- 1The Fifth Clinical College of Guangzhou University of Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Department of Orthopedics, Guangdong Provincial Second Hospital of Traditional Chinese Medicine, Guangzhou, China
Background: Cellular senescence is associated with age-related pathological changes, senescent cells promote the development of knee osteoarthritis. A better understanding between knee osteoarthritis and cellular senescence may enhance the effectiveness of therapies that aim to slow or stop the progression of this disease.
Purpose: This study aimed to systematically analyze and visualize the publication trends, research frontiers and current research hotspots of knee osteoarthritis and cellular senescence by using bibliometrics.
Methods: The publication search was performed on the Web of Science Core Collection database for documents published from 1992 to 2023. VOSviewer, Citespace, R package Bibliometrix and Microsoft Office Excel were used to study the characteristics of the publications. The publication number, countries, institutions, authors, journals, citations and co-citations, keywords were analyzed.
Results: A total of 1,074 publications were analyzed, with an average annual growth rate of 29.89%. United States accounted for the biggest contributor, ranked first in publications and citations. Publications of this field were published in 420 journals, OSTEOARTHRITIS and CARTILAGE was the most influential. A total of 5,657 authors contributed to this research. The most productive author was Lotz, MK (n = 31, H-index = 22, Total citation = 2,619), followed by Loeser, R.F (n = 16, H-index = 14, Total citation = 2,825). However, the collaboration between authors was relatively weak. Out of the 1,556 institutions involved, 60% were from the United States. Scripps Research ranked first with 25 papers and a total of 2,538 citations. The hotspots of this field had focused on the pathomechanisms (e.g., expression, inflammation, apoptosis, autophagy, oxidative stress) and therapeutics (e.g., stem cell, platelet-rich plasma, transplantation, autologous chondrocytes, repair), and the exploration of Senolytics might be the important direction of future research.
Conclusion: Research on the cross field of knee osteoarthritis and cellular senescence is flourishing. Age-related pathomechanism maps of various cells in the joint and the targeted medicines for the senescent cells may be the future trends. This bibliometric study provides a comprehensive analysis of this cross field and new insights into future research.
1 Introduction
Knee osteoarthritis (KOA) is a chronic, degenerative disease commonly seen in clinic practice. Pathological changes involved the entire knee joint, including hyaluronic articular cartilage, subchondral bone, ligaments, joint capsule, synovium, sub-patellar fat pad, and periarticular muscles (Sharma, 2021). KOA progresses slowly and eventually leads to loss of mobility due to pain and impaired joint motion, especially in the elderly (Katz et al., 2021). KOA is not a disease driven by a single factor, but rather a heterogeneous syndrome. Risk factors such as joint injury, obesity, genetics, and metabolic bone disease are all associated with KOA (Yao et al., 2023), but the most common risk factor is age (Jeon et al., 2018). Numerous studies in recent years have shown that cellular senescence plays an important role in the development and progression of OA.
Cellular senescence was first proposed by Hayflick and Moorhead in 1961 (Hayflick and Moorhead, 1961). Cellular senescence is a stable terminal state in which the cell irreversibly leaves the cell cycle and enters a growth arrest (Gorgoulis et al., 2019). Telomere shortening, oxidative stress, and abnormal chromatin structure lead to nuclear DNA damage, resulting in cell cycle arrest and eventually cellular senescence (Roupakia et al., 2021). Cellular senescence is a stress response, mainly to remove damaged cells and eventually achieve tissue regeneration; however, with aging or continuous stimulation, the balance between senescence and regeneration is disrupted, and cellular senescence then becomes a problem rather than a normal procedure in tissue regeneration (Huang et al., 2022).
Numerous studies have found that cells in KOA exhibit a variety of aging-related phenotypes, like other organs, cells in the joint also show senescence and degeneration over time, and the number of senescent cells increases with aging (Diekman et al., 2018). However, the specific mechanism of cellular senescence related to KOA remains unclear, and many researchers have begun to explore targeting senescent cells as a treatment for KOA.
Bibliometrics analysis was first introduced by Prichard in 1969, has been widely utilized for assessing and quantifying publication data, including researchers. Countries and their cooperation, analysis of keywords, references, and co-citation can reveal the global trends and research hotspots. Outcomes from the bibliometric study can help researchers to identify the current research concerns to guide future research directions (Abramo et al., 2011).
However, bibliometric features have not been explored in the cross field of cellular senescence and KOA. The research on KOA and cellular senescence is progressing rapidly, it can be challenging for novice researchers to quickly grasp a thorough understanding of this field. Consequently, it is crucial to provide an overarching overview of research trends, focal areas, and significant contributions made by institutions and authors in this field. In this study, we aimed to analyze the publications in the cross field of KOA and cellular senescence from 1992 to 2023 through bibliometric methods. We conducted a thorough analysis to determine the current research status and knowledge base, aiming to assist researchers in understanding the historical hotspots and future research trends in this field.
2 Methods
2.1 Data collection and retrieval strategies
Web of Science Core Collection (WOSCC) is currently considered as one of the most comprehensive and authoritative databases for bibliometrics compared to databases such as Scopus and Pubmed (Zhu et al., 2020; Wu et al., 2021). We conducted a comprehensive search of all literature on KOA and cellular senescence using WOSCC on 10 May 2023. The search strategy was TS = Cellular Senescence OR Cell Aging AND TS = Knee Osteoarthritis, literature type = (Article OR Review), language = English. Upon completion of the search, the information data of the retrieved literature was selected as “Full Record and Cited References” and downloaded from the WOSCC database for further analysis. The literature information included authors’ name, publication year, institutions, countries, keywords, journals, citations and H-index, etc.
2.2 Data analysis and visualization
We used R package Bibliometrix, VOSviewer 1.6.18, Microsoft Office Excel 2021 and Citespace 5.8r5 for bibliometric analysis of the final included publications. R package Bibliometrix was used to extract basic information of all publications, such as the number of publications, types of publications, number of publications by authors, number of publications by journals, H-index of authors and journals, etc. VOSviewer was used for co-occurrence, co-authorship and co-citation analysis of keywords, countries, institutions and citations. The study utilized Microsoft Office Excel 2021 to analyze annual publication statistics and predict future trends. CiteSpace was used to detect the keywords and references with strong citation bursts, which were used as markers to identify research hotspots in different periods. Co-occurrence, co-authorship and co-citation analysis could identify high-frequency items and the links between them. Hotspots represented research directions that received significant attention from researchers. The citation burst analysis could identify references and keywords that experienced a notable increase in citations within a short period, indicated researchers’ interest during that time. Figure 1A showed the workflow diagram of this study.
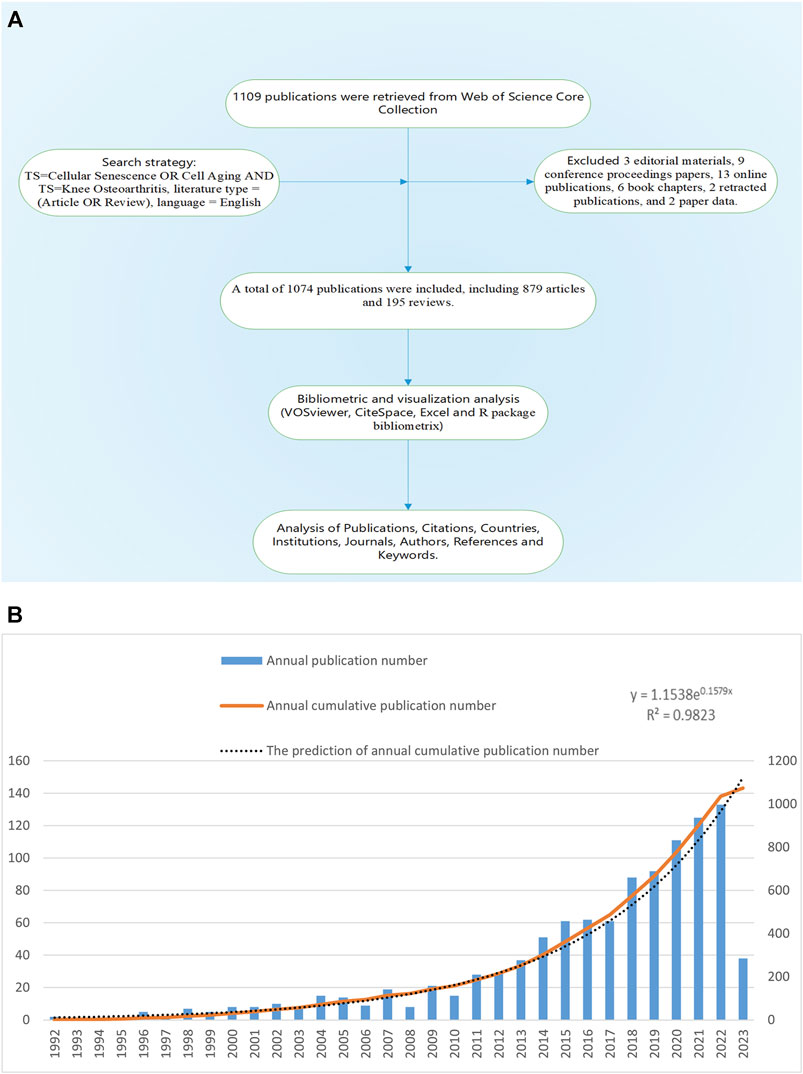
FIGURE 1. (A) Workflow diagram of this study. (B) The annual number of publications of KOA and cellular senescence from 1992 to 2023.
