
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Physiol. , 07 February 2023
Sec. Skeletal Physiology
Volume 14 - 2023 | https://doi.org/10.3389/fphys.2023.1105303
Objective: To systematically review the effects of different resistance training (RT) protocols on bone mineral density (BMD) in postmenopausal women.
Methods: Randomized controlled trials (RCTs) on the resistance training in improving bone mineral density for postmenopausal women were searched in databases including ProQuest, PubMed, Cochrane Library, Embase, and Web of Science. The retrieval time range was from the establishment of the database to May 2022. The included literature was independently screened and relevant data was extracted by two reviewers. The systematic review followed the Joanna Briggs Institute (JBI) methodology for reviews of quantitative evidence. Quality of risk was assessed using the Physical Therapy Evidence Database (PEDro) scale, risk of bias was assessedusing the Cochrane RoB2 tool and a network Meta-analysis was performed on the data using Stata 16.0.
Results: A total of 19 studies, which included 919 subjects, were eventually acquired. The results of the network Meta-analysis showed that moderate intensity resistance training was superior in improving lumbar spine bone mineral density (LS BMD) and femoral neck bone mineral density (FN BMD) compared to the control group (as per usual daily life), with a statistically significant difference (p < 0.05). There was, however, no statistically significant difference between the groups in terms of increasing total hip bone mineral density (TH BMD) and trochanter bone mineral density (Troch BMD), although moderate intensity training tends to increase bone mineral density (p > 0.05). In addition, when training frequency is taken into consideration, 3 days/week of moderate intensity training (3MI) was superior to 2 days/week (2MI) in improving lumbar spine bone mineral density , and moderate intensity training was superior to low and high intensity resistance trainings at training frequency of 3 day/week, with statistically significant differences (p < 0.05). The cumulative probability ranking results indicated that 3MI was the optimal option in improving lumbar spine, femoral neck, total hip and Troch bone mineral density. Subgroup analyses combining interventions time showed that for lumbar spine and femoral neck bone mineral density, 3MI protocol with intervention duration within 1 year (≤48 weeks) had a significant advantage over other interventions, while this advantage was no longer significant with the intervention duration of more than 1 year (>48 weeks).
Conclusion: Current evidence shows that moderate intensity resistance training for 3 days/week can be preferred clinically to improve bone mineral density in postmenopausal women, and it is recommended that the duration of the same training should not exceed 1 year. Nevertheless, more high-quality studies are needed to verify the above conclusion.
Bone loss is significantly accelerated with the loss of estrogen after menopause in elderly women. Studies have shown that during 1–10 years after menopause, the annual loss rate of human bone mass is 1.5%–2.5% (Cheng et al., 2020). BMD and bone mass reduction above a certain range is prone to osteoporosis, which increases the risk of fracture by 2.6 times (Sathyapalan et al., 2017). Approximately 200 million women worldwide suffer from osteoporosis after menopause, because the destruction of trabecular bone structure results in increased bone fragility and decreased bone mechanical strength, which consequently adds the risk of fracture (Levin et al., 2018). Osteoporosis and fall risk are determinants of fragility fractures. According to the Iolascon study, women with higher fall risk exhibit more osteoporotic fractures and poorer physical performance, leading to more medication intake. Besides, patients with osteoporosis and fractures are more prone to disability and highly dependent on others in daily activities, which significantly undermines their life qualities (Global Burden of Disease Study 2013 Collaborators, 2015). Accordingly, it is essential to find ways that effectively prevent and treat osteoporosis (Reginster and Burlet, 2006; Jackson and Mysiw, 2014). Despite the effective role of drugs that improve bone mineral density (BMD) in treating osteoporosis (Crandall et al., 2014), the non-adherence and lack of persistence of anti-osteoporosis therapy lead to poor outcomes in practice, such as less reduction in bone turnover rate, smaller increase in BMD, and a significant rise in fracture risk (Migliaccio et al., 2013; Ringe and Farahmand, 2014; Lagari et al., 2015).
It is proved that exercise training, such as weight bearing, progressive resistance training, strength training, etc., can significantly reduce fall rate to improve function recovery and decrease fracture risk (Benedetti et al., 2018). In addition, the theoretical basis for osteoporosis prevention is to furthest increase bone mass during the peak period of bone mass balance and maintain it for a longer time. Meanwhile, physical exercise is required during the period of rapid bone mass loss to slow down its loss rate. Accordingly, exercise training for the elderly should be strengthened to prevent bone loss and increase BMD, so as to effectively prevent osteoporosis and reduce fall risk. The Clinical Practice Guidelines (CGP) advocates exercise of moderate-to-high intensity to prevent bone loss (Füzéki and Banzer, 2018), which, however, differs in recommended exercise intensity, and fails to make specific indications on the type, frequency, intensity, and duration of exercise. Therefore, relevant investigation should be conducted to improve the quality of evidence regarding the application of this intervention in the management of patients with osteoporosis. Previous studies have demonstrated the obvious advantages of resistance training (RT) over other exercise methods in increasing BMD (Palombaro et al., 2013), however, no consensus has been reached on the optimal training intensity and frequency. Therefore, this study designed various combinations of training intensity and frequency using a network Meta-analysis to find the optimal scheme, so as to compare the effects of different schemes on BMD in postmenopausal women, and provide a scientific basis for exercise prescriptions that can improve BMD in the elderly population.
The systematic review of this paper was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA); this study has been registered with the International Prospective Registerof Systematic Reviews (PROSPERO) under the registration number: CRD42020212253.
Using the PICO (Population/patients, Intervention/exposure, Control/comparison, and Outcome) strategy, the studies that meet the following criteria were included in the study. Based on the previous studies (Kistler-Fischbacher et al., 2021), the training intensity was divided into high intensity (≥80% 1RM), moderate intensity (65%–80% 1RM) and low intensity (≤65% 1RM), and frequency was divided into high frequency (3 days/week) and low frequency (2 days/week). The PICO strategy is presented in Table 1.
