- 1Automatic Control Department (ESAII), Barcelona East School of Engineering (EEBE), Universitat Politècnica de Catalunya (UPC), Barcelona, Spain
- 2Institute for Bioengineering of Catalonia (IBEC), The Barcelona Institute of Science and Technology, Barcelona, Spain
- 3Institute of Innovative Health Technologies, Jena, Germany
- 4Hospital de la Santa Creu i Sant Pau, Barcelona, Spain
- 5CIBER de Bioengenieria, Biomateriales y Nanomedicina (CIBER-BBN), Barcelona, Spain
A large portion of the elderly population are affected by cardiovascular diseases. Early prognosis of cardiomyopathies remains a challenge. The aim of this study was to classify cardiomyopathy patients by their etiology based on significant indexes extracted from the characterization of the baroreflex mechanism in function of the influence of the cardio-respiratory activity over the blood pressure. Forty-one cardiomyopathy patients (CMP) classified as ischemic (ICM—24 patients) and dilated (DCM—17 patients) were considered. In addition, thirty-nine control (CON) subjects were used as reference. The beat-to-beat (BBI) time series, from the electrocardiographic (ECG) signal, the systolic (SBP), and diastolic (DBP) time series, from the blood pressure signal (BP), and the respiratory time (TT), from the respiratory flow (RF) signal, were extracted. The three-dimensional representation of the cardiorespiratory and vascular activities was characterized geometrically, by fitting a polygon that contains 95% of data, and by statistical descriptive indices. DCM patients presented specific patterns in the respiratory response to decreasing blood pressure activity. ICM patients presented more stable cardiorespiratory activity in comparison with DCM patients. In general, CMP shown limited ability to regulate changes in blood pressure. In addition, patients also shown a limited ability of their cardiac and respiratory systems response to regulate incremental changes of the vascular variability and a lower heart rate variability. The best classifiers were used to build support vector machine models. The optimal model to classify ICM versus DCM patients achieved 92.7% accuracy, 94.1% sensitivity, and 91.7% specificity. When comparing CMP patients and CON subjects, the best model achieved 86.2% accuracy, 82.9% sensitivity, and 89.7% specificity. When comparing ICM patients and CON subjects, the best model achieved 88.9% accuracy, 87.5% sensitivity, and 89.7% specificity. When comparing DCM patients and CON subjects, the best model achieved 87.5% accuracy, 76.5% sensitivity, and 92.3% specificity. In conclusion, this study introduced a new method for the classification of patients by their etiology based on new indices from the analysis of the baroreflex mechanism.
1 Introduction
One of the most relevant challenges in biomedical research is the analysis of system response variability. This is due not only because a system’s response variability represents the description of the patient’s state, but also because it can mask comorbidities. Diseases like cardiomyopathies, which affect a large segment of the elderly population, are of particular interest, as they constitute one of the most common causes of death. The clinical diagnosis of these patients, especially the ones with multiple comorbidities, are still challenging.
These pathologies may originate in an ischemic (ICM) or dilated (DCM) process of the heart. The reaction to this process, however, may be related to the variability of the cardiorespiratory and cardiovascular system response, which is associated with the baroreflex mechanism. Therefore, these systems can help in differentiating between these cardiomyopathies, which can lead to an improved and earlier diagnosis for these patients (Medina et al., 1985; Sawada MD et al., 1992). For instance, patients that suffers from ischemic related cardiomyopathies develops a worse prognosis and are candidates for a more specialized therapy. Some authors suggest that there is a high prevalence of etiologic misclassification in advanced heart failure (Mehra and Uber, 2013). According to their research, at least 20% of the patients were misclassified.
Despite the symptomatic similarities between ischemic and dilated cardiomyopathies, they differ in etiology. For instance, ischemic cardiomyopathy (ICM) is related to coronary artery disease, and is characterized by myocyte loss, a compensatory tissue hypertrophy, and ventricular scarring (Beltrami et al., 1994). ICM patients may be treated according to their heart damage with medications such as nitrates, beta blockers, angiotensin-converting enzyme inhibitors, among others. In more critical cases, the treatment can include procedures like angioplasty or coronary artery bypass. On the other hand, dilated cardiomyopathy (DCM) is characterized by the enlargement and weakening of the left ventricle and contractile dysfunction (McNally Elizabeth and Mestroni, 2017). DCM may be associated with an increased risk of severe arrhythmia. Treatment of DCM patients could include medications like angiotensin II beta blockers, diuretics, digoxin, among others. Additionally, in some cases is necessary to implant devices such as the implantable cardioverter-defibrillator, left ventricular assist devices or a biventricular peacemaker. Considering the differences mentioned, new knowledge that could help improving the diagnosis of these type of patients is desired.
Several linear and non-linear techniques based on the analysis of biomedical signals have been explored in earlier studies (Davos et al., 2002; Stein et al., 2005; Voss et al., 2010). Deep learning methods have been developed to predict ventricular fibrillation episodes in cardiomyopathy patients (Tseng and Tseng, 2020), and to predict heart failure in patients with different levels of left ventricular ejection fraction (Alkhodari1 et al., 2021). Machine learning techniques have been used to predict cardiac-related prognosis in different types of patients (Guisen et al., 2021), for the prediction of complications after cardiovascular surgery (Jiang1 et al., 2021), and for the prediction of the progression of heart failure in hypertrophic cardiomyopathy (Fahmy et al., 2021). Support vector machine-based algorithms have been explored for the detection of cardiac disorders (Wang and Zheng, 2009; Borkar and Annadate, 2018). Differences in the cardiovascular autonomic regulation of ischemic and dilated cardiomyopathy patients have been hypothesized (Freeman et al., 2006). The calcium release during the excitation-contraction coupling of the heart was investigated in both ischemic and dilated cardiomyopathy patients (Brillantes et al., 1992). The authors found that sarcoplasmic reticulum calcium release channel levels were 28% lower in ICM patients, suggesting that ischemic hearts exhibit diminished calcium release during excitation-contraction. This pathological behavior could be caused by the abnormal calcium processing of myopathic cardiac muscle in ischemic heart disease.
Other authors measured the content and activities of components of the β-adrenergic receptor-G protein-adenylate cyclase complex and adrenergic neurotransmitter levels in the left and right ventricular myocardium of patients with ischemic and dilated cardiomyopathy. They revealed that there are differences between the regulatory β-receptor-effector related mechanisms in ICM vs. DCM patients (Bristow et al., 1991). ICM patients were characterized by less β-adrenergic receptor downregulation in both ventricles, a decreased coupling of β-adrenergic receptors mediating a contractile response in right ventricular tissue, and decreased coupling of β-adrenergic receptors mediating adenylate cyclase stimulation in the left ventricular tissue.
