- 1College of Plant Protection, MARA Key Laboratory of Soybean Disease and Pest Control, Jilin Agricultural University, Changchun, China
- 2MARA-CABI Joint Laboratory for Bio-safety, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China
- 3Institute of Entomology, College of Life Sciences, Nankai University, Tianjin, China
- 4The New Zealand Institute for Plant and Food Research Limited, Auckland Mail Centre, Auckland, New Zealand
- 5Hubei Engineering Technology Center for Forewarning and Management of Agricultural and Forestry Pests, Institute of Entomology, College of Agriculture, Yangtze University, Jingzhou, China
Introduction: The genus Trissolcus includes a number of egg parasitoids that are known to contribute to the control of Halyomorpha halys. The number of progenies, particularly females, is important for the efficient mass rearing of species used in augmentative biological control programs. Cold storage is an important technique for extending the shelf life of natural enemies used in such programs.
Methods: We assessed how fecundity, sex ratio, lifespan, and the number of hosts parasitized within 24 h were affected by host density for T. japonicus and T. cultratus when offered fresh H. halys eggs and how these parameters were affected if adult parasitoids were first placed in cold storage (11°C in the dark) for 19 weeks before being used for propagation.
Results: The fecundity were 110.2 and 84.2 offspring emerged at 25°C, for parasitoids not placed in cold storage; among the offspring that emerged, 82.6% and 85.6% were female for T. japonicus and T. cultratus, respectively. If first placed in cold storage, T. japonicus and T. cultratus produced 35.1 and 24.6 offspring per female, respectively, although cold storage significantly extended the shelf life. The survival rates of parasitoids that were placed in cold storage were 90.3% and 81.3% for females, and 3.2% and 0.9% for males of T. japonicus and T. cultratus, respectively. The number of hosts parasitized within 24 h was not shown to be density dependent, but it was significantly lower after cold storage.
Discussion: This information can be used to estimate the likely production for augmented rearing colonies for use in biological control programs.
1 Introduction
The brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), is an invasive and polyphagous pest native to China, Japan, and Korea (Lee et al., 2013). Invasive populations of H. halys have been detected in North America (Hoebeke and Carter, 2003), Europe (Wermelinger et al., 2008), and South America (Faúndez and Rider, 2017). Adults and nymphs of H. halys feed on economically important crops, including vegetables, fruits, and ornamental trees. The most serious damage is to a number of fruits in crops, such as apple and peach (Acebes-Doria et al., 2016), pear (Bariselli et al., 2016), and kiwifruit (Bernardinelli et al., 2017). This stink bug has been reported to cause losses of 30%–90% in pears and 50% in peaches in China, which are within the insect’s native range (Zhao et al., 2019). Because conventional insecticide management of this pest can have negative impacts on the environment (Goff and Giraudo, 2019), the use of environment-friendly control measures based on integrated pest management (IPM) practices is desirable, including the use of egg parasitoids.
Biological control is generally regarded as an environmentally safe method of pest management that aims to reduce targeted pest populations below economic threshold levels by the use of natural enemies (Waage and Greathead, 1988). Natural enemies of H. halys include parasitoids and predators. Investigations in Asia, North America, and Europe have focused on native and exotic natural enemies of H. halys, especially egg parasitoids. Three families of hymenopteran parasitoids have been found to attack H. halys eggs: Scelionidae (Telenomus spp., Trissolcus spp., and Gryon spp.), Eupelmidae (Anastatus spp.), and Encyrtidae (Ooencyrtus spp.) (Abram et al., 2017; Zhang et al., 2017). The relative prevalence of different parasitoid species associated with H. halys eggs seems to be habitat dependent. For example, Telenomus podisi Ashmead is the most abundant species in field crops and vegetable crops, while Anastatus spp. and Trissolcus spp. dominate in ornamentals, forests, and seminatural or urban habitats (Abram et al., 2017). Trissolcus japonicus has been identified as the most common parasitoid species attacking H. halys, and it is regarded as the most promising natural enemy for classical biological control of H. halys (Lara et al., 2019). Adventive populations of T. japonicus occur in the United States (Leskey and Nielsen, 2018), Italy (Peverieri et al., 2018), Switzerland (Stahl et al., 2019), and Canada (Abram et al., 2019; Gariepy and Talamas, 2019). Trissolcus cultratus is another common egg parasitoid of H. halys in China (Yang et al., 2009; Yang et al., 2015), with mean parasitism rates of 84.2% in laboratory choice tests, a level that was not significantly different from that of T. japonicus (94.1%) (Yang et al., 2015). However, a Swiss population of T. cultratus was found to be unable to attack fresh egg masses of H. halys. The Swiss population of this parasitoid is believed to be a different geographical strain of T. cultratus from that found attacking H. halys in China and other parts of the bug’s native range and, therefore, not co-evolved with H. halys (Haye et al., 2021). Anyhow, T. cultratus is still under consideration as a parasitoid that could contribute to the control H. halys in the long-term (Haye et al., 2015). A recent study looking at the abundance and diversity of egg parasitoids of H. halys in kiwifruit orchards in China showed that parasitism rates by T. cultratus and T. japonicus were very similar (Avila et al., 2021). This finding adds further support that parasitoid composition depends on the habitat type and suggests that T. cultratus is also a potentially important biological control agent of H. halys.
