
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 01 June 2023
Sec. Gastrointestinal Sciences
Volume 14 - 2023 | https://doi.org/10.3389/fphys.2023.1186665
Objective: To explore the clinical value of fecal calprotectin (FC) for evaluating disease activity in patients with Crohn’s disease (CD) and its relationship with disease location.
Methods: Patients with CD were enrolled retrospectively, and clinical data, including FC levels, were collected. Clinical activity was assessed using the Crohn’s disease activity index (CDAI). Endoscopic activity was assessed using a simple endoscopic score for Crohn’s disease (SES-CD). The partial SES-CD (pSES-CD) was scored for the size of ulcers in each segment as defined by the SES-CD and was calculated as the sum of segmental ulcer scores.
Results: This study included 273 CD patients. The FC level was significantly positively correlated with the CDAI and SES-CD, with correlation coefficients of 0.666 and 0.674, respectively. The median FC levels in patients with clinical remission and mildly active and moderately–severely active disease were 41.01, 164.20, and 444.45 μg/g. These values were 26.94, 66.77, and 327.22 μg/g during endoscopic remission and mildly and moderately–severely active stages, respectively. Compared with c-reactive protein (CRP), the erythrocyte sedimentation rate (ESR), and other biomarker parameters, FC was better at predicting disease activity for CD patients. For an FC <74.52 μg/g, the area under the curve (AUC) for predicting clinical remission was 0.86, with a sensitivity of 89.47% and a specificity of 71.70%. Moreover, endoscopic remission was predicted with a sensitivity of 68.02% and a specificity of 85.53%. The AUC was 0.83, and the cutoff value was 80.84 μg/g. In patients with ileal and (ileo) colonic CD, FC was significantly correlated with the CDAI, SES-CD, and pSES-CD. The correlation coefficients were 0.711 (CDAI), 0.473 (SES-CD), and 0.369 (pSES-CD) in patients with ileal CD and 0.687, 0.745, and 0.714 in patients with (ileo) colonic CD, respectively. For patients in remission, those in the active stage, and those with large or very large ulcers, differences in FC levels were not significant between patients with ileal and (ileo) colonic CD.
Conclusion: FC is a reliable predictor of disease activity in patients with CD, including those with ileal CD. FC is thus recommended for the routine follow-up of patients with CD.
Crohn’s disease (CD) is a persistent and recurrent inflammatory disorder of the gastrointestinal tract, which affects the entire digestive tract, particularly the terminal ileum and colon (Ayling and Kok, 2018; Ricciuto and Griffiths, 2019). Clinical manifestations are heterogeneous, and some patients develop complications such as intestinal stenosis, fistula, or abscess with disease progression, even requiring surgical intervention, which severely affects their quality of life (Wehkamp et al., 2016; Torres et al., 2017). The therapeutic goal is to induce and maintain remission and prevent long-term complications. Therefore, the accurate and objective assessment of disease activity is crucial.
Among common methods used to assess disease activity in patients with CD, endoscopy is used to visualize the gastrointestinal mucosa and perform a biopsy. However, this is an unpleasant experience for patients, both financially and physically, and it can lead to endoscope-related complications. Commonly used biomarkers, such as c-reactive protein (CRP) and the erythrocyte sedimentation rate (ESR), lack sensitivity and specificity. Therefore, a better biomarker for intestinal inflammation is required in clinical practice.
Calprotectin is mainly derived from neutrophils, with a small proportion from monocytes and macrophages, and is a 36 kDa protein (Kalla et al., 2014). It was first described as an L1 protein in 1980 and is a heterodimer composed of S100A8 and S100A9. The antimicrobial effects of calprotectin are associated with its ability to chelate metal ions. And its concentration in feces is approximately six times higher than that in plasma (Baumgart and Sandborn, 2012; Rokkas et al., 2018).
Fecal calprotectin (FC) is currently used for CD diagnosis, differential diagnosis, disease recurrence evaluations, and disease activity in patients (García-Sánchez et al., 2010; Caviglia et al., 2018). However, the utility of its use to evaluate disease activity, including the best thresholds, varies among studies. Moreover, whether FC can be used to evaluate patients with ileal CD remains controversial. Therefore, the aim of this study was to investigate the clinical value of FC in assessing disease activity in patients with CD and whether its level is affected by disease location.
We retrospectively enrolled 273 patients with CD admitted to the Division of Gastroenterology at Wuhan Union Hospital between December 2020 and July 2022. Inclusion criteria were as follows: hospitalized CD patients diagnosed via endoscopy and histopathology; clinical data and biochemical parameters, including FC, CRP, ESR, hemoglobin (Hb), platelet count (PLT), white blood cell count (WBC), neutrophil percentage (N%), neutrophil-to-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), platelet-to-lymphocyte percentage ratio (PLpR), serum albumin (ALB), and D-dimer (D-D), detected within 1 week before endoscopy. The exclusion criteria were as follows: patients with other intestinal ulcerative diseases, including ulcerative colitis (UC), infectious (bacterial, fungal, or viral) enteritis, and intestinal tuberculosis; patients with colorectal cancer; patients treated with non-steroidal anti-inflammatory drugs or proton pump inhibitors within 4 weeks; patients with severe cardiovascular, respiratory, or urinary dysfunction; and pregnant or lactating patients. This study was approved by the Tongji Medical College Ethics Committee (Ethics No. 2022 [0827]).
