- 1Department of Clinical Laboratory, The 4th Hospital of Harbin Medical University, Harbin, Heilongjiang, China
- 2Department of Hepatopancreatobiliary Surgery, Second Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, China
Background: Limited information is currently available on the natural history and prognosis of two distinct histological subtypes of adenocarcinoma (AC) in the colon: mucinous adenocarcinoma (MAC) and signet-ring cell carcinoma (SRCC). Therefore, the aim of this study is to examine the clinicopathological characteristics of colon MAC and SRCC, comparing them to classical AC, using a large cohort of cases from the United States.
Methods: Patients diagnosed with colon AC, MAC, or SRCC from the SEER database between 2000 and 2018 were included in our study. Incidence trends, patient demographics, tumor characteristics, treatment, and survival were analyzed.
Results: In our study, we analyzed a total of 310,813 patients with colon cancers, including 271,382 cases of classical AC, 34,750 cases of MAC, and 4,681 cases of SRCC. Over the study period, we observed a decline in the age-adjusted incidence rates of colon AC, MAC, and SRCC. Notably, the MAC and SRCC cohorts differed significantly from AC in terms of patient characteristics, tumor locations, and treatment patterns. Patients with MAC and SRCC had poorer survival outcomes compared to those with AC. Factors associated with worse survival included older age, male sex, poorly differentiated tumors, advanced stage, and the presence of MAC or SRCC histology. On the other hand, surgical intervention was associated with improved survival.
Conclusion: Our study underscores the significance of recognizing the distinct features and outcomes associated with different histological subtypes of colon cancer. Further research is warranted to delve into the underlying biological traits that contribute to these differences and to develop more tailored treatment strategies.
Introduction
Gastrointestinal cancers pose a significant global health burden, with over 5.1 million new cases and 3.6 million deaths reported in 2020 alone (Bordry et al., 2021; Sung et al., 2021; Davila and Davila, 2022). Among these cancers, colon cancers are among the most common affecting the gastrointestinal tract. While adenocarcinoma (AC) is the predominant subtype, there are two distinct and relatively rare variants known as mucinous adenocarcinoma (MAC) and signet-ring cell carcinoma (SRCC), characterized by mucin secretion (Arai, 2019; Nagtegaal et al., 2020; Ahadi et al., 2021; Washington et al., 2021). The signet-ring cell component that occupies 50% or more of the lesion distinguishes SRCC from MAC. The histological grade of a tumor can significantly impact its biology and survival, leading to the possibility that these two distinct variants represent different diseases with unique clinical features and prognoses. However, due to their rarity, investigating the clinicopathological characteristics and survival outcomes of colon MAC and SRCC has been challenging, and their clinical significance remains uncertain. In the era of precision medicine, management strategies of malignancies are customized to suit patient and tumor characteristics, rather than using a one-size-fits-all approach. While histological classification is readily available, its clinical implications have been subject to conflicting findings in the literature (Overman et al., 2013; Widmann et al., 2016).
Therefore, this study aims to analyze a large population-based cohort from the United States to comprehensively characterize and compare the clinicopathological features and outcomes of colon AC, MAC, and SRCC. By conducting an in-depth analysis of the clinical and pathological characteristics of these cancers, the study aims to offer valuable insights into their etiology, progression, and therapeutic approaches. Ultimately, the findings from this research may contribute to the development of more targeted and effective diagnostic and treatment strategies specific to the distinct characteristics of these colon cancer subtypes.
Methods
This study analyzed patients diagnosed with colon AC, MAC and SRCC from the Surveillance, Epidemiology and End Results (SEER)-18 program between 2000 and 2018. The inclusion criteria encompassed cases with primary tumors located in the colon (excluding appendix). Patient data regarding clinicopathological characteristics were collected for comprehensive analysis, including variables such as age at diagnosis, gender, race, year of diagnosis, tumor grade, stage, treatment, overall survival (OS), and cancer-specific survival (CSS). Patients with incomplete survival outcome information were excluded from the study. The study received approval from the institutional review board (IRB) of the fourth Hospital of Harbin Medical University, and informed consent was waived due to the observational design.
Statistical analysis
Incidence rates were calculated using SEER*Stat software and were age-adjusted to the 2000 U.S. standard population and expressed per 100,000 person-years. Categorical variables were presented as number and percentages, and compared by chi-square test. Survival outcomes were estimated using the Kaplan-Meier method with log-rank test. Univariable and multivariable Cox regression analyses were performed to identify potential factors associated with overall survival in colon cancers. Schoenfeld residuals were examined to identify any time-dependent biases. The SPSS and R software was used to perform all tests of statistical significance, with a significance level established at p < 0.05. Proportionality of hazards was evaluated for each variable, and schoenfeld residuals were examined to identify any time-dependent biases.
Results
Between 2000 and 2018, our analysis included 310,813 colon cancer patients who met the inclusion criteria. Of these patients, 271,382 (87.3%) were diagnosed with classical AC, 34,750 (11.2%) were diagnosed with MAC, and 4,681 (1.5%) were diagnosed with SRCC (Table 1).