In the development of augmentative biocontrol programs, cold storage is commonly used to stockpile hosts and natural enemies (Colinet and Boivin, 2011; Rathee and Ram, 2018), which can be used to maximize the number of parasitoids available at a given time (Leopold, 2019). Initial studies reported that T. japonicus can be reared on previously frozen (−80°C) H. halys egg masses (Haye et al., 2015). However, further research found both lethal and sublethal negative effects on parasitism and the production of offspring when using frozen H. halys egg masses to rear T. japonicus. The use of frozen eggs reduced the number of wasps produced by 56%–65% and increased production time due to slow development compared to fresh eggs (Mcintosh et al., 2019). Parasitism rates and progeny production of T. japonicus were higher when host eggs offered for parasitism had been stored at higher temperatures. For example, parasitoid progeny production was higher using host eggs stored at 6°C than that of eggs stored at minus 24°C for up to 2 months prior to exposure to parasitism (Bittau et al., 2021). Similarly, parasitoid emergence from egg masses refrigerated at 8°C for up to 2 months before parasitism was higher than that from frozen egg masses (Wong et al., 2021). A recent study conducted by Cira et al. (2021) reported high mortality for all immature ages of T. japonicus when stored at various cold temperatures and for various times, and only adults showed high levels of survival. Qiu (2007) reported that the survival rate of female T. japonicus was approximately 90% when stored at 11°C for 19 weeks, and Bittau et al. (2021) reported survival rates of 87.1% when T. japonicus adults were stored at 16°C for 12 weeks. Longer duration cold storage of adult Trissolcus seems to be the best way to accumulate quantities of this parasitoid and extend the product shelf life of parasitoids reared for field releases. However, little is known about the implications of cold storage for the whole-life fecundity of T. japonicus or T. cultratus adults. High parasitoid fecundity is important for efficient mass rearing and development of effective biological control programs (Gündüz and Gülel, 2005).
In this study, we compared the survival rate, lifetime fecundity, sex ratio, and the number of hosts parasitized within 24 h (on different host densities) of T. japonicus and T. cultratus before and after being cold stored at 11°C for 19 weeks.
2 Materials and methods
2.1 Halyomorpha halys rearing
The H. halys laboratory colony was established using adults collected from an organic kiwifruit orchard located at the Northwest Agriculture and Forestry University kiwifruit experimental field station (34°07′27″N; 107°59′31″E) in Mei County, Baoji city, Shaanxi Province, China. The colony was continuously reared on green beans (Phaseolus vulgaris L.) and corn (Zea mays L.) in gauze cages (60 × 60 × 60 cm3) and kept in the laboratory at a constant climatic condition 25°C ± 1°C, 60% ± 1% RH, and a 16:8 h L:D cycle. Food was changed every 3 to 4 days. Newly laid eggs were collected from the cages daily and maintained under the same conditions in separate rearing cages or provided to parasitoids.
2.2 Parasitoid rearing
Trissolcus japonicus and T. cultratus colonies were started from field collections of parasitized H. halys eggs conducted in the same kiwifruit orchard where the H. halys were collected in Mei County, Baoji city, Shaanxi Province. Both populations were reared separately in transparent acrylic rearing cages (25 × 25 × 25 cm3). Pure honey soaked into cotton wicks was provided weekly for nutrition. For colony production, adult females of either T. japonicus or T. cultratus were provided with fresh H. halys egg masses (preliminary data showed eggs 1 and 3 days old were not distinguished, but fewer eggs were parasitized if host eggs were 5 days old (Yang et al., 2018), and parasitoids were allowed to parasitize eggs for 2 days. After the exposure period, parasitized egg masses were removed from the cages and reared individually per egg mass in a Petri dish (d = 5 cm) at 25°C ± 1°C, 60% ± 1% RH, and a 16:8 h L:D photoperiod. Egg masses were checked daily for parasitoid emergence. Once female parasitoids emerged (day 1), they were placed with males for 3 days to mate, and pure honey was provided for adult feeding. Identification of individuals from newly established laboratory colonies of T. japonicus and T. cultratus was verified by E. Talamas (Systematic Entomology Laboratory, USDA/ARS c/o NMNH, Smithsonian Institution, Washington DC.)
2.3 Cold storage of T. japonicus and T. cultratus
All the mated (males that emerged ahead by one or two days had to wait for females to emerge, and they mated at once when they met during 3 days of mixed rearing) 3-day-old female and male parasitoids were grouped based on each egg mass and placed in a 10-mL tube (16 × 81 mm), and stored in an incubator (Ningbo Haishu Saifu Experimental Instrument Factory, SPX-250, Ningbo city, Zhejiang province) at 11°C ± 1°C, 60% ± 1% RH, and continuous darkness for 19 weeks (133 days). All groups were checked weekly for mortality, and honey droplets in tubes were renewed weekly. The same procedure was carried out for each parasitoid species. The total number was 582 (30 tubes) T. japonicus and 563 (28 tubes) T. cultratus for cold storage.
2.4 Fecundity bioassays of T. japonicus and T. cultratus
Fecundity trials were conducted with parasitoids under ambient (non-cold storage) rearing conditions (25°C ± 1°C, 60% ± 5% RH, and 16 h light). Three-day-old mated female parasitoids were randomly selected and placed individually in Petri dishes (d = 5 cm). Honey was provided for adult nutrition, and H. halys egg masses (each egg mass of around 28 eggs) were provided for parasitoid oviposition. Egg masses were replaced every 2 days: three egg masses for the first three consecutive times, then one egg mass was provided from the fourth time to refresh host eggs until the female wasp died. Exposed egg masses were placed and reared individually in Petri dishes, under laboratory conditions (as described above) until all eggs produced either parasitoid progeny or stink bug nymphs. In total, 23 replicates (one female parasitoid as one replicate) were conducted for T. japonicus and 26 for T. cultratus. The numbers of emerging parasitoids or H. halys nymphs were recorded. Unhatched eggs were dissected under a stereomicroscope and classified as dead immature parasitoids, dead H. halys nymphs, or failed eggs (i.e., nothing found). The proportion of adult parasitoid progeny that were female was calculated, and the average oviposition period and longevity (from parasitoids emergence to death) at 25°C ± 1°C, 60% ± 5% RH, and 16 h light conditions were also recorded. The parasitism rate was defined as (number of emerged parasitoids + number of dead parasitoids within host egg)/total number of H. halys eggs × 100. The rate of nymph was defined as (number of emerged nymphs + number of dead nymphs within host egg)/total number of H. halys eggs × 100. The rate of egg mortality was defined as number of dead eggs/total number of H. halys eggs × 100.
