- 1School of Sport Science, Qufu Normal University, Qufu, Shandong, China
- 2School of Physical Education, Anyang Normal University, Anyang, Henan, China
- 3School of Sport, Guangxi University of Science and Technology, Liuzhou, Guangxi, China
Background: Exercise has emerged as an effective approach to promote individual health and has shown potential in aiding smoking cessation. However, the specific benefits of exercise in smoking cessation remain unclear, and conflicting findings across studies may be attributed to variations in study populations and intervention characteristics. This study aims to conduct a meta-analysis to evaluate the impact of exercise interventions on tobacco dependence in smokers and assess the effectiveness of exercise in facilitating smoking cessation.
Methods: A comprehensive search was performed in databases including PubMed, Web of Science, Embase, The Cochrane Library, and Scopus to identify relevant randomized controlled trials published before 30 October 2022. The Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines were followed during the review process. The quality of evidence (QoE) was assessed with GRADE (grading of recommendations, assessment, development and evaluations) methodology.
Results: Acute exercise was found to significantly reduce smoking cravings [MD = −1.84, 95% CI (−2.92, −0.76), p < 0.001; SMD = −1.64, 95% CI (−2.22, −1.05), p < 0.001] and alleviate most withdrawal symptoms in smokers. However, there was no significant difference in the smoking cessation rate between the exercise group and the control group (p > 0.05). Exercise was associated with increased positive mood [SMD = 0.36, 95% CI (0.14, 0.58), p = 0.001] and reduced negative mood in smokers [SMD = −0.26, 95% CI (−0.39, −0.12), p < 0.001].
Conclusion: Acute exercise interventions effectively reduce cravings and withdrawal symptoms in smokers. However, long-term exercise interventions do not significantly improve the smoking cessation rate. Exercise can help reduce negative mood and enhance positive mood in smokers. Smokers with high levels of tobacco dependence may derive less benefit from exercise. Factors such as literature quality, exercise intervention characteristics, and exercise adherence may influence the effectiveness of interventions.
Trial registration: This research protocol was registered in the International Prospective Register for Systematic Reviews (PROSPERO https://www.crd.york.ac.uk/PROSPERO/). Registration number: CRD42022326109.
1 Introduction
Smoking remains one of the leading preventable causes of premature death worldwide, resulting in approximately 8 million deaths annually (World Health Organization, 2021). The “China Smoking Health Report 2020” released by China’s National Health Commission revealed that China has over 300 million smokers, with a smoking rate of 26.6% among individuals aged 15 years and older (Council, 2019). Smoking is a significant contributing factor to various diseases, including lung cancer, chronic respiratory diseases, coronary heart disease, stroke, and diabetes (West, 2017). Recent studies have also demonstrated that smokers face a significantly higher risk of COVID-19 progression and mortality compared to nonsmokers (Li et al., 2021). The “Health China Action (2019–2030)” issued by China’s National Health and Wellness Commission aims to reduce the smoking prevalence among individuals aged 15 years and older to less than 24.5% by 2022 and 20% by 2030 (Opinions of the State Council on the Implementation of Health China Action, 2019). Despite the implementation of effective tobacco control measures such as policy bans, health education, and medical consultations, quitting smoking remains challenging due to the highly addictive nature of nicotine, psychological and behavioral habits, and sociocultural factors associated with tobacco use. Approximately 75%–95% of smokers relapse within 6 months of attempting to quit (Livingstone-Banks et al., 2019). Therefore, it is crucial to actively explore effective strategies to reduce tobacco dependence.
Previous interventions to address tobacco dependence have primarily focused on psychological interventions, self-management techniques, and medication. Although these approaches demonstrate relatively significant short-term effects, their long-term efficacy is limited, with success rates ranging from only 7%–9% (Hughes et al., 2014). Moreover, self-management and psychological interventions require specific psychological skills that smokers often find difficult to navigate on their own (García-Gómez et al., 2019), Medication, on the other hand, may lead to side effects and potential dependence (Batra et al., 2016).
In recent years, with the increasing focus on the relationship between exercise and health, the role of exercise in tobacco dependence cessation has garnered significant attention. Exercise has been proposed as a standalone or adjunctive treatment for smoking cessation due to its potential to alleviate withdrawal symptoms, cigarette cravings, concerns about weight gain, as well as improve mood and mitigate the adverse effects of smoking on cardiorespiratory function (Haasova et al., 2014; Klinsophon et al., 2017). However, the findings from academic research on the effects of exercise interventions on tobacco dependence have been inconclusive, particularly regarding the long-term effects of such interventions (Ho et al., 2014; Ussher et al., 2019). A review of recent meta-analyses in the field of exercise interventions for smoking cessation has identified the following key characteristics: 1) Many studies lacked strict inclusion criteria. For instance, pregnant women (Klinsophon et al., 2017) and individuals with psychiatric disorders (Santos et al., 2021) were not consistently excluded, and literature on exercise counseling as an intervention was not consistently excluded either. Considering that physiological differences during pregnancy may affect nicotine withdrawal symptoms, including changes in hormone levels, which may affect nicotine metabolism and withdrawal response s (Míguez et al., 2019). In addition, pregnant women typically have higher motivation to quit smoking than non-pregnant adults because they have to consider not only their health but also the health of their fetus (Tong et al., 2008; Miyazaki et al., 2013; Patnode et al., 2021; Jackson et al., 2022). These may affect their willingness and success in trying to quit smoking. Therefore, Pregnant women were excluded from this study. Individuals with mental illness often experience unstable mental health, which can make smoking cessation or reduction particularly challenging (Schöttl et al., 2022). The inclusion of these studies may have influenced the overall results of the meta-analysis. 2) The use of a single outcome measure makes it difficult to obtain a comprehensive understanding of the effects of exercise (Klinsophon et al., 2017; Santos et al., 2021). 3) Most studies did not investigate potential moderating variables between exercise and tobacco dependence, such as exercise intervention characteristics. These variables are crucial in the development of effective intervention programs, and their absence leads to inadequate guidance for existing exercise programs. Furthermore, the search deadline for the most recent reviews in this field was January 2018, and several new randomized controlled trials have been published since then. Therefore, a new review is necessary to build upon the observations from previous meta-analyses and incorporate the latest evidence. In this study, a comprehensive set of outcome measures was employed, and the literature was screened more rigorously. Subgroup analysis was conducted to identify potential factors that influence the effects of exercise interventions, aiming to achieve a more comprehensive understanding of the effects of exercise. Meta-analysis was employed to systematically review existing studies and clarify the impact of exercise on tobacco dependence among smokers.
2 Materials and methods
The review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Page et al., 2021), and the protocol had previously been registered in PROSPERO (CRD42022326109). It is important to note that the statement “screening literature pre-dates registration” in the study registration form was an error made by the registrant. The official search for this study commenced on 30 October 2022. We want to emphasize that our study was not biased as a result of this error.
2.1 Search strategy
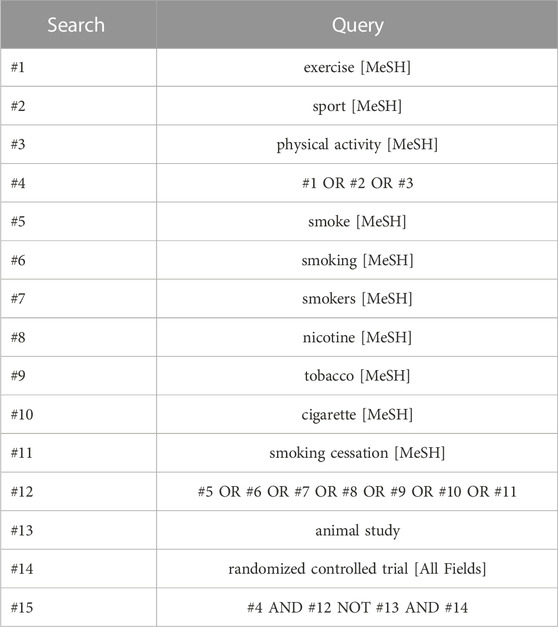
Randomized controlled trials (RCTs) of exercise interventions for smoking cessation were searched in the PubMed, Web of Science, Embase, The Cochrane Library, and Scopus databases. The search period ranged from the inception of each database to 30 October 2022. Additional sources, including previously published reviews, gray literature, expert documents, reference lists of eligible studies, and relevant reviews, were also searched. The search employed a combination of subject terms and free words. YH was responsible for identifying search terms and developing search strategies, while WX executed the specific search implementation. The search terms encompassed topics such as smoking, tobacco, nicotine, cigarettes, exercise, sport, physical activity, and randomized controlled trial. To illustrate, Table 1 presents the specific search strategy used for the PubMed database.
2.2 Inclusion and exclusion criteria
2.2.1 Inclusion criteria
Participant Type: We included individuals who were dependent on tobacco, without any restrictions on age or gender.
Design Type: This analysis included randomized controlled trials (RCTs).
Intervention Types: We included interventions aimed at increasing exercise, either as a standalone approach or as an adjunct to smoking cessation interventions. These were compared with smoking cessation programs alone or other non-exercise control groups. The control and exercise groups differed in their interventions only in whether or not they exercised, e.g., the control group intervention was nicotine replacement therapy and the exercise group intervention was exercise + nicotine replacement therapy.
Outcome Types: The study considered several outcome indicators, including craving, withdrawal symptoms, smoking cessation rate, and mood. The trials reported at least one of these available data.
2.2.2 Exclusion criteria
The exclusion criteria were as follows:
(1) Studies involving patients with concurrent substance dependence other than tobacco (e.g., alcohol, drugs, etc.), pregnant women, or individuals with psychiatric disorders.
(2) Studies without a control group or with a control group that included an exercise intervention.
(3) Studies with incomplete or unquantified data for outcome indicators or lack of appropriate outcome indicators.
(4) Qualitative studies, reviews, case studies, animal studies, and duplicate publications.
(5) Articles for which the full text was not accessible through various channels.
