Corrigendum: Fat oxidation rates and cardiorespiratory responses during exercise in different subject populations with post-acute sequelae of SARS-CoV-2 infection: a comparison with normative percentile values
- 1Department of Biomedical Sciences for Health, Università degli Studi di Milano, Milan, Italy
- 2Department of Endocrinology, Nutrition and Metabolic Diseases, IRCCS MultiMedica, Milan, Italy
- 3Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Mother-Child Sciences, Università degli Studi di Genova, Genova, Italy
Introduction: Post-acute sequelae of SARS-CoV-2 infection (PASC) presents a spectrum of symptoms following acute COVID-19, with exercise intolerance being a prevalent manifestation likely linked to disrupted oxygen metabolism and mitochondrial function. This study aims to assess maximal fat oxidation (MFO) and exercise intensity at MFO (FATmax) in distinct PASC subject groups and compare these findings with normative data.
Methods: Eight male subjects with PASC were involved in this study. The participants were divided into two groups: “endurance-trained” subjects (
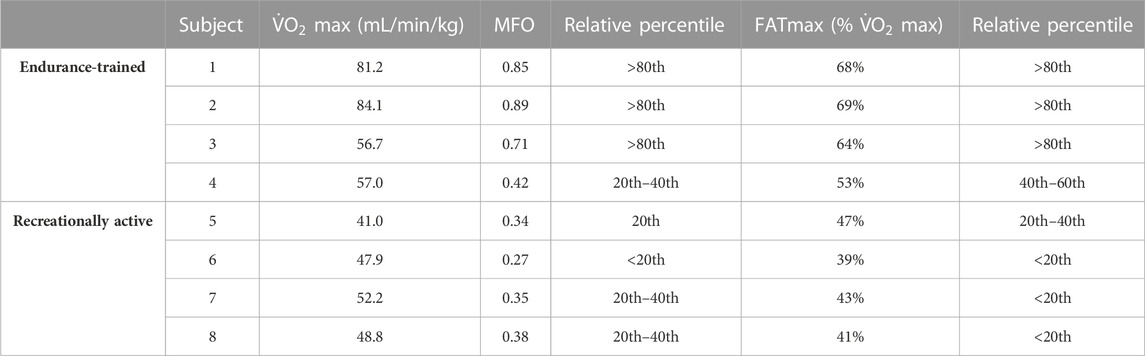
Results: The MFO and FATmax of “endurance-trained” subjects were 0.85, 0.89, 0.71, and 0.42 and 68%, 69%, 64%, and 53%, respectively. Three out of four subjects showed both MFO and FATmax values placed over the 80th percentile of normative data. The MFO and FATmax of “recreationally active” subjects were 0.34, 0.27, 0.35, and 0.38 and 47%, 39%, 43%, and 41%, respectively. All MFO and FATmax values of those subjects placed below the 20th percentile or between the 20th and 40th percentile.
Discussion: Significant differences in MFO and FATmax values between ‘endurance-trained’ and “recreationally active” subjects suggest that specific endurance training, rather than simply an active lifestyle, may provide protective effects against alterations in mitochondrial function during exercise in subjects with PASC.