Parasitoid fecundity after cold storage was assessed using the same methods as described for wasps not subject to cold storage. Wasps tested were female parasitoids that had survived 19 weeks of cold storage period. These females were allowed 2 days to adapt to warm ambient conditions before testing. At least ten replicates (one female as a replicate) were run for T. japonicus and T. cultratus.
2.5 Effect of host numbers on the per-female rate of oviposition
Three-day-old mated females of T. japonicus and T. cultratus were offered different numbers of H. halys egg masses (1, 2, 3, 4, or 5, each with 26–28 eggs). Females (one female per Petri dish) were allowed to oviposit for 24 h at 25°C and were then removed from Petri dishes (d = 5 cm). Exposed egg masses were held and reared individually in Petri dishes, under laboratory conditions (as described above), until all eggs produced either parasitoid progeny, live stink bug nymphs, or failed. The numbers of emerging parasitoids or H. halys nymphs were recorded. Failed eggs were dissected under a stereomicroscope and classified as immature parasitoids, dead H. halys nymphs, or failed eggs (i.e., nothing found). Ten replicates were conducted for each treatment. The per-female rate of oviposition was defined as the number of emerged parasitoids + immature parasitoids.
Trissolcus japonicus and T. cultratus, after cold storage for 19 weeks, were also parallely tested, and the female rate of oviposition depends on five host densities. The method was the same as that mentioned previously. Three to nine replicates were conducted for each treatment.
2.6 Data analysis
Differences in lifetime fecundity (i.e., number of parasitoids that emerged), the duration of the oviposition period and the parasitoid lifespan, the female proportion of parasitoid offspring, parasitism rate, rate of nymph, rate of egg mortality, and female oviposition rate were compared between the two parasitoids species (T. japonicus and T. cultratus), and treatments (control wasps and one subjected to cold storage) were analyzed using GLM with Poisson distribution followed by LSD post-hoc tests. All statistical analyses were carried out using SPSS 21.0 statistical software. All figures were made using Origin 2022 software.
3 Results
3.1 Fecundity parameters of T. japonicus and T. cultratus under ambient temperature (25°C) and after cold storage
In tests conducted with parasitoids reared under ambient temperature conditions (25°C), a total of 12,776 and 11,122 host eggs were provided to T. japonicus (n = 23) and T. cultratus (n = 26), respectively, to assess the number of parasitoids produced over their lifetime. An average of 555.5 ± 12.4 or 427.8 ± 7.5 fresh H. halys eggs were offered to each T. japonicus and T. cultratus female, respectively. The average number of parasitoids that emerged from T. japonicus per parental female was 110.2 ± 6.6, while for T. cultratus, it was 84.2 ± 3.2. The former was significantly higher (GLM χ2 = 84.957, df = 1, p < 0.001) (Figure 1).
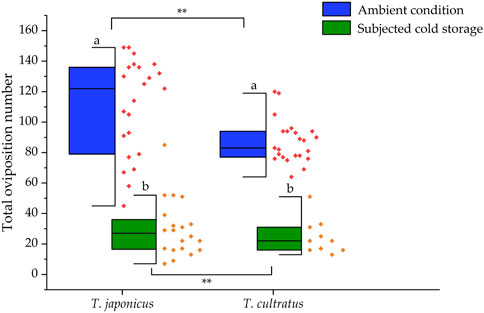
FIGURE 1. Number of parasitoids produced per parental female of T. japonicus and T. cultratus placed under ambient (25°C) versus subjected to cold storage. Asterisks represent significant differences between T. japonicus and T. cultratus within the same treatment (**p < 0.01). Different letters represent significant differences between ambient temperature (25°C) and subjected to cold storage conditions within each parasitoid species (p < 0.05).
For tests conducted with female adult parasitoids that survived from cold storage (at 11°C for 19 weeks), a total of 7,171 and 7,327 host eggs were offered to T. japonicus (n = 10) and T. cultratus (n = 10), respectively. The number of parasitoids produced per parental females (surviving cold storage) of T. japonicus was 35.1 ± 7.4, which was a significant decrease of 68.1% compared to the control (GLM χ2 = 403.379, df = 1, p < 0.001). Similarly, the number of parasitoids produced per parental female of T. cultratus was 24.6 ± 3.6, which was a significant decrease of 70.8% compared to the control (females not subject to cold storage) (GLM χ2 = 334.768, df = 1, p < 0.001). There were significant differences in the number of parasitoids produced by T. japonicus versus T. cultratus after cold storage (GLM χ2 = 18.274, df = 1, p < 0.001) (Figure 1).
At ambient conditions (25°C), there was no significant difference between T. japonicus and T. cultratus in the parasitism rate (GLM χ2 = 0.231, df = 1, p = 0.630), rate of nymphal (GLM χ2 = 1.767, df = 1, p = 0.184), or the rate of egg mortality (GLM χ2 = 3.636, df = 1, p = 0.057) (Table 1).
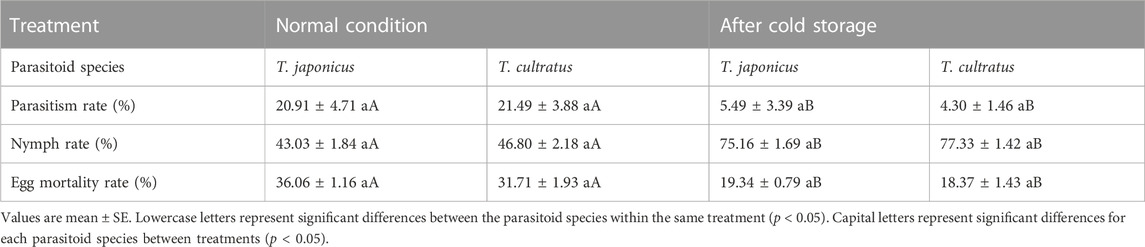
TABLE 1. Parameters for T. japonicus and T. cultratus when reared under normal conditions (25°C) and after a cold storage period of 19 weeks at constant 11°C.
