
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Physiol., 27 March 2023
Sec. Membrane Physiology and Membrane Biophysics
Volume 14 - 2023 | https://doi.org/10.3389/fphys.2023.1182455
This article is part of the Research TopicThe Evolving Picture of Ca2+ Leak from Endoplasmic Reticulum in Health and DiseasesView all 9 articles
Editorial on the Research Topic
The evolving picture of Ca2+leak from endoplasmic reticulum in health and diseases
The endoplasmic reticulum (ER) is one of the two main reservoirs for releasable Ca2+ in the cell and usually maintains free Ca2+ concentrations of 100–800 μM, which amounts to at least three orders of magnitude higher than in the cytosol (Berridge et al., 2000; Berridge, 2002) (Figure 1A). Therefore, it is remarkable that the ER membrane is not tight to ions; it has indeed a distinct permeability to ions and even small molecules. When the sarcoplasmic/endoplasmic reticulum Ca2+ ATP-ases (SERCA), which pump Ca2+ into the ER, is blocked, e.g., by thapsigargin, the Ca2+ concentration in the ER decreases, unmasking the Ca2+ leak/leakage or passive Ca2+ efflux from the ER. In the absence of extracellular Ca2+, the SERCA inhibition typically leads to a decrease in ER Ca2+ with the corresponding transient increase of cytosolic Ca2+ (Gamayun et al., 2019). Within several molecular pathways for Ca2+ leakage that co-exist in ER membranes, Sec61 translocons are unparalleled because they support both translocation of proteins into the ER and Ca2+ leakage from the ER, suggesting a dynamic coupling between ER membrane permeability and protein synthesis (Figure 1B). Therefore, it is not surprising that the Sec61-mediated Ca2+ leakage from the ER has been implicated in the etiology of various cancers, neurodegeneration, and infectious diseases (such as Buruli ulcer) as well as inherited diseases, such as immunodeficiency, neutropenia and tubulointerstitial kidney disease (Bolar et al., 2016; Schubert et al., 2018; Van Nieuwenhove et al., 2020; Bhadra et al., 2021; Sicking et al., 2022). Notably, the other ER membrane resident Ca2+ leak channels are, in alphabetical order, Bcl-2 (Pinton et al., 2001; Chami et al., 2004), CALHM1 (Gallego-Sandín et al., 2011), Pannexin 1 (Abeele et al., 2006), Presenillins 1 and 2 (Tu et al., 2006), truncated SERCA variants (Chami et al., 2001; Chami et al., 2008) and transient receptor potential superfamily members TRPC1 (Berbey et al., 2009) and TRPP2 (see below). In contrast to the latter proteins, however, the Sec61 translocons are ubiquitous and highly abundant, depending on secretory capacity of the cell, i.e., the extension of the ER (Pick et al., 2021). In HeLa cells, for example, the concentration of heterotrimeric Sec61 complexes is between 139 and 456 nM (judging from the concentration of the subunit with the lowest and highest cellular concentration, respectively, (Lang et al., 2017), and Sec61 channels support about 60% of the Ca2+ leakage from the ER (Lang et al., 2011; Gamayun et al., 2019).