Clinical activity was evaluated using the Crohn’s disease activity index (CDAI). CDAI <150 was considered remission, 150–220 was mildly active, 221–450 was moderately active, and ≥450 was severely active. Based on the simple endoscopic score for Crohn’s disease (SES-CD), endoscopic activity was determined as follows: 0–2 indicated remission, 3–6 indicated mild activity, 7–15 indicated moderate activity, and ≥16 indicated severely active disease. According to the SES-CD, ulcers were classified and scored as absent (0), aphthous (1), large (0.5–2 cm) (2), or very large (>2 cm) (3). Partial SES-CD (pSES-CD) was determined based on the sum of the ulcer scores in each segment (terminal ileum, right colon, transverse colon, left colon, and rectum), which was between 0 and 15.
The first stool sample was collected in the morning and transferred to a research laboratory. FC levels were measured using a commercially available rapid kit (WIZ BIOTECH). The kit measures the FC concentration via fluorescence immunochromatography with a detection range of 10–2,400 μg/g. Therefore, all values below 10 μg/g and above 2,400 μg/g were considered equal to 10 and 2,400 μg/g, respectively.
Statistical analyses were performed using SPSS version 23.0. Graphs were drawn using GraphPad Prism software (version 8.0). Data conforming to a normal distribution are described as the mean ± standard deviation. The median values were used to represent data with skewed distributions. A t-test was used for data conforming to a normal distribution, whereas a non-parametric test was used for data conforming to a skewed distribution. We used the Mann–Whitney U test for nonparametric comparisons between two groups and the Kruskal–Wallis H test for comparisons among multiple groups. Categorical variables were presented as numbers and percentages. Spearman’s rank correlation was used for the correlation analysis. A receiver operating characteristic (ROC) curve was drawn to estimate the area under the curve (AUC), and the optimal cutoff level was estimated using Youden’s index. A two-tailed p-value < 0.05 was considered statistically significant.
In this study, 273 CD patients (213 males and 60 females), with a median age of 29 years and a median duration of 12 months, were enrolled. According to the CDAI, 159 patients (58.24%) were in the clinical remission stage and 114 patients (41.76%) were in the active stage. According to the SES-CD, 76 patients (27.84%) were in the endoscopic remission stage, and 197 (72.16%) were in the endoscopic active stage. The baseline characteristics are shown in Table 1.
The CDAI and SES-CD were positively correlated with the FC level, with correlation coefficients of 0.666 and 0.674, respectively. In addition, the FC level was positively correlated with CRP, ESR, PLT, WBC, N%, NLR, PLR, PLpR, and D-D and negatively correlated with Hb and ALB. Compared to that with CRP, ESR, and other biochemical parameters, FC showed the strongest correlation with CDAI and SES-CD (Table 2).
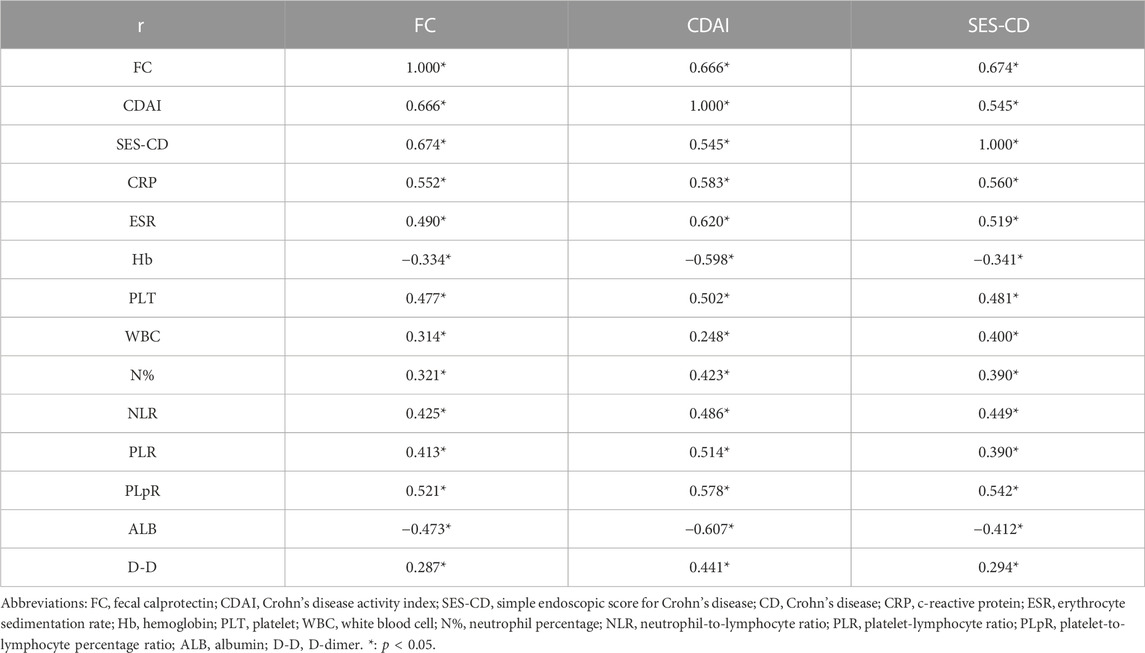
TABLE 2. Correlation analysis of FC levels with the CDAI, SES-CD, and biochemical parameters in patients with CD.
