PIP2 as essential cofactors for the complete activation of Gαi3 (Won et al., 2023).
Neural circuits regulate organ function to stabilize physiological conditions, providing homeostasis to the body’s internal environment (Figure 1). The vagus nerve travels to the internal visceral and cardiovascular organs, where it regulates physiological responses to environmental changes and damages (Rosas-Ballina et al., 2011). ACh released from the vagus nerve binds to the muscarinic receptors. mAChRs comprise a family of five GPCRs, M1 to M5. Three of these receptor subtypes (M1, M3, and M5) have been shown to mainly couple to G proteins of the Gq/11 family, whereas the remaining two subtypes (M2 and M4) preferentially signal through the Gi/o family of G proteins (Hulme et al., 1990). The most well-known example of regulating effects on organs by mAChRs is in the heart, where the activation of M2 receptor results in the activation of Gβγ-dimer, thereby stimulating the GIRK channel to causing membrane hyperpolarization, ultimately slowing pacemaker depolarization (Harvey and Belevych, 2003).
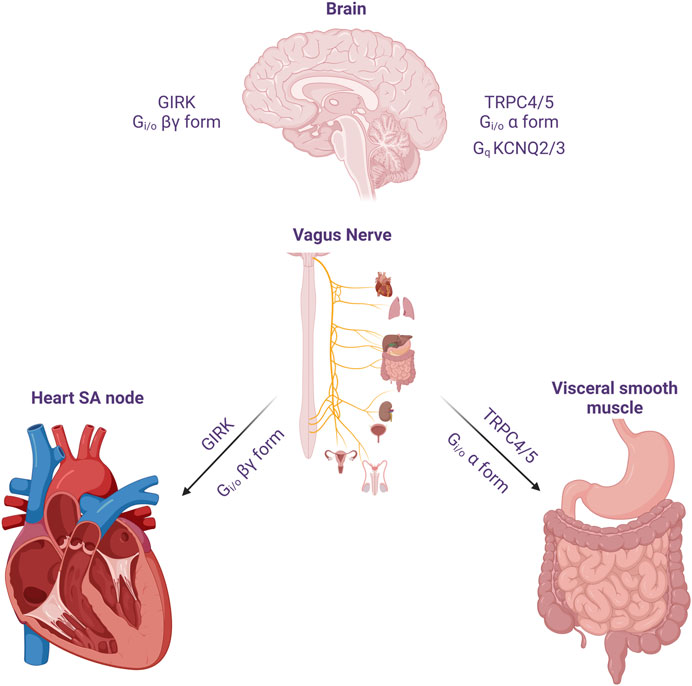
FIGURE 1. The effect of acetylcholine released from cholinergic neuron on heart, visceral smooth muscles and the brain. The vagus nerve acts on the heart to reduce heart rate and reduce cardiac contractility. One of its important actions is to cause hyperpolarization and reduce heart rate through Gi/o protein βγ subunits. In the GI smooth muscle, it activates mIcat cation channels through Gi/o protein alpha subunit, causing depolarization and increasing the frequency of pacemaker potential and contraction. In addition, cholinergic nerves increase neuronal excitability by suppressing M current (IM) at the superior cervical ganglion sympathetic neurons. Later, the molecular candidate for each ion channels were identified as GIRK(Kir3), TRPC4/5 and KCNQ2/3. An emphasis on the role of TRPC in the Gi signaling pathway should be considered in the brain, as well as M channels and GIRK channels.
In the GI tract and many other visceral organs, release of ACh from autonomic nerves triggers excitation and contraction of smooth muscle by activating mAChRs. Although various types of mAChRs contribute to concurrent signals for mIcat generation, the activation of M2 receptors predominantly induces the opening of cationic channels. These channels are also subject to modulation by M3 receptors (Bolton and Zholos, 1997; Zholos and Bolton, 1997). Only M2 and M3 receptors mediate contraction in all studied visceral smooth muscles, and M2 receptors contribute to contraction by inhibiting relaxation caused by agents that increase cAMP (Tanahashi et al., 2021). However, some evidences suggest that increase in intracellular Ca2+ concentration eliminates the influence of Ca2+ release, leading to 1) mIcat inhibition and 2) Gαo-regulated depression (Zholos and Bolton, 1997; Yan et al., 2003). In the smooth muscles of various visceral organs, ACh serves as the primary neurotransmitter for excitation (Beech, 1997). It is released from short postganglionic nerves providing parasympathetic innervations to the smooth muscles of organs such as urinary bladder or myometrium (Zholos et al., 2024). The GI tract is equipped with inherent neural plexuses, where ACh is discharged by stimulating motor neurons within the enteric nervous system (Zholos et al., 2024).
Moreover, in the lingual artery, peripheral nerve stimulation resulted in relaxation and membrane hyperpolarization, which inhibitory responses were hindered by atropine (Bevan and Brayden, 1987). ACh plays an important role of endothelium dependent vascular relaxation in the aorta tissue preparation (Freichel et al., 2001). The relaxation was partially blocked in TRPC4 knockout mice (Freichel et al., 2001). On the other hand, Mori group showed that TRPC5 could be nitrosylated by G protein-coupled ATP stimulation in the endothelium. In addition, TRPC1/5 heteromer perform a major role on the NO formation from eNOS in the endothelium via a physical interaction of TRPC5 with eNOS (Yoshida et al., 2006). Interestingly, PKD1 activates TRPC4 in the endothelium through the Gi/o protein activation and controls endothelial cell migration and proliferation (Kwak et al., 2018).
The signaling of G protein-coupled receptors (GPCR) through G protein-gated inwardly rectifying potassium channels (GIRK) is confined to the cell membrane (Benham et al., 1985; Logothetis et al., 1987). Release of ACh from postganglionic parasympathetic nerve terminals activates muscarinic receptors in the heart. All parts of the mammalian heart are innervated by parasympatheric vagal nerves; vagal activation stimulates the cardiac muscarinic ACh receptors (Capilupi et al., 2020). Stimulation of muscarinic receptors within the heart, specifically the M2 subtype, modulates pacemaker activity and AV conduction, and directly (in atria) or indirectly (in ventricles) effects the force of contraction (Dhein et al., 2001). Mice lacking functional M2 was tested to confirm that M2 subtype is important in the regulation of heart rate as well as anti-nociceptive responses (Gomeza et al., 1999). M1/M3/M5 receptors are also localized in the heart but only M2 are known to mediate significant impacts on heart rate; M2 is the major subtype in cardiac tissue membranes in mammalian heart (Dhein et al., 2001; Willmy-Matthes et al., 2003; Andersson et al., 2011). c-AMP dependent ion channel alteration by M2 muscarinic receptors significantly regulates cardiac function (Harvey and Belevych, 2003). The cardiac GIRK channel, commonly known as Ach-regulated potassium current (IKACh), is composed of a heterotetramer comprising GIRK1 and GIRK4 subunits (Luscher and Slesinger, 2010). GIRK channels mediate inhibitory neurotransmission through G protein-coupled receptors (GPCR) in heart and brain; GIRK channels are known to be expressed in the ventricle (Liang et al., 2014). When an agonist binds to GPCR, GDP is substituted to GTP and dissociates Gα and Gβγ (Lambert, 2008). Then, Gβγ activates GIRK channel by binding to its cytoplasmic region.
There was a historical controversy regarding which subunits were involved in the activation of GIRK, α or βγ. However, the βγ subunit turned out to be the channel modulator (Logothetis et al., 1987). Decades of years later, the atomic structure of Gβγ-bounded GIRK channel obtained by X-ray crystallography and cryo-EM provided clear insights (Figure 2). The 3.5 Å resolution crystal structure of the mammalian GIRK2 channel in complex with Gβγ protein subunits suggest that the GIRK channel complex with Gβγ differ from the structure without Gβγ, representing the channel state from G protein activation to pre-open conformation and implying the functional pathway from closed to open (Whorton and MacKinnon, 2013). The Gβγ-GIRK interaction sites have mostly been researched in GIRK1 and GIRK2 (Yokogawa et al., 2011). Gβγ protein binds to multiple contact sites in the complex with GIRK. Several studies based on mutagenesis suggest that extra amino acid residues within Gβ might be involved in the regulation of basal or induced activities in GIRK (Albsoul-Younes et al., 2001; Zhao et al., 2003). Unlike Gβγ, there is still no crystal structures of GIRK-Gα and the interaction between the two are determined as GDP-bound, that is considered inactive. When the GIRK channel is open, the rate of membrane depolarization slows down due to the hyperpolarization of membrane potential.
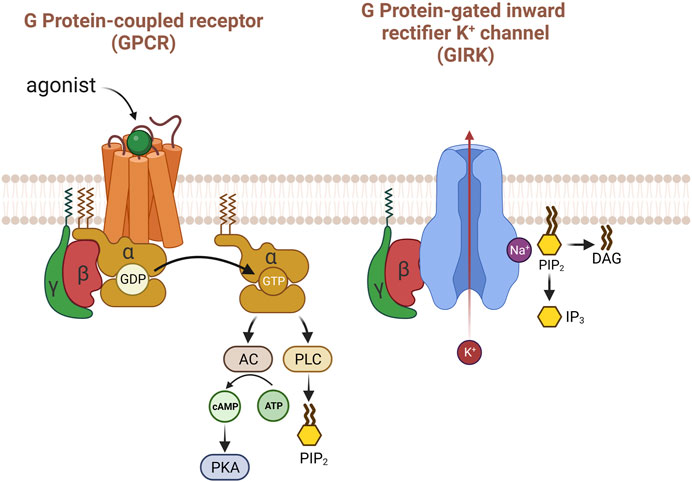
FIGURE 2. The activation of GIRK with G protein βγ subunits. When acetylcholine binds to the M2 receptor in the heart, several pathways are activated, but the most well-known is that Gβγ mainly and directly activates the GIRK potassium channel. PIP2 causes structural changes in GIRK potassium channels to enhance their response to Gβγ. Uniquely, sodium is important for the activity of this potassium channel.