Another study compared the myocardial tissue from ischemic and dilated cardiomyopathy patients (Böhm et al., 1990). The authors found a reduction in positive inotropic effects, leading to weaker contractions in DCM patients compared to ICM patients. This result suggests that increased levels of heterotrimeric G-proteins and reduced basal adenylate cyclase activity give rise to the abnormal regulation of contractility in dilated hearts. Other authors have used both neural networks and fuzzy methods to classify different heart diseases, including ischemic and dilated cardiomyopathies (Acharya et al., 2004).
Analyzing the interactions between the cardiovascular and respiratory systems could provide new insights into these cardiomyopathies and contribute to the more accurate diagnosis of these diseases. The coupled influence between the respiratory and cardiovascular systems under pathological conditions have been explored before (Hernando et al., 2013). In our previous work, we proposed analyzing the cardiovascular activity using coupling analysis (Rodriguez et al., 2019). In this work, we introduce cardiorespiratory interaction as a means by which to analyze the behavior of the systems associated with ICM and DCM cardiomyopathies. We proposed a three-dimensional analysis that considered the relationship between the cardiac, respiratory, and vascular systems. Based on vascular activity as the input and output of the baroreflex response, we evaluated the variability of these interactions. The aim of this study was to analyze the suitability of cardiorespiratory and vascular interactions for the classification of ICM and DCM patients. However, we also characterized these interactions through features extracted from electrocardiography (ECG), respiratory flow and blood pressure signals.
2 Data acquisition
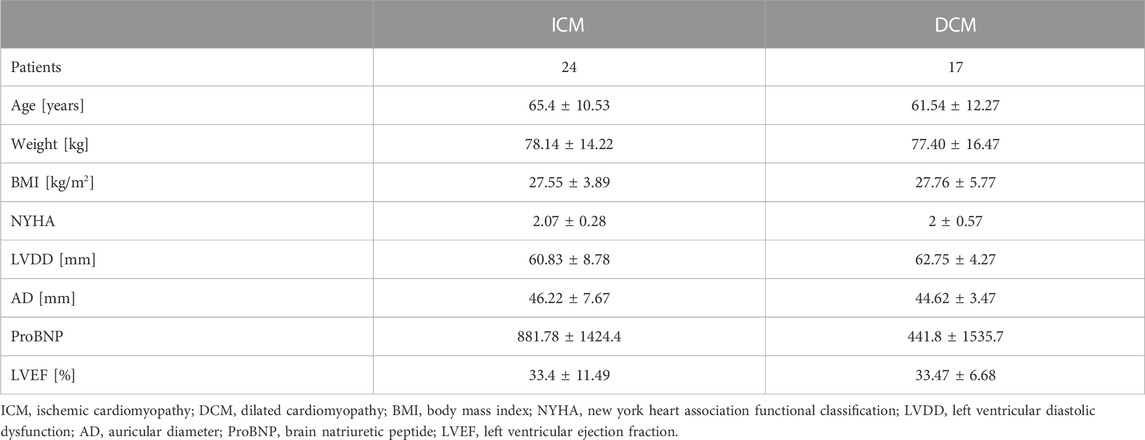
Noninvasive electrocardiographic (ECG), blood pressure (BP) and respiratory flow (RF) signals from 41 cardiomyopathy patients were registered at the Santa Creu i Sant Pau Hospital in Barcelona, Spain. All records were performed according to a protocol, previously approved by the Hospital ethics committee. Forty-one cardiomyopathy patients were used to explore the method. The patients were characterized by New York Heart Association functional classification (NYHA)
3 Signal processing
The ECG, BP and RF signals linear trend were removed, and in-house preprocessing tools were used to reduce noise, artifacts and spikes. Any sample with a value equal to or greater than 3 times the standard deviation of the series was considered an outlier. All outliers were eliminated. The beat-to-beat interval time series (BBI) were extracted from the ECG signal using an in-house algorithm that detects the R peaks and calculate the time between peaks, milliseconds. The systolic (SBP) and diastolic (DBP) blood pressure time series were calculated as the maximum and minimum values of the BP in each heartbeat, in mmHg. The total respiratory cycle time series (TT) was assessed as the time between two successive breath periods, in seconds. Thereafter, all-time series were inspected and edited, if necessary.
In order to analyze the cardiac, respiratory and vascular interactions, the time series were synchronized using linear interpolation method with a sample frequency of 1 Hz. To properly observe and analyze changes in the respiratory activity, the time series were decimated to 0.25 Hz.
4 Methodology
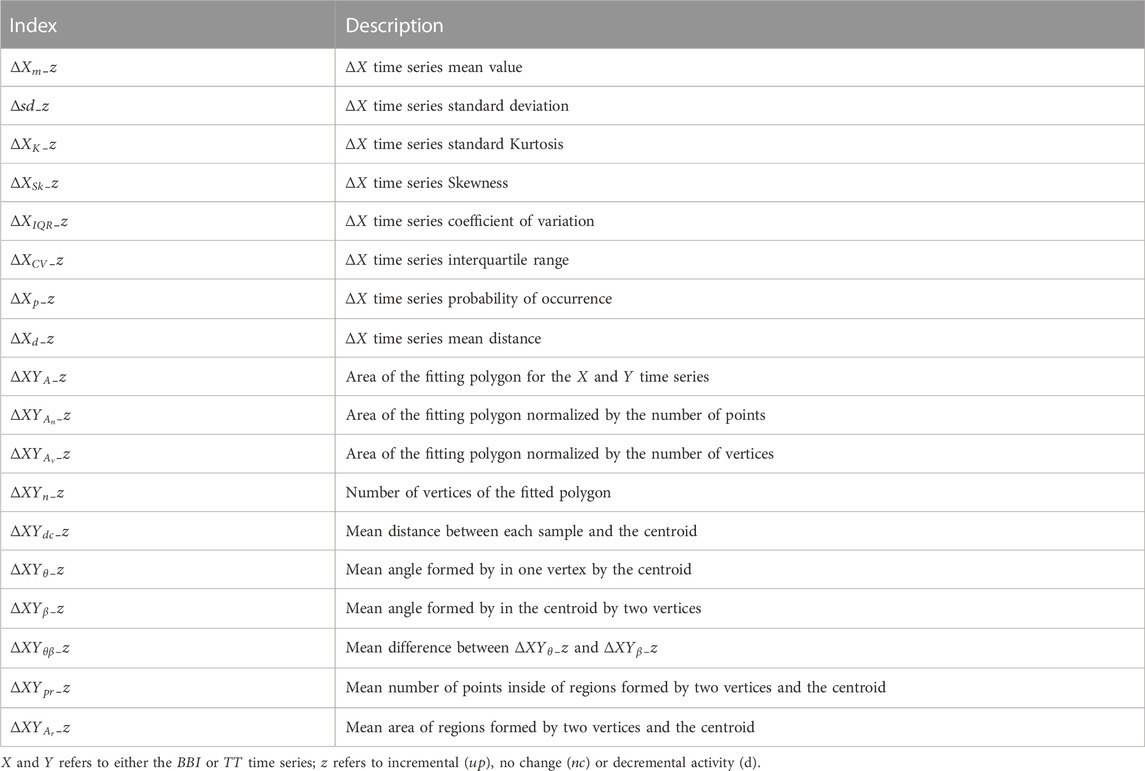
To analyze the interaction between the cardiac, respiratory and vascular systems, we took the difference between two consecutive events
being