In our study, patients with severely active disease (clinically and endoscopically) were analyzed and discussed together with the moderately active group, as there were only two and 20 cases with severe clinical and endoscopic activity, respectively. As shown in Figure 1A, the median FC levels in clinical remission, mild activity, and moderate–severe activity groups were 41.01 (17.31, 96.62), 164.20 (84.49, 274.54), and 444.45 (202.47, 708.82) µg/g, with significant differences noted (p < 0.001). The median FC levels of CD patients in the endoscopic remission, mildly active, and moderately–severely active stages were 26.94 (10.00, 59.56), 66.77 (35.40, 147.89), and 327.22 (150.64, 526.93) µg/g, with significant differences observed (p < 0.001). The median CRP levels were 3.30 (2.98, 6.42), 6.30 (3.34, 20.15), and 30.30 (11.78, 54.93) mg/L in clinical remission, mildly active, and moderately–severely active groups, respectively, as shown in Figure 1B. Regarding endoscopic activity, a significant difference was observed between patients with mild activity (3.34 mg/L) and those with moderately–severely active stages (16.90 mg/L, p < 0.001), but not between patients with mild activity and those in remission (3.13 mg/L, p = 0.074). There were also significant differences in ESR among patients with different disease activities (Figure 1C). The median ESR levels were 5.00 (2.00, 11.00), 16.00 (6.00, 24.25), and 30.00 (13.25, 46.25) mm/h for clinical remission, mildly active, and moderately–severely active groups, respectively. Moreover, these values were 3.50 (2.00, 9.75), 6.00 (3.00, 14.00), and 19.00 (9.75, 40.00) mm/h for endoscopic remission, mildly active, and moderately–severely active cases, respectively. The median levels of other biochemical parameters in patients with CD with different disease activities are shown in Supplementary Tables S1A, B.
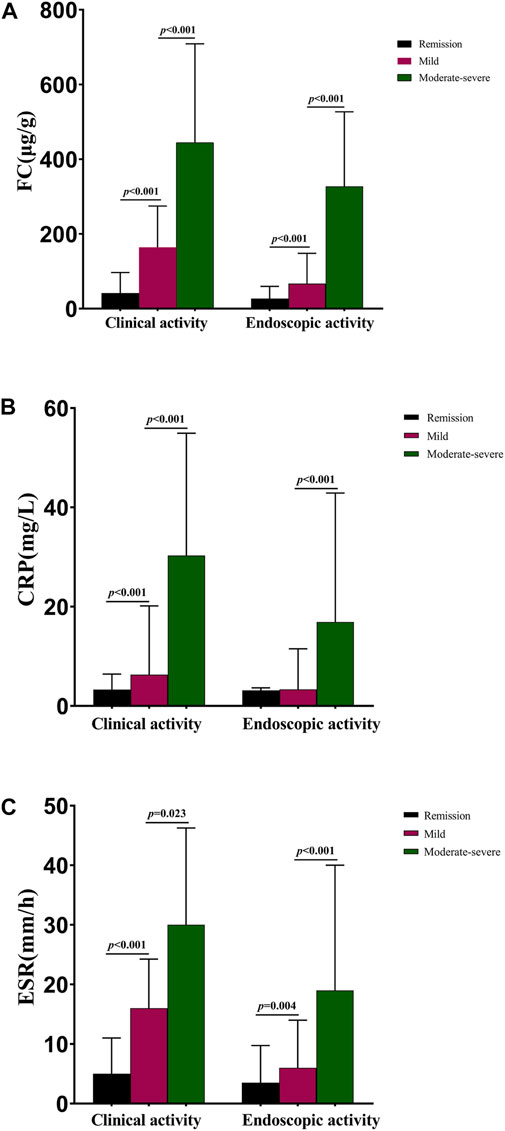
FIGURE 1. Median FC, CRP, and ESR levels in CD patients with different disease activities. (A) Median FC levels in CD patients with different disease activities; (B) Median CRP levels in patients with different disease activities; (C) Median ESR levels in patients with different disease activities. Abbreviations: FC, fecal calprotectin; CRP, c-reactive protein; ESR, erythrocyte sedimentation rate; CD, Crohn’s disease.
As shown in Table 3, when the FC was <74.52 μg/g, clinical remission was predicted with a sensitivity of 89.47% and a specificity of 71.70%. When predicting endoscopic remission, the AUC was 0.83, and 80.84 μg/g was the optimal cutoff value, with a sensitivity and specificity of 68.02% and 85.53%, respectively. Compared with the CRP level and ESR, the FC level was a better predictor of clinical and endoscopic remission for CD patients. ROC curves for FC, CRP, and ESR, in terms of predicting clinical and endoscopic remission, are shown in Figures 2A, B, respectively. The ROC analysis of other biochemical parameters for the prediction of clinical and endoscopic remission is shown in Supplementary Table S2.