The exclusion criteria adopted in the present study are as follows:
(1) Concurrent drug therapy during resistance training;
(2) Mixed combination of exercise interventions apart from resistance training;
(3) Had undergone a high intensity physical training in the 6 months prior to the trial;
(4) Exercise intervention intensity not marked in detail or intensity expressed in a non-RM way;
(5) Liver, kidney and endocrine system diseases that affecting bone metabolism;
(6) Abstracts, reviews, conference reports, etc.;
(7) Multiple publications of the literature;
(8) Non-extractable data;
(9) Full text not available;
(10) Lack of outcome measures
In this study, the search strategy was developed and implemented according to PRISMA. A systematic search was conducted in databases including ProQuest, PubMed, Cochrane Library, Embase, and Web of Science with a retrieval time range from the establishment of the database to May 2022.
The search terms included bone mineral density (BMD), resistance training, strength training, and postmenopausal. The specific search strategy was as follows (PubMed was used as an example): (Bone Mineral Density OR Density, Bone OR bone density OR BMD) AND (exercise OR resistance training OR resistance OR weight lift OR strength training OR strength exercise OR weight training) AND (post-menopausal OR post menopause OR menopausal).
After duplicate data was removed using EndnoteX9, two reviewers independently analyzed the titles and abstracts of all literature retrieved from the database. Then, they browsed through the entire articles, further screened out those that met the inclusion criteria, downloaded the full text, and divided them into three groups-included, possibly included, and excluded. For any disagreement, articles were reevaluated. If disagreements persisted, group discussions were held to resolve it.
The following references were extracted from each study by two reviewers using pre-assigned tables, including the first author, year of publication, country, intervention time, sample size (enrollment/disenrollment), mean age of participants, exercise intervention plan (group count setting, frequency, intensity, periodicity and Resistance training type) and outcome measures, the main result and complication.
Methodological quality and risk of bias of the included studies were assessed using the Joanna Briggs Institute (JBI) Checklists Critical Appraisal Tool for Randomized Controlled Trials (RCTs) (Sukan et al., 2022), PEDro scale (Le et al., 2014) and Cochrane RoB2 (Minozzi et al., 2020). The PEDro scale has been widely used to classify the quality of evidence in randomized controlled trials, aiming to enhance application of the best evidence in clinic treatment to make physical therapy more effective. The PEDro scale is a RCT rating scale based on the Delphi list (Maher et al., 2003). It includes 11 items, each item is worth one point, and the total score is 10 points (the final score excludes any “inclusion criteria” item), from 0 (high risk of bias) to 10 (low risk of bias), where a value ≥ 6 represents the critical value for low risk of bias studies. The risk of bias in the studies was classified into three types such as low risk, uncertain risk, and high risk according to the JBI assessment results. Based on the number of “yes” responses, <40% was considered “high risk,” 40%–80% “moderate risk,” and >80% “low risk. “The risk of bias was assessed using Cochrane RoB 2 (Sterne et al., 2019). Five domains were included in this tool to assessthe risk of bias, including bias because of 1) randomizationprocess, 2) deviations from intended interventions, 3) missing outcome data, 4) measurement of the outcome, and 5) selection of the reported results. Each domain could bescored as low, moderate, or high risk of bias. Finally, an overall risk of bias score was provided.
Data preprocessing and analysis were performed by two reviewers. The raw data was preprocessed using Microsoft Office Excel and results were indicated as the difference value between the endpoint and base values. All results were converted into a mean value and its standard deviation (ΔMean ± SD) in uniform unit of g/cm2.
Network Meta-analysis and graphical plotting were performed for relevant data using Stata 16.0 software. The outcome measures were continuous variables and rated by the same scale, so the weighted mean difference (WMD) and 95% confidence interval (CI) were taken as effect sizes. The network evidence diagram of direct comparison between intervention intensity-frequency combinations was plotted; then the consistency of each outcome indicator’s closed loop was evaluated using the loop inconsistency test, where it indicated a good consistency between the direct and indirect evidence if 95% CI of the loop inconsistency factor (IF) contained 0 (Jackson et al., 2016). The results of network Meta-analysis were presented through pairwise comparison of forest plots. Cumulative ranking probability plots drawn based on the surface under the cumulative ranking curve (SUCRA) were used to determine the optimal training intensity and frequency. Comparison-correction funnel plots were used to test for publication bias and small sample effect.
Apart from the above, stability of the study results was verified using subgroup and sensitivity analyses. Subgroup analyses was performed according to the duration of intervention, while sensitivity analyses was performed by excluding studies with a sample size of less than 10.
A total of 4,208 studies were retrieved, and based on multiple screenings, 19 studies with a total of 919 patients were finally included. The flow chart of selection process in this study is shown in Figure 1.
A total of 19 studies (Pruitt et al., 1992; Nelson et al., 1994; Nichols KPN et al., 1995; Pruitt et al., 1995; Hartard et al., 1996; Kerr et al., 1996; Bemben et al., 2000; Maddalozzo and Snow, 2000; Rhodes et al., 2000; Chilibeck et al., 2002; Milliken et al., 2003; Liu-Ambrose et al., 2004; Siegrist CL, 2006; Maddalozzo et al., 2007; Bocalini et al., 2009; Chuin et al., 2009; Bemben et al., 2010; Bocalini et al., 2010; Marques et al., 2012) with 919 subjects were included in this study. Countries where the studies were published involved the United States (n = 9), Brazil (n = 2), Australia (n = 2), Canada (n = 4), Germany (n = 1), and Portugal (n = 1). In the intervention group, eight studies involved 3HI, five involved 3MI, six involved 3LI, two involved 2HI, three involved 2MI, and 0 involved 2LI. BMD of four sites were included in the outcome measures, respectively LS (n = 16), FN (n = 19), TH (n = 8) and Troch (n = 8). The detailed basic characteristics of the included studies are as shown in Table 2.