3 Results
3.1 Publication summary
Based on the search criteria, 1,109 publications from 1992 to 2023 were retrieved, excluding 3 editorial materials, 9 conference proceedings papers, 13 online publications, 6 book chapters, 2 retracted publications, and 2 paper data. A total of 1,074 publications were included, written by 5,756 authors from 1,556 institutions and published in 420 journals, including 879 articles and 195 reviews.
The annual publication number of this field was low before 2001, rose slowly from 10 to 28 in 2002–2011, and entered a rapid growth period in 2011–2022, the annual publication number increased from 29 to 133, with an average annual growth rate of 29.89% (Figure 1B). The growth prediction model based on Microsoft Office Excel 2021:
The prediction model was highly consistent with the actual situation. It should be noted that 2023 was only 5 months away, so we did not include the publications of 2023. According to the prediction model, the number of publications in 2023 will reach 145, while the actual number of publications from January 1 to May 10 was 38, basically in line with the predicted amount.
3.2 Analysis of countries’ contribution
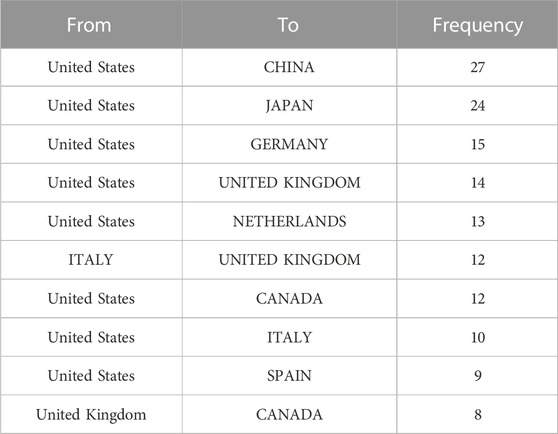
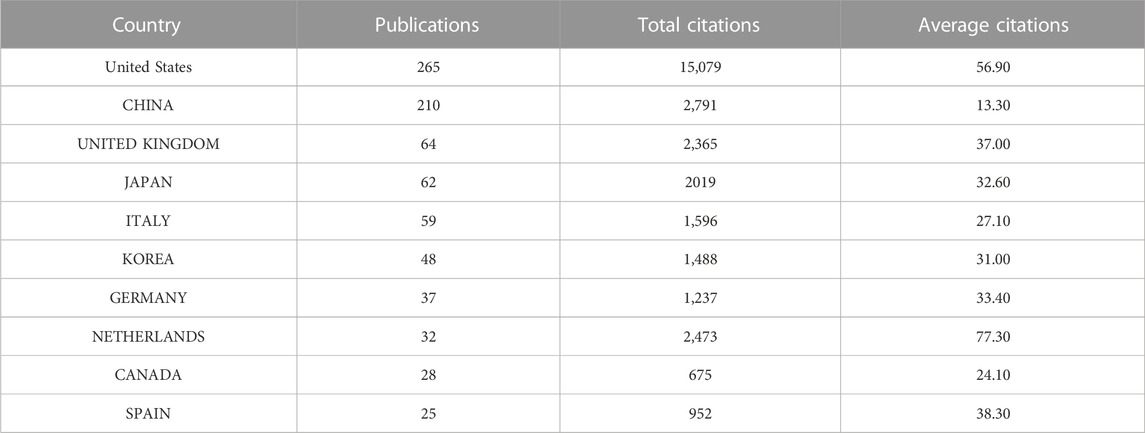
The publication number of different country/region was shown in Table 1, United States contributed the most (n = 265,24.7%), followed by China (n = 210,19.6%), the United Kingdom (n = 64, 6%), Japan (n = 62, 5.8%), and Italy (n = 59, 5.5%). Regarding the number of national citations, United States remained the leader in this area with 15,079 total citations and 56.90 average citations, China with 2,791 total citations and 13.30 average citations, the United Kingdom with 2,365 total citations and 37.00 average citations, and Japan with 20,19 total citations and 32.60 average citations. It was worth noting that the publication number of Netherlands was only 32, but with 2,473 total citations and 77.30 average citations, ranking first among all countries. As shown in Figure 2A, United States cooperated the most with other countries, including China, Japan, Germany, the United Kingdom, and the Netherlands. Table 2 showed the information of the top ten countries with most cooperation frequency.
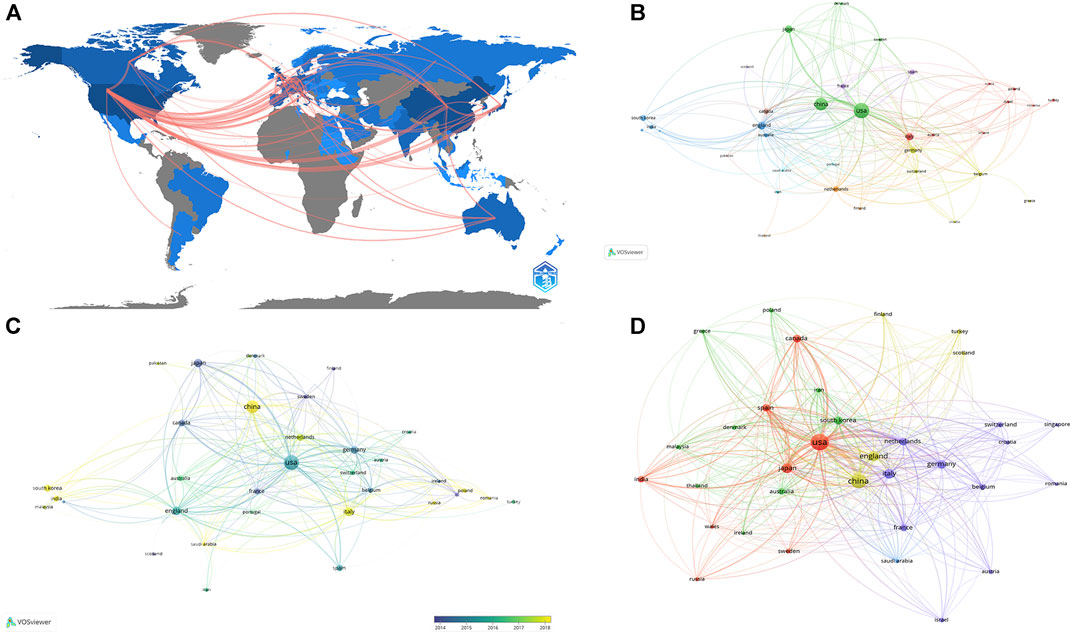
FIGURE 2. The analysis of countries (A) Shades of the color represented the publication number, the thickness of the line represents the frequency of cooperation. (B, C) The network map of countries, the size of the nodes represents the number of publications (B). The color represents the average appearing year (C). (D) The co-citation analysis of countries, the size of the nodes represents the number of total citations.
3.3 Analysis of higher-impact journals
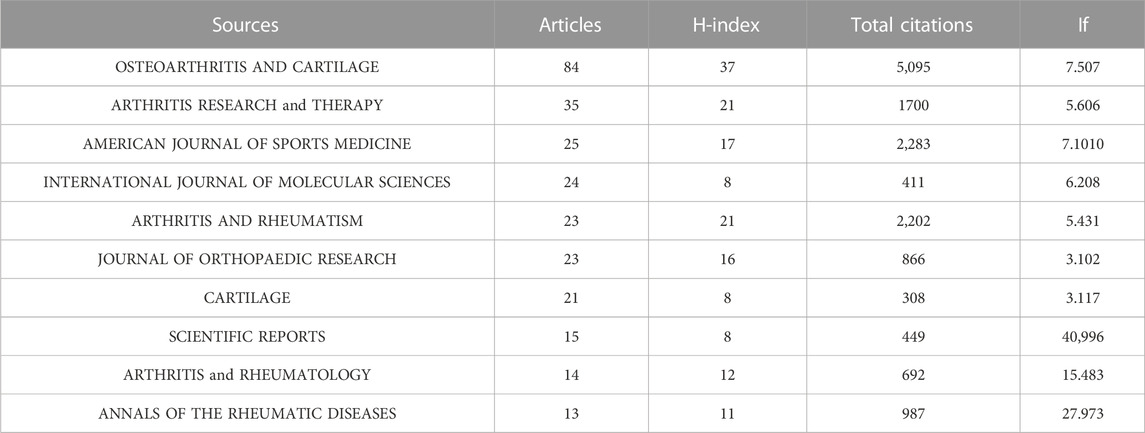
Research of KOA and cellular senescence was published in 420 journals. According to Bradford’s theorem (Figure 3C), the 17 core journals were those with 9 or more publications. Table 3 summarized the basic information of the top ten journals with high publication number. Based on publication number and H-index of journals, OSTEOARTHRITIS and CARTILAGE was the most influential, with 84 publications (H-index = 37, Total citations = 5,095, UK), including 76 articles and 8 reviews, indicated that the journal always paid attention to high-quality research and had been widely recognized in the cross field of KOA and cellular senescence.
