- Department of Respiratory and Critical Care Medicine, West China School of Medicine and West China Hospital, Sichuan University, Chengdu, China
Background: The predictive ability of the ventilatory ratio (VR) for extubation failure risk in critically ill patients on mechanical ventilation is unclear. This study aims to examine the predictive ability of VR for extubation failure risk.
Methods: This retrospective study was based on the MIMIC-IV database. The MIMIC-IV database consists of the clinical information of patients who were admitted to the intensive care unit at the Beth Israel Deaconess Medical Center between 2008 and 2019. With extubation failure as the primary outcome and in-hospital mortality as the secondary outcome, we assessed the predictive value of VR 4 hours before extubation using a multivariate logistic regression model.
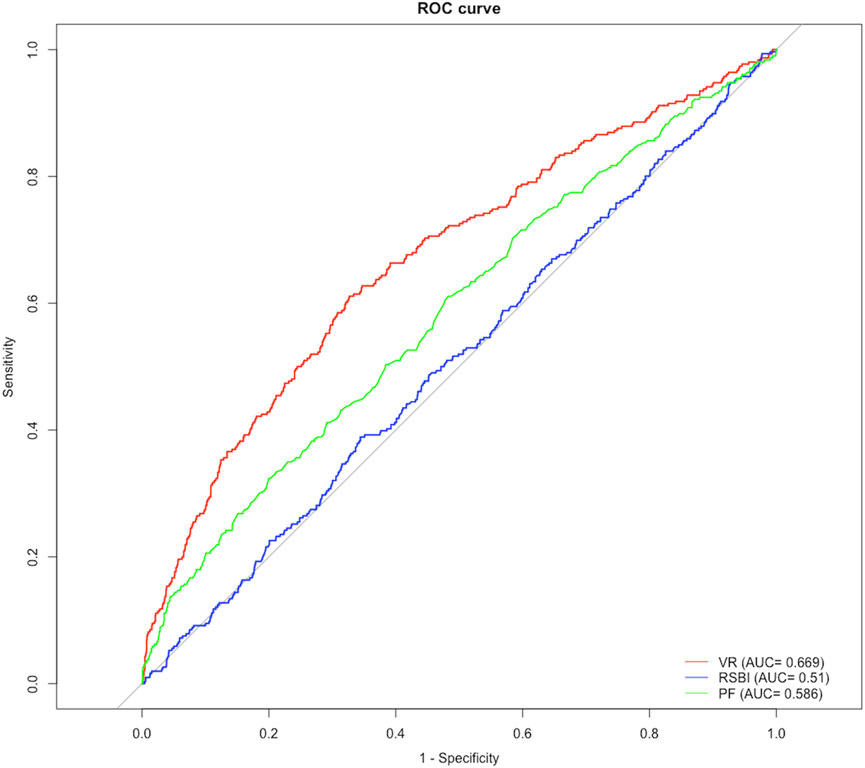
Results: Of 3,569 ventilated patients who were included, the rate of extubation-failure was 12.7% and the median Sequential Organ Failure Assessment (SOFA) score was 6 before extubation. Increased VR, elevated heart rate, greater positive end-expiratory pressure, higher blood urea nitrogen level, higher platelet count, greater SOFA score, decreased pH, decreased tidal volume, presence of chronic pulmonary disease, paraplegia, and metastatic solid tumor were independent predictors for extubation failure. A threshold of 1.595 of VR was associated with prolonged intensive care unit length of stay, higher risk of mortality and extubation failure. The area under the receiver operating characteristic curve (ROC) for VR was 0.669 [0.635–0.703], which was significantly larger than the rapid shallow breathing index [0.510 (0.476–0.545)] and the partial pressure of oxygen to the fraction of inspired oxygen [0.586 (0.551–0.621)].
Conclusion: VR 4 hours before extubation was associated with extubation failure, mortality, and prolonged length of stay in the intensive care unit. VR provides good predictive performance for extubation failure (measured by ROC) than the rapid shallow breathing index. Further prospective studies are warranted to confirm these findings.
Background
Invasive mechanical ventilation (IMV) is an advanced respiratory support widely used in intensive care units (ICUs). The process of removing an endotracheal tube to liberate a patient from mechanical ventilation (MV) is referred to as extubation. Previous studies have found that reintubation after extubation failure was associated with higher morbidity and mortality (Boles et al., 2007; Thille et al., 2013). To avoid prematurely failed extubation attempts while avoiding unnecessary prolonged MV days, many factors such as rapid shallow breathing index (RSBI), maximum occlusion pressure, etc., were assessed by prior studies (Frutos-Vivar et al., 2006; Eskandar and Apostolakos, 2007; Hsieh et al., 2018). However, no ideal parameters were proven to have a high predictive value for extubation. Moreover, those studies focused on oxygenation-related indices other than ventilation-related indices in most cases, while the ability to eliminate carbon dioxide (CO2) was found to be associated with disease progression and prognosis (Nuckton et al., 2002; Cepkova et al., 2007; Siddiki et al., 2010). Therefore, it is meaningful to accurately predict the risk of extubation failure and add additional ventilation-related information to facilitate the extubation process.