Introduction
The syndrome of post-acute sequelae of SARS-CoV-2 infection (PASC) is a clinical condition characterized by a wide range of symptoms for 4 weeks or more following acute COVID-19 (World Health Organization, 2023). Exercise intolerance is one of the most widespread manifestations among subjects with PASC. This condition may be the result of impaired oxygen metabolic homeostasis and altered mitochondrial function (Astin et al., 2023). In general, mitochondrial disorders are a complex group of diseases caused by impairment of the mitochondrial respiratory chain (or electron transport chain), which in some patients can lead to an unexplained post-viral illness and myalgic encephalomyelitis/chronic fatigue syndrome (Wood et al., 2021). From a biochemical perspective, mitochondria may utilize various substrates depending on the load and duration of exercise (McArdle WD et al., 2001). During exercise lasting more than 1 minute, adenosine triphosphate (ATP) production is mainly generated via oxidative phosphorylation in the tricarboxylic acid cycle (TCA). The substrates utilized in the TCA to generate reducing equivalents fuel the electron transport chain inside mitochondria are fatty acids (FAs) and carbohydrates (CAs). In this energy production system, called the aerobic system, oxygen (O2) represents the final electron acceptor in mitochondria (McArdle WD et al., 2001). During a submaximal exercise, FA and CA are the main substrates to produce energy, and their contribution depends on exercise intensity. In this sense, rates of b-oxidation of FA (FATox rate) and CA oxidation rates (CHOox rate) are inversely proportional throughout an incremental exercise. Specifically, after reaching the maximal rate of fat oxidation (MFO), throughout an incremental test, the FATox rate tends to decrease, while the CHOox rate tends to increase. Thus, a more detailed analysis in the plasma of metabolites involved in the aerobic system was conducted recently in order to detect the presence of a metabolic dysfunction (Guntur et al., 2022). Results showed that the plasma of PASC subjects exhibited significantly higher levels of acyl-carnitines and free FAs and lower levels of pyruvate, lactate, and TCA metabolites such as succinate, malate, and citrate, compared to an healthy group and a group of subjects recovered from COVID-19, without PASC. These data are indicative of ongoing mobilization of FA but show impaired ability for oxidation due to mitochondrial dysfunction. In this contest, other research groups (de Boer et al., 2022) investigated whether patients with PASC had compromised mitochondrial function during graded exercise. A cardiopulmonary exercise test (CPET) has been used to calculate the FATox rate and lactate clearance, providing insight into mitochondrial function. Results showed inappropriately high arterial lactate levels and reduced FATox rate at relatively low exercise intensity in this type of subjects. Those data indicate that the transition from the FATox rate to the CHOox rate occurs earlier, suggesting, also in this case, dysfunctional mitochondria. Specifically, the premature lactate accumulation suggests either a metabolic shift in increased glycolysis or the inability to utilize lactate in the mitochondria as an alternative source of energy during exercise (de Boer et al., 2022). Normally, during an incremental exercise, lactate values and oxygen consumption (
Materials and methods
Eight male subjects were involved in this study. The investigation lasted 3 months, and it was a pilot study that will serve as a basis for future more in-depth research on the topic at hand. Following the classification of a recent review (Maunder et al., 2018), regarding normative values of MFO and FATmax, subjects were divided into two groups: “endurance-trained” and “recreationally active.” “Endurance-trained” was defined as a subject with VO2max >55 mL/min/kg and active engagement in training for endurance events. “Recreationally active” was defined as physically active, not training for specific endurance events, and with VO2max <55 mL/min/kg (Maunder et al., 2018). Specifically, recreationally active subjects were involved in activities such as tennis, soccer, and gym fitness. Their anthropometric, physiological, and training characteristics are reported in Table 1. Before viral infection, both groups had been maintaining the same training or physical activity status compared to the post-COVID-19 period. The subjects had been reported a positive diagnosis of SARS-CoV-2 within 12 months before, with mild symptoms and no need for hospital care. The therapy was limited to taking NSAIDs for a few days. The subjects suffered PASC with preserved pulmonary and cardiac function and presented brain fog, insomnia, and memory impairment with the main symptoms. In order to detect the MFO and FATmax, each subject performed the “FATmax test” (Achten and Jeukendrup, 2004), a graded exercise test on an indoor roller (Direto XR-T, ELITE), with their own bike. Workload roller, expressed in watt (W), was monitored by software My E-Training, ELITE. Pedaling cadence was freely chosen and maintained constant ( ± five repetitions per minute (rpm)). Each participant completed the test at the same time of the day (±2 h) after 7–8 h of sleep and under similar environmental conditions (18–20 °C). Participants were asked to consume the same meals and drinks during the 24 h prior to testing and to fast overnight before the test. Specifically, after a free warm-up period of 5 min, the subjects performed a continuous ramp test for
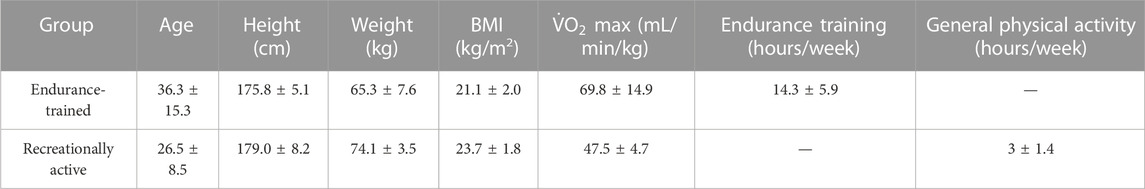
TABLE 1. Anthropometric, physiological, and training characteristics for each group. Data are reported as mean values ±SD.
