- 1Department of Cardiology, Bispebjerg Hospital, University of Copenhagen, Copenhagen, Denmark
- 2Electrophysiology and Heart Modeling Institute, University of Bordeaux, Bordeaux, France
- 3Amsterdam UMC, location Academic Medical Centre, Department of Experimental Cardiology, University of Amsterdam, Amsterdam, Netherlands
- 4Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands
- 5Department of Cardiology, Catharina Hospital, Eindhoven, Netherlands
Atrial fibrillation (AF) often requires invasive treatment by ablation to decrease symptom burden. The pulmonary veins (PV) are thought to trigger paroxysms of AF, and ablative PV isolation (PVI) is a cornerstone in AF treatment. However, incomplete PVI, where electrical conduction between the PV and left atrium (LA) is maintained, is curative of AF in a subset of patients. This implies that an antiarrhythmic effect other than electrical isolation between the PV and LA plays a role in AF prevention in these patients. We reason that the PV myocardium constitutes an arrhythmogenic substrate conducive to reentry in the patients with curative incomplete PVI. This PV substrate is amenable to ablation, even when conduction between the LA and PV persists. We propose that PV ablation strategies are differentiated to fit the arrhythmogenic mechanisms in the individual patient. PV substrate modification in patients with PV reentry may constitute a new therapeutic approach that is potentially simpler and more effective, in this subgroup of patients.
Introduction
In 1998, Haïssaguerre et al. (1998) attributed atrial fibrillation (AF) paroxysms to ectopy from the pulmonary veins (PV). Since, ablative PV isolation (PVI) has been a cornerstone in the invasive antiarrhythmic treatment of patients with paroxysmal AF (episodes of AF shorter than 1 week) in whom pharmacological treatment is ineffective (Calkins et al., 2018). The PVI lesions are created to prevent conduction of ectopic activations from the PV to the left atrium (LA). PV ablation strategies have higher success rate of AF prevention than ablation elsewhere in the atria (Sau et al., 2019). However, the long-term success rate of a single PVI procedure is about 60% (Kis et al., 2017).
Although it has been attempted to completely isolate each PV from the atrium, long-term clinically successful ablation also occurs in paroxysmal AF patients with incomplete isolation or electrical reconnection between the PV and LA (Cappato et al., 2003; Verma et al., 2005; Pratola et al., 2008; Jiang et al., 2014; Kuck et al., 2016). This implies that therapeutic mechanisms other than electrical isolation between the PV and LA play a role in a subset of patients.
In this perspective paper, we elaborate on the idea that the antiarrhythmic effect of incomplete PVI is caused by modification of a local arrhythmogenic PV substrate, rather than sole isolation of ectopic PV foci, at least in some patients. This is in agreement with the current guidelines for AF treatment that point out an improved personalized therapy can be achieved by assessing the pathophysiological processes in the individual patient (Hindricks et al., 2021). We, therefore, do not challenge the therapeutic use of ablative PVI in AF patient but rather argue for a patient-specific mechanism-driven PV ablation therapy. We intend to discuss the basic arrhythmogenic mechanisms likely to occur in the PV (focal and re-entry arrhythmogenesis) and not potential modulating factors, which have been substantially reviewed elsewhere (Nattel, 2017; Linz et al., 2019; Gottlieb et al., 2021b; Gottlieb et al., 2022).
As of now, we lack the precise knowledge how to select the patients who respond to PVI, to PV ablation, or not at all to AF ablation. Matching the ablation strategy to the paroxysmal AF mechanism in a personalized patient-specific manner may improve patient care (Figure 1).