The ventilatory ratio (VR) is a simple bedside index of ventilation efficiency (Sinha et al., 2009). VR is defined as the ratio of the observed minute volume and observed partial pressure of arterial carbon dioxide (PaCO2) over the predicted minute volume and predicted PaCO2. The predicted minute volume is calculated with the predicted body weight, and a value that lies close to the mean PaCO2 in healthy individuals represents the predicted PaCO2. A value of VR approximating one represents matched perfusion ventilation that can clear CO2 efficiently (Sinha et al., 2009). Several studies reported that higher VR was associated with higher mortality and shorter ventilator-free days in patients with acute respiratory distress syndrome (ARDS), reflecting the severity of the disease (Sinha et al., 2013a; Sinha et al., 2013b; Sinha et al., 2014; Sinha et al., 2019; Ende et al., 2021; Torres et al., 2021). Previous studies also showed the potential value of VR to adjust strategies to liberate from ventilators. One study found that patients were finally liberated from ventilators only when VR fell under 2 and higher VR was related to a prolonged duration of the weaning process (Proklou et al., 2021). However, to our knowledge, there is no study discussing the predictive ability of VR obtained before extubation for the risk of extubation failure. This study aims to identify the association between VR and extubation failure and explore the cut-off value of VR as a tool to facilitate the decision-making process of extubation in clinical situations.
Methods
Source of data
This study was a retrospective cohort analysis using the open-source critical care database MIMIC-IV (Version 1.0) (Goldberger et al., 2000; Johnson et al., 2011). The MIMIC-IV database consists of the clinical information of patients who were admitted to the ICU at the Beth Israel Deaconess Medical Center between 2008 and 2019. One author, Huan Yang, completed the Collaborative Institutional Training Initiative examination (certification number: 45308698) to achieve access to the database for data extraction.
Outcome definition and data collection
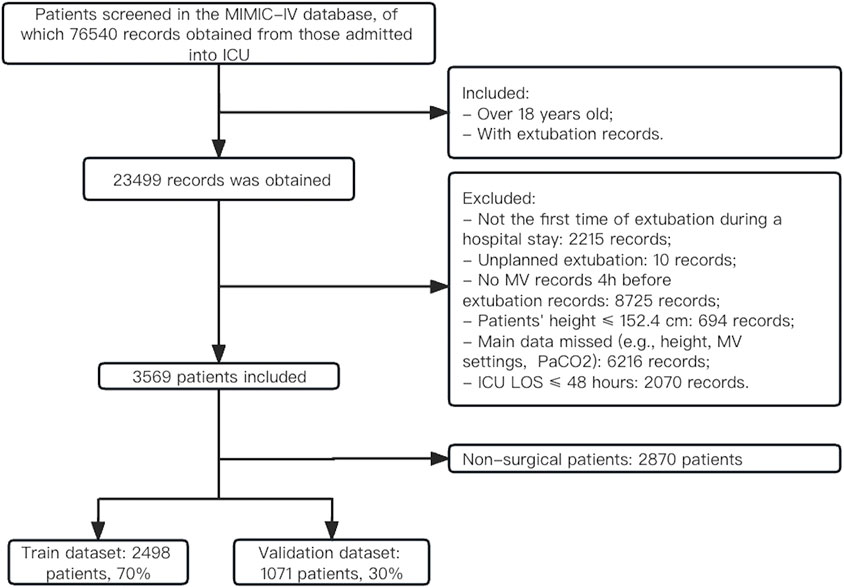
Extubation failure was defined as the need for ventilatory support [noninvasive ventilation (NIV) or reintubation] or death within 48 h following extubation (Boles et al., 2007). We extracted variables with PostgreSQL (Version 14.0; PostgreSQL Global Development Group) and pgAdmin 4 (Version 6.11). The inclusion criteria were as follows: i) age > 18 years, ii) height > 152.4 cm, iii) with extubation records. The exclusion criteria were as follows: i) not the first time of extubation during a hospital stay, ii) unplanned extubation, iii) ICU length of stay (LOS) ≤ 48 h, iv) main data missed: MV settings and PaCO2 4 hours before extubation.