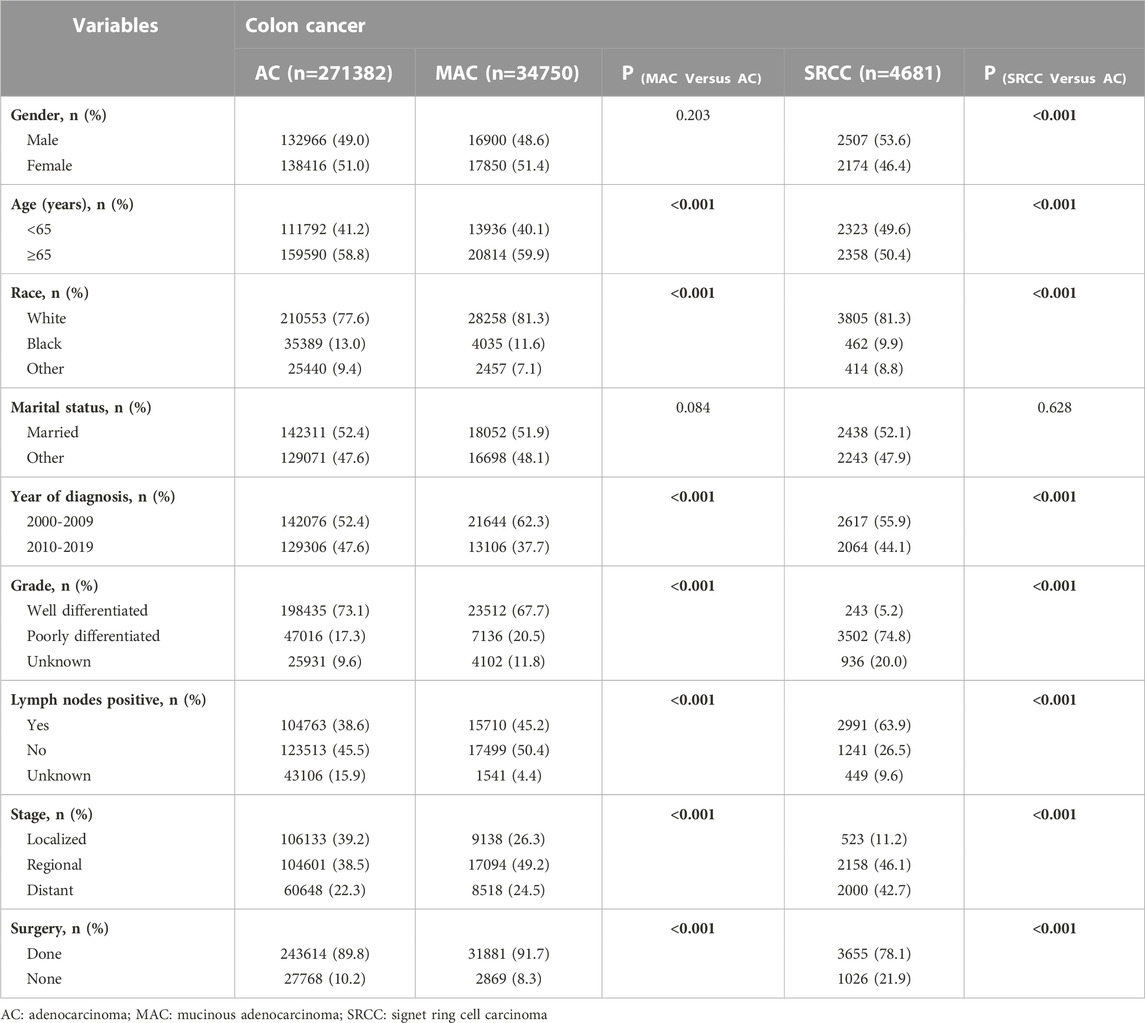
TABLE 1. Comparison of the clinicopathological characteristics of patients with colon AC, MAC, and SRCC.
Incidence
The overall age-adjusted incidence of AC during the study period was 28.04 per 100,000 person-years. We observed a 1.6-fold decrease in the incidence, from 35.48 per 100,000 person-years in 2000 to 22.30 per 100,000 person-years in 2018. Similarly, the incidence of MAC showed a 3.2-fold decrease, declining from 4.74 per 100,000 person-years in 2000 to 1.50 per 100,000 person-years in 2018. As for SRCC, the age-adjusted incidence was 0.48 per 100,000 person-years in 2000, which decreased to 0.23 per 100,000 person-years by 2018 (Figure 1).
