
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol. , 29 March 2023
Sec. Metabolic Physiology
Volume 14 - 2023 | https://doi.org/10.3389/fphys.2023.1150232
This article is part of the Research Topic Primary Cilia as Therapeutic Targets View all 8 articles
Neuronal primary cilia have recently emerged as important contributors to the central regulation of energy homeostasis. As non-motile, microtubule-based organelles, primary cilia serve as signaling antennae for metabolic status. The impairment of ciliary structure or function can produce ciliopathies for which obesity is a hallmark phenotype and global ablation of cilia induces non-syndromic adiposity in mouse models. This organelle is not only a hub for metabolic signaling, but also for catecholamine neuromodulation that shapes neuronal circuitry in response to sensory input. The objective of this review is to highlight current research investigating the mechanisms of primary cilium-regulated metabolic drives for maintaining energy homeostasis.
Obesity and overnutrition are common metabolic challenges of the 21st century. Obesity induces blood-brain barrier damage that is associated with lipid peroxidation and inflammation (Salameh et al., 2019). With time, this leads to neuronal dysfunction and behavioral phenotypes like anxiety and memory dysfunction that are deleterious to healthy cognitive aging (Takechi et al., 2017). Identifying mechanisms of metabolic-induced neuropathology are critical to our increasing aged and obese population.
Obesity is multifactorial: it can develop from an imbalance of social and environmental factors such as exercise or palatable diets and is influenced by the genetic landscape in which it occurs. Common obesity is polygenic, but in rarer circumstances can be associated with a single gene. Among efforts to elucidate the complete story of obesity pathology, the central nervous system has become a crucial investigative focus. The brain is the master regulator of energy homeostasis as it coordinates responses to humoral, metabolic, and neural signals from the periphery (Lee and Mattson, 2014). The hypothalamus is a particularly important integration hub that was first implicated in energy regulation by obesity-inducing lesions in the ventromedial nucleus (Brobeck, 1946). Neuronal response to metabolic cues is modulated by signaling at the primary cilium.
When ciliary assembly, maintenance, or function are compromised, clinically relevant ciliopathies develop at a combined frequency of 1:1000 live births (Zaghloul and Katsanis, 2009). Importantly, ciliopathies exhibit an extensive and overlapping spectrum of phenotypes—such as obesity—which are common in the general population. For example, between 72% and 86% of Bardet-Biedl Syndrome patients and 100% of Alström Syndrome patients develop early-onset obesity (Marshall et al., 2011; Forsythe and Beales, 2013). The role of ciliary dysfunction in obesity is supported by findings that the global ablation of primary cilia in rodent models induces adiposity characterized by excessive eating (hyperphagia) and hyperleptinemia (Davenport et al., 2007).
The primary cilium acts as an antenna amplifying signals via G-protein coupled receptor (GPCR) activity and high concentrations of intraciliary calcium that shift membrane polarization, allowing the neuron to integrate local environmental metabolic cues to inform downstream activity. As part of its signaling function the primary cilium uses unique trafficking intraflagellar transport machinery to insert/remove proteins into/from the ciliary membrane as trafficked proteins must contend with steric hindrance at the ciliary base due to fence-like scaffolding at the periciliary diffusion barrier (Berbari et al., 2008; Schou et al., 2015). The presence or absence of specific GPCRs at the ciliary membrane, i.e., inserted into the plasma membrane instead of the ciliary membrane, affects the cellular phenotype (Cook et al., 2021). Because dopamine and serotonin receptors are enriched in the primary cilium, this organelle is not only a hub for metabolic signaling, but also for catecholamine neuromodulation linking reward and motivation with metabolic requirements (Atkinson et al., 2015; Schou et al., 2015; Sheu et al., 2022). The objective of this review is to highlight current research investigating the role of the neuronal primary cilium in integrating metabolic function with behavioral drives.
Neuronal primary cilia are non-motile, ∼0.2–0.5 µm wide, 2–12 μm long organelles that project from the soma. A primary cilium is composed of a microtubule-based axoneme that emanates out of a basal body anchored via transition fibers. The basal body is composed of several Bardet–Biedl syndrome (BBS) proteins that make up the BBSome. The BBSome is tethered to the actin cytoskeleton and is critical for intraflagellar transport (IFT)-regulated trafficking of ciliary cargo (Berbari et al., 2008; Hernandez-Hernandez et al., 2013; Williams et al., 2014; Smith et al., 2020). Although the ciliary membrane appears to be continuous with the plasma membrane, lateral movement of membrane proteins is restricted (Vieira et al., 2006). Proteins destined for the ciliary membrane must dock at adapters near the ciliary base to pass through the ciliary transition zone, a diffusion barrier that provides steric hurdles to non-ciliary proteins (Nachury et al., 2010). Finally, the distinct lipid composition of the primary cilium alters its biophysical properties that determine signaling and protein trafficking (Nachury and Mick, 2019). The ciliary membrane is enriched with GPCRs embedded within lipid rafts next to calcium channels, making this organelle a highly dynamic sensor for the extracellular environment.
Primary cilium length can vary in response to homeostatic states or nutrient availability. In rat neurons, overexpression of serotonin receptor 5-HT6, a GPCR that localizes to the primary cilium, increases ciliary length, and this is associated in reduced dendritic arborization (Guadiana et al., 2013). Neuropeptide signaling at the primary cilium is classically represented by the GPCR for melanin-concentrating hormone (MCH), an orexigenic neuropeptide that links sleep, metabolism, and reward. Application of MCH decreases MCHR1+ primary cilia length which was blocked with application of Gi/o inhibitors (Kobayashi et al., 2021). Length changes and resorption occur in primary cilia in response to cell cycle transition. Cilia shrink during G1 to S phase until almost resorption during mitosis. Cell culture experiments demonstrate that nutrient state regulates length and ciliary resorption can be induced by nutrient availability (Lim et al., 2015). Resorption occurs with microtubule deacetylation that destabilizes the axoneme in addition to actin cytoskeletal destabilization (Kim et al., 2015; Lim et al., 2015; Ran et al., 2015). In some contexts, disassembly occurs in response to decapitation in which actin-dependent processes pinch off the tip of the primary cilium yielding a ciliary vesicle (Phua et al., 2017). However, because neurons are post-mitotic, their primary cilia have not been shown to undergo resorption.
Ciliogenesis occurs when membrane- bound vesicles emerging from the Golgi are recruited to the mother centriole where they fuse with proteins on the plasma membrane. The mother centriole is converted into the basal body, and proteins are recruited to establish the transition zone. Microtubules form at the basal body to become the growing axoneme, and cilia protein assembly is dependent on IFT and kinesin/dynein activity (Conduit and Vanhaesebroeck, 2020).
The ciliary membrane is distinct from the rest of the plasma membrane in its lipid composition. The relative proportions and spatial distribution of sphingolipids, phosphoinositides, and cholesterol in the ciliary membrane are essential for proper primary cilium function. Many GPCRs are embedded within lipid rafts, microdomains enriched with cholesterol and sphingolipids (Figure 1).
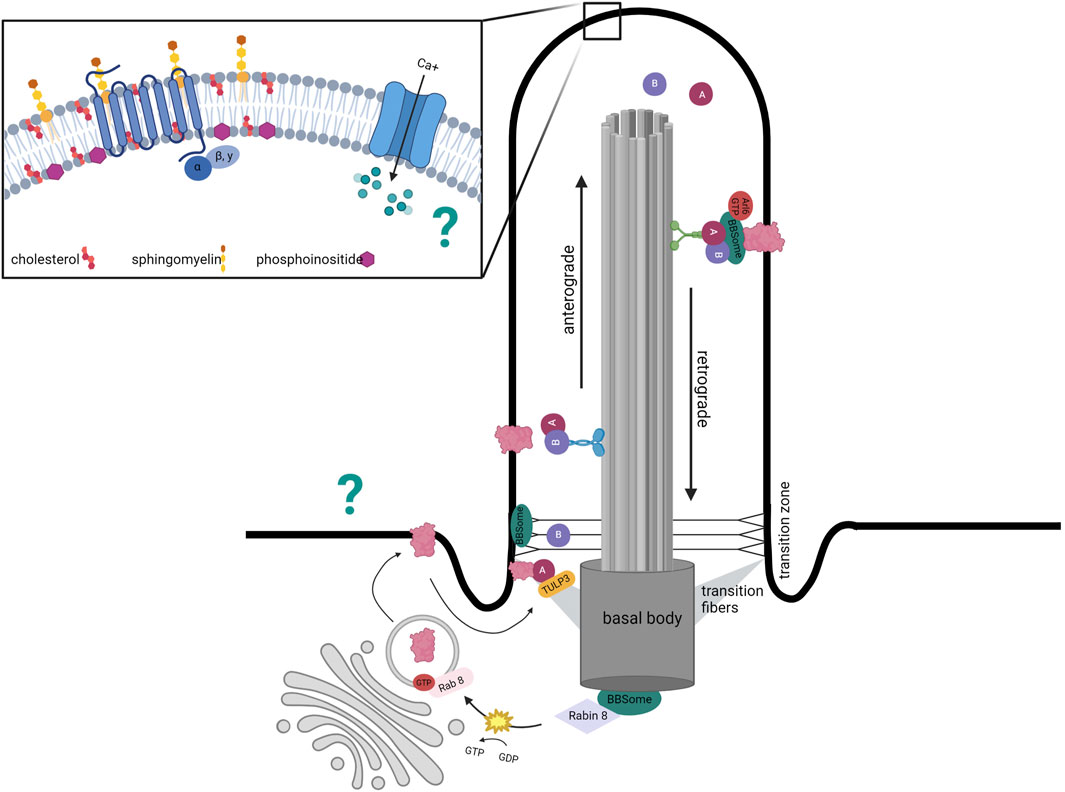
FIGURE 1. Distinct mechanism of ciliary protein transport. Structure of primary cilium to highlight distinct ciliary membrane composition in inset. Ciliary trafficking of receptors such as GPCRs involves vesicle budding from the Golgi that docks at the base of the cilium with intraflagellar (IFT)- mediated transportation via anterograde kinesin motors. IFT-A-mediated entry requires TUB like protein 3 (TULP3), a member of the Tubby family of proteins that interacts with the IFT-A core as an effector. The BBSome localizes to the basal body and transition zone and represents a docking point for proteins destined to enter the cilium. The BBSome has also been implicated in the exit of ciliary GPCRs out of the cilium as disruption of its functions leads to accumulation of GPCRs within the cilium. In addition to the BBSome, removal of proteins is mediated by retrograde transport with IFT complexes coupled to dynein motors. The question mark represents the uncertainty regarding how GPCR- associated cAMP and calcium oscillations are transmitted from the cilium to the rest of the neuron.
Sphingolipids have a ceramide building block that is modified at the endoplasmic reticulum depending on the plasma membrane destination (Olsen and Færgeman, 2017). One of the resulting derivatives, sphingomyelin is abundant in the nervous system plasma membrane and vital for dendritic arborization and myelination. Sphingolipids are also heavily represented in the ciliary membrane (Kaiser et al., 2020). The sphingomyelin: cholesterol ratio is especially high in the ciliary membrane where sphingomyelin sequesters accessible cholesterol in a mechanism that regulates canonical ciliary GPCR signaling (Myers et al., 2017; Kinnebrew et al., 2019). When sphingolipid metabolism is disrupted by blocking ceramide synthesis, organization of microdomains is also disrupted due to alterations in protein-lipid interactions. This can result in changes to membrane polarization when microdomains critical for ion channel function are altered by interfering with sphingolipid metabolism (Olsen and Færgeman, 2017).
