- 1Department of Hepatopancreatobiliary Surgery, The People’s Hospital of Leshan, Leshan, Sichuan, China
- 2Diagnosis and Treatment Center for Liver, Gallbladder, Pancreas and Spleen System Diseases of Leshan, Leshan, Sichuan, China
- 3Liver Transplantation Center, State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University and Collaborative Innovation Center of Biotherapy, Chengdu, Sichuan, China
Objective: Pancreatic sinistral portal hypertension (PSPH) is a common complication of acute pancreatitis (AP) and can cause massive gastrointestinal bleeding, which is one of the causes of AP-related mortality. However, there is currently no predictive model for AP concurrent with PSPH. This study aimed to identify the risk factors for AP concurrent with PSPH and use these factors to build a related predictive model.
Materials and methods: We collected clinical data from 282 patients with AP. 192 patients were used as a training group and 90 patients as a validation group. Univariate and multivariate analyses were used to identify independent risk factors for AP complicated with PSPH, and then a nomogram was established. The models are cross verification and Internal verification. The predictive ability and accuracy of the model were evaluated based on the working curve of the subjects and the calibration curve, respectively. The clinical value of the model was evaluated using decision curve analysis (DCA).
Results: The univariate analysis revealed significant differences in the occurrence of PSPH with respect to sex, recurrent AP, history of hypertension, smoking history, patency of the splenic vein, pancreatic necrosis or pancreatic pseudocyst formation, the most significant site of pancreatic swelling, presence of a Dmure D polymer, MCTSI, and involvement of lipase and amylase. The logistic multivariate regression analysis showed that male sex, splenic-vein stenosis or occlusion and swelling were located in the body-tail, and MCTSI was an independent risk factor for PSPH. The nomogram and ROC curve were constructed. The area under the working curve of the subjects was 0.91, and the sensitivity and specificity were 82.5% and 89.1%, respectively. In the validation group, the C-index is 0.826. The nomogram was internally validated using 1,000 bootstrap samples, and the c-index was 0.898. The calibration curve demonstrated that the predicted probability was concordant with the observed probability, and the DCA confirmed that the model had robust clinical utility.
Conclusion: Male sex, splenic-vein stenosis or occlusion, recurrent AP, and swelling are located in the body-tail, and MCTSI is an independent risk factor for the occurrence of PSPH. The predictive model developed for AP complicated with PSPH may serve toward developing preventive and therapeutic approaches for PSPH.
Introduction
Acute pancreatitis (AP) is a local inflammatory disease of the exocrine glands of the pancreas and is one of the most common digestive-tract diseases. The global incidence rate is (4.9–73.4)/100000 (Boxhoorn et al., 2020; Spagnolo et al., 2022). For AP patients with systemic inflammatory-response syndrome and/or multiple-organ failure, the disease progresses rapidly, the treatment is difficult, and the case fatality rate is as high as 20%–40% (Lee and Papachristou, 2019; Trikudanathan et al., 2019). Peripancreatic fluid accumulation, acute necrotic accumulation, pancreatic pseudocyst formation, pancreatic sinistral portal hypertension (PSPH), gastrointestinal or abdominal bleeding, and intestinal fistula are common complications of AP (Jiang et al., 2020; Vogel et al., 2022).
PSPH is a common complication of AP and accounts for 5%–10% of cases of extrahepatic portal hypertension (Gyoten et al., 2017; Ru et al., 2020). It results from pancreatic diseases that affect the portal vein and the associated branches. The most commonly affected vein in pancreatic diseases leading to PSPH is the splenic vein, which may experience vascular obstruction and blood-reflux disturbance. This vascular pathology can lead to splenomegaly and increased splenogastric venous pressure, which are the main clinical manifestations of regional portal hypertension. PSPH is a common complication of AP and the splenomegaly accompanied by hypersplenism and massive gastrointestinal hemorrhage caused by PSPH is one of the causes of death in AP patients. At present, about 4%–17% of patients with PSPH will develop gastrointestinal bleeding, and about 1.2%–14.5% will die as a result (Ru et al., 2020).
Therefore, early identification and intervention, or effective treatment of AP complicated with PSPH is of great significance to improve the prognosis and minimize associated medical costs. Although the risk factors for AP complicated with PSPH have recently been probed by several groups, no optimal predictive model for this condition has been established (Wang et al., 2022; Yu et al., 2022). Thus, this study aimed to identify the major risk factors and use them to construct a predictive model of AP complicated with PSPH.