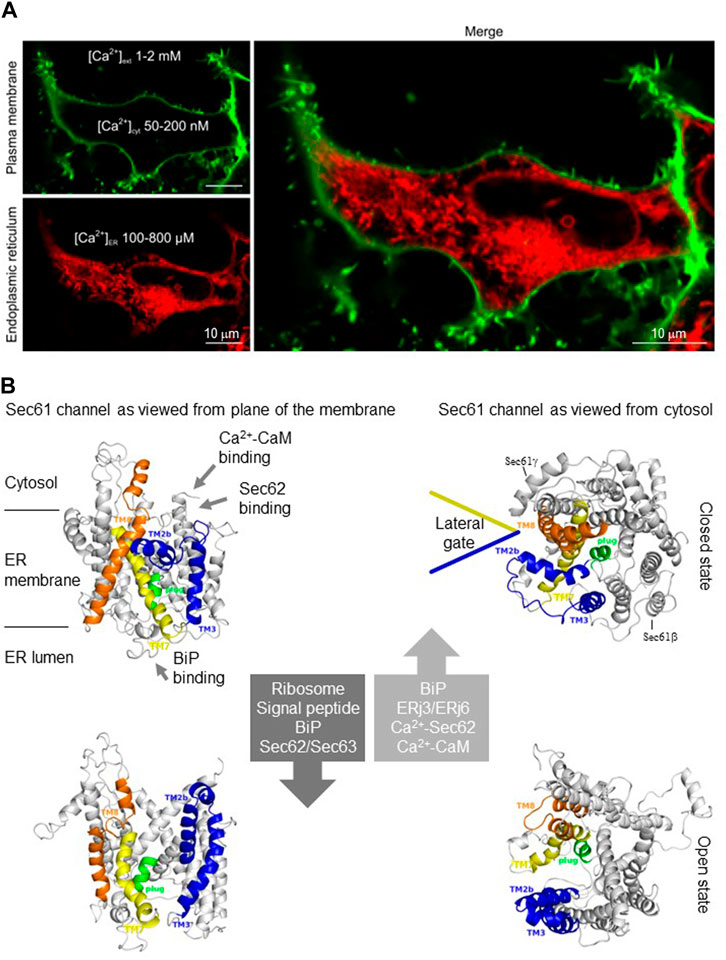
FIGURE 1. I The endoplasmic reticulum (ER) of nucleated human cells has major functions in cellular calcium homeostasis and contains the abundant and ubiquitous Sec61 channel. (A) The ER is shown here in a HEK293 cell after fluorescence microscopy after staining with ER-Tracker™ Red (BODIPY™ TR Glibenclamide), the plasma membrane was stained with CellMask™ Green Plasma Membrane Stain (details are given by Pick et al.). The image was kindly provided by Tillman Pick (Experimental and Clinical Pharmacology and Toxicology, Saarland University). (B) The Sec61 channel is shown in its modeled closed (top) and open (bottom) conformational states, as indicated (adopted from Lang et al., 2017). These two states are proposed to be in a dynamic equilibrium with each other. The fully open state of the Sec61 channel allows the initial entry of precursor polypeptides from the cytosol into the ER lumen and ER membrane, respectively. In addition, it allows the passive efflux of Ca2+ from the ER lumen into the cytosol after termination of the translocation process and, therefore, it can be quantified in live cell Ca2+ imaging in cytosol and ER lumen using ratiometric dyes and fluorescent proteins. Ca2+ efflux may also be possible in the transition state (not shown), which can be detected in the presence of Sec61 channel inhibitors such as Eeyarestatins or Mycolactone and may be identical to the so-called primed state that can be induced by ribosomes in co-translational- and by the Sec62/Sec63 complex in post-translational-transport (Gamayun et al., 2019; Bhadra et al., 2021).
Originally, the Ca2+ leakage from the ER and specifically, the Sec61-mediated Ca2+ leakage from the ER represented a new and unexpected mechanisms of the ER Ca2+ homeostasis. It first came up in the early 2,000 years in seminal papers on human cells (Camello et al., 2002; Lomax et al., 2002; Van Coppenolle et al., 2004; Flourakis et al., 2006; Giunti et al., 2007) and, subsequently, was confirmed in vivo by a global RNAi screen for genes that are involved in store-operated Ca2+ entry (SOCE) in Drosophila (Zhang et al., 2006) as well as by biochemical and biophysical approaches (Wirth et al., 2003; Erdmann et al., 2011; Lang et al., 2011; Schäuble et al., 2012). The latter experimental approaches involved single channel recordings from purified and reconstituted Sec61 complexes and live cell calcium imaging in cytosol and ER lumen of human cells in combination with siRNA treatment or plasmid driven mutant variant expression. Several studies also identified various interaction partners of the Sec61 channel that are involved in tight control of the Ca2+ leak (Figure 1B), i.e., the ER-lumenal chaperone BiP and its cochaperones ERj3 and ERj6 (Schäuble et al., 2012; Schorr et al., 2015) as well as cytosolic calmodulin (CaM) and the ER membrane protein Sec62 (Erdmann et al., 2011; Linxweiler et al., 2013), thereby preventing excessive Ca2+ leakage that may lead to apoptosis (Hara et al., 2013; Feliziani et al., 2020). Furthermore, three inhibitors of the Sec61 channel, Eeyarestatins ES1 and ES24 as well as Mycolactone have been characterized as enhancers of Ca2+ leakage (Gamayun et al., 2019; Bhadra et al., 2021). As further readings on the subject of Sec61 inhibitors we recommend recent reports on the cryo-EM structures of the mammalian Sec61 translocon inhibited by various small molecules (Gérard et al., 2020; Itskanov et al., 2022; Rehan et al., 2022).