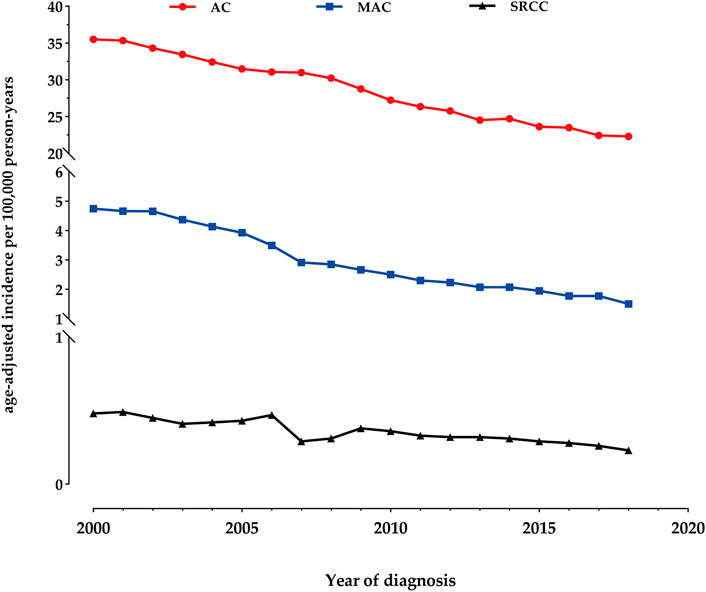
FIGURE 1. Incidence Trends of colon adenocarcinoma (AC), mucinous adenocarcinoma (MAC), and signet-ring cell carcinoma (SRCC) fom 2000 to 2018.
Patient characteristics
The marital status did not significantly differ among patients with classical AC, MAC, and SRCC. The distribution of sex was similar between AC and MAC patients, while there was a higher proportion of males in the SRCC cohort. Patients with SRCC were more likely to be younger than 65 years old compared to those with AC (49.6% VS. 41.2%, p < 0.001). There was a higher percentage of MAC diagnoses between 2000 and 2009 compared to AC diagnoses (62.3% VS. 37.7%, p < 0.001). Both MAC and SRCC were more likely to be diagnosed with poorly differentiated tumors and at more advanced stages upon presentation, especially SRCC (p < 0.001 for each). Lymph node metastasis was found in 38.6% of AC patients, 45.2% of MAC patients, and 63.9% of SRCC patients. In terms of treatment, patients with SRCC had a significantly lower rate of surgical interventions compared to those with AC (Table 1).
Stage and histology distribution
Figure 2 presents the stage and histology distribution of colon cancers for each year during the study period. In the overall cohort, the proportions of distant disease showed a slight increase from 2000 to 2018 (Figure 2A). However, in terms of histology distribution, the proportions of classical AC increased from 2000 to 2018, while the proportions of MAC decreased. On the other hand, the proportions of SRCC in the colon remained relatively stable (Figure 2B).
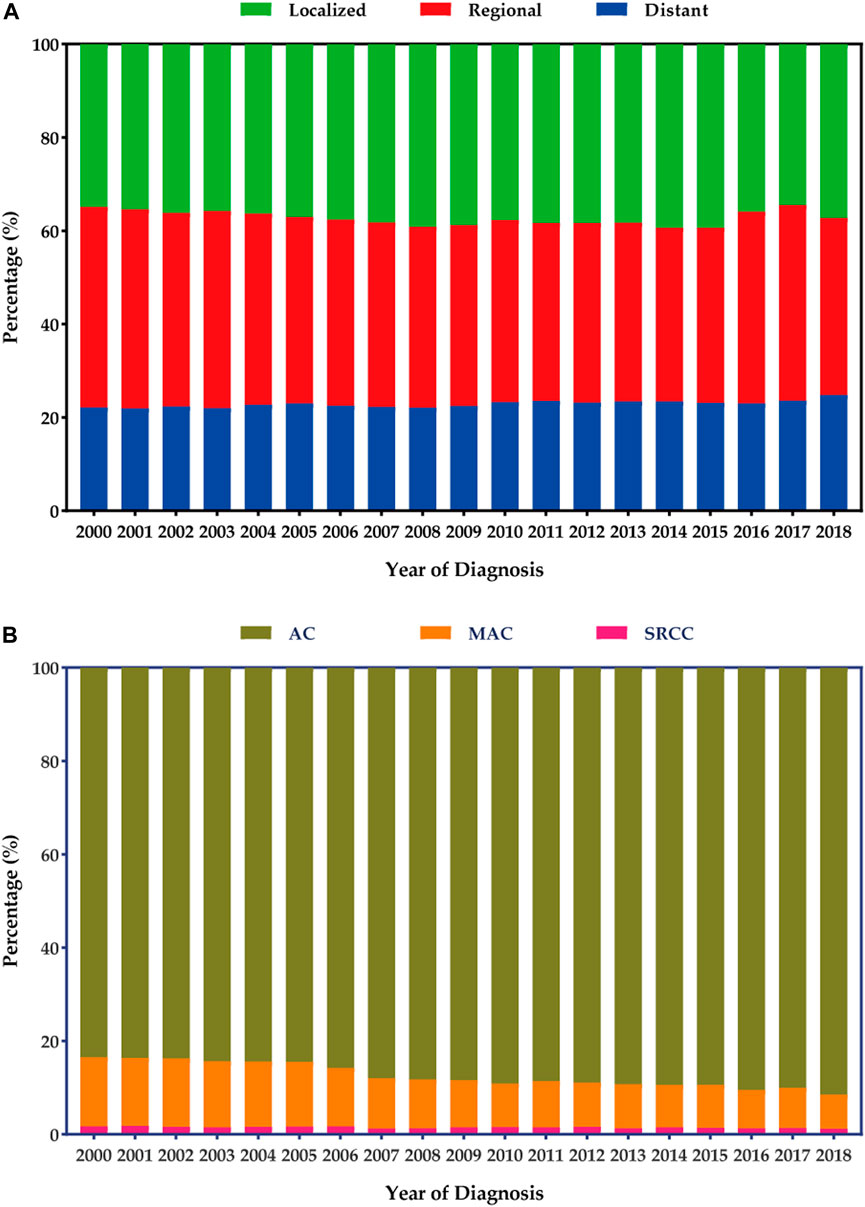
FIGURE 2. Stage and histology distribution among patients with colon cancers. (A) Stage distribution. (B) Histology distribution.