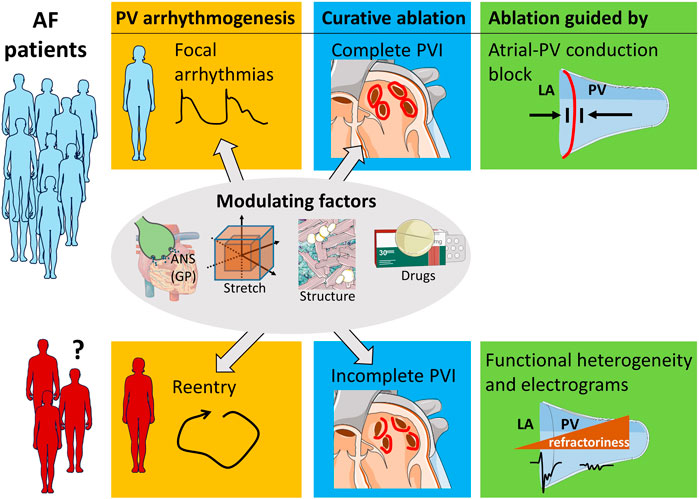
FIGURE 1. PV ablation strategy dependent on arrhythmia mechanism. Patients with focal arrhythmogenesis in the PV benefit from complete PVI lesions that inhibit atrial-PV conduction. A subset of patients restores sinus rhythm by incomplete PVI. The clinical characteristics of these patients are unknown, and future research can help to identify them. The arrhythmogenic mechanism of their AF likely is reentry involving the PV myocardium. Ablation of this PV substrate can be guided by the morphology of the localized electrograms and by functional properties, such as refractoriness. Modulating factors, such as localized autonomic nervous system (ANS) involving the ganglionated plexuses (GP), atrial stretch, structural remodeling, and pharmacology influence both focal arrhythmogenesis and re-entry.
PV reconnection and ablation success
Reestablishment of the atrial-PV conduction (reconnection) in one or more PV occurs in the majority of patients after PVI (Jefairi et al., 2019; Daimee et al., 2021). Because recurrence of AF, either early during the 3-month blinding period following the PVI procedure or late (> 3 months), is thought to be caused by re-establishment of electrical atrial-PV reconnection, a new PVI procedure is considered justified in patients with AF recurrence (Haissaguerre et al., 2000; Callans et al., 2004; Gottlieb et al., 2021b). In this case, the reconnected PV are re-isolated. Electrical reconnection also is observed after a second PVI that was performed in reconnected PV following the initial PVI (Cappato et al., 2003). Cappato et al. (2003) even reported that at least 1 PV was reconnected in 3 patients undergoing a fourth PVI ablation procedure. Thus, electrical reconnection after a procedural complete PVI ablation is common.
Several observational studies confirm that stable restoration of sinus rhythm also occurs in patients with electrical atrial-PV reconnection after PVI (Table 1) (Cappato et al., 2003; Ouyang et al., 2005; Verma et al., 2005; Pratola et al., 2008; Jiang et al., 2014; Kuck et al., 2016). Overall, electrical atrial-PV reconnection was present in 110 of 159 patients with an initial, clinically successful ablation (Cappato et al., 2003; Verma et al., 2005; Pratola et al., 2008; Jiang et al., 2014; Kuck et al., 2016). Reconnection had even occurred in all 4 PV in 31% of the patients without AF in one study (Jiang et al., 2014). Moreover, the number of reconnected PV was similar in patients with and without AF recurrence (Pratola et al., 2008; Jiang et al., 2014). Indeed, a meta-analysis including 7 studies conclude that the number of reconnected PV does not correlate with the risk of AF recurrence after PVI (Nery et al., 2016). These observations indicate that the arrhythmogenic mechanisms of AF in a subset of patients are amenable to an ablation strategy that does not prevent focal PV arrhythmias from entering the LA.