However, after a cold storage period, both species of parasitoids experienced a significant decrease in their parasitism rates (T. japonicus GLM χ2 = 92.361, df = 1, p < 0.001; T. cultratus GLM χ2 = 193.876, df = 1, p < 0.001) and the egg mortality rate (T. japonicus GLM χ2 = 87.723, df = 1, p < 0.001; T. cultratus GLM χ2 = 17.706, df = 1, p < 0.001) compared to those in the ambient condition (25°C). Conversely, the nymph rate increased significantly for both parasitoid species after cold storage (T. japonicus GLM χ2 = 120.616, df = 1, p < 0.001; T. cultratus GLM χ2 = 74.036, df = 1, p < 0.001) (Table 1).
Between T. japonicus and T. cultratus after cold storage, there were no significant differences in the parasitism rate (GLM χ2 = 1.163, df = 1, p = 0.281), the nymph rate (GLM χ2 = 1.068, df = 1, p = 0.301), and egg mortality rate (GLM χ2 = 0.391, df = 1, p = 0.532) (Table 1).
3.2 Proportion of female parasitoids
Under ambient rearing conditions, the proportion of female parasitoids produced was 82.6% ± 1.6% and 85.6% ± 1.2% for T. japonicus and T. cultratus, respectively. After cold storage, the proportion of females parasitoids was 78.0% ± 3.9% of T. japonicus, which was not significantly different from wasps reared under ambient conditions (GLM χ2 = 1.769, df = 1, p = 0.183). However, the percentage of females produced by T. cultratus in the offspring was 72.4% ± 4.2%, which is significantly lower after cold storage than wasps reared under ambient conditions (GLM χ2 = 18.976, df = 1, p < 0.001) (Figure 2).
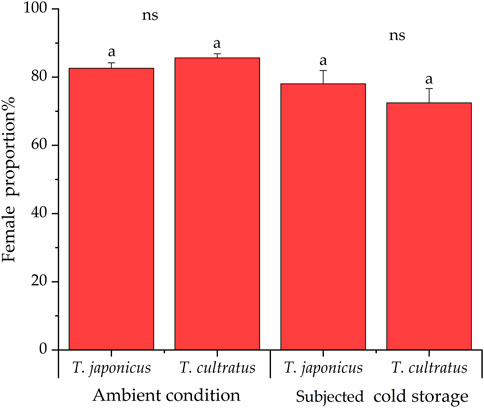
FIGURE 2. Proportion of females produced by T. japonicus and T. cultratus under ambient rearing conditions (25°C) and after cold storage for 19 weeks at 11°C for offspring. Same marked by lowercase letters represent no significant differences for each parasitoid species between ambient and after cold storage (p > 0.05), and ns denotes no significant difference between T. japonicus and T. cultratus within the same treatment.
3.3 Oviposition number variation during lifetime of T. japonicus and T. cultratus
At ambient rearing conditions (25°C), the mean oviposition duration was 21.5 ± 0.9 days for T. japonicus, which was significantly different (GLM χ2 = 53.627, df = 1, p < 0.001) from that for T. cultratus (12.8 ± 0.40 days). The number of ovipositions decreased with female age, and the highest level of oviposition was on the first day of access of new parasitoids to hosts when there were 29.5 ± 2.1 eggs/female/day for T. japonicus and 25.1 ± 1.4 eggs for T. cultratus (Figure 3).
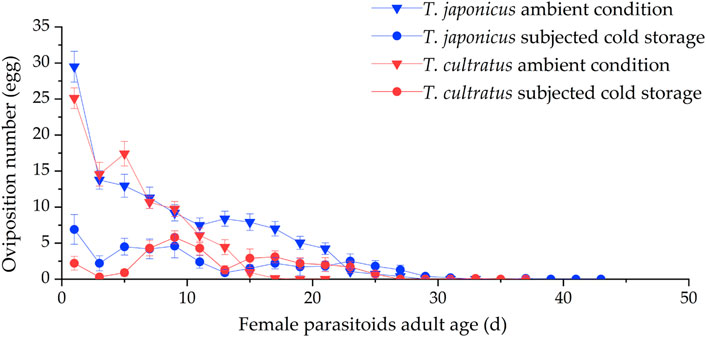
FIGURE 3. Variation in number of ovipositions by T. japonicus and T. cultratus under ambient rearing condition (25°C) and after cold storage for 19 weeks at 11°C. Values are the mean ± SE.
After cold storage, the mean oviposition duration was 24.4 ± 2.0 days for T. japonicus and 17.8 ± 1.9 days for T. cultratus. Oviposition occurred from the first day that parasitoids were exposed to host eggs. The oviposition duration was significantly different between the two species after cold storage (GLM χ2 = 10.237, df = 1, p < 0.01) (Figure 3).
3.4 Effect of cold storage on lifespan of T. japonicus and T. cultratus
Mean lifespan values for T. japonicus and T. cultratus females under ambient rearing conditions (25°C) were 30.7 ± 0.7 and 18.9 ± 0.5 days, respectively, which differed significantly between the two species (GLM χ2 = 67.848, df = 1, p < 0.001). Adults of both parasitoids were still alive and fertile after 19 weeks of cold storage. The lifespan (period from parasitoid emergence to death) showed no significant difference between the two parasitoid species after cold storage (GLM χ2 = 1.908, df = 1, p = 0.167) (Figure 4).

FIGURE 4. Mean lifespan of T. japonicus and T. cultratus when under ambient rearing conditions (25°C) and extended by cold storage. Values are means ± SE. Asterisks represent statistically significant differences, and ns present no significant difference between T. japonicus and T. cultratus within the same treatment (***p < 0.001).