Survival
Table 2; Figure 3 present the survival outcomes for different histological subtypes of colon cancers. For the overall cohort, compared with patients with classical AC (median overall survival, 77.0 months), patients with MAC and SRCC had less favorable survival outcomes, with median overall survivals of 53.0 months and 16.0 months, respectively (HRMAC = 1.20, p < 0.001; HRSRCC = 2.27, p < 0.001) (Figure 3A). Likewise, patients with loco-regional MAC or SRCC experienced worse survival outcomes when compared to those with loco-regional AC (Figures 3B, C). Among patients with distant disease, those with MAC had similar overall survival to those with AC, both having a median overall survival of 12.0 months (p = 0.473). However, the survival outcomes for patients with SRCC were still less favorable, with a median survival of 8.0 months (Figure 3D).
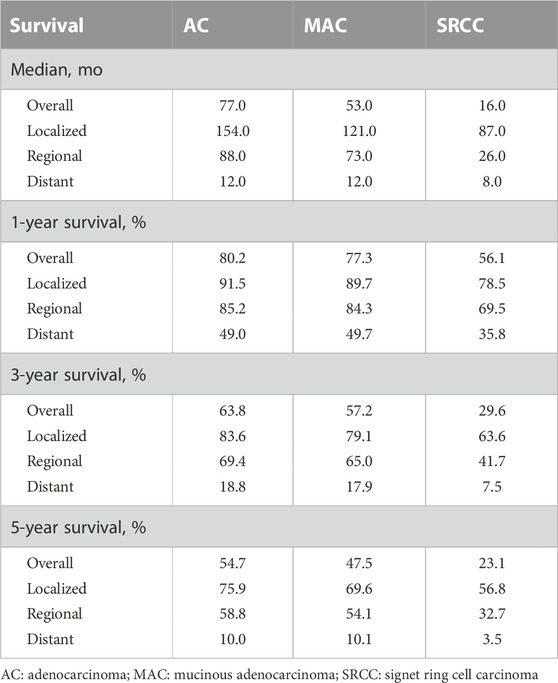
TABLE 2. Median, 1-year, 3-year, and 5-year survival rate of Patients with Colon Cancers by histological subtypes.
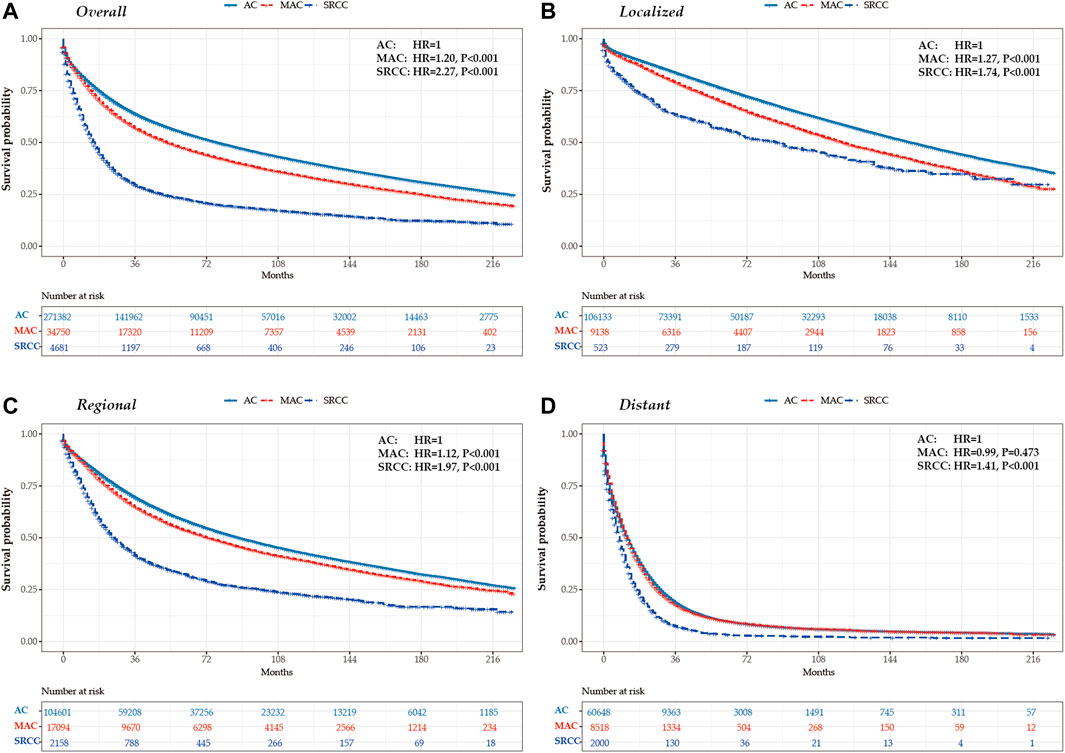
FIGURE 3. Kaplan-Meier survival curves for colon cancers by histological subtypes and tumor stage in the United States between 2000 and 2018. (A) overall (B) localized stage (C) regional stage (D) distant stage.