In the last 5 years, a picture started to evolve in which the Sec61-mediated Ca2+ leakage from the ER is not only a major player in various pathophysiological settings but also provides a link between energetic requirements of protein translocation into and folding and assembly within the ER under physiological conditions (Klein et al., 2018; Yong et al., 2019; reviewed by Zimmermann and Lang, 2020). Briefly, human SLC35B1 apparently imports ATP into the ER in exchange for ADP and was named AXER (ATP/ADP exchanger in the ER membrane) (Klein et al., 2018; Schwarzbaum et al., 2022). Furthermore, an ER low energy response (termed lowER) was characterized as a central reulatory circuit for maintaining ATP supply to the ER. This regulatory circuit was proposed to involve BiP dissociation from the Sec61 channel under conditions of a low ATP/ADP ratio in the ER lumen, thus allowing Ca2+ leakage from the ER (Klein et al., 2018). Accordingly, Ca2+ binds to CaM in the cytosol and activates AMP-activated protein kinase via Ca2+/CaM dependent kinase 2 and, eventually, 6-phospho-fructo-2-kinase. Activated 6-phospho-fructo-2-kinase stimulates ADP phosphorylation in glycolysis, subsequently allowing ATP import into the ER via AXER (in exchange for ADP), which is futher activated by Ca2+ efflux from the ER. Normalization of the ER ATP/ADP ratio allows BiP to limit the Ca2+ leakage via binding to Sec61 channels and thus inactivates the regulatory circuit. However, the details of this signal transduction pathway are still somewhat controversial (Yong et al., 2019; Zimmermann and Lang, 2020).
Goal of this Research Topic is to present a combination of review articles and state-of-the-art studies that cover aspects of the Ca2+ leak from ER in health and diseases. Considering that Sec61 translocons function as ion channels in the ER membrane, it appeared to be interesting to explore the pore structure and eventually the open-closed kinetics of these unusual ion channels. It is remarkable that the Sec61-mediated Ca2+ leak from the ER has been implicated in the etiology of diseases such as cancer and inherited as well as infectious diseases. A good proportion of the papers in the Research Topic will therefore focus on ER Ca2+ leakage in diseases. Finally, a number of small molecules that inhibit protein translocation have been described and we would like to draw the attention to papers looking for their mode of action with focus on the ER Ca2+ leak (Gamayun et al., 2019; Gérard et al., 2020; Bhadra et al., 2021).
In this Research Topic, renowned international experts in the area of cell biology and human medicine report on their mechanistic and medical insights into various aspects of the Ca2+ leak/leakage or passive Ca2+ efflux from the ER. Schulte and Blum set the stage and provide a comprehensive overview about the Ca2+ homeostasis in human neuronal cells, specifically, the surprisingly dynamic Ca2+ fluxes between the ER, the cytosol and the extracellular space as well as how the ER Ca2+ leak contributes to evolutionary conserved Ca2+ phenomena such as SOCE, ER Ca2+ induced Ca2+ release (CICR) and Ca2+ oscillations. Next, Pick et al. present their quantitative data and kinetics of thapsigargin-induced Ca2+ efflux from the ER and SOCE as its consequence on the Ca2+ dynamics in HEK293 cells. Parys and Van Coppenolle focus our attention on the Sec61 channel as the most abundant and ubiquitous ER Ca2+ leak channel and its various roles in health and disease. This brilliant review also introduces the various interaction partners of the Sec61 channel that are involved in tight control of the leak (including Sec62). Dagnino-Acosta and Guerrero-Hernandez add another control mechanism of the Sec61 channel, i.e., phosphorylation, which at least in smooth muscle cells is mediated by protein kinase C (PKC). Coming back to the role of the Sec61 channel and its modulators in disease, the contribution by Zimmermann et al. on tumor diseases is highly recommended to bridge the gap from bench to bedside. This paper is also an excellent introduction for two original articles by Radosa et al. as well as Körner et al. respectively, which deal with Ca2+ efflux from the ER as a novel target of anti-metastatic and anti-proliferative therapy in head and neck cancer and the oncogene SEC62 as a prognostic marker in patients with ovarian malignancies, respectively. Staying with human medicine, the Research Topic is finished off by Liu et al. who discuss the current views on the biophysical and physiological properties of the ER membrane protein PKD2, which is also termed polycystin-2 or TRPP2, and on how PKD2 contributes to ER Ca2+ homeostasis in cell physiology and to autosomal polycystic kidney disease in pathophysiology. Notably, PKD2 represents one of the additional channels for Ca2+ efflux from the ER, which be highlighted to exist in addition to the Sec61 channel in the Introduction to this editorial.