Table 3 provides the quality evaluation results of the included studies. In PEDro rating scale, an article was considered “good” if it was rated six scores or more. In this systematic review, the median PEDro score of the included studies was 5, within the range of (4–7), and 10 of the 19 studies scored at the predetermined level (≥6). All studies stated the inclusion criteria, All the studies were randomly assigned subjects; no studies revealed detailed allocation concealment; all studies were comparable at baseline, no studies blinded the researchers and subjects, four blinded the outcome raters, nine had a clinical dropout rate >15%, and three contained no intentional analysis. Added to that, between-group statistics, point measures, and difference value statistics were conducted on all the included studies.
The JBI (Checklists Critical assessment Tool) was employed to determine the quality of articles in randomized controlled trials, which revealed the absence of blinding of participants (19/19), treatment providers (19/19), and outcome evaluators (15/19). This may lead to performance or detection bias, especially for subjective outcome measures (Supplementary Table S1).
Using ROB two to assess methodological quality and bias in the included studies (Supplementary Figure S1A, B). The majority of studies were assessed as “some concerns”, with only three studies being at low risk of bias (Nelson et al., 1994; Liu-Ambrose et al., 2004; Bocalini et al., 2009) and two studies at high risk (Milliken et al., 2003; Maddalozzo et al., 2007). The main source of concern was potential bias due to the selection of the reported result. Two studies were at high risk of bias due to the randomization process (Milliken et al., 2003; Maddalozzo et al., 2007). These issues may be due, in part, to lack of clarity in reporting rather than study conduct, as many studies did not publish a protocolor analysis plan or there was a lack of clarity in reporting method of randomization. Other sources of concern were potential deviations from the stated interventions (Nichols KPN et al., 1995; Hartard et al., 1996; Rhodes et al., 2000; Maddalozzo et al., 2007) and Only one study (Chilibeck et al., 2002) had some concerns about the risks in the outcome measures. One study (Pruitt et al., 1995) has some concerns about missing outcome data of the included study, and one study (Milliken et al., 2003) shows a high risk.
A total of 16 studies with 722 subjects were included in this part. The network evidence diagram is shown as Figure 2A. The loop inconsistency test manifested that the 95%CI of the loop in consistency factor (IF) contained 0, indicating good consistency between the direct and indirect evidence in Figure 2B. The pairwise comparison of forest plot in Figure 2C showed that 3MI was superior in improving LS BMD compared to the control group, and the difference was statistically significant [WMD = 0.48, 95%CI(0.22,0.74),p < 0.05]; 3MI was superior to 2MI [WMD = −0.46,95%CI(−0.86, −0.07),p < 0.05], 3LI [WMD = 0.48,95%CI(0.11,0.84),p < 0.05], and 3HI [WMD = −0.47,95%CI(−0.80, −0.14),p < 0.05], which indicated that RT at moderate intensity was better than the other two intensities and that high frequency training at moderate intensity was better than low frequency training. Moreover, the SUCRA results in Figure 2D showed the highest SUCRA value appeared at 3MI (98.8%). Based on the above research, 3MI may be the optimal option.
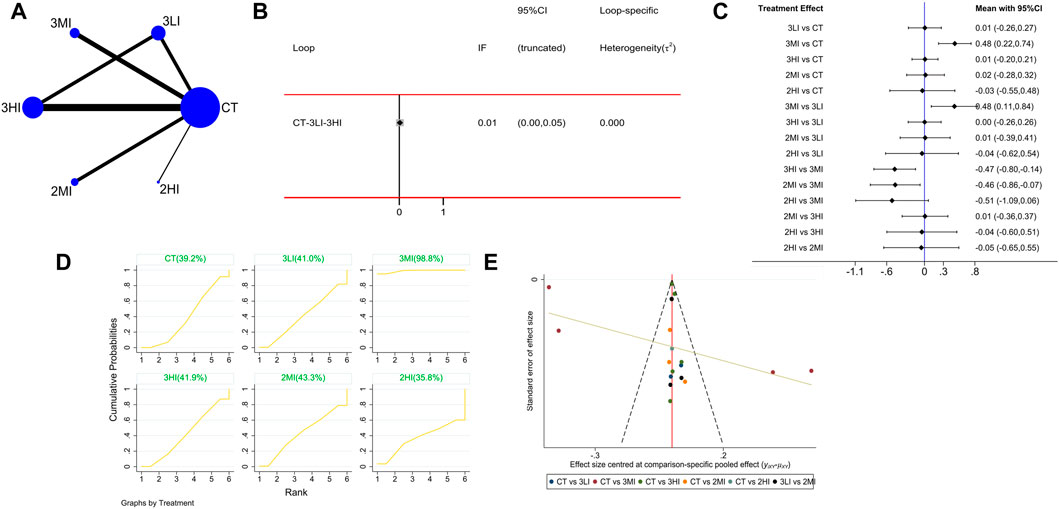
FIGURE 2. Network meta-analysis results forlumbar spine (LS) BMD. (A) Network evidence diagram; (B) loop inconsistency test; (C) forest plot; (D) the figure of cumulative probability ranking; (E) funnel plot.
A total of 19 studies with 767 subjects were included. The network evidence diagram is shown as Figure 3A. The loop inconsistency test in Figure 3B indicated that 95% CI of the loop inconsistency factor (IF) contained 0, indicating good consistency between the direct and indirect evidence. The pairwise comparison of the forest plot in Figure 3C manifested that 3MI was effective in improving FN BMD compared to the control group, and the difference was statistically significant [WMD = 0.31,95% CI (0.01,0.62), p < 0.05]; in the pairwise comparison of the other groups, although 3MI had a significant advantage on FN BMD, the difference was not statistically significant (p > 0.05). Besides, the cumulative probability ranking in Figure 3D showed that 3MI ranked first (SUCRA value of 87.2%). For the above reason, 3MI may be the optimal option to improve FN BMD.