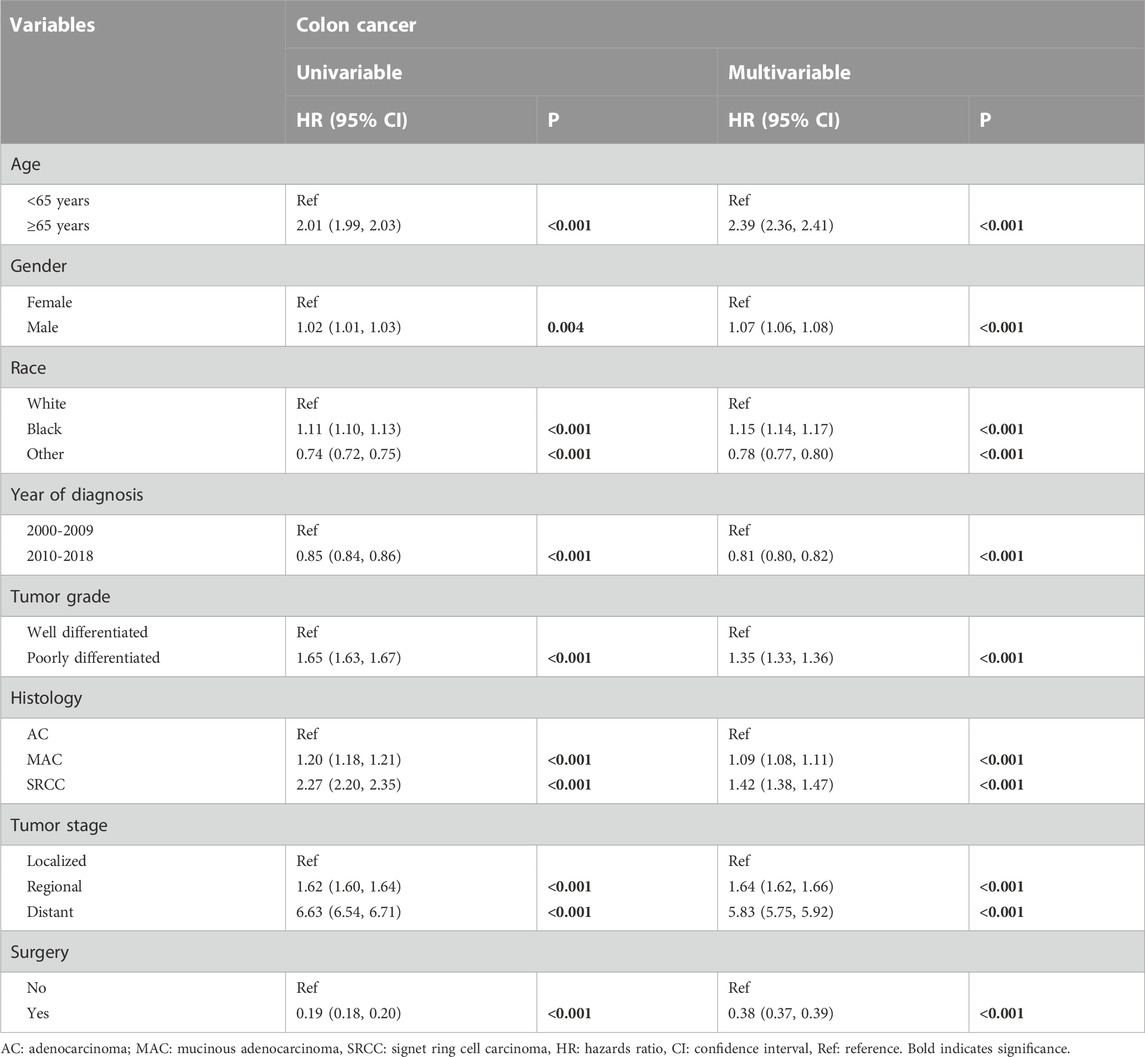
Factors associated with OS
Factors impacting overall survival were determined using univariable and multivariable Cox regression analyses. No evidence of violation of the proportional hazard assumption was found. Table 3 presents the clinicopathological variables associated with overall survival in colon cancers. In the multivariable analyses, patients aged ≥65 years were found to have a worse prognosis (HR, 2.39; 95%CI, 2.36–2.41; p < 0.001). Female sex was associated with significantly better survival outcomes compared to male sex. It is not surprising that patients with poorly differentiated tumors or more advanced diseases exhibited worse overall survival. Moreover, patients with a histology of MAC or SRCC had less favorable outcomes compared to those with classical AC. Surgical intervention was associated with improved survival outcomes for patients with colon cancers (HR, 0.38; 95%CI, 0.37–0.39; p < 0.001).