Gas exchange and lactate measurements
Expiratory ventilation (VE),
FATox rates and
Results
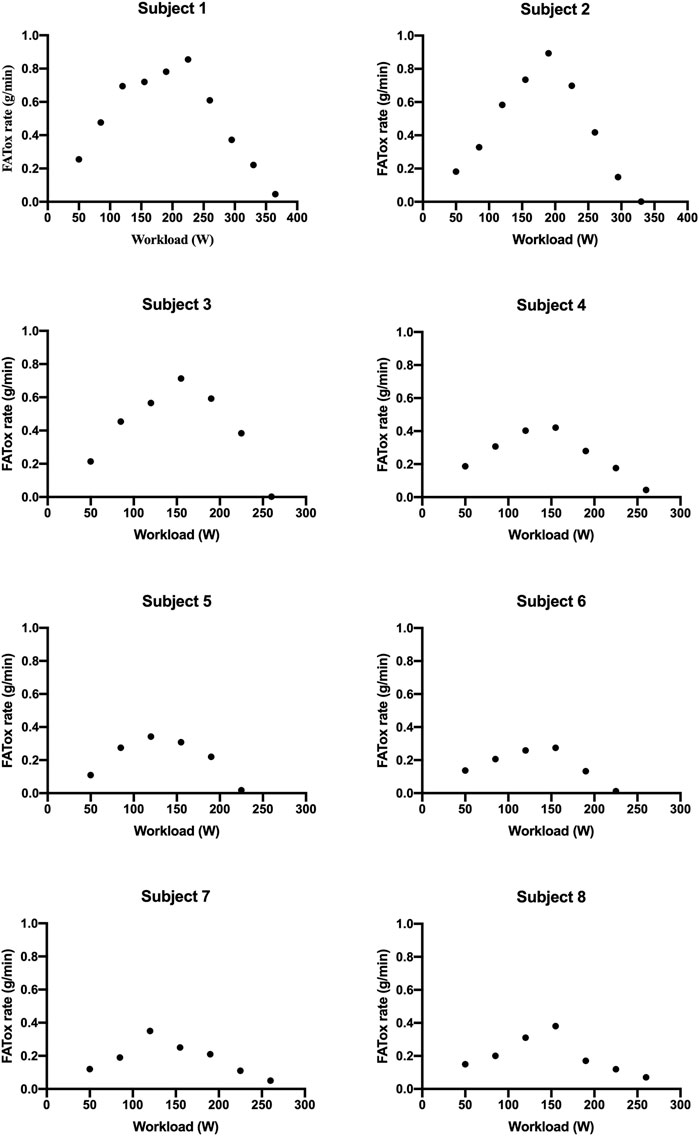
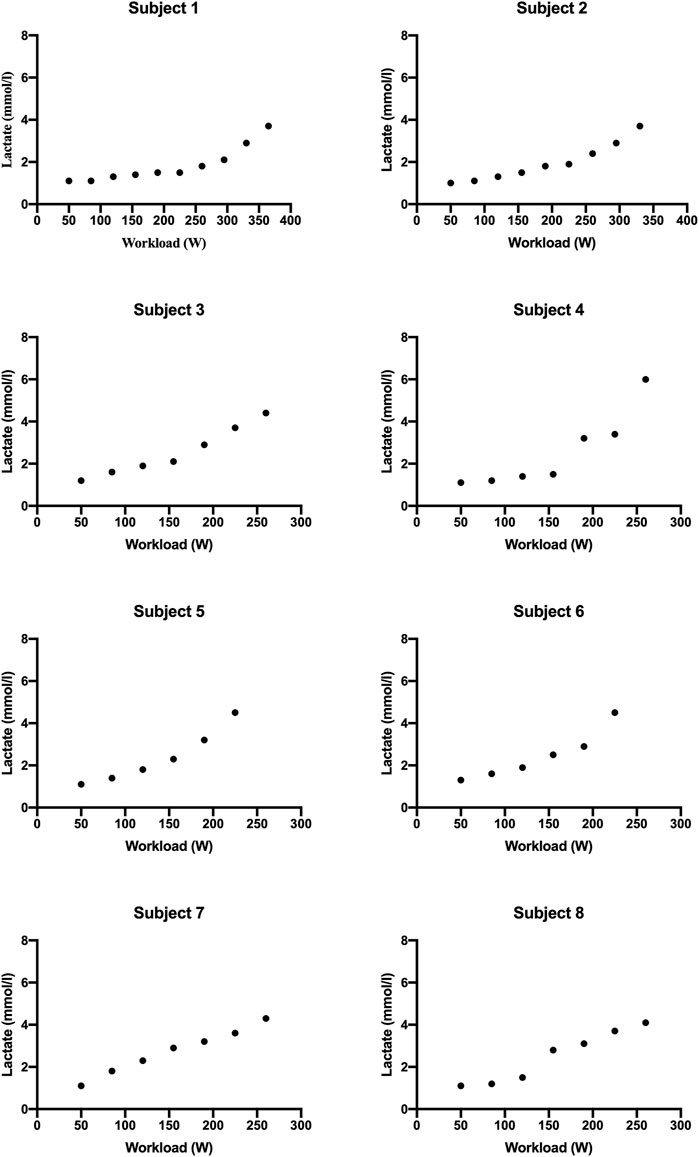
MFO and FATmax values for “endurance-trained” and “recreationally active” subjects are summarized in Table 2. The subjects’ FATox rate for each step is described in Figure 1. Lactate kinetics until the workload step with the lowest FATox rate is described in Figure 2. The MFO and FATmax of “endurance-trained” subjects were 0.85, 0.89, 0.71, and 0.42 and 68%, 69%, 64%, and 53%, respectively. Subjects 1, 2, and 3 showed high MFO and FATmax, and those values both placed over the highest percentile (80th percentile) of normative data. MFO and FATmax of subject 4 placed between the 20th and 40th percentile of MFO normative data and between the 40th and 60th percentile of FATmax normative data. The MFO and FATmax of “recreationally active” subjects were 0.34, 0.27, 0.35, and 0.38 and 47%, 39%, 43%, and 41%, respectively. MFO and FATmax of subject 5 placed on the 20th percentile and between the 20th and 40th percentile, respectively. MFO and FATmax of subject 6 both placed below the 20th percentile. MFO and FATmax of subject 7 placed between the 20th and 40th percentile and below the 20th percentile, respectively. MFO and FATmax of subject 8 placed between the 20th and 40th percentile and below the 20th percentile, respectively. In the four “endurance-trained” subjects, lactate values at MFO were 1.5, 1.8, 2.1, and 1.5, respectively. In the four “recreationally active” subjects, lactate values at MFO were 1.8, 2.5, 2.3, and 2.8, respectively.
Discussion
PASC presents a spectrum of symptoms following acute COVID-19, with exercise intolerance being a prevalent manifestation likely linked to disrupted oxygen metabolism and mitochondrial function (Astin et al., 2023). Specifically, it seems that metabolic disfunction leads to a reduction of the FATox rate at relatively low exercise (de Boer et al., 2022). At the same time, it is well known that endurance training stimulates massive FA mobilization, and in this way, it is capable