Respiratory parameters, arterial blood gas (ABG), and vital signs were extracted 4 hours before extubation. To reduce missing data, other clinical and laboratory variables were extracted within 24 h before extubation, including Glasgow Coma Scales (GCS), the Sequential Organ Failure Assessment (SOFA), blood routines, liver and kidney functions, coagulations, cardiac markers, etc. Reasonable ranges were set for filtering out extreme values (Supplementary Table S10). Observed values outside these ranges were treated as missing data. For some variables with multiple measurements, the worst values were assessed based on clinical relevance. In addition, features (e.g., age, sex, comorbidities) were selected within 24 h after ICU admission. Finally, NIV use, reintubation, and death within 48 h after extubation, in-hospital mortality, ICU LOS, and hospital LOS were also assessed.
Predicted body weight was calculated using the NHLBI ARDS network formula (Acute Respiratory Distress Syndrome Network et al., 2000).
VR was calculated as follows (Sinha et al., 2009):
Statistical analysis
Continuous variables are presented as medians with their interquartile ranges (IQRs), and categorical variables are presented as total numbers and percentages. Normal distribution for continuous variables was determined using the Shapiro‒Wilk test (Razali and Wah, 2011). Continuous variables (median) were compared using Student’s test (normal distribution) (Mishra et al., 2019) or the Wilcoxon rank sum test (nonnormal distribution) (McKnight et al., 2010), and proportions were compared using χ2 or Fisher exact tests when the numbers were small (Hess and Hess, 2017). Statistical significance was considered to be at two-sided p < 0.05. The MIMIC-IV dataset was first randomly split into the training set (70%) and the internal validation set (30%). In the training set, a receiver operating characteristic (ROC) curve analysis was used to confirm the best cut-off values of VR, RSBI, and the partial pressure of oxygen to the fraction of inspired oxygen (PaO2/FiO2) at 4 h before extubation. The predictive ability of cut-off values was assessed for discrimination using C-statistics in the validation set. Variables with a two-sided p-value of < 0.05 from the independent Student’s t-test/Wilcoxon rank sum test and χ2/Fisher exact tests were considered for multivariate logistic regression. Lasso regression was performed for automatic feature selection. Multicollinearity was calculated through the analysis of the variance inflation factors. Thereafter, factors with clinical plausibility were selected and further analyzed in stepwise multiple logistic regression to identify the association between extubation failure and VR. To test the robustness of any association between extubation failure and VR, interactions with other covariates [i.e., age, RSBI, PaO2/FiO2, sex, positive end-expiratory pressure (PEEP), SOFA] that increased extubation failure were tested (Thille et al., 2013). Furthermore, a sensitivity analysis was conducted by excluding patients who were admitted for surgical reasons. The results were summarized with the odds ratio (OR), 95% confidence interval (CI), and p-value. To avoid bias introduced by missing data, variables with more than 10% missing data were excluded, and the analysis of the primary outcome was imputed using multiple imputation chain equations using the “MICE” package in R. All statistical analyses were performed with R version 4.0.5 (http://www.R-project.org) and Stata/SE 15.1.
Results
Study population
As shown in Figure 1, a total of 76,540 records were obtained from patients who were admitted to the ICU. Of those, 23,499 records were extracted from patients (over 18 years old) who had extubation records. 3,569 patients who underwent planned extubation were ultimately extracted from the MIMIC-IV database according to prespecified inclusion criteria. Overall, the majority of patients (67.1%) were male, and the mean age was 65.29 ± 13.98 years (Supplementary Table S1). The Charlson comorbidity index was 6 (IQR, 4–7) and the median SOFA score was 6 (IQR, 5–9) before extubation. The detailed clinical information for the included patients before extubation is listed in Supplementary Table S1, and the baseline characteristics on ICU admission are presented in Supplementary Table S2. 1839 (56.0%, n = 3,284) patients had VR > 2 on admission, and 496 patients (13.9%, n = 3,569) had VR > 2 before extubation. The median duration of ICU LOS was 4 days (IQR, 3–7) and that of hospital LOS was 10 days (IQR, 7–16). There were 454 (12.7%) patients who failed extubation within 48 h, and the overall in-hospital mortality was 7.3%. The extracted dataset was randomly divided into the training set (n = 2,498) and the internal validation set (n = 1,071). The baseline characteristics were similar between the training set and validation set (Supplementary Table S3). For sensitivity analysis, 2,870 non-surgical patients were obtained. The rate of extubation failure was 13.6% (391) and the rate of mortality was 8.7% (250) in this population (see Supplementary Table S4).
VR and other risk factors
In the training set, patients in the extubation failure group had significantly higher VRs (1.71 [1.36, 2.10] vs. 1.41 [1.19, 1.71], p < 0.001). Moreover, the extubation failure group exhibited a greater prevalence of comorbidities, required higher ventilation settings, had higher mean values for heart rate, GCS, SOFA, PaCO2, anion gap, and a lower mean value of pH, PaO2/FiO2, and peripheral arterial oxygen saturation (SpO2). Notable differences in laboratory variables included higher blood urea nitrogen level (BUN), creatinine level, and platelet count among the extubation failure group (all p < 0.05), as shown in Table 1.