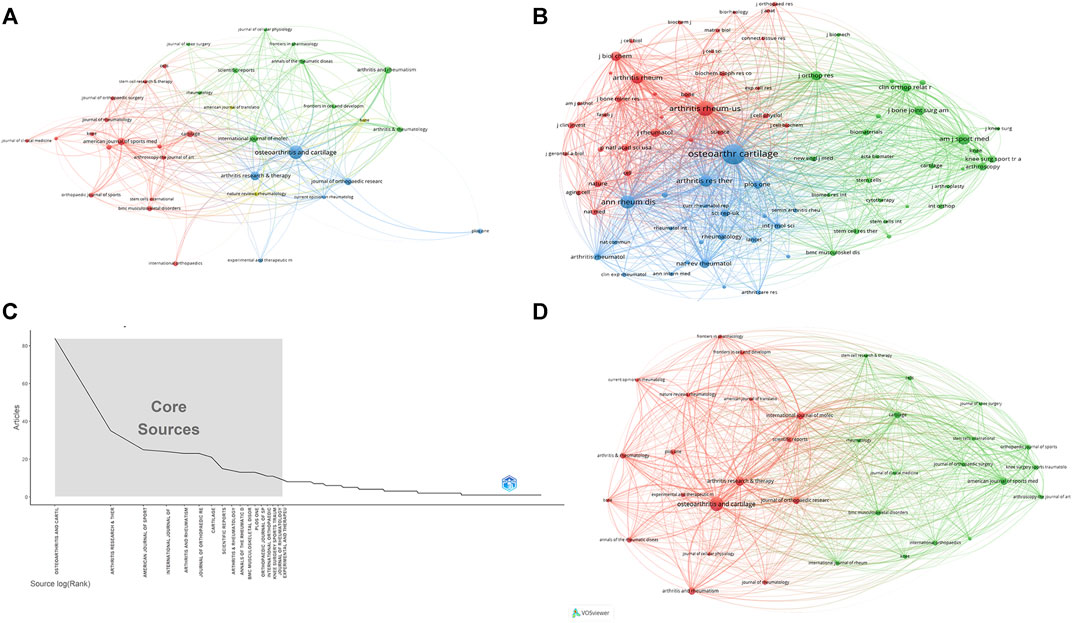
FIGURE 3. The analysis of journals (A) The analysis of publications, the size of the nodes represents the publication number of journals. (B) The analysis of citations, the size of the nodes represents the total citations of journals. (C) Core journals according to the Bradford’s Law. (D) The co-citation analysis of journals, the size of the nodes represents the total citations of journals.
3.4 Analysis of institutions
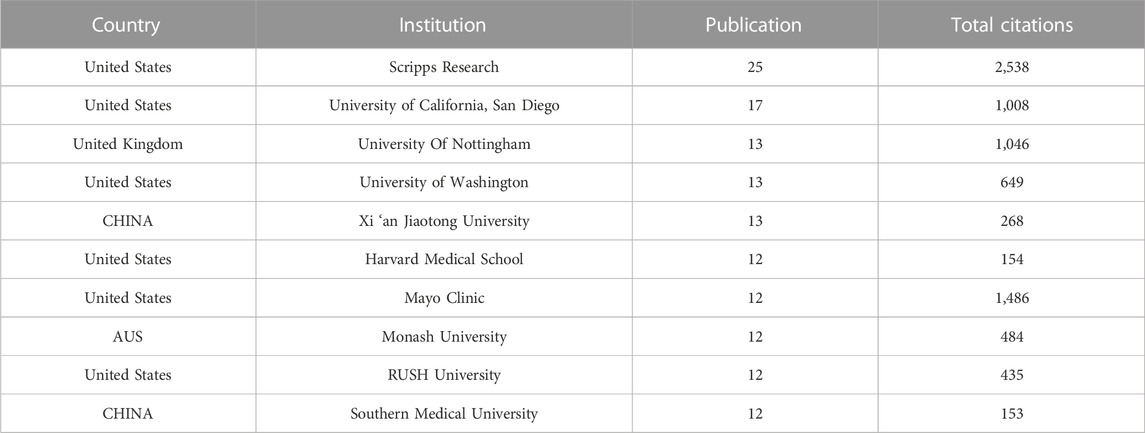
A total of 1,556 institutions published 1,074 publications. Table 4 showed the top ten institutions with highest publication number. Among these institutions, 60% were from United States, and the others were two from China, one from the United Kingdom, and one from Australia. Scripps Research ranked first with 25 papers and 2,538 total citations, followed by the University of California, San Diego (n = 17,TC = 1,008), and the University of Nottingham (n = 13, Total citations = 1,046), the University of Washington (n = 13,TC = 649), and Xi ‘an Jiaotong University (n = 13,TC = 268). Table 5 showed the difference in high publications institutions and high citations institutions, High citation institutions generally started their research earlier, such as Wake Forest University, Johns Hopkins University, Scripps Research, Mayo Clinic, Cornell University, etc. Figure 4A showed a strong cooperative relationship between Scripps Research, University of California, San Diego, and University of Washington, which had a higher number of citations and publications.
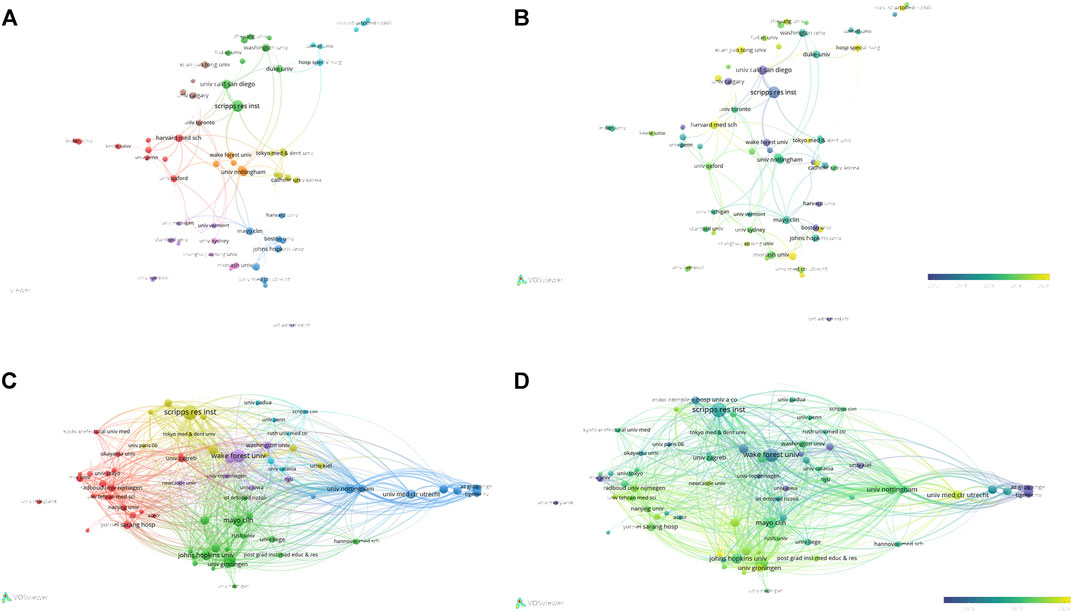
FIGURE 4. The analysis of institutions (A, B) The network map of institutions, the size of the nodes represents the number of publications. The color of the nodes represents the average appearing year (B). (C, D) The analysis of institutions’ citations, the size of the nodes represents the number of citations. The color represents the average appearing year (D).
3.5 Analysis of authors
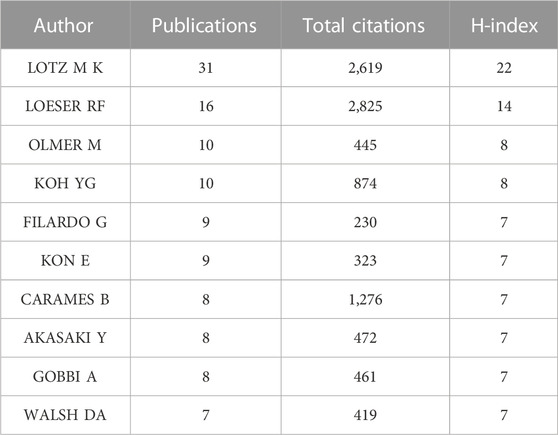
5,676 authors had published 1,074 literature in this field. Table 6 showed the information of top ten authors with the highest publication number. Lotz M.K was the leader in this field based on publication number, citations, and H-index, and had long been deeply involved in the research of KOA and cellular senescence. Figure 5A showed the analysis of authors, with some collaboration only between highly influential authors Lotz M.K, Loeser R.F, Carames Blanco F.J. The collaboration between other authors was weak, indicated that researchers in this field need to cooperate more closely and frequently to promote the development of this field.
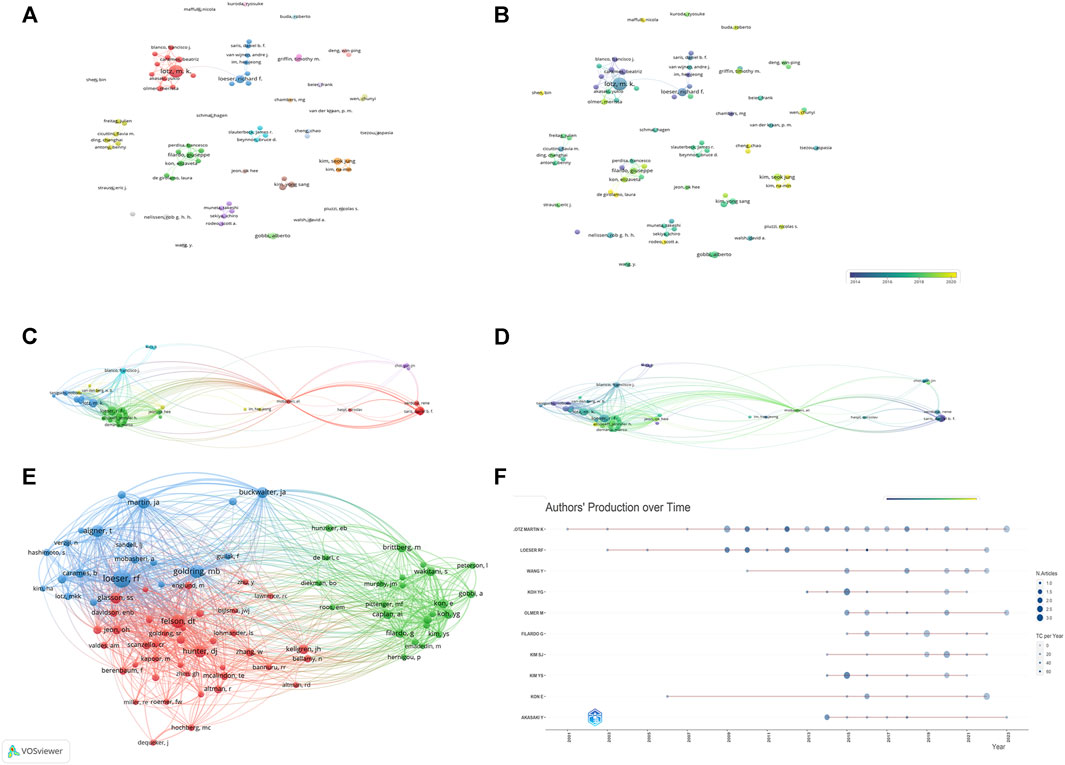
FIGURE 5. The analysis of authors (A, B) The network map of authors, the size of the nodes represents the number of publications. The color of the nodes represents the average appearing year (B). (C, D) The citation number of authors, the size of the nodes represents the number of citations. The color represents the average appearing year (D). (E) The bibliographic coupling of authors, the size of the nodes represents the number of publications. (F) The publication over time of top ten productive authors.