to improve both MFO and FATmax. Thus, the aim of this study is to analyze MFO and FATmax in different types of subjects with PASC and compare them with normative percentile values. All subjects presented mild symptoms of PASC with preserved pulmonary and cardiac function; thus, they were able to conduct a regular daily work activity. A recent review described normative data for different subject populations, including “endurance-trained,” engaged in training for endurance events, and “recreationally active,” physically active but not trained for endurance events (Maunder et al., 2018). In this context, this study showed how MFO and FATmax of subjects 3 from 4 of “endurance-trained” participants placed over the 80th percentile of their corresponding cohort. Only one “endurance-trained” subject presented MFO and FATmax values below the 60th percentile. These data could be the consequence of the infection of the subject by the virus two times within 6 months. Meanwhile, all “recreationally active” subjects showed MFO and FATmax values placed below the 40th percentile of their corresponding population. From lactate kinetics analysis, it appears how “endurance-trained” subjects show, generally, constant low values throughout the first steps of the test. Meanwhile, in the “recreationally active” group, there emerges, since the early steps, a more linear correlation between lactate values and workload. Indeed, lactate values at MFO in “endurance-trained” subjects were tendentially lower than in “recreationally active” subjects. These data strengthen the hypothesis that specific endurance training improves FA oxidation at relatively low exercise intensity, delaying, in this way, the increase of CHOox and, consequently, the accumulation of blood lactate. Taking into account all these preliminary findings, it could be suggestive of assuming that a training focus on endurance capacity may offer greater protection, compared to other types of exercise, against alterations caused by viral infection, such as mitochondrial dysfunction or, more broadly, anomalies within the pathway of FA oxidation.