Recent study demonstrates that neurons generate specific signals, that are produced by activating TRPC4 and GIRK channels, reflecting concurrent stimulation of Gq/11 and Gi/o pathways. The simultaneous transmission of neurotransmitters via the Gq/11 and Gi/o pathways is translated into distinct electrical responses through the collaborative functions of TRPC4 and GIRK, facilitating communication to downstream neurons (Tian et al., 2022). On the other hand, Gβγ subunits are barely involved in the direct activation of TRPC4 or TRPC5 by Gαi unlike GIRK channels. PIP2 has been identified as a regulator of the gating of GIRK channel, and GIRK’s x-ray crystal structure of GIRK revealed that each channel interacts with four PIP2 molecules. Additionally, the interaction between the TRPC4 channel and PIP2 is well-established, emphasizing the crucial role of PIP2 in maintaining these channels (Kim et al., 2012). In addition, As PIP2 has been recognized to regulate membrane-associated proteins and act as a signal molecule in phospholipase C-linked Gq-coupled receptor (GqPCR) pathways and GqPCR-induced inhibition of ion channels by means of PIP2 depletion occurs in a receptor-specific manner (Cho et al., 2005a; Cho et al., 2005b).
The cholinergic GI smooth muscle contraction is regarded as an M3 response mediated by the Ca2+ signaling pathway, which includes Gq/11-coupled activation of phospholipase C-β (PLC-β) (So and Kim, 2003). PLC cleaves the membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2) into the second messengers diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), leading to Ca2+ release. As the M3/Gq/PLCβ pathway is ubiquitous in the GI smooth muscle, the DAG-dependent mechanism might as well contribute to mIcat activation in guinea-pig ileum and stomach and mouse ileum (Unno et al., 2006).
In all types of visceral smooth muscles, ACh serves as the primary excitatory neurotransmitter. It is released from short postganglionic nerves providing parasympathetic innervation to the smooth muscles of visceral organs. Over time, research on all types of visceral smooth muscle has dramatically increased as work on GI Smooth muscle increased concurrently. Smooth muscle researches established direct correlation between membrane depolarization, action potential frequency and the force of ACh -induced contractions (Bulbring, 1954). The effects ascribed to non-selective increase of membrane permeability to Na+, K+, and Ca2+, but not Cl−. Thus, patch clamp technique was used to directly record and characterize mIcat (muscarinic cation current) as a nonselective, voltage-sensitive cation current that switches on by ACh stimulation on single smooth muscle cells of the rabbit jejunum in 1985 (Benham et al., 1985). After this first publication of directly recorded patch clamp data was published, numerous investigations revealed the role of a pertussis-toxin sensitive G protein (Inoue and Isenberg, 1990a; Komori and Bolton, 1990; Komori et al., 1992; Zholos et al., 1994; Zholos et al., 2004) and intracellular Ca2+ on mIcat potentiation (Inoue and Isenberg, 1990b; Komori et al., 1993). The activation of mIcat is inhibited by anti-Gi/o protein antibodies in GI smooth muscle (Kim et al., 1998a; Yan et al., 2003), indicating the involvement of Gi/o protein in the activation of mIcat.
These initial discoveries have indicated a mutual reliance of mIcat on the activation of both M2R and M3R. As mentioned earlier, M2R couples to pertussis-toxin sensitive Gi/o proteins and M3R is coupled to phospholipase C(PLC)/IP3 pathway of Gq/11 proteins (Figure 3). The concurrent oscillations of intracellular Ca2+ concentration and mIcat activation disclosed the PLC/IP3 pathway to IP3-induced Ca2+ release, which was observed in single guinea-pig ileal smooth muscle cells (Komori et al., 1993; Zholos et al., 1994). Such potentiation of mIcat during peaks of IP3-induced Ca2+ release enhances membrane depolarization, reaching the action potential threshold and causing voltage-dependent Ca2+ entry via voltage gated Ca2+ channel. When combined with a concurrent peak of IP3-induced Ca2+ release, it elicits smooth muscle contraction. The fact is, the change in intracellular Ca2+ concentration induced by L-type Ca2+ channel is remarkably higher than the changes induced by muscarinic receptor-operated cation channels (Kim et al., 1998b). Synthetic smooth muscle cells within the vascular system reduce the expression of L-type voltage-gated Ca2+ channels while simultaneously elevating the expression of low voltage-activated Ca2+ channels and TRPC channels (House et al., 2008). TRPC4/5 and TRPC6 have been known to be related to the Ca2+ responsive pathways that play a role in the transcriptional regulation (Freichel et al., 2001; Tiruppathi et al., 2002; Kuwahara et al., 2006). In addition, TRPC4/6 have been suggested to have a role in the in vivo regulation of GI motility by influencing the contraction of smooth muscle cells (Tsvilovskyy et al., 2009), producing the nonselective cationic currents through muscarinic receptor stimulation in intestine smooth muscle cells (Figure 3).
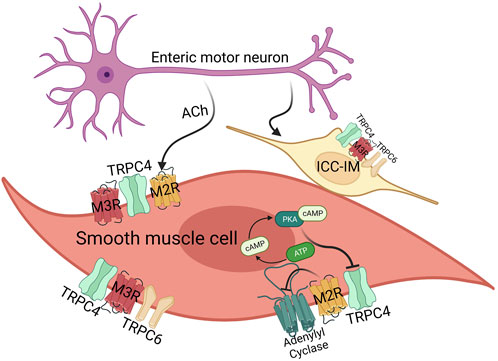
FIGURE 3. The effects of acetylcholine on smooth muscle cells and ICC-IM via M2/3 receptors. GI smooth muscle mainly expresses M2 (80%) and M3 (20%) receptor, and ICC-IM expresses M3 receptor. In case of TRPC channels, smooth muscles mainly express TRPC4 and TRPC6. Both M2/3 receptor are important for TRPC4 function via Gi and Gq proteins, whereas M3 receptor activates TRPC4 and TRPC6 via the Gq-PLC-DAG pathway. M2 receptor alone activates TRPC, but in this case, it also works through Gi-AC-cAMP-PKA as well as Gi protein itself.
Since interstitial cells of Cajal (ICCs) play a crucial role in cholinergic neurotransmission within visceral smooth muscles, these cells can be considered as an additional target contributing to smooth muscle complications following general anesthesia (Zholos et al., 2024). There was a study of the transcriptome in ICCs uncovered the presence of 550 ion channel isoforms in jejunal and colonic ICCs (Figure 3). This includes channels that have been previously identified as responsive to general anesthetics in various cell types (Lee et al., 2017). Notably, mouse intestinal ICCs express TRPC4 and TRPC5 channels. Experimental evidence using the specific TRPC4/5 blocker ML204 and the direct agonist EA has highlighted the significance of these channels in modulating spontaneous intracellular Ca2+ oscillations and pacemaker activity (Lee et al., 2020). ICCs serve as the pacemaker cells that initiate and propagate electrical slow waves in the GI smooth muscles. Although the pacemaker activity originates from Ano-1 or TRPM7, TRPC channels induce depolarization after eating and increase the frequency of the pacemaker activity (Figure 3). Along with TRPC4, TRPC6 have also been identified in ICCs in the same preparation (Epperson et al., 2000; Lee et al., 2017).
TRPC5 is expressed in a variety of smooth muscle cell types and TRPC4 has been demonstrated to exhibit broad expression within the endothelial tissue, suggesting its potential role in orchestrating the regulation of vascular smooth muscle through endothelium-dependent mechanisms (Freichel et al., 2001; Tiruppathi et al., 2002). TRPC4 came out to be the most important TRPC channel regarding the smooth muscle cells as they have been found in a widespread of smooth muscle cells from different vascular beds and has response to ACh triggered muscarinic receptor activation in smooth muscle cells of the GI tract. The ACh -activated TRPC channels would result in the depolarization of smooth muscle cells in the intestine, leading to subsequent activation of L-type Ca2+ channels and inducing contraction (Tsvilovskyy et al., 2009). The impact of muscarinic effects on numerous channels poses the complex challenge of discerning their respective significance, particularly within the interactions involving M2 and M3 receptors. Nonetheless, the activation of mIcat undeniably stands out as a primary mechanism for exciting GI smooth muscle (Zholos, 2006).
Many studies have suggested that the enteric nervous system plays an important role in normal GI smooth muscle development (Chamley-Campbell et al., 1979; Langer et al., 1994; McHugh, 1995). The bidirectional communications between the evolving enteric nervous system and GI smooth muscle seem to have a crucial impact on the regular differentiation, maturation, and functioning of both tissue types. The significance of specific receptor ligand pathways in regulating these essential cell-to-cell interactions throughout GI development has been confirmed, which may lead to clinical importance of certain GI diseases and disorders (McHugh, 1995).
TRPC4/5 channel is a molecular candidate for mIcat (Zhu et al., 2003; Lee et al., 2005) and can be directly activated by constitutively active GαiQL proteins. TRPC4 and TRPC5 belong to the same subfamily and both are activated by Gi/o proteins. Gi2 prefers to bind with TRPC4 whereas Gi3 prefers TRPC5 (Jeon et al., 2008; Jeon et al., 2012). Initial studies suggested that the binding sites for G protein exist at the rib helix of TRPC4/5 channels or the CIRB domain (Jeon et al., 2012). However, recent cryo-EM structure showed that IYY58-60 amino acids at ARD bind with Gi3 protein (Won et al., 2023). Main debate concerns with the role of PIP2. We showed that PIP2 is essential for maintaining TRPC5 channel activity. Recently, we directly applied PIP2 with inside-out patch mode and activated TRPC5 channels. When the binding sites was mutated, the mutants did not respond to intracellularly applied PIP2. The role of Gαi protein was to enhance the affinity of TRPC5 channels to PIP2 at the physiological PIP2 range (Won et al., 2023). Other research groups showed that PIP2 inhibited TRPC5 tonically at the basal level, and depletion of PIP2 decreased the activation time constant and rapidly increased the TRPC5 current (Thakur et al., 2016). Furthermore, Gudermann group suggest that DAG is a real activator for TRPC4 and TRPC5 channels because PIP2 depletion cause TRPC4/5 to respond to DAG (Storch et al., 2017). Another important point is the roles of PLCδ1. We showed that PLCδ1 was activated by Ca2+ influx through TRPC4 and played a negative role on TRPC4 currents (Ko et al., 2023). Zhu group showed the contrary results. They needed Gi protein and PLCδ1 to activate TRPC4 channels (Thakur et al., 2016). When PLCδ1 was inhibited, TRPC4 was not activated by agonists, even in the presence of Gi proteins. Ca2+ and H+ ion were suggested as activators (Jeon et al., 2020a; Thakur et al., 2020). The exact roles of PIP2 would be revealed when the cryo-EM structure of the PIP2-bounded TRPC5 channel is obtained.