Materials and methods
Patients selection
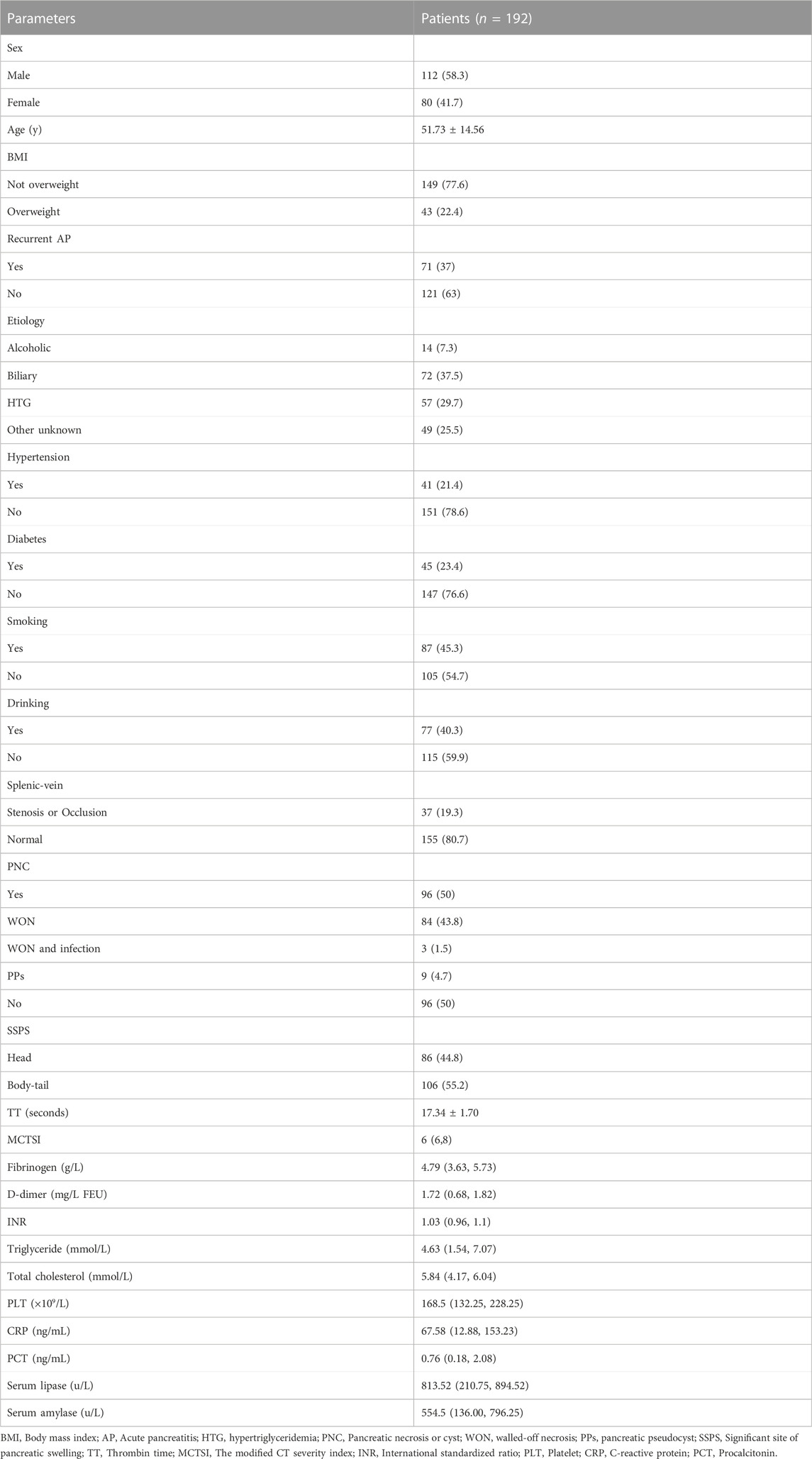
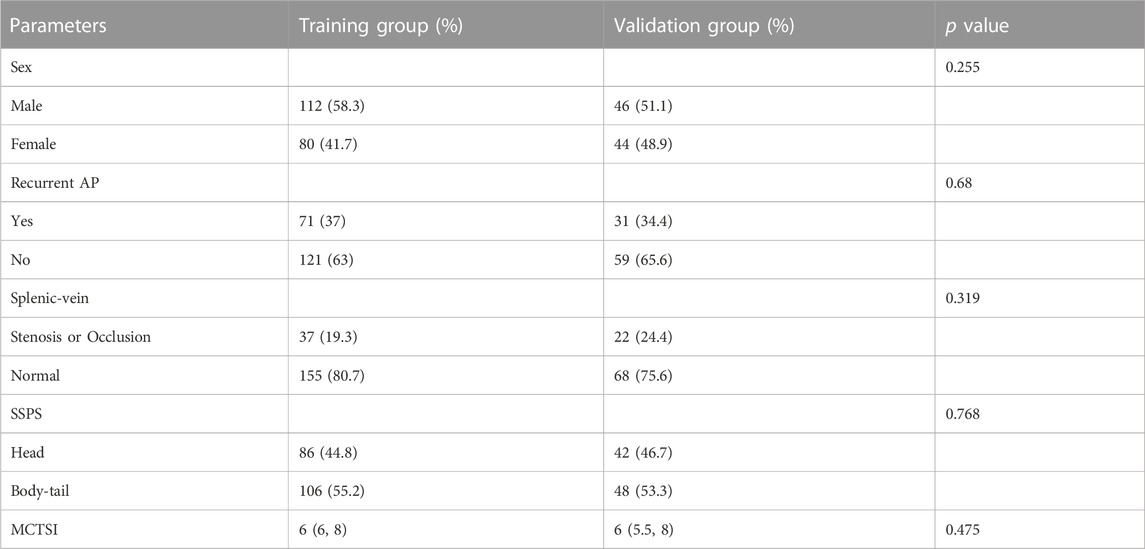
Clinical data from 2,572 patients with AP registered at the Leshan People’s Hospital between January 2018 and July 2023 were collected retrospectively. The inclusion criteria were as follows: 1) a clear history of non-tumor–related AP; 2) no history of cirrhosis, hematological diseases, or schistosomiasis; 3) cases with complete clinical data; 4) inpatients; and 5) cases that were followed up. The exclusion criteria were 1) history of pancreatitis caused by tumor; 2) presence of chronic pancreatitis; 3) history of cirrhosis, hematological diseases, or schistosomiasis; 4) incomplete clinical data; 5) emergency or outpatient cases; and 6) cases that were lost during the follow-up (Figure 1). Finally, 292 patients were included, of which 192 patients from January 2018 to December 2022 were used as the training group to build the model, and 90 patients from January 2023 to July 2023 were used as the validation group to verify the model (Table 1).