To determine vascular activity, we defined a threshold associated with the increase, decrease or absence of change of the
where
To analyze the variability of the vascular system, three new sub-spaces were defined in accordance with the
To evaluate the variability of cardiorespiratory activity associated with vascular behavior, a three-dimensional representation was generated for each sub-space: “up”, “no change” and “down”. This representation presents the relationship between changes in the
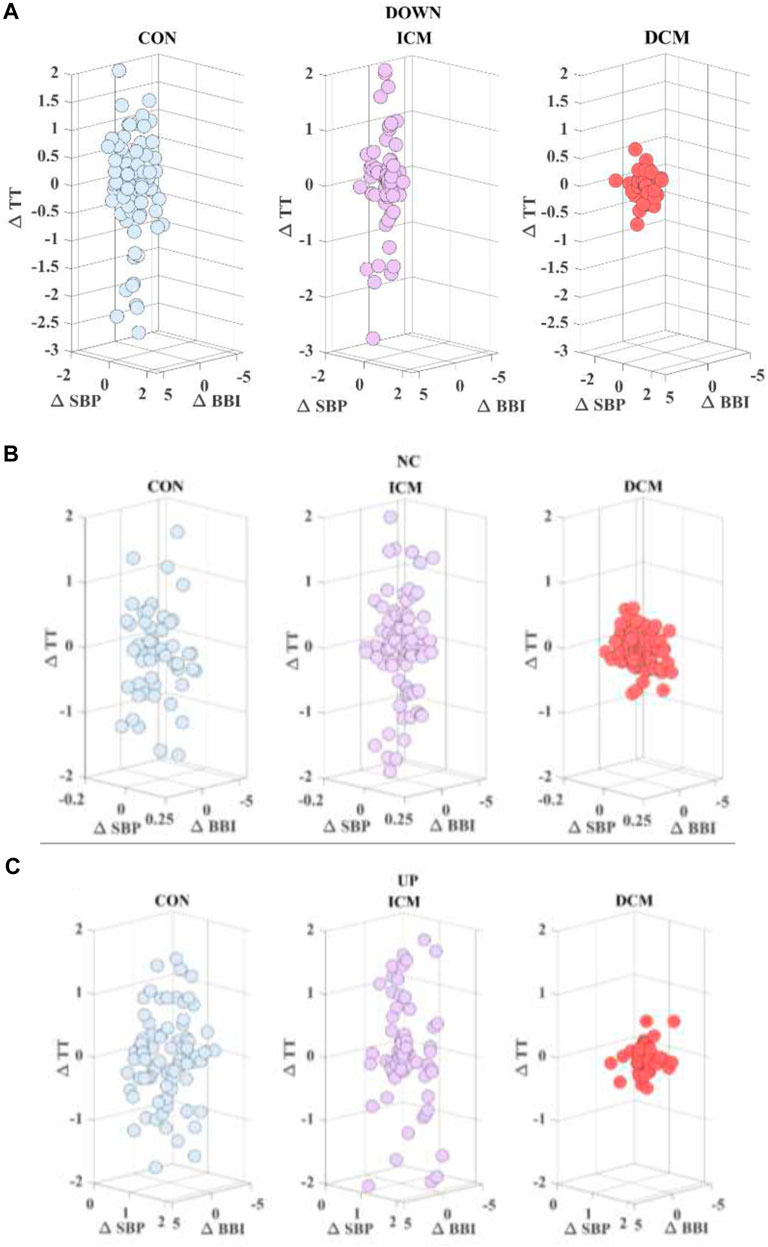
FIGURE 1. Cardiac (∆BBI) vs. respiratory (∆TT) time series considering (∆SBP) (A) down, (B) no change, and (C) up activity, for a CON subject, an ICM patient and a DCM patient.
Several parameters were extracted from this three-dimensional representation in order to characterize their interactions. For each subject/patient scatterplot, a polygon that represents the projection of the cardiac and respiratory activity based on vascular behavior (
where
The morphology of the polygon was also analyzed using all the triangles that form it. First, the mean area of all triangles (
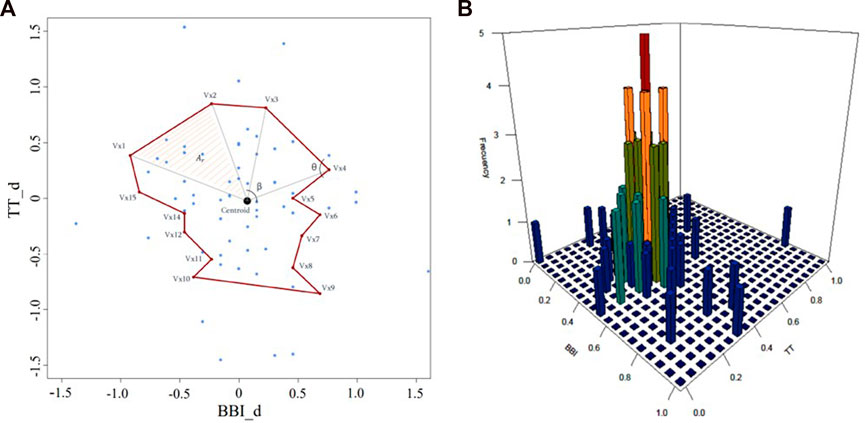
FIGURE 2. Scatterplot of cardiorespiratory activity for decreasing systolic vascular activity in a CON subject. (A) morphological characterization including
5 Classification and validation
Support vector machines (SVM) method is useful for classification tasks where the classes are not linearly separable in the original space. By transforming the data into a higher dimensional space, SVM can transform a complex non-linear problem into a simple linear one. This is achieved through the optimization of a hyperplane defined by the SVM function, being
where
The Gaussian kernel is often used to model radially distributed data and is given by,
where
The Laplace kernel is a less
where
The ANOVA kernel is used on multidimensional support vector regression models and given by,
where
The classification problem is then solved by maximizing the margin while minimizing the training error. Using the Lagrange multipliers method, a dual formulation is obtained:
where
Each feature was scaled and normalized (zero mean and unit variance) in order to avoid scaling biases. For each iteration of features, the model was built by optimizing the value of
A Mann-Whitney non-parametric statistical test was applied to evaluate the statistically significant differences between all the indices. Any index with a p-value
6 Results and discussion
This work aimed to characterize the cardiovascular and cardiorespiratory activity of patients with ischemic or dilated cardiomyopathy. A total of 168 indices were obtained during the characterization process. The results were analyzed considering the following comparisons:
- Ischemic vs. dilated cardiomyopathy patients (ICM vs. DCM)
- Cardiomyopathy patients vs. control subjects (CMP vs. CON)
- Ischemic cardiomyopathy patients vs. control subjects (ICM vs. CON)
- Dilated cardiomyopathy patients vs. control subjects (DCM vs. CON)
Figure 3 provides an example of the statistical characterization of cardiorespiratory activity based on systolic blood pressure activity according to the different sub-spaces: down (a, b, c), no-change (d, e, f), and up (g, h, i) for a CON subject (a, d, g), an ICM patient (b, e, h), and a DCM patient (c, f, i). Figure 4 illustrates an example of the morphological characterization of cardiorespiratory activity based on systolic blood pressure activity according to the different sub-spaces: down (a, b, c), no-change (d, e, f), and up (g, h, i) for a CON subject (a, d, g), an ICM patient (b, e, h), and a DCM patient (c, f, i).