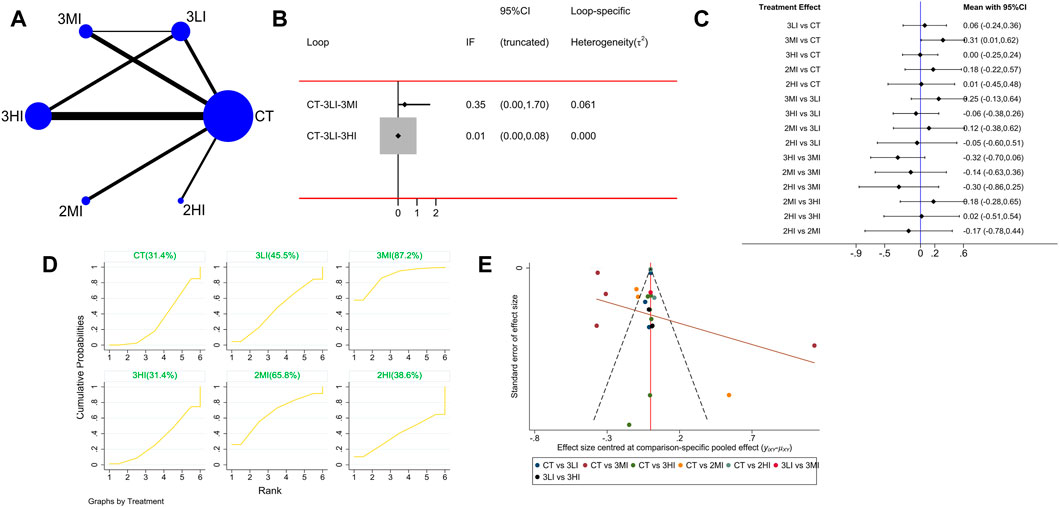
FIGURE 3. Network meta-analysis results for femoral neck (FN) BMD. (A) Network evidence diagram; (B) loop inconsistency test; (C) forest plot; (D) the figure of cumulative probability ranking; (E) funnel plot.
A total of eight studies with 282 subjects were included in this part. The network evidence diagram is shown as Figure 4A. The loop inconsistency test showed in Figure 4B that 95% CI of the loop inconsistency factor (IF) contained 0, indicating good consistency between the direct and indirect evidence. The forest plot in Figure 4C presented no statistical difference in the pairwise comparisons (p > 0.05). Meanwhile, the cumulative probability ranking in Figure 4D showed that 3MI ranked first (SUCRA value of 76.6%). Based on the above study, 3MI may be the best protocol to improve TH BMD.
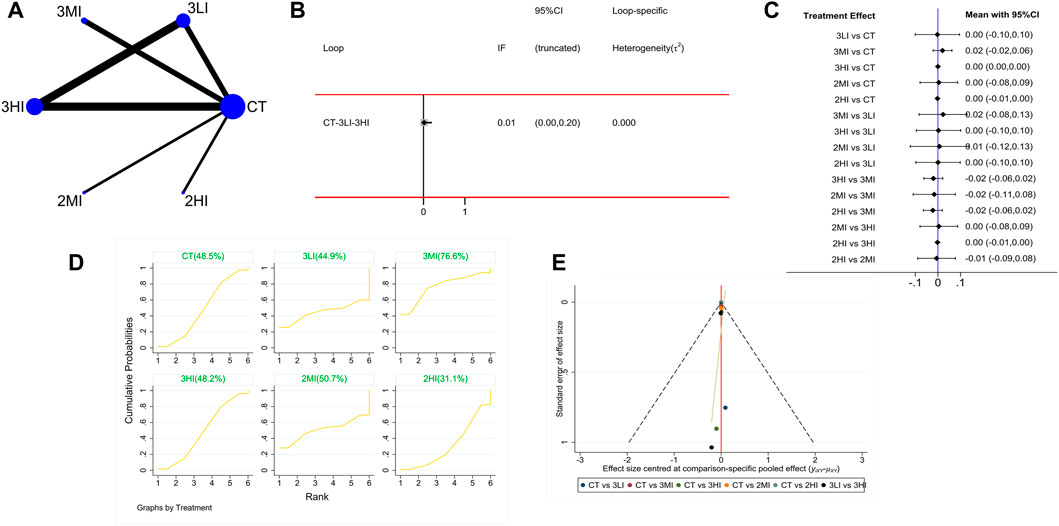
FIGURE 4. Network meta-analysis results for total hip (TH) BMD. (A) Network evidence diagram; (B) loop inconsistency test; (C) forest plot; (D) the figure of cumulative probability ranking; (E) funnel plot.
A total of eight studies with 393 subjects were included in this part. The network evidence diagram is shown in Figure 5A. The loop inconsistency test in Figure 5B showed good consistency between the direct and indirect evidence. The forest plot in Figure 5C indicated no statistically significant difference between the pairwise comparisons (p > 0.05). Meanwhile, the cumulative probability ranking in Figure 5D showed that 3MI ranked first (SUCRA value of 74.1%). To sum up, 3MI may be the best protocol to improve Troch BMD.