AC and RZ drafted, edited, and approved the final version of the manuscript.
The authors acknowledge financial support from the German Research Foundation (DFG) via the Collaborative Research Centers SFB 530 and SFB 894 in the course of the last two decades.
The guest editors are grateful to the authors of the Research Topic for their insightful, comprehensive and timely contributions as well as to Specialty Chief Editor Christoph Fahlke for his continuous support of this Research Topic. Furthermore, they acknowledge the continuous intellectual support by their colleagues and spokespersons of SFB 530 and SFB 894, Drs. Veit Flockerzi and Jens Rettig.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abeele, F. V., Bidaux, G., Gordienko, D., Beck, B., Panchin, Y. V., Barnova, A. V., et al. (2006). Functional implications of calcium permeability of the channel formed by pannexin 1. J. Cell Biol. 174, 535–546. doi:10.1083/jcb.200601115
Berbey, C., Weiss, N., Legrand, C., and Allard, B. (2009). Transient receptor potential canonical type 1 (TRPC1) operates as a sarcoplasmic reticulum calcium leak channel in skeletal muscle. J. Biol. Chem. 284, 36387–36394. doi:10.1074/jbc.M109.073221
Berridge, M. J., Lipp, P., and Bootman, M. D. (2000). The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell. Biol. 1, 11–21. doi:10.1038/35036035
Berridge, M. J. (2002). The endoplasmic reticulum: A multifunctional signaling organelle. Cell Calcium 32, 235–249. doi:10.1016/s0143416002001823
Bhadra, P., Dos Santos, S., Gamayun, I., Pick, T., Neumann, C., Ogbechi, J., et al. (2021). Mycolactone enhances the Ca2+ leak from endoplasmic reticulum by trapping Sec61 translocons in a Ca2+ permeable state. Biochem. J. 478, 4005–4024. doi:10.1042/BCJ20210345
Bolar, N. A., Golzio, C., Živná, M., Hayot, G., Van Hemelrijk, C., Schepers, D., et al. (2016). Heterozygous loss-of-function SEC61A1 mutations cause autosomal-dominant tubulo-interstitial and glomerulocystic kidney disease with anemia. Am. J. Hum. Genet. 299, 174–187. doi:10.1016/ajhg.2016.05.28
Camello, C., Lomax, R., Petersen, O. H., and Tepikin, A. V. (2002). Calcium leak from intracellular stores-the enigma of calcium signalling. Cell Calcium 32, 355–361. doi:10.1016/s0143416002001926
Chami, M., Gozuacik, D., Lagorce, D., Brini, M., Falson, P., Peaucellier, G., et al. (2001). SERCA1 truncated proteins unable to pump calcium reduce the endoplasmic reticulum calcium concentration and induce apoptosis. J. Cell Biol. 153, 1301–1314. doi:10.1083/jcb.153.6.1301
Chami, M., Oules, B., Szabadkai, G., Tacine, R., Rizzuto, R., and Paterlini-Bréchot, P. (2008). Role of SERCA1 truncated isoform in the proapoptotic calcium transfer from ER to mitochondria during ER stress. Mol. Cell. 32, 641–651. doi:10.1016/j.molcel.2008.11.014
Chami, M., Prandini, A., Campanella, M., Pinton, P., Szabadkai, G., Reed, J. C., et al. (2004). Bcl-2 and Bax exert opposing effects on Ca2+ signaling, which do not depend on their putative pore-forming region. J. Biol. Chem. 279, 54581–54589. doi:10.1074/jbc.M409663200
Erdmann, F., Schäuble, N., Lang, S., Jung, M., Honigmann, A., Ahmad, M., et al. (2011). Interaction of calmodulin with Sec61a limits Ca2+ leakage from the endoplasmic reticulum. EMBO J. 30, 17–31. doi:10.1038/emboj.2010.284
Feliziani, C., Quasollo, G., Holstein, D., Fernandez, M., Paton, J. C., Paton, A. W., et al. (2020). Endoplasmic reticulum stress induces Ca2+ signaling system initiated by Ca2+ microdomains. bioRxiv. 2020.07.10.328849. doi:10.1101/2020.10.07.328849