2.3 Study selection and data extraction
Literature search records were managed using EndNote X8 software (Clarivate Analytics, Philadelphia, PA, United States). The results of the database searches were imported and combined with EndNote X8, and duplicates were removed. Two researchers (YH and WX) independently screened the study titles and abstracts, retrieved and assessed the full text for compliance with inclusion criteria. Any discrepancies were resolved through discussion with YG if necessary, until a consensus was reached. Once the screening was complete, the full text was reviewed again, and data extraction was performed. Two researchers (YH and WX) independently extracted and entered the data. YG intervened to review and verify the data in cases of disagreement or inconsistency. The extracted information included three aspects (World Health Organization, 2021): basic information about the included literature, such as the first author, year of publication, characteristics of the study population (sample size, age, sex, tobacco dependence), content of the interventions in the experimental and control groups, intervention protocol (intensity, duration, frequency, period, etc.), and outcome indicators used (Council, 2019); information on the quality evaluation of the included literature; and (West, 2017) data indicators included in the literature, such as means and standard deviations of pre- and post-tests for each outcome and the number of events. If figures were reported graphically without providing the required data, GetData Graph Digitizer software (version 2.22) was used for data extraction.
2.4 Quality assessment
The Cochrane Risk of Bias tool was utilized to assess the quality of the included RCTs based on seven indicators: method of random allocation, allocation concealment, blinding of participants (and personnel), blinding of outcome assessment, completeness of outcome data, selective reporting of study results, and other sources of bias. The quality of the study was categorized into 3 grades: high (low risk for 4 or more entries), moderate (low risk for 2 or 3 entries), and low (low risk for 1 or no entries, potential for bias) (Wu et al., 2017). Risk of bias assessment was performed independently by two review authors (YH and WX), and any disagreements were resolved through discussion with a third author (YG).
2.5 Data analysis
Data were collated and analyzed using RevMan software version 5.4 and Stata software version 16.0. For continuous outcomes such as craving and withdrawal symptoms, weighted mean differences (WMDs) or standardized mean differences (SMDs) with 95% confidence intervals (CIs) were used as effect sizes. WMD was estimated when outcome measurements across all studies used the same scale, while SMD was employed when outcomes were measured using different quantitative scales (Higgins et al., 2019). Reported effect sizes were classified as trivial (<0.2), small (0.2 to <0.5), moderate (0.5 to <0.8), and large (≥0.8) (Cohen, 2013). For dichotomous outcomes, including adverse events, relative risks (RRs) with 95% CIs were pooled. I2 was used to test the heterogeneity of the included studies, in which 25, 50% and 75% of the I2 value were the judgment thresholds of low, medium and high heterogeneity, respectively (Higgins et al., 2003). Fixed-effects models were employed when heterogeneity was low; otherwise, random-effects models were used for the analysis. Sensitivity analysis was conducted by excluding trials with an assessed risk of bias to test the robustness of the pooled results. Exploratory subgroup analyses were performed to examine whether various factors influenced the effect size estimates. Publication bias tests could be conducted using funnel plots and quantified using Egger’s method (Sterne et al., 2011). For the evaluation of the quality of evidence (QoE), the GRADE methodology was used, evaluating five domains: inconsistency, risk of bias, imprecision, indirectness, and publication bias. Finally, QoE was presented in summary tables (SoF) using GRADEpro GDT (https://gradepro.org/, accessed on 12 July 2023. Any decisions to downgrade the certainty of studies were justified in footnotes.
3 Results
3.1 Literature selection
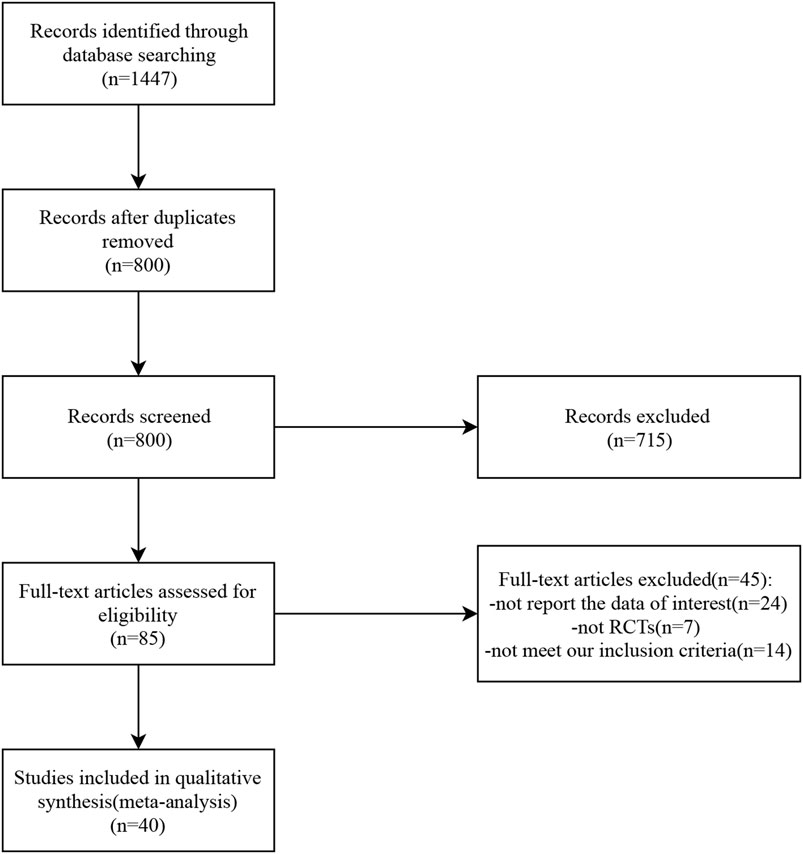
A total of 1,447 studies related to the topic of this study were retrieved from seven databases. After eliminating duplicates, 800 studies remained. The titles and abstracts of these studies were reviewed, and 85 studies were selected for further assessment. Among them, 45 studies were excluded: 24 did not report relevant data, seven were not randomized controlled trials (RCTs), and 14 did not meet the inclusion criteria. Finally, 40 studies were included in the analysis. The detailed selection process is presented in Figure 1.
3.2 Characteristics of the included studies
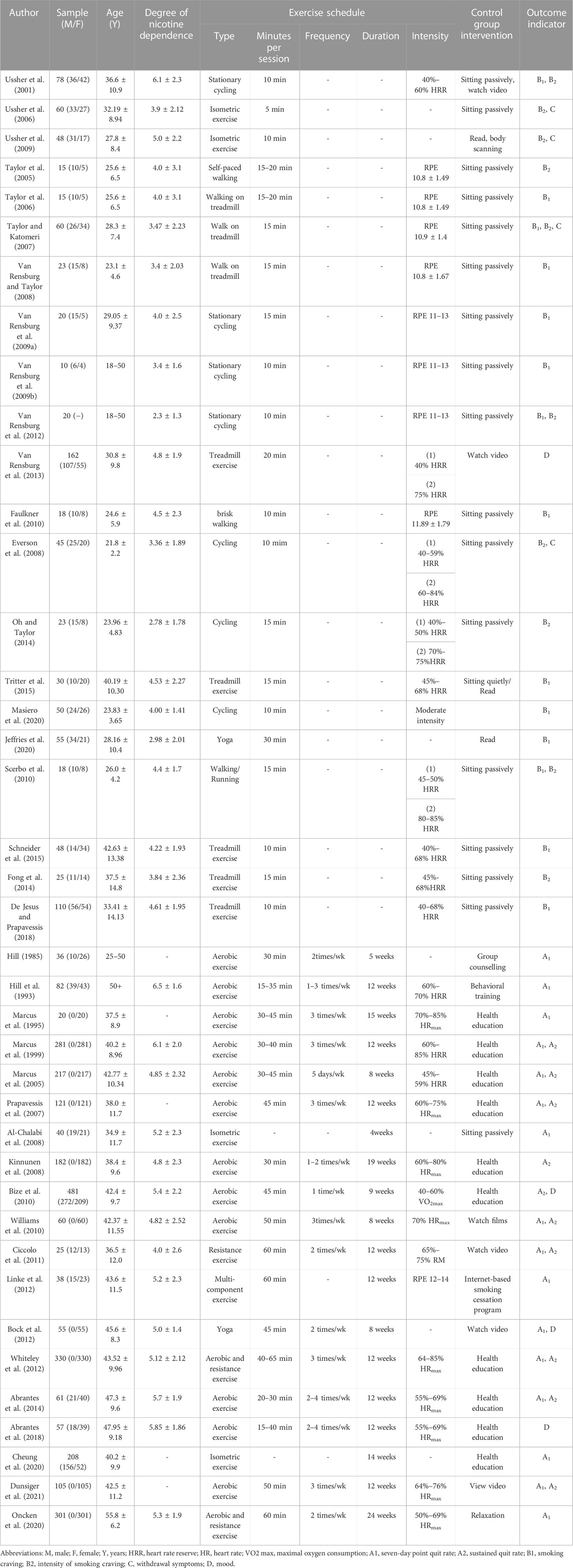
Table 2 provides an overview of the included studies. The 40 studies encompassed a total of 43 RCTs, with three studies comprising 2 RCTs each [denoted as (World Health Organization, 2021; Council, 2019), respectively]. The meta-analysis included a total of 3,427 smokers. Ten studies exclusively enrolled female participants, while the remaining studies included both sexes. The age range of participants in the included studies was 18–65 years.
Of the included studies, 21 focused on acute exercise interventions with a duration of 5–30 min (Ussher et al., 2001; Taylor et al., 2005; Taylor et al., 2006; Ussher et al., 2006; Taylor and Katomeri, 2007; Everson et al., 2008; Van Rensburg and Taylor, 2008; Van Rensburg et al., 2009a; Van Rensburg et al., 2009b; Ussher et al., 2009; Faulkner et al., 2010; Scerbo et al., 2010; Van Rensburg et al., 2012; Van Rensburg et al., 2013; Fong et al., 2014; Oh and Taylor, 2014; Schneider et al., 2015; Tritter et al., 2015; De Jesus and Prapavessis, 2018; Jeffries et al., 2020; Masiero et al., 2020). The remaining 19 studies involved long-term exercise interventions ranging from 4 to 19 weeks (Hill, 1985; Hill et al., 1993; Marcus et al., 1995; Marcus et al., 1999; Marcus et al., 2005; Prapavessis et al., 2007; Al-Chalabi et al., 2008; Kinnunen et al., 2008; Bize et al., 2010; Williams et al., 2010; Ciccolo et al., 2011; Bock et al., 2012; Linke et al., 2012; Whiteley et al., 2012; Abrantes et al., 2014; Abrantes et al., 2018; Cheung et al., 2020; Oncken et al., 2020; Dunsiger et al., 2021). The included studies incorporated various exercise modalities, including aerobic exercise, isometric exercise, resistance exercise, yoga, and multicomponent training (combinations of different exercises such as aerobic exercise, resistance exercise, balance, and flexibility exercises). Among these, aerobic exercise was the most frequently utilized, with cycling, walking, and treadmill activities being the predominant forms.