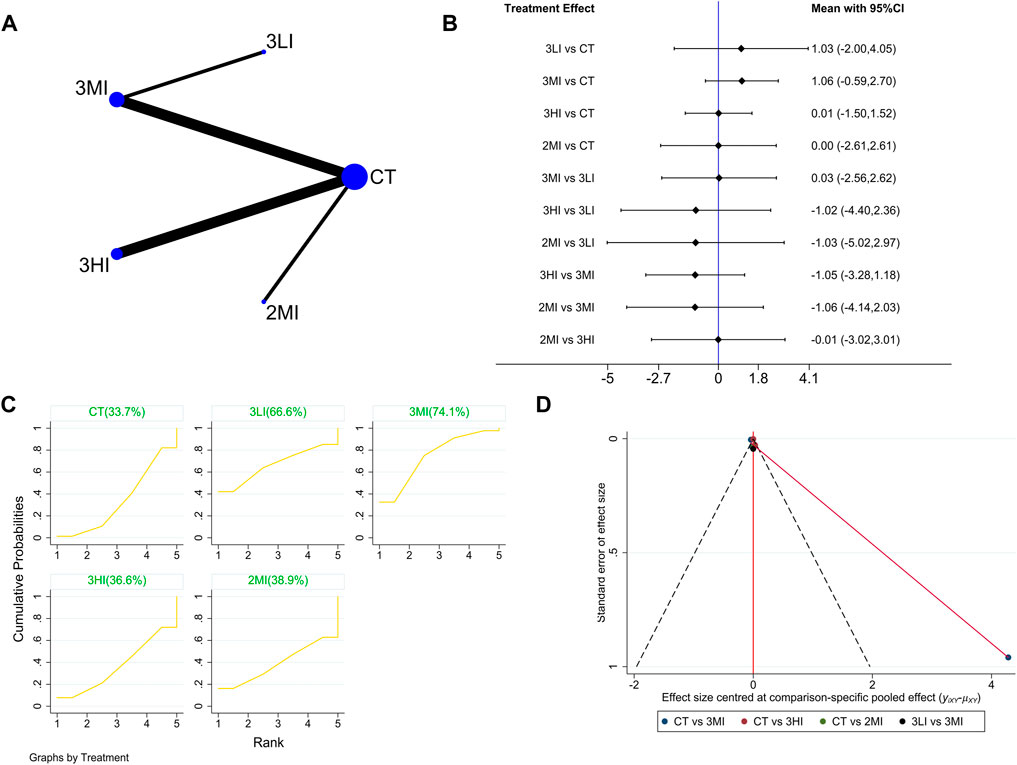
FIGURE 5. Network meta-analysis results forTroch BMD. (A) Network evidence diagram; (B) forest plot; (C) the figure of cumulative probability ranking; (D) funnel plot.
It can be seen from Figures 2E–5E that the funnel plot of each indicator was basically symmetrical and most of the points were in the upper part of the funnel, while only a few points of LS and FN BMD fell in the outer part of the funnel. The overall results revealed that publication bias was less likely contained in this study, but the interpretation of the results still needs to be treated with caution.
In this part, sensitivity analyses were also performed by removing studies with sample sizes less than 10 to verify the robustness of our results.
In regard to LS BMD, studies including Bemben-2000 and Maddalozzo-2000 were removed. The network evidence diagram and loop inconsistency test are shown in Supplementary Figure S2A, B. The pairwise comparisons in the forest plot (Supplementary Figure S2C) and the cumulative probability ranking (Supplementary Figure S2D) were not significantly different from the overall results. Hence, it can be concluded that our results are stable and reliable.
In regard to FN BMD, studies including Bemben-2000, Pruitt1995 and Maddalozzo-2000 were excluded. The network evidence diagram and loop inconsistency test are shown in Supplementary Figure S3A, B. The pairwise comparisons in the forest plot (Supplementary Figure S3C) and the cumulative probability ranking (Supplementary Figure S3D) were not significantly different from the overall results. Therefore, it can be concluded that the results are stable and reliable.
With respect to TH BMD, studies including Bemben-2000 and Maddalozzo-2000 were removed. The network evidence diagram and loop inconsistency test are shown in Supplementary Figure S4A, B. The pairwise comparisons in the forest plot (Supplementary Figure S4C) and the cumulative probability ranking (Supplementary Figure S4D) were not significantly different from the overall results. Consequently, it can be concluded that our results are reliable.
Furthermore, considering that differences in intervention time might have an impact on the results of this study, subgroup analyses of LS BMD and FN BMD were performed on the basis of different intervention time. Two subgroups were set up: Intervention time ≤48 weeks group and intervention time>48 weeks group. Whereas, TH BMD, Troch BMD was not available for subgroup analysis due to the number of included studies.
In intervention time ≤48 weeks group of LS BMD, the network evidence diagram and loop inconsistency test are shown in Supplementary Figure S5A, B. The results of the forest plot (Supplementary Figure S5C) showed that 3MI was superior to CT in improving LS BMD (p < 0.05), and was also superior to 3LI and 3HI (p < 0.05). For RT at moderate intensity, 3MI was better than 2MI in improving BMD, with a statistically significant difference (p < 0.05). In intervention time>48 weeks group, the network evidence diagram and loop inconsistency test are shown in Supplementary Figure S6A, B. The results of the forest plot (Supplementary Figure S6C) showed that the difference was not statistically significant in either two comparisons, despite of the relative advantage of 3MI (p > 0.05). Additionally, the cumulative probability ranking results of both subgroups (Supplementary Figures S5D, 6D) showed that 3MI was the best option to improve LS BMD.
Network evidence diagram and loop inconsistency test for both subgroups in FN BMD are shown in Supplementary Figures 7A–B, 8A–B, respectively. Forest plot results (Supplementary Figure S7C) in intervention time ≤48 weeks group showed that resistance training at moderate intensity (either 3MI or 2MI) was superior to other RT protocols in improving FN BMD, with a statistically significant difference (p < 0.05). In contrast, in the subgroup with intervention time>48 weeks, the results of forest plot (Supplementary Figure S8C) showed no statistically significant difference between the groups in pairwise comparisons (p < 0.05). The results of the cumulative probability ranking (Supplementary Figures S7D, 8D) showed that the best protocol with intervention duration within 1 year (≤48 weeks) was 3MI, whereas the best protocol with intervention time>48 weeks was 2HI (68.0%).
This study provides evidence for selecting optimal resistance training for postmenopausal women. As is known to all, the effect of RT on BMD is related to resistance form, resistance intensity, training frequency and training duration (Sañudo et al., 2017). This Meta-analysis explored the effect of resistance training at different intensities and frequencies on BMD at different sites (lumbar spine, femoral neck, total hip and trochanter) in postmenopausal women and performed a subgroup analyses based on intervention time to find the optimal combination at different intervention time through comparison. The network Meta-analysis showed that resistance training at moderate intensity for 3 days a week (3MI), was relatively effective in improving LS, FN, TH and Troch BMD in postmenopausal women, especially LS and FN BMD which was more significant.