3.6 Analysis of citations and co-citations
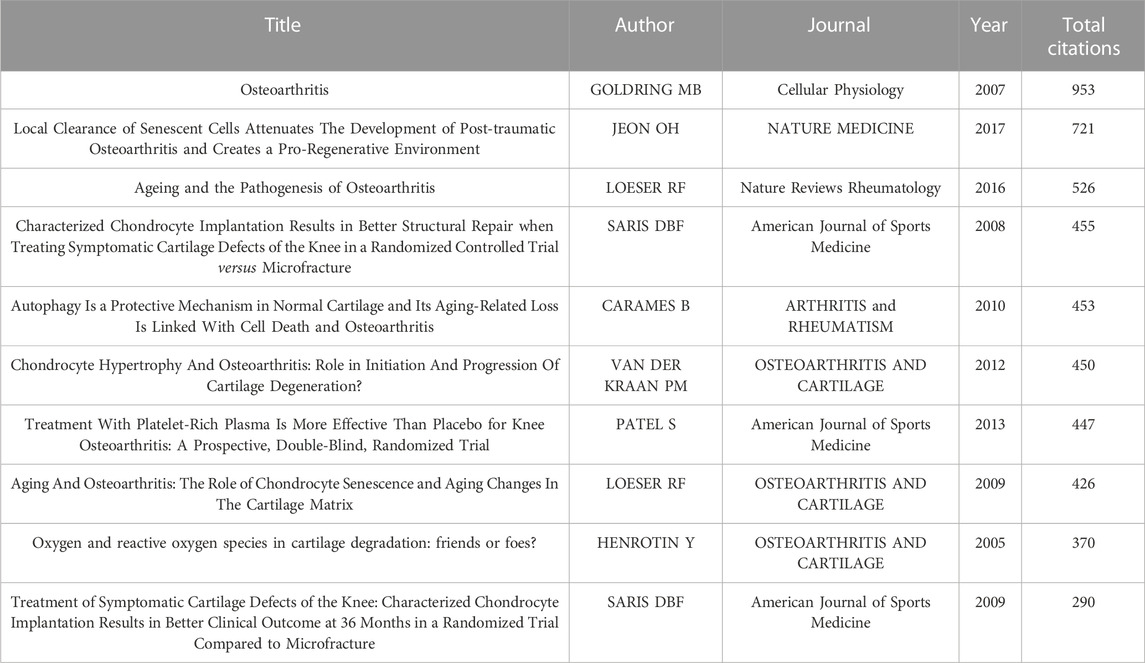
A total of 205 publications with more than 50 citations were identified. Table 7 showed the top ten publications with highest citations. Figure 6A showed the network map of total citations of publications. Figure 6B showed the network map of co-cited references. Additionally, a co-citation analysis of 39,069 references was conducted, and 99 literatures were cited more than 20 times. The first was published by Kellgren J.H and Lawrence J.S in 1957, proposed the Kellgren-Lawrence grading scoring system of osteoarthritis, which classified KOA from mild to severe as grade 0, I, II, III, and IV based on the features of x-ray (Kellgren and Lawrence, 1957). The second was published by Loeser R.F et al., in 2012, which outlined the pathological changes of joint tissues in KOA and the mechanisms of these changes (Loeser et al., 2012). The third was published by Brittberg M et al., in 1994, the author used cultured autologous chondrocytes for the repair of deep cartilage defects on the tibiofemoral articular, achieved good results (Brittberg et al., 1994). The fourth was published by Jeoh et al., in 2017, which found that selective removal of local senescent cells can significantly promote the regeneration of patient cartilage (Jeon et al., 2017). The fifth was published by Glasson S.S et al., in 2010, which proposed the OARSI score of mouse osteoarthritis tissue (Glasson et al., 2010). We also conducted the top 25 references with citation burst, to identify Figure 6D showed the top ten references with most local citations the references with a remarkable citation in a relatively short period of time (Figure 6C).
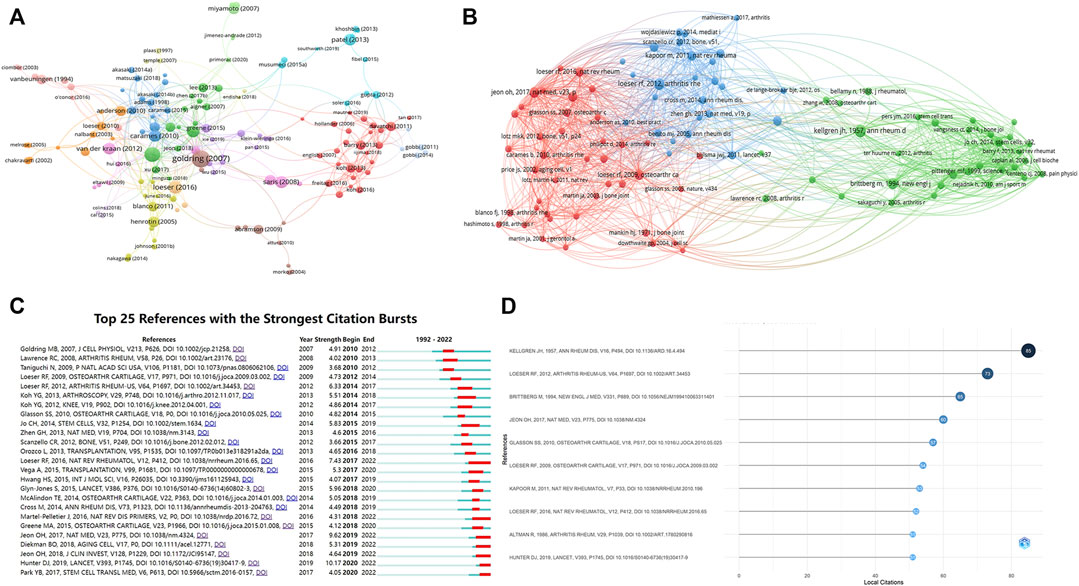
FIGURE 6. The analysis of citation and Co-citation (A) Network map of total citations of publications. (B) Network map of co-cited references. (C) Top 25 references with strongest citation bursts. (D) The top ten references with most local citations.
3.7 Analysis of keywords
Figure 7A illustrated the network analysis of keywords, the node size represented the occurrence frequency, the distance of two nodes reflected their association strength, and the nodes with a closer distance were classified as the same cluster. Knee osteoarthritis and cell senescence which appeared most frequently in this study were not shown. Red represented cluster I, which showed the tissues, cells, and metabolic processes associated with cell senescence. Such as cartilage, subchondral bone, chondrocytes, anterior cruciate ligament, synovial fluid, collagen, and matrix, etc. Blue represented cluster II, the main nodes were inflammation, progression, mechanism, etiology, epidemic, risk. Green represented cluster III, mainly focused on clinical treatments, such as stem cells, intra-articular injections, autologous chondrocyte transplantation, repair, and platelet-rich plasma.
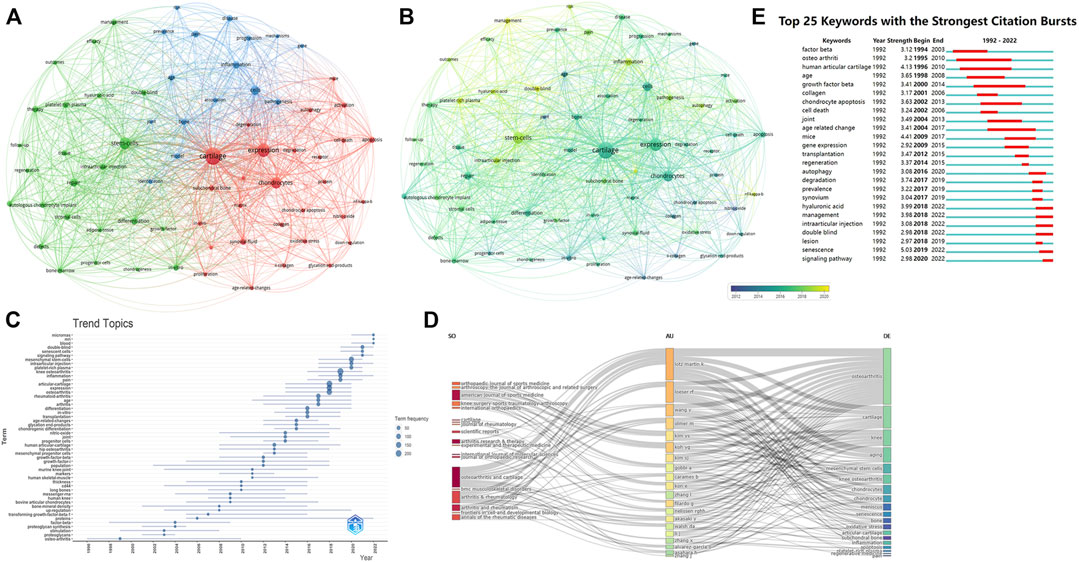
FIGURE 7. The analysis of keywords (A, B) Network map of keywords, the size of the nodes represents the frequency. The color represents the average appearing year (B). (C) The trend topics based on keywords generated by R package Bibliometric. (D) The three-plot field of journals, authors and keywords. (E) Top 25 keywords with strongest citation bursts.