Microdomain composition is critical for associating with lipid-binding domains on receptors/channels, and lipid modification of proteins determines membrane association (Resh, 2013). For example, when myristoylation (Emmer et al., 2009; Tao et al., 2009), palmitoylation (Emmer et al., 2009), or SUMOylation (McIntyre et al., 2015) is blocked, ciliary proteins fail to localize to the ciliary membrane. Palmitoylated acetylated tubulin colocalizes with ceramide-rich regions in the ciliary membrane, and inhibition of this post-translational modification was sufficient to impair ciliogenesis (Tripathi et al., 2021). However, not all lipid modifications have this effect as geranylgeranylation (Williams et al., 2014) of GFP restricted its trafficking to the ciliary proximal region of olfactory sensory neurons rather than to the ciliary membrane. Mislocalization of ciliary proteins can have robust behavioral effects (Yamakawa et al., 2021). When a ciliary GPCR that regulates energy homeostasis, melanocortin-4 receptor (MC4R), is retained subcellularly due to a mutation in the ciliary localization signal domain, ligand-induced activation is hindered. Mice with this mutation are obese and are unable to appropriately integrate metabolic signals at the hypothalamus (Siljee et al., 2018).
Although not particularly abundant compared to other membrane lipids, phosphoinositides are phospholipids enriched in the ciliary membrane. Phosphatidylinositol 4-phosphate [PtdIns(4)P], PtdIns (4,5)P2, PtdIns (3,4,5)P3, and PtdIns (3,4)P2 are phosphoinositides that define subdomains in the cilia. This is in part through their varied lipid-binding domains that determine recruitment of different effector proteins (Conduit and Vanhaesebroeck, 2020). One such lipid-binding domain critical to ciliary lipid membrane maintenance is the Pleckstrin Homology (PH). These domains are also present in phosphatidylinositol-4-phosphate adaptor protein-2 (FAPP2), which binds PtdIns (4)P via PH domains at the Golgi for transport. In cells, FAPP2 depletion hindered the formation of a primary cilium and was associated with vesicle accumulation at the microtubule organizing center (Vieira et al., 2006).
PtdIns (4)P provides structure to the ciliary membrane, and PtdIns (4,5)P2 levels regulate ciliary length and stability (Garcia-Gonzalo et al., 2015). Phosphatidylinositol 4,5-bisposphate [PtdIns (4,5)P2] exists along a gradient within the primary cilium with levels comparable to the plasma membrane at the base, but undetectable at the tip (Stilling et al., 2022). The compartmentalization occurs due to the coordinated activity of Tctn1 and Inpp5e. Tctn1 is a transition zone protein critical for ciliary localization of phosphoinositide 5-phosphatase (Inpp5e) which converts PtdIns (4,5)P2 into PtdIns(4)P. Inpp5e −/− mouse embryonic fibroblasts have a robust reduction in PtdIns(4)P levels and PtdIns (4,5)P2 is distributed along the ciliary membrane rather than restricted to the ciliary base. This shift in membrane lipid composition was associated with dysfunctional trafficking of ciliary proteins, including IFT-A whose ciliary levels depends on PtdIns (4,5)P2, and IFT-A is critical for intraflagellar transport of GPCRs (Garcia-Gonzalo et al., 2015). In humans, loss of Inpp5e function is associated with Joubert Syndrome, a multisystem ciliopathy characterized by organ dysfunction and cognitive impairment due to ciliary transition zone dysfunction (Dyson et al., 2017). Unsurprisingly, Tctn1 mutations also cause Joubert Syndrome (Garcia-Gonzalo et al., 2011). Membrane lipid constitution is therefore essential to GPCR composition and length of the primary cilium.
The axoneme is encased by a phospholipid membrane studded with multiple GPCRs that are preferentially enriched in cilia trafficked via IFT trains. IFT trains are composed of subcomplexes—IFT-A and IFT-B—and protein motors that together carry cargo along the axoneme microtubules. Trains a) assemble at the base and undergo anterograde transport driven by IFT-B and kinesin-2 movement towards the positive end, b) are then remodeled at the ciliary tip, and finally c) exit through retrograde transport driven by IFT-A and dynein-2 movement towards the negative end. Many of the IFT components are necessary for cilia assembly (Lechtreck, 2015). Mutations in kinesin family member 3A (Kif3a)—a subunit of kinesin-2—or Ift88/Tg737—a subunit of the IFT-B complex—are commonly employed to conditionally ablate cilia in the laboratory (Davenport et al., 2007).
Considering that primary cilia are signaling hubs, IFT is also important for functional maintenance by trafficking receptors and downstream effectors. Ciliary protein trafficking is currently suspected to be a combination of IFT and diffusion with differences likely based on protein and cell type (Ye et al., 2013). IFT-dependent vesicular trafficking involves packaging proteins into vesicles, docking at the base of the cilium, and IFT movement to the tip (Rosenbaum and Witman, 2002). In addition to its role in anterograde transport, IFT-A has also been implicated in the entry of ciliary GPCRs. IFT-A-mediated entry requires TUB like protein 3 (TULP3), a member of the Tubby family of proteins that interacts with the IFT-A core as an effector (Figure 1) (Mukhopadhyay et al., 2010). siRNA depletion of Tulp3 inhibits the localization of ciliary GPCRs such as somatostatin receptor 3 (SSTR3) and melanin concentrating hormone receptor 1 (MCHR1) (Mukhopadhyay et al., 2010). However, IFT-A and TULP3 are not the only mediators of ciliary protein trafficking. For example, a complex of ciliary localizing proteins called the BBSome is also involved in other aspects of the vesicular trafficking pathway as will be discussed.
Overall, elucidating the mechanisms by which receptors and their effectors are trafficked to primary cilia is central to delineating how ciliary structure contributes to cilia-regulated energy homeostasis. For example, tubby mice—which exhibit a loss-of-function mutation in the TUB gene, the founding member of the Tubby family—manifest an obese phenotype (Coleman and Eicher, 1990), and homozygous mutation in TUB has been associated with early-onset obesity in humans (Borman et al., 2014).
The BBSome complex is named for Bardet-Biedl Syndrome (BBS), a pleiotropic, autosomal recessive ciliopathy. BBS is characterized by retinal degeneration, renal dysfunction, and early-onset obesity, but also presents with hypogonadism, polydactyly, and learning difficulties (Forsythe and Beales, 2013). Renal dysfunction and obesity are the primary clinical concerns. Newborns typically exhibit normal birth weights; however, diffuse adiposity rapidly ensues by early childhood and adult patients exhibit truncal obesity (Forsythe and Beales, 2013; Pomeroy et al., 2021). Heterozygous carriers are also predisposed to obesity (Croft et al., 1995).
BBS genes either encode the subunits of the BBSome or are involved in its formation and activation. The BBSome is a heterooctameric protein complex implicated in ciliary structure and function (Loktev et al., 2008). The core complex is composed of subunits BBS1/2/4/5/7/8/9/18 and is assembled by a separate chaperonin-like complex containing BBS6/10/12 (Nachury et al., 2007; Loktev et al., 2008; Seo et al., 2010). During formation, BBS7 is stabilized by the chaperonin-complex so that it can interact with BBS2. The remaining core forms through protein-protein interactions. BBS1 and BBS4 are the most peripheral core subunits, suggesting that they may mediate any functional BBSome interactions (Zhang et al., 2012). Due to this assembly process, the entire BBSome can become compromised by the absence of nearly any of the core subunits or chaperonin-complex components.
Disruption of BBS2 is specifically associated with adult obesity whereas BBS4 and BBS6 have been linked to both adult and childhood obesity (Benzinou et al., 2006). However, BBS1 and its prevalent missense mutation (M390R) are not associated with adiposity (Fan et al., 2004; Benzinou et al., 2006). Mouse models both support and conflict with these associations. At around 4 months, Bbs1M390R knock-in, Bbs2 −/−, Bbs4 −/−, Bbs6 −/−, and Bbs7 −/− mice developed obesity characterized by hyperphagia (Nishimura et al., 2004; Eichers et al., 2006; Davis et al., 2007; Zhang et al., 2013). Similarly, human BBS patients exhibit hyperphagic behaviors (Sherafat-Kazemzadeh et al., 2013). However, disruption of BBS3/ARL6—an activator of the BBSome complex—induced limited weight gain without hyperphagia (Zhang et al., 2011). Bbs2 −/− and Bbs4 −/− mice displayed decreased energy expenditure (Rahmouni et al., 2008). Currently, it is unclear if altered food intake or energy expenditure are causes or consequences of obesity in BBS. For example, Bbs2 −/− and Bbs4 −/− mice still gained significant weight when hyperphagia was eliminated by pair-feeding, suggesting that decreased energy expenditure may underlie the phenotype (Rahmouni et al., 2008). However, a cohort of human BBS patients exhibited no significant differences in energy metabolism when compared to other obese individuals (Grace et al., 2003).
In the search for the underlying mechanisms of the BBS obesity phenotype, a parsimonious role for the BBSome has not yet been identified. First, the BBSome is not required for ciliogenesis, revealing a dichotomy in which IFT mutations hinder cilia formation whereas BBS mutations do not (Mykytyn et al., 2004; Nishimura et al., 2004; Davis et al., 2007; Zhang et al., 2013). Importantly, the BBSome localizes to the basal body and transition zone (Blacque et al., 2004; Nachury et al., 2007; Williams et al., 2014). The BBSome supports ciliary signaling through trafficking ciliary proteins.
The BBSome regulates multiple steps of the ciliary vesicular trafficking process. It has been implicated in regulating the enrichment of GPCRs within the cilium. For example, SSTR3, NPY2R and MCHR1 fail to accumulate in neuronal cilia in multiple brain regions in the absence of BBS1, BBS2, and BBS4 (Berbari et al., 2008; Loktev et al., 2013), and BBSome disruption induces ciliary accumulation of DR1R, GRP19, and hedgehog pathway receptors (Domire et al., 2011; Ye et al., 2018; Stubbs et al., 2023). Overall, it appears that the BBSome may select proteins for IFT-dependent trafficking and contribute to IFT train formation at the base and tip, potentially through stabilizing interactions between IFT-A and IFT-B (Blacque et al., 2004; Wei et al., 2012; Williams et al., 2014). The process of GPCR enrichment in neuronal primary cilia also relies on TUB and TULP3 proteins (Sun et al., 2012; Loktev et al., 2013). Similarly to BBS mutations, mice with TUB or TULP3 also develop obese phenotypes (Sun et al., 2012). A synthesized model of ciliary trafficking suggests that a) BBS1 in association with Rabin8—a guanine nucleotide exchange factor—activates Rab8 to promote vesicle docking at the ciliary base followed by b) direct protein entry into the cilia via IFT-A/TULP3 and finally c) the formation of a BBSome complex mediates the retrieval and export of specific proteins through the transition zone (Jin et al., 2010; Ye et al., 2018; Nachury, 2018). Thus, the underpinnings of the BBS obesity phenotype likely stem from the mislocalization and impaired signaling of ciliary membrane proteins whose trafficking is BBSome-dependent.
An attractive mechanism is the impaired localization of LepRb. First, hyperleptinemia and leptin resistance are present in many BBS mouse models such as Bbs2 −/−, Bbs4 −/−, and Bbs6 −/− (Rahmouni et al., 2008). In humans, BBS patients exhibit leptin levels that are greater than what would be expected for their degree of adiposity (Feuillan et al., 2011; Büscher et al., 2012). Moreover, the hyperleptinemia appears causative considering that leptin levels were increased both before the onset of obesity and after weight normalization by calorie restriction (Rahmouni et al., 2008; Seo et al., 2010). Experiments have further demonstrated that resistance was not due to the inability of leptin to cross the blood-brain barrier but could rather be attributed to blunted STAT3 phosphorylation (Seo et al., 2009). BBS1 interacts with the C-terminal domain of LepRb—and the M390R mutation greatly impairs this interaction (Seo et al., 2009). Although BBS1 is the point of interaction, the entire BBSome was recently found to be necessary for trafficking LepRb not to the cilia as had been predicted, but rather to the periciliary plasma membrane (Guo et al., 2016). The mislocalization of LepRb underlies leptin resistance and is sufficient to induce obesity as demonstrated by significant weight gain in mice lacking BBS1 in neurons expressing LepRb (Guo et al., 2016). Thus, these studies have illuminated a role for the BBSome in trafficking proteins not only into cilia, but also to periciliary regions.