3.5 Survival rates of parasitoids during cold storage
The survival rates declined during cold storage for both parasitoid species (Figure 5). However, survival rates for females were dramatically higher than survival rates of males (T. japonicus GLM χ2 = 883.283, df = 1, p < 0.001; T. cultratus GLM χ2 = 612.088, df = 1, p < 0.001), with mean female survival rates of 90.3% ± 1.6% and 81.3% ± 3.1% for T. japonicus and T. cultratus after 19 weeks of cold storage, respectively. Male survival rates, in contrast, were very low after cold storage, being 3.2% ± 2.5% and 0.9% ± 0.9% for T. japonicus and T. cultratus, respectively (Figure 5).
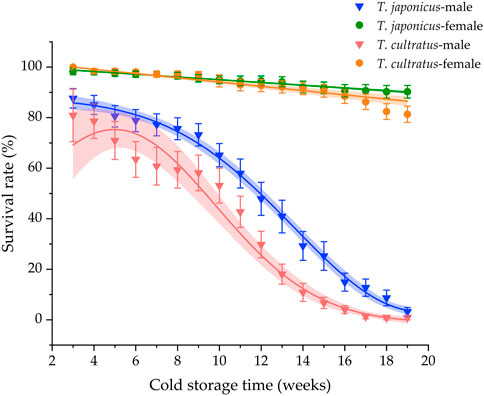
FIGURE 5. Survival rate of T. japonicus and T. cultratus during 19 weeks of cold storage at 11°C. Values are mean ± SE.
3.6 Oviposition number within 24 h at different host densities
At the normal condition, the average oviposition numbers were 27.4 ± 0.40, 23.0 ± 2.61, 22.5 ± 1.84, 23.2 ± 2.56, and 26.0 ± 4.63 eggs when providing one to five egg masses to T. japonicus, and there were no significant differences between the different densities tested (GLM χ2 = 3.321, df = 4, p = 0.506) (Figure 6A). The oviposition rates of T. cultratus were 26.6 ± 0.68, 22.7 ± 4.16, 25.6 ± 4.72, 23.9 ± 4.79, and 26.3 ± 5.11 eggs at five host densities tested, and no differences were observed between different densities (GLM χ2 = 0.924, df = 4, p = 0.921) (Figure 6B).
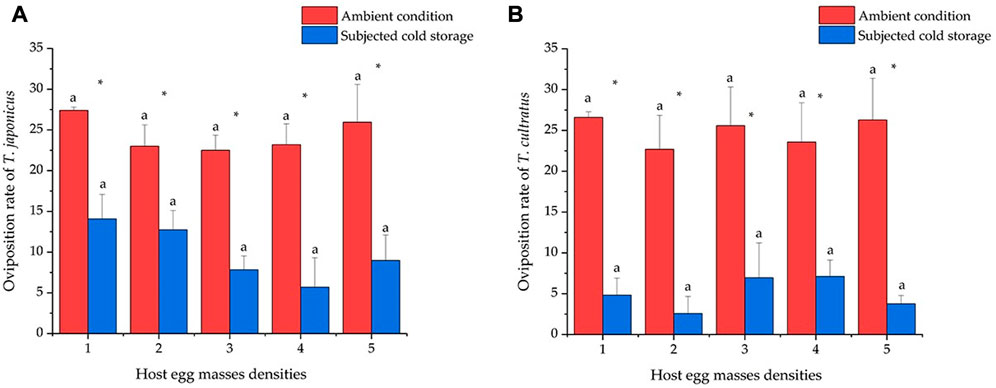
FIGURE 6. Daily per female oviposition rate of T. japonicus (6A) and T. cultratus (6B) when individual females were offered one to five host egg masses. Values are means ± SE. Asterisks represent statistically significant differences between wasps placed in ambient conditions (25°C) and wasps subjected to 19 weeks of cold storage (11°C) at the same host density level. Lowercase letters represent no significant difference between different host densities by each parasitoid species (p > 0.05).
The oviposition rate significantly decreased after cold storage for both species of parasitoids. The oviposition rates of T. japonicus were 14.1 ± 3.01, 12.7 ± 2.37, 7.8 ± 1.69, 5.7 ± 3.60, and 9.0 ± 3.11 eggs for the five host densities, which did not differ significantly (GLM χ2 = 7.871, df = 4, p = 0.096) (Figure 6A). The oviposition rates of T. cultratus were 4.8 ± 2.07, 2.6 ± 2.09, 7.0 ± 4.25, 7.1 ± 1.99, and 3.8 ± 1.02 eggs for the five host densities and did not differ significantly (GLM χ2 = 3.299, df = 4, p = 0.509) (Figure 6B).
Comparisons for the number of oviposition within 24 h between two species after cold storage show that the number of oviposition by T. japonicus was significantly higher than T. cultratus at low host egg densities, one egg mass (GLM χ2 = 7.944, df = 1, p < 0.01), and two egg masses (GLM χ2 = 11.168, df = 1, p < 0.001). No significant differences were found between the two species of parasitoids when provided with three, four, and five host egg masses.
4 Discussion
High fecundity and a female-biased progeny sex ratio are important factors for efficient mass rearing to support programs of augmentative biological control (Gündüz and Gülel, 2005). Egg parasitoids in the genera Trissolcus are well known as control agents of H. halys, especially T. japonicus, whose release has been widely recommended to control H. halys in field crops, both in its native and adventive ranges. For example, Mi et al. (2017) reported releases of T. japonicus and Anastatus sp. for suppression of H. halys in kiwifruit orchards in China. T. japonicus was intentionally released across four eco-regions in Oregon (United States) for investigation of its dispersal capacity (Lowenstein et al., 2019) as part of a classical biocontrol program in North America (Leskey and Nielsen, 2018). Based on a risk analysis of T. japonicus, a petition for its release in the field was approved in 2020, with plans to rear and release over 120,000 female T. japonicus in Italy (Decreto, 2020; Conti et al., 2021). Research on the fecundity of T. japonicus and its cold storage supports the logistics accumulation of enough parasitoids to support field release on such large scales. We found that when the parasitoids T. japonicus and T. cultratus were reared at 25°C, their lifetime fecundities, respectively, were 110.2 and 84.2, and the proportion female values in their progeny were 82.6% and 85.6%. In addition, we found that the female oviposition number was 22.5 to 27.4 for T. japonicus and 22.7 to 26.2 for T. cultratus within 24 h on various host densities. We deduced that T. cultratus did not perform as well as T. japonicus as a potential biological control agent against H. halys based on these fecundity and related factors.