In overly visualization the color from blue to yellow represented the early and late appearance of the keywords. The research on cartilage, chondrocytes, collagen, and nitric oxide appeared earlier, and researchers had recently targeted autophagy, inflammation, stem cells. Figure 7C showed the topic trends, and the results were generally consistent with the overly visualization. The top 25 keywords with the strongest citation burst were also conducted, in order to show the historical research trend of this field (Figure 7E).
In addition, we also constructed a three-field plot of journals, authors and keywords (Figure 7D), and the results were in line with the above analysis. Lotz M.k and Loeser R.F were most closely related to this cross field, and their research was mainly focused on cartilage changes. The journals most closely associated with this field were Osteoarthritis and Cartilage, American Journal of Sports Medicine, and Arthritis and Rheumatism.
3.8 Analysis of research topic and types in different countries
In order to show the research topics and types among countries, we separately extracted the bibliometric information of articles, reviews and countries with a large number of publications. Due to the publication number of countries other than the United States and China were relatively small, we conducted keywords network analysis of the United States and China. Figure 8 illustrated the co-authorship analysis of articles and reviews among countries, and keywords network analysis for United States and China. Our findings showed that the United States contributed the largest number of articles, while the review number of China was in the same level with the United States. Indicated that China should focus on improving the quality and quantity of studies. Keywords network analysis indicated that the United States had conducted more extensive research in this field, with deep cultivation in pathomechanisms and therapeutics, while the research topics of China were mainly focused on pathomechanisms.
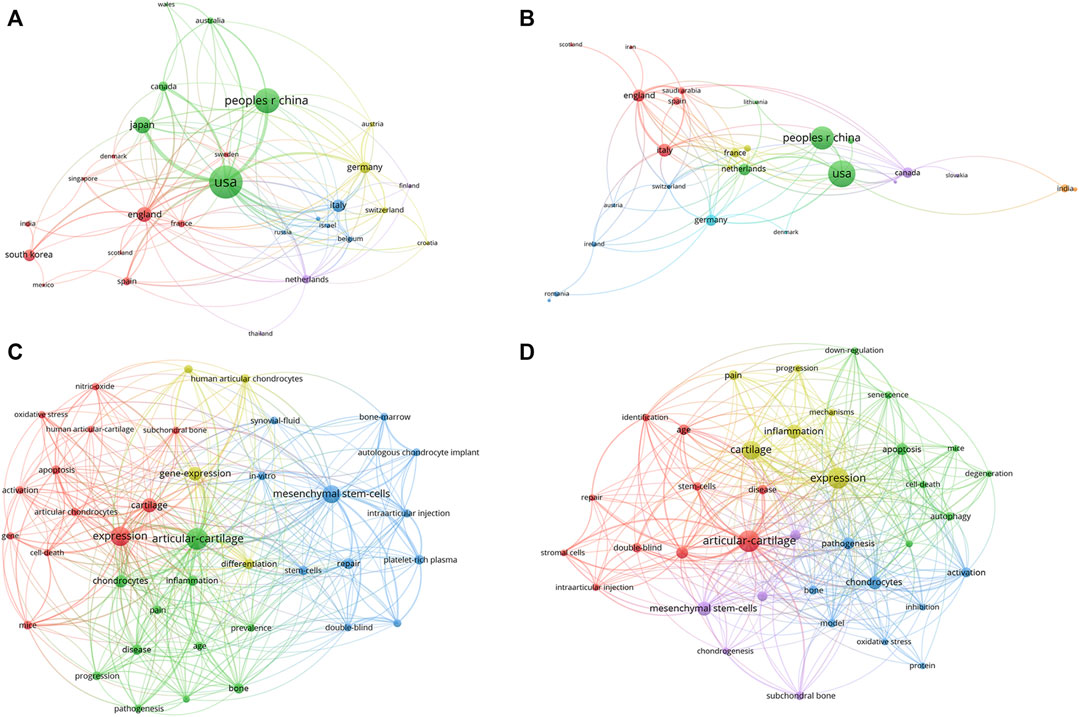
FIGURE 8. The analysis of research topic and types in different countries (A) Countries’ network map of articles, the size of the nodes represents the frequency. (B) Countries’ network map of reviews, the size of the nodes represents the frequency. (C) The keywords network map of United States, the size of the nodes represents the frequency. (D) The keywords network map of China, the size of the nodes represents the frequency.
4 Discussion
4.1 Summary of findings
Bibliometric analysis was a method of evaluating importance and influence of publications from various aspects, providing a reliable analysis of bibliometric parameter for researchers in a certain field (Ellegaard and Wallin, 2015). The analysis of the annual publication number represented the popularity of research in this field (Wen et al., 2022). The annual publication number in this field began to grow rapidly after 2010 Until the date of our bibliometric data was downloaded, the number of publications in 2023 was basically in line with the prediction model, indicated that this field had been paid more and more attention from researchers.
With the continuous progress of medical technology and social productivity, many countries had entered the stage of aging population, and the incidence of KOA increased year by year, which brought heavy economic burden on both society and patients (Giorgino et al., 2023). The top ten countries in terms of publication number and citations were all developed countries except China, which might be related to the investment of scientific research funding and the increase of incidence in these countries. As the biggest developing country and the second largest population in the world, China had a large number of KOA patients. The number of publications in China might be related to these aspects. Although the number of publications in China had been growth in recent years, the citations was relatively low, suggested that Chinese researchers might need to focus on improving the quality of their research to enhance their impact and contribution in this field.
The country with the largest contribution in this cross field was United States, which was far ahead of other countries, and had conducted more collaborations with other countries. This might be related to the fact that United States had the highest healthcare spending globally. It was worth noting that the number of publications of Netherland was not prominent, but the average citations of Netherland ranked first, indicated that the quality of these research was relatively high.
Among the top ten institutions, six were from the United States, two were from China, and the rest were from the United Kingdom and Australia. The institution with the highest publications number was Scripps Research (n = 25, Total citation = 2,538), which was the absolute leader in this field. It was worth noting that Wake Forest University, with only ten publications, but the citation number had reached 1989. The network map of institutions showed that the institutions from the United States and Europe had conducted a longer period of time in this cross field. In recent years, some institutions in China had also made a lot of exploration in this field, such as Xi ‘an Jiaotong University and Southern Medical University. Although the publication number of these institutions was high, but the citation number was quite low. On the one hand, it might be due to the short time since publication, which had not form a large number of citations. On the other hand, it might be due to the low quality of publications, which had not form a huge impact in this filed.
The publication number of Scripps Research might be related to the core author in this field, Lotz M.K (n = 31, H-index = 22, Total citation = 2,619), who mainly focused on joint senescence, the pathogenesis of osteoarthritis and drug discovery. The second highest contributor in this field was Loeser R.F (n = 16, H-index = 14, Total citation = 2,825). It was worth noting that although the publication number of Loeser R.F was much lower than Lotz M.K, there were 2,825 citations of Loeser’s publications, ranking first among all authors. The two reviews in 2009 and 2016 were cited for 426 times and 526 times, respectively (Loeser, 2009; Loeser et al., 2016). Loeser mainly focused on the mechanisms related to joint tissue destruction in osteoarthritis. The analysis of co-authorship showed that the correlation between authors was low. Indicated that it was necessary to establish stronger cooperation between researchers in this field, which might be crucial to the development of this field.
The publications of KOA and cellular senescence were published in 420 different journals. According to Bradford Theorem, 18 core journals accounted for 4.28% of all journals, with a total of 356 publications accounting for 33% of the total publications. The most influential was OSTEOARTHRITIS and CARTILAGE (n = 84, H-index = 37, Total citation = 5,095), ranking first among all the journals. Among the other journals, ARTHRITIS and RHEUMATISM and AMERICAN JOURNAL of SPORTS MEDICINE had high impact, with large number of publications and citations. Among the top ten journals in terms of publication number, only ARTHRITIS and RHEUMATOLOGY had an impact factor higher than 10. Indicated that it was challenging to publish literature of this field on high-impact factor journals.
4.2 Hot topic in basic and clinical research
Since Hayflick and Moorhead proposed the concept of cellular senescence in 1961, many researchers had investigated the pleiotropic role of cellular senescence as a driver of diseases, including tumors, atherosclerosis, cardiac dysfunction, and renal insufficiency (Wang and Bennett, 2012; Docherty et al., 2019; Chen et al., 2022b; Schmitt et al., 2022). As one of the hallmarks of aging, the increased burden of senescent cells in various tissues was the main pathogenic factors of aging-related diseases (Tominaga, 2015). The current understanding of cellular senescence in KOA was that the senescence of all tissues would impact on the degenerative changes of the whole joint (Wu et al., 2022). The network analysis of keywords revealed that the most frequent keywords were cartilage, expression, stem cells, and chondrocytes. And all the keywords were divided into three clusters, in which red was cluster I, green was cluster II, and blue was cluster III.