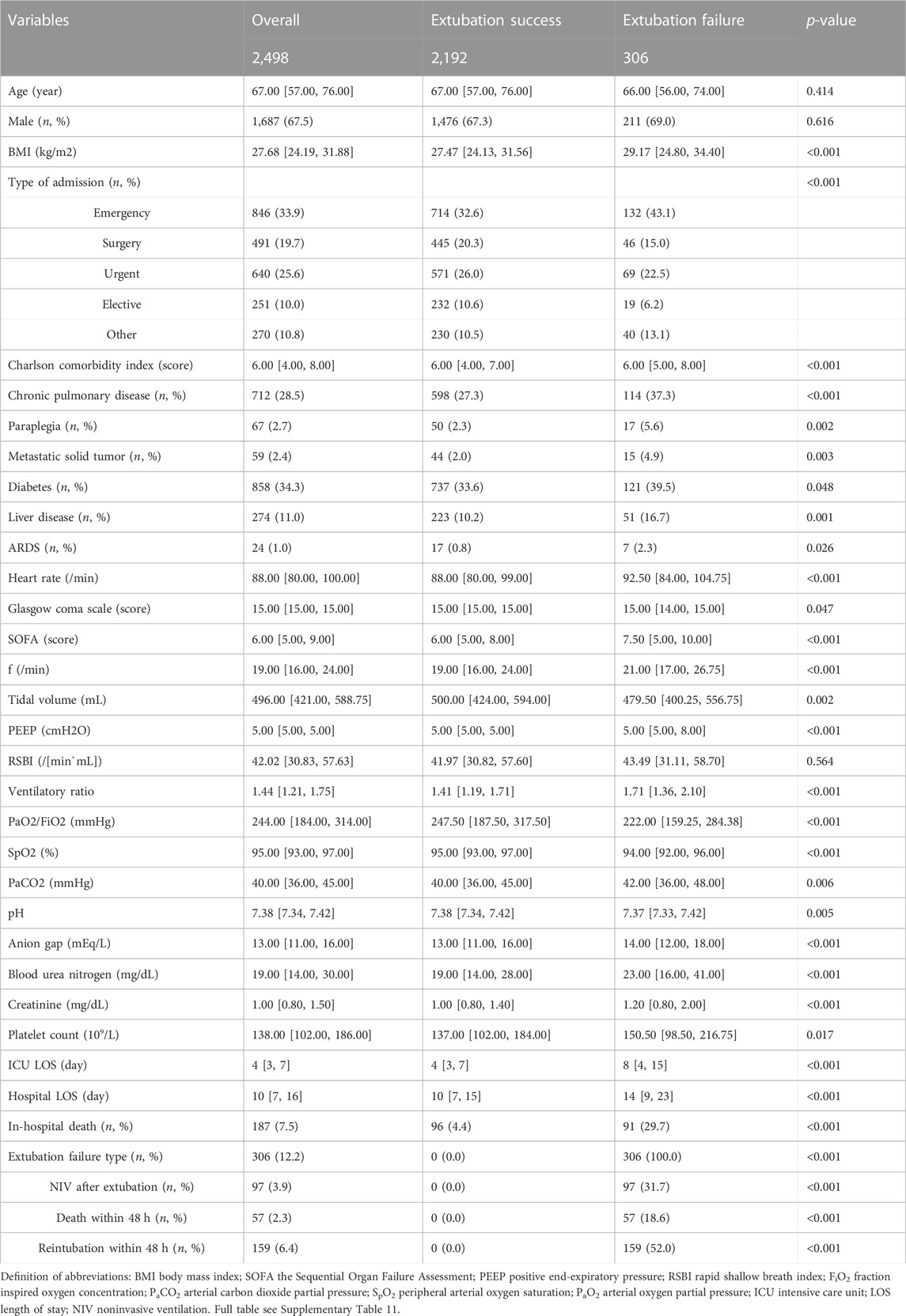
TABLE 1. Baseline population characteristics with stratification by extubation failure before extubation in the train set.