Discussion
To our knowledge, this study represents the largest population-based analysis comparing the epidemiology, clinicopathological characteristics, and outcomes of colon AC, MAC, and SRCC in the United States from 2000 to 2018. The majority of cases in our study were classical AC (87.3%), with MAC and SRCC comprising smaller proportions. During the study period, we observed a 1.6-fold decrease in the overall age-adjusted incidence of AC, from 35.48 per 100,000 person-years in 2000 to 22.30 in 2018. Similarly, the incidence of MAC showed a 3.2-fold decrease, declining from 4.74 per 100,000 person-years in 2000 to 1.50 in 2018. As for SRCC, the age-adjusted incidence was 0.48 per 100,000 person-years in 2000, which decreased to 0.23 by 2018. Furthermore, MAC and SRCC exhibited significantly distinct clinical features compared to classical AC. Both MAC and SRCC were more likely to be diagnosed with poorly differentiated tumors and at more advanced stages upon presentation, particularly SRCC. Patients with SRCC also experienced significantly worse survival outcomes compared to those with AC, even after adjusting for tumor stage.
Gastrointestinal MAC and SRCC are two rare histological subtypes of cancers associated with abundant mucous production (Gopalan et al., 2011; Imai et al., 2013). MAC is presented with a predominant extracellular mucin accumulation, while SRCC, on the other hand, is characterized by excessive intracytoplasmic mucin (Hartman et al., 2013; Barresi and Pedrazzani, 2020). Previous studies have shown significant differences in the clinicopathological features between MAC and SRCC, as well as associations between tumor biology, treatment sensitivities, and survival results (Yang et al., 2004; Nitsche et al., 2013; Nitsche et al., 2016; Kermanshahi et al., 2017; Tang et al., 2020). However, whether these biological traits carry the same weight in cases with colon MAC versus SRCC remains unclear and requires further investigation. In addition, the relative paucity of MAC and SRCC cases as compared to typical AC in digestive system has limited characterization of MAC and SRCC to small case series. Our study based on the SEER-18 database met the requirement to ensure the presence of sufficiently large cohorts of study population upon which outcome analyses can be performed.
The impact of MAC and SRCC on different organs of the gastrointestinal tract is unclear, including whether they share similar clinical and pathological features or outcomes. Generally, SRCC tends to be more aggressive, diagnosed at later stages, and have lower survival rates than classical AC (Pernot et al., 2015). Poor tumor differentiation and delayed diagnosis are more common in MAC and SRCC than in conventional AC, which may contribute to these unfavorable characteristics, especially in SRCC (Mekenkamp et al.; Zaafouri et al., 2022). When comparing SRCC to MAC by location, differences in their behavior and prognoses are significant, partially due to the variations in tumor distribution between the two histological subtypes. Consistent with most studies, patients with SRCC have a higher incidence of lymph node metastasis and distant disease (Chew et al., 2010).
The incidence of colon cancer has significantly decreased over the past 2 decades, indicating that the peak of colon cancer may have already been reached. Unhealthy lifestyle habits, including excessive consumption of fat, sugar, red meat, and alcohol, as well as having a high body mass index, are well-known risk factors for gastrointestinal tumors. The observed decline in incidence in our study can be attributed to the promotion of healthier lifestyles and the implementation of effective colon cancer screening programs, enabling the early detection and treatment of precancerous lesions. As a result, the overall incidence of colon cancer has gradually decreased over time.
Although traditional staging systems are effective in predicting survival in colon cancers, tumor histology and pathological grading can provide valuable information about clinical behavior. For instance, a retrospective study by Song et al. demonstrated that the presence of signet-ring cell component in MAC was associated with worse overall and recurrence-free survival (Song et al., 2019). Further research is needed to explore the biological distinctions among these rare subtypes. To the best of our knowledge, our study represents the largest population-level analysis in the United States regarding the clinicopathological characteristics of gastrointestinal MAC and SRCC, confirming previous nationwide epidemiological data from other sources.