Diagnosis and definitions
The diagnosis of AP was based on persistent epigastric pain, serum amylase level > 3-folds of the normal level, and computed tomography (CT) or magnetic resonance imaging (MRI) findings suggestive of AP (Banks et al., 2013). The diagnosis of PSPH was based on enhanced CT or MRI findings indicating portal-vein obstruction, solitary gastroesophageal varices, splenomegaly, and the absence of cirrhosis or abnormal liver function. Splenic-vein occlusion was defined as complete occlusion with no blood flow, whereas the thinnest point of the splenic vein is defined as stenosis if it is less than or equal to 50% of the normal mean value (Easler et al., 2014; Yu et al., 2022). Body mass index (BMI) ≥ 25 kg/m2 was defined as overweight, and BMI <25 kg/m2 was defined as not overweight (Caballero, 2019). Patients with a prior history of acute pancreatitis admitted to the hospital for treatment were defined as Recurrent AP (Hu et al., 2022).
Clinical-data collection
General information
The information about patient sex, age, BMI, and any history of pancreatitis, etiology of pancreatitis, hypertension, diabetes, smoking, or drinking was collected.
CT or MRI findings
On admission or diagnosis with pancreatitis, all the patients underwent CT or MRI to assess the patency of the splenic vein. The imaging was used to detect any thrombus, stenosis, or occlusion, as well as necrotic accumulation or pseudocyst formation, and identify the most significant site of pancreatic swelling. The severity of pancreatitis was graded by radiographers according to the modified CT severity index (MCTSI).
Serological tests
Serological tests were performed on admission, and thrombin time (TT), international standardized ratio (INR), and D-dimer, fibrinogen, triglyceride, total cholesterol, platelet (PLT), C-reactive protein (CRP), procalcitonin (PCT), blood lipase, and hemodiastase levels were recorded.
Follow-up
For patients with unclear history of pancreatitis, cirrhosis, hematological diseases, and schistosomiasis, data were obtained by telephone follow-up. Follow up with CT or MRI every 3–6 months after discharge, with a duration of 12 months.
Statistical analysis
The SPSS (v26.0) software was used for statistical analysis. For normally distributed measurement data, descriptive statistics were presented as mean ± SD, and group comparisons were made using the t-test. For skewed distribution data, descriptive statistics were presented as median (±interquartile range), and group comparisons were made using the Mann-Whitney U test. Categorical data were presented as number of cases (percentage), and group comparisons were made using the