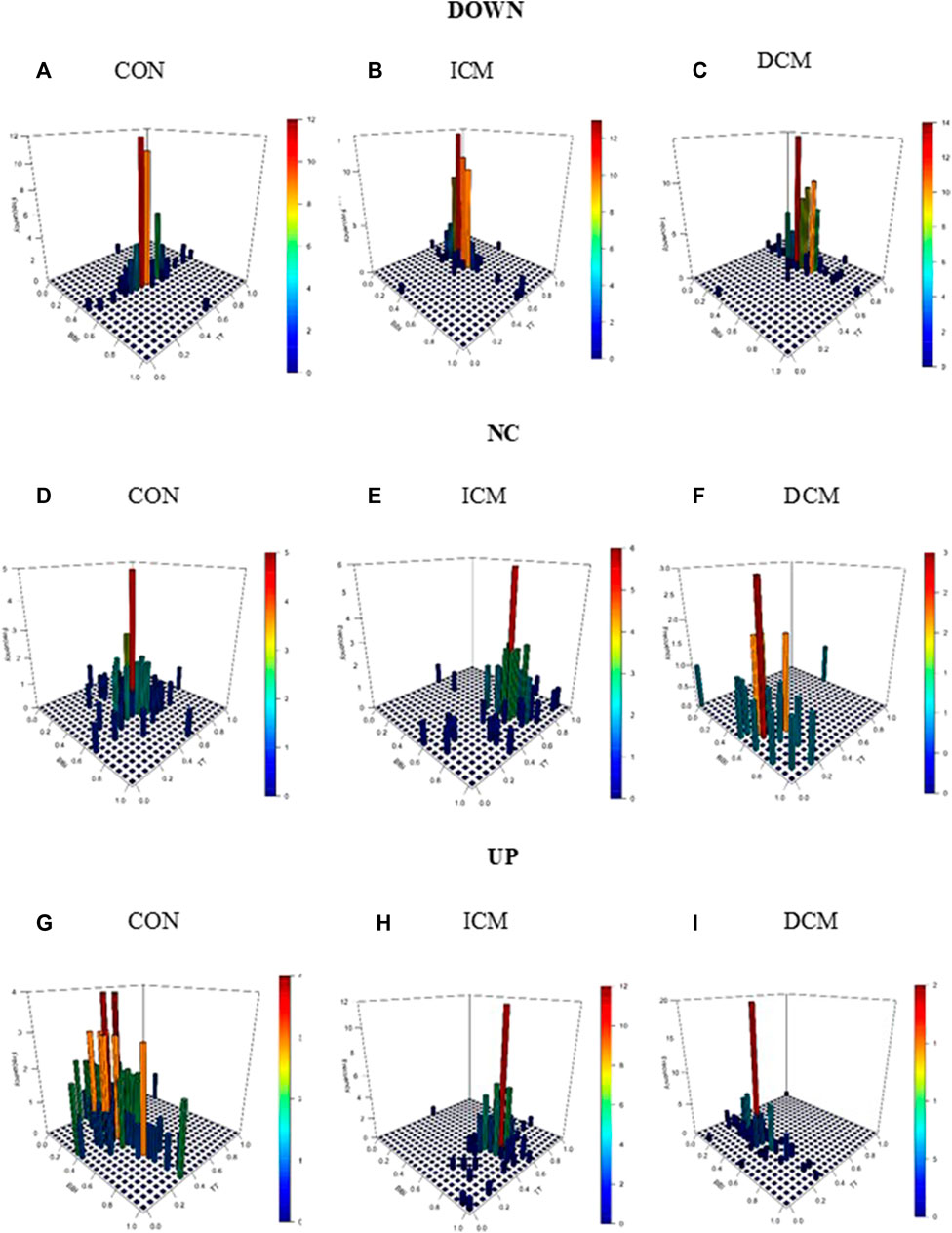
FIGURE 3. Statistical characterization of cardiorespiratory activity based on the sub-space for systolic blood pressure activity: down for (A) CON subject, (B) ICM and (C) DCM patients; no-change for (D) CON subject, (E) ICM and (F) DCM patients; and up for (G) CON subject, (H) ICM and (I) DCM patients.