Managing MAC and SRCC remains challenging due to their rarity and heterogeneous nature. Treatment typically involves a combination of surgery, chemotherapy, and radiation therapy, tailored based on the tumor location, stage, and the patient’s overall health. However, prior studies have suggested that MAC and SRCC may exhibit an inferior response to commonly used therapies, such as neoadjuvant chemoradiotherapy, when compared to classical AC (Kim et al., 2013; McCawley et al., 2016). Due to its more aggressive behavior and tendency for early metastasis, SRCC often necessitates a more aggressive management approach. Therefore, patients diagnosed with these subtypes may be suitable candidates for personalized treatment, including a multidisciplinary approach and more frequent follow-up. Additional strengths of our study include the extensive longitudinal follow-up spanning nearly 20 years and the analysis of clinicopathological characteristics using individual-level data on a national scale, which allowing us to gain a unique insight into the differences and disparities among colon AC, MAC, and SRCC. Nevertheless, it is important to acknowledge the limitations of our study. Being a retrospective design, we cannot completely eliminate selection biases inherent in the data. Furthermore, the absence of detailed treatment information is another potential limitation that may impact the robustness of our findings.
Our study provides a detailed picture of the clinicopathological features of colon AC, MAC and SRCC based on a large cohort of patients from US. While they share some similarities, they differ significantly in terms of their histological features, clinical behavior, and prognosis. Our findings highlight the importance of distinguishing these subtypes from classical AC and underscore the need for further research to explore the variations in their biological traits. It is important to acknowledge that our study has certain limitations, including potential selection biases and incomplete data on treatment details. Nevertheless, it provides valuable insights into the differences and disparities among colon AC, MAC, and SRCC.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: SEER program.
Author contributions
PK contributed to the conception and methodology. YL and WY made data collection and analysis. FL and BL revised the manuscript. YL and XL designed the study and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the China Postdoctoral Science Foundation (Grant No. 2019M651299) and Heilongjiang Postdoctoral Fund (Grant No. LBHZ18133).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahadi, M., Sokolova, A., Brown, I., Chou, A., and Gill, A. J. (2021). The 2019 world health organization classification of appendiceal, colorectal and anal canal tumours: An update and critical assessment. Pathology 53 (4), 454–461. doi:10.1016/j.pathol.2020.10.010
Arai, T. (2019). Where does signet-ring cell carcinoma come from and where does it go? Gastric cancer official J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 22 (4), 651–652. doi:10.1007/s10120-019-00960-w
Barresi, V., and Pedrazzani, C. (2020). Colorectal signet ring cell carcinoma: Advancing research in a rare cancer. Future Oncol. Lond. Engl. 16 (17), 1161–1163. doi:10.2217/fon-2020-0242
Bordry, N., Astaras, C., Ongaro, M., Goossens, N., Frossard, J. L., and Koessler, T. (2021). Recent advances in gastrointestinal cancers. World J. gastroenterology 27 (28), 4493–4503. doi:10.3748/wjg.v27.i28.4493
Chew, M. H., Yeo, S. A., Ng, Z. P., Lim, K. H., Koh, P. K., Ng, K. H., et al. (2010). Critical analysis of mucin and signet ring cell as prognostic factors in an Asian population of 2,764 sporadic colorectal cancers. Int. J. colorectal Dis. 25 (10), 1221–1229. doi:10.1007/s00384-010-1033-3
Davila, R. E., and Davila, M. L. (2022). Recent advancements in the diagnosis and treatment of gastrointestinal cancers. Gastroenterology Clin. N. Am. 51 (3), xiii–xiv. doi:10.1016/j.gtc.2022.07.009
Gopalan, V., Smith, R. A., Ho, Y. H., and Lam, A. K. (2011). Signet-ring cell carcinoma of colorectum-current perspectives and molecular biology. Int. J. colorectal Dis. 26 (2), 127–133. doi:10.1007/s00384-010-1037-z
Hartman, D. J., Nikiforova, M. N., Chang, D. T., Chu, E., Bahary, N., Brand, R. E., et al. (2013). Signet ring cell colorectal carcinoma: A distinct subset of mucin-poor microsatellite-stable signet ring cell carcinoma associated with dismal prognosis. Am. J. Surg. pathology 37 (7), 969–977. doi:10.1097/PAS.0b013e3182851e2b
Imai, Y., Yamagishi, H., Fukuda, K., Ono, Y., Inoue, T., and Ueda, Y. (2013). Differential mucin phenotypes and their significance in a variation of colorectal carcinoma. World J. gastroenterology 19 (25), 3957–3968. doi:10.3748/wjg.v19.i25.3957