chi-squared test. The factors found to be related to the occurrence of PSPH in the univariate analysis were included in the multivariate logistic regression model analysis to identify the independent risk factors for PSPH. The identified independent risk factors were used to construct an nomogram and calibration curve by using “rms,” and “regplot” programs in the R (v4.2.2) software, respectively. “pROC,” “plotROC,” and “rmda” were used to draw the receiver operating characteristic (ROC) and decision curves of the predictive model, and the area under the ROC curve (AUC) was calculated to evaluate the effectiveness of the predictive model. Finally, 1,000 bootstrap samples were used for internal verification.
Results
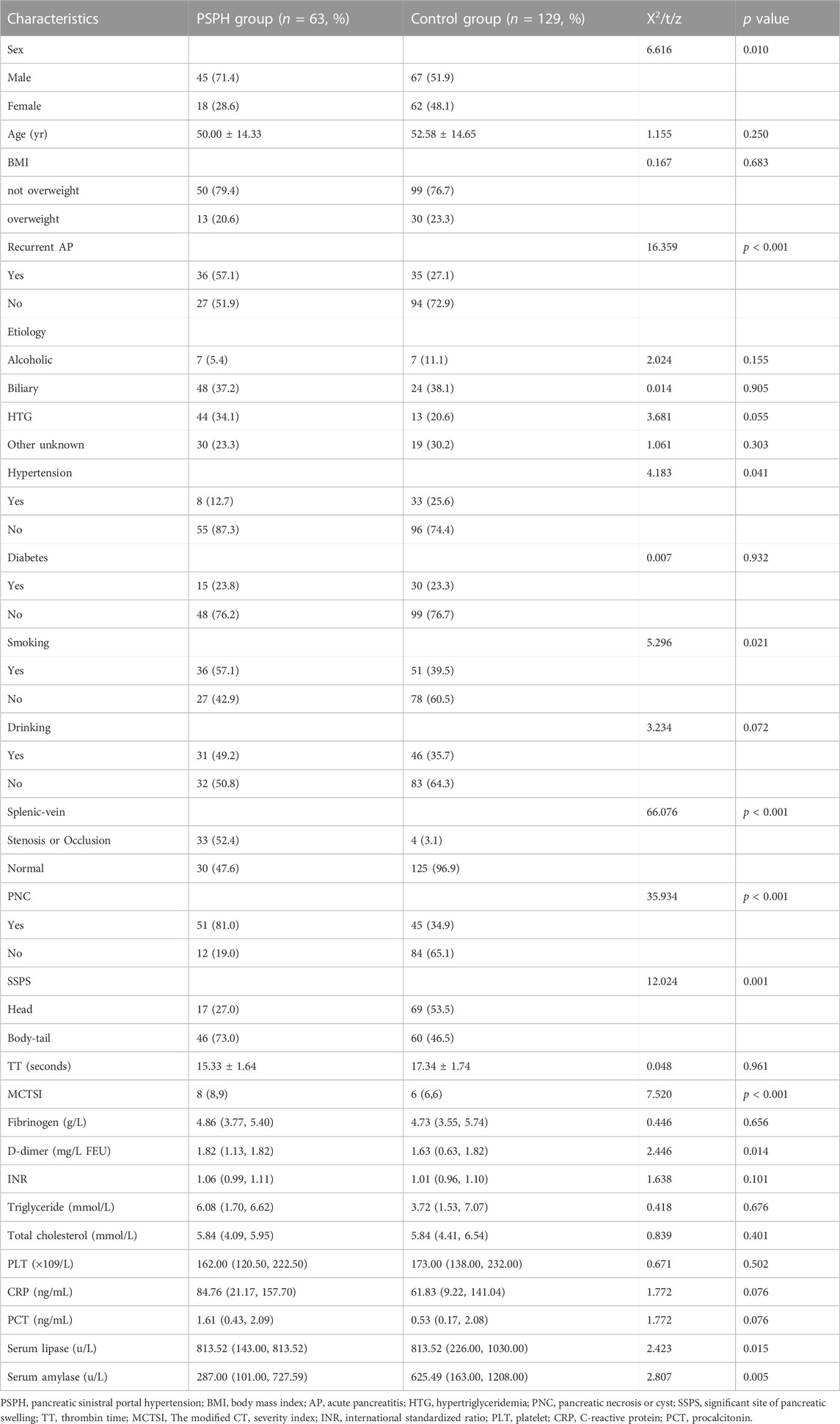
In the training group, the patients were divided into PSPH (n = 63) and non-PSPH (n = 129) groups according to the occurrence of PSPH. Univariate analysis showed that age, BMI, etiology of pancreatitis, history of diabetes, history of drinking, TT, INR, platelet count, or serum level of fibrinogen, CRP, triglyceride, or total cholesterol had no significant effect on the occurrence of PSPH (p > 0.05). Sex (p = 0.010), hypertension history (p = 0.041), recurrent AP (p < 0.001), smoking history (p = 0.021), splenic-vein stenosis or occlusion (p < 0.001), pancreatic necrosis or cyst (p < 0.001), significant site of pancreatic swelling (p = 0.001), D-dimer (p = 0.014), MCTSI (p < 0.001), serum lipase (p = 0.015), and serum amylase (p = 0.005) had significant effect on the occurrence of PSPH (p < 0.05) (Table 2).
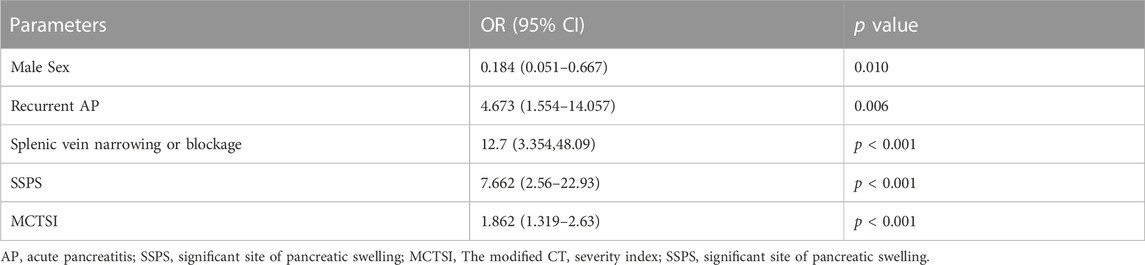
The above indices were used to perform a logistic multivariate regression analysis, which revealed male sex (p = 0.010), recurrent AP (p = 0.006), splenic-vein stenosis or occlusion (p < 0.001), swelling are located in the body-tail (p < 0.001), and MCTSI (p < 0.001) as independent risk factors for PSPH (Table 3). The above indexes were included in the verification group and analyzed with the training group, and the results were not statistically significant (p > 0.05) (Table 4).
Construction of a nomogram
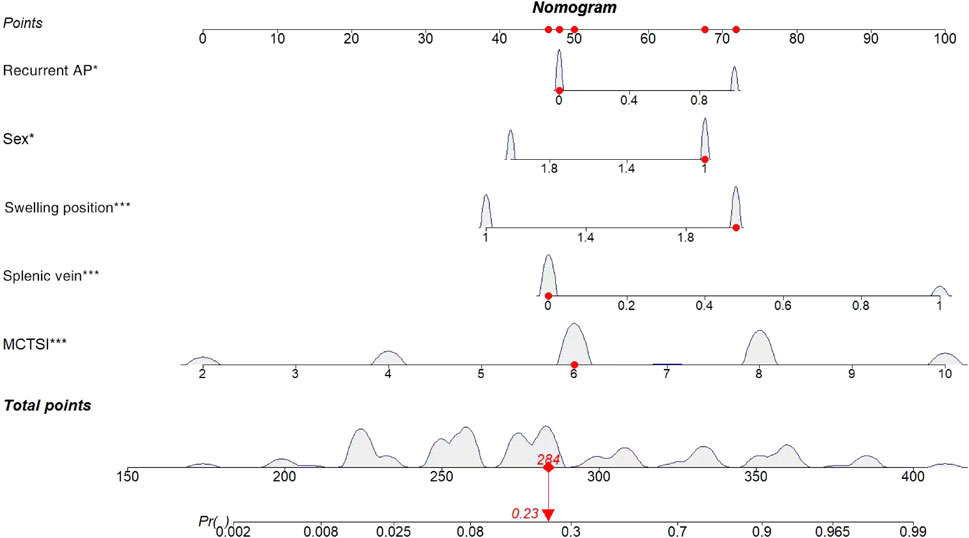
Based on the results of the logistic multivariate regression analysis, a nomogram that comprised five important factors for predicting the occurrence of PSPH (sex, recurrent AP, patency of the splenic vein, site of swelling, and MCTSI) was constructed. Each value of these variables was assigned a score on the scale axis, and the total score was calculated by summing up each score. By projecting the total score on the probability axis, the likelihood of PSPH occurrence was estimated (Figure 2).