Recent cryo-EM structure supports the fact that PIP2 binding site on TRPC5 is located near the S2-S3 linker, S4-S5 linker, TRP helix, and helix-loop-helix region. As Gαi protein binds to TRPC5, increase in PIP2 affinity leads to the increase as well. This means that Gαi protein is not necessary to open the TRPC5 channel, but the intracellular Ca2+ concentration and PIP2 affinity (or binding) may be the direct trigger for opening the TRPC5 channel. Both Ca2+ and PIP2 have the potential to serve as a cofactor in the activation of the channel at the intracellular leaflet, consistent with findings from previous studies (Ningoo et al., 2021). Full activation of Gαi3 to the channel may require the involvement of all three factors: Ca2+, PIP2, and Gαi3 (Figure 4). Also, PLCδ1 does not bind to TRPC5 unlike it does with TRPC4, which causes TRPC5 to have basal current with a high concentration of PIP2.
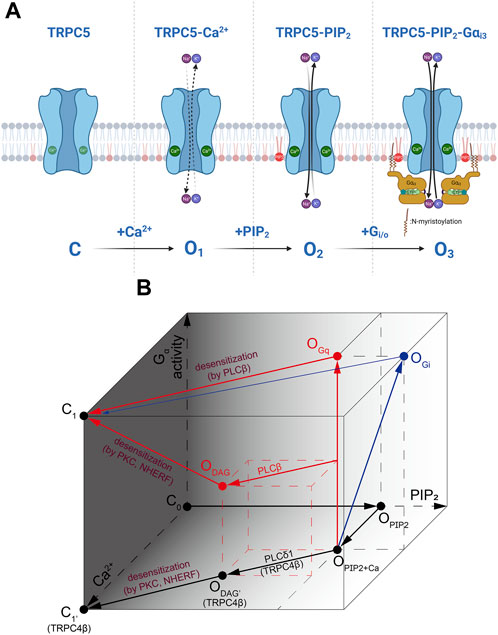
FIGURE 4. The activation process of TRPC4/5 via Ca2+, PIP2 and Gα. (A) During the inside-out patch clamp recordings, we induced initial TRPC5 activity with Ca2+ and subsequently activated the TRPC5 current by applying Gαi3 protein or PIP2. Gαi and/or Gαq proteins are considered to directly induce further activation of the TRPC5 channel. However, in actual physiological situations, it might be assumed that PIP2 always tends to be attached to the TRPC5 ion channel. (B) A cube-shaped schematic diagram describing the overall gating mechanism of TRPC4/5 channels. Three axes represent PIP2 (X-axis) or Ca2+ (Y-axis) binding with the channels, and the strength and/or progress of the Gα activity (Z-axis). Red and blue arrows represent sequences mediated by Gq and Gi, respectively.
We summarizes the complex interaction of G protein, DAG, PIP2 and calcium as in Figure 4. First, to indicate that the channel is not open without PIP2, the side consisting of the Gα and Ca2+ axes is darkened and points on the side set to be closed (C0, C1). In the presence of PIP2 and Ca2+, channels are partially open (OPIP2+Ca). As Gαi activity increases and Gαi bind directly to channels (OPIP2+Ca→OGi), the PIP2 sensitivity of the channel increases. In the diagram, this change is depicted by the increase in PIP2 concentration, although it does not imply the actual elevation in PIP2 concentration. Gq activity also opens TRPC4/5 channels potently (OPIP2+Ca→OGq). Both open states induced by Gq and Gi reach to closed state through activation of PLCβ (OGq or OGi→C1). However, the transition from Gi-open state is not powerful, as depicted. This process is accompanied by an increase in Ca2+ and a decrease in PIP2. TRPC4/5 channels can open by under specific conditions when diacylglycerol (DAG) is generated from PIP2 molecule (ODAG). PIP2 hydrolysis occurs in TRPC4β by PLCδ1. At this time, PLCδ1 becomes active due to an increase in Ca2+ independent of any Gα activities. Therefore, the process is drawn at the bottom (OPIP2+Ca→ODAG’). Since PLCβ is activated by Gq, it is plotted diagonally to reflect the Ca2+ increase and PIP2 depletion at the time point in which Gq activity has progressed to some extent along the vetical OPIP2+Ca–OGq line (middle of OPIP2+Ca–OGq line→ODAG). DAG-induced open states become closed when the C-terminus of the channel is phosphorylated by PKC, followed by binding with PDZ motif of Na+/H+ exchanger regulatory factor (NHERF). The process occurs concurrently with the advancement of Gq activity, depletion in PIP2, and an increase in Ca2+ levels, reaching the dark side mentioned first and entering a closed state (ODAG→C1). But Gα activity is not needed in the case of TRP4β (ODAG’→C1).
GPCR-Gi/o protein signaling pathway in neurons is an essential component of the complex network of signaling mechanisms that regulate neuronal function (Figure 5). Recent studies indicated the direct relationship between neurological disorders and TRPC4/5 channels. Increased TRPC5 S-glutathionylation by oxidative stress contribute to neuronal damage in striatum that may result in Huntington’s disease (Hong et al., 2015). Dysfunction in TRPC4 may lead to epilepsy or autism spectrum disorder (Zheng, 2017; Gupta et al., 2023; Zhou et al., 2023). Freichel group showed that heteromeric TRPC1/4/5 channels are involved in depression and anxiety (Broker-Lai et al., 2017; Chu et al., 2020) TRPC1/4/5 channels play a role in the development of morphine tolerance and hyperalgesia. Prolonged exposure to morphine results in an increase in the expression of TRPC1/4/5 channels in the spinal cord (Chu et al., 2020). TRPC1/4/5 channels also possess developmental functions in neurons. TRPC5 regulates hippocampal neurite development (Greka et al., 2003), and dendrite patterning (Puram et al., 2011). TRPC4 in rat dorsal root ganglion neurons are known to be necessary for neurite outgrowth. Suppression of TRPC4 immuno-reactivity resulted in decrease in the length of neurites in cultured dorsal root ganglion neurons, confirming the necessity of TRPC4. Nerve injury causes increase in TRPC4 as well (Wu et al., 2008). Later research reported activation of TRPC4β, TRPC4 splice variants, through Gαi regulates the morphogenesis of dendrites in cultured hippocampal neurons (Jeon et al., 2013).
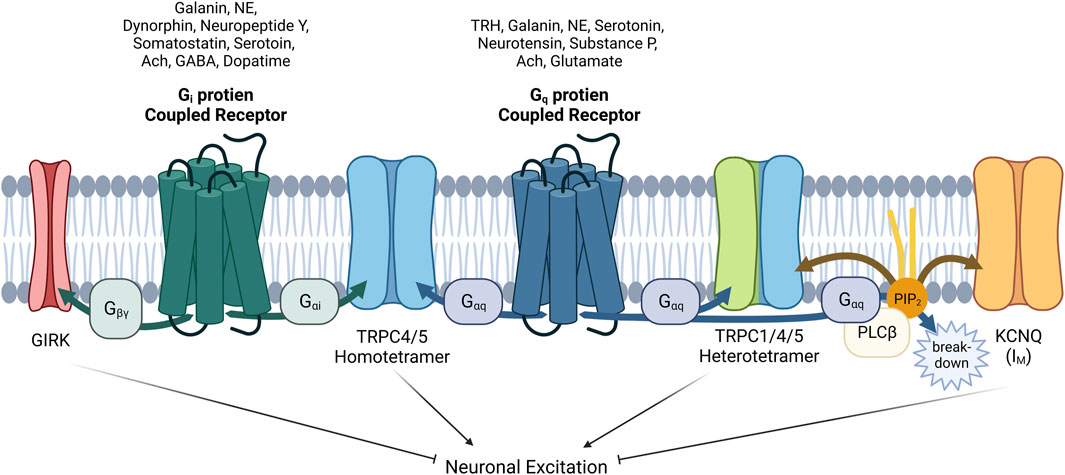
FIGURE 5. Complex interactions among GIRK, TRPC1/4/5 and KCNQ channels with GPCRs via G proteins α or βγ subunits and PIP2 in neurons. The role of GPCRs in neurons is intricate and complicated. When acetylcholine acts on neuronal cells, the physiological function of at least three ion channels (TRPC1/4/5, GIRK, KCNQ) must be analyzed considering their distribution and expression. In addition, βγ subunit also inhibits CaV channels, contrary to activation of GIRK. As for TRPC1/4/5, heteromers seems to play a major role in the brain recently, so heteromers should always be considered together as well as homomers. In addition to acetylcholine, galanin, norepinephrine (NE), and serotonin must always be considered as a neurotransmitter acting on both Gi and Gq proteins. Furthermore we must remember that GABA, dopamine, somatostatin, neuropeptide Y, and dynorphin, which act on Gi-coupled GPCR pathway, can work inducing the direct binding of Gαi proteins to TRPC4/5 homomer. In our hands, Gαq also binds directly (Myeong et al., 2018), but the structure of Gαq bound TRPC4/5 has not yet been revealed. GPCRs that suppress the M current have been demonstrated to utilize Gq/11 proteins for the activation of phospholipase C, leading to the hydrolysis of PIP2. PIP2 serves as a diffusible second messenger within the membrane, directly influencing the activity of KCNQ currents.