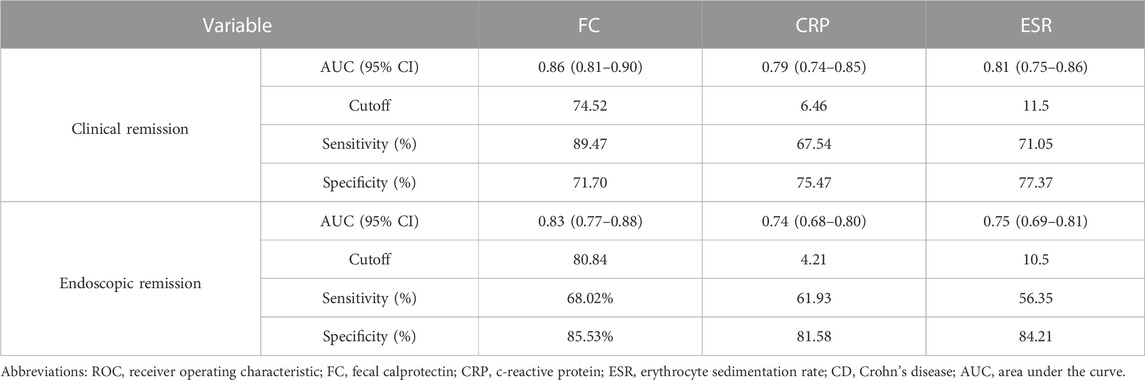
TABLE 3. ROC analysis of FC, CRP, and ESR for predicting clinical and endoscopic remission in patients with CD.
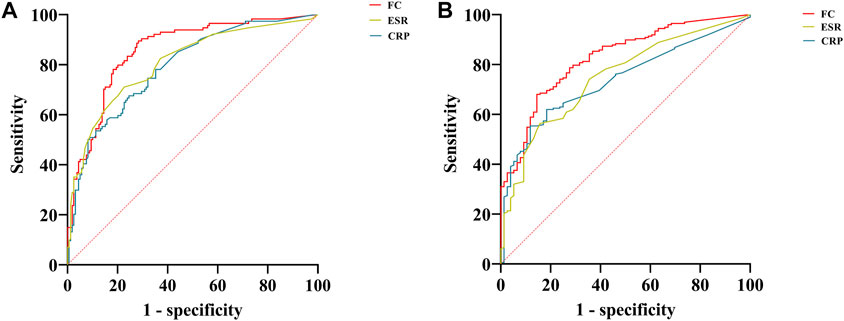
FIGURE 2. ROC curves of FC, CRP, and ESR for predicting clinical and endoscopic remission in patients with CD. (A) ROC curves of FC, CRP, and ESR for predicting clinical remission; (B) ROC curves of FC, CRP, and ESR for predicting endoscopic remission. Abbreviations: ROC, receiver operating characteristic; FC, fecal calprotectin; CRP, c-reactive protein; ESR, erythrocyte sedimentation rate; CD, Crohn’s disease.
Based on the Montreal classification, 41 patients with ileal CD and 189 patients with (ileo) colonic CD were enrolled in our study. As shown in Table 4, FC was positively correlated with the CDAI, SES-CD, and pSES-CD in both groups. Correlations of 0.711 and 0.687 were found between the CDAI and FC in patients with ileal and (ileo) colonic CD, respectively. In patients with (ileo) colonic CD, the correlation coefficients between FC with SES-CD or pSES-CD were 0.745 and 0.714, respectively. For patients with ileal CD, they were 0.473 and 0.369, which were weaker than those for (ileo) colonic CD patients. Table 5 shows the median FC levels based on different Montreal disease locations, which did not differ significantly for patients in remission between ileal and (ileo) colonic CD. Similarly, the median FC levels in active patients did not differ significantly. In addition, in patients with ileal and (ileo) colonic CD, the FC level in the clinically or endoscopically active stage was significantly higher than that in patients in remission. The median levels of other biochemical parameters based on different Montreal disease locations in patients with ileal and (ileo) colonic CD are shown in Supplementary Tables 3A, B.
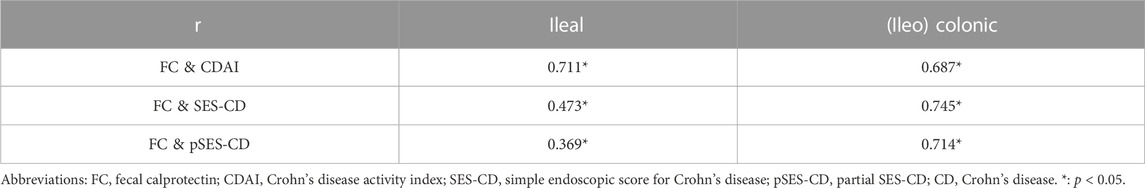
TABLE 4. Correlation analysis of FC levels with CDAI, SES-CD, and pSES-CD based on different Montreal disease locations in patients with CD.
Figures 3A, B show the ROC curves of FC for the prediction of clinical and endoscopic remission in patients with ileal and (ileo) colonic CD. When predicting clinical remission in patients with ileal CD, the optimal cutoff value of FC was 59.55 μg/g. Moreover, the AUC was 0.93 (95% CI: 0.86–1.00), with a sensitivity and specificity of 100% and 76.19%, respectively. For patients with (ileo) colonic CD, the optimal cutoff value of FC was 138.90 μg/g. The AUC was 0.84 (95% CI: 0.78–0.90), with a sensitivity and specificity of 81.93% and 81.13%, respectively (Figure 3A). When predicting endoscopic remission in patients with ileal CD, the optimal FC cutoff value was 72.04 μg/g. Here, the AUC was 0.73 (95% CI: 0.57–0.89), with a sensitivity and specificity of 68.75% and 77.78%, respectively. For (ileo) colonic CD cases, the optimal cutoff value was 80.84 μg/g. Further, the AUC was 0.84 (95% CI: 0.79–0.99), with a sensitivity and specificity of 67.88% and 86.57%, respectively (Figure 3B). Among the 41 patients with ileal CD, 19 (46.34%) had large or very large ulcers with a median FC level of 258.96 (84.11, 627.51) µg/g. Among the 189 patients with (ileo) colonic CD, 78 (61.26%) had large or very large ulcers with a median FC level of 351.37 (168.17, 527.66) µg/g. The differences in the FC levels between the two groups were not significant (Figure 4).