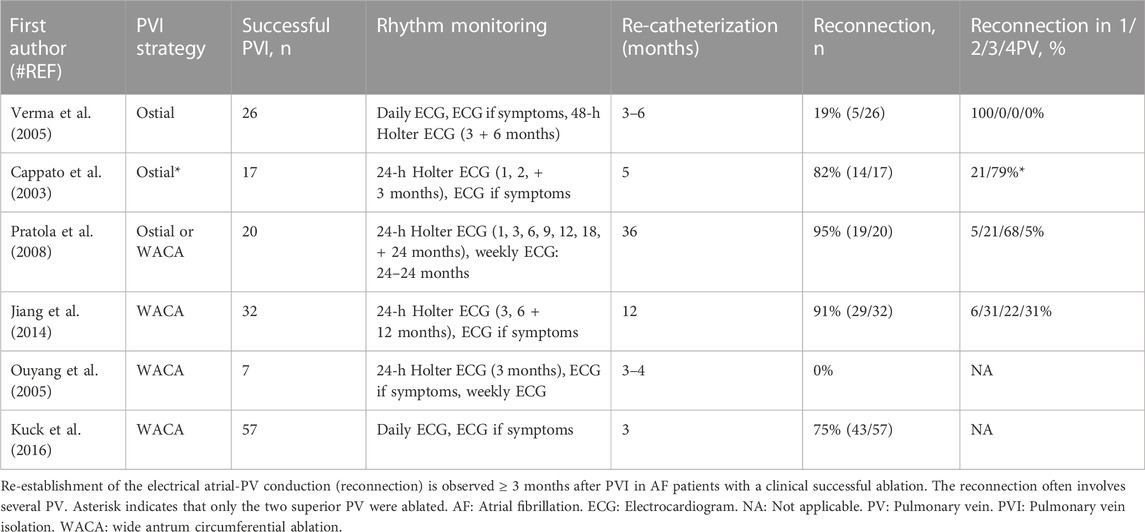
TABLE 1. Incidence of electrical atrial-PV reconnection after initial PVI and its association with ablation success.
It can be argued that the clinical outcome of incomplete PVI depends on the arrhythmic activity of the reconnected PV. Thus, reconnection of a PV with lesser arrhythmic activity would play a minor role in AF recurrence. Conversely, development of arrhythmic activity in a reconnected PV could cause ablation failure later on. However, a lower functional arrhythmogenicity in reconnected PV does not seem to be the reason for clinically successful incomplete PVI because randomized trials testing incomplete PVI in all PV have shown similar success rates to complete PVI ablation.
Randomized trials: incomplete vs. complete PVI
The difference in ablation outcome of complete PVI and intentionally incomplete PVI has been tested in randomized trials (Zhao et al., 2013; Gula et al., 2016; Kuck et al., 2016). Zhao et al. placed 3 radial ablation lines in each PV and observed an increased success rate compared with wide antrum catheter ablation (WACA) PVI (74% vs. 50%, respectively, p = 0.025) (Zhao et al., 2013). A success rate of 93% was achieved 6 months after ablation therapy using ablation lines between the PV and the mitral annulus (Kottkamp et al., 2002), whereas similar success rates were achieved between AF patients randomized to either complete WACA or ablation of segments around the PV until bidirectional conduction block (the remaining segments were left non-ablated) (Gula et al., 2016).
Kuck et al. (2016) created incomplete WACA by cessation of radiofrequency energy at the moment of loss of the PV potential at one point in the circular isolation lesion. Clinically successful ablation occurred in both the complete and incomplete PVI groups. Re-catheterization 3 months post-ablation was performed in all patients and revealed that, in the presence of atrial-PV reconnection, the procedural complete PVI was superior in AF prevention compared with procedural incomplete PVI (Kuck et al., 2016). This difference in success rates may also be explained by administration of more ablative energy in the complete than incomplete PVI group (because both groups eventually had PV reconnection) and thereby by extensive modification of the PV.
Thus, complete abolition of atrial-PV conduction is not necessary for restoration of sinus rhythm in all patients. We surmise that this is potentially explained by modification of an arrhythmogenic substrate.
PV arrhythmogenesis
The arrhythmogenic mechanisms operative in the PV region encompass automaticity, triggered activity, and reentry. The first two mechanisms emanate as “focal” arrhythmias and are sensitive to complete PVI.
Focal arrhythmogenesis
Solid and direct evidence of automaticity in native PV is scarce but cells staining positive for the ion channel carrying pacemaker current (HCN4) are observed in the PV myocardium of 4 of 5 AF patients in a post mortem study and not in 3 patients in sinus rhythm and also not in PV from healthy rats (Yamamoto et al., 2006; Nguyen et al., 2009). The embryonic development of the PV myocardium depends on the transcription factor Pitx2c that suppress automaticity (Rivaud et al., 2021). However, no mutations exist in the PITX2 gene of 96 idiopathic AF patients (Boldt et al., 2010). Instead, a lower level of Pitx2c mRNA in atrial myocytes is associated with recurrence of AF after AF ablation (Reyat et al., 2020). This suggests that automaticity in the PV, if present, may arise from acquired changes in expression profile rather than inborn genetic variations.