The combination of cluster I and cluster II were roughly the internal structure of the joint and the pathological changes in the development of KOA. Such as cartilage, chondrocytes, bone, subchondral bone, joint synovial fluid, anterior cruciate ligament, expression, inflammation, oxidative stress, autophagy, apoptosis, mechanism, pathology, etc. Initially, researchers considered KOA as a matter of mechanical damage, in contrast, KOA was caused by an imbalance of the whole joint tissues. The focus of researchers was on the senescent associated secretory phenotype (SASP) of senescent cells; the effect of senescent cells on surrounding cells; the upstream regulatory mechanisms of cellular senescence. In this process, the pathological changes of articular cartilage associated with aging were most concerned by researchers, because the most significant pathological changes in KOA were degeneration of chondrocytes and degradation of the extracellular matrix. Articular chondrocytes were usually in a state of low proliferation, after joint injury, chondrocytes could enhance their proliferation capacity to repair cartilage, but it would also make chondrocytes more prone to cell senescence, thereby promoting the development of KOA (Ashraf et al., 2016; Loeser et al., 2016; He and Sharpless, 2017).
However, some researchers believed that the effect of chondrocyte senescence on KOA might be driven by SASP factors, rather than the decline of chondrocytes proliferation (Greene and Loeser, 2015; Chen et al., 2022a). SASP referred to the secretion of specific bioactive molecules by senescent cells that could induce a range of physiological responses in the surrounding microenvironment, including inflammation, cell cycle arrest, and tumors (Birch and Gil, 2020). Several common SASP factors such as MCP1, IL1, IL6, MMP3, and MMP13, which contributed to the inflammatory microenvironment of KOA (Liu et al., 2021). Chronic inflammation might lead to the senescence of other cells in the joint, extracellular matrix breakdown, synovial inflammation and subchondral bone remodeling (Cai et al., 2020). Moreover, some inflammatory cytokines in synovial fluid might also be the source of pain in KOA (Nees et al., 2019).
Xu and others found that the transplantation of senescent fibroblasts into the knee joint of mice leads to cartilage degeneration, osteophytes formation and affect the mobility of mice, indicated that the knee joint microenvironment was altered by senescent cells through paracrine effects and induce pathological changes (Xu et al., 2017). Therefore, many researchers believed that a better understanding of the pathogenesis of KOA and the expression of SASP factors in the joint tissue might be a promising research direction.
Oxidative stress was also a hot topic in this field, as mitochondrial dysfunction in senescent cells led to an increase in reactive oxygen species (ROS), which in turn caused oxidative stress (Erusalimsky, 2020; Ebert et al., 2022). Oxidative stress was thought to be a driver of catabolic and anabolic imbalance in cartilage, leading to degradation of cartilage extracellular matrix and causing apoptosis (Bolduc et al., 2019; Kulkarni et al., 2021). Some researchers had found that the progression of KOA can be delayed by inhibiting oxidative stress (Feng et al., 2019; Wang et al., 2020). Indicated that inhibiting oxidative stress was also a potential strategy for the treatment of KOA.
Autophagy protected the body from cellular senescence by removing damaged organelles and long-lived macromolecules (Mizushima and Levine, 2020; Gong et al., 2023). Autophagy was an indispensable mechanism to maintain the stability of the intracellular environment. In articular cartilage, the role of autophagy was particularly important in maintaining chondrocyte homeostasis and function due to the low proliferation rate of chondrocytes (Kao et al., 2022). Carames and Martin Lotz found that autophagy was a self-protective mechanism of cartilage and that impaired autophagy with cellular senescence led to chondrocyte apoptosis (Carames et al., 2010). And they found that rapamycin could reduce the severity of osteoarthritis by activating autophagy, indicated that activating autophagy in chondrocytes through drugs might be an effective treatment for KOA (Lopez de Figueroa et al., 2015). In 2023, Irene Lorenzo-Gómez and others found a key chaperone for chondrocyte autophagy, HSP90A, defective chaperone mediated autophagy might be crucial to KOA (Lorenzo-Gomez et al., 2023).
In the pathological process of KOA, senescent chondrocytes might affect the chondrogenic differentiation potential of bone marrow derived Mesenchymal stem cells (MSCS), and senescent MSCs might lose their immunomodulatory effect, thereby promoting the development of KOA (Cao et al., 2019; Malaise et al., 2019). Mateos J and Arufe MC revealed that the chondrogenic differentiation of human MSCs was negatively affected by Lamin A deregulation, which in turn disrupted the oxidative stress balance in MSCs (Mateos et al., 2013). Murphy and others used Luciferase to label stem cells in different types of mouse articular cartilage and found that the number of stem cells decreased significantly with age (Murphy et al., 2020).
The cluster III mainly focused on therapeutic modalities, such as stem cells, repair, platelet-rich plasma, intra-articular injection, and transplantation. Many researchers had revealed the important pathological role of stem cell senescence in KOA and also provided a theoretical basis for endogenous stem cell therapy for KOA (Prajwal et al., 2022). In recent years, more and more researchers believed that exosomes secreted by MSCs also play a role in the treatment of osteoarthritis (Yu et al., 2022). Miriam Morente-López and others found that the extracellular vesicles treatment of MSCs was more effective than miR-21−MSCs themselves in reducing systemic inflammation in KOA (Morente-Lopez et al., 2022). Researchers found that local intra-articular injection of MSCs can promote the regeneration and repair of cartilage tissue and reduce the degeneration caused by KOA (Tong et al., 2020). MSCs were able to regulate local inflammation, apoptosis and proliferation of cells and promote the repair of bone and cartilage by secreting exosomes, growth factors, cytokines, anti-inflammatory factors and other bioactive molecules (Zhang et al., 2021). There are currently 140 clinical studies of stem cell therapy for KOA registered on clinicaltrial.gov (Clinicaltrials, 2023).
Platelet-rich plasma (PRP) was a platelet concentrate extracted from autologous blood by centrifugation, intra-articular injection of PRP could induce cell migration and proliferation, promote the formation of cell matrix and cartilage regeneration, inhibit chondrocyte apoptosis, ameliorate inflammatory microenvironment (Andia et al., 2021; Szwedowski et al., 2021). Although many researchers had found the positive effect of PRP for the treatment of KOA, some researchers found that there were some heterogeneities in the clinical efficacy of PRP, it might be related to the preparation of PRP, concentration of each component and frequency of treatment, rest time, etc (Harrison, 2018). Therefore, it was of great significance to formulate an international standard for the preparation and application of PRP (Anitua and Prado, 2019).
Some researchers had also explored cartilage and chondrocyte transplantation. However, there were some problems in clinical application, such as the difficulty of obtaining autologous cartilage and the rejection of allogeneic cartilage (Lindahl, 2015; Sato et al., 2019).
4.3 The future research trends
To further explore the research dynamics in the cross field of KOA and cellular senescence, we conducted the analysis of keywords and citations bursts. After comprehensive analysis, we found that a publication by Joen H and others in 2017, had received widespread attention (Jeon et al., 2017). This publication had been cited multiple times in a relatively short period of time, with 60 local citations and 721 global citations. They found that intra-articular injection of the Senolytic UBX0101 could selectively removes local senescent cells, thereby alleviating post-traumatic osteoarthritis and creating an environment for cartilage regeneration. Since then, many researchers had explored the therapeutic effect of other Senolytics. In 2019 Garrett A and others found that navitoclax (ABT-263), a dual BCL-2 and BCL-XL inhibitor, could eliminate senescent chondrocytes expressing high levels of p16 by apoptosis (Sessions et al., 2019). George Batshon et al. found that combined application of Navitoclax and UBX0101 could reduce the Serum NT/CT SIRT1 ratio in ACLT mice by eliminating senescent cells, thus alleviating KOA (Batshon et al., 2020). High-throughput drug screening could be used to discover new Senolytics, unveiling new mechanisms that contribute to the treatment of KOA. Nogueira-Recalde U et al. screened over 1,000 compounds for Senolytic therapies in human chondrocytes, found that Fenofibrate, an agonist of flavonoids and peroxisome proliferator-activated receptor-α (PPARα), was able to induce apoptosis in SA-β-gal-positive chondrocytes (Nogueira-Recalde et al., 2019). This discovery led the authors to investigate PPARα expression in KOA, and they found that the expression of PPARα was reduced in the blood and knee cartilage of patients with OA. Flavonoids that could activate sirtuins, such as Fisetin, were linked to longevity and inhibit IL-1β induced inflammation in osteoarthritic chondrocytes. Fisetin was evaluated in clinical trials for efficacy in alleviating osteoarthritis symptoms by reducing senescence burden in cartilage.
Although pharmacological approaches to treat cellular senescence related KOA seemed promising, potential side effects and differences in drug efficacy remained concerns (Bai et al., 2022). Even if senescent cells were responsible for KOA progression, prematurely eliminating these cells or preventing paracrine signaling might impede the initial healing of tissues (Yun et al., 2015). Several studies had shown that Senolytics can target to eliminate senescent chondrocytes, the subsequent maintenance of cartilage homeostasis was still unknown (Dai et al., 2020; Miura et al., 2022). Although Senolytic had achieved positive effects in the laboratory, there was still a certain distance from clinical application. Therefore, the construction of aging mechanism maps of various tissues and cells such as cartilage, synovium, subchondral bone and stem cells might be of great significance to reveal the mechanism of KOA and find new therapeutic targets.
5 Limitations
There were some limitations to our study. Firstly, the publications were only retrieved from one database, no search was conducted in other databases such as Pubmed, Embase, Cochrane library, so there might be a certain degree of omission in the data. Secondly, we only collected English publications, which might cause selection bias, leading to certain errors in the results. Finally, the cross field of cellular senescence and KOA was constantly evolving and new publications were being published every day, some high-quality studies published in recent years might have been overlooked due to limited citation.