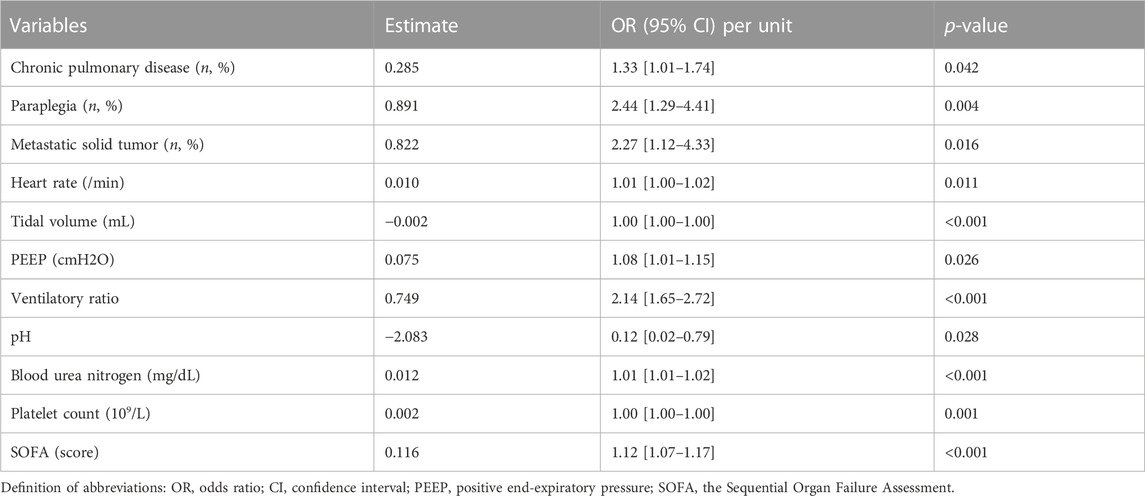
Increased VR was an independent risk factor for extubation failure after multivariable adjustment (OR 2.14 [1.65–2.72], p < 0.001). Other factors, including elevated heart rate (OR 1.01 [1.00–1.02]), lower tidal volume (OR 1.00 [1.00–1.00]), higher PEEP (OR 1.08 [1.01–1.15]), increased BUN (OR 1.01 [1.01–1.02]), increased platelet count (OR 1.00 [1.00–1.00]), greater SOFA (OR 1.12 [1.07–1.17]), presence of chronic pulmonary disease (OR 1.33 [1.01–1.74]), presence of paraplegia (OR 2.44 [1.29–4.41]), presence of metastatic solid tumor (OR 2.27 [1.12–4.33]), and decreased pH (OR 0.12 [0.02–0.79]), were also significantly associated with an increased risk for extubation failure (Table 2).
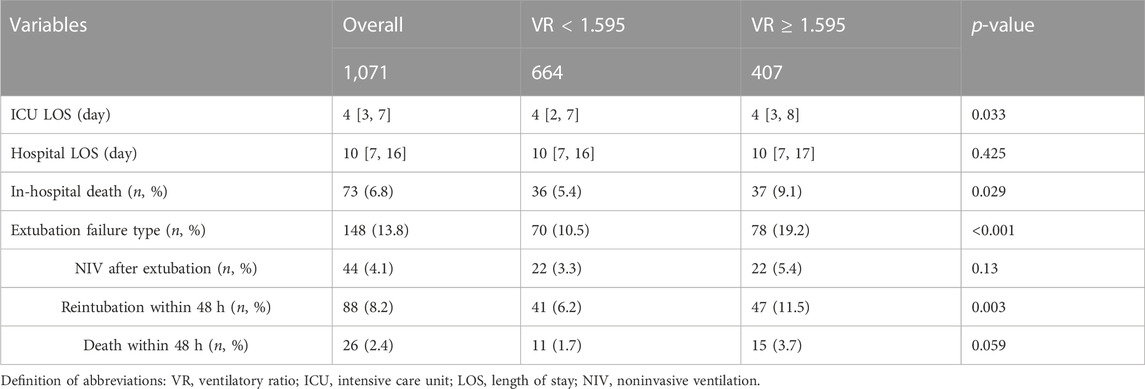
Figure 2 shows ROC curves for VR at 4 hours before extubation (as a continuous variable) in the training set. The area under the ROC curve (AUC) maximized was 0.669 with a cut-off value of 1.595 using the Youden index (Supplementary Table S5). VR ≥ 1.595 remained an independent factor of extubation failure even after adjusting for age, different age groups, type of admission, PaO2/FiO2, RSBI, sex, PEEP, and SOFA (Supplementary Table S6), which was confirmed in the validation set (C-index: 0.585 [0.542, 0.628]) and non-surgical group (C-index: 0.632 [0.605–0.658]). Compared with patients with VR < 1.595, patients with VR ≥ 1.595 had prolonged ICU LOS (4 [3, 8] vs. 4 [2, 7] days, p = 0.033), a higher risk of extubation failure [78 (19.2%) vs. 70 (10.5%), p < 0.001], and higher risk of in-hospital mortality (37 (9.1%) vs. 36 (5.4%), see Table 3). Similar results were found in non-surgical patients (see Supplementary Table S7).
Secondary outcomes
We also tested the predictive ability of RSBI and PaO2/FiO2 in this population, and the AUC of VR was significantly higher than that of RSBI (0.510 [IQR, 0.476–0.545]) and PaO2/FiO2 (0.586 [IQR, 0.551–0.621]) at 4 hours before extubation for predicting reintubation (all p-value < 0.05, Supplementary Table S5). In addition, significant differences were found between survivors and nonsurvivors in VR (1.44 [1.20, 1.74] vs. 1.49 [1.19, 1.96], p = 0.032, see Supplementary Table S8).