3.3 Results of risk of bias
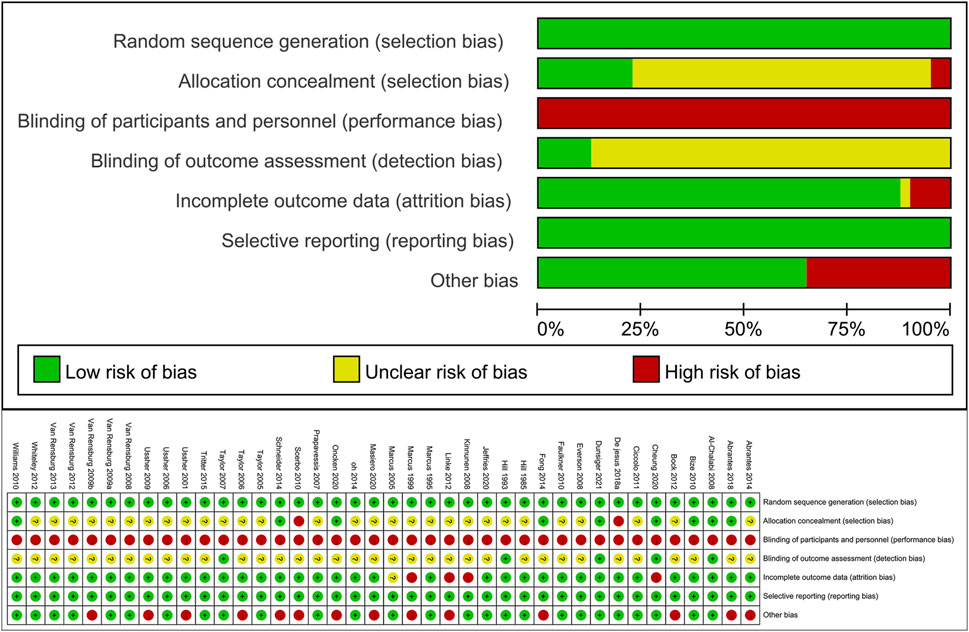
The assessment of risk of bias in the included trials is summarized in Figure 2. All 40 included studies employed random allocation and did not selectively report study outcomes. However, ensuring participant blinding was challenging due to the nature of exercise interventions, and most studies did not provide specific details on assessor blinding, resulting in a high risk of bias. Some of the included studies had missing outcome data, and although some studies had similar proportions of missing data between intervention groups or provided acceptable explanations, three studies still had a high risk of bias. Additionally, 12 (32%) studies reported other sources of bias, primarily including small sample sizes and significant baseline differences (p < 0.05). Based on the Cochrane Risk of Bias Assessment Tool, 7 studies were classified as low quality, 21 as moderate quality, and 12 as high quality. Overall, the methodological quality of the included studies was moderate.
3.4 Effects of acute exercise on smoking cravings
3.4.1 Meta-analysis results and heterogeneity test
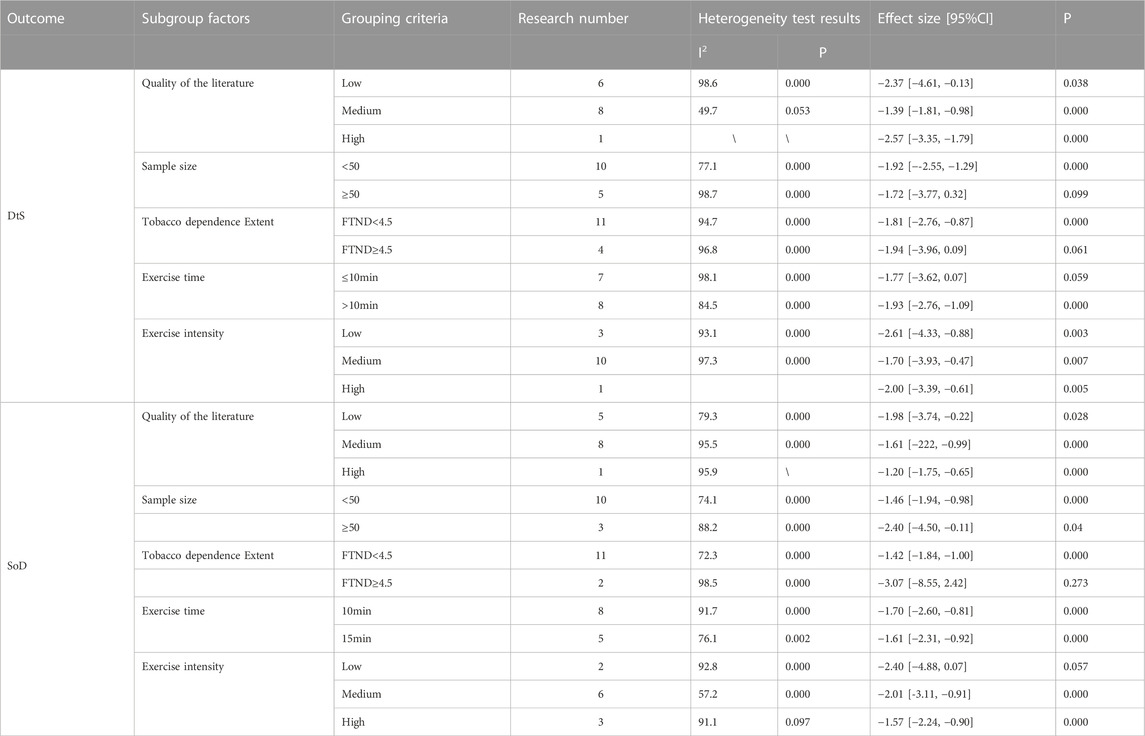
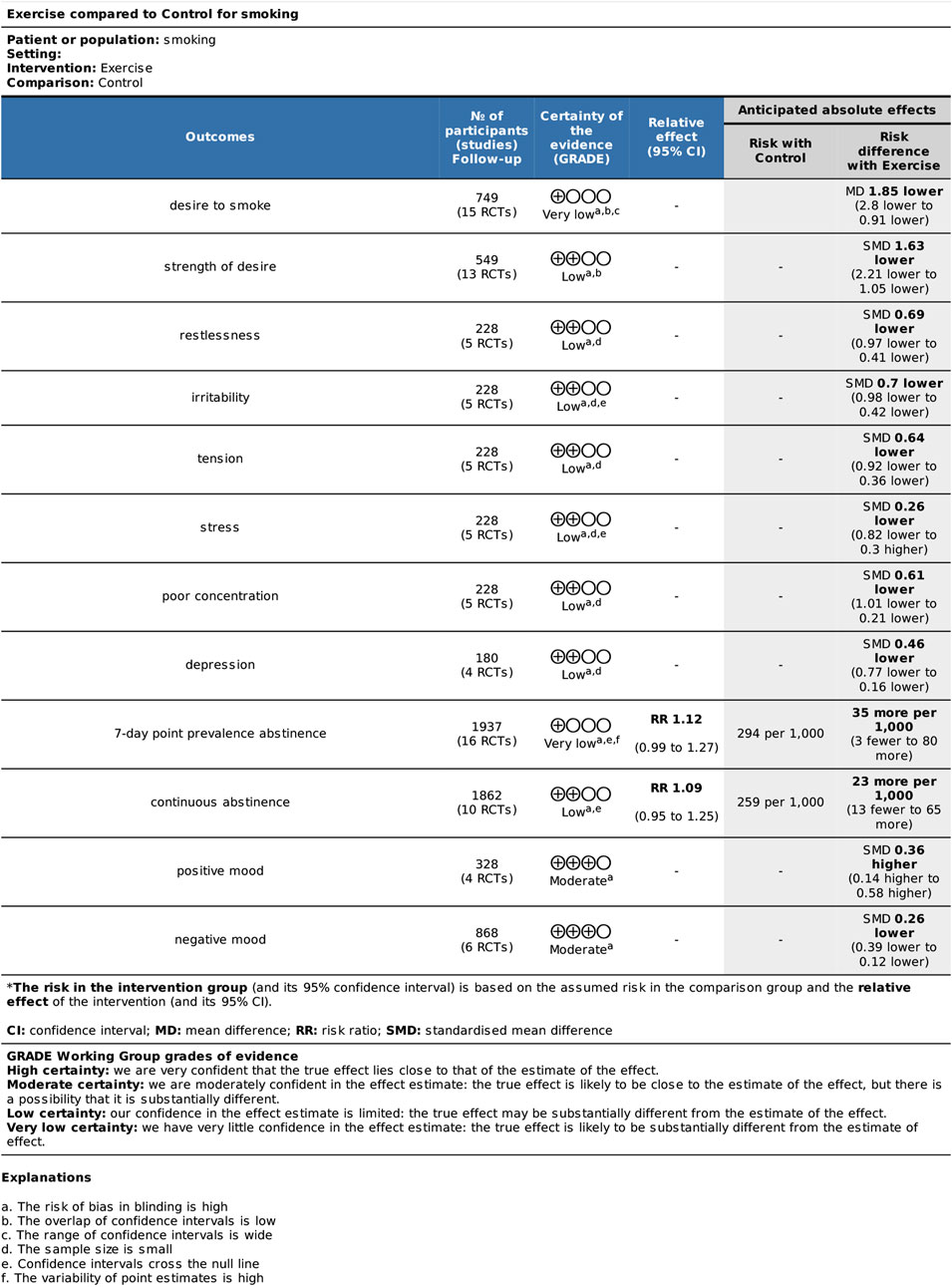
Smokers’ cravings were assessed using two Likert scales: desire to smoke (DtS) and strength of desire (SoD). A total of 15 RCTs (749 participants in total, with 385 in the exercise group and 364 in the control group) were included for DtS (Ussher et al., 2001; Taylor et al., 2006; Taylor and Katomeri, 2007; Van Rensburg and Taylor, 2008; Van Rensburg et al., 2009a; Van Rensburg et al., 2009b; Faulkner et al., 2010; Scerbo et al., 2010; Schneider et al., 2015; Tritter et al., 2015; De Jesus and Prapavessis, 2018; Jeffries et al., 2020; Masiero et al., 2020). Ten studies measuring SoD were included (Ussher et al., 2001; Taylor et al., 2005; Ussher et al., 2006; Taylor and Katomeri, 2007; Everson et al., 2008; Ussher et al., 2009; Scerbo et al., 2010; Van Rensburg et al., 2012; Fong et al., 2014; Oh and Taylor, 2014), involving a total of 554 participants (273 in the exercise group and 281 in the control group). The meta-analysis revealed that exercise interventions reduced DtS among smokers with a large effect size, based on a random-effects model [mean difference (MD) = −1.85, 95% confidence interval (CI) (−2.80, −0.91), p < 0.001]. The exercise interventions also had aa large effect size on SoD compared to the control group, as indicated by a standardized mean difference (SMD) of −1.63, 95% CI (−2.21, −1.05), p < 0.001 (Figure 3).