Kermanshahi, T. R., Magge, D., Choudry, H., Ramalingam, L., Zhu, B., Pingpank, J., et al. (2017). Mucinous and signet ring cell differentiation affect patterns of metastasis in colorectal carcinoma and influence survival. Int. J. Surg. pathology 25 (2), 108–117. doi:10.1177/1066896916664990
Kim, S. H., Shin, S. J., Lee, K. Y., Kim, H., Kim, T. I., Kang, D. R., et al. (2013). Prognostic value of mucinous histology depends on microsatellite instability status in patients with stage III colon cancer treated with adjuvant FOLFOX chemotherapy: A retrospective cohort study. Ann. Surg. Oncol. 20 (11), 3407–3413. doi:10.1245/s10434-013-3169-1
McCawley, N., Clancy, C., O'Neill, B. D., Deasy, J., McNamara, D. A., and Burke, J. P. (2016). Mucinous rectal adenocarcinoma is associated with a poor response to neoadjuvant chemoradiotherapy: A systematic review and meta-analysis. Dis. colon rectum 59 (12), 1200–1208. doi:10.1097/DCR.0000000000000635
Mekenkamp, L. J., Heesterbeek, K. J., Koopman, M., Tol, J., Teerenstra, S., Venderbosch, S., et al. (2012). Mucinous adenocarcinomas: Poor prognosis in metastatic colorectal cancer. Eur. J. cancer 48(4), 501–509. doi:10.1016/j.ejca.2011.12.004
Nagtegaal, I. D., Odze, R. D., Klimstra, D., Paradis, V., Rugge, M., Schirmacher, P., et al. (2020). The 2019 WHO classification of tumours of the digestive system. Histopathology 76 (2), 182–188. doi:10.1111/his.13975
Nitsche, U., Friess, H., Agha, A., Angele, M., Eckel, R., Heitland, W., et al. (2016). Prognosis of mucinous and signet-ring cell colorectal cancer in a population-based cohort. J. cancer Res. Clin. Oncol. 142 (11), 2357–2366. doi:10.1007/s00432-016-2224-2
Nitsche, U., Zimmermann, A., Späth, C., Müller, T., Maak, M., Schuster, T., et al. (2013). Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann. Surg. 258 (5), 775–782. doi:10.1097/SLA.0b013e3182a69f7e
Overman, M. J., Fournier, K., Hu, C. Y., Eng, C., Taggart, M., Royal, R., et al. (2013). Improving the AJCC/TNM staging for adenocarcinomas of the appendix: The prognostic impact of histological grade. Ann. Surg. 257 (6), 1072–1078. doi:10.1097/SLA.0b013e318269d680
Pernot, S., Voron, T., Perkins, G., Lagorce-Pages, C., Berger, A., and Taieb, J. (2015). Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J. gastroenterology 21 (40), 11428–11438. doi:10.3748/wjg.v21.i40.11428
Song, I. H., Hong, S. M., Yu, E., Yoon, Y. S., Park, I. J., Lim, S. B., et al. (2019). Signet ring cell component predicts aggressive behaviour in colorectal mucinous adenocarcinoma. Pathology 51 (4), 384–391. doi:10.1016/j.pathol.2019.03.001
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tang, C. T., Chen, Y., and Zeng, C. (2020). Prognostic analysis of gastric signet ring cell carcinoma and mucinous carcinoma: A propensity score-matched study and competing risk analysis. Aging 12 (21), 22059–22077. doi:10.18632/aging.104048
Washington, M. K., Goldberg, R. M., Chang, G. J., Limburg, P., Lam, A. K., Salto-Tellez, M., et al. (2021). Diagnosis of digestive system tumours. Int. J. cancer 148 (5), 1040–1050. doi:10.1002/ijc.33210
Widmann, B., Warschkow, R., Schmied, B. M., Marti, L., and Steffen, T. (2016). Impact of mucinous histology on the prognosis of stage I-iii adenocarcinomas of the appendix: A population-based, propensity score-matched analysis. J. Gastrointest. Surg. official J. Soc. Surg. Alimentary Tract 20 (8), 1493–1502. doi:10.1007/s11605-016-3148-5
Yang, X. F., Yang, L., Mao, X. Y., Wu, D. Y., Zhang, S. M., and Xin, Y. (2004). Pathobiological behavior and molecular mechanism of signet ring cell carcinoma and mucinous adenocarcinoma of the stomach: A comparative study. World J. gastroenterology 10 (5), 750–754. doi:10.3748/wjg.v10.i5.750
Zaafouri, H., Jouini, R., Khedhiri, N., Khanchel, F., Cherif, M., Mesbahi, M., et al. (2022). Comparison between signet-ring cell carcinoma and non-signet-ring cell carcinoma of the stomach: Clinicopathological parameters, epidemiological data, outcome, and prognosis-a cohort study of 123 patients from a non-endemic country. World J. Surg. Oncol. 20 (1), 238. doi:10.1186/s12957-022-02699-8
Keywords: colon cancers, AC, MAC, SRCC, incidence, characteristics, survival
Citation: Liu Y, Yin W, Li X, Li B, Liu F and Kang P (2023) Comparative analysis of tumor biology and prognosis in mucinous and signet-ring cell colon cancers versus classical adenocarcinoma. Front. Physiol. 14:1199211. doi: 10.3389/fphys.2023.1199211
Received: 03 April 2023; Accepted: 12 July 2023;
Published: 31 July 2023.
Edited by:
Giuliano Ramadori, University of Göttingen, GermanyReviewed by:
Bernhard W. Renz, Ludwig Maximilian University of Munich, GermanyTuomas Kaprio, Hospital District of Helsinki and Uusimaa, Finland
Copyright © 2023 Liu, Yin, Li, Li, Liu and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengcheng Kang, MTU5MDQ1MDYxOThAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yang Liu
Yang Liu Wenxin Yin2†
Wenxin Yin2† Pengcheng Kang
Pengcheng Kang