6 Conclusion
In conclusion, this study is the first bibliometric analysis of the intersection of KOA and cellular senescence, we summarize the current research status, countries/regions, authors, keywords, and hotspots of this field. A strong ascending trend for the publication number is observed, and it may be a hint that researchers’ interest and attention in this field are increasing. Senolytics may be the up-to-date research frontiers. Our analysis provides a scientific perspective on the cross filed of KOA and cellular senescence for relevant researchers, funding agencies, and policymakers.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SW: Conceptualization, Formal Analysis, Visualization, Writing–original draft, Writing–review and editing. JY: Data curation, Formal Analysis, Visualization, Writing–review and editing. RX: Visualization, Writing–review and editing. CL: Software, Writing–review and editing. JL: Methodology, Writing–review and editing. XS: Data curation, Writing–review and editing. WL: Supervision, Writing–review and editing. XX: Supervision, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Guangzhou Science and Technology Plan Project (No. 202206010048), Guangdong Provincial Science and Technology Plan Project (No. 2021B1111610007), Natural Science Foundation of Guangdong Province (No. 2021A1515011545), and Guangdong Provincial Science and Technology Plan Project (No. 2018B020207009), Guangdong Provincial Enterprise Joint Foundation for Basic and Applied Basic Research General Project (No. 2022A1515220157), and Guangdong Provincial Bureau of Traditional Chinese Medicine research project (No. 20211023).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abramo G., D'angelo C. A., Viel F. (2011). The field-standardized average impact of national research systems compared to world average: the case of Italy. Scientometrics 88, 599–615. doi:10.1007/s11192-011-0406-x
Andia I., Atilano L., Maffulli N. (2021). Moving toward targeting the right phenotype with the right platelet-rich plasma (PRP) formulation for knee osteoarthritis. Ther. Adv. Musculoskelet. Dis. 13, 1759720X211004336. doi:10.1177/1759720X211004336
Anitua E., Prado R. (2019). Addressing reproducibility in stem cell and PRP therapies. Trends Biotechnol. 37, 340–344. doi:10.1016/j.tibtech.2018.11.010
Ashraf S., Cha B. H., Kim J. S., Ahn J., Han I., Park H., et al. (2016). Regulation of senescence associated signaling mechanisms in chondrocytes for cartilage tissue regeneration. Osteoarthr. Cartil. 24, 196–205. doi:10.1016/j.joca.2015.07.008
Bai Z., Yang P., Yu F., Li Z., Yao Z., Martinez J., et al. (2022). Combining adoptive NK cell infusion with a dopamine-releasing peptide reduces senescent cells in aged mice. Cell Death Dis. 13, 305. doi:10.1038/s41419-022-04562-w
Batshon G., Elayyan J., Qiq O., Reich E., Ben-Aderet L., Kandel L., et al. (2020). Serum NT/CT SIRT1 ratio reflects early osteoarthritis and chondrosenescence. Ann. Rheum. Dis. 79, 1370–1380. doi:10.1136/annrheumdis-2020-217072
Birch J., Gil J. (2020). Senescence and the SASP: many therapeutic avenues. Genes Dev. 34, 1565–1576. doi:10.1101/gad.343129.120
Bolduc J. A., Collins J. A., Loeser R. F. (2019). Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 132, 73–82. doi:10.1016/j.freeradbiomed.2018.08.038
Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. (1994). Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 331, 889–895. doi:10.1056/NEJM199410063311401
Cai Y., Zhou H., Zhu Y., Sun Q., Ji Y., Xue A., et al. (2020). Elimination of senescent cells by beta-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res. 30, 574–589. doi:10.1038/s41422-020-0314-9
Cao X., Luo P., Huang J., Liang C., He J., Wang Z., et al. (2019). Intraarticular senescent chondrocytes impair the cartilage regeneration capacity of mesenchymal stem cells. Stem Cell Res. Ther. 10, 86. doi:10.1186/s13287-019-1193-1
Carames B., Taniguchi N., Otsuki S., Blanco F. J., Lotz M. (2010). Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 62, 791–801. doi:10.1002/art.27305
Chen H., Lee R. T., Garbern J. C. (2022b). Senescence mechanisms and targets in the heart. Cardiovasc Res. 118, 1173–1187. doi:10.1093/cvr/cvab161
Chen H., Qin J., Shi H., Li Q., Zhou S., Chen L. (2022a). Rhoifolin ameliorates osteoarthritis via the Nrf2/NF-κB axis: in vitro and in vivo experiments. Osteoarthr. Cartil. 30, 735–745. doi:10.1016/j.joca.2022.01.009
Clinicaltrials (2023). ClinicalTrials.gov. https://clinicaltrials.gov/search?cond=knee%20osteoarthritis&intr=stem%20cell.
Dai H., Chen R., Gui C., Tao T., Ge Y., Zhao X., et al. (2020). Eliminating senescent chondrogenic progenitor cells enhances chondrogenesis under intermittent hydrostatic pressure for the treatment of OA. Stem Cell Res. Ther. 11, 199. doi:10.1186/s13287-020-01708-5
Diekman B. O., Sessions G. A., Collins J. A., Knecht A. K., Strum S. L., Mitin N. K., et al. (2018). Expression of p16 (INK) (4a) is a biomarker of chondrocyte aging but does not cause osteoarthritis. Aging Cell 17, e12771. doi:10.1111/acel.12771
Docherty M. H., O'sullivan E. D., Bonventre J. V., Ferenbach D. A. (2019). Cellular senescence in the kidney. J. Am. Soc. Nephrol. 30, 726–736. doi:10.1681/ASN.2018121251
Ebert T., Tran N., Schurgers L., Stenvinkel P., Shiels P. G. (2022). Ageing - oxidative stress, PTMs and disease. Mol. Asp. Med. 86, 101099. doi:10.1016/j.mam.2022.101099
Ellegaard O., Wallin J. A. (2015). The bibliometric analysis of scholarly production: how great is the impact? Scientometrics 105, 1809–1831. doi:10.1007/s11192-015-1645-z
Erusalimsky J. D. (2020). Oxidative stress, telomeres and cellular senescence: what non-drug interventions might break the link? Free Radic. Biol. Med. 150, 87–95. doi:10.1016/j.freeradbiomed.2020.02.008
Feng K., Chen Z., Pengcheng L., Zhang S., Wang X. (2019). Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J. Cell Physiol. 234, 18192–18205. doi:10.1002/jcp.28452
Giorgino R., Albano D., Fusco S., Peretti G. M., Mangiavini L., Messina C. (2023). Knee osteoarthritis: epidemiology, pathogenesis, and mesenchymal stem cells: what else is new? An update. Int. J. Mol. Sci. 24, 6405. doi:10.3390/ijms24076405
Glasson S. S., Chambers M. G., Van Den Berg W. B., Little C. B. (2010). The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 18 (3), S17–S23. doi:10.1016/j.joca.2010.05.025
Gong Y., Li S., Wu J., Zhang T., Fang S., Feng D., et al. (2023). Autophagy in the pathogenesis and therapeutic potential of post-traumatic osteoarthritis. Burns Trauma 11, tkac060. doi:10.1093/burnst/tkac060
Gorgoulis V., Adams P. D., Alimonti A., Bennett D. C., Bischof O., Bishop C., et al. (2019). Cellular senescence: defining a path forward. Cell 179, 813–827. doi:10.1016/j.cell.2019.10.005
Greene M. A., Loeser R. F. (2015). Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 23, 1966–1971. doi:10.1016/j.joca.2015.01.008
Harrison P.Subcommittee on Platelet Physiology (2018). The use of platelets in regenerative medicine and proposal for a new classification system: guidance from the SSC of the ISTH. J. Thromb. Haemost. 16, 1895–1900. doi:10.1111/jth.14223
Hayflick L., Moorhead P. S. (1961). The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621. doi:10.1016/0014-4827(61)90192-6
He S., Sharpless N. E. (2017). Senescence in Health and disease. Cell 169, 1000–1011. doi:10.1016/j.cell.2017.05.015
Huang W., Hickson L. J., Eirin A., Kirkland J. L., Lerman L. O. (2022). Cellular senescence: the good, the bad and the unknown. Nat. Rev. Nephrol. 18, 611–627. doi:10.1038/s41581-022-00601-z
Jeon O. H., David N., Campisi J., Elisseeff J. H. (2018). Senescent cells and osteoarthritis: a painful connection. J. Clin. Invest. 128, 1229–1237. doi:10.1172/JCI95147
Jeon O. H., Kim C., Laberge R. M., Demaria M., Rathod S., Vasserot A. P., et al. (2017). Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781. doi:10.1038/nm.4324
Kao W. C., Chen J. C., Liu P. C., Lu C. C., Lin S. Y., Chuang S. C., et al. (2022). The role of autophagy in osteoarthritic cartilage. Biomolecules 12, 1357. doi:10.3390/biom12101357
Katz J. N., Arant K. R., Loeser R. F. (2021). Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA 325, 568–578. doi:10.1001/jama.2020.22171
Kellgren J. H., Lawrence J. S. (1957). Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 16, 494–502. doi:10.1136/ard.16.4.494