With regard to training intensity, numerous studies have hypothesized that there was a threshold at which BMD values increased with the increase of training intensity, and beyond which it didn’t (Vainionpää et al., 2006). However, some studies proposed that excessive training intensity can even lead to a decrease in BMD and negatively affect bone health. This may be caused by the fact that prolonged high intensity training disrupts the endocrine system, which interferes with the hypothalamic-pituitary-gonadal axis and indirectly inhibits the production and release of estrogen from the ovaries, further reducing the concentration of estrogen in the blood and weakening bone formation.
Results of network Meta-analysis showed that moderate intensity was more superior in increasing BMD compared to high intensity, and the cumulative probability ranking also showed that moderate intensity was the optimal solution, similar to the results of studies such as MICHL (Verschueren et al., 2004). The possible reason was that the strain of bone generated by the stimulation of mechanical loading at moderate intensity led to increasing activity of osteoblasts, greater bone formation than bone resorption, and continuous accumulation of bone minerals, which all resulted in the increased BMD. Hence, it can be extrapolated that moderate intensity training may be just enough to produce strain on the bone without excessive stress causing subtle damage, and thus it stimulated osteoclast proliferation and increased bone formation. Furthermore, some studies revealed that low intensity training had an insufficiently significant effect on BMD increase (Kistler-Fischbacher et al., 2021). The reason for this may be that lower loads mostly did not reach the stress threshold of bone strain and not effectively stimulate bone tissue.
In this paper, the training frequency was also considered, and research results revealed that 3 days/week of moderate intensity training (3MI) was mostly better than 2 days/week (2MI), and this difference was more pronounced in LS BMD. This was because the frequency of loading was the main influence factor that induced a bone adaptive response that could directly affect bone formation. PINHEIRO (Borba-Pinheiro et al., 2016) demonstrated that resistance training performed 3 times/week by postmenopausal women was better than 2 times/week in improving LS and FN BMD, which was generally consistent with the results of this study. In terms of this study, sensitivity analysis was conducted by excluding studies with a single group sample size of less than 10, and the results similarly presented that 3MI was the optimal choice. It suggested that our findings are more reliable.
Previous studies have shown that exercise produces adaptive bone development only when it achieved cumulative time and volume. The bone reconstruction cycle generally takes 3–4 months, and the bone needs 7–9 months to achieve a new stable level of bone volume after alteration (Fuchs et al., 2001); hence, clinical trials of ≥6 months were selected for this study on the included literature in order to make the results stable and realistic. Additionally, considering the influence of intervention time on the effect of RT, subgroup analyses were performed. Two subgroups were set up: Intervention time ≤48 weeks group and intervention time>48 weeks group. Subgroup analyses results showed that for LS and FN BMD, 3MI protocol with intervention duration within 1 year (≤48 weeks) had a significant advantage over other interventions, while this advantage was no longer significant with the intervention duration of more than 1 year (>48 weeks). The reason may be related to the reduced sensitivity of bone to stress stimuli due to prolonged adoption of exercise at the same intensity, which led to slower bone plasticity building (Benedetti et al., 2018). Apart from this, prolonged endurance training interfered with the secretion of hormones and other functions of the hypothalamus, resulting in low levels of sex hormones or a severe lack of them, breaking the balance of osteoblast and osteoclast activity and causing bone resorption greater than bone formation. In conclusion, when designing resistance training prescriptions for postmenopausal women, the duration and intensity of exercise should be fully considered. As a consequence, when developing training program, the training duration should not be too long unless a new strain distribution forms, and either progressive resistance or alternating exercises at different intensities can be selected.
The results of this meta analysis are helpful to refine the exercise prescription of resistance exercise and provide evidence for improving bone mineral density in postmenopausal women. There are a large number of postmenopausal women in the world and a lot of money is spent on the fight against osteoporosis. The development of resistance movement does not need to invest expensive equipment and large areas, which can save a lot of medical expenses. In addition, resistance exercise has good safety, and participants will feel the pleasure of dopamine secretion during exercise (Nybo et al., 2003). To sum up, resistance exercise is of great clinical significance.
Although we included all interventions in this network meta-analysis to obtain comprehensive results, the study had certain limitations. Firstly, the population included in the study was not identical in terms of daily exercise habits and exercise sites for RT, and most RCTs did not describe details of the randomized scheme, allocation concealment and blinding of subjects and researchers with regard to article quality assessment, which could lead to potential heterogeneity. Secondly, this analysis included only studies that quantified exercise intensify using 1RM. However, only 19 studies were qualified through screening under the precise classification. The lack of direct evidence may limit the reliability of the results and it’s necessary to treat it with caution. Finally, since the included studies ended up being available only in English, there may be language bias.
In conclusion, moderate intensity and high frequency (at 65%–80% 1RM, 3 days/week) may be the optimal training protocol to improve LS, FN, TH and Troch BMD in postmenopausal women. Considering the effect of the exercise cycle, it is recommended that the same training protocol should preferably last no longer than 1 year in order to reasonably regulate bone adaptation and produce new strain distribution. However, due to study limitations, the conclusion drawn in this paper need to be further validated by more high-quality studies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
ZW and YL drafted the manuscript. All authors participated in article search, study selection, data extraction, and review on risk of bias. What’s more, all authors provided final approval.