The numbers of ovipositions on the first day of life after access to hosts were the highest for both T. japonicus (29.5) and T. cultratus (25.1). This suggests that one egg mass of H. halys (about 28 eggs) per day would be sufficient to support the daily oviposition of T. japonicus or T. cultratus. Subsequently, daily oviposition declined with female age. Age-related declines in parasitoid fecundity were similarly noted Sabbatini-Peverieri et al. (2020) for T. japonicus and T. mitsukurii.
T. japonicus are ideally reared on fresh newly laid unfertilized H. halys eggs, which leads to the highest parasitoid development rate and fecundity (Gündüz and Gülel, 2005). However, mass-rearing facilities are often constrained by the limited availability of such eggs. Cold storage is an important method for extending the shelf life of parasitoids used as biological control agents (Bittau et al., 2021). Cold storage of each life stage of T. japonicus has been studied, and immature parasitoid development on H. halys eggs was clearly described (Giovannini et al., 2021). The eggs and larvae of Trissolcus halyomorphae (syn T. japonicus) could not tolerate storage temperature at either 7 or 11°C. Storage of pupae was feasible, but storage of adult females was optimal, with adult survival being 90% (Qiu, 2007), making cold storage of adults a viable method to extend parasitoid lifespan and match the timing of T. japonicus production with release needs (Cira et al., 2021). Storage of adults of T. japonicus at 8°C and short photoperiod (8L:14D) resulted in lower realized fertility of stored females and fewer female progeny than those in storage at 13°C and 18°C (Gündüz and Gülel, 2005). Lee and Denlinger (2010) reported that low temperatures affected insects differently based on the severity of the cold and the duration of exposure. Photoperiod during storage can also affect parasitoid fitness but was not investigated in our present study. In our study, female parasitoid adults were stored at 11°C for nearly 5 months (19 weeks) in darkness, and those conditions reduced fecundity by 31.9% for T. japonicus and 29.2% for T. cultratus compared to rearing at 25°C without cold storage. We deduced that T. cultratus did not perform as well as T. japonicus as a potential biological control agent against H. halys based on these fecundity and related factors. One more positive aspect is that the proportion of female offspring was not low level, 78.0% and 72.4% for T. japonicus and T. cultratus after storage. So, we deduced that the triple number of storage T. japonicus would be of equal efficiency with the non-cold stored one, both for calculating accumulation numbers during mass rearing and parasitism in field biological control.
For male parasitoids of both species, survival rates declined quickly after 9 weeks of storage, suggesting that 9 weeks may be a better storage time for stockpiling parasitoids at 11°C. The general pattern of high survival rates for females of T. japonicus and T. cultratus and low survival rates for males, after 19 weeks of cold storage, agrees with results from previous studies (Qiu, 2007). Further research is warranted to assess additional combinations of cold storage temperatures, storage duration, and light regimes to determine the optional condition for stockpiling T. japonicus and T. cultratus without a significant decline in the reproductive rate.
5 Conclusion
Lifetime fecundity of T. japonicus and T. cultratus were 110.2 and 84.2, respectively, when reared at 25°C, 16L:8D, 60% ± 1% RH (ambient conditions). The lifespan of both species was significantly extended by cold storage at 11°C for 19 weeks. Both parasitoid species remained fertile after cold storage, although the lifetime fecundity was significantly reduced to around one-third of that of wasps not subjected to cold storage. Under ambient conditions (no cold storage), both wasp species laid about 28 eggs within 24 h of contact with hosts, but this value was much lower (less than 14.1) after cold storage.
Data availability statement
The original contribution presented in the study is included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
J-PZ, S-SS, FZ, and W-JL conceived and designed the experiments. W-JL, J-HC, X-YT, and Z-YL conducted experiments. W-JL, J-HC, and M-YA analyzed data. GA, J-PZ, and S-SS wrote the original draft. FZ, M-YA, and GA reviewed and edited the manuscript. All authors read and approved the manuscript.
Funding
This research was funded by China’s donation to the CABI Development Fund, grant number VM10051.
Acknowledgments
CABI is an international intergovernmental organization and we gratefully acknowledge the core financial support from our member countries (and lead agencies) including the United Kingdom (Department for International Development), China (Chinese Ministry of Agriculture and Rural Affairs), Australia (Australian Centre for International Agricultural Research), Canada (Agriculture and Agri-Food Canada), the Netherlands (Directorate-General for International Cooperation), and Switzerland (Swiss Agency for Development and Cooperation). See https://www.cabi.org/about-cabi/whowe-work-with/key-donors/ for full details.