The primary cilium structure is ideal for maintaining differential calcium gradients. The cytoplasmic volume is about 30,000x that of the primary cilium, and intraciliary calcium concentration is about 6X the cytoplasmic concentration ([Ca]cilia ∼ 600 nM and [Ca]cytosol ∼ 100 nM). The ciliary membrane is highly enriched in calcium channels like polycystin (PC)- and transient receptor potential cation (TRP)- family members (Decaen et al., 2013; Delling et al., 2013; Saternos et al., 2020). The small volume coupled with the density of calcium-permeant channels allows 200–300 calcium ions to generate a molarity 1 µM [Ca]cilia making this an ideal environment for amplifying Gαq – coupled receptors that when activated generate diacylglycerol and Ins (1,4,5)P3 in addition to the opening of TRP channels (Ritter and Hall, 2009).
Not only do intraciliary calcium levels regulate adenylyl cyclase 3 (ADYC3) signaling, but ADCY3 regulates intraciliary calcium activity. ADCY3 is a downstream effector of GPCR activation concentrated in primary cilia of olfactory neurons and critical to metabolism and contains a calcium-binding domain (Moore et al., 2018). This ADCY isoform is also found in osteoclast primary cilia where it regulates to fluid shear stress-induced calcium activity during bone growth (Halls and Cooper, 2011; Moore et al., 2018). Calcium bound- calmodulin and calcium alone have been shown to inhibit ADCY3 activity (Sadana and Dessauer, 2009).
ADCY3 levels in primary cilia control intraciliary cyclic adenosine monophosphate (cAMP) levels—whether or not intraciliary cAMP is distinct from cytosolic cAMP is unclear as reports have been conflicting (Moore et al., 2016; Jiang et al., 2019; Sherpa et al., 2019). Sequestration of these second messengers facilitates temporal and spatial signal integration. Transmission of both cAMP and calcium waves from the primary cilium signaling into the cytosol regulate neuronal excitability although the mechanism by which this occurs remains to be clarified (Green and Mykytyn, 2010). It is tempting to speculate that these signals are transmitted via inward rectifying potassium channels as G-protein coupled receptor inward rectifying potassium channels (GIRKs) are in close proximity to GPCRs and allows an efflux of potassium that hyperpolarizes the neuron (Lüscher and Slesinger, 2010). Similarly, PC channels in the primary cilium can be primed with high intraciliary calcium levels and are permeable to Na+ and K+ (Liu et al., 2018). An ideal location for such channels to propagate second messenger signals from the ciliary GPCR activity to the rest of the cell is at the base of the cilium, and potassium channels that regulate neuronal excitability have been found in the ciliary pocket and at the primary cilium base. Mutations to inward rectifying- and voltage-gated potassium channels are associated with impaired ciliogenesis (Sánchez et al., 2016; Napoli et al., 2022) indicating that sensitivity to local changes in voltage is a requirement for primary cilia.
Although not localized to the primary cilium per se, hypercholesteremia led to upregulation of membrane-bound cholesterol- associated GIRK activity in rat hippocampus that modified neuronal excitability. GIRK localization within cholesterol-enriched lipid rafts at membrane can be explained by their multiple cholesterol-binding motifs (Bukiya et al., 2017). Associated with Gq activity, PtdIns (4,5)P2 levels also regulate inward rectifying potassium channels. As previously mentioned, PtdIns (4,5)P2 levels exist along a gradient with the highest concentration at the ciliary base. Depletion of PtdIns (4,5)P2 from Gq – associated phospholipase C activity inhibits GIRKs in atrial myocytes, and supplementation with PtdIns (4,5)P2 reduced this inhibitory effect (Meyer et al., 2001).
The functional organization and relative size constraints of the primary cilium with its densely packed GPCRs in close apposition to calcium channels within cholesterol-rich lipid rafts allows for nuanced transmission to the neuronal soma. Ciliary activity must be integrated with inward-rectifying potassium channels and fluctuating PtdIns (4,5)P2 levels at the ciliary base to before spreading to the rest of the plasma membrane to mediate polarization. Primary cilium- induced hyper- or depolarization leads to downstream adjustments to intercellular circuitry.
In the brain, several neuromodulators have ciliary GPCRs, including the serotonergic receptor 5-HT6R; the dopaminergic receptors D1R, D2R, D5R; neuropeptidergic receptors for neuropeptide Y NPY2R, NPY5R and somatostatin receptors SSTR1-5 (Green and Mykytyn, 2010; Lukomska et al., 2020). Disruption of these signaling pathways via alterations to ciliary structure or function shifts neuronal connectivity. Dopaminergic tone is essential for short- and long-term maintenance of neuronal health as dopamine metabolites are oxidants (Meiser et al., 2013) and dopaminergic and GABA-ergic interneurons have high tonic activity. Therefore, ciliary localized receptors are poised to be integral to interneuron activity regulation.
This is elegantly depicted by selective deletion of Arl13b, a GTPase critical for ciliary signaling. Deletion of Arl13b disrupts signaling without affecting ciliary structure. Arl13b deletion from striatal parvalbumin+ and somatostatin+ interneurons was associated with altered morphology of inhibitory interneuronal networks that reduced perisomatic synaptic contact sites on medium spiny neurons (MSNs). Decreased perisomatic contacts from interneurons onto MSNs shifted the excitability/inhibitory tone with reduced miniature inhibitory postsynaptic currents without affecting miniature excitatory postsynaptic currents from sampled MSNs (Guo et al., 2017). Similarly, mutations in Arl13b and Inpp5e cause ciliopathies that fall under Joubert Syndrome related disorders and lead to aberrant axonal tracts in mouse models that mimics human axonal tract defects (Guo et al., 2019a). ARL13b is also dependent on TULP3 for its trafficking to the cilium and its mislocalization may underlie some of the neural defects in Tulp3 KO mice (Palicharla et al., 2023).
Disruption of BBSome signaling in Bbs4−/− mice inhibits proper D1R trafficking causing accumulation of D1R in the primary cilium (Domire et al., 2011). Dysfunctional D1R at the primary cilium due to either conditional knock-out of primary cilia in D1R+ neurons or by eliminating Bbs1 from D1R+ neurons in which loss of D1R from primary cilia or abnormal accumulation of D1R in the primary cilia lead to inappropriate striatal signaling. These mice are obese in part due to reduced locomotor activity in the absence of hyperphagia (Stubbs et al., 2022). Removing primary cilia from dorsal striatal neurons had several interesting behavioral outcomes including increased repetitive behaviors and deficits in sensorimotor gating (Alhassen et al., 2022a) indicating atypical striatal circuitry. However, not all neuronal cilia loss models cause locomotor changes and obesity phenotypes. Removal of cilia from GAD2-expressing GABAergic neurons resulted in no changes to basal locomotor activity, however mutant mice showed reduced body weight compared to wildtype littermates (Ramos et al., 2021).
The contribution of impaired ciliary signaling to obesity via disrupted energy expenditure suggests that primary cilia are important for hypothalamic responses to leptin. Leptin is an afferent signaling molecule that is released in proportion to fat stores to communicate a fed state, evoking a net anorexigenic response via short-term suppression of food intake and long-term stimulation of energy expenditure. It is secreted directly from adipose tissue, enters the bloodstream, and crosses the blood-brain barrier to bind to the LepRb splice form of leptin receptors which is primarily expressed in the hypothalamus (Gorska et al., 2010). Polymorphisms within LepRb have been associated with increased adiposity in multiple populations (Thompson et al., 1997; de Oliveira et al., 2013; Marginean et al., 2016), and mice heterozygous for loss-of-function mutations in the genes that encode for leptin and leptin receptors develop increased body fat (Chung et al., 1998).
The well-characterized action of leptin occurs within the leptin-melanocortin pathway (Baldini and Phelan, 2019). Here, leptin binds directly to the LepRb receptors of neurons in the arcuate nucleus (ARC). First, it activates proopiomelanocortin (POMC)-expressing neurons to stimulate the release of anorexigenic peptides such as alpha melanocyte stimulating hormone (α-MSH). Simultaneously, it inhibits agouti-related peptide (AgRP)-and neuropeptide Y (NPY)-expressing neurons to suppress the release of orexigenic peptides such as AgRP and NPY. AgRP, NPY, and α-MSH are released in the paraventricular nucleus (PVN) where α-MSH activates melanocortin-4 receptors (MC4R) and AgRP inhibits the constitutive activity of MC4R. When activated, LepRb can engage with several different pathways through the autophosphorylation of JAK2. The STAT3 pathway, initiated by phosphorylation of Tyr1138 on LepRb, promotes POMC expression to regulate food intake (Bates et al., 2003; Baldini and Phelan, 2019).
Leptin prepares the hypothalamus for its anorexigenic communication by modulating neuronal structure both in development and adulthood. Support for this comes from obese leptin-deficient (ob/ob) mice which exhibit a reduced density of ARC projections that can be rescued by leptin treatment during a postnatal period, but not in adulthood (Bouret et al., 2004). This suggests that leptin acts within a critical period to drive aspects of feeding circuitry formation. The disruption of ARC projections is paralleled in LepRb deficient (db/db) mice, indicating that LepRb mediates leptin’s developmental actions (Bouret et al., 2012). The modulation of ciliary length in adulthood is also a critical component of leptin’s anorexigenic signaling. For example, leptin treatment of N1 hypothalamic cells in vitro increased ciliary length up to 3 μm. One mechanism of leptin-induced cilia elongation is mediated through reduced density of F-actin fibers, which act as a blockade to both ciliary vesicular transport and membrane remodeling (Kang et al., 2015; Smith et al., 2020). Conversely, the length of cilia is reduced under conditions of low leptin or leptin insensitivity such as diet-induced obesity, ob/ob and db/db mice, or after 36 h of fasting (Han et al., 2014). Thus, longer cilia seem to be important for sufficient leptin signaling in a subset of hypothalamic neurons.
Logically, obesity might be expected to be correlated with low leptin levels; however, this is only the case for 10% of obese individuals (Ahima, 2008). Instead, hyperleptinemia develops into leptin resistance in an overwhelming number of obese patients and rodent models. Resistance is characterized by a lack of anorexigenic response despite high levels of circulating leptin and can either be primary or secondary to the development of obesity (Klok et al., 2007). Attractive mechanisms for causative resistance include defective leptin transport across the blood-brain barrier, underdeveloped hypothalamic circuitry, LepRb signaling cascade dysfunction or defects in downstream projections. Impairment of ciliary structure and signaling appears to contribute to leptin resistance in obesity pathology.
The leptin-melanocortin pathway begins in the arcuate nucleus (ARC) with the stimulation of POMC-expressing neurons and the inhibition of AgRP/NPY-expressing neurons (Figure 2). Signaling through POMC neurons is particularly necessary for leptin’s anorexigenic effects due to the production of α-MSH, an anorexigenic neuropeptide cleaved from the POMC precursor. The absence of leptin receptors from POMC neurons induces obesity associated with decreased POMC mRNA, and POMC deficiency in humans is characterized by early-onset obesity (Krude et al., 1998; Krude et al., 2003). Disruption of BBS1 in POMC or AgRP neurons was sufficient to induce adiposity through a decrease in POMC expression and induced pluripotent stem cell-derived hypothalamic arcuate-like neurons with the most common BBS1 and BBS10 mutations subjected to leptin failed to increase p-STAT3 (Guo et al., 2019b; Wang et al., 2021a).