This work was supported by the Science and Technology Fund Project of Guizhou Health Commission (No: gzwkj 2022-306)
Thanks to all authors for their contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2023.1105303/full#supplementary-material
Bemben D. A., Fetters N. L., Bemben M. G., Nabavi N., Koh E. T. (2000). Musculoskeletal responses to high- and low-intensity resistance training in early postmenopausal women. Med. Sci. Sports Exerc 32 (11), 1949–1957. doi:10.1097/00005768-200011000-00020
Bemben D. A., Palmer I. J., Bemben M. G., Knehans A. W. (2010). Effects of combined whole-body vibration and resistance training on muscular strength and bone metabolism in postmenopausal women. Bone 47, 650–656. doi:10.1016/j.bone.2010.06.019
Benedetti M. G., Furlini G., Zati A., Letizia Mauro G. (2018). The effectiveness of physical exercise on bone density in osteoporotic patients. Biomed. Res. Int. 2018, 4840531. doi:10.1155/2018/4840531
Bocalini D. S., Serra A. J., Dos Santos L. (2010). Moderate resistive training maintains bone mineral density and improves functional fitness in postmenopausal women. J. Aging Res. 2010, 760818. doi:10.4061/2010/760818
Bocalini D. S., Serra A. J., dos Santos L., Murad N., Levy R. F. (2009). Strength training preserves the bone mineral density of postmenopausal women without hormone replacement therapy. J. Aging Health 21, 519–527. doi:10.1177/0898264309332839
Borba-Pinheiro C. J., Dantas E. H., Vale R. G., Drigo A. J., Carvalho M. C. G., Tonini T., et al. (2016). Resistance training programs on bone related variables and functional independence of postmenopausal women in pharmacological treatment: A randomized controlled trial. Arch. Gerontol. Geriatr. 65, 36–44. doi:10.1016/j.archger.2016.02.010
Cheng C., Wentworth K., Shoback D. M. (2020). New Frontiers in osteoporosis therapy. Annu. Rev. Med. 71, 277–288. doi:10.1146/annurev-med-052218-020620
Chilibeck P. D., Davison K. S., Whiting S. J., Suzuki Y., Janzen C. L., Peloso P. (2002). The effect of strength training combined with bisphosphonate (etidronate) therapy on bone mineral, lean tissue, and fat mass in postmenopausal women. Can. J. Physiol. Pharmacol. 80, 941–950. doi:10.1139/y02-126
Chuin A., Labonté M., Tessier D., Khalil A., Bobeuf F., Doyon C. Y., et al. (2009). Effect of antioxidants combined to resistance training on BMD in elderly women: A pilot study. Osteoporos. Int. 20, 1253–1258. doi:10.1007/s00198-008-0798-5
Crandall C. J., Newberry S. J., Diamant A., Lim Y. W., Gellad W. F., Booth M. J., et al. (2014). Comparative effectiveness of pharmacologic treatments to prevent fractures: An updated systematic review. Ann. Intern Med. 161 (10), 711–723. doi:10.7326/M14-0317
Fuchs R. K., Bauer J. J., Snow C. M. (2001). Jumping improves hip and lumbar spine bone mass in prepubescent children: A randomized controlled trial. J. Bone Min. Res. 16 (1), 148–156. doi:10.1359/jbmr.2001.16.1.148
Füzéki E., Banzer W. (2018). Physical activity recommendations for health and beyond in currently inactive populations. Int. J. Environ. Res. Public Health 15, 1042. doi:10.3390/ijerph15051042
Global Burden of Disease Study 2013 Collaborators (2015). Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: A systematic analysis for the global burden of disease study 2013. Lancet 386, 743–800. doi:10.1016/S0140-6736(15)60692-4
Hartard M., Haber P., Ilieva D., Preisinger E., Seidl G., Huber J. (1996). Systematic strength training as a model of therapeutic intervention. A controlled trial in postmenopausal women with osteopenia. Am. J. Phys. Med. Rehabil. 75, 21–28. doi:10.1097/00002060-199601000-00006
Jackson D., Boddington P., White I. R. (2016). The design-by-treatment interaction model: A unifying framework for modelling loop inconsistency in network meta-analysis. Res. Synth. Methods 7 (3), 329–332. doi:10.1002/jrsm.1188
Jackson R. D., Mysiw W. J. (2014). Insights into the epidemiology of postmenopausal osteoporosis: The women's health initiative. Semin. Reprod. Med. 32 (6), 454–462. doi:10.1055/s-0034-1384629
Kerr D., Morton A., Dick I., Prince R. (1996). Exercise effects on bone mass in postmenopausal women are site-specific and load-dependent. J. Bone Min. Res. 11 (2), 218–225. doi:10.1002/jbmr.5650110211
Kistler-Fischbacher M., Weeks B. K., Beck B. R. (2021). The effect of exercise intensity on bone in postmenopausal women (part 1): A systematic review. Bone 143, 115696. doi:10.1016/j.bone.2020.115696
Lagari V. S., McAninch E., Baim S. (2015). Considerations regarding adherence of anti-osteoporosis therapy. Postgrad. Med. 127, 92–98. doi:10.1080/00325481.2015.993278
Le Q., Qu Y., Tao Y., Zhu S. (2014). Effects of repetitive transcranial magnetic stimulation on hand function recovery and excitability of the motor cortex after stroke: A meta-analysis. Am. J. Phys. Med. Rehabil. 93 (5), 422–430. doi:10.1097/PHM.0000000000000027
Levin V. A., Jiang X., Kagan R. (2018). Estrogen therapy for osteoporosis in the modern era. Osteoporos. Int. 29 (5), 1049–1055. doi:10.1007/s00198-018-4414-z
Liu-Ambrose T. Y., Khan K. M., Eng J. J., Heinonen A., McKay H. A. (2004). Both resistance and agility training increase cortical bone density in 75- to 85-year-old women with low bone mass: A 6-month randomized controlled trial. J. Clin. Densitom. 7 (4), 390–398. doi:10.1385/jcd:7:4:390
Maddalozzo G. F., Snow C. M. (2000). High intensity resistance training: Effects on bone in older men and women. Calcif. Tissue Int. 66, 399–404. doi:10.1007/s002230010081
Maddalozzo G. F., Widrick J. J., Cardinal B. J., Winters-Stone K. M., Hoffman M. A., Snow C. M. (2007). The effects of hormone replacement therapy and resistance training on spine bone mineral density in early postmenopausal women. Bone 40, 1244–1251. doi:10.1016/j.bone.2006.12.059