Flourakis, M., Van Coppenolle, F., Lehen'kyi, V., Beck, B., Skryma, R., and Prevarskaya, N. (2006). Passive calcium leak via translocon is a first step for iPLA2-pathway regulated store operated channels activation. FASEB J. 20, 1215–1217. doi:10.1096/fj.05-5254fje
Gallego-Sandín, S., Alonso, M. T., and García-Sancho, J. (2011). Calcium homoeostasis modulator 1 (CALHM1) reduces the calcium content of the endoplasmic reticulum (ER) and triggers ER stress. Biochem. J. 437, 469–475. doi:10.1042/BJ20110479
Gamayun, I., 0´Keefe, S., Pick, T., Klein, M.-C., Nguyen, D., McKibbin, C., et al. (2019). Eeyarestatin compounds selectively enhance Sec61-mediated Ca2+ leakage from the endoplasmic reticulum. Cell Chem. Biol. 26, 571–583. e6. doi:10.1016/j.chembiol.2019.01.010
Gérard, S. F., Hall, B. S., Zaki, A. M., Corfield, K. A., Mayerhofer, P. U., Costa, C., et al. (2020). Structure of the inhibited state of the Sec translocon. Mol. Cell 79, 406–415. e7. doi:10.1016/j.molcel.2020.06.013
Giunti, R., Gamberucci, A., Fulceri, R., Banhegyi, G., and Benedetti, A. (2007). Both translocon and a cation channel are involved in the passive Ca2+ leak from the endoplasmic reticulum: A mechanistic study on rat liver microsomes. Arch. Biochem. Biophys. 462, 115–121. doi:10.1016/j.abb.2007.03.039
Hara, T., Mahadevan, J., Kanekura, K., Hara, M., Lu, S., and Urano, F. (2013). Calcium efflux from the endoplasmic reticulum leads to ß cell death. Endo 1519. doi:10.1210/en.2013-1519
Itskanov, S., Wang, L., Junne, T., Sheriff, R., Xiao, L., Blanchard, N., et al. (2022). A common mechanism of Sec61 translocon inhibition by small molecules. bioRxiv. 2022.08.11.503542. doi:10.1101/2022.08.11.503542
Klein, M.-C., Zimmermann, K., Schorr, S., Landini, M., Klemens, P., Altensell, J., et al. (2018). AXER is an ATP/ADP exchanger in the membrane of the endoplasmic reticulum. Nat. Commun. 9, 3489. doi:10.1038/s41467-018-06003-9
Lang, S., Erdmann, F., Jung, M., Wagner, R., Cavalié, A., and Zimmermann, R. (2011). Sec61 complexes form ubiquitous ER Ca2+ leak channels. Channels 5, 228–235. doi:10.4161/chan.5.3.15314
Lang, S., Pfeffer, S., Lee, P.-H., Cavalié, A., Helms, V., Förster, F., et al. (2017). An update on Sec61 channel functions, mechanisms, and related diseases. Front. Physiol. 8, 887. doi:10.3389/fphys.2017.00887
Linxweiler, M., Schorr, S., Jung, M., Schäuble, N., Linxweiler, J., Langer, F., et al. (2013). Targeting cell migration and the endoplasmic reticulum stress response with calmodulin antagonists: A clinically tested small molecule phenocopy of SEC62 gene silencing in human tumor cells. BMC Cancer 13, 574. doi:10.1186/1471-2407-13-574
Lomax, R. B., Camello, C., Van Coppenolle, F., Petersen, O. H., and Tepikin, A. V. (2002). Basal and physiological Ca2+ leak from the endoplasmic reticulum of pancreatic acinar cells. Second messenger-activated channels and translocons. J. Biol. Chem. 277, 26479–26485. doi:10.1074/jbc.M201845200
Pick, T., Beck, A., Gamayun, I., Schwarz, Y., Schirra, C., Jung, M., et al. (2021). Remodelling of Ca2+ homeostasis is linked to enlarged endoplasmic reticulum in secretory cells. Cell Calcium 99, 102473. doi:10.1016/j.ceca.2021.102473
Pinton, P., Ferrari, D., Rapizzi, E., Di Virgilio, F., Pozzan, T., and Rizzuto, R. (2001). The Ca2+ concentration of the endoplasmic reticulum is a key determinant of the ceramide-induced apoptosis: Significance for the molecular mechanism of bcl-2 action. EMBO J. 20, 2690–2701. doi:10.1093/embo.j/20.11.2690