Conflict of interest
GA was employed by The New Zealand Institute for Plant and Food Research Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abram, P. K., Hoelmer, K. A., Acebes-Doria, A., Andrews, H., Beers, E. H., Bergh, J. C., et al. (2017). Indigenous arthropod natural enemies of the invasive Brown marmorated stink bug in North America and Europe. J. Pest Sci. 90, 1009–1020. doi:10.1007/s10340-017-0891-7
Abram, P. K., Talamas, E. J., Acheampong, S., Mason, P. G., and Gariepy, T. D. (2019). First detection of the samurai wasp, Trissolcus japonicus (Ashmead) (hymenoptera, Scelionidae), in Canada. J. Hymenoptera Res. 68, 29–36. doi:10.3897/jhr.68.32203
Acebes-Doria, A. L., Leskey, T. C., and Bergh, J. C. (2016). Injury to apples and peaches at harvest from feeding by Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) nymphs early and late in the season. Crop Prot. 89, 58–65. doi:10.1016/j.cropro.2016.06.022
Avila, G. A., Chen, J., Li, W., Alavi, M., Mi, Q., Sandanayaka, M., et al. (2021). Seasonal abundance and diversity of egg parasitoids of Halyomorpha halys in kiwifruit orchards in China. Insects 12, 428. doi:10.3390/insects12050428
Bariselli, M., Bugiani, R., and Maistrello, L. (2016). Distribution and damage caused by Halyomorpha halys in Italy. Eppo Bull. 46, 332–334. doi:10.1111/epp.12289
Bernardinelli, I., Malossini, G., and Benvenuto, L. (2017). Halyomorpha halys: Risultati preliminari di alcune attività sperimentali condotte in Friuli Venezia Giulia nel 2016. Not. Ersal 1, 24–26.
Bittau, B., Dindo, M. L., Burgio, G., Sabbatini-Peverieri, G., Hoelmer, K. A., Roversi, P. F., et al. (2021). Implementing mass rearing of Trissolcus japonicus (hymenoptera: Scelionidae) on cold-stored host eggs. Insects 12, 840. doi:10.3390/insects12090840
Cira, T., Santacruz, E. N., and Koch, R. L. (2021). Optimization of Trissolcus japonicus cold storage methods for biological control of Halyomorpha halys. Biol. Control 156, 104534. doi:10.1016/j.biocontrol.2021.104534
Colinet, H., and Boivin, G. (2011). Insect parasitoids cold storage: A comprehensive review of factors of variability and consequences. Biol. Control 58, 83–95. doi:10.1016/j.biocontrol.2011.04.014
Conti, E., Avila, G., Barratt, B., Cingolani, F., Colazza, S., Guarino, S., et al. (2021). Biological control of invasive stink bugs: Review of global state and future prospects. Entomologia Exp. Appl. 169, 28–51. doi:10.1111/eea.12967
Decreto (2020). Immissione in natura della specie non autoctona Trissolcus japonicus quale Agente di Controllo Biologico del fitofago Halyomorpha halys ai sensi del Decreto del Presidente della Repubblica 8 settembre 1997. del 9 giugno 42967n. 357, art. 12. 2020.
Faúndez, E. I., and Rider, D. A. (2017). The Brown marmorated stink bug Halyomorpha halys (Stål, 1855) (Heteroptera: Pentatomidae) in Chile. Arq. Entomolóxicos 17, 305–307.
Gariepy, T. D., and Talamas, E. J. (2019). Discovery of Trissolcus japonicus (hymenoptera: Scelionidae) in ontario, Canada. Can. Entomologist 151, 824–826. doi:10.4039/tce.2019.58
Giovannini, L., Sabbatini-Peverieri, G., Tillman, P. G., Hoelmer, K. A., and Roversi, P. F. (2021). Reproductive and developmental biology of Acroclisoides sinicus, a hyperparasitoid of scelionid parasitoids. Biology 10 (3), 229. doi:10.3390/biology10030229
Goff, G. L., and Giraudo, M. (2019). “Effects of pesticides on the environment and insecticide resistance,” in Olfactory concepts of insect control-alternative to insecticides. Editor J. F. Picimbon (Cham: Springer), 51–78.
Gündüz, E. A., and Gülel, A. (2005). Investigation of fecundity and sex ratio in the parasitoid Bracon hebetor Say (Hymenoptera: Braconidae) in relation to parasitoid age. Turkish J. Zoology 29, 291–294.
Haye, T., Fischer, S., Zhang, J., and Gariepy, T. (2015). Can native egg parasitoids adopt the invasive Brown marmorated stink bug, Halyomorpha halys (Heteroptera: Pentatomidae), in Europe? J. Pest Sci. 88, 693–705. doi:10.1007/s10340-015-0671-1
Haye, T., Zhang, J. P., Risse, M., and Gariepy, T. (2021). A temporal trophic shift from primary parasitism to facultative hyperparasitism during interspecific competition between two co-evolved scelionid egg parasitoids. Ecol. Evol. 11, 18708–18718. doi:10.1002/ece3.8483
Hoebeke, E. R., and Carter, M. E. (2003). Halyomorpha halys (stål) (heteroptera: Pentatomidae): A polyphagous plant pest from Asia newly detected in North America. Proc. Entomological Soc. Wash. 105, 225–237.
Lara, J. R., Pickett, C. H., Kamiyama, M. T., Figueroa, S., Romo, M., Cabanas, C., et al. (2019). Physiological host range of Trissolcus japonicus in relation to Halyomorpha halys and other pentatomids from California. BioControl 64, 513–528. doi:10.1007/s10526-019-09950-4
Lee, D. H., Short, B. D., Joseph, S. V., Bergh, J. C., and Leskey, T. C. (2013). Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ. Entomol. 42, 627–641. doi:10.1603/EN13006
Lee, R., and Denlinger, D. (2010). “Rapid cold-hardening: Ecological significance and underpinning mechanisms,” in Low temperature biology of insects. Editors D. L. Denlinger, and R. E. Lee 2nd ed (Cambridge: Cambridge University Press), 35–58.
Leopold, R. A. (2019). “Cold storage of insects for integrated pest management,” in Temperature sensitivity in insects and application in integrated pest management. Editors G. J. Hallman, and D. L. Denlinger 1st ed (New York: CRC Press), 235–267.