Observations of afterdepolarizations and thereby triggered activity in the PV myocardium are limited to animal studies and are scarce in absence of modulating factors, such as autonomic nervous modulation. Chen et al. reported early afterdepolarizations in isolated PV from 7 of 17 dogs, whereas Wang et al. (2003) did not observe early afterdepolarizations in the PV from 50 dogs (Chen et al., 2000).
Re-entry
For reentrant mechanisms an arrhythmogenic substrate is required. The complex structure in the PV facilitates conduction heterogeneity and together with gradients of refractoriness provide the basis for unidirectional block (Saito et al., 2000; Ho et al., 2001; Hocini et al., 2002; Hamabe et al., 2003; Hassink et al., 2003). Combined, this sets the stage for reentry (Moe et al., 1964; Spach and Dolber, 1986). Although intramural reentry is difficult to document (also experimentally), the PV myocardial sleeve is likely thick enough to sustain an intramural re-entrant circuit (Gottlieb et al., 2021a). Modification of the substrate for reentry does not require complete PVI. A transection of the reentry pathway at any point of the circle suffices (Mines, 1914).
Arrhythmia mechanism and ablation strategy
The observation that electrical dissociation between the PV and the LA is not necessary for clinically successful AF ablation brings forward the possibility that focal arrhythmias from the PV myocardium are not always responsible for AF arrhythmogenesis and that rapid activations in the PV do not mean that the arrhythmia also is triggered within in the PV. Often, provocative maneuvers, including atrial pacing, have been used to elicit arrhythmias in the PV (Haissaguerre et al., 1998; Haïssaguerre et al., 2000; Haissaguerre et al., 2000). Such premature stimuli can trigger reentrant activations in an arrhythmogenic substrate (Moe et al., 1964). Indeed, premature atrial complexes are common in AF patients and may arise in both the right and left atrium (Schmitt et al., 2002; Cho et al., 2020). Reentry can be the origin of premature beats as well (de Bakker et al., 2002).
Spontaneous focal arrhythmias in the PV likely are the cause of paroxysmal AF in many patients. In these patients, complete long-lasting PVI should be the therapeutic goal. In case of AF recurrence in these patient, re-isolation of reconnected PV should be attempted. On the hand, reentry involving the PV myocardium can also underlie the mechanism of paroxysmal AF in some patients. Patients with PV reentry could be treated by incomplete PVI that modulates the arrhythmogenic substrate. Consequently, a shift in emphasis from isolation of focal PV arrhythmias to ablative modification of an arrhythmogenic PV substrate may be warranted in these selected patients. Tools to single out the AF mechanism in the individual patient need to be developed. Local fractionated potentials recorded from the atrial-PV interface may play a role (Figure 1) (Gottlieb et al., 2021a).
Hypothesis and perspective
We hypothesize that various arrhythmogenic mechanisms (focal, reentrant) are operative in the PV of patients with paroxysmal AF and ablation strategies directed at the dominant patient specific arrhythmia mechanism can increase ablation success rates.
Identification of the subset of patients with PV arrhythmogenesis by reentry is required (Figure 1). Demographic parameters, such as age and biological sex may play a role in the selection process. Sex-related differences exist in calcium handling, action potential duration, and extracellular matrix structure (Odening et al., 2019). Moreover, the modulating arrhythmogenic factors (body position, obstructive sleep apnea, alcohol dependence, left atrial stretch) can be evaluated before the ablation procedure by history taking, heart rate variability, PV morphology, and mechanics through imaging.
During the ablative procedure, functional properties of the PV myocardium, such as large gradients in refractoriness and conduction heterogeneity may be assessed by an electrophysiological study immediately before delivery of ablative energy.