Discussion
The current retrospective study focused on a large cohort of ventilated patients who were admitted to the ICU for at least 48 h. The rate of extubation-failure was 12.7% and the median SOFA score was 6 before extubation. We found that higher VR at 4 hours before extubation was an independent factor for predicting extubation failure even after adjusting for age, sex, type of admission, PaO2/FiO2, RSBI, PEEP, and SOFA. The threshold of VR was 1.595 with a predictive value of 0.585 in predicting extubation failure. Other variables, increased heart rate, lowerr tidal volume, greater PEEP levels, decreased pH levels, higher blood urea nitrogen level, elevated platelet count, presence of chronic pulmonary disease, paraplegia, and metastatic solid tumor were also independent factors associated with extubation failure.
We found that a threshold of 1.595 of VR was an independent risk factor for extubation failure in this study. This result was consistent with one previous study that showed that patients were finally liberated from assisted mechanical ventilation only when their VR fell below 2 (16). In addition, we found that the predictability of VR was better than that of RSBI for predicting extubation failure. Although RSBI is commonly used to support decision-making during weaning, it might be significantly affected by the level of ventilator support (Patel et al., 2009). VR was a rather reliable variable even after adjusting variables that increased extubation failure and testing in a non-surgical population. This further supports the ability of VR to identify increased ventilatory demands and the potential to be a better tool to facilitate the decision-making process of extubation.
Several studies reported that VR in the first days of mechanical ventilation was significantly associated with a higher risk of mortality (Sinha et al., 2019; Ende et al., 2021; Torres et al., 2021). In a recent multicenter study involving patients with coronavirus disease 2019 (COVID-19)-related ARDS, it was observed that the VR was significantly different between survivors and non-survivors on the second day (1.95 ± 0.68 vs. 2.09 ± 0.60) and third day (2.06 ± 0.70 vs. 2.26 ± 0.68) of mechanical ventilation (Morales-Quinteros et al., 2021). Moreover, one study showed the dynamic change of VR from ICU admission to day 3 was independently associated with death (OR 1.04 [1.01–1.07], p = 0.030) (Torres et al., 2021). In line with those studies, we also found VR 4 hours before extubation was higher in nonsurvivors.
Other factors, such as increased heart rate, evaluated PEEP, higher BUN, higher platelet count, greater SOFA score, decreased pH, decreased tidal volume, and presence of chronic pulmonary disease, paraplegia, and metastatic solid tumor were also significantly associated with an increased risk for extubation failure in the current study. Heart rate and SOFA score were comprehensive factors to evaluate the disease progression of the patient. Patients who required higher PEEP and exhibited a lower tidal volume might be associated with impaired lung compliance and inadequate gas exchange. The levels of BUN and pH reflect the accumulation of waste products in the systemic circulation and the imbalance of the acid-base status. Suraseranivong et al. also reported higher levels of BUN in the group of patients who experienced extubation failure (Suraseranivong et al., 2018). Patients with chronic pulmonary disease, paraplegia, and metastatic solid tumor are often accompanied by respiratory muscle weakness and impaired respiratory functions, including weakened cough reflex and decreased ability to clear secretions. A previous study reported that patients older than 65 years with pre-existing chronic cardiac or respiratory diseases had a significantly higher reintubation rate of 34% compared to 9% in patients without these conditions (Thille et al., 2011).
There are several limitations of this study. First, our study was a retrospective study based on the MIMIC-IV database. The ventilation strategies and settings used before extubation and the protocols of extubation were not standardized. Second, we failed to calculate the duration of ventilation due to the lack of intubation records before extubation. However, we excluded patients after surgery following routine ventilation in sensitivity analysis, as those patients had a shorter ventilation duration, a low risk of extubation failure, and a low incidence of ARDS. Therefore, the incidence of extubation failure in the current study was 12.7% which was similar to that in previous studies, ranging from 10%–20% (Thille et al., 2013). The low fraction of ARDS in the current study might be related to the fact that the diagnosis of ARDS is underestimated in clinical settings. We could not reclassify the included patients according to the Berlin definition since this was a retrospective study, which might lead to biased results. Third, we failed to capture data on levels of sedation, which may have influenced ventilatory impairment. In addition, the acute physiology and chronic health evaluation II score was not calculated because of insufficient data. The amount of missing data in the variables assessed in the study is a potential limitation. However, the analyses after multiple imputations yielded similar results (see Supplementary Table S8). Although our VR cut-off value is validated using data from a multicentre database, we still need prospective studies for further exploration.