FIGURE 2. Nomogram for predicting incidence rate among patients with AP. AP, Acute pancreatitis; MCTSI, The modified CT severity index.
The following is an example of how the predictive model can be used: A male patient (67 points) with acute pancreatitis, no history of acute pancreatitis (48 points), and no stenosis or occlusion of the splenic vein (47 points) based on CT or MRI had the most significant site of swelling in the body-tail of the pancreas (72 points) and an MCTSI of 6 points (50 points), reaching a total score of 284 points. Based on the model, the probability of this patient to develop PSPH in the future is estimated at 23%. Conversely, for a male patient with pancreatitis and a previous history of AP who also exhibits splenic-vein stenosis or occlusion with the most obvious swelling of the body-tail, and an MCTSI of 10, the probability of developing PSPH in the future is very high (Figure 3).
Verification of the model
Based on the results of cross verification, the predictive model showed good consistency, as evidenced by a c-index of 0.826, In the internal verification, the C-index was 0.898. Additionally, the calibration curve demonstrated good agreement between the predicted and actual PSPH values (Figures 4–6).

FIGURE 4. The receiver operating characteristic curve (ROC) was plotted based on the p values of the patients calculated using the screening model. Area under curve value was 0.910, with a sensitivity of 89.1% and a specificity of 82.5%. In the validation group, the C-index is 0.826. AUC, Area under curve.
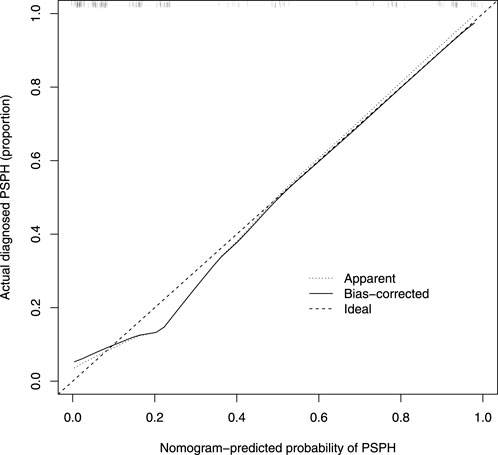
FIGURE 5. Calibration curve of the nomogram. The X-axis represented the predicted possible Pancreatic sinistral portal hypertension (PSPH) risk. The Y-axis represented the actual diagnosed PSPH. The diagonal dotted line meant a perfect prediction by an ideal model. The short-dashed line represented the apparent prediction of nomogram, and the solid line was bias-corrected by bootstrapping (B = 1,000 repetitions), indicating observed nomogram performance.
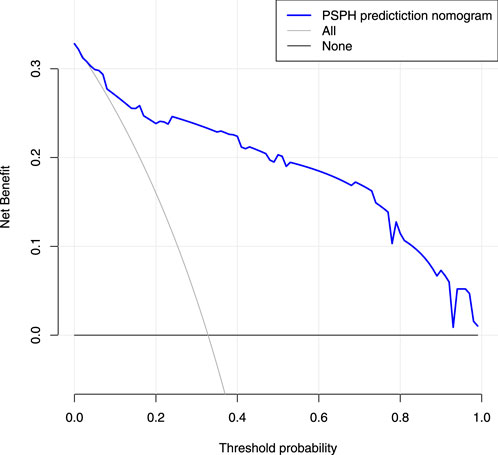
FIGURE 6. Decision curve analysis for the nomogram. The X-axis showed the threshold probability. The Y-axis measured the net benefit. The blue solid line represented the nomogram. The gray solid line represented the assumption that all subjects were Pancreatic sinistral portal hypertension.
Discussion
PSPH is a disorder that causes regional increases in portal vein pressure due to splenic-vein reflux. It is commonly associated with pancreatitis, and can lead to isolated gastric varices and splenomegaly. If left untreated, PSPH can lead to life-threatening upper gastrointestinal bleeding (Köklü et al., 2007; Xie et al., 2019; Ono et al., 2021). Preventing the onset of PSPH is crucial, as it is a curable form of portal hypertension. However, there are few studies that have assessed the risk factors for PSPH, and no predictive model of AP complicated with PSPH has been reported yet (Li et al., 2019). In this study, we identified male sex, recurrent AP, splenic-vein patency, swelling are located in the body-tail, and MCTSI as independent risk factors for PSPH onset. We used these findings to construct a nomogram that visualizes the results of the regression analysis. The model was internally verified and found to perform well, allowing for an accurate prediction of the probability of AP patients developing PSPH. Thus, the model developed in this study has significant implications for the prognosis of AP patients.