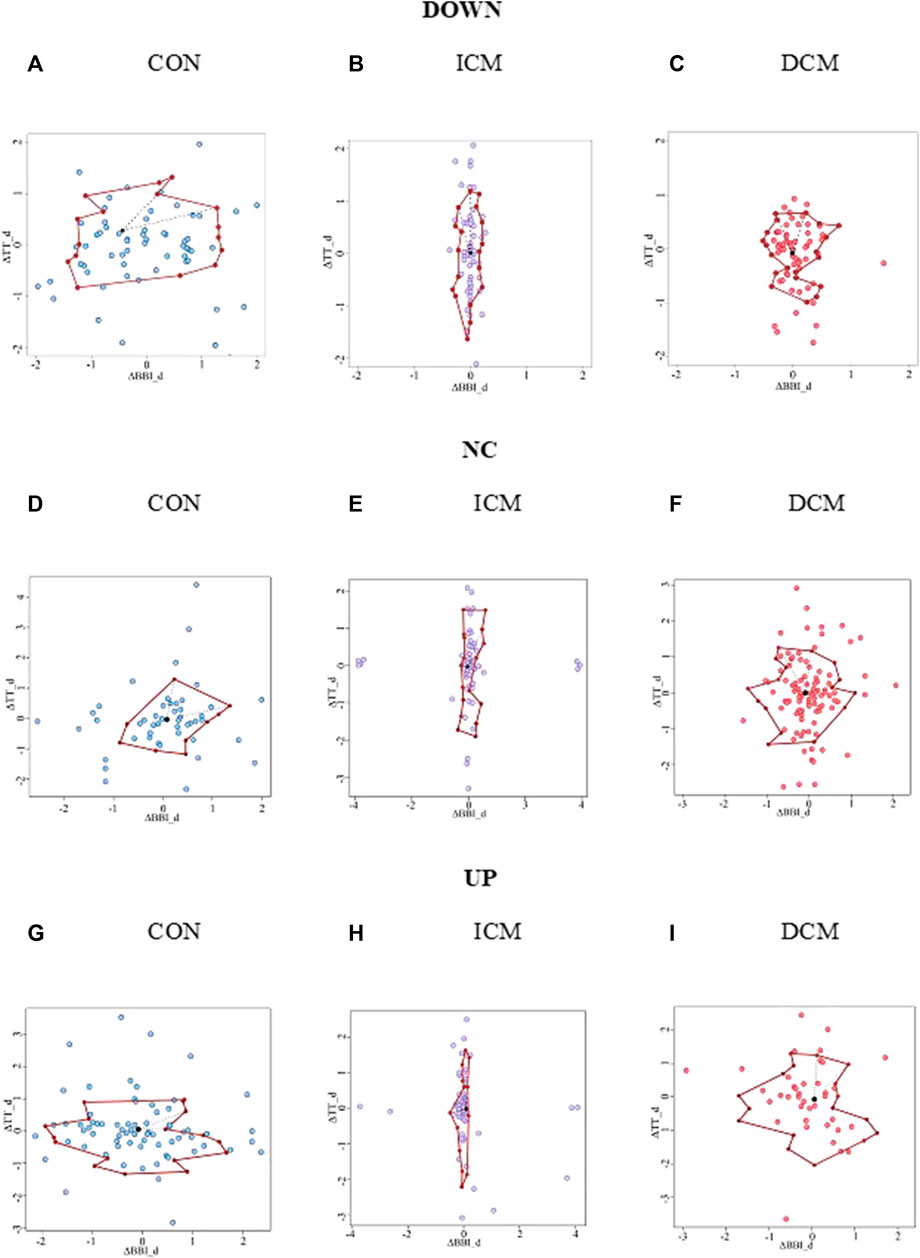
FIGURE 4. Morphological characterization of cardiorespiratory activity based on the sub-space for systolic blood pressure activity: down for (A) CON subject, (B) ICM and (C) DCM patients; no-change for (D) CON subject, (E) ICM and (F) DCM patients; and up for (G) CON subject, (H) ICM and (I) DCM patients.
In the comparison of ICM and DCM patients, 9 indices presented statistically significant differences, and relatively low correlation. For the CMP patients vs. CON group, 13 indices presented statistical differences. When each pathology was compared to the CON group, 10 indices presented differences when compared to ICM patients, and 19 when compared to DCM patients. These indices were used to build different SVM models, determining the best classifiers in each case. Table 3 presents the most relevant indices for each comparison in terms of mean value and standard deviation.
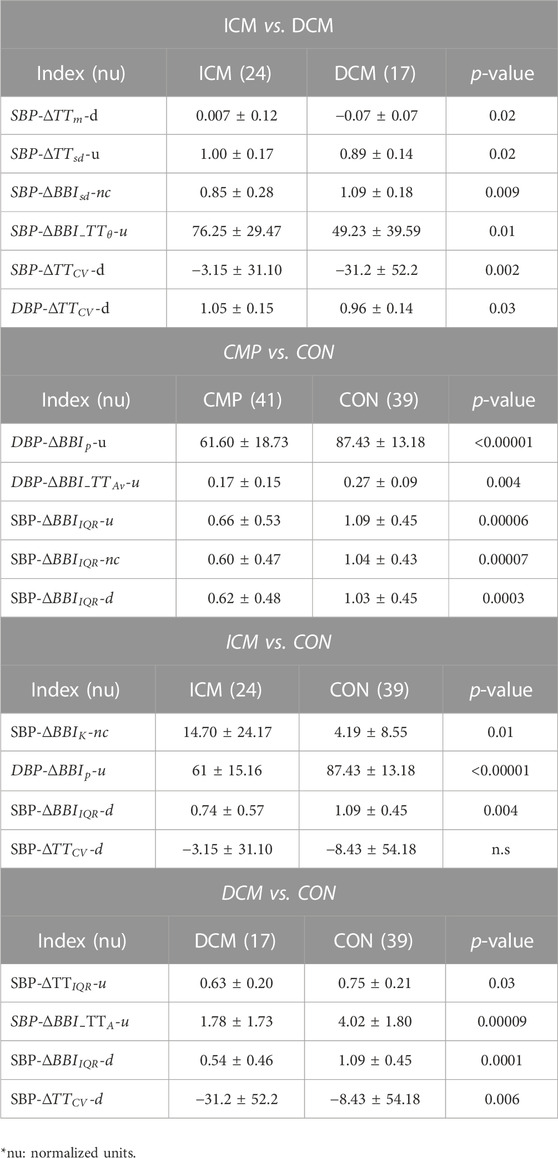
TABLE 3. Significant indices for ICM vs. DCM, CMP vs. CON, ICM vs. CON, and DCM vs. CON comparisons, presented in terms of mean value and standard deviation.
The
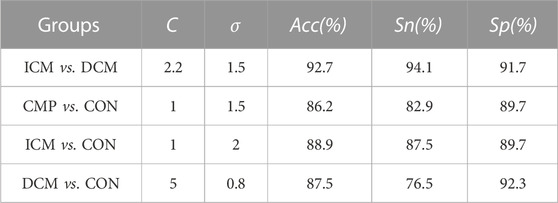
TABLE 4. Accuracy (Acc), sensitivity (Sn), and specificity (Sp), obtained with the best SVM model for each classification group.