Limitations of this study and perspectives
Due to the absence of the subjects’ physiological data prior to SARS-CoV-2 infection, it was not possible comparing metabolic variables before and after infection in this preliminary report. In the future, several studies will be necessary to contribute to a deeper understanding of the relationship between exercise, mitochondrial function, and metabolic dysregulation in PASC subjects. Specifically, it might be useful to design a longitudinal study to investigate the effects of a structured exercise intervention, focusing on endurance training, on individuals with PASC and assess changes in exercise tolerance, mitochondrial function, and metabolic parameters over time. This study could include subgroups with varying exercise intensities and durations to determine the optimal training regimen for improving post-viral exercise intolerance in PASC patients. Additionally, it would be necessary conducting a comprehensive study to analyze metabolic profiles and mitochondrial function in different subtypes of PASC. Through this investigation, it will be possible to understand whether there are distinct metabolic signatures associated with varying symptomatology within PASC. This could involve advanced metabolomics profiling, including plasma metabolites and mitochondrial biomarkers, to identify specific metabolic dysfunctions in subgroups of PASC patients and tailor interventions accordingly. Finally, it could be interesting to compare the impact of different exercise modalities, such as aerobic exercise, resistance training, and a combination of both, on the metabolic and mitochondrial function as well. In this way, considerations for individualized exercise prescriptions based on the severity and nature of PASC symptoms would be valuable in developing targeted rehabilitation strategies.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the University of Milan. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AM: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, resources, writing–original draft, and writing–review and editing. RC: data curation, formal analysis, investigation, supervision, and writing–review and editing. DG: data curation, formal analysis, investigation, software, visualization, and writing–review and editing. SD: data curation, formal analysis, investigation, software, visualization, and writing–review and editing. LL: formal analysis, investigation, methodology, supervision, visualization, and writing–review and editing. LF: conceptualization, formal analysis, investigation, methodology, visualization, writing–original draft, and writing–review and editing.
Funding
The authors declare financial support was received for the publication of this article. This work has been supported by the Italian Ministry of Health–Ricerca Corrente–IRCCS MultiMedica.