TRPC4 as well as TRPC1 support the repetitive neural spiking in brain, confirming the various functions on the neuronal pathway. Relatively high expression level of TRPC4 in lateral septum promotes firing rate. Lateral septum receives signals from various brain regions, extending from hippocampus to amygdala, where diverse neurotransmitters such as ACh, dopamine, glutamate, GABA, and serotonin converge. The depolarization of plateau potential, responsive to electrical stimulation in the presence of blockers for inotropic GABA and glutamate receptors, were shown to be mediated by the Gq/11-coupled group 1 metabotropic glutamate receptors (Gallagher et al., 1995). Later, Zhu group showed that both Gi- and Gq-coupled signaling pathways are important for the spike firing in lateral septal nucleus and the response differs from Gq-only or Gi-only signaling (Yang et al., 2015; Jeon et al., 2020b; Tian et al., 2022). Two interconvertible depolarization responses (below-threshold-depolarization and above-plateu-depolarization) of TRPC4-group 1 metabotropic glutamate receptor activation contribute to patterns in lateral septal neuron firing activities (Phelan et al., 2012; Tian et al., 2014; Phelan et al., 2023). Another research has shown that not only TRPC4 but also TRPC1 are essential for an intrinsic membrane conductance mediating the plateau potential in lateral septal neurons (Phelan et al., 2012).
Flockerzi group elaborately showed that heteromeric TRPC1/4/5 channels are the major functional channels in the brain using multiple specific antibodies for TRPC1/4/5, multi-epitope affinity purifications, and high resolution liquid mass spectrometry (nano-LC-MS/MS) (Kollewe et al., 2022). The amount of TRPC proteins determined in each sample by nano-LC-MS/MS were finally combined to deduce the abundance of each isoform in all possible tetrameric configurations. The importance of hetero-tetramers is rising, given that only minor portions of the TRPC1, TRPC4, and TRPC5 proteins in the brain are present in homomers (13%, 6%, 9%, respectively). The majority is incorporated into three categories of heteromers: TRPC1/C4, TRPC1/C5, and TRPC1/C4/C5 (Kollewe et al., 2022). These findings are notable since homo- or hetero-tetramers modify channel properties significantly, such as Ca2+ permeability, PIP2 sensitivity, and I-V curve. Further studies of the heteromeric TRPC1/4/5 channels in GPCR signaling pathway are necessary to understand their activity under physiological conditions.
Shapiro group put an emphasis on the role of TRPC in the Gi signaling pathway in the brain, as well as M channels and GIRK channels (Carver and Shapiro, 2019; Carver et al., 2020; Carver et al., 2021). Sohn group also suggested that TRPC5 mediates the effects of leptin and serotonin via POMC neurons (Gao et al., 2017), and this effect is independent of altering GIRK channel activity (Sohn et al., 2011). Recently, Gi/o-coupled GPCR in the paraventricular nucleus of the hypothalamus was found to antagonize the anorexic effect of serotonin agents via KATP channels (Yoo et al., 2021). Melanocortin 4 receptors (MC4Rs) in parasympathetic preganglionic neurons activate KATP channels via Gs signaling, but in sympathetic preganglionic neurons, they activate putative nonselective cation channels (Sohn et al., 2013). In case of this neuronal circuit regulating feeding behavior and energy metabolism (involving POMC or NPY/AgRP neurons), TRPC5 channels are more crucial than GIRK and CaV channels. Most importantly and recently, Zhu group suggested that the lateral septal nucleus utilizes a minimum of two channels, TRPC4 and GIRK, both of which are modulated by Gi/o and Gq/11 pathways. While the Gi/o and Gq/11 pathways compete in their effects on GIRK, they cooperate in producing a self-propagating all-or-none activation of TRPC4. Zhu group emphasized that these nonlinear interactions allow for the encoding of coincident signaling, particularly the relative degrees to which the 2 G protein pathways are being activated, resulting in discernible action potential firing patterns (Tian et al., 2022).
In case of TRPC5, it exhibits the highest expression in the brain, mostly in CA1 pyramidal cell, amygdala, cingulate gyrus, and cerebellar nuclei (Riccio et al., 2002). M2R and M4R, the Gi/o-coupled GPCRs, are localized to both presynaptic and postsynaptic terminals, where they inhibit neuronal excitation with the coupled-Gi/o proteins. Gi/o-coupled GPCRs mediate inhibitory signals. For example, activation of these receptors can lead to a decrease in cAMP levels, which, in turn, can modulate ion channel activity and neurotransmitter release. This inhibition is crucial for maintaining the balance of excitatory and inhibitory signals in various processes in nervous system, such as synaptic transmission and neuronal excitability. Knockout mice of M4R, not M2R, show increased basal ACh release in the hippocampus (Tzavara et al., 2003). Considering with the recent finding that TRPC5 is the direct effector of Gi/o protein triggered by GPCR activation (Won et al., 2023), researchers should particularly consider the novel GPCR-Gαi-TRPC5 pathway, especially in studies related to neuronal diseases.
TRP channels are known to be transducers of exogenous and endogenous noxious cues. The last decade has been superb with dramatically high resolution of molecular structures that have allowed us to learn the molecular intricacies of TRP channels using cryogenic electron microscopy. These findings, in combination with functional studies, have provided insights into the role played by these channels in the generation and maintenance of pain (Rosenbaum et al., 2022). The expression pattern in brain nuclei of TRPC4 and TRPC5 also show possibilities to become a novel TRP targets involved in pain processing. While the emphasis on the generation of pain has traditionally been centered on sensory neurons, there is a high possibility of discovering new drugs based on non-neuronal cell types, which can also impact pain perception (Rosenbaum et al., 2022). Research on TRPC4 knockout rats showed tolerance to visceral pain responses, whereas somatic pain responses were uninfluenced (Koivisto et al., 2022). In addition, the non-selective TRPC4/5 antagonist, 4-methyl-2-(1-piperidinyl)quinoline (ML-204), inhibited visceral pain responses in wild-type rats, confirming the role of TRPC4 in visceral pain. The application of ML-204 to amygdala results in suppression of mechanical hypersensitivity and attenuated neuropathic pain behavior in rats with spared nerve injury (Wei et al., 2015). Another TRPC4/5 antagonist HC-070 developed by Hydra and Boehringer Ingelheim is currently in clinical trial for the treatment of anxiety disorder and depression (Wulff et al., 2019). HC-070 also had a significant anti-hypersensitivity effect in the established phase of the chronic constriction injury model (Jalava et al., 2023). TRPC5 inhibitor GFB-887, currently in phase 2 clinical trial, is being developed by Goldfinch Bio for the treatment of kidney disease. GFB-887 was first developed as a treatment for diabetic nephropathy but GFB-887 showed the best result in patients with focal segmental glomerulosclerosis, a rare kidney disease marked by blood vessel scarring in the glomerulus (NCT number: NCT04387448). These findings suggest that centrally mediated TRPC4 and TRPC5 antagonists could relieve visceral and neuropathic pain (Blum et al., 2019). On the other hand, TRPC4 and TRPC5 activator Englerin A has been developed for cancer therapy as it can inhibit growth of tumor cell lines at nanomolar concentrations (Carson et al., 2015). Englerin A is a selective inhibitor of renal cancer cell growth compared to normal kidney cells and cancer cell lines of different origin (Akbulut et al., 2015). Selectivity turns out to be one of the most important factors in drug development, meaning that a discovery of a precise structure of Englerin A binding site in TRPC4/5 would be crucial for further research (Jeong et al., 2019; Kim et al., 2019).
Recent TRPC4/5 inhibitors block both TRPC4/5 with relatively similar potency, which confirms that they are not ready for pharmaceutical use (Zheng, 2022). The zinc binding site and CIRB site in the more variable cytosolic domain may also be promising for developing drugs that can differentiate TRPC4 and TRPC5 (Zheng, 2022). Furthermore, the PIP2 binding site may be a novel site to target as well. Activators or inhibitors for TRPC4/5 can be classified into three types, extracellular type, transmembrane (TM) type and cytosolic type (Figure 6). Ions like H+ ion, La3+ or Gd3+, binds to extracellular sites and activates TRPC4/5 channels (Jung et al., 2003; Semtner et al., 2007). This sites might be suitable for drugs which are water soluble and have charges. Many drugs, like riluzole, pico145, HC-070 or clemizole, binds to TM area (Wright et al., 2020; Song et al., 2021; Yang et al., 2022). Physiological modulators like Ca2+, DAG, or PIP2 also binds to TM domain. The posttranslational modification, like PKA or PKC phosphorylation and glutathionylation, occurs on cytosolic area. We showed that G protein binds to cytosolic ARD and activates TRPC5 channels. For specific effect of drugs, multiple sites should be considered like G protein binding and PKA phosphorylation sites, PIP2 and NHERF binding sites, or DAG binding and PKC phosphorylation sites (Zhu et al., 2005; Sung et al., 2011; Storch et al., 2017). Focusing only on the TM sites might not be enough for visualizing specific effect of drugs on TRPC4/5. Recent genetic study showed that R175C gain of function mutation in TRPC5 cause an impaired intellectual ability (Leitao et al., 2022). In this case, the drug affecting glutathionylation might improve said symptoms.