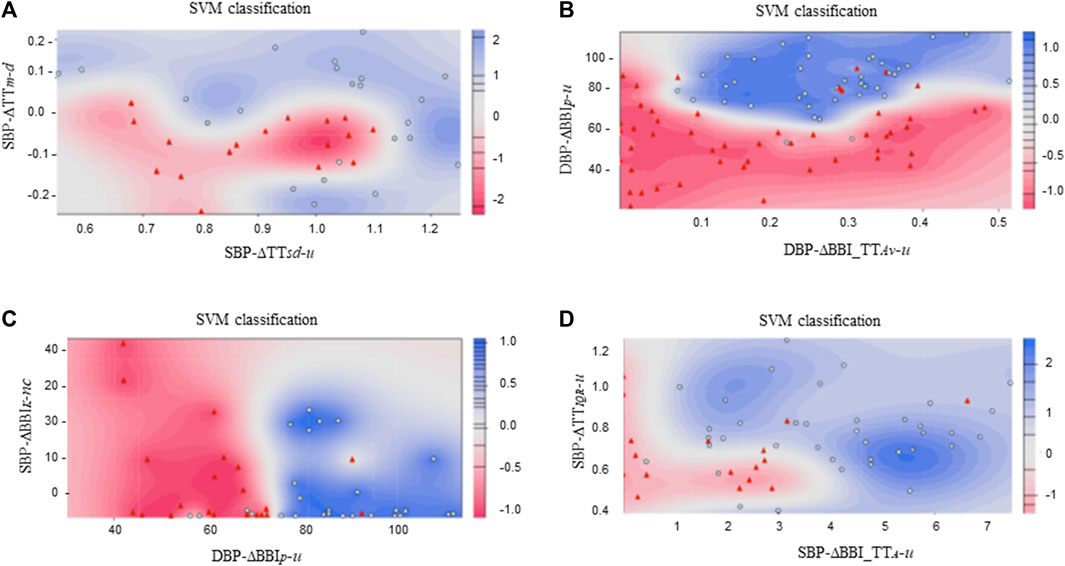
FIGURE 5. SVM classification plots considering: (A) ICM ( ) vs. DCM (
) vs. DCM ( ) patients, (B) CMP (
) patients, (B) CMP ( ) patients vs. CON (
) patients vs. CON ( ) subjects, (C) ICM (
) subjects, (C) ICM ( ) patients vs. CON (
) patients vs. CON ( ) subjects, and (D) DCM (
) subjects, and (D) DCM ( ) patients vs. CON (
) patients vs. CON ( ) subjects.
) subjects.
The interaction between cardiorespiratory and vascular activity was explored and characterized to analyze the behavior of these systems in patients diagnosed with ischemic or dilated cardiomyopathy. Afterwards, the best indices were used to classify these patients, through the introduction of new information about the response of the cardiac and respiratory systems, based on vascular activity.
Our results indicate a decreasing respiratory response in DCM patients when systolic blood pressure activity decreases (
The decreased respiratory activity observed during drops in systolic blood pressure in DCM patients suggests a faster breathing response to decreasing blood pressure. Changes in breathing rhythm are known to affect blood pressure levels (Nuckowska et al., 2019). In addition, several respiratory abnormalities have been associated before with left ventricular impairment (Mortara et al., 1999). On the other hand, it have been stated before that respiratory performance may be dependent on cardiac function, especially in heart failure patients (Nishimura et al., 1994). We hypothesize that the respiratory activity in DCM patients compensates for the impaired cardiac-dependent regulation of blood pressure during the baroreflex response.
The comparison of SBP activity when the values correspond to “no change” revealed that cardiac response was more stable in ICM patients (
In the presence of increased SBP activity, the respiratory response is more disperse in ICM patients than in DCM patients (
In addition, in cases of increased in SBP activity, the mean angle formed by each vertex and the centroid of its fitting cloud of points associated with cardiorespiratory activity (
An analysis of overall cardiac behavior among CMP patients, in general showed a reduced response to increasing DBP activity (
A similar behavior was observed for increased DBP activity, when ICM patients were compared to the CON subjects (
Cardiac and respiratory variability differences were also found when the DCM patients were compared to the CON subjects for increased SBP activity (
This study has some limitations that should be taken into consideration. One of them is the age disparity between the control group and the patient group. However, the influence of age on the study of cardiac activity variability has been extensively studied in previous works (Porta et al., 2014; Voss et al., 2015). Nonetheless, this limitation does not affect our results when comparing variations among the different parameters that describe cardiorespiratory and vascular relationships, thereby mitigating possible biases derived from the passing of time.
Another limitation is related to the comorbidities and confounding factors presented by the patients, which may influence the autonomic regulation of the systems. Nevertheless, this study allows independent quantification of cardiorespiratory interactions as a function of vascular activity. Assessing the relative variations between these systems can contribute to the early analysis of the evolution of the patients.
7 Conclusion
In conclusion, the analysis of the interaction between cardiac and respiratory activity and blood pressure provides novel insight into the classification of patients with this type of disease. We found respiratory patterns that are more prevalent in dilated cardiomyopathy patients. In general, cardiac and respiratory variability showed lower values in these patients compared to the patterns observed in healthy subjects. Furthermore, these results suggest more specific patterns in the respiratory response with decreased blood pressure activity for patients with dilated cardiomyopathy.
The work presented here may provide physicians with new symptom-independent information about these types of cardiomyopathies, which may help improving the diagnosis of these patients. This method not only serves to analyze the behavior of ischemic and dilated cardiomyopathy patients, but also introduces a new procedure with which to analyze the dynamical behavior between other coupled systems. However, additional features with clinical information about the patients should be considered to increase the discrimination between the groups. This study is limited by the size of the dataset employed and, consequently, the results discussed in this work are of an exploratory nature and should be validated using a larger dataset.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Data supporting the findings of this study are available from BG, but restrictions apply to the availability of these data, which were used under license for the current study, by what are not publicly available. The data set is not publicly available due to the conditions that were established when the protocol was defined and the patients were registered. Requests to access these datasets should be directed to BG, QmVhdHJpei5HaXJhbGRvQHVwYy5lZHU=.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethical committee of Santa Creu i Sant Pau Hospital in Barcelona, Spain- Biomedical signals recorded between 2010—2011. The patients/participants provided their written informed consent to participate in this study.
Author contributions
BG and AV provided the conceptualization of the study. BG, JR, and AV designed the study and analyzed the results. BG and JR developed the software, performed the required computations, and wrote most of the manuscript. BG, AV, and SB provided the datasets. SS and SH contributed to the interpretation of the results. All authors contributed to the article and approved the submitted version.