FIGURE 3. Meta-analysis of the effect of acute exercise on craving in tobacco -dependent individuals: (A) DtS and (B) SoD.
Heterogeneity tests showed high levels of heterogeneity for both DtS (I2 = 97%, p < 0.001) and SoD (I2 = 88%, p < 0.001). Sensitivity analysis, which involved removing individual studies, did not yield significant changes in the effect sizes for either outcome, indicating the robustness of the data.
3.4.2 Subgroup analysis
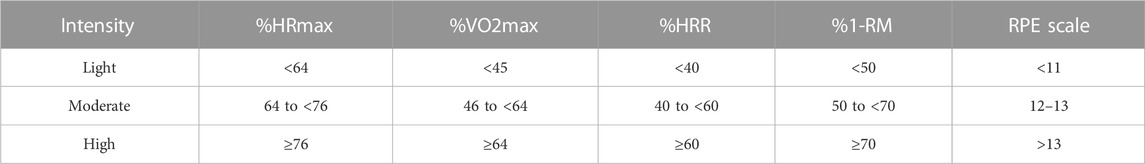
Subgroup analyses were conducted to analyze exercise intervention characteristics, including exercise duration (≤10 min or >10 min) and exercise intensity (exercise intensity reported in the original studies prevailed; if not reported, the criteria in Table 3 for exercise intensity classification were applied). Since most of the included studies focused on aerobic exercise, the number of studies investigating other types of exercise was limited (less than two). Therefore, exercise types were not used as a covariate. The random-effects model was employed for the analysis. The subgroup analysis results for DtS indicated that (1) exercise duration of more than 10 min had a significant effect in reducing DtS [MD = −1.93, 95% CI (−2.76, −1.09), p < 0.001], whereas exercise durations of ≤10 min did not significantly reduce DtS [MD = −1.77, 95% CI (−3.62, 0.07), p = 0.059], and (Council, 2019) all exercise intensities had a significant effect on DtS. Notably, the high-intensity group had only one included study, and its results require further exploration.
For SoD, the subgroup analysis results showed that 1) the included RCTs focused on exercise durations of 10 min and 15 min, and both durations yielded significant effects, and (Council, 2019) three studies compared different exercise intensities in relation to SoD. Heterogeneity tests within each intensity group revealed substantial heterogeneity between studies. Thus, the random-effects model was used to estimate the effect sizes. The difference between the low-intensity group and the control group was not statistically significant [SMD = −2.40, 95% CI (−4.88, 0.07), p = 0.057], whereas both the medium-intensity group [SMD = −2.01, 95% CI (−3.11, −0.91), p < 0.001] and high-intensity group showed significant differences compared to the control group [SMD = −1.57, 95% CI (−2.24, −0.90), p < 0.001].
Subgroup analyses were performed to investigate the impact of various study characteristics on the effectiveness of the intervention, including literature quality (risk of bias categorized as low, medium, or high), sample size (≤50 or >50), and the level of tobacco dependence (FTND <4.5 or FTND ≥4.5) among participants. The analysis was conducted using a random-effects model. The results revealed the following findings (World Health Organization, 2021): Medium- and high-quality literature demonstrated more favorable effects of exercise interventions on both DtS and SoD among individuals with tobacco dependence compared to low-quality literature (Council, 2019). Sample size had an influence on the intervention effects. Studies with sample sizes greater than 50 showed less significant effects of exercise interventions on craving production [SMD = −1.72, 95% CI (−3.77, 0.32), p = 0.099; SMD = −2.40, 95% CI (−4.50, −0.11), p = 0.040] (West, 2017). Smokers with higher levels of tobacco dependence [SMD = −1.94, 95% CI (−3.96, 0.09), p = 0.061; SMD = −3.07, 95% CI (−8.55, 2.42), p = 0.273] were less likely to derive benefits from exercise compared to smokers with lower levels of tobacco dependence (refer to Table 4 for detailed results).
3.4.3 Publication bias test
Egger’s test was used for the publication bias test, and the results showed that SoD indicators might have publication bias (p < 0.05); Dts indicators were less likely to have publication bias (p > 0.05).
3.5 Effects of acute exercise on withdrawal symptoms
3.5.1 Meta-analysis results and heterogeneity test
This study utilized six indicators to assess withdrawal symptoms: restlessness, irritability, tension, stress, poor concentration, and depression. The analysis included four studies with a total of 228 participants (Ussher et al., 2006; Taylor and Katomeri, 2007; Everson et al., 2008; Ussher et al., 2009), with 95 in the exercise group and 133 in the control group. The meta-analysis revealed that exercise interventions were more effective than the control group in alleviating withdrawal symptoms, except for stress, with both medium and small effect sizes.
The heterogeneity test indicated that restlessness (I2 = 40%, p = 0.640), irritability (I2 = 36%, p = 0.180), tension (I2 = 28%, p = 0.240), and depression (I2 = 50%, p = 0.110) had moderate heterogeneity, whereas stress (I2 = 75%, p = 0.003) and poor concentration (I2 = 50%, p = 0.090) exhibited substantial level of heterogeneity. Due to the small sample size, meta-regression and subgroup analysis could not be conducted to identify the sources of heterogeneity. However, through sensitivity analysis, two studies, namely, Taylor and Katomeri, (2007) and Everson et al., (2008), were identified as sources of heterogeneity, and their exclusion resulted in heterogeneity reduced to 0 for both indicators (refer to Figure 4).

FIGURE 4. Meta-analysis of the effect of acute exercise on withdrawal symptoms in tobacco-dependent individuals.
3.6 Effect of long-term exercise on smoking cessation rates
3.6.1 Meta-analysis results and heterogeneity test
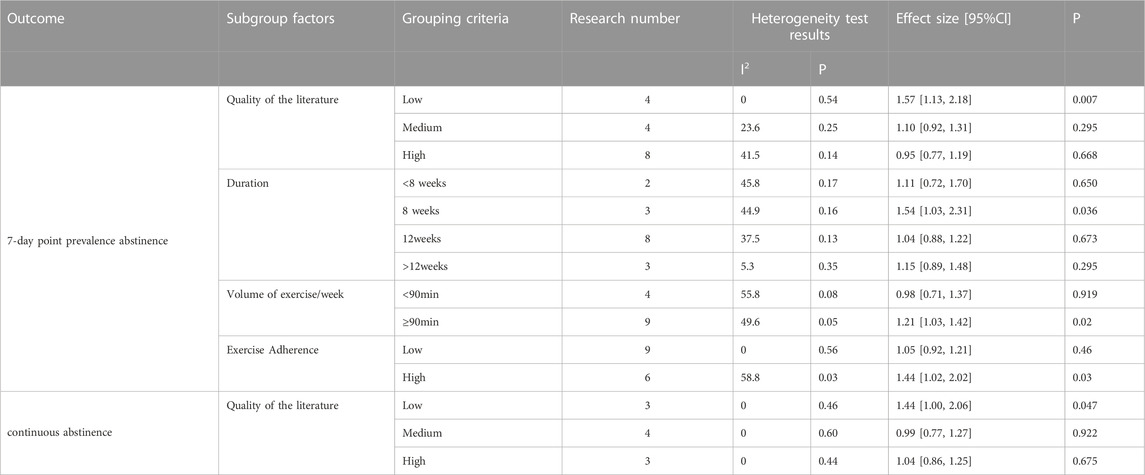
The analysis included a total of 16 randomized controlled trials (RCTs) involving 1937 participants, with 976 in the exercise group and 961 in the control group, to assess the 7-day point prevalence abstinence as the outcome (Hill, 1985; Hill et al., 1993; Marcus et al., 1995; Marcus et al., 1999; Marcus et al., 2005; Prapavessis et al., 2007; Al-Chalabi et al., 2008; Williams et al., 2010; Ciccolo et al., 2011; Bock et al., 2012; Linke et al., 2012; Whiteley et al., 2012; Abrantes et al., 2014; Cheung et al., 2020; Oncken et al., 2020; Dunsiger et al., 2021). Additionally, 10 RCTs comprising 1862 participants, with 922 in the exercise group and 940 in the control group, were included to evaluate continuous abstinence (Marcus et al., 1999; Marcus et al., 2005; Prapavessis et al., 2007; Kinnunen et al., 2008; Bize et al., 2010; Williams et al., 2010; Ciccolo et al., 2011; Whiteley et al., 2012; Abrantes et al., 2014; Dunsiger et al., 2021). The meta-analysis found no significant difference in the change of 7-day point prevalence abstinence [RR = 1.12, 95% CI (0.99, 1.27), p = 0.080] and continuous abstinence [RR = 1.09, 95% CI (0.95, 1.25), p = 0.220] between the exercise group and the control group. This suggests that long-term exercise interventions did not significantly enhance smoking cessation rates. As the seven-day point prevalence indicator for smoking cessation is moderate level of heterogeneity (I2 = 35%, p = 0.070) and the sustained point prevalence indicator for smoking cessation is low level of heterogeneity (I2 = 0%, p = 0.450), a fixed-effects model was employed for the analysis. Sensitivity analysis conducted for each indicator by excluding individual studies demonstrated no significant change in effect size or heterogeneity, indicating the stability of the outcome data (see Figure 5).