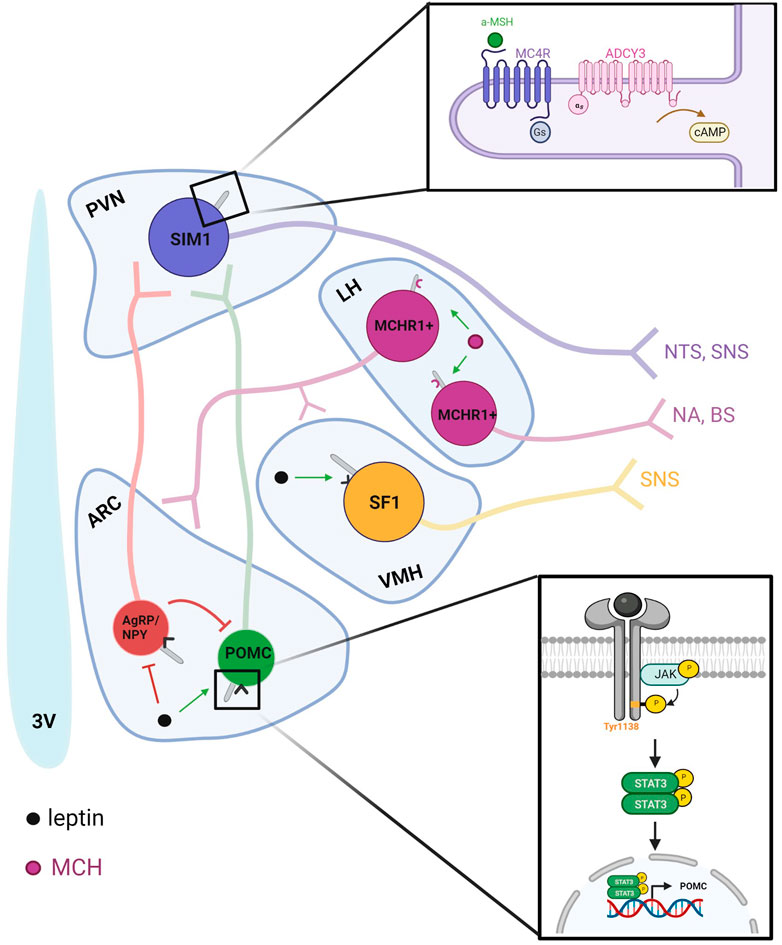
FIGURE 2. Ciliary-specific signaling in the hypothalamus integrates peripheral nutrient signals with neuronal output and connectivity. Leptin released from the periphery is detected by primary cilia in the hypothalamus. The leptin-melanocortin pathway begins in the ARC with the stimulation of POMC-expressing neurons and the inhibition of AgRP/NPY-expressing neurons. POMC-expressing neurons release α-MSH, which then binds to MC4R to increase satiety. Within the hypothalamus, MCH also regulates energy homeostasis as an orexigenic peptide that is upregulated during fasted states to increase food intake. In addition to the zona incerta, MCH is expressed in the lateral hypothalamus and sends projections locally to the ARC and VMH and to regions such as the brain stem and nucleus accumbens to modulate autonomic and hedonic aspects of food intake.
Embryonic ciliogenesis is important for the leptin-driven formation of POMC-associated feeding circuits which are unwired at birth. Ciliogenesis was disrupted in rodent POMC neurons at embryonic day 10 by conditionally knocking-out Ift88, and the subsequent reduction in dendrite formation and arborization impaired circuit development leading to hyperphagic-associated obesity. Ciliogenesis could also be disrupted by postnatal exposure to a leptin antagonist, suggesting that the postnatal leptin surge may stimulate hypothalamic ciliogenesis as a necessity for its regulation of POMC circuit development (Lee et al., 2020).
Because inhibiting ciliogenesis reduces lysosomal protein degradation, impaired autophagy may underlie the obesity phenotype (Lee et al., 2020). When autophagy is disrupted in POMC neurons, axon growth is impaired, and adiposity subsequently develops (Coupé et al., 2012)– indicating a bidirectional relationship between ciliogenesis and autophagy regulation (Yamamoto and Mizushima, 2021). High-fat diets, specifically enriched with palmitic acid blunts autophagy by increasing levels of SQSTM1 and reducing the transformation of LC3I-LC3II in POMC neurons. This is associated with lysosomal swelling but decreased autophagosome-lysosomal fusion critical for cellular turnover of lipids (Hernández-Cáceres et al., 2020). Further work from this same group found that application of palmitic acid also led to insulin resistance through increased Free Fatty Acid Receptor 1 (FFAR1), and FFAR1 inhibition reversed the palmitic acid-induced insulin resistance in hypothalamic neurons (Hernández-Cáceres et al., 2019). Unlike cholesterol, palmitic acid can enter through the blood-brain barrier and accumulates in hypothalamic neurons leading to inflammation only in male mice. Interestingly, male mice with palmitic acid-induced hypothalamic inflammation also developed myocardial dysfunction (Morselli et al., 2014). Whether this was due to a shift in hypothalamic control of sympathetic tone was not elucidated, but high-fat diets have been shown to induce autonomic disturbances (Kim et al., 2021).
Neuropeptides from ARC neurons are released in the paraventricular nucleus (PVN) where α-MSH agonizes melanocortin 4 receptors (MC4R) via Gs-coupled cascades to inhibit feeding (Marsh et al., 1999). Heterozygous loss-of-function mutations in MC4R are the most common monogenic form of severe obesity in humans (Vaisse et al., 2000); Mc4r −/− mice develop obesity associated with hyperphagia and hyperleptinemia (Huszar et al., 1997). Obesity-inducing lesions within the PVN have implicated local neurons in autonomic regulation which may underlie the hypothalamic modulation of feeding behavior (Leibowitz et al., 1981; Sakaguchi et al., 1988).
MC4R is localized to the primary cilia of Single-minded 1 (Sim1) neurons (Siljee et al., 2018). Cilia are necessary for the anorexigenic action of MC4R as ablation of cilia in the PVN reduces their response to agonists (Wang et al., 2021b). MC4R signaling also requires cAMP production by adenylyl cyclases. Compounding evidence has specifically implicated Type 3 Adenylyl Cyclase (Adyc3) and its protein product ADCY3 in hypothalamic energy homeostasis. First, polymorphisms in Adyc3 have been associated with obesity in multiple populations (Nordman et al., 2008; Wang et al., 2010; Grarup et al., 2018). A global knockout of Adcy3 in mice induces obesity characterized by hyperphagia and decreased physical activity (Wang et al., 2009). These mice also exhibit hyperleptinemia and a blunted response to leptin prior to significant weight gain, suggesting that leptin resistance may underlie the obese phenotype. Conversely, a gain-of-function mutation (M279I) protects mice against diet-induced obesity (Pitman et al., 2014). ADCY3 is enriched to primary cilia throughout the brain and is commonly used as a nearly ubiquitous marker for neuronal primary cilia (Bishop et al., 2007). Recently, ADCY3 was found to colocalize with MC4R in Sim1+ neurons (Siljee et al., 2018). Specific inhibition of ciliary ADCY3 using GPR88, a Gi-coupled receptor specific to cilia that was artificially constructed to be constitutively active, resulted in a blunted response to an MC4R agonist and subsequent weight gain associated with hyperphagia (Siljee et al., 2018). Thus, ADCY3 is suspected to be the downstream cAMP producer necessary for the MC4R signaling pathway that reduces food intake.
Currently, both MC4R and ADCY3 localization appear to be BBSome-independent (Berbari et al., 2008). For example, Tectonic1 (TCTN1), a transition zone complex, and SUMOylation have been implicated in ADCY3 trafficking in mouse embryonic fibroblasts and olfactory sensory neurons respectively (Garcia-Gonzalo et al., 2011; McIntyre et al., 2015). The role of SUMOylation in ADCY3 trafficking supports nucleoporins and processes analogous to nuclear import in ciliary entry (Kee and Verhey, 2013; Lynne Blasius et al., 2019). Considering that some Adcy3 −/− mice display primary leptin resistance, it is tempting to propose that cAMP deficiency may underlie obesity by blunting anorexigenic downstream responses.
Although the melanocortin system is a primary focus for leptin’s anorexigenic action in the hypothalamus, obesity in POMC LepRb −/− mice is only a fraction of that observed after global LepRb depletion. The ventromedial nucleus (VMH) of the hypothalamus has long been implicated in energy homeostasis as evidenced by studies in which lesions induce obesity. This adiposity is associated with secondary hyperphagia as lesioned mice still gain significant weight compared to controls even when pair-fed. However, lesions do produce decreased sympathetic nervous activity in brown adipose tissue, suggesting that adiposity may develop out of decreased energy expenditure (vander Tuig et al., 1982). Steroidogenic factor-1 (SF-1)-expressing neurons have been identified as another type of neuron directly activated by leptin (Dhillon et al., 2006). Ablation of LepRb in SF-1 neurons in mice induced mild obesity which was not characterized by hyperphagia. Like POMC ablation from LepRb positive neurons, this phenotype produced only 20% of the weight gain resulting from global LepRb depletion (Dhillon et al., 2006). In another study, mice that similarly lacked LepRb in SF-1 neurons did not show significant weight gain on a standard diet but did exhibit increased susceptibility to diet-induced obesity (Bingham et al., 2008). These studies support a role for first order SF-1 neurons in leptin’s regulation of energy homeostasis that is likely additive to POMC neurons.
Primary cilia may modulate the sympathetic tone that the VMH exerts upon brown adipose tissue as a mechanism for controlling energy expenditure. Ciliary ablation on SF-1 neurons in mice induced obesity associated with decreased energy expenditure but not hyperphagia (Sun et al., 2021). Interestingly, the BBSome is also implicated in sympathetic regulation. Knocking out Bbs1 in SF-1 neurons produced a similar obesity phenotype characterized by decreased energy expenditure (Rouabhi et al., 2021). These hyperphagia-independent obesity models are distinct from the phenotypes that are produced by damage to other hypothalamic nuclei and neurons, suggesting that obesity induced by VMH dysfunction is characterized by a causative decrease in energy expenditure.
An Adcy3 knockdown in the VMH using Cre-recombinase methods produced hyperphagia-associated obesity in mice on a standard diet (Cao et al., 2016). In a separate study, ciliary ADCY3 knockdown in the VMH using CRISPR-Cas9 did not induce significant weight gain on a standard diet but did result in increased susceptibility to a high-fat diet in mice (Yang et al., 2022). Altered autophagy due to a deficiency of ciliary ADCY3 may contribute to the development of obesity. GABA type A receptor-associated protein (GABARAP), a cAMP-regulated protein implicated in substrate recruitment to autophagosomes, was shown to interact with ADCY3 in a cilia-dependent manner. Furthermore, GABARAP knockout produced comparable susceptibility to diet-induced obesity (Yang et al., 2022). Considering that reduced autophagy hinders the development of POMC neurons in the ARC, it would be intriguing for future studies to explore if reduced ADCY3-dependent recruitment to autophagosomes has a structural impact on SF1 development and VMH sympathetic projections.
Finally, mutations in Rpgrip1l, a ciliary transition zone associated protein, are also associated with obese phenotypes. Mutations in Rpgrip1l underlie Joubert related disorders in humans, and lead to obese phenotypes in mice (Wolf et al., 2007; Stratigopoulos et al., 2014; Stratigopoulos et al., 2016). Hypomorphism of RPGRIPl1, leads to a reduction in ADCY3 positive cilia, increased food consumption and decreased sensitivity to leptin signaling (Stratigopoulos et al., 2016). The effects of Rpgrip1l mutation may be in part, neurodevelopmental in nature. Congenital targeting of Rpgrip1l in POMC neurons results in decreased neuronal number and obesity, while adult onset of hypomorphic mutations does not cause adiposity. Teasing apart changes in neurodevelopment and signaling in adult neurons will be important for understanding the role of ciliary signaling in controlling feeding behaviors (Cox and Powley, 1981).