Conclusion
In conclusion, higher VR at 4 hours before extubation was an independent factor for predicting extubation failure within 48 h. VR showed the ability to identify increased ventilatory demands and might be a useful tool to support decision-making during weaning.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://mimic.mit.edu/.
Ethics statement
Research use of MIMIC-IV data has been approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center (BIDMC) and Massachusetts Institute of Technology (MIT). The MIMIC-IV database was built by MIT and was approved to waive the documentation of informed consent by the Institutional Review Board of the BIDMC. One author, HY, completed the Collaborative Institutional Training Initiative (CITI) training program “Human Research, Data or Specimens Only Research” to gain access to the database. All methods were executed under relevant guidelines and regulations.
Author contributions
HY designed the study, HY and YN drafted the manuscript. HY, YN, and DH conducted the literature search and data analysis. HY and YN contributed equally to the study. ZL revised the manuscript critically for important intellectual content, and ZL gave the final version for publication. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Sichuan Science and Technology Agency Grant (22QYCX0105).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2023.1137115/full#supplementary-material
Abbreviations
ABG, arterial blood gas; ARDS: acute respiratory distress syndrome; AUC, area under receiver operating characteristic curve; BMI, body mass index; BUN, blood urea nitrogen; CI, confidence interval; CO2, carbon dioxide; COVID-19, Coronavirus disease 2019; GCS, glasgow coma scales; HFNC, high-flow nasal cannula; ICU, intensive care unit; IMV, invasive mechanical ventilation; IQR, interquartile range; LOS, length of stay; NIV, noninvasive ventilation; MV, mechanical ventilation; OR, odd ratio; PaCO2, the arterial partial pressure of carbon dioxide; PEEP, positive end-expiratory pressure; PaO2/FiO2, the partial pressure of oxygen to the fraction of inspired oxygen; RBC, red blood cell; ROC, receiver-operating characteristic; RSBI: rapid shallow breathing index; SpO2, peripheral arterial oxygen saturation; SOFA, the sequential organ failure assessment; VR, ventilatory ratio.
References
Acute Respiratory Distress Syndrome Network Brower, R. G., Matthay, M. A., Morris, A., Schoenfeld, D., Thompson, B. T., et al. (2000). Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 342 (18), 1301–1308. doi:10.1056/NEJM200005043421801
Boles, J. M., Bion, J., Connors, A., Herridge, M., Marsh, B., Melot, C., et al. (2007). Weaning from mechanical ventilation. Eur. Respir. J. 29 (5), 1033–1056. doi:10.1183/09031936.00010206
Cepkova, M., Kapur, V., Ren, X., Quinn, T., Zhuo, H., Foster, E., et al. (2007). Pulmonary dead space fraction and pulmonary artery systolic pressure as early predictors of clinical outcome in acute lung injury. Chest 132 (3), 836–842. doi:10.1378/chest.07-0409
Ende, V. J., Singh, G., Babatsikos, I., Hou, W., Li, H., Thode, H. C., et al. (2021). Survival of COVID-19 patients with respiratory failure is related to temporal changes in gas exchange and mechanical ventilation. J. Intensive Care Med. 36 (10), 1209–1216. doi:10.1177/08850666211033836
Eskandar, N., and Apostolakos, M. J. (2007). Weaning from mechanical ventilation. Crit. Care Clin. 23 (2), 263–274. doi:10.1016/j.ccc.2006.12.002
Frutos-Vivar, F., Ferguson, N. D., Esteban, A., Epstein, S. K., Arabi, Y., Apezteguía, C., et al. (2006). Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest 130 (6), 1664–1671. doi:10.1378/chest.130.6.1664
Goldberger, A. L., Amaral, L. A., Glass, L., Hausdorff, J. M., Ivanov, P. C., Mark, R. G., et al. (2000). PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. circulation 101 (23), e215–e220. doi:10.1161/01.cir.101.23.e215
Hess, A. S., and Hess, J. R. (2017). Understanding tests of the association of categorical variables: The pearson chi-square test and Fisher’s exact test. Transfusion 57 (4), 877–879. doi:10.1111/trf.14057
Hsieh, M. H., Hsieh, M. J., Chen, C. M., Hsieh, C. C., Chao, C. M., and Lai, C. C. (2018). An artificial neural network model for predicting successful extubation in intensive care units. J. Clin. Med. 7 (9), E240. doi:10.3390/jcm7090240
McKnight, P. E., Najab, J., and Mann-Whitney, U. (2010). “Test,” in The corsini encyclopedia of psychology [internet] (John Wiley & Sons, Ltd), 1. [cited 2022 Aug 13] Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470479216.corpsy0524.