Funding
This study has received funding from by the CERCA Program/Generalitat de Catalunya, from the Secretaria d’Universitats i Recerca de la Generalitat de Catalunya GRC 2021 SGR 01390, and from the Spanish Ministry of Science and Innovation (PID2021-126455OB-I00 MCIN/AEI/FEDER).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acharya, R., Kumar, A., Bhat, P. S., Lim, C. M., lyengar, S. S., Kannathal, N., et al. (2004). Classification of cardiac abnormalities using heart rate signals. Med. Biol. Eng. Comput. 42 (3), 288–293. doi:10.1007/BF02344702
Alkhodari1, M., Jelinek, H., Karlas, A., Soulaidopoulos, S., Arsenos, P., Doundoulakis, I., et al. (2021). Deep learning predicts heart failure with preserved, mid-range, and reduced left ventricular ejection fraction from patient clinical profiles. Front. Cardiovasc. Med. 2021. doi:10.3389/fcvm.2021.755968
Baumert, M., Baier, V., Truebner, S., Schirdewan, A., and Voss, A. (2005). Short- and long-term joint symbolic dynamics of heart rate and blood pressure in dilated cardiomyopathy. IEEE Trans. Biomed. Eng. 52 (12), 2112–2115. doi:10.1109/TBME.2005.857636
Beltrami, C. A., Finato, N., Rocco, M., Feruglio, G. A., Puricelli, C., Cigola, E., et al. (1994). Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation 89 (1), 151–163. doi:10.1161/01.CIR.89.1.151
Böhm, M., Gierschik, P., H Jakobs, K., Pieske, B., Schnabel, P., Ungerer, M., et al. (1990). Increase of Gi alpha in human hearts with dilated but not ischemic cardiomyopathy. Circulation 82 (4), 1249–1265. doi:10.1161/01.CIR.82.4.1249
Borkar, S., and Annadate, M. N. “Supervised machine learning algorithm for detection of cardiac disorders,” in Proceedings of the 2018 Fourth International Conference on Computing Communication Control and Automation (ICCUBEA), Pune, India, August 2018, 1–4. doi:10.1109/ICCUBEA.2018.8697795
Brillantes, A. M., Allen, P., Takahashi, T., Izumo, S., and Marks, A. R. (1992). Differences in cardiac calcium release channel (ryanodine receptor) expression in myocardium from patients with end-stage heart failure caused by ischemic versus dilated cardiomyopathy. Circulation Res. 71 (1), 18–26. doi:10.1161/01.RES.71.1.18
Bristow, M. R., Anderson, F. L., Port, J. D., Skerl, L., Hershberger, R. E., Larrabee, P., et al. (1991). Differences in beta-adrenergic neuroeffector mechanisms in ischemic versus idiopathic dilated cardiomyopathy. Circulation 84 (3), 1024–1039. doi:10.1161/01.CIR.84.3.1024
Claria, F., Vallverdu, M., Martinez, A., Viñolas, X., Zareba, W., Bayés de Luna, A., et al. (2001). Sympatho-vagal activity described by the complex and deterministic behavior of heart rate variability. Comput. Cardiol. 28, 473–476. doi:10.1109/CIC.2001.977695
Cuponne, A. V., Vallverdú, M., Gomis, P., Porta, A., Voss, A., Bayes de Luna, A., et al. “Symbolic dynamics of QT interval series in ischemic cardiomyopathy,” in Proceedings of the 2012 Computing in Cardiology, Krakow, Poland, September 2012, 617–620.
Davos, C., Davies, L., and Piepoli, M. (2002). The effect of baroreceptor activity on cardiovascular regulation. Hellemoc J. Cardiol. 43, 145–155.
Fahmy, A. S., Rowin, E. J., Manning, W. J., Maron, M. S., and Nezafat, R. (2021). Machine learning for predicting heart failure progression in hypertrophic cardiomyopathy. Front. Cardiovasc. Med. 13 (8), 647857. doi:10.3389/fcvm.2021.647857
Freeman, J. V., Dewey, F. E., Hadley, D. M., Myers, J., and Froelicher, V. F. (2006). Autonomic nervous system interaction with the cardiovascular system during exercise. Prog. Cardiovasc. Dis. 48 (5), 342–362. doi:10.1016/j.pcad.2005.11.003
Giraldo, B. F., Pericàs, M. F., Schröeder, R., and Voss, A. (2018). “Respiratory sinus arrhythmia quantified with linear and non-linear techniques to classify dilated and ischemic cardiomyopathy,” in Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Hawai, USA, July 2018, 4860–4863. doi:10.1109/EMBC.2018.8513199
Guisen, L., Liu, Q., Chen, Y., Zong, X., Xi, Y., Li, T., et al. (2021). Machine learning to predict long-term cardiac-relative prognosis in patients with extra-cardiac vascular disease. Front. Cardiovasc. Med. 25. doi:10.3389/fcvm.2021.771504
Hernando, D., Alcaine, A., Pueyo, E., Laguna, P., Orini, M., Arcentales, A., et al. (2013). Influence of respiration in the very low frequency modulation of QRS slopes and heart rate variability in cardiomyopathy patients. Comput. Cardiol., 117–120.