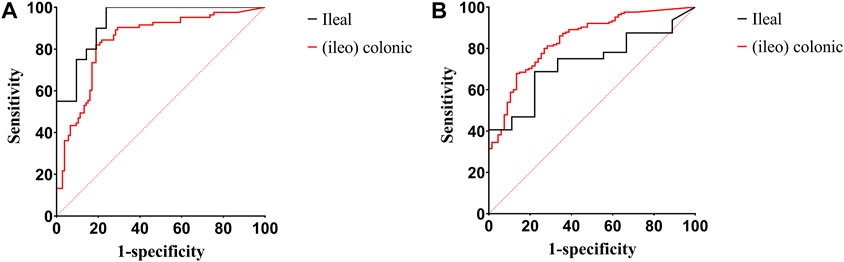
FIGURE 3. ROC curves of FC for predicting clinical and endoscopic remission in patients with ileal and (ileo) colonic CD. (A) ROC curves of FC for predicting clinical remission in patients with ileal and (ileo) colonic CD; (B) ROC curves of FC for predicting endoscopic remission in patients with ileal and (ileo) colonic CD. Abbreviations: ROC, receiver operating characteristic; FC, fecal calprotectin; CD, Crohn’s disease.
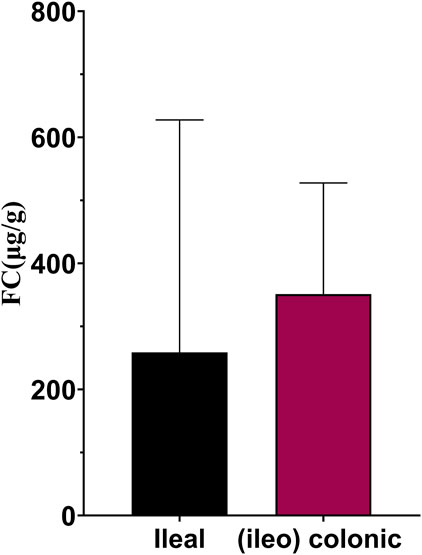
FIGURE 4. FC levels in ileal and (ileo) colonic CD in patients with large or very large ulcers. Abbreviations: FC, fecal calprotectin; CD, Crohn’s disease.
In recent years, FC has been gradually applied to the evaluation and management of patients with CD, including clinical and endoscopic activity evaluations, the prediction of disease recurrence, and assessing treatment responses (Boschetti et al., 2015; Veyre et al., 2021). However, the capacity of using FC to assess disease activity and whether the level of FC is affected by disease location remain controversial. Some studies indicated that FC levels in CD patients are positively correlated with endoscopic activity, with correlation coefficients of 0.6–0.8 (Sipponen et al., 2008; Schaffer et al., 2014; Iwamoto et al., 2018; Somwaru et al., 2019) and the median FC level in patients in the remission stage being lower (Schoepfer et al., 2010; Zittan et al., 2016). Moreover, compared to that with CRP, a widely used inflammatory marker, the correlation between SES-CD and FC was stronger (Matsuura et al., 2018; Knyazev et al., 2019). Different studies have reported different optimal FC thresholds for evaluating endoscopic activity. A cross-sectional study of 41 patients with CD showed that the optimal cutoff value for predicting endoscopic remission was 96 μg/g, and the sensitivity and specificity were 75.0% and 84.4%, respectively (Cannatelli et al., 2021). In another study, the sensitivity and specificity for distinguishing endoscopic remission from the active stage were 70% and 92%, respectively, with a FC level of 200 μg/g (Sipponen et al., 2008). In a study from China, a FC <156.09 μg/g predicted endoscopic remission with a sensitivity of 78.24% and a specificity of 83.61% (Han et al., 2022). The FC level when evaluating clinical activity has been reported to be significantly correlated in patients with UC but not in CD patients (D'Haens et al., 2012). However, most studies have suggested that FC can be used to evaluate clinical activity in patients with CD. For example, a meta-analysis included 727 patients with CD, and the AUC of FC for distinguishing patients in clinical remission from those in the active stage was 0.88 (Lin et al., 2014). A prospective study in Korea reported a significant correlation between the CDAI and FC in 38 CD patients (r = 0.520) (Lee et al., 2019). Further, a low FC level was found to predict clinical remission in CD patients, and the specificity was 100% when FC was <56 μg/g (Mooiweer et al., 2015).