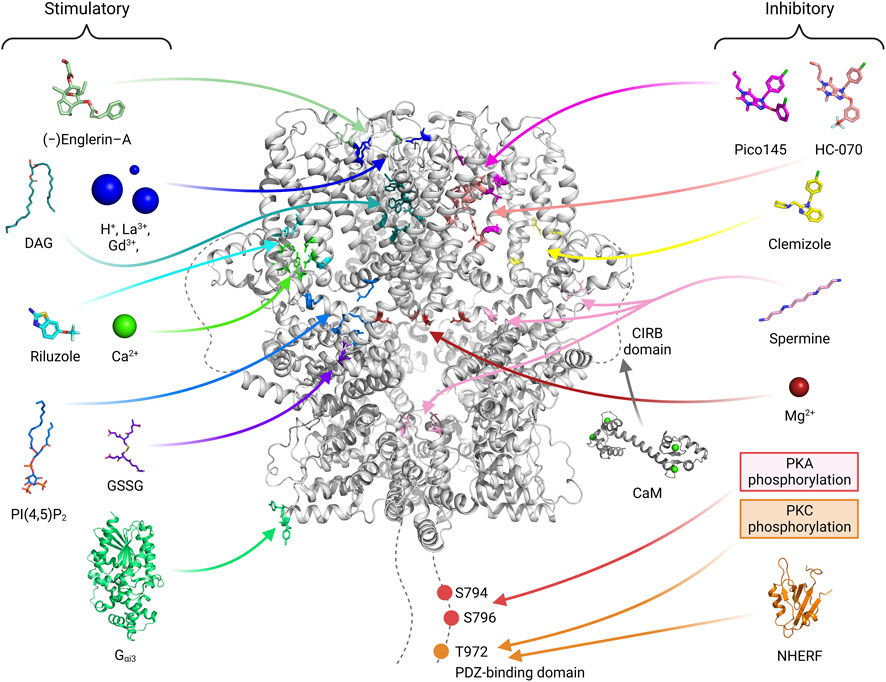
FIGURE 6. The binding sites of activators or inhibitors on the TRPC4/5 channels. The binding or regulating sites of various substances that modulate the channel activity are shown with a human TRPC5 channel structure (PDB ID: 7X6I). Stimulatory molecules or atoms are placed on the left side, and their binding sites are depicted with residues of blue-toned color. On the right side, inhibitory molecules, atoms, or modifications are illustrated, and their binding sites are indicated with red-toned color. Given that the effect of calmodulin (CaM) varies depending on the research group (Kim et al., 2006; Vinayagam et al., 2020), the binding site of the molecule is indicated with a gray color. In drug development, effective drugs can be developed by using computers to predict binding to various sites and then verifying these predictions through experiments. The drugs made so far are concentrated in the membrane area. The GSSG glutathionylation site will be a good target considering the recent results showing the relation of TRPC5 R175C mutation and impaired intellectual ability (Leitao et al., 2022). It is also connected to zinc, which in turn connected to redox sensing and zinc poisoning. For each substance, references are added. DAG (storch et al., 2016; Song et al., 2021; Wright et al., 2020; PDB ID: 7E4T), H+ ion (Semtner et al., 2007), La3+, Gd3+ (Jung et al., 2003), Gαi, PIP2 (Won et al., 2023; PDB ID: 7X6I), (−)-Englerin A (Jung et al., 2003; Kim et al., 2020), Riluzole (Yang et al., 2022; PDB ID: 7WDB), GSSG (Hong et al., 2015), Ca2+ (Duan et al., 2018; Duan et al., 2019; Vinayagam et al., 2020; Song et al., 2021; Won et al., 2023) are stimulatory. Pico145 (Wright et al., 2020; PDB ID: 6YSN), HC-070, Clemizole (Song et al., 2021; PDB ID: 7D4Q, 7D4P), spermine (Kim et al., 2016; 2020), Mg2+ (Obukhov and Nowycky, 2005), PKA (Sung et al., 2011), PKC (Zhu et al., 2005) and NHERF (Storch et al., 2017; Otsuguro et al., 2008; PDB ID of PDZ domain: 1G04) are inhibitory.
Ion channels, especially TRPC channels are now considered as novel target to be directly regulated by Gαi proteins. GPCR-Gi protein pathways are involved in the regulation of vagus muscarinic pathway under physiological conditions and are closely associated with the regulation of internal visceral organs. The direct and indirect modulations of TRPC channel by G protein play an important role in the muscarinic stimulation that is known to involve the GPCR-Gi protein pathway including dopamine, μ-opioid, serotonin, glutamate, GABA, and the complex interaction between GIRK and TRPC4/5 should be considered in the field of neuroscience. However, two big questions need to be further addressed: the structure and functional involvement of heteromeric TRPC channels and the PIP2 binding site regarding the TRPC channel and G protein complex. Heteromeric TRPC channels are naturally expressed at relatively high levels in the brain, which may be a key for a drug development in the field.
HK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. JK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–review and editing, Writing–original draft. CP: Formal Analysis, Writing–review and editing. BJ: Formal Analysis, Writing–review and editing. IS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Research Foundation of Korea grant 2020R1A2C1012670 and 2021R1A4A2001857 (IS), Seoul National University Hospital Research Fund 04-2020-0220 (IS), Education and Research Encouragement Fund of Seoul National University Hospital (IS), BK21 FOUR education program scholarship (HK, JK, BJ).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agretti P., De Marco G., Pinchera A., Vitti P., Bernal J., Tonacchera M. (2007). Ras homolog enriched in striatum inhibits the functional activity of wild type thyrotropin, follicle-stimulating hormone, luteinizing hormone receptors and activating thyrotropin receptor mutations by altering their expression in COS-7 cells. J. Endocrinol. Invest. 30 (4), 279–284. doi:10.1007/BF03346294
Akbulut Y., Gaunt H. J., Muraki K., Ludlow M. J., Amer M. S., Bruns A., et al. (2015). (-)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew. Chem. Int. Ed. Engl. 54 (12), 3787–3791. doi:10.1002/anie.201411511
Albsoul-Younes A. M., Sternweis P. M., Zhao P., Nakata H., Nakajima S., Nakajima Y., et al. (2001). Interaction sites of the G protein beta subunit with brain G protein-coupled inward rectifier K+ channel. J. Biol. Chem. 276 (16), 12712–12717. doi:10.1074/jbc.M011231200
Andersson K. E., Campeau L., Olshansky B. (2011). Cardiac effects of muscarinic receptor antagonists used for voiding dysfunction. Br. J. Clin. Pharmacol. 72 (2), 186–196. doi:10.1111/j.1365-2125.2010.03813.x
Beech D. J. (1997). Actions of neurotransmitters and other messengers on Ca2+ channels and K+ channels in smooth muscle cells. Pharmacol. Ther. 73 (2), 91–119. doi:10.1016/s0163-7258(97)87271-3
Benham C. D., Bolton T. B., Lang R. J. (1985). Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature 316 (6026), 345–347. doi:10.1038/316345a0
Bernal J., Crespo P. (2006). Analysis of Rhes activation state and effector function. Methods Enzymol. 407, 535–542. doi:10.1016/S0076-6879(05)07043-6
Bernal L., Sotelo-Hitschfeld P., Konig C., Sinica V., Wyatt A., Winter Z., et al. (2021). Odontoblast TRPC5 channels signal cold pain in teeth. Sci. Adv. 7 (13), eabf5567. doi:10.1126/sciadv.abf5567
Bevan J. A., Brayden J. E. (1987). Nonadrenergic neural vasodilator mechanisms. Circ. Res. 60 (3), 309–326. doi:10.1161/01.res.60.3.309
Blum T., Moreno-Perez A., Pyrski M., Bufe B., Arifovic A., Weissgerber P., et al. (2019). Trpc5 deficiency causes hypoprolactinemia and altered function of oscillatory dopamine neurons in the arcuate nucleus. Proc. Natl. Acad. Sci. U. S. A. 116 (30), 15236–15243. doi:10.1073/pnas.1905705116
Bolton T. B., Zholos A. V. (1997). Activation of M2 muscarinic receptors in Guinea-pig ileum opens cationic channels modulated by M3 muscarinic receptors. Life Sci. 60 (13-14), 1121–1128. doi:10.1016/s0024-3205(97)00056-8
Broker-Lai J., Kollewe A., Schindeldecker B., Pohle J., Nguyen Chi V., Mathar I., et al. (2017). Heteromeric channels formed by TRPC1, TRPC4 and TRPC5 define hippocampal synaptic transmission and working memory. EMBO J. 36 (18), 2770–2789. doi:10.15252/embj.201696369
Bulbring E. (1954). Membrane potentials of smooth muscle fibres of the taenia coli of the Guinea-pig. J. Physiol. 125 (2), 302–315. doi:10.1113/jphysiol.1954.sp005159
Capilupi M. J., Kerath S. M., Becker L. B. (2020). Vagus nerve stimulation and the cardiovascular system. Cold Spring Harb. Perspect. Med. 10 (2), a034173. doi:10.1101/cshperspect.a034173
Carson C., Raman P., Tullai J., Xu L., Henault M., Thomas E., et al. (2015). Englerin A agonizes the TRPC4/C5 cation channels to inhibit tumor cell line proliferation. PLoS One 10 (6), e0127498. doi:10.1371/journal.pone.0127498
Carver C. M., DeWitt H. R., Stoja A. P., Shapiro M. S. (2021). Blockade of TRPC channels limits cholinergic-driven hyperexcitability and seizure susceptibility after traumatic brain injury. Front. Neurosci. 15, 681144. doi:10.3389/fnins.2021.681144
Carver C. M., Hastings S. D., Cook M. E., Shapiro M. S. (2020). Functional responses of the hippocampus to hyperexcitability depend on directed, neuron-specific KCNQ2 K(+) channel plasticity. Hippocampus 30 (5), 435–455. doi:10.1002/hipo.23163
Carver C. M., Shapiro M. S. (2019). Gq-coupled muscarinic receptor enhancement of KCNQ2/3 channels and activation of TRPC channels in multimodal control of excitability in dentate gyrus granule cells. J. Neurosci. 39 (9), 1566–1587. doi:10.1523/JNEUROSCI.1781-18.2018
Chamley-Campbell J., Campbell G. R., Ross R. (1979). The smooth muscle cell in culture. Physiol. Rev. 59 (1), 1–61. doi:10.1152/physrev.1979.59.1.1
Cho H., Kim Y. A., Yoon J. Y., Lee D., Kim J. H., Lee S. H., et al. (2005a). Low mobility of phosphatidylinositol 4,5-bisphosphate underlies receptor specificity of Gq-mediated ion channel regulation in atrial myocytes. Proc. Natl. Acad. Sci. U. S. A. 102 (42), 15241–15246. doi:10.1073/pnas.0408851102
Cho H., Lee D., Lee S. H., Ho W. K. (2005b). Receptor-induced depletion of phosphatidylinositol 4,5-bisphosphate inhibits inwardly rectifying K+ channels in a receptor-specific manner. Proc. Natl. Acad. Sci. U. S. A. 102 (12), 4643–4648. doi:10.1073/pnas.0408844102