Mishra, P., Singh, U., Pandey, C. M., Mishra, P., and Pandey, G. (2019). Application of student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 22 (4), 407–411. doi:10.4103/aca.ACA_94_19
Morales-Quinteros, L., Neto, A. S., Artigas, A., Blanch, L., Botta, M., Kaufman, D. A., et al. (2021). Dead space estimates may not be independently associated with 28-day mortality in COVID-19 ARDS. Crit. Care 25 (1), 171. doi:10.1186/s13054-021-03570-0
Nuckton, T. J., Alonso, J. A., Kallet, R. H., Daniel, B. M., Pittet, J. F., Eisner, M. D., et al. (2002). Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N. Engl. J. Med. 346 (17), 1281–1286. doi:10.1056/NEJMoa012835
Patel, K. N., Ganatra, K. D., Bates, J. H. T., and Young, M. P. (2009). Variation in the rapid shallow breathing index associated with common measurement techniques and conditions. Respir. Care 54 (11), 1462–1466.
Proklou, A., Papadakis, E., Kondili, E., Tserlikakis, N., Karageorgos, V., Konstantinou, I., et al. (2021). Ventilatory ratio threshold for unassisted breathing: A retrospective exploratory analysis. Respir. Care 66 (11), 1699–1703. doi:10.4187/respcare.09208
Razali, N. M., and Wah, Y. B. (2011). Power comparisons of shapiro-wilk, Kolmogorov-smirnov, lilliefors and anderson-darling tests. J. Stat. Model. Anal. 2 (1), 21–33.
Siddiki, H., Kojicic, M., Li, G., Yilmaz, M., Thompson, T. B., Hubmayr, R. D., et al. (2010). Bedside quantification of dead-space fraction using routine clinical data in patients with acute lung injury: Secondary analysis of two prospective trials. Crit. Care 14 (4), R141. doi:10.1186/cc9206
Sinha, P., Calfee, C. S., Beitler, J. R., Soni, N., Ho, K., Matthay, M. A., et al. (2019). Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 199 (3), 333–341. doi:10.1164/rccm.201804-0692OC
Sinha, P., Fauvel, N. J., Singh, P., and Soni, N. (2013). Analysis of ventilatory ratio as a novel method to monitor ventilatory adequacy at the bedside. Crit. Care 17 (1), R34–R38. doi:10.1186/cc12541
Sinha, P., Fauvel, N. J., Singh, S., and Soni, N. (2009). Ventilatory ratio: A simple bedside measure of ventilation. Br. J. Anaesth. 102 (5), 692–697. doi:10.1093/bja/aep054
Sinha, P., Sanders, R. D., Soni, N., Vukoja, M. K., and Gajic, O. (2013). Acute respiratory distress syndrome: The prognostic value of ventilatory ratio—a simple bedside tool to monitor ventilatory efficiency. Am. J. Respir. Crit. Care Med. 187 (10), 1150–1153. doi:10.1164/rccm.201211-2037LE
Sinha, P., Singh, S., Hardman, J. G., Bersten, A. D., and Soni, N. (2014). Evaluation of the physiological properties of ventilatory ratio in a computational cardiopulmonary model and its clinical application in an acute respiratory distress syndrome population. Br. J. Anaesth. 112 (1), 96–101. doi:10.1093/bja/aet283
Suraseranivong, R., Krairit, O., Theerawit, P., and Sutherasan, Y. (2018). Association between age-related factors and extubation failure in elderly patients. PLoS One 13 (11), e0207628. doi:10.1371/journal.pone.0207628
Thille, A. W., Harrois, A., Schortgen, F., Brun-Buisson, C., and Brochard, L. (2011). Outcomes of extubation failure in medical intensive care unit patients. Crit. Care Med. 39 (12), 2612–2618. doi:10.1097/CCM.0b013e3182282a5a
Thille, A. W., Richard, J. C. M., and Brochard, L. (2013). The decision to extubate in the intensive care unit. Am. J. Respir. Crit. Care Med. 187 (12), 1294–1302. doi:10.1164/rccm.201208-1523CI
Keywords: ventilatory ratio, extubation failure, mechanical ventilation, intensive care unit, prediction
Citation: Yang H, Ni Y, Huang D and Liang Z (2023) Ventilatory ratio as a predictor for extubation failure in critical ill patients based on MIMIC-IV database (from 2008 to 2019). Front. Physiol. 14:1137115. doi: 10.3389/fphys.2023.1137115
Received: 04 January 2023; Accepted: 22 May 2023;
Published: 01 June 2023.
Edited by:
Christina Maria Pabelick, Mayo Clinic, United StatesReviewed by:
Katerina Vaporidi, University of Crete, GreeceGazi Arslan, Dokuz Eylül University, Türkiye
Copyright © 2023 Yang, Ni, Huang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zongan Liang, bGlhbmd6YUBzY3UuZWR1LmNu
†These authors have contributed equally to this work
 Huan Yang
Huan Yang Yuenan Ni
Yuenan Ni Dong Huang
Dong Huang Zongan Liang
Zongan Liang