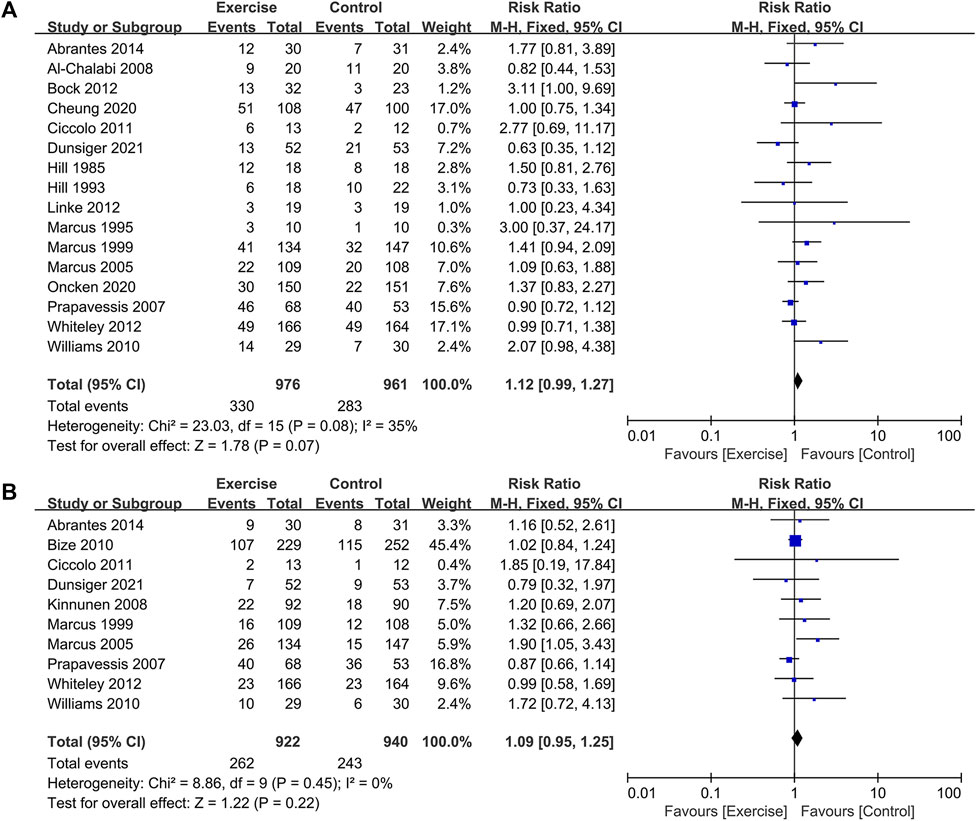
FIGURE 5. Effect of long-term exercise on smoking cessation rates in tobacco-dependent individuals: (A) 7-day point prevalence abstinence and (B) continuous abstinence.
3.6.2 Meta-regression analysis
In the meta-regression analysis, the effect size served as the dependent variable, while the heterogeneous explanatory variables (covariates) included sample size, sex, intervention period, exercise intensity, exercise duration, frequency, and exercise adherence. The results indicated that only exercise adherence significantly explained the heterogeneity in 7-day point prevalence abstinence following the physical activity intervention (p < 0.05), whereas none of the other explanatory variables reached a significant level of explanation for the heterogeneity observed in the meta-analysis groups (p > 0.05).
3.6.3 Subgroup analysis
The impact of exercise may be influenced by various characteristics of the exercise intervention program. A subgroup analysis was conducted to explore differences in the effect of exercise intensity (low, medium, or high), intervention duration (<8 weeks, 8 weeks, 12 weeks, or >12 weeks), and volume of exercise (<90 min/week or ≥90 min/week) on smoking cessation rates among smokers. Subgroup analyses were also performed to investigate other study characteristics that might influence the intervention effect, such as literature quality (low, medium, or high) and exercise adherence (low: <70% or high: ≥70%). The analysis employed a fixed-effects model for assessing literature quality and exercise duration, while a random-effects model was used for exercise volume and adherence. The following results were obtained (see Table 5): (World Health Organization, 2021) Regarding literature quality, the exercise intervention group exhibited higher quitting rates compared to the control group, with statistically significant differences [RR = 1.57, 95% CI (1.13, 2.18), p = 0.007; RR = 1.44, 95% CI (1.00, 2.06), p = 0.047] (Council, 2019). Concerning exercise intervention characteristics, the 8-week exercise intervention demonstrated higher 7-day point prevalence abstinence rates compared to the control group [RR = 1.54, 95% CI (1.03, 2.31), p = 0.036]. For the remaining intervention durations, no significant differences in quitting rates were observed between the intervention and control groups. Positive intervention effects on quitting rates were observed when the exercise volume was ≥90 min per week [RR = 1.21, 95% CI (1.03, 1.42), p = 0.020] (West, 2017). Only studies with high exercise adherence exhibited higher cessation rates in the intervention group compared to the control group [RR = 1.44, 95% CI (1.02, 2.02), p = 0.036].
3.6.4 Publication bias analysis
The RCTs included in the two indicators of long-term exercise amounted to 10. Ten RCTs were included for the two indicators related to long-term exercise. Therefore, a funnel plot was employed to assess publication bias. As depicted in Figure 6, the funnel plot exhibited a symmetrical distribution for the present meta-analysis. The Egger test results revealed that for 7-day point prevalence abstinence, t = −1.59, P > |t| = 0.137 > 0.05, and for continuous abstinence, t = −0.99, P > |t| = 0.349 > 0.05. These findings suggest the absence of significant publication bias.
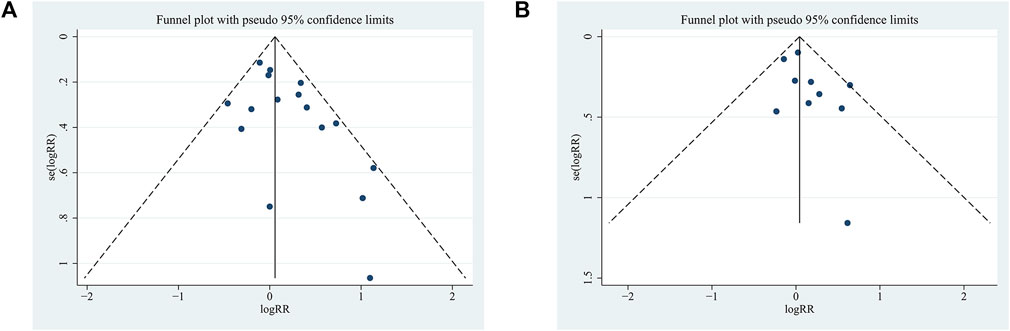
FIGURE 6. Funnel plot of the meta-analysis of a long-term exercise intervention on smoking cessation rates in tobacco-dependent individuals: (A) 7-day point prevalence abstinence and (B) continuous abstinence.
3.7 Effects of exercise intervention on mood
This study provides a brief exploration of moods, specifically positive and negative moods as separate domains. The impact of exercise interventions on smokers’ mood was evaluated through six randomized controlled trials (RCTs) from five studies (Bize et al., 2010; Bock et al., 2012; Van Rensburg et al., 2013; Abrantes et al., 2014; Abrantes et al., 2018). The heterogeneity test results, as shown in Figure 7, indicated low levels of heterogeneity for both positive mood (I2 = 0%, p = 0.910) and negative mood (I2 = 1%, p = 0.410). Consequently, a fixed-effects model was employed for the analysis. The combined effect sizes revealed that exercise interventions effectively improved smokers’ mood, with an increase in positive mood [SMD = 0.36, 95% CI (0.14, 0.58), p = 0.001] and a decrease in negative mood [SMD = −0.26, 95% CI (−0.39, −0.12), p < 0.001]. Sensitivity analysis conducted on the included studies demonstrated the relative stability of the outcome data.
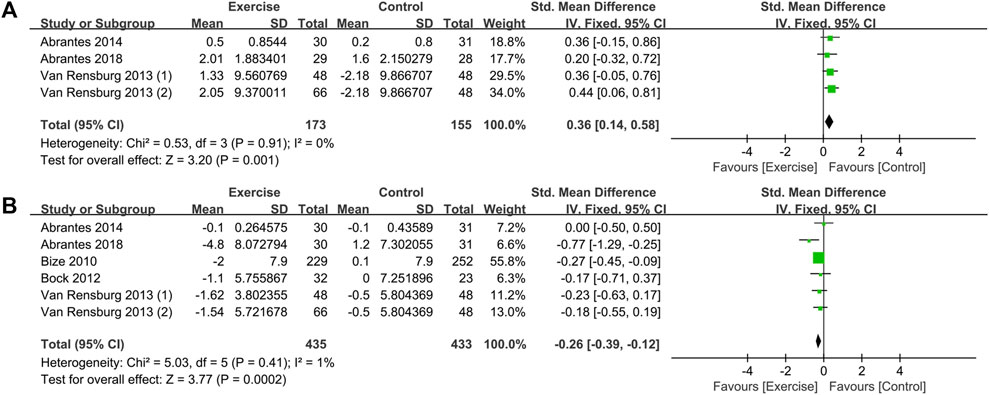
FIGURE 7. Meta-analysis of the effect of exercise on the mood of tobacco-dependent individuals: (A) positive mood and (B) negative mood.
3.8 Quality of evidence
Results of the GRADE analyses are provided in Figure 8. The outcomes DtS, and 7-day point prevalence abstinence had very low QoE; SoD, withdrawal symptoms, and continuous abstinence had low QoE; and mood had moderate QoE quality of evidence.
4 Discussion
The effectiveness of exercise interventions for smoking cessation has been a subject of controversy, and previous reviews have yielded mixed results. This study aimed to provide a more rigorous and comprehensive meta-analysis to evaluate the effects of exercise interventions on smoking cessation. The findings of this study indicate that short-term exercise can effectively reduce cravings for cigarettes and alleviate most withdrawal symptoms. However, long-term exercise does not appear to improve the rates of 7-day point prevalence abstinence and continuous abstinence among smokers. Additionally, exercise interventions were found to have a positive impact on mood in smokers or those attempting to quit.