Leskey, T. C., and Nielsen, A. L. (2018). Impact of the invasive Brown marmorated stink bug in North America and Europe: History, biology, ecology, and management. Annu. Rev. Entomology 63, 599–618. doi:10.1146/annurev-ento-020117-043226
Lowenstein, D. M., Andrews, H., Hilton, R. J., Kaiser, C., and Wiman, N. G. (2019). Establishment in an introduced range: Dispersal capacity and winter survival of Trissolcus japonicus, an adventive egg parasitoid. Insects 10 (12), 443. doi:10.3390/insects10120443
Mcintosh, H., Lowenstein, D. M., Wiman, N. G., Wong, J. S., and Lee, J. C. (2019). Parasitism of frozen Halyomorpha halys eggs by Trissolcus japonicus: Applications for rearing and experimentation. Biocontrol Sci. Technol. 29, 478–493. doi:10.1080/09583157.2019.1566439
Mi, Q. Q., Zhang, J. P., Han, Y. X., Yan, Y. C., Zhang, B. X., Li, D. S., et al. (2017). “Releases of Trissolcus japonicus and Anastatus sp. for suppression of Halyomorpha halys in kiwifruit orchards,” in Proceedings of the 5th international symposium on biological control of arthropods. Editors P. G. Mason, D. R. Gillespie, and C. Vincent (Wallingford UKLangkawi, Malaysia: CABI), 297.
Peverieri, G. S., Talamas, E., Bon, M. C., Marianelli, L., Bernardinelli, I., Malossini, G., et al. (2018). Two Asian egg parasitoids of Halyomorpha halys (stål) (Hemiptera, Pentatomidae) emerge in northern Italy: Trissolcus mitsukurii (Ashmead) and Trissolcus japonicus (Ashmead) (hymenoptera, Scelionidae). J. Hymenoptera Res. 67, 37–53. doi:10.3897/jhr.67.30883
Qiu, L. F. (2007). Studies on biology of the brown marmorated stink bug Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), an important pest for pome trees in China and its biological control. Ph.D thesis (Beijing, China: Chinese Academy of Forestry).
Rathee, M., and Ram, P. (2018). Retracted article: Impact of cold storage on the performance of entomophagous insects: An overview. Phytoparasitica 46, 421–449. doi:10.1007/s12600-018-0683-5
Sabbatini-Peverieri, G., Dieckhoff, C., Giovannini, L., Marianelli, L., Roversi, P. F., and Hoelmer, K. (2020). Rearing Trissolcus japonicus and Trissolcus mitsukurii for biological control of Halyomorpha halys. Insects 11, 787. doi:10.3390/insects11110787
Stahl, J., Tortorici, F., Pontini, M., Bon, M. C., Hoelmer, K., Marazzi, C., et al. (2019). First discovery of adventive populations of Trissolcus japonicus in Europe. J. Pest Sci. 92, 371–379. doi:10.1007/s10340-018-1061-2
Waage, J. K., and Greathead, D. J. (1988). Biological control: Challenges and opportunities. Philosophical transactions of the royal society of london. Biol. Sci. 318, 111–128.
Wermelinger, B., Wyniger, D., and Forster, B. (2008). First records of an invasive bug in Europe: Halyomorpha halys stal (heteroptera: Pentatomidae), a new pest on woody ornamentals and fruit trees? Mittl. Entomol. Ges. 81, 1–8.
Wong, W. H., Walz, M. A., Oscienny, A. B., Sherwood, J. L., and Abram, P. K. (2021). An effective cold storage method for stockpiling Halyomorpha halys (Hemiptera: Pentatomidae) eggs for field surveys and laboratory rearing of Trissolcus japonicus (hymenoptera: Scelionidae). J. Econ. Entomology 114, 571–581. doi:10.1093/jee/toaa307
Yang, S. Y., Zhan, H. X., Zhang, F., Babendreier, D., Zhong, Y. Z., Lou, Q. Z., et al. (2018). Development and fecundity of Trissolcus japonicus on fertilized and unfertilized eggs of the Brown marmorated stink bug, Halyomorpha halys. J. Pest Sci. 91, 1335–1343. doi:10.1007/s10340-018-0998-5
Yang, Y. L., Zhong, Y. Z., Zhang, F., Zhou, C. Q., Yang, S. Y., and Zhang, J. P. (2015). Parasitic capacity of Trissolcus halyomorphae and T. flavipes (hymenoptera: Scelionidae) on eggs of Halyomorpha halys. J. Environ. Entomology 6, 1257–1262.
Yang, Z. Q., Yao, Y. X., Qiu, L. F., and Li, Z. X. (2009). A new species of Trissolcus (Hymenoptera: Scelionidae) parasitizing eggs of Halyomorpha halys (Heteroptera: Pentatomidae) in China with comments on its biology. Ann. Entomological Soc. Am. 102, 39–47. doi:10.1603/008.102.0104
Zhang, J. P., Zhang, F., Gariepy, T., Mason, P., Gillespie, D., Talamas, E., et al. (2017). Seasonal parasitism and host specificity of Trissolcus japonicus in northern China. J. Pest Sci. 90, 1127–1141. doi:10.1007/s10340-017-0863-y
Keywords: Halyomorpha halys, biological control, Trissolcus japonicus, Trissolcus cultratus, fecundity, natural enemy, cold storage
Citation: Li W-J, Chen J-H, Avila GA, Ali M-Y, Tian X-Y, Luo Z-Y, Zhang F, Shi S-S and Zhang J-P (2023) Performance of two egg parasitoids of brown marmorated stink bug before and after cold storage. Front. Physiol. 14:1102216. doi: 10.3389/fphys.2023.1102216
Received: 18 November 2022; Accepted: 14 February 2023;
Published: 02 March 2023.
Edited by:
Feng Shang, Southwest University, ChinaCopyright © 2023 Li, Chen, Avila, Ali, Tian, Luo, Zhang, Shi and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu-Sen Shi, c3NzLTYzQDI2My5uZXQ=; Jin-Ping Zhang, ai56aGFuZ0BjYWJpLm9yZw==
 Wen-Jing Li
Wen-Jing Li Ju-Hong Chen2,3
Ju-Hong Chen2,3 Gonzalo A. Avila
Gonzalo A. Avila Muhammad-Yasir Ali
Muhammad-Yasir Ali Feng Zhang
Feng Zhang Jin-Ping Zhang
Jin-Ping Zhang