MCH plays a role in energy homeostasis as an orexigenic peptide that is upregulated during fasted states to increase food intake (Qu et al., 1996). In addition to the zona incerta, MCH is expressed in the lateral hypothalamus and sends projections locally to the ARC and VMH and to regions such as the brain stem and nucleus accumbens to modulate autonomic and hedonic aspects of food intake (Figure 2) (Luthin, 2007). These neurons also project to several olfactory regions; the olfactory bulb (OB), anterior olfactory nucleus (AON), piriform cortex (PC), and olfactory tubercle (OT), where it is poised to modulate olfactory information that could influence feeding behaviors. Interestingly, MCH knockout mice show some defects in olfactory driven behaviors, such as reduced ability to locate a food source, and decreased maternal behaviors (Vaisse et al., 2000). MCH also promotes sleep, and MCH neurons exhibit increased activity during rapid eye movement (REM) sleep (Morselli et al., 2014; Kim et al., 2021). Optogenetically stimulating MCH neurons can prolong REM sleep, while their inhibition can shorten sleep cycles in rodents (Marsh et al., 1999; Morselli et al., 2014). MCH activity at the primary cilium offers another dimension for integration of peripheral input in hypothalamic neurons through the olfactory system. Peripherally, MCHR1 is found on adipocytes and the pancreas (Macneil et al., 2013), linking metabolic states in the brain with energy supplies in the form of fats and insulin. Localization of MCHR1 to the primary cilium is essential for its function and mislocalization to the plasma membrane yields an altered transcriptional profile in cell models (Cook et al., 2021).
MCH levels are elevated in diet-induced obese rats, and chronic infusion is sufficient to produce obesity in mice under either standard or high-fat diets (Gomori et al., 2003; Elliott et al., 2004). Conversely, Mch −/− and Mchr1 −/− mice are both lean (Shimada et al., 1998; Marsh et al., 2002). Considering this, it seems that for MCHR1 mislocalization to contribute to obesity in BBS, the receptor must still be able to signal outside of the cilia and may even exhibit a gain of function to promote hyperphagia (Vaisse et al., 2017). MCH signaling at MCHR1 has been shown to modulate ciliary length. MCH shortens primary cilia in hTERT-RPE1 cells and in vivo work has replicated these results with optogenetic and chemogenetic stimulation of MCH neurons in CA1, striatum, prefrontal cortex, and nucleus accumbens (Hamamoto et al., 2016; Alhassen et al., 2022b). Ciliary length modulation by MCH opposes leptin’s anorexigenic signaling which elongates cilia. Cilia are shortened in obese ob/ob mice where perhaps orexigenic signaling experiences greater disinhibition due to the absence of leptin. This raises the question of how shortening cilia is beneficial for orexigenic signaling and how its inhibition may contribute to obesity.
The median eminence (ME) is a circumventricular organ known for its blood-brain barrier (BBB) permeability and as such represents a critical node for peripheral-central metabolic communication. MCH neurons in the lateral hypothalamus that regulate feeding behavior project to the ME where they regulate blood-brain barrier permeability through their contacts with tanycytes and endothelial cells. Tanycytes are specialized ependymal/glial cells lining the ventricles that regulate cerebrospinal fluid flow. The vasculature of the ME is distinct from the rest of the brain and is organized into fenestrated capillary loops in contact with tanycytic processes. During fasted states, in which MCH levels are high, ME capillary fenestration is increased due to vascular endothelial growth factor-A (VEGF-A) release from MCH+ neurons. In mice, optogenetic activation of MCH+ neurons increased leptin sensitivity measured by reduced food intake as well as permeability of microvessel loops in the ME (Jiang et al., 2020). MCH+ neurons also project into the cerebroventricular space. Clever trapping experiments for immunosequestration of MCH in the third ventricle that reduced MCH levels in the cerebrospinal fluid also reduced food intake in rats. This work was critical in demonstrating activity of MCH outside of its receptor through volume transmission rather than synaptically (Noble et al., 2018).
Neuronal primary cilia regulate metabolic regulation via specific localization of trafficking proteins, receptors, and secondary messengers. Research has indicated that aberrant ciliary signaling through mutations that affect ciliary length, protein localization, and ciliary membrane composition have robust, global effects on energy homeostasis. This is particularly true when signaling of hypothalamic neuronal primary cilia are disrupted due to their bidirectional relationship with peripheral nutrient signals at circumventricular organs. Targeted inhibition of ciliary-specific receptors may therefore represent an attractive tool for mitigating neuropathology related to metabolic dysfunction.
KD, MR, and AS, and wrote the review and prepared figures under the guidance of JCM.
This work was supported by funding from NIH R01DC019379 awarded to JCM.
We thank Lori Driscoll, Ph.D., and Darrell Killian, Ph.D. for feedback on early versions of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahima R. S. (2008). Revisiting leptin’s role in obesity and weight loss. J. Clin. Investigation 118, 2380–2383. doi:10.1172/jci36284
Alhassen W., Alhassen S., Chen J., Monfared R. V., Alachkar A. (2022). Cilia in the striatum mediate timing-dependent functions. Mol. Neurobiol. 60, 545–565. doi:10.1007/s12035-022-03095-9
Alhassen W., Kobayashi Y., Su J., Robbins B., Nguyen H., Myint T., et al. (2022). Regulation of brain primary cilia length by MCH signaling: Evidence from pharmacological, genetic, optogenetic, and chemogenic manipulations. Mol. Neurobiol. 59 (1), 245–265. doi:10.1007/s12035-021-02511-w
Atkinson K. F., Kathem S. H., Jin X., Muntean B. S., AbouAlaiwi W. A., Nauli A. M., et al. (2015). Dopaminergic signaling within the primary cilia in the renovascular system. Front. Physiol. 6, 103. doi:10.3389/fphys.2015.00103
Brobeck J. R. (1946). Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol. Rev. 26 (4), 541–559. doi:10.1152/physrev.1946.26.4.541
Baldini G., Phelan K. D. (2019). The melanocortin pathway and control of appetite-progress and therapeutic implications. J. Endocrinol. 241, R1–R33. doi:10.1530/JOE-18-0596
Bates S. H., Stearns W. H., Dundon T. A., Schubert M., Tso A. W. K., Wang Y., et al. (2003). STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421 (6925), 856–859. doi:10.1038/nature01388
Benzinou M., Walley A., Lobbens S., Charles M. A., Jouret B., Fumeron F., et al. (2006). Bardet-Biedl syndrome gene variants are associated with both childhood and adult common obesity in French Caucasians. Diabetes 55 (10), 2876–2882. doi:10.2337/db06-0337
Berbari N. F., Lewis J. S., Bishop G. A., Askwith C. C., Mykytyn K. (2008). Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc. Natl. Acad. Sci. U. S. A. 105 (11), 4242–4246. doi:10.1073/pnas.0711027105
Bingham N. C., Anderson K. K., Reuter A. L., Stallings N. R., Parker K. L. (2008). Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology 149 (5), 2138–2148. doi:10.1210/en.2007-1200
Bishop G. A., Berbari N. F., Lewis J., Mykytyn K. (2007). Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J. Comp. Neurology 505 (5), 562–571. doi:10.1002/cne.21510
Blacque O. E., Reardon M. J., Li C., McCarthy J., Mahjoub M. R., Ansley S. J., et al. (2004). Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes. Dev. 18 (13), 1630–1642. doi:10.1101/gad.1194004
Borman A. D., Pearce L. R., Mackay D. S., Nagel-Wolfrum K., Davidson A. E., Henderson R., et al. (2014). A homozygous mutation in the TUB gene associated with retinal dystrophy and obesity. Hum. Mutat. 35 (3), 289–293. doi:10.1002/humu.22482
Bouret S. G., Bates S. H., Chen S., Myers M. G., Simerly R. B. (2012). Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. J. Neurosci. 32 (4), 1244–1252. doi:10.1523/JNEUROSCI.2277-11.2012
Bouret S. G., Draper S. J., Simerly R. B. (2004). Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304, 108–110. doi:10.1126/science.1095004
Bukiya A. N., Durdagim S., Noskov S., Rosenhouse-Dantske A. (2017). Cholesterol up-regulates neuronal G protein-gated inwardly rectifying potassium (GIRK) channel activity in the hippocampus. J. Biol. Chem. 292 (15), 6135–6147. doi:10.1074/jbc.M116.753350
Büscher A. K., Cetiner M., Büscher R., Wingen A. M., Hauffa B. P., Hoyer P. F. (2012). Obesity in patients with Bardet-Biedl syndrome: Influence of appetite-regulating hormones. Pediatr. Nephrol. 27 (11), 2065–2071. doi:10.1007/s00467-012-2220-y
Cao H., Chen X., Yang Y., R Storm D. (2016). Disruption of type 3 adenylyl cyclase expression in the hypothalamus leads to obesity. Integr. Obes. Diabetes 2 (3), 225–228. doi:10.15761/IOD.1000149
Chung W. K., Belfi K., Chua M., Wiley J., Mackintosh R., Nicolson M., et al. (1998). Heterozygosity forLep<sup>ob</sup>orLepr<sup>db</sup>affects body composition and leptin homeostasis in adult mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 274 (4 43-4), R985–R990. doi:10.1152/ajpregu.1998.274.4.r985
Coleman D. L., Eicher E. M. (1990). Fat (fat) and tubby (tub): Two autosomal recessive mutations causing obesity syndromes in the mouse. J. Hered. 81 (6), 424–427. doi:10.1093/oxfordjournals.jhered.a111019
Conduit S. E., Vanhaesebroeck B. (2020). Phosphoinositide lipids in primary cilia biology. Biochem. J. 477, 3541–3565. doi:10.1042/BCJ20200277
Cook L. B., Ophardt H. D., Shen R., Pratt B. H., Galbier L. A. (2021). Transcriptome analysis of ciliary-dependent MCH signaling in differentiating 3T3-L1 pre-adipocytes. Sci. Rep. 11, 4880. doi:10.1038/s41598-021-84138-4
Coupé B., Ishii Y., Dietrich M. O., Komatsu M., Horvath T. L., Bouret S. G. (2012). Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell. Metab. 15 (2), 247–255. doi:10.1016/j.cmet.2011.12.016
Cox J. E., Powley T. L. (1981). Intragastric pair feeding fails to prevent VMH obesity or hyperinsulinemia. Am. J. Physiol. Endocrinol. Metab. 3, 5.