4.1 Methodological quality assessment
Ensuring participant blinding was challenging due to the specific nature of exercise interventions, and most studies did not provide specific information on assessor blinding, resulting in a high risk of bias. Some of the included studies had missing outcome data, and although some had similar proportions of missing data across intervention groups or valid explanations, three studies still carried a high risk of bias. Furthermore, 12 studies (32%) reported other risks of bias, primarily related to small sample sizes and uneven baseline data (p < 0.05). Overall, the included studies were deemed to be of low quality, potentially impacting the credibility of the meta-analysis results to some extent. Subgroup analysis considering the quality of the literature as a covariate revealed that the results of low-quality literature contradicted the overall findings (i.e., long-term exercise improving smoking cessation rates versus long-term training not improving smoking cessation rates), indicating the influence of literature quality on the results. Future studies should strive to improve overall study quality by implementing allocation concealment, assessor blinding, increasing sample sizes, and addressing missing data issues.
4.2 Analysis of the effect of exercise interventions on tobacco dependence in smokers and possible mechanisms
4.2.1 Craving, and withdrawal symptoms
Smoking craving and withdrawal symptoms are important indicators for assessing tobacco dependence. Most studies have utilized smoking craving to evaluate the effectiveness of acute exercise interventions for smoking cessation and have shown that a higher desire to smoke is associated with a shorter time to the next cigarette. Withdrawal symptoms experienced during the cessation process are the primary drivers of relapse, characterized by heightened negative emotions and difficulties with concentration. Consequently, reducing withdrawal symptoms is crucial for enhancing smoking cessation success rates. This study confirmed that acute exercise interventions can aid smoking cessation by reducing cravings and withdrawal symptoms.
The selection of exercise intensity is a prominent topic in the realm of acute exercise interventions for smoking cessation. The subgroup analysis conducted in this study revealed that low, medium, and high-intensity exercise all alleviated cravings and withdrawal symptoms in smokers. However, low-intensity exercise was not found to be effective in reducing symptoms of depression. Previous studies comparing the effects of different exercise intensities on craving interventions have shown no significant difference in craving changes between medium- and high-intensity exercise at the end of the intervention. However, high-intensity exercise exhibited certain advantages, including a longer-lasting intervention effect and preventing smokers from shifting their attention to smoking-related cues (attentional bias) (Everson et al., 2008; Scerbo et al., 2010; Oh and Taylor, 2014). Conversely, Robert et al. found that, compared to moderate-intensity exercise, high-intensity exercise can significantly reduce the desire for cigarettes in the short term (Roberts et al., 2015). The findings of this study suggest that this discrepancy may be attributed to the characteristics of the study sample, which included individuals with a high level of tobacco dependence. This implies that high-intensity exercise may have a more pronounced effect on reducing cravings than moderate-intensity exercise in smokers with high levels of tobacco dependence.
These findings are consistent with the results of the present meta-analysis, which demonstrated a strong association between the level of tobacco dependence and the effectiveness of acute exercise interventions. Smokers with high levels of tobacco dependence were less likely to benefit from exercise. In conclusion, for tobacco-dependent patients, acute exercise interventions should primarily consist of moderate intensity, taking into account the degree of dependence. When the level of dependence is high, appropriately increasing the intensity could lead to better intervention effects.
Exercise interventions may impact cravings in smokers through the following mechanisms:
Cognitive improvement: Exercise plays a role in modulating attentional bias, increasing cognitive load on the brain’s information processing capacity, and reducing activation in brain regions associated with reward processing and visuospatial attention (Van Rensburg et al., 2009b). Additionally, exercise increases activation in brain regions of the medial prefrontal cortex related to the brain’s default mode, shifting attention away from tobacco-related cues (Van Rensburg et al., 2012). Moreover, brief periods of exercise induce physiological changes that cause structural (increase in white matter and gray matter volumes) (Suo et al., 2016; Bashir et al., 2021) and functional (cerebral metabolism and cerebral blood flow) (Renke et al., 2022) alterations in the brain. These changes promote the remodeling of brain structures like the prefrontal lobe and striatum, enhance inhibitory control in tobacco-dependent individuals, and consequently reduce tobacco dependence.
Reward substitution: Exercise effectively increases the levels of substances such as endorphins in tobacco-dependent individuals, compensating for the rewarding pleasure derived from smoking through neurohumoral regulation (Georgakouli et al., 2020). Furthermore, exercise enhances dopaminergic activity in the limbic reward system, improves the function of the midbrain dopaminergic system, and stabilizes the structure of the midbrain dopamine system. This normalization of reward and treatment pathways in the brains of tobacco-dependent patients helps overcome the lack of euphoria caused by nicotine withdrawal, thereby enhancing their psychological level of pleasure and promoting positive emotional experiences (Georgakouli et al., 2020).
4.2.2 Smoking cessation rate
A total of 19 of the included studies addressed the effect of long-term exercise interventions on tobacco dependence in smokers. Only three of the 17 studies reported a positive effect of exercise on smoking cessation (Marcus et al., 1991; Marcus et al., 1999; Bock et al., 2012). The results of meta-analysis showed that no significant difference in quit rates between the exercise and control groups in long-term exercise interventions, which is generally consistent with previous results (Ussher et al., 2019).
Exercise adherence and frequency are potential factors influencing the effectiveness of long-term exercise interventions. The majority of in-home exercise studies have shown poor exercise adherence. Kinnunen’s study (Kinnunen et al., 2008) revealed that less than 50% of participants followed the prescribed exercise regimen during the initial 5 weeks, and this percentage dropped to 6.5% by the end of the treatment period. Marcus (Marcus et al., 2005) found that only 15.2% of participants adhered to the exercise prescription. A study investigating the relationship between exercise effects and adherence discovered a moderate association between higher exercise frequency and improved 7-day quit rates as well as longer quit times during and after treatment (Williams et al., 2010). Consequently, the positive impact of exercise may be compromised by a lack of strict adherence to the exercise prescription. A subgroup analysis based on exercise adherence in this study indicated that the exercise group with high adherence demonstrated a higher 7-day point quitting rate. However, a more recent study in the higher quality literature revealed that despite approximately 85% of sessions being attended across different treatment conditions and 88% of exercise sessions being completed within the prescribed moderate intensity range, this level of compliance was insufficient to demonstrate an improvement in smoking cessation rates through exercise intervention (Dunsiger et al., 2021). Considering that the intervention effect of acute exercise on craving gradually diminishes after approximately 30 min, Williams’ study showed that exercising three times per week resulted in favorable acute changes in affect and cigarette cravings from pre-to post-exercise, but exercise did not consistently influence affect or craving on a session-to-session basis. Dunsiger (Dunsiger et al., 2021) also implemented an intervention protocol involving exercise three times per week with extended intervals between interventions. Long-term exercise at lower intervention frequencies may not sustainably reduce cravings and withdrawal symptoms, necessitating frequent and consistent exercise over time to achieve benefits. Therefore, future studies could investigate whether ensuring both frequency and exercise compliance in long-term exercise interventions could enhance smoking cessation outcomes.
4.2.3 Mood
The study findings indicate that exercise has a greater impact on mood improvement, encompassing both an increase in positive mood and a decrease in negative mood. However, upon reviewing the literature, we identified a lack of differentiation between “emotion,” “affect,” and “mood” in current research on exercise interventions for smoking cessation. These three concepts were often conflated, which was also evident in the literature used as an outcome indicator in our study. Additionally, referencing the relevant literature by Ekkekakis (Ekkekakis, 2003; Ekkekakis, 2013), and a study on PANAS (Watson et al., 1988), one of the selected emotion rating scales, it became evident that the scale itself has certain limitations. Despite being described as a mood rating scale by its developers, the scale’s name suggests an affect rating scale, and the internal items encompass both mood, emotions, and affect. It is important to emphasize that “mood,” “emotion,” and “affect” are distinct terms and cannot be used interchangeably. Therefore, it is recommended that future studies make clear distinctions and provide a comprehensive understanding of the research framework.
5 Limitations
The study has several limitations that may impact the reliability of the findings. These limitations include difficulties in contacting authors of some literature, lack of standardization in physical activity variables (e.g., frequency, intensity, duration, and type), and potential confounding factors such as adherence. Firstly, the inability to reach authors for specific literature sources hinders data verification. Additionally, due to the nature of exercise interventions, achieving blinding is challenging, and most studies inadequately describe whether assessor blinding and allocation concealment were implemented, resulting in increased methodological heterogeneity. Secondly, during data processing, it was observed that there was considerable heterogeneity among the studies that used craving as an outcome indicator. However, after conducting regression and subgroup analyses to explore potential contributing factors to heterogeneity, no specific source of heterogeneity could be identified. Lastly, although cardiopulmonary function and mood are presumed to be important regulatory variables in the relationship between exercise and tobacco dependence, only a limited number of studies have provided clarification on the participants’ level of cardiorespiratory function and mood status, which prevented their data from being included in this study.
6 Conclusion
Based on this meta-analysis, it is evident that acute exercise significantly reduces cravings and withdrawal symptoms in smokers, thus supporting its potential role in smoking cessation. However, the effectiveness of long-term exercise interventions remains inconclusive, as long-term exercise did not yield higher quit rates. Exercise can help reduce negative mood and enhance positive mood in smokers. Therefore, future research of higher quality is required. Furthermore, greater attention should be given to strategies aimed at improving exercise adherence in long-term interventions, as well as reevaluating the intervention effects by reducing the interval between interventions. Additionally, it is recommended that future studies accurately differentiate between mood, emotion, and affect.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
YH and JH were responsible for the study concept and design, reviewed the manuscript for intellectual contents. WX performed the analysis and wrote the first draft. YG were involved in the data analysis. All authors critically read, revised, and approved the final version for publication.