Rehan, S., Tranter, D., and Sharp, P. P. (2022). Signal peptide mimicry primes Sec61 for client-selective inhibition. bioRxiv. 2022.07.03.498529. doi:10.1101/2022.07.03.498529
Schäuble, N., Lang, S., Jung, M., Cappel, S., Schorr, S., Ulucan, Ö., et al. (2012). BiP-mediated closing of the Sec61 channel limits Ca2+ leakage from the ER. EMBO J. 31, 3282–3296. doi:10.1038/emboj.2012.189
Schorr, S., Klein, M.-C., Gamayun, I., Melnyk, A., Jung, M., Schäuble, N., et al. (2015). Co-chaperone specificity in gating of the polypeptide conducting channel in the membrane of the human endoplasmic reticulum. J. Biol. Chem. 290, 18621–18635. doi:10.1074/jbc.M115.636639
Schubert, D., Klein, M.-C., Haßdenteufel, S., Caballero-Oteyza, A., Yang, L., Proietti, M., et al. (2018). Plasma cell deficiency in human subjects with heterozygous mutations in Sec61 translocon alpha 1 (SEC61A1). J. Allergy Clin. Immunol. 141, 1427–1438. doi:10.1016/j.jaci.2017.06.042
Schwarzbaum, P. J., Schachter, J., and Bredeston, L. M. (2022). The broad range di- and tri-nucleotide exchanger SLC35B1 displays asymmetrical affinities for ATP transport across the ER membrane. J. Biol. Chem. 298, 101537. doi:10.1016/j.jbc.2021.101537
Sicking, M., Živná, M., Bhadra, P., Barešová, V., Tirincsi, A., Hadzibeganovic, D., et al. (2022). Phenylbutyrate rescues the transport defect of the Sec61α mutations V67G and T185A for renin. Live Sci. Alliance 5, e202101150. doi:10.26508/lsa.202101150
Tu, H., Nelson, O., Bezprozvanny, A., Wang, Z., Lee, S.-F., Hao, Y.-H., et al. (2006). Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer´s disease-linked mutations. Cell 126, 981–993. doi:10.1016/j.cell.2006.06.059
Van Coppenolle, F., Van den Abeele, F., Slomianny, C., Flourakis, M., Hesketh, J., Dewailly, E., et al. (2004). "The stress of dying": The role of heat shock proteins in the regulation of apoptosis. J. Cell Sci. 117, 2641–2651. doi:10.1242/jcs.01284
Van Nieuwenhove, E., Barber, J., Smeets, E., Neumann, J., Willemsen, M., Pasciuto, E., et al. (2020). Defective Sec61α1 underlies a novel cause of autosomal dominant severe congenital neutropenia. J. Allergy Clin. Immunol. 146, 1180–1193. doi:10.1016/j.jaci.2020.03.034
Wirth, A., Jung, M., Bies, C., Frien, M., Tyedmers, J., Zimmermann, R., et al. (2003). The Sec61p complex is a dynamic precursor activated channel. Mol. Cell 12, 261–268. doi:10.1016/s1097-2765(03)00283-1
Yong, J., Bischof, H., Burgstaller, S., Siirin, M., Murphy, A., Malli, R., et al. (2019). Mitochondria supply ATP to the ER through a mechanism antagonized by cytosolic Ca2+. eLife 8, e49682. doi:10.7554/eLife.49682
Zhang, S. L., Yeromin, A. V., Zhang, X. H.-F., Yu, Y., Safrina, O., Penna, A., et al. (2006). Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc. Natl. Acad. Sci. U. S. A. 103, 9357–9362. doi:10.1073/pnas.0603161103
Keywords: calcium homeostasis, calcium leak, endoplasmic reticulum, Sec61 channel, translocon, tumor driver gene Sec62
Citation: Cavalié A and Zimmermann R (2023) Editorial: The evolving picture of Ca2+ leak from endoplasmic reticulum in health and diseases. Front. Physiol. 14:1182455. doi: 10.3389/fphys.2023.1182455
Received: 08 March 2023; Accepted: 20 March 2023;
Published: 27 March 2023.
Edited and reviewed by:
Christoph Fahlke, Helmholtz Association of German Research Centres (HZ), GermanyCopyright © 2023 Cavalié and Zimmermann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adolfo Cavalié, YWRvbGZvLmNhdmFsaWVAdWtzLmV1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.