In our study, the FC levels of patients in remission (26.94 μg/g) or with mild (66.77 μg/g) or moderate–severe activity (327.22 μg/g), endoscopically, differed significantly. FC could be used to predict endoscopic remission with a cutoff value of 80.84 μg/g, suggesting that endoscopy could be postponed. Further, the median FC levels were 41.01, 164.20, and 444.45 μg/g in patients in clinical remission or with mild activity or moderate–severe activity, respectively, with statistically significant differences. The cutoff value for evaluating clinical remission was found to be 74.52 μg/g, with an AUC of 0.86. The cutoff value of FC in predicting endoscopic remission was lower in our study than in other studies, which could be related to the detection method, the criteria of endoscopic activity assessment, and the subjects included. The AUC of FC for predicting clinical and endoscopic remission was higher than that of the CRP level, ESR, and other biochemical parameters, which is consistent with previous studies (Kawashima et al., 2017). In some studies, several biochemical parameters were applied to assess and predict disease activity in CD patients, such as CRP, ESR, ALB, NLR, WBC, and PLT counts (Vermeire et al., 2006; Fu et al., 2021). In our study, the SES-CD and CDAI were significantly correlated with FC, CRP, ESR, Hb, PLT, WBC, N%, NLR, PLR, PLpR, ALB, and D-D, among which the correlation with FC was the strongest, and this finding was consistent with previous studies. In addition, FC levels in patients were positively correlated with CRP levels, ESR, and WBC (Gecse et al., 2015). In our study, in addition to CRP, ESR, and WBC, FC exhibited a positive correlation with PLT, N%, NLR, PLR, PLpR, and D-D and a negative correlation with Hb and ALB.
The association between the lesion location and FC remains controversial. Some studies have suggested that the FC level in patients with ileal CD does not represent the severity of intestinal inflammation, with a weak or even no correlation (Koulaouzidis et al., 2016; Zittan et al., 2018). For example, a retrospective study showed that the sensitivity of FC in diagnosing endoscopically active stages in patients with ileal CD was only 36%, indicating that it is not a satisfactory biomarker for these patients (D'Arcangelo et al., 2021). In another study, FC levels did not increase significantly in patients with L1 CD even if prominent ulcers were observed during endoscopy (Gecse et al., 2015). However, other studies have suggested a significant correlation between FC and SES-CD in patients with ileal CD and have accurately predicted endoscopic activity (Arai et al., 2017; Monteiro et al., 2018; Romero-Mascarell et al., 2022). According to Buisson et al. (2021), FC can be used to effectively detect ulcers in patients with ileal and (ileo) colonic CD (Jensen et al., 2011). In a small prospective study in Japan, compared with that in (ileo) colonic CD patients, FC and SES-CD were more strongly correlated in patients with ileal CD (r = 0.78 vs. 0.67) (Inokuchi et al., 2016). In our study, FC was significantly correlated with the CDAI, SES-CD, and pSES-CD in two types of patients. However, compared with that in (ileo) colonic CD patients, the correlation among SES-CD, pSES-CD, and FC in ileal CD patients was inferior. The FC levels of patients with ileal and (ileo) colonic CD did not differ significantly in the remission or active stages. Among patients with the same lesion location, those in the active stage had significantly higher FC levels than those in remission. In addition, in patients with large or very large ulcers, the differences in FC levels were not significant between patients with ileal and (ileo) colonic CD. Therefore, FC is a reliable predictor of disease activity in patients with CD, including those with ileal CD. Nonetheless, caution should be exercised when evaluating endoscopic activity in such patients with ileal CD.
Our study has some limitations. First, owing to its retrospective nature, there is a possibility of selection bias. Second, the number of patients with clinically and endoscopically severe activity was small; therefore, the FC levels of patients in this group could not be compared and analyzed separately. Finally, the detection range of the FC level was 10–2,400 μg/g, and the FC level was <10 μg/g or >2,400 μg/g in a small number of patients, which could have influenced the results.
In conclusion, FC is a reliable predictor of disease activity in patients with CD, including those with ileal CD. Further, 74.52 μg/g and 80.84 μg/g are the best cutoff values when predicting clinical and endoscopic remission, respectively. Therefore, FC is recommended for the routine follow-up of patients with CD.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Tongji Medical College Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
JL collected, analyzed the data and completed the article; MX helped with data collection and analysis. WQ provided the results of fecal calprotectin; FL, YidC, SL, and YiyC contributed to data analysis; LZ designed the study and provided critical review of the manuscript. All authors contributed to the article and approved the submitted version.