Funding
The study was supported by grants from the Projects of Science and Technology in Henan Province (No. 212102310263 to YG), and the project of Humanities and Social Science of Guangxi colleges and universities thousand young and middle-aged backbone teachers training (2022QGRW036).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2023.1221898/full#supplementary-material
References
Abrantes, A. M., Bloom, E. L., Strong, D. R., Riebe, D., Marcus, B. H., Desaulniers, J., et al. (2014). A preliminary randomized controlled trial of a behavioral exercise intervention for smoking cessation. Nicotine Tob. Res. 16 (8), 1094–1103. doi:10.1093/ntr/ntu036
Abrantes, A. M., Farris, S. G., Minami, H., Strong, D. R., Riebe, D., and Brown, R. A. (2018). Acute effects of aerobic exercise on affect and smoking craving in the weeks before and after a cessation attempt. Nicotine Tob. Res. 20 (5), 575–582. doi:10.1093/ntr/ntx104
Al-Chalabi, L., Prasad, N., Steed, L., Stenner, S., Aveyard, P., Beach, J., et al. (2008). A pilot randomised controlled trial of the feasibility of using body scan and isometric exercises for reducing urge to smoke in a smoking cessation clinic. BMC public health 8, 349. doi:10.1186/1471-2458-8-349
Bashir, S., Al-Sultan, F., Jamea, A. A., Almousa, A., Alnafisah, M., Alzahrani, M., et al. (2021). Physical exercise keeps the brain connected by increasing white matter integrity in healthy controls. Medicine 100 (35), e27015. doi:10.1097/MD.0000000000027015
Batra, A., Petersen, K. U., Hoch, E., Mann, K., Kröger, C., Schweizer, C., et al. (2016). Psychotherapy and pharmacotherapy for harmful tobacco use and tobacco dependency. Der Nervenarzt 87 (1), 35–45. doi:10.1007/s00115-015-0037-1
Bize, R., Willi, C., Chiolero, A., Stoianov, R., Payot, S., Locatelli, I., et al. (2010). Participation in a population-based physical activity programme as an aid for smoking cessation: A randomised trial. Tob. control 19 (6), 488–494. doi:10.1136/tc.2009.030288
Bock, B. C., Fava, J. L., Gaskins, R., Morrow, K. M., Williams, D. M., Jennings, E., et al. (2012). Yoga as a complementary treatment for smoking cessation in women. J. Women's Health 21 (2), 240–248. doi:10.1089/jwh.2011.2963
Cheung, Y. T., Lam, T. H., Chan, C. H. H., Ho, K. S., Fok, W. Y. P., Wang, M. P., et al. (2020). Brief handgrip and isometric exercise intervention for smoking cessation: A pilot randomized trial. Addict. Behav. 100, 106119. doi:10.1016/j.addbeh.2019.106119
Ciccolo, J. T., Dunsiger, S. I., Williams, D. M., Bartholomew, J. B., Jennings, E. G., Ussher, M. H., et al. (2011). Resistance training as an aid to standard smoking cessation treatment: A pilot study. Nicotine Tob. Res. 13 (8), 756–760. doi:10.1093/ntr/ntr068
Council, T. S. (2019). State council measures to enhance people's fitness, health. Available at: http://english.gov.cn/policies/latest_releases/2019/07/15/content_281476765851704.htm ((Accessed May 21, 2022).
De Jesus, S., and Prapavessis, H. (2018). Affect and cortisol mechanisms through which acute exercise attenuates cigarette cravings during a temporary quit attempt. Addict. Behav. 80, 82–88. doi:10.1016/j.addbeh.2018.01.007
Dunsiger, S., Emerson, J. A., Ussher, M., Marcus, B. H., Miranda, R., Monti, P. M., et al. (2021). Exercise as a smoking cessation treatment for women: A randomized controlled trial. J. Behav. Med. 44, 794–802. doi:10.1007/s10865-021-00236-8
Ekkekakis, P. (2003). Pleasure and displeasure from the body: Perspectives from exercise. Cogn. Emot. 17 (2), 213–239. doi:10.1080/02699930302292
Ekkekakis, P. (2013). The measurement of affect, mood, and emotion: A guide for health-behavioral research. Cambridge: Cambridge University Press.
Everson, E. S., Daley, A. J., and Ussher, M. (2008). The effects of moderate and vigorous exercise on desire to smoke, withdrawal symptoms and mood in abstaining young adult smokers. Ment. health Phys. activity 1 (1), 26–31. doi:10.1016/j.mhpa.2008.06.001
Faulkner, G. E., Arbour-Nicitopoulos, K. P., and Hsin, A. (2010). Cutting down one puff at a time: The acute effects of exercise on smoking behaviour. J. Smok. Cessat. 5 (2), 130–135. doi:10.1375/jsc.5.2.130
Fong, A. J., De Jesus, S., Bray, S. R., and Prapavessis, H. (2014). Effect of exercise on cigarette cravings and ad libitum smoking following concurrent stressors. Addict. Behav. 39 (10), 1516–1521. doi:10.1016/j.addbeh.2014.05.027
García-Gómez, L., Hernández-Pérez, A., Noé-Díaz, V., Riesco-Miranda, J. A., and Jiménez-Ruiz, C. (2019). Smoking cessation treatments: Current psychological and pharmacological options. Rev. Investig. clinica; organo del Hosp. Enfermedades Nutr. 71 (1), 7–16. doi:10.24875/RIC.18002629
Georgakouli, K., Manthou, E., Georgoulias, P., Ziaka, A., and Jamurtas, A. Z. (2020). HPA axis responses to acute exercise differ in smokers and non-smokers. Physiology Behav. 229 (8), 113258. doi:10.1016/j.physbeh.2020.113258
Haasova, M., Warren, F. C., Ussher, M., Van Rensburg, K. J., Faulkner, G., Cropley, M., et al. (2014). The acute effects of physical activity on cigarette cravings: Exploration of potential moderators, mediators and physical activity attributes using individual participant data (IPD) meta-analyses. Psychopharmacology 231 (7), 1267–1275. doi:10.1007/s00213-014-3450-4
Higgins, J. P., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (2019). Cochrane handbook for systematic reviews of interventions. Chichester (UK): John Wiley and Sons.
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Br. Med. J. 327, 557–560. doi:10.1136/bmj.327.7414.557
Hill, J. S. (1985). Effect of a program of aerobic exercise on the smoking behaviour of a group of adult volunteers. Can. J. public health = Revue Can. de sante publique 76 (3), 183–186.
Hill, R. D., Rigdon, M., and Johnson, S. (1993). Behavioral smoking cessation treatment for older chronic smokers. Behav. Ther. 24 (2), 321–329. doi:10.1016/s0005-7894(05)80272-2
Ho, J. Y., Kraemer, W. J., Volek, J. S., Vingren, J. L., Fragala, M. S., Flanagan, S. D., et al. (2014). Effects of resistance exercise on the HPA axis response to psychological stress during short-term smoking abstinence in men. Addict. Behav. 39 (3), 695–698. doi:10.1016/j.addbeh.2013.10.027
Hughes, J. R., Stead, L. F., Hartmann-Boyce, J., Cahill, K., and Lancaster, T. (2014). Antidepressants for smoking cessation. Cochrane database Syst. Rev. 2014 (1), CD000031. doi:10.1002/14651858.CD000031.pub4
Jackson, S., Brown, J., Norris, E., Livingstone-Banks, J., Hayes, E., and Lindson, N. (2022). Mindfulness for smoking cessation. Cochrane database Syst. Rev. 4 (4), Cd013696. doi:10.1002/14651858.CD013696.pub2
Jeffries, E. R., Zvolensky, M. J., and Buckner, J. D. (2020). The acute impact of hatha yoga on craving among smokers attempting to reduce or quit. Nicotine Tob. Res. 22 (3), 446–451. doi:10.1093/ntr/nty263
Kinnunen, T., Leeman, R. F., Korhonen, T., Quiles, Z. N., Terwal, D. M., Garvey, A. J., et al. (2008). Exercise as an adjunct to nicotine gum in treating tobacco dependence among women. Nicotine Tob. Res. 10 (4), 689–703. doi:10.1080/14622200801979043
Klinsophon, T., Thaveeratitham, P., Sitthipornvorakul, E., and Janwantanakul, P. (2017). Effect of exercise type on smoking cessation: A meta-analysis of randomized controlled trials. BMC Res. notes 10 (1), 442. doi:10.1186/s13104-017-2762-y
Li, X., Zhong, X., Wang, Y., Zeng, X., Luo, T., and Liu, Q. (2021). Clinical determinants of the severity of COVID-19: A systematic review and meta-analysis. PloS one 16 (5), e0250602. doi:10.1371/journal.pone.0250602
Linke, S. E., Rutledge, T., and Myers, M. G. (2012). Intermittent exercise in response to cigarette cravings in the context of an Internet-based smoking cessation program. Ment. health Phys. activity 5 (1), 85–92. doi:10.1016/j.mhpa.2012.02.001
Livingstone-Banks, J., Norris, E., Hartmann-Boyce, J., West, R., Jarvis, M., Chubb, E., et al. (2019). Relapse prevention interventions for smoking cessation. Cochrane database Syst. Rev. 2019 (10). doi:10.1002/14651858.CD003999.pub6
Marcus, B. H., Albrecht, A. E., King, T. K., Parisi, A. F., Pinto, B. M., Roberts, M., et al. (1999). The efficacy of exercise as an aid for smoking cessation in women: A randomized controlled trial. Archives Intern. Med. 159 (11), 1229–1234. doi:10.1001/archinte.159.11.1229
Marcus, B. H., Albrecht, A. E., Niaura, R. S., Abrams, D. B., and Thompson, P. D. (1991). Usefulness of physical exercise for maintaining smoking cessation in women. Am. J. Cardiol. 68 (4), 406–407. doi:10.1016/0002-9149(91)90843-a
Marcus, B. H., Albrecht, A. E., Niaura, R. S., Taylor, E. R., Simkin, L. R., Feder, S. I., et al. (1995). Exercise enhances the maintenance of smoking cessation in women. Addict. Behav. 20 (1), 87–92. doi:10.1016/0306-4603(94)00048-4
Marcus, B. H., Lewis, B. A., Hogan, J., King, T. K., Albrecht, A. E., Bock, B., et al. (2005). The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: A randomized controlled trial. Nicotine Tob. Res. 7 (6), 871–880. doi:10.1080/14622200500266056
Masiero, M., Keyworth, H., Pravettoni, G., Cropley, M., and Bailey, A. (2020). Short bouts of physical activity are associated with reduced smoking withdrawal symptoms, but perceptions of intensity may Be the key. Healthc. (Basel, Switz. 8 (4), 425. doi:10.3390/healthcare8040425
Míguez, M. C., Pereira, B., Pinto, T. M., and Figueiredo, B. (2019). Continued tobacco consumption during pregnancy and women's depression and anxiety symptoms. Int. J. Public Health 64 (9), 1355–1365. doi:10.1007/s00038-019-01308-y
Miyazaki, Y., Hayashi, K., Mizunuma, H., Lee, J. S., Katanoda, K., Imazeki, S., et al. (2013). Smoking habits in relation to reproductive events among Japanese women: Findings of the Japanese nurses' health study. Prev. Med. 57 (5), 729–731. doi:10.1016/j.ypmed.2013.08.004
Oh, H., and Taylor, A. H. (2014). Self-regulating smoking and snacking through physical activity. Health Psychol. 33 (4), 349–359. doi:10.1037/a0032423
Oncken, C., Allen, S., Litt, M., Kenny, A., Lando, H., Allen, A., et al. (2020). Exercise for smoking cessation in postmenopausal women: A randomized, controlled trial. Nicotine Tob. Res. 22 (9), 1587–1595. doi:10.1093/ntr/ntz176
Opinions of the State Council on the Implementation of Health China Action (2019). Bulletin of the state Council of the people's Republic of China, 6.