Croft J. B., Morrell D., Chase C. L., Swift M. (1995). Obesity in heterozygous carriers of the gene for the Bardet-Biedl syndrome. Am. J. Med. Genet. 55 (1), 12–15. doi:10.1002/ajmg.1320550105
Davenport J. R., Watts A. J., Roper V. C., Croyle M. J., van Groen T., Wyss J. M., et al. (2007). Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 17 (18), 1586–1594. doi:10.1016/j.cub.2007.08.034
Davis R. E., Swiderski R. E., Rahmouni K., Nishimura D. Y., Mullins R. F., Agassandian K., et al. (2007). A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc. Natl. Acad. Sci. U. S. A. 104 (49), 19422–19427. doi:10.1073/pnas.0708571104
de Oliveira R., Cerda A., Genvigir F. D. V., Sampaio M. F., Armaganijan D., Bernik M. M. S., et al. (2013). Leptin receptor gene polymorphisms are associated with adiposity and metabolic alterations in Brazilian individuals. Arq. Bras. Endocrinol. Metabol. 57 (9), 677–684. doi:10.1590/s0004-27302013000900002
Decaen P. G., Delling M., Vien T. N., Clapham D. E. (2013). Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504 (7479), 315–318. doi:10.1038/nature12832
Delling M., Decaen P. G., Doerner J. F., Febvay S., Clapham D. E. (2013). Primary cilia are specialized calcium signalling organelles. Nature 504 (7479), 311–314. doi:10.1038/nature12833
Dhillon H., Zigman J. M., Ye C., Lee C. E., McGovern R. A., Tang V., et al. (2006). Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49 (2), 191–203. doi:10.1016/j.neuron.2005.12.021
Domire J. S., Green J. A., Lee K. G., Johnson A. D., Askwith C. C., Mykytyn K. (2011). Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cell. Mol. Life Sci. 68 (17), 2951–2960. doi:10.1007/s00018-010-0603-4
Dyson J. M., Conduit S. E., Feeney S. J., Hakim S., DiTommaso T., Fulcher A. J., et al. (2017). INPP5E regulates phosphoinositide-dependent cilia transition zone function. J. Cell. Biol. 216 (1), 247–263. doi:10.1083/jcb.201511055
Eichers E. R., Abd-El-Barr M. M., Paylor R., Lewis R. A., Bi W., Lin X., et al. (2006). Phenotypic characterization of Bbs4 null mice reveals age-dependent penetrance and variable expressivity. Hum. Genet. 120 (2), 211–226. doi:10.1007/s00439-006-0197-y
Elliott J. C., Harrold J. A., Brodin P., Enquist K., Bäckman A., Byström M., et al. (2004). Increases in melanin-concentrating hormone and MCH receptor levels in the hypothalamus of dietary-obese rats. Mol. Brain Res. 128 (2), 150–159. doi:10.1016/j.molbrainres.2004.06.010
Emmer B. T., Souther C., Toriello K. M., Olson C. L., Epting C. L., Engman D. M. (2009). Identification of a palmitoyl acyltransferase required for protein sorting to the flagellar membrane. J. Cell. Sci. 122 (6), 867–874. doi:10.1242/jcs.041764
Fan Y., Rahman P., Peddle L., Hefferton D., Gladney N., Moore S. J., et al. (2004). Bardet-Biedl syndrome 1 genotype and obesity in the Newfoundland population. Int. J. Obes. 28 (5), 680–684. doi:10.1038/sj.ijo.0802601
Feuillan P. P., Ng D., Han J. C., Sapp J. C., Wetsch K., Spaulding E., et al. (2011). Patients with Bardet-Biedl syndrome have hyperleptinemia suggestive of leptin resistance. J. Clin. Endocrinol. Metabolism 96 (3), E528–E535. doi:10.1210/jc.2010-2290
Forsythe E., Beales P. L. (2013). Bardet-Biedl syndrome. Eur. J. Hum. Genet. 21 (1), 8–13. doi:10.1038/ejhg.2012.115
Garcia-Gonzalo F. R., Corbit K. C., Sirerol-Piquer M. S., Ramaswami G., Otto E. A., Noriega T. R., et al. (2011). A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat. Genet. 43 (8), 776–784. doi:10.1038/ng.891
Garcia-Gonzalo F. R., Phua S. C., Roberson E. C., Garcia G., Abedin M., Schurmans S., et al. (2015). Phosphoinositides regulate ciliary protein trafficking to modulate hedgehog signaling. Dev. Cell. 34 (4), 400–409. doi:10.1016/j.devcel.2015.08.001
Gomori A., Ishihara A., Ito M., Mashiko S., Matsushita H., Yumoto M., et al. (2003). Characterization of MCH-mediated obesity in mice. Am. J. Physiol. Endocrinol. Metab. 284 (3 47-3), E940–E945. doi:10.1152/ajpendo.00529.2002
Gorska E., Popko K., Stelmaszczyk-Emmel A., Ciepiela O., Kucharska A., Wasik M. (2010). Leptin receptors. Eur. J. Med. Res. 15, 3–12. doi:10.1007/978-3-642-24716-3_1
Grace C., Beales P., Summerbell C., Jebb S. A., Wright A., Parker D., et al. (2003). Energy metabolism in Bardet-Biedl syndrome. Int. J. Obes. 27 (11), 1319–1324. doi:10.1038/sj.ijo.0802420
Grarup N., Moltke I., Andersen M. K., Dalby M., Vitting-Seerup K., Kern T., et al. (2018). Loss-of-function variants in ADCY3 increase risk of obesity and type 2 diabetes. Nat. Genet. 50 (2), 172–174. doi:10.1038/s41588-017-0022-7
Green J. A., Mykytyn K. (2010). Neuronal ciliary signaling in homeostasis and disease. Cell. Mol. Life Sci. 67, 3287–3297. doi:10.1007/s00018-010-0425-4
Guadiana S. M., Semple-Rowland S., Daroszewski D., Madorsky I., Breunig J. J., Mykytyn K., et al. (2013). Arborization of dendrites by developing neocortical neurons is dependent on primary cilia and type 3 adenylyl cyclase. J. Neurosci. 33 (6), 2626–2638. doi:10.1523/JNEUROSCI.2906-12.2013
Guo D. F., Cui H., Zhang Q., Morgan D. A., Thedens D. R., Nishimura D., et al. (2016). The BBSome controls energy homeostasis by mediating the transport of the leptin receptor to the plasma membrane. PLoS Genet. 12 (2), e1005890. doi:10.1371/journal.pgen.1005890
Guo D. F., Lin Z., Wu Y., Searby C., Thedens D. R., Richerson G. B., et al. (2019). The BBSome in POMC and AgRP neurons is necessary for body weight regulation and sorting of metabolic receptors. Diabetes 68 (8), 1591–1603. doi:10.2337/db18-1088
Guo J., Otis J. M., Higginbotham H., Monckton C., Cheng J. G., Asokan A., et al. (2017). Primary cilia signaling shapes the development of interneuronal connectivity. Dev. Cell. 42 (3), 286–300.e4. doi:10.1016/j.devcel.2017.07.010
Guo J., Otis J. M., Suciu S. K., Catalano C., Xing L., Constable S., et al. (2019). Primary cilia signaling promotes axonal tract development and is disrupted in Joubert syndrome-related disorders models. Dev. Cell. 51 (6), 759–774.e5. doi:10.1016/j.devcel.2019.11.005
Halls M. L., Cooper D. M. F. (2011). Regulation by Ca2+-signaling pathways of adenylyl cyclases. Cold Spring Harb. Perspect. Biol. 3 (1), a004143. doi:10.1101/cshperspect.a004143
Hamamoto A., Yamato S., Katoh Y., Nakayama K., Yoshimura K., Takeda S., et al. (2016). Modulation of primary cilia length by melanin-concentrating hormone receptor 1. Cell. Signal 28 (6), 572–584. doi:10.1016/j.cellsig.2016.02.018
Han Y. M., Kang G. M., Byun K., Ko H. W., Kim J., Shin M. S., et al. (2014). Leptin-promoted cilia assembly is critical for normal energy balance. J. Clin. Investigation 124 (5), 2193–2197. doi:10.1172/JCI69395
Hernández-Cáceres M. P., Cereceda K., Hernández S., Li Y., Narro C., Rivera P., et al. (2020). Palmitic acid reduces the autophagic flux in hypothalamic neurons by impairing autophagosome-lysosome fusion and endolysosomal dynamics. Mol. Cell. Oncol. 7 (5), 1789418. doi:10.1080/23723556.2020.1789418
Hernández-Cáceres M. P., Toledo-Valenzuela L., Díaz-Castro F., Ávalos Y., Burgos P., Narro C., et al. (2019). Palmitic acid reduces the autophagic flux and insulin sensitivity through the activation of the free fatty acid receptor 1 (FFAR1) in the hypothalamic neuronal cell line N43/5. Front. Endocrinol. (Lausanne) 10 (MAR), 176. doi:10.3389/fendo.2019.00176
Hernandez-Hernandez V., Pravincumar P., Diaz-Font A., May-Simera H., Jenkins D., Knight M., et al. (2013). Bardet-biedl syndrome proteins control the cilia length through regulation of actin polymerization. Hum. Mol. Genet. 22 (19), 3858–3868. doi:10.1093/hmg/ddt241
Huszar D., Lynch C. A., Fairchild-Huntress V., Dunmore J. H., Fang Q., Berkemeier L. R., et al. (1997). Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 88 (1), 131–141. doi:10.1016/s0092-8674(00)81865-6
Jiang H., Gallet S., Klemm P., Scholl P., Folz-Donahue K., Altmüller J., et al. (2020). MCH neurons regulate permeability of the median eminence barrier. Neuron 107 (2), 306–319. doi:10.1016/j.neuron.2020.04.020
Jiang J. Y., Falcone J. L., Curci S., Hofer A. M. (2019). Direct visualization of cAMP signaling in primary cilia reveals up-regulation of ciliary GPCR activity following Hedgehog activation. Proc. Natl. Acad. Sci. U. S. A. 116 (24), 12066–12071. doi:10.1073/pnas.1819730116
Jin H., White S. R., Shida T., Schulz S., Aguiar M., Gygi S. P., et al. (2010). The conserved bardet-biedl syndrome proteins assemble a coat that traffics membrane proteins to Cilia. Cell. 141 (7), 1208–1219. doi:10.1016/j.cell.2010.05.015
Kaiser F., Huebecker M., Wachten D. (2020). Sphingolipids controlling ciliary and microvillar function. FEBS Lett. 594, 3652–3667. doi:10.1002/1873-3468.13816
Kang G. M., Han Y., Ko H. W., Kim J., Chul Oh B., Kwon I., et al. (2015). Leptin elongates hypothalamic neuronal cilia via transcriptional regulation and actin destabilization. J. Biol. Chem. 290 (29), 18146–18155. doi:10.1074/jbc.M115.639468
Kee H. L., Verhey K. J. (2013). Molecular connections between nuclear and ciliary import processes. Cilia 2, 11. doi:10.1186/2046-2530-2-11
Kim D., Lee Y., Kim H. R., Park Y. J., Hwang H., Rhim H., et al. (2021). Hypothalamic administration of sargahydroquinoic acid elevates peripheral thermogenic signaling and ameliorates high fat diet-induced obesity through the sympathetic nervous system. Sci. Rep. 11 (1), 21315. doi:10.1038/s41598-021-00074-3
Kim J., Jo H., Hong H., Kim M. H., Kim J. M., Lee J. K., et al. (2015). Actin remodelling factors control ciliogenesis by regulating YAP/TAZ activity and vesicle trafficking. Nat. Commun. 6, 6781. doi:10.1038/ncomms7781
Kinnebrew M., Iverson E. J., Patel B. B., Pusapati G. v., Kong J. H., Johnson K. A., et al. (2019). Cholesterol accessibility at the ciliary membrane controls hedgehog signaling. Elife 8, e50051. doi:10.7554/eLife.50051
Klok M. D., Jakobsdottir S., Drent M. L. (2007). The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 8, 21–34. doi:10.1111/j.1467-789X.2006.00270.x
Kobayashi Y., Okada T., Miki D., Sekino Y., Koganezawa N., Shirao T., et al. (2021). Properties of primary cilia in melanin-concentrating hormone receptor 1-bearing hippocampal neurons in vivo and in vitro. Neurochem. Int. 142, 104902. doi:10.1016/j.neuint.2020.104902