Kulkarni P., Martson A., Vidya R., Chitnavis S., Harsulkar A. (2021). Pathophysiological landscape of osteoarthritis. Adv. Clin. Chem. 100, 37–90. doi:10.1016/bs.acc.2020.04.002
Lindahl A. (2015). From gristle to chondrocyte transplantation: treatment of cartilage injuries. Philos. Trans. R. Soc. Lond B Biol. Sci. 370, 20140369. doi:10.1098/rstb.2014.0369
Liu W., Brodsky A. S., Feng M., Liu Y., Ding J., Jayasuriya C. T., et al. (2021). Senescent tissue-resident mesenchymal stromal cells are an internal source of inflammation in human osteoarthritic cartilage. Front. Cell Dev. Biol. 9, 725071. doi:10.3389/fcell.2021.725071
Loeser R. F. (2009). Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 17, 971–979. doi:10.1016/j.joca.2009.03.002
Loeser R. F., Collins J. A., Diekman B. O. (2016). Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 12, 412–420. doi:10.1038/nrrheum.2016.65
Loeser R. F., Goldring S. R., Scanzello C. R., Goldring M. B. (2012). Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 64, 1697–1707. doi:10.1002/art.34453
Lopez De Figueroa P., Lotz M. K., Blanco F. J., Carames B. (2015). Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis Rheumatol. 67, 966–976. doi:10.1002/art.39025
Lorenzo-Gomez I., Nogueira-Recalde U., Garcia-Dominguez C., Oreiro-Villar N., Lotz M., Pinto-Tasende J. A., et al. (2023). Defective chaperone-mediated autophagy is a hallmark of joint disease in patients with knee osteoarthritis. Osteoarthr. Cartil. 31, 919–933. doi:10.1016/j.joca.2023.02.076
Malaise O., Tachikart Y., Constantinides M., Mumme M., Ferreira-Lopez R., Noack S., et al. (2019). Mesenchymal stem cell senescence alleviates their intrinsic and seno-suppressive paracrine properties contributing to osteoarthritis development. Aging (Albany NY) 11, 9128–9146. doi:10.18632/aging.102379
Mateos J., De La Fuente A., Lesende-Rodriguez I., Fernandez-Pernas P., Arufe M. C., Blanco F. J. (2013). Lamin A deregulation in human mesenchymal stem cells promotes an impairment in their chondrogenic potential and imbalance in their response to oxidative stress. Stem Cell Res. 11, 1137–1148. doi:10.1016/j.scr.2013.07.004
Miura Y., Endo K., Komori K., Sekiya I. (2022). Clearance of senescent cells with ABT-263 improves biological functions of synovial mesenchymal stem cells from osteoarthritis patients. Stem Cell Res. Ther. 13, 222. doi:10.1186/s13287-022-02901-4
Mizushima N., Levine B. (2020). Autophagy in human diseases. N. Engl. J. Med. 383, 1564–1576. doi:10.1056/NEJMra2022774
Morente-Lopez M., Mato-Basalo R., Lucio-Gallego S., Silva-Fernandez L., Gonzalez-Rodriguez A., De Toro F. J., et al. (2022). Therapy free of cells vs human mesenchymal stem cells from umbilical cord stroma to treat the inflammation in OA. Cell Mol. Life Sci. 79, 557. doi:10.1007/s00018-022-04580-z
Murphy M. P., Koepke L. S., Lopez M. T., Tong X., Ambrosi T. H., Gulati G. S., et al. (2020). Articular cartilage regeneration by activated skeletal stem cells. Nat. Med. 26, 1583–1592. doi:10.1038/s41591-020-1013-2
Nees T. A., Rosshirt N., Zhang J. A., Reiner T., Sorbi R., Tripel E., et al. (2019). Synovial cytokines significantly correlate with osteoarthritis-related knee pain and disability: inflammatory mediators of potential clinical relevance. J. Clin. Med. 8, 1343. doi:10.3390/jcm8091343
Nogueira-Recalde U., Lorenzo-Gomez I., Blanco F. J., Loza M. I., Grassi D., Shirinsky V., et al. (2019). Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine 45, 588–605. doi:10.1016/j.ebiom.2019.06.049
Prajwal G. S., Jeyaraman N., Kanth V. K., Jeyaraman M., Muthu S., Rajendran S. N. S., et al. (2022). Lineage differentiation potential of different sources of mesenchymal stem cells for osteoarthritis knee. Pharm. (Basel) 15, 386. doi:10.3390/ph15040386
Roupakia E., Markopoulos G. S., Kolettas E. (2021). Genes and pathways involved in senescence bypass identified by functional genetic screens. Mech. Ageing Dev. 194, 111432. doi:10.1016/j.mad.2021.111432
Sato M., Yamato M., Mitani G., Takagaki T., Hamahashi K., Nakamura Y., et al. (2019). Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. NPJ Regen. Med. 4, 4. doi:10.1038/s41536-019-0069-4
Schmitt C. A., Wang B., Demaria M. (2022). Senescence and cancer - role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 19, 619–636. doi:10.1038/s41571-022-00668-4
Sessions G. A., Copp M. E., Liu J. Y., Sinkler M. A., D'costa S., Diekman B. O. (2019). Controlled induction and targeted elimination of p16 (INK4a)-expressing chondrocytes in cartilage explant culture. FASEB J. 33, 12364–12373. doi:10.1096/fj.201900815RR
Sharma L. (2021). Osteoarthritis of the knee. N. Engl. J. Med. 384, 51–59. doi:10.1056/NEJMcp1903768
Szwedowski D., Szczepanek J., Paczesny L., Zabrzynski J., Gagat M., Mobasheri A., et al. (2021). The effect of platelet-rich plasma on the intra-articular microenvironment in knee osteoarthritis. Int. J. Mol. Sci. 22, 5492. doi:10.3390/ijms22115492
Tominaga K. (2015). The emerging role of senescent cells in tissue homeostasis and pathophysiology. Pathobiol. Aging Age Relat. Dis. 5, 27743. doi:10.3402/pba.v5.27743
Tong W., Zhang X., Zhang Q., Fang J., Liu Y., Shao Z., et al. (2020). Multiple umbilical cord-derived MSCs administrations attenuate rat osteoarthritis progression via preserving articular cartilage superficial layer cells and inhibiting synovitis. J. Orthop. Transl. 23, 21–28. doi:10.1016/j.jot.2020.03.007
Wang F. S., Kuo C. W., Ko J. Y., Chen Y. S., Wang S. Y., Ke H. J., et al. (2020). Irisin mitigates oxidative stress, chondrocyte dysfunction and osteoarthritis development through regulating mitochondrial integrity and autophagy. Antioxidants (Basel) 9, 810. doi:10.3390/antiox9090810
Wang J. C., Bennett M. (2012). Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ. Res. 111, 245–259. doi:10.1161/CIRCRESAHA.111.261388
Wen P., Liu R., Wang J., Wang Y., Song W., Zhang Y. (2022). Bibliometric insights from publications on subchondral bone research in osteoarthritis. Front. Physiol. 13, 1095868. doi:10.3389/fphys.2022.1095868
Wu C. J., Liu R. X., Huan S. W., Tang W., Zeng Y. K., Zhang J. C., et al. (2022). Senescent skeletal cells cross-talk with synovial cells plays a key role in the pathogenesis of osteoarthritis. Arthritis Res. Ther. 24, 59. doi:10.1186/s13075-022-02747-4
Wu H., Li Y., Tong L., Wang Y., Sun Z. (2021). Worldwide research tendency and hotspots on hip fracture: a 20-year bibliometric analysis. Arch. Osteoporos. 16, 73. doi:10.1007/s11657-021-00929-2
Xu M., Bradley E. W., Weivoda M. M., Hwang S. M., Pirtskhalava T., Decklever T., et al. (2017). Transplanted senescent cells induce an osteoarthritis-like condition in mice. J. Gerontol. A Biol. Sci. Med. Sci. 72, 780–785. doi:10.1093/gerona/glw154
Yao Q., Wu X., Tao C., Gong W., Chen M., Qu M., et al. (2023). Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target Ther. 8, 56. doi:10.1038/s41392-023-01330-w
Yu H., Huang Y., Yang L. (2022). Research progress in the use of mesenchymal stem cells and their derived exosomes in the treatment of osteoarthritis. Ageing Res. Rev. 80, 101684. doi:10.1016/j.arr.2022.101684
Yun M. H., Davaapil H., Brockes J. P. (2015). Recurrent turnover of senescent cells during regeneration of a complex structure. Elife 4, e05505. doi:10.7554/eLife.05505
Zhang Q., Xiang E., Rao W., Zhang Y. Q., Xiao C. H., Li C. Y., et al. (2021). Intra-articular injection of human umbilical cord mesenchymal stem cells ameliorates monosodium iodoacetate-induced osteoarthritis in rats by inhibiting cartilage degradation and inflammation. Bone Jt. Res. 10, 226–236. doi:10.1302/2046-3758.103.BJR-2020-0206.R2
Keywords: knee osteoarthritis, cellular senescence, publication trends, WoSCC, bibliometric
Citation: Wang S, Yang J, Xiang R, Li C, Li J, Shen X, Liu W and Xu X (2023) Research and publication trends on knee osteoarthritis and cellular senescence: a bibliometric analysis. Front. Physiol. 14:1269338. doi: 10.3389/fphys.2023.1269338
Received: 29 July 2023; Accepted: 08 November 2023;
Published: 17 November 2023.
Edited by:
Xiaolin Wang, Capital Medical University, National Center for Children’s Health (NCCH), ChinaReviewed by:
Juan Antonio Fafian Labora, University of A Coruña, SpainEmilio A Cafferata, Goethe University, Carolinum, Germany
Copyright © 2023 Wang, Yang, Xiang, Li, Li, Shen, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuemeng Xu, eHV4dWVtZW5nQDE2My5jb20=
 Shuai Wang
Shuai Wang Jiyong Yang1
Jiyong Yang1 Congcong Li
Congcong Li Junyi Li
Junyi Li Xuemeng Xu
Xuemeng Xu