With the development of AP, various factors affecting the blood flow of the splenic vein appear, such as splenic-vein thrombosis, mechanical compression by pancreatic pseudocysts, and vascular-wall fibrosis resulting from repeated inflammatory stimulation. As a consequence of these factors, splenic-vein stenosis or occlusion may occur, leading to the occurrence of PSPH (Moossa and Gadd, 1985; Ito et al., 2008; Kul et al., 2018). It has been reported that the interval from the onset of AP to the onset of PSPH ranges from 10 days to 9 years. In the early stage, there may be splenic-vein stenosis or occlusion caused by inflammatory necrotic vascular-wall fibrosis, and in the late stage, thrombus and pseudocyst formations may mechanically oppress the splenic vein (Yu et al., 2022). We collected the AP history of each patient and conducted univariate analysis, which revealed a possible association between previous AP occurrences and PSPH (p < 0.001). Many studies have shown a strong correlation between the incidence of PSPH and male sex, and this may be related to the fact that men are more likely to develop splenic vein thrombosis (Roach et al., 2014). Furthermore, men often smoke (Shoar et al., 2018), which can cause atherosclerosis of the vascular-wall and is more likely to cause the formation of thrombus. Smoking has been found to increase the risk of vascular-wall atherosclerosis and thrombus formation (Flora and Nayak, 2019). In this univariate analysis, significant differences were observed between the male and female patients (p = 0.010), as well as between the patients with a history of smoking and those without (p = 0.021). These findings suggest that thrombus formation is one of the key factors contributing to the development of PSPH.
CT or MRI highly facilitates the diagnosis of pancreatic lesions (Kul et al., 2018; Xie et al., 2018; Xie et al., 2019). Complications of pancreatitis, such as necrotic accumulation, pseudocyst or thrombus formation, and patency of the splenic vein, depend on its evaluation. These factors are related to the occurrence of PSPH (Li et al., 2019). Currently, there is no established association between PSPH and the site of pancreatic swelling. However, our multivariate regression analysis revealed that the location of the lesion in the pancreatic body-tail (p < 0.001) is an independent factor linked to the occurrence of PSPH. It is considered that the splenic vein is tighter when the pancreatic body and tail are shaped, and it is easier to oppress the splenic vein when the pancreatic body and tail are swollen, and it is also related to the reflux of some small branches of the pancreatic vein located in the pancreatic body and tail into the splenic vein.
Yu et al. (2022) posited that the development of PSPH is independent of the etiology of AP. Conversely, Li et al. (2019) suggested a specific association between lipid-derived AP and PSPH, highlighting hypertriglyceridemia as a risk factor for moderate to severe AP. This study’s findings align with those of Chen, indicating that the emergence of PSPH is not directly linked to the etiology of AP. This led to the consideration that the incidence of PSPH might be more closely associated with the severity of pancreatitis. Of the seven studies that have investigated high serum triglyceride level as a potential factor affecting the severity of pancreatitis, five have found a possible association (Carr et al., 2016). The results of other studies have shown that BMI >25 kg/m2 increases the risk for critical AP (Dobszai et al., 2019; İnce et al., 2022). Although these indicators may increase the severity of AP, there was no significant relationship between PSPH and PSPH in this study. In addition, in a predictive model study based on thrombus and inflammatory markers, PT, D-dimer, CRP, and PCT were associated with the severity of pancreatitis (Han et al., 2022), only CRP was found to be significantly associated in our study. MCTSI scores have been widely used to assess the severity of AP (Alberti et al., 2021), and the PSPH group (p < 0.001) in our study scored higher among the data we included.
The identified independent risk factors for PSPH were utilized to develop a nomogram with high sensitivity and specificity. For individuals at elevated risk of PSPH, preemptive clinical intervention is recommended. Splenic vein thrombosis, a known complication of AP, has been associated with risk factors such as male gender, smoking, and elevated CRP levels (Toqué et al., 2015), findings that align with those of this study. Accordingly, clinicians should advise patients to cease smoking. In cases of splenic venous thrombosis, the use of anticoagulant therapy, previously viewed as having a low recurrence rate but high bleeding risk, remains contentious (De Stefano and Martinelli, 2010; Harris et al., 2013). Recent studies by Sissingh et al. (2023), however, advocate for the active use of low molecular weight heparin in treatment. The necessity of prophylactic low molecular weight heparin in AP patients without thrombosis formation has not been reported. In addition, we searched the time interval between the most recent AP episode and the diagnosis of PSPH. 16.7% of patients were diagnosed with PSPH at the time of hospitalization, 65.3% were diagnosed within 1 year after the most recent AP, and 18% were diagnosed more than 1 year after the most recent AP. In this study, more than 80% of PSPH patients can be diagnosed by abdominal CT or gastroscopy within 1 year. Therefore, for those who are at high risk of PSPH as indicated by the nomogram, we recommend that patients undergo semi-annual CT and gastrointestinal endoscopy, and pay attention to the situation of blood stool, avoid bleeding upper digestive tract massive bleeding, life-threatening.
There are also some limitations in this study. MCTSI is highly subjective, and different radiologists may assign different scores; hence, an internationally unified method is necessary for scoring the severity of pancreatitis. Moreover, the samples in this study were obtained from single centers, and data from multiple centers should be obtained in the future to verify the model externally.