The study was supported by National Natural Science Foundation of China (No. 82170547 and No. 81873558).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2023.1186665/full#supplementary-material
Arai, T., Takeuchi, K., Miyamura, M., Ishikawa, R., Yamada, A., Katsumata, M., et al. (2017). Level of fecal calprotectin correlates with severity of small bowel Crohn's disease, measured by balloon-assisted enteroscopy and computed tomography enterography. Clin. Gastroenterol. Hepatol. 15 (1), 56–62. doi:10.1016/j.cgh.2016.08.015
Ayling, R. M., and Kok, K. (2018). Fecal calprotectin. Adv. Clin. Chem. 87, 161–190. doi:10.1016/bs.acc.2018.07.005
Baumgart, D. C., and Sandborn, W. J. (2012). Crohn's disease. Lancet 380 (9853), 1590–1605. doi:10.1016/S0140-6736(12)60026-9
Boschetti, G., Laidet, M., Moussata, D., Stefanescu, C., Roblin, X., Phelip, G., et al. (2015). Levels of fecal calprotectin are associated with the severity of postoperative endoscopic recurrence in asymptomatic patients with Crohn's disease. Am. J. Gastroenterol. 110 (6), 865–872. doi:10.1038/ajg.2015.30
Buisson, A., Mak, W. Y., Andersen, M. J., Lei, D., Pekow, J., Cohen, R. D., et al. (2021). Fecal calprotectin is highly effective to detect endoscopic ulcerations in Crohn's disease regardless of disease location. Inflamm. Bowel Dis. 27 (7), 1008–1016. doi:10.1093/ibd/izaa269
Cannatelli, R., Bazarova, A., Zardo, D., Nardone, O. M., Shivaji, U., Smith, S. C. L., et al. (2021). Fecal calprotectin thresholds to predict endoscopic remission using advanced optical enhancement techniques and histological remission in IBD patients. Inflamm. Bowel Dis. 27 (5), 647–654. doi:10.1093/ibd/izaa163
Caviglia, G. P., Ribaldone, D. G., Rosso, C., Saracco, G. M., Astegiano, M., and Pellicano, R. (2018). Fecal calprotectin: Beyond intestinal organic diseases. Panminerva Med. 60 (1), 29–34. doi:10.23736/S0031-0808.18.03405-5
D'Arcangelo, G., Imondi, C., Terrin, G., Catassi, G., and Aloi, M. (2021). Is fecal calprotectin a useful marker for small bowel Crohn disease? J. Pediatr. Gastroenterol. Nutr. 73 (2), 242–246. doi:10.1097/MPG.0000000000003151
D'Haens, G., Ferrante, M., Vermeire, S., Baert, F., Noman, M., Moortgat, L., et al. (2012). Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 18 (12), 2218–2224. doi:10.1002/ibd.22917
Fu, W., Fu, H., Ye, W., Han, Y., Liu, X., Zhu, S., et al. (2021). Peripheral blood neutrophil-to-lymphocyte ratio in inflammatory bowel disease and disease activity: A meta-analysis. Int. Immunopharmacol. 101, 108235. doi:10.1016/j.intimp.2021.108235
García-Sánchez, V., Iglesias-Flores, E., González, R., Gisbert, J. P., Gallardo-Valverde, J. M., González-Galilea, A., et al. (2010). Does fecal calprotectin predict relapse in patients with Crohn's disease and ulcerative colitis? J. Crohns Colitis 4 (2), 144–152. doi:10.1016/j.crohns.2009.09.008
Gecse, K. B., Brandse, J. F., van Wilpe, S., Löwenberg, M., Ponsioen, C., van den Brink, G., et al. (2015). Impact of disease location on fecal calprotectin levels in Crohn's disease. Scand. J. Gastroenterol. 50 (7), 841–847. doi:10.3109/00365521.2015.1008035
Han, W., Wu, J., Zhang, P., Hu, N., Mei, Q., and Hu, J. (2022). Fecal calprotectin predicts endoscopic activity and mucosal healing of small bowel Crohn's disease evaluated by double-balloon endoscopy. Int. J. Colorectal Dis. 37 (9), 1953–1961. doi:10.1007/s00384-022-04232-5
Inokuchi, T., Kato, J., Hiraoka, S., Takashima, S., Nakarai, A., Takei, D., et al. (2016). Fecal immunochemical test versus fecal calprotectin for prediction of mucosal healing in Crohn's disease. Inflamm. Bowel Dis. 22 (5), 1078–1085. doi:10.1097/MIB.0000000000000728
Iwamoto, F., Matsuoka, K., Motobayashi, M., Takenaka, K., Kuno, T., Tanaka, K., et al. (2018). Prediction of disease activity of Crohn's disease through fecal calprotectin evaluated by balloon-assisted endoscopy. J. Gastroenterol. Hepatol. 33 (12), 1984–1989. doi:10.1111/jgh.14310
Jensen, M. D., Kjeldsen, J., and Nathan, T. (2011). Fecal calprotectin is equally sensitive in Crohn's disease affecting the small bowel and colon. Scand. J. Gastroenterol. 46 (6), 694–700. doi:10.3109/00365521.2011.560680
Kalla, R., Ventham, N. T., Satsangi, J., and Arnott, I. D. (2014). Crohn's disease. BMJ 349, g6670. doi:10.1136/bmj.g6670
Kawashima, K., Ishihara, S., Yuki, T., Fukuba, N., Sonoyama, H., Kazumori, H., et al. (2017). Fecal calprotectin more accurately predicts endoscopic remission of Crohn's disease than serological biomarkers evaluated using balloon-assisted enteroscopy. Inflamm. Bowel Dis. 23 (11), 2027–2034. doi:10.1097/MIB.0000000000001202
Knyazev, O. V., Kagramanova, A. V., Korneeva, I. A., Noskova, K. K., Belousov, S. V., and Parfenov, A. I. (2019). The use of fecal calprotectin in monitoring activity of inflammatory bowel diseases. Ter. Arkh 91 (4), 53–61. doi:10.26442/00403660.2019.04.000229