Jiang1, H., Liu, L., Wang, Y., Ji, H., Ma, X., Wu, J., et al. (2021). Machine learning for the prediction of complications in patients after cardiac valvular surgery. Front. Cardiovasc. Med. 8. doi:10.3389/fcvm.2021.771246
Kaplan, D. T., Furman, M. I., Pincus, S. M., Ryan, S. M., Lipsitz, L. A., and Goldberger, A. L. (1991). Aging and the complexity of cardiovascular dynamics. Biophysical J. 59 (4), 945–949. (in eng). doi:10.1016/S0006-3495(91)82309-8
Kuroda, K., Kato, T. S., and Amano, A. (2015). Hypertensive cardiomyopathy: A clinical approach and literature review. World J. Hypertens. 5 (2), 41–52. doi:10.5494/wjh.v5.i2.41
Lombardi, F., Casalone, C., Malfatto, G., Pagani, M., and Malliani, A. (1984). Global versus regional myocardial ischaemia: differences in cardiovascular and sympathetic responses in cats. Cardiovasc. Res. 18 (1), 14–23. doi:10.1093/cvr/18.1.14
Malfatto, G., Branzi, G., Gritti, S., Sala, L., Bragato, R., Perego, G. B., et al. (2001). Different baseline sympathovagal balance and cardiac autonomic responsiveness in ischemic and non-ischemic congestive heart failure. Eur. J. Heart Fail. 3 (2), 197–202. doi:10.1016/S1388-9842(00)00139-2
McNally Elizabeth, M., and Mestroni, L. (2017). Dilated cardiomyopathy: genetic determinants and mechanisms. Circulation Res. 121 (7), 731–748. doi:10.1161/CIRCRESAHA.116.309396
Medina, R., Panidis, I. P., Morganroth, J., Kotler, M. N., and Mintz, G. S. (1985). The value of echocardiographic regional wall motion abnormalities in detecting coronary artery disease in patients with or without a dilated left ventricle. Am. Heart J. 109 (4), 799–803. doi:10.1016/0002-8703(85)90641-6
Mehra, L. M., and Uber, P. A. (2013). Preponderance and implications of etiologic misclassification in advanced heart failure: A clinical-pathologic investigation. J. Heart Lung Transplant. 32 (2), 268–269. doi:10.1016/j.healun.2012.12.003
Mortara, A., Sleight, P., Pinna, G., Maestri, R., et al. “Association between hemodynamic impairment and cheyne-Stokes respiration and periodic breathing in chronic stable congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy”, Am. J. Cardiol., vol. 84, no. 8, pp. 900–904. 1999 doi:10.1016/S0002-9149(99)00462-2
Mohammed, F. H., Shafei, E. M., El-Garhy, A. M., and El-Dosoky, M. A. (2018). “Modeling of the effect of dilated cardiomyopathy on the behavior of the heart,” in Proceedings of the 2018 9th Cairo International Biomedical Engineering Conference (CIBEC), Cairo, Egypt, December 2018, 17–20. doi:10.1109/CIBEC.2018.8641792
Momose, M., Tyndale-Hines, L., Bengel, F. M., and Schwaiger, M. (2001). How heterogeneous is the cardiac autonomic innervation? Basic Res. Cardiol. 96 (6), 539–546. doi:10.1007/s003950170004
Nishimura, Y., Maeda, H., Tanaka, K., Nakamura, H., Hashimoto, Y., and Yokohama, M. (1994). Respiratory muscle strength and hemodynamics in chronic heart failure. Chest 105 (2), 355–359. doi:10.1378/chest.105.2.355
Nuckowska, M. K., Gruszecki, M., Kot, J., Wolf, J., Guminski, W., Frydrychowski, A. F., et al. (2019). Impact of slow breathing on the blood pressure and subarachnoid space width oscillations in humans. Sci. Rep. 9 (1), 6232. doi:10.1038/s41598-019-42552-9
Porta, A., Faes, L., Bari, V., Marchi, A., Bassani, T., Nollo, G., et al. (2014). Effect of age on complexity and causality of the cardiovascular control: comparison between model-based and model-free approaches. PloS one 9, e89463. doi:10.1371/journal.pone.0089463
Rodriguez, J., Schulz, S., Voss, A., and Giraldo, B. F. “Cardiovascular coupling-based classification of ischemic and dilated cardiomyopathy patients,” in Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, July 2019, 2007–2010. doi:10.1109/EMBC.2019.8857875
Sawada Md, S. G., Ryan Md, T., Segar, D. M. D., Atherton, L. M. D., Fineberg PhD, N., Davis, C., et al. (1992). Distinguishing ischemic cardiomyopathy from nonischemic dilated cardiomyopathy with coronary echocardiography. J. Am. Coll. Cardiol. 19 (6), 1223–1228. doi:10.1016/0735-1097(92)90328-K
Stein, P. K., Domitrovich, P. P., Hui, N., Rautaharju, P., and Gottdiener, J. (2005). Sometimes higher heart rate variability is not better heart rate variability: results of graphical and nonlinear analyses. J. Cardiovasc. Electrophysiol. 16 (9), 954–959. doi:10.1111/j.1540-8167.2005.40788.x
Tseng, L. M., and Tseng, V. S. (2020). Predicting ventricular fibrillation through deep learning. IEEE Access 8, 221886–221896. doi:10.1109/ACCESS.2020.3042782
van de Borne, P., Oren, R., Abouassaly, C., Anderson, E., and Somers, V. K. (1998). Effect of Cheyne-Stokes respiration on muscle sympathetic nerve activity in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 81 (4), 432–436. doi:10.1016/S0002-9149(97)00936-3
Voss, A., Schroeder, R., Truebner, S., Goernig, M., Schirdewan, A., and Figulla, H. R., "Alternans of blood pressure and heart rate in patients with dilated cardiomyopathy," 2006, in Proceedings of the 2006 Computers in Cardiology, September 2006, Valencia, Spain, pp. 421–424.
Voss, A., Schroeder, R., Heitmann, A., Peters, A., and Perz, S. (2015). Short-term heart rate variability--influence of gender and age in healthy subjects. PLoS One 10 (3), e0118308. doi:10.1371/journal.pone.0118308
Voss, A., Schroeder, R., Caminal, P., Vallverdú, M., Brunel, H., Cygankiewicz, I., et al. (2010). Segmented symbolic dynamics for risk stratification in patients with ischemic heart failure. Cardiovasc. Eng. Technol. 1 (4), 290–298. doi:10.1007/s13239-010-0025-3
Wang, H., and Zheng, H. “A bayesian approach to improving decision making in support vector machine and its application in bioinformatics,” in Proceedings of the 2009 Fifth International Conference on Natural Computation, Tianjian, China, August 2009, 89–93. doi:10.1109/ICNC.2009.626
Keywords: baroreflex activity, cardio-respiratory variability, blood pressure variability, morphology-relative change, ischemic-dilated cardiomyopathy
Citation: Rodriguez J, Schulz S, Voss A, Herrera S, Benito S and Giraldo BF (2023) Baroreflex activity through the analysis of the cardio-respiratory variability influence over blood pressure in cardiomyopathy patients. Front. Physiol. 14:1184293. doi: 10.3389/fphys.2023.1184293
Received: 11 March 2023; Accepted: 01 June 2023;
Published: 10 August 2023.
Edited by:
Stefano Severi, University of Bologna, ItalyReviewed by:
Fabio Badilini, AMPS LLC, United StatesAgnese Sbrollini, Marche Polytechnic University, Italy
Copyright © 2023 Rodriguez, Schulz, Voss, Herrera, Benito and Giraldo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beatriz F. Giraldo, QmVhdHJpei5HaXJhbGRvQHVwYy5lZHU=
†Present address: Steffen Schulz, Division of Oncology and Hematology, Department of Pediatrics, Berlin Institute of Health, Charité – Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin, Berlin, Germany
 Javier Rodriguez
Javier Rodriguez Steffen Schulz
Steffen Schulz Andreas Voss
Andreas Voss Sergio Herrera4
Sergio Herrera4 Beatriz F. Giraldo
Beatriz F. Giraldo