In summary, this study identified male sex, splenic-vein stenosis or occlusion, recurrent AP, and swelling are located in the body-tail, and MCTSI is an independent risk factor for the occurrence of PSPH. The predictive model developed for AP complicated with PSPH may serve toward developing related preventive and therapeutic approaches.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The People’s Hospital of Leshan. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
XZ and T-YM contributed equally as co-first authors. XZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Software, Writing–original draft. T-YM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Software, Writing–original draft. K-YJ: Conceptualization, Formal Analysis, Writing–review and editing. Q-YX: Conceptualization, Resources, Writing–review and editing. JY: Data curation, Funding acquisition, Writing–review and editing. BD: Data curation, Funding acquisition, Writing–review and editing. Z-XW: Formal Analysis, Methodology, Writing–review and editing. J-QF: Formal Analysis, Methodology, Writing–review and editing. F-WG: Supervision, Funding acquisition, Validation, Visualization, Writing–review and editing. Z-HL: Supervision, Funding acquisition, Resources, Validation, Visualization, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by Leshan key science and technology project, No. 21SZD069.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alberti P., Pando E., Mata R., Vidal L., Roson N., Mast R., et al. (2021). Evaluation of the modified computed tomography severity index (MCTSI) and computed tomography severity index (CTSI) in predicting severity and clinical outcomes in acute pancreatitis. J. Dig. Dis. 22, 41–48. doi:10.1111/1751-2980.12961
Banks P. A., Bollen T. L., Dervenis C., Gooszen H. G., Johnson C. D., Sarr M. G., et al. (2013). Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 62, 102–111. doi:10.1136/gutjnl-2012-302779
Boxhoorn L., Voermans R. P., Bouwense S. A., Bruno M. J., Verdonk R. C., Boermeester M. A., et al. (2020). Acute pancreatitis. Lancet 396, 726–734. doi:10.1016/s0140-6736(20)31310-6
Caballero B. (2019). Humans against obesity: who will win? Adv. Nutr. 10, S4-S9–s9. doi:10.1093/advances/nmy055
Carr R. A., Rejowski B. J., Cote G. A., Pitt H. A., Zyromski N. J. (2016). Systematic review of hypertriglyceridemia-induced acute pancreatitis: a more virulent etiology? Pancreatology 16, 469–476. doi:10.1016/j.pan.2016.02.011
De Stefano V., Martinelli I. (2010). Splanchnic vein thrombosis: clinical presentation, risk factors and treatment. Intern Emerg. Med. 5 (6), 487–494. doi:10.1007/s11739-010-0413-6
Dobszai D., Mátrai P., Gyöngyi Z., Csupor D., Bajor J., Erőss B., et al. (2019). Body-mass index correlates with severity and mortality in acute pancreatitis: a meta-analysis. World J. Gastroenterol. 25, 729–743. doi:10.3748/wjg.v25.i6.729
Easler J., Muddana V., Furlan A., Dasyam A., Vipperla K., Slivka A., et al. (2014). Portosplenomesenteric venous thrombosis in patients with acute pancreatitis isassociated with pancreatic necrosis and usually has a benign course. Clin. GastroenterolHepatol 12, 854–862. doi:10.1016/j.cgh.2013.09.068
Flora G. D., Nayak M. K. (2019). A brief review of cardiovascular diseases, associated risk factors and current treatment regimes. Curr. Pharm. Des. 25, 4063–4084. doi:10.2174/1381612825666190925163827
Gyoten K., Mizuno S., Nagata M., Ogura T., Usui M., Isaji S. (2017). Significance of simultaneous splenic artery resection in left-sided portal hypertension after pancreaticoduodenectomy with combined portal vein resection. World J. Surg. 41, 2111–2120. doi:10.1007/s00268-017-3916-8
Han T., Cheng T., Liao Y., He Y., Liu B., Lai Q., et al. (2022). Development and validation of a novel prognostic score based on thrombotic and inflammatory biomarkers for predicting28-day adverse outcomes in patients with acute pancreatitis. J. Inflamm. Res. 15, 395–408. doi:10.2147/jir.S344446
Harris S., Nadkarni N. A., Naina H. V., Vege S. S. (2013). Splanchnic vein thrombosis in acute pancreatitis: a single-center experience. Pancreas 42 (8), 1251–1254. doi:10.1097/MPA.0b013e3182968ff5
Hu Y., Liu N., Tang L., Liu Q., Pan K., Lei L., et al. (2022). Three-dimensional radiomics features of magnetic resonance T2-weighted imaging combined with clinical characteristics to predict the recurrence of acute pancreatitis. Front. Med. (Lausanne) 9, 777368. doi:10.3389/fmed.2022.777368
Ince A. T., Seven G., Koçhan K., Kiremitçi S., Yıldız K., Şentürk H. (2022). The course of acute pancreatitis in patients with different BMI groups. Pancreatology 22, 348–355. doi:10.1016/j.pan.2022.03.009
Ito K., Kudo A., Nakamura N., Tanaka S., Teramoto K., Arii S. (2008). Left-sided portal hypertension caused by serous cystadenoma of the pancreas: report of a case. Surg. Today 38, 184–187. doi:10.1007/s00595-007-3600-y