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ Clin. Res. ed) 372, 790–799. doi:10.1016/j.rec.2021.07.010
Patnode, C. D., Henderson, J. T., Melnikow, J., Coppola, E. L., Durbin, S., and Thomas, R. U. S. (2021). “Preventive services task force evidence syntheses, formerly systematic evidence reviews,” in Interventions for tobacco cessation in adults, including pregnant women: An evidence update for the US preventive services task force (Rockville (MD): Agency for Healthcare Research and Quality).
Prapavessis, H., Cameron, L., Baldi, J. C., Robinson, S., Borrie, K., Harper, T., et al. (2007). The effects of exercise and nicotine replacement therapy on smoking rates in women. Addict. Behav. 32 (7), 1416–1432. doi:10.1016/j.addbeh.2006.10.005
Renke, M. B., Marcinkowska, A. B., Kujach, S., and Winklewski, P. J. (2022). A systematic review of the impact of physical exercise-induced increased resting cerebral blood flow on cognitive functions. Front. Aging Neurosci. 14, 803332. doi:10.3389/fnagi.2022.803332
Roberts, V., Gant, N., Sollers, J. J., Bullen, C., Jiang, Y., and Maddison, R. (2015). Effects of exercise on the desire to smoke and physiological responses to temporary smoking abstinence: A crossover trial. Psychopharmacology 232 (6), 1071–1081. doi:10.1007/s00213-014-3742-8
Santos, C. P., Proença, M., Gouveia, T. D. S., Soares de Oliveira, C. B., Tacao, G. Y., Trevisan, I. B., et al. (2021). Effectiveness of aerobic exercise on smoking cessation in adults: A systematic review and meta-analysis. J. Phys. activity health 18 (2), 230–242. doi:10.1123/jpah.2019-0339
Scerbo, F., Faulkner, G., Taylor, A., and Thomas, S. (2010). Effects of exercise on cravings to smoke: The role of exercise intensity and cortisol. J. sports Sci. 28 (1), 11–19. doi:10.1080/02640410903390089
Schneider, T., De Jesus, S., and Prapavessis, H. (2015). The effect of acute exercise on smoking topography: No evidence for cutting down one puff at a time. J. Smok. Cessat. 10 (02), 146–153. doi:10.1017/jsc.2014.2
Schöttl, S. E., Niedermeier, M., Kopp-Wilfling, P., Frühauf, A., Bichler, C. S., Edlinger, M., et al. (2022). Add-on exercise interventions for smoking cessation in people with mental illness: A systematic review and meta-analysis. BMC sports Sci. Med. rehabilitation 14 (1), 115. doi:10.1186/s13102-022-00498-y
Sterne, J. A., Sutton, A. J., Ioannidis, J. P., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ Clin. Res. ed) 343, d4002. doi:10.1136/bmj.d4002
Suo, C., Singh, M. F., Gates, N., Wen, W., Sachdev, P., Brodaty, H., et al. (2016). Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol. Psychiatry 21 (11), 1633–1642. doi:10.1038/mp.2016.19
Taylor, A., Katomeri, M., and Ussher, M. (2006). Effects of walking on cigarette cravings and affect in the context of nesbitt's paradox and the circumplex model. J. Sport and Exerc. Psychol. 28 (1), 18–31. doi:10.1123/jsep.28.1.18
Taylor, A., and Katomeri, M. (2007). Walking reduces cue-elicited cigarette cravings and withdrawal symptoms, and delays ad libitum smoking. Nicotine Tob. Res. 9 (11), 1183–1190. doi:10.1080/14622200701648896
Taylor, A. H., Katomeri, M., and Ussher, M. (2005). Acute effects of self-paced walking on urges to smoke during temporary smoking abstinence. Psychopharmacology 181 (1), 1–7. doi:10.1007/s00213-005-2216-4
Tong, V. T., England, L. J., Dietz, P. M., and Asare, L. A. (2008). Smoking patterns and use of cessation interventions during pregnancy. Am. J. Prev. Med. 35 (4), 327–333. doi:10.1016/j.amepre.2008.06.033
Tritter, A., Fitzgeorge, L., and Prapavessis, H. (2015). The effect of acute exercise on cigarette cravings while using a nicotine lozenge. Psychopharmacology 232 (14), 2531–2539. doi:10.1007/s00213-015-3887-0
Ussher, M., Cropley, M., Playle, S., Mohidin, R., and West, R. (2009). Effect of isometric exercise and body scanning on cigarette cravings and withdrawal symptoms. Addict. (Abingdon, Engl. 104 (7), 1251–1257. doi:10.1111/j.1360-0443.2009.02605.x
Ussher, M., Nunziata, P., Cropley, M., and West, R. (2001). Effect of a short bout of exercise on tobacco withdrawal symptoms and desire to smoke. Psychopharmacology 158 (1), 66–72. doi:10.1007/s002130100846
Ussher, M., West, R., Doshi, R., and Sampuran, A. K. (2006). Acute effect of isometric exercise on desire to smoke and tobacco withdrawal symptoms. Hum. Psychopharmacol-Clin Exp. 21 (1), 39–46. doi:10.1002/hup.744
Ussher, M. H., Faulkner, G. E. J., Angus, K., Hartmann-Boyce, J., and Taylor, A. H. (2019). Exercise interventions for smoking cessation. Cochrane Database Syst. Rev. 2019 (10). doi:10.1002/14651858.CD002295.pub6
Van Rensburg, K., and Taylor, A. H. (2008). The effects of acute exercise on cognitive functioning and cigarette cravings during temporary abstinence from smoking. Hum. Psychopharmacol. 23 (3), 193–199. doi:10.1002/hup.925
Van Rensburg, K. J., Elibero, A., Kilpatrick, M., and Drobes, D. J. (2013). Impact of aerobic exercise intensity on craving and reactivity to smoking cues. Exp. Clin. Psychopharmacol. 21 (3), 196–203. doi:10.1037/a0032768
Van Rensburg, K. J., Taylor, A., Benattayallah, A., and Hodgson, T. (2012). The effects of exercise on cigarette cravings and brain activation in response to smoking-related images. Psychopharmacology 221 (4), 659–666. doi:10.1007/s00213-011-2610-z
Van Rensburg, K. J., Taylor, A., Hodgson, T., and Benattayallah, A. (2009b). Acute exercise modulates cigarette cravings and brain activation in response to smoking-related images: An fMRI study. Psychopharmacology 203 (3), 589–598. doi:10.1007/s00213-008-1405-3
Van Rensburg, K. J., Taylor, A., and Hodgson, T. (2009a). The effects of acute exercise on attentional bias towards smoking-related stimuli during temporary abstinence from smoking. Addict. (Abingdon, Engl. 104 (11), 1910–1917. doi:10.1111/j.1360-0443.2009.02692.x
Watson, D., Clark, L. A., and Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 54 (6), 1063–1070. doi:10.1037//0022-3514.54.6.1063
West, R. (2017). Tobacco smoking: Health impact, prevalence, correlates and interventions. Psychol. health 32 (8), 1018–1036. doi:10.1080/08870446.2017.1325890
Whiteley, J. A., Williams, D. M., Dunsiger, S., Jennings, E. G., Ciccolo, J. T., Bock, B. C., et al. (2012). YMCA commit to quit: Randomized trial outcomes. Am. J. Prev. Med. 43 (3), 256–262. doi:10.1016/j.amepre.2012.05.025
Williams, D. M., Whiteley, J. A., Dunsiger, S., Jennings, E. G., Albrecht, A. E., Ussher, M. H., et al. (2010). Moderate intensity exercise as an adjunct to standard smoking cessation treatment for women: A pilot study. Psychol. Addict. Behav. 24 (2), 349–354. doi:10.1037/a0018332
World Health Organization (2021). Tobacco. Available at: http://www.who.int/ihr/en/((Accessed May 21, 2022).
Keywords: effect, exercise, meta-analysis, smoking cessation, mood, tobacco dependence, cravings
Citation: Zhou Y, Feng W, Guo Y and Wu J (2023) Effect of exercise intervention on smoking cessation: a meta-analysis. Front. Physiol. 14:1221898. doi: 10.3389/fphys.2023.1221898
Received: 13 May 2023; Accepted: 28 July 2023;
Published: 08 August 2023.
Edited by:
Yan Li, Beijing Sport University, ChinaReviewed by:
Junhao Huang, Guangzhou Sport University, ChinaJiaqing Tong, Medical College of Wisconsin, United States
Copyright © 2023 Zhou, Feng, Guo and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juhua Wu, juhuahf@163.com
†These authors have contributed equally to this work and share first authorship
 Yuehui Zhou
Yuehui Zhou Wenxia Feng
Wenxia Feng Yugang Guo
Yugang Guo Juhua Wu3*
Juhua Wu3*