We showed that the structural composites of the PV myocardium are reflected in the local unipolar electrograms: a large steep deflection and small fractionation corresponded to the larger myocardial mass interspersed with collagen and fat in the proximal PV (Gottlieb et al., 2021a). In addition, arrhythmias inducibility was only possible following pacing in the proximal PV and not in the distal PV, supporting a reentrant mechanism. Hocini and colleagues have similarly shown that electrogram fractionation in the PV myocardium is associated with conduction delay (Hocini et al., 2002). Therefore, ablative modification of the PV substrate prone to reentry guided by the electrogram morphology and repolarization differences may constitute a new therapeutic strategy meriting further research.
Because a critical myocardial mass in combination with the anisotropic structure is needed to accommodate a reentrant activation wave, a critical limit of the extent of ablated PV substrate as antiarrhythmic treatment exists (Garrey, 1914; Allessie et al., 1984). Accordingly, the procedural endpoints of such PV substrate modification by ablation differ from the complete PVI strategy, possibly by including a reduction in electrical activity in the atrial-PV junction and within the PV, instead of conduction block between the LA and PV. Disappearance of local electrogram characteristics may also be a novel end-point. This remains to be tested in AF patients.
Thus, differentiation of the ablation strategy involving the PV based on underlying arrhythmia mechanisms may prove to be beneficial in terms of clinical success rates. Patients with focal PV arrhythmias should have complete PVI without gaps in the encircling lesions, whereas patients with PV reentry may benefit more from ablation targeting reduction of the localized substrate maintaining the reentrant activations than from a conventional PVI.
In conclusion, PV reentry likely underlies arrhythmogenesis in some paroxysmal AF patients, who would benefit more from a targeted PV substrate modification than PVI. A future challenge is to match ablation strategy to arrhythmia mechanism in a personalized patient specific manner, but tools to do this are presently lacking.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LG wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by the Leducq foundation Rhythm (Rhythm [16CVD02]) to RC, a Medtronic unrestricted research grant to LD, and a Catharina Hospital Eindhoven research grant to LD.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allessie, M. A., Lammers, W. J., Bonke, I. M., and Hollen, J. (1984). Intra-atrial reentry as a mechanism for atrial flutter induced by acetylcholine and rapid pacing in the dog. Circulation 70 (1), 123–135. doi:10.1161/01.cir.70.1.123
Boldt, L. H., Posch, M. G., Perrot, A., Polotzki, M., Rolf, S., Parwani, A. S., et al. (2010). Mutational analysis of the PITX2 and NKX2-5 genes in patients with idiopathic atrial fibrillation. Int. J. Cardiol. 145 (2), 316–317. doi:10.1016/j.ijcard.2009.11.023
Calkins, H., Hindricks, G., Cappato, R., Kim, Y. H., Saad, E. B., Aguinaga, L., et al. (2018). 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 20 (1), e1–e160. doi:10.1093/europace/eux274
Callans, D. J., Gerstenfeld, E. P., Dixit, S., Zado, E., Vanderhoff, M., Ren, J. F., et al. (2004). Efficacy of repeat pulmonary vein isolation procedures in patients with recurrent atrial fibrillation. J. Cardiovasc Electrophysiol. 15 (9), 1050–1055. doi:10.1046/j.1540-8167.2004.04052.x
Cappato, R., Negroni, S., Pecora, D., Bentivegna, S., Lupo, P. P., Carolei, A., et al. (2003). Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation 108 (13), 1599–1604. doi:10.1161/01.Cir.0000091081.19465.F1