Krude H., Biebermann H., Luck W., Horn R., Brabant G., Grüters A. (1998). Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet. 19 (2), 155–157. doi:10.1038/509
Krude H., Biebermann H., Schnabel D., Tansek M. Z., Theunissen P., Mullis P. E., et al. (2003). Obesity due to proopiomelanocortin deficiency: Three new cases and treatment trials with thyroid hormone and ACTH4-10. J. Clin. Endocrinol. Metabolism 88 (10), 4633–4640. doi:10.1210/jc.2003-030502
Lechtreck K. F. (2015). IFT-cargo interactions and protein transport in cilia. Trends Biochem. Sci. 40, 765–778. doi:10.1016/j.tibs.2015.09.003
Lee C. H., Song D. K., Park C. B., Choi J., Kang G. M., Shin S. H., et al. (2020). Primary cilia mediate early life programming of adiposity through lysosomal regulation in the developing mouse hypothalamus. Nat. Commun. 11 (1), 5772. doi:10.1038/s41467-020-19638-4
Lee E. B., Mattson M. P. (2014). The neuropathology of obesity: Insights from human disease. Acta Neuropathol. 127, 3–28. doi:10.1007/s00401-013-1190-x
Leibowitz S. F., Hammer N. J., Chang K. (1981). Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol. Behav. 27 (6), 1031–1040. doi:10.1016/0031-9384(81)90366-8
Lim Y. C., McGlashan S. R., Cooling M. T., Long D. S. (2015). Culture and detection of primary cilia in endothelial cell models. Cilia 4 (1), 11. doi:10.1186/s13630-015-0020-2
Liu X., Vien T., Duan J., Sheu S. H., DeCaen P. G., Clapham D. E. (2018). Polycystin-2 is an essential ion channel subunit in the primary cilium of the renal collecting duct epithelium. Elife 7, e33183. doi:10.7554/eLife.33183
Loktev A. V., Jackson P. K., Neuropeptide Y. (2013). Neuropeptide Y family receptors traffic via the Bardet-Biedl syndrome pathway to signal in neuronal primary cilia. Cell. Rep. 5 (5), 1316–1329. doi:10.1016/j.celrep.2013.11.011
Loktev A. v., Zhang Q., Beck J. S., Searby C. C., Scheetz T. E., Bazan J. F., et al. (2008). A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev. Cell. 15 (6), 854–865. doi:10.1016/j.devcel.2008.11.001
Lukomska A., Dobrzanski G., Liguz-Lecznar M., Kossut M. (2020). Somatostatin receptors (SSTR1-5) on inhibitory interneurons in the barrel cortex. Brain Struct. Funct. 225 (1), 387–401. doi:10.1007/s00429-019-02011-7
Lüscher C., Slesinger P. A. (2010). Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat. Rev. Neurosci. 11, 301–315. doi:10.1038/nrn2834
Luthin D. R. (2007). Anti-obesity effects of small molecule melanin-concentrating hormone receptor1 (MCHR1) antagonists. Life Sci. 81, 423–440. doi:10.1016/j.lfs.2007.05.029
Lynne Blasius T., Takao D., Verhey K. J. (2019). NPHP proteins are binding partners of nucleoporins at the base of the primary cilium. PLoS One 14 (9), e0222924. doi:10.1371/journal.pone.0222924
Macneil D. J., Vaudry H., Boutin J. A. (2013). The role of melanin-concentrating hormone and its receptors in energy homeostasis. Front. Endocrinol. (Lausanne) 4, 49. doi:10.3389/fendo.2013.00049
Marginean C. O., Marginean C., Voidazan S., Melit L., Crauciuc A., Duicu C., et al. (2016). Correlations between leptin gene polymorphisms 223 A/G, 1019 G/A, 492 G/C, 976 C/A, and anthropometrical and biochemical parameters in children with obesity. Med. (United States) 95 (12), e3115. doi:10.1097/md.0000000000003115
Marsh D. J., Hollopeter G., Huszar D., Laufer R., Yagaloff K. A., Fisher S. L., et al. (1999). Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat. Genet. 21 (1), 119–122. doi:10.1038/5070
Marsh D. J., Weingarth D. T., Novi D. E., Chen H. Y., Trumbauer M. E., Chen A. S., et al. (2002). Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc. Natl. Acad. Sci. U. S. A. 99 (5), 3240–3245. doi:10.1073/pnas.052706899
Marshall J. D., Maffei P., Collin G. B., Naggert J. K. (2011). Alström syndrome: Genetics and clinical overview. Curr. Genomics 12 (3), 225–235. doi:10.2174/138920211795677912
McIntyre J. C., Joiner A. M., Zhang L., Iñiguez-Lluhí J., Martens J. R. (2015). SUMOylation regulates ciliary localization of olfactory signaling proteins. J. Cell. Sci. 128 (10), 1934–1945. doi:10.1242/jcs.164673
Meiser J., Weindl D., Hiller K. (2013). Complexity of dopamine metabolism. Cell. Commun. Signal. 11, 34. doi:10.1186/1478-811X-11-34
Meyer T., Wellner-Kienitz M. C., Biewald A., Bender K., Eickel A., Pott L. (2001). Depletion of phosphatidylinositol 4,5-bisphosphate by activation of phospholipase C-coupled receptors causes slow inhibition but not desensitization of G protein-gated inward rectifier K+ current in atrial myocytes. J. Biol. Chem. 276 (8), 5650–5658. doi:10.1074/jbc.M009179200
Moore B. S., Stepanchick A. N., Tewson P. H., Hartle C. M., Zhang J., Quinn A. M., et al. (2016). Cilia have high cAMP levels that are inhibited by Sonic Hedgehog-regulated calcium dynamics. Proc. Natl. Acad. Sci. U. S. A. 113 (46), 13069–13074. doi:10.1073/pnas.1602393113
Moore E. R., Ryu H. S., Zhu Y. X., Jacobs C. R. (2018). Adenylyl cyclases and TRPV4 mediate Ca2+/cAMP dynamics to enhance fluid flow-induced osteogenesis in osteocytes. J. Mol. Biochem. 7, 48–59.
Morselli E., Fuente-Martin E., Finan B., Kim M., Frank A., Garcia-Caceres C., et al. (2014). Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell. Rep. 9 (2), 633–645. doi:10.1016/j.celrep.2014.09.025
Mukhopadhyay S., Wen X., Chih B., Nelson C. D., Lane W. S., Scales S. J., et al. (2010). TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes. Dev. 24 (19), 2180–2193. doi:10.1101/gad.1966210
Myers B. R., Neahring L., Zhang Y., Roberts K. J., Beachy P. A. (2017). Rapid, direct activity assays for Smoothened reveal Hedgehog pathway regulation by membrane cholesterol and extracellular sodium. Proc. Natl. Acad. Sci. U. S. A. 114 (52), E11141–E11150. doi:10.1073/pnas.1717891115
Mykytyn K., Mullins R. F., Andrews M., Chiang A. P., Swiderski R. E., Yang B., et al. (2004). Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc. Natl. Acad. Sci. U. S. A. 101 (23), 8664–8669. doi:10.1073/pnas.0402354101
Nachury M. v., Loktev A. v., Zhang Q., Westlake C. J., Peränen J., Merdes A., et al. (2007). A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 129 (6), 1201–1213. doi:10.1016/j.cell.2007.03.053
Nachury M. v., Mick D. U. (2019). Establishing and regulating the composition of cilia for signal transduction. Nat. Rev. Mol. Cell. Biol. 20, 389–405. doi:10.1038/s41580-019-0116-4
Nachury M. v., Seeley E. S., Jin H. (2010). Trafficking to the ciliary membrane: How to get across the periciliary diffusion barrier? Annu. Rev. Cell. Dev. Biol. 26, 59–87. doi:10.1146/annurev.cellbio.042308.11333
Nachury M. v. (2018). The molecular machines that traffic signaling receptors into and out of cilia. Curr. Opin. Cell. Biol. 51, 124–131. doi:10.1016/j.ceb.2018.03.004
Napoli G., Panzironi N., Traversa A., Catalanotto C., Pace V., Petrizzelli F., et al. (2022). Potassium Channel KCNH1 activating variants cause altered functional and morphological ciliogenesis. Mol. Neurobiol. 59 (8), 4825–4838. doi:10.1007/s12035-022-02886-4
Nishimura D. Y., Fath M., Mullins R. F., Searby C., Andrews M., Davis R., et al. (2004). Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc. Natl. Acad. Sci. U. S. A. 101 (47), 16588–16593. doi:10.1073/pnas.0405496101
Noble E. E., Hahn J. D., Konanur V. R., Hsu T. M., Page S. J., Cortella A. M., et al. (2018). Control of feeding behavior by cerebral ventricular volume transmission of melanin-concentrating hormone. Cell. Metab. 28 (1), 55–68.e7. doi:10.1016/j.cmet.2018.05.001
Nordman S., Abulaiti A., Hilding A., Långberg E. C., Humphreys K., Östenson C. G., et al. (2008). Genetic variation of the adenylyl cyclase 3 (AC3) locus and its influence on type 2 diabetes and obesity susceptibility in Swedish men. Int. J. Obes. 32 (3), 407–412. doi:10.1038/sj.ijo.0803742
Olsen A. S. B., Færgeman N. J. (2017). Sphingolipids: Membrane microdomains in brain development, function and neurological diseases. Open Biol. 7, 170069. doi:10.1098/rsob.170069
Palicharla V., Hwang S. H., Bandarigoda S., Legue E., Shimada I., Familiari N., et al. (2023). Interactions between TULP3 tubby domain and ARL13B amphipathic helix promote lipidated protein transport to cilia. Mol. Biol. Cell. 34 (3), ar18. doi:10.1091/mbc.E22-10-0473
Phua S. C., Chiba S., Suzuki M., Su E., Roberson E. C., Pusapati G. v., et al. (2017). Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell. 168 (1–2), 264–279.e15. doi:10.1016/j.cell.2016.12.032
Pitman J. L., Wheeler M. C., Lloyd D. J., Walker J. R., Glynne R. J., Gekakis N. (2014). A gain-of-function mutation in Adenylate Cyclase 3 protects mice from diet-induced obesity. PLoS One 9 (10), e110226. doi:10.1371/journal.pone.0110226
Pomeroy J., Krentz A. D., Richardson J. G., Berg R. L., VanWormer J. J., Haws R. M. (2021). Bardet-Biedl syndrome: Weight patterns and genetics in a rare obesity syndrome. Pediatr. Obes. 16 (2), e12703. doi:10.1111/ijpo.12703
Qu D., Ludwig D. S., Gammeltoft S., Piper M., Pelleymounter M. A., Cullen M. J., et al. (1996). A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 380 (6571), 243–247. doi:10.1038/380243a0
Rahmouni K., Fath M. A., Seo S., Thedens D. R., Berry C. J., Weiss R., et al. (2008). Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J. Clin. Investigation 118 (4), 1458–1467. doi:10.1172/JCI32357
Ramos C., Roberts J. B., Jasso K. R., ten Eyck T. W., Everett T., Pozo P., et al. (2021). Neuron-specific cilia loss differentially alters locomotor responses to amphetamine in mice. J. Neurosci. Res. 99 (3), 827–842. doi:10.1002/jnr.24755
Ran J., Yang Y., Li D., Liu M., Zhou J. (2015). Deacetylation of α-tubulin and cortactin is required for HDAC6 to trigger ciliary disassembly. Sci. Rep. 5, 12917. doi:10.1038/srep12917
Resh M. D. (2013). Covalent lipid modifications of proteins. Curr. Biol. 23, 419–425. doi:10.1016/j.bbrc.2016.10.086
Ritter S. L., Hall R. A. (2009). Fine-tuning of GPCR activity by receptor-interacting proteins. Nat. Rev. Mol. Cell. Biol. 10, 819–830. doi:10.1038/nrm2803
Rosenbaum J. L., Witman G. B. (2002). Intraflagellar transport. Nat. Rev. Mol. Cell. Biol. 3, 813–825. doi:10.1038/nrm952
Rouabhi M., Guo D. F., Morgan D. A., Zhu Z., López M., Zingman L., et al. (2021). BBSome ablation in SF1 neurons causes obesity without comorbidities. Mol. Metab. 48, 101211. doi:10.1016/j.molmet.2021.101211