Chu W. G., Wang F. D., Sun Z. C., Ma S. B., Wang X., Han W. J., et al. (2020). TRPC1/4/5 channels contribute to morphine-induced analgesic tolerance and hyperalgesia by enhancing spinal synaptic potentiation and structural plasticity. FASEB J. 34 (6), 8526–8543. doi:10.1096/fj.202000154RR
Clapham D. E. (1994). Direct G protein activation of ion channels? Annu. Rev. Neurosci. 17, 441–464. doi:10.1146/annurev.ne.17.030194.002301
Dhein S., van Koppen C. J., Brodde O. E. (2001). Muscarinic receptors in the mammalian heart. Pharmacol. Res. 44 (3), 161–182. doi:10.1006/phrs.2001.0835
Duan J., Li J., Chen G. L., Ge Y., Liu J., Xie K., et al. (2019). Cryo-EM structure of TRPC5 at 2.8-A resolution reveals unique and conserved structural elements essential for channel function. Sci. Adv. 5 (7), eaaw7935. doi:10.1126/sciadv.aaw7935
Duan J., Li J., Zeng B., Chen G. L., Peng X., Zhang Y., et al. (2018). Structure of the mouse TRPC4 ion channel. Nat. Commun. 9 (1), 3102. doi:10.1038/s41467-018-05247-9
Epperson A., Hatton W. J., Callaghan B., Doherty P., Walker R. L., Sanders K. M., et al. (2000). Molecular markers expressed in cultured and freshly isolated interstitial cells of Cajal. Am. J. Physiol. Cell Physiol. 279 (2), C529–C539. doi:10.1152/ajpcell.2000.279.2.C529
Freichel M., Suh S. H., Pfeifer A., Schweig U., Trost C., Weissgerber P., et al. (2001). Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4-/- mice. Nat. Cell Biol. 3 (2), 121–127. doi:10.1038/35055019
Gallagher J. P., Zheng F., Hasuo H., Shinnick-Gallagher P. (1995). Activities of neurons within the rat dorsolateral septal nucleus (DLSN). Prog. Neurobiol. 45 (5), 373–395. doi:10.1016/0301-0082(95)98600-a
Gao Y., Yao T., Deng Z., Sohn J. W., Sun J., Huang Y., et al. (2017). TrpC5 mediates acute leptin and serotonin effects via pomc neurons. Cell Rep. 18 (3), 583–592. doi:10.1016/j.celrep.2016.12.072
Gomeza J., Shannon H., Kostenis E., Felder C., Zhang L., Brodkin J., et al. (1999). Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. U. S. A. 96 (4), 1692–1697. doi:10.1073/pnas.96.4.1692
Greka A., Navarro B., Oancea E., Duggan A., Clapham D. E. (2003). TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat. Neurosci. 6 (8), 837–845. doi:10.1038/nn1092
Gupta V., Ben-Mahmoud A., Ku B., Velayutham D., Jan Z., Yousef Aden A., et al. (2023). Identification of two novel autism genes, TRPC4 and SCFD2, in Qatar simplex families through exome sequencing. Front. Psychiatry 14, 1251884. doi:10.3389/fpsyt.2023.1251884
Harvey R. D., Belevych A. E. (2003). Muscarinic regulation of cardiac ion channels. Br. J. Pharmacol. 139 (6), 1074–1084. doi:10.1038/sj.bjp.0705338
Hong C., Seo H., Kwak M., Jeon J., Jang J., Jeong E. M., et al. (2015). Increased TRPC5 glutathionylation contributes to striatal neuron loss in Huntington's disease. Brain 138 (10), 3030–3047. doi:10.1093/brain/awv188
House S. J., Potier M., Bisaillon J., Singer H. A., Trebak M. (2008). The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflugers Arch. 456 (5), 769–785. doi:10.1007/s00424-008-0491-8
Hulme E. C., Birdsall N. J., Buckley N. J. (1990). Muscarinic receptor subtypes. Annu. Rev. Pharmacol. Toxicol. 30, 633–673. doi:10.1146/annurev.pa.30.040190.003221
Inoue R., Isenberg G. (1990a). Acetylcholine activates nonselective cation channels in Guinea pig ileum through a G protein. Am. J. Physiol. 258 (1), C1173–C1178. doi:10.1152/ajpcell.1990.258.6.C1173
Inoue R., Isenberg G. (1990b). Intracellular calcium ions modulate acetylcholine-induced inward current in Guinea-pig ileum. J. Physiol. 424, 73–92. doi:10.1113/jphysiol.1990.sp018056
Jalava N., Kaskinoro J., Chapman H., Morales M., Metsankyla H., Heinonen S. M., et al. (2023). Inhibition of canonical transient receptor potential channels 4/5 with highly selective and potent small-molecule HC-070 alleviates mechanical hypersensitivity in rat models of visceral and neuropathic pain. Int. J. Mol. Sci. 24 (4), 3350. doi:10.3390/ijms24043350
Jeon J., Bu F., Sun G., Tian J. B., Ting S. M., Li J., et al. (2020a). Contribution of TRPC channels in neuronal excitotoxicity associated with neurodegenerative disease and ischemic stroke. Front. Cell Dev. Biol. 8, 618663. doi:10.3389/fcell.2020.618663
Jeon J., Tian J. B., Zhu M. X. (2020b). TRPC4 as a coincident detector of G(i/o) and G(q/11) signaling: mechanisms and pathophysiological implications. Curr. Opin. Physiol. 17, 34–41. doi:10.1016/j.cophys.2020.06.008
Jeon J. P., Hong C., Park E. J., Jeon J. H., Cho N. H., Kim I. G., et al. (2012). Selective Gαi subunits as novel direct activators of transient receptor potential canonical (TRPC)4 and TRPC5 channels. J. Biol. Chem. 287 (21), 17029–17039. doi:10.1074/jbc.M111.326553
Jeon J. P., Lee K. P., Park E. J., Sung T. S., Kim B. J., Jeon J. H., et al. (2008). The specific activation of TRPC4 by Gi protein subtype. Biochem. Biophys. Res. Commun. 377 (2), 538–543. doi:10.1016/j.bbrc.2008.10.012
Jeon J. P., Roh S. E., Wie J., Kim J., Kim H., Lee K. P., et al. (2013). Activation of TRPC4β by Gαi subunit increases Ca2+ selectivity and controls neurite morphogenesis in cultured hippocampal neuron. Cell Calcium 54 (4), 307–319. doi:10.1016/j.ceca.2013.07.006
Jeong S., Ko J., Kim M., Park K. C., Park E. Y. J., Kim J., et al. (2019). Englerin A-sensing charged residues for transient receptor potential canonical 5 channel activation. Korean J. Physiol. Pharmacol. 23 (3), 191–201. doi:10.4196/kjpp.2019.23.3.191
Jung S., Muhle A., Schaefer M., Strotmann R., Schultz G., Plant T. D. (2003). Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J. Biol. Chem. 278 (6), 3562–3571. doi:10.1074/jbc.M211484200
Kim H., Kim J., Jeon J. P., Myeong J., Wie J., Hong C., et al. (2012). The roles of G proteins in the activation of TRPC4 and TRPC5 transient receptor potential channels. Channels (Austin) 6 (5), 333–343. doi:10.4161/chan.21198
Kim J., Ko J., Hong C., So I. (2019). Structure-function relationship and physiological roles of transient receptor potential canonical (TRPC) 4 and 5 channels. Cells 9 (1), 73. doi:10.3390/cells9010073
Kim J., Moon S. H., Kim T., Ko J., Jeon Y. K., Shin Y. C., et al. (2020). Analysis of interaction between intracellular spermine and transient receptor potential canonical 4 channel: multiple candidate sites of negatively charged amino acids for the inward rectification of transient receptor potential canonical 4. Korean J. Physiol. Pharmacol. 24 (1), 101–110. doi:10.4196/kjpp.2020.24.1.101
Kim J., Moon S. H., Shin Y. C., Jeon J. H., Park K. J., Lee K. P., et al. (2016). Intracellular spermine blocks TRPC4 channel via electrostatic interaction with C-terminal negative amino acids. Pflugers Arch. 468 (4), 551–561. doi:10.1007/s00424-015-1753-x
Kim M. T., Kim B. J., Lee J. H., Kwon S. C., Yeon D. S., Yang D. K., et al. (2006). Involvement of calmodulin and myosin light chain kinase in activation of mTRPC5 expressed in HEK cells. Am. J. Physiol. Cell Physiol. 290 (4), C1031–C1040. doi:10.1152/ajpcell.00602.2004
Kim S. J., Koh E. M., Kang T. M., Kim Y. C., So I., Isenberg G., et al. (1998a). Ca2+ influx through carbachol-activated non-selective cation channels in Guinea-pig gastric myocytes. J. Physiol. 513 (3), 749–760. doi:10.1111/j.1469-7793.1998.749ba.x
Kim Y. C., Kim S. J., Sim J. H., Jun J. Y., Kang T. M., Suh S. H., et al. (1998b). Protein kinase C mediates the desensitization of CCh-activated nonselective cationic current in Guinea-pig gastric myocytes. Pflugers Arch. 436 (1), 1–8. doi:10.1007/s004240050597
Ko J., Kim J., Myeong J., Kwak M., So I. (2023). Negative self-regulation of transient receptor potential canonical 4 by the specific interaction with phospholipase C-δ1. Korean J. Physiol. Pharmacol. 27 (2), 187–196. doi:10.4196/kjpp.2023.27.2.187
Koivisto A. P., Belvisi M. G., Gaudet R., Szallasi A. (2022). Advances in TRP channel drug discovery: from target validation to clinical studies. Nat. Rev. Drug Discov. 21 (1), 41–59. doi:10.1038/s41573-021-00268-4
Kollewe A., Schwarz Y., Oleinikov K., Raza A., Haupt A., Wartenberg P., et al. (2022). Subunit composition, molecular environment, and activation of native TRPC channels encoded by their interactomes. Neuron 110 (24), 4162–4175.e7. doi:10.1016/j.neuron.2022.09.029
Komori S., Bolton T. B. (1990). Role of G proteins in muscarinic receptor inward and outward currents in rabbit jejunal smooth muscle. J. Physiol. 427, 395–419. doi:10.1113/jphysiol.1990.sp018178
Komori S., Kawai M., Pacaud P., Ohashi H., Bolton T. B. (1993). Oscillations of receptor-operated cationic current and internal calcium in single Guinea-pig ileal smooth muscle cells. Pflugers Arch. 424 (5-6), 431–438. doi:10.1007/BF00374905
Komori S., Kawai M., Takewaki T., Ohashi H. (1992). GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in Guinea-pig ileal muscle. J. Physiol. 450, 105–126. doi:10.1113/jphysiol.1992.sp019118
Kuwahara K., Wang Y., McAnally J., Richardson J. A., Bassel-Duby R., Hill J. A., et al. (2006). TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J. Clin. Invest. 116 (12), 3114–3126. doi:10.1172/JCI27702
Kwak M., Hong C., Myeong J., Park E. Y. J., Jeon J. H., So I. (2018). Gαi-mediated TRPC4 activation by polycystin-1 contributes to endothelial function via STAT1 activation. Sci. Rep. 8 (1), 3480. doi:10.1038/s41598-018-21873-1
Lambert N. A. (2008). Dissociation of heterotrimeric g proteins in cells. Sci. Signal 1 (25), re5. doi:10.1126/scisignal.125re5
Langer J. C., Betti P. A., Blennerhassett M. G. (1994). Smooth muscle from aganglionic bowel in Hirschsprung's disease impairs neuronal development in vitro. Cell Tissue Res. 276 (1), 181–186. doi:10.1007/BF00354798
Lee J. H., Wu W. H., Huang X. Y., Jun J. Y., Choi S. (2020). Transient receptor potential canonical 4 and 5 channel antagonist ML204 depolarized pacemaker potentials of interstitial cells of cajal. J. Neurogastroenterol. Motil. 26 (4), 521–528. doi:10.5056/jnm20064
Lee K. P., Jun J. Y., Chang I. Y., Suh S. H., So I., Kim K. W. (2005). TRPC4 is an essential component of the nonselective cation channel activated by muscarinic stimulation in mouse visceral smooth muscle cells. Mol. Cells 20 (3), 435–441. doi:10.1016/s1016-8478(23)13250-x
Lee M. Y., Ha S. E., Park C., Park P. J., Fuchs R., Wei L., et al. (2017). Transcriptome of interstitial cells of Cajal reveals unique and selective gene signatures. PLoS One 12 (4), e0176031. doi:10.1371/journal.pone.0176031
Leitao E., Schroder C., Parenti I., Dalle C., Rastetter A., Kuhnel T., et al. (2022). Systematic analysis and prediction of genes associated with monogenic disorders on human chromosome X. Nat. Commun. 13 (1), 6570. doi:10.1038/s41467-022-34264-y
Liang B., Nissen J. D., Laursen M., Wang X., Skibsbye L., Hearing M. C., et al. (2014). G-protein-coupled inward rectifier potassium current contributes to ventricular repolarization. Cardiovasc Res. 101 (1), 175–184. doi:10.1093/cvr/cvt240
Liu N., Wang Y., Li T., Feng X. (2021). G protein coupled receptors (GPCRs): signaling pathways, characterization, and functions in insect Physiology and toxicology. Int. J. Mol. Sci. 22 (10), 5260. doi:10.3390/ijms22105260
Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. (1987). The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325 (6102), 321–326. doi:10.1038/325321a0
Luscher C., Slesinger P. A. (2010). Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat. Rev. Neurosci. 11 (5), 301–315. doi:10.1038/nrn2834
Lyon A. M., Dutta S., Boguth C. A., Skiniotis G., Tesmer J. J. (2013). Full-length Gα(q)-phospholipase C-β3 structure reveals interfaces of the C-terminal coiled-coil domain. Nat. Struct. Mol. Biol. 20 (3), 355–362. doi:10.1038/nsmb.2497
McHugh K. M. (1995). Molecular analysis of smooth muscle development in the mouse. Dev. Dyn. 204 (3), 278–290. doi:10.1002/aja.1002040306
Minke B., Wu C., Pak W. L. (1975). Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature 258 (5530), 84–87. doi:10.1038/258084a0
Myeong J., Ko J., Kwak M., Kim J., Woo J., Ha K., et al. (2018). Dual action of the Gαq-PLCβ-PI(4,5)P2 pathway on TRPC1/4 and TRPC1/5 heterotetramers. Sci. Rep. 8 (1), 12117. doi:10.1038/s41598-018-30625-0
Neves S. R., Ram P. T., Iyengar R. (2002). G protein pathways. Science 296 (5573), 1636–1639. doi:10.1126/science.1071550
Ningoo M., Plant L. D., Greka A., Logothetis D. E. (2021). PIP(2) regulation of TRPC5 channel activation and desensitization. J. Biol. Chem. 296, 100726. doi:10.1016/j.jbc.2021.100726
Obukhov A. G., Nowycky M. C. (2005). A cytosolic residue mediates Mg2+ block and regulates inward current amplitude of a transient receptor potential channel. J. Neurosci. 25 (5), 1234–1239. doi:10.1523/JNEUROSCI.4451-04.2005
Oldham W. M., Hamm H. E. (2008). Heterotrimeric G protein activation by G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 9 (1), 60–71. doi:10.1038/nrm2299
Otsuguro K., Tang J., Tang Y., Xiao R., Freichel M., Tsvilovskyy V., et al. (2008). Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 283 (15), 10026–10036. doi:10.1074/jbc.M707306200
Phelan K. D., Mock M. M., Kretz O., Shwe U. T., Kozhemyakin M., Greenfield L. J., et al. (2012). Heteromeric canonical transient receptor potential 1 and 4 channels play a critical role in epileptiform burst firing and seizure-induced neurodegeneration. Mol. Pharmacol. 81 (3), 384–392. doi:10.1124/mol.111.075341
Phelan K. D., Shwe U. T., Zheng F. (2023). Pharmacological differences between native homomeric transient receptor potential canonical type 4 channels and heteromeric transient receptor potential canonical type 1/4 channels in lateral septal neurons. Pharm. (Basel) 16 (9), 1291. doi:10.3390/ph16091291
Puram S. V., Riccio A., Koirala S., Ikeuchi Y., Kim A. H., Corfas G., et al. (2011). A TRPC5-regulated calcium signaling pathway controls dendrite patterning in the mammalian brain. Genes Dev. 25 (24), 2659–2673. doi:10.1101/gad.174060.111
Riccio A., Medhurst A. D., Mattei C., Kelsell R. E., Calver A. R., Randall A. D., et al. (2002). mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res. Mol. Brain Res. 109 (1-2), 95–104. doi:10.1016/s0169-328x(02)00527-2
Rosas-Ballina M., Olofsson P. S., Ochani M., Valdes-Ferrer S. I., Levine Y. A., Reardon C., et al. (2011). Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334 (6052), 98–101. doi:10.1126/science.1209985
Rosenbaum T., Morales-Lazaro S. L., Islas L. D. (2022). TRP channels: a journey towards a molecular understanding of pain. Nat. Rev. Neurosci. 23 (10), 596–610. doi:10.1038/s41583-022-00611-7
Semtner M., Schaefer M., Pinkenburg O., Plant T. D. (2007). Potentiation of TRPC5 by protons. J. Biol. Chem. 282 (46), 33868–33878. doi:10.1074/jbc.M702577200
So I., Kim K. W. (2003). Nonselective cation channels activated by the stimulation of muscarinic receptors in mammalian gastric smooth muscle. J. Smooth Muscle Res. 39 (6), 231–247. doi:10.1540/jsmr.39.231
Sohn J. W., Harris L. E., Berglund E. D., Liu T., Vong L., Lowell B. B., et al. (2013). Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell 152 (3), 612–619. doi:10.1016/j.cell.2012.12.022
Sohn J. W., Xu Y., Jones J. E., Wickman K., Williams K. W., Elmquist J. K. (2011). Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 71 (3), 488–497. doi:10.1016/j.neuron.2011.06.012
Song K., Wei M., Guo W., Quan L., Kang Y., Wu J. X., et al. (2021). Structural basis for human TRPC5 channel inhibition by two distinct inhibitors. Elife 10, e63429. doi:10.7554/eLife.63429
Storch U., Forst A. L., Pardatscher F., Erdogmus S., Philipp M., Gregoritza M., et al. (2017). Dynamic NHERF interaction with TRPC4/5 proteins is required for channel gating by diacylglycerol. Proc. Natl. Acad. Sci. U. S. A. 114 (1), E37–E46. doi:10.1073/pnas.1612263114
Sung T. S., Jeon J. P., Kim B. J., Hong C., Kim S. Y., Kim J., et al. (2011). Molecular determinants of PKA-dependent inhibition of TRPC5 channel. Am. J. Physiol. Cell Physiol. 301 (4), C823–C832. doi:10.1152/ajpcell.00351.2010
Tanahashi Y., Komori S., Matsuyama H., Kitazawa T., Unno T. (2021). Functions of muscarinic receptor subtypes in gastrointestinal smooth muscle: a review of studies with receptor-knockout mice. Int. J. Mol. Sci. 22 (2), 926. doi:10.3390/ijms22020926
Thakur D. P., Tian J. B., Jeon J., Xiong J., Huang Y., Flockerzi V., et al. (2016). Critical roles of Gi/o proteins and phospholipase C-δ1 in the activation of receptor-operated TRPC4 channels. Proc. Natl. Acad. Sci. U. S. A. 113 (4), 1092–1097. doi:10.1073/pnas.1522294113
Thakur D. P., Wang Q., Jeon J., Tian J. B., Zhu M. X. (2020). Intracellular acidification facilitates receptor-operated TRPC4 activation through PLCδ1 in a Ca2+ -dependent manner. J. Physiol. 598 (13), 2651–2667. doi:10.1113/JP279658
Tian J., Thakur D. P., Lu Y., Zhu Y., Freichel M., Flockerzi V., et al. (2014). Dual depolarization responses generated within the same lateral septal neurons by TRPC4-containing channels. Pflugers Arch. 466 (7), 1301–1316. doi:10.1007/s00424-013-1362-5
Tian J. B., Yang J., Joslin W. C., Flockerzi V., Prescott S. A., Birnbaumer L., et al. (2022). TRPC4 and GIRK channels underlie neuronal coding of firing patterns that reflect G(q/11)-G(i/o) coincidence signals of variable strengths. Proc. Natl. Acad. Sci. U. S. A. 119 (20), e2120870119. doi:10.1073/pnas.2120870119
Tiruppathi C., Freichel M., Vogel S. M., Paria B. C., Mehta D., Flockerzi V., et al. (2002). Impairment of store-operated Ca2+ entry in TRPC4(-/-) mice interferes with increase in lung microvascular permeability. Circ. Res. 91 (1), 70–76. doi:10.1161/01.res.0000023391.40106.a8
Tsvilovskyy V. V., Zholos A. V., Aberle T., Philipp S. E., Dietrich A., Zhu M. X., et al. (2009). Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology 137 (4), 1415–1424. doi:10.1053/j.gastro.2009.06.046
Tu Y., Wu C. (1999). Cloning, expression and characterization of a novel human Ras-related protein that is regulated by glucocorticoid hormone. Biochim. Biophys. Acta 1489 (2-3), 452–456. doi:10.1016/s0167-4781(99)00197-9
Tzavara E. T., Bymaster F. P., Felder C. C., Wade M., Gomeza J., Wess J., et al. (2003). Dysregulated hippocampal acetylcholine neurotransmission and impaired cognition in M2, M4 and M2/M4 muscarinic receptor knockout mice. Mol. Psychiatry 8 (7), 673–679. doi:10.1038/sj.mp.4001270
Unno T., Matsuyama H., Okamoto H., Sakamoto T., Yamamoto M., Tanahashi Y., et al. (2006). Muscarinic cationic current in gastrointestinal smooth muscles: signal transduction and role in contraction. Auton. Autacoid Pharmacol. 26 (3), 203–217. doi:10.1111/j.1474-8673.2006.00366.x
Vinayagam D., Quentin D., Yu-Strzelczyk J., Sitsel O., Merino F., Stabrin M., et al. (2020). Structural basis of TRPC4 regulation by calmodulin and pharmacological agents. Elife 9, e60603. doi:10.7554/eLife.60603
Wei H., Sagalajev B., Yuzer M. A., Koivisto A., Pertovaara A. (2015). Regulation of neuropathic pain behavior by amygdaloid TRPC4/C5 channels. Neurosci. Lett. 608, 12–17. doi:10.1016/j.neulet.2015.09.033
Whorton M. R., MacKinnon R. (2013). X-ray structure of the mammalian GIRK2-βγ G-protein complex. Nature 498 (7453), 190–197. doi:10.1038/nature12241
Wie J., Kim B. J., Myeong J., Ha K., Jeong S. J., Yang D., et al. (2015). The Roles of Rasd1 small G proteins and leptin in the activation of TRPC4 transient receptor potential channels. Channels (Austin) 9 (4), 186–195. doi:10.1080/19336950.2015.1058454
Willmy-Matthes P., Leineweber K., Wangemann T., Silber R. E., Brodde O. E. (2003). Existence of functional M3-muscarinic receptors in the human heart. Naunyn Schmiedeb. Arch. Pharmacol. 368 (4), 316–319. doi:10.1007/s00210-003-0796-2
Won J., Kim J., Jeong H., Kim J., Feng S., Jeong B., et al. (2023). Molecular architecture of the Gαi-bound TRPC5 ion channel. Nat. Commun. 14 (1), 2550. doi:10.1038/s41467-023-38281-3
Wright D. J., Simmons K. J., Johnson R. M., Beech D. J., Muench S. P., Bon R. S. (2020). Human TRPC5 structures reveal interaction of a xanthine-based TRPC1/4/5 inhibitor with a conserved lipid binding site. Commun. Biol. 3 (1), 704. doi:10.1038/s42003-020-01437-8
Wu D., Huang W., Richardson P. M., Priestley J. V., Liu M. (2008). TRPC4 in rat dorsal root ganglion neurons is increased after nerve injury and is necessary for neurite outgrowth. J. Biol. Chem. 283 (1), 416–426. doi:10.1074/jbc.M703177200
Wulff H., Christophersen P., Colussi P., Chandy K. G., Yarov-Yarovoy V. (2019). Antibodies and venom peptides: new modalities for ion channels. Nat. Rev. Drug Discov. 18 (5), 339–357. doi:10.1038/s41573-019-0013-8
Yan H. D., Okamoto H., Unno T., Tsytsyura Y. D., Prestwich S. A., Komori S., et al. (2003). Effects of G protein-specific antibodies and G beta gamma subunits on the muscarinic receptor-operated cation current in Guinea-pig ileal smooth muscle cells. Br. J. Pharmacol. 139 (3), 605–615. doi:10.1038/sj.bjp.0705289
Yang L. P., Jiang F. J., Wu G. S., Deng K., Wen M., Zhou X., et al. (2015). Acute treatment with a novel TRPC4/C5 channel inhibitor produces antidepressant and anxiolytic-like effects in mice. PLoS One 10 (8), e0136255. doi:10.1371/journal.pone.0136255
Yang Y., Wei M., Chen L. (2022). Structural identification of riluzole-binding site on human TRPC5. Cell Discov. 8 (1), 67. doi:10.1038/s41421-022-00410-5
Yokogawa M., Osawa M., Takeuchi K., Mase Y., Shimada I. (2011). NMR analyses of the Gbetagamma binding and conformational rearrangements of the cytoplasmic pore of G protein-activated inwardly rectifying potassium channel 1 (GIRK1). J. Biol. Chem. 286 (3), 2215–2223. doi:10.1074/jbc.M110.160754
Yoo E. S., Li L., Jia L., Lord C. C., Lee C. E., Birnbaum S. G., et al. (2021). Gαi/o-coupled Htr2c in the paraventricular nucleus of the hypothalamus antagonizes the anorectic effect of serotonin agents. Cell Rep. 37 (7), 109997. doi:10.1016/j.celrep.2021.109997
Yoshida T., Inoue R., Morii T., Takahashi N., Yamamoto S., Hara Y., et al. (2006). Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2 (11), 596–607. doi:10.1038/nchembio821
Zhang H., Craciun L. C., Mirshahi T., Rohacs T., Lopes C. M., Jin T., et al. (2003). PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron 37 (6), 963–975. doi:10.1016/s0896-6273(03)00125-9
Zhang M., Ma Y., Ye X., Zhang N., Pan L., Wang B. (2023). TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target Ther. 8 (1), 261. doi:10.1038/s41392-023-01464-x
Zhao Q., Kawano T., Nakata H., Nakajima Y., Nakajima S., Kozasa T. (2003). Interaction of G protein beta subunit with inward rectifier K(+) channel Kir3. Mol. Pharmacol. 64 (5), 1085–1091. doi:10.1124/mol.64.5.1085
Zheng F. (2017). TRPC channels and epilepsy. Adv. Exp. Med. Biol. 976, 123–135. doi:10.1007/978-94-024-1088-4_11
Zheng F. (2022). “Canonical transient receptor potential channels as novel targets for antiepileptic drugs,” in Epilepsy. Editor S. J. Czuczwar (Brisbane (AU): Exon Publications).
Zholos A. V. (2006). Regulation of TRP-like muscarinic cation current in gastrointestinal smooth muscle with special reference to PLC/InsP3/Ca2+ system. Acta Pharmacol. Sin. 27 (7), 833–842. doi:10.1111/j.1745-7254.2006.00392.x
Zholos A. V., Bolton T. B. (1997). Muscarinic receptor subtypes controlling the cationic current in Guinea-pig ileal smooth muscle. Br. J. Pharmacol. 122 (5), 885–893. doi:10.1038/sj.bjp.0701438
Zholos A. V., Komori S., Ohashi H., Bolton T. B. (1994). Ca2+ inhibition of inositol trisphosphate-induced Ca2+ release in single smooth muscle cells of Guinea-pig small intestine. J. Physiol. 481 (1), 97–109. doi:10.1113/jphysiol.1994.sp020421
Zholos A. V., Melnyk M. I., Dryn D. O. (2024). Molecular mechanisms of cholinergic neurotransmission in visceral smooth muscles with a focus on receptor-operated TRPC4 channel and impairment of gastrointestinal motility by general anaesthetics and anxiolytics. Neuropharmacology 242, 109776. doi:10.1016/j.neuropharm.2023.109776
Zholos A. V., Zholos A. A., Bolton T. B. (2004). G protein-gated TRP-like cationic channel activated by muscarinic receptors: effect of potential on single-channel gating. J. Gen. Physiol. 123 (5), 581–598. doi:10.1085/jgp.200309002
Zhou Q., Fu X., Xu J., Dong S., Liu C., Cheng D., et al. (2023). Hypothalamic warm-sensitive neurons require TRPC4 channel for detecting internal warmth and regulating body temperature in mice. Neuron 111 (3), 387–404.e8. doi:10.1016/j.neuron.2022.11.008
Zhou Y., Castonguay P., Sidhom E. H., Clark A. R., Dvela-Levitt M., Kim S., et al. (2017). A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science 358 (6368), 1332–1336. doi:10.1126/science.aal4178
Zhu M. H., Lee Y. M., Jin N., So I., Kim K. W. (2003). The transient receptor potential protein homologue TRPC4/5 as a candidate for the nonselective cationic channel activated by muscarinic stimulation in the murine stomach. Neurophysiology 35 (3/4), 302–307. doi:10.1023/b:neph.0000008790.75165.d6
Keywords: G protein, GPCR, Gi pathway, ion channel, TRPC4, TRPC5
Citation: Kang H, Kim J, Park CH, Jeong B and So I (2024) Direct modulation of TRPC ion channels by Gα proteins. Front. Physiol. 15:1362987. doi: 10.3389/fphys.2024.1362987
Received: 29 December 2023; Accepted: 26 January 2024;
Published: 07 February 2024.
Edited by:
Susumu Ohya, Nagoya City University, JapanReviewed by:
Takuro Numaga-Tomita, Shinshu University, JapanCopyright © 2024 Kang, Kim, Park, Jeong and So. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Insuk So, aW5zdWtAc251LmFjLmty
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.