Chen, Y. J., Chen, S. A., Chang, M. S., and Lin, C. I. (2000). Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog: Implication for the Genesis of atrial fibrillation. Cardiovasc Res. 48 (2), 265–273. doi:10.1016/s0008-6363(00)00179-6
Cho, S., Kim, J., Kim, J. B., Park, J., Park, J. K., Kang, K. W., et al. (2020). The difference of burden of ectopic beats in different types of atrial fibrillation and the effect of atrial fibrillation type on stroke risk in a prospective cohort of patients with atrial fibrillation (CODE-AF registry). Sci. Rep. 10 (1), 6319. doi:10.1038/s41598-020-63370-4
Daimee, U. A., Akhtar, T., Boyle, T. A., Jager, L., Arbab-Zadeh, A., Marine, J. E., et al. (2021). Repeat catheter ablation for recurrent atrial fibrillation: Electrophysiologic findings and clinical outcomes. J. Cardiovasc Electrophysiol. 32, 628–638. doi:10.1111/jce.14867
de Bakker, J. M., Ho, S. Y., and Hocini, M. (2002). Basic and clinical electrophysiology of pulmonary vein ectopy. Cardiovasc Res. 54 (2), 287–294. doi:10.1016/s0008-6363(01)00532-6
Garrey, W. E. (1914). The nature of fibrillary contraction of the heart.—its relation to tissue mass and form. Am. J. Physiology-Legacy Content 33 (3), 397–414. doi:10.1152/ajplegacy.1914.33.3.397
Gottlieb, L. A., Belterman, C., van Amersfoorth, S., Loyer, V., Constantin, M., Hocini, M., et al. (2021a). Profibrillatory structural and functional properties of the atrial-pulmonary junction in the absence of remodeling. Front. Physiol. 12, 748203. doi:10.3389/fphys.2021.748203
Gottlieb, L. A., Coronel, R., and Dekker, L. R. C. (2022). Reduction in atrial and pulmonary vein stretch as a therapeutic target for prevention of atrial fibrillation. Heart rhythm. 20, 291–298. doi:10.1016/j.hrthm.2022.10.009
Gottlieb, L. A., Dekker, L. R. C., and Coronel, R. (2021b). The blinding period following ablation therapy for atrial fibrillation: Proarrhythmic and antiarrhythmic pathophysiological mechanisms. JACC Clin. Electrophysiol. 7 (3), 416–430. doi:10.1016/j.jacep.2021.01.011
Gula, L. J., Leong-Sit, P., Manlucu, J., Hillock, L., Yee, R., Tang, A. S., et al. (2016). Pulmonary vein isolation with incomplete antral ablation lines: Is more ablation necessary? Results of a randomized trial. J. Cardiovasc Electrophysiol. 27 (3), 298–302. doi:10.1111/jce.12876
Haïssaguerre, M., Jaïs, P., Shah, D. C., Garrigue, S., Takahashi, A., Lavergne, T., et al. (2000). Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation 101 (12), 1409–1417. doi:10.1161/01.cir.101.12.1409
Haissaguerre, M., Jais, P., Shah, D. C., Takahashi, A., Hocini, M., Quiniou, G., et al. (1998). Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 339 (10), 659–666. doi:10.1056/nejm199809033391003
Haissaguerre, M., Shah, D. C., Jais, P., Hocini, M., Yamane, T., Deisenhofer, I., et al. (2000). Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation 102 (20), 2463–2465. doi:10.1161/01.cir.102.20.2463
Hamabe, A., Okuyama, Y., Miyauchi, Y., Zhou, S., Pak, H. N., Karagueuzian, H. S., et al. (2003). Correlation between anatomy and electrical activation in canine pulmonary veins. Circulation 107 (11), 1550–1555. doi:10.1161/01.cir.0000056765.97013.5e
Hassink, R. J., Aretz, H. T., Ruskin, J., and Keane, D. (2003). Morphology of atrial myocardium in human pulmonary veins: A postmortem analysis in patients with and without atrial fibrillation. J. Am. Coll. Cardiol. 42 (6), 1108–1114. doi:10.1016/s0735-1097(03)00918-5
Hindricks, G., Potpara, T., Dagres, N., Arbelo, E., Bax, J. J., Blomström-Lundqvist, C., et al. (2021). 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 42 (5), 373–498. doi:10.1093/eurheartj/ehaa612