Jiang X., Shi J. Y., Wang X. Y., Hu Y., Cui Y. F. (2020). The impacts of infectious complications on outcomes in acute pancreatitis: a retrospective study. Mil. Med. Res. 7, 38. doi:10.1186/s40779-020-00265-5
Köklü S., Coban S., Yüksel O., Arhan M. (2007). Left-sided portal hypertension. Dig. Dis. Sci. 52, 1141–1149. doi:10.1007/s10620-006-9307-x
Kul M., Haliloğlu N., Hürsoy N., Erden A. (2018). Sinistral portal hypertension: computed tomography imaging findings and clinical appearance-A descriptive case series. Can. Assoc. Radiol. J. 69, 417–421. doi:10.1016/j.carj.2018.07.006
Lee P. J., Papachristou G. I. (2019). New insights into acute pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 16, 479–496. doi:10.1038/s41575-019-0158-2
Li H., Yang Z., Tian F. (2019). Clinical characteristics and risk factors for sinistral portal hypertension associated with moderate and severe acute pancreatitis: a seven-year single-center retrospective study. Med. Sci. Monit. 25, 5969–5976. doi:10.12659/msm.916192
Moossa A. R., Gadd M. A. (1985). Isolated splenic vein thrombosis. World J. Surg. 9, 384–390. doi:10.1007/bf01655272
Ono Y., Inoue Y., Kato T., Matsueda K., Oba A., Sato T., et al. (2021). Sinistral portal hypertension after pancreaticoduodenectomy with splenic vein resection: pathogenesis and its prevention. Cancers (Basel) 13, 5334. doi:10.3390/cancers13215334
Roach R. E., Cannegieter S. C., Lijfering W. M. (2014). Differential risks in men and women for first and recurrent venous thrombosis: the role of genes and environment. J. Thromb. Haemost. 12, 1593–1600. doi:10.1111/jth.12678
Ru N., He C. H., Ren X. L., Chen J. Y., Yu F. F., Yan Z. J., et al. (2020). Risk factors for sinistral portal hypertension and related variceal bleeding in patients with chronic pancreatitis. J. Dig. Dis. 21, 468–474. doi:10.1111/1751-2980.12916
Shoar S., Saber A. A., Rubenstein R., Safari S., Brethauer S. A., Al-Thani H., et al. (2018). Portomesentric and splenic vein thrombosis (PMSVT) after bariatric surgery: a systematic review of 110 patients. Surg. Obes. Relat. Dis. 14, 47–59. doi:10.1016/j.soard.2017.09.512
Sissingh N. J., Groen J. V., Timmerhuis H. C., Besselink M. G., Boekestijn B., Bollen T. L., et al. (2023). Therapeutic anticoagulation for splanchnic vein thrombosis in acute pancreatitis: a national survey and case-vignette study. World J. Gastroenterol. 29 (21), 3328–3340. doi:10.3748/wjg.v29.i21.3328
Spagnolo D. M., Greer P. J., Ohlsen C. S., Mance S., Ellison M., Breze C., et al. (2022). Acute and chronic pancreatitis disease prevalence, classification, and comorbidities: a cohort study of the UK BioBank. Clin. Transl. Gastroenterol. 13, e00455. doi:10.14309/ctg.0000000000000455
Toqué L., Hamy A., Hamel J. F., Cesbron E., Hulo P., Robert S., et al. (2015). Predictive factors of splanchnic vein thrombosis in acute pancreatitis: a 6-year single-center experience. J. Dig. Dis. 16 (12), 734–740. doi:10.1111/1751-2980.12298
Trikudanathan G., Wolbrink D. R. J., van Santvoort H. C., Mallery S., Freeman M., Besselink M. G. (2019). Current concepts in severe acute and necrotizing pancreatitis: an evidence-based approach. Gastroenterology 156, 1994–2007. doi:10.1053/j.gastro.2019.01.269
Vogel M., Ehlken H., Kluge S., Roesch T., Lohse A. W., Huber S., et al. (2022). High risk of complications and acute-on-chronic liver failure in cirrhosis patients with acute pancreatitis. Eur. J. Intern Med. 102, 54–62. doi:10.1016/j.ejim.2022.05.034
Wang Z., Li M., Huang X., Xiong J., Tian B. (2022). Preoperative splenic artery embolism followed by splenectomy is safe and effective in patients with sinistral portal hypertension. Langenbecks Arch. Surg. 407, 313–319. doi:10.1007/s00423-021-02329-z
Xie C. L., Wu C. Q., Chen Y., Chen T. W., Xue H. D., Jin Z. Y., et al. (2019). Sinistral portal hypertension in acute pancreatitis: a magnetic resonance imaging study. Pancreas 48, 187–192. doi:10.1097/mpa.0000000000001242
Xie C. L., Zhang M., Chen Y., Hu R., Tang M. Y., Chen T. W., et al. (2018). Spleen and splenic vascular involvement in acute pancreatitis: an MRI study. Quant. Imaging Med. Surg. 8, 291–300. doi:10.21037/qims.2018.03.04
Keywords: acute pancreatitis, pancreatic sinistral, portal hypertension, nomogram, predictive model
Citation: Zhao X, Mao T-Y, Jiang K-Y, Xie Q-Y, Yang J, Du B, Wang Z-X, Fu J-Q, Gao F-W and Lei Z-H (2024) Analysis of risk factors for acute pancreatitis complicated with pancreatic sinistral portal hypertension and construction of predictive model. Front. Physiol. 14:1256615. doi: 10.3389/fphys.2023.1256615
Received: 12 July 2023; Accepted: 18 December 2023;
Published: 08 January 2024.
Edited by:
Stephen J. Pandol, Cedars Sinai Medical Center, United StatesCopyright © 2024 Zhao, Mao, Jiang, Xie, Yang, Du, Wang, Fu, Gao and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng-Wei Gao, NzA3ODAyNTVAcXEuY29t; Ze-Hua Lei, bGVpdHNlaHVhQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Xin Zhao
Xin Zhao Tian-Yang Mao
Tian-Yang Mao Kang-Yi Jiang1,2
Kang-Yi Jiang1,2 Ze-Hua Lei
Ze-Hua Lei