Maher C. G., Sherrington C., Herbert R. D., Moseley A. M., Elkins M. (2003). Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 83 (8), 713–721. doi:10.1093/ptj/83.8.713
Marques E. A., Mota J., Carvalho J. (2012). Exercise effects on bone mineral density in older adults: A meta-analysis of randomized controlled trials. Age (Dordr) 34, 1493–1515. doi:10.1007/s11357-011-9311-8
Migliaccio S., Resmini G., Buffa A., Fornari R., Di Pietro G., Cerocchi I., et al. (2013). Evaluation of persistence and adherence to teriparatide treatment in patients affected by severe osteoporosis (PATT): A multicenter observational real life study. Clin. Cases Min. Bone Metab. 10, 56–60. doi:10.11138/ccmbm/2013.10.1.056
Milliken L. A., Going S. B., Houtkooper L. B., Flint-Wagner H. G., Figueroa A., Metcalfe L. L., et al. (2003). Effects of exercise training on bone remodeling, insulin-like growth factors, and bone mineral density in postmenopausal women with and without hormone replacement therapy. Calcif. Tissue Int. 72, 478–484. doi:10.1007/s00223-001-1128-5
Minozzi S., Cinquini M., Gianola S., Gonzalez-Lorenzo M., Banzi R. (2020). The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J. Clin. Epidemiol. 126, 37–44. doi:10.1016/j.jclinepi.2020.06.015
Nelson M. E., Fiatarone M. A., Morganti C. M., Trice I., Greenberg R. A., Evans W. J. (1994). Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures. A randomized controlled trial. JAMA 272, 1909–1914. doi:10.1001/jama.1994.03520240037038
Nichols Kpn J. F., Peterson K. K., Sartoris D. J. (1995). Bone mineral density responses to high-intensity strength training in active older women. Aging Phys. 5 (3), 26–38. doi:10.1123/japa.3.1.26
Nybo L., Nielsen B., Blomstrand E., Moller K., Secher N. (2003). Neurohumoral responses during prolonged exercise in humans. J. Appl. Physiol. 95, 1125–1131. doi:10.1152/japplphysiol.00241.2003
Palombaro K. M., Black J. D., Buchbinder R., Jette D. U. (2013). Effectiveness of exercise for managing osteoporosis in women postmenopause. Phys. Ther. 93, 1021–1025. doi:10.2522/ptj.20110476
Pruitt L. A., Jackson R. D., Bartels R. L., Lehnhard H. J. (1992). Weight-training effects on bone mineral density in early postmenopausal women. J. Bone Min. Res. 7, 179–185. doi:10.1002/jbmr.5650070209
Pruitt L. A., Taaffe D. R., Marcus R. (1995). Effects of a one-year high-intensity versus low-intensity resistance training program on bone mineral density in older women. J. Bone Min. Res. 10, 1788–1795. doi:10.1002/jbmr.5650101123
Reginster J. Y., Burlet N. (2006). Osteoporosis: A still increasing prevalence. Bone 38 (2), S4–S9. doi:10.1016/j.bone.2005.11.024
Rhodes E. C., Martin A. D., Taunton J. E., Donnelly M., Warren J., Elliot J. (2000). Effects of one year of resistance training on the relation between muscular strength and bone density in elderly women. Br. J. Sports Med. 34, 18–22. doi:10.1136/bjsm.34.1.18
Ringe J. D., Farahmand P. (2014). Improved real-life adherence of 6-monthly denosumab injections due to positive feedback based on rapid 6-month BMD increase and good safety profile. Rheumatol. Int. 34, 727–732. doi:10.1007/s00296-012-2663-2
Sañudo B., de Hoyo M., Del Pozo-Cruz J., Carrasco L., Del Pozo-Cruz B., Tejero S., et al. (2017). A systematic review of the exercise effect on bone health: The importance of assessing mechanical loading in perimenopausal and postmenopausal women. Menopause 24 (10), 1208–1216. doi:10.1097/GME.0000000000000872
Sathyapalan T., Aye M., Rigby A. S., Fraser W. D., Thatcher N. J., Kilpatrick E. S., et al. (2017). Soy reduces bone turnover markers in women during early menopause: A randomized controlled trial. J. Bone Min. Res. 32 (1), 157–164. doi:10.1002/jbmr.2927
Siegrist Cl M. (2006). Strength training using conventional or vibration machines and spinal gymnastics in the prevention of osteoporosis in post-menopausal women. Dtsch. Z Sportmed 57, 182–188. doi:10.1001/jamaneurol.2019.0534
Sterne J., Savović J., Page M. J., Elbers R. G., Blencowe N. S., Boutron I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Sukan B., Akdevelioğlu Y., Sukan V. N. (2022). Effect of antioxidant supplementation on endometriosis-related pain: A systematic review. Curr. Nutr. Rep. 11, 753–764. doi:10.1007/s13668-022-00432-1
Vainionpää A., Korpelainen R., Vihriälä E., Rinta-Paavola A., Leppäluoto J., Jämsä T., et al. (2006). Intensity of exercise is associated with bone density change in premenopausal women. Osteoporos. Int. 17 (3), 455–463. doi:10.1007/s00198-005-0005-x
Verschueren S. M., Roelants M., Delecluse C., Swinnen S., Vanderschueren D., Boonen S. (2004). Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: A randomized controlled pilot study. J. Bone Min. Res. 19 (3), 352–359. doi:10.1359/JBMR.0301245
Keywords: resistance training, bone mineral density, postmenopausal, meta analysis, protocol
Citation: Wang Z, Zan X, Li Y, Lu Y, Xia Y and Pan X (2023) Comparative efficacy different resistance training protocols on bone mineral density in postmenopausal women: A systematic review and network meta-analysis. Front. Physiol. 14:1105303. doi: 10.3389/fphys.2023.1105303
Received: 22 November 2022; Accepted: 23 January 2023;
Published: 07 February 2023.
Edited by:
Anjali P. Kusumbe, University of Oxford, United KingdomReviewed by:
Dario Calafiore, Azienda Socio Sanitaria Territoriale di Mantova, ItalyCopyright © 2023 Wang, Zan, Li, Lu, Xia and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjie Li, eWpsMjAyMTAyMDFAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.