Ho, S. Y., Cabrera, J. A., Tran, V. H., Farre, J., Anderson, R. H., and Sanchez-Quintana, D. (2001). Architecture of the pulmonary veins: Relevance to radiofrequency ablation. Heart 86 (3), 265–270. doi:10.1136/heart.86.3.265
Hocini, M., Ho, S. Y., Kawara, T., Linnenbank, A. C., Potse, M., Shah, D., et al. (2002). Electrical conduction in canine pulmonary veins: Electrophysiological and anatomic correlation. Circulation 105 (20), 2442–2448. doi:10.1161/01.cir.0000016062.80020.11
Jefairi, N. A., Camaioni, C., Sridi, S., Cheniti, G., Takigawa, M., Nivet, H., et al. (2019). Relationship between atrial scar on cardiac magnetic resonance and pulmonary vein reconnection after catheter ablation for paroxysmal atrial fibrillation. J. Cardiovasc Electrophysiol. 30 (5), 727–740. doi:10.1111/jce.13908
Jiang, R. H., Po, S. S., Tung, R., Liu, Q., Sheng, X., Zhang, Z. W., et al. (2014). Incidence of pulmonary vein conduction recovery in patients without clinical recurrence after ablation of paroxysmal atrial fibrillation: Mechanistic implications. Heart rhythm. 11 (6), 969–976. doi:10.1016/j.hrthm.2014.03.015
Kis, Z., Muka, T., Franco, O. H., Bramer, W. M., De Vries, L. J., Kardos, A., et al. (2017). The short and long-term efficacy of pulmonary vein isolation as a sole treatment strategy for paroxysmal atrial fibrillation: A systematic review and meta-analysis. Curr. Cardiol. Rev. 13 (3), 199–208. doi:10.2174/1573403x13666170117125124
Kottkamp, H., Hindricks, G., Autschbach, R., Krauss, B., Strasser, B., Schirdewahn, P., et al. (2002). Specific linear left atrial lesions in atrial fibrillation: Intraoperative radiofrequency ablation using minimally invasive surgical techniques. J. Am. Coll. Cardiol. 40 (3), 475–480. doi:10.1016/s0735-1097(02)01993-9
Kuck, K. H., Hoffmann, B. A., Ernst, S., Wegscheider, K., Treszl, A., Metzner, A., et al. (2016). Impact of complete versus incomplete circumferential lines around the pulmonary veins during catheter ablation of paroxysmal atrial fibrillation: Results from the gap-atrial fibrillation-German atrial fibrillation competence network 1 trial. Circ. Arrhythm. Electrophysiol. 9 (1), e003337. doi:10.1161/circep.115.003337
Linz, D., Elliott, A. D., Hohl, M., Malik, V., Schotten, U., Dobrev, D., et al. (2019). Role of autonomic nervous system in atrial fibrillation. Int. J. Cardiol. 287, 181–188. doi:10.1016/j.ijcard.2018.11.091
Mines, G. R. (1914). On circulating excitations in heart muscle and their possible relation to tachycardia and fibrillation. Trans. R. Soc. Can. 8, 43–52.
Moe, G. K., Rheinboldt, W. C., and Abildskov, J. A. (1964). A computer model of atrial fibrillation. Am. Heart J. 67, 200–220. doi:10.1016/0002-8703(64)90371-0
Nattel, S. (2017). Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. JACC Clin. Electrophysiol. 3 (5), 425–435. doi:10.1016/j.jacep.2017.03.002
Nery, P. B., Belliveau, D., Nair, G. M., Bernick, J., Redpath, C. J., Szczotka, A., et al. (2016). Relationship between pulmonary vein reconnection and atrial fibrillation recurrence: A systematic review and meta-analysis. JACC Clin. Electrophysiol. 2 (4), 474–483. doi:10.1016/j.jacep.2016.02.003
Nguyen, B. L., Fishbein, M. C., Chen, L. S., Chen, P. S., and Masroor, S. (2009). Histopathological substrate for chronic atrial fibrillation in humans. Heart rhythm. 6 (4), 454–460. doi:10.1016/j.hrthm.2009.01.010
Odening, K. E., Deiß, S., Dilling-Boer, D., Didenko, M., Eriksson, U., Nedios, S., et al. (2019). Mechanisms of sex differences in atrial fibrillation: Role of hormones and differences in electrophysiology, structure, function, and remodelling. Europace 21 (3), 366–376. doi:10.1093/europace/euy215