Koulaouzidis, A., Sipponen, T., Nemeth, A., Makins, R., Kopylov, U., Nadler, M., et al. (2016). Association between fecal calprotectin levels and small-bowel inflammation score in capsule endoscopy: A multicenter retrospective study. Dig. Dis. Sci. 61 (7), 2033–2040. doi:10.1007/s10620-016-4104-7
Lee, Y. W., Lee, K. M., Lee, J. M., Chung, Y. Y., Kim, D. B., Kim, Y. J., et al. (2019). The usefulness of fecal calprotectin in assessing inflammatory bowel disease activity. Korean J. Intern Med. 34 (1), 72–80. doi:10.3904/kjim.2016.324
Lin, J. F., Chen, J. M., Zuo, J. H., Yu, A., Xiao, Z. J., Deng, F. H., et al. (2014). Meta-analysis: Fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm. Bowel Dis. 20 (8), 1407–1415. doi:10.1097/MIB.0000000000000057
Matsuura, R., Watanabe, O., Nakamura, M., Yamamura, T., Matsushita, M., Suhara, H., et al. (2018). Fecal calprotectin reflects endoscopic activity in patients with small-bowel Crohn's disease according to double-balloon endoscopy findings. Nagoya J. Med. Sci. 80 (2), 257–266. doi:10.18999/nagjms.80.2.257
Monteiro, S., Barbosa, M., Cúrdia Gonçalves, T., Boal Carvalho, P., Moreira, M. J., Rosa, B., et al. (2018). Fecal calprotectin as a selection tool for small bowel capsule endoscopy in suspected Crohn's disease. Inflamm. Bowel Dis. 24 (9), 2033–2038. doi:10.1093/ibd/izy098
Mooiweer, E., Severs, M., Schipper, M. E., Fidder, H. H., Siersema, P. D., Laheij, R. J., et al. (2015). Low fecal calprotectin predicts sustained clinical remission in inflammatory bowel disease patients: A plea for deep remission. J. Crohns Colitis 9 (1), 50–55. doi:10.1093/ecco-jcc/jju003
Ricciuto, A., and Griffiths, A. M. (2019). Clinical value of fecal calprotectin. Crit. Rev. Clin. Lab. Sci. 56 (5), 307–320. doi:10.1080/10408363.2019.1619159
Rokkas, T., Portincasa, P., and Koutroubakis, I. E. (2018). Fecal calprotectin in assessing inflammatory bowel disease endoscopic activity: A diagnostic accuracy meta-analysis. J. Gastrointestin Liver Dis. 27 (3), 299–306. doi:10.15403/jgld.2014.1121.273.pti
Romero-Mascarell, C., Fernández-Esparrach, G., Rodríguez-De Miguel, C., Masamunt, M. C., Rodríguez, S., Rimola, J., et al. (2022). Fecal calprotectin for small bowel Crohn's disease: Is it a cutoff issue? Diagn. (Basel) 12 (9), 2226. doi:10.3390/diagnostics12092226
Schaffer, T., Schoepfer, A. M., and Seibold, F.Swiss IBD Cohort Study Group (2014). Serum ficolin-2 correlates worse than fecal calprotectin and CRP with endoscopic Crohn's disease activity. J. Crohns Colitis 8 (9), 1125–1132. doi:10.1016/j.crohns.2014.02.014
Schoepfer, A. M., Beglinger, C., Straumann, A., Trummler, M., Vavricka, S. R., Bruegger, L. E., et al. (2010). Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am. J. Gastroenterol. 105 (1), 162–169. doi:10.1038/ajg.2009.545
Sipponen, T., Savilahti, E., Kolho, K. L., Nuutinen, H., Turunen, U., and Färkkilä, M. (2008). Crohn's disease activity assessed by fecal calprotectin and lactoferrin: Correlation with Crohn's disease activity index and endoscopic findings. Inflamm. Bowel Dis. 14 (1), 40–46. doi:10.1002/ibd.20312
Somwaru, A. S., Khanijow, V., and Katabathina, V. S. (2019). Magnetic resonance enterography, colonoscopy, and fecal calprotectin correlate in colonic Crohn's disease. BMC Gastroenterol. 19 (1), 210. doi:10.1186/s12876-019-1125-7
Torres, J., Mehandru, S., Colombel, J. F., and Peyrin-Biroulet, L. (2017). Crohn's disease. Lancet 389 (10080), 1741–1755. doi:10.1016/S0140-6736(16)31711-1
Vermeire, S., Van Assche, G., and Rutgeerts, P. (2006). Laboratory markers in IBD: Useful, magic, or unnecessary toys? Gut 55 (3), 426–431. doi:10.1136/gut.2005.069476
Veyre, F., Boschetti, G., Meunier, C., Cuerq, C., Gay, C., Charlois, A. L., et al. (2021). Low levels of fecal calprotectin 3 Months after surgery predict subsequent endoscopic postoperative remission in Crohn's disease. Dig. Dis. Sci. 66 (12), 4429–4435. doi:10.1007/s10620-020-06751-z
Wehkamp, J., Götz, M., Herrlinger, K., Steurer, W., and Stange, E. F. (2016). Inflammatory bowel disease. Dtsch. Arztebl Int. 113 (5), 72–82. doi:10.3238/arztebl.2016.0072
Zittan, E., Kelly, O. B., Gralnek, I. M., Silverberg, M. S., and Hillary Steinhart, A. (2018). Fecal calprotectin correlates with active colonic inflammatory bowel disease but not with small intestinal Crohn's disease activity. JGH Open 2 (5), 201–206. doi:10.1002/jgh3.12068
Keywords: fecal calprotectin, crohn’s disease, clinical value, disease activity, cutoff value
Citation: Li J, Xu M, Qian W, Ling F, Chen Y, Li S, Cheng Y and Zhu L (2023) Clinical value of fecal calprotectin for evaluating disease activity in patients with Crohn’s disease. Front. Physiol. 14:1186665. doi: 10.3389/fphys.2023.1186665
Received: 15 March 2023; Accepted: 24 May 2023;
Published: 01 June 2023.
Edited by:
Giuliano Ramadori, University of Göttingen, GermanyReviewed by:
Shenghong Zhang, The First Affiliated Hospital of Sun Yat-sen University, ChinaCopyright © 2023 Li, Xu, Qian, Ling, Chen, Li, Cheng and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liangru Zhu, emh1bGlhbmdydUBodXN0LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.