Sadana R., Dessauer C. W. (2009). Physiological roles for G protein-regulated adenylyl cyclase isoforms: Insights from knockout and overexpression studies. NeuroSignals 17, 5–22. doi:10.1159/000166277
Sakaguchi T., Bray G. A., Eddlestone G. (1988). Sympathetic activity following paraventricular or ventromedial hypothalamic lesions in rats. Brain Res. Bull. 20 (4), 461–465. doi:10.1016/0361-9230(88)90135-9
Salameh T. S., Mortell W. G., Logsdon A. F., Butterfield D. A., Banks W. A. (2019). Disruption of the hippocampal and hypothalamic blood-brain barrier in a diet-induced obese model of type II diabetes: Prevention and treatment by the mitochondrial carbonic anhydrase inhibitor, topiramate. Fluids Barriers CNS 16, 1. doi:10.1186/s12987-018-0121-6
Sánchez A., Urrego D., Pardo L. A. (2016). Cyclic expression of the voltage-gated potassium channel K V 10.1 promotes disassembly of the primary cilium. EMBO Rep. 17 (5), 708–723. doi:10.15252/embr.201541082
Saternos H., Ley S., Aboualaiwi W. (2020). Primary cilia and calcium signaling interactions. Int. J. Mol. Sci. 21, 7109. doi:10.3390/ijms21197109
Schou K. B., Pedersen L. B., Christensen S. T. (2015). Ins and outs of GPCR signaling in primary cilia. EMBO Rep. 16 (9), 1099–1113. doi:10.15252/embr.201540530
Seo S., Baye L. M., Schulz N. P., Beck J. S., Zhang Q., Slusarski D. C., et al. (2010). BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly. Proc. Natl. Acad. Sci. U. S. A. 107 (4), 1488–1493. doi:10.1073/pnas.0910268107
Seo S., Guo D. F., Bugge K., Morgan D. A., Rahmouni K., Sheffield V. C. (2009). Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum. Mol. Genet. 18 (7), 1323–1331. doi:10.1093/hmg/ddp031
Sherafat-Kazemzadeh R., Ivey L., Kahn S. R., Sapp J. C., Hicks M. D., Kim R. C., et al. (2013). Hyperphagia among patients with Bardet-Biedl syndrome. Pediatr. Obes. 8 (5), e64–e67. doi:10.1111/j.2047-6310.2013.00182.x
Sherpa R. T., Mohieldin A. M., Pala R., Wachten D., Ostrom R. S., Nauli S. M. (2019). Sensory primary cilium is a responsive cAMP microdomain in renal epithelia. Sci. Rep. 9 (1), 6523. doi:10.1038/s41598-019-43002-2
Sheu S. H., Upadhyayula S., Dupuy V., Pang S., Deng F., Wan J., et al. (2022). A serotonergic axon-cilium synapse drives nuclear signaling to alter chromatin accessibility. Cell. 185 (18), 3390–3407. doi:10.1016/j.cell.2022.07.026
Shimada M., Tritos N. A., Lowell B. B., Flier J. S., Maratos-Flier E. (1998). Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature 396 (6712), 670–674. doi:10.1038/25341
Siljee J. E., Wang Y., Bernard A. A., Ersoy B. A., Zhang S., Marley A., et al. (2018). Subcellular localization of MC4R with ADCY3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nat. Genet. 50 (2), 180–185. doi:10.1038/s41588-017-0020-9
Smith C. E. L., Lake A. V. R., Johnson C. A. (2020). Primary cilia, ciliogenesis and the actin cytoskeleton: A little less resorption, A little more actin please. Front. Cell. Dev. Biol. 8, 622822. doi:10.3389/fcell.2020.622822
Stilling S., Kalliakoudas T., Benninghoven-Frey H., Inoue T., Falkenburger B. H. (2022). PIP2 determines length and stability of primary cilia by balancing membrane turnovers. Commun. Biol. 5 (1), 93. doi:10.1038/s42003-022-03028-1
Stratigopoulos G., Burnett L. C., Rausch R., Gill R., Penn D. B., Skowronski A. A., et al. (2016). Hypomorphism of Fto and Rpgrip1l causes obesity in mice. J. Clin. Investigation 126 (5), 1897–1910. doi:10.1172/JCI85526
Stratigopoulos G., Martin Carli J. F., O’Day D. R., Wang L., Leduc C. A., Lanzano P., et al. (2014). Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the fto locus, causes increased adiposity in mice. Cell. Metab. 19 (5), 767–779. doi:10.1016/j.cmet.2014.04.009
Stubbs T., Bingman J. I., Besse J., Mykytyn K. (2023). Ciliary signaling proteins are mislocalized in the brains of Bardet-Biedl syndrome 1-null mice. Front. Cell Dev Biol. 10, 1092161.
Stubbs T., Koemeter-Cox A., Bingman J. I., Zhao F., Kalyanasundaram A., Rowland L. A., et al. (2022). Disruption of dopamine receptor 1 localization to primary cilia impairs signaling in striatal neurons. J. Neurosci. 42 (35), 6692–6705. doi:10.1523/JNEUROSCI.0497-22.2022
Sun J. S., Yang D. J., Kinyua A. W., Yoon S. G., Seong J. K., Kim J., et al. (2021). Ventromedial hypothalamic primary cilia control energy and skeletal homeostasis. J. Clin. Investigation 131 (1), e138107. doi:10.1172/JCI138107
Sun X., Haley J., Bulgakov O. V., Cai X., McGinnis J., Li T. (2012). Tubby is required for trafficking G protein-coupled receptors to neuronal cilia. Cilia 1, 21. doi:10.1186/2046-2530-1-21
Takechi R., Lam V., Brook E., Giles C., Fimognari N., Mooranian A., et al. (2017). Blood-brain barrier dysfunction precedes cognitive decline and neurodegeneration in diabetic insulin resistant mouse model: An implication for causal link. Front. Aging Neurosci. 9, 399. doi:10.3389/fnagi.2017.00399
Tao B., Bu S., Yang Z., Siroky B., Kappes J. C., Kispert A., et al. (2009). Cystin localizes to primary cilia via membrane microdomains and a targeting motif. J. Am. Soc. Nephrol. 20 (12), 2570–2580. doi:10.1681/ASN.2009020188
Thompson D. B., Ravussin E., Bennett P. H., Bogardus C. (1997). Structure and sequence variation at the human leptin receptor gene in lean and obese Pima Indians. Hum. Mol. Genet. 6 (5), 675–679. doi:10.1093/hmg/6.5.675
Tripathi P., Zhu Z., Qin H., Elsherbini A., Crivelli S. M., Roush E., et al. (2021). Palmitoylation of acetylated tubulin and association with ceramide-rich platforms is critical for ciliogenesis. J. Lipid Res. 62, 100021. doi:10.1194/jlr.RA120001190
Vaisse C., Clement K., Durand E., Hercberg S., Guy-Grand B., Froguel P. (2000). Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J. Clin. Investigation 106 (2), 253–262. doi:10.1172/JCI9238
Vaisse C., Reiter J. F., Berbari N. F. (2017). Cilia and obesity. Cold Spring Harb. Perspect. Biol. 9 (7), a028217. doi:10.1101/cshperspect.a028217
vander Tuig J. G., Knehans A. W., Romsos D. R. (1982). Reduced sympathetic nervous system activity in rats with ventromedial hypothalamic lesions. Life Sci. 30 (11), 913–920. doi:10.1016/0024-3205(82)90619-1
Vieira O. v., Gaus K., Verkade P., Fullekrug J., Vaz W. L. C., Simons K. (2006). FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc. Natl. Acad. Sci. U. S. A. 103 (49), 18556–18561. doi:10.1073/pnas.0608291103
Wang H., Wu M., Zhu W., Shen J., Shi X., Yang J., et al. (2010). Evaluation of the association between the AC3 genetic polymorphisms and obesity in a Chinese Han population. PLoS One 5 (11), e13851. doi:10.1371/journal.pone.0013851
Wang L., Liu Y., Stratigopoulos G., Panigrahi S., Sui L., Zhang Y., et al. (2021). Bardet-Biedl syndrome proteins regulate intracellular signaling and neuronal function in patient-specific iPSC-derived neurons. J. Clin. Investigation 131 (8), e146287. doi:10.1172/JCI146287
Wang Y., Bernard A., Comblain F., Yue X., Paillart C., Zhang S., et al. (2021). Melanocortin 4 receptor signals at the neuronal primary cilium to control food intake and body weight. J. Clin. Investigation 131 (9), e142064. doi:10.1172/JCI142064
Wang Z., Li V., Chan G. C. K., Phan T., Nudelman A. S., Xia Z., et al. (2009). Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS One 4 (9), e6979. doi:10.1371/journal.pone.0006979
Wei Q., Zhang Y., Li Y., Zhang Q., Ling K., Hu J. (2012). The BBSome controls IFT assembly and turnaround in cilia. Nat. Cell. Biol. 14 (9), 950–957. doi:10.1038/ncb2560
Williams C. L., Mcintyre J. C., Norris S. R., Jenkins P. M., Zhang L., Pei Q., et al. (2014). Direct evidence for BBSome-associated intraflagellar transport reveals distinct properties of native mammalian cilia. Nat. Commun. 5, 5813. doi:10.1038/ncomms6813
Wolf M. T. F., Saunier S., O’Toole J. F., Wanner N., Groshong T., Attanasio M., et al. (2007). Mutational analysis of the RPGRIP1L gene in patients with Joubert syndrome and nephronophthisis. Kidney Int. 72 (12), 1520–1526. doi:10.1038/sj.ki.5002630
Yamakawa D., Katoh D., Kasahara K., Shiromizu T., Matsuyama M., Matsuda C., et al. (2021). Primary cilia-dependent lipid raft/caveolin dynamics regulate adipogenesis. Cell. Rep. 34 (10), 108817. doi:10.1016/j.celrep.2021.108817
Yamamoto Y., Mizushima N. (2021). Autophagy and ciliogenesis. JMA J. 4 (3), 207–215. doi:10.31662/jmaj.2021-0090
Yang D., Wu X., Wang W., Zhou Y., Wang Z. (2022). Ciliary type III adenylyl cyclase in the VMH is crucial for high-fat diet-induced obesity mediated by autophagy. Adv. Sci. 9 (3), e2102568. doi:10.1002/advs.202102568
Ye F., Breslow D. K., Koslover E. F., Spakowitz A. J., Nelson W. J., Nachury M. V. (2013). Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. Elife 2, e00654. doi:10.7554/eLife.00654
Ye F., Nager A. R., Nachury M. V. (2018). BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. J. Cell Biol. 217 (5), 1847–1868. doi:10.1083/jcb.201709041
Zaghloul N. A., Katsanis N. (2009). Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J. Clin. Investigation 119, 428–437. doi:10.1172/JCI37041
Zhang Q., Nishimura D., Seo S., Vogel T., Morgan D. A., Searby C., et al. (2011). Bardet-Biedl syndrome 3 (Bbs3) knockout mouse model reveals common BBS-associated phenotypes and Bbs3 unique phenotypes. Proc. Natl. Acad. Sci. U. S. A. 108 (51), 20678–20683. doi:10.1073/pnas.1113220108
Zhang Q., Nishimura D., Vogel T., Shao J., Swiderski R., Yin T., et al. (2013). BBS7 is required for BBSome formation and its absence in mice results in bardet-biedl syndrome phenotypes and selective abnormalities in membrane protein trafficking. J. Cell. Sci. 126 (11), 2372–2380. doi:10.1242/jcs.111740
Keywords: primary cilium, hypothalamus, metabolism, obesity, BBSome, intraflagellar transport
Citation: DeMars KM, Ross MR, Starr A and McIntyre JC (2023) Neuronal primary cilia integrate peripheral signals with metabolic drives. Front. Physiol. 14:1150232. doi: 10.3389/fphys.2023.1150232
Received: 23 January 2023; Accepted: 20 March 2023;
Published: 29 March 2023.
Edited by:
Masaki Saito, Teikyo University, JapanReviewed by:
Hu Huang, East Carolina University, United StatesCopyright © 2023 DeMars, Ross, Starr and McIntyre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeremy C. McIntyre, Jmcin@ufl.edu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.