Ouyang, F., Antz, M., Ernst, S., Hachiya, H., Mavrakis, H., Deger, F. T., et al. (2005). Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: Lessons from double lasso technique. Circulation 111 (2), 127–135. doi:10.1161/01.cir.0000151289.73085.36
Pratola, C., Baldo, E., Notarstefano, P., Toselli, T., and Ferrari, R. (2008). Radiofrequency ablation of atrial fibrillation: Is the persistence of all intraprocedural targets necessary for long-term maintenance of sinus rhythm? Circulation 117 (2), 136–143. doi:10.1161/circulationaha.106.678789
Reyat, J. S., Chua, W., Cardoso, V. R., Witten, A., Kastner, P. M., Kabir, S. N., et al. (2020). Reduced left atrial cardiomyocyte PITX2 and elevated circulating BMP10 predict atrial fibrillation after ablation. JCI Insight 5 (16), e139179. doi:10.1172/jci.insight.139179
Rivaud, M. R., Blok, M., Jongbloed, M. R. M., and Boukens, B. J. (2021). How cardiac embryology translates into clinical arrhythmias. J. Cardiovasc Dev. Dis. 8 (6), 70. doi:10.3390/jcdd8060070
Saito, T., Waki, K., and Becker, A. E. (2000). Left atrial myocardial extension onto pulmonary veins in humans: Anatomic observations relevant for atrial arrhythmias. J. Cardiovasc. Electrophysiol. 11 (8), 888–894. doi:10.1111/j.1540-8167.2000.tb00068.x
Sau, A., Howard, J. P., Al-Aidarous, S., Ferreira-Martins, J., Al-Khayatt, B., Lim, P. B., et al. (2019). Meta-analysis of randomized controlled trials of atrial fibrillation ablation with pulmonary vein isolation versus without. JACC Clin. Electrophysiol. 5 (8), 968–976. doi:10.1016/j.jacep.2019.05.012
Schmitt, C., Ndrepepa, G., Weber, S., Schmieder, S., Weyerbrock, S., Schneider, M., et al. (2002). Biatrial multisite mapping of atrial premature complexes triggering onset of atrial fibrillation. Am. J. Cardiol. 89 (12), 1381–1387. doi:10.1016/s0002-9149(02)02350-0
Spach, M. S., and Dolber, P. C. (1986). Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ. Res. 58 (3), 356–371. doi:10.1161/01.res.58.3.356
Verma, A., Kilicaslan, F., Pisano, E., Marrouche, N. F., Fanelli, R., Brachmann, J., et al. (2005). Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation 112 (5), 627–635. doi:10.1161/circulationaha.104.533190
Wang, T. M., Chiang, C. E., Sheu, J. R., Tsou, C. H., Chang, H. M., and Luk, H. N. (2003). Homogenous distribution of fast response action potentials in canine pulmonary vein sleeves: A contradictory report. Int. J. Cardiol. 89 (2-3), 187–195. doi:10.1016/s0167-5273(02)00474-6
Yamamoto, M., Dobrzynski, H., Tellez, J., Niwa, R., Billeter, R., Honjo, H., et al. (2006). Extended atrial conduction system characterised by the expression of the HCN4 channel and connexin45. Cardiovasc Res. 72 (2), 271–281. doi:10.1016/j.cardiores.2006.07.026
Keywords: ablation, focal arrhythmias, paroxysmal atrial fibrillation, pulmonary vein, pulmonary vein isolation, reentry, trigger
Citation: Gottlieb LA, Dekker LRC and Coronel R (2023) Arrhythmia mechanism dependent pulmonary vein ablation in paroxysmal atrial fibrillation. Front. Physiol. 14:1157338. doi: 10.3389/fphys.2023.1157338
Received: 02 February 2023; Accepted: 16 May 2023;
Published: 24 May 2023.
Edited by:
Tamas Szili-Torok, Erasmus Medical Center, NetherlandsReviewed by:
Andreas Rillig, University Medical Center Hamburg-Eppendorf, GermanyCopyright © 2023 Gottlieb, Dekker and Coronel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lukas R. C. Dekker, bHVrYXMuZGVra2VyQGNhdGhhcmluYXppZWtlbmh1aXMubmw=
 Lisa A. Gottlieb
Lisa A. Gottlieb Lukas R. C. Dekker
Lukas R. C. Dekker Ruben Coronel
Ruben Coronel