Acknowledgments
The authors thank the ‘Fondazione Romeo ed Enrica Invernizzi’ for supporting AM.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Achten J., Jeukendrup A. E. (2004). Relation between plasma lactate concentration and fat oxidation rates over a wide range of exercise intensities. Int. J. Sports Med. 25, 32–37. doi:10.1055/s-2003-45231
Astin R., Banerjee A., Baker M. R., Dani M., Ford E., Hull J. H., et al. (2023). Long COVID: mechanisms, risk factors and recovery. Exp. Physiol. 108, 12–27. doi:10.1113/EP090802
Bircher S., Knetchle B. (2004). Relation between plasma lactate concentration and fat oxidation rates over a wide range of exercise intensities. Int. J. Sports Med. 25, 32–37. doi:10.1055/s-2003-45231
de Boer E., Petrache I., Goldstein N. M., Olin J. T., Keith R. C., Modena B., et al. (2022). Decreased fatty acid oxidation and altered lactate production during exercise in patients with post-acute COVID-19 syndrome. Am. J. Respir. Crit. Care Med. 205, 126–129. doi:10.1164/rccm.202108-1903LE
Frandsen J., Vest S., Larsen S., Dela F., Helge J. (2017). Maximal fat oxidation is related to performance in an ironman triathlon. Int. J. Sports Med. 38, 975–982. doi:10.1055/s-0043-117178
Frayn K. N. (1983). Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 55, 628–634. doi:10.1152/jappl.1983.55.2.628
Guntur V. P., Nemkov T., de Boer E., Mohning M. P., Baraghoshi D., Cendali F. I., et al. (2022). Signatures of mitochondrial dysfunction and impaired fatty acid metabolism in plasma of patients with post-acute sequelae of COVID-19 (PASC). Metabolites 12, 1026. doi:10.3390/metabo12111026
Hurley B. F., Nemeth P. M., Martin W. H., Hagberg J. M., Dalsky G. P., Holloszy J. O. (1986). Muscle triglyceride utilization during exercise: effect of training. J. Appl. Physiol. 60, 562–567. doi:10.1152/jappl.1986.60.2.562
Martin W. H., Dalsky G. P., Hurley B. F., Matthews D. E., Bier D. M., Hagberg J. M., et al. (1993). Effect of endurance training on plasma free fatty acid turnover and oxidation during exercise. Am. J. Physiology-Endocrinology Metabolism 265, E708–E714. doi:10.1152/ajpendo.1993.265.5.E708
Maunder E., Plews D. J., Kilding A. E. (2018). Contextualising maximal fat oxidation during exercise: determinants and normative values. Front. Physiol. 9, 599. doi:10.3389/fphys.2018.00599
McArdle W. D., Katch F. I., Katch V. L. (2001). Exercise Physiology: energy, nutrition, and human performance. 5th ed. Philadelphia: Lippincott Williams and Wilkins.
Midgley A. W., McNaughton L. R., Polman R., Marchant D. (2007). Criteria for determination of maximal oxygen uptake: a brief critique and recommendations for future research. Sports Med. 37, 1019–1028. doi:10.2165/00007256-200737120-00002
Midgley A. W., Mc Naughton L. R., Wilkinson M. (2006). Criteria and other methodological considerations in the evaluation of time at V.O2max. J. Sports Med. Phys. Fit. 46, 183–188.
Phillips S. M., Green H. J., Tarnopolsky M. A., Heigenhauser G. J. F., Hill R. E., Grant S. M. (1996). Effects of training duration on substrate turnover and oxidation during exercise. J. Appl. Physiol. 81, 2182–2191. doi:10.1152/jappl.1996.81.5.2182
Wood E., Hall K. H., Tate W. (2021). Role of mitochondria, oxidative stress and the response to antioxidants in myalgic encephalomyelitis/chronic fatigue syndrome: a possible approach to SARS-CoV-2 ‘long-haulers. Chronic Dis. Transl. Med. 7, 14–26. doi:10.1016/j.cdtm.2020.11.002
Keywords: post-acute sequelae of SARS-CoV-2 infection, fat oxidation, metabolic dysfunction, cycling, exercise performance
Citation: Meloni A, Codella R, Gotti D, Di Gennaro S, Luzi L and Filipas L (2023) Fat oxidation rates and cardiorespiratory responses during exercise in different subject populations with post-acute sequelae of SARS-CoV-2 infection: a comparison with normative percentile values. Front. Physiol. 14:1310319. doi: 10.3389/fphys.2023.1310319
Received: 09 October 2023; Accepted: 27 November 2023;
Published: 08 December 2023.
Edited by:
Simone Luti, University of Florence, ItalyReviewed by:
Samuel Honório, Polytechnic Institute of Castelo Branco, PortugalKarin Vonbank, Medical University of Vienna, Austria
Copyright © 2023 Meloni, Codella, Gotti, Di Gennaro, Luzi and Filipas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Meloni, YW5kcmVhLm1lbG9uaUB1bmltaS5pdA==
 Andrea Meloni
Andrea Meloni Roberto Codella
Roberto Codella Daniel Gotti
Daniel Gotti Simone Di Gennaro
Simone Di Gennaro Livio Luzi
Livio Luzi